Abstract
Background
Antidepressants are widely used to treat chronic neuropathic pain (pain due to nerve damage), usually in doses below those at which they exert antidepressant effects. An earlier review that included all antidepressants for neuropathic pain is being replaced by new reviews of individual drugs examining individual neuropathic pain conditions.
Desipramine is a tricyclic antidepressant that is occasionally used for treating neuropathic pain.
Objectives
To assess the analgesic efficacy of desipramine for chronic neuropathic pain in adults, and to assess the associated adverse events.
Search methods
We searched CENTRAL, MEDLINE, and EMBASE from inception to 29 April 2014, and the reference lists of retrieved papers and other reviews. We also used our own hand searched database to identify older studies, and two clinical trials databases for ongoing or unpublished studies.
Selection criteria
We included randomised, double‐blind studies of at least two weeks duration comparing desipramine with placebo or another active treatment in chronic neuropathic pain. Participants were adults aged 18 years and over. We included only full journal publication articles.
Data collection and analysis
Two review authors independently extracted the efficacy and adverse event data, and examined issues of study quality. We performed analysis using three tiers of evidence. First tier evidence was derived from data meeting current best standards and subject to minimal risk of bias (outcome equivalent to substantial pain intensity reduction, intention‐to‐treat analysis without imputation for dropouts, at least 200 participants in the comparison, 8 to 12 weeks duration, parallel design); second tier from data that failed to meet one or more of these criteria and were considered at some risk of bias but with adequate numbers in the comparison; and third tier from data involving small numbers of participants and considered very likely to be biased or that used outcomes of limited clinical utility, or both.
Main results
Five studies treated 177 participants with painful diabetic neuropathy (104) or postherpetic neuralgia (73). The mean or median ages in the studies were 55 to 72 years. Four studies used a cross‐over, and one a parallel group design; 145 participants were randomised to receive desipramine 12.5 mg to 250 mg daily, with most taking 100 mg to 150 mg daily following titration. Comparators were placebo in three studies (an 'active placebo' in two studies), fluoxetine, clomipramine (one study each), and amitriptyline (two studies), and treatment was for two to six weeks. All studies had one or more sources of potential major bias.
No study provided first or second tier evidence for any outcome. No data were available on the proportion of people with at least 50% or 30% reduction in pain, but data were available from three studies for our other primary outcome of Patient Global Impression of Change, reported as patient evaluation of pain relief that was 'complete' or 'a lot'. No pooling of data was possible, but third tier evidence in individual studies indicated some improvement in pain relief with desipramine compared with placebo, although this was very low quality evidence, derived mainly from group mean data and completer analyses in small, short duration studies where major bias was possible. There were too few participants in comparisons of desipramine with another active treatment to draw any conclusions.
All studies reported some information about adverse events, but reporting was inconsistent and fragmented. Participants taking desipramine experienced more adverse events, and a higher rate of withdrawal due to adverse events, than did participants taking placebo (very low quality evidence).
Authors' conclusions
This review found little evidence to support the use of desipramine to treat neuropathic pain. There was very low quality evidence of benefit and harm, but this came from studies that were methodologically flawed and potentially subject to major bias. Effective medicines with much greater supportive evidence are available. There may be a role for desipramine in patients who have not obtained pain relief from other treatments.
Plain language summary
Desipramine for neuropathic pain in adults
Neuropathic pain is pain coming from damaged nerves. It is different from pain messages carried along healthy nerves from damaged tissue (a fall, cut, or arthritic knee). Neuropathic pain is treated by different medicines than pain from damaged tissue. Medicines like paracetamol or ibuprofen are not usually effective in neuropathic pain, while medicines that are sometimes used to treat depression or epilepsy can be very effective in some people with neuropathic pain.
Desipramine is a tricyclic antidepressant from the same class of medicines as amitriptyline, which is widely recommended for treating neuropathic pain. Desipramine may also be useful in these painful conditions. In 2014, we performed searches to look for clinical trials where desipramine was used to treat neuropathic pain.
Five small studies, each with 24 to 54 participants, included 177 participants in total with painful diabetic neuropathy or postherpetic neuralgia. Studies were randomised and double‐blind, but all had one or more sources of potential major bias that could lead to overestimation of efficacy. It was not possible to combine information from the different studies, but individually they indicated some benefit from desipramine (usually at a dose between 100 mg and 150 mg daily), compared with placebo, at the expense of increased adverse events. There was not enough information about other comparators to draw any conclusions.
There was too little information, which was of inadequate quality, to be sure that desipramine works as a pain medicine in painful diabetic neuropathy or postherpetic neuralgia, and no information about other types of neuropathic pain. Other medicines have been shown to be effective as treatments of first choice.
Summary of findings
for the main comparison.
| Desipramine compared with placebo for painful diabetic neuropathy and postherpetic neuralgia | ||||||
|
Patient or population: adults with neuropathic pain (3 studies in painful diabetic neuropathy and postherpetic neuralgia) Settings: community Intervention: Desipramine 12.5 mg to 250 mg daily Comparison: Placebo (or active placebo ‐ benztropine) | ||||||
| Outcomes | Outcome with comparator (placebo) | Outcome with intervention | RR (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments |
| At least 50% reduction in pain | No data | No data | n/a | n/a | ||
| At least 30% reduction in pain | No data | No data | n/a | n/a | ||
| Proportion below 30/100 mm on VAS | No data | No data | n/a | n/a | ||
| Patient Global Impression of Change very much improved (PGPR "complete" or "a lot") | 3.8 % and 8.3% (3 events) | 17% and 30% (12 events) |
Not calculated | 50 participants, 2 studies | Very low | Small numbers of participants in 2 studies with short duration, cross‐over design, different painful neuropathy condition |
| Patient Global Impression of Change much or very much improved (PGPR "complete", "a lot", or "moderate") | 7.7% and 8.3% (4 events) |
46% in both studies (23 events) |
Not calculated | 50 participants, 2 studies | Very low | Small numbers of participants in 2 studies with short duration, cross‐over design, different painful neuropathy condition |
| Adverse event withdrawals | 0% to 12% (4 events) |
0% to 19% (10 events) |
Not calculated | 76 participants, 3 studies | Very low | Small numbers of participants in 3 studies with short duration, cross‐over design |
| Serious adverse events | None reported | None reported | n/a | 177 participants, 5 studies | n/a | Small numbers of participants in 5 studies of short duration (placebo‐ and active‐controlled) |
| Death | None reported | None reported | n/a | 177 participants, 5 studies | n/a | Small numbers of participants in 5 studies of short duration (placebo‐ and active‐controlled) |
CI: confidence interval; PGPR: Patient global evaluation of pain relief; RR: risk ratio; VAS: visual analogue scale; n/a: not applicable
Background
This review is based on a template for reviews of drugs used to relieve neuropathic pain. The aim is for all reviews to use the same methods, based on new criteria for what constitutes reliable evidence in chronic pain (Moore 2010a) (Appendix 1).
Desipramine is a tricyclic antidepressant (TCA) that is sometimes used to treat chronic neuropathic pain (pain due to nerve damage or changes in the central nervous system (CNS)). While its use is not specifically recommended, it is listed alongside other tricyclic antidepressants in some treatment guidelines, although this is an unlicensed indication (Attal 2010; Dworkin 2010; Finnerup 2010; Moulin 2007). It is an active metabolite of imipramine, the subject of another Cochrane review (Hearn 2014).
Description of the condition
The 2011 International Association for the Study of Pain (IASP) definition of neuropathic pain is "pain caused by a lesion or disease of the somatosensory system" (Jensen 2011), based on an earlier consensus meeting (Treede 2008). Neuropathic pain may be caused by nerve damage, but is often followed by changes in the CNS (Moisset 2007). It is complex (Apkarian 2011; Tracey 2011), and neuropathic pain features can be found in patients with joint pain (Soni 2013). Many people with neuropathic pain conditions are significantly disabled with moderate or severe pain for many years.
Chronic painful conditions comprise five of the 11 top‐ranking conditions for years lived with disability in 2010 (Vos 2012), and are responsible for considerable loss of quality of life, employment, and increased health costs (Moore 2014a).
In primary care in the UK, the incidences, per 100,000 person‐years observation, have been reported as 28 (95% confidence interval (CI) 27 to 30) for postherpetic neuralgia, 27 (95% CI 26 to 29) for trigeminal neuralgia, 0.8 (95% CI 0.6 to 1.1) for phantom limb pain, and 21 (95% CI 20 to 22) for painful diabetic neuropathy (Hall 2008). The incidence of postherpetic neuralgia in the UK appears to be increasing (Hall 2013). Estimates vary between studies, often because of small numbers of cases. The incidence of trigeminal neuralgia has been estimated at 4 in 100,000 per year (Katusic 1991; Rappaport 1994), while more recently, a study of facial pain in the Netherlands found incidences per 100,000‐person years of 12.6 for trigeminal neuralgia and 3.9 for postherpetic neuralgia (Koopman 2009). A systematic review of chronic pain demonstrated that some neuropathic pain conditions, such as painful diabetic neuropathy, can be more common, with prevalence rates up to 400 per 100,000 person‐years (McQuay 2007), illustrating how common the condition is, as well as its chronicity. The prevalence of neuropathic pain was reported as being 3.3% in Austria (Gustorff 2008), 6.9% in France (Bouhassira 2008), as high as 8% in the UK (Torrance 2006), and about 7% in a systematic review of studies published since 2000 (Moore 2014a). The incidence of some forms of neuropathic pain, such as diabetic neuropathy and postsurgical chronic pain (which is often neuropathic in origin), is increasing (Hall 2008).
Neuropathic pain is known to be difficult to treat effectively, with only a minority of individuals experiencing a clinically relevant benefit from any one intervention. A multidisciplinary approach is now advocated, with pharmacological interventions being combined with physical or cognitive interventions, or both. Conventional analgesics are usually not effective. Some patients may derive some benefit from a topical lidocaine patch or low concentration topical capsaicin, though evidence about benefits is uncertain (Derry 2012; Derry 2014). High‐concentration topical capsaicin may benefit some patients with postherpetic neuralgia (Derry 2013). Treatment is more usually by so‐called unconventional analgesics such as antidepressants like duloxetine and amitriptyline (Lunn 2014; Moore 2012a; Sultan 2008), or antiepileptics like gabapentin or pregabalin (Moore 2009; Moore 2014b). An overview of treatment guidelines shows general similarities based on the evidence available, but guidelines are not always consistent with one another (O'Connor 2009). The proportion of patients who achieve worthwhile pain relief (typically at least 50% pain intensity reduction (Moore 2013a)) is small, generally 10% to 25% more than with placebo, with numbers needed to treat to benefit (NNTs) usually between 4 and 10 (Moore 2013b).
Description of the intervention
Desipramine is a tricyclic antidepressant, and is an active metabolite of imipramine, which is the subject of a separate review (Hearn 2014). While these medicines are still used to treat depressive illness, they have also been used to treat neuropathic pain, although they are not licensed for use in this context in the UK or USA.
Desipramine is available as tablets (10 mg, 25 mg, 50 mg, 75 mg, 100 mg, and 150 mg). For treating neuropathic pain, typical starting dosages are between 10 mg and 25 mg daily, usually taken at night, increasing to 150 mg daily if necessary. Common adverse events associated with its use include dry mouth, constipation, weight gain, blurred vision, and orthostatic hypotension. It is less sedating than amitriptyline.
How the intervention might work
Desipramine is a very strong reuptake inhibitor of norepinephrine, and to a lesser extent, serotonin. It is also a metabolite of imipramine. Its mechanism of action in the treatment of neuropathic pain remains uncertain, but it is thought that norepinephrine reuptake inhibition causes activation of descending pathways in the spinal cord, which then block ascending signals to the brain. Tricyclic antidepressants are also known to inhibit sodium channels in nerve membranes, with an efficacy comparable to conventional local anaesthetics (Lenkey 2006; Sudoh 2003). This would predict a rapid onset of pain relief. TCAs are known to block sodium channels, binding at the local anaesthetic site at plasma levels found with therapeutically relevant doses.
That the mechanism differs from that in treating depression is confirmed by the observation that analgesia with antidepressants is often achieved at a lower dosage than for the onset of any antidepressant effect, and more promptly. In addition, there is no correlation between the effects of antidepressants on mood and pain, and antidepressants produce analgesia in patients with and without depression (Onghena 1992).
Why it is important to do this review
The earlier review of antidepressants for neuropathic pain is being updated with separate reviews for individual drugs due to the larger amount of data now available for some of them (Saarto 2007). The individual reviews (including amitriptyline (Moore 2012a), imipramine (Hearn 2014), and duloxetine (Lunn 2014)) will be included in an overview review of antidepressant drugs for neuropathic pain.
The standards used to assess evidence in chronic pain trials have changed substantially, with particular attention being paid to trial duration, withdrawals, and statistical imputation following withdrawal, all of which can substantially alter estimates of efficacy (Appendix 1). The most important change is the move from using average pain scores, or average change in pain scores, to the number of patients who have a large decrease in pain (by at least 50%) and who continue their treatment, ideally in trials of 8 to 12 weeks or longer. Pain intensity reduction of 50% or more has been shown to correlate with improvements in comorbid symptoms, function, and quality of life. These standards are set out in the reference guide for pain studies (AUREF 2012).
This Cochrane systematic review assesses evidence in ways that make both statistical and clinical sense, and uses developing criteria for what constitutes reliable evidence in chronic pain (Moore 2010a). Trials included and analysed meet a minimum of reporting quality (blinding, randomisation), validity (duration, dose and timing, diagnosis, outcomes) and size (ideally at least 500 participants in a comparison in which the NNT is four or above (Moore 1998)). This does set high standards and marks a departure from how reviews have been done previously.
Objectives
To assess the analgesic efficacy of desipramine for chronic neuropathic pain in adults, and to evaluate adverse events reported in the studies.
Methods
Criteria for considering studies for this review
Types of studies
We included studies if they were randomised controlled trials (RCTs) with double‐blind assessment of participant outcomes following two weeks of treatment or longer, although the emphasis of the review was on studies of eight weeks or longer. We required full journal publication, with the exception of online clinical trial results summaries of otherwise unpublished clinical trials and abstracts with sufficient data for analysis. We did not include short abstracts (usually meeting reports). We excluded studies that were non‐randomised, studies of experimental pain, case reports, and clinical observations.
Types of participants
Participants were aged 18 years and above and could have one or more of a wide range of chronic neuropathic pain conditions including (but not limited to):
cancer‐related neuropathy;
complex regional pain syndrome (CRPS) Type II;
human immunodeficiency virus (HIV) neuropathy;
painful diabetic neuropathy;
phantom limb pain;
postherpetic neuralgia;
postoperative or traumatic neuropathic pain;
spinal cord injury;
trigeminal neuralgia;
and
CRPS Type I.
We would include studies of participants with more than one type of neuropathic pain; in such cases we would analyse results according to the primary condition. Migraine and headache studies were excluded.
Types of interventions
Oral desipramine at any dose, administered for the relief of neuropathic pain and compared to placebo or any active comparator.
Types of outcome measures
Studies used a variety of outcome measures, with the majority using subjective scales (numerical rating scale (NRS) or visual analogue scale (VAS)) for pain intensity or pain relief, or both. We were particularly interested in the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) definitions for moderate and substantial benefit in chronic pain studies (Dworkin 2008). These are defined as at least 30% pain relief over baseline (moderate), at least 50% pain relief over baseline (substantial), much or very much improved on the Patient Global Impression of Change (PGIC) (moderate), and very much improved on the PGIC (substantial). These outcomes concentrate on dichotomous outcomes in circumstances where pain responses do not follow a normal (Gaussian) distribution. People with chronic pain desire high levels of pain relief, ideally more than 50%, and with pain not worse than mild (O'Brien 2010).
We have included a 'Summary of findings' table as set out in the author guide (AUREF 2012), which includes outcomes of at least 50% and at least 30% pain intensity reduction, PGIC, adverse event withdrawals, serious adverse events and death (Table 1).
Primary outcomes
Patient‐reported pain relief of 30% or greater
Patient‐reported pain relief of 50% or greater
PGIC much or very much improved
PGIC very much improved
Secondary outcomes
Any pain‐related outcome indicating some improvement
Withdrawals due to lack of efficacy
Participants experiencing any adverse event
Participants experiencing any serious adverse event. Serious adverse events typically include any untoward medical occurrence or effect that at any dose results in death, is life‐threatening, requires hospitalisation or prolongation of existing hospitalisation, results in persistent or significant disability or incapacity, is a congenital anomaly or birth defect, is an ‘important medical event’ that may jeopardise the patient, or may require an intervention to prevent one of the above characteristics or consequences
Withdrawals due to adverse events
Specific adverse events, particularly somnolence and dizziness
These outcomes were not eligibility criteria for this review, but outcomes of interest within the included studies.
Search methods for identification of studies
Electronic searches
We searched the following databases:
the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2014, Issue 4 of 12);
MEDLINE (via Ovid) (1946 to 29 April 2014);
EMBASE (via Ovid) (1974 to 29 April 2014).
Search strategies for CENTRAL, MEDLINE, and EMBASE are in Appendix 2, Appendix 3, and Appendix 4. There were no language restrictions.
Searching other resources
We reviewed the bibliographies of any randomised trials identified and review articles, and searched clinical trial databases (ClinicalTrials.gov (http://clinicaltrials.gov/) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal (http://apps.who.int/trialsearch/)) to identify additional published or unpublished data. We did not contact investigators or study sponsors because of the age of the studies identified and because this is not an area of active research.
Data collection and analysis
The intention was to perform separate analyses according to particular neuropathic pain conditions. Analyses combining different neuropathic pain conditions would be done for exploratory purposes only.
Selection of studies
We determined eligibility by reading the abstract of each study identified by the search. We eliminated studies that clearly did not satisfy the inclusion criteria, and we obtained full copies of the remaining studies; decisions were made by two review authors. Two review authors then read these studies independently and reached agreement by discussion. We did not anonymise the studies in any way before assessment. A Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow chart shows the selection process (Moher 2009).
Data extraction and management
Two review authors independently extracted data using a standard form and checked for agreement before entry into the Cochrane Collaboration's statistical software (Review Manager 2013) or any other analysis tool. We included information about the pain condition and number of participants treated, drug and dosing regimen, study design (placebo or active control), study duration and follow‐up, analgesic outcome measures and results, withdrawals and adverse events (participants experiencing any adverse event, or serious adverse event).
Assessment of risk of bias in included studies
We used the Oxford Quality Score as the basis for inclusion (Jadad 1996), limiting inclusion to studies that were randomised and double‐blind as a minimum.
Two authors independently assessed risk of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and adapted from those used by the Cochrane Pregnancy and Childbirth Group, with any disagreements resolved by discussion.
We assessed the following for each study.
Random sequence generation (checking for possible selection bias). We assessed the method used to generate the allocation sequence as: low risk of bias (any truly random process, for example random number table; computer random number generator); unclear risk of bias (method used to generate sequence not clearly stated). We excluded studies using a non‐random process (for example, odd or even date of birth; hospital or clinic record number).
Allocation concealment (checking for possible selection bias). The method used to conceal allocation to interventions prior to assignment determines whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. We assessed the methods as: low risk of bias (for example, telephone or central randomisation; consecutively numbered sealed opaque envelopes); unclear risk of bias (method not clearly stated). We excluded studies that did not conceal allocation (for example, open list).
Blinding of outcome assessment (checking for possible detection bias). We assessed the methods used to blind study participants and outcome assessors from knowledge of which intervention a participant received. We assessed the methods as: low risk of bias (study stated that it was blinded and described the method used to achieve blinding, for example, identical tablets; matched in appearance and smell); unclear risk of bias (study stated that it was blinded but did not provide an adequate description of how it was achieved). We excluded studies that were not double‐blind.
Incomplete outcome data (checking for possible attrition bias due to the amount, nature, and handling of incomplete outcome data). We assessed the methods used to deal with incomplete data as: low risk (< 10% of participants did not complete the study or used ‘baseline observation carried forward' (BOCF) analysis, or both); unclear risk of bias (used 'last observation carried forward' (LOCF) analysis); high risk of bias (used 'completer' analysis).
Size of study (checking for possible biases confounded by small size). We assessed studies as being at low risk of bias (≥ 200 participants per treatment arm); unclear risk of bias (50 to 199 participants per treatment arm); high risk of bias (< 50 participants per treatment arm).
Measures of treatment effect
We planned to calculate numbers needed to treat (NNTs) as the reciprocal of the absolute risk reduction (ARR) (McQuay 1998). For unwanted effects, the NNT becomes the number needed to treat to harm (NNH) and is calculated in the same manner. We planned to use dichotomous data to calculate risk ratio (RR) with 95% CI using a fixed‐effect model unless significant statistical heterogeneity was found (Assessment of heterogeneity). Continuous data were not used in the analyses.
Unit of analysis issues
The control treatment arm would be split between active treatment arms in a single study if the active treatment arms were not combined for analysis.
For cross‐over studies we planned to use first period data only wherever possible. Where this was not reported we analysed the data as if the treatment periods were parallel, drawing attention to the potential bias this may introduce, and interpreting the results accordingly.
Dealing with missing data
Where possible we have used intention‐to‐treat (ITT) analysis where the ITT population consists of participants who were randomised, took at least one dose of the assigned study medication, and provided at least one post‐baseline assessment. Missing participants were assigned zero improvement. Where this was not possible we have used the results as reported, but drawn attention to the potential bias this may introduce. These data were not used in any analyses.
Assessment of heterogeneity
We planned to deal with clinical heterogeneity by combining studies that examined similar conditions. We would have assessed statistical heterogeneity visually (L'Abbé 1987), and with the use of the I2 statistic.
Assessment of reporting biases
The aim of this review was to use dichotomous data of known utility (Moore 2010c). The review did not depend on what authors of the original studies chose to report or not, though clearly difficulties arose in studies failing to report any dichotomous results. We have extracted and reported continuous data, which probably poorly reflect efficacy and utility, where useful, for illustrative purposes only.
We planned to assess publication bias using a method designed to detect the amount of unpublished data with a null effect required to make any result clinically irrelevant (usually taken to mean an NNT of 10 or higher) (Moore 2008).
Data synthesis
We planned to use a fixed‐effect model for meta‐analysis or use a random‐effects model for meta‐analysis if there was significant clinical heterogeneity and it was considered appropriate to combine studies.
We analysed data for each painful condition in three tiers, according to outcome and freedom from known sources of bias.
The first tier uses data meeting current best standards, where studies report the outcome of at least 50% pain intensity reduction over baseline (or its equivalent), without the use of LOCF or other imputation method for dropouts, report an ITT analysis, last eight or more weeks, have a parallel‐group design, and have at least 200 participants (preferably at least 400) in the comparison (Moore 2010a; Moore 2012b). These top tier results are reported first.
The second tier uses data from at least 200 participants but where one or more of the above conditions is not met (for example reporting at least 30% pain intensity reduction, using LOCF or a completer analysis, or lasting four to eight weeks).
The third tier of evidence relates to data from fewer than 200 participants, or where there are expected to be significant problems because, for example, of very short duration studies of less than four weeks, where there is major heterogeneity between studies, or where there are shortcomings in allocation concealment, attrition, or incomplete outcome data. For this third tier of evidence, no data synthesis is reasonable, and may be misleading, but an indication of beneficial effects might be possible.
Subgroup analysis and investigation of heterogeneity
We planned all analyses to be according to individual painful conditions, because placebo response rates with the same outcome can vary between conditions, as can the drug‐specific effects (Moore 2009). If there had been sufficient data we would have carried out subgroup analysis for dose of desipramine and duration of study.
Sensitivity analysis
If there had been sufficient data we would have examined details of dose escalation schedules to investigate if this could provide some basis for a sensitivity analysis.
Results
Description of studies
Results of the search
Searches of bibliographic databases found 33 titles in CENTRAL, 160 in MEDLINE, and 140 in EMBASE, which we examined for inclusion. After screening titles and abstracts, we obtained full copies and examined seven reports in detail. We included five studies and excluded two (Figure 1). Searches of trial databases and study or review reference lists did not identify any additional studies.
1.
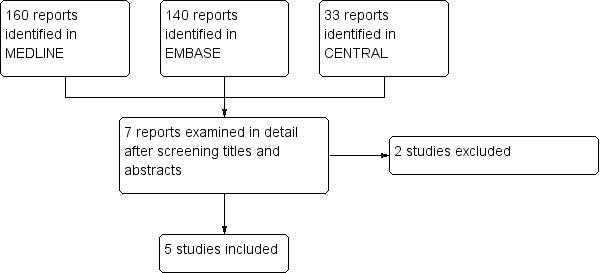
Study flow diagram.
Included studies
Five studies treated 177 participants, of whom 145 were randomised to desipramine (Kishore‐Kumar 1990; Max 1991; Max 1992; Rowbotham 2005; Sindrup 1990); in the cross‐over studies, however, it was not clear that all randomised participants received all treatments, and most studies did not report results for all randomised participants (that is, completer analyses reported). In four studies, there was a desipramine dose‐finding phase (Kishore‐Kumar 1990; Max 1991; Max 1992; Rowbotham 2005): in the first of these, 3 out of 32 participants withdrew because of adverse effects. Participants took oral desipramine for between two and six weeks. Daily doses were between 12.5 mg and 250 mg daily (mostly 100 mg to 200 mg). The licensed maximum dose is 300 mg. In one study, poor metabolisers were treated with lower doses (Sindrup 1990).
Study participants were aged between 20 and 84 years (study means or medians from 55 to 72 years), with an approximately five to four ratio of men to women (94 men; 76 women; 7 not stated (Sindrup 1990)). Participants had experienced pain associated with postherpetic neuralgia (Kishore‐Kumar 1990; Rowbotham 2005) (73 participants) or diabetic neuropathy (Max 1991; Max 1992; Sindrup 1990) (104 participants) for at least three months. Common grounds for exclusion from studies were severe depression, severe pain of other cause, and medical contra‐indication.
In three studies, desipramine was compared to placebo (Sindrup 1990) or active placebo (benztropine) (Kishore‐Kumar 1990; Max 1991). The active placebo was chosen to mimic dry mouth, which is associated with TCAs, and help maintain blinding.
In three studies, other analgesics were compared to desipramine: clomipramine (Sindrup 1990); amitriptyline (Max 1992; Rowbotham 2005); fluoxetine (Rowbotham 2005).
One study used a parallel‐group design (Rowbotham 2005), while the other four used a cross‐over design (Kishore‐Kumar 1990; Max 1991; Max 1992; Sindrup 1990). One study had washout periods between cross‐over periods (Sindrup 1990).
Stable medication for diabetes was maintained.
Excluded studies
We excluded two studies after reading the full papers. Coquoz 1991 used participants who were healthy volunteers, while Raja 2002 randomised participants to drug classes, not to individual drugs, and results for individual drugs were not reported separately.
Risk of bias in included studies
Comments on potential biases in individual studies are reported in the risk of bias section of the Characteristics of included studies table. The findings are displayed in Figure 2 and Figure 3; no sensitivity analysis was undertaken. The greatest risk of bias came from small study size.
2.
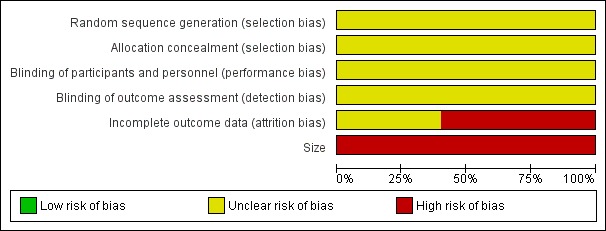
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
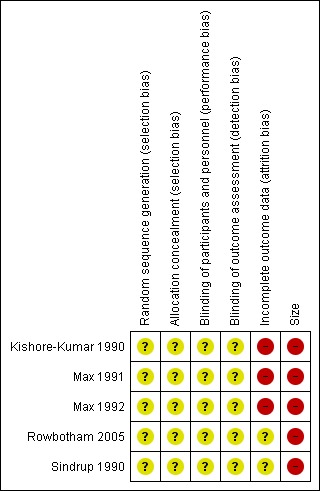
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All studies were randomised. No studies adequately described how allocation to treatment groups was concealed.
Blinding
All studies were double blind. Only one study (Sindrup 1990) adequately described the methods used to ensure that participants and interacting investigators were unable to differentiate between active and control groups; another referred to use of placebo capsules to maintain the same dosing regime (Rowbotham 2005).
Incomplete outcome data
All four cross‐over studies (Kishore‐Kumar 1990; Max 1991; Max 1992; Sindrup 1990) performed efficacy analyses only on those participants who completed all treatment phases of the study (completer analyses), and did not report results for the first phase only. The parallel group study performed an intention‐to‐treat (ITT) analysis (imputation not reported) (Rowbotham 2005).
Proportions of withdrawals with desipramine varied: 5/26 (Kishore‐Kumar 1990); 2/24 (Max 1991); 7/54 (Max 1992); 2/15 (Rowbotham 2005); 3/26 (Sindrup 1990). Therefore, some 13% of participants randomised to desipramine dropped out.
Missing participants can sometimes be added back in (analysed as non‐responders) for dichotomous outcomes, but this is not possible when mean data are reported.
Selective reporting
All studies reported the outcomes specified in their methods but these were usually not our preferred (primary) outcomes. Pain was not reported separately in Sindrup 1990.
Other potential sources of bias
None of the studies randomised sufficient numbers of participants to minimise the bias associated with small studies (Nüesch 2010). The greatest number randomised was 54 (although only 38 provided data) (Max 1992) so the risk of bias is high for this item.
Effects of interventions
See: Table 1
Efficacy
There was no first or second tier evidence of efficacy. Evidence was downgraded primarily because of the short duration of the studies, small numbers of participants in comparisons, reporting of completer analyses in cross‐over studies, and lack of desirable primary outcomes.
Details of data from individual studies are shown in Appendix 5.
Third tier evidence
None of the studies reported our primary outcome of participants with at least 30% or at least 50% reduction in pain intensity. Four studies used a six‐point scale to assess the Patient Global evaluation of Pain Relief (PGPR) (complete, a lot, moderate, slight, none, worse). We considered that PGPR complete or a lot was equivalent to our primary outcome of PGIC very much improved, and PGPR complete, a lot, or moderate was equivalent to PGIC much or very much improved.
Kishore‐Kumar 1990 reported that PGPR was complete or a lot for 8/19 (ITT 8/26) taking desipramine (1/19 with placebo; ITT 1/26) and at least moderate for 12/19 with desipramine (2/19 with placebo). Participants' weekly rating of pain intensity was significantly lower with desipramine than with placebo by the end of the study. Some pain components were also reduced compared with placebo (steady: 9/19 compared with 1/19; brief: 4/8 compared with 0/8; mechanical allodynia: 7/18 compared with 1/18).
Max 1991 reported similar findings that PGPR was complete or a lot for 4/20 (ITT 4/24) taking desipramine (2/20 with placebo; ITT 2/24) and at least moderate for 11/20 with desipramine (2/20 with placebo). Participants' weekly rating of pain intensity was significantly lower with desipramine than with placebo by the end of the study. Some pain components were also reduced compared with placebo: pain described as "steady, burning pain" was significantly reduced).
Max 1992 found that PGPR was similar for desipramine and amitriptyline (placebo not used): complete or a lot for 15/38 (ITT 15/54) and 18/38 (ITT 18/54) participants respectively, and at least moderate for 23/38 and 28/38 respectively. Participants' weekly rating of pain intensity was not significantly different.
Rowbotham 2005 reported that Visual Analogue Scale (VAS) readings fell by 47% with desipramine, compared with 38% and 35% falls with amitriptyline and fluoxetine respectively. PGPR was at least moderate for 12/15 with desipramine, 9/17 with amitriptyline, and 5/15 with fluoxetine (ITT analysis).
In Sindrup 1990, pain was not reported separately but as part of a set of neuropathy symptoms. Both observers and participants reported a significant reduction in symptom scores with desipramine compared to placebo; there was a non‐significant fall in the individual pain item score with desipramine.
Adverse events
Details of adverse events reported in individual studies are in Appendix 6. All studies reported some information about adverse events, but reporting was inconsistent and fragmented.
Participants experiencing any adverse event
One study reported the number of participants who experienced one or more adverse events for all those who were treated (ITT) (Max 1992). Two studies reported this number for completers (Kishore‐Kumar 1990; Max 1991). Two studies reported on mean or median adverse event scores (based on the intensity of the event) for completers only (Rowbotham 2005; Sindrup 1990).
Kishore‐Kumar 1990 reported that all completers had experienced at least one adverse event with desipramine (19/19), while 15/19 had done so with placebo. The most common events with desipramine were dry mouth, constipation, dizziness, sedation, micturition difficulty, insomnia, and sweating. The most common with placebo were dry mouth, dizziness, and constipation, but these were experienced at lower levels than with desipramine.
Max 1991 reported that nearly all completers had experienced at least one adverse event with desipramine (18/20), while nearly as many had done so with placebo (15/19). The most common (over 10%) events with desipramine were dry mouth, sedation, insomnia, constipation, orthostatic symptoms, palpitations, and increased sweating. The most common with placebo were dry mouth, sedation, constipation, and insomnia. Insomnia, constipation, and dry mouth were slightly more frequent with desipramine.
Max 1992 reported similar numbers of participants with one or more adverse events with both desipramine (29/54) and amitriptyline (31/54). The most common (over 10%) events with desipramine were dry mouth, tiredness, constipation, insomnia, increased sweating, headache, lightheadedness. Figures were similar for amitriptyline.
Rowbotham 2005 reported that completers' scores (presumably means) on a symptom checklist increased by more than 1 (for a range of 0 to 3) for adverse events as follows. Desipramine and amitriptyline: dry mouth, constipation, bitter taste; fluoxetine: sleepiness and lightheadedness.
Sindrup 1990 reported medians of completers' total scores for 14 side effects, with observers assigning values to each symptom (5‐point scale from 0 to 2.0). For desipramine and clomipramine the medians of the total scores were 4.5 and 4.0 respectively. They reported a median of 0.02 for placebo scores but this must be erroneous.
Participants experiencing any serious adverse event
No serious adverse events were reported during desipramine treatment, though one participant was hospitalised for nausea and weakness with hyponatraemia during fluoxetine treatment (Rowbotham 2005).
There were no deaths.
Withdrawals
All five studies reported on withdrawals. Details of withdrawals reported in individual studies are in Appendix 6.
Withdrawals due to adverse events
All five studies reported on withdrawals due to adverse events.
In four studies (Kishore‐Kumar 1990; Max 1991; Max 1992; Sindrup 1990) 17/130 participants withdrew during desipramine treatment, 4/76 during placebo treatment (Kishore‐Kumar 1990; Max 1991; Sindrup 1990), 7/54 during amitriptyline treatment (Max 1992), and 3/26 during clomipramine treatment (Sindrup 1990).
There was a variety of reasons for these withdrawals: three were due to rash, two to fever, two to bundle branch block, five to possible symptoms of low blood pressure, and one each to tiredness, insomnia, palpitations, tremor, jitteriness, chest pain, and "seizure".
The four placebo withdrawals were due to combinations of vertigo, nausea, rash, unsteadiness, mental fogginess, and chest pain. All occurred with the 'active' placebo treatment.
Rowbotham 2005 reported nine withdrawals but gave reasons for only five of these. There were 2/15 with desipramine and 2/17 with amitriptyline: adverse events were cited for three of these but the drug involved was not specified. There were 5/15 withdrawals with fluoxetine, adverse events being cited for two of these and no reason given for the others.
Withdrawals due to lack of efficacy
There were no withdrawals due to lack of efficacy with desipramine, one with placebo (Max 1991), and one with clomipramine (Sindrup 1990).
Withdrawals for other reasons
Kishore‐Kumar 1990 reported 2/26 withdrawals for "intercurrent illness", and Max 1992 reported two "voluntary" withdrawals.
As noted above, Rowbotham 2005 gave reasons for only 5/9 withdrawals. Three from the fluoxetine arm remain unexplained, as did one from either the desipramine or amitriptyline arms.
Discussion
Summary of main results
We found five studies enrolling 177 participants with chronic neuropathic pain, 59% of whom had painful diabetic neuropathy and 41% of whom had postherpetic neuralgia.
None of the studies reported our primary outcomes of at least 30% or at least 50% pain relief, but three reported outcomes we considered equivalent to our other primary outcomes of PGIC much or very much improved, and PGIC very much improved. No first or second tier evidence was available. No pooling of data was possible, but third tier evidence in individual studies indicated some improvement in pain relief with desipramine compared with placebo, although this was derived mainly from group mean data and completer analyses (see Appendix 1), in small, short duration studies where major bias is possible. Participants taking desipramine experienced more adverse events, and a higher rate of withdrawal due to adverse events, than did participants taking placebo. See Table 1.
Comparison with alternative treatments indicate more effective and safer medicines are available (Lunn 2014; Moore 2009; Moore 2014b).
Overall completeness and applicability of evidence
Desipramine was tested only in painful diabetic neuropathy and postherpetic neuralgia, so results cannot be reliably extrapolated to other neuropathic conditions.
Since desipramine is a TCA, depression among participants may conceivably affect the results. Three studies excluded people with severe depression (Kishore‐Kumar 1990; Max 1991; Max 1992). One reported on the incidence of depression in participants and its progress during the trial (Rowbotham 2005); the small numbers involved do not lend themselves to any conclusion about the effect of depression on pain relief. Sindrup 1990 do not mention depression. Three studies showed a rapid onset of pain relief (Kishore‐Kumar 1990; Max 1991; Max 1992), consistent with prompt action on sodium channels, and a different mechanism of action than that of its antidepressant effect. Rowbotham 2005 do not describe the speed of onset; Sindrup 1990 only tested for two weeks and, while there is evidence of pain relief, changes were not significant.
Short‐term studies (less than six weeks) may not accurately predict longer‐term efficacy in chronic conditions: four studies were of six weeks' duration, while one was of only two weeks duration. Furthermore, caution is required in interpreting adverse event data from short duration studies for real world clinical practice, particularly where so few participants have been studied.
Quality of the evidence
Reporting quality in the studies was generally poor by current standards. While all the studies were randomised and double‐blind, none provided data that met predefined criteria for first or second tier analysis. All the studies were small (with a maximum of 54 participants in any treatment arm). Four of the five were of short duration (six weeks), used cross‐over design without separate reporting of first period data, and reported only on participants who completed more than one phase of treatment. One was of very short duration (two weeks).
Differential rates of adverse events between placebo and treatment arms can suggest unblinding, but in these studies adverse events were assessed inconsistently, making comparisons difficult. None of the studies reported asking participants to guess their allocation at the end of the trial to check for unblinding. The methods used to collect adverse events could usefully include symptom checklists for self‐completion at baseline and at assessment points; these can even be graded for severity. Three studies did this (Kishore‐Kumar 1990; Max 1991; Max 1992), giving proportions reporting particular symptoms, but not reporting the time course of adverse events. Rowbotham 2005 used a checklist but only reported qualitatively. Sindrup 1990 seems to have used a checklist but reported only median scores (and those incorrectly).
Potential biases in the review process
The review was restricted to randomised double‐blind studies, thus limiting the potential for bias. Other possible sources of bias that could have affected the review included the following.
Duration: NNT estimates of efficacy in chronic pain studies tend to increase (get worse) with increasing duration (Moore 2010b). None of the studies lasted more than six weeks, which may lead to an overestimate of efficacy.
The degree of exaggeration of treatment effects in cross‐over trials compared to parallel‐group designs, as has been seen in some circumstances (Khan 1996), is unclear but unlikely to be the source of major bias (Elbourne 2002). Withdrawals meant that any results were more likely to be per protocol for completers than for a true ITT analysis. The majority of data in this review were from cross‐over studies.
All four cross‐over studies reported results only for those who completed at least two treatment periods, which is likely to overestimate efficacy. Where possible, we have indicated results for an ITT analysis (adding missing participants back into the denominator) for one primary outcome, to give a more conservative estimate.
The absence of publication bias (unpublished trials showing no benefit of desipramine over placebo) can never be proven. We carried out a broad search for studies and feel it is unlikely that significant amounts of data remain unknown to us.
Agreements and disagreements with other studies or reviews
This new review does not change the results of the previous Cochrane review (Saarto 2007).
Guidelines to treat neuropathic pain in Europe and UK do not specifically recommend use of desipramine (Attal 2010; NICE 2013). In USA, Dworkin 2010 recommend desipramine as an alternative to nortriptyline as a first‐line TCA treatment for neuropathic pain.
Authors' conclusions
Implications for practice.
This review found little evidence to support the use of desipramine to treat neuropathic pain. There was very low quality evidence of some effect from studies that were methodologically flawed and potentially subject to major bias. There are more effective and safer medicines available. There may be a role for use of desipramine in patients who have not obtained pain relief from other treatments.
Implications for research.
Larger, better‐designed studies would provide more definitive conclusions on the efficacy of desipramine, but it is unlikely that these will be carried out, given the age of the drug and the alternatives available, or that they could be justified on the evidence available.
Reasonable levels of evidence exist for the benefit of other antiepileptic and antidepressant drugs in the treatment of chronic neuropathic pain. There is a need to develop new treatments for neuropathic pain conditions, given that at present we have limited efficacy for a limited number of drugs, and some people remain inadequately treated.
What's new
| Date | Event | Description |
|---|---|---|
| 4 October 2019 | Review declared as stable | See Published notes. |
History
Protocol first published: Issue 2, 2014 Review first published: Issue 9, 2014
| Date | Event | Description |
|---|---|---|
| 1 October 2014 | Review declared as stable | This review will be assessed for further updating in 2019 as it is unlikely that new evidence will be published. |
Notes
A restricted search in September 2019 did not identify any potentially relevant studies likely to change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. We will update the review if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Acknowledgements
Institutional support was provided by the Oxford Pain Relief Trust.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane PaPaS Group. The views and opinions expressed in this review are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS) or the Department of Health.
Appendices
Appendix 1. Methodological considerations for chronic pain
There have been several recent changes in how efficacy of conventional and unconventional treatments is assessed in chronic painful conditions. The outcomes are now better defined, particularly with new criteria of what constitutes moderate or substantial benefit (Dworkin 2008); older trials may only report participants with 'any improvement'. Newer trials tend to be larger, avoiding problems from the random play of chance. Newer trials also tend to be longer, up to 12 weeks, and longer trials provide a more rigorous and valid assessment of efficacy in chronic conditions. New standards have evolved for assessing efficacy in neuropathic pain, and we are now applying stricter criteria for inclusion of trials and assessment of outcomes, and are more aware of problems that may affect our overall assessment. To summarise some of the recent insights that must be considered in this new review.
Pain results tend to have a U‐shaped distribution rather than a bell‐shaped distribution. This is true in acute pain (Moore 2011a; Moore 2011b), back pain (Moore 2010c), arthritis (Moore 2010b), as well as in fibromyalgia (Straube 2010); in all cases average results usually describe the experience of almost no‐one in the trial. Data expressed as averages are potentially misleading, unless they can be proven to be suitable.
As a consequence, we have to depend on dichotomous results (the individual either has or does not have the outcome) usually from pain changes or patient global assessments. The IMMPACT group has helped with their definitions of minimal, moderate, and substantial improvement (Dworkin 2008). In arthritis, trials shorter than 12 weeks, and especially those shorter than eight weeks, overestimate the effect of treatment (Moore 2010b); the effect is particularly strong for less effective analgesics, and this may also be relevant in neuropathic‐type pain.
The proportion of patients with at least moderate benefit can be small, even with an effective medicine, falling from 60% with an effective medicine in arthritis, to 30% in fibromyalgia (Moore 2009; Moore 2010b; Straube 2008; Sultan 2008). A Cochrane systematic review of pregabalin in neuropathic pain demonstrated different response rates for different types of chronic pain (higher in diabetic neuropathy and postherpetic neuralgia and lower in central pain) (Moore 2009). This indicates that different neuropathic pain conditions should be treated separately from one another, and that pooling should not be done unless there are good grounds for doing so.
Finally, presently unpublished individual patient analyses indicate that patients who get good pain relief (moderate or better) have major benefits in many other outcomes, affecting quality of life in a significant way (Moore 2010d).
Appendix 2. Search strategy for CENTRAL (The Cochrane LIbrary)
#1 MeSH descriptor Pain explode all trees (32778)
#2 MeSH descriptor Peripheral Nervous System Diseases explode all trees (2993) #3 MeSH descriptor Somatosensory Disorders explode all trees (718) #4 ((pain* or discomfort*) and (central or complex or rheumat* or muscl* or muscul* or myofasci* or nerv* or neuralg* or neuropath*)):it,ab,kw (16907) #5 ((neur* or nerv*) and (compress* or damag*)):it,ab,kw (2029) #6 (1 or 2 or 3 or 4 or 5) (46034) #7 MeSH descriptor Desipramine, this term only (382) #8 (desipramine or Norpramin or Pertofrane):it,ab,kw (635) #9 7 or 8 (635) #10 6 and 9 (36) #11 Limit 10 to CENTRAL (33)
Appendix 3. Search strategy for MEDLINE (via Ovid)
exp PAIN/ (302655)
exp PERIPHERAL NERVOUS SYSTEM DISEASES/ (113897)
exp SOMATOSENSORY DISORDERS/ (15847)
((pain* or discomfort*) adj10 (central or complex or nerv* or neuralg* or neuropath*)).mp. (37625)
((neur* or nerv*) adj6 (compress* or damag*)).mp. (47198)
1 or 2 or 3 or 4 or 5 (444403)
Desipramine/ (5411)
(desipramine or Norpramin or Pertofrane).mp. (7445)
7 or 8 (7445)
randomized controlled trial.pt. (370572)
randomized.ab. (269403)
placebo.ab. (145013)
drug therapy.fs. (1688999)
randomly.ab. (191615)
trial.ab. (279322)
groups.ab. (1232736)
10 or 11 or 12 or 13 or 14 or 15 or 16 (3135037)
6 and 9 and 17 (160)
Appendix 4. Search strategy for EMBASE (via Ovid)
exp neuralgia/ (71974)
((pain* or discomfort*) adj10 (central or complex or nerv* or neuralg* or neuropath*)).mp. (78492)
((neur* or nerv*) adj6 (compress* or damag*)).mp. (66848)
1 or 2 or 3 (176956)
desipramine/ (20432)
(desipramine or Norpramin or Pertofrane).mp. (21023)
5 or 6 (21023)crossover‐procedure/ (38616)
double‐blind procedure/ (115238)
randomized controlled trial/ (342543)
(random* or factorial* or crossover* or cross over* or cross‐over* or placebo* or (doubl* adj blind*) or assign* or allocat*).tw. (1188683)
8 or 9 or 10 or 11 (1265261)
4 and 7 and 12 (140)
Appendix 5. Summary of outcomes in individual studies: efficacy
| Study |
Treatment (taken at night, unless stated) |
Pain outcome | Other efficacy outcome |
| Kishore‐Kumar 1990 | 18:00 single dose Desipramine 12.5 mg daily initially, increasing to 250 mg daily or maximum tolerated Active placebo (benztropine) 0.5 mg daily , increasing to 1 mg daily |
Participants' global evaluation of pain relief (6 items: worse to complete): moderate or better Desipramine 12/19; Placebo 2/19 a lot or complete Desipramine 8/19; Placebo 1/19 Weekly participant rating of pain intensity: desipramine superior to placebo by end of week 6 (p<0.001) (also by end of week 3: p<0.05) (inclusion of drop‐outs did not change conclusion) |
Reduction in pain components (desipramine:placebo) Steady 9/19:1/19 Brief 4/8:0/8 Mechanical allodynia 7/18:1/18 |
| Max 1991 | 18:00 single dose Desipramine 12.5 mg daily initially, increasing to 250 mg daily or maximum tolerated Active placebo (benztropine) 0.5 mg daily , increasing to 1 mg daily |
Participants' global evaluation of pain relief (6 items: worse to complete): moderate or better Desipramine 11/20; Placebo 2/20 a lot or complete Desipramine 4/20; Placebo 2/20 Weekly participant rating of pain intensity: Desipramine superior to placebo by end of week 6 (p<0.01) (also by start of week 5: p<0.05) (inclusion of one drop‐out who completed at least part of both phases did not change conclusion) |
Reduction in pain components: Steady burning significantly reduced with desipramine (P<0.05) "Trend" towards reduction with steady aching and brief pains |
| Max 1992 | 21:00 single dose Desipramine 12.5 mg daily initially, increasing to 250 mg daily or maximum tolerated Amitriptyline 12.5 mg daily initially, increasing to 250 mg daily or maximum tolerated |
Participants' global evaluation of pain relief (6 items: worse to complete): moderate or better Desipramine 23/38; Amitriptyline 28/38 a lot or complete Desipramine 15/38; Amitriptyline 18/38 ITT analysis results "similar" Weekly mean of participant rating of pain intensity: no significant difference between desipramine and amitriptyline |
|
| Rowbotham 2005 | Two doses per day (times not given) Desipramine 25‐150 mg daily, titrated to maximum tolerable Amitriptyline 25‐150 mg daily, titrated to maximum tolerable Fluoxetine 10‐60 mg daily, titrated to maximum tolerable |
Weekly results from Visual Analogue Scale of pain intensity: baseline to end Desipramine 50 to 29mm (47% decrease) Amitriptyline 59 to 39mm (38%) decrease) Fluoxetine 53 to 36mm (35% decrease) 6 item pain relief scale (worse to complete): at least moderate relief Desipramine 12/15 Amitriptyline 9/17 Fluoxetine 5/15 Relief category scale (0‐5: 3 = moderate relief) Desipramine 3.1 Amitriptyline 2.7 Fluoxetine 2.1 |
|
| Sindrup 1990 | 20:00 single dose Desipramine 200 mg daily (EM); 50 mg daily (PM) Clomipramine 75 mg daily (EM); 50 mg daily (PM) Placebo |
Pain not assessed separately but as one of set of neuropathy symptoms Neuropathy symptom scale (6 item, 0 to 2): pain, paraesthesia, dysaesthesia, numbness, nightly deterioration, sleep disturbance) Performed daily by participants and at end of treatment period by physician Scores significantly better than placebo with both observer and self‐rating: desipramine 0.05<P<0.10 Median reduction with observer scores >25% reduction in score compared with placebo: Desipramine 10/25 Clomipramine 14/25 |
Single items: desipramine not significantly lower than placebo for pain Median [sic but probably mean] 1.02 versus 1.50 (0‐2 scale) (P>0.30) |
| EM: extensive metaboliser; PM: poor metaboliser | |||
Appendix 6. Summary of outcomes in individual studies: adverse events and withdrawals
| Study |
Treatment (taken at night, unless stated) |
Adverse events | Withdrawals |
| Kishore‐Kumar 1990 | 18:00 single dose Desipramine 12.5 mg daily initially, increasing to 250 mg daily or maximum tolerated Active placebo (benztropine) 0.5 mg daily , increasing to 1 mg daily |
Any AE:
Desipramine 19/19 completers
Placebo 15/19 completers
Most common with desipramine: dry mouth, constipation, dizziness, sedation, micturition difficulty, insomnia, sweating Most common with placebo: dry mouth, dizziness, constipation |
Due to AEs: Desipramine 5/26 (syncope, palpitations/left bundle branch block, jitteriness/atypical chest pain, fever, vertigo) Placebo 3/26 (vertigo/nausea, skin rash, unsteadiness/mental fogginess) Intercurrent illness: 2 |
| Max 1991 | 18:00 single dose Desipramine 12.5 mg daily initially, increasing to 250 mg daily or maximum tolerated Active placebo (benztropine) 0.5 mg daily , increasing to 1 mg daily |
Any AE:
Desipramine 18/20 completers
Placebo 17/20 completers
Most common with desipramine: dry mouth, sedation, insomnia, constipation, orthostatic symptoms, palpitations, sweating Most common with placebo: dry mouth, sedation, constipation, insomnia |
Due to AEs: Desipramine 2/24 (seizure, insomnia) Placebo 1/24 (chest pain) Lack of efficacy: Desipramine 0/24 Placebo 1/24 |
| Max 1992 | 21:00 single dose Desipramine 12.5 mg daily initially, increasing to 250 mg daily or maximum tolerated Amitriptyline 12.5 mg daily initially, increasing to 250 mg daily or maximum tolerated |
Any AE:
Desipramine 29/54
Amitriptyline 31/54
Most common with desipramine: dry mouth, tiredness, constipation, insomnia, sweating, headache, lightheadedness, orthostatic symptoms, palpitations Most common with amitriptyline: dry mouth, tiredness, headache, palpitations, sweating, constipation, lightheadedness, orthostatic symptoms |
Due to AEs: Desipramine 7/54 (rash (3), orthostatic hypotension, fever, tremor, bundle branch block) Amitriptyline 7/54 (confusion (2), orthostatic hypotension, fatigue, malaise, rash, hypomania) Two other withdrawals were described as "voluntary" |
| Rowbotham 2005 | Two doses per day (times not given) Desipramine 25‐150 mg daily, titrated to maximum tolerable Amitriptyline 25‐150 mg daily, titrated to maximum tolerable Fluoxetine 10‐60 mg daily, titrated to maximum tolerable |
Ratings were obtained weekly for a 28‐item side effects checklist Scores increased for adverse events as follows: Desipramine: dry mouth, constipation, bitter taste Amitriptyline: dry mouth, constipation, bitter taste Fluoxetine: sleepiness, lightheadedness | For all causes*: Desipramine 2/15 Amitriptyline 2/17 Fluoxetine 5/15 *reasons given for only five withdrawals Due to AEs: Desipramine and amitriptyline combined: 2 sedation/cognitive impairment; 1 symptomatic orthostasis Fluoxetine: 1 recurrence of atrial fibrillation; 1 hospitalised for nausea and weakness with hyponatraemia Non‐completers had less pain relief, lower maximum tolerated dose, and higher adverse event symptom score |
| Sindrup 1990 | 20:00 single dose Desipramine 200 mg daily (EM); 50 mg daily (PM) Clomipramine 75 mg daily (EM); 50 mg daily (PM) Placebo |
A list of side effects was scored: median of total score was significantly higher for desipramine (4.5) and clomipramine (4.0) than placebo (0.02*)
*clearly, this figure cannot be correct: if only 6 participants (out of 19 completers) report adverse events, the median value must be zero. By contrast the lowest possible value for the mean would be 0.2. Most common adverse events: dry mouth, sweating, orthostatic dizziness, fatigue 6 participants reported one or more of these during placebo |
Due to AEs: Desipramine 3/26 (nausea, tiredness, dizziness) Clomipramine 3/26 (nausea, tiredness, dizziness, confusion) Lack of efficacy: Desipramine 0/26 Clomipramine 1/26 |
| AE: adverse event; EM: extensive metaboliser; PM: poor metaboliser | |||
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised, double‐blind, placebo‐controlled, cross‐over study After clinical assessment, 2 x 6 weeks of desipramine or placebo. Dose titrated to maximum tolerated over 4 weeks, then stable for 2 weeks. No washout between treatment periods |
|
| Participants | Postherpetic neuralgia for at least 3 months; normal cognition and communication ability Exclusions: other severe pain; severe depression; medical contra‐indication N = 26 (19 completed) M 17; F 9 Median age 62 years (range 38 ‐ 79) |
|
| Interventions | Desipramine 12.5 mg ‐ 250 mg daily Benztropine (active placebo) 0.5 mg ‐ 1.0 mg daily |
|
| Outcomes | Patient Global evaluation of Pain Relief (PGPR): 6‐point categorical scale, from complete to worse at end of treatment period PI: 4‐point scale for steady, brief, allodynia (mechanical, heat, cold), before and after treatment Daily PI diary, using 13 descriptors. Converted into numerical scores and weekly means calculated |
|
| Notes | Oxford Quality Score: R = 1; DB = 1; W = 1. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details of randomisation method not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Details of blinding not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Details of blinding not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer analysis reported |
| Size | High risk | < 50 participants per treatment arm (≤ 26) |
| Methods | Randomised, double‐blind, placebo‐controlled, cross‐over study After clinical assessment, 2 x 6 weeks of desipramine or placebo. Dose titrated to maximum tolerated over 4 weeks, then stable for 2 weeks. No washout between treatment periods |
|
| Participants | Painful diabetic neuropathy for at least 3 months; normal cognition and communication ability Exclusions: other etiology for neuropathy; other severe pain; severe depression; medical contra‐indication N = 24 (20 completed) M 15; F 9 Median age 62 years (range 21 ‐ 71) |
|
| Interventions | Desipramine 12.5 mg ‐ 250 mg daily Benztropine (active placebo) 0.5 mg ‐ 1.0 mg daily |
|
| Outcomes | PGPR at end of treatment period: 6‐point categorical scale, from complete to worse Comparison of PI before and after treatment: 4‐point scale for steady burning, steady aching, brief, allodynia (mechanical, warm), cold hyperalgesia Daily PI diary, using 13 descriptors. Converted into numerical scores and weekly means calculated |
|
| Notes | Oxford Quality Score: R = 1; DB = 1; W = 1. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details of randomisation method not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Details of blinding not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Details of blinding not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer analysis reported |
| Size | High risk | < 50 participants per treatment arm (≤ 24) |
| Methods | Randomised, double‐blind, cross‐over study After clinical assessment, 2 x 6 weeks of desipramine or amitriptyline. Dose titrated to maximum tolerated over 4 weeks, then stable for 2 weeks. No washout between treatment periods |
|
| Participants | Painful diabetic neuropathy for at least 3 months; stable glycaemic control Exclusions: other more severe pain; severe depression; postural hypotension; vascular disease; nephropathy; medical contra‐indication N = 54 (38 completed) M 33; F 21 Median age 58 years (range 20 ‐ 84) |
|
| Interventions | Desipramine 12.5 mg ‐ 150 mg daily Amitriptyline 12.5 mg ‐ 150 mg daily |
|
| Outcomes | PGPR at end of treatment period: 6‐point categorical scale, from complete to worse Daily PI diary, using 13 descriptors. Converted into numerical scores and weekly means calculated |
|
| Notes | Oxford Quality Score: R = 1; DB = 1; W = 0. Total = 2/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details of randomisation method not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Details of blinding not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Details of blinding not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer analysis reported. Claims ITT analysis "similar". Withdrawals > 10% (but evenly distributed between groups) |
| Size | High risk | < 50 participants evaluated per treatment arm (54 randomised, but only 38 completers) |
| Methods | Randomised, double‐blind, parallel group study Titration to maximum tolerated dose over 3 weeks, then stable dose for further 3 weeks |
|
| Participants | Postherpetic neuralgia for >3 months; normal cognition and communication ability; never had adequate trial of antidepressant
Exclusions: previous withdrawal due to antidepressant adverse event; other more severe pain; medical contra‐indication N = 47 (38 completed) M 20; F 27 Mean age 72 years (range 40 ‐ 84) |
|
| Interventions | Desipramine 25 mg ‐ 150 mg daily Amitriptyline 25 mg ‐ 150 mg daily Fluoxetine 10 mg ‐ 60 mg daily |
|
| Outcomes | PI: Visual Analogue Scale of pain intensity obtained weekly PR: 6‐point scale, from 0 = worse to 5 = complete relief |
|
| Notes | Oxford Quality Score: R = 1; DB = 1; W = 1. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details of randomisation method not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Details of blinding not reported Probably satisfactory as it is stated that placebo capsules were used to maintain the dose of 2 capsules twice daily |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Details of blinding not reported, probably satisfactory |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Imputation not reported. ITT analysis is similar to completer analysis |
| Size | High risk | < 50 participants per treatment arm (≤ 47) |
| Methods | Randomised, double‐blind, placebo‐controlled, cross‐over study 3 x 2 weeks of desipramine, clomipramine or placebo. Washout between treatment periods: 1 week for extensive metabolisers of sparteine (EM); 3 weeks for poor metabolisers (PM) |
|
| Participants | Painful diabetic neuropathy N = 26 (19 completers) Mean age 55 years (29 to 78) (completers) M 9, F 10 (completers) |
|
| Interventions | Desipramine 200 mg daily (EM) or 50 mg daily (PM) Clomipramine 75 mg daily (EM) or 50 mg daily (PM) Placebo | |
| Outcomes | Neuropathy symptom scale: 6 item (pain, paraesthesia, dysaesthesia, numbness, nightly deterioration, sleep disturbance); 0 to 2, maximum score 12
Greater than 25% reduction in neuropathy score from baseline (participant daily self‐rating) |
|
| Notes | Oxford Quality Score: R = 1; DB = 2; W = 1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details of randomisation method not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Placebo tablets were of identical size and colour" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Placebo tablets were of identical size and colour" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Completer analysis |
| Size | High risk | < 50 participants per treatment arm (≤ 26) |
DB: double blind; F: female; ITT: intention‐to‐treat; M: male; N: number of participants in study; PGPR: Patient Global evaluation of Pain Relief; PI: pain intensity; PR: pain relief; R: randomised; W: withdrawals
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Coquoz 1991 | Participants were healthy volunteers |
| Raja 2002 | Participants randomised to drug class, not individual drug, and results for individual drugs not reported separately |
Differences between protocol and review
The protocol specified any route of administration of desipramine, but since formulations are available for oral administration only, this was specified in the full review.
Contributions of authors
LH, SD and RAM wrote the protocol. LH and SD carried out searches, assessed studies for inclusion, and extracted data. PW acted as arbitrator. All authors were involved in writing the review. RAM will be responsible for updating the review.
Sources of support
Internal sources
-
Oxford Pain Relief Trust, UK.
General institutional support
External sources
No sources of support supplied
Declarations of interest
LH has no known conflicts of interest.
TP, SD, PW, and RAM have no conflicts relating to this review or any similar product.
For transparency, TP, SD, PW, and RAM have received research support from charities, government, and industry sources at various times, but none relate to this review. SD, PW and RAM are funded by the NIHR for work on a series of reviews informing the unmet need of chronic pain and providing the evidence for treatments of pain.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Kishore‐Kumar 1990 {published data only}
- Kishore‐Kumar R, Max MB, Schafer SC, Gaughan AM, Smoller B, Gracely RH, Dubner R. Desipramine relieves postherpetic neuralgia. Clinical Pharmacology and Therapeutics 1990;47(3):305‐12. [DOI] [PubMed] [Google Scholar]
Max 1991 {published data only}
- Max MB, Kishore‐Kumar R, Schafer SC, Meister B, Gracely RH, Smoller B, Dubner R. Efficacy of desipramine in painful diabetic neuropathy: a placebo‐controlled trial. Pain 1991;45:3‐9. [DOI] [PubMed] [Google Scholar]
Max 1992 {published data only}
- Max MB, Lynch SA, Muir J, Shoaf SE, Smoller B, Dubner R. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. New England Journal of Medicine 1992;326(19):1250‐6. [DOI] [PubMed] [Google Scholar]
Rowbotham 2005 {published data only}
- Rowbotham MC, Reisner LA, Davies PS, Fields HL. Treatment response in antidepressant‐naïve postherpetic neuralgia patients: double‐blind, randomized trial. Journal of Pain 2005;6(11):741‐6. [DOI] [PubMed] [Google Scholar]
Sindrup 1990 {published data only}
- Sindrup SH, Gram LF, Skjold T, Grodum E, Brøsen K, Beck‐Nielsen H. Clomipramine vs desipramine vs placebo in the treatment of diabetic neuropathy symptoms. A double‐blind cross‐over study. British Journal of Clinical Pharmacology 1990;30(5):683‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Coquoz 1991 {published data only}
- Coquoz D, Porchet HC, Dayer P. [Central analgesic effects of antidepressant drugs with various mechanisms of action: desipramine, fluvoxamine and moclobemide] [Effet analgesique central d'antidepresseurs a mode d'action distinct: desipramine, fluvoxamine et moclobemide]. Schweizerische Medizinische Wochenschrift 1991;121(49):1843‐5. [PubMed] [Google Scholar]
Raja 2002 {published data only}
- Raja SN, Haythornthwaite JA, Pappagallo M, Clark MR, Travison TG, Sabeen S, et al. Opioids versus antidepressants in postherpetic neuralgia: a randomized, placebo‐controlled trial. Neurology 2002;59(7):1015‐21. [DOI] [PubMed] [Google Scholar]
Additional references
Apkarian 2011
- Apkarian AV, Hashmi JA, Baliki MN. Pain and the brain: specificity and plasticity of the brain in clinical chronic pain. Pain 2011;152(3 Suppl):S49‐64. [DOI: 10.1016/j.pain.2010.11.010] [DOI] [PMC free article] [PubMed] [Google Scholar]
Attal 2010
- Attal N, Cruccu G, Baron R, Haanpää M, Hansson P, Jensen TS, et al. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. European Journal of Neurology 2010;17(9):1113‐e88. [DOI: ] [DOI] [PubMed] [Google Scholar]
AUREF 2012
- Cochrane Pain, Palliative and Supportive Care Group. PaPaS Author and Referee Guidance. http://papas.cochrane.org/sites/papas.cochrane.org/files/uploads/L%20‐%20PaPaSAuthor%26RefereeGuidance.pdf (accessed 22 January 2013).
Bouhassira 2008
- Bouhassira D, Lantéri‐Minet M, Attal N, Laurent B, Touboul C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain 2008;136(3):380‐7. [DOI: 10.1016/j.pain.2007.08.013] [DOI] [PubMed] [Google Scholar]
Derry 2012
- Derry S, Moore RA. Topical capsaicin (low concentration) for chronic neuropathic pain in adults. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD010111] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2013
- Derry S, Sven‐Rice A, Cole P, Tan T, Moore RA. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD007393.pub3] [DOI] [PubMed] [Google Scholar]
Derry 2014
- Derry S, Moore RA. Topical lidocaine for neuropathic pain in adults. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD010958] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dworkin 2008
- Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. Journal of Pain 2008;9(2):105‐21. [DOI: 10.1016/j.jpain.2007.09.005] [DOI] [PubMed] [Google Scholar]
Dworkin 2010
- Dworkin RH, O'Connor AB, Audette J, Baron R, Gourlay GK, Haanpää ML, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clinic Proceedings 2010;85(3 Suppl):S3‐14. [DOI: 10.4065/mcp.2009.0649] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI: 10.1093/ije/31.1.140] [DOI] [PubMed] [Google Scholar]
Finnerup 2010
- Finnerup NB, Sindrup SH, Jensen TS. The evidence for pharmacological treatment of neuropathic pain. Pain 2010;150(3):573‐81. [DOI: 10.1016/j.pain.2010.06.019] [DOI] [PubMed] [Google Scholar]
Gustorff 2008
- Gustorff B, Dorner T, Likar R, Grisold W, Lawrence K, Schwarz F, et al. Prevalence of self‐reported neuropathic pain and impact on quality of life: a prospective representative survey. Acta Anaesthesiologica Scandinavica 2008;52(1):132‐6. [DOI: ] [DOI] [PubMed] [Google Scholar]
Hall 2008
- Hall GC, Carroll D, McQuay HJ. Primary care incidence and treatment of four neuropathic pain conditions: a descriptive study, 2002‐2005. BMC Family Practice 2008;9:26. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hall 2013
- Hall GC, Morant SV, Carroll D, Gabriel ZL, McQuay HJ. An observational descriptive study of the epidemiology and treatment of neuropathic pain in a UK general population. BMC Family Practice 2013;14:28. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hearn 2014
- Hearn L, Wiffen PJ, Moore RA, Derry S. Imipramine for neuropathic pain in adults. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD010769.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. The Cochrane Collaboration.
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [DOI: 10.1016/0197‐2456(95)00134‐4] [DOI] [PubMed] [Google Scholar]
Jensen 2011
Katusic 1991
- Katusic S, Williams DB, Beard CM, Bergstralh EJ, Kurland LT. Epidemiology and clinical features of idiopathic trigeminal neuralgia and glossopharyngeal neuralgia: similarities and differences, Rochester, Minnesota,1945‐1984. Neuroepidemiology 1991;10:276‐81. [DOI] [PubMed] [Google Scholar]
Khan 1996
- Khan KS, Daya S, Jadad A. The importance of quality of primary studies in producing unbiased systematic reviews. Archives of Internal Medicine 1996;156(6):661‐6. [PubMed] [Google Scholar]
Koopman 2009
- Koopman JS, Dieleman JP, Huygen FJ, Mos M, Martin CG, Sturkenboom MC. Incidence of facial pain in the general population. Pain 2009;147(1‐3):122‐7. [DOI: 10.1016/j.pain.2009.08.023] [DOI] [PubMed] [Google Scholar]
L'Abbé 1987
- L'Abbé KA, Detsky AS, O'Rourke K. Meta‐analysis in clinical research. Annals of Internal Medicine 1987;107:224‐33. [DOI] [PubMed] [Google Scholar]
Lenkey 2006
- Lenkey N, Karoly R, Kiss JP, Szasz BK, Vizi ES, Mike A. The mechanism of activity‐dependent sodium channel inhibition by the antidepressants fluoxetine and desipramine. Molecular Pharmacology 2006;70(6):2052‐63. [DOI: 10.1124/mol.106.026419] [DOI] [PubMed] [Google Scholar]
Lunn 2014
- Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD007115.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
McQuay 1998
- McQuay H, Moore R. An evidence‐based resource for pain relief. Oxford: Oxford University Press, 1998. [Google Scholar]
McQuay 2007
- McQuay HJ, Smith LA, Moore RA. Chronic Pain. In: Stevens A, Raftery J, Mant J, Simpson S editor(s). Health Care Needs Assessment. 3rd Edition. Oxford: Radcliffe Publishing, 2007. [ISBN: 978‐1‐84619‐063‐6] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, the PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moisset 2007
- Moisset X, Bouhassira D. Brain imaging of neuropathic pain. NeuroImage 2007;37 Suppl 1:S80‐8. [DOI: 10.1016/j.neuroimage.2007.03.054] [DOI] [PubMed] [Google Scholar]
Moore 1998
- Moore RA, Gavaghan D, Tramèr MR, Collins SL, McQuay HJ. Size is everything ‐ large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain 1998;78(3):209‐16. [DOI: ] [DOI] [PubMed] [Google Scholar]
Moore 2008
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: Moore RA, Kalso E, McQuay HJ editor(s). Systematic Reviews in Pain Research: Methodology Refined. 1st Edition. Seattle: IASP Press, 2008:15‐24. [ISBN: 978‐0931092695] [Google Scholar]
Moore 2009
- Moore RA, Straube S, Wiffen PJ, Derry S, McQuay HJ. Pregabalin for acute and chronic pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD007076.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2010a
- Moore RA, Eccleston C, Derry S, Wiffen P, Bell RF, Straube S, et al. "Evidence" in chronic pain ‐ establishing best practice in the reporting of systematic reviews. Pain 2010;150(3):386‐9. [DOI: 10.1016/j.pain.2010.05.011] [DOI] [PubMed] [Google Scholar]
Moore 2010b
- Moore RA, Moore OA, Derry S, Peloso PM, Gammaitoni AR, Wang H. Responder analysis for pain relief and numbers needed to treat in a meta‐analysis of etoricoxib osteoarthritis trials: bridging a gap between clinical trials and clinical practice. Annals of the Rheumatic Diseases 2010;69(2):374‐9. [DOI: 10.1136/ard.2009.107805] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2010c
- Moore RA, Straube S, Paine J, Phillips CJ, Derry S, McQuay HJ. Fibromyalgia: moderate and substantial pain intensity reduction predicts improvement in other outcomes and substantial quality of life gain. Pain 2010;149(2):360‐4. [DOI: 10.1016/j.pain.2010.02.039] [DOI] [PubMed] [Google Scholar]
Moore 2010d
- Moore RA, Smugar SS, Wang H, Peloso PM, Gammaitoni A. Numbers‐needed‐to‐treat analyses ‐ do timing, dropouts, and outcome matter? Pooled analysis of two randomized, placebo‐controlled chronic low back pain trials. Pain 2010;151(3):592‐7. [DOI: 10.1016/j.pain.2010.07.013] [DOI] [PubMed] [Google Scholar]
Moore 2011a
- Moore RA, Straube S, Paine J, Derry S, McQuay HJ. Minimum efficacy criteria for comparisons between treatments using individual patient meta‐analysis of acute pain trials: examples of etoricoxib, paracetamol, ibuprofen, and ibuprofen/paracetamol combinations after third molar extraction. Pain 2011;152(5):982‐9. [DOI: 10.1016/j.pain.2010.11.030] [DOI] [PubMed] [Google Scholar]
Moore 2011b
- Moore RA, Mhuircheartaigh RJ, Derry S, McQuay HJ. Mean analgesic consumption is inappropriate for testing analgesic efficacy in post‐operative pain: analysis and alternative suggestion. European Journal of Anaesthesiology 2011;28(6):427‐32. [DOI: 10.1097/EJA.0b013e328343c569] [DOI] [PubMed] [Google Scholar]
Moore 2012a
- Moore RA, Derry S, Aldington D, Cole P, Wiffen PJ. Amitriptyline for neuropathic pain and fibromyalgia in adults. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD008242.pub2] [DOI] [PubMed] [Google Scholar]
Moore 2012b
- Moore RA, Straube S, Eccleston C, Derry S, Aldington D, Wiffen P, et al. Estimate at your peril: imputation methods for patient withdrawal can bias efficacy outcomes in chronic pain trials using responder analyses. Pain 2012;153(2):265‐8. [DOI] [PubMed] [Google Scholar]
Moore 2013a
- Moore RA, Straube S, Aldington D. Pain measures and cut‐offs ‐ 'no worse than mild pain' as a simple, universal outcome. Anaesthesia 2013;68(4):400‐12. [DOI: 10.1111/anae.12148] [DOI] [PubMed] [Google Scholar]
Moore 2013b
- Moore A, Derry S, Eccleston C, Kalso E. Expect analgesic failure; pursue analgesic success. BMJ 2013;346:f2690. [DOI: 10.1136/bmj.f2690] [DOI] [PubMed] [Google Scholar]
Moore 2014a
- Moore RA, Derry S, Taylor RS, Straube S, Phillips CJ. The costs and consequences of adequately managed chronic non‐cancer pain and chronic neuropathic pain. Pain Practice 2014;14(1):79‐94. [DOI: 10.1111/papr.12050] [DOI] [PubMed] [Google Scholar]
Moore 2014b
- Moore RA, Wiffen PJ, Derry S, Toelle T, Rice AS. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database of Systematic Reviews 2014, Issue 4. [DOI: 10.1002/14651858.CD007938.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moulin 2007
- Moulin DE, Clark AJ, Gilron I, Ware MA, Watson CP, Sessle BJ, et al. Pharmacological management of chronic neuropathic pain ‐ consensus statement and guidelines from the Canadian Pain Society. Pain Research and Management 2007;12(1):13‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2013
- NICE clinical guideline 173. Neuropathic pain – pharmacological management. www.nice.org.uk/guidance/CG173 (accessed 24 March 2014) 2013:1‐138.
Nüesch 2010
- Nüesch E, Trelle S, Reichenbach S, Rutjes AW, Tschannen B, Altman DG, et al. Small study effects in meta‐analyses of osteoarthritis trials: meta‐epidemiological study. BMJ 2010;341:c3515. [DOI: 10.1136/bmj.c3515] [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Brien 2010
- O'Brien EM, Staud RM, Hassinger AD, McCulloch RC, Craggs JG, Atchison JW, et al. Patient‐centered perspective on treatment outcomes in chronic pain. Pain Medicine 2010;11(1):6‐15. [DOI: ] [DOI] [PubMed] [Google Scholar]
O'Connor 2009
- O'Connor AB, Dworkin RH. Treatment of neuropathic pain: an overview of recent guidelines. American Journal of Medicine 2009;122(10 Suppl):S22‐32. [DOI: 10.1016/j.amjmed.2009.04.007] [DOI] [PubMed] [Google Scholar]
Onghena 1992
- Onghena P, Houdenhove B. Antidepressant‐induced analgesia in chronic non‐malignant pain: a meta‐analysis of 39 placebo‐controlled studies. Pain 1992;49:205‐20. [DOI] [PubMed] [Google Scholar]
Rappaport 1994
- Rappaport ZH, Devor M. Trigeminal neuralgia: the role of self‐sustaining discharge in the trigeminal ganglion. Pain 1994;56:127‐38. [DOI: ] [DOI] [PubMed] [Google Scholar]
Review Manager 2013 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2013.
Soni 2013
- Soni A, Batra R, Gwilym S, Spector T, Hart D, Arden N, et al. Neuropathic features of joint pain: A community‐based study. Arthritis & Rheumatism 2013;April 1:Epub ahead of print. [DOI: 10.1002/art.37962] [DOI] [PMC free article] [PubMed] [Google Scholar]
Straube 2008
- Straube S, Derry S, McQuay HJ, Moore RA. Enriched enrolment: definition and effects of enrichment and dose in trials of pregabalin and gabapentin in neuropathic pain. A systematic review. British Journal of Clinical Pharmacology 2008;66(2):266‐75. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Straube 2010
- Straube S, Derry S, Moore RA, Paine J, McQuay HJ. Pregabalin in fibromyalgia ‐ responder analysis from individual patient data. BMC Musculoskeletal Disorders 2010;11:150. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sudoh 2003
- Sudoh Y, Cahoon EE, Gerner P, Wang GK. Tricyclic antidepressants as long‐acting local anesthetics. Pain 2003;103(1‐2):49‐55. [DOI: ] [DOI] [PubMed] [Google Scholar]
Sultan 2008
- Sultan A, Gaskell H, Derry S, Moore RA. Duloxetine for painful diabetic neuropathy and fibromyalgia pain: systematic review of randomised trials. BMC Neurology 2008;8:29. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Torrance 2006
- Torrance N, Smith BH, Bennett MI, Lee AJ. The epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population survey. Journal of Pain 2006;7(4):281‐9. [DOI: 10.1016/j.jpain.2005.11.008] [DOI] [PubMed] [Google Scholar]
Tracey 2011
- Tracey I. Can neuroimaging studies identify pain endophenotypes in humans?. Nature Reviews Neurology 2011;7(3):173‐81. [DOI: 10.1038/nrneurol.2011.4] [DOI] [PubMed] [Google Scholar]
Treede 2008
- Treede RD, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, Griffin JW, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology 2008;70(18):1630‐5. [DOI: 10.1212/01.wnl.0000282763.29778.59] [DOI] [PubMed] [Google Scholar]
Vos 2012
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990‐2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380(9859):2163‐96. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Saarto 2007
- Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD005454.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


