Aeromonas species can cause severe infections in immunocompromised individuals upon exposure to virulent pathogens in the environment, but the characteristics of environmental Aeromonas species remain unclear. Our study showed that several pathogenic Aeromonas species possessing virulence traits and antimicrobial resistance similar to those of Aeromonas isolates causing clinical diseases were present in fish intended for human consumption in Tainan City, Taiwan.
KEYWORDS: Aeromonas, virulence factors, β-lactam, antimicrobial susceptibility
ABSTRACT
The present study aimed to isolate Aeromonas from fish sold in the markets as well as in sushi and seafood shops and compare their virulence factors and antimicrobial characteristics with those of clinical isolates. Among the 128 fish isolates and 47 clinical isolates, Aeromonas caviae, A. dhakensis, and A. veronii were the principal species. A. dhakensis isolates carried at least 5 virulence genes, more than other Aeromonas species. The predominant genotype of virulence genes was hlyA lip alt col ela in both A. dhakensis and A. hydrophila isolates, alt col ela in A. caviae isolates, and act in A. veronii isolates. A. dhakensis, A. hydrophila, and A. veronii isolates more often exhibited hemolytic and proteolytic activity and showed greater virulence than A. caviae isolates in Caenorhabditis elegans and the C2C12 cell line. However, the link between the genotypes and phenotypes of the studied virulence genes in Aeromonas species was not evident. Among the four major clinical Aeromonas species, nearly all (99.0%) A. dhakensis, A. hydrophila, and A. veronii isolates harbored blaCphA, which encodes a carbapenemase, but only a minority (6.7%, 7/104) were nonsusceptible to carbapenem. Regarding AmpC β-lactamase genes, blaAQU-1 was exclusively found in A. dhakensis isolates, and blaMOX3 was found only in A. caviae isolates, but only 7.6% (n = 6) of the 79 Aeromonas isolates carrying blaAQU-1 or blaMOX3 exhibited a cefotaxime resistance phenotype. In conclusion, fish Aeromonas isolates carry a variety of combinations of virulence and β-lactamase resistance genes and exhibit virulence phenotypes and antimicrobial resistance profiles similar to those of clinical isolates.
IMPORTANCE Aeromonas species can cause severe infections in immunocompromised individuals upon exposure to virulent pathogens in the environment, but the characteristics of environmental Aeromonas species remain unclear. Our study showed that several pathogenic Aeromonas species possessing virulence traits and antimicrobial resistance similar to those of Aeromonas isolates causing clinical diseases were present in fish intended for human consumption in Tainan City, Taiwan.
INTRODUCTION
Aeromonas species are widespread in aquatic creatures (1–4) and have been isolated from a variety of seafood (5, 6), such as in frozen fish ready for human consumption and in market-sold sushi products containing raw fish (7, 8). Moreover, Aeromonas species have been isolated from stool samples obtained from persons with diarrhea (9). However, certain individuals, especially those with biliary diseases or cancers, are at high risk of extraintestinal Aeromonas infections after consumption of contaminated food or associated food products (10, 11).
Fish constitute one of the protein food sources in southern Taiwan, where the prevalence of chronic liver diseases and Aeromonas bacteremia is high (12). In an earlier study conducted in northern Taiwan, Aeromonas isolates were found in 88% of seafood from markets and supermarkets, and among these isolates, 98% of Aeromonas hydrophila isolates and 94% of A. sobria isolates produced β-hemolysin (13). In a survey of virulence markers in A. veronii and A. hydrophila isolates recovered from freshwater fish and human stool samples, this virulence trait was obvious even at 4°C (14), suggesting that the presence of A. hydrophila and A. veronii in ice-stored freshwater fish conferred potential health risks. These results suggest that susceptible individuals consuming fish contaminated by pathogenic Aeromonas species may develop invasive extraintestinal infections, such as bacteremia or biliary tract infections.
Molecular identification of Aeromonas species is regarded as a standard method due to the inaccuracy of conventional phenotypic tests. For example, several isolates originally phenotypically identified as A. hydrophila were reclassified as A. dhakensis using rpoD or gyrB sequencing (15, 16). Therefore, the clinical significance of some Aeromonas species should be reevaluated, especially in areas of endemicity with a high prevalence of Aeromonas infections. Therefore, microbiological surveillance for Aeromonas species by molecular methods is warranted in Taiwan.
Three classes of chromosomally mediated β-lactamases, i.e., AmpC β-lactamases, metallo-β-lactamases (MBLs), and penicillinases, are present in clinical Aeromonas isolates (10, 17). Another important class of β-lactamases, the class A extended-spectrum β-lactamases (ESBLs), has been increasingly reported in clinical and environmental aeromonads (18, 19). The presence of ESBLs among pathogenic aeromonads raises the concern of inappropriate cephalosporin monotherapy for severe Aeromonas infection if the expression of β-lactamase can be induced by cephalosporin. In addition, data on the phenotypes and genotypes of antimicrobial resistance among pathogenic Aeromonas isolates in the environment or in food sources are very limited in Taiwan. Therefore, the present study aimed to survey Aeromonas species in fish from markets and from sushi and seafood shops in Tainan City, Taiwan, and to compare their virulence genes and antimicrobial resistance profiles with those of clinical isolates from the same geographical area.
RESULTS
Prevalence of Aeromonas species in fish.
A total of 235 samples were collected, and 78.7% (185) grew bacteria on Aeromonas selective medium (LabM 167). Of those isolates, 128 (69.2%) were identified as Aeromonas species and the others were identified as Enterobacter cloacae (n = 1), Shewanella species (n = 1), Vibrio species (n = 2), and Pseudomonas species (n = 53) by sequencing of the rpoD genes. A. caviae (n = 43, 33.6%) was the most common species, followed by A. veronii (n = 33, 25.8%), A. bivalvium (n = 13, 10.2%), A. dhakensis (n = 11, 8.6%), and A. hydrophila (n = 9, 7.0%). Other species included A. enteropelogenes (n = 6), A. taiwanensis (n = 6), A. media (n = 4), A. salmonicida (n = 2), and A. jandaei (n = 1).
Prevalence of Aeromonas species in humans.
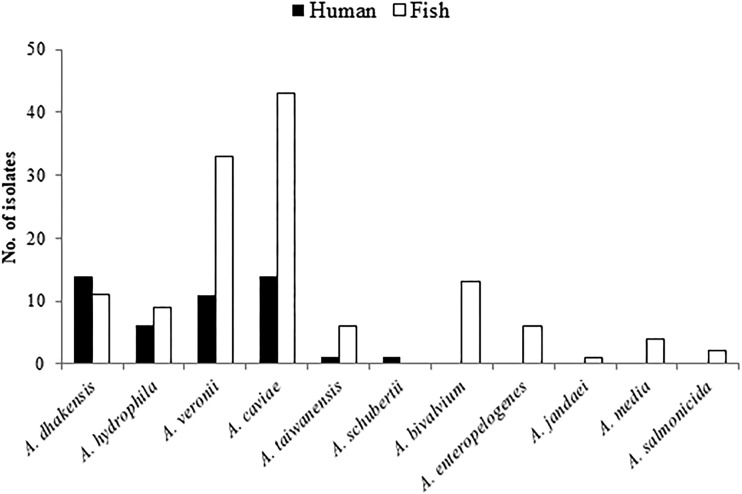
In 2011, a total of 47 clinical isolates were available for further analysis (Table 1). Blood was the most common source, with 61.7% of the clinical isolates being recovered from the blood of 29 patients, and of these isolates, 27.6% were from 8 patients with concurrent bacteremia caused by other bacterial species, including Escherichia coli, Klebsiella pneumoniae, E. cloacae, Acinetobacter baumannii, Acinetobacter lwoffii, Pseudomonas aeruginosa, and Clostridium perfringens. The patients had a mean age ± standard deviation of 70 ± 14 years, with a slight male predominance (male to female ratio, 1.1). Common underlying diseases were cancer (n = 11 patients), diabetes mellitus (n = 8), biliary or gallbladder stones (n = 7), and liver cirrhosis (n = 5). The infection sources of Aeromonas bacteremia were identified in 15 patients and included biliary tract infection (n = 9 patients), skin and soft tissue infection (n = 3), vascular catheter-associated infection (n = 2), and intra-abdominal infection (n = 1). All patients were hospitalized and received antimicrobial therapy, and four died in the hospital, resulting in an in-hospital mortality rate of 13.8%. Overall, the major Aeromonas species noted in humans and fish were A. dhakensis, A. caviae, and A. veronii; A. bivalvium, A. media, A. salmonicida, and A. jandaei were not present in any of the clinical samples (Fig. 1).
TABLE 1.
Clinical sources of the 47 clinical Aeromonas isolates
| Source | No. of isolates |
||||||
|---|---|---|---|---|---|---|---|
| A. dhakensis (n = 14) | A. hydrophila (n = 6) | A. veronii (n = 11) | A. caviae (n = 14) | A. schubertii (n = 1) | A. taiwanensis (n = 1) | Total (n = 47) | |
| Blood | 9 | 3 | 5 | 10 | 1 | 1 | 29 |
| Ascites | 1 | 0 | 3 | 0 | 0 | 0 | 4 |
| Bile | 1 | 0 | 2 | 1 | 0 | 0 | 4 |
| Wound | 1 | 1 | 0 | 2 | 0 | 0 | 4 |
| Pus | 1 | 1 | 1 | 0 | 0 | 0 | 3 |
| Urine | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Other | 0 | 1 | 0 | 1 | 0 | 0 | 2 |
FIG 1.
Distribution of Aeromonas species in clinical samples and in fish obtained from markets and from sushi and seafood shops.
β-Hemolysis and exoprotease assays.
A total of 145 (82.9%) isolates showed beta-hemolysis, and 88% expressed proteolytic activity, as shown in Table 2. All four A. media isolates lacked hemolytic activity on agar plates. Notably, the hemolytic phenotype and exoprotease activity were less common in A. caviae isolates (77.2% and 68.4%, respectively) than in A. veronii isolates (97.7% and 95.5%, respectively), A. dhakensis isolates (100% and 100%, respectively), or A. hydrophila isolates (100% and 100%, respectively) (P values were <0.05 for all comparisons). No significant difference in beta-hemolytic or proteolytic activity was found between fish and clinical isolates of A. caviae, A. veronii, A. dhakensis, and A. hydrophila.
TABLE 2.
Prevalence of hemolytic and proteolytic activity and virulence genes in fish and clinical Aeromonas isolates
| Species and source | No. (%) of isolatesa
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Hemolysis | Proteolysis | act | aerA | alt | ascF-ascG | aexT | ascV | ast | col | ela | hlyA | lip | |
| A. caviae | 57 | |||||||||||||
| Fish | 43 | 31 (72.1) | 30 (69.8) | 0 | 0 | 21 (48.8)** | 0 | 0 | 0 | 0 | 30 (69.8) | 43 (100) | 0 | 11 (25.6) |
| Human | 14 | 13 (92.9) | 9 (64.3) | 1 (7.1) | 0 | 0 | 0 | 0 | 0 | 0 | 14 (100)* | 14 (100) | 0 | 14 (100)*** |
| A. veronii | 44 | |||||||||||||
| Fish | 33 | 32 (97.0) | 31 (93.9) | 26 (78.8) | 0 | 12 (36.4) | 0 | 4 (12.1) | 2 (6.1) | 5 (15.2) | 30 (90.9)*** | 5 (15.2) | 0 | 2 (6.1) |
| Human | 11 | 11 (100) | 11 (100) | 11 (100) | 0 | 2 (18.2) | 1 (9.1) | 2 (18.2) | 7 (63.6)*** | 0 | 2 (18.2) | 0 | 0 | 3 (27.3) |
| A. dhakensis | 24 | |||||||||||||
| Fish | 10 | 10 (100) | 10 (100) | 4 (40) | 3 (30) | 10 (100) | 2 (20) | 0 | 1 (10) | 1 (10) | 10 (100) | 10 (100) | 10 (100) | 10 (100) |
| Human | 14 | 14 (100) | 14 (100) | 4 (28.6) | 4 (28.6) | 14 (100) | 2 (14.3) | 0 | 2 (14.3) | 0 | 14 (100) | 14 (100) | 13 (92.9) | 14 (100) |
| A. hydrophila | 16 | |||||||||||||
| Fish | 10 | 10 (100) | 10 (100) | 1 (10) | 1 (10) | 10 (100) | 3 (30) | 1 (10) | 0 | 1 (10) | 10 (100) | 10 (100) | 10 (100) | 10 (100) |
| Human | 6 | 6 (100) | 6 (100) | 2 (33.3) | 2 (33.3) | 6 (100) | 3 (50) | 1 (16.7) | 3 (50) | 6 (100)*** | 6 (100) | 6 (100) | 6 (100) | 6 (100) |
| A. taiwanensis | 7 | |||||||||||||
| Fish | 6 | 3 (50) | 6 (100) | 0 | 0 | 2 (33.3) | 0 | 6 (100) | 0 | 0 | 0 | 6 (100) | 0 | 0 |
| Human | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (100) | 0 | 1 (100) |
| A. schubertii, human | 1 | 1 (100) | 1 (100) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (100) | 0 | 0 |
| A. bivalvium, fish | 13 | 5 (38.5) | 13 (100) | 0 | 0 | 4 (30.8) | 0 | 0 | 0 | 0 | 10 (76.9) | 13 (100) | 0 | 8 (61.5) |
| A. enteropelogenes, fish | 6 | 6 (100) | 6 (100) | 0 | 0 | 3 (50) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 (50) |
| A. media, fish | 4 | 0 | 4 (100) | 0 | 0 | 2 (50) | 0 | 0 | 0 | 0 | 2 (50) | 4 (100) | 0 | 0 |
| A. salmonicida, fish | 2 | 2 (100) | 2 (100) | 2 (100) | 0 | 1 (50) | 0 | 0 | 0 | 0 | 2 (100) | 2 (100) | 2 (100) | 2 (100) |
| A. jandaei, fish | 1 | 1 (100) | 1 (100) | 0 | 0 | 0 | 0 | 0 | 1 (100) | 0 | 0 | 0 | 1 (100) | 0 |
| Total | 175 | 145 (82.9) | 154 (88.0) | 51 (29.1) | 10 (5.7) | 87 (49.7) | 11 (6.3) | 14 (8.0) | 16 (9.1) | 13 (7.4) | 130 (74.3) | 129 (73.7) | 42 (24.0) | 84 (48.0) |
act, gene encoding a heat-labile cytotoxin; aerA, gene encoding an aerolysin; alt, gene encoding a heat-labile cytotoxin; ascF-ascG, aexT, and ascV, components of the type III secretion system; ela, gene encoding elastase; col, gene encoding collagenase; hlyA, gene encoding hemolysin; lip, gene encoding lipase; *, P < 0.05; **, P < 0.001; ***, P < 0.0001.
Distribution of virulence genes.
The distribution of virulence genes in Aeromonas species is shown in Table 2. The collagenase gene (col) was the most commonly found gene and was found in 74.3% of the 175 isolates, followed by the gene for elastase (ela) (n = 129, 73.7%). The genes encoding the ADP-ribosyltransferase toxin (aexT), aerolysin (aerA), components of the type III secretion system (ascF-ascG and ascV), and heat-stable cytotoxin (ast) were identified in less than 10% of the studied isolates. Although the number of isolates was low, few of the virulence genes studied were detected in one clinical isolate of A. schubertii (only ela), one fish isolate of A. jandaei (ascV and hlyA), and all six fish isolates of A. enteropelogenes (alt and lip).
The distribution of some virulence genes was heterogeneous in the fish and clinical isolates of Aeromonas species. Notably, five virulence genes were noted in >90% of fish and clinical isolates of A. dhakensis and A. hydrophila, and the distribution of virulence genes was similar regardless of the origin of the isolates, except that ast was predominant in clinical isolates of A. hydrophila (100% in clinical isolates versus 10% of fish isolates; P < 0.0001). More clinical A. caviae isolates than fish A. caviae isolates carried col (100% versus 69.8%, P = 0.025) and lip (100% versus 25.6%, P < 0.0001). Similarly, more clinical A. veronii isolates than fish isolates had ascV (63.6% versus 6.1%, P < 0.0001), but col was more commonly found in the fish A. veronii isolates than in the clinical counterparts (90.9% versus 18.2%, P < 0.0001).
The common genotypes, as indicated by the combination of putative virulence factors in the fish and clinical isolates, are summarized in Table 3. Among the A. dhakensis isolates, the most common genotype was hlyA lip alt col ela, and the act genotype was the most common among A. veronii isolates. The major genotype in A. caviae isolates was lip col ela, which was more common in clinical isolates than in fish isolates (64.3% versus 15.2%, P = 0.0015).
TABLE 3.
Common genotypes in the fish and clinical isolates of different Aeromonas species
| Species and genotypea | No. (%) of isolates |
|
|---|---|---|
| Fish isolates | Clinical isolates | |
| Aeromonas dhakensis | ||
| Total | 10 | 14 |
| hlyA lip alt col ela | 3 (30) | 5 (35.7) |
| Aeromonas hydrophila | ||
| Total | 10 | 6 |
| hlyA lip ascF-ascG alt col ela | 3 (30) | 0 |
| hlyA lip alt col ela | 3 (30) | 0 |
| act hlyA aerA lip ascF-ascG alt ast col ela | 0 | 2 (33.3) |
| hlyA lip ascF-ascG alt ast col ela | 0 | 2 (33.3) |
| Aeromonas caviae | ||
| Total | 33 | 14 |
| lip col ela | 5 (15.2) | 9 (64.3)b |
| Aeromonas veronii | ||
| Total | 43 | 14 |
| act | 8 (18.6) | 3 (21.4) |
act, gene encoding heat-labile cytotoxin; aerA, gene encoding aerolysin; alt, gene encoding heat-labile cytotoxin; ascF-ascG, aexT, and ascV, gene encoding components of the type III secretion system; ela, gene encoding elastase; col, gene encoding collagenase; hlyA, gene encoding hemolysin; lip, gene encoding lipase.
P < 0.005.
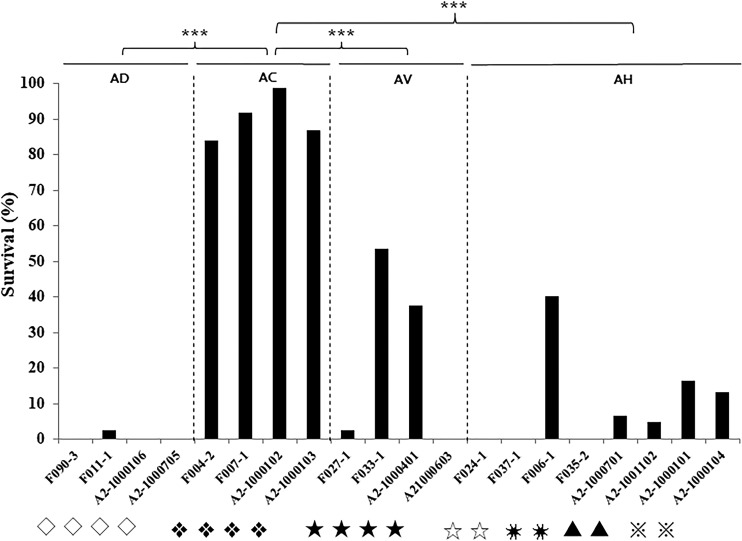
Caenorhabditis elegans LT assays for the major genotypes.
Twenty isolates of the major genotypes belonging to four Aeromonas species were selected for the liquid toxicity (LT) assay. Almost all C. elegans worms fed A. dhakensis isolates died within 24 h (336/338, 99.4%; Fig. 2). In contrast, the survival rates of C. elegans worms infected with A. hydrophila, A. veronii, and A. caviae were 9.1% (57/623), 24.3% (76/313), and 90.7% (263/290), respectively. Overall, A. caviae isolates were less lethal than the isolates of the other species to C. elegans (P < 0.0001 for all comparisons).
FIG 2.
Twenty-four-hour survival rates of Caenorhabditis elegans worms infected by the major genotypes of fish and human isolates of four Aeromonas species, Aeromonas dhakensis (AD), Aeromonas hydrophila (AH), Aeromonas veronii (AV), and Aeromonas caviae (AC), in the liquid toxicity assay. ***, P < 0.0001 compared with A. caviae. F, fish isolates; A2, clinical isolates. Symbols for genotypes: ◇, hlyA lip alt col ela; ❖, lip col ela; ★, act; ☆, hlyA lip ascF-ascG alt col ela; ✷, hlyA lip alt col ela; ▲, act hlyA aerA lip ascF-ascG alt ast col ela; ※, hlyA lip ascF-ascG alt ast col ela.
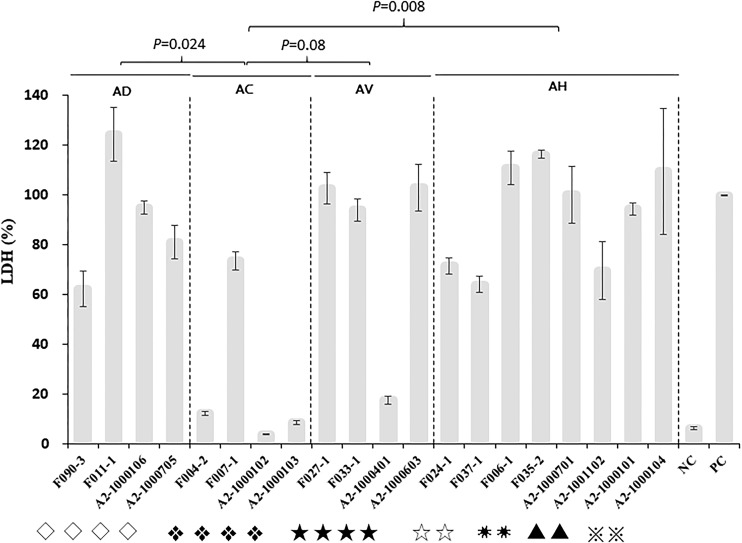
Cytotoxicity of the major genotypes.
The cytotoxicity of 20 selected isolates of the major genotypes belonging to four Aeromonas species was assessed in the C2C12 mouse myoblast cell line (Fig. 3). The mean level of lactate dehydrogenase (LDH) release induced by A. dhakensis isolates was 90.7% ± 26.1%, that induced by A. hydrophila was 92.1% ± 20.8%, that induced by A. veronii was 79.3% ± 41.3%, and that induced by A. caviae was 24.7% ± 32.8% (one-way analysis of variance [ANOVA], P < 0.0001). The post hoc Tukey’s honest significant difference (HSD) test demonstrated that A. caviae isolates were less cytotoxic to the C2C12 cell line than A. dhakensis (P = 0.024), A. hydrophila (P = 0.008), or A. veronii (P = 0.08) isolates.
FIG 3.
Cytotoxicity of Aeromonas dhakensis (AD), Aeromonas hydrophila (AH), Aeromonas veronii (AV), and Aeromonas caviae (AC) isolates to the C2C12 mouse fibroblast cell line, expressed as the proportion of the level of LDH release induced by the Aeromonas isolates compared with the level of LDH release induced by lysis solution (the value for the positive control was 100%). F, fish isolates; A2, clinical isolates; PC, positive control; NC, negative control. Symbols for genotypes: ◇, hlyA lip alt col ela; ❖, lip col ela; ★, act; ☆, hlyA lip ascF-ascG alt col ela; ✷, hlyA lip alt col ela; ▲, act hlyA aerA lip ascF-ascG alt ast col ela; ※, hlyA lip ascF-ascG alt ast col ela.
Antimicrobial susceptibility.
The antimicrobial susceptibility rates of Aeromonas isolates to 18 antimicrobial agents are shown in Table 4. Two species (A. bivalvium and A. enteropelogenes) found only in fish were susceptible to the tested antibiotics, with the exception of ampicillin, cefazolin, co-trimoxazole, and ampicillin-sulbactam. In general, clinical isolates of A. dhakensis, A. hydrophila, and A. caviae were less susceptible than their counterpart fish isolates to the commonly prescribed β-lactams, such as penicillins (piperacillin and piperacillin-tazobactam), broad-spectrum cephalosporins (cefuroxime, cefotaxime, and ceftazidime), and carbapenems (ertapenem and imipenem). Most (95.4%) Aeromonas isolates were susceptible to ertapenem and imipenem, but five A. veronii isolates, two A. dhakensis isolates, and one A. caviae isolate were nonsusceptible to a carbapenem. Although 88% (n = 154) of the 175 Aeromonas isolates were resistant to cefazolin, 51.5% (n = 17) of 33 A. veronii fish isolates were cefazolin susceptible, but none of the clinical A. veronii isolates were susceptible to cefazolin (51.5% versus 0%, P = 0.003). All fish A. hydrophila isolates were susceptible to cefuroxime, cefotaxime, and ceftazidime, but only half of the clinical A. hydrophila isolates were susceptible (100% versus 50%, P = 0.04). However, the co-trimoxazole susceptibility rate was significantly higher for clinical isolates than for their fish counterparts for A. dhakensis (92.9% versus 0%, P < 0.00001), A. caviae (57.1% versus 7%, P = 0.002), and A. veronii (100% versus 15.1%, P < 0.00001).
TABLE 4.
Antimicrobial susceptibility of Aeromonas isolates from humans and fisha
| Drug | No. (%) of susceptible isolates or susceptibility |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
A. dhakensis |
A. hydrophila |
A. caviae |
A. veronii |
A. bivalvium | A. enteropelogenes |
A. taiwanensis |
A. media | A. jandaei | A. salmonicida | A. schubertii | ||||||
| Fish (n = 10) | Human (n = 14) | Fish (n = 10) | Human (n = 6) | Fish (n = 43) | Human (n = 14) | Fish (n = 33) | Human (n = 11) | Fish (n = 13) | Fish (n = 6) | Fish (n = 6) | Human (n = 1) | Fish (n = 4) | Fish (n = 1) | Fish (n = 2) | Human (n = 1) | |
| AM | 2 (20) | 0 | 0 | 0 | 0 | 0 | 1 (3.0) | 0 | 0 | 4 (66.7) | 0 | R | 0 | R | 0 | R |
| SAM | 2 (20) | 0 | 0 | 0 | 4 (9.3) | 0 | 1 (3.0) | 0 | 0 | 5 (83.3) | 0 | R | 0 | R | 0 | R |
| PIP | 9 (90) | 12 (85.7) | 9 (90) | 3 (50) | 37 (86.0) | 11 (78.6) | 28 (84.8) | 10 (90.9) | 10 (76.9) | 6 (100) | 5 (83.3) | S | 3 (75.0) | S | 2 (100) | S |
| TZP | 10 (100) | 12 (85.7) | 10 (100) | 6 (100) | 42 (97.7) | 12 (85.7) | 32 (97.0) | 11 (100) | 13 (100) | 6 (100) | 5 (83.3) | S | 3 (75.0) | R | 2 (100) | S |
| CZ | 0 | 0 | 0 | 0 | 2 (4.7) | 0 | 17 (51.5) | 0 | 0 | 2 (33.3) | 0 | R | 0 | I | 0 | I |
| CXM | 10 (100) | 12 (85.7) | 10 (100) | 3 (50) | 43 (100) | 10 (71.4) | 33 (100) | 11 (100) | 13 (100) | 6 (100) | 6 (100) | I | 4 (100) | S | 2 (100) | S |
| CTX | 10 (100) | 12 (85.7) | 10 (100) | 3 (50) | 43 (100) | 10 (71.4) | 33 (100) | 11 (100) | 13 (100) | 6 (100) | 6 (100) | I | 4 (100) | S | 2 (100) | S |
| CAZ | 10 (100) | 12 (85.7) | 10 (100) | 3 (50) | 43 (100) | 11 (78.6) | 33 (100) | 11 (100) | 13 (100) | 6 (100) | 6 (100) | S | 4 (100) | S | 2 (100) | S |
| FEP | 10 (100) | 14 (100) | 10 (100) | 6 (100) | 43 (100) | 13 (92.9) | 33 (100) | 11 (100) | 13 (100) | 6 (100) | 6 (100) | S | 4 (100) | S | 2 (100) | S |
| ATM | 10 (100) | 14 (100) | 10 (100) | 5 (83.3) | 43 (100) | 12 (85.7) | 33 (100) | 11 (100) | 13 (100) | 6 (100) | 6 (100) | S | 4 (100) | S | 2 (100) | S |
| ETP | 10 (100) | 12 (85.7) | 10 (100) | 6 (100) | 43 (100) | 13 (92.9) | 30 (90.9) | 9 (81.8) | 13 (100) | 6 (100) | 6 (100) | S | 4 (100) | S | 2 (100) | S |
| IMP | 10 (100) | 12 (85.7) | 10 (100) | 6 (100) | 43 (100) | 14 (100) | 33 (100) | 10 (90.9) | 13 (100) | 6 (100) | 6 (100) | S | 4 (100) | S | 2 (100) | S |
| LVX | 10 (100) | 14 (100) | 10 (100) | 5 (83.3) | 43 (100) | 13 (92.9) | 33 (100) | 11 (100) | 13 (100) | 6 (100) | 6 (100) | S | 4 (100) | S | 2 (100) | S |
| GM | 10 (100) | 14 (100) | 9 (100) | 6 (100) | 43 (100) | 13 (92.9) | 33 (100) | 11 (100) | 13 (100) | 6 (100) | 6 (100) | S | 4 (100) | S | 2 (100) | S |
| AN | 10 (100) | 14 (100) | 10 (100) | 5 (83.3) | 43 (100) | 14 (100) | 33 (100) | 11 (100) | 13 (100) | 6 (100) | 6 (100) | S | 4 (100) | S | 2 (100) | S |
| SXT | 0 | 13 (92.9) | 1 (10) | 6 (100) | 3 (7.0) | 8 (57.1) | 5 (15.1) | 11 (100) | 1 (7.7) | 2 (33.3) | 3 (50) | S | 0 | I | 1 (50) | S |
| DXY | 10 (100) | 14 (100) | 9 (90) | 6 (100) | 40 (93.0) | 13 (92.9) | 31 (93.9) | 11 (100) | 13 (100) | 6 (100) | 6 (100) | S | 4 (100) | S | 2 (100) | S |
| TET | 9 (90) | 7 (50) | 7 (70) | 5 (83.3) | 36 (83.7) | 11 (78.6) | 17 (51.5) | 8 (72.7) | 13 (100) | 6 (100) | 5 (83.3) | S | 4 (100) | S | 2 (100) | S |
AM, ampicillin; SAM, ampicillin-sulbactam; PIP, piperacillin; TZP, piperacillin-tazobactam; CZ, cefazolin; CXM, cefuroxime; CTX, cefotaxime; CAZ, ceftazidime; FEP, cefepime; ATM, aztreonam; ETP, ertapenem; IMP, imipenem; LVX, levofloxacin; GM, gentamicin; AN, amikacin; SXT, co-trimoxazole; DXY, doxycycline; TET, tetracycline; S, susceptible; I, intermediate; R, resistant.
β-Lactamase genes.
The targeted β-lactamase genes were found in only four species: A. dhakensis, A. hydrophila, A. veronii, and A. caviae (Table 5). The AmpC β-lactamase-encoding gene, blaAQU-1, was present in all 14 clinical A. dhakensis isolates and 60% of 10 fish isolates. Almost all A. dhakensis (24/24), A. hydrophila (16/16), and A. veronii (43/44) isolates but none of the 57 A. caviae isolates carried blaCphA. However, blaMOX3 was present in only two A. caviae isolates, and blaPER3 was present in one A. caviae isolate. ESBL genes, such as blaSHV12, blaCTX1, blaCTX2, blaCTX3, blaCTX9, blaCTX13, blaCTX14, and blaCTX151, were not detected in the studied Aeromonas isolates. The association of the MBL gene (blaCphA) and AmpC β-lactamase genes (blaAQU-1 and blaMOX3) with antimicrobial resistance is shown in Table 6. The presence of the blaMOX3 gene was not associated with cefotaxime nonsusceptibility (6.8% versus 3.7%, P = 0.40), and the blaCphA gene was not associated with ertapenem resistance (4.8% versus 1.7%, P = 0.65). The proportion of isolates nonsusceptible to cefotaxime was higher among blaAQU-1-positive isolates than among blaAQU-1-negative isolates (10% versus 0%, P = 0.019).
TABLE 5.
Prevalence of four β-lactamase genes in Aeromonas isolates from humans and fish
| Species and source | No. (%) of isolates carryinga
: |
||||
|---|---|---|---|---|---|
| Total | blaPER3 | blaAQU-1 | blaMOX3 | blaCphA | |
| Aeromonas dhakensis | |||||
| Fish | 10 | 0 | 6 (60) | 0 | 10 (100) |
| Human | 14 | 0 | 14 (100) | 0 | 14 (100) |
| Aeromonas hydrophila | |||||
| Fish | 10 | 0 | 0 | 0 | 10 (100) |
| Human | 6 | 0 | 0 | 0 | 6 (100) |
| Aeromonas veronii | |||||
| Fish | 33 | 0 | 0 | 2 (6.1) | 33 (100) |
| Human | 11 | 0 | 0 | 0 | 10 (90.9) |
| Aeromonas caviae | |||||
| Fish | 43 | 0 | 0 | 43 (100) | 0 |
| Human | 14 | 1 (7.1) | 0 | 14 (100) | 0 |
These genes encode metallo-β-lactamase (CphA), AmpC β-lactamase (AQU-1 or MOX3), or an extended spectrum β-lactamase (PER3).
TABLE 6.
Association between susceptibility to ertapenem or the 3rd-generation cephalosporin cefotaxime and the presence of blaCphA, blaAQU-1, or blaMOX3 in Aeromonas dhakensis, Aeromonas hydrophila, Aeromonas veronii, and Aeromonas caviae isolates
| Susceptibility | Ertapenem |
Cefotaxime |
|||||||
|---|---|---|---|---|---|---|---|---|---|
|
blaCphA |
blaAQU-1 |
blaMOX3 |
|||||||
| No. (%) of isolates |
P value | No. (%) of isolates |
P value | No. (%) of isolates |
P value | ||||
| Positive (n = 83) | Negative (n = 58) | Positive (n = 20) | Negative (n = 141) | Positive (n = 59) | Negative (n = 82) | ||||
| Nonsusceptible | 4 (4.8) | 1 (1.7) | 0.65 | 2 (10) | 0 | 0.019 | 4 (6.8) | 3 (3.7) | 0.40 |
| Susceptible | 79 (95.2) | 57 (98.3) | 18 (90) | 121 (85.8) | 55 (93.2) | 79 (96.3) | |||
DISCUSSION
Aeromonas is an important pathogen of community-acquired infections in southern Taiwan (12). Our data show that Aeromonas species causing clinical diseases, i.e., A. dhakensis, A. hydrophila, A. veronii, and A. caviae, can be isolated from fish and share virulence properties and antimicrobial resistance profiles similar to those of isolates of human origin. In a study conducted in Mexico City, Mexico, 82 Aeromonas isolates were discovered in 250 frozen fish ready for human consumption (7), and another study in Norway identified multiple Aeromonas species in fresh retail sushi (8). In these two studies, A. veronii, A. hydrophila, and A. media were the major species. A substantial proportion of our patients with bacteremia had underlying cancer, diabetes mellitus, biliary or gallbladder stones, or liver cirrhosis. Despite a lack of direct evidence supporting the foodborne nature of Aeromonas infection in case clusters or outbreaks, the above-described susceptible hosts carry the risk of invasive Aeromonas infection after the consumption of seafood contaminated with pathogenic Aeromonas species (20–22).
In the present study, the virulence traits of fish or clinical Aeromonas isolates were compared by hemolytic and proteolytic activity assays, C. elegans LT assays, and cytotoxicity assays. According to a previous report, the virulence manifested in the LT and cytotoxicity assays was correlated with the virulence traits demonstrated in a mouse infection model (23). Our results indicated that the A. dhakensis, A. hydrophila, and A. veronii isolates were more virulent than the A. caviae isolates, as evidenced by the hemolytic and proteolytic phenotypes and as seen in the two infection models with C. elegans and C2C12 cells. Furthermore, the fish isolates of A. dhakensis, A. hydrophila, and A. veronii were as virulent as their human counterparts.
Although a variety of combinations of virulence genes among clinical and fish Aeromonas isolates were found in the present work, there was no clear link between the presence of a specific gene and the virulence phenotype, suggesting the need for more research to identify the virulence factors or regulatory mechanisms of Aeromonas species. Consistent with previous findings in A. hydrophila isolates, the virulence phenotype is the result of a molecular symphony from the cumulative effect of multiple contributing virulence factors (24). However, some virulence traits have been identified in specific Aeromonas species. A. hydrophila often carries aerA, hlyA, and alt (25, 26). Since previous studies did not distinguish A. dhakensis from A. hydrophila by the molecular typing method, it is possible that A. dhakensis may be misidentified as A. hydrophila. Our work showed that aerA and hlyA were distributed mostly in A. hydrophila and A. dhakensis isolates. A. caviae infrequently carries these genes (7, 25, 27). Aerolysin has been considered a potential virulence factor in A. caviae (28) but was not detected in our A. caviae isolates. A. salmonicida, an Aeromonas species prevalent in fish and seafood samples (1, 7, 8) but rare in clinical samples, has been reported to carry pathogenic genes, such as act, alt, and hlyA (8). A significant proportion of A. veronii isolates (84%, 37/44) carried act, and the act-positive isolates exhibited virulence phenotypes in the LT and cytotoxicity assays. Taken together, our findings indicate that the absence of aerA, hly, or act may be associated with the low virulence of A. caviae compared with that of other pathogenic Aeromonas species.
Variations in antimicrobial susceptibility among clinical and fish Aeromonas isolates suggest the presence of different clones or the existence of selective pressure from antibiotics in the aquatic environment or clinical practice. Previous studies have shown that the genes encoding MBLs and AmpC β-lactamases are widely distributed among clinical Aeromonas species (29, 30) and that their corresponding resistance phenotypes did not manifest unless their expression was induced under specific circumstances (31–33). Genetic modifications, such as an extra insertion sequence in blaCphA, may alter the expression of CphA (33). The emergence of resistance to cefotaxime was reported in a patient with A. hydrophila infection treated with cefotaxime (34), and imipenem therapy can induce carbapenem resistance in patients with severe A. hydrophila and A. veronii infections (35, 36). These observations emphasize that carbapenems should be used with caution for Aeromonas infections unless severe infections due to non-blaCphA-carrying A. caviae are verified. Similarly, cefepime, a 4th-generation cephalosporin, should be considered for invasive infections caused by Aeromonas species expressing the AmpC β-lactamase, e.g., A. hydrophila and A. caviae (17).
In conclusion, multiple Aeromonas species were isolated from fish intended for human consumption in Tainan City. Fish isolates carry certain combinations of genes encoding putative virulence factors and β-lactam resistance that are also present in clinical isolates.
MATERIALS AND METHODS
Fish and clinical sample collection, storage, and preparation.
Apparently healthy fish were purchased from traditional markets, supermarkets, and sushi and seafood shops in Tainan City between 1 and 30 June 2011. Each fish sample was individually packed in a clean polyethylene bag and transferred in a cooler to the laboratory for bacterial culture. Culture samples were collected from fish gills or surfaces using sterile swabs; plated on a selective Aeromonas selective medium, LabM 167 (Lab M Ltd., Lancashire, UK), as described previously (37); and cultivated at 37°C for 24 h.
Clinical Aeromonas isolates were obtained between January and December 2011 from the microbiological laboratory at National Cheng Kung University Hospital, a medical center in southern Taiwan, and stored at −70°C. Aeromonas isolates were identified by a positive oxidase test, d-glucose fermentation, a motility test, the absence of growth in 6.5% sodium chloride, resistance to the vibriostatic agent O/129 (150 μg), and identification by the Vitek GNI Plus system (bioMérieux, Marcy l’Etoile, France). The final species identification was determined based on the partial sequence of rpoD (38).
Antimicrobial susceptibility tests.
The antimicrobial susceptibility of each Aeromonas isolate was measured on Mueller-Hinton agar (CMP, Creative Media Products, Ltd., New Taipei City, Taiwan) using the disc diffusion method (Becton, Dickinson Microbiology Systems, Sparks, MD, USA), and the interpretative criteria followed the Clinical and Laboratory Standards Institute (CLSI) recommendations for Aeromonas species (CLSI M45-A2, 2010) (39). The antimicrobial agents tested included ampicillin, ampicillin-sulbactam, piperacillin, piperacillin-tazobactam, cefazolin, cefuroxime, cefotaxime, ceftazidime, cefepime, aztreonam, ertapenem, imipenem, levofloxacin, gentamicin, amikacin, co-trimoxazole, doxycycline, and tetracycline.
PCR of virulence and drug resistance genes.
The studied isolates were screened for the drug resistance genes reported in Aeromonas species, including those encoding ESBLs (CTX-M and PER-3), AmpC β-lactamases (AQU-1 and MOX-3), and MBL (CphA), by PCR. It has been reported that Aeromonas species can secrete a broad range of exotoxins and exoenzymes, which are responsible for clinical infections in humans (40). In the present study, we studied the genes encoding exotoxins or secretion system components and extracellular enzymes, including the genes for aerolysin (aerA), hemolysin (hlyA), type III secretion system components (ascV and ascF-ascG), ADP-ribosyltransferase toxin (aexT), heat-labile enterotoxin (act), heat-stable cytotoxin (ast), heat-labile cytotoxin (alt), lipase (lip), elastase (ela), and collagenase (col). All primers used for the detection of virulence and antimicrobial resistance genes and the associated references are summarized in Table S1 in the supplemental material (20, 41, 42).
Hemolysis and exoprotease assays.
The degree of beta-hemolysis was assessed on Luria-Bertani (LB) agar containing 5% (vol/vol) sheep blood agar (Difco Laboratories, Detroit, MI, USA). Qualitative assays of exoprotease activity were performed on LB agar containing 2% (wt/vol) skim milk (Difco Laboratories, Detroit, MI, USA). The presence of clear zones surrounding the streaks indicated positive reactions in exoprotease and hemolytic tests (23).
LT assay in Caenorhabditis elegans.
To compare the virulence of the fish isolates with the virulence of the clinical isolates, the isolates of different species with major genotypes according to the presence of virulence genes, including hlyA lip alt col ela (2 fish and 2 clinical A. dhakensis isolates), lip col ela (2 fish and 2 clinical A. caviae isolates), act (2 fish and 2 clinical A. veronii isolates), hlyA lip ascF-ascG alt col ela (2 fish A. hydrophila isolates), hlyA lip alt col ela (2 fish A. hydrophila isolates), act hlyA aerA lip ascF-ascG alt ast col ela (2 clinical A. hydrophila isolates), and hlyA lip ascF-ascG alt ast col ela (2 clinical A. hydrophila isolates), were selected for the C. elegans liquid toxicity (LT) assay. The detailed procedures for the LT assay were described previously (43). The survival rates of the worms were determined by dividing the number of live worms by the total number of worms after infection for 24 h.
Cytotoxicity assay.
Cytotoxicity assays were conducted in a mouse C2C12 fibroblast cell line (American Type Culture Collection no. CRL-1772; BCRC no. 60083) obtained from the Bioresource Collection and Research Center, Hsinchu, Taiwan, and the levels of lactate dehydrogenase (LDH) release were measured (23). Cells were cultured in complete medium, consisting of Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Grand Island, NY, USA) and 10% fetal bovine serum (FBS; Gibco, Grand Island, NY, USA), and incubated at 37°C in the presence of 5% CO2. Aeromonas isolates were grown in 2 ml of LB medium for 16 h, and 50 μl of the bacterial solution was transferred to 5 ml of LB medium and cultivated for another 3 h at 37°C. C2C12 myoblast cells were separated by centrifugation and seeded into 12-well plates (5 × 104 cells/well). The cells were incubated with the bacterial cultures at a multiplicity of infection (MOI) of 20. After incubation at 37°C for 2 h, the culture medium was examined for LDH levels by use of a CytoTox 96 kit (Promega, Madison, WI). A group treated with lysis solution (Promega, Madison, WI) was used as the positive control, and an untreated group was used as the negative control. The cytotoxic activity was expressed as the percentage of the mean of triplicate measurements of the released LDH level induced by Aeromonas isolates compared with that induced by lysis solution (defined as 100% cytotoxicity).
Statistical analysis.
Statistical analysis was performed to compare the differences in the variables between different Aeromonas isolates with the Statistical Package for the Social Sciences (version 21.0; SPSS, Chicago, IL, USA). Categorical variables were compared by the chi-square test or Fisher’s exact test. Cytotoxicity was compared by one-way analysis of variance (ANOVA) with Tukey’s honest significant difference (HSD) post hoc test.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Ministry of Science and Technology of Taiwan (grant MOST 105-2628-B-006-017-MY3), National Health Research Institute, Taiwan (grant ID-100-PP-17), and the National Cheng Kung University Hospital (grants NCKUH-10705001 and NCKUH-10802036).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.01360-19.
REFERENCES
- 1.Hanninen ML, Oivanen P, Hirvela-Koski V. 1997. Aeromonas species in fish, fish-eggs, shrimp and freshwater. Int J Food Microbiol 34:17–26. doi: 10.1016/S0168-1605(96)01163-4. [DOI] [PubMed] [Google Scholar]
- 2.Huys G, Pearson M, Kampfer P, Denys R, Cnockaert M, Inglis V, Swings J. 2003. Aeromonas hydrophila subsp. ranae subsp. nov., isolated from septicaemic farmed frogs in Thailand. Int J Syst Evol Microbiol 53:885–891. doi: 10.1099/ijs.0.02357-0. [DOI] [PubMed] [Google Scholar]
- 3.Minana-Galbis D, Farfan M, Fuste MC, Loren JG. 2004. Aeromonas molluscorum sp. nov., isolated from bivalve molluscs. Int J Syst Evol Microbiol 54:2073–2078. doi: 10.1099/ijs.0.63202-0. [DOI] [PubMed] [Google Scholar]
- 4.Yi SW, You MJ, Cho HS, Lee CS, Kwon JK, Shin GW. 2013. Molecular characterization of Aeromonas species isolated from farmed eels (Anguilla japonica). Vet Microbiol 164:195–200. doi: 10.1016/j.vetmic.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Abd-El-Malek AM. 2017. Incidence and virulence characteristics of Aeromonas spp. in fish. Vet World 10:34–37. doi: 10.14202/vetworld.2017.34-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramadan H, Ibrahim N, Samir M, Abd El-Moaty A, Gad T. 2018. Aeromonas hydrophila from marketed mullet (Mugil cephalus) in Egypt: PCR characterization of beta-lactam resistance and virulence genes. J Appl Microbiol 124:1629–1637. doi: 10.1111/jam.13734. [DOI] [PubMed] [Google Scholar]
- 7.Castro-Escarpulli G, Figueras MJ, Aguilera-Arreola G, Soler L, Fernández-Rendón E, Aparicio GO, Guarro J, Chacón MR. 2003. Characterisation of Aeromonas spp. isolated from frozen fish intended for human consumption in Mexico. Int J Food Microbiol 84:41–49. doi: 10.1016/S0168-1605(02)00393-8. [DOI] [PubMed] [Google Scholar]
- 8.Hoel S, Vadstein O, Jakobsen AN. 2017. Species distribution and prevalence of putative virulence factors in mesophilic Aeromonas spp. isolated from fresh retail sushi. Front Microbiol 8:931. doi: 10.3389/fmicb.2017.00931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Graevenitz A. 2007. The role of Aeromonas in diarrhea: a review. Infection 35:59–64. doi: 10.1007/s15010-007-6243-4. [DOI] [PubMed] [Google Scholar]
- 10.Janda JM, Abbott SL. 2010. The genus Aeromonas: taxonomy, pathogenicity, and infection. Clin Microbiol Rev 23:35–73. doi: 10.1128/CMR.00039-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chao CM, Lai CC, Tang HJ, Ko WC, Hsueh PR. 2013. Biliary tract infections caused by Aeromonas species. Eur J Clin Microbiol Infect Dis 32:245–251. doi: 10.1007/s10096-012-1736-1. [DOI] [PubMed] [Google Scholar]
- 12.Wu CJ, Chen PL, Tang HJ, Chen HM, Tseng FC, Shih HI, Hung YP, Chung CH, Ko WC. 2014. Incidence of Aeromonas bacteremia in southern Taiwan: Vibrio and Salmonella bacteremia as comparators. J Microbiol Immunol Infect 47:145–148. doi: 10.1016/j.jmii.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 13.Yaun SS, Lin LP. 1993. Isolation and characterization of Aeromonas from seafoods in Taipei. Zhonghua Min Guo Wei Sheng Wu Ji Mian Yi Xue Za Zhi 26:78–85. [PubMed] [Google Scholar]
- 14.Gonzalez-Serrano CJ, Santos JA, Garcia-Lopez ML, Otero A. 2002. Virulence markers in Aeromonas hydrophila and Aeromonas veronii biovar sobria isolates from freshwater fish and from a diarrhoea case. J Appl Microbiol 93:414–419. doi: 10.1046/j.1365-2672.2002.01705.x. [DOI] [PubMed] [Google Scholar]
- 15.Aravena-Roman M, Harnett GB, Riley TV, Inglis TJ, Chang BJ. 2011. Aeromonas aquariorum is widely distributed in clinical and environmental specimens and can be misidentified as Aeromonas hydrophila. J Clin Microbiol 49:3006–3008. doi: 10.1128/JCM.00472-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morinaga Y, Yanagihara K, Eugenin FL, Beaz-Hidalgo R, Kohno S, Figueras Salvat MJ. 2013. Identification error of Aeromonas aquariorum: a causative agent of septicemia. Diagn Microbiol Infect Dis 76:106–109. doi: 10.1016/j.diagmicrobio.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 17.Chen PL, Ko WC, Wu CJ. 2012. Complexity of beta-lactamases among clinical Aeromonas isolates and its clinical implications. J Microbiol Immunol Infect 45:398–403. doi: 10.1016/j.jmii.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Girlich D, Poirel L, Nordmann P. 2011. Diversity of clavulanic acid-inhibited extended-spectrum beta-lactamases in Aeromonas spp. from the Seine River, Paris, France. Antimicrob Agents Chemother 55:1256–1261. doi: 10.1128/AAC.00921-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu SY, Zhang YL, Geng SN, Li TY, Ye ZM, Zhang DS, Zou F, Zhou HW. 2010. High diversity of extended-spectrum beta-lactamase-producing bacteria in an urban river sediment habitat. Appl Environ Microbiol 76:5972–5976. doi: 10.1128/AEM.00711-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu CJ, Wu JJ, Yan JJ, Lee HC, Lee NY, Chang CM, Shih HI, Wu HM, Wang LR, Ko WC. 2007. Clinical significance and distribution of putative virulence markers of 116 consecutive clinical Aeromonas isolates in southern Taiwan. J Infect 54:151–158. doi: 10.1016/j.jinf.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Rhee JY, Jung DS, Peck KR. 2016. Clinical and therapeutic implications of Aeromonas bacteremia: 14 years nation-wide experiences in Korea. Infect Chemother 48:274–284. doi: 10.3947/ic.2016.48.4.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Batra P, Mathur P, Misra MC. 2016. Aeromonas spp.: an emerging nosocomial pathogen. J Lab Physicians 8:1–4. doi: 10.4103/0974-2727.176234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen PL, Wu CJ, Tsai PJ, Tang HJ, Chuang YC, Lee NY, Lee CC, Li CW, Li MC, Chen CC, Tsai HW, Ou CC, Chen CS, Ko WC. 2014. Virulence diversity among bacteremic Aeromonas isolates: ex vivo, animal, and clinical evidences. PLoS One 9:e111213. doi: 10.1371/journal.pone.0111213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rasmussen-Ivey CR, Figueras MJ, McGarey D, Liles MR. 2016. Virulence factors of Aeromonas hydrophila: in the wake of reclassification. Front Microbiol 7:1337. doi: 10.3389/fmicb.2016.01337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Granum PE, O'Sullivan K, Tomás JM, Ormen O. 1998. Possible virulence factors of Aeromonas spp. from food and water. FEMS Immunol Med Microbiol 21:131–137. doi: 10.1111/j.1574-695X.1998.tb01158.x. [DOI] [PubMed] [Google Scholar]
- 26.Castilho MC, Castro TL, Araujo VS, Trajano RS, Santos PA, Pimenta PM, Lucheze K, Melo JT, Goncalves AM, Nogueira RT, de Luna MG, Freitas-Almeida AC. 2009. High frequency of hemolytic and cytotoxic activity in Aeromonas spp. isolated from clinical, food and environmental in Rio de Janeiro, Brazil. Antonie Van Leeuwenhoek 96:53–61. doi: 10.1007/s10482-009-9335-6. [DOI] [PubMed] [Google Scholar]
- 27.Pablos M, Remacha M-A, Rodríguez-Calleja J-M, Santos JA, Otero A, García-López M-L. 2010. Identity, virulence genes, and clonal relatedness of Aeromonas isolates from patients with diarrhea and drinking water. Eur J Clin Microbiol Infect Dis 29:1163–1172. doi: 10.1007/s10096-010-0982-3. [DOI] [PubMed] [Google Scholar]
- 28.Ottaviani D, Parlani C, Citterio B, Masini L, Leoni F, Canonico C, Sabatini L, Bruscolini F, Pianetti A. 2011. Putative virulence properties of Aeromonas strains isolated from food, environmental and clinical sources in Italy: a comparative study. Int J Food Microbiol 144:538–545. doi: 10.1016/j.ijfoodmicro.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 29.Rossolini GM, Zanchi A, Chiesurin A, Amicosante G, Satta G, Guglielmetti P. 1995. Distribution of cphA or related carbapenemase-encoding genes and production of carbapenemase activity in members of the genus Aeromonas. Antimicrob Agents Chemother 39:346–349. doi: 10.1128/aac.39.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fosse T, Giraud-Morin C, Madinier I, Labia R. 2003. Sequence analysis and biochemical characterisation of chromosomal CAV-1 (Aeromonas caviae), the parental cephalosporinase of plasmid-mediated AmpC ‘FOX’ cluster. FEMS Microbiol Lett 222:93–98. doi: 10.1016/S0378-1097(03)00253-2. [DOI] [PubMed] [Google Scholar]
- 31.Walsh TR, Payne DJ, MacGowan AP, Bennett PM. 1995. A clinical isolate of Aeromonas sobria with three chromosomally mediated inducible beta-lactamases: a cephalosporinase, a penicillinase and a third enzyme, displaying carbapenemase activity. J Antimicrob Chemother 35:271–279. doi: 10.1093/jac/35.2.271. [DOI] [PubMed] [Google Scholar]
- 32.Walsh TR, Stunt RA, Nabi JA, MacGowan AP, Bennett PM. 1997. Distribution and expression of beta-lactamase genes among Aeromonas spp. J Antimicrob Chemother 40:171–178. doi: 10.1093/jac/40.2.171. [DOI] [PubMed] [Google Scholar]
- 33.Wu CJ, Chen PL, Wu JJ, Yan JJ, Lee CC, Lee HC, Lee NY, Chang CM, Lin YT, Chiu YC, Ko WC. 2012. Distribution and phenotypic and genotypic detection of a metallo-beta-lactamase, CphA, among bacteraemic Aeromonas isolates. J Med Microbiol 61:712–719. doi: 10.1099/jmm.0.038323-0. [DOI] [PubMed] [Google Scholar]
- 34.Ko WC, Wu HM, Chang TC, Yan JJ, Wu JJ. 1998. Inducible beta-lactam resistance in Aeromonas hydrophila: therapeutic challenge for antimicrobial therapy. J Clin Microbiol 36:3188–3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee CH, Liu MS, Hsieh SH. 2003. Aeromonas hydrophila bacteremia presenting as non-traumatic acute osteomyelitis in a cirrhotic patient. Chang Gung Med J 26:520–524. [PubMed] [Google Scholar]
- 36.Sanchez-Cespedes J, Figueras MJ, Aspiroz C, Aldea MJ, Toledo M, Alperi A, Marco F, Vila J. 2009. Development of imipenem resistance in an Aeromonas veronii biovar sobria clinical isolate recovered from a patient with cholangitis. J Med Microbiol 58:451–455. doi: 10.1099/jmm.0.47804-0. [DOI] [PubMed] [Google Scholar]
- 37.Chen PL, Tsai PJ, Chen CS, Lu YC, Chen HM, Lee NY, Lee CC, Li CW, Li MC, Wu CJ, Ko WC. 2015. Aeromonas stool isolates from individuals with or without diarrhea in southern Taiwan: predominance of Aeromonas veronii. J Microbiol Immunol Infect 48:618–624. doi: 10.1016/j.jmii.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 38.Soler L, Yáñez MA, Chacon MR, Aguilera-Arreola MG, Catalán V, Figueras MJ, Martínez-Murcia AJ. 2004. Phylogenetic analysis of the genus Aeromonas based on two housekeeping genes. Int J Syst Evol Microbiol 54:1511–1519. doi: 10.1099/ijs.0.03048-0. [DOI] [PubMed] [Google Scholar]
- 39.Clinical and Laboratory Standards Institute. 2010. Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria; approved guideline, 2nd ed M45-A2 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 40.Tomas JM. 2012. The main Aeromonas pathogenic factors. ISRN Microbiol 2012:256261. doi: 10.5402/2012/256261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sen K, Rodgers M. 2004. Distribution of six virulence factors in Aeromonas species isolated from US drinking water utilities: a PCR identification. J Appl Microbiol 97:1077–1086. doi: 10.1111/j.1365-2672.2004.02398.x. [DOI] [PubMed] [Google Scholar]
- 42.Wu CJ, Chuang YC, Lee MF, Lee CC, Lee HC, Lee NY, Chang CM, Chen PL, Lin YT, Yan JJ, Ko WC. 2011. Bacteremia due to extended-spectrum-beta-lactamase-producing Aeromonas spp. at a medical center in Southern Taiwan. Antimicrob Agents Chemother 55:5813–5818. doi: 10.1128/AAC.00634-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen PL, Wu CJ, Chen CS, Tsai PJ, Tang HJ, Ko WC. 2014. A comparative study of clinical Aeromonas dhakensis and Aeromonas hydrophila isolates in southern Taiwan: A. dhakensis is more predominant and virulent. Clin Microbiol Infect 20:O428–O434. doi: 10.1111/1469-0691.12456. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.