Abstract
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system (CNS) in which the immune system damages the protective insulation surrounding the nerve fibers that project from neurons. A hallmark of MS and its animal model, experimental autoimmune encephalomyelitis (EAE), is autoimmunity against proteins of the myelin sheath. Most studies in this field have focused on the roles of CD4+ T lymphocytes, which form part of the adaptive immune system as both mediators and regulators in disease pathogenesis. Consequently, the treatments for MS often target the inflammatory CD4+ T-cell responses. However, many other lymphocyte subsets contribute to the pathophysiology of MS and EAE, and these subsets include CD8+ T cells and B cells of the adaptive immune system, lymphocytes of the innate immune system such as natural killer cells, and subsets of innate-like T and B lymphocytes such as γδ T cells, natural killer T cells, and mucosal-associated invariant T cells. Several of these lymphocyte subsets can act as mediators of CNS inflammation, whereas others exhibit immunoregulatory functions in disease. Importantly, the efficacy of some MS treatments might be mediated in part by effects on lymphocytes other than CD4+ T cells. Here we review the contributions of distinct subsets of lymphocytes on the pathogenesis of MS and EAE, with an emphasis on lymphocytes other than CD4+ T cells. A better understanding of the distinct lymphocyte subsets that contribute to the pathophysiology of MS and its experimental models will inform the development of novel therapeutic approaches.
Keywords: Multiple sclerosis, Experimental autoimmune encephalomyelitis, Innate lymphoid cells, Innate-like lymphocytes, Innate-like T and B cells, Adaptive lymphocytes
Subject terms: Autoimmunity, Innate lymphoid cells
Introduction
Multiple sclerosis (MS) is a chronic autoimmune disease caused by demyelination of the neurons in the central nervous system (CNS), which results in highly variable symptoms, such as muscle weakness, gait difficulties, fatigue, visual disturbances, and loss of coordination and speech, and might ultimately cause paralysis.1–3 Approximately 2.5 million people worldwide are affected by this disease and the incidence of MS is two- to threefold higher in women than in men. MS can take multiple forms and the most common presentation of MS, which is referred to as relapsing–remitting MS, involves defined attacks of new or increasing neurological symptoms that are followed by periods of partial or complete recovery. Progressive MS, which can occur following the onset of the disease (primary progressive MS) or after an initial relapsing–remitting course (secondary progressive MS), is characterized by a progressive worsening of neurological function over time. The treatments of MS aim to reduce inflammation and return function after an acute attack to the baseline levels, to modify the disease course for the prevention of future attacks, and to manage the diverse symptoms of MS.4
Although the etiology of MS remains unknown, it is thought that this disease occurs in genetically predisposed individuals following exposure to an environmental trigger.5 Genome-wide association studies have revealed a critical role of immune factors in disease pathogenesis and many of the genes associated with MS susceptibility are also observed in other autoimmune diseases, such as type 1 diabetes, rheumatoid arthritis, and Crohn’s disease.6,7 Environmental factors that can predispose individuals to MS include low levels of vitamin D, smoking, obesity, and infection with Epstein–Barr virus.8,9
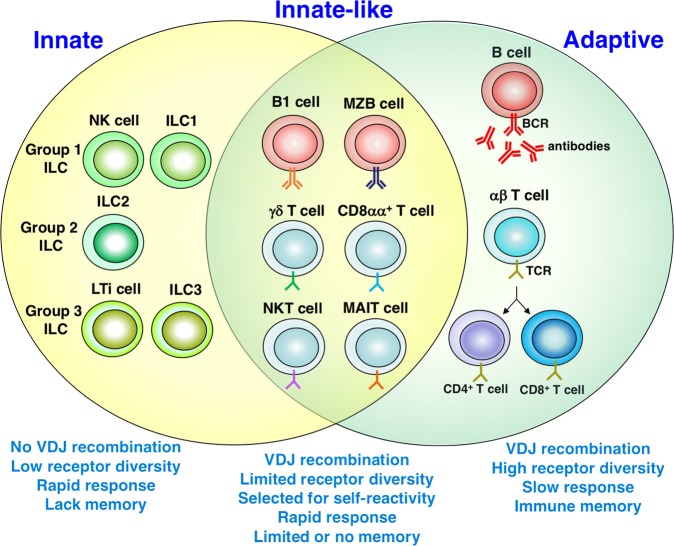
The pathogenesis of MS is mediated primarily by T cells.1,10 T cells are primed to CNS autoantigens in the periphery and then cross the blood–brain barrier to activate microglia and macrophages. In concert, these cells induce the death of myelin-producing oligodendrocytes and directly damage the myelin sheath around nerve fibers to generate active lesions in the CNS. The key role of T cells, particularly CD4+ T cells, in MS has been confirmed in experimental autoimmune encephalomyelitis (EAE), which is the main animal model of MS and can be induced in a variety of mammalian species through immunization with myelin antigens accompanied by adjuvant or through the adoptive transfer of myelin-reactive T cells.11 Studies on MS and EAE have largely focused on CD4+ T cells and many treatments for MS are based on targeting these cells. However, many other lymphocyte subsets have been implicated in disease pathogenesis12,13 and these subsets include lymphocytes other than CD4+ T cells that belong to the adaptive immune system, lymphocytes that belong to the innate immune system, and innate-like B and T lymphocytes that exhibit properties of both innate and adaptive immunity (Fig. 1). Several of these cell types infiltrate the CNS of MS patients, have been implicated in the efficacy of disease-modifying treatments, and contribute to the pathogenesis of EAE. Here we review the role of distinct subsets of innate, innate-like, and adaptive lymphocytes in the pathogenesis of MS and EAE, with an emphasis on cells other than CD4+ T cells.
Fig. 1.
Subsets of innate, innate-like, and adaptive lymphocytes. The main subsets of innate, innate-like, and adaptive lymphocytes, and their key properties. Abbreviations: BCR B-cell receptor, ILC innate lymphoid cell, LTi lymphoid tissue inducer, MAIT mucosal-associated invariant T, MZB marginal zone B, NK natural killer, NKT natural killer T, TCR T-cell receptor, VDJ variable diversity joining
Adaptive lymphocytes
In addition to CD4+ T cells, which have been the main focus of studies on MS and EAE, the lymphocytes of the adaptive immune system include CD8+ T cells and B cells. Although each of these cell types can mediate disease, they also contain regulatory subsets that can suppress disease.14
CD4+ T cells
CD4+ T cells are found in the brain lesions of patients with MS.15,16 The key role of these cells as effectors in disease was confirmed by the identification of human leukocyte antigen (HLA)-DR15 as the key genetic factor associated with disease susceptibility.17 In addition, CD4+ T cells have a critical role in the pathogenesis of EAE. The susceptibility of different mouse strains to EAE is correlated with their major histocompatibility complex (MHC) haplotype. The adoptive transfer of CD4+ T cells from mice immunized with myelin antigens can often induce disease in recipient animals. Both interferon (IFN)-γ-producing T helper (Th) 1 and interleukin (IL)-17-producing Th17 cells are pathogenic, and most studies have indicated that Th17 cells are the main culprits.1,10 In EAE, these T cells are specific for peptides derived from immunizing myelin antigens, which include myelin basic protein (MBP), proteolipid protein, and myelin oligodendrocyte glycoprotein,18 and these target antigens might also have a role in MS.1,10 Although the frequency of circulating myelin-reactive CD4+ T cells in patients with MS is similar to that in healthy individuals, the T cells from MS patients exhibit a pro-inflammatory phenotype characterized by Th17 cytokines19 and often exhibit spontaneous proliferation ex vivo.20
Considering the critical role of CD4+ T cells in mediating CNS inflammation, it was surprising that a phase II trial with a monoclonal anti-CD4 antibody failed to reduce MS activity.21 This failure might have been due, in part, to the critical role of CD4+Foxp3+ regulatory T cells (Tregs) in suppressing autoreactive T-cell responses.22,23 Tregs produce the anti-inflammatory cytokines IL-10 and transforming growth factor (TGF)-β, which suppress myelin-reactive CD4+ T cells. Consistent with their suppressive role in disease progression, CD4+ Tregs from MS patients exhibit impaired functions24 and myelin-specific T cells from patients show a reduced capacity to produce IL-10 compared with those obtained from healthy controls.19
CD8+ T cells
One of the main findings identifying CD8+ T cells as effectors in MS is that these cells are found in MS lesions at greater numbers than CD4+ T cells.15,16,25 Furthermore, although MHC class II alleles are the main genetic factors that predispose individuals to MS, MHC class I alleles have also been associated with MS.26–29 HLA-A3 and -B7 are associated with a higher risk of MS, whereas HLA-A2 is associated with a reduced risk of MS. These findings suggest that CD8+ T cells might have pathogenic and protective functions in MS.
The CD8+ T cells in the CNS lesions and cerebrospinal fluid (CSF) of MS patients exhibit oligoclonal expansions,30–32 suggesting antigen-driven selection. These cells express granzyme B, which suggests that they have cytotoxic activity, and produce the pro-inflammatory cytokines IFN-γ and IL-17.33–35 One challenge in interpreting these findings is that some of the CD8+ T cells investigated in these studies likely represent MR1-restricted mucosal-associated invariant T (MAIT) cells expressing CD8α chains (see below).36,37
The adoptive transfer of myelin antigen-specific CD8+ T cells in the EAE model induces disease in the recipient animals and causes CNS lesions and symptoms that are more similar to those found in human disease than those observed in CD4+ T-cell-mediated EAE.38,39 Similar to the results found for CD4+ T cells, IL-17 was the relevant pathogenic cytokine produced by myelin-reactive CD8+ T cells. Furthermore, IL-17-producing CD8+ T cells are required for the development of Th17-cell-mediated EAE, which suggests the involvement of critical interactions between pathogenic CD8+ and CD4+ T cells in disease progression.40
The possibility that CD8+ T cells, which have a pathogenic role, might also have suppressive functions in CNS autoimmunity is reinforced by the finding that the depletion of CD8+ T cells before the induction of EAE in mice results in disease exacerbation.41 This result was further supported by studies with MHC class I- and CD8α-deficient animals, which developed more severe disease than wild-type animals.42–44 In some studies, neuroantigen-specific CD8+ T cells were shown to suppress EAE induction and even ameliorate established EAE upon adoptive transfer through a mechanism involving the direct lysis of pathogenic CD4+ T cells.43,45 The majority of regulatory CD8+ Tregs responsible for these effects are restricted by classical MHC class I molecules. However, CD8+ Tregs restricted by the nonclassical MHC class I molecule Qa-1 that protect against EAE have also been described.46 In some of these studies, Qa-1-restricted CD8+ Tregs mediate their effects by recognizing peptides derived from specific T-cell receptor (TCR) Vβ molecules on myelin antigen-reactive CD4+ T cells and thereby directly cause their lysis.47,48 In addition, some Qa-1-restricted CD8+ T cells react with myelin-derived peptide antigens.49 In mice, Qa-1-restricted CD8+ Tregs are induced during treatment with glatiramer acetate,50 which is a peptide that mimics MBP and an Food and Drug Administration-approved MS treatment. The disease-ameliorating effects of glatiramer acetate are dependent on Qa-1-restricted CD8+ Tregs.50
CD8+ T cells with regulatory functions in MS were identified many years ago.51–53 Phenotypically distinct subsets of CD8+ Tregs have been identified in humans and these include IL-10-producing cells and cells with cytotoxic properties.51,54 Some of these cells might be restricted by the nonclassical MHC class I protein HLA-E, which is the functional homolog of murine Qa-1. Glatiramer acetate induces HLA-E-restricted CD8+ Tregs in treated patients and these cells have strong suppressive potential against CD4+ T cells.55
B cells
B cells, which are best known for their capacity to produce antibodies, infiltrate MS lesions in the CNS.56,57 Many of these cells have a memory phenotype and are found in cellular immune aggregates that resemble tertiary lymphoid structures.58 The abnormal presence of antibodies in the CNS represents the most consistent feature of patients with MS.59,60 These antibodies show evidence of clonotypic expansion, which suggests an antigen-driven process. Some of these antibodies are directed against myelin antigens, which can cause demyelination and axonal damage in a complement-dependent manner.61,62 In some patients, the removal of antibodies by plasmapheresis and immunoadsorption is beneficial, which suggests that antibodies can contribute to disease pathogenesis.63,64 However, the finding that B-cell depletion with the anti-CD20 antibody rituximab limits relapses in MS but does not influence abnormalities in CSF antibodies65 indicates that antibody-independent roles of B cells are involved in the beneficial effects of B-cell depletion.66 Indeed, an important role of B cells is the presentation of antigens to T lymphocytes in the context of MHC class II.67 Memory B cells in MS patients can efficiently present neuronal antigens to T cells.68 A recent study identified RASGRP2, a guanine nucleotide exchange factor expressed by B cells and enriched in the brain, as an autoantigen presented by memory B cells in the periphery to self-reactive brain-homing T cells,69 which suggests that B cell/T cell interactions outside of the brain might induce pathogenic T cells that can then enter the brain to attack CNS-resident antigens.70 Another function of B cells is cytokine production and subsets of pro-inflammatory B cells that produce cytokines such as tumor necrosis factor (TNF)-α, lymphotoxin α, IL-6, and granulocyte-macrophage colony-stimulating factor (GM-CSF), as well as anti-inflammatory regulatory B cells (Bregs) that produce cytokines such as IL-10, IL-35, and/or TGF-β have been identified.71,72 Multiple B-cell populations with regulatory properties have been described62 and these include cells with phenotypic markers of immature or mature B cells, plasma cells, or B1a cells, which constitute a subset of innate-like B cells (see below). In MS, B cells appear to be polarized toward a pro-inflammatory phenotype and defects in Bregs have been observed, at least in some studies.73 Some MS therapies, including IFN-β, glatiramer acetate, and fingolimod (FTY720, which sequesters lymphocytes in lymph nodes), are associated with an anti-inflammatory shift in B-cell cytokine responses,73 which suggests that the balance between pro- and anti-inflammatory B-cell activities is relevant to disease pathogenesis and treatment success.
In EAE, B cells have distinct pathogenic or regulatory functions, depending on the stage of disease.74 B-cell-deficient mice exhibit an exacerbated disease course, which is due to the regulatory functions of B cells and their capacity to produce IL-10.75,76 B-cell depletion using anti-CD20 antibodies before EAE induction causes exacerbated disease and this effect is associated with the loss of IL-10-producing Bregs.77 However, B-cell depletion after EAE induction ameliorates disease severity due to the pathogenic role of memory B cells, which facilitate T-cell reactivation during the later disease stages.77–81 In adoptive transfer studies, Bregs reduce EAE initiation but not ongoing EAE disease.82 Similarly, the adoptive transfer of Bregs expanded ex vivo from splenic B cells through the provision of CD40 and IL-21 receptor signals can inhibit disease, even in mice with established disease.83 In similar experiments, Bregs induced in vitro or in vivo from bone marrow cells through the provision of Toll-like receptor (TLR) 9 signals can protect against disease when administered at the onset of clinical symptoms.84,85 Bregs have also been implicated in the beneficial effects of glatiramer acetate against EAE.86,87 In addition to IL-10, other mechanisms have been proposed for the suppressive functions of Bregs in EAE88,89 and these include the induction of IL-10-producing Tregs90 and the production of TGF-β, IL-35, programmed death 1 ligand 1 or Fas ligand.88,89 Although most studies have implicated adaptive Bregs in these activities, they were unable to exclude a potential contribution of innate-like Bregs.
Innate lymphocytes
It is now appreciated that the innate immune system includes a variety of lymphoid subsets, called innate lymphoid cells (ILCs), which are strategically positioned in many tissues of the body to provide protection against infections and to control tissue inflammation.91,92 ILCs have been divided into three distinct groups (Fig. 1) based on their expression of transcription factors, cytokine profiles, and functional properties. Group 1 ILCs include classical natural killer (NK) cells and ILC1 cells that produce IFN-γ, group 2 ILCs include ILC2 cells that produce IL-4, IL-5, and IL-13, and group 3 ILCs include lymphoid tissue inducer (LTi) cells implicated in the development of secondary lymphoid organs and ILC3 cells that produce IL-17 and IL-22. These cells generally have roles very early in the immune system, are highly responsive to activation by certain cytokines, and can interact with adaptive lymphocytes.93 Several of these cell types, particularly NK cells, have been implicated in the progression of MS and EAE.
NK cells
NK cells are innate lymphocytes with cytolytic activity that is controlled via a variety of activating and inhibitory receptors.94 These cells can produce the pro-inflammatory cytokines IFN-γ and TNF-α, the anti-inflammatory cytokine IL-10, and the growth factor GM-CSF in response to activation by cytokines such as IL-12, IL-15, and IL-18.95 These cells can engage in extensive reciprocal interactions with autoreactive T cells.96
In humans (but not in mice), NK cells can be distinguished by the expression of CD56, a neural cell adhesion molecule.97 CD56dim NK cells are more effective killers, whereas CD56bright NK cells produce greater amounts and a wider variety of cytokines, including immunoregulatory cytokines.98 In the CSF of MS patients, the CD56bright population is expanded compared with the CD56dim population99 and this expansion likely serves as a countermeasure in response to the inflammatory process. In contrast, in the peripheral blood, the CD56dim population is enhanced.100 Several therapies, including IFN-β,101 daclizumab (an antibody directed against the IL-2 receptor α subunit, CD25),102,103 and natalizumab (an antibody directed against the α4 integrin, which is part of the adhesion molecule very late antigen-4 expressed by effector lymphocytes),104 induce an increase in the CD56bright NK cell population in the circulation of patients. These findings are consistent with the suppressive effect of the CD56bright NK cell population observed in MS.105 These CD56bright NK cells can lyse activated (but not resting) CD4+ T cells106,107 and this finding suggests a mechanism for their suppressive activities in MS.
In EAE, the depletion of NK cells has resulted in contradictory results: some studies have provided evidence for disease exacerbation upon NK cell depletion108–111 and other studies have demonstrated that NK cell depletion results in disease amelioration.112 This contradiction is likely due to differences in the experimental systems employed, particularly the reagents used to deplete NK cells.113 CX3CL1 (fractalkine)-deficient mice, in which the homing of NK cells to the CNS is prevented, develop an exaggerated form of EAE,114 which suggests that NK cells exert protective effects in EAE. Several subsequent studies have provided evidence for a protective role of NK cells in EAE.105 Mechanistically, it has been shown that a blocking antibody directed against the NK cell inhibitory receptor NKG2A, which interacts with Qa-1, can increase the killing of T cells, microglia, and macrophages.115,116 As Qa-1 is downregulated during EAE, these findings suggest that the progression of EAE is associated with a loss of the tolerance of pathogenic T cells and myeloid cells to NK cell lysis. The expansion of NK cells by treatment with IL-2 in complex with anti-IL-2 antibodies ameliorates EAE,111 which suggests a potential approach for the therapeutic targeting of NK cells in MS.
A recent study provided evidence for a role of NK cells in delaying the repair process in CNS inflammation.117 In particular, the study showed that NK cells are retained for an extended period of time within the CNS during the chronic phase of MS and EAE. Moreover, NK cells suppress the reparative properties of neural stem cells during EAE through a mechanism that involves Qa-1 downregulation in these cells, resulting in their lysis by NK cells.
Collectively, the findings discussed here are consistent with a suppressive role of NK cells during the initiation and progression of CNS inflammation, but these cells might hinder recovery from brain inflammation.
LTi cells
LTi cells are a subset of ILCs that are critically important for the development of secondary lymphoid organs.118 These cells also control the generation of organized follicle-like structures called tertiary lymphoid follicles that might develop during chronic inflammation in tissues, including the CNS.58,119,120 Several studies have provided evidence showing that LTi cells are expanded in the CNS and circulation of patients with MS,121–124 which suggests that these cells have a pathogenic role by promoting the generation of tertiary lymphoid follicles. Consistent with these findings, the treatment of patients with daclizumab decreases the number of LTi cells compared with untreated patients.121
LTi cells have also been identified in the meninges of the CNS of mice and these cells accumulate and become activated during EAE.125 It has been proposed that LTi cells promote the progression of EAE,125,126 even though conclusive studies remain to be performed.
Other ILC cells
ILC2 cells have been implicated in sex differences associated with EAE in the Swiss Jim Lambert (SJL) mouse strain127,128 and the related studies proposed that a deficit in Th2-promoting ILC2 cells in female SJL mice, compared with male mice, is responsible for the increased susceptibility of female animals to EAE.
A recent study provided evidence for a pathogenic role of a subset of ILCs that is dependent on the transcription factor T-bet and expresses the natural cytotoxicity receptor NKp46 in EAE.129,130 These cells, which localize to the meninges, induce the expression of pro-inflammatory mediators that facilitate the entry of pathogenic T cells into the CNS. The relevant ILCs responsible for these effects include both ILC1 and ILC3 cells (but not NK cells).
Innate-like T and B cells
In addition to subsets of lymphocytes that belong to either the innate or the adaptive immune system, several subsets of lymphocytes exhibit phenotypic and functional properties of both the innate and adaptive immune system, and are referred to as innate-like T and B cells or “inbetweeners” (Fig. 1).131,132 These cells exhibit several common features, including the expression of antigen-specific TCRs or B-cell receptors with limited diversity, the expression of surface markers typically found on memory cells, a tendency for autoreactivity, a rapid and robust response to antigen and cytokines, and limited memory responses. Multiple T-cell subsets that belong to this family of lymphocytes, including γδ T cells, NKT cells, and MAIT cells, have been implicated in the pathogenesis of MS and EAE. Few studies have investigated the potential role of innate-like B cells in CNS autoimmunity.
γδ T cells
T cells expressing γδ TCRs represent ~2% of all T cells in peripheral blood and secondary lymphoid organs but represent a major lymphocyte population in the epithelia of the gut, skin, lung, and other organs.133,134 Distinct subsets of γδ T cells characterized by the expression of specific Vγ TCR chains as well as unique functional properties are found in distinct mucosal locations. The antigen specificity of γδ T cells remains largely unknown and rarely involves antigen presentation via classical MHC molecules, although some γδ T cells recognize stress-induced nonclassical MHC class I molecules.135 Unlike αβ T cells, which are dependent on cognate antigens for their activation, γδ T cells can be readily stimulated with TLR ligands and cytokines such as IL-1 and IL-23. In turn, these cells can produce a variety of pro- and anti-inflammatory cytokines.
γδ T cells have been found in MS lesions in the brain and CSF of patients with recent onset disease, where they are clonally expanded, which is suggestive of antigenic stimulation.136,137 A potential role of γδ T cells in the pathogenesis of MS has been further suggested by the capacity of these cells to lyse oligodendrocytes.138,139
In mice, γδ T cells can be found in the CNS during steady-state conditions140,141 and their number increases during the development of EAE.142 This population is enriched in cells expressing the Vγ4, Vγ1, and Vγ6 chains. The Vγ4 subset primarily produces IL-17 and has pro-inflammatory functions, whereas the Vγ1 subset primarily produces IFN-γ and has immunoregulatory functions.143 Although initial studies provided evidence for a pathogenic role of γδ T cells in EAE, more recent studies have suggested that these cells can have both pathogenic and beneficial effects on disease.113,144,145 The depletion of γδ T cells using monoclonal antibodies suppresses EAE severity.146–148 Consistent with these findings, mice that are genetically deficient in γδ T cells are protected against EAE.149,150 A subsequent study investigated the role of γδ T cell subsets through the use of activating, Vγ chain-specific antibodies.151 Activated Vγ4 cells exacerbate EAE, whereas activated Vγ1 cells suppress EAE. Although the related mechanisms remain unclear, it has been proposed that Vγ4 cells promote the activity of encephalitogenic Th cells while suppressing Tregs,149,152 whereas Vγ1 cells enhance Treg cell function during EAE.151
NKT cells
NKT cells are a subset of T lymphocytes that express TCRs together with surface markers that are characteristic of the NK cell lineage.153–155 These cells recognize glycolipid antigens that are presented to them by antigen-presenting cells in the context of the MHC class I-related protein CD1d. Two subsets of NKT cells have been identified: type 1 or invariant NKT (iNKT) cells express semi-invariant TCRs (Vα14-Jα18/Vβ8, -7, or -2 in mice and Vα24-Jα18/Vβ11 in humans), whereas type 2 or diverse NKT (dNKT) cells express more diverse or oligoclonal TCRs. Although iNKT cells are predominant in mice, dNKT cells are predominant in humans. iNKT cells are specific for a variety of microbial and autologous glycolipid antigens and include subsets with polarized cytokine production profiles, which is a property acquired during their development in the thymus. Although the antigen specificity of dNKT cells remains less well defined, a substantial proportion of these cells react with sulfatide, a self-glycolipid found in myelin sheaths.156 In addition to TCR-mediated activation, NKT cells are also highly responsive to stimulation by cytokines. These cells have been implicated in a variety of infectious, autoimmune, and inflammatory diseases.157,158 Curiously, iNKT and dNKT cells often have opposing roles in an immune response.159
The numbers and functional activities of iNKT cells are decreased in the circulation of MS patients,160,161 and the numbers and functions of these cells are restored in patients in remission due to treatment with IFN-β.162 In patients treated with corticosteroids163 or patients in remission in the absence of treatment,164 iNKT cells adopt a Th2-biased cytokine production profile, which suggests potential suppressive activities against the disease process. Studies on the status of dNKT cells in MS have not yet been performed.
SJL mice, which develop a relapsing–remitting form of EAE, have defects in iNKT cell numbers and functions due to absence of the Vβ8 gene segment,165,166 which is the main Vβ chain of murine iNKT cells. iNKT cells are found in the healthy CNS and remain unchanged following EAE induction.167 In contrast, sulfatide-reactive dNKT cells become expanded by several fold in the CNS of mice with EAE.156
EAE studies with NKT cell-deficient mice have provided divergent results. In CD1d-deficient mice, which lack both iNKT and dNKT cells, either no effect167–169 or disease exacerbation has been observed.170 In Jα18-deficient animals, which only lack iNKT cells, disease amelioration,171 no effect,168 or disease exacerbation172 has been reported. Vα14-Jα18 TCR transgenic mice that overproduce iNKT cells are partially protected against EAE through a mechanism that involves iNKT cell infiltration into the CNS and inhibition of autoantigen-specific Th cell responses in the spleen.173,174 Thus, the role of iNKT and dNKT cells in the natural progression of EAE remains unclear. Potential reasons for these divergent findings include differences in the mouse strains and experimental procedures employed, as well as altered effects on iNKT cells exerted by the gut microflora present in different housing facilities, which is a possibility that is supported by one EAE study.175
Due to their expression of semi-invariant (for iNKT cells) or oligoclonal (for dNKT cells) TCRs, it has been possible to investigate the effects of specific glycolipid ligands for these cells on the progression of EAE.176 For iNKT cells, many studies have employed the glycolipid α-galactosylceramide (α-GalCer), a synthetic version of a marine sponge-derived natural product that activates iNKT cells from a variety of mammalian species.177 These studies were performed with a variety of EAE models, mouse strains, and treatment doses, routes, and schedules.158,178 Using B6, PL/J, or B10.PL strains, treatment before or close to the time of EAE induction protected against disease in most studies, whereas treatment following EAE induction usually resulted in disease exacerbation.167–170,179 In SJL mice, which have numerical deficits in iNKT cells as well as a Th1 bias in iNKT cell cytokine production,165,166 α-GalCer exacerbated disease.169 Disease protection is typically associated with a shift in the autoantigen-specific T-cell response from Th1/Th17 toward Th2 and the opposite was observed in the cases of disease exacerbation.165,166 A role for the Th2 cytokines IL-4 and IL-10 has also been reported.167,169,180 The role of Th2 cytokines is further supported by the finding that α-GalCer analogs that induce a Th2-biased cytokine profile in iNKT cells exhibit improved suppressive activities in EAE.181,182 Additional mechanisms that have been proposed to contribute to the therapeutic activities of α-GalCer against EAE include the induction of immune-suppressive myeloid lineage cells (dendritic cells, M2 macrophages, and myeloid-derived suppressor cells)172,183–185 and CD4+ Tregs.176,186
The capacity of sulfatide, the ligand for a major subset of dNKT cells, to modulate EAE has also been investigated.176 A single treatment with sulfatide before, during, or after EAE induction exerts protective effects in B6 and B10.PL mice.156 This ligand has also been found to effectively reverse ongoing relapsing–remitting disease in SJL mice.187 Disease protection is associated with reduced autoantigen-specific Th1 and Th17 responses. Surprisingly, the treatment efficacy also required the participation of iNKT cells.187 A model in which sulfatide-activated dNKT cells induce an anergic response in iNKT cells and a tolerant response in peripheral dendritic cells and CNS-resident microglia has been proposed.
MAIT cells
MAIT cells are a subset of innate-like T cells that show substantial similarities to NKT cells.36,37 These cells, which were originally identified in association with the gut mucosa, express semi-invariant TCRs (Vα19-Jα33 in mice and Vα7.2-Jα33 in humans) that are specific for bacterial-derived riboflavin (vitamin B2) antigens presented in the context of the MHC class I-related protein MR1. MR1-restricted T cells that express more diverse receptors have also been identified.188 MAIT cells are rare in mice but are quite abundant in the peripheral blood of humans, where they constitute ~5% of the T-cell population. Similar to NKT cells, these cells are often activated by cytokines in an MR1-independent manner. These cells can produce a variety of predominantly pro-inflammatory cytokines and have been implicated in immune responses against microorganisms and in inflammatory and autoimmune diseases, including CNS autoimmunity.189,190
MAIT cells have been identified in the CNS lesions of patients with MS.191–195 Most studies have found a reduction in all or subsets of MAIT cells in the circulation of MS patients,193,195–197 although one study found an increase in these cells.34 Another study also reported that MAIT cells in untreated MS patients show a reduced capacity to produce IFN-γ and TNF-α, and that this defect is overcome by treatment with fingolimod.197 Finally, a recent study revealed that MAIT cells in the peripheral blood of MS patients produce increased levels of IL-17.198 Collectively, these studies are most consistent with a pathogenic role for MAIT cells in MS.
In EAE, the available evidence points toward a protective role of MAIT cells in CNS inflammation.199 MR1-deficient mice develop more severe EAE than wild-type animals and, conversely, Vα19-Jα33 TCR transgenic animals develop less severe EAE. Furthermore, the adoptive transfer of MAIT cells protects wild-type mice against EAE. The protective effects of murine MAIT cells in this model are associated with reduced Th1 and Th17 responses against CNS autoantigens and increased secretion of IL-10. As there is little evidence showing that human MAIT cells produce IL-10, it is unclear whether these mouse studies can be extrapolated to human MS patients.
Innate-like B cells
This group of lymphocytes includes B1 cells, which are enriched in the peritoneal and pleural cavities,200 and marginal zone B (MZB) cells, which segregate into the marginal zone of the spleen.201 Both of these B-cell subsets can produce antibodies in a T-cell-independent manner. Some of these cells, particularly B1 cells that express the CD5 surface marker (B1a cells), are also capable of producing IL-10 and can thus function as Bregs.72,202
B1 cells accumulate in the brain-draining cervical lymph nodes during EAE.203 The depletion of these cells during the induction phase of EAE exacerbates the pathology of the disease, whereas their depletion during the effector phase decreases the disease pathology.204 Whether these effects are directly mediated by IL-10-producing B1a cells remains unclear. The counts of MZB cells are substantially reduced in the brain-draining cervical lymph nodes of mice with EAE.203
Concluding remarks
The reviewed studies reveal that multiple different lymphocyte subsets contribute to the initiation, progression, and repair of CNS autoimmunity, and these subsets include cells that belong to either the innate or adaptive immune systems and cells that straddle these systems (Fig. 1). Several of these cell types contain subpopulations with pathogenic or suppressive functions, which need to be considered when investigating the contribution of individual subsets to disease pathogenesis. For example, the divergent roles of particular lymphocyte subsets often observed in the initiation, progression, or reparative stages of disease might be explained, at least in some cases, by the opposing contributions of pro- and anti-inflammatory subpopulations.
Most treatments for MS and EAE have been developed based on the critical role of pathogenic CD4+ T cells (Th1 and particularly Th17 cells) in disease, but accumulating evidence indicates that successful treatments are often associated with alterations in many other lymphocyte subsets. These findings not only suggest the involvement of a wide variety of lymphocyte subsets in disease pathogenesis but also identify these cells as potential targets for immunotherapy. Many of these cell types can influence the induction, CNS recruitment, and/or activity of pathogenic Th1/Th17 cells and protective Tregs. Several of these lymphocyte subsets are amenable to therapeutic targeting by biologics such as monoclonal antibodies or, in some cases (e.g., NKT cells), with cognate antigens. It is therefore critically important to investigate the precise functions of individual lymphocyte subsets in CNS autoimmunity, including EAE. Notably, when attempting to translate findings obtained in mice with EAE to human MS patients, it is important to keep in mind that several of the lymphocyte subsets that have emerged as novel immunotherapeutic targets exhibit divergent phenotypic and functional properties in mice and humans.
Acknowledgements
The work in the researchers’ lab was supported by grants from the NIH (DK104817 to L.V.K.), the Department of Defense (W81XWH-15-1-0543 to L.V.K.), and the National Multiple Sclerosis Society (60006625 to L.V.K.). J.L.P. was supported by predoctoral NIH training grants (T32HL069765 and T32AR059039).
Competing interests
The authors declare no conflict of interest.
References
- 1.Goverman J. Autoimmune T cell responses in the central nervous system. Nat. Rev. Immunol. 2009;9:393–407. doi: 10.1038/nri2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dobson R, Giovannoni G. Multiple sclerosis - a review. Eur. J. Neurol. 2019;26:27–40. doi: 10.1111/ene.13819. [DOI] [PubMed] [Google Scholar]
- 3.Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N. Engl. J. Med. 2018;378:169–180. doi: 10.1056/NEJMra1401483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gholamzad M, et al. A comprehensive review on the treatment approaches of multiple sclerosis: currently and in the future. Inflamm. Res. 2019;68:25–38. doi: 10.1007/s00011-018-1185-0. [DOI] [PubMed] [Google Scholar]
- 5.Olsson T, Barcellos LF, Alfredsson L. Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat. Rev. Neurol. 2017;13:25–36. doi: 10.1038/nrneurol.2016.187. [DOI] [PubMed] [Google Scholar]
- 6.Baranzini SE, Oksenberg JR. The genetics of multiple sclerosis: from 0 to 200 in 50 years. Trends Genet. 2017;33:960–970. doi: 10.1016/j.tig.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.International Multiple Sclerosis Genetics C, Wellcome Trust Case Control C. Sawcer S, et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 2011;476:214–219. doi: 10.1038/nature10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part II: noninfectious factors. Ann. Neurol. 2007;61:504–513. doi: 10.1002/ana.21141. [DOI] [PubMed] [Google Scholar]
- 9.Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part I: the role of infection. Ann. Neurol. 2007;61:288–299. doi: 10.1002/ana.21117. [DOI] [PubMed] [Google Scholar]
- 10.Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev. Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 11.Ransohoff RM. Animal models of multiple sclerosis: the good, the bad and the bottom line. Nat. Neurosci. 2012;15:1074–1077. doi: 10.1038/nn.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rangachari M, Kerfoot SM, Arbour N, Alvarez JI. Editorial: Lymphocytes in MS and EAE: more than just a CD4( + ) World. Front Immunol. 2017;8:133. doi: 10.3389/fimmu.2017.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rahmanzadeh R., Bruck W., Minagar A., Sahraian M. A. Multiple sclerosis pathogenesis: missing pieces of an old puzzle. Rev. Neurosci. 30, 67–83 (2018). [DOI] [PubMed]
- 14.Vasileiadis GK, et al. Regulatory B and T lymphocytes in multiple sclerosis: friends or foes? Auto. Immun. Highlights. 2018;9:9. doi: 10.1007/s13317-018-0109-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Booss J, Esiri MM, Tourtellotte WW, Mason DY. Immunohistological analysis of T lymphocyte subsets in the central nervous system in chronic progressive multiple sclerosis. J. Neurol. Sci. 1983;62:219–232. doi: 10.1016/0022-510X(83)90201-0. [DOI] [PubMed] [Google Scholar]
- 16.Hauser SL, et al. Immunohistochemical analysis of the cellular infiltrate in multiple sclerosis lesions. Ann. Neurol. 1986;19:578–587. doi: 10.1002/ana.410190610. [DOI] [PubMed] [Google Scholar]
- 17.Sawcer S, Franklin RJ, Ban M. Multiple sclerosis genetics. Lancet Neurol. 2014;13:700–709. doi: 10.1016/S1474-4422(14)70041-9. [DOI] [PubMed] [Google Scholar]
- 18.Krishnamoorthy G, Wekerle H. EAE: an immunologist’s magic eye. Eur. J. Immunol. 2009;39:2031–2035. doi: 10.1002/eji.200939568. [DOI] [PubMed] [Google Scholar]
- 19.Cao Y, et al. Functional inflammatory profiles distinguish myelin-reactive T cells from patients with multiple sclerosis. Sci. Transl. Med. 2015;7:287ra274. doi: 10.1126/scitranslmed.aaa8038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohme M, et al. HLA-DR15-derived self-peptides are involved in increased autologous T cell proliferation in multiple sclerosis. Brain. 2013;136:1783–1798. doi: 10.1093/brain/awt108. [DOI] [PubMed] [Google Scholar]
- 21.van Oosten BW, et al. Treatment of multiple sclerosis with the monoclonal anti-CD4 antibody cM-T412: results of a randomized, double-blind, placebo-controlled, MR-monitored phase II trial. Neurology. 1997;49:351–357. doi: 10.1212/WNL.49.2.351. [DOI] [PubMed] [Google Scholar]
- 22.Zhang H, Podojil JR, Luo X, Miller SD. Intrinsic and induced regulation of the age-associated onset of spontaneous experimental autoimmune encephalomyelitis. J. Immunol. 2008;181:4638–4647. doi: 10.4049/jimmunol.181.7.4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lafaille JJ, Nagashima K, Katsuki M, Tonegawa S. High incidence of spontaneous autoimmune encephalomyelitis in immunodeficient anti-myelin basic protein T cell receptor transgenic mice. Cell. 1994;78:399–408. doi: 10.1016/0092-8674(94)90419-7. [DOI] [PubMed] [Google Scholar]
- 24.O’Connor RA, Anderton SM. Foxp3 + regulatory T cells in the control of experimental CNS autoimmune disease. J. Neuroimmunol. 2008;193:1–11. doi: 10.1016/j.jneuroim.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 25.Salou M, Nicol B, Garcia A, Laplaud DA. Involvement of CD8( + ) T cells in multiple sclerosis. Front Immunol. 2015;6:604. doi: 10.3389/fimmu.2015.00604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jersild C, Svejgaard A, Fog T. HL-A antigens and multiple sclerosis. Lancet. 1972;1:1240–1241. doi: 10.1016/S0140-6736(72)90962-2. [DOI] [PubMed] [Google Scholar]
- 27.Naito S, Namerow N, Mickey MR, Terasaki PI. Multiple sclerosis: association with HL-A3. Tissue Antigens. 1972;2:1–4. doi: 10.1111/j.1399-0039.1972.tb00111.x. [DOI] [PubMed] [Google Scholar]
- 28.Fogdell-Hahn A, Ligers A, Gronning M, Hillert J, Olerup O. Multiple sclerosis: a modifying influence of HLA class I genes in an HLA class II associated autoimmune disease. Tissue Antigens. 2000;55:140–148. doi: 10.1034/j.1399-0039.2000.550205.x. [DOI] [PubMed] [Google Scholar]
- 29.Harbo HF, et al. Genes in the HLA class I region may contribute to the HLA class II-associated genetic susceptibility to multiple sclerosis. Tissue Antigens. 2004;63:237–247. doi: 10.1111/j.0001-2815.2004.00173.x. [DOI] [PubMed] [Google Scholar]
- 30.Salou M, et al. Expanded CD8 T-cell sharing between periphery and CNS in multiple sclerosis. Ann. Clin. Transl. Neurol. 2015;2:609–622. doi: 10.1002/acn3.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Babbe H, et al. Clonal expansions of CD8( + ) T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J. Exp. Med. 2000;192:393–404. doi: 10.1084/jem.192.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Junker A, et al. Multiple sclerosis: T-cell receptor expression in distinct brain regions. Brain. 2007;130:2789–2799. doi: 10.1093/brain/awm214. [DOI] [PubMed] [Google Scholar]
- 33.Ifergan I, et al. Central nervous system recruitment of effector memory CD8 + T lymphocytes during neuroinflammation is dependent on alpha4 integrin. Brain. 2011;134:3560–3577. doi: 10.1093/brain/awr268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Annibali V, et al. CD161(high)CD8+T cells bear pathogenetic potential in multiple sclerosis. Brain. 2011;134:542–554. doi: 10.1093/brain/awq354. [DOI] [PubMed] [Google Scholar]
- 35.Jilek S, et al. CSF enrichment of highly differentiated CD8 + T cells in early multiple sclerosis. Clin. Immunol. 2007;123:105–113. doi: 10.1016/j.clim.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 36.Keller AN, Corbett AJ, Wubben JM, McCluskey J, Rossjohn J. MAIT cells and MR1-antigen recognition. Curr. Opin. Immunol. 2017;46:66–74. doi: 10.1016/j.coi.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 37.Lantz O, Legoux F. MAIT cells: an historical and evolutionary perspective. Immunol. Cell Biol. 2018;96:564–572. doi: 10.1111/imcb.1034. [DOI] [PubMed] [Google Scholar]
- 38.Sun D, et al. Myelin antigen-specific CD8+T cells are encephalitogenic and produce severe disease in C57BL/6 mice. J. Immunol. 2001;166:7579–7587. doi: 10.4049/jimmunol.166.12.7579. [DOI] [PubMed] [Google Scholar]
- 39.Huseby ES, et al. A pathogenic role for myelin-specific CD8( + ) T cells in a model for multiple sclerosis. J. Exp. Med. 2001;194:669–676. doi: 10.1084/jem.194.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huber M, et al. IL-17A secretion by CD8 + T cells supports Th17-mediated autoimmune encephalomyelitis. J. Clin. Invest. 2013;123:247–260. doi: 10.1172/JCI63681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Najafian N, et al. Regulatory functions of CD8 + CD28- T cells in an autoimmune disease model. J. Clin. Invest. 2003;112:1037–1048. doi: 10.1172/JCI17935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Linker RA, et al. EAE in beta-2 microglobulin-deficient mice: axonal damage is not dependent on MHC-I restricted immune responses. Neurobiol. Dis. 2005;19:218–228. doi: 10.1016/j.nbd.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 43.Ortega SB, et al. The disease-ameliorating function of autoregulatory CD8 T cells is mediated by targeting of encephalitogenic CD4 T cells in experimental autoimmune encephalomyelitis. J. Immunol. 2013;191:117–126. doi: 10.4049/jimmunol.1300452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weiss HA, Millward JM, Owens T. CD8 + T cells in inflammatory demyelinating disease. J. Neuroimmunol. 2007;191:79–85. doi: 10.1016/j.jneuroim.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 45.York NR, et al. Immune regulatory CNS-reactive CD8 + T cells in experimental autoimmune encephalomyelitis. J. Autoimmun. 2010;35:33–44. doi: 10.1016/j.jaut.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang H, Braunstein NS, Yu B, Winchester R, Chess L. CD8 + T cells control the TH phenotype of MBP-reactive CD4 + T cells in EAE mice. Proc. Natl Acad. Sci. USA. 2001;98:6301–6306. doi: 10.1073/pnas.101123098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang X, et al. Regulation of immunity by a novel population of Qa-1-restricted CD8alphaalpha + TCRalphabeta + T cells. J. Immunol. 2006;177:7645–7655. doi: 10.4049/jimmunol.177.11.7645. [DOI] [PubMed] [Google Scholar]
- 48.Varthaman A, et al. Physiological induction of regulatory Qa-1-restricted CD8 + T cells triggered by endogenous CD4 + T cell responses. PLoS ONE. 2011;6:e21628. doi: 10.1371/journal.pone.0021628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang X, et al. Targeting non-classical myelin epitopes to treat experimental autoimmune encephalomyelitis. Sci. Rep. 2016;6:36064. doi: 10.1038/srep36064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tyler AF, Mendoza JP, Firan M, Karandikar NJ. CD8( + ) T cells are required for glatiramer acetate therapy in autoimmune demyelinating disease. PLoS ONE. 2013;8:e66772. doi: 10.1371/journal.pone.0066772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sinha S, Boyden AW, Itani FR, Crawford MP, Karandikar NJ. CD8( + ) T-cells as immune regulators of multiple sclerosis. Front Immunol. 2015;6:619. doi: 10.3389/fimmu.2015.00619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Antel JP, et al. Comparison of T8 + cell-mediated suppressor and cytotoxic functions in multiple sclerosis. J. Neuroimmunol. 1986;12:215–224. doi: 10.1016/S0165-5728(86)80005-4. [DOI] [PubMed] [Google Scholar]
- 53.Balashov KE, Khoury SJ, Hafler DA, Weiner HL. Inhibition of T cell responses by activated human CD8 + T cells is mediated by interferon-gamma and is defective in chronic progressive multiple sclerosis. J. Clin. Invest. 1995;95:2711–2719. doi: 10.1172/JCI117973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Van Kaer L. Comeback kids: CD8( + ) suppressor T cells are back in the game. J. Clin. Invest. 2010;120:3432–3434. doi: 10.1172/JCI44395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tennakoon DK, et al. Therapeutic induction of regulatory, cytotoxic CD8 + T cells in multiple sclerosis. J. Immunol. 2006;176:7119–7129. doi: 10.4049/jimmunol.176.11.7119. [DOI] [PubMed] [Google Scholar]
- 56.Krumbholz M, Derfuss T, Hohlfeld R, Meinl E. B cells and antibodies in multiple sclerosis pathogenesis and therapy. Nat. Rev. Neurol. 2012;8:613–623. doi: 10.1038/nrneurol.2012.203. [DOI] [PubMed] [Google Scholar]
- 57.Claes N, Fraussen J, Stinissen P, Hupperts R, Somers V. B cells are multifunctional players in multiple sclerosis pathogenesis: insights from therapeutic interventions. Front Immunol. 2015;6:642. doi: 10.3389/fimmu.2015.00642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mitsdoerffer M, Peters A. Tertiary lymphoid organs in central nervous system autoimmunity. Front Immunol. 2016;7:451. doi: 10.3389/fimmu.2016.00451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McLaughlin KA, Wucherpfennig KW. B cells and autoantibodies in the pathogenesis of multiple sclerosis and related inflammatory demyelinating diseases. Adv. Immunol. 2008;98:121–149. doi: 10.1016/S0065-2776(08)00404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Staun-Ram E, Miller A. Effector and regulatory B cells in multiple sclerosis. Clin. Immunol. 2017;184:11–25. doi: 10.1016/j.clim.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 61.Piddlesden SJ, Lassmann H, Zimprich F, Morgan BP, Linington C. The demyelinating potential of antibodies to myelin oligodendrocyte glycoprotein is related to their ability to fix complement. Am. J. Pathol. 1993;143:555–564. [PMC free article] [PubMed] [Google Scholar]
- 62.Elliott C, et al. Functional identification of pathogenic autoantibody responses in patients with multiple sclerosis. Brain. 2012;135:1819–1833. doi: 10.1093/brain/aws105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Heigl F, et al. Immunoadsorption in steroid-refractory multiple sclerosis: clinical experience in 60 patients. Atheroscler. Suppl. 2013;14:167–173. doi: 10.1016/j.atherosclerosissup.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 64.Keegan M, et al. Relation between humoral pathological changes in multiple sclerosis and response to therapeutic plasma exchange. Lancet. 2005;366:579–582. doi: 10.1016/S0140-6736(05)67102-4. [DOI] [PubMed] [Google Scholar]
- 65.Piccio L, et al. Changes in B- and T-lymphocyte and chemokine levels with rituximab treatment in multiple sclerosis. Arch. Neurol. 2010;67:707–714. doi: 10.1001/archneurol.2010.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Michel L, et al. B cells in the multiple sclerosis central nervous system: trafficking and contribution to CNS-compartmentalized inflammation. Front. Immunol. 2015;6:636. doi: 10.3389/fimmu.2015.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Adler LN, et al. The other function: class II-Restricted Antigen Presentation by B Cells. Front. Immunol. 2017;8:319. doi: 10.3389/fimmu.2017.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harp CT, Lovett-Racke AE, Racke MK, Frohman EM, Monson NL. Impact of myelin-specific antigen presenting B cells on T cell activation in multiple sclerosis. Clin. Immunol. 2008;128:382–391. doi: 10.1016/j.clim.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 69.Jelcic I, et al. Memory B cells activate Brain-Homing, autoreactive CD4( + ) T cells in multiple sclerosis. Cell. 2018;175:85–100 e123. doi: 10.1016/j.cell.2018.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ransohoff RM. Immune-cell crosstalk in multiple sclerosis. Nature. 2018;563:194–195. doi: 10.1038/d41586-018-07063-z. [DOI] [PubMed] [Google Scholar]
- 71.Shen P, Fillatreau S. Antibody-independent functions of B cells: a focus on cytokines. Nat. Rev. Immunol. 2015;15:441–451. doi: 10.1038/nri3857. [DOI] [PubMed] [Google Scholar]
- 72.Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev. Immunol. 2012;30:221–241. doi: 10.1146/annurev-immunol-020711-074934. [DOI] [PubMed] [Google Scholar]
- 73.Li R, et al. Cytokine-defined B cell responses as therapeutic targets in multiple sclerosis. Front Immunol. 2015;6:626. doi: 10.3389/fimmu.2015.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kurosaki T. Paradox of B cell-targeted therapies. J. Clin. Invest. 2008;118:3260–3263. doi: 10.1172/JCI37099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wolf SD, Dittel BN, Hardardottir F, Janeway CA., Jr. Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. J. Exp. Med. 1996;184:2271–2278. doi: 10.1084/jem.184.6.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat. Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 77.Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J. Clin. Invest. 2008;118:3420–3430. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pierson ER, Stromnes IM, Goverman JM. B cells promote induction of experimental autoimmune encephalomyelitis by facilitating reactivation of T cells in the central nervous system. J. Immunol. 2014;192:929–939. doi: 10.4049/jimmunol.1302171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Molnarfi N, et al. MHC class II-dependent B cell APC function is required for induction of CNS autoimmunity independent of myelin-specific antibodies. J. Exp. Med. 2013;210:2921–2937. doi: 10.1084/jem.20130699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Parker Harp CR, et al. B cell antigen presentation is sufficient to drive neuroinflammation in an animal model of multiple sclerosis. J. Immunol. 2015;194:5077–5084. doi: 10.4049/jimmunol.1402236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barr TA, et al. B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6-producing B cells. J. Exp. Med. 2012;209:1001–1010. doi: 10.1084/jem.20111675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Matsushita T, Horikawa M, Iwata Y, Tedder TF. Regulatory B cells (B10 cells) and regulatory T cells have independent roles in controlling experimental autoimmune encephalomyelitis initiation and late-phase immunopathogenesis. J. Immunol. 2010;185:2240–2252. doi: 10.4049/jimmunol.1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yoshizaki A, et al. Regulatory B cells control T-cell autoimmunity through IL-21-dependent cognate interactions. Nature. 2012;491:264–268. doi: 10.1038/nature11501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Korniotis S, et al. Treatment of ongoing autoimmune encephalomyelitis with activated B-cell progenitors maturing into regulatory B cells. Nat. Commun. 2016;7:12134. doi: 10.1038/ncomms12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hong J, et al. TLR9 mediated regulatory B10 cell amplification following sub-total body irradiation: implications in attenuating EAE. Mol. Immunol. 2017;83:52–61. doi: 10.1016/j.molimm.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 86.Kala M, et al. B cells from glatiramer acetate-treated mice suppress experimental autoimmune encephalomyelitis. Exp. Neurol. 2010;221:136–145. doi: 10.1016/j.expneurol.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 87.Van Kaer L. Glatiramer acetate for treatment of MS: regulatory B cells join the cast of players. Exp. Neurol. 2011;227:19–23. doi: 10.1016/j.expneurol.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ray A, Wang L, Dittel BN. IL-10-independent regulatory B-cell subsets and mechanisms of action. Int Immunol. 2015;27:531–536. doi: 10.1093/intimm/dxv033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ray A, Dittel BN. Mechanisms of regulatory B cell function in autoimmune and inflammatory diseases beyond IL-10. J. Clin. Med. 2017;6:E12. doi: 10.3390/jcm6010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pennati A, et al. Regulatory B cells induce formation of IL-10-expressing T cells in mice with autoimmune neuroinflammation. J. Neurosci. 2016;36:12598–12610. doi: 10.1523/JNEUROSCI.1994-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Klose CS, Artis D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat. Immunol. 2016;17:765–774. doi: 10.1038/ni.3489. [DOI] [PubMed] [Google Scholar]
- 92.Simoni Y, Newell EW. Dissecting human ILC heterogeneity: more than just three subsets. Immunology. 2018;153:297–303. doi: 10.1111/imm.12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gasteiger G, Rudensky AY. Interactions between innate and adaptive lymphocytes. Nat. Rev. Immunol. 2014;14:631–639. doi: 10.1038/nri3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat. Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vivier E, et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shi FD, Van Kaer L. Reciprocal regulation between natural killer cells and autoreactive T cells. Nat. Rev. Immunol. 2006;6:751–760. doi: 10.1038/nri1935. [DOI] [PubMed] [Google Scholar]
- 97.Cooper MA, et al. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood. 2001;97:3146–3151. doi: 10.1182/blood.V97.10.3146. [DOI] [PubMed] [Google Scholar]
- 98.Shi FD, Ljunggren HG, La Cava A, Van Kaer L. Organ-specific features of natural killer cells. Nat. Rev. Immunol. 2011;11:658–671. doi: 10.1038/nri3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rodriguez-Martin E, et al. Natural killer cell subsets in cerebrospinal fluid of patients with multiple sclerosis. Clin. Exp. Immunol. 2015;180:243–249. doi: 10.1111/cei.12580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Plantone D, et al. Circulating CD56dim NK cells expressing perforin are increased in progressive multiple sclerosis. J. Neuroimmunol. 2013;265:124–127. doi: 10.1016/j.jneuroim.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 101.Saraste M, Irjala H, Airas L. Expansion of CD56Bright natural killer cells in the peripheral blood of multiple sclerosis patients treated with interferon-beta. Neurol. Sci. 2007;28:121–126. doi: 10.1007/s10072-007-0803-3. [DOI] [PubMed] [Google Scholar]
- 102.Bielekova B, et al. Regulatory CD56(bright) natural killer cells mediate immunomodulatory effects of IL-2Ralpha-targeted therapy (daclizumab) in multiple sclerosis. Proc. Natl Acad. Sci. USA. 2006;103:5941–5946. doi: 10.1073/pnas.0601335103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Elkins J, et al. CD56(bright) natural killer cells and response to daclizumab HYP in relapsing-remitting MS. Neurol. Neuroimmunol. Neuroinflamm. 2015;2:e65. doi: 10.1212/NXI.0000000000000065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Putzki N, Baranwal MK, Tettenborn B, Limmroth V, Kreuzfelder E. Effects of natalizumab on circulating B cells, T regulatory cells and natural killer cells. Eur. Neurol. 2010;63:311–317. doi: 10.1159/000302687. [DOI] [PubMed] [Google Scholar]
- 105.Gross CC, et al. Regulatory functions of natural killer cells in multiple sclerosis. Front Immunol. 2016;7:606. doi: 10.3389/fimmu.2016.00606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nielsen N, Odum N, Urso B, Lanier LL, Spee P. Cytotoxicity of CD56(bright) NK cells towards autologous activated CD4 + T cells is mediated through NKG2D, LFA-1 and TRAIL and dampened via CD94/NKG2A. PLoS ONE. 2012;7:e31959. doi: 10.1371/journal.pone.0031959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gross CC, et al. Impaired NK-mediated regulation of T-cell activity in multiple sclerosis is reconstituted by IL-2 receptor modulation. Proc. Natl Acad. Sci. USA. 2016;113:E2973–E2982. doi: 10.1073/pnas.1524924113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang B, Yamamura T, Kondo T, Fujiwara M, Tabira T. Regulation of experimental autoimmune encephalomyelitis by natural killer (NK) cells. J. Exp. Med. 1997;186:1677–1687. doi: 10.1084/jem.186.10.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xu W, Fazekas G, Hara H, Tabira T. Mechanism of natural killer (NK) cell regulatory role in experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2005;163:24–30. doi: 10.1016/j.jneuroim.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 110.Matsumoto Y, et al. Role of natural killer cells and TCR gamma delta T cells in acute autoimmune encephalomyelitis. Eur. J. Immunol. 1998;28:1681–1688. doi: 10.1002/(SICI)1521-4141(199805)28:05<1681::AID-IMMU1681>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 111.Hao J, et al. Central nervous system (CNS)-resident natural killer cells suppress Th17 responses and CNS autoimmune pathology. J. Exp. Med. 2010;207:1907–1921. doi: 10.1084/jem.20092749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Winkler-Pickett R, et al. In vivo regulation of experimental autoimmune encephalomyelitis by NK cells: alteration of primary adaptive responses. J. Immunol. 2008;180:4495–4506. doi: 10.4049/jimmunol.180.7.4495. [DOI] [PubMed] [Google Scholar]
- 113.Edwards SC, McGinley AM, McGuinness NC, Mills KH. gammadelta T cells and NK cells - distinct pathogenic roles as innate-like immune cells in CNS autoimmunity. Front Immunol. 2015;6:455. doi: 10.3389/fimmu.2015.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Huang D, et al. The neuronal chemokine CX3CL1/fractalkine selectively recruits NK cells that modify experimental autoimmune encephalomyelitis within the central nervous system. FASEB J. 2006;20:896–905. doi: 10.1096/fj.05-5465com. [DOI] [PubMed] [Google Scholar]
- 115.Leavenworth JW, et al. Analysis of the cellular mechanism underlying inhibition of EAE after treatment with anti-NKG2A F(ab’)2. Proc. Natl Acad. Sci. USA. 2010;107:2562–2567. doi: 10.1073/pnas.0914732107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jiang W, et al. Acetylcholine-producing NK cells attenuate CNS inflammation via modulation of infiltrating monocytes/macrophages. Proc. Natl Acad. Sci. USA. 2017;114:E6202–E6211. doi: 10.1073/pnas.1705491114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu Q, et al. Neural stem cells sustain natural killer cells that dictate recovery from brain inflammation. Nat. Neurosci. 2016;19:243–252. doi: 10.1038/nn.4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Withers DR. Lymphoid tissue inducer cells. Curr. Biol. 2011;21:R381–R382. doi: 10.1016/j.cub.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 119.Strober W. The LTi cell, an immunologic chameleon. Immunity. 2010;33:650–652. doi: 10.1016/j.immuni.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pikor NB, Prat A, Bar-Or A, Gommerman JL. Meningeal tertiary lymphoid tissues and multiple sclerosis: a gathering place for diverse types of immune cells during CNS autoimmunity. Front. Immunol. 2015;6:657. doi: 10.3389/fimmu.2015.00657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Perry JS, et al. Inhibition of LTi cell development by CD25 blockade is associated with decreased intrathecal inflammation in multiple sclerosis. Sci. Transl. Med. 2012;4:145ra106. doi: 10.1126/scitranslmed.3004140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Serafini B, et al. RORgammat expression and lymphoid neogenesis in the brain of patients with secondary progressive multiple sclerosis. J. Neuropathol. Exp. Neurol. 2016;75:877–888. doi: 10.1093/jnen/nlw063. [DOI] [PubMed] [Google Scholar]
- 123.Degn M, et al. Increased prevalence of lymphoid tissue inducer cells in the cerebrospinal fluid of patients with early multiple sclerosis. Mult. Scler. 2016;22:1013–1020. doi: 10.1177/1352458515609795. [DOI] [PubMed] [Google Scholar]
- 124.Gross CC, et al. Distinct pattern of lesion distribution in multiple sclerosis is associated with different circulating T-helper and helper-like innate lymphoid cell subsets. Mult. Scler. 2017;23:1025–1030. doi: 10.1177/1352458516662726. [DOI] [PubMed] [Google Scholar]
- 125.Hatfield JK, Brown MA. Group 3 innate lymphoid cells accumulate and exhibit disease-induced activation in the meninges in EAE. Cell Immunol. 2015;297:69–79. doi: 10.1016/j.cellimm.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 126.Columba-Cabezas S, et al. Suppression of established experimental autoimmune encephalomyelitis and formation of meningeal lymphoid follicles by lymphotoxin beta receptor-Ig fusion protein. J. Neuroimmunol. 2006;179:76–86. doi: 10.1016/j.jneuroim.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 127.Russi AE, Walker-Caulfield ME, Ebel ME, Brown MA. Cutting edge: c-Kit signaling differentially regulates type 2 innate lymphoid cell accumulation and susceptibility to central nervous system demyelination in male and female SJL mice. J. Immunol. 2015;194:5609–5613. doi: 10.4049/jimmunol.1500068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Russi AE, Ebel ME, Yang Y, Brown MA. Male-specific IL-33 expression regulates sex-dimorphic EAE susceptibility. Proc. Natl Acad. Sci. USA. 2018;115:E1520–E1529. doi: 10.1073/pnas.1710401115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kwong B, et al. T-bet-dependent NKp46( + ) innate lymphoid cells regulate the onset of TH17-induced neuroinflammation. Nat. Immunol. 2017;18:1117–1127. doi: 10.1038/ni.3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Brown MA, Russi AE. (.T)Betting on innate lymphoid cells in CNS inflammatory disease. Nat. Immunol. 2017;18:1063–1064. doi: 10.1038/ni.3839. [DOI] [PubMed] [Google Scholar]
- 131.Bendelac A, Bonneville M, Kearney JF. Autoreactivity by design: innate B and T lymphocytes. Nat. Rev. Immunol. 2001;1:177–186. doi: 10.1038/35105052. [DOI] [PubMed] [Google Scholar]
- 132.Lanier LL. Shades of grey--the blurring view of innate and adaptive immunity. Nat. Rev. Immunol. 2013;13:73–74. doi: 10.1038/nri3389. [DOI] [PubMed] [Google Scholar]
- 133.Chien YH, Meyer C, Bonneville M. gammadelta T cells: first line of defense and beyond. Annu Rev. Immunol. 2014;32:121–155. doi: 10.1146/annurev-immunol-032713-120216. [DOI] [PubMed] [Google Scholar]
- 134.Vantourout P, Hayday A. Six-of-the-best: unique contributions of gammadelta T cells to immunology. Nat. Rev. Immunol. 2013;13:88–100. doi: 10.1038/nri3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Van Kaer L, et al. Recognition of MHC TL gene products by gamma delta T cells. Immunol. Rev. 1991;120:89–115. doi: 10.1111/j.1600-065X.1991.tb00589.x. [DOI] [PubMed] [Google Scholar]
- 136.Shimonkevitz R, Colburn C, Burnham JA, Murray RS, Kotzin BL. Clonal expansions of activated gamma/delta T cells in recent-onset multiple sclerosis. Proc. Natl Acad. Sci. USA. 1993;90:923–927. doi: 10.1073/pnas.90.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wucherpfennig KW, et al. Gamma delta T-cell receptor repertoire in acute multiple sclerosis lesions. Proc. Natl Acad. Sci. USA. 1992;89:4588–4592. doi: 10.1073/pnas.89.10.4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Freedman MS, Ruijs TC, Selin LK, Antel JP. Peripheral blood gamma-delta T cells lyse fresh human brain-derived oligodendrocytes. Ann. Neurol. 1991;30:794–800. doi: 10.1002/ana.410300608. [DOI] [PubMed] [Google Scholar]
- 139.Zeine R, et al. Mechanism of gammadelta T cell-induced human oligodendrocyte cytotoxicity: relevance to multiple sclerosis. J. Neuroimmunol. 1998;87:49–61. doi: 10.1016/S0165-5728(98)00047-2. [DOI] [PubMed] [Google Scholar]
- 140.Ponomarev ED, et al. Gamma delta T cell regulation of IFN-gamma production by central nervous system-infiltrating encephalitogenic T cells: correlation with recovery from experimental autoimmune encephalomyelitis. J. Immunol. 2004;173:1587–1595. doi: 10.4049/jimmunol.173.3.1587. [DOI] [PubMed] [Google Scholar]
- 141.Reynolds JM, Martinez GJ, Chung Y, Dong C. Toll-like receptor 4 signaling in T cells promotes autoimmune inflammation. Proc. Natl Acad. Sci. USA. 2012;109:13064–13069. doi: 10.1073/pnas.1120585109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Olive C. Gamma delta T cell receptor variable region usage during the development of experimental allergic encephalomyelitis. J. Neuroimmunol. 1995;62:1–7. doi: 10.1016/0165-5728(95)00081-C. [DOI] [PubMed] [Google Scholar]
- 143.O’Brien RL, Born WK. gammadelta T cell subsets: a link between TCR and function? Semin. Immunol. 2010;22:193–198. doi: 10.1016/j.smim.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Malik S, Want MY, Awasthi A. The emerging roles of gamma-delta T cells in tissue inflammation in experimental autoimmune encephalomyelitis. Front. Immunol. 2016;7:14. doi: 10.3389/fimmu.2016.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.McGinley A. M., Edwards S. C., Raverdeau M., Mills K. H. G. Th17cells, gammadelta T cells and their interplay in EAE and multiple sclerosis. J. Autoimmun. pii: S0896-8411, 30007-6 (2018). [DOI] [PubMed]
- 146.Odyniec A, et al. Gammadelta T cells enhance the expression of experimental autoimmune encephalomyelitis by promoting antigen presentation and IL-12 production. J. Immunol. 2004;173:682–694. doi: 10.4049/jimmunol.173.1.682. [DOI] [PubMed] [Google Scholar]
- 147.Rajan AJ, Gao YL, Raine CS, Brosnan CF. A pathogenic role for gamma delta T cells in relapsing-remitting experimental allergic encephalomyelitis in the SJL mouse. J. Immunol. 1996;157:941–949. [PubMed] [Google Scholar]
- 148.Dandekar AA, Perlman S. Virus-induced demyelination in nude mice is mediated by gamma delta T cells. Am. J. Pathol. 2002;161:1255–1263. doi: 10.1016/S0002-9440(10)64402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Petermann F, et al. gammadelta T cells enhance autoimmunity by restraining regulatory T cell responses via an interleukin-23-dependent mechanism. Immunity. 2010;33:351–363. doi: 10.1016/j.immuni.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Spahn TW, Issazadah S, Salvin AJ, Weiner HL. Decreased severity of myelin oligodendrocyte glycoprotein peptide 33 - 35-induced experimental autoimmune encephalomyelitis in mice with a disrupted TCR delta chain gene. Eur. J. Immunol. 1999;29:4060–4071. doi: 10.1002/(SICI)1521-4141(199912)29:12<4060::AID-IMMU4060>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 151.Blink SE, et al. gammadelta T cell subsets play opposing roles in regulating experimental autoimmune encephalomyelitis. Cell Immunol. 2014;290:39–51. doi: 10.1016/j.cellimm.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sutton CE, et al. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 153.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu. Rev. Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 154.Taniguchi M, Harada M, Kojo S, Nakayama T, Wakao H. The regulatory role of Valpha14 NKT cells in innate and acquired immune response. Annu. Rev. Immunol. 2003;21:483–513. doi: 10.1146/annurev.immunol.21.120601.141057. [DOI] [PubMed] [Google Scholar]
- 155.Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu. Rev. Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 156.Jahng A, et al. Prevention of autoimmunity by targeting a distinct, noninvariant CD1d-reactive T cell population reactive to sulfatide. J. Exp. Med. 2004;199:947–957. doi: 10.1084/jem.20031389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Van Kaer L, Parekh VV, Wu L. Invariant natural killer T cells as sensors and managers of inflammation. Trends Immunol. 2013;34:50–58. doi: 10.1016/j.it.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Van Kaer L, Wu L. Therapeutic potential of invariant natural killer T cells in autoimmunity. Front. Immunol. 2018;9:519. doi: 10.3389/fimmu.2018.00519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kumar V, Delovitch TL. Different subsets of natural killer T cells may vary in their roles in health and disease. Immunology. 2014;142:321–336. doi: 10.1111/imm.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Illes Z, et al. Differential expression of NK T cell V alpha 24J alpha Q invariant TCR chain in the lesions of multiple sclerosis and chronic inflammatory demyelinating polyneuropathy. J. Immunol. 2000;164:4375–4381. doi: 10.4049/jimmunol.164.8.4375. [DOI] [PubMed] [Google Scholar]
- 161.van der Vliet HJ, et al. Circulating V(alpha24 + ) Vbeta11 + NKT cell numbers are decreased in a wide variety of diseases that are characterized by autoreactive tissue damage. Clin. Immunol. 2001;100:144–148. doi: 10.1006/clim.2001.5060. [DOI] [PubMed] [Google Scholar]
- 162.Gigli G, Caielli S, Cutuli D, Falcone M. Innate immunity modulates autoimmunity: type 1 interferon-beta treatment in multiple sclerosis promotes growth and function of regulatory invariant natural killer T cells through dendritic cell maturation. Immunology. 2007;122:409–417. doi: 10.1111/j.1365-2567.2007.02655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sakuishi K, Miyake S, Yamamura T. Role of NK cells and invariant NKT cells in multiple sclerosis. Results Probl. Cell Differ. 2010;51:127–147. doi: 10.1007/400_2009_11. [DOI] [PubMed] [Google Scholar]
- 164.Araki M, et al. Th2 bias of CD4 + NKT cells derived from multiple sclerosis in remission. Int. Immunol. 2003;15:279–288. doi: 10.1093/intimm/dxg029. [DOI] [PubMed] [Google Scholar]
- 165.Yoshimoto T, Bendelac A, Hu-Li J, Paul WE. Defective IgE production by SJL mice is linked to the absence of CD4+, NK1.1+T cells that promptly produce interleukin 4. Proc. Natl Acad. Sci. USA. 1995;92:11931–11934. doi: 10.1073/pnas.92.25.11931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Singh AK, et al. The natural killer T cell ligand alpha-galactosylceramide prevents or promotes pristane-induced lupus in mice. Eur. J. Immunol. 2005;35:1143–1154. doi: 10.1002/eji.200425861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Jahng AW, et al. Activation of natural killer T cells potentiates or prevents experimental autoimmune encephalomyelitis. J. Exp. Med. 2001;194:1789–1799. doi: 10.1084/jem.194.12.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Furlan R, et al. Activation of invariant NKT cells by alphaGalCer administration protects mice from MOG35-55-induced EAE: critical roles for administration route and IFN-gamma. Eur. J. Immunol. 2003;33:1830–1838. doi: 10.1002/eji.200323885. [DOI] [PubMed] [Google Scholar]
- 169.Singh AK, et al. Natural killer T cell activation protects mice against experimental autoimmune encephalomyelitis. J. Exp. Med. 2001;194:1801–1811. doi: 10.1084/jem.194.12.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Teige A, et al. CD1-dependent regulation of chronic central nervous system inflammation in experimental autoimmune encephalomyelitis. J. Immunol. 2004;172:186–194. doi: 10.4049/jimmunol.172.1.186. [DOI] [PubMed] [Google Scholar]
- 171.Viale R, Ware R, Maricic I, Chaturvedi V, Kumar V. NKT cell subsets can exert opposing effects in autoimmunity, tumor surveillance and inflammation. Curr. Immunol. Rev. 2012;8:287–296. doi: 10.2174/157339512804806224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Denney L, et al. Activation of invariant NKT cells in early phase of experimental autoimmune encephalomyelitis results in differentiation of Ly6Chi inflammatory monocyte to M2 macrophages and improved outcome. J. Immunol. 2012;189:551–557. doi: 10.4049/jimmunol.1103608. [DOI] [PubMed] [Google Scholar]
- 173.Mars LT, et al. Cutting edge: V alpha 14-J alpha 281 NKT cells naturally regulate experimental autoimmune encephalomyelitis in nonobese diabetic mice. J. Immunol. 2002;168:6007–6011. doi: 10.4049/jimmunol.168.12.6007. [DOI] [PubMed] [Google Scholar]
- 174.Mars LT, et al. Invariant NKT cells regulate experimental autoimmune encephalomyelitis and infiltrate the central nervous system in a CD1d-independent manner. J. Immunol. 2008;181:2321–2329. doi: 10.4049/jimmunol.181.4.2321. [DOI] [PubMed] [Google Scholar]
- 175.Yokote H, et al. NKT cell-dependent amelioration of a mouse model of multiple sclerosis by altering gut flora. Am. J. Pathol. 2008;173:1714–1723. doi: 10.2353/ajpath.2008.080622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Van Kaer L, Wu L, Parekh VV. Natural killer T cells in multiple sclerosis and its animal model, experimental autoimmune encephalomyelitis. Immunology. 2015;146:1–10. doi: 10.1111/imm.12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kawano T, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 178.Van Kaer L. alpha-Galactosylceramide therapy for autoimmune diseases: prospects and obstacles. Nat. Rev. Immunol. 2005;5:31–42. doi: 10.1038/nri1531. [DOI] [PubMed] [Google Scholar]
- 179.Qian G, et al. High doses of alpha-galactosylceramide potentiate experimental autoimmune encephalomyelitis by directly enhancing Th17 response. Cell Res. 2010;20:480–491. doi: 10.1038/cr.2010.6. [DOI] [PubMed] [Google Scholar]
- 180.Oh SJ, Chung DH. Invariant NKT cells producing IL-4 or IL-10, but not IFN-gamma, inhibit the Th1 response in experimental autoimmune encephalomyelitis, whereas none of these cells inhibits the Th17 response. J. Immunol. 2011;186:6815–6821. doi: 10.4049/jimmunol.1003916. [DOI] [PubMed] [Google Scholar]
- 181.Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 2001;413:531–534. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- 182.Shiozaki M, et al. Synthesis and biological activity of hydroxylated analogues of KRN7000 (alpha-galactosylceramide) Carbohydr. Res. 2013;370:46–66. doi: 10.1016/j.carres.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 183.Kojo S, et al. Induction of regulatory properties in dendritic cells by Valpha14 NKT cells. J. Immunol. 2005;175:3648–3655. doi: 10.4049/jimmunol.175.6.3648. [DOI] [PubMed] [Google Scholar]
- 184.Wang J, et al. Ligand-dependent induction of noninflammatory dendritic cells by anergic invariant NKT cells minimizes autoimmune inflammation. J. Immunol. 2008;181:2438–2445. doi: 10.4049/jimmunol.181.4.2438. [DOI] [PubMed] [Google Scholar]
- 185.Parekh VV, Wu L, Olivares-Villagomez D, Wilson KT, Van Kaer L. Activated invariant NKT cells control central nervous system autoimmunity in a mechanism that involves myeloid-derived suppressor cells. J. Immunol. 2013;190:1948–1960. doi: 10.4049/jimmunol.1201718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.La Cava A, Van Kaer L, Fu Dong S. CD4 + CD25 + Tregs and NKT cells: regulators regulating regulators. Trends Immunol. 2006;27:322–327. doi: 10.1016/j.it.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 187.Maricic I, Halder R, Bischof F, Kumar V. Dendritic cells and anergic type I NKT cells play a crucial role in sulfatide-mediated immune regulation in experimental autoimmune encephalomyelitis. J. Immunol. 2014;193:1035–1046. doi: 10.4049/jimmunol.1302898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gherardin NA, McCluskey J, Rossjohn J, Godfrey DI. The Diverse Family of MR1-Restricted T Cells. J. Immunol. 2018;201:2862–2871. doi: 10.4049/jimmunol.1801091. [DOI] [PubMed] [Google Scholar]
- 189.Chiba A, Murayama G, Miyake S. Mucosal-associated invariant T cells in autoimmune diseases. Front Immunol. 2018;9:1333. doi: 10.3389/fimmu.2018.01333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Treiner E, Liblau RS. Mucosal-associated invariant T cells in multiple sclerosis: the jury is still out. Front Immunol. 2015;6:503. doi: 10.3389/fimmu.2015.00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Illes Z, Shimamura M, Newcombe J, Oka N, Yamamura T. Accumulation of Valpha7.2-Jalpha33 invariant T cells in human autoimmune inflammatory lesions in the nervous system. Int Immunol. 2004;16:223–230. doi: 10.1093/intimm/dxh018. [DOI] [PubMed] [Google Scholar]
- 192.Abrahamsson SV, et al. Non-myeloablative autologous haematopoietic stem cell transplantation expands regulatory cells and depletes IL-17 producing mucosal-associated invariant T cells in multiple sclerosis. Brain. 2013;136:2888–2903. doi: 10.1093/brain/awt182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Willing A, et al. CD8( + ) MAIT cells infiltrate into the CNS and alterations in their blood frequencies correlate with IL-18 serum levels in multiple sclerosis. Eur. J. Immunol. 2014;44:3119–3128. doi: 10.1002/eji.201344160. [DOI] [PubMed] [Google Scholar]
- 194.Held K, et al. alphabeta T-cell receptors from multiple sclerosis brain lesions show MAIT cell-related features. Neurol. Neuroimmunol. Neuroinflamm. 2015;2:e107. doi: 10.1212/NXI.0000000000000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Salou M, et al. Neuropathologic, phenotypic and functional analyses of mucosal associated invariant T cells in multiple sclerosis. Clin. Immunol. 2016;166-167:1–11. doi: 10.1016/j.clim.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 196.Miyazaki Y, Miyake S, Chiba A, Lantz O, Yamamura T. Mucosal-associated invariant T cells regulate Th1 response in multiple sclerosis. Int Immunol. 2011;23:529–535. doi: 10.1093/intimm/dxr047. [DOI] [PubMed] [Google Scholar]
- 197.Sugimoto C, et al. The dynamics of mucosal-associated invariant T cells in multiple sclerosis + 2016;5:1259. doi: 10.1186/s40064-016-2923-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Willing A, Jager J, Reinhardt S, Kursawe N, Friese MA. Production of IL-17 by MAIT cells is increased in multiple sclerosis and is associated with IL-7 receptor expression. J. Immunol. 2018;200:974–982. doi: 10.4049/jimmunol.1701213. [DOI] [PubMed] [Google Scholar]
- 199.Croxford JL, Miyake S, Huang YY, Shimamura M, Yamamura T. Invariant V(alpha)19i T cells regulate autoimmune inflammation. Nat. Immunol. 2006;7:987–994. doi: 10.1038/ni1370. [DOI] [PubMed] [Google Scholar]
- 200.Hardy RR, Hayakawa K. Perspectives on fetal derived CD5 + B1 B cells. Eur. J. Immunol. 2015;45:2978–2984. doi: 10.1002/eji.201445146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Martin F, Kearney JF. Marginal-zone B cells. Nat. Rev. Immunol. 2002;2:323–335. doi: 10.1038/nri799. [DOI] [PubMed] [Google Scholar]
- 202.Zhang X. Regulatory functions of innate-like B cells. Cell Mol. Immunol. 2013;10:113–121. doi: 10.1038/cmi.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lee-Chang C, et al. Susceptibility to experimental autoimmune encephalomyelitis is associated with altered B-cell subsets distribution and decreased serum BAFF levels. Immunol. Lett. 2011;135:108–117. doi: 10.1016/j.imlet.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 204.Peterson LK, Tsunoda I, Fujinami RS. Role of CD5 + B-1 cells in EAE pathogenesis. Autoimmunity. 2008;41:353–362. doi: 10.1080/08916930801890280. [DOI] [PMC free article] [PubMed] [Google Scholar]