Abstract
Objectives
To examine trends in inequality in life expectancy and age-specific death rates across 40 US spatial units from 1990 to 2016.
Methods
We use multiple cause-of-death data from vital statistics to estimate measures of inequality in mortality across metropolitan status and geographic region. We consider trends for 5-year age intervals and examine inequality in cause-specific mortality.
Results
For both sexes, spatial inequality in life expectancy and all-cause mortality above age 25 rose between 2002-04 and 2014–16. During this period, the standard deviation in life expectancy at birth increased by 19% for males and by 44% for females. Areas that had higher life expectancy at the beginning of the period enjoyed larger gains in life expectancy. Especially noteworthy are divergent trends between large central metropolitan areas on the coasts and non-metropolitan areas in Appalachia and the South. Spatial inequality in mortality from lung cancer/respiratory diseases rose substantially, particularly for older women. Spatial inequality in mortality from the combination of drug overdose, alcohol use, and suicide increased at ages 30–34, but declined at ages 50–54 and 70–74. Inequality in mortality from circulatory diseases, the largest cause of death, grew for some groups, particularly 30-34 year-old women. Mortality from screenable cancers, an indicator of the performance of medical systems, showed relatively little spatial disparity during the period.
Conclusions
Spatial inequality in life expectancy at birth and adult mortality has increased in recent decades.
Highlights
-
•
Spatial inequality in mortality has risen since 2003 at all adult ages.
-
•
Large cities on the coasts are diverging from rural areas in Appalachia and the South.
-
•
At younger ages, patterns due to growing inequality in deaths from drugs, suicide, and alcohol.
-
•
At older ages, patterns driven by divergent trends in smoking-related mortality.
1. Introduction
Where one lives structures the life course in ways that affect length of life and ultimate cause of death. These influences include the quality and accessibility of health services (Association of American Medical Colleges, 2017), ecological features that affect disease incidence and transmission (Dalziel et al., 2018), interpersonal influences on health behaviors like smoking and obesity (Christakis and Fowler, 2007, Christakis and Fowler, 2008), and major changes in the structure of employment opportunities (Charles, Hurst, & Schwartz, 2018; United States Department of Agriculture 2017).
One commonly studied spatial dimension of mortality is the rural/urban divide. By most accounts, rural areas were disadvantaged in 1990 and had slower subsequent mortality improvements than urban areas so that rural/urban differences in mortality have widened (Cosby et al., 2019; Singh & Siahpush, 2014; Stein, Gennuso, Ugboaja, & Remington, 2017). Uncertainty is added to this assessment by several studies that find a “rural paradox”, with rural mortality rates lower than urban rates (Hayward, Pienta, & McLaughlin, 1997; McLaughlin, Stokes, & Nonoyama, 2001; Yang, Jensen, & Haran, 2011). South/Non-South differences have widened during the past several decades (Fenelon, 2013). While studies utilizing these dichotomies are valuable, there is a great deal of spatial variation within each of these broad categories (Case & Deaton, 2017; Elo, Hendi, Ho, Vierboom, & Preston, 2019). This variation can be better captured by a comprehensive index of inequality applied to a more detailed set of spatial units.
In this paper, we take advantage of the huge volume of annual vital statistics produced by the National Center for Health Statistics to investigate the extent of mortality inequality among 40 spatial units over the period 1990–2016. Our units of analysis are combinations of metropolitan status and geographic region that highlight these two separable dimensions. The spatial detail available on several million annual deaths enables us to identify levels and trends in spatial inequality by 5-year age groups for the first time. It also permits a consideration of spatial trends in life expectancy at birth, for which detailed age-specific data are required. To shed light on the social and biomedical processes that contribute to levels and trends in inequality, we examine inequality by cause of death. Changes in geographic patterns of mortality signal whether factors determining health outcomes are converging or diverging across the nation.
2. Data and methods
We examine inequality in mortality across 40 spatial units between 1990 and 2016, considering trends in life expectancy at birth and in the Index of Dissimilarity applied to 5-year age intervals. We use age-, sex-, and county-specific data on annual deaths and underlying cause of death from Multiple Cause of Death files provided by the National Center for Health Statistics (NCHS). We estimate person-years of exposure using vintage 2016 NCHS/Census bridged-race population estimates by age, sex, and county (postcensal for 2011 forward and intercensal for earlier years).
We classify counties into 40 spatial units (Appendix A Table 1), consisting of 10 broad geographic regions, each of which is further divided into 4 metropolitan statuses. Our 10 geographic regions include the 9 Census divisions, as well as Appalachia, as defined by the Appalachian Regional Commission. Appalachian counties, which include all of West Virginia and counties from 12 other states, are excluded from their overlapping Census regions.
We determine a county's metro status using the Economic Research Service (ERS) classification, which was modified by NCHS (Ingram & Franco, 2014). Metro status consists of 4 categories: large central metros, large metro suburbs (hereafter “suburbs”), small metros, and non-metropolitan areas. To maintain consistency over time, we use counties' metropolitan category as of 2013. We use these spatial categories, rather than states or counties, because states would tend to obscure salient rural/urban differences and the excessive detail produced by using 3000 + counties may not permit clear generalizations to emerge. Like all studies involving geographic variation, our results depend on our choice of areal unit and our inferences could differ at different levels of aggregation. Though the metropolitan/nonmetropolitan classification of some counties changed over the study period, we show that our conclusions are robust to changes in counties' status over time.
We begin by examining trends in life expectancy at birth in the 40 spatial units. Life expectancy at birth is the expected length of life for a newborn subject for all of his or her life to the age-specific death rates of a particular period and spatial unit. It is calculated using standard methods (Preston, Guillot, & Heuveline, 2001) and takes account of the growth rate of the population at ages 85+ (Horiuchi & Coale, 1982).
Our primary measure of spatial inequality is the Index of Dissimilarity in age group x to x + n (nIDx), defined as:
where is the percentage of deaths and the percentage of the population aged x to x + n in spatial unit i. We pool data into three three-year periods (1990–1992, 2002–2004, and 2014–2016) and focus primarily on the period between 2002-04 and 2014-16 when trends in US mortality were especially problematic (Case & Deaton, 2017). The index is interpretable as the minimum percentage of deaths that would have to be reallocated within that age interval to equalize the spatial distributions of deaths and population (Mackenbach & Kunst, 1997; Regidor, 2004). Such an equalization would produce identical death rates across all spatial units. The summation is multiplied by 0.5 so that the value of ID can potentially range from 0%, when there is no disparity between the distributions of deaths and population, to 100%, when the disparity is complete.
The ID is a relative measure of inequality rather than an absolute one because it is scale-invariant; all death rates could be multiplied by the same factor without changing the value of the measure (Mackenbach & Kunst, 1997). Relative measures are used roughly four times more frequently in health research than absolute measures (King, Harper, & Young, 2012). The Index of Dissimilarity is very closely related to the Gini coefficient since both indexes can be derived from the Lorenz curve. We prefer the ID to the Gini coefficient because of its more straightforward interpretation, as cited in the above paragraph, and its ease of graphical display (Harper & Lynch, 2005). It is the most widely used measure of spatial unevenness (Iceland, Weinberg, & Steinmetz, 2002).
In addition to calculating the index for all causes of death, we also consider levels and trends in inequality for specific cause-of-death categories. We focus on nine mutually exclusive and exhaustive cause-of-death categories:
-
•
Breast, prostrate, cervical, and colorectal cancers;
-
•
Circulatory diseases (includes heart disease and stroke);
-
•
Drug overdose, alcohol-related causes, and suicide;
-
•
HIV/AIDS;
-
•
Homicide;
-
•
Lung cancer and respiratory diseases;
-
•
Other external causes (including transport accidents, falls, drownings); and
-
•
All other causes
The ICD-9 and ICD-10 codes for these causes are listed in Appendix A Table 2. The list above includes three categories which are combinations of several causes. First, we aggregate screenable cancers—breast, prostate, colorectal, and cervical—to create an indicator of access to and quality of health services. A large percentage of deaths from these cancers can be prevented by timely diagnosis and proper treatment (Ginsberg, Edejer, Lauer, & Sepulveda, 2009; Ginsberg, Lim, Lauer, Johns, & Sepulveda, 2010; Heijnsdijk et al., 2015; Plevritis et al., 2018). We also consider the role of a category that combines alcohol-attributable deaths, drug overdoses, and suicides. This aggregate, often termed “deaths of despair”, has been hypothesized to play a key role in the recent adverse US mortality trends (Case and Deaton, 2015, Case and Deaton, 2017). We use ICD-9 and ICD-10 codes recommended by CDC, which are not identical to those used by Case and Deaton. Finally, we combine lung cancer and respiratory diseases to serve as an indicator of the mortality effects of smoking. In 2014–2016, age-standardized death rates from lung cancer and respiratory diseases at ages 25–64 were correlated at 0.86 for females and 0.88 for males across the 40 units.
We focus the cause of death analysis on three age intervals. First, we examine ages 30–34 since, as we show below, it is the age interval in which inequality is highest. We also consider the age interval 50–54 because it is one of the primary age categories used by Case and Deaton (2015; 2017) to describe and analyze “deaths of despair”, an analysis that has initiated a large and vigorous scholarly response. Finally, we examine mortality by cause of death at ages 70–74, which are representative of older ages, but whose cause of death categories are not yet highly distorted by co-morbidities (Tinetti et al., 2012).
3. Results
3.1. Levels and trends in spatial inequality in life expectancy at birth
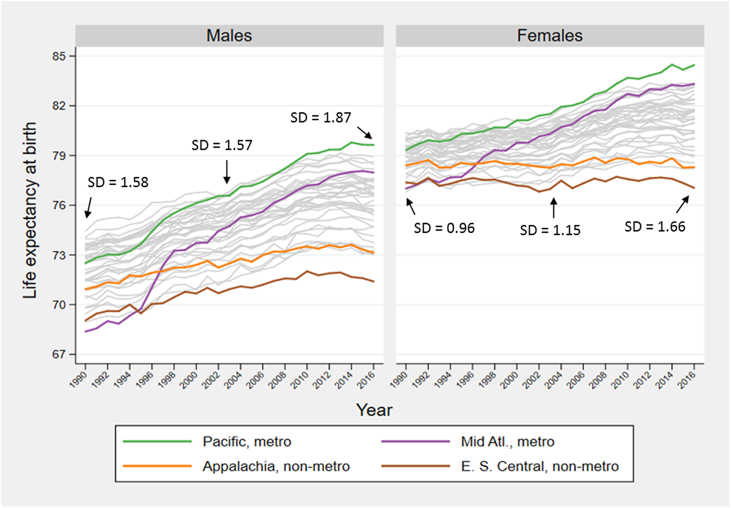
Fig. 1 shows trends in life expectancy at birth for the 40 spatial units during 1990–2016. Based on the mean life expectancy of males and females, we identify four outliers, labeled in Fig. 1 and subsequent figures: large central metropolitan areas in the Pacific region, which had the highest life expectancy in 2016; large central metropolitan areas in the Mid-Atlantic region, which had the greatest gain in life expectancy over the period 1990–2016; non-metropolitan areas in the East South Central region, which had the lowest life expectancy in 2016; and non-metropolitan areas in Appalachia, which had the smallest gain in life expectancy over the period. To give a visual impression of trends, the remaining 36 regions are represented in gray. The outliers are chosen for expositional purposes only; their choice has no bearing on the estimation of inequality. Each unit's life expectancy at birth for 1991, 2003, and 2015, along with each unit's rank, is given in the online supplement, in Appendix A Table 3.
Fig. 1.
Trends in life expectancy at birth in 40 spatial units, 1990-2016
SD = standard deviation. E. S. Central: East South Central; Mid Atl: Mid Atlantic.
Fig. 1 also presents data on one indicator of inequality, the unweighted standard deviation in life expectancy at birth among the 40 units. Between 2003 and 2016, the standard deviation rose 44% among women (1.66/1.15) and 19% among men (1.87/1.57). Inequality among males exceeded that among females throughout the period.
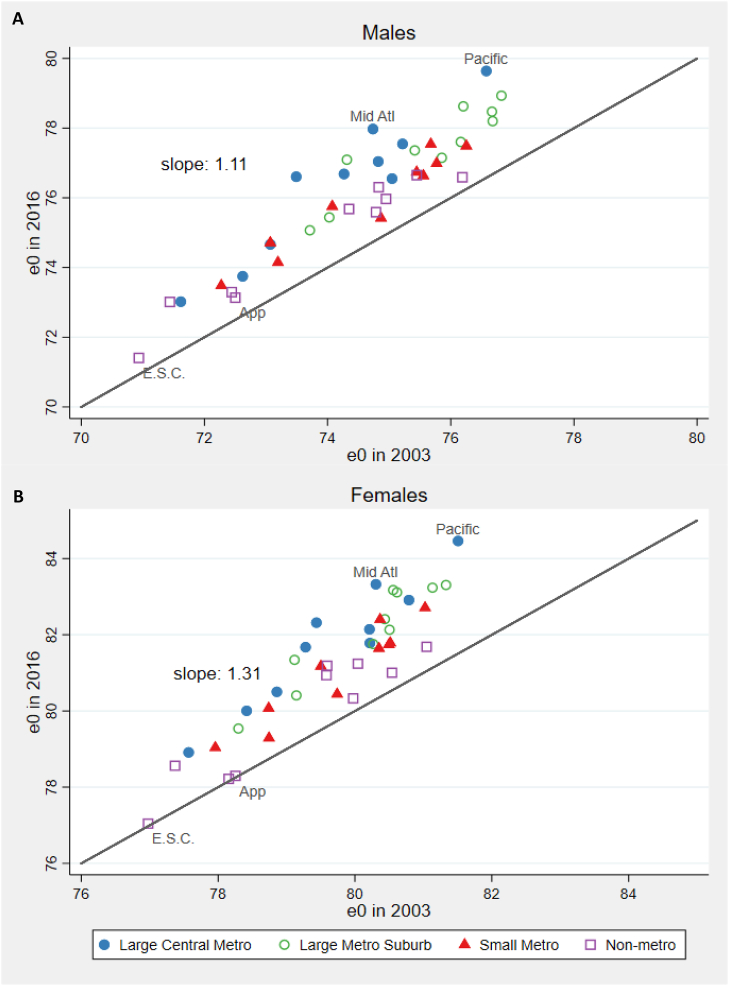
Fig. 2 plots each unit's life expectancy in 2003 and 2016, with points falling above the diagonal line indicating an increase over time. The Figure shows that areas with higher life expectancies in 2003 experienced, on average, larger gains in life expectancy between 2003 and 2016. The slope of the relationship between the two life expectancies implies that, among women, each incremental year of life expectancy in 2003 was associated with an additional gain of 0.31 years of life expectancy by 2016. Among men, the additional gain was 0.11 years.
Fig. 2.
Life expectancy at birth (years) in 2003 and 2016.
App: Appalachia; E.S.C.: East South Central; Mid Atl: Mid Atlantic.
Fig. 2 shows that areas that gained the most years of life over the period were primarily large central metropolitan areas and their suburbs. By 2016, the seven highest life expectancies for women and the eight highest for men belonged to these categories. The smallest gains were experienced by non-metropolitan areas, which solidified their position as the highest mortality category. In 2016, the four lowest life expectancies for women and four of the five lowest for men were observed in non-metropolitan areas. One exception to the good performance of large central metropolitan areas were those in the East South Central region, which ranked in the bottom five life expectancies in 2016 for both men and women.
The key role of metro status in the divergence of life expectancy trajectories is discernible from Appendix A Table 4. The Table lists the percentage of the variation in life expectancy at birth among the 40 units that is associated with either region or metro status, as measured by the r-squared term in bivariate regressions of life expectancy on region or metro status. Although the regional variable is associated with a greater share of the variance than metro status in both 2003 and 2016, metro status is associated with a greater share of the change between these two years.
3.2. Levels and trends in spatial inequality in all-cause mortality by age
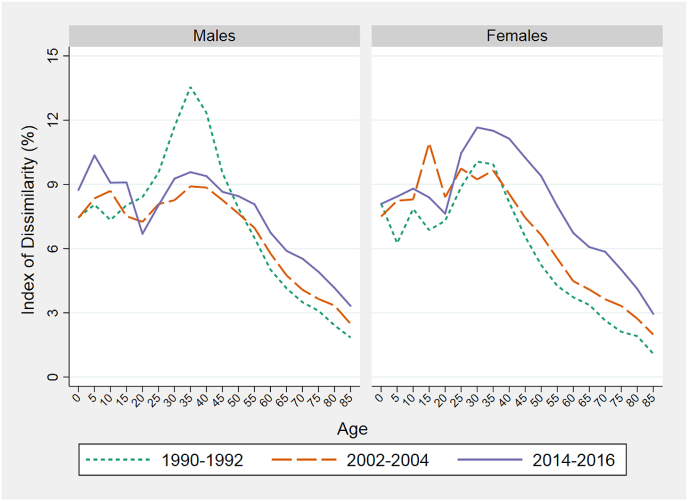
Life expectancy at birth combines death rates at all ages into a single index. To shed light on inequality levels and trends by age, we apply the Index of Dissimilarity to death rates in 5-year age groups among the 40 spatial units. Fig. 3 presents results for 1990–92, 2002–04, and 2014–16. For both sexes, the age-pattern of inequality peaks in the age interval 25–39 and declines steadily thereafter. Early to mid-adulthood is clearly associated with the greatest geographic disparity in survival.
Fig. 3.
Index of Dissimilarity in all-cause mortality by age in three periods.
Trends in levels of inequality vary by age and sex. At the peak ages for males, inequality declined sharply between 1990-92 and 2002-04 while it rose at older ages. The relatively small change in inequality in male life expectancy between 1990 and 2003 shown in Fig. 1 was clearly a product of offsetting trends at younger vs older adult ages.
For both sexes, inequality rose between 2002-04 and 2014-16 at all ages above 25. The increase was particularly sharp among women, consistent with Fig. 1, Fig. 2. Below age 10, inequality rose for both sexes between 1990-92 and 2014–16, although trends are less distinct in the second half of the period for females. For the remaining analyses, we focus on adult mortality.
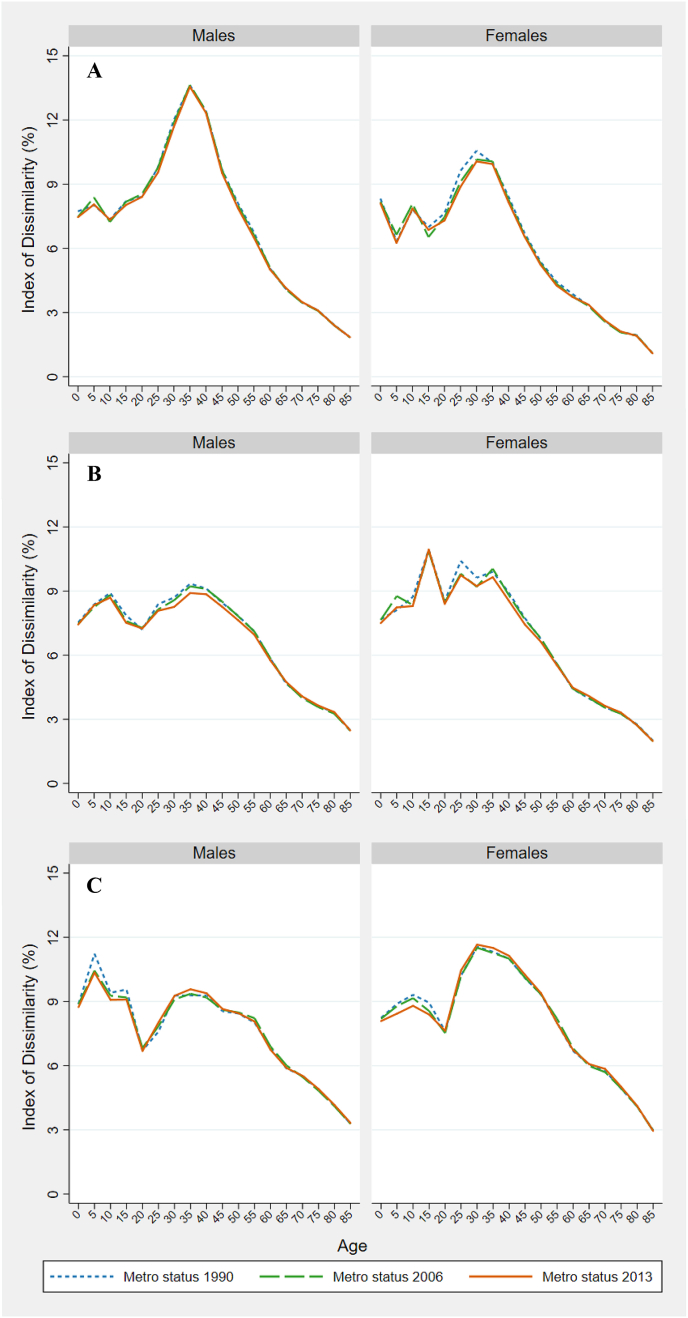
To test our findings' sensitivity to the decision to anchor a county's metro status to its characteristics in 2013, we re-estimate the results in Fig. 3 using counties' metro status in 1990 and 2006 (in addition to 2013). Appendix A Fig. 1 presents these results and indicates that, while using 2013 metro status results in a lower estimate of inequality at some ages, the differences are negligible. On average, the difference between the highest and lowest ID was 0.2 percentage points, with differences ranging from 0.004 to 0.877 percentage points.
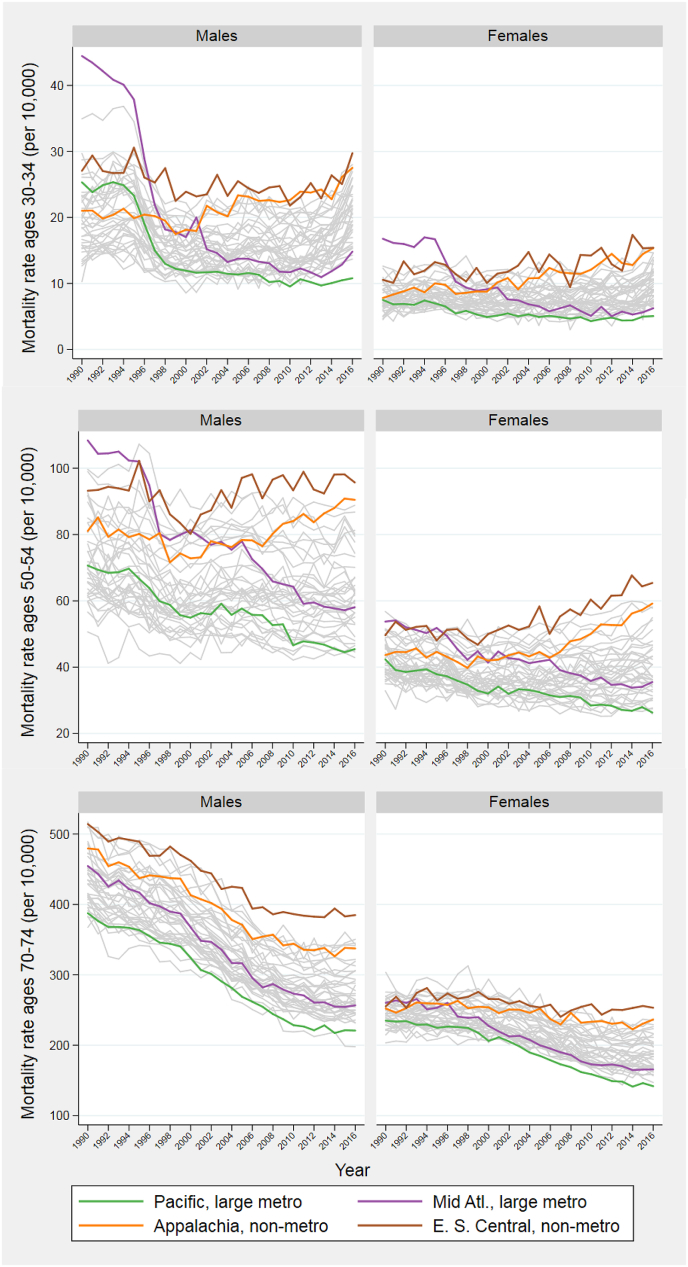
Fig. 4 provides detail on the regional mortality patterns producing the changes in inequality observed in Fig. 3. The Figure presents trends in age-specific all-cause death rates at ages 30–34, 50–54, and 70–74. Using colored trend lines, we distinguish the four outlier areas identified earlier. Appendix A Table 3 lists each unit's value, as well as ranking, for 1991, 2003, and 2015.
Fig. 4.
All-cause mortality rates at three ages.
E. S. Central: East South Central; Mid Atl = Mid Atlantic.
At ages 30–34, the huge reduction in the ID between 1990-92 and 2002-04 among males is led by large central metropolitan areas in the Mid-Atlantic region. While mortality rates at ages 30–34 in this unit far exceeded those in any of the other 39 units at the beginning of the study, rates were near the middle of the distribution by 2002-04 (see Appendix Table 3 for ranking). Large central metropolitan areas in the Mid-Atlantic include New York City, where massive declines in mortality from HIV/AIDS and homicide between 1990 and 2000 at these ages were key factors in an extremely rapid gain in life expectancy (Preston & Elo, 2014). By 2014–16, large central metropolitan areas in the Mid-Atlantic had among the lowest mortality levels at ages 30–34. Large central metropolitan areas in the Pacific also showed substantial mortality gains relative to other areas. For both sexes, non-metropolitan areas in Appalachia and East-South Central had among the highest death rates by 2014–2016. Mortality at ages 30–34 has been rising in both regions since 2000.
The story is similar at ages 50–54. After increases beginning around 2000, death rates in non-metropolitan areas in Appalachia and the East South Central region finished the period higher than those in any other regional grouping. Large central metropolitan areas in the Pacific and Mid-Atlantic regions enjoyed systematic mortality reductions throughout the period. The dispersion in female mortality rates at ages 50–54 rose throughout this period. Regional mortality trends at ages 70–74 are similar, with disparities that widen over time more for women than for men.
3.3. Levels and trends in spatial inequality by cause of death and age
The ID for all causes combined is a product of inequalities in the underlying causes of death, the distribution of causes, and interactions among the causes. Table 1 shows the ID for the nine causes of death, as well as for all causes, in 2002-04 and 2014–16. As above, we distinguish three age groups: 30–34, 50–54, and 70–74. We do not present results when there are fewer than 1000 deaths in a cause-sex-age-period grouping.
Table 1.
Index of Dissimilaritya by cause of death at three ages, 2002-04 and 2014–16.
| Cause of death | 2002–2004 |
2014–2016 |
Change (2014–2016) - (2002–2004) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 30-34 y. | 50-54 y. | 70-74 y. | 30-34 y. | 50-54 y. | 70-74 y. | 30-34 y. | 50-54 y. | 70-74 y. | ||
| Males | HIV/AIDS | 28.0 | 33.6 | --b | – | 25.6 | – | – | −8.0 | – |
| Homicide | 22.4 | 21.9 | – | 19.9 | 20.2 | – | −2.4 | −1.8 | – | |
| Lung cancer, resp. | – | 10.7 | 6.2 | – | 16.0 | 9.1 | – | 5.3 | 2.9 | |
| Other external | 14.8 | 12.4 | 8.5 | 15.5 | 12.6 | 7.8 | 0.6 | 0.2 | −0.7 | |
| Circ disease | 11.4 | 9.3 | 5.3 | 11.3 | 10.0 | 6.7 | −0.1 | 0.7 | 1.5 | |
| Alz, mental, nervous system | 10.5 | 7.7 | 5.9 | 13.2 | 9.3 | 6.0 | 2.7 | 1.6 | 0.2 | |
| All other | 7.5 | 6.8 | 3.6 | 9.2 | 8.1 | 4.2 | 1.7 | 1.3 | 0.6 | |
| Drug, alcohol, suicide | 8.2 | 9.9 | 10.8 | 13.1 | 7.3 | 9.0 | 5.0 | −2.6 | −1.8 | |
| Screenable cancersc | – | 6.9 | 3.7 | – | 7.1 | 4.4 | – | 0.2 | 0.7 | |
| All causes | 8.3 | 7.6 | 4.1 | 9.3 | 8.4 | 5.5 | 1.0 | 0.8 | 1.5 | |
| Females | HIV/AIDS | 39.8 | 42.6 | – | – | – | – | – | – | – |
| Homicide | 15.5 | – | – | 16.7 | – | – | 1.1 | – | – | |
| Lung cancer, resp | – | 6.9 | 4.2 | – | 16.6 | 8.6 | – | 9.7 | 4.4 | |
| Other external | 18.2 | 12.9 | 7.7 | 19.1 | 14.0 | 7.8 | 0.9 | 1.1 | 0.1 | |
| Circ disease | 13.3 | 12.4 | 5.7 | 16.5 | 13.6 | 7.6 | 3.2 | 1.2 | 1.9 | |
| Alz, mental, nervous system | – | 7.0 | 6.9 | 16.3 | 10.8 | 7.4 | – | 3.8 | 0.5 | |
| Drug, alcohol, suicide | 11.3 | 13.5 | 18.4 | 14.3 | 9.4 | 14.1 | 2.9 | −4.1 | −4.3 | |
| All other | 8.4 | 6.4 | 3.3 | 10.1 | 8.4 | 4.9 | 1.7 | 2.0 | 1.6 | |
| Screenable cancersc | 8.3 | 4.0 | 3.7 | 7.0 | 4.3 | 3.5 | −1.3 | 0.2 | −0.2 | |
| All causes | 9.2 | 6.6 | 3.6 | 11.7 | 9.4 | 5.9 | 2.4 | 2.8 | 2.2 | |
Sorted from largest to smallest at age 50–54 in 2014–2016.
Minimum percentage of deaths that would have to be reallocated in an age interval to equalize the spatial distribution of deaths and population.
Causes with less than 1000 deaths in each age, sex, and period group are excluded.
Screenable cancers include breast, prostrate, colorectal, and cervical cancers.
Among males aged 30–34 and 50–54, HIV/AIDS and homicide, two causes of death with strong behavioral and socio-structural risk factors, exhibit the greatest inequality. The high ID values are a product of exceptionally high death rates from these causes in large central metropolitan areas. Large central metropolitan areas in the East South Central region (Alabama, Kentucky, Mississippi, Tennessee) stand out as especially hazardous with respect to these causes (results not shown).
In contrast, Table 1 shows very low inequality among screenable cancers. At ages 50–54 and 70–74 for both sexes, screenable cancers have the lowest or second lowest ID of any cause in both 2002-04 and 2014–16, ranging from 3.5% to 7.1%. The relative spatial equality of mortality from this disease category reduced spatial inequality from all causes combined; removing deaths from this cause in Table 2 raises the all-cause ID, especially for women.
Table 2.
Index of Dissimilarity in all-cause mortality with select causes removed.
| Cause removed | 2002–2004 |
2014–2016 |
|||||
|---|---|---|---|---|---|---|---|
| 30-35 y. | 50-54 y. | 70-74 y. | 30-35 y. | 50-54 y. | 70-74 y. | ||
| Males | Lung cancer, resp. | –a | 7.48 | 3.99 | – | 7.91 | 4.88 |
| Circ. disease | 8.30 | 7.03 | 3.92 | 9.27 | 8.07 | 5.21 | |
| Other external | 8.16 | 7.77 | 4.06 | 9.05 | 8.25 | 5.53 | |
| Homicide | 7.85 | 7.52 | – | 9.94 | 8.46 | – | |
| Alz, mental, nervous system | 8.40 | 7.69 | 4.10 | 9.26 | 8.46 | 5.57 | |
| Screenable cancersb | – | 7.67 | 4.17 | – | 8.55 | 5.69 | |
| HIV/AIDS | 8.04 | 7.28 | – | – | 8.60 | – | |
| All other | 9.05 | 8.11 | 4.49 | 9.40 | 8.73 | 6.28 | |
| Drugs, alcohol, suicide | 9.72 | 8.34 | 4.18 | 9.71 | 9.36 | 5.68 | |
| None (all-cause ID) | 8.26 | 7.63 | 4.07 | 9.27 | 8.45 | 5.53 | |
| Females | Lung cancer, resp. | – | 6.86 | 3.86 | – | 8.52 | 5.45 |
| Circ. disease | 9.05 | 5.08 | 3.23 | 11.25 | 8.61 | 5.55 | |
| Other external | 8.91 | 6.72 | 3.63 | 11.08 | 9.25 | 5.85 | |
| Alz, mental, nervous system | – | 6.80 | 3.60 | 11.51 | 9.35 | 5.80 | |
| Homicide | 9.20 | – | – | 11.69 | – | – | |
| HIV/AIDS | 9.12 | 6.39 | – | – | – | – | |
| Drugs, alcohol, suicide | 10.25 | 7.19 | 3.70 | 12.13 | 9.94 | 5.96 | |
| All other | 10.17 | 6.95 | 3.88 | 12.40 | 9.98 | 6.50 | |
| Screenable cancersb | 9.50 | 7.18 | 3.77 | 12.09 | 10.20 | 6.09 | |
| None (all-cause ID) | 9.23 | 6.63 | 3.63 | 11.66 | 9.38 | 5.85 | |
Sorted from smallest to largest ID with cause removed at age 50–54 in 2014-2016. A smaller new ID indicates that had there been no inequality in deaths from that given cause, inequality in all-cause mortality would have been lower.
Causes with less than 1000 deaths in each age, sex, and period group are excluded.
Screenable cancers include breast, prostrate, colorectal, and cervical cancers.
Circulatory diseases, which here include both heart disease and cerebrovascular disease, comprise the largest cause of death in the United States (Heron, 2018). Relative to other causes, spatial inequality in deaths from circulatory diseases generally falls in the middle of the distribution, with the ID ranging from 5.3% to 12.6%.
Consistent with Fig. 3, Table 1 shows that inequality in all-cause mortality rose at all three ages for both sexes between 2002-02 and 2014–16. The increases were larger for women than for men.
Ages 30–34. Cause-specific inequality among 30-34 year-old women grew most for circulatory diseases, followed by drug/alcohol/suicide mortality. For men, the largest change occurred for drug/alcohol/suicide mortality. In fact, the increase in inequality in all-cause mortality in this demographic group is entirely attributable to growing inequality in deaths from drugs/alcohol/suicide; the so-called deaths of despair. If we remove these deaths and recalculate the ID as in Table 2, the value is basically unchanged at 9.72% in 2002-04 and 9.71% in 2014-16. For women, the removal of these deaths reduces the increase in the all-cause ID from 2.43 to 1.88 percentage points, or by 23%.
Ages 50–54 and 70–74. In contrast, inequality in drugs/alcohol/suicide among the older adults in Table 1 declined more than for any cause. Declines were especially pronounced among women. Inequality declined because women in non-metropolitan areas, which began the period with the lowest mortality levels from drugs/alcohol/suicide, had the largest increase in mortality during the period (not shown). Among males, all metropolitan types had large increases in mortality from this cause, reducing the relative differences among them. By 2014–16, the relative spatial equality of drug/alcohol/suicide deaths suppresses the all-cause ID for both sexes at both age intervals (Table 2).
The largest increases in cause-specific inequality at ages 50–54 and 70–74 occurred for lung cancer/respiratory diseases. For women at ages 50–54, inequality increased more for this category than for any other cause of death for either sex at any age; the ID for this category rose from 6.9% in 2002-04 to 16.6% in 2014-16.
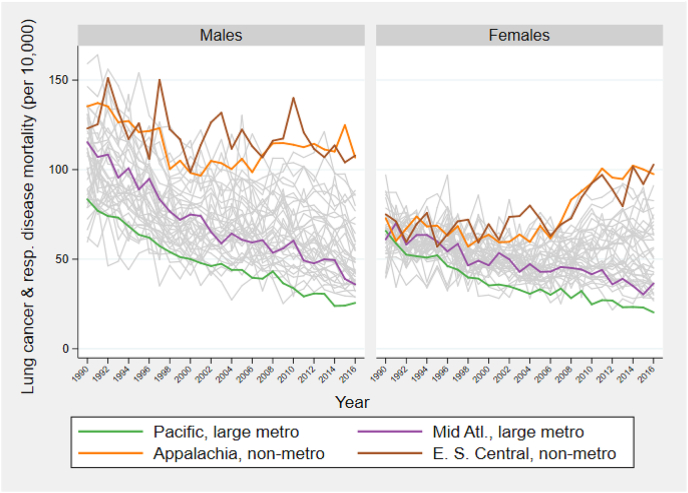
Appendix A Fig. 2 shows trends in mortality from lung cancer/respiratory disease at ages 50–54 in the 40 units. Among males, death rates since 2000 are high and relatively flat in non-metropolitan East South Central and Appalachia, while death rates decline steadily in large central metropolitan Pacific and Mid-Atlantic regions. Among women, the four outlier areas begin with similar death rates from this category in 1990 and diverge rapidly thereafter; mortality falls in the two large central metropolitan regions and actually rises in the two non-metropolitan regions. Smoking-related diseases are an important contributor to the rise in inequality from all causes combined; if their death rates were set at zero throughout the period, the rise in ID between 2002-04 and 2014-16 would be reduced by 48% for men and 55% for women (Table 2).
The changes in inequality in life expectancy that are examined in the first part of the paper are a product of changes in the age- and cause-specific changes in mortality that are studied in the second part. These two elements can be combined by decomposing changes in the variance in life expectancy across our 40 units into changes in mortality by age and cause. Such an analysis is presented for the period 2000–2016 in Appendix B. Results in Appendix B Table 1, Appendix B Table 2 show that ages 50–54 and 55–59 contributed the greatest share to changes in the variance in life expectancy at birth for women and men, respectively. Seventy-eight percent of the change for females and 87% for males is attributable to the age range 30–74 on which we have focused attention. Confirming the importance of smoking patterns for increasing inequality for women, the combination of lung cancer and respiratory diseases was the single largest contributor (save for the residual category) to rising variance among women (20%), followed closely by circulatory diseases (19%). Among men, the equivalent percentages are 12% and 23%. At 16%, drugs, alcohol, and suicide, heavily concentrated in the age interval 30–39, were the third leading cause of increased inequality for men, following other external causes (17%). Changing death rates from screenable cancers contributed minimally (2–3%) to rising inequality, while the spatial pattern of decline in death rates from HIV/AIDS over the period reduced inequality.
4. Discussion
We have shown in Fig. 3 that spatial inequality in mortality declines steadily with age above ages 35–39. One possible explanation for that decline is that causes of death with low levels of inequality, like screenable cancers, become more prominent as age advances relative to causes that are associated with behavior and exhibit more dispersion such as homicide, HIV/AIDS, and deaths from drugs/alcohol/suicide. That this is not a completely satisfactory answer is suggested by the fact that nearly all cause-of-death categories themselves show declines in inequality with increasing age in Table 1. Such declines are consistent with environmental influences on mortality that become less important relative to aging influences as age advances. In an article reviewing individual-level relations between socioeconomic status, health, and mortality, O'Rand and Lynch (2018) conclude that nearly every study finds that health disparities narrow with age. They refer to this relationship as “age as leveler”. Our results suggest that such a leveling process pertains to geographic disparities as well.
Although we document rising spatial inequality in mortality at most ages, trends vary by cause of death. We chose screenable cancers as an indicator of the quality of medical care since a large percentage of deaths from breast, prostate, colorectal, and cervical cancers can be prevented by timely diagnosis and proper treatment. The low spatial inequality in mortality from screenable cancers is one indicator that the quality of medical services is not a large source of regional disparity, at least relative to other factors at work. This result is consistent with Chetty et al.’s (2016) finding that life expectancy for individuals in the lowest quartile of income was not spatially correlated with measures of access to health care.
Given the coincidence of extraordinary increases in mortality from drugs/alcohol/suicide and the increases in spatial inequality in mortality that we have documented, one might expect the two phenomena to be closely related. While this category did contribute substantially to rising inequality at ages 30–34, geographic convergence occurred for this category at ages 50–54 and 70–74. One must look elsewhere for explanations of changing spatial divergence at these ages, which are associated with many more deaths.
Smoking is clearly an important driver of rising spatial inequality at older ages. This may seem surprising, given that smoking prevalence has declined in the United States. But trends in smoking prevalence are highly differentiated by geography, especially for women. The first national survey of US smoking behavior in 1955 showed that the prevalence of current smoking among women in urbanized areas of 1 + million was 27.8%, compared to only 9.4% in rural farm areas (Haenszel, Shimkin, & Miller, 1956: Table 14b). Women in the West were most likely to smoke and those in the Midwest and South least likely. The metropolitan and regional patterns are now reversed. In 2013–14, rural women were much more likely to smoke than urban women (Roberts et al., 2017) and women in the Midwest and South had the highest prevalence of current cigarette smoking (Jamal et al., 2015). It is clear that the trend lines for smoking prevalence by metropolitan status and region have crossed sometime during the past 60 years. Appendix A Fig. 2, which shows little differentiation in women's mortality rates at ages 50–54 from lung cancer and respiratory diseases among our four outliers in 1990 and rapid dispersion thereafter, helps to locate the approximate period of that cross-over.
Among men, differences in smoking prevalence by metropolitan type and region were much smaller in 1955 than among women; the regional range was only 45.2%–47.4% (Haenszel et al., 1956). By 2005, the West and Northeast had lower smoking prevalence than the Midwest and the South (Roberts et al., 2017). The faster decline in male smoking in the West and Northeast is reflected in their much more rapid mortality declines from lung cancer and respiratory diseases. Consistent with our results, Chetty et al. (2016) conclude that smoking prevalence is one of the strongest spatial correlates of life expectancy for low-income individuals.
We found that non-metropolitan areas have been lagging further behind large metro areas. While these results are consistent with work finding an increasing rural mortality disadvantage (Cosby et al., 2019; Singh & Siahpush, 2014; Stein et al., 2017), they also stand in contrast to other research documenting a rural advantage (Hayward et al., 1997; McLaughlin et al., 2001; Yang et al., 2011). One reason for this contradiction may be that the superior performance of large metro areas is a relatively recent phenomenon. While male life expectancy in large central metros in the Mid-Atlantic, for example, was 40th out of 40 in 1991, it was catapulted to 6th place in 2015 (and from 38th to 3rd place among women). Large metro areas in other regions also made considerable strides, with both male and female life expectancy rising an average 11 places over the period (Appendix A Table 3).
Our findings regarding spatial inequality in US mortality are echoed in international comparisons. We find that spatial inequality in mortality is greatest in early to mid-adulthood. These are also the ages at which the US ranks most poorly relative to other OECD countries (Ho, 2013; Ho & Hendi, 2018). Our findings indicating the important role of smoking in US spatial inequality echo the result of a large international study of mortality at ages 50+, which concludes that the history of heavy smoking in the US is the single most important factor in producing the large US disadvantage in life expectancy at age 50 (National Research Council, 2011). And one area in which we find spatial mortality inequalities to be quite small, screenable cancers, is consistent with the US having the best trends among OECD countries in breast and prostate cancer mortality, a ranking that has been attributed to exceptionally aggressive screening and treatment in the US (Preston & Ho, 2011).
Rising spatial inequality in US mortality is coincident with rising mortality differentials by education and income (Chetty et al., 2016; Congressional Budget Office, 2008; Hayward, Hummer, & Sasson, 2015; Hendi 2015, 2017). The mortality patterns that we describe may also be connected to other spatial patterns. Many writers have referred to increasing social and cultural bifurcation between “coastal elites” and residents in “the heartland” (e.g., Chua, 2018). Our results indicate that mortality trends also adhere to this bifurcation.
The patterns that we describe are products of spatial variation in the factors that affect mortality. Our analysis of causes of death has shed light on some of the major influences, e.g., smoking at older ages and "deaths of despair" at younger ages. But the growing magnitude of spatial variation in recent years should stimulate additional efforts to identify causal factors. Some of these will refer to characteristics of places (e.g., health facilities and industrial changes), while others will refer to characteristics of people who live in these places (e.g., the distribution of educational attainment). One especially interesting issue is how spatial disparities have evolved for different racial groups as mortality differences between blacks and whites have narrowed (Arias & Xu, 2018). The vast diversity of conditions in this broad ranging country, combined with effective national monitoring of its vital statistics, creates an ideal laboratory for investigating major influences on mortality and the length of life.
Ethical statement
The authors certify that this material has not been published in whole or in part elsewhere, the manuscript is not currently being considered for publication in another journal, and that all authors were involved in the creation of the present manuscript. The authors have no conflict of interests to disclose.
Acknowledgements
We thank Irma Elo, Jessica Ho, and Nick Graetz for helpful suggestions on earlier versions of this manuscript. We also thank four anonymous reviewers for their time and comments. This work was supported by grant #74439 from the Robert Wood Johnson Foundation; awards P30AG012836 and R01AG060115 (“Causes of Geographic Divergence in American Mortality between 1990 and 2015”) from the National Institute on Aging; and by the National Institutes of Health's Eunice Kennedy Shriver National Institute of Child Health and Human Development under award numbers T32HD007242, R24HD044964, and P2CHD047879.
Appendix A.
Appendix A Table 1.
Definitions of region and metropolitan status.
| Region | New England (CT, ME, MA, NH, RI, VT) |
| Middle Atlantic (NJ, NY, PA) | |
| East North Central (IL, IN, MI, OH, WI) | |
| West North Central (IA, KS, MN, MO, NE, ND, SD) | |
| South Atlantic (DE, DC, FL, GA, MD, NC, SC, VA) | |
| East South Central (AL, KY, MS, TN) | |
| West South Central (AR, LA, OK, TX) | |
| Mountain (AZ, CO, ID, MT, NV, NM, UT, WY) | |
| Pacific (AK, CA, HW, OR, WA) | |
| Appalachiaa | |
| Metro Statusb | Large central metro (counties of MSAs with a population of at least 1 million, including counties that contain all or a part of the area's inner cities) |
| Large metro suburb (surrounding counties of large central metro) | |
| Medium & small metro (counties with MSAs of 50,000–999,999 population) | |
| Non-metropolitan areas | |
MSA: Metropolitan Statistical Area.
a. As defined by the Appalachian Regional Commission and used by Elo et al. (2019). Appalachia includes all of WV and certain counties in AL, GA, KY, MD, MS, NY, NC, OH, PA, SC, TN, and VA. These counties are excluded from their overlapping census divisions.
b. Based on the NCHS metropolitan classification scheme (Ingram & Franco, 2014).
Appendix A Table 2.
Cause of death classifications.
| Cause of death | ICD-9 | ICD-10 |
|---|---|---|
| Breast, prostate, colorectal, and cervical cancers | 174-175, 180, 185, 153-154 | C50, C53, C61, C18–C21 |
| Circulatory diseases | 390-459 (excluding 425.5) | I00–I99 (excluding I42.6) |
| Drug overdose, alcohol-related causes, and suicide | E850–E858, E860, E950-E959, E962, E980.0–E980.5, 291, 303, 305.0, 357.5, 425.5, 535.3, 571.0–571.3, 790.3 | E24.4, F10, G31.2, G62.1, G72.1, I42.6, K29.2, K70, K85.2, K86.0, R78.0, X40–X45, X60–X85, Y10–Y15, Y870.0 |
| HIV/AIDS | 042–044 | B20–B24 |
| Homicide* | E960-E969 (excluding E962) | X86–Y09, Y87.1 |
| Lung cancer and respiratory diseases (excl. influenza and pneumonia) | 162, 460–519 (excluding 480–487) | C33, C34, J00–J98 (excluding J09–J18) |
| Mental and nervous system disorders, including Alzheimer's Disease | 290-389 (excluding 291, 303, 305.0, 357.5) | F01–F99 (excluding F10), G00–G98 (excluding G31.2, G62.1, G72.1) |
| Other external causes | E800-E999 (excluding E850–E858, E860, E950–E969, E980.0–E980.5) | V01–Y89 (excluding X40-X45, X60-Y15, Y87.0-Y87.1) |
| All other causes | 001-289 (excluding 042–044, 174–175, 180, 185, 153–154), 520–799 (excluding 535.3, 571.0–571.3, 790.3) | A00–E90 (excluding B20–B24, C33, C34, C50, C53, C61, C18–C21, E24.4), G99, H00–H93, J09-J18, J99, K00–R99 (excluding K29.2, K70, K85.2, K86.0, R78.0), U00–U99, Y90–Y98 |
* Except assault by drugs, medicaments, and biological substances, which is included in drug overdose.
Appendix A Table 3.
Values and ranking of life expectancy at birth and age-specific mortality rates for 40 spatial units.
| Region | Metro |
Life expectancy at birth (years) |
Age-specific mortality rate (per 10,000) |
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Ages 30–34 |
Ages 50–54 |
Ages 70–74 |
|||||||||||||||||||||||
|
1991 |
2003 |
2015 |
1991 |
2003 |
2015 |
1991 |
2003 |
2015 |
1991 |
2003 |
2015 |
||||||||||||||
| Value | Rank | Value | Rank | Value | Rank | Value | Rank | Value | Rank | Value | Rank | Value | Rank | Value | Rank | Value | Rank | Value | Rank | Value | Rank | Value | Rank | ||
| Appalachia | Large Central Metro | 71.15 | 30 | 72.62 | 34 | 73.94 | 34 | 23 | 27 | 15 | 20 | 27 | 40 | 82 | 29 | 79 | 34 | 82 | 34 | 453 | 32 | 365 | 31 | 297 | 30 |
| Appalachia | Large Metro Suburb | 72.16 | 23 | 74.03 | 28 | 75.71 | 28 | 16 | 10 | 12 | 11 | 22 | 31 | 76 | 24 | 63 | 20 | 58 | 17 | 453 | 31 | 365 | 30 | 296 | 28 |
| Appalachia | Small Metro | 71.59 | 27 | 73.19 | 31 | 74.27 | 33 | 21 | 22 | 18 | 31 | 23 | 33 | 77 | 26 | 74 | 28 | 80 | 33 | 466 | 35 | 376 | 33 | 317 | 33 |
| Appalachia | Nonmetro | 71.09 | 31 | 72.50 | 35 | 73.33 | 37 | 21 | 23 | 21 | 36 | 26 | 39 | 85 | 34 | 77 | 30 | 91 | 39 | 478 | 36 | 394 | 35 | 338 | 35 |
| East North Central | Large Central Metro | 70.01 | 35 | 73.07 | 33 | 75.03 | 31 | 27 | 36 | 18 | 29 | 18 | 20 | 89 | 36 | 78 | 33 | 70 | 30 | 466 | 34 | 370 | 32 | 305 | 31 |
| East North Central | Large Metro Suburb | 73.54 | 11 | 75.85 | 9 | 77.33 | 14 | 15 | 5 | 12 | 9 | 17 | 18 | 57 | 4 | 51 | 6 | 50 | 7 | 425 | 21 | 342 | 23 | 259 | 19 |
| East North Central | Small Metro | 72.89 | 17 | 74.87 | 19 | 75.79 | 27 | 16 | 11 | 13 | 15 | 20 | 26 | 63 | 10 | 62 | 16 | 63 | 24 | 435 | 25 | 347 | 25 | 286 | 25 |
| East North Central | Nonmetro | 72.86 | 18 | 74.79 | 22 | 75.65 | 29 | 16 | 8 | 14 | 17 | 21 | 28 | 67 | 18 | 59 | 14 | 62 | 23 | 432 | 23 | 351 | 26 | 297 | 29 |
| East South Central | Large Central Metro | 69.93 | 36 | 71.62 | 38 | 73.41 | 36 | 26 | 35 | 21 | 35 | 21 | 29 | 83 | 31 | 90 | 39 | 85 | 35 | 495 | 38 | 437 | 40 | 343 | 37 |
| East South Central | Large Metro Suburb | 71.75 | 26 | 73.71 | 29 | 75.21 | 30 | 17 | 13 | 16 | 26 | 23 | 35 | 68 | 20 | 70 | 27 | 70 | 29 | 463 | 33 | 398 | 37 | 309 | 32 |
| East South Central | Small Metro | 70.30 | 34 | 72.27 | 37 | 73.51 | 35 | 24 | 31 | 22 | 38 | 24 | 36 | 87 | 35 | 82 | 36 | 86 | 36 | 509 | 40 | 400 | 38 | 342 | 36 |
| East South Central | Nonmetro | 69.46 | 38 | 70.93 | 40 | 71.60 | 40 | 29 | 38 | 26 | 40 | 25 | 38 | 93 | 37 | 93 | 40 | 98 | 40 | 503 | 39 | 422 | 39 | 383 | 40 |
| Middle Atlantic | Large Central Metro | 68.57 | 40 | 74.74 | 23 | 78.07 | 6 | 43 | 40 | 15 | 19 | 13 | 2 | 104 | 40 | 78 | 31 | 57 | 16 | 443 | 27 | 336 | 19 | 254 | 14 |
| Middle Atlantic | Large Metro Suburb | 73.86 | 4 | 76.67 | 3 | 78.82 | 3 | 17 | 15 | 11 | 2 | 15 | 10 | 57 | 5 | 49 | 3 | 44 | 2 | 409 | 15 | 315 | 10 | 232 | 3 |
| Middle Atlantic | Small Metro | 73.45 | 12 | 75.56 | 12 | 76.78 | 19 | 16 | 9 | 13 | 12 | 17 | 15 | 65 | 16 | 58 | 10 | 54 | 12 | 418 | 18 | 327 | 16 | 272 | 22 |
| Middle Atlantic | Nonmetro | 73.09 | 15 | 75.45 | 13 | 77.36 | 12 | 17 | 14 | 10 | 1 | 15 | 11 | 64 | 15 | 53 | 7 | 52 | 8 | 418 | 20 | 318 | 13 | 257 | 17 |
| Mountain | Large Central Metro | 72.69 | 20 | 74.82 | 21 | 77.40 | 11 | 22 | 26 | 16 | 24 | 16 | 14 | 76 | 25 | 67 | 24 | 56 | 13 | 382 | 6 | 309 | 6 | 253 | 12 |
| Mountain | Large Metro Suburb | 75.06 | 1 | 76.82 | 1 | 79.03 | 2 | 15 | 6 | 12 | 10 | 14 | 4 | 49 | 1 | 44 | 1 | 44 | 1 | 367 | 2 | 296 | 2 | 199 | 1 |
| Mountain | Small Metro | 73.72 | 6 | 75.45 | 14 | 76.94 | 18 | 18 | 18 | 16 | 25 | 20 | 24 | 64 | 14 | 63 | 18 | 64 | 25 | 387 | 7 | 307 | 4 | 248 | 8 |
| Mountain | Nonmetro | 72.67 | 21 | 74.35 | 24 | 75.82 | 26 | 23 | 28 | 23 | 39 | 24 | 37 | 64 | 13 | 63 | 19 | 65 | 26 | 376 | 3 | 309 | 5 | 255 | 15 |
| New England | Large Central Metro | 72.28 | 22 | 75.22 | 16 | 77.44 | 10 | 22 | 25 | 14 | 18 | 14 | 5 | 68 | 21 | 70 | 25 | 56 | 15 | 412 | 16 | 339 | 21 | 242 | 6 |
| New England | Large Metro Suburb | 74.54 | 2 | 76.68 | 2 | 78.21 | 5 | 14 | 2 | 12 | 8 | 20 | 23 | 52 | 2 | 49 | 4 | 45 | 4 | 393 | 10 | 317 | 12 | 238 | 5 |
| New England | Small Metro | 73.65 | 9 | 76.25 | 5 | 77.55 | 9 | 18 | 16 | 11 | 4 | 21 | 27 | 61 | 6 | 54 | 8 | 53 | 9 | 392 | 9 | 311 | 8 | 248 | 9 |
| New England | Nonmetro | 73.74 | 5 | 76.19 | 7 | 77.00 | 16 | 14 | 3 | 12 | 7 | 22 | 32 | 66 | 17 | 47 | 2 | 54 | 11 | 418 | 19 | 339 | 20 | 238 | 4 |
| Pacific | Large Central Metro | 72.83 | 19 | 76.58 | 4 | 79.65 | 1 | 24 | 29 | 12 | 6 | 10 | 1 | 69 | 22 | 59 | 13 | 45 | 3 | 376 | 4 | 291 | 1 | 221 | 2 |
| Pacific | Large Metro Suburb | 73.67 | 8 | 76.20 | 6 | 78.55 | 4 | 17 | 12 | 13 | 13 | 13 | 3 | 62 | 8 | 56 | 9 | 47 | 6 | 393 | 11 | 310 | 7 | 245 | 7 |
| Pacific | Small Metro | 73.69 | 7 | 75.68 | 11 | 77.35 | 13 | 19 | 19 | 13 | 16 | 15 | 8 | 64 | 12 | 60 | 15 | 58 | 18 | 379 | 5 | 319 | 14 | 252 | 11 |
| Pacific | Nonmetro | 72.95 | 16 | 74.83 | 20 | 75.98 | 24 | 20 | 20 | 19 | 32 | 19 | 22 | 63 | 11 | 65 | 23 | 69 | 28 | 358 | 1 | 306 | 3 | 256 | 16 |
| South Atlantic | Large Central Metro | 69.84 | 37 | 73.49 | 30 | 76.76 | 20 | 36 | 39 | 17 | 28 | 16 | 12 | 95 | 38 | 82 | 35 | 60 | 21 | 426 | 22 | 346 | 24 | 273 | 23 |
| South Atlantic | Large Metro Suburb | 73.15 | 14 | 75.42 | 15 | 77.63 | 7 | 20 | 21 | 15 | 21 | 17 | 16 | 67 | 19 | 62 | 17 | 53 | 10 | 388 | 8 | 312 | 9 | 250 | 10 |
| South Atlantic | Small Metro | 71.81 | 25 | 74.08 | 27 | 75.90 | 25 | 24 | 32 | 18 | 30 | 20 | 25 | 84 | 32 | 78 | 32 | 74 | 31 | 396 | 12 | 317 | 11 | 262 | 20 |
| South Atlantic | Nonmetro | 69.17 | 39 | 71.44 | 39 | 73.16 | 38 | 26 | 34 | 21 | 37 | 21 | 30 | 97 | 39 | 88 | 38 | 87 | 37 | 494 | 37 | 394 | 36 | 345 | 38 |
| West North Central | Large Central Metro | 71.30 | 29 | 75.05 | 17 | 76.49 | 22 | 25 | 33 | 13 | 14 | 16 | 13 | 77 | 27 | 64 | 21 | 60 | 22 | 432 | 24 | 341 | 22 | 259 | 18 |
| West North Central | Large Metro Suburb | 73.97 | 3 | 76.16 | 8 | 77.62 | 8 | 14 | 4 | 11 | 3 | 15 | 9 | 54 | 3 | 50 | 5 | 47 | 5 | 408 | 14 | 334 | 18 | 254 | 13 |
| West North Central | Small Metro | 73.61 | 10 | 75.77 | 10 | 76.97 | 17 | 14 | 1 | 12 | 5 | 14 | 6 | 61 | 7 | 58 | 11 | 59 | 19 | 416 | 17 | 330 | 17 | 270 | 21 |
| West North Central | Nonmetro | 73.31 | 13 | 74.95 | 18 | 75.98 | 23 | 15 | 7 | 15 | 22 | 17 | 19 | 62 | 9 | 59 | 12 | 66 | 27 | 401 | 13 | 324 | 15 | 288 | 27 |
| West South Central | Large Central Metro | 70.66 | 33 | 74.27 | 26 | 76.70 | 21 | 29 | 37 | 16 | 27 | 15 | 7 | 83 | 30 | 70 | 26 | 59 | 20 | 443 | 26 | 362 | 27 | 286 | 26 |
| West South Central | Large Metro Suburb | 72.11 | 24 | 74.31 | 25 | 77.02 | 15 | 18 | 17 | 15 | 23 | 17 | 17 | 71 | 23 | 64 | 22 | 56 | 14 | 446 | 29 | 362 | 28 | 273 | 24 |
| West South Central | Small Metro | 71.54 | 28 | 73.07 | 32 | 74.52 | 32 | 21 | 24 | 19 | 33 | 19 | 21 | 78 | 28 | 77 | 29 | 80 | 32 | 449 | 30 | 364 | 29 | 320 | 34 |
| West South Central | Nonmetro | 71.01 | 32 | 72.44 | 36 | 73.05 | 39 | 24 | 30 | 20 | 34 | 23 | 34 | 84 | 33 | 84 | 37 | 87 | 38 | 446 | 28 | 387 | 34 | 361 | 39 |
Appendix A Table 4.
Percentage of variation in life expectancy associated with region or metro status.
| Year | Region | Metro Status | |
|---|---|---|---|
| Males | |||
| 2003 | 69.0 | 19.0 | |
| 2016 | 63.3 | 26.1 | |
| Change 2003–2016 | 36.1 | 46.1 | |
| Females | |||
| 2003 | 77.7 | 9.5 | |
| 2016 | 64.5 | 27.2 | |
| Change 2003–2016 | 35.1 | 54.3 | |
The percentage of variation is the r-squared value from bivariate regressions of life expectancy at birth on region/metro status in a given year, by sex.
Appendix A Fig. 1.
Index of Dissimilarity in all-cause mortality by age in 1990–1992 (panel A), 2002–2004 (panel B), and 2014–2015 (panel C), using counties' metro status in 1990, 2006, and 2013.
Appendix A Fig. 2.
Lung cancer and respiratory disease mortality trends at ages 50–54.
E. S. Central: East South Central; Mid Atl: Mid Atlantic.
Appendix B.
Age and Cause of Death Decomposition of the Increase in Variance in Life Expectancy Across 40 Spatial Units, 2000–2016
In this appendix we decompose the increase in variance in life expectancy at birth across the 40 spatial units between 2000 and 2016. The variance decomposition method used is similar to that used by Timonin et al. (2016). Their method first computes the variance in life expectancy and then substitutes the national average death rate for each geographic unit's death rate for a given age and cause of death. After this substitution, the variance in life expectancy is re-computed. The difference between this new variance and the variance before substitution is the contribution of a specific age and cause of death grouping to total variance. This substitution approach is then systematically applied to each age and cause of death grouping with total variance being recomputed at each step. The change in the computed variance with each step is the contribution of that age and cause of death grouping to total variance.
This approach will yield different results depending on the order in which age and cause of death groupings are selected for substitution. Substituting, for example, lung cancer at ages 60–64 prior to circulatory diseases at ages 55–59 will generally yield different results than following the reverse order when substituting. Timonin et al. suggest computing this decomposition for every possible permutation of cause of death groupings and in ascending age order and then reporting the average of all of the decompositions as the final decomposition. This method has the advantage of being independent of cause of death ordering. Its main limitation is the computational complexity involved in computing the decomposition when there are many causes of death. For example, to compute the decomposition of the change in variance between two time periods in our data would require over 1 billion life expectancy calculations.
We instead compute a similar but less computationally demanding two-stage decomposition. The two stages are (1) a series of independent decompositions (i.e., decompositions involving one cause of death at a time) and (2) a sequence of dependent decompositions (i.e., replacing each cause of death sequentially as described in the paragraphs above). Our decomposition uses the same stepwise substitution method described above, but instead of computing the decomposition for all possible cause of death permutations, we compute it for only one permutation. This permutation is determined by first substituting out one cause of death at a time and then ranking causes of death by their independent contributions to total variance. We substitute the residual category last. For example, we find that lung cancer and respiratory diseases, when considered independently of other causes of death, made the largest contribution to variance for both men and women, so in the stepwise substitution decomposition we replace lung cancer and respiratory disease mortality first. We compute the stepwise substitution decomposition for each cause of death and each age group in ascending age order. In other words, we first substitute lung cancer and respiratory diseases for age 0, then circulatory diseases for age 0, and so on, until we have exhausted all causes of death. Then we move on to perform the substitutions at ages 1–4, 5–9, …, and finally ages 85+, iterating through the 9 causes of death for each age group. The ordering of causes of death for the decomposition is: lung cancer and respiratory diseases, circulatory diseases, Alzheimer's and mental/nervous system disesases, other external injuries, drugs/alcohol/suicide, homicide, screenable cancers, HIV/AIDS, and all other causes (the residual category). The end result is a decomposition of the change in variance in life expectancy into 19 age groups cross-classified with 9 cause of death groups for each sex.
Results
Our findings for men and women are reported in Appendix B Table 1, Appendix B Table 2, respectively. These tables report how much of the increase between 2000 and 2016 in variance in life expectancy across our 40 geographic units is due to each age/cause of death grouping. The contributions sum to 100%. For scale, variance increased for men by 1.09 units (from 2.51 to 3.60) and for women by 1.63 units (from 1.20 to 2.83).
In short, the variance decomposition findings mirror the mortality inequality findings reported in the main text. The largest contributions to increases in variance occur at ages 30–74 for both men and women. Increases in variance are attributable mostly to mortality from the residual category, lung cancer and respiratory diseases, and circulatory diseases. The “other external” category makes the next largest contribution. The drugs, alcohol, and suicide category makes a smaller contribution, while screenable cancers make a contribution of almost zero. Alzheimer's and mental/nervous system diseases and homicide make contributions that lie in the middle. Improvements in HIV/AIDS mortality appear to have reduced variance in life expectancy (i.e., they make negative contributions). The single largest age-cause contribution is circulatory disease mortality at ages 50–54 for women (3.3% of the total variance) and ages 55–59 for men (4.5% of the total increase in variance).
Overall, these results are highly suggestive of smoking-attributable mortality being a major contributor to increases in geographic inequality in life expectancy. The increases load most heavily on lung cancer and respiratory diseases and circulatory diseases at ages 50–74. These are precisely the ages and causes of death that are most associated with mortality from smoking. Also notable is that the single largest age group contribution to the increase in variance comes from ages 50–54 for women. This is precisely the age group singled out by Case and Deaton (2017) in support of their “deaths of despair” thesis. However, the causes of death contributing to increases in variance at these ages aren't drugs, alcohol, and suicide, but are instead the causes associated most with cigarette smoking: lung cancer, respiratory diseases, and circulatory diseases. This same pattern is evident for men at ages 50–54 and 55–59. The largest contribution to inequality from drugs, alcohol, and suicide comes at ages 30–34 for men and 35–39 for women, contributing to the relatively large increases in inequality among younger adults.
Appendix B Table 1.
Age and cause of death decomposition of change in variance in life expectancy at birth across 40 spatial units, 2000–2016. Males.
| Age x | Lung cancer, resp. dis. | Circulatory diseases | Alz, mental, nervous system | Other external | Drugs, alcohol, suicide | Homicide | Screenable cancers | HIV/AIDS | All others | TOTAL |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | −0.83% | 0.22% | −0.35% | 2.02% | 0.11% | −0.58% | 0.00% | 0.00% | −1.59% | −1% |
| 1 | 0.30% | 0.11% | 0.00% | −0.63% | 0.01% | −0.04% | 0.00% | −0.05% | 0.89% | 1% |
| 5 | 0.08% | −0.02% | 0.01% | −0.09% | 0.00% | 0.08% | 0.00% | −0.02% | 0.27% | 0% |
| 10 | −0.04% | −0.34% | 0.30% | 0.49% | 0.45% | −0.28% | −0.03% | 0.02% | −0.21% | 0% |
| 15 | −0.13% | 0.15% | −0.27% | 1.52% | 1.04% | 0.45% | 0.01% | 0.03% | −0.03% | 3% |
| 20 | −0.29% | −0.31% | −0.11% | 1.31% | 1.05% | 0.01% | −0.07% | −0.25% | 0.06% | 1% |
| 25 | −0.24% | 0.59% | −0.18% | 0.61% | 1.62% | 0.71% | −0.07% | −0.79% | 0.71% | 3% |
| 30 | 0.41% | 0.50% | 0.00% | 2.75% | 4.10% | 1.18% | 0.03% | −2.08% | 1.70% | 9% |
| 35 | 0.41% | 3.48% | 0.73% | 2.28% | 2.05% | 0.88% | 0.00% | −2.50% | 2.86% | 10% |
| 40 | −0.47% | 1.69% | 0.23% | 1.61% | 1.98% | 1.05% | 0.02% | −1.41% | 1.89% | 7% |
| 45 | −0.44% | 1.91% | 0.42% | 1.51% | 1.68% | 0.06% | 0.31% | −0.91% | 3.33% | 8% |
| 50 | 1.75% | 3.46% | 0.42% | 1.60% | 1.54% | 0.04% | 0.16% | −0.16% | 5.88% | 15% |
| 55 | 2.48% | 4.48% | 0.33% | 0.82% | 0.45% | 0.22% | 0.74% | −0.23% | 5.60% | 15% |
| 60 | 2.17% | 2.74% | 0.31% | 0.79% | −0.32% | 0.03% | 0.56% | −0.17% | 4.61% | 11% |
| 65 | 1.31% | 1.92% | 0.49% | 0.28% | −0.23% | 0.00% | 0.26% | −0.05% | 2.20% | 6% |
| 70 | 2.56% | 1.09% | 0.57% | 0.15% | 0.05% | 0.02% | −0.12% | −0.02% | 1.62% | 6% |
| 75 | 1.48% | 0.05% | 0.47% | 0.06% | 0.01% | −0.01% | −0.13% | −0.01% | 0.97% | 3% |
| 80 | 0.71% | 0.33% | 0.41% | 0.09% | −0.01% | 0.00% | −0.02% | 0.00% | 0.53% | 2% |
| 85+ | 0.73% | 0.54% | 0.47% | 0.03% | 0.00% | 0.00% | −0.01% | 0.00% | 0.26% | 2% |
| TOTAL | 12% | 23% | 4% | 17% | 16% | 4% | 2% | −9% | 32% | 100% |
Appendix B Table 2.
Age and cause of death decomposition of change in variance in life expectancy at birth across 40 spatial units, 2000–2016. Females.
| Age x | Lung cancer, resp. dis. | Circulatory diseases | Alz, mental, nervous system | Other external | Drugs, alcohol, suicide | Homicide | Screenable cancers | HIV/AIDS | All others | TOTAL |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.05% | 0.15% | −0.05% | 0.51% | 0.01% | 0.02% | 0.00% | 0.00% | 3.43% | 4% |
| 1 | −0.05% | 0.15% | −0.16% | 0.14% | 0.03% | −0.05% | 0.00% | 0.01% | 0.50% | 1% |
| 5 | −0.06% | −0.04% | 0.14% | 0.84% | −0.02% | −0.10% | 0.00% | −0.04% | 0.10% | 1% |
| 10 | −0.05% | −0.08% | 0.09% | 0.25% | 0.01% | 0.03% | −0.02% | 0.00% | 0.17% | 0% |
| 15 | 0.02% | −0.07% | 0.16% | 0.97% | −0.26% | 0.11% | −0.02% | −0.02% | 0.31% | 1% |
| 20 | 0.25% | 0.02% | 0.17% | 0.95% | 0.09% | 0.23% | −0.04% | −0.07% | 0.56% | 2% |
| 25 | 0.38% | 0.36% | −0.01% | 0.68% | 0.84% | 0.19% | 0.06% | −0.44% | 0.93% | 3% |
| 30 | 0.15% | 0.99% | 0.55% | 1.12% | 2.02% | 0.04% | 0.38% | −0.28% | 1.86% | 7% |
| 35 | 0.33% | 1.73% | 0.50% | 1.11% | 2.58% | 0.15% | 0.41% | 0.03% | 2.98% | 10% |
| 40 | 0.51% | 2.69% | 0.74% | 0.87% | 1.52% | 0.04% | −0.02% | −0.02% | 2.51% | 9% |
| 45 | 1.55% | 2.41% | 0.43% | 0.46% | 1.39% | 0.08% | 0.25% | 0.02% | 3.10% | 10% |
| 50 | 3.13% | 3.27% | 0.42% | 0.65% | 0.78% | 0.07% | 0.63% | 0.00% | 3.57% | 13% |
| 55 | 2.95% | 2.35% | 0.49% | 0.44% | 0.22% | 0.00% | 0.38% | −0.03% | 3.26% | 10% |
| 60 | 2.50% | 1.71% | 0.60% | 0.20% | −0.18% | 0.01% | 0.36% | −0.01% | 2.53% | 8% |
| 65 | 2.58% | 1.05% | 0.62% | 0.09% | −0.09% | 0.02% | 0.21% | −0.03% | 2.06% | 7% |
| 70 | 2.76% | 0.84% | 0.68% | 0.18% | −0.04% | 0.00% | 0.22% | −0.01% | 1.59% | 6% |
| 75 | 1.73% | 0.13% | 0.86% | 0.10% | −0.01% | 0.00% | 0.11% | −0.01% | 0.89% | 4% |
| 80 | 0.97% | 0.19% | 1.06% | 0.05% | −0.01% | 0.00% | 0.03% | 0.00% | 0.45% | 3% |
| 85+ | 0.66% | 0.64% | 1.27% | 0.04% | 0.00% | 0.00% | 0.00% | 0.00% | 0.34% | 3% |
| TOTAL | 20% | 19% | 9% | 10% | 9% | 1% | 3% | −1% | 31% | 100% |
References
- Arias E., Xu J. United States life tables 2015. National Vital Statistics Reports. 2018;67(7) [PubMed] [Google Scholar]
- Association of American Medical Colleges 2017 state physician workforce data report. 2017. https://store.aamc.org/2017-state-physician-workforce-data-report.html Retrieved from.
- Case A., Deaton A. Rising morbidity and mortality in midlife among white non-hispanic americans in the 21st century. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(49):15078–15083. doi: 10.1073/pnas.1518393112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case A., Deaton A. Mortality and morbidity in the 21st century. Brookings Papers on Economic Activity. 2017:397–476. doi: 10.1353/eca.2017.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles K., Hurst E., Schwartz M. 2018. The transformation of manufacturing and the decline in U.S. employment. National Bureau of Economic Research Working Paper 24468. Cambridge, Mass. [Google Scholar]
- Chetty R., Stepner M., Abraham S., Lin S., Scuderi B., Turner N.…Cutler D. The association between income and life expectancy in the United States, 2001-2014. Jama-Journal of the American Medical Association. 2016;315(16):1750–1766. doi: 10.1001/jama.2016.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakis N.A., Fowler J.H. The spread of obesity in a large social network over 32 years. New England Journal of Medicine. 2007;357(4):370–379. doi: 10.1056/NEJMsa066082. [DOI] [PubMed] [Google Scholar]
- Christakis N.A., Fowler J.H. The collective dynamics of smoking in a large social network. New England Journal of Medicine. 2008;358(21):2249–2258. doi: 10.1056/NEJMsa0706154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua A. Penguin Press; New York: 2018. Political tribes: Group instinct and the fate of nations. [Google Scholar]
- Congressional Budget Office . 2008. Growing disparities in life expectancy. [Google Scholar]
- Cosby A.G., McDoom-Echebiri M.M., James W., Khandekar H., Brown W., Hanna H.L. Growth and persistence of place-based mortality in the United States: The rural mortality penalty. American Journal of Public Health. 2019;109(1):155–162. doi: 10.2105/AJPH.2018.304787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalziel B.D., Kissler S., Gog J.R., Viboud C., Bjornstad O.N., Metcalf C.J.E. Urbanization and humidity shape the intensity of influenza epidemics in US cities. Science. 2018;362(6410):75–79. doi: 10.1126/science.aat6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elo I.T., Hendi A.S., Ho J.Y., Vierboom Y.V., Preston S.H. Trends in non-Hispanic white mortality in the United States by metropolitan nonmetropolitan status and region, 1990-2016. Population and Development Review. 2019;45(3):549–583. doi: 10.1111/padr.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenelon A. Geographic divergence in mortality in the United States. Population and Development Review. 2013;39(4):611–634. doi: 10.1111/j.1728-4457.2013.00630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg G.M., Edejer T.T., Lauer J.A., Sepulveda C. Screening, prevention and treatment of cervical cancer: A global and regional generalized cost-effectiveness analysis. Vaccine. 2009;27(43):6060–6079. doi: 10.1016/j.vaccine.2009.07.026. [DOI] [PubMed] [Google Scholar]
- Ginsberg G.M., Lim S.S., Lauer J.A., Johns B.P., Sepulveda C.R. Prevention, screening and treatment of colorectal cancer: A global and regional generalized cost effectiveness analysis. Cost Effectiveness and Resource Allocation. 2010;8(2):1–16. doi: 10.1186/1478-7547-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenszel W., Shimkin M., Miller H. United States Department of Health, Education, and Welfare; Washington, DC: 1956. Public health monograph no. 45: Tobacco smoking patterns in the United States. [PubMed] [Google Scholar]
- Harper S., Lynch J. National Cancer Institute; Bethesda, MD: 2005. Methods for measuring cancer disparities: Using data relevant to healthy people 2010 cancer-related objectives. [Google Scholar]
- Hayward M.D., Hummer R.A., Sasson I. Trends and group differences in the association between educational attainment and US adult mortality: Implications for understanding education's causal influence. Social Science & Medicine. 2015;127:8–18. doi: 10.1016/j.socscimed.2014.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward M.D., Pienta A.M., McLaughlin D.K. Inequality in men's mortality: The socioeconomic status gradient and geographic context. Journal of Health and Social Behavior. 1997:313–330. [PubMed] [Google Scholar]
- Heijnsdijk E.A.M., de Carvalho T.M., Auvinen A., Zappa M., Nelen V., Kwiatkowski M.…de Koning H.J. Cost-effectiveness of prostate cancer screening: A simulation study based on ERSPC data. Journal of the National Cancer Institute. 2015;107(1):dju366. doi: 10.1093/jnci/dju366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendi A.S. Trends in U.S. Life expectancy gradients: The role of changing educational composition. International Journal of Epidemiology. 2015;44(3):946–955. doi: 10.1093/ije/dyv062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendi A.S. Trends in education-specific life expectancy, data quality, and shifting education distributions: A note on recent research. Demography. 2017;54(3):1203–1213. doi: 10.1007/s13524-017-0574-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron M. Leading causes for 2016. National Vital Statistics Reports. 2018;67(6) (National Center for Health Statistics) [PubMed] [Google Scholar]
- Ho J.Y. Mortality under age 50 accounts for much of the fact that US life expectancy lags that of other high-income countries. Health Affairs. 2013;32(3):459–467. doi: 10.1377/hlthaff.2012.0574. [DOI] [PubMed] [Google Scholar]
- Ho J.Y., Hendi A.S. Recents trends in life expectancy across high income countries: Retrospective observational study. BMJ. 2018;362 doi: 10.1136/bmj.k2562. k2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi S., Coale Ansley J. A simple equation for estimating the expectation of life at old ages. Population Studies. 1982;36(2):317–326. doi: 10.1080/00324728.1982.10409034. [DOI] [PubMed] [Google Scholar]
- Iceland J., Weinberg D.H., Steinmetz E. U.S. Government Printing Office; Washington, DC: 2002. U.S. Census bureau, series CENSR-3, racial and ethnic residential segregation in the United States: 1980-2000. [Google Scholar]
- Ingram D.D., Franco S.F. 2013 NCHS urban-rural classification scheme for counties. National Center for Health Statistics Vital Health Statistics. 2014;2(166) [PubMed] [Google Scholar]
- Jamal A., Homa D.M., O'Connor E., Babb S.D., Caraballo R.S., Singh T.…King B.A. Current cigarette smoking among adults--United States 2005-2014. Centers for Disease Control and Prevention Morbidity and Mortality Weekly Report. 2015;64(44):1233–1240. doi: 10.15585/mmwr.mm6444a2. [DOI] [PubMed] [Google Scholar]
- King N.B., Harper S., Young M.E. Use of relative and absolute effect measures in reporting health inequalities: Structured review. British Medical Journal. 2012;345 doi: 10.1136/bmj.e5774. e5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenbach J., Kunst A. Measuring the magnitude of socio-economic inequalities in health: An overview of available measures illustrated with two examples from Europe. Social Science & Medicine. 1997;44(6):757–771. doi: 10.1016/s0277-9536(96)00073-1. [DOI] [PubMed] [Google Scholar]
- McLaughlin D.K., Stokes C.S., Nonoyama A. Residence and income inequality: Effects on mortality among US counties. Rural Sociology. 2001;66(4):579–598. [Google Scholar]
- National Research Council . Explaining divergent levels of longevity in high-income countries. In: Crimmins E.M., Preston S.H., Cohen B., editors. The National Academies Press; Washington, DC: 2011. (Panel on understanding divergent trends in longevity in high-income countries. Committee on population, division of behavioral and social sciences and education). [PubMed] [Google Scholar]
- O'Rand A.M., Lynch S.M. Socioeconomic status, health and mortality in aging populations. In: Hayward M.D., Majmundar M.K., editors. Future directions for the demography of aging. The National Academies Press; Washington, DC: 2018. [PubMed] [Google Scholar]
- Plevritis S.K., Munoz D., Kurian A.W., Stout N.K., Alagoz O., Near A.M.…Mandelblatt J.S. Association of screening and treatment with breast cancer mortality by molecular subtype in US women, 2000-2012. Jama-Journal of the American Medical Association. 2018;319(2):154–164. doi: 10.1001/jama.2017.19130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston S.H., Elo I.T. Anatomy of a municipal triumph: New York city's upsurge in life expectancy. Population and Development Review. 2014;40(1):1–29. doi: 10.1111/j.1728-4457.2014.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston S.H., Guillot M., Heuveline P. Blackwell Publishers; Malden, MA: 2001. Demography: Measuring and modeling population processes. [Google Scholar]
- Preston S.H., Ho J.Y. Low life expectancy in the United States: Is the health care system at fault? In: Crimmins E.M., Preston S.H., Cohen B., editors. International differences in mortality at older ages: Dimensions and sources. National Academies Press; Washington, DC: 2011. pp. 259–298. [PubMed] [Google Scholar]
- Regidor E. Measures of health inequalities: Part 1. Journal of Epidemiology & Community Health. 2004;58(10):858–861. doi: 10.1136/jech.2003.015347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M.E., Doogan N.J., Stanton C.A., Quisenberry A.J., Villanti A.C., Gaalema D.E.…Higgins S.T. Rural versus urban use of traditional and emerging tobacco products in the United States, 2013-2014. American Journal of Public Health. 2017;107(10):1554–1559. doi: 10.2105/AJPH.2017.303967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh G.K., Siahpush M. Widening rural-urban disparities in life expectancy, US, 1969-2009. American Journal of Preventive Medicine. 2014;46(2):E19–E29. doi: 10.1016/j.amepre.2013.10.017. [DOI] [PubMed] [Google Scholar]
- Stein E.M., Gennuso K.P., Ugboaja D.C., Remington P.L. The epidemic of despair among white Americans: Trends in the leading causes of premature death, 1999-2015. American Journal of Public Health. 2017;107(10):1541–1547. doi: 10.2105/AJPH.2017.303941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timonin S., Shkolnikov V.M., Jasilionis D., Grigoriev P., Jdanov D.A., Leon D.A. Disparities in length of life across developed countries: Measuring and decomposing changes over time within and between country groups. Population Health Metrics. 2016;14(29) doi: 10.1186/s12963-016-0094-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinetti M.E., McAvay G.J., Murphy T.E., Gross C.P., Lin H., Allore H.G. Contribution of individual diseases to death in older adults with multiple diseases. Journal of the American Geriatric Society. 2012;60(8):1448–1456. doi: 10.1111/j.1532-5415.2012.04077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Department of Agriculture (USDA) Rural employment and unemployment. 2017. https://www.ers.usda.gov/topics/rural-economy-population/employment-education/rural-employment-and-unemployment/ Accessed 09/18.
- Yang T.C., Jensen L., Haran M. Social capital and human mortality: Explaining the rural paradox with county-level mortality data. Rural Sociology. 2011;76(3):347–374. doi: 10.1111/j.1549-0831.2011.00055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]