Abstract
There exist a large number of cohort studies that have been used to identify genetic and biological risk factors for developing Alzheimer's disease (AD). However, there is a disagreement between studies as to how strongly these risk factors affect the rate of progression through diagnostic groups toward AD. We have calculated the probability of transitioning through diagnostic groups in six studies and considered how uncertainty around the strength of the effect of these risk factors affects estimates of the distribution of individuals in each diagnostic group in an AD clinical trial simulator. In this work, we identify the optimal choice of widely collected variables for comparing data sets and calculating probabilities of progression toward AD. We use the estimated transition probabilities to inform stochastic simulations of AD progression that are based on a Markov model and compare predicted incidence rates to those in a community-based study, the Cardiovascular Health Study.
Keywords: Epidemiology, Dementia, Statistical analysis, Mild cognitive impairment, Mixed regression, Modeling
1. Introduction
Alzheimer's disease (AD) is a progressive, neurodegenerative disease that is characterized clinically by a progressive decline in cognitive function. To date, clinical trials of AD therapies have been largely unsuccessful [1]. Several factors are thought to contribute to these failures, including incorrect target selection and poor trial design [1], [2]. As AD is a condition associated with the aging population, disease-modifying treatments aimed at slowing the rate of progression toward AD would have considerable impact in reducing the social and economic burden of this disease worldwide.
Mathematical and statistical models of AD progression are increasingly focused on modeling the disease as a continuum of cognitive states toward AD [3], [4], [5], [6]. We have previously presented a 3-state model that describes disease progression from cognitively normal (CN) to a mild cognitively impaired (MCI) state and an AD state [7]. In addition, other models have been developed to include substates of mild, moderate, or severe AD and also those that focus on biomarker [8] or cognitive and functional states [4], [5]. Most of these models have been developed to describe progression rates using transition probabilities that have been derived from a single data set using a range of different statistical and epidemiological methods. However, there remains a need for convincing results, which have been generated from individual studies that are applicable to other data sets or to a general population. A meta-analysis by Neu et al. [9] compared the odds ratios for the risk of developing disease with the risk factors such as age, sex, and apolipoprotein E4 (APOE ε4) from 27 independent research studies and found wide differences in the estimates for developing MCI and AD. Furthermore, a study by Qian et al. [10] which compared risk estimates for the incidence of MCI or dementia among cognitively unimpaired individuals stratified by APOE ε4 and age found that, while the effect of age and APOE ε4 was consistent between studies, there were differences in the cumulative incidence of AD between the data sets.
In this study, we calculate and compare transition probabilities from six data sets namely the Australian Imaging, Biomarkers and Lifestyle study (AIBL), AddNeuroMed, Alzheimer's Disease NeuroImaging study (ADNI), BIOCARD: Predictors of Cognitive Decline Among Normal Individuals (BIOCARD), the Framingham Heart Study (FHS), and National Alzheimer's Coordinating Center (NACC), which represent 22,993 individuals with 2639 reported cases of dementia when combined. We use these probabilities to inform stochastic simulations of AD progression that are based on a Markov model. In particular, we simulate the progression from CN to AD in an artificial population and compare the results to a subset of the Cardiovascular Heart Study (CHS) cohort. The key strength of this work is the calculation of transition probabilities from a large number of data sets using identical data analysis protocols. This allows us to eliminate heterogeneity in results that arise as a result of using different statistical methods between data sets.
2. Methods
2.1. Data sets used
2.1.1. Alzheimer's Disease NeuroImaging study
The ADNI was launched in 2003 with the goal of testing whether serial magnetic response imaging (MRI), positron emission tomography, other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression to the disease states MCI and AD. The study data available in this analysis consist of 1737 individuals with data acquired at multiple sites across the United States and Canada and are composed of three stages of recruitment: ADNI1, ADNIGO, and ADNI2. One of the primary goals of the ongoing development of the ADNI database is to enable investigators to define the progression of AD for individuals at risk with a lack of cognitive or functional concerns. The data used in this study were downloaded on October 31, 2016.
2.1.2. National Alzheimer's Coordinating Center
The NACC database was set up in 1999 and is based out of approximately 39 Alzheimer's Disease Centers across the United States. Participant-enrollment methods at Alzheimer's Disease Centers include but are not limited to clinic samples, public recruitment methods, participant referrals, other ongoing studies, and, occasionally, population-based samples. Data are available for 9927 individuals and were collected in a cumulative database across a range of measures including clinical assessments, MRI scans, and other markers of neuropathology [11]. The present study is based on data that have been recorded between September 2005 and June 2016.
2.1.3. Framingham Heart Study
The FHS was established as a prospective cohort study in 1948 with an original cohort of 5209 individuals that were sampled randomly to be representative of the community. These individuals have undergone up to 32 examinations that include physical examinations and laboratory testings [12]. Two further cohorts were sampled in 1971 and 2002, which recruited 5124 offspring and spouses of offspring of the original cohort, as well as the children of the offspring cohort. Surveillance for dementia began in 1975, and individuals were seen at regular intervals of between 2 to 4 years, details of which are published elsewhere [13], [14]. The data/analyses presented in the current publication are based on the use of study data downloaded from the dbGaP Web site, under phs000007.v30.p1 (https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000007.v30.p1)
2.1.4. BIOCARD
The BIOCARD study was conceptualized to investigate the predictors of cognitive decline among normal individuals. The study was designed to investigate variables that could predict future progression to AD among CN individuals [15]. With this end, extensive neuropsychological, MRI, cerebrospinal fluid, and blood markers were recorded longitudinally for 349 individuals. The study has been underway since 1995 and is currently being led by investigators at John Hopkins University after a break in funding between 2005 and 2009. By design, the cohort is middle-aged, and approximately three quarters of individuals had a family history of dementia at enrollment.
2.1.5. Australian Imaging, Biomarkers and Lifestyle study
The AIBL study follows a prospective Australian cohort of more than 1000 individuals living in Melbourne and Perth while collecting extensive neuropsychological, imaging (both MRI and positron emission tomography), and biological markers of AD over more than 126 months [16]. The aim of this study is to identify the predictive value of biomarkers, cognitive variables, and lifestyle factors for future progression to AD with a focus on early detection and building toward lifestyle interventions. Data were collected by the AIBL study group. AIBL study methodology has been reported previously [16].
2.1.6. AddNeuroMed
The AddNeuroMed study was established in 2006 and enrolled 781 individuals at baseline. The primary goal of AddNeuroMed has been to assess longitudinal MRI changes in AD, MCI, and healthy controls using image-acquisition protocols compatible with those used in ADNI [17]. To do so, individuals are followed up and diagnosed as CN/MCI/AD longitudinally at 3- to 12-month intervals at centers across 6 European countries—Finland, Italy, Greece, Poland, France, and the UK [18].
2.1.7. Cardiovascular Heart Study
The CHS is a prospective study that was designed primarily to investigate and quantify the association between previously known and hypothesized risk factors, with coronary heart disease and stroke in community-dwelling adults aged 65 years and older in the United States [19]. Individuals included in the CHS were randomly sampled from medical records in the US cities of Pittsburgh, Sacramento, Hagerstown, and Winston-Salem. The cohort consists of 5888 individuals of which 687 comprise the cohort of African-Americans who were recruited in 1992–1993. At baseline, clinical examinations and in-person interviews in the home using a series of standard questionnaires were conducted. The status of dementia was assessed from the completion of a cranial MRI and The Modified Mini-Mental State (3MS) Examination assessment in 1992–1994 onwards [20]. Participants were followed up annually at clinic visits and semiannually with telephone interviews thereafter, up to and including year 11 of the study (1998–1999). A further wave of follow-up took place in year 18 as part of the ancillary All Stars study of the remaining 1677 survivors of the CHS cohort which was designed to assess risk factors associated with healthy physical and cognitive aging.
2.2. Estimation of transition probabilities and odds ratios
Transition probabilities and odds ratios of the likelihood of transitioning to a more severe diagnostic state were calculated, taking account of three key confounding variables namely gender, APOE ε4 carrier status, and age. We used a generalized linear mixed model (GLMM) with a logit link function to estimate the odds ratios associated with progressing to a more severe disease state with which we were able to calculate the probabilities of doing so (transition probabilities). The model took the form
where pij is the probability of participant j transitioning backwards at observation i; β0 is the mean intercept and βk is the log odds ratio associated with a one unit increase in variable xk; represents a specific deviance from β0 for individual j which accounts for the variability in the likelihood of transitioning between individuals; represents the random error that accounts for the variability within individuals. Hence, parameters and represent the variance between and within individuals, respectively.
Models were based on three factors that were recorded (in a comparable manner) between the six data sets; age (continuous, centered at 50 years and scaled by a factor of 100), gender, APOE ε4 carrier status (defined dichotomously as homozygous/heterozygous). Additional terms were included in the model to account for the current diagnostic state to differentiate between CN > MCI and MCI > AD transition probabilities using the same model and the time between visits (centered at 12 months) that we normalized to 12 months when calculating the transition probabilities from odds ratios. The R package R2MLwiN was used to estimate the model parameters using Monte-Carlo Markov Chains and goodness of fit statistics [21]. To identify the combination of variables that fits best across data sets, we explored a number of models (Table 1) and based the model-selection procedure on the deviance information criterion. The model with the smallest deviance information criterion was selected and used to calculate transition probabilities [22].
Table 1.
Model inclusion table
| Variable | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 |
|---|---|---|---|---|---|---|
| TBV | • | • | • | • | • | • |
| DX | • | • | • | • | • | • |
| APOE ε4 | • | • | • | • | • | |
| Gender | • | • | • | • | • | |
| Age | • | • | • | |||
| Age^2 | • | • | • | |||
| Age^3 | • | • | ||||
| Age:DX | • | • | • | |||
| Age^2:DX | • | • | • | |||
| Age^3:DX | • | • |
NOTE. Variables included in each of the six models.
Abbreviations: APOE, apolipoprotein E; TBV, time between consecutive visits; DX, current diagnostic group, interaction between two variables.
2.3. Incorporation of transition probabilities in a stochastic individual–based model of AD
We developed a stochastic individual-based model of AD from an existing Markov model of disease progression described in the study by Hadjichrysanthou et al. [7]. In the model presented here, the transition probabilities are updated at every time step (time step t was set at t = 0.001 yrs) and are calculated by application of the inverse logit function to the estimated odds ratios, which themselves are based on age, gender, current diagnostic state, and APOE ε4 carrier status. On initialization, a random intercept term is drawn from an distribution for each patient, where is the variance of the random intercept term in the fitted mixed-effects model. The full repertoire of β coefficients in the mixed-effect model was sampled 200 times from the stable Monte-Carlo Markov Chain estimates for each data set, and 500 simulations were performed with each parameter set to eliminate aleatory uncertainty. Simulations were performed using 1000 simulated patients for a total of 15 years (Fig. 1). In each simulation, patient demographics were drawn from the distributions of the baseline population of the CHS study.
Fig. 1.
Steps toward the development of a clinical trial simulator for AD. Abbreviations: AD, Alzheimer's disease; MCMC, Monte-Carlo Markov Chains.
3. Results
3.1. Comparing risk estimates and predicting the rate of progression through clinical states toward AD in multiple data sets
We identified eight risk factors for AD that had previously been used in other studies to calculate the rates of progression to be considered in our analysis. These variables were time between visits, age, Age^2, Age^3, APOE ε4 carrier status, gender, and current diagnostic group (CN or MCI). We used GLMMs to estimate the odds ratios for progression to the next (more severe disease) state within the data sets using six distinct combinations of the available variables (Table 1) and estimating their parameters using each of the six data sets (Supplementary Table 1). Although it is widely considered to be a modifier of risk, education was excluded from our study due to inconsistencies in data availability and methodology of measuring level of education within the different data sets. However, of the five data sets in which education could be compared, no significant association was found with the rate of transition (Supplementary Table 2). We used the model deviance to select the model that was the best universal fit to the data sets. For data sets AIBL, AddNeuroMed, BIOCARD, and FHS, the model with the lowest deviance contained time between visits, gender, diagnostic group, age^2, and the interaction between ageˆ2 and diagnostic group (Supplementary Table 1, Model 3). For ADNI and NACC, the best fitting model contained time between visits, gender, diagnostic group, age, and the interaction between age and the diagnostic group (Supplementary Table 1, Model 2). For ADNI and NACC, the relative difference in deviance between model 2 and model 3 was minimal compared with the other data sets. Thus, to preserve homogeneity between analysis methods, we determined that model 3 was the most appropriate model for calculating the 12-month probability of transitioning toward AD, in the multiple data sets that were examined. It should be noted that deviances could not be statistically compared since the models were not nested.
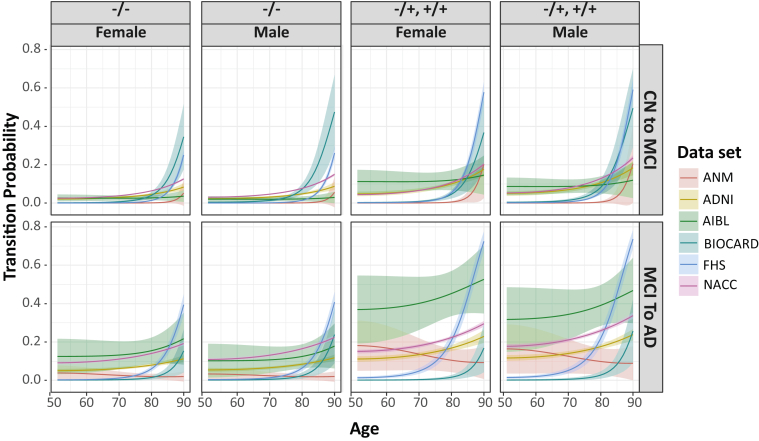
Subsequently, we calculated transition probabilities for rates of progression toward AD using model 3. In this model, the term representing time between visits significantly increased the likelihood of transitioning to a more severe diagnostic state in the data sets that had a longer average time span between visits (i.e., in ADNI, BIOCARD, FHS, and NACC [P < .001]). The odds ratio for an increase of one year in the time-between-visits variable in these four data sets ranged from 1.24 in FHS to 1.87 in ADNI (Supplementary Table 1). The probability of progressing toward AD increased with Age^2, and as expected, the highest odds ratio in all data sets was associated with the Age^2 term; however, this was significantly reduced in individuals who were already diagnosed with MCI. In other words, individuals are more likely to transition from MCI to AD than they are from CN to MCI (i.e., acceleration of disease progression clinically). This effect appears to be less pronounced as individuals enter the oldest ages. APOE ε4 carriage (heterozygous or homozygous) confers significantly increased risk in every data set, except for AIBL where the odds ratio is positive but nonsignificant (odds ratio [OR], 1.138; P = .741). Only NACC demonstrated an effect of gender, with females less likely to transition to a more severe state (OR, 0.820; P < .001). The transition probabilities are displayed graphically in Fig. 2.
Fig. 2.
Transition probabilities in the data sets. Shown are the mean (line), and standard deviation (cloud), of transition probabilities from CN to MCI (top row) and MCI to AD (bottom row). Probabilities are stratified by age, gender, and APOE ε4 carrier status (−/−, noncarrier, +/− homozyte, +/+, heterozyte). Abbreviations: CN, cognitively normal; MCI, mild cognitively impaired; AD, Alzheimer's disease; APOE, apolipoprotein E; ADNI, Alzheimer's Disease Neuroimaging Initiative; AIBL, Australian Imaging Biomarkers and Lifestyle; ANM, AddNeuroMed; FHS, the Framingham Heart Study; NACC, National Alzheimer's Coordinating Center.
3.2. Comparing cumulative incidence of AD incidence between data sets using simulations of a standardized population of individuals
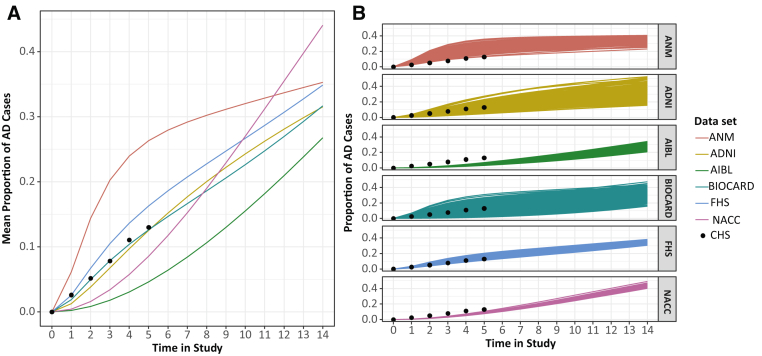
We used our clinical trial simulator to explore how incidence rates predicted in each data set differed in a standardized population. We first created a virtual population by sampling demographic variables using the baseline distribution from those in the CHS study who were CN at baseline and remained in the study for 6 years. This study was not included in our estimation of transition probabilities (Supplementary Table 3). Following this, we calculated 12-month transition probabilities, adjusting for the known risk factors that were shared between the cohorts, and the results are presented in Supplementary Table 1. The 10-year cumulative AD incidence rates in the simulations range from 15.4% using AIBL-derived probabilities to 32.0% using probabilities derived from AddNeuroMed. The biggest variation in incidence rate predictions came from AddNeuroMed and BIOCARD, and the smallest came from AIBL, FHS, and NACC (Fig. 3A).
Fig. 3.
Predicted incidence rates in a generalized population. Two hundred parameter samples from each data set were generated and simulated 500 times to produce a range of estimates of the incidence rate of AD in a population that was cognitively normal at baseline and had demographics similar to that from CHS. Shown are mean from each parameter sample (A) and population level means (B). Abbreviations: AD, Alzheimer's disease; ANM, AddNeuroMed; ADNI, Alzheimer's Disease Neuroimaging Initiative; AIBL, Australian Imaging Biomarkers and Lifestyle; FHS, the Framingham Heart Study; NACC, National Alzheimer's Coordinating Center.
We then compared these results to the actual incidence rates in the CHS population from which the baseline demographics were derived (Fig. 3B). We found that the predictions made using the AddNeuroMed-derived transition probabilities overestimated the incidence rate in a generalized population. The best predictions were made using ADNI-, BIOCARD-, and FHS-derived transition probabilities, with NACC and AIBL both underestimating the incidence rate.
4. Discussion
In this study, we have presented a comparison of the odds ratios and the rate of transitioning through the cognitive decline spectrum in AD for multiple data sets, using a standardized analysis method. Our results highlight the vast differences in odds ratios and 12-month transition probabilities determined from six major AD cohorts. However, it provides a tool or platform that can be used to further understand the reasons behind these differences.
We acknowledge that the studies considered here differ greatly in design especially in the criteria for subject recruitment. We, therefore, accept that methods that may be ideal for one study may be not optimal for another. However, the purpose of the present study was not to model transition probabilities as accurately as possible within specific studies but to begin eliminating the variability that is introduced using different methods for their calculation. Moreover, standardized analyses also highlight which design aspects and data characteristics of cohort studies should be considered when conducting more detailed analyses on a single study basis.
Qian et al. [10] have previously identified differences in odds ratios and cumulative incidence rates between studies through meta-analyses. However, the study could only estimate lifetime incidence for individuals that had a maximum age within the 80–85 years age band and only for two data sets. From the calculation of transition probabilities using continuous age as a risk factor, we found that the probability of developing AD increases substantially from age 80 in the three largest data sets, all of which had a large number of patients from individuals older than 80 years (Supplementary Fig. 1). We considered age (Age, Age^2, and Age^3), current diagnostic state (CN or MCI), APOE ε4 carrier status (positive for at least one allele or negative), and gender as potential risk factors for developing AD. These were based on measures that were shared between the data sets, and as expected, we identified age as the most significant risk factor. Combinations of Age, Age^2, and Age^3 were significantly associated with an increase in the risk of transitioning from CN to MCI in all data sets. However, the effect of age on the transition from MCI to AD is unclear because the interaction between age and diagnosis had differential effects, both between models and data sets under the same model. An important extension of this work would be to perform the same analysis using a well-defined metric of biological age such as those proposed by Belsky et al., Gott et al., and Petkovich et al. [23], [24], [25] in place of age. If the variables required for predicting biological age were measured in a variety of data sets, combined with exploring differential clinical endpoints, it should be possible to define suitable endpoints that could be agreed upon within the Alzheimer's research field.
The results presented here also demonstrate which of the six data sets analyzed are suitable for combining in a pooled analysis to increase sample size and therefore the robustness of inferences drawn from analyses. For example, ADNI, BIOCARD, and NACC have similar transition probabilities with respect to age.
By using simulation to extend these results, we found that the cumulative incidence of AD in a standardized population differed with a magnitude greater than the variability within cohort data sets (Fig. 3B). Of considerable interest is the observation that the probabilities generated from ADNI, a so-called “convenience cohort”, better predicted incidence in a standardized population than community-based studies such as FHS. A possible reason for this is that factors such as education and biomarker values that were not included in the model could be more similar between ADNI and CHS than between FHS and CHS; however, both data sets provide an adequate job estimate of the CHS probabilities. Furthermore, because we are using only individuals that are retained for 16 years in CHS rather than the full population, it is possible that the incidence rates reported are increased from what would be expected in a general population. Additionally, from the transition probabilities recorded in Fig. 2, it appears that individuals in the convenience cohort transition more quickly than individuals in FHS at younger ages but slower at older ages. Thus, it is plausible that the incidence rates in ADNI and FHS are similar because of a balance between the two sets of probabilities over time.
The statistical approach in using GLMMs rather than observed transition rates stratified by confounding factors increases sample size and allows for the inclusion of multiple risk factors both independently and combined. This approach also allows us to consider the population-level effects as well as quantify variance at the individual level. While we also acknowledge significant limitations to using diagnostic state as an endpoint for our analysis, it is important to note that there is no currently agreed upon gold standard endpoint in the AD field to use in its place as yet.
These results have important implications for both clinical trial design and public health policies. First, they highlight the degree to which incidence varies between seemingly similar populations. All the data sets included in this study comprised of individuals in the United States and Canada, demographic factors of which were accounted for in the calculation of the transition probabilities. Furthermore, this study highlights the considerable increase in the probability of developing AD in individuals older than 80 years compared with younger individuals. Another important conclusion arising from this work is that when predicting how interventions might impact the general population, a variety of data sets should be used to produce more reliable results of what is the background likelihood of transitioning between disease states in the control untreated group. For example, the work by Davis et al. [4] predicted that a 20% reduction in the number of MCI cases due to AD would translate to a 5% difference in AD cases in NACC. However, it is unclear if this would still be the case in a population such as FHS or ADNI where the rate of developing MCI was generally higher, but the rate of converting from MCI to AD was lower in older age groups.
An important future extension to this work would be to repeat this analysis with a wider range of data sets, incorporating some of the developing cohort studies from non-US sites such as Chariot-Pro (UK), the Gothenburg Study, and the Rotterdam cohort study [26].
Research in Context.
-
1.
Systematic review: The authors searched PubMed to identify previous research articles calculating or comparing incidence and prevalence of Alzheimer's Disease (AD) between Aging US cohorts.
-
2.
Interpretation: We have calculated the probability of transitioning toward AD in six studies and considered how the uncertainty around strength of these risk factors affects estimates of the distribution of individuals in each diagnostic group in an AD clinical trial simulator. We identify the optimal choice of widely collected variables for comparing data sets and calculating probabilities of progression toward AD and compare predicted incidence rates to those in a community-based study. This study highlights the degree to which incidence varies between seemingly similar populations and implies that when predicting how interventions might impact the general population, a variety of datasets should be used to produce more reliable results of what is the background likelihood of transitioning between disease states in the control untreated group.
-
3.
Future directions: An important future extension to this work would be to repeat this analysis with a wider range of datasets, incorporating some of the developing cohort studies from non-US sites such as Chariot-Pro (UK), the Gothenburg Study, and the Rotterdam cohort study.
Acknowledgments
Funding: This study was funded by the Janssen Prevention Center.
Alzheimer's Disease Neuroimaging Initiative (ADNI) is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following organizations: AbbVie; Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for NeuroImaging at the University of Southern California.
The Australian Imaging Biomarkers and Lifestyle (AIBL) study (www.AIBL.csiro.au) is a consortium between Austin Health, CSIRO, Edith Cowan University, the Florey Institute (The University of Melbourne), and the National Aging Research Institute. Partial financial support was provided by the Alzheimer's Association (US), the Alzheimer's Drug Discovery Foundation, an anonymous foundation, the Science and Industry Endowment Fund, the Dementia Collaborative Research Centres, the Victorian Government's Operational Infrastructure Support program, the McCusker Alzheimer's Research Foundation, the National Health and Medical Research Council, and the Yulgilbar Foundation. Numerous commercial interactions have supported data collection and analysis. In-kind support has also been provided by Sir Charles Gairdner Hospital, Cogstate Ltd., Hollywood Private Hospital, the University of Melbourne, and St. Vincent's Hospital.
The National Alzheimer's Coordinating Center (NACC) database is funded by National Institute on Aging (NIA)/National Institutes of Health (NIH) grant U01 AG016976. NACC data are contributed by the NIA-funded ADCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Steven Ferris, PhD), P30 AG013854 (PI M. Marsel Mesulam, MD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG016570 (PI Marie-Francoise Chesselet, MD, PhD), P50 AG005131 (PI Douglas Galasko, MD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P50 AG005136 (PI Thomas Montine, MD, PhD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD), and P50 AG047270 (PI Stephen Strittmatter, MD, PhD).
The Framingham Heart Study is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with Boston University (contract no.: N01-HC-25195 and HHSN268201500001I). This manuscript was not prepared in collaboration with investigators of the Framingham Heart Study and does not necessarily reflect the opinions or views of the Framingham Heart Study, Boston University, or NHLBI.
The BIOCARD study is supported by a grant from the National Institute on Aging: U19-AG03365. The BIOCARD Study consists of 7 Cores with the following members: (1) the Administrative Core (Marilyn Albert, Barbara Rodzon, Corinne Pettigrew), (2) the Clinical Core (Marilyn Albert, Anja Soldan, Rebecca Gottesman, Ned Sacktor, Scott Turner, Leonie Farrington, Maura Grega, Gay Rudow, Scott Rudow, Rostislav Brichko), (3) the Imaging Core (Michael Miller, Susumu Mori, Tilak Ratnanather, Timothy Brown, Hayan Chi, Anthony Kolasny, Kenichi Oishi, Laurent Younes), (4) the Biospecimen Core (Richard O'Brien, Abhay Moghekar, Jacqueline Darrow, Alexandria Lewis), (5) the Informatics Core (Roberta Scherer, David Shade, Ann Ervin, Jennifer Jones, Hamadou Coulibaly, April Broadnax, Lisa Lassiter), (6) the Biostatistics Core (Mei-Cheng Wang, Jiangxia Wang, Yuxin Zhu), and (7) the Neuropathology Core (Juan Troncoso, Olga Pletnikova, Gay Rudow, Karen Fisher). The authors would like to acknowledge the contributions to BIOCARD of the Geriatric Psychiatry Branch (GPB) of the intramural program of the National Institute of Mental Health who initiated the study (PI Dr. Trey Sunderland).
Data used in the preparation of this study were obtained from the AddNeuroMed study that was supported by InnoMed, (Innovative Medicines in Europe) an Integrated Project funded by the European Union of the Sixth Framework program priority FP6-2004-LIFESCIHEALTH-5, Life Sciences, Genomics and Biotechnology for Health, Health Research Council of Academy of Finland (HS), The Gamla Tjanarinnor Foundation, The Swedish Alzheimer's Association and Swedish Brain Power.
The CHS study was supported by contracts HHSN268201200036 C, HHSN268200800007 C, HHSN268201800001 C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, and N01HC85086 and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by grants R01AG023629, P50-AG005133, and R01-AG020098 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org.
Disclosures: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.trci.2019.04.005.
Supplementary Data
References
- 1.Mehta D., Jackson R., Paul G., Shi J., Sabbagh M. Why do trials for Alzheimer's disease drugs keep failing? A discontinued drug perspective for 2010–2015. Expert Opin Investig Drugs. 2017;26:735–739. doi: 10.1080/13543784.2017.1323868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson R.M., Hadjichrysanthou C., Evans S., Wong M.M. Why do so many clinical trials of therapies for Alzheimer's disease fail? Lancet. 2017;390:2327–2329. doi: 10.1016/S0140-6736(17)32399-1. [DOI] [PubMed] [Google Scholar]
- 3.Abner E.L., Kryscio R.J., Cooper G.E., Fardo D.W., Jicha G.A., Mendiondo M.S. 2012. Mild Cognitive Impairment: Statistical Models of Transition Using Longitudinal Clinical Data. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis M., O Connell T., Johnson S., Cline S., Merikle E., Martenyi F. Estimating Alzheimer's disease progression rates from normal cognition through mild cognitive impairment and stages of dementia. Curr Alzheimer Res. 2018;15:777–788. doi: 10.2174/1567205015666180119092427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Green C., Zhang S. Predicting the progression of Alzheimer's disease dementia: a multidomain health policy model. Alzheimers Dement. 2016;12:776–785. doi: 10.1016/j.jalz.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spackman D.E., Kadiyala S., Neumann P.J., Veenstra D.L., Sullivan S.D. Measuring Alzheimer disease progression with transition probabilities: estimates from NACC-UDS. Curr Alzheimer Res. 2012;9:1050–1058. doi: 10.2174/156720512803569046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hadjichrysanthou C., Ower A.K., de Wolf F., Anderson R.M., Alzheimer's Disease Neuroimaging Initiative The development of a stochastic mathematical model of Alzheimer's disease to help improve the design of clinical trials of potential treatments. PLoS One. 2018;13:e0190615. doi: 10.1371/journal.pone.0190615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jack C.R., Therneau T.M., Wiste H.J., Weigand S.D., Knopman D.S., Lowe V.J. Transition rates between amyloid and neurodegeneration biomarker states and to dementia: a population-based, longitudinal cohort study. Lancet Neurol. 2016;15:56–64. doi: 10.1016/S1474-4422(15)00323-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neu S.C., Pa J., Kukull W., Beekly D., Kuzma A., Gangadharan P. Apolipoprotein E genotype and sex risk factors for Alzheimer disease: a Meta-analysis. JAMA Neurol. 2017;74:1178–1189. doi: 10.1001/jamaneurol.2017.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qian J., Wolters F.J., Beiser A., Haan M., Ikram M.A., Karlawish J. APOE-related risk of mild cognitive impairment and dementia for prevention trials: an analysis of four cohorts. PLoS Med. 2017;14:e1002254. doi: 10.1371/journal.pmed.1002254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beekly D.L., Ramos E.M., Lee W.W., Deitrich W.D., Jacka M.E., Wu J. The National Alzheimer's Coordinating Center (NACC) database: the uniform data set. Alzheimer Dis Assoc Disord. 2007;21:249–258. doi: 10.1097/WAD.0b013e318142774e. [DOI] [PubMed] [Google Scholar]
- 12.Satizabal C.L., Beiser A.S., Chouraki V., Chêne G., Dufouil C., Seshadri S. Incidence of dementia over three decades in the Framingham Heart Study. N Engl J Med. 2016;374:523–532. doi: 10.1056/NEJMoa1504327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seshadri S., Wolf P.A., Beiser A., Au R., McNulty K., White R. Lifetime risk of dementia and Alzheimer's disease. The impact of mortality on risk estimates in the Framingham Study. Neurology. 1997;49:1498–1504. doi: 10.1212/wnl.49.6.1498. [DOI] [PubMed] [Google Scholar]
- 14.Seshadri S., Beiser A., Au R., Wolf P.A., Evans D.A., Wilson R.S. Operationalizing diagnostic criteria for Alzheimer's disease and other age-related cognitive impairment—Part 2. Alzheimers Dement. 2011;7:35–52. doi: 10.1016/j.jalz.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albert M., Soldan A., Gottesman R., McKhann G., Sacktor N., Farrington L. Cognitive changes preceding clinical symptom onset of mild cognitive impairment and relationship to ApoE genotype. Curr Alzheimer Res. 2014;11:773–784. doi: 10.2174/156720501108140910121920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellis K.A., Bush A.I., Darby D., De Fazio D., Foster J., Hudson P. The Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging: methodology and baseline characteristics of 1112 individuals recruited for a longitudinal study of Alzheimer's disease. Int Psychogeriatr. 2009;21:672–687. doi: 10.1017/S1041610209009405. [DOI] [PubMed] [Google Scholar]
- 17.Simmons Andrew, Westman Eric, Sebastian Muehlboeck, Patrizia Mecocci, Bruno Vellas, Magda Tsolaki. MRI Measures of Alzheimer's disease and the AddNeuroMed Study. Ann N Y Acad Sci. 2009;1180:47–55. doi: 10.1111/j.1749-6632.2009.05063.x. [DOI] [PubMed] [Google Scholar]
- 18.Simmons A., Westman E., Muehlboeck S., Mecocci P., Vellas B., Tsolaki M. The AddNeuroMed framework for multi-centre MRI assessment of Alzheimer's disease : experience from the first 24 months. Int J Geriatr Psychiatry. 2011;26:75–82. doi: 10.1002/gps.2491. [DOI] [PubMed] [Google Scholar]
- 19.Fried L.P., Borhani N.O., Enright P., Furberg C.D., Gardin J.M., Kronmal R.A. 1991. The Cardiovascular Health Study: Design and Rationale. [DOI] [PubMed] [Google Scholar]
- 20.Fitzpatrick A.L., Kuller L.H., Ives D.G., Lopez O.L., Jagust W., Breitner J.C.S. Incidence and prevalence of dementia in the Cardiovascular Health Study. J Am Geriatr Soc. 2004;52:195–204. doi: 10.1111/j.1532-5415.2004.52058.x. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z., Parker R., Charlton C., Leckie G., Browne W. R2MLwiN: A Package to Run MLwiN from within R. J Stat Softw Artic. 2016;72:1–43. [Google Scholar]
- 22.Lunn D, Jackson C, Best N, Thomas A, Spiegelhalter D. The BUGS book: a practical introduction to Bayesian analysis. Boca Raton, FL: Chapman and Hall/CRC; 2012.
- 23.Belsky D.W., Caspi A., Houts R., Cohen H.J., Corcoran D.L., Danese A. Quantification of biological aging in young adults. Proc Natl Acad Sci U S A. 2015;112:E4104–E4110. doi: 10.1073/pnas.1506264112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gott A., Andrews C., Larriva Hormigos M., Spencer K., Bateson M., Nettle D. Chronological age, biological age, and individual variation in the stress response in the European starling: a follow-up study. PeerJ. 2018;6:e5842. doi: 10.7717/peerj.5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petkovich D.A., Podolskiy D.I., Lobanov A.V., Lee S.-G., Miller R.A., Gladyshev V.N. Using DNA methylation profiling to evaluate biological age and longevity interventions. Cell Metab. 2017;25:954–960.e6. doi: 10.1016/j.cmet.2017.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hofman A., Grobbee D.E., de Jong P.T., van den Ouweland F.A. Determinants of disease and disability in the elderly: the Rotterdam Elderly Study. Eur J Epidemiol. 1991;7:403–422. doi: 10.1007/BF00145007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.