Invasive Staphylococcus aureus infections account for 15 to 50% of fatal bloodstream infections annually. These disseminated infections often arise without a defined portal of entry into the host but cause high rates of mortality. The fungus Candida albicans and the Gram-positive bacterium S. aureus can form polymicrobial biofilms on epithelial tissue, facilitated by the C. albicans adhesin encoded by ALS3.
KEYWORDS: Candida albicans, Staphylococcus aureus, biofilms, innate immunity, polymicrobial
ABSTRACT
Invasive Staphylococcus aureus infections account for 15 to 50% of fatal bloodstream infections annually. These disseminated infections often arise without a defined portal of entry into the host but cause high rates of mortality. The fungus Candida albicans and the Gram-positive bacterium S. aureus can form polymicrobial biofilms on epithelial tissue, facilitated by the C. albicans adhesin encoded by ALS3. While a bacterium-fungus interaction is required for systemic infection, the mechanism by which bacteria disseminate from the epithelium to internal organs is unclear. In this study, we show that highly immunogenic C. albicans hyphae attract phagocytic cells, which rapidly engulf adherent S. aureus and subsequently migrate to cervical lymph nodes. Following S. aureus-loaded phagocyte translocation from the mucosal surface, S. aureus produces systemic disease with accompanying morbidity and mortality. Our results suggest a novel role for the host in facilitating a bacterium-fungus infectious synergy, leading to disseminated staphylococcal disease.
INTRODUCTION
Within environmental systems and hosts, microorganisms exist in complex multispecies communities rather than as isolated organisms. The formation of polymicrobial biofilms, which protects numerous bacteria and fungi within a polysaccharide extracellular matrix (1, 2), involves complex interspecies and interkingdom interactions (3). The formation of biofilms on devitalized tissues, on medical devices, and within immunocompromised patients continues to burden health care communities worldwide; biofilm-mediated infections account for about 25% of all nosocomial infections (4, 5). The incidence of polymicrobial implant-associated infections underwent an alarming 5-fold increase in 6 years (from 2004 to 2010, a period during which clinical protocols for detection and treatment were improved), highlighting the importance of this etiology in clinical care (6).
The Gram-positive bacterium Staphylococcus aureus and the polymorphic fungus Candida albicans are biofilm-forming, opportunistic pathogens capable of cocolonizing multiple niches in humans (7–9). S. aureus has remained in the spotlight due to the burgeoning increase in methicillin-resistant Staphylococcus aureus (MRSA) strains and the narrowing spectrum of effective antibiotics (10). The Centers for Disease Control and Prevention (CDC) estimates that 33% of the population carry S. aureus in their nares, while 2% harbor MRSA (7). This bacterium possesses a wide range of virulence factors, including immunoavoidance mechanisms, toxins, antimicrobial resistance determinants, and adherence proteins, which allow for increased pathogenesis (11). The production of toxins by community-associated strains, which tend to infect healthy individuals, is predicted to be regulated by unique genetic mechanisms, which enable rapid dissemination in vivo and increase the risk of necrotizing pneumonia (12). Despite these virulence mechanisms, most humans carry S. aureus as a commensal; establishment of invasive disease requires breaks in mucosal or integumental surfaces (13, 14).
C. albicans is also a commensal of humans, colonizing the gut, skin, and mucosal surfaces, but can act as a pathogen colonizing medical devices, such as dentures and catheters (15, 16). This polymorphic fungal pathogen can detect changes in environmental parameters and shift from the single-cell yeast morphology to the multicellular hyphal morphology. Using epithelial receptors and secreted factors, hyphae can actively penetrate mucosal barriers (17, 18). The dysfunction of immune functions related to systemic conditions, such as HIV infection/AIDS, cancer, and immunosuppression for organ transplant, enables this fungus to transition into an invasive pathogen, causing bloodstream infections with a high rate of mortality (19–21). The rate of oral candidiasis in AIDS and cancer patients is 9 to 31% and 20%, respectively (8, 22). As the frequency of Candida strains resistant to azole- and echinocandin-class drugs increases, the direct hospitalization costs are predicted to increase by millions of U.S. dollars annually (23).
S. aureus and C. albicans have been coisolated from patients with a range of biofilm-associated diseases, from noninvasive colonization of denture surfaces leading to tissue inflammation (denture stomatitis) to complex and life-threatening burn-wound infections, ventilator-associated pneumonia, and cystic fibrosis (24–28). Both organisms are consistently isolated from bloodstream infections (4, 8, 29–31), and up to 24% of patients with confirmed candidemia have concurrent bacteremia; S. aureus was isolated in 20% of these cases (32). As Klotz and colleagues noted, detection of candidemia is difficult, with most inquiries underestimating the true burden of disease; these clinical findings suggest that C. albicans is a risk factor for S. aureus bacteremia and that their synergistic interaction increases mortality rates (32).
We have investigated the mechanisms of the C. albicans and S. aureus association and reported that S. aureus can strongly adhere to C. albicans hyphae (33). This specific interaction is mediated by the Als3p protein of C. albicans (34) and is expressed exclusively in the hyphal morphology (35). These studies, as well as others, suggest that both organisms benefit from this interaction, which may modulate the host immune response differently than monospecies infections. Peters and Noverr (2013) utilized a murine model to compare monospecies and polymicrobial (C. albicans and S. aureus) peritonitis, the incidence of which is rising due to the increased use of peritoneal dialysis (36). Their results demonstrated increased neutrophil trafficking into the peritoneal cavity and heightened levels of proinflammatory cytokines associated with innate immune responses, including interleukin-6 (IL-6) and granulocyte colony-stimulating factor. However, the immunological mechanisms and signaling behind this infectious synergy remain unclear.
Using an established murine model of oral coinfection, we showed that a physical interaction between C. albicans hyphae and S. aureus is required for bacterial invasion (37). The association of S. aureus with Als3p-expressing C. albicans in the oral cavity resulted in S. aureus bacteremia and the isolation of S. aureus bacteria from kidney tissue. Histology demonstrated that C. albicans hyphae penetrated the tongue epithelium and that S. aureus comigrated with the hyphae. Therefore, we proposed the following hitchhiking hypothesis: S. aureus adhesion to C. albicans hyphae (mediated by Als3p) enables S. aureus to utilize the invasive potential of C. albicans (37). However, S. aureus rarely adheres to the growing tip of Candida hyphae and remains static as the hyphae grow at their tip, growth that mimics the traversal of epithelial layers of the tongue (see Fig. S1 in the supplemental material).
S. aureus can take up residence within phagocytes, such as macrophages and neutrophils, and persist at low levels until escaping (38–40). S. aureus can also replicate and survive within liver macrophages (Kupffer cells) by preventing macrophages from using reactive oxygen species (ROS) to clear the infection (40). Therefore, we now hypothesize that host phagocytic cells are recruited to the highly immunogenic C. albicans hyphae but are unable to engulf the relatively large (>20 μm) hyphal elements. Instead, hypha-attached S. aureus cells are easily taken up by activated phagocytes and disseminated systemically, resulting in infection. Thus, S. aureus may subvert the compromised oral innate immune system in critically ill patients and use phagocyte mobility to spread to other organs, causing infections that result in significant morbidity and mortality in those with immune dysfunction.
In this study, we examined the hypothesized mechanism by which C. albicans hyphae facilitate bacterial dissemination by analyzing in vitro phagocytosis by macrophages and neutrophils in the presence of S. aureus and C. albicans by time-lapse microscopy. Using our murine model of oral coinfection, we demonstrate that the draining cervical lymph nodes can function as a reservoir for S. aureus during coinfection. Our results suggest that the interaction between S. aureus and C. albicans enables the former to hitchhike within the innate phagocytic immune response and that the oral cavity can be an intrinsic portal for systemic disease.
RESULTS
S. aureus does not travel along growing C. albicans hyphae.
To evaluate how nonmotile S. aureus can utilize C. albicans to traverse epithelial tissue, we used time-lapse microscopy to visualize the association of S. aureus with C. albicans. We found that as hyphae extended at their tips, adherent S. aureus did not travel along the length of the hypha but remained at the initial attachment site (Fig. 1A; see also Video S1 in the supplemental material). This suggests that S. aureus, when it is strongly bound to Als3p on hyphae of C. albicans, does not travel alongside the growing hypha and may not utilize the penetrating potential of C. albicans hyphae to traverse epithelial tissue. In addition, under conditions simulating venous blood flow, S. aureus remained firmly attached to C. albicans (Video S2), preventing dissemination through the host.
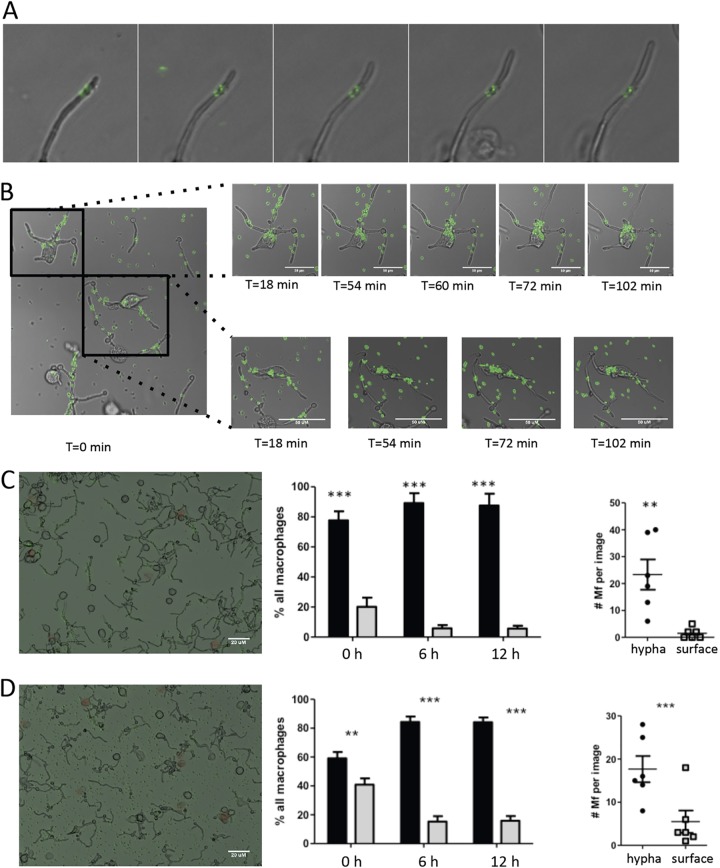
FIG 1.
S. aureus cells remain adherent to a single point on C. albicans hyphae in coculture in vitro. (A) Time-lapse microscopy was used to visualize S. aureus adhering to growing hyphae of C. albicans. (B) Active phagocytosis of adherent S. aureus from the hyphae of C. albicans was monitored for over 1 h after the introduction of macrophages. Macrophages can be seen extracting S. aureus from distant areas (top series) or crawling along hypha and removing nearby S. aureus (bottom series). (C) The impact of serum on immune cell targeting was tested by coating with 10% serum prior to macrophage application. In total, macrophages preferentially attached to C. albicans hyphae (center, black bars) over PS (center, gray bars), and after 6 h, 90% of all macrophages were attached to hyphae, where phagocytic activity occurred. Within individual views, an average of 25 macrophages (Mf) could be seen attaching to hyphae (right, black dots) and only a select few were detected still attached to PS (right, open squares). (D) The absence of serum did not impact the phagocytosis of S. aureus on the hyphae of C. albicans. Actively phagocytosing macrophages were scored relative to their hypha-associated state (center, black bars, and right, black dots) or surface-associated state (center, gray bars, and right, open squares). Bars = 20 μm. P values were determined by Student's t test. **, P <0.001; ***, P < 0.0001.
Innate immune cells are highly attracted to C. albicans yet phagocytose S. aureus.
Previous research has shown that innate immune cells, particularly macrophages, can attempt to engulf C. albicans yeast blastospheres but that this action triggers hyphal formation, leading to macrophage death (41, 42). Strikingly, phagocytes are generally more attracted to hyphae than to yeast cells due to significant differences in cell wall composition, including glucan production and expression (43). The adhesion forces of S. aureus on the hyphae of C. albicans are particularly strong (44); this prompted us to ask the question whether S. aureus strongly bound to the hyphae of C. albicans is protected against phagocytosis. We sought to determine if this occurs following the introduction of macrophages to C. albicans hyphae to more accurately simulate an innate immune response in the host. J774 murine macrophages applied to coculture immediately targeted hyphae (Fig. 1B; time zero) but clung to the appendage as they reached out to grab nearby green fluorescent protein (GFP)-labeled S. aureus (Fig. 1B; time [T] = 18 min and T = 54 min). Macrophages were capable of removing a significant amount of adherent S. aureus bacteria for a prolonged period (72 min) and climbed extending hyphae to reach distant bacteria (Fig. 1B, bottom, T = 54 and 72 min, and Video S3).
The interaction between S. aureus and surface interfaces, including C. albicans hyphae, can be modified by addition of fetal bovine serum (FBS). On polystyrene (PS) surfaces, serum inhibits the adhesion of S. aureus to most surfaces but not to C. albicans hyphae (45). Introduction of large quantities of serum (>25%) to hyphae results in the nonspecific binding of S. aureus and C. albicans (34, 46). We used the differential effect that serum has on the interaction of S. aureus with hyphae and PS surfaces to gain insight into the preference of macrophages for hypha-bound S. aureus or PS surface-bound S. aureus. Macrophages preferentially sought out hyphae irrespective of the presence of serum, and by 6 h, most macrophages were attached to hyphae (Fig. 1C and D, middle, black bars versus gray bars). Similar to previous results, we noted that S. aureus binding to C. albicans increased with just the addition of 10% serum (Fig. 1C and 1D, left). In the absence of serum, with S. aureus adhering to both PS surfaces and hyphae, macrophages preferably phagocytosed Candida-adherent S. aureus (Fig. 1D, left; the data are quantified in Fig. 1D, right) rather than surface-adherent S. aureus. These data suggest that macrophages are primarily recruited by C. albicans hyphae, leading or even facilitating the phagocytosis of hypha-adherent S. aureus.
S. aureus escapes the oral cavity by traversing draining cervical lymph nodes.
S. aureus typically requires a portal of entry, i.e., a breach of mucosal or skin integrity, to cause life-threatening disease (14). In light of our in vitro observations using a macrophage cell line, we used our murine model of oral coinfection to determine whether phagocytes facilitate staphylococcal dissemination. As described previously (37), using corticosteroid injections, we achieved consistent immunosuppression of C57BL/6 mice, which enabled colonization by C. albicans, with and without S. aureus, of the tongue tissue (Fig. 2A). This colonization was stable 1 and 2 days after coinfection with S. aureus (Fig. 2B). We noted persistent and heavy S. aureus infection of underlying tongue tissue only in the coinfected mice, supporting previous findings that S. aureus cannot actively penetrate the tongue epithelium (i.e., an intact mucosal barrier), requiring the presence of C. albicans (37).
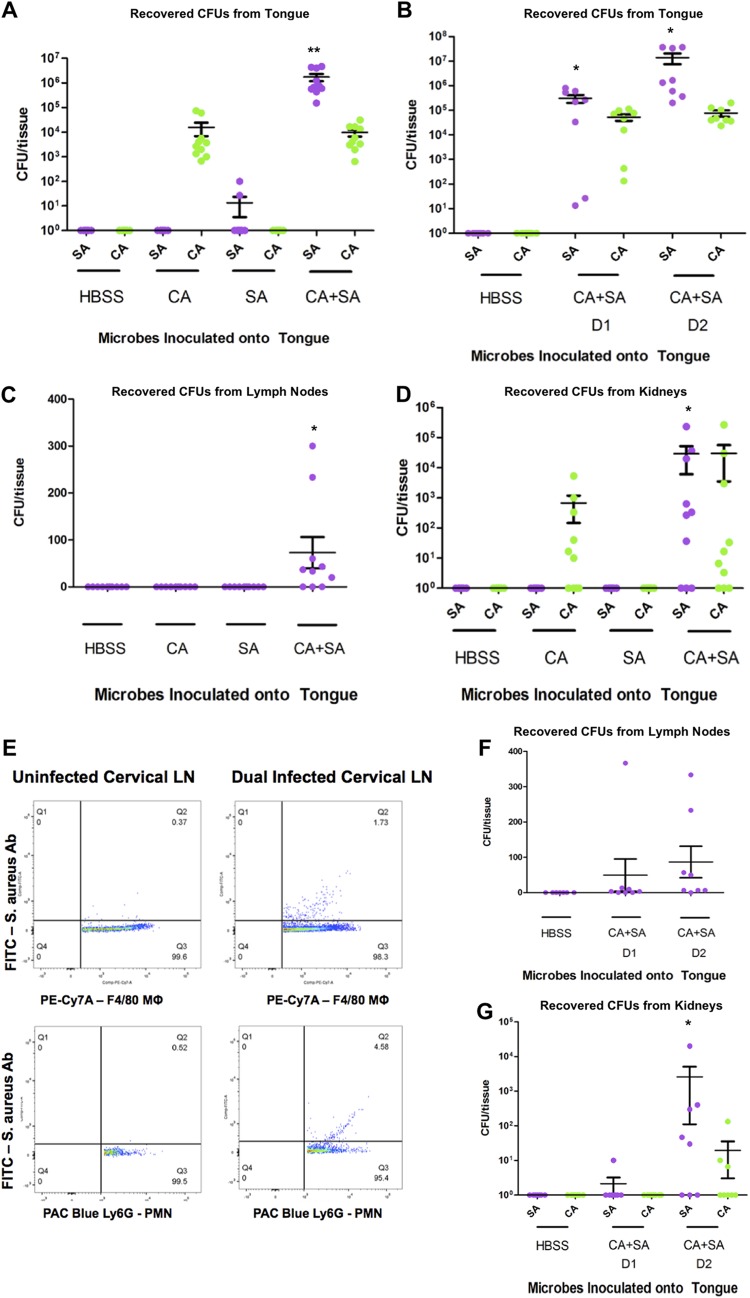
FIG 2.
In vivo oral coinfection demonstrates trafficking of phagocytes from tongue tissue to draining cervical lymph nodes and progression of systemic MRSA infection. (A and B) Numbers of CFU in tongue tissue following administration of Hanks balanced salt solution (HBSS), Candida albicans SC5314 alone (CA), methicillin-resistant Staphylococcus aureus USA300 alone (SA), or Candida albicans SC5314 alone and methicillin-resistant Staphylococcus aureus USA300 (CA+SA) to the oral cavity of C57BL/6 mice at 1 to 2 days (B) or 3 days (A) following oral inoculation of S. aureus. (C and D) Numbers of CFU in the draining cervical lymph nodes (C) and kidneys (D) at 3 days post-oral inoculation with S. aureus. (E) Flow cytometry analysis of cervical lymph node (LN) cell suspensions following fixation, host macrophage (PE-Cy7A-F4/80) or neutrophil (Pacific Blue [PAC Blue]-Ly6G) antibody labeling, permeabilization, and methicillin-resistant S. aureus (FITC-S. aureus antibody [Ab]) labeling 3 days following oral inoculation of uninfected controls or S. aureus and C. albicans dual species-infected mice. (F and G) Numbers of CFU in cervical lymph nodes (F) and kidneys (G) at 1 to 2 days postinoculation of S. aureus. P values were determined by one-way analysis of variance with Dunnett’s multiple-comparison test. *, P < 0.05; **, P < 0.001.
Viable S. aureus was detected exclusively in the draining cervical lymph nodes, which handle the lymph circulation for the oral cavity, of coinfected mice (Fig. 2C). Similarly, S. aureus disseminated into the kidney tissue only of coinfected mice, and coinfected mice that lacked disseminated disease also had no viable S. aureus in their lymph nodes (Fig. 2D). Fluorescence-activated cell sorting (FACS) analysis of lymph node cells of uninfected and coinfected mice gated on macrophages (F4/80 marker) and neutrophils (Ly6G marker) showed that those of coinfected mice contained intracellular S. aureus (Fig. 2E). At 1 and 2 days post-S. aureus infection, the bacterial burdens in both the lymph nodes and kidneys increased (Fig. 2F and G), demonstrating the temporal progression of S. aureus infection from the cervical lymph nodes to the kidneys. Mice infected solely with S. aureus had no lymph node or kidney bacterial burdens, supporting the findings of our previous studies that coinfection must be present for systemic disease (37). Taken together, these data support a role for host phagocytes in the development of systemic staphylococcal disease following oral coinfection by S. aureus and C. albicans.
Phagocytic cells converge on C. albicans hyphae in vivo early in infection and cause inflammatory destruction of the tongue structure.
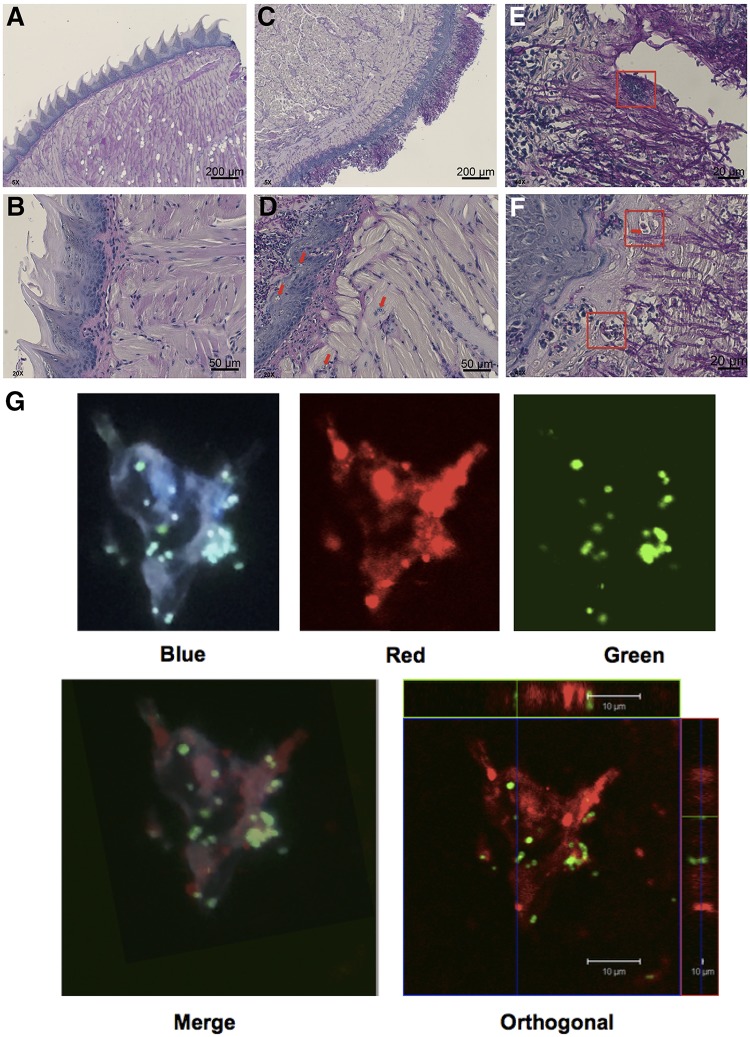
To link our earlier in vitro observations with the in vivo attraction of phagocytic cells by C. albicans, we used immunohistochemistry to visualize the impact of coinfection on tongue tissue and its innate defenses. We stained uninfected and infected tongue tissue sections from our murine model with periodic acid-Schiff (PAS) stain and counterstained with hematoxylin for nuclei. PAS stain stains the glucans and carbohydrates that compose fungal membranes, allowing the visualization of both yeast and hyphae. The overall tongue musculature was strikingly different between uninfected (Fig. 3A and B) and coinfected (Fig. 3C to F) mice. The loss of keratinized tissue peaks was prominent in coinfected mice, and the deep muscular layers of the tongue were highly disrupted, showing evidence of inflammation (Fig. 3C and D). Uninfected mice showed few inflammatory cells in the keratinized epithelium and muscle layers (Fig. 3A and B). Closer examination of infected tissue 1 day after coinfection demonstrated infiltration of polymorphonuclear leukocytes (PMNs) throughout the muscular layers and into the squamous epithelium on the dorsum of the tongue (Fig. 3D, red arrows). Bacterial clumps were associated with the hyphae of C. albicans, even at the early stages of infection (Fig. 3E, red box), and PMNs and monocytes were observed to be swelling and phagocytizing staphylococci (Fig. 3F, red boxes and red arrows). Immunohistochemical labeling of macrophages in tongue tissue at 5 days postinfection showed these cells actively engulfing the S. aureus bacteria within the keratinized layer (Fig. 3G and Fig. S4). Confocal imaging focusing on single macrophages demonstrated S. aureus within anti-CD68-labeled macrophages from orthogonal projections (Fig. 3G, bottom). These images provide support for the suggestion that, in vivo, phagocytes are attracted by C. albicans and can act as reservoirs for S. aureus, enabling the bacteria to access multiple organ systems in the host.
FIG 3.
Innate immune cells enter tongue tissue to colocalize at hyphae and bacteria within 1 day of coinfection. Tongues were excised from immunocompromised animals, embedded in paraffin, and stained with PAS. Images represent tongues from two uninfected mice (A and B) and two infected mice (C and D) at 1 day after coinfection with S. aureus and C. albicans. Red arrows indicate PMN invasion up hyphae (D), and red boxes indicate staphylococci clumping tightly with C. albicans hyphae and cells (E) or monocytes surrounding hyphae to seize bacteria (F; the red box and the red box with a red arrow indicate ingested staphylococci). Magnifications, ×5 (A and B), ×20 (C and D), and ×40 (E and F). (G) Immunohistochemically labeled macrophages (red filter) were seen engulfing S. aureus (green filter) only in coinfected mice. The fluorescent images were counterstained with DAPI to show nuclei (blue filter). An orthogonal image (bottom right) shows S. aureus within a macrophage. Magnifications, ×100.
DISCUSSION
In this study, we revisited our previous hypothesis that C. albicans facilitates staphylococcal disease by a mechanism of bacterial hitchhiking (37). We observed in vitro that adherent S. aureus does not progress with hyphal extension, thereby eliminating the possibility that staphylococci are actively transported through tissue as a bacterial hitchhiker. When phagocytic cells, the first lines of defense against infection, were added to these in vitro cultures, macrophages where highly attracted to C. albicans hyphae yet selectively engulfed the hypha-attached S. aureus. Therefore, we hypothesized that in vivo, highly immunogenic C. albicans hyphae attract phagocytic cells, which mediate staphylococcal uptake and dissemination, essentially providing S. aureus with a porte d’entrée.
To test this hypothesis, we used our established model of oral coinfection in immunocompromised C57BL/6 mice (37). FACS analysis of macrophages and neutrophils revealed intracellular S. aureus cells within the lymph nodes of only those hosts with oral coinfection with both S. aureus and C. albicans. Culture of lymph nodes from coinfected hosts showed that these phagocyte-associated staphylococci were viable. In addition, the data indicated a temporal progression of infection from the cervical lymph nodes to the kidneys. Finally, we visualized macrophages actively engulfing S. aureus in tongue tissue using immunohistochemistry and confocal microscopy. These results provide evidence that S. aureus bacteria attached to C. albicans hyphae are phagocytosed and are transported in this protected intracellular environment to distal host sites, promoting disseminated staphylococcal disease.
Macrophages are highly attracted to C. albicans, and the fungus is capable of inhibiting their respiratory burst by secreting molecules to disable nitric oxide production and alkalinizing the macrophage intracellular pH through fungal ammonia production, preventing fungal death. This allows engulfed yeast to transition into hyphae, perforate the phagosome, and escape back into the extracellular environment (41, 42, 47, 48). Multiple studies have previously shown the plasticity of the survival responses from C. albicans in the presence of macrophages through in vitro experiments; these studies focused on how yeast cells within a macrophage rely on transcription factors encoding amino acid metabolism to sense the phagosome environment and, in turn, induce hyphal formation (42, 48). Our in vitro studies aimed to examine how this interaction would change if the macrophage encountered penetrating hyphae and bacteria, representing invasion through tongue tissue, as seen in oropharyngeal candidiasis. Instead of hyphae perforating the immune cell and killing it, macrophages used the hyphae as a scaffold and targeted attached S. aureus (Fig. 1). This interaction was not inhibited by the exclusion of serum (Fig. 1C and D). These findings suggest that in the interaction of C. albicans with S. aureus, attraction of macrophages by hyphae facilitates phagocytosis of attached bacteria.
In mice with oral coinfection, we found viable S. aureus colonizing the lymph nodes and kidneys, suggesting that phagocytes do not kill S. aureus upon endocytosis of attached bacteria (Fig. 2C and D). We did not find viable C. albicans within lymph nodes, possibly due to an inability of macrophages and neutrophils to engulf large hyphal segments that would be present from an established case of candidiasis. Macrophage-mediated phagocytosis of bacteria is associated with the release of oxygen radicals into the phagosome to create an inhospitable environment (49). This oxidative burst is a vital component of innate immunity against bacteria (50). To sustain candidiasis, we administered corticosteroid injections to the mice prior to infection. The usage of corticosteroids remains common practice for transplant patients to prevent graft-versus-host disease and increases the risk of oropharyngeal and disseminated candidiasis in these patients (19). The impacts of corticosteroids on the immune system have long been explored; rat studies have shown that their application dampens immune responses and impairs the activity of alveolar macrophages against bacteria (51). In particular, IL-1β signaling is downregulated during corticosteroid usage, decreasing the effectiveness of the respiratory burst in these innate immune cells (52). Surewaard and colleagues (2016) noted that the loss of NADPH oxidase through the use of Ncf1m1J and Cybb−/− knockout mouse strains prevented innate immune cells from producing the needed reactive oxygen species and allowed MRSA to replicate to high levels in Kupffer cells of otherwise immunocompetent animals during intravenously induced S. aureus bacteremia (40). Only by fusing the antibiotic vancomycin to liposomes to make “vancosomes” that could be taken up by macrophages were the authors able to decrease liver colonization and increase survival (40). The intracellular survival of S. aureus has been described in macrophages and PMNs in immunocompetent hosts using in vitro and in vivo monospecies infections (reviewed in references 53 and 54). It is likely that in our mice, the macrophages and neutrophils that engulf S. aureus cells no longer function optimally, further enhancing the probability of bacterial survival. This finding, together with previous reports of the difficulty of treating pathogens hidden within these cells, is of great clinical importance (40, 55). S. aureus and C. albicans are often coisolated from immunocompromised patients (28, 32), and the coculture of both organisms from the blood is associated with a 50% mortality rate. This finding also suggests that treatment of polymicrobial infections may require targeting of immune cells to prevent reseeding after antibiotic treatment.
Our previous studies have established that Als3p expression by C. albicans is required for the binding of S. aureus and the subsequent dissemination from the oral cavity to distant organs, such as the kidney (34, 37, 56). Als3p is not the only mechanism of staphylococcal adherence to C. albicans, as others have noted that nonspecific interactions can be introduced by high levels of serum (46, 57), suggesting that serum components can assist with the binding between these pathogens. In the serum-limited oral cavity, such nonspecific binding is unlikely to occur; moreover, we demonstrated here that the interaction between C. albicans and S. aureus is strong and is unbroken by conditions simulating blood flow (see Fig. S2 in the supplemental material). As such, adherent bacteria could survive as hyphae traverse through tissue. However, we showed in vitro that attached bacteria are sedentary and are not passed along Als3p receptors or other binding mechanisms as the hyphae grow (Fig. 1A and Fig. S1). Therefore, C. albicans hypha formation does not represent a direct transport mechanism for S. aureus but, rather, represents a method to start infection and recruit macrophages into epithelial tissue. This is further supported by the massive inflammation at the epithelial border of the tongue (Fig. 3D and F) and confocal imaging of internalized S. aureus within tongue macrophages (Fig. 3G and Fig. S3). How S. aureus escapes the macrophage after phagocytosis during polymicrobial infection is unknown, but this bacterium possesses several leukocidins and toxins that can lyse cells (58). Identification of the staphylococcal factors that promote survival during the innate immune response will be the focus of future studies.
Phagocytosis is a complex process involving an interplay between phagocytic receptors and pattern recognition receptors (PRRs) that detect broad classes of pathogens (59). Initially, phagocytes are recruited to mucosal surfaces infected by Candida under the direction of host antimicrobial peptides (e.g., β-defensin 2) and chemokines (e.g., CCL20) (60–63). Phagocytes recognize the pathogen-associated molecular patterns (PAMPs), including N-linked and O-linked mannans and β-glucans, present on the fungal cell wall through host PRRs and attach to hyphae (64–67). Following this recognition, the relatively large hyphae are not effectively phagocytosed. However, the PAMPs (including peptidoglycan, lipoteichoic acid, and lipoproteins) of the hypha-adherent S. aureus bacteria are also readily identified by the host PRRs. The signaling pathways within host phagocytes that are influenced during phagocytosis of coinfecting C. albicans and S. aureus remain largely unknown. Extracellular ATP is recognized as a danger signal, released upon infection or cell death. Pérez-Flores and colleagues (2016) showed that extracellular ATP and Ca2+ play a role in phagocytosis by coating nanoparticles with S. aureus or live Candida glabrata yeast cells and feeding them to murine macrophages in the presence of exogenous ATP and Ca2+ (68). Membrane ionotropic P2X receptor 1 (P2X1) to P2X7 sense danger molecules, such as ATP, and P2X7 is abundant on the surface of macrophages (69). Activation of P2X7 is important in the response to infections caused by intracellular bacteria (70, 71). Pérez-Flores et al. reported that activation of P2X7 inhibited the phagocytosis of bacteria but not C. glabrata by J774 macrophages (68). This supports the notion that P2X receptors are required to sense the cellular damage caused by a bacterium-fungus coinfection and initiate the immune response. However, the mechanisms of phagocytosis, intracellular survival, and phagocytic escape of S. aureus during coinfection remain to be elucidated. Therefore, future studies will focus on the blockade of phagocyte signaling and its impact on bacterial dissemination during coinfection.
In summary, C. albicans hyphae attract phagocytes that target attached S. aureus cells, which are actively removed from these hyphae and phagocytosed by macrophages and PMNs. Following ingestion, S. aureus escapes intracellular killing and is trafficked to the draining host lymph nodes and further distal sites, resulting in life-threatening disseminated infections. To our knowledge, this is the first report of a critical role for the innate immune system in promoting the development of systemic disease following polymicrobial infection of the oral cavity. The impact of these findings reaches beyond S. aureus and C. albicans interactions at the oral mucosal surfaces. These findings may be applicable to other mucosal surfaces where these microbes coexist (e.g., the craniofacial, respiratory, gastrointestinal, and urogenital tracts) as well as in infections associated with indwelling medical devices (e.g., intravenous catheters, ventilator tubes), burn wounds, or local host immune dysfunction (e.g., diabetic foot ulcers, traumatic musculoskeletal infections, or decubitus ulcers). It is also possible that the oral innate immune cells transport periodontal pathogens, as periodontal disease is correlated with systemic diseases, such as diabetes, atherosclerosis, myocardial infarction, and stroke, with periodontal pathogens being detected in atherosclerotic and cardiac lesions (72–78). However, the direct implications of periodontal pathogens in these diseases remain debated, and further study is needed. Regardless, the results of this study highlight the importance of studying the systemic implications of localized polymicrobial infections as well as the complicity of the host immune response in the infectious synergism seen in some polymicrobial infections.
MATERIALS AND METHODS
Strains and growth conditions.
Candida albicans strain SC5314 (79) was maintained from glycerol freezer stocks on yeast-peptone-dextrose agar. Methicillin-resistant Staphylococcus aureus (MRSA) strain USA300 JE2, from the Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA) (80), was maintained from glycerol freezer stocks on sheep’s blood agar (BBL). GFP-labeled S. aureus ATCC 12600 (81) was used for in vitro assays and grown in brain heart infusion (BHI) broth supplemented with tetracycline (10 μg/ml; Sigma-Aldrich) at 37°C. For in vitro assays, C. albicans was grown in BHI broth at 30°C at an oblique angle to ensure growth in the yeast form only.
For animal studies, single colonies of C. albicans were grown in BHI broth (Teknova) supplemented with 10 μg/ml chloramphenicol (Sigma-Aldrich) and subcultured for 3 days prior to inoculation into mice to ensure the presence of yeast blastospores. Cultures were grown at 30°C with shaking at 225 rpm under aerobic conditions and on the day of inoculation were washed and diluted to 6 × 106 CFU/ml in Hanks balanced salt solution (HBSS). Single colonies of S. aureus JE2 were grown in Trypticase soy broth (TSB; Remel) overnight at 37°C and subcultured on the day of inoculation to mid-log phase. Cultures were washed and diluted to 6 × 106 CFU/ml in HBSS. All inocula were kept at 30°C (C. albicans) or 37°C (S. aureus) prior to infection and were enumerated using serial dilution and viable counting.
J774 macrophages.
J774 murine macrophages (ATCC TIB-67) were maintained in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum (FBS; Sigma-Aldrich) and an antibiotic-antimycotic solution (100 U/ml penicillin, 100 μg/ml streptomycin, and 250 ng/ml amphotericin B [PSA]; Sigma-Aldrich). For phagocytosis assays, 80% confluent macrophages were removed from the culture flasks using a sterile cell scraper and diluted 10-fold in DMEM–10% FBS without PSA. To determine macrophage viability during assays, 0.2 μl/ml propidium iodide (PI; Molecular Probes, Life Technologies) was added to diluted suspensions of macrophages.
In vitro phagocytosis.
Overnight cultures of C. albicans were diluted to an optical density at 600 nm (OD600) of ∼0.003 in phosphate-buffered saline (PBS; Gibco BRL). Tissue culture-treated 12-well plates were seeded with 1 ml C. albicans suspension (Greiner Bio-One) and incubated for 3 h at 37°C to allow initial attachment and hyphal growth. After 3 h, the wells were washed with PBS and coated with serum (1 ml of 50% FBS diluted in PBS) or PBS alone (1 ml) for 1 h. Following coating, the wells were washed with PBS and 1 ml of GFP-labeled S. aureus suspension diluted to an OD600 of ∼0.01 in PBS was added. C. albicans and S. aureus were then incubated for 1 h under gentle agitation to allow S. aureus to adhere to hyphae. Nonadherent S. aureus cells were removed by vigorous washing in PBS twice, and 1 ml of diluted (1:10) macrophage suspension in DMEM–10% FBS with or without PI was added. This dilution was determined to yield approximately 105 cells/ml. The plates were immediately placed under a microscope to visualize phagocytosis.
Time-lapse microscopy.
(i) Confocal microscopy. The plates were placed under a Leica IR-BE infrared confocal microscope (Leica Microsystems) at room temperature. Phase-contrast and fluorescence images were acquired at 6-min intervals for 100 min, and z-stacks were created to span single-cell layers (8 μm). Images were processed using Leica Confocal software (Leica Microsystems) and ImageJ software (http://imagej.nih.gov/ij/).
(ii) Inverted fluorescence microscopy. Plates were placed under an Axio Observer Z1 automated microscope in a heated (37°C) plate holder (Zeiss). C. albicans and macrophages were visualized using bright-field illumination, S. aureus was visualized by imaging the GFP signal, and viability was monitored by PI staining. Images of at least two randomly selected fixed plate positions per experimental condition per run were taken every 10 min. Macrophages were imaged at a ×20 magnification for a maximum of 12 h. PI staining showed that 75% of the macrophages remained viable at 12 h, while most PMNs had died after 6 h. The obtained images were further processed using Montage software (Molecular Devices) and ImageJ software (http://imagej.nih.gov/ij/).
(iii) Quantification of macrophages. To calculate the percentage of macrophages adhering to hyphae or plastic, macrophages in time-lapse stack images were counted at three time points: 1 (0 min), 37 (6 h), and 73 (12 h). Their positions and viability were determined and divided by the total number of cells per image. The numbers of actively phagocytosing macrophages were determined by creating time-lapse movies from stack images and counting the number of active cells per movie. Quantification was performed in six independent experiments, using images from at least two different plate positions per experimental condition. Comparisons of the macrophage location between treated plates and hyphae were performed using Student's t test (two-tailed, unequal variance).
Murine model of oral coinfection.
The murine immune response to a dual-species infection was tested using an oral coinfection model as described previously (37), with some modifications (see Fig. S5 in the supplemental material). The animal studies were approved by the University of Maryland Institutional Animal Care and Use Committee. Briefly, C57BL/6 mice were obtained from The Jackson Laboratory (Bar Harbor, ME) and maintained under pathogen-free conditions. Mice were given water treated daily with ampicillin (300 μg/ml) to reduce oral flora carriage and three subcutaneous shots of cortisone acetate (225 mg/kg of body weight) to suppress their immune system. Cortisone shots were given 1 day prior to the first inoculation (day 1) and every other day (days 3 and 5) thereafter to ensure continuous immunosuppression. The animals were anesthetized and laid supine while receiving a calcium alginate swab soaked in a C. albicans suspension (day 2) or an S. aureus suspension (day 4) for 75 min. Mice in the dual-species infection groups were inoculated on both days and, along with S. aureus-inoculated mice, were exposed to S. aureus (6 × 106 CFU/ml) in the drinking water for the remainder of the study. On day 6 or 7, depending on weight loss, mice were euthanized and their tongues, lymph nodes, and kidneys were harvested. A 2-mm slice of tongue was removed for determination of the microbial burden (number of CFU per tissue specimen). All tissues were homogenized in 500 μl of sterile PBS and serially diluted on CHROMagar for S. aureus and C. albicans. A total of 40 mice (10 mice per experimental condition) were used in three independent experiments (4 animals per group for 2 independent experiments, 2 animals per group for the immunofluorescence imaging independent experiment). Lymph nodes from uninfected and coinfected mice (four mice per group) were pooled from a separate experiment for analysis via flow cytometry.
Lymph nodes were placed in cold sterile flow cytometry buffer (eBioscience), and cells were released by gentle grinding between two sterile frosted-glass microscope slides. Cells were pipetted repeatedly through a 25-gauge needle to produce single-cell suspensions. The suspensions were flushed through a 40-μm-pore-size basket filter and washed before being labeled with phycoerythrin (PE)-Cy7 conjugated to anti-F4/80 (clone BM8; BioLegend), Pacific Blue conjugated to anti-Ly6G (clone 1A8; BD Biosciences), and Alexa Fluor 674 conjugated to anti-CD11b (clone M1/70; BioLegend) for 1 h at 4°C. The cells were then washed and fixed for intracellular straining for 30 min at room temperature. Suspensions were permeabilized by incubating three times for 5 min each time with permeabilization buffer (eBioscience) at room temperature. Intracellular S. aureus was labeled using a fluorescein isothiocyanate (FITC)-conjugated anti-S. aureus antibody (polyclonal anti-rabbit immunoglobulin; GeneTex) and incubated for 30 min at room temperature. Cells were washed, resuspended in flow cytometry buffer on ice, and read on a Becton, Dickinson LSRII instrument using FACSDiva software. The populations were gated on macrophages and neutrophils to determine the presence of intracellular S. aureus bacteria using FlowJo software (Tree Star, Inc.).
Tongue histology and immunohistochemistry.
Tongues were removed from euthanized uninfected mice and from mice euthanized 1 day after C. albicans-S. aureus infection. A small portion of tongue tissue was retained for CFU enumeration, and tongues were placed in neutral buffered formalin for paraffin embedding at the University of Maryland School of Medicine Pathology Associates. Sections were stained with periodic acid-Schiff (PAS) and examined by bright-field microscopy using a Zeiss Axio Imager microscope (Carl Zeiss). For immunohistochemistry, tongues were placed in Optimum Cutting Temperature (OCT) compound (Sakura Tissue-Tek) and snap-frozen in liquid nitrogen. Frozen tongue tissues were sectioned (10 μm) using a Leica AM1925 cryomicrotome and fixed in 4% paraformaldehyde solution. Nonspecific staining was blocked with bovine serum albumin (BSA), and the slides were stained with an FITC-conjugated anti-S. aureus antibody (as used in flow cytometry) and an eFluor 660-conjugated anti-CD68 antibody (clone FA-11; eBioscience) overnight at 4°C. On the following day, the slides were washed in PBS and counterstained with DAPI (4′,6-diamidino-2-phenylindole; ProLong Gold antifade mountant with DAPI; Molecular Probes), before being viewed under a Zeiss Meta confocal fluorescence microscope (Carl Zeiss) and analyzed using LSMIX software (Carl Zeiss).
Supplementary Material
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00137-19.
REFERENCES
- 1.Brogden KA, Guthmiller JM, Taylor CE. 2005. Human polymicrobial infections. Lancet 365:253–255. doi: 10.1016/S0140-6736(05)17745-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lynch AS, Robertson GT. 2008. Bacterial and fungal biofilm infections. Annu Rev Med 59:415–428. doi: 10.1146/annurev.med.59.110106.132000. [DOI] [PubMed] [Google Scholar]
- 3.Deveau A, Bonito G, Uehling J, Paoletti M, Becker M, Bindschedler S, Hacquard S, Herve V, Labbe J, Lastovetsky OA, Mieszkin S, Millet LJ, Vajna B, Junier P, Bonfante P, Krom BP, Olsson S, van Elsas JD, Wick LY. 2018. Bacterial-fungal interactions: ecology, mechanisms and challenges. FEMS Microbiol Rev 42:335–352. doi: 10.1093/femsre/fuy008. [DOI] [PubMed] [Google Scholar]
- 4.Lockhart SR, Etienne KA, Vallabhaneni S, Farooqi J, Chowdhary A, Govender NP, Colombo AL, Calvo B, Cuomo CA, Desjardins CA, Berkow EL, Castanheira M, Magobo RE, Jabeen K, Asghar RJ, Meis JF, Jackson B, Chiller T, Litvintseva AP. 2017. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis 64:134–140. doi: 10.1093/cid/ciw691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, Lynfield R, Maloney M, McAllister-Hollod L, Nadle J, Ray SM, Thompson DL, Wilson LE, Fridkin SK, Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team . 2014. Multistate point-prevalence survey of health care-associated infections. N Engl J Med 370:1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benito N, Franco M, Coll P, Gálvez ML, Jordán M, López-Contreras J, Pomar V, Monllau JC, Mirelis B, Gurguí M. 2014. Etiology of surgical site infections after primary total joint arthroplasties. J Orthop Res 32:633–637. doi: 10.1002/jor.22581. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. 2012. Active bacterial core surveillance report, Emerging Infections Program Network, methicillin-resistant Staphylococcus aureus, 2011. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 8.Morgan J, Meltzer MI, Plikaytis BD, Sofair AN, Huie-White S, Wilcox S, Harrison LH, Seaberg EC, Hajjeh RA, Teutsch SM. 2005. Excess mortality, hospital stay, and cost due to candidemia: a case-control study using data from population-based candidemia surveillance. Infect Control Hosp Epidemiol 26:540–547. doi: 10.1086/502581. [DOI] [PubMed] [Google Scholar]
- 9.Kim J, Sudbery P. 2011. Candida albicans, a major human fungal pathogen. J Microbiol 49:171–177. doi: 10.1007/s12275-011-1064-7. [DOI] [PubMed] [Google Scholar]
- 10.Mostofsky E, Lipsitch M, Regev-Yochay G. 2011. Is methicillin-resistant Staphylococcus aureus replacing methicillin-susceptible S. aureus? J Antimicrob Chemother 66:2199–2214. doi: 10.1093/jac/dkr278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.David MZ, Daum RS. 2010. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev 23:616–687. doi: 10.1128/CMR.00081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeLeo FR, Otto M, Kreiswirth BN, Chambers HF. 2010. Community-associated meticillin-resistant Staphylococcus aureus. Lancet 375:1557–1568. doi: 10.1016/S0140-6736(09)61999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Acton DS, Plat-Sinnige MJ, van Wamel W, de Groot N, van Belkum A. 2009. Intestinal carriage of Staphylococcus aureus: how does its frequency compare with that of nasal carriage and what is its clinical impact? Eur J Clin Microbiol Infect Dis 28:115–127. doi: 10.1007/s10096-008-0602-7. [DOI] [PubMed] [Google Scholar]
- 14.del Rio A, Cervera C, Moreno A, Moreillon P, Miro JM. 2009. Patients at risk of complications of Staphylococcus aureus bloodstream infection. Clin Infect Dis 48(Suppl 4):S246–S253. doi: 10.1086/598187. [DOI] [PubMed] [Google Scholar]
- 15.Cannon RD, Chaffin WL. 2001. Colonization is a crucial factor in oral candidiasis. J Dent Educ 65:785–787. [PubMed] [Google Scholar]
- 16.Budtz-Jorgensen E. 2000. Ecology of Candida-associated denture somatitis. Microb Ecol Health Dis 12:170–185. doi: 10.1080/089106000750051846. [DOI] [Google Scholar]
- 17.Phan QT, Fratti RA, Prasadarao NV, Edwards JE Jr, Filler SG. 2005. N-cadherin mediates endocytosis of Candida albicans by endothelial cells. J Biol Chem 280:10455–10461. doi: 10.1074/jbc.M412592200. [DOI] [PubMed] [Google Scholar]
- 18.Phan QT, Myers CL, Fu Y, Sheppard DC, Yeaman MR, Welch WH, Ibrahim AS, Edwards JE Jr, Filler SG. 2007. Als3 is a Candida albicans invasin that binds to cadherins and induces endocytosis by host cells. PLoS Biol 5:e64. doi: 10.1371/journal.pbio.0050064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dongari-Bagtzoglou A, Dwivedi P, Ioannidou E, Shaqman M, Hull D, Burleson J. 2009. Oral Candida infection and colonization in solid organ transplant recipients. Oral Microbiol Immunol 24:249–254. doi: 10.1111/j.1399-302X.2009.00505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Repentigny L, Lewandowski D, Jolicoeur P. 2004. Immunopathogenesis of oropharyngeal candidiasis in human immunodeficiency virus infection. Clin Microbiol Rev 17:729–759. doi: 10.1128/CMR.17.4.729-759.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. 1992. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recommend Rep 41(RR-17):1–19. [PubMed] [Google Scholar]
- 22.Schelenz S, Abdallah S, Gray G, Stubbings H, Gow I, Baker P, Hunter PR. 2011. Epidemiology of oral yeast colonization and infection in patients with hematological malignancies, head neck and solid tumors. J Oral Pathol Med 40:83–89. doi: 10.1111/j.1600-0714.2010.00937.x. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. 2013. Antibiotic resistance threats in the United States, 2013. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 24.Cuesta AI, Jewtuchowicz V, Brusca MI, Nastri ML, Rosa AC. 2010. Prevalence of Staphylococcus spp and Candida spp in the oral cavity and periodontal pockets of periodontal disease patients. Acta Odontol Latinoam 23:20–26. [PubMed] [Google Scholar]
- 25.Timsit JF, Cheval C, Gachot B, Bruneel F, Wolff M, Carlet J, Regnier B. 2001. Usefulness of a strategy based on bronchoscopy with direct examination of bronchoalveolar lavage fluid in the initial antibiotic therapy of suspected ventilator-associated pneumonia. Intensive Care Med 27:640–647. doi: 10.1007/s001340000840. [DOI] [PubMed] [Google Scholar]
- 26.Valenza G, Tappe D, Turnwald D, Frosch M, Konig C, Hebestreit H, Abele-Horn M. 2008. Prevalence and antimicrobial susceptibility of microorganisms isolated from sputa of patients with cystic fibrosis. J Cystic Fibrosis 7:123–127. doi: 10.1016/j.jcf.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Baena-Monroy T, Moreno-Maldonado V, Franco-Martinez F, Adalpe-Barrios B, Quindos G, Sanchez-Vargas LO. 2005. Candida albicans, Staphylococcus aureus and Streptococcus mutans colonization in patients wearing dental prosthesis. Med Oral Patol Oral Cir Bucal 10(Suppl 1):E27–E39. [PubMed] [Google Scholar]
- 28.Gupta N, Haque A, Mukhopadhyay G, Narayan RP, Prasad R. 2005. Interactions between bacteria and Candida in the burn wound. Burns 31:375–378. doi: 10.1016/j.burns.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 29.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK, Active Bacterial Core Surveillance (ABCs) MRSA Investigators . 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 30.Perlroth J, Choi B, Spellberg B. 2007. Nosocomial fungal infections: epidemiology, diagnosis, and treatment. Med Mycol 45:321–346. doi: 10.1080/13693780701218689. [DOI] [PubMed] [Google Scholar]
- 31.Kock R, Becker K, Cookson B, van Gemert-Pijnen JE, Harbarth S, Kluytmans J, Mielke M, Peters G, Skov RL, Struelens MJ, Tacconelli E, Navarro Torne A, Witte W, Friedrich AW. 2010. Methicillin-resistant Staphylococcus aureus (MRSA): burden of disease and control challenges in Europe. Euro Surveill 15(41):pii=19688 10.2807/ese.15.41.19688-en. [DOI] [PubMed] [Google Scholar]
- 32.Klotz SA, Chasin BS, Powell B, Gaur NK, Lipke PN. 2007. Polymicrobial bloodstream infections involving Candida species: analysis of patients and review of the literature. Diagn Microbiol Infect Dis 59:401–406. doi: 10.1016/j.diagmicrobio.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Peters BM, Jabra-Rizk MA, Scheper MA, Leid JG, Costerton JW, Shirtliff ME. 2010. Microbial interactions and differential protein expression in Staphylococcus aureus-Candida albicans dual-species biofilms. FEMS Immunol Med Microbiol 59:493–503. doi: 10.1111/j.1574-695X.2010.00710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peters BM, Ovchinnikova ES, Krom BP, Schlecht LM, Zhou H, Hoyer LL, Busscher HJ, van der Mei HC, Jabra-Rizk MA, Shirtliff ME. 2012. Staphylococcus aureus adherence to Candida albicans hyphae is mediated by the hyphal adhesin Als3p. Microbiology 158:2975–2986. doi: 10.1099/mic.0.062109-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beaussart A, Alsteens D, El-Kirat-Chatel S, Lipke PN, Kucharíková S, Van Dijck P, Dufrêne YF. 2012. Single-molecule imaging and functional analysis of Als adhesins and mannans during Candida albicans morphogenesis. ACS Nano 6:10950–10964. doi: 10.1021/nn304505s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peters BM, Noverr MC. 2013. Candida albicans-Staphylococcus aureus polymicrobial peritonitis modulates host innate immunity. Infect Immun 81:2178–2189. doi: 10.1128/IAI.00265-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schlecht LM, Peters BM, Krom BP, Freiberg JA, Hansch GM, Filler SG, Jabra-Rizk MA, Shirtliff ME. 2015. Systemic Staphylococcus aureus infection mediated by Candida albicans hyphal invasion of mucosal tissue. Microbiology 161:168–181. doi: 10.1099/mic.0.083485-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gresham HD, Lowrance JH, Caver TE, Wilson BS, Cheung AL, Lindberg FP. 2000. Survival of Staphylococcus aureus inside neutrophils contributes to infection. J Immunol 164:3713–3722. doi: 10.4049/jimmunol.164.7.3713. [DOI] [PubMed] [Google Scholar]
- 39.Tuchscherr L, Medina E, Hussain M, Volker W, Heitmann V, Niemann S, Holzinger D, Roth J, Proctor RA, Becker K, Peters G, Loffler B. 2011. Staphylococcus aureus phenotype switching: an effective bacterial strategy to escape host immune response and establish a chronic infection. EMBO Mol Med 3:129–141. doi: 10.1002/emmm.201000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Surewaard BG, Deniset JF, Zemp FJ, Amrein M, Otto M, Conly J, Omri A, Yates RM, Kubes P. 2016. Identification and treatment of the Staphylococcus aureus reservoir in vivo. J Exp Med 213:1141–1151. doi: 10.1084/jem.20160334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lorenz MC, Bender JA, Fink GR. 2004. Transcriptional response of Candida albicans upon internalization by macrophages. Eukaryot Cell 3:1076–1087. doi: 10.1128/EC.3.5.1076-1087.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vylkova S, Lorenz MC. 2014. Modulation of phagosomal pH by Candida albicans promotes hyphal morphogenesis and requires Stp2p, a regulator of amino acid transport. PLoS Pathog 10:e1003995. doi: 10.1371/journal.ppat.1003995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ballou ER, Avelar GM, Childers DS, Mackie J, Bain JM, Wagener J, Kastora SL, Panea MD, Hardison SE, Walker LA, Erwig LP, Munro CA, Gow NA, Brown GD, MacCallum DM, Brown AJ. 2016. Lactate signalling regulates fungal beta-glucan masking and immune evasion. Nat Microbiol 2:16238. doi: 10.1038/nmicrobiol.2016.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ovchinnikova ES, Krom BP, Busscher HJ, van der Mei HC. 2012. Evaluation of adhesion forces of Staphylococcus aureus along the length of Candida albicans hyphae. BMC Microbiol 12:281. doi: 10.1186/1471-2180-12-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ovchinnikova ES, van der Mei HC, Krom BP, Busscher HJ. 2013. Exchange of adsorbed serum proteins during adhesion of Staphylococcus aureus to an abiotic surface and Candida albicans hyphae—an AFM study. Colloids Surf B Biointerfaces 110:45–50. doi: 10.1016/j.colsurfb.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 46.Harriott MM, Noverr MC. 2010. Ability of Candida albicans mutants to induce Staphylococcus aureus vancomycin resistance during polymicrobial biofilm formation. Antimicrob Agents Chemother 54:3746–3755. doi: 10.1128/AAC.00573-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Collette JR, Zhou H, Lorenz MC. 2014. Candida albicans suppresses nitric oxide generation from macrophages via a secreted molecule. PLoS One 9:e96203. doi: 10.1371/journal.pone.0096203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vylkova S, Lorenz MC. 2017. Phagosomal neutralization by the fungal pathogen Candida albicans induces macrophage pyroptosis. Infect Immun 85:e00832-16. doi: 10.1128/IAI.00832-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Segal AW. 2008. The function of the NADPH oxidase of phagocytes and its relationship to other NOXs in plants, invertebrates, and mammals. Int J Biochem Cell Biol 40:604–618. doi: 10.1016/j.biocel.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sibille Y, Reynolds HY. 1990. Macrophages and polymorphonuclear neutrophils in lung defense and injury. Am Rev Respir Dis 141:471–501. doi: 10.1164/ajrccm/141.2.471. [DOI] [PubMed] [Google Scholar]
- 51.Gross NT, Chinchilla M, Camner P, Jarstrand C. 1996. Anticryptococcal activity by alveolar macrophages from rats treated with cortisone acetate during different periods of time. Mycopathologia 136:1–8. doi: 10.1007/BF00436653. [DOI] [PubMed] [Google Scholar]
- 52.Brattsand R, Linden M. 1996. Cytokine modulation by glucocorticoids: mechanisms and actions in cellular studies. Aliment Pharmacol Ther 10(Suppl 2):81–90. doi: 10.1046/j.1365-2036.1996.22164025.x. [DOI] [PubMed] [Google Scholar]
- 53.Flannagan RS, Heit B, Heinrichs DE. 2015. Antimicrobial mechanisms of macrophages and the immune evasion strategies of Staphylococcus aureus. Pathogens 4:826–868. doi: 10.3390/pathogens4040826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Kessel KP, Bestebroer J, van Strijp JA. 2014. Neutrophil-mediated phagocytosis of Staphylococcus aureus. Front Immunol 5:467. doi: 10.3389/fimmu.2014.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guidi-Rontani C. 2002. The alveolar macrophage: the Trojan horse of Bacillus anthracis. Trends Microbiol 10:405–409. doi: 10.1016/S0966-842X(02)02422-8. [DOI] [PubMed] [Google Scholar]
- 56.Peters BM, Jabra-Rizk MA, O'May GA, Costerton JW, Shirtliff ME. 2012. Polymicrobial interactions: impact on pathogenesis and human disease. Clin Microbiol Rev 25:193–213. doi: 10.1128/CMR.00013-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harriott MM, Noverr MC. 2009. Candida albicans and Staphylococcus aureus form polymicrobial biofilms: effects on antimicrobial resistance. Antimicrob Agents Chemother 53:3914–3922. doi: 10.1128/AAC.00657-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Diep BA, Otto M. 2008. The role of virulence determinants in community-associated MRSA pathogenesis. Trends Microbiol 16:361–369. doi: 10.1016/j.tim.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Freeman SA, Grinstein S. 2014. Phagocytosis: receptors, signal integration, and the cytoskeleton. Immunol Rev 262:193–215. doi: 10.1111/imr.12212. [DOI] [PubMed] [Google Scholar]
- 60.Dieu-Nosjean MC, Massacrier C, Homey B, Vanbervliet B, Pin JJ, Vicari A, Lebecque S, Dezutter-Dambuyant C, Schmitt D, Zlotnik A, Caux C. 2000. Macrophage inflammatory protein 3alpha is expressed at inflamed epithelial surfaces and is the most potent chemokine known in attracting Langerhans cell precursors. J Exp Med 192:705–718. doi: 10.1084/jem.192.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Feng Z, Jiang B, Chandra J, Ghannoum M, Nelson S, Weinberg A. 2005. Human beta-defensins: differential activity against candidal species and regulation by Candida albicans. J Dent Res 84:445–450. doi: 10.1177/154405910508400509. [DOI] [PubMed] [Google Scholar]
- 62.Thorley AJ, Goldstraw P, Young A, Tetley TD. 2005. Primary human alveolar type II epithelial cell CCL20 (macrophage inflammatory protein-3alpha)-induced dendritic cell migration. Am J Respir Cell Mol Biol 32:262–267. doi: 10.1165/rcmb.2004-0196OC. [DOI] [PubMed] [Google Scholar]
- 63.Sierro F, Dubois B, Coste A, Kaiserlian D, Kraehenbuhl JP, Sirard JC. 2001. Flagellin stimulation of intestinal epithelial cells triggers CCL20-mediated migration of dendritic cells. Proc Natl Acad Sci U S A 98:13722–13727. doi: 10.1073/pnas.241308598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brown GD, Taylor PR, Reid DM, Willment JA, Williams DL, Martinez-Pomares L, Wong SY, Gordon S. 2002. Dectin-1 is a major beta-glucan receptor on macrophages. J Exp Med 196:407–412. doi: 10.1084/jem.20020470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Taylor PR, Brown GD, Reid DM, Willment JA, Martinez-Pomares L, Gordon S, Wong SY. 2002. The beta-glucan receptor, dectin-1, is predominantly expressed on the surface of cells of the monocyte/macrophage and neutrophil lineages. J Immunol 169:3876–3882. doi: 10.4049/jimmunol.169.7.3876. [DOI] [PubMed] [Google Scholar]
- 66.Pietrella D, Bistoni G, Corbucci C, Perito S, Vecchiarelli A. 2006. Candida albicans mannoprotein influences the biological function of dendritic cells. Cell Microbiol 8:602–612. doi: 10.1111/j.1462-5822.2005.00651.x. [DOI] [PubMed] [Google Scholar]
- 67.Netea MG, Marodi L. 2010. Innate immune mechanisms for recognition and uptake of Candida species. Trends Immunol 31:346–353. doi: 10.1016/j.it.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 68.Pérez-Flores G, Hernández-Silva C, Gutiérrez-Escobedo G, De Las Peñas A, Castaño I, Arreola J, Pérez-Cornejo P. 2016. P2X7 from J774 murine macrophages acts as a scavenger receptor for bacteria but not yeast. Biochem Biophys Res Commun 481:19–24. doi: 10.1016/j.bbrc.2016.11.027. [DOI] [PubMed] [Google Scholar]
- 69.Burnstock G. 2016. P2X ion channel receptors and inflammation. Purinergic Signal 12:59–67. doi: 10.1007/s11302-015-9493-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fairbairn IP, Stober CB, Kumararatne DS, Lammas DA. 2001. ATP-mediated killing of intracellular mycobacteria by macrophages is a P2X(7)-dependent process inducing bacterial death by phagosome-lysosome fusion. J Immunol 167:3300–3307. doi: 10.4049/jimmunol.167.6.3300. [DOI] [PubMed] [Google Scholar]
- 71.Miller CM, Boulter NR, Fuller SJ, Zakrzewski AM, Lees MP, Saunders BM, Wiley JS, Smith NC. 2011. The role of the P2X(7) receptor in infectious diseases. PLoS Pathog 7:e1002212. doi: 10.1371/journal.ppat.1002212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nakano K, Nemoto H, Nomura R, Inaba H, Yoshioka H, Taniguchi K, Amano A, Ooshima T. 2009. Detection of oral bacteria in cardiovascular specimens. Oral Microbiol Immunol 24:64–68. doi: 10.1111/j.1399-302X.2008.00479.x. [DOI] [PubMed] [Google Scholar]
- 73.Kebschull M, Demmer RT, Papapanou PN. 2010. “Gum bug, leave my heart alone!”—epidemiologic and mechanistic evidence linking periodontal infections and atherosclerosis. J Dent Res 89:879–902. doi: 10.1177/0022034510375281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Haraszthy VI, Zambon JJ, Trevisan M, Zeid M, Genco RJ. 2000. Identification of periodontal pathogens in atheromatous plaques. J Periodontol 71:1554–1560. doi: 10.1902/jop.2000.71.10.1554. [DOI] [PubMed] [Google Scholar]
- 75.Kozarov E, Sweier D, Shelburne C, Progulske-Fox A, Lopatin D. 2006. Detection of bacterial DNA in atheromatous plaques by quantitative PCR. Microbes Infect 8:687–693. doi: 10.1016/j.micinf.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 76.Kozarov EV, Dorn BR, Shelburne CE, Dunn WA Jr, Progulske-Fox A. 2005. Human atherosclerotic plaque contains viable invasive Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis. Arterioscler Thromb Vasc Biol 25:e17–e18. doi: 10.1161/01.ATV.0000155018.67835.1a. [DOI] [PubMed] [Google Scholar]
- 77.Nakano K, Wada K, Nomura R, Nemoto H, Inaba H, Kojima A, Naka S, Hokamura K, Mukai T, Nakajima A, Umemura K, Kamisaki Y, Yoshioka H, Taniguchi K, Amano A, Ooshima T. 2011. Characterization of aortic aneurysms in cardiovascular disease patients harboring Porphyromonas gingivalis. Oral Dis 17:370–378. doi: 10.1111/j.1601-0825.2010.01759.x. [DOI] [PubMed] [Google Scholar]
- 78.Gaetti-Jardim E Jr, Marcelino SL, Feitosa AC, Romito GA, Avila-Campos MJ. 2009. Quantitative detection of periodontopathic bacteria in atherosclerotic plaques from coronary arteries. J Med Microbiol 58:1568–1575. doi: 10.1099/jmm.0.013383-0. [DOI] [PubMed] [Google Scholar]
- 79.Gillum AM, Tsay EY, Kirsch DR. 1984. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet 198:179–182. doi: 10.1007/BF00328721. [DOI] [PubMed] [Google Scholar]
- 80.Kennedy AD, Porcella SF, Martens C, Whitney AR, Braughton KR, Chen L, Craig CT, Tenover FC, Kreiswirth BN, Musser JM, DeLeo FR. 2010. Complete nucleotide sequence analysis of plasmids in strains of Staphylococcus aureus clone USA300 reveals a high level of identity among isolates with closely related core genome sequences. J Clin Microbiol 48:4504–4511. doi: 10.1128/JCM.01050-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li J, Busscher HJ, van der Mei HC, Norde W, Krom BP, Sjollema J. 2011. Analysis of the contribution of sedimentation to bacterial mass transport in a parallel plate flow chamber: part II: use of fluorescence imaging. Colloids Surf B Biointerfaces 87:427–432. doi: 10.1016/j.colsurfb.2011.06.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.