Summary
Plants are able to effectively cope with invading pathogens by activating an immune response based on the detection of invasion patterns (IPs) originating from the pathogen or released by the plant after infection. At a first level, this perception takes place at the plasma membrane through cell surface immune receptors and although the involvement of proteinaceous pattern recognition receptors (PRRs) is well established, increasing data are also pointing out the role of membrane lipids in the sensing of IPs. In this review, we discuss the evolution of various conceptual models describing plant immunity and present an overview of well‐characterized IPs from different natures and origins. We summarize the current knowledge on how they are perceived by plants at the plasma membrane, highlighting the increasingly apparent diversity of sentinel‐related systems in plants.
Keywords: invasion patterns, lipids, pattern‐triggered immunity, plasma membrane, PRR
Introduction
At the base of the food web, plants are targeted by a wide range of organisms belonging to multiple branches of the evolutionary tree. To counter pathogen attack, plants use two distinct mechanisms: (i) constitutive defences, including pre‐existing physical and chemical barriers, and (ii) inducible defences activated after perception of the invader (Heath, 2000; Lee et al., 2017; Veronese et al., 2003).
In the 2000s, a general concept was proposed to describe plant immunity as a ‘zigzag model’ (Jones and Dangl, 2006). In this model, the recognition by the plant of pathogen‐ or microbial‐associated molecular patterns (PAMPs or MAMPs) that are typically essential components of whole classes of pathogens/microorganisms, results in PAMP‐triggered immunity (PTI). The concept of PTI was then extended to molecules that may arise from the plant itself because of the damage caused by microbes and called damage‐associated molecular patterns (DAMPs) (Boller and Felix, 2009). PTI activation classically involves cell surface‐localized pattern recognition receptors (PRRs), including membrane receptor kinases (RKs) or receptor‐like proteins (RLPs) (Boutrot and Zipfel, 2017). To proliferate, pathogens have developed the capacity to block PTI (now also called pattern‐ or PRR‐triggered immunity) by secreting effectors that interfere with perception or immunity‐related signaling (Białas et al., 2018; Varden et al., 2017). In the zigzag model, resistant plants are able to directly or indirectly sense effectors through intracellular nucleotide‐binding domain and leucine‐rich repeat (LRR) receptors (NLRs; a.k.a. nucleotide binding and oligomerization domain (NOD)‐like receptors) (Adachi et al., 2019; Zhang et al., 2017). Sensing of effectors by NLRs leads to NLR‐triggered immunity (NTI; a.k.a. effector‐triggered immunity (ETI) (Boutrot and Zipfel, 2017). Pathogens may then evolve new effector(s) able to suppress NTI and plants can evolve new NLRs in order to counter pathogen infection. Although this zigzag model is mainly valid and still largely used, it does not take into account some cases of plant immunity, suggesting that there may be no clear distinction between PTI and NTI or MAMPs/DAMPs and effectors. For instance, some apoplastic avirulence (Avr) proteins secreted by microorganisms and also considered as effectors are recognized by PRRs (de Wit, 2016b). To address the limitations and inconsistences of the zigzag model, alternative concepts of plant immunity have been proposed recently and are based on the recognition of microbial invasion via invasion patterns (IPs) that include all microbe‐derived or plant‐derived molecules (Cook et al., 2015; Kanyuka and Rudd, 2019). IPs therefore encompass MAMPs, effectors (both apoplastic and cytosolic) and DAMPs, as well as any other microbe‐derived or plant‐derived evolutionary conserved or variable molecules that signal the pathogen invasion and trigger an immune response (Kanyuka and Rudd, 2019). In these models, IPs are sensed by IP receptors (IPRs). In the most recent model, IPRs are divided either in cell surface immune receptors (CSIRs) synonymous to PRRs that include RKs and RLPs or intracellular immune receptors (IIRs), mainly synonymous to NLRs (Kanyuka and Rudd, 2019). In the present review, to avoid any ambiguity, we will therefore use the general term of IPs that encompasses all molecules perceived by the plant as a danger without distinction of their origin or their potential function.
Although IP sensing at the plasma membrane through proteinaceous PRRs is well documented, an increasing number of studies has highlighted a key role of membrane lipids in the direct or indirect recognition of some IPs by plant cells. Indeed, several molecules such as necrosis and ethylene‐inducing peptide 1‐like (NLP) proteins, harpins, rhamnolipids and lipopeptides trigger plant immunity through lipid receptors and/or potential membrane lipid‐raft structure perturbations (Farace et al., 2015; Henry et al., 2011; Klemptner et al., 2014; Sanchez et al., 2012). Moreover, several synthetic compounds that mimic IP‐triggered plant signalling or perception are known to interact with biological or biomimetic lipid‐based membranes and trigger plant immunity (Bektas and Eulgem, 2015; Luzuriaga‐Loaiza et al., 2018; Nasir et al., 2017).
In this context, the main goal of this review is to present an overview of well‐characterized apoplastic IPs (either microbe‐ or plant‐derived) and summarize the current knowledge on their mode of perception at the plasma membrane through proteinaceous PRRs and/or lipid‐driven processes.
IPs sensed by PRRs or involving known RK co‐receptors
Apoplastic IPs display a large diversity of biochemical nature, including (glyco)protein‐, polysaccharide‐ and lipid‐based structures (summarized in Table 1). Most of these IPs characterized to date are known to be sensed by PRRs. For the majority of these IP/PRR couples, evidence for direct binding has been given through biochemical experiments. Proteinaceous PRRs are generally composed of an extracellular domain that binds the IP, a transmembrane domain and for some of them an intracellular kinase domain. A first distinction between PRRs can be made based on the nature of the extracellular domain such as LRR‐, LysM‐, Lectin‐, EGF‐like‐based domains (Fig. 1). A second distinction among PRRs can be made between RKs that display an intracellular kinase domain and RLPs without a kinase domain. In addition, IP perception through PRR activation has been linked to RK co‐receptors involved in signal transduction. The RK co‐receptors BAK1, SOBIR1 (both with an extracellular LRR‐type domain) and CERK1 (with an extracellular LysM‐type domain) are the best known and are major modulators of PTI (Couto and Zipfel, 2016) (Fig. 1).
Table 1.
Examples of apoplastic invasion patterns (IPs) with known or putative perception systems in plants
| IPs | Perception | Phylogenetic origin | Cellular origin | References |
|---|---|---|---|---|
| IPs sensed by PRRs or involving known RKs | ||||
| Protein‐derived IPs sensed by LRR‐RK‐type PRRs | ||||
| Flg22 | LRR‐RK FLS2 | Bacteria | Flagella | Chinchilla et al. (2006); Gómez‐Gómez and Boller (2000); Gómez‐Gómez et al. (1999) |
| FlgII‐28 | LRR‐RK FLS3 | Bacteria | Flagella | Hind et al. (2016) |
| Elf18 | LRR‐RK EFR | Bacteria | Cytoplasm, secretome, cell surface | Kunze et al. (2004); Zipfel et al. (2006) |
| CSP | LRR‐RK CORE | Bacteria | Cytoplasm | Felix and Boller, (2003); Wang et al. (2016), Wei et al. (2018) |
| XUP25 | LRR‐RK XPS1 | Bacteria | Plasma membrane | Mott et al. (2016) |
| RaxX | LRR‐RK XA21 | Bacteria | Secretome | Pruitt et al. (2015) |
| (Glyco)protein‐derived IPs sensed by RLP‐type PRRs | ||||
| PGNs | LysM‐RLPs LYM1/LYM3 and LYP4/LYP6 | Bacteria | Cell wall | Gust et al. (2007) |
| NLPs | LRR‐RLP RLP23 | Bacteria, oomycete, fungi | Secretome | Albert et al. (2019); Böhm et al. (2014) |
| Elicitins | LRR‐RLP ELR (INF1) and S‐domain lectin RK NgRLK1 (capsicein) | Oomycete | Secretome | Du et al. (2015); Kim et al. (2010) |
| Ave1 | LRR‐RLP Ve1 | Fungi, bacteria | Secretome | de Jonge et al. (2012) |
| Avr4 | LRR‐RLP Cf‐4 | Fungi | Secretome | Postma et al. (2016) |
| Avr9 | LRR‐RLP Cf‐9 | Fungi | Secretome | Rowland et al. (2005) |
| Avr2 | LRR‐RLP Cf‐2 by targeting Rcr3 | Fungi | Secretome | de Wit (2016b); Rooney et al. (2005); Van't Klooster et al. (2011) |
| Gr‐VAP1 | LRR‐RLP Cf‐2 by targeting Rcr3 | Nematode | Secretome | Lozano‐Torres et al. (2012) |
| EIX | LRR‐RLP LeEix2 | Fungi | Secretome | Bar et al. (2010); Ron and Avni (2004) |
| PGs | LRR‐RLP RBPG1 | Fungi | Secretome | Zhang et al. (2014) |
| Protein‐derived IPs sensed by unidentified PRRs but requiring RK co‐receptors | ||||
| CBEL | Co‐receptor BAK1 | Oomycete | Cell wall | Larroque et al. (2013) |
| GroEL | Co‐receptor BAK1 | Bacteria (aphid endosymbiont) | Cytoplasm | Chaudhary et al. (2014) |
| BcSpl1 | Co‐receptor BAK1 | Fungi | Secretome | Frías et al. (2011, 2013) |
| RcCDI1 | Co‐receptors BAK1 and SOBIR1 | Fungi | Secretome | Franco‐Orozco et al. (2017) |
| VmE02 | Co‐receptor BAK1 | Fungi | Secretome | Nie et al. (2019) |
| BcXyl1 | Co‐receptors BAK1 and SOBIR1 | Fungi | Secretome | Yang et al. (2018) |
| XEG1 | Co‐receptor BAK1 | Oomycete | Secretome | Ma et al. (2015) |
| Lipid‐derived and polysaccharide‐derived IPs from microbial origin | ||||
| LPS/LOS | Co‐receptor CERK1 | Bacteria | Cell wall | Desaki et al. (2018) |
| Medium‐chain 3‐hydroxy fatty acids | Bulb‐type lectin RK LORE | Bacteria | Unknown | Kutschera et al. (2019) |
| Chitin |
LysM‐RLPs CEBIP and LYP4/LYP6 LysM‐RKs LYK5 and VvLYK1‐1 /VvLYK1‐2 |
Fungi, arthropod, oomycete | Cell wall, exoskeleton | Cao et al. (2014); Hayafune et al. (2014); Liu et al. (2012) |
| Chitosan |
Potential WAK1 and GsSRK receptors LysM‐RKs VvLYK1‐1 /VvLYK1‐2 |
Fungi | Cell wall | Brulé et al. (2018); Liu et al. (2018) |
| IPs from plant origin | ||||
| AtPep1/2/3 | LRR‐RKs PEPR1/PEPR2 | Plant | Cytoplasm | Krol et al. (2010) |
| PIP1/2 | LRR‐RK RLK7 | Plant | Secretome | Hou et al. (2014) |
| Systemin | LRR‐RKs SYR1/SYR2 and LRR‐RK PORK1 | Plant | Cytoplasm | Santamaria et al. (2018); Wang et al. (2018); Xu et al. (2018) |
| eATP | L‐type lectin RK DORN1 | All reign | Cytoplasm | Choi et al. (2014) |
| β‐(1,3)‐glucans | Co‐receptor CERK1 | Plant | Vacuole | Mélida et al. (2018) |
| OGs | WAK1 RK | Plant | Cell wall | Brutus et al. (2010) |
| IPs sensed through plasma membrane lipid interaction | ||||
| Proteinaceous IPs | ||||
| NLPs | GIPCs | Bacteria, oomycete, fungi | Secretome | Lenarčič et al. (2017) |
| Harpins | Lipids | Bacteria | Secretome | Choi et al. (2013); Lee et al. (2001) |
| Elicitins | Sterols | Oomycete | Secretome | Derevnina et al. (2016); Gerbeau‐Pissot et al. (2014) |
| Lipid‐based IPs | ||||
| Ergosterol | Lipid raft disturbance | Fungi | Plasma membrane | Rossard et al. (2010); Xu et al. (2001) |
| Lipopeptides | Phospholipids | Bacteria | Secretome | Henry et al. (2011) |
| RLs |
Phosphatidylcholines/POPC/phosphatidylinositol/ phosphatidylglycerol/β‐sitosterol/glucosylceramide |
Bacteria | Secretome | Sanchez et al. (2012); Monnier et al. (2019) |
| SRBs | PLPC/β‐sitosterol | Synthetic | / | Luzuriaga‐Loiaza et al. (2018) |
| Ac‐RL/Alk‐RL | PLPC/β‐sitosterol | Synthetic | / | Nasir et al. (2017) |
| Polysaccharide‐derived structures | ||||
| Chitosan | Phospholipids | Fungi | Cell wall | Amborabé et al. (2000); Rossard et al. (2010) |
GIPC, glycosyl inositol phosphoryl ceramide (sphingolipid); PLPC, 1‐palmitoyl‐2‐linoleoyl‐sn‐glycero‐3‐phosphocholine (phospholipid); POPC, 1‐palmitoyl‐2‐oleoyl‐glycero‐3‐phosphocholine (phospholipid).
Figure 1.
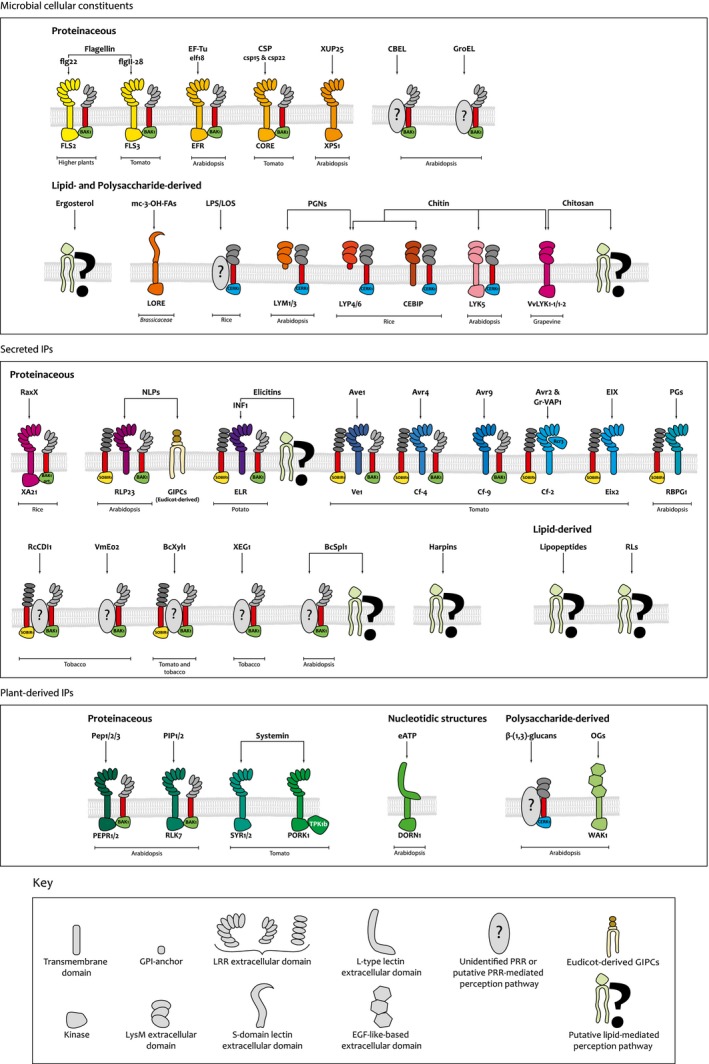
Representation of invasion pattern (IP) perception through known or potential pattern recognition receptors (PRRs) or involving plasma membrane lipids. CBEL, cellulose binding elicitor lectin; CSP, cold shock protein; EIX, ethylene‐inducing xylanase; GIPC, glycosyl inositol phosphoryl ceramide; GPI, glycophosphatidylinositol; LOS, lipooligosaccharides; LPS, lipopolysaccharides; LRR, leucine‐rich repeat; NLP, necrosis and ethylene‐inducing peptide 1‐like; OGs, oligogalacturonides; ort., orthologue; PGNs, peptidoglycans; PGs, endopolygalacturonases; RLs, rhamnolipids.
Protein‐derived IPs sensed by LRR‐RK‐type PRRs
Bacterial protein‐derived IPs are the most studied apoplastic plant immune‐inducing molecules. Among them, flagellin, a subunit protein of the flagellum present in many bacteria including Pseudomonas and Xanthomonas species, is the best characterized. As flagella are essential structures for bacterial fixation to host cells and motility, flagellin is an optimal target for the plant immune system. Flagellin monomers can be found in the extracellular space either during flagellum construction or due to damage to flagellar filaments (Gómez‐Gómez and Boller, 2002). It was recently demonstrated in Nicotiana benthamiana that plant‐secreted β‐galactosidase 1 (BGAL1) promotes hydrolytic release of the active IP from glycosylated flagellin. BGAL1 acts in immunity against pathogenic Pseudomonas syringae strains only when they carry a terminal modified viosamine in the flagellin O‐glycan. Interestingly, P. syringae pathovars are able to evade host immunity by using BGAL1‐resistant O‐glycans or by producing a BGAL1 inhibitor (Buscaill et al., 2019). Although flagellin is perceived by a large set of plants, the peptide sequence necessary for recognition and the cognate PRRs vary among plant species (Boller and Felix, 2009; Trdá et al., 2015). For instance, a 22‐amino acid peptide (flg22), originating from the N‐terminal extremity of flagellin is perceived by FLS2, a LRR‐RK present in several monocots and eudicots (Chinchilla et al., 2006; Gómez‐Gómez and Boller, 2000; Gómez‐Gómez et al., 1999). In tomato, FLS3 senses another part of flagellin, flgII‐28, independently of FLS2 (Hind et al., 2016).
Elf18, an 18‐amino acid‐long sequence originating from the N‐terminal part of bacterial elongation factor Tu (EF‐Tu), has also been widely studied for its ability to induce a plant immune response (Zipfel et al., 2006). EF‐Tu is localized at the bacterial surface (in outer membrane vesicles) and in the secretome of several bacteria (Katsir and Bahar, 2017; Zipfel et al., 2006). EF‐Tu and its elf18 peptide are perceived by the LRR‐RK EFR in Arabidopsis thaliana (hereafter, Arabidopsis). Interestingly, elf18 does not trigger immunity‐related mechanisms in plants outside the Brassicaceae, unlike flg22 (Kunze et al., 2004; Zipfel et al., 2006). Similarly, the conserved domains of bacterial cold shock protein (CSP), csp15 and csp22, are perceived by the LRR‐RK CORE that is only present in Solanaceae (Felix and Boller, 2003; Wang et al., 2016; Wei et al., 2018). CSPs are intracellular (cytoplasmic) proteins and how they become available for perception by PRRs is still nebulous. XUP25 peptide, originating from P. syringae uracil/xanthine permease, induces immunity through the LRR‐RK XPS1 in Arabidopsis (Mott et al., 2016). How the peptide is liberated from the main protein remains unknown. Plant proteases that participate in plant immunity could be involved in the process (Balakireva and Zamyatnin, 2018). Until now, no RK co‐receptor required for signal transduction has been associated with XPS1. It should be noted that the majority of the protein‐derived IPs originating from microbes and sensed by LRR‐RK‐type PRRs are not secreted but are microbial constituents. To date, the RaxX tyrosine‐sulphated protein that is secreted by Gram‐negative bacteria (in particular Xanthomonas species) and sensed by the rice LRR‐RK XA21 (Pruitt et al., 2015) is the only exception. Interestingly, XA21 interacts with the rice BAK1 orthologue, OsSERK2. All these PRRs (excepted XPS1 for which it has not yet been investigated) require the co‐receptor BAK1 or an orthologue to trigger signal transduction.
(Glyco)protein‐derived IPs sensed by RLP‐type PRRs
The bacterial cell wall is well known to be a source of IPs. Peptidoglycans (PGNs), which provide rigidity and structure to the bacterial cell wall in both Gram‐negative and Gram‐positive bacteria, induce innate immunity in monocots and eudicots. Muropeptides that are PGN breakdown products from Agrobacterium and Xanthomonas are sensed by Arabidopsis (Erbs et al., 2008). In addition, Staphylococcus aureus PGNs mediate immune stimulation in Arabidopsis based on recognition of the PGN sugar backbone (Gust et al., 2007). Perception of PGNs in Arabidopsis involves the co‐receptor CERK1 and the LysM domain RLPs LYM1 and LYM3 (Willmann et al., 2011). Notably, CERK1 is also necessary for signal transduction upon chitin perception in this plant (Miya et al., 2007). In rice, LYP4 and LYP6, the homologous RLPs of LYM1 and LYM3, mediate PGNs and chitin sensing by interacting with CERK1 (Cao et al., 2014; Liu et al., 2012). Whereas the majority of protein‐derived IPs is perceived by LRR‐type PRRs, it is interesting to notice that PGNs are sensed by LysM‐type PRRs, clearly suggesting that it is the glycan part of the molecule that is recognized by plants. Although PGNs are present in the outer leaflet of Gram‐positive bacteria, they are localized in the periplasmic space under the outer membrane in Gram‐negative bacteria and are therefore hardly available for perception by PRRs (Silipo et al., 2010). However, plants can produce apoplastic PGN hydrolases such as LYS1 that could release elicitor PGN fragments from insoluble bacterial cell walls (Liu et al., 2014).
Numerous protein‐derived IPs are secreted in the apoplast by pathogens. Their direct accessibility to PRRs makes them interesting targets for the plant immune system. NLPs form a family of proteins secreted by phytopathogenic bacteria, oomycetes and fungi (Gijzen and Nurnberger, 2006; Lenarčič et al., 2017). These proteins, characterized by their necrosis‐inducing Phytophthora protein1 (NPP1) domain, induce plant immunity and cell death in eudicot plant species (Lenarčič et al., 2017; Qutob et al., 2006). The PRR‐mediated sensing of nlp20, a conserved 20‐mer fragment from NLPs, has been identified in Arabidopsis and involves the LRR‐RLP RLP23 and the SOBIR1/BAK1 complex (Albert et al., 2015; Böhm et al., 2014). Elicitins, first characterized in the 1980s, are IPs secreted by the oomycetes Phytophthora sp. and Pythium sp. (Ricci et al., 1989). They induce defence responses and a localized programmed cell death called hypersensitive response (HR) in various plant species (Derevnina et al., 2016). The LRR‐RLP ELR from potato is able to sense the elicitin INF1 and associates with BAK1 and SOBIR1 to activate defence responses (Domazakis et al., 2018; Du et al., 2015). In tomato, elicitin perception involves the co‐receptor/adaptor kinase SOBIR1 that associates with several RLPs to induce plant immunity (Gust and Felix, 2014; Peng et al., 2015). Interestingly, unlike INF1, the Phytophthora capsici capsicein was shown to interact in vitro with the S‐domain lectin RK NgRLK1 from Nicotiana glutinosa (Kim et al., 2010).
Several IPs, often referred to as apoplastic effectors according to the zigzag model, are perceived by RLP‐type PRRs. Ave1 secreted by Verticillium dahliae is sensed by the RLP Ve1 in tomato (de Jonge et al., 2012) and this perception requires BAK1 (Fradin et al., 2009) and SOBIR1 (Liebrand et al., 2013). Interestingly, Ave1 is homologous to plant natriuretic peptides (PNPs), which are mobile signalling hormones secreted in the apoplast under biotic and abiotic stresses and for which a cognate plasma membrane LRR‐RK has been characterized in Arabidopsis (Turek and Gehring, 2016). Avr4 and Avr9 are two other fungal proteins secreted by Cladosporium fulvum. They promote, respectively, Cf‐4 and Cf‐9 RLP association with BAK1 to initiate an immune response in tomato. Avr2, another C. fulvum effector, is recognized by the RLP Cf‐2. Cf‐4‐ and Cf‐2‐mediated immunity both require SOBIR1 as co‐receptor (Liebrand et al., 2013; Postma et al., 2016). Interestingly, Avr2 is also able to inhibit the apoplastic tomato cysteine protease Rcr3 and the direct interaction is necessary to trigger Cf‐2‐dependent HR and resistance to C. fulvum (Rooney et al., 2005; Van't Klooster et al., 2011). Reminiscent of the Avr2/Rcr3/Cf‐2 mechanism of perception, the nematode venom allergen‐like protein Gr‐VAP1, involved in Globodera rostochiensis virulence, targets the Rcr3 protein from Solanum pimpinellifolium (Lozano‐Torres et al., 2012).
Some phytopathogenic fungi, mostly necrotrophs, secrete cell wall‐degrading enzymes (CWDE) (Kubicek et al., 2014). Some of these enzymes are directly recognized by plant cells and not through the DAMPs that could be produced by their lytic activity. This is the case of xylanases involved in hemicellulose degradation. The fungal ethylene‐inducing xylanase (EIX) is directly perceived by tomato cells through the RLP Eix2, leading to activation of defence responses and induction of an HR (Bar et al., 2010; Ron and Avni, 2004). Moreover, Eix2 interacts with SOBIR1 (Liebrand et al., 2013). Endopolygalacturonases (PGs) are other fungal CWDEs that degrade pectins and act as virulence factors for several fungal pathogens. In Arabidopsis, the LRR‐RLP RBPG1 has been identified as a receptor for BcPG2, BcPG3, BcPG4 and BcPG6 originating from Botrytis cinerea and Aspergillus niger. This recognition process involves the co‐receptor SOBIR1 (Zhang et al., 2014). In grapevine, BcPG1, an endopolygalacturonase from B. cinerea, was shown to activate plant defence responses independently of its enzymatic activity, also suggesting a direct recognition in this plant (Poinssot et al., 2003). Remarkably, all these LRR‐type RLPs lacking the cytoplasmic signalling competent moiety require the RK co‐receptors SOBIR1 and/or BAK1 for signal transduction (Liebrand et al., 2014).
Protein‐derived IPs sensed by unidentified PRRs but requiring RK co‐receptors
Many of the previously described RK or RLP PRRs have been identified through forward or reverse genetic approaches and for most of them a direct interaction with the corresponding IP has been confirmed by receptor‐ligand binding experiments. However, for a number of IPs known to induce plant immunity, their cognate receptor is not yet identified albeit the involvement of RK co‐receptors has been highlighted.
CBEL (cellulose binding elicitor lectin), a glycoprotein from the cell wall of Phytophthora nicotianae, is perceived by Arabidopsis and tobacco. In Arabidopsis, BAK1 is necessary for CBEL‐mediated immunity (Larroque et al., 2013). Interestingly, its cellulose‐binding domain (CBD) is sufficient to induce plant defence responses (Gaulin et al., 2006). The intracellular chaperonin GroEL from the aphid endosymbiont Buchnera aphidicola was also shown to induce a BAK1‐dependent immune response in Arabidopsis (Chaudhary et al., 2014). BcSpl1, an abundant cerato‐platanin protein present in the secretome of the necrotrophic fungus B. cinerea, triggers a BAK1‐dependent HR in tomato, tobacco and Arabidopsis (Frías et al., 2011, 2013). Similarly, the small cysteine‐rich protein VmE02 secreted by another necrotrophic fungus Valsa mali and XEG1, a Phytophthora sojae secreted glycoside hydrolase, triggers plant defences and cell death in a BAK1‐dependent manner in tobacco (Ma et al., 2015; Nie et al., 2019). For some IPs, the involvement of both BAK1 and SOBIR1 in plant immunity associated with an HR has been demonstrated. This is the case for RcCDI1, a small protein with an unknown function secreted by Rhynchosporium commune, which is only recognized by eudicots (Franco‐Orozco et al., 2017) and the B. cinerea xylanase BcXyl1 active on tobacco and tomato (Yang et al., 2018). It should be noted that the co‐receptors BAK1 and SOBIR1 are only involved in the perception of protein‐derived IPs so far. In addition, SOBIR1 has only been linked to the perception of secreted IPs.
Lipid‐derived and polysaccharide‐derived IPs from microbial origin
Lipopolysaccharides (LPS) and lipooligosaccharides (LOS) are major constituents of bacterial Gram‐negative cell walls and are well known for inducing immunity in several plant species (Desender et al., 2006; Dow et al., 2000). LPS consist of a lipid A portion, an oligosaccharide core and an O‐polysaccharidic extremity. In tobacco, treatment with lipid A induces late‐phase defence responses while the oligosaccharide core induces early immune responses (Erbs and Newman, 2012; Silipo et al., 2005). In Arabidopsis, the lipid A fragment carries the minimal molecular pattern recognized by plants (Ranf et al., 2015). Recently, it was shown that the Brassicaceae specific bulb‐type lectin RK LORE recognizes medium‐chain 3‐hydroxy fatty acid (mc‐3‐OH‐FA) metabolites present in the lipid A structures from Pseudomonas and Xanthomonas bacteria (Kutschera et al., 2019). mc‐3‐OH‐FAs are sensed in a chain length‐ and hydroxylation‐specific manner. Interestingly, bacterial compounds comprising mc‐3‐OH‐acyl building blocks but devoid of free mc‐3‐OH‐FAs (such as lipid A but also lipopolysaccharides, rhamnolipids, lipopeptides and acyl‐homoserine‐lactones) do not trigger LORE‐dependent immunity (Kutschera et al., 2019). Recently, it has also been shown that LPS can induce LORE‐independent immunity in Arabidopsis (Shang‐Guan et al., 2018). Interestingly, in rice the perception of LPS is partly dependent on CERK1, while this co‐receptor is not involved in LPS sensing in Arabidopsis (Desaki et al., 2018; Ranf et al., 2015). This indicates that LPS perception mechanisms in monocots and eudicots could require different receptor complexes and potentially different molecular patterns. LPS, as constituents of the cell wall, are hardly available for plant cell perception and the exact mechanisms of LPS delivery to plant cells is not fully understood. Surfactants were shown to promote the release of LPS from bacterial cells (Al‐Tahhan et al., 2000) and plant lipid binding protein (LBP)‐like proteins or bacterial outer membrane vesicles could also be involved in LPS delivery (Iizasa et al., 2016; Katsir and Bahar, 2017).
Plant genomes encode several hydrolytic enzymes, including chitinases and glucanases commonly known as pathogenesis‐related (PR) proteins that can use the fungal cell wall as a substrate to release chitin and glucans (Pusztahelyi, 2018). Chitin, an homopolymer of β‐(1,4)‐linked N‐acetyl‐d‐glucosamine (GlcNAc) present in fungi and oomycete cell wall and arthropod exoskeleton, is a widespread IP perceived by monocot and eudicot plants. Perception of chitin and chitin‐derived oligosaccharide structures by plants depends on the acetylation and/or the polymerization degree of these compounds (Pusztahelyi, 2018). Chitin perception in rice and Arabidopsis implies PRRs sharing a similar extracellular LysM domain (Cao et al., 2014). In rice, the RLP OsCEBIP binds chitin and interacts with OsCERK1 to trigger signalling events (Hayafune et al., 2014). The LYP4 and LYP6 CEBiP‐like RLPs from rice are also able to bind chitin and are partially involved in its recognition by the plants (Liu et al., 2012). In Arabidopsis, CERK1 is also necessary for chitin sensing. This LysM‐containing RK interacts with chitin and LYK5, another LysM RK, to induce defence responses (Cao et al., 2014). Chitosan, a modified form of chitin only found in fungi that possess deacetylase enzymes, is also perceived by several plants (Hadwiger, 2013; Zuppini et al., 2004). Chitosan oligosaccharide binding proteins were recently identified from the plasma membrane of wheat leaf cell and include W5G2U8, a potential WAK1 receptor protein, and W5HY42 and W5I0R4, which are potential GsSRK (G‐type lectin S‐receptor‐like serine/threonine‐protein kinases) receptor proteins (Liu et al., 2018). Whether these proteins directly interact with chitosan is still unknown. Recently, it was demonstrated that chitin and chitosan perception involves VvLYK1‐1 and VvLYK1‐2 in grapevine (Brulé et al., 2018). β‐(1,6)‐glucans, important polysaccharides from fungi and oomycetes, have been extensively studied for their capacity to induce an immune response in plants. How these molecules are sensed by plants, however, is poorly understood (Fesel and Zuccaro, 2016). Remarkably, the extracellular domains of PRRs involved in the recognition of polysaccharide‐derived IPs from microbes and characterized to date are only from LysM‐types.
IPs from plant origin
Plants are able to detect damage caused by pathogens or pests (Gust et al., 2017). Among IP endogen peptides, AtPeps are the best known. AtPep1, AtPep2 and AtPep3 are perceived by the LRR‐RKs PEPR1 and PEPR2 in Arabidopsis (Krol et al., 2010). AtPep1 is derived from the C‐terminus part of PROPEP1 protein and is over‐expressed following wounding, cell wall degradation and IP perception (Krol et al., 2010). Similarly, PIP1 and PIP2, two other pathogen‐inducible endogen peptides, are recognized by plants. After infection, prePIP1 and prePIP2 are secreted in the extracellular space and cleaved at the C‐terminus. The LRR‐RK RLK7 participates in the perception of PIP1 (Hou et al., 2014).
Systemin is an 18 amino acid peptide from Solanaceae released in the apoplast by an unknown mechanism. Systemin interacts with the LRR‐RKs SYR1 and SYR2 to induce defence against insect herbivory (Wang et al., 2018). PORK1, another LRR‐RK from tomato closely related to the SYR1 and SYR2 proteins, is also required for systemin‐induced defences (Xu et al., 2018). Whether SYR1/2 could act in concert with PORK1 remains to be investigated. PORK1 interacts and phosphorylates the protein kinase TPK1b to induce systemin‐driven immunity. Interestingly, systemin treatment does not induce ROS production and phosphorylation cascade activation is not reduced in the PORK1 RNAi line (Xu et al., 2018), suggesting that systemin also induces PORK1‐independent defence mechanisms in plants.
DORN1, a purinoreceptor from the L‐type lectin RK family, senses extracellular ATP (eATP) in Arabidopsis and is necessary for eATP‐mediated defence induction. Interestingly, it only perceives eATP and no other eNTPs (Choi et al., 2014). How eATP is available for perception by the PRR remains unknown. One hypothesis could be a release by cellular lysis upon pathogen infection. Even though other nucleotidic molecules like bacterial RNAs (Lee et al., 2016) and extracellular small DNA fragments (Duran‐flores and Heil, 2018) are known to induce plant immunity, only eATP was identified with a cognate PRR.
Although β‐(1,6)‐glucans are generally specific of fungi and oomycetes, β‐(1,3)‐glucans are naturally present in plant cell walls (Fesel and Zuccaro, 2016). In Arabidopsis, it was recently shown that non‐branched β‐(1,3)‐glucans sensing requires CERK1, the co‐receptor also involved in chitin perception (Mélida et al., 2018). Laminarin, a β‐(1,3)‐glucan polymer with β‐(1,6) branches produced by Laminaria digitata alga, induces a PTI in several plants, including grapevine (Aziz et al., 2003), tobacco (Klarzynski et al., 2000) and Arabidopsis (Ménard et al., 2004). Interestingly, a sulphated‐derived structure of this β‐(1,3)‐glucan is even more active (Menard et al., 2004; Trouvelot et al., 2008). Oligogalacturonides (oligomers of α‐(1,4)‐linked galacturonosyl residues, OGs), released from pectin after degradation by fungal polygalacturonases, associate with WAK1, a singular EGF‐like‐RK to trigger plant immunity (Brutus et al., 2010). Overall, plant protein‐derived IPs are sensed by receptors carrying an LRR domain (like microbial IPs) and more specifically belonging to the RK family. In addition, plant β‐(1,3)‐glucan recognition requires the co‐receptor CERK1, which is mainly associated with LysM‐type PRRs and is also involved in the sensing of polysaccharide‐derived IPs from microbes.
IPs sensed through plasma membrane lipid interaction
Like proteins, lipids are major components of plasma membranes. Lipids, as the first components encountered by IPs able to bind or insert into plant plasma membranes, could participate in their initial sensing and the establishment of a ‘danger’‐related immune response. Various studies suggest that some IPs may directly interact with lipids (and not PRRs) either modulating plasma membrane physical properties (driven by insertion between lipids and/or membrane damages) or, as demonstrated more recently, using lipid decoration as the receptor/target (Mamode‐Cassim et al., 2019). In all cases, this interaction of IPs with plasma membrane lipids could change the behaviour and functions of membrane microdomains/nanodomains containing a variety of integral membrane proteins, such as mechanoreceptors, ion channels, membrane receptors and enzymes. The changes in location and/or activity of membrane proteins after lipid binding could lead to immune signalling activation (Fig. 1). IPs interacting with plasma membrane lipids are often of hydrophobic or amphiphilic nature and include proteinaceous, lipid‐derived or polysaccharide‐derived structures (Table 1). Moreover, some of these compounds can be classified as or are related to toxin‐like compounds.
Proteinaceous IPs
The best recent example of IPs directly binding to lipids is represented by cytotoxic NLPs. NLP‐mediated phytotoxicity and plant defence gene expression are closely related, suggesting that toxin‐mediated interference with host integrity triggers plant immunity‐associated responses. This phytotoxin‐mediated activation of plant immunity is reminiscent of microbial toxin‐induced inflammasome activation in vertebrates, which results in secretion of cytokines and programmed pro‐inflammatory cell death (Ottmann et al., 2009; Qutob et al., 2006). Even though a 20‐mer conserved fragment from NLPs induces plant immunity through RLP23/SOBIR1/BAK1 protein complex, cytotoxic NLPs also directly bind to glycosyl inositol phosphoryl ceramide (GIPC) (Lenarčič et al., 2017). GIPCs are the most abundant sphingolipids in plant membranes and comprise 60–80% lipids in the outer leaflet of the plasma membrane (Lenarčič et al., 2017; Van den Ackerveken, 2017). NLPs can bind terminal monomeric hexose moieties of GIPCs, resulting in conformational changes within the protein. The NLP/GIPC binding has been quantified by surface plasmon resonance analysis with a dissociation constant of around 300 nM in Arabidopsis. Only eudicot plants are affected by NLPs. Insensitivity of monocot plants to NLPs may be explained by the length of the GIPC headgroup, consisting of three‐terminal hexoses instead of two for eudicots (Lenarčič et al., 2017; Van den Ackerveken, 2017). NLPs are known as toxin‐like or virulence factors, therefore sphingolipids described as ‘receptors’ could also be considered as ‘targets’ for NLPs. However, it can be assumed that both the PRR recognition and the toxin‐like effects through lipid binding could act in concert to be used by the plant to perceive a ‘danger’ and to activate a strong innate immune response leading to an active plant cell death process (Qutob et al., 2006). Similarly, the cerato‐platanin BcSpl1 secreted by a necrotrophic fungus could be recognized as a ‘danger’ signal via a BAK1‐dependent process and a less specific and indirect sensing through lipid‐driven perturbation. Accordingly, BcSpl1 was shown to associate with the plant plasma membrane, triggering rapid morphological changes at the cellular level (Frías et al., 2014).
Harpins are proteins secreted by type III secretion systems of Gram‐negative bacteria like Erwinia amylovora and have been very well known as IPs since the 1990s (Baker et al., 1993; Wei et al., 1992). Harpins induce defence responses in several plant species (Choi et al., 2013). They are able to interact with lipids to form pores in artificial membranes and they participate in virulence to several bacteria (Choi et al., 2013). Interestingly, a non‐proteinaceous harpin binding site has been characterized in tobacco plasma membranes. It mediates activation of the PR gene HIN1 through mitogen‐activated protein kinase activity, independently of extracellular calcium fluxes (Lee et al., 2001). Elicitins and cryptogein in particular are known to bind sterols and other lipids with various affinities. Independent studies have revealed that elicitins can act as sterol carriers by scavenging sterols from synthetic liposomes and plant plasma membranes (Derevnina et al., 2016). Elicitin‐induced cell death could be due to disruption of plant plasma membrane integrity upon interaction. Interestingly, long‐chain bases sphingolipids (LCBs) and their phosphorylated derivatives present in the plasma membrane differentially regulate cryptogein‐induced production of reactive oxygen species (ROS) in tobacco cells (Coursol et al., 2015). In addition, the use of fluorescence recovery after photobleaching revealed an increase in plasma membrane fluidity induced by cryptogein, but not by flagellin (Gerbeau‐Pissot et al., 2014). As for NLPs, it can be postulated that both PRR recognition and lipid‐driven perturbations could act participate in elicitin‐related strong immune responses.
Lipid‐based IPs
Several lipid‐derived IPs have already been discovered, including arachidonic acid, eicosapolyenoic acid (Bostock et al., 1981, 2011; Robinson and Bostock, 2015) and cerebroside (Umemura et al., 2002, 2004). However, little is known about their perception by plants.
Ergosterol, a fungi‐specific sterol, induces immunity‐related markers in tobacco and tomato (Klemptner et al., 2014). Interestingly, this molecule triggers apoplastic medium alkalinization in tomato, unlike plant sterols (Granado et al., 1995). It has been hypothesized that plants either possess an ergosterol receptor or that ergosterol uptake could lead to perturbations of lipid raft structures because of their ability to form very stable microdomains (Klemptner et al., 2014; Xu et al., 2001). In this respect, ergosterol directly affects Beta vulgaris plasma membrane H+‐ATPase activities, indicating that it could impact the structural organization of lipid rafts from this plant plasma membrane (Rossard et al., 2010).
Cyclic lipopeptides are amphiphilic molecules produced by a large variety of bacteria such as Streptomyces, Pseudomonas and Bacillus (Raaijmakers et al., 2010). Lipopeptides have emerged as key players in the induction of plant immunity driven by beneficial microorganisms (Falardeau et al., 2013; Raaijmakers et al., 2010). Bacillus subtilis is known to produce three main families of cyclic lipopeptides, namely surfactins, iturins and fengycins (Falardeau et al., 2013). Mycosubstilin, a lipopeptide from the iturin family, plipastatin from the fengycin family and surfactin activate immunity‐related markers in grapevine, cotton and Arabidopsis (Debois et al., 2015; Farace et al., 2015; Han et al., 2015). Mycosubtilin and fengycin are known to interact with membrane lipids (Deleu et al., 2005, 2008; Maget‐Dana and Ptak, 1990; Nasir et al., 2010). Massetolide A, secreted by Pseudomonas fluorescens SS101, also induces plant defence mechanisms in tomato (Tran et al., 2007) and orfamide produced by Pseudomonas spp. has recently been shown to induce rice and bean immunity (Ma et al., 2016, 2017). Surfactin studies highlighted that the lipopeptide structure strongly impacts its ability to trigger an immune response. Surfactins with C14 and C15 chain length induce extracellular medium alkalinization, unlike C12 and C13 (Jourdan et al., 2009). Importantly, it was demonstrated that surfactin has to target the lipid fraction of the plant plasma membrane in order to induce immune‐related defence responses (Henry et al., 2011). Longer chain length surfactins displayed stronger interactions with membranes compared to C12 and C13 variants. Moreover, there was no refractory state induced by repeated stimulations with surfactin. It was therefore proposed that surfactin perception relies on a lipid‐driven process rather than a direct sensing by high‐affinity protein receptors (Henry et al., 2011). Rhamnolipids (RLs) are amphiphilic molecules secreted by Pseudomonas and Burkholderia species and involved in bacterial motility and biofilm formation (Abdel‐Mawgoud et al., 2010). RLs are able to induce Brassica napus, Arabidopsis and Vitis vinifera immunity (Monnier et al., 2018; Sanchez et al., 2012; Varnier et al., 2009). Rhamnose alone is not responsible for this immune response (Varnier et al., 2009). Given their amphiphilic nature, it was postulated that RLs could interact with plant membrane lipids (Sanchez et al., 2012). Recently it has been demonstrated that RLs fit into plant lipid‐based membrane models and are located near the lipid phosphate group of the phospholipid bilayers, near phospholipid glycerol backbones (Monnier et al., 2019). RL insertion inside the lipid bilayer does not strongly affect lipid dynamics but the nature of the phytosterols could influence the effect of RLs on plant plasma membrane destabilization. These subtle changes in lipid dynamics could be linked with plant defence induction (Monnier et al., 2019). Interestingly, synthetic molecules derived from the RL structure are also known to induce plant immunity. Synthetic rhamnolipid bolaforms (SRBs), composed of two rhamnoses separated by a fatty acid chain (Obounou Akong et al., 2015), trigger an immune response in Arabidopsis that varies according to fatty acid chain length (Luzuriaga‐Loaiza et al., 2018). Ac‐RLs and Alk‐RLs, only differing from natural RLs by the terminal group of the carbon chain (with a methyl for Alk‐RLs and a carboxylic acid for Ac‐RLs), induce ROS production in Arabidopsis (Nasir et al., 2017). Interestingly, these synthetic RLs are able to interact with membrane lipids, suggesting that perception of these molecules could involve a lipid‐driven process (Luzuriaga‐Loaiza et al., 2018; Nasir et al., 2017). Alk‐RLs were more favourably inserted into model membranes and induced a higher response than Ac‐RL, suggesting that differences in the biological activity of these molecules could be linked to their amphiphilic nature and their capacity to interact with the membrane (Nasir et al., 2017). The synthetic 3‐tetradecylamino‐tert‐butyl‐N‐tetradecylpropionamidine (diC14) lipid is known to induce TLR4‐dependent mechanisms in mammals. It has also been studied for its eliciting properties in Arabidopsis and interestingly the plant defence response induced by the molecule is independent from CERK1 (Cambiagno et al., 2015). The chain length of the lipid influences the immune response in Arabidopsis. diC14 and diC16 induce defence‐related gene expression in this plant, but diC16 leads to weaker responses. diC14 pretreatment triggers Arabidopsis resistance against P. syringae, unlike diC16 (Cambiagno et al., 2015). It was therefore hypothesized that the interaction of diC14 with plant plasma membrane lipids may alter the organization, compartmentalization or composition of this membrane to somehow boost the activity of the plant defence system (Cambiagno et al., 2015).
Polysaccharide‐derived IPs
Some studies have shown that the LysM domain of CERK1 has very weak binding affinity to chitosan (Iizasa et al., 2010; Petutschnig et al., 2010). Moreover, defence genes are up‐regulated by chitosan, both in wild‐type Arabidopsis and chitin‐insensitive cerk1 mutant, demonstrating that chitosan is perceived through a CERK1‐independent pathway (Povero et al., 2011). In addition, chitosan can interact with phospholipids bilayers (Fang et al., 2001). As proposed for several IPs, chitosan could induce membrane structure modifications, stimulating plant immunity (Iriti and Varoni, 2015). As for ergosterol, it was also demonstrated that chitosan directly affects plasma membrane H+‐ATPase, giving rise to a possible link between chitosan‐triggered plant innate immunity and its putative impact on the structural organization of lipid rafts from the plant plasma membrane (Amborabé et al., 2008; Rossard et al., 2010).
Conclusion
Apoplastic IPs are diverse in their molecular nature: some are kingdom‐specific or even specific to species, while others are present in several kingdoms, such as chitin, which is found in fungi, bacteria and arthropods. The majority of apoplastic IPs characterized to date are perceived by plants at the plasma membrane through PRRs. Interestingly, more and more studies are also suggesting a new perception system based on direct sensing through membrane lipids without the involvement of specific proteinaceous PRRs. This sensing system monitors membrane perturbations and is driven by amphiphilic compounds or toxin‐like compounds. Interestingly, some IPs, such as NLPs, elicitins or chitosan, can be perceived through direct interaction with PRRs and/or by lipid‐mediated mechanisms. In addition, IPs known or suspected to be perceived through membrane lipids are only from microbial origin. Many studies are still required to understand how IPs are sensed by plants and it seems that a large variety of processes are involved. Further integrative investigations, including biophysical approaches and functional biology on plasma membrane lipids, are required to characterize lipid‐based IP perception and its potential relationship with PRR‐mediated mechanisms. The understanding of IP‐triggered immunity is a first step in the development of new plant breeding strategies. PRR engineering and modification of the lipid composition of the plasma membrane could impact the ability of plants to perceive IPs and are examples of biotechnologies that could be used to optimize plant resistance.
Acknowledgements
This work was supported by the grants from the Rhamnoprot and ResIGLi projects, both co‐funded by Region Grand Est and the European Union FEDER programme. Support from the MESRI (Ministère de l’Enseignement Supérieur, de la Recherche et de l’Innovation) and the Federative Research Structure SFR Condorcet are gratefully acknowledged. We thank the reviewers for critical reading and very useful comments to improve the manuscript.
References
- Abdel‐Mawgoud, A.M. , Lépine, F. and Déziel, E. (2010) Rhamnolipids: diversity of structures, microbial origins and roles. Appl. Microbiol. Biotechnol. 86, 1323–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Ackerveken, G. (2017) How plants differ in toxin‐sensitivity. Science, 358, 1383–1384. [DOI] [PubMed] [Google Scholar]
- Adachi, H. , Derevnina, L. and Kamoun, S. (2019) NLR singletons, pairs, and networks: evolution, assembly, and regulation of the intracellular immunoreceptor circuitry of plants. Curr. Opin. Plant Biol. 50, 121–131. [DOI] [PubMed] [Google Scholar]
- Albert, I. , Böhm, H. , Albert, M. , Feiler, C.E. , Imkampe, J. , Wallmeroth, N. , Brancato, C. , Raaymakers, T.M. , Oome, S. , Zhang, H. , Krol, E. , Grefen, C. , Gust, A.A. , Chai, J. , Hedrich, R. , Van den Ackerveken, G. and Nürnberger, T. (2015) An RLP23‐SOBIR1‐BAK1 complex mediates NLP‐triggered immunity. Nat. Plants, 1, 15140. [DOI] [PubMed] [Google Scholar]
- Al‐Tahhan, R.A. , Sandrin, T.R. , Bodour, A.A. and Maier, R.M. (2000) Rhamnolipid‐induced removal of lipopolysaccharide from Pseudomonas aeruginosa: effect on cell surface properties and interaction with hydrophobic substrates. Appl. Environ. Microbiol. 66, 3262–3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amborabé, B.E. , Bonmort, J. , Fleurat‐Lessard, P. and Roblin, G. (2008) Early events induced by chitosan on plant cells. J. Exp. Bot. 59, 2317–2324. [DOI] [PubMed] [Google Scholar]
- Aziz, A. , Poinssot, B. , Daire, X. , Adrian, M. , Bézier, A. , Lambert, B. , Joubert, J.M. and Pugin, A. (2003) Laminarin elicits defense responses in grapevine and induces protection against Botrytis cinerea and Plasmopara viticola . Mol. Plant–Microbe Interact. 16, 1118–1128. [DOI] [PubMed] [Google Scholar]
- Baker, C.J. , Orlandi, E.W. and Mock, N.M. (1993) Harpin, an elicitor of the hypersensitive response in tobacco caused by Erwinia amylovora, elicits active oxygen production in suspension cells. Plant Physiol. 102, 1341–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakireva, A.V. and Zamyatnin, A.A. (2018) Indispensable role of proteases in plant innate immunity. Int. J. Mol. Sci. 19, 629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar, M. , Sharfman, M. , Ron, M. and Avni, A. (2010) BAK1 is required for the attenuation of ethylene‐inducing xylanase (Eix)‐induced defense responses by the decoy receptor LeEix1. Plant J. 63, 791–800. [DOI] [PubMed] [Google Scholar]
- Bektas, Y. and Eulgem, T. (2015) Synthetic plant defense elicitors. Front. Plant Sci. 5, 804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Białas, A. , Zess, E.K. , De la Concepcion, J.C. , Franceschetti, M. , Pennington, H.G. , Yoshida, K. , Upson, J.L. , Chanclud, E. , Wu, C.H. , Langner, T. , Maqbool, A. , Varden, F.A. , Derevnina, L. , Belhaj, K. , Fujisaki, K. , Saitoh, H. , Terauchi, R. , Banfield, M.J. and Kamoun, S. (2018) Lessons in effector and NLR biology of plant‐microbe systems. Mol. Plant–Microbe Interact. 31, 34–45. [DOI] [PubMed] [Google Scholar]
- Böhm, H. , Albert, I. , Oome, S. , Raaymakers, T.M. , Van den Ackerveken, G. and Nürnberger, T. (2014) A conserved peptide pattern from a widespread microbial virulence factor triggers pattern-induced immunity in Arabidopsis . PLoS pathogens, 10, e1004491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller, T. and Felix, G. (2009) A renaissance of elicitors: perception of microbe‐associated molecular patterns and danger signals by pattern‐recognition receptors. Annu. Rev. Plant Biol. 60, 379–406. [DOI] [PubMed] [Google Scholar]
- Bostock, R.M. , Kuc, J.A. and Laine, R.A. (1981) Eicosapentaenoic and arachidonic acids from Phytophthora infestans elicit fungitoxic sesquiterpenes in the potato. Science, 212, 67–69. [DOI] [PubMed] [Google Scholar]
- Bostock, R.M. , Savchenko, T. , Lazarus, C. and Dehesh, K. (2011) Eicosapolyenoic acids. Plant Signal. Behav. 6, 531–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutrot, F. and Zipfel, C. (2017) Function, discovery, and exploitation of plant pattern recognition receptors for broad‐spectrum disease resistance. Annu. Rev. Phytopathol. 55, 257–286. [DOI] [PubMed] [Google Scholar]
- Brulé, D. , Villano, C. , Davies, L.J. , Trdá, L. , Claverie, J. , Héloir, M.C. , Chiltz, A. , Adrian, M. , Darblade, B. , Tornero, P. , Stransfeld, L. , Boutrot, F. , Zipfel, C. , Dry, I.B. and Poinssot, B. (2018) The grapevine (Vitis vinifera) LysM receptor kinases VvLYK1‐1 and VvLYK1‐2 mediate chitooligosaccharide‐triggered immunity. Plant Biotechnol. J. 17, 812–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brutus, A. , Sicilia, F. , Macone, A. , Cervone, F. and Lorenzo, G.D. (2010) A domain swap approach reveals a role of the plant wall‐associated kinase 1 (WAK1) as a receptor of oligogalacturonides. Proc. Natl. Acad. Sci. USA. 107, 9452–9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buscaill, P. , Chandrasekar, B. , Sanguankiattichai, N. , Kourelis, J. , Kaschani, F. , Thomas, E.L. , Morimoto, K. , Kaiser, M. , Preston, G.M. , Ichinose, Y. and van der Hoorn, R.A.L. (2019) Glycosidase and glycan polymorphism control hydrolytic release of immunogenic flagellin peptides. Science, 364, Available at 10.1126/science.aav0748. [DOI] [PubMed] [Google Scholar]
- Cambiagno, D.A. , Lonez, C. , Ruysschaert, J.M. and Alvarez, M.E. (2015) The synthetic cationic lipid diC14 activates a sector of the Arabidopsis defence network requiring endogenous signalling components. Mol. Plant Pathol. 16, 963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, Y. , Liang, Y. , Tanaka, K. , Nguyen, C.T. , Jedrzejczak, R.P. , Joachimiak, A. and Stacey, G. (2014). The kinase LYK5 is a major chitin receptor in Arabidopsis and forms a chitin‐induced complex with related kinase CERK1. eLife, 3 10.7554/eLife.03766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary, R. , Atamian, H.S. , Shen, Z. , Briggs, S.P. and Kaloshian, I. (2014) GroEL from the endosymbiont Buchnera aphidicola betrays the aphid by triggering plant defense. Proc. Natl. Acad. Sci. USA. 111, 8919–8924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinchilla, D. , Bauer, Z. , Regenass, M. , Boller, T. and Felix, G. (2006) The Arabidopsis receptor kinase FLS2 binds flg22 and determines the specificity of flagellin perception. Plant Cell, 18, 465–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, M.S. , Kim, W. , Lee, C. and Oh, C.S. (2013) Harpins, multifunctional proteins secreted by gram‐negative plant‐pathogenic bacteria. Mol. Plant–Microbe Interact. 26, 1115–1122. [DOI] [PubMed] [Google Scholar]
- Choi, J. , Tanaka, K. , Cao, Y. , Qi, Y. , Qiu, J. , Liang, Y. , Lee, S.Y. and Stacey, G. (2014) Identification of a plant receptor for extracellular ATP. Science, 343, 290–294. [DOI] [PubMed] [Google Scholar]
- Cook, D.E. , Mesarich, C.H. and Thomma, B.P.H.J. (2015) Understanding plant immunity as a surveillance system to detect invasion. Annu. Rev. Phytopathol. 53, 541–563. [DOI] [PubMed] [Google Scholar]
- Coursol, S. , Fromentin, J. , Noirot, E. , Brière, C. , Robert, F. , Morel, J. , Liang, Y.K. , Lherminier, J. and Simon‐Plas, F. (2015) Long‐chain bases and their phosphorylated derivatives differentially regulate cryptogein‐induced production of reactive oxygen species in tobacco (Nicotiana tabacum) BY‐2 cells. New Phytol. 205, 1239–1249. [DOI] [PubMed] [Google Scholar]
- Couto, D. and Zipfel, C. (2016) Regulation of pattern recognition receptor signalling in plants. Nat. Rev. Immunol. 16, 537–552. [DOI] [PubMed] [Google Scholar]
- Debois, D. , Fernandez, O. , Franzil, L. , Jourdan, E. , de Brogniez, A. , Willems, L. , Clément, C. , Dorey, S. , De Pauw, E. and Ongena, M. (2015) Plant polysaccharides initiate underground crosstalk with bacilli by inducing synthesis of the immunogenic lipopeptide surfactin. Environ. Microbiol. Rep. 7, 570–582. [DOI] [PubMed] [Google Scholar]
- Deleu, M. , Paquot, M. and Nylander, T. (2005) Fengycin interaction with lipid monolayers at the air–aqueous interface – implications for the effect of fengycin on biological membranes. J. Colloid. Interface Sci. 283, 358–365. [DOI] [PubMed] [Google Scholar]
- Deleu, M. , Paquot, M. and Nylander, T. (2008) Effect of fengycin, a lipopeptide produced by Bacillus subtilis, on model biomembranes. Biophys. J. 94, 2667–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derevnina, L. , Dagdas, Y.F. , la Concepcion, J.C.D. , Bialas, A. , Kellner, R. , Petre, B. , Domazakis, E. , Du, J. , Wu, C.H. , Lin, X. , Aguilera‐Galvez, C. , Cruz‐Mireles, N. , Vleeshouwers, V.G.A.A. and Kamoun, S. (2016) Nine things to know about elicitins. New Phytol. 212, 888–895. [DOI] [PubMed] [Google Scholar]
- Desaki, Y. , Kouzai, Y. , Ninomiya, Y. , Iwase, R. , Shimizu, Y. , Seko, K. , Molinaro, A. , Minami, E. , Shibuya, N. , Kaku, H. and Nishizawa, Y. (2018) OsCERK1 plays a crucial role in the lipopolysaccharide‐induced immune response of rice. New Phytol. 217, 1042–1049. [DOI] [PubMed] [Google Scholar]
- Desender, S. , Klarzynski, O. , Potin, P. , Barzic, M.R. , Andrivon, D. and Val, F. (2006) Lipopolysaccharides of Pectobacterium atrosepticum and Pseudomonas corrugata induce different defence response patterns in tobacco, tomato, and potato. Plant Biol. 8, 636–645. [DOI] [PubMed] [Google Scholar]
- Dodds, P.N. and Rathjen, J.P. (2010) Plant immunity: towards an integrated view of plant–pathogen interactions. Nat. Rev. Genet. 11, 539–548. [DOI] [PubMed] [Google Scholar]
- Domazakis, E. , Wouters, D. , Visser, R.G.F. , Kamoun, S. , Joosten, M.H.A.J. and Vleeshouwers, V.G.A.A. (2018) The ELR‐SOBIR1 complex functions as a two‐component receptor‐like kinase to mount defense against Phytophthora infestans . Mol. Plant–Microbe Interact. 31, 795–802. [DOI] [PubMed] [Google Scholar]
- Dow, M. , Newman, M.A. and von Roepenack, E. (2000) The induction and modulation of plant defense responses by bacterial lipopolysaccharides. Annu. Rev. Phytopathol. 38, 241–261. [DOI] [PubMed] [Google Scholar]
- Du, J. , Verzaux, E. , Chaparro‐Garcia, A. , Bijsterbosch, G. , Keizer, L.C.P. , Zhou, J. , Liebrand, T.W.H. , Xie, C. , Govers, F. , Robatzek, S. , van der Vossen, E.A.G. , Jacobsen, E. , Visser, R.G.F. , Kamoun, S. and Vleeshouwers, V.G.A.A. (2015) Elicitin recognition confers enhanced resistance to Phytophthora infestans in potato. Nat. Plants, 1, 15034. [DOI] [PubMed] [Google Scholar]
- Duran‐Flores, D. and Heil, M. (2018) Extracellular self‐DNA as a damage‐associated molecular pattern (DAMP) that triggers self‐specific immunity induction in plants. Brain Behav. Immun. 72, 78–88. [DOI] [PubMed] [Google Scholar]
- Erbs, G. and Newman, M.A. (2012) The role of lipopolysaccharide and peptidoglycan, two glycosylated bacterial microbe‐associated molecular patterns (MAMPs), in plant innate immunity. Mol. Plant Pathol. 13, 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbs, G. , Silipo, A. , Aslam, S. , De Castro, C. , Liparoti, V. , Flagiello, A. , Pucci, P. , Lanzetta, R. , Parrilli, M. , Molinaro, A. , Newman, M.A. and Cooper, R.M. (2008) Peptidoglycan and muropeptides from pathogens Agrobacterium and Xanthomonas elicit plant innate immunity: structure and activity. Chem. & Biol. 15, 438–448. [DOI] [PubMed] [Google Scholar]
- Falardeau, J. , Wise, C. , Novitsky, L. and Avis, T.J. (2013) Ecological and mechanistic insights into the direct and indirect antimicrobial properties of Bacillus subtilis lipopeptides on plant pathogens. J. Chem. Ecol. 39, 869–878. [DOI] [PubMed] [Google Scholar]
- Fang, N. , Chan, V. , Mao, H.Q. and Leong, K.W. (2001) Interactions of phospholipid bilayer with chitosan: effect of molecular weight and pH. Biomacromol. 2, 1161–1168. [DOI] [PubMed] [Google Scholar]
- Farace, G. , Fernandez, O. , Jacquens, L. , Coutte, F. , Krier, F. , Jacques, P. , Clément, C. , Barka, E.A. , Jacquard, C. and Dorey, S. (2015) Cyclic lipopeptides from Bacillus subtilis activate distinct patterns of defence responses in grapevine. Mol. Plant Pathol. 16, 177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix, G. and Boller, T. (2003) Molecular sensing of bacteria in plants. The highly conserved RNA‐binding motif RNP‐1 of bacterial cold shock proteins is recognized as an elicitor signal in tobacco. J. Biol. Chem. 278, 6201–6208. [DOI] [PubMed] [Google Scholar]
- Fesel, P.H. and Zuccaro, A. (2016) β‐glucan: Crucial component of the fungal cell wall and elusive MAMP in plants. Fungal Genet. Biol. 90, 53–60. [DOI] [PubMed] [Google Scholar]
- Fradin, E.F. , Zhang, Z. , Juarez Ayala, J.C. , Castroverde, C.D.M. , Nazar, R.N. , Robb, J. , Liu, C.M. and Thomma, B.P.H.J. (2009) Genetic dissection of Verticillium wilt resistance mediated by tomato Ve1. Plant Physiol. 150, 320–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco‐Orozco, B. , Berepiki, A. , Ruiz, O. , Gamble, L. , Griffe, L.L. , Wang, S. , Birch, P.R.J. , Kanyuka, K. and Avrova, A. (2017) A new proteinaceous pathogen‐associated molecular pattern (PAMP) identified in Ascomycete fungi induces cell death in Solanaceae. New Phytol. 214, 1657–1672. [DOI] [PubMed] [Google Scholar]
- Frías, M. , González, C. and Brito, N. (2011) BcSpl1, a cerato‐platanin family protein, contributes to Botrytis cinerea virulence and elicits the hypersensitive response in the host. New Phytol. 192, 483–495. [DOI] [PubMed] [Google Scholar]
- Frías, M. , Brito, N. and González, C. (2013) The Botrytis cinerea cerato‐platanin BcSpl1 is a potent inducer of systemic acquired resistance (SAR) in tobacco and generates a wave of salicylic acid expanding from the site of application. Mol. Plant Pathol. 14, 191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frías, M. , Brito, N. , González, M. and González, C. (2014) The phytotoxic activity of the cerato‐platanin BcSpl1 resides in a two‐peptide motif on the protein surface. Mol. Plant Pathol. 15, 342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaulin, E. , Dramé, N. , Lafitte, C. , Torto‐Alalibo, T. , Martinez, Y. , Ameline‐Torregrosa, C. , Khatib, M. , Mazarguil, H. , Villalba‐Mateos, F. , Kamoun, S. , Mazars, C. , Dumas, B. , Bottin, A. , Esquerré‐Tugayé, M.T. and Rickauer, M. (2006) Cellulose binding domains of a Phytophthora cell wall protein are novel pathogen‐associated molecular patterns. Plant Cell, 18, 1766–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbeau‐Pissot, P. , Der, C. , Thomas, D. , Anca, I.A. , Grosjean, K. , Roche, Y. , Perrier‐Cornet, J.M. , Mongrand, S. and Simon‐Plas, F. (2014) Modification of plasma membrane organization in tobacco cells elicited by cryptogein. Plant Physiol. 164, 273–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gijzen, M. and Nürnberger, T. (2006) Nep1‐like proteins from plant pathogens: recruitment and diversification of the NPP1 domain across taxa. Phytochem. 67, 1800–1807. [DOI] [PubMed] [Google Scholar]
- Gómez‐Gómez, L. and Boller, T. (2000) FLS2: an LRR receptor‐like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis . Mol. Cell, 5, 1003–1011. [DOI] [PubMed] [Google Scholar]
- Gómez‐Gómez, L. and Boller, T. (2002) Flagellin perception: a paradigm for innate immunity. Trends Plant Sci. 7, 251–256. [DOI] [PubMed] [Google Scholar]
- Gómez‐Gómez, L. , Felix, G. and Boller, T. (1999) A single locus determines sensitivity to bacterial flagellin in Arabidopsis thaliana . Plant J. 18, 277–284. [DOI] [PubMed] [Google Scholar]
- Granado, J. , Felix, G. and Boller, T. (1995) Perception of fungal sterols in plants (subnanomolar concentrations of ergosterol elicit extracellular alkalinization in Tomato cells). Plant Physiol. 107, 485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gust, A.A. and Felix, G. (2014) Receptor like proteins associate with SOBIR1‐type of adaptors to form bimolecular receptor kinases. Curr. Opin. Plant Biol. 21, 104–111. [DOI] [PubMed] [Google Scholar]
- Gust, A.A. , Biswas, R. , Lenz, H.D. , Rauhut, T. , Ranf, S. , Kemmerling, B. , Götz, F. , Glawischnig, E. , Lee, J. , Felix, G. and Nürnberger, T. (2007) Bacteria‐derived peptidoglycans constitute pathogen‐associated molecular patterns triggering innate immunity in Arabidopsis . J. Biol. Chem. 282, 32338–32348. [DOI] [PubMed] [Google Scholar]
- Gust, A.A. , Pruitt, R. and Nürnberger, T. (2017) Sensing danger: key to activating plant immunity. Trends Plant Sci. 22, 779–791. [DOI] [PubMed] [Google Scholar]
- Hadwiger, L.A. (2013) Multiple effects of chitosan on plant systems: solid science or hype. Plant Sci. 208, 42–49. [DOI] [PubMed] [Google Scholar]
- Han, Q. , Wu, F. , Wang, X. , Qi, H. , Shi, L. , Ren, A. , Liu, Q. , Zhao, M. and Tang, C. (2015) The bacterial lipopeptide iturins induce Verticillium dahliae cell death by affecting fungal signalling pathways and mediate plant defence responses involved in pathogen‐associated molecular pattern‐triggered immunity. Environ. Microbiol. 17, 1166–1188. [DOI] [PubMed] [Google Scholar]
- Hayafune, M. , Berisio, R. , Marchetti, R. , Silipo, A. , Kayama, M. , Desaki, Y. , Arima, S. , Squeglia, F. , Ruggiero, A. , Tokuyasu, K. , Molinaro, A. , Kaku, H. and Shibuya, N. (2014) Chitin‐induced activation of immune signaling by the rice receptor CEBiP relies on a unique sandwich‐type dimerization. Proc. Natl. Acad. Sci. USA. 111, E404–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath, M.C. (2000) Nonhost resistance and nonspecific plant defenses. Curr. Opin. Plant Biol. 3, 315–319. [DOI] [PubMed] [Google Scholar]
- Henry, G. , Deleu, M. , Jourdan, E. , Thonart, P. and Ongena, M. (2011) The bacterial lipopeptide surfactin targets the lipid fraction of the plant plasma membrane to trigger immune‐related defence responses. Cell. Microbiol. 13, 1824–1837. [DOI] [PubMed] [Google Scholar]
- Hind, S.R. , Strickler, S.R. , Boyle, P.C. , Dunham, D.M. , Bao, Z. , O’Doherty, I.M. , Baccile, J.A. , Hoki, J.S. , Viox, E.G. , Clarke, C.R. , Vinatzer, B.A. , Schroeder, F.C. and Martin, G.B. (2016) Tomato receptor FLAGELLIN‐SENSING 3 binds flgII‐28 and activates the plant immune system. Nat. Plants, 2, 16128. [DOI] [PubMed] [Google Scholar]
- Hou, S. , Wang, X. , Chen, D. , Yang, X. , Wang, M. , Turrà, D. , Di Pietro, A. and Zhang, W. (2014) The secreted peptide PIP1 amplifies immunity through receptor‐like kinase 7. PLoS Pathog. 10, e1004331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizasa, E. , Mitsutomi, M. and Nagano, Y. (2010) Direct binding of a plant LysM receptor‐like kinase, LysM RLK1/CERK1, to chitin in vitro. J. Biol. Chem. 285, 2996–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizasa, S. , Iizasa, E. , Matsuzaki, S. , Tanaka, H. , Kodama, Y. , Watanabe, K. and Nagano, Y. (2016) Arabidopsis LBP/BPI related‐1 and ‐2 bind to LPS directly and regulate PR1 expression. Sci. Rep. 6, 27527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iriti, M. and Varoni, E.M. (2015) Chitosan‐induced antiviral activity and innate immunity in plants. Environ Sci. Pollut. Res. 22, 2935–2944. [DOI] [PubMed] [Google Scholar]
- Jones, J.D. and Dangl, J.L. (2006) The plant immune system. Nature, 444, 323–329. [DOI] [PubMed] [Google Scholar]
- de Jonge, R. , Peter van Esse, H. , Maruthachalam, K. , Bolton, M.D. , Santhanam, P. , Saber, M.K. , Zhang, Z. , Usami, T. , Lievens, B. , Subbarao, K.V. and Thomma, B.P.H.J. (2012) Tomato immune receptor Ve1 recognizes effector of multiple fungal pathogens uncovered by genome and RNA sequencing. Proc. Natl. Acad. Sci. USA. 109, 5110–5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdan, E. , Henry, G. , Duby, F. , Dommes, J. , Barthélemy, J.P. , Thonart, P. and Ongena, M. (2009) Insights into the defense‐related events occurring in plant cells following perception of surfactin‐type lipopeptide from Bacillus subtilis . Mol. Plant–Microbe Interact. 22, 456–468. [DOI] [PubMed] [Google Scholar]
- Kanyuka, K. and Rudd, J.J. (2019) Cell surface immune receptors: the guardians of the plant's extracellular spaces. Curr. Opin. Plant Biol. 50, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsir, L. and Bahar, O. (2017) Bacterial outer membrane vesicles at the plant‐pathogen interface. PLoS Pathog. 13, e1006306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Y.T. , Oh, J. , Kim, K.H. , Uhm, J.Y. and Lee, B.M. (2010) Isolation and characterization of NgRLK1, a receptor‐like kinase of Nicotiana glutinosa that interacts with the elicitin of Phytophthora capsici . Mol. Biol. Rep. 37, 717–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarzynski, O. , Plesse, B. , Joubert, J.M. , Yvin, J.C. , Kopp, M. , Kloareg, B. and Fritig, B. (2000) Linear beta‐1,3 glucans are elicitors of defense responses in tobacco. Plant Physiol. 124, 1027–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemptner, R.L. , Sherwood, J.S. , Tugizimana, F. , Dubery, I.A. and Piater, L.A. (2014) Ergosterol, an orphan fungal microbe‐associated molecular pattern (MAMP). Mol. Plant Pathol. 15, 747–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol, E. , Mentzel, T. , Chinchilla, D. , Boller, T. , Felix, G. , Kemmerling, B. , Postel, S. , Arents, M. , Jeworutzki, E. , Al‐Rasheid, K.A.S. , Becker, D. and Hedrich, R. (2010) Perception of the Arabidopsis danger signal peptide 1 involves the pattern recognition receptor AtPEPR1 and its close homologue AtPEPR2. J. Biol. Chem. 285, 13471–13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicek, C.P. , Starr, T.L. and Glass, N.L. (2014) Plant cell wall‐degrading enzymes and their secretion in plant‐pathogenic fungi. Annu. Rev. Phytopathol. 52, 427–451. [DOI] [PubMed] [Google Scholar]
- Kunze, G. , Zipfel, C. , Robatzek, S. , Niehaus, K. , Boller, T. and Felix, G. (2004) The N terminus of bacterial elongation factor Tu elicits innate immunity in Arabidopsis plants. Plant Cell, 16, 3496–3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutschera, A. , Dawid, C. , Gisch, N. , Schmid, C. , Raasch, L. , Gerster, T. , Schäffer, M. , Smakowska‐Luzan, E. , Belkhadir, Y. , Vlot, A.C. , Chandler, C.E. , Schellenberger, R. , Schwudke, D. , Ernst, R.K. , Dorey, S. , Hückelhoven, R. , Hofmann, T. and Ranf, S. (2019) Bacterial medium‐chain 3‐hydroxy fatty acid metabolites trigger immunity in Arabidopsis plants. Science, 364, 178–181. [DOI] [PubMed] [Google Scholar]
- Larroque, M. , Belmas, E. , Martinez, T. , Vergnes, S. , Ladouce, N. , Lafitte, C. , Gaulin, E. and Dumas, B. (2013) Pathogen‐associated molecular pattern‐triggered immunity and resistance to the root pathogen Phytophthora parasitica in Arabidopsis . J. Exp. Bot. 64, 3615–3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. , Klessig, D.F. and Nürnberger, T. (2001) A harpin binding site in tobacco plasma membranes mediates activation of the pathogenesis‐related gene HIN1 independent of extracellular calcium but dependent on mitogen‐activated protein kinase activity. Plant Cell, 13, 1079–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, B. , Park, Y.S. , Lee, S. , Song, G.C. and Ryu, C.M. (2016) Bacterial RNAs activate innate immunity in Arabidopsis . New Phytol. 209, 785–797. [DOI] [PubMed] [Google Scholar]
- Lee, H.A. , Lee, H.Y. , Seo, E. , Lee, J. , Kim, S.B. , Oh, S. , Choi, Eunbi , Choi, Eunhye , Lee, S.E. and Choi, D. (2017) Current understandings of plant nonhost resistance. Mol. Plant–Microbe Interact. 30, 5–15. [DOI] [PubMed] [Google Scholar]
- Lenarčič, T. , Albert, I. , Böhm, H. , Hodnik, V. , Pirc, K. , Zavec, A.B. , Podobnik, M. , Pahovnik, D. , Žagar, E. , Pruitt, R. , Greimel, P. , Yamaji‐Hasegawa, A. , Kobayashi, T. , Zienkiewicz, A. , Gömann, J. , Mortimer, J.C. , Fang, L. , Mamode‐Cassim, A. , Deleu, M. , Lins, L. , Oecking, C. , Feussner, I. , Mongrand, S. , Anderluh, G. and Nürnberger, T. (2017) Eudicot plant‐specific sphingolipids determine host selectivity of microbial NLP cytolysins. Science, 358, 1431–1434. [DOI] [PubMed] [Google Scholar]
- Liebrand, T.W. , van den Berg, G.C. , Zhang, Z. , Smit, P. , Cordewener, J.H. , America, A.H. , Sklenar, J. , Jones, A.M. , Tameling, W.I. , Robatzek, S. , Thomma, B.P. and Joosten, M.H. (2013) Receptor‐like kinase SOBIR1/EVR interacts with receptor‐like proteins in plant immunity against fungal infection. Proc. Natl. Acad. Sci. USA. 110, 10010–10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebrand, T.W. , van den Burg, H.A. and Joosten, M.H. (2014) Two for all: receptor‐associated kinases SOBIR1 and BAK1. Trends Plant Sci. 19, 123–132. [DOI] [PubMed] [Google Scholar]
- Liu, B. , Li, J.F. , Ao, Y. , Qu, J. , Li, Z. , Su, J. , Zhang, Y. , Liu, J. , Feng, D. , Qi, K. , He, Y. , Wang, J. and Wang, H.B. (2012) Lysin motif‐containing proteins LYP4 and LYP6 play dual roles in peptidoglycan and chitin perception in rice innate immunity. Plant Cell, 24, 3406–3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , Grabherr, H.M. , Willmann, R. , Kolb, D. , Brunner, F. , Bertsche, U. , Kühner, D. , Franz‐Wachtel, M. , Amin, B. , Felix, G. , Ongena, M. , Nürnberger, T. and Gust, A.A. (2014). Host‐induced bacterial cell wall decomposition mediates pattern‐triggered immunity in Arabidopsis. eLife, 3 10.7554/eLife.01990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, D. , Jiao, S. , Cheng, G. , Li, X. , Pei, Z. , Pei, Y. , Yin, H. and Du, Y. (2018) Identification of chitosan oligosaccharides binding proteins from the plasma membrane of wheat leaf cell. Int. J. Biol. Macromol. 111, 1083–1090. [DOI] [PubMed] [Google Scholar]
- Lozano‐Torres, J.L. , Wilbers, R.H.P. , Gawronski, P. , Boshoven, J.C. , Finkers‐Tomczak, A. , Cordewener, J.H.G. , America, A.H.P. , Overmars, H.A. , Van ‘t Klooster, J.W. , Baranowski, L. , Sobczak, M. , Ilyas, M. , van der Hoorn, R.A.L. , Schots, A. , de Wit, P.J.G.M. , Bakker, J. , Goverse, A. and Smant, G. (2012) Dual disease resistance mediated by the immune receptor Cf‐2 in tomato requires a common virulence target of a fungus and a nematode. Proc. Natl. Acad. Sci. USA. 109, 10119–10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzuriaga‐Loaiza, W.P. , Schellenberger, R. , Gaetano, Y.D. , Akong, F.O. , Villaume, S. , Crouzet, J. , Haudrechy, A. , Baillieul, F. , Clément, C. , Lins, L. , Allais, F. , Ongena, M. , Bouquillon, S. , Deleu, M. and Dorey, S. (2018) Synthetic rhamnolipid bolaforms trigger an innate immune response in Arabidopsis thaliana . Sci. Rep. 8, 8534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, Y. , Han, C. , Chen, J. , Li, H. , He, K. , Liu, A. and Li, D. (2015) Fungal cellulase is an elicitor but its enzymatic activity is not required for its elicitor activity. Mol. Plant Pathol. 16, 14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, Z. , Hua, G.K.H. , Ongena, M. and Höfte, M. (2016) Role of phenazines and cyclic lipopeptides produced by Pseudomonas sp. CMR12a in induced systemic resistance on rice and bean. Env. Microbiol. Reports, 8, 896–904. [DOI] [PubMed] [Google Scholar]
- Ma, Z. , Ongena, M. and Höfte, M. (2017) The cyclic lipopeptide orfamide induces systemic resistance in rice to Cochliobolus miyabeanus but not to Magnaporthe oryzae . Plant Cell Rep. 36, 1731–1746. [DOI] [PubMed] [Google Scholar]
- Maget‐Dana, R. and Ptak, M. (1990) Iturin lipopeptides: interactions of mycosubtilin with lipids in planar membranes and mixed monolayers. Biochim. Biophys. Acta, 1023, 34–40. [DOI] [PubMed] [Google Scholar]
- Mamode‐Cassim, A. , Gouguet, P. , Gronnier, J. , Laurent, N. , Germain, V. , Grison, M. , Boutté, Y. , Gerbeau‐Pissot, P. , Simon‐Plas, F. and Mongrand, S. (2019) Plant lipids: Key players of plasma membrane organization and function. Prog. Lipid Res. 73, 1–27. [DOI] [PubMed] [Google Scholar]
- Mélida, H. , Sopeña‐Torres, S. , Bacete, L. , Garrido‐Arandia, M. , Jordá, L. , López, G. , Muñoz‐Barrios, A. , Pacios, L.F. and Molina, A. (2018) Non‐branched β‐1,3‐glucan oligosaccharides trigger immune responses in Arabidopsis . Plant J. 93, 34–49. [DOI] [PubMed] [Google Scholar]
- Ménard, R. , Alban, S. , de Ruffray, P. , Jamois, F. , Franz, G. , Fritig, B. , Yvin, J.‐C. and Kauffmann, S. (2004) Beta‐1,3 glucan sulfate, but not beta‐1,3 glucan, induces the salicylic acid signaling pathway in tobacco and Arabidopsis . Plant Cell, 16, 3020–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miya, A. , Albert, P. , Shinya, T. , Desaki, Y. , Ichimura, K. , Shirasu, K. , Narusaka, Y. , Kawakami, N. , Kaku, H. and Shibuya, N. (2007) CERK1, a LysM receptor kinase, is essential for chitin elicitor signaling in Arabidopsis . Proc. Natl. Acad. Sci. USA. 104, 19613–19618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnier, N. , Furlan, A. , Botcazon, C. , Dahi, A. , Mongelard, G. , Cordelier, S. , Clément, C. , Dorey, S. , Sarazin, C. and Rippa, S. (2018) Rhamnolipids from Pseudomonas aeruginosa are elicitors triggering Brassica napus protection against Botrytis cinerea without physiological disorders. Front. Plant Sci. 9, 1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnier, N. , Furlan, A.L. , Buchoux, S. , Deleu, M. , Dauchez, M. , Rippa, S. and Sarazin, C. (2019) Exploring the dual interaction of natural rhamnolipids with plant and fungal biomimetic plasma membranes through biophysical studies. Int. J. Mol. Sci. Available at 10.3390/ijms20051009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott, G.A. , Thakur, S. , Smakowska, E. , Wang, P.W. , Belkhadir, Y. , Desveaux, D. and Guttman, D.S. (2016) Genomic screens identify a new phytobacterial microbe‐associated molecular pattern and the cognate Arabidopsis receptor‐like kinase that mediates its immune elicitation. Gen. Biol. 17, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasir, M.N. , Thawani, A. , Kouzayha, A. and Besson, F. (2010) Interactions of the natural antimicrobial mycosubtilin with phospholipid membrane models. Colloids Surf. B. Biointerfaces, 78, 1723. [DOI] [PubMed] [Google Scholar]
- Nasir, M.N. , Lins, L. , Crowet, J.‐M. , Ongena, M. , Dorey, S. , Dhondt‐Cordelier, S. , Clément, C. , Bouquillon, S. , Haudrechy, A. , Sarazin, C. , Fauconnier, M.L. , Nott, K. and Deleu, M. (2017) Differential interaction of synthetic glycolipids with biomimetic plasma membrane lipids correlates with the plant biological response. Langmuir, 33, 9979–9987. [DOI] [PubMed] [Google Scholar]
- Nie, J. , Yin, Z. , Li, Z. , Wu, Y. and Huang, L. (2019) A small cysteine‐rich protein from two kingdoms of microbes is recognized as a novel pathogen‐associated molecular pattern. New Phytol, 222, 995–1011. [DOI] [PubMed] [Google Scholar]
- Obounou Akong, F. and Bouquillon, S. (2015) Efficient syntheses of bolaform surfactants from l-rhamnose and/or 3-(4-hydroxyphenyl)propionic acid. Green Chem. 17, 3290–3300. [Google Scholar]
- Ottmann, C. , Luberacki, B. , Küfner, I. , Koch, W. , Brunner, F. , Weyand, M. , Mattinen, L. , Pirhonen, M. , Anderluh, G. , Seitz, H.U. , Nürnberger, T. and Oecking, C. (2009) A common toxin fold mediates microbial attack and plant defense. Proc. Natl. Acad. Sci. USA. 106, 10359–10364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, K.C. , Wang, C.W. , Wu, C.H. , Huang, C.T. and Liou, R.F. (2015) Tomato SOBIR1/EVR homologs are involved in elicitin perception and plant defense against the oomycete pathogen Phytophthora parasitica . Mol. Plant–Microbe Interact. 28, 913–926. [DOI] [PubMed] [Google Scholar]
- Petutschnig, E.K. , Jones, A.M.E. , Serazetdinova, L. , Lipka, U. and Lipka, V. (2010) The lysin motif receptor‐like kinase (LysM‐RLK) CERK1 is a major chitin‐binding protein in Arabidopsis thaliana and subject to chitin‐induced phosphorylation. J. Biol. Chem. 285, 28902–28911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poinssot, B. , Vandelle, E. , Bentéjac, M. , Adrian, M. , Levis, C. , Brygoo, Y. , Garin, J. , Sicilia, F. , Coutos‐Thévenot, P. and Pugin, A. (2003) The endopolygalacturonase 1 from Botrytis cinerea activates grapevine defense reactions unrelated to its enzymatic activity. Mol. Plant–Microbe Interact. 16, 553–564. [DOI] [PubMed] [Google Scholar]
- Postma, J. , Liebrand, T.W.H. , Bi, G. , Evrard, A. , Bye, R.R. , Mbengue, M. , Kuhn, H. , Joosten, M.H.A.J. and Robatzek, S. (2016) Avr4 promotes Cf‐4 receptor‐like protein association with the BAK1/SERK3 receptor‐like kinase to initiate receptor endocytosis and plant immunity. New Phytol. 210, 627–642. [DOI] [PubMed] [Google Scholar]
- Povero, G. , Loreti, E. , Pucciariello, C. , Santaniello, A. , Di Tommaso, D. , Di Tommaso, G. , Kapetis, D. , Zolezzi, F. , Piaggesi, A. and Perata, P. (2011) Transcript profiling of chitosan‐treated Arabidopsis seedlings. J. Plant Res. 124, 619–629. [DOI] [PubMed] [Google Scholar]
- Pruitt, R.N. , Schwessinger, B. , Joe, A. , Thomas, N. , Liu, F. , Albert, M. , Robinson, M.R. , Chan, L.J.G. , Luu, D.D. , Chen, H. , Bahar, O. , Daudi, A. , De Vleesschauwer, D. , Caddell, D. , Zhang, W. , Zhao, X. , Li, X. , Heazlewood, J.L. , Ruan, D. , Majumder, D. , Chern, M. , Kalbacher, H. , Midha, S. , Patil, P.B. , Sonti, R.V. , Petzold, C.J. , Liu, C.C. , Brodbelt, J.S. , Felix, G. and Ronald, P.C. (2015) The rice immune receptor XA21 recognizes a tyrosine‐sulfated protein from a Gram‐negative bacterium. Sci. Adv, 1, e1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusztahelyi, T. (2018) Chitin and chitin‐related compounds in plant‐fungal interactions. Mycology, 9, 189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qutob, D. , Kemmerling, B. , Brunner, F. , Küfner, I. , Engelhardt, S. , Gust, A.A. , Luberacki, B. , Seitz, H.U. , Stahl, D. , Rauhut, T. , Glawischnig, E. , Schween, G. , Lacombe, B. , Watanabe, N. , Lam, E. , Schlichting, R. , Scheel, D. , Nau, K. , Dodt, G. , Hubert, D. , Gijzen, M. and Nürnberger, T. (2006) Phytotoxicity and innate immune responses induced by Nep1‐like proteins. Plant Cell, 18, 3721–3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raaijmakers, J.M. , De Bruijn, I. , Nybroe, O. and Ongena, M. (2010) Natural functions of lipopeptides from Bacillus and Pseudomonas: more than surfactants and antibiotics. FEMS Microbiol. Rev. 34, 1037–1062. [DOI] [PubMed] [Google Scholar]
- Ranf, S. , Gisch, N. , Schäffer, M. , Illig, T. , Westphal, L. , Knirel, Y.A. , Sánchez‐Carballo, P.M. , Zähringer, U. , Hückelhoven, R. , Lee, J. and Scheel, D. (2015) A lectin S‐domain receptor kinase mediates lipopolysaccharide sensing in Arabidopsis thaliana . Nat. Immunol. 16, 426–433. [DOI] [PubMed] [Google Scholar]
- Ricci, P. , Bonnet, P. , Huet, J.C. , Sallantin, M. , Beauvais‐Cante, F. , Bruneteau, M. , Billard, V. , Michel, G. and Pernollet, J.C. (1989) Structure and activity of proteins from pathogenic fungi Phytophthora eliciting necrosis and acquired resistance in tobacco. Eur. J. Biochem. 183, 555–563. [DOI] [PubMed] [Google Scholar]
- Robinson, S.M. and Bostock, R.M. (2015) β‐glucans and eicosapolyenoic acids as MAMPs in plant–oomycete interactions: past and present. Front. Plant Sci, 5, 797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron, M. and Avni, A. (2004) The receptor for the fungal elicitor ethylene‐inducing xylanase is a member of a resistance‐like gene family in tomato. Plant Cell, 16, 1604–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney, H.C.E. , Klooster, J.W. , van’t Hoorn, R.A.L. van der Joosten, M.H.A.J. , Jones, J.D.G. and de Wit, P.J.G.M. (2005) Cladosporium Avr2 inhibits tomato Rcr3 protease required for Cf‐2‐dependent disease resistance. Science, 308, 1783–1786. [DOI] [PubMed] [Google Scholar]
- Rossard, S. , Roblin, G. and Atanassova, R. (2010) Ergosterol triggers characteristic elicitation steps in Beta vulgaris leaf tissues. J. Exp. Bot. 61, 1807–1816. [DOI] [PubMed] [Google Scholar]
- Rowland, O. , Ludwig, A.A. , Merrick, C.J. , Baillieul, F. , Tracy, F.E. , Durrant, W.E. , Fritz‐Laylin, L. , Nekrasov, V. , Sjölander, K. , Yoshioka, H. and Jones, J.D.G. (2005) Functional analysis of Avr9/Cf‐9 rapidly elicited genes identifies a protein kinase, ACIK1, that is essential for full Cf‐9‐dependent disease resistance in tomato. Plant Cell, 17, 295–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez, L. , Courteaux, B. , Hubert, J. , Kauffmann, S. , Renault, J.H. , Clément, C. , Baillieul, F. and Dorey, S. (2012) Rhamnolipids elicit defense responses and induce disease resistance against biotrophic, hemibiotrophic, and necrotrophic pathogens that require different signaling pathways in Arabidopsis and highlight a central role for salicylic acid. Plant Physiol. 160, 1630–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santamaria, M.E. , Arnaiz, A. , Gonzalez‐Melendi, P. , Martinez, M. and Diaz, I. (2018) Plant perception and short‐term responses to phytophagous insects and mites. Int. J. Mol. Sci. 19, 1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang‐Guan, K. , Wang, M. , Htwe, N.M.P.S. , Li, P. , Li, Y. , Qi, F. , Zhang, D. , Cao, M. , Kim, C. , Weng, H. , Cen, H. , Black, I.M. , Azadi, P. , Carlson, R.W. , Stacey, G. and Liang, Y. (2018) Lipopolysaccharides trigger two successive bursts of reactive oxygen species at distinct cellular locations. Plant Physiol. 176, 2543–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu, V.K. , Vorhölter, F.J. , Niehaus, K. and Watt, S.A. (2008) Analysis of outer membrane vesicle associated proteins isolated from the plant pathogenic bacterium Xanthomonas campestris pv. campestris . BMC Microbiol. 8, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silipo, A. , Molinaro, A. , Sturiale, L. , Dow, J.M. , Erbs, G. , Lanzetta, R. , Newman, M.A. and Parrilli, M. (2005) The elicitation of plant innate immunity by lipooligosaccharide of Xanthomonas campestris . J. Biol. Chem. 280, 33660–33668. [DOI] [PubMed] [Google Scholar]
- Silipo, A. , Erbs, G. , Shinya, T. , Dow, J.M. , Parrilli, M. , Lanzetta, R. , Shibuya, N. , Newman, M.‐A. and Molinaro, A. (2010) Glyco‐conjugates as elicitors or suppressors of plant innate immunity. Glycobiol. 20, 406–419. [DOI] [PubMed] [Google Scholar]
- Tran, H. , Ficke, A. , Asiimwe, T. , Höfte, M. and Raaijmakers, J.M. (2007) Role of the cyclic lipopeptide massetolide A in biological control of Phytophthora infestans and in colonization of tomato plants by Pseudomonas fluorescens . New Phytol. 175, 731–742. [DOI] [PubMed] [Google Scholar]
- Trdá, L. , Boutrot, F. , Claverie, J. , Brulé, D. , Dorey, S. and Poinssot, B. (2015) Perception of pathogenic or beneficial bacteria and their evasion of host immunity: pattern recognition receptors in the frontline. Front. Plant Sci. 6, 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouvelot, S. , Varnier, A.‐L. , Allègre, M. , Mercier, L. , Baillieul, F. , Arnould, C. , Gianinazzi‐Pearson, V. , Klarzynski, O. , Joubert, J.M. , Pugin, A. and Daire, X. (2008) A beta‐1,3 glucan sulfate induces resistance in grapevine against Plasmopara viticola through priming of defense responses, including HR‐like cell death. Mol. Plant–Microbe Interact. 21, 232–243. [DOI] [PubMed] [Google Scholar]
- Turek, I. and Gehring, C. (2016) The plant natriuretic peptide receptor is a guanylyl cyclase and enables cGMP‐dependent signaling. Plant Mol. Biol. 91, 275–286. [DOI] [PubMed] [Google Scholar]
- Umemura, K. , Ogawa, N. , Koga, J. , Iwata, M. and Usami, H. (2002) Elicitor activity of cerebroside, a sphingolipid elicitor, in cell suspension cultures of rice. Plant Cell Physiol. 43, 778–784. [DOI] [PubMed] [Google Scholar]
- Umemura, K. , Tanino, S. , Nagatsuka, T. , Koga, J. , Iwata, M. , Nagashima, K. and Amemiya, Y. (2004) Cerebroside elicitor confers resistance to fusarium disease in various plant species. Phytopathology. 94, 813–818. [DOI] [PubMed] [Google Scholar]
- Van’t Klooster, J.W. , Van der Kamp, M.W. , Vervoort, J. , Beekwilder, J. , Boeren, S. , Joosten, M.H.A.J. , Thomma, B.P.H.J. and De Wit, P.J.G.M. (2011) Affinity of Avr2 for tomato cysteine protease Rcr3 correlates with the Avr2‐triggered Cf‐2‐mediated hypersensitive response. Mol. Plant Pathol. 12, 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varden, F.A. , De la Concepcion, J.C. , Maidment, J.H. and Banfield, M.J. (2017) Taking the stage: effectors in the spotlight. Curr. Opin. Plant Biol. 38, 25–33. [DOI] [PubMed] [Google Scholar]
- Varnier, A.L. , Sanchez, L. , Vatsa, P. , Boudesocque, L. , Garcia‐Brugger, A. , Rabenoelina, F. , Sorokin, A. , Renault, J.H. , Kauffmann, S. , Pugin, A. , Clement, C. , Baillieul, F. and Dorey, S. (2009) Bacterial rhamnolipids are novel MAMPs conferring resistance to Botrytis cinerea in grapevine. Plant Cell Environ. 32, 178–193. [DOI] [PubMed] [Google Scholar]
- Veronese, P. , Ruiz, M.T. , Coca, M.A. , Hernandez‐Lopez, A. , Lee, H. , Ibeas, J.I. , Damsz, B. , Pardo, J.M. , Hasegawa, P.M. , Bressan, R.A. and Narasimhan, M.L. (2003) In defense against pathogens. Both plant sentinels and foot soldiers need to know the enemy. Plant Physiol. 131, 1580–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Albert, M. , Einig, E. , Fürst, U. , Krust, D. and Felix, G. (2016) The pattern‐recognition receptor CORE of Solanaceae detects bacterial cold‐shock protein. Nat. Plants, 2, 16185. [DOI] [PubMed] [Google Scholar]
- Wang, L. , Einig, E. , Almeida‐Trapp, M. , Albert, M. , Fliegmann, J. , Mithöfer, A. , Kalbacher, H. and Felix, G. (2018) The systemin receptor SYR1 enhances resistance of tomato against herbivorous insects. Nat. Plants, 4, 152–156. [DOI] [PubMed] [Google Scholar]
- Wei, Z.M. , Laby, R.J. , Zumoff, C.H. , Bauer, D.W. , He, S.Y. , Collmer, A. and Beer, S.V. (1992) Harpin, elicitor of the hypersensitive response produced by the plant pathogen Erwinia amylovora . Science, 257, 85–88. [DOI] [PubMed] [Google Scholar]
- Wei, Y. , Caceres‐Moreno, C. , Jimenez‐Gongora, T. , Wang, K. , Sang, Y. , Lozano‐Duran, R. and Macho, A.P. (2018) The Ralstonia solanacearum csp22 peptide, but not flagellin‐derived peptides, is perceived by plants from the Solanaceae family. Plant Biotech. J. 16, 1349–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmann, R. , Lajunen, H.M. , Erbs, G. , Newman, M.‐A. , Kolb, D. , Tsuda, K. , Katagiri, F. , Fliegmann, J. , Bono, J.J. , Cullimore, J.V. , Jehle, A.K. , Götz, F. , Kulik, A. , Molinaro, A. , Lipka, V. , Gust, A.A. and Nürnberger, T. (2011) Arabidopsis lysin‐motif proteins LYM1 LYM3 CERK1 mediate bacterial peptidoglycan sensing and immunity to bacterial infection. Proc. Natl. Acad. Sci. USA. 108, 19824–19829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit, P.J. (2016a) Cladosporium fulvum effectors: weapons in the arms race with tomato. Annu. Rev. Phytopathol. 54, 1–23. [DOI] [PubMed] [Google Scholar]
- de Wit, P.J.G.M. (2016b) Apoplastic fungal effectors in historic perspective; a personal view. New Phytol. 212, 805–813. [DOI] [PubMed] [Google Scholar]
- Xu, X. , Bittman, R. , Duportail, G. , Heissler, D. , Vilcheze, C. and London, E. (2001) Effect of the structure of natural sterols and sphingolipids on the formation of ordered sphingolipid/sterol domains (rafts). Comparison of cholesterol to plant, fungal, and disease‐associated sterols and comparison of sphingomyelin, cerebrosides, and ceramide. J. Biol. Chem. 276, 33540–33546. [DOI] [PubMed] [Google Scholar]
- Xu, S. , Liao, C.J. , Jaiswal, N. , Lee, S. , Yun, D.J. , Lee, S.Y. , Garvey, M. , Kaplan, I. and Mengiste, T. (2018) Tomato PEPR1 ORTHOLOGUE RECEPTOR‐LIKE KINASE1 regulates responses to systemin, necrotrophic fungi and insect herbivory. Plant Cell, 30, 2214–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y. , Yang, X. , Dong, Y. and Qiu, D. (2018) The Botrytis cinerea xylanase BcXyl1 modulates plant immunity. Front. Microbiol. 9, 2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L. , Kars, I. , Essenstam, B. , Liebrand, T.W.H. , Wagemakers, L. , Elberse, J. , Tagkalaki, P. , Tjoitang, D. , van den Ackerveken, G. and van Kan, J.A.L. (2014) Fungal endopolygalacturonases are recognized as microbe‐associated molecular patterns by the Arabidopsis receptor‐like protein RESPONSIVENESS TO BOTRYTIS POLYGALACTURONASES. Plant Physiol. 164, 352–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. , Dodds, P.N. and Bernoux, M. (2017) What do we know about NOD‐like receptors in plant immunity? Annu. Rev. Phytopathol. 55, 205–229. [DOI] [PubMed] [Google Scholar]
- Zipfel, C. , Kunze, G. , Chinchilla, D. , Caniard, A. , Jones, J.D.G. , Boller, T. and Felix, G. (2006) Perception of the bacterial PAMP EF‐Tu by the receptor EFR restricts Agrobacterium‐mediated transformation. Cell, 125, 749–760. [DOI] [PubMed] [Google Scholar]
- Zuppini, A. , Baldan, B. , Millioni, R. , Favaron, F. , Navazio, L. and Mariani, P. (2004) Chitosan induces Ca2+‐mediated programmed cell death in soybean cells. New Phytol. 161, 557–568. [DOI] [PubMed] [Google Scholar]


