Abstract
The prevalence of cardiovascular disease (CVD) is rising worldwide, remaining the major cause of death in developed countries. Polyphenols have been shown to have cardioprotective properties; however, their impact on iron bioavailability and potential impact on other aspects of health is unclear. A systematic review was undertaken to evaluate the current status of the relationship between habitual polyphenol consumption, iron status, and circulating biomarkers of CVD. Following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) 2009 guidelines, searches were performed across 5 electronic databases (PubMed, Cochrane Library, Scopus, Web of Science, and CINAHL) to identify randomized controlled trials which investigated the effects of polyphenol consumption on inflammatory markers, serum lipid profile, and iron absorption and bioavailability. In total, 1174 records were identified, with only 7 studies meeting the inclusion criteria. The selected studies involved 133 participants and used a variety of foods and supplements, including olive oil and cherries, rich in polyphenols including hydroxytyrosol, quercetin, and resveratrol, as well as catechin enriched drinks. The duration of the studies ranged from between 56 and 145 days, with total polyphenolic content of the food items and supplements ranging from 45 to 1015 mg (per 100 g). Polyphenols did not appear to interfere with iron status, and most studies reported improvements in inflammatory markers and lipid profile. While these results are promising, the limited number of studies and considerable heterogeneity across the interventions support the need for more extensive trials assessing the relationship between polyphenol intake, iron bioavailability, and CVD risk.
Keywords: Polyphenol, CVD, atherosclerosis, iron status
Introduction
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality globally, being accountable for almost one-third of all deaths worldwide.1 It is a collective term for diseases of the heart and blood vessels, most of which are life-threatening if not adequately treated.2 The pathological condition underlying the majority of CVD is atherosclerosis: an inflammatory disease orchestrated through an intrinsic activation system of vasoactive mediators, coagulation factors, complement pathways, and chemotactic factors.3 Atherosclerosis develops as a response to the combination of acquired and inherited risk factors. In addition to modifiable risk factors such as sedentary lifestyle, high circulating triglycerides (TG) and cholesterol levels (particularly small low-density lipoprotein cholesterol [LDL-C] particles), smoking, obesity, hypertension, and diabetes, there are also genetic risk factors comprising specific mutations in the LDL receptor (LDL-R) which disrupt LDL-C uptake, metabolism, and cholesterol homeostasis.4-6
Exposure to the aforementioned risk factors can cause intimal lesions and initiate atherogenesis.4,7 Particularly, oxidized LDL-C within the subendothelial space plays a key role in atherogenesis, being taken up by macrophages which, subsequently, transform to foam cells thereby promoting the formation of an atheromatous core.7 Once this response is initiated, the development of these plaques induces the narrowing and weakening of the vessel wall and can ultimately progress to rupture, leading to the development of an obstructive vascular thrombosis or ischemic injury.4,7 Hypercholesterolemia is a major risk factor for atherosclerosis, being able to initiate atheromatous plaque development even in the absence of other risk factors.4,8 Small LDL-C particles, the lipoprotein subclass that delivers cholesterol to peripheral tissues, have been proposed as the main culprit in the onset and progression of atherosclerosis in light of the ability of LDL to deliver cholesterol to the artery intima and support the formation of foam cells thereby contributing to the inflammatory response and build-up of the atheromatous plaques.3,4 On the contrary, high-density lipoproteins (HDLs), synthesized in the liver and intestine, transport around 20% to 30% of the total plasma cholesterol from the periphery back to the liver and are reported to be inversely related with the development and progression of CVD.9
The role of inflammation is pivotal in all stages of atherogenesis, from initiation and formation of atherosclerotic plaque to plaque development and rupture.3,4,8,10 Inflammatory markers, such as tumor necrosis factor-alpha (TNF-α) and interleukin 6 (IL-6), play a fundamental role in both reducing cardiac function and initiating myocardial cell pathology.11,12 It is well established that lowering levels of TNF-α can exert beneficial effects on cardiovascular health, including protection against ischemia and pressure overload.13-15 Inflammation contributes to the pathogenesis of CVD through an increase in inflammatory markers, such as C-reactive protein (CRP) which are also associated with increased CVD risk.3,4,8 As some of the major risk factors associated with atherosclerosis and CVD are modifiable, it is well established that lifestyle interventions targeting dietary habits and physical activity are promising therapeutic tools to prevent and/or manage CVD risk.8,15 Considering the cardiometabolic “insult” perpetuated by a Western diet, identifying potential food bioactive derivatives able to offset the deleterious effects of ultra-processed foods often rich in sugars, long-chain saturated fatty acids, and those with increased omega-6:omega-3, represents novel nutritional tools to improve cardiometabolic health. In this regard, polyphenols have emerged due to their valuable therapeutic benefits on human health.10,16
Polyphenols are secondary plant metabolites and bioactive compounds naturally occurring in plants and plant-derived products, including commonly consumed foods and beverages.10,16,17 They are reported to possess strong antioxidative properties in vitro and can be grouped into different classes based on their function and chemical structure.18 These classes of bioactive compounds are distinguished by the number of phenol rings they contain18 and can be divided into groups such as anthocyanins (red, purple, or blue plant-based foods); cinnamic acids (most fruits, coffee, oats, and rice); flavanols and flavonoids (teas, cocoas, fruits, vegetables, and wine); and stilbenoids such as resveratrol (grapes, nuts, and berries).17,19 Polyphenols found within the food matrix are abundant in many healthy dietary patterns and may play a putatively beneficial role in human health,20-22 including the prevention of non-communicable diseases associated with oxidative stress and inflammation.10,18 The cardioprotective properties of polyphenols were also linked with their antioxidant activity, although in recent years they have been researched for their anti-atherosclerotic and immunomodulatory properties18,23 paving the way for novel nutritional approaches for reduction of CVD risk.10,16,18,23 Despite these health-promoting qualities, it has also been postulated that some polyphenols may be associated with negative health consequences, including decreasing the absorption and bioavailability of essential nutrients in the body—in particular, iron.24
Iron is an essential micronutrient that plays an important role in several physiological functions such as transport and storage of oxygen, ATP (adenosine triphosphate) synthesis, and as a co-factor for several enzymes including cytochrome P450 complexes that function as monooxygenases.25,26 Although iron is abundant in some food sources, deficiency can still occur despite adequate dietary intake24,27-29 with iron bioavailability depending on the form in which iron is consumed.26 Heme iron is derived from hemoglobulin and myoglobulin of animal food sources and accounts for around 15% to 30% of absorbability, contributing to at least 10% of total absorbed iron, while non-heme iron (derived from plants and iron-fortified foods) is characterized by a lower bioavailability due to much less effective intestinal absorption.30 In addition, micronutrient status and the interaction between iron and other dietary factors, including food constituents, play an important role in determining the adequacy of iron nutrition, affecting the ability to either inhibit or enhance absorption in the duodenum.31-33
The consumption of foods containing high levels of heme iron has been reported to be more consistently associated with an increased risk of CVD and CVD-related mortality, in comparison with foods containing iron from plant sources.27,28 These differences could be potentially attributed to the additional phytochemicals and the overall composition of foods, with non-heme iron-containing foods being generally rich in antioxidants. Heme iron sources typically contain long-chain saturated fatty acids which, in turn, have been associated with CVD or more recently metabolites of heme which have been also associated with increased risk of CVD by promoting metabolic inflammation and an unfavorable blood lipid profile.28 Independently of its dietary origins, iron status has been speculated to be related to the development of CVD, with some propositions that increased circulating iron levels may promote atherogenesis by increasing free radical formation and oxidative stress.28,34 High circulating iron levels being recognized as a putative risk factor for CVD, ferropenia and anemia have also been postulated as risk factors for CVD.27 On the contrary, iron deficiency has been associated with increased risk of thrombosis, contributing to a hypercoagulable state due to the increased viscosity of red blood cells (RBC) and, in turn, affecting blood flow within the vessels.28 As such, the role of polyphenols as mediators of CVD risk based on iron levels outside of the recommended ranges is relatively unexplored.
Several reviews have focused on polyphenol intake and inflammatory markers of CVD risk,35,36 individual polyphenols and polyphenolic compounds20-22 as well as the relationship of non-heme iron absorption inhibition by polyphenols.37,38 However, there is evidence to support that some polyphenols might influence iron bioavailability,33 which, in turn, might potentially increase the risk of development of CVD. Although several reviews have described the individual relationships between polyphenols and CVD risk markers/iron status as 2 independent components35-38 to the best of our knowledge, there have not been pooled studies assessing the effect that polyphenols may have on both of these variables, and whether they might be interrelated. Therefore, a systematic review was conducted to examine the effects of dietary polyphenols on iron status and CVD risk markers.
Materials and Methods
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines 2009.39 In addition, this systematic literature review is registered with the international prospective register of systematic reviews (PROSPERO: CRD42018116571).
Search strategy
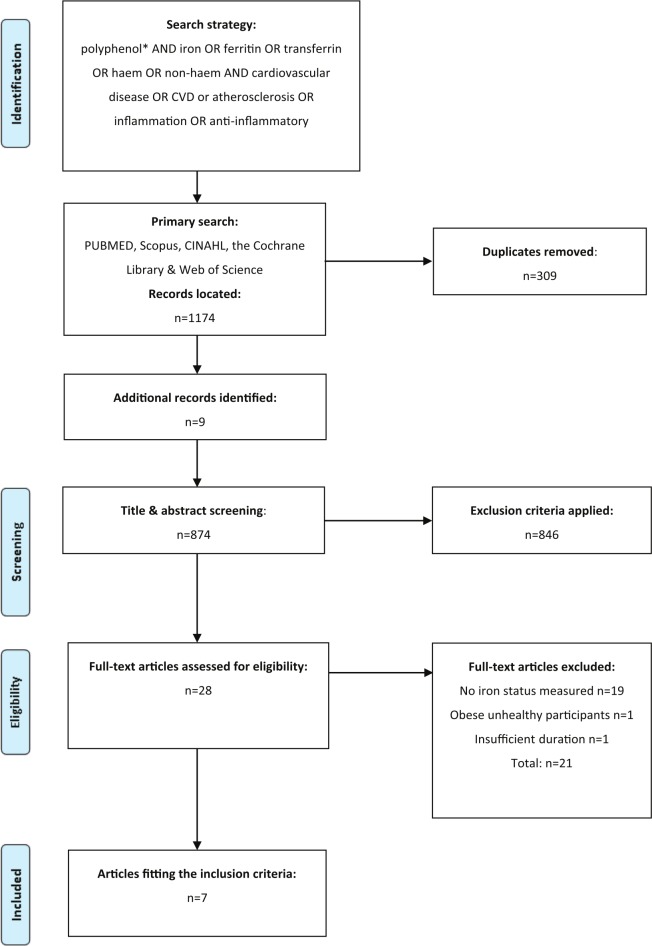
A comprehensive literature search was performed by three authors (H.S., N.M.D. and N.N.) across 5 databases (CINAHL, Cochrane Library, PubMed, Scopus, and Web of Science) to identify relevant randomized controlled trials that assessed the effect of polyphenols on iron status and CVD risk markers from the time of database inception until May 12, 2019. Searches were conducted using the search strategy (((polyphenol*) AND (iron OR ferritin OR transferrin OR haem OR non-haem)) AND (“cardiovascular disease” OR CVD OR atherosclerosis OR inflammation OR “anti-inflammatory”)). Furthermore, studies were retrieved through manual searches of included articles and reference lists of previous review articles. Figure 1 illustrates the search strategy used for this systematic review.
Figure 1.
PRISMA flow chart summary of systematic review search process.
Selection criteria
Selected articles were limited to healthy participants, or those individuals considered to be at an increased risk of CVD without being on prescription medications, and studies where the intervention period lasted for at least 28 days and did not measure an acute effect. Studies were included if they measured both CVD and iron-related outcomes, and only studies that provided quantifiable levels of polyphenols (as a supplement or food source) were considered eligible for this review. Studies were excluded if the polyphenols were used as combined therapy with other medications. Studies were also excluded if participants had been diagnosed with a chronic illness such as diabetes, impaired renal function, liver or gastrointestinal diseases, or cancer within the last 5 years.
Outcome measures
The primary outcome measures were iron levels as measured by ferritin or hepcidin in the blood, or as iron absorption measured by the determination of RBC activity after orally consuming radioiron. Due to the proposed relationship between overall iron status and its influence on CVD risk, secondary outcome measures were a change in blood lipid levels, biomarkers of oxidative damage to lipids, and levels of inflammatory markers including, but not limited to, TNF-α, IL-6, and CRP as well as biomarkers of antioxidant status. Any adverse events reported in studies were included in the evaluation, with outcomes based on a statistically significant change (P < .05) after polyphenol consumption compared with non-treatment groups in accordance with the PRISMA harms checklist.40
Assessment of risk of bias
Risk of bias was independently assessed by 2 reviewers (H.S. and N.N.) using the Cochrane risk of bias criteria.41 This tool includes criteria for assessing sequence generation; allocation concealment; blinding of participants, personnel, and outcome assessors; incomplete outcome data, and selective outcome reporting. For each criterion, studies were assessed for risk of bias as low, high, or unclear. Country of origin and source of funding were also considered to determine whether bias may have been introduced into the studies. Any differences in opinion between reviewers were resolved through discussion using the third reviewer (N.M.D.).
Results
Description of studies
In total, 1174 records were identified from the initial electronic database searches. Following additional manual searches (n = 9) and removal of duplicates (n = 309), a total of 28 records were identified for full-text screening, with only 7 studies42-48 meeting the inclusion criteria (Figure 1). All included studies were clinical trials that assessed the consumption of polyphenols or phenolic compounds derived from a food source or a supplement (such as hydroxytyrosol) on risk markers for CVD, chronic disease or inflammation, and iron status. Polyphenols used as part of the selected studies were originating from olive oil,44 aronia,48 pomegranate,47 cherry fruit,42,43 or fortified apple juice,45 with the main polyphenol components being anthocyanins, catechins, ellagitannins, diterpenes, flavonoids, and stilbenoids.42-48 Doses of the dietary polyphenol supplements varied in their method of delivery and ranged in their intervention period for between 28 and 90 days, with the average duration of the included studies being 69.5 days. Total polyphenol content within the daily supplements ranged between 45 and 1015 mg (per 100 g of supplement). In total, 133 individuals participated in the selected studies, with sample sizes ranging from 14 to 46 participants, and an age range of 20 to 58 years (Table 1). All studies42-48 assessed the role of polyphenols on iron status, and findings relative to CVD risk factors are synthesized below.
Table 1.
Summary of findings of the included studies.
| Reference | Aims | Study design | Participants (n) | Age (range) mean ± SD | Duration | Polyphenol composition (mg/100 g) | Dose | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Kelley et al42 | Assess the effect of sweet bing cherries on plasma lipids and markers of inflammation | Human clinical trial | Healthy M (n = 2) and F (n = 16) All (n = 18) |
50 ± 1 | 64d Baseline 8d; Intervention 28d; post-intervention 28d |
Total 135; Hydroxycinnamates 72.2; Anthocyanins 35.6; Procyanidins 21.3; Flavanols 5.9 |
280 g/d | IG: Decrease in CRP (P < .05); no change in IL-6, HDL-C, LDL-C VLDL-C, and TG or particle size (all Ps > .05). No change in hemoglobin. No change in NO (P = .07). |
| Kelley et al43 | Examine the effects of cherry consumption on risk factors for multiple chronic diseases in plasma samples from humans with modestly elevated CRP, using a proteomic approach | Human clinical trial | Healthy M (n = 2) and F (n = 16) All (n = 18) |
All 50 ± 1 | 64d Baseline 8d; Intervention 28d; post-intervention 28d |
Total 135; Hydroxycinnamates 72.2; Anthocyanins 35.6; Procyanidins 21.3; Flavanols 5.9 |
280 g/d | IG: No change in plasma concentrations of ferritin, CRP, IL-18. No change in TNF-α (P = .07). |
| Lopez-Huertas and Fonolla44 | Assess the effects of HT supplementation on markers of CVD risk and several clinical conditions | Human clinical trial | Healthy M (n = 11) and F (n = 3) All (n = 14) |
34.1±a | 56d | HT; composition not stated in original manuscript | 45 mg/d | Decrease in ferritin (P < .05), increase in VC (P < .05). No change from baseline for CRP, LDL-C, TG, or ox-LDL (all Ps > .05). |
| Soriano-Maldonado et al45 | Investigate the effects of consumption of 2 cloudy apple juices with different polyphenol and VC content on antioxidant status, cardiometabolic and inflammatory markers | Human randomized cross-over study | Healthy M (n = 8) and F (= 12) All (n = 20) |
23.7 ± 2.3 | 77d; Introduction 7d; Intervention 28d; Washout 14d; Intervention 28d |
VC-rich juice: catechin 510; Polyphenol-rich juice: catechin 993 |
500 mL/d | No difference in blood markers between groups (all Ps > .05), except ferritin, which was lower in initial VC juice group compared with the polyphenol juice (P = .024). FRAP increased in VC juice group (P = .031). No change in plasma VC concentration between groups (both Ps > .05). No change in iron analysis after consumption of either juice (both Ps > .05). No change in cholesterol except TC (P = .037) post-intervention. |
| Suliburska et al46 | To examine the effects of GTE on the mineral, body mass, lipid profile, glucose, and antioxidant status of obese patients | Human prospective, randomized, double-blind control trial | Obese (BMI ⩾ 30 kg/m2) M (n = 23) and F (n = 23) All (n = 46) |
50.4 ± 8.3 | 84d | GTE 379; EGCG 208 | 379 mg/d | IG: Decrease in TC (P < .01), TG (P < .01), HDL (P = .044) and LDL (P = .02). Decrease in Ferritin (P = .048) |
| Urbaniak et al47 | Analyze the effect of POM supplementation on selected pro-inflammatory cytokines, hepcidin, and markers of iron metabolism in well-trained rowers | Human double-blind, placebo-controlled trial | Healthy M (n = 19) | 20.8 ± 0.8 | 61d | Total 220; Individual composition not stated in original manuscript |
50 mL/d | IG: No effect on serum hepcidin, myoglobin, or creatine kinase levels (all Ps > .05) no change in ferritin levels between groups, post-exercise iron levels increased in both IG and PG (P < .002). |
| Villaño et al48 | Investigate a citrus-based juice, rich in polyphenols and VC, along with long-term exercise on iron status | Human cross-over study | Healthy M (n = 10) and F (n = 6) All (n = 16) |
(19–21) | 145d Baseline 15d; Control training 15d; Intervention 45d; Washout 10d; Intervention 45d; Post-intervention 15d |
Total 125; Individual composition not stated in original manuscript |
200 mL/d | IG: No effect on plasma hepcidin concentration in either groups and no significant difference observed in iron absorption between IG and PG (all Ps > .05). |
Abbreviations: BMI, body mass index; CRP, C-reactive protein; CVD, cardiovascular disease; d, day; EGCG, epigallocatechin gallate; F, female; FRAP, ferric reducing antioxidant power; GTE, green tea extract; HDL-C, high-density lipoprotein cholesterol; HFA, hydroxy fatty acids; HT, hydroxytyrosol; IG, intervention group; IL-6, interleukin 6; LDL-C, low-density lipoprotein cholesterol; M, male; NO, nitric oxide; ox-LDL, oxidized low-density lipoprotein; PG, placebo group; POM, pomegranate juice; TC, total cholesterol; TG, triglycerides; TNF-α, tumor necrosis factor-alpha; VC, Vitamin C; VLDL-C, very-low-density lipoprotein cholesterol.
SD not reported in original manuscript.
Effect of polyphenols on iron status
All studies42-48 examined the hematological variables related to iron status, including hemoglobin, hepcidin, and ferritin. Three studies43,44,46 reported a significant decrease (all Ps < .05) in ferritin after supplementing with catechin-rich supplements. Interestingly, in a study by Soriano-Maldonado et al,45 serum ferritin was reported to have decreased significantly after the first arm of the study with 1 intervention group supplementing with a Vitamin C-rich apple juice when compared with a catechin-rich apple juice (P = .024). No significant difference, however, was reported between the groups in relation to ferritin or iron metabolism on completion of the cross-over study (both groups P > .05). Urbaniak et al47 reported a significant post-exercise increase in soluble transferrin receptors (P < .04) at baseline, and circulating iron levels in both the intervention group (supplementing with 50 mL pomegranate juice) and placebo groups (P < .002) after an exercise test on a rowing ergometer (2000 m), but no change in ferritin levels were reported between groups at the end of the study (P > .05).
Effect of polyphenols on markers of inflammation
Inflammatory markers relating to CVD were assessed in 4 of the selected studies after supplementing polyphenols. The studies by Kelley et al42,43 reported a decrease in CRP (P < .05); however, IL-6 and IL-18 levels were not affected and did not change from baseline (P > .05), whereas TNF-α tended to decrease (P = .07) after the consumption of 280 g/day cherries for 28 days. Lopez-Huertas and Fonolla44 observed no change in CRP throughout the testing period after administration of hydroxytyrosol for 56 days (P > .05). The results from Soriano-Maldonado et al45 regarding inflammation-related parameters in healthy young adults showed no significant effects of either Vitamin C-rich apple juice or polyphenol-rich apple juice consumption when compared with the baseline data (P > .05).
Effect of polyphenols on cholesterol levels
Changes in cholesterol levels associated with polyphenol consumption were assessed in 4 of the eligible studies43-46 (Table 1). Suliburska et al46 observed a decrease in total cholesterol (TC; P < .01), TG (P < .01), HDL (P = .044), and LDL (P = .035) after supplementing with green tea extract in an obese (body mass index [BMI] > 30 kg/m2) sample population. One study assessing cherry fruit consumption43 found no significant change in plasma cholesterol concentrations of TC, HDL, LDL, very-low-density lipoprotein cholesterol (VLDL-C), and TG (all Ps > .05), or their particle size and number relative to baseline measurements in healthy middle-late age (50 ± 1) men and women. The second study, also by Kelley et al,44 observed no significant differences from baseline for LDL-C and TG. Soriano-Maldonado et al45 reported no significant change in cholesterol levels after daily consumption of either Vitamin C-rich apple juice or a catechin-rich apple juice, except for TC (P = .037).
Effect of polyphenols on oxidative stress and antioxidant status
Oxidative stress and antioxidant status were assessed in 2 studies44,45 measuring nitric oxide (NO), ox-LDL, and plasma Vitamin C. No significant difference was reported from baseline in the study assessing ox-LDL (P > .05); however, the same study reported a significant difference (P < .05) in Vitamin C after 56 days of hydroxytyrosol administration.44 Soriano-Maldonado et al45 noted a significant increase in ferric reducing antioxidant power (FRAP) in the Vitamin C-rich apple juice group (P = .031). However, there was no significant change in plasma Vitamin C concentration between either intervention groups on completion of the study (both Ps > .05).
Adverse effects
None of the included studies reported any adverse effects related to polyphenol consumption during the intervention periods.
Risk of bias
Risk of bias, including country of origin and the role of funding, is summarized in Table 2. The studies reviewed were at low risk of bias, with some qualities being unclear. One study43 was considered at an elevated risk of bias for one aspect—which was incomplete outcome data. This study did not show data for reduced circulating concentrations of NO. Overall, the risk of bias did not seem to influence the study with respect to the other primary outcome measures (blood lipid levels, and levels of the inflammatory markers TNF-α, IL-6, and CRP). The studies were undertaken in 3 different countries consisting of the United States, Spain, and Poland. Two studies42,43 from the United States, as well as one46 from Poland, were unclear in their sources of funding.
Table 2.
Risk of bias of the included studies.
| Bias category | Kelley et al42 | Kelley et al43 | Lopez-Huertas and Fonolla44 | Soriano-Maldonado et al45 | Suliburska et al46 | Urbaniak et al47 | Villaño et al48 |
|---|---|---|---|---|---|---|---|
| Random sequence generation (selection bias) | Low | Low | Low | Low | Low | Low | Low |
| Allocation concealment (selection bias) | Low | Low | Low | Low | Low | Low | Low |
| Blinding of participants and personnel (performance bias) | Low | Low | Low | Low | Low | Low | Low |
| Incomplete outcome data (attrition bias) | Low | Low/unclear | Low | Low | Low | Low | Low |
| Blinding outcome assessment (detection bias) | Low | Low | Low | Low | Low | Low | Low |
| Selective reporting | Low/unclear | Low | Low | Low | Low | Low/Unclear | Low |
| Other bias | Low | Low | Low | Low | Low | Low | Low |
| Country | The United States | The United States | Spain | Spain | Poland | Poland | Spain |
| Source of funding | Unclear | Unclear | Government/Institution | Government/Institution | Unclear | Government | Institution |
Discussion
This systematic review evaluated the effects of dietary polyphenol consumption, particularly anthocyanins, flavanols, flavonoids, stilbenoids, and tyrosols commonly found in a variety of different food products, on iron status and CVD risk markers. The impact on circulating inflammatory mediators given their role as markers of, and pathogenic factors contributing to, CVD was the focus of this review, as well as the effect of polyphenols on iron bioavailability and status. Although polyphenol consumption has been shown to provide health benefits, particularly by exerting an anti-inflammatory effect, the results of this systematic review identified a gap in the literature, with more evidence required to address the effects of polyphenol consumption on both CVD risk and the putative associations with iron status, which can have both protective and detrimental effects on CVD in a population.
Iron deficiency is a common concern in both the developed and the developing world, with particular population groups, such as young females, and elderly individuals being at higher risk compared with others.49,50 The effects of the diet on the bioavailability of iron and its metabolism have been independently assessed in animal51 and human trials,52 providing evidence that non-heme iron absorption and iron status are influenced by certain foods or food constituents, such as polyphenols.33 Tuntipopipat et al53 investigated the effect of polyphenols found in 6 herbs and spices on iron bioavailability in humans, reporting a reduction in iron bioavailability in response to the consumption of all herbs and spices tested, with the exception of tamarind. A study by Lesjak et al32 also highlights how polyphenol consumption has the potential to attenuate iron levels in certain at-risk populations.
Polyphenols and polyphenolic compounds are rather ubiquitous molecules found naturally in different foods and food products.16,17 However, when taking into consideration that there are over 8000 already identified compounds, it is difficult to establish a direct link between the individual dietary polyphenols (or even their classes) and the health outcomes as in the case of inflammatory responses22,54-56 while also considering the different bioavailability of diverse dietary polyphenols.55,57 Nevertheless, there is evidence in support of diets rich in polyphenols or phenolic compounds, such as the Mediterranean diet, in regulating the low-grade chronic inflammation typical of the metabolic syndrome and CVD.58,59
Numerous animal, in vitro, and acute intervention studies have proposed that iron stores may play a role in the beneficial vascular effects of some polyphenols.60,61 Hemoglobin is an iron-containing protein, which facilitates the transport of oxygen to cells, as well as CO2, that are heavily involved in the body’s respiratory exchange.4 Iron absorbed from the gut is bound to plasma transferrin and transported to the marrow, where it is delivered to developing cells and incorporated into hemoglobin.4,62 After an average of 120 days, iron is extracted from hemoglobin in erythrocytes and recycled back to plasma transferrin. It is important to appreciate the role that hemoglobin plays in iron metabolism, as there has been increasing investigation into the relationship between hemoglobin deficiency, and disturbed iron supply and intestinal absorption.4,62 Animal studies have demonstrated that mice with hemoglobin anemia led to a reduction in iron uptake by developing erythrocyte cells.62 Previous reviews, epidemiological, animal, and in vitro studies have assessed polyphenol-iron interactions, especially those found in tea.18,60,61 The degree of inhibiting iron absorption is related to the amount and type of iron, and phenolic compounds present.61 Acute studies have found that the consumption of tea with an iron-rich meal can reduce absorption by 90%.60 Literature suggests that polyphenols significantly decrease non-heme iron absorption in the duodenum, affecting the overall iron status and harboring potential long-term health effects.32 All studies included in this review42-48 examined hematological variables relating to iron status. Interestingly, Urbainiak et al47 observed an increase in plasma ferritin in both the placebo and the intervention groups (supplementing with 50 mL pomegranate juice daily) after an exercise test involving a 2000-m row on a rowing ergometer; this is probably due to the role of iron in anaerobic metabolism and the sample population being well-trained athletes.47,63 Three43,44,46 of the included studies reported a decrease in iron-related parameters such as hepcidin, after consumption of polyphenols (especially catechin-rich supplements); however, it cannot be completely isolated that polyphenol intake does not affect iron status as a whole due to the vast evidence showing otherwise.24,31,60 Rather though, the habitual consumption of polyphenols during iron supplementation should be considered as potentially beneficial to prevent the development of negative CVD effects associated with the iron supplementation.
Overweight and obesity have been widely associated with increased risk of developing CVD, type 2 diabetes mellitus, fatty liver disease, certain types of cancer, and various neurological disorders.64-67 Obesity is characterized by a state of low-grade chronic inflammation which is a shared pathophysiological feature with atherosclerosis.68,69 While this response is predominately driven by adipocytes and the recruitment of macrophages and other immune cells in the adipose tissue in obesity, regarding atherosclerosis, inflammation affects the vessel wall with macrophages and T-cells being recruited in the arterial intima.67,68 Regarding the triggers of this inflammatory response, nutrient overload, and particularly long-chain saturated fatty acids, sugar and an elevated omega-3:omega-6 ratio, alongside adipocyte hypertrophy and adipose tissue hypoxia, have been proposed as the main culprit.67-69 This atypical inflammatory response, despite having been widely described as the joining link between obesity and insulin resistance, also contributes to promoting atherogenesis and CVD.65,70 While distinctly separate pathologies, obesity and atherosclerosis share similar pathophysiological responses, with obesity triggering vascular dysfunction, inflammation, and hypercoagulation, which are pivotal pathological features in atherogenesis.68-70 Thus, inflammation not only represents a marker of CVD but also a pathogenetic factor mediating all stages of atherogenesis and promotion of metabolic disorders, such as insulin resistance and dyslipidemia which, in turn, can promote atherosclerosis.68,70 This substantially provides the rationale for the inclusion of inflammatory biomarkers as a predictive assessment for individual risk of CVD.65,70,71
In light of this, the prominent inflammatory markers IL-6, TNF-α, and CRP are studied for their integral involvement in the atherosclerotic process and progression, attributable to the chronic inflammatory reaction within the intima of the blood vessel wall, as well as within obesity.4,5,71 The results from the included studies generally showed a decrease in the inflammatory markers IL-6, and CRP after polyphenol consumption.42,43 It is well established that elevated circulating and intra-cardiac IL-6 levels may contribute to the progression of myocardial damage and dysfunction, as well as inducing cardiac hypertrophy.72 As CRP is synthesized by hepatocytes in response to IL-6, its mediated effects have been demonstrated to strongly predict adverse cardiac events.73 Previous studies12 have identified IL-6 as being an independent predictor of plaque progression and a marker of prevalent progressive atherosclerosis in a general population. Hepcidin is the principal regulator of iron absorption and increased concentrations can lead to pathology including anemia.74 Some literature has also suggested that IL-6 causes a significant decrease in serum iron levels, and as acting as an antimicrobial peptide, it is induced by IL-6 during inflammation.75 Based on the findings from in vitro and animal studies, it was shown that quercetin, 1 of the intervention flavonoids found in 2 of the selected studies,42,43 can attenuate lipid peroxidation, platelet aggregation, and capillary permeability, showing a clinically valuable perspective for reducing inflammation, and CVD development and risk.76 A study by Kelley et al43 observed a reduction in concentrations of some markers of inflammation, including CRP; however, there were no changes in circulating lipids after the intervention period. Importantly, several other inflammatory markers, such as intercellular adhesion molecule-1 (ICAM-1), IL-6 soluble receptor (IL-6sR), and tissue inhibitor of metalloproteinases-2 (TIMP-2), were not altered after the intervention period, and this may be due to the low levels of these markers present in already healthy individuals.43
Polyphenol consumption has shown promise in clinically attenuating pathways associated with cholesterol regulation.56 The liver has 3 tightly related mechanisms to control plasma cholesterol levels. Hepatocytes remove LDL-C from the plasma through the LDL-R, releasing cholesterol into the cytoplasm.4 An increase in intracellular cholesterol negatively regulates the activity of sterol regulatory element-binding proteins (SREBPs), which in turn regulates the expression of the LDL receptor and 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, a key enzyme in the biosynthetic pathway to cholesterol.4,6 As intracellular cholesterol falls, the synthesis of HMG-CoA and cholesterol synthesis increases.4,6 The changes in cholesterol levels (TC, LDL-C, and HDL-C) after polyphenol intervention were assessed in four43-46 of the studies included as part of this systematic review. The inhibition of LDL oxidation is a major anti-atherogenic property of HDL,77 and animal studies have assessed the influence of polyphenols on HDL-C composition and functional state.9 In the study by Kelley et al,43 there were no changes observed in plasma concentrations of either LDL-C or HDL-C nor their particle size and number, after an intervention period of daily cherry consumption. This could be potentially due to the participants already having cholesterol levels within the normal ranges or due to the potentially insufficient duration of the treatment to exhibit any further cholesterol-lowering effect. It has been proposed that iron catalyzes the highly reactive forms of free oxygen species, promoting LDL oxidation.78 Several studies have indicated that polyphenols may inhibit the oxidation of LDL.9,79 Two studies44,45 used for this systematic review assessed polyphenol intake on antioxidant status and markers of oxidative stress. No significant difference was reported from baseline in the study assessing ox-LDL.44 As the oxidation of LDL-C leads to a change in conformation, meaning that LDL-C can easily enter the monocyte-macrophage system of the arterial wall and promote atherogenesis, inhibiting this oxidized state may prove clinically useful for reducing lipid CVD risk.80 The study by Soriano-Maldonado et al45 noted an increase in antioxidant power in participants consuming an apple juice fortified with 60 mg/L of Vitamin C, important in measuring the FRAP and estimating its antioxidant effect.81 It is worth mentioning that an acute effect of reduced NO concentration was observed initially in the study by Kelley et al,43 in participants immediately after cherry consumption. However, the concentrations returned to baseline levels at the follow-up measurements. As previously outlined, a reduction in body iron stores can result from a diet low in iron, or when dietary factors inhibiting iron (such as polyphenols and phytates) are more predominate over those which enhance iron (such as ascorbic acid and red meat).82 In addition, the role of the iron stores in the pathogenesis of atherosclerosis and further development of CVD was primarily proposed through the development of reactive oxygen species (ROS) while much later it was evident that these mechanisms are much more complex.83 One of the putative mechanisms of action could be through the oxidative modification of lipoproteins, in particular, LDL as both lipid and protein components can be affected.83-85
While it is proposed that some dietary polyphenols inhibit iron absorption,29,31,60 it is often neglected in human interventions as having both potentially beneficial and detrimental effects on CVD.34 Despite economic and scientific development, iron deficiency remains the most common micronutrient deficiency globally.86 Care should be taken to ensure that any individual supplementing with polyphenols for CVD benefit, or those consuming a diet naturally high in polyphenolic products, is taking up adequate bioavailable iron to meet the body’s requirements and ensure overall optimum health to avoid the discussed adverse effects. In addition, consumption of food products that are rich in polyphenols, such as green tea, has also been associated with successful aging, indicating an important role of high polyphenol products as a component of the overall healthy diet.87 Contrariwise, iron excess and its association with ROS generation have been examined in both in vivo and in vitro studies,88 suggesting that underlying cellular injury could be the cause of fibrosis and cirrhosis of the liver.89 Hepatotoxicity is the most consistent finding in patients suffering iron-overload, followed by CVD.89 This reflects the need for consideration of iron’s role in CVD, as well as the subsequent outcome of iron status after polyphenol consumption, for CVD risk as well as for overall health.90 Future research should also consider polyphenol consumption along with specific iron requirement in relation to gender, age, and physiological states—such as later life, adolescence, and pre- and post-menopause, as well as during lactation.50,91
Conclusions
Diet remains an integral part of CVD prevention as well as overall health promotion. Polyphenols have shown benefit in the reduction of inflammation and through their anti-atherogenic properties, and based on the present study, do not influence iron status based on the doses reported herein. Therefore, polyphenol supplementation poses potential for alternatives to traditional pharmacological approaches for CVD risk management, with relatively limited side-effects. However, there remains a lack of evidence convincingly linking polyphenol supplementation, iron status, and CVD risk. These results indicate that more extensive studies are needed to consider the effect of long-term polyphenol supplementation on both iron status and CVD, particularly in subjects with high iron requirements.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: NMD is supported by Dementia Australia Research Foundation PhD Scholarship.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: NN conceived and designed the research question; HS, NN and NMD performed the searches; HS, NMD, and NN analyzed the data; HS drafted the paper; NMD, MB, AJMK, EG, DDM, DS, and NN reviewed the manuscript and provided input in the final manuscript. All authors contributed significantly to this manuscript.
ORCID iDs: Duane D Mellor  https://orcid.org/0000-0002-1369-3868
https://orcid.org/0000-0002-1369-3868
Nenad Naumovski  https://orcid.org/0000-0002-2841-4497
https://orcid.org/0000-0002-2841-4497
References
- 1. World Health Organization. Cardiovascular diseases (CVDs). World Health Organization (WHO). http://www.who.int/cardiovascular_diseases/en/. Published 2018. Accessed February 28, 2018.
- 2. Department of Health. Cardiovascular disease. Department of Health, Australian Government. http://www.health.gov.au/internet/main/publishing.nsf/content/chronic-cardio. Published 2016. Updated November 28, 2016. Accessed April 15, 2018.
- 3. Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis. Annu Rev Immunol. 2009;27:165-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vinay K, Abbas AK, Aster JC. Robbins and Cotran Pathologic Basis of Disease. 9th ed Philadelphia, PA: Elsevier Saunders; 2015. [Google Scholar]
- 5. Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109:27-32. [DOI] [PubMed] [Google Scholar]
- 6. Hobbs HH, Brown MS, Goldstein JL. Molecular genetics of the LDL receptor gene in familial hypercholesterolemia. Hum Mutat. 1992;1:445-466. [DOI] [PubMed] [Google Scholar]
- 7. Bobryshev YV. Monocyte recruitment and foam cell formation in atherosclerosis. Micron. 2006;37:208-222. [DOI] [PubMed] [Google Scholar]
- 8. Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32: 2045-2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zagayko AL, Kravchenko GB, Krasilnikova OA, Ogai YO. Grape polyphenols increase the activity of HDL enzymes in old and obese rats. Oxid Med Cell Longev. 2013;2013:593761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tangney C, Rasmussen H. Polyphenols, inflammation, and cardiovascular disease. Curr Atheroscler Rep. 2013;15:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ridker PM, Rifai N, Pfeffer M, Sacks F, Lepage S, Braunwald E. Elevation of Tumor Necrosis Factor-α and increased risk of recurrent coronary events after myocardial infarction. Circulation. 2000;101:2149-2153. [DOI] [PubMed] [Google Scholar]
- 12. Eltoft A, Arntzen KA, Wilsgaard T, Mathiesen EB, Johnsen SH. Interleukin-6 is an independent predictor of progressive atherosclerosis in the carotid artery: the Tromsø Study. Atherosclerosis. 2018;271:1-8. [DOI] [PubMed] [Google Scholar]
- 13. Sack MN. Tumor necrosis factor-α in cardiovascular biology and the potential role for anti-tumor necrosis factor-α therapy in heart disease. Pharmacol Ther. 2002;94:123-135. [DOI] [PubMed] [Google Scholar]
- 14. Bilsborough W, O’Driscoll G, Stanton K, et al. Effect of lowering tumour necrosis factor-alpha on vascular endothelial function in Type II diabetes. Clin Sci (Lond). 2002;103:163-169. [DOI] [PubMed] [Google Scholar]
- 15. Oikonomou E, Siasos G, Zaromitidou M, et al. Atorvastatin treatment improves endothelial function through endothelial progenitor cells mobilization in ischemic heart failure patients. Atherosclerosis. 2015;238:159-164. [DOI] [PubMed] [Google Scholar]
- 16. Santhakumar A, Battino M, Alvarez-Suarez J. Dietary polyphenols: structures, bioavailability and protective effects against atherosclerosis. Food Chem Toxicol. 2018;113:49-65. [DOI] [PubMed] [Google Scholar]
- 17. Cvejic HJ, Russo G, Godos J, et al. Beneficial effects of polyphenols on chronic diseases and ageing. In: Galanakis CM, ed., Polyphenols: Properties, Recovery, and Applications. Amsterdam, The Netherlands: Elsevier Inc.; 2018:69-102. [Google Scholar]
- 18. Manach C, Scalbert A, Morand C, Remesy C, Jimenez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727-747. [DOI] [PubMed] [Google Scholar]
- 19. Travica N, D’Cunha NM, Naumovski N, et al. The effect of blueberry interventions on cognitive performance and mood: a systematic review of randomized controlled trials [published online ahead of print April 15, 2019]. Brain Behav Immun. doi: 10.1016/j.bbi.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 20. Christenson J, Whitby SJ, Mellor D, et al. The effects of resveratrol supplementation in overweight and obese humans: a systematic review of randomized trials. Metab Syndr Relat Disord. 2016;14:323-333. [DOI] [PubMed] [Google Scholar]
- 21. D’Cunha NM, Georgousopoulou EN, Dadigamuwage L, et al. Effect of long-term nutraceutical and dietary supplement use on cognition in the elderly: a 10-year systematic review of randomised controlled trials. Br J Nutr. 2018;119: 280-298. [DOI] [PubMed] [Google Scholar]
- 22. Nash V, Ranadheera CS, Georgousopoulou EN, et al. The effects of grape and red wine polyphenols on gut microbiota—a systematic review. Food Res Int. 2018; 113:277-287. [DOI] [PubMed] [Google Scholar]
- 23. Ramprasath V, Jones P. Anti-atherogenic effects of resveratrol. Eur J Clin Nutr. 2010;64:660-668. [DOI] [PubMed] [Google Scholar]
- 24. Siegenberg D, Baynes R, Bothwell TH, et al. Ascorbic acid prevents the dose-dependent inhibitory effects of polyphenols and phytates on nonheme-iron absorption. Am J Clin Nutr. 1991;53:537-541. [DOI] [PubMed] [Google Scholar]
- 25. Reddy MB, Armah SM, Stewart JW, O’Brien KO. Iron absorption from iron-enriched aspergillus oryzae is similar to ferrous sulfate in healthy female subjects. Curr Dev Nutr. 2018;2:nzy004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Beard JL. Iron biology in immune function, muscle metabolism and neuronal functioning. J Nutr. 2001;131:568S-579S; discussion 580S. [DOI] [PubMed] [Google Scholar]
- 27. von Haehling S, Jankowska EA, van Veldhuisen DJ, Ponikowski P, Anker SD. Iron deficiency and cardiovascular disease. Nat Rev Cardiology. 2015;12:659-669. [DOI] [PubMed] [Google Scholar]
- 28. Lapice E, Masulli M, Vaccaro O. Iron deficiency and cardiovascular disease: an updated review of the evidence. Curr Atheroscler Rep. 2013;15:358. [DOI] [PubMed] [Google Scholar]
- 29. Abbaspour N, Hurrell R, Kelishadi R. Review on iron and its importance for human health. J Res Med Sci. 2014;19:164-174. [PMC free article] [PubMed] [Google Scholar]
- 30. Ems T, Huecker MR. Biochemistry, Iron Absorption. Treasure Island, FL: StatPearls; 2018. [PubMed] [Google Scholar]
- 31. Zijp IM, Korver O, Tijburg LBM. Effect of tea and other dietary factors on iron absorption. Crit Rev Food Sci Nutr. 2000;40:371-398. [DOI] [PubMed] [Google Scholar]
- 32. Lesjak M, Hoque R, Balesaria S, et al. Quercetin inhibits intestinal iron absorption and ferroportin transporter expression in vivo and in vitro. PLoS ONE. 2014;9:e102900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lynch SR. Interaction of iron with other nutrients. Nutr Rev. 1997;55:102-110. [DOI] [PubMed] [Google Scholar]
- 34. Corti MC, Gaziano M, Hennekens CH. Iron status and risk of cardiovascular disease. Ann Epidemiol. 1997;7:62-68. [DOI] [PubMed] [Google Scholar]
- 35. Chiva-Blanch G, Arranz S, Lamuela-Raventos RM, Estruch R. Effects of wine, alcohol and polyphenols on cardiovascular disease risk factors: evidences from human studies. Alcohol Alcohol. 2013;48:270-277. [DOI] [PubMed] [Google Scholar]
- 36. McKay DL, Blumberg JB. Cranberries (Vaccinium macrocarpon) and cardiovascular disease risk factors. Nutr Rev. 2007;65:490-502. [DOI] [PubMed] [Google Scholar]
- 37. Ahmad Fuzi SF, Koller D, Bruggraber S, Pereira DI, Dainty JR, Mushtaq S. A 1-h time interval between a meal containing iron and consumption of tea attenuates the inhibitory effects on iron absorption: a controlled trial in a cohort of healthy UK women using a stable iron isotope. Am J Clin Nutr. 2017;106:1413-1421. [DOI] [PubMed] [Google Scholar]
- 38. Bignell T. Iron inhibition by plant polyphenols: an adjunct to treatment in hereditary haemochromatosis. Aust J Herb Med. 2012;24:125-127. [Google Scholar]
- 39. Moher D, Liberati A, Tetzlaff J, Altman GA, The PRIMSA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zorzela Liliane Z, Loke YK, Ioannidis JP, et al. PRISMA harms checklist: improving harms reporting in systematic reviews. BMJ. 2016;352:i157. [DOI] [PubMed] [Google Scholar]
- 41. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2011. http://handbook.cochrane.org.
- 42. Kelley DS, Adkins Y, Reddy A, Woodhouse LR, Mackey BE, Erickson KL. Sweet bing cherries lower circulating concentrations of markers for chronic inflammatory diseases in healthy humans. J Nutr. 2013;143:340-344. [DOI] [PubMed] [Google Scholar]
- 43. Kelley DS, Rasooly R, Jacob RA, Kader AA, Mackey BE. Consumption of bing sweet cherries lowers circulating concentrations of inflammation markers in healthy men and women. J Nutr. 2006;136:981. [DOI] [PubMed] [Google Scholar]
- 44. Lopez-Huertas E, Fonolla J. Hydroxytyrosol supplementation increases vitamin C levels in vivo. A human volunteer trial. Redox Biol. 2017;11:384-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Soriano-Maldonado A, Hidalgo M, Arteaga P, de Pascual-Teresa S, Nova E. Effects of regular consumption of vitamin C-rich or polyphenol-rich apple juice on cardiometabolic markers in healthy adults: a randomized crossover trial. Eur J Nutr. 2014;53:1645-1657. [DOI] [PubMed] [Google Scholar]
- 46. Suliburska J, Bogdanski P, Szulinska M, Stepien M, Pupek-Musialik D, Jablecka A. Effects of green tea supplementation on elements, total antioxidants, lipids, and glucose values in the serum of obese patients. Biol Trace Elem Res. 2012;149: 315-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Urbaniak A, Basta P, Ast K, et al. The impact of supplementation with pomegranate fruit (Punica granatum L.) juice on selected antioxidant parameters and markers of iron metabolism in rowers. J Int Soc Sports Nutr. 2018;15:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Villaño D, Vilaplana C, Medina S, et al. Relationship between the ingestion of a polyphenol-rich drink, hepcidin hormone, and long-term training. Molecules. 2016;21:1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Beck KL, Conlon CA, Kruger R, Coad J. Dietary determinants of and possible solutions to iron deficiency for young women living in industrialized countries: a review. Nutrients. 2014;6:3747-3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ramakrishnan U, Kuklina E, Stein AD. Iron stores and cardiovascular disease risk factors in women of reproductive age in the United States. Am J Clin Nutr. 2002;76:1256-1260. [DOI] [PubMed] [Google Scholar]
- 51. Brown RC, Klein A, Simmons WK, Hurrell RF. The influence of jamaican herb teas and other polyphenol-containing beverages on iron absorption in the rat. Nutr Res. 1990;10:343-353. [Google Scholar]
- 52. Cook JD, Reddy MB, Hurrell RF. The effect of red and white wines on nonheme-iron absorption in humans. Am J Clin Nutr. 1995;61:800-804. [DOI] [PubMed] [Google Scholar]
- 53. Tuntipopipat S, Zeder C, Siriprapa P, Charoenkiatkul S. Inhibitory effects of spices and herbs on iron availability. Int J Food Sci Nutr. 2009;60:43-55. [DOI] [PubMed] [Google Scholar]
- 54. Naumovski N. Bioactive composition of plants and plant foods. In: Scarlett CJ, Vuong QV, eds., Plant Bioactive Compounds for Pancreatic Cancer Prevention and Treatment. New York, NY: Nova Science Publishers; 2015:81-116. [Google Scholar]
- 55. Lau SO, Georgousopoulou EN, Kellett J, et al. The effect of dietary supplementation of green tea catechins on cardiovascular disease risk markers in humans: a systematic review of clinical trials. Beverages. 2016;2:16. [Google Scholar]
- 56. González-Sarrías A, Combet E, Pinto P, et al. A systematic review and meta-analysis of the effects of flavanol-containing tea, cocoa and apple products on body composition and blood lipids: exploring the factors responsible for variability in their efficacy. Nutrients. 2017;9:746. [Google Scholar]
- 57. Naumovski N, Blades BL, Roach PD. Food inhibits the oral bioavailability of the major green tea antioxidant epigallocatechin gallate in humans. Antioxidants (Basel). 2015;4:373-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bonaccio M, Pounis G, Cerletti C, Donati MB, Iacoviello L, de Gaetano G. Mediterranean diet, dietary polyphenols and low grade inflammation: results from the MOLI-SANI study. Br J Clin Pharmacol. 2017;83:107-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Garcia-Conesa MT, Chambers K, Combet E, et al. Meta-analysis of the effects of foods and derived products containing ellagitannins and anthocyanins on cardiometabolic biomarkers: analysis of factors influencing variability of the individual responses. Int J Mol Sci. 2018;19:694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Disler PB, Lynch SR, Charlton RW, et al. The effect of tea on iron absorption. Gut. 1975;16:193-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dueik V, Chen BK, Diosady LL. Iron-polyphenol interaction reduces iron bioavailability in fortified tea: competing complexation to ensure iron bioavailability. J Food Qual. 2017;2017:7. [Google Scholar]
- 62. Wilkins SJ, Frazer DM, Millard KN, McLaren GD, Anderson GJ. Iron metabolism in the hemoglobin-deficit mouse: correlation of diferric transferrin with hepcidin expression. Blood. 2006;107:1659-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Stugiewicz M, Tkaczyszyn M, Kasztura M, Banasiak W, Ponikowski P, Jankowska EA. The influence of iron deficiency on the functioning of skeletal muscles: experimental evidence and clinical implications. Eur J Heart Fail. 2016;18:762-773. [DOI] [PubMed] [Google Scholar]
- 64. Akil L, Ahmad HA. Relationships between obesity and cardiovascular diseases in four southern states and Colorado. J Health Care Poor Underserved. 2011;22: 61-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jiang S-Z, Lu W, Zong X-F, Ruan H-Y, Liu Y. Obesity and hypertension. Exp Ther Med. 2016;12:2395-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chan RSM, Woo J. Prevention of overweight and obesity: how effective is the current public health approach. Int J Environ Res Public Health. 2010;7: 765-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Luchsinger JA, Gustafson DR. Adiposity, type 2 diabetes, and Alzheimer’s disease. J Alzheimers Dis. 2009;16:693-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444: 860-867. [DOI] [PubMed] [Google Scholar]
- 69. Brown WV, Fujioka K, Wilson PWF, Woodworth KA. Obesity: why be concerned? Am J Med. 2009;122:S4-S11. [DOI] [PubMed] [Google Scholar]
- 70. Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009;6:399. [DOI] [PubMed] [Google Scholar]
- 71. Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003; 112:1821-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kanda T, Takahashi T. Interleukin-6 and cardiovascular diseases. Jpn Heart J. 2004;45:183-193. [DOI] [PubMed] [Google Scholar]
- 73. Shrivastava AK, Singh HV, Raizada A, Singh SK. C-reactive protein, inflammation and coronary heart disease. Egypt Heart J. 2015;67:89-97. [Google Scholar]
- 74. Ganz T, Nemeth E. Hepcidin and disorders of iron metabolism. Annu Rev Med. 2011;62:347-360. [DOI] [PubMed] [Google Scholar]
- 75. Nakagawa H, Tamura T, Mitsuda Y, et al. Inverse correlation between serum interleukin-6 and iron levels among Japanese adults: a cross-sectional study. BMC Hematol. 2014;14:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Li Y, Yao J, Han C, et al. Quercetin, inflammation and immunity. Nutrients. 2016;8:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Rye KA, Bursill CA, Lambert G, Tabet F, Barter PJ. The metabolism and anti-atherogenic properties of HDL. J Lipid Res. 2009;50:S195-S200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kraml PJ, Klein RL, Huang Y, Nareika A, Lopes-Virella MF. Iron loading increases cholesterol accumulation and macrophage scavenger receptor I expression in THP-1 mononuclear phagocytes. Metabolism. 2005;54:453-459. [DOI] [PubMed] [Google Scholar]
- 79. Pokimica B, Garcia-Conesa MT, Zec M, et al. Chokeberry juice containing polyphenols does not affect cholesterol or blood pressure but modifies the composition of plasma phospholipids fatty acids in individuals at cardiovascular risk. Nutrients. 2019;11:850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Covas MI, Nyyssonen K, Poulsen HE, et al. The effect of polyphenols in olive oil on heart disease risk factors. Ann Intern Med. 2006:333-341. [DOI] [PubMed] [Google Scholar]
- 81. Benzie IFF, Strain JJ. The Ferric Reducing Ability of Plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70-76. [DOI] [PubMed] [Google Scholar]
- 82. Mascitelli M, Goldstein MR. Vascular beneficial effects of polyphenol-rich olive oil and reduced body iron stores. Eur J Nutr. 2013;52:1961-1962. [DOI] [PubMed] [Google Scholar]
- 83. Kraml P. The role of iron in the pathogenesis of atherosclerosis. Physiol Res. 2017;66:S55-S67. [DOI] [PubMed] [Google Scholar]
- 84. Mahmoudi MJ, Mahmoudi M, Siassi F, et al. Lymphocyte cytotoxicity of oxLDL in patients with atherosclerosis. Iran J Immunol. 2011;8:27-33. [PubMed] [Google Scholar]
- 85. Pirillo A, Norata GD, Catapano AL. LOX-1, OxLDL, and atherosclerosis. Mediators Inflamm. 2013;2013:152786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Pasricha S, Drakesmith H, Black J, Hipgrave D, Biggs B. Control of iron deficiency anemia in low- and middle-income countries. Blood. 2013;121:2607-2617. [DOI] [PubMed] [Google Scholar]
- 87. Naumovski N, Foscolou A, D’Cunha NM, et al. The association between green and black tea consumption on successful aging: a combined analysis of the ATTICA and MEDiterranean ISlands (MEDIS) epidemiological studies. Molecules. 2019;24:E1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Galleano M, Puntarulo S. Role of antioxidants on the erythrocytes resistance to lipid peroxidation after acute iron overload in rats. Biochim Biophys Acta. 1995; 1271:321-326. [DOI] [PubMed] [Google Scholar]
- 89. Puntarulo S. Iron, oxidative stress and human health. Mol Aspects Med. 2005; 26:299-312. [DOI] [PubMed] [Google Scholar]
- 90. Milenkovic D, Morand C, Cassidy A, et al. Interindividual variability in biomarkers of cardiometabolic health after consumption of major plant-food bioactive compounds and the determinants involved. Adv Nutr. 2017;8:558-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Jian J, Pelle E, Huang X. Iron and menopause: does increased iron affect the health of postmenopausal women? Antioxid Redox Signal. 2009;11:2939-2943. [DOI] [PMC free article] [PubMed] [Google Scholar]