Abstract
Osteoporosis is characterized by a decrease in bone mass and strength, rendering people prone to osteoporotic fractures caused by low-energy forces. The primary treatment strategy for osteoporotic fractures is surgery; however, the compromised and comminuted bones in osteoporotic fracture sites are not conducive to optimum reduction and rigid fixation. In addition, these patients always exhibit accompanying aging-related disorders, including high inflammatory status, decreased mechanical loading and abnormal skeletal metabolism, which are disadvantages for fracture healing around sites that have undergone orthopedic procedures. Since the incidence of osteoporosis is expected to increase worldwide, orthopedic surgeons should pay more attention to comprehensive strategies for improving the poor prognosis of osteoporotic fractures. Herein, we highlight the molecular basis of osteoimmunology and bone mechanosensation in different healing phases of elderly osteoporotic fractures, guiding perioperative management to alleviate the unfavorable effects of insufficient mechanical loading, high inflammatory levels and pathogen infection. The well-informed pharmacologic and surgical intervention, including treatment with anti-inflammatory drugs and sufficient application of antibiotics, as well as bench-to-bedside strategies for bone augmentation and hardware selection, should be made according to a comprehensive understanding of bone biomechanical properties in addition to the remodeling status of osteoporotic bones, which is necessary for creating proper biological and mechanical environments for bone union and remodeling. Multidisciplinary collaboration will facilitate the improvement of overall osteoporotic care and reduction of secondary fracture incidence.
Subject terms: Bone, Bone quality and biomechanics, Metabolism
Introduction
The major characteristic of osteoporosis is a decrease in bone mass and quality,1 rendering people prone to osteoporotic fracture (fragility fracture) caused by low-energy trauma.2 Osteoporosis is a prevailing skeletal disease of the elderly; nearly 200 million osteoporotic patients are diagnosed annually, and almost 9 million osteoporotic fractures occur worldwide.3 Surgery is the primary treatment strategy for osteoporotic fracture; however, poor prognoses are presented due to the combination of biological and surgical factors.4 The common sites of osteoporotic bones are usually compromised and comminuted, which makes it hard to achieve an optimum reduction and stable fixation.3,5 Osteoporotic fractures occur mostly in elderly patients, who exhibit underlying, unfavorable systemic conditions that are prone to complications.6 The abnormal remodeling status of bone with osteoporosis would deteriorate after bed braking, which poses a disadvantage with respect to fracture healing and bone callus strength; furthermore, the refracture risk following surgery increases significantly.7 In terms of the complexity of treatment and poor prognosis, the annual facility-related hospital cost of osteoporotic fractures is the highest (up to $5.1 billion), followed by that of myocardial infarction and stroke.8
Although the results of the clinical studies remain controversial, the majority have demonstrated that decreased callus area (20%–40%) and bone mineral density (BMD) occur in the fracture sites of elderly osteoporotic patients4. Studies have indicated that the delayed or nonunion of osteoporotic fractures is implicated in the scarce capacity of bone regeneration with aging.9,10 Additionally, the bone properties of such patients are quite different from those of normal individuals and are manifested in the decrease of bone mechanics and mechanosensation, as well as the abnormal bone metabolism caused by immune disorders.11 To improve the current unsatisfactory status of osteoporotic fracture treatment, we must first gain an in-depth understanding of the mechanism of fracture healing in elderly patients with osteoporosis. Herein, we highlight the pivotal roles of mechanical loading and osteoimmunology in aging-related osteoporotic fractures, guiding the intervention in osteoporotic fracture patients combined with an optimal treatment strategy for improving the overall standard of care and reducing the incidence of secondary fracture.
Static and dynamic changes in osteoporotic bone
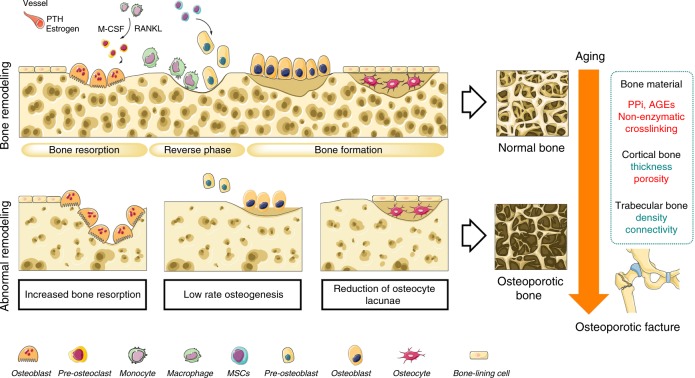
Bone is a unique tissue due to its elasticity and strength that permits deformation under a certain level of loading stress before failing.12 The strength of bone is mainly dependent on the distribution and density of the inorganic matrix mineralization.13 Cortical bone consisting of dense and well-organized lamellae has higher strength but a lower capacity to withstand a load that exceeds the elastic deformation range compared with that of trabecular bone, which is composed of unparallel lamellar units with variable porosity (50%–90%).14 The mechanical competence of cancellous bone is based largely on the BMD, while the stiffness of cortical bone is highly dependent on its porosity.3,15 In contrast to calcified matrix mineralization, the organic matrix (e.g., collagen and noncollagenous proteins) is thought to control bone ductility and its capacity to withstand an impact without cracking.16 A large proportion (90%) of the organic matrix is composed of type I collagen, which undergoes numerous posttranslational modifications.17Among them, enzymatic modifications positively affect the biomechanical stability of bone, while nonenzymatic crosslinking is associated with a deterioration in these properties.16 Noncollagenous proteins, including osteopontin (OPN) and osteocalcin (OCN), account for 10% of the organic matrix and limit crack energy through the control of hydroxyapatite size and orientation.18 Whereas bone material properties provide only a static snapshot of bone quality, the abilities of self-regeneration and remodeling provide a dynamic profile of bone health.19 The cortical and trabecular bone both undergo lifelong remodeling coupled with bone resorption, which is mediated by osteoclasts following osteoblastic bone formation.20 Osteoclasts are of hematopoietic stem cell (HSC) origin and share precursors with macrophages.21 In the presence of macrophage colony-stimulating factor (M-CSF), osteoclast precursors differentiate to preosteoclasts by the binding of receptor activator of nuclear factor kappa-B ligand (RANKL) to its cognate receptor, receptor activator of nuclear factor kappa-B (RANK). These mononuclear preosteoclasts then fuse to form multinuclear bone-resorbing osteoclasts.21 In contrast, osteoblasts are derived from mesenchymal stem cells (MSCs),22 and osteoblastic bone formation is separated from resorption by a reversal phase for several weeks.23 Mature osteoblasts then differentiate into osteocytes, which reside in small lacunae inside the calcified bone matrix.24 The long dendritic extensions of osteocytes together with the cell bodies form the lacuno-canalicular network (LCN), which allows direct signal transduction. The speed of mineral accumulation in the bone remodeling cycle is also affected by numerous endocrine factors, such as parathyroid hormone (PTH) and estrogen, which are supplied by the bone vascular systems.25,26 However, the normal regulation of bone remodeling could be interrupted as a consequence of skeletal senescence,27,28 which impact the integrity and biomechanical properties of both cortical and cancellous bones.29 The abnormal bone remodeling shifts toward bone resorption, which is either due to excessive activation of osteoclasts or to a low capacity of bone regeneration.30 In addition, age-related loss of proteostasis and increased levels of oxidants result in the overaccumulation of inorganic pyrophosphate (PPi) or advanced glycation end-products (AGEs). During the development of osteoporosis, osteocyte numbers per unit of bone area are gradually reduced,31 resulting in decreased trabecular thickness and more intracortical porosity.32 These considerable changes in the matrix composition and structure cause deterioration of bone quality and compromise its resistance to mechanical loading.33 Thus, osteoporotic fractures are the macroscopic result of microstructural alterations that increase the susceptibility of bone to the applied load34 (Fig. 1).
Fig. 1.
Static and dynamic changes in osteoporotic bone. An osteoporotic fracture is the macroscopic result of microstructural alterations that change the response of bone to the applied load. The aging process in osteoporotic bone would lead to overaccumulation of PPi, AGEs, and nonenzymatic crosslinking of collagen, which disturb the normal organization of bone material. With the increase of bone resorption and low rate osteogenesis, the osteocyte lacunae reduction leads to decreased trabecular thickness and more porous cortical bone. PTH parathyroid hormone, M-CSF macrophage colony-stimulating factor, RANKL receptor activator of nuclear factor kappa-B ligand, PPi inorganic pyrophosphate, AGEs advanced glycation end-products, MSCs mesenchymal stem cells. “Red” refers to upregulation; “Green” refers to downregulation
Osteoimmunology in hematoma and inflammatory phases
Secondary fracture healing occurs after a fracture without rigid fixation. Under the influence of active loading, an external callus is initiated to bridge the fracture gap35 in a three-stage process consisting of inflammation, repair, and remodeling.36 The first two of these partially overlapping phases restore bone structure and continuity over a period of 3 months to allow full weight bearing. The last phase involves gradual remodeling of bone to withstand the usual strains of daily life.37 In contrast, primary fracture healing without formation of a periosteal callus usually requires direct contact of compact bone or rigid surgical intervention that makes the fracture gap <200 μm. However, elderly osteoporotic bones, such as metaphyseal sites, which are highly susceptible to bone degradation, make it difficult to maintain anatomical reduction and rigid fixation using traditional screws due to inadequate insertional torque.38 In this situation, the healing process will be more like indirect bony union with the response of loading and inflammation, forming a periosteal callus bridging the fracture gap. The specific osteoimmunology and mechanosensation status of patients with osteoporotic fractures affect these healing phases in different manners.39
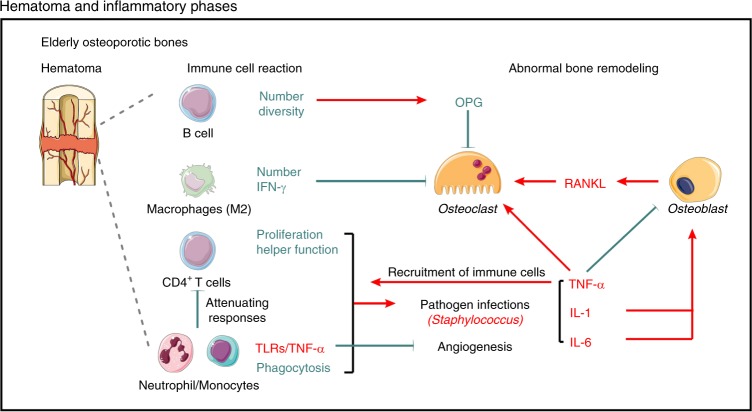
Bone fracture induces immediate inflammation and bleeding around bone extremities and within the medulla, where a template is formed for callus formation, called a hematoma.40 Around the hematoma sites, inflammatory cells, such as macrophages/monocytes or B/T cells, are activated to release inflammatory cytokines, including tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), and interleukin-6 (IL-6) into the systemic circulation.41 These cytokines are responsible for the initiation of immune and inflammatory responses,42 including enhancement of blood flow and vessel permeability, as well as the recruitment of immune cells for pathogen clearance.43 The limited inflammatory response is required to initiate the repair cascade and mobilize all the required factors involved in the early bridging of the fracture gap, especially in indirect bony unions without rigid fixation.44 The interactions between the skeletal system and immune function, comprising osteoimmunology, in osteoporotic fractures are altered with age.45 It has been reported that an age-associated decline in the absolute numbers of human B cell precursors in bone marrow46 leads to a significant decrease in the number of mature human B cells.47,48 Compared with young adults, the B cell repertoire is less diverse in elderly individuals.49 As to T cells, studies exhibit reductions of proliferation and helper function in CD4+ T cells that recruit neutrophils and macrophages to infected sites of elderly individuals.50 Consistent with this finding, the impaired neutrophil51/monocytes52-mediated phagocytosis also showed an age-dependent reduction.53 In contrast, the expression of Toll-like receptors (TLRs), a group of pattern recognition receptors (PRRs) that trigger pro-inflammatory responses,54 is increased in monocytes and dendritic cells in elderly people, accompanied by increased production of IL-1 and TNF-α.55 In vitro and in vivo studies have shown that persistent tumor necrosis factor (TNF) expression impairs cell-mediated immune responses and Th2 differentiation from naïve T cells.56–58 Moreover, constant stimulation by TNF-α elevates the threshold for T cell activation via the T-cell receptor (TCR), attenuating T cell responses to antigen59 and negatively affecting angiogenesis during fracture healing.60 Thus, the early immune responses and pathogen clearance of aged patients with osteoporotic fractures would be impaired or delayed due to the insufficient acquired immunity and dysfunction of the innate immune system.61 Furthermore, pathogen infections induce host inflammation and contribute to local bone loss. The most frequent pathogen identified in bone infection is Staphylococcus.62 Staphylococcus aureus protein A induces the production of inflammatory cytokines, such as TNF-α,63 IL-6, interleukin-1 alpha (IL-1α),64 interleukin-1 beta (IL-1β),64 and neutrophil-attracting chemokines in local tissues. On the one hand, short-term (24 h) upregulated cytokines, such as TNF-α are essential for local recruitment of neutrophils,41 macrophages, and T cells for pathogen clearance.65,66 However, the long-term presence of these cytokines, especially TNF-α, IL-1, and IL-6, activates CD4+ T cells, promoting RANKL expression by osteoblasts67 and synergizing directly with RANK to amplify osteoclastogenesis68 and bone resorption.69
In general, high levels of pro-inflammatory cytokines, either in the circulation or local tissues, are found in the aged population.70 Serum IL-1, IL-6, and/or TNF-α levels have been shown to be upregulated in elderly patients with bone loss,70 supporting the hypothesis of increased inflammation with aging.71 In fact, TNF-α promotes bone resorption by both directly inducing osteoclast differentiation72 and inhibiting osteoblast differentiation and function.73,74 IL-1 drives osteoclast differentiation via a RANKL/RANK-independent mechanism.75 IL-6 indirectly plays a positive role in osteoclast differentiation by binding IL-6 receptors expressed on osteoblastic cells to induce RANKL expression.76 Neutrophils stimulate osteoclastogenesis by upregulating cell surface RANKL expression under TLR stimulation77 or by inducing osteoblast retraction.78 Interferon gamma (IFN-γ), secreted by anti-inflammatory macrophages (M2), inhibits osteoclast differentiation via rapid degradation of TRAF6.79 However, macrophage polarization shows a shift toward macrophages (M1) that promote inflammatory cytokines as a consequence of aging.80 In contrast, mature B cells are important regulators of a decoy receptor for RANKL, osteoprotegerin (OPG). In total, 40% of the OPG in bone marrow is produced by mature B cells alone.81 The increased bone resorption and low levels of bone marrow OPG were demonstrated in B cell-deficient mice; this defect can be normalized by the transplantation of B cells. As a result of the decreased number of mature human B cells, the supply of OPG is low in patients with osteoporosis. Thus, current evidence supports that the high RANKL/OPG ratio caused by aging-related inflammation and the lack of mature B cells is associated with the hyperactivation of osteoclastogenesis and aggravation of bone resorption in elderly patients with bone loss, which increases the incidence of further intraoperative or postoperative fractures (Fig. 2). Moreover, Nagae et al. concluded that overactivation of osteoclasts plays an important role in chronic pain after osteoporotic fracture by creating acidosis.82 Hyper osteoclast activity may lead to pathological modifications of bone sensory nerve fibers, with an overexpression of acid-sensitive pain receptors, which contributes to generating and maintaining pain in osteoporosis.83
Fig. 2.
Osteoimmunology in elderly osteoporotic bones. Hematoma and inflammatory phases are the immediate reactions to a fracture. The limited inflammatory response at the fracture site is essential to initiate repair processes and mobilize all the required factors involved in the early bridging of the fracture gap, especially in indirect bony unions without rigid fixation. The high RANKL/OPG ratio caused by aging-related inflammation and the lack of mature B cells is associated with the hyperactivation of osteoclastogenesis and aggravation of bone resorption in elderly patients with bone loss, which increases the incidence of intra- or postoperative further fractures. OPG osteoprotegerin, IFN-γ interferon gamma, RANKL receptor activator of nuclear factor kappa-B ligand, TLRs toll-like receptors, TNF-α tumor necrosis factor alpha, IL-1 interleukin-1, IL-6 interleukin-6. “Red” refers to upregulation; “Green” refers to downregulation
Molecular basis of bone mechanosensation
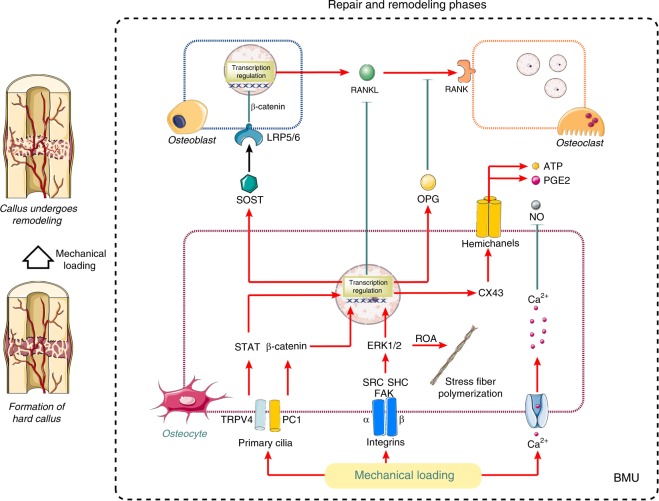
Primary fracture healing occurs when the fracture site achieves rigid anatomical and mechanical fixation. Under these conditions, a soft callus enveloping the bone extremities subsequently calcifies to a peripheral solid callus by intramembranous ossification.35 However, in elderly osteoporotic bones, the healing process will be more like indirect bony union by forming a periosteal callus bridging the fracture gap, since it is usually difficult for the compromised bones to maintain enough stress stimulation.84 Among the bone multicellular units (BMUs), which consist of various cells involved in bone remodeling, the osteocytes embedded in the matrix function as major mechanosensitive cells.85 Substantial evidence indicates that the mechanosensation of osteocytes is mediated by signaling molecules, such as Wnts, bone morphogenetic proteins (BMPs), nitric oxide (NO), and prostaglandin E2 (PGE2) in response to mechanical stimulation.86 Furthermore, altered enzyme activity and RNA synthesis have been reported in osteoclasts after mechanical loading of intact bone, which further supports the mechanosensory role of osteocytes in bone.87 Thus, adequate mechanical loading and mechanotransduction are pivotal factors in the repair and remodeling phase of fracture healing.
Mechanical forces, including fluid flow as well as compressive/tensile forces in the LCN,13 induce cell-level physical signals of shear stress, electric/streaming potentials, and substrate strain by acting on cell surface sensors and within the signaling pathways.88 To date, evidence strongly suggests that integrins on the surface of bone cells are ubiquitous sensors of mechanical forces capable of detecting alterations in the mechanical environment in the extracellular milieu.89 Shear strain is detected by primary cilia via polycystin 1 (PC1) and transient receptor potential cation channel subfamily V member 4 (TRPV4), which activate signal transducer and activator of transcription (STAT) signals to induce ion flux.90 Wnt signaling is also activated by cilia via the noncanonical pathway, resulting in β-catenin degradation.91,92 The role of canonical Wnt in the suppression of the SOST gene (sclerostin) has also been demonstrated.93 Furthermore, fluid shear stress can activate voltage-sensitive calcium channels on the plasma membrane, leading to influx of Ca2+, which induces PGE2 synthesis via ATP and inhibits NO generation as a second messenger.94,95 PGE2 and ATP are released via connexin hemichanels formed following extracellular signal-regulated kinase1/2 (ERK1/2)-induced transcription of connexin-43 (Cx43).96,97 Compressive/tensile forces impose hydraulic pressure in the lacunar-canalicular system,89 which increases cellular deformation of osteocytes98. The substrate strain at the membrane can be sensed by integrins that transmit force to the cell cytoskeleton via ERK, proto-oncogene tyrosine-protein kinase Src (SRC) and replication origin activator (ROA) to induce stress fiber polymerization.98 The cell nucleus plays crucial roles in response to cellular mechanotransduction. Transcriptional regulation in the cell nucleus converts incoming mechanoresponsive signals into biological signaling and even directly responds to cellular deformation.99 These intracellular signaling pathways converge to modulate osteogenic transcription factors in addition to regulators of growth factors and matrix proteins required for osteogenesis. Evidence suggests that mechanical signals induce OPG and suppress RANKL to inhibit osteoclast differentiation.100
The morphological changes of osteocytes with aging have been reported to influence their mechanosensitivity and the response to loads.101 Changes in LCN volume due to the increased rate of osteocytic osteolysis with aging or trauma have been shown to affect local bone mechanosensation.102 Additionally, age-related changes in periosteal modeling arise from cell function/signaling deficits combined with increased marrow adiposity leading to a reduced pool of osteoblast progenitors.103,104 Furthermore, periosteal lining cell numbers and osteoblast life-span are reduced by an increased rate of apoptosis.105 There is an age-related switch in macrophage differentiation from the anti-inflammatory (M2) phenotype that mediates tissue repair to the inflammatory (M1) phenotype.53 As a consequence of the decline in the secretion of anti-inflammatory and osteogenic cytokines, the bone regeneration capability could be impaired in the process of remodeling osteoporotic fractures.7 In osteoporotic fractures, the inevitable immobilization and stress shielding achieved by orthopedic surgery reduce the mechanical loading compared with that at normal sites.106 The deficiency of stress loading on surface sensors of bone cells is accompanied by NF-κB activation of osteoblasts and neighboring immune cells that promotes RANKL production to trigger osteoclastogenesis and bone resorption.107,108 This process results in the excess removal of bone mass,93 which therefore leads to a coarse trabecular pattern and thinning of cortical bone. Estrogen controls the adaptation of osteoblasts and osteocytes to mechanical loads via binding to the estrogen receptor (ER) or activation of TGF1 receptors.109 Delayed ER expression was shown to be correlated with impaired callus formation capacity in the healing process.110 A study in humans suggested that mechanical interventions enhance periosteal modeling and bone strength in the young skeleton,111 while the effects are markedly diminished in the elderly skeleton.111,112 In vitro studies indicate that the age-related increase in osteocyte degradation and reduction in the basal level of mechanosensation significantly affect second messenger signaling to modulate bone regeneration111 (Fig. 3). An optimal strategy for improving the treatment of osteoporotic fractures must address both biological and mechanical issues based on the molecular mechanisms of mechanical loading in fracture healing.93
Fig. 3.
Molecular basis of bone mechanosensation. The bone multicellular unit (BMU), which consists of osteocytes, osteoblasts, and osteoclasts, functions as a large mechanosensitive organ. Mechanical loading can be sensed by primary cilia, integrins, and Ca2+ channels on the surface of bone cells, then transcribed in the nucleus with inhibition of RANKL production and promotion of sclerostin and OPG. LRP5/6, low-density lipoprotein receptor-related protein 5/6; SOST sclerostin, RANKL receptor activator of nuclear factor kappa-B ligand, RANK receptor activator of nuclear factor kappa-B, OPG osteoprotegerin, ATP adenosine triphosphate, PGE2 prostaglandin E2, NO nitric oxide, CX43 connexin-43, STAT signal transducer and activator of transcription, ERK1/2 extracellular signal-regulated kinase1/2, ROA replication origin activator, TRPV4 transient receptor potential cation channel subfamily V member 4, PC1 polycystin 1, SRC proto-oncogene tyrosine-protein kinase Src, SHC Shc-transforming protein, FAK focal adhesion kinase, BMU bone multicellular unit. “Red” refers to upregulation; “Green” refers to downregulation
Anti-inflammatory effects of mechanical loading
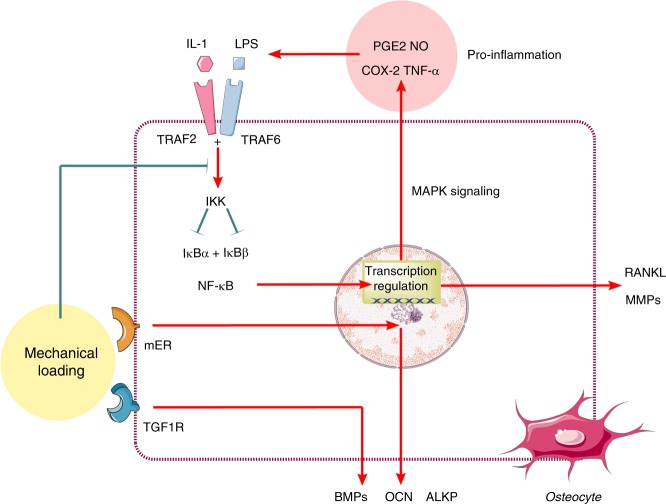
After the fracture gap has been bridged by a callus, the woven bone is slowly replaced with lamellar bone structures. Balanced resorption and formation of new bone require a normal environment without excessive inflammation.37 In these long-term phases, mechanics play a pivotal role, not only as the forces driving remodeling but also as the regulators that function to inhibit inflammation and abrogate the associated repression of growth factors and matrix synthesis.113 The traumatic signals caused by fracture and surgery initiate pro-inflammatory signaling cascades via activation of NF-κB transcription factors.114,115 NF-κB activation leads to the production of high levels of NO and superoxide that mediate both bone damage and matrix degradation.116 Mediators such as TNF-α, IL-1β, and matrix metalloproteinases (MMPs) play key roles in the pathogenesis of inflammatory bone diseases and injuries.117 In IL-1β-treated osteoblast-like cells, mechanical signals have been shown to rapidly (within 10 min) and dramatically inhibit NF-κB nuclear translocation.118 The mechanism involves the inhibition of TNF receptor-associated factor 6 (TRAF6) phosphorylation and subsequent activation of the inhibitor of NF-κB kinase (IKK) complex.119 This process prevents the proteosomal degradation of NF-κB inhibitor alpha (IκBα) and NF-κB inhibitor beta (IκBβ) phosphorylation, which in turn inhibits nuclear translocation of NF-κB and subsequent pro-inflammatory gene transcription.120 In fact, mechanotransduction at low magnitudes is a potent anti-inflammatory signal121 that counters the NF-κB signaling cascade.122 In vitro studies in osteoblasts have shown that the pro-inflammatory mediators suppressed by mechanical signals (tensile, compressive, and shear) include IL-1β-induced NO, COX-2, PGE2, cytokines (IL-1β and TNF-α), and MMPs.123 Simultaneously, mechanical signals upregulate the expression of growth factors, such as BMPs, OCN, and alkaline phosphatase (ALKP), which are inhibited during inflammation.124 Several anti-inflammatory cytokines (IL-10) and tissue inhibitors of metalloproteinases (TIMPs) that are inhibited during inflammation are upregulated by mechanical signals. For instance, IL-10 and TIMP-II synthesized by low magnitudes of mechanical signals can suppress inflammation and matrix breakdown in osteoblast and osteoblast-like cells.125 In contrast, exogenous PGE2 was demonstrated to function as an intercellular messenger for enhancement of the mechanosensitivity of bone to loading forces both in vitro and in vivo.126 Furthermore, in the presence of PGE2 signaling, osteocytes release NO in response to mechanical stimulation via cytoskeletal adaptation and mitogen-activated protein kinase (MAPK) pathways.127 Additionally, mechanical loading increases ER-α expression at the fracture callus, which is beneficial for mechanical signal transduction and fracture repair.128,129 These data indicate that rigid fixation and adequate mechanical loading are a means of improving the immune environment that benefits bone healing130 (Fig. 4).
Fig. 4.
Anti-inflammatory effects of mechanical loading. The mechanotransduction at low magnitudes is a potent signal to counter inflammation activated by the NF-κB signaling cascade. IL-1 interleukin-1, LPS lipopolysaccharide, TRAF2 TNF receptor-associated factor 2, TRAF6 TNF receptor-associated factor 6, PGE2 prostaglandin E2, NO nitric oxide, COX-2 cyclooxygenase-2, TNF-α tumor necrosis factor alpha, IKK inhibitor of NF-κB kinase, IκBα NF-κB inhibitor alpha, IκBβ NF-κB inhibitor beta, BMPs bone morphogenetic proteins; OCN osteocalcin; ALKP alkaline phosphatase; RANKL receptor activator of nuclear factor kappa-B ligand, MAPK mitogen-activated protein kinase, MMPs matrix metalloproteinases. “Red” refers to upregulation; “Green” refers to downregulation
Management of hematoma and perioperative infection
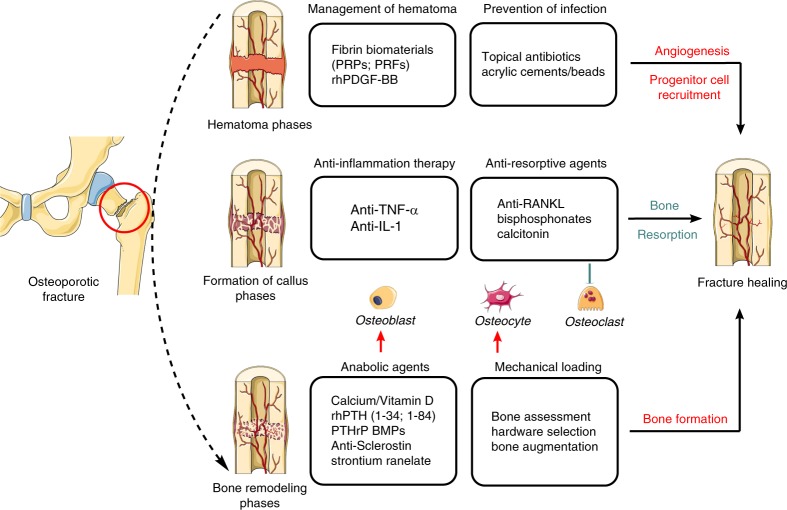
The most satisfactory bone healing depends on a good biological environment and appropriate mechanical loading for bone repair and remodeling. Orthopedic surgeons are encouraged to familiarize themselves with the molecular basis of skeletal senescence, mechanical loading and osteoimmunology in osteoporotic fractures, which is critical for determining an appropriate surgical technique or nonsurgical intervention3. In terms of the decrease in early immune responses and pathogen clearance in aged patients with osteoporotic fractures, special preoperative management is required to achieve a better local healing environment. Previous studies have revealed the osteoimmunological role of hematoma in fracture healing, especially in the inflammatory phase.42,131 However, there is still no consensus on hematoma management among surgeons. Grundnes and Reikeras reported that early removal of the hematoma (2–7 days) after fracture greatly prohibited bone healing in an animal fracture model.132 Other researchers, however, found that hematoma without normal fibrinolysis was an obstacle to cellular trafficking, which subsequently inhibited fracture healing by impeding macrophage accumulation.133 Thus, in clinical practice, early hematoma in the fracture site should be preserved and induced to “mature”. Indeed, the use of fibrin biomaterials, including platelet-rich plasma (PRPs), platelet-rich fibrin (PRFs) and other treatments,134 mimicking the structure of the natural hematoma, has demonstrated promising effects on the improvement of fracture healing. In addition, early-stage treatment with recombinant human platelet-derived growth factor-BB (rhPDGF-BB) could be beneficial for vascularization and angiogenesis in local sites,135 which would promote the recruitment of progenitors and accelerate bone remodeling in fracture healing of the elderly.136 In short, hematoma is a natural factor that enhances fracture healing and should be preserved in fracture sites, although thorough mechanisms are needed to be well investigated. Due to the decline of immune responses and pathogen clearance dysfunction in elderly patients with osteoporotic fractures, the prevention of perioperative infection through the use of adequate doses of antibiotics is important for maintaining a normal environment for initiating fracture healing (Fig. 5). Consequently, in orthopedic surgery, antibiotics, such as antibiotic-augmented acrylic cements/beads are increasingly used in topical form.137 However, the enhanced antibiotic treatment doses are thousands of times higher than those required to inhibit bacterial growth.137 Current evidence suggests that this concentration is detrimental to abnormal bone remodeling as a result of negative effects on mitochondrial physiology.137 Thus, local antibiotic vehicles must be designed to deliver sufficiently high concentrations to inhibit bacterial growth without affecting bone cell metabolism.138
Fig. 5.
Bench-to-bedside strategies for osteoporotic fracture. The most satisfactory bone healing depends on two pivotal factors: a good biological environment and appropriate mechanical loading for bone repair and homeostasis. Bench-to-bedside strategies, including management of hematomas and perioperative infections, anti-inflammation and regulation of bone resorption, and rigid fixation and mechanical loading enhancement would benefit the creation of the proper environment for fracture healing of osteoporotic bones. PRPs platelet-rich plasma, PRFs platelet-rich fibrin, rhPDGF-BB recombinant human platelet-derived growth factor-BB, TNF-α tumor necrosis factor alpha, IL-1 interleukin-1, RANKL receptor activator of nuclear factor kappa-B ligand, rhPTH recombinant human parathyroid hormone, PTHrP parathyroid hormone-related protein, BMPs bone morphogenetic proteins. “Red” refers to upregulation; “Green” refers to downregulation
Anti-inflammation and regulation of bone remodeling
Increased RANKL/OPG ratios caused by aging-related inflammation are associated with hyperactivation of osteoclastogenesis and exacerbation of bone resorption in elderly patients, leading to subsequent impairment of bone healing and inflammatory pain. Anti-inflammation therapy is a potential strategy that may benefit aging-related osteoporotic fracture by reducing inflammation and providing protection against bone loss. Studies have shown that healthy transgenic mice injected with anti-TNF-α repeatedly promote T cell responses to cognate peptide antigen.139 In the clinical setting, anti-TNF-α (infliximab, Remicade) rapidly and remarkably restores the responses of T cells from rheumatoid arthritis (RA) patients.56 Treatment with infliximab protects against bone loss and improves the formation/resorption marker ratio in this population, suggesting beneficial systemic and local bone effects.140,141 Although anti-inflammatory therapies have not been used clinically to treat osteoporosis, they have shown good promise in mouse models. Indeed, pharmacological or genetic ablation of TNF142 and IL-1143 by somatic gene therapy144 has been used effectively to prevent ovariectomy-induced bone loss in mice. Thus, anti-inflammation therapy is a potential strategy that may benefit osteoporosis patients because of reduced inflammation in addition to protection against bone loss. However, the risk of infection is increased in patients undergoing anti-inflammation treatment, suggesting that anti-inflammatory drugs should be discontinued for a period of time before surgery.145 Because chronic inflammation affects bone healing, the anti-inflammatory drug should be reused in osteoporotic fracture after an acute immune response to alleviate inflammation-induced bone loss.
The recent development of antiresorptive agents (e.g., bisphosphonates, RANKL inhibitor) represents a significant advance in therapeutic options for improving bone quality and metabolism.146 Bisphosphonates are commonly used in osteoporosis to prevent and reduce pain by modifying osteoclast activity.147 Following an osteoporotic fracture, early intervention with anti-resorptive drugs after surgery would not affect fracture union.148 However, bisphosphonate-dependent repair processes become progressively dominant in the late phases, suggesting that continuous administration of alendronate causes delayed healing in mechanically compromised situations.149 Denosumab, a RANKL inhibitor, can significantly reduce the high RANKL/OPG ratio in the inflammatory and repair phases of fracture healing with aging-related osteoporosis150 and has been identified as an efficacious osteoporosis treatment option with low rates of adverse events.151 Calcitonin effectively relieves bone pain and can reduce bone loss in osteoporotic fractures, although short-term (3 months) use is recommended.152,153 In summary, reducing the frequency of postoperative syndromes in patients with osteoporosis requires not only regulation of the immune response but also balanced bone resorption and osteogenesis (Fig. 5). Studies have demonstrated that anti-inflammation therapy combined with a bone resorption blocking drug154 reverses systemic bone loss,155 while the timing and extent of immune intervention require further clinical exploration.
Strategies for mechanical loading enhancement and rigid fixation
The biochemical responses of osteocytes to mechanical loads are mediated by signals induced via a variety of mechanosensitive proteins, such as primary cilia, integrins, and activated ion channels.156 However, it is as yet unclear how osteocytes perceive and differentiate responses to two drastically opposite magnitudes of mechanical signals, that is, those of physiological magnitudes that initiate regenerative responses and of traumatic signals that initiate bone damage and resorption.87 Appropriate use of bone formation promoters (e.g., calcium/vitamin D), mainly for osteoblasts and osteocytes, helps to further enhance the mechanical induction and repair of bone structure. Patients over 65 years old with BMD less than −2.5SD or postmenopausal women with multiple osteoporotic vertebral fractures or hip fractures who have not responded to bisphosphonate therapy should be switched to the available anabolic agents,7,131 including recombinant human parathyroid hormone (rhPTH,[1–34] [1–84]) and parathyroid hormone-related protein (PTHrP).30 Strontium ranelate is now considered effective in enhancing the biomechanical properties of bone for resistance fragility fractures. Strontium ranelate increases bone formation and decreases bone resorption, thereby rebalancing bone remodeling, which is conducive to new bone formation.157 Numerous studies have shown that strontium ranelates functions in improvement in all parameters related to bone quality and strength.158 The sclerostin monoclonal antibody, such as romosozumab, has been shown to lead to gains in hip BMD.159 In addition, BMPs, which belong to transforming growth factor-beta (TGF-β) family members,160 lead to synergistic induction of downstream TGFβ signaling for osteogenesis combined with physical microenvironment.161 Tricalcium phosphate and polymethylmethacrylate (PMMA) are usually employed to augment bone cement and increase the stability of implant fixation in osteoporotic bone.162,163 These cements undergo interdigitation in porous bone38 to increase the surface area of contact and provide additional resistance against the screw threads. PMMA has also been used for the delivery of drugs, such as antibiotics, via bone cements.164 However, PMMA undergoes an exothermic reaction during the drying process, with the potential to initiate thermal bone necrosis.165 In addition, PMMA is difficult to remove in cases of revision or infection without integrating into the bony architecture.166 In contrast, the integration of tricalcium phosphate into the bone provides a potential scaffold for biological activity and cell growth in a demineralized bone matrix.167 Allograft fibulas are used in bone with low BMD as tools for reduction as well as the provision of medial calcar support.168 As mechanical stimulation is a potent anti-inflammatory signal, sufficient postsurgery mechanical loading interventions, including physical therapy and rehabilitation, are helpful for building a supportive mechanical and biological environment around the local fracture sites for bone healing. Low intensity vibration (LIV) improves bone quality by activating cells responsible for bone remodeling and biasing the differentiation of mesenchymal and HSC progenitors toward osteoblastogenesis.169,170 However, current evidence is insufficient to support the benefit of ultrasound and extracorporeal shockwave therapies (ECSW) for fracture healing in clinical practice171 (Fig. 5).
Mechanical bone strength is vital for the stable anchorage of hardware required for fracture repair. Due to the impaired bone strength and complicated immunology environment in elderly individuals with osteoporosis, more suitable implants with better mechanical characteristics are required to improve aging-related osteoporotic fracture healing. Measurement of the thickness and porosity of cortical bone prior to surgery is important in guiding hardware selection for the repair of osteoporotic fractures. Thus, it is of great importance to identify parameters for evaluating bone quality (Fig. 5). Only 60% of the variation in bone densitometry was measured by dual-energy X-ray absorptiometry (DXA) because it is hard to recognize differences in both trabecular and cortical bone geometrical macrostructure.172 Both trabecular connectivity and cortical porosity significantly influence bone strength parameters, including stiffness to resist deformation and elasticity to absorb energy.173 To determine a better intervention, state-of-the-art clinical imaging techniques will help in measuring bone structural parameters, instead of focusing on BMD alone.174 Evaluation of the grayscale intensity map of DXA imaging can provide more precise information for bone structural parameters compared with BMD measurement.175 The trabecular bone score (TBS) correlates positively with trabecular connectivity based on evaluation of the DXA image.176 Combining TBS and BMD measurements provides an improved prediction of bone strength compared with BMD alone.177,178 Evaluations of structural, material, and mechanical properties based on bone biopsy specimens provide a reliable assessment of local bone characteristics, which are vital independent determinants of bone strength.179 The DensiProbe can be a helpful tool for intraoperative assessment of mechanical peak torque in mechanical testing setups,180 providing information that can be valuable in choosing implants. Furthermore, this approach does not increase the risk to the patient or increase the surgeon’s workload since the central peg hole can be used for the next procedure.180 Cortical and cancellous screws are traditional designs, with the former having relatively narrower outer diameters and decreased thread pitch.181 In both cases, the fixation strength depends on the torque generated between the bone and thread that resists shear.182 During insertion of a cancellous bone screw into the osteoporotic bone, the torque reaches the plateau prior to the contact of all the screw threads.183 The changes in screw geometry that confer an advantage on cancellous screws are lost below a threshold BMD of 0.4 g·cm–1.184 The plateau torque (T Plateau), which is an efficient predictor of insertion failure at the femoral head, is significantly dependent on aspects of the bone microarchitecture, such as the structure model index (SMI) and bone volume fraction (BV/TV).185 Previous studies suggest that a more plate-like bone structure, a higher BV/TV, and a higher surface-to-volume ratio provide a structural environment that favors cutting of the screw threads into the bone, resulting in an increased T Plateau.186 Unstable and comminuted fracture patterns as well as early implant-bone fatigue in osteoporotic bones lead to implant loosening and fixation failure.3 Locking-plate technology provides a more advantageous biomechanical environment that facilitates the formation of a fixed angle between the plate and screw.187 Despite the greater overall stability, locking plates may create an excessively rigid construct, which is predisposed to peri-implant fracture.188 In proximal humeral fractures with low BMD,189 computed tomography (CT) assessments suggest that locking plates do not reduce the rate of mechanical failure. In elderly patients with low BMD, tibial plateau fracture is associated with increased comminution and compromised fixation, suggesting that external fixation might be a more effective option than dual plating.190 An intramedullary nail (IMN) is a load-sharing device with the advantage of promoting secondary bone healing while preserving the surrounding soft tissues and minimizing fracture-induced hematomas.191 The loss of interlocking screw fixation can be mitigated through a number of strategies, including the application of washers and interlocking screws in multiple planes. However, cortical thinning of osteoporotic bone increases the intramedullary canal diameter, and a larger-diameter nail is required to achieve a diaphyseal fit and stability. Therefore, an early quantitative computed tomography (QCT) assessment of the cortical thickness is critical in using IMN in osteoporotic fractures. Intra-articular and complex fractures in patients with osteoporosis pose unique challenges for surgeons. These patients have inadequate subchondral bone quality to allow for anatomic reductions, and the stability of the implant is difficult to maintain after the reintroduction of weight-bearing and increased range of motion.192 Primary arthroplasty (total hip/knee/elbow arthroplasty) has been adopted to obtain adequate weight-bearing and early mobilization, which has a superior prognosis compared to internal fixation in acute acetabular fractures, displaced intra-articular tibial plateau fractures and complex distal humeral fractures.193 Despite the advent of locked anatomic plates, a majority of experts recommend arthroplasty in the context of poor bone quality and small fracture fragments (Table 1).
Table 1.
Clinical options in osteoporotic fractures
| Clinical options | Characteristic | Index | Methods | Fracture site and pattern | Disadvantage in osteoporotic bone | Ref. |
|---|---|---|---|---|---|---|
| Cortical bone screws | Narrow outer diameters and decreased thread pitch compared to cancellous bone screw |
BMD Thickness and porosity of cortical bone |
DXA QCT |
Femoral heads Femoral neck fractures |
50% reduction of the holding strength per 1 mm decrease of cortical thickness | 175, 194, 195 |
| Cancellous bone screw | Reach the plateau torque level prior to contact of all the screw threads |
BMD, TBS SMI BV/TV |
DXA HR-pQCT μMRI |
Femoral metaphysis Distal radius Femoral heads |
Reduction of thread-bone interface that produces torque | 183, 184, 196, 197 |
| Bicortical lag screw | Potential improvement of thread purchase |
BMD, TBS SMI BV/TV |
DXA HR-pQCT |
Medial malleolus fractures | 185, 198, 199 | |
| Traditional plates | Compress the fracture fragments between bone implant interface to create fixation strength |
BMD Bone stiffness and strength |
DXA | Regular fractures | Decrease of the axial and torsional stiffness | 190, 200, 201 |
| Locking plate | Fixed-angle construct between screw and plate |
BMD, TBS Proximal cortical thickness Failure load |
DXA QCT |
Proximal humerus fractures | Reduction of callus formation without micromotion across the fracture site; Loss of fixation and screw cut-out | 5, 187, 188, 198, 202 |
| Intramedullary nail | Preserving the soft tissues around fracture site |
BMD Cortical thickness |
DXA QCT |
Proximal humerus fractures | A larger-diameter nail is required to achieve a diaphyseal fit and stability | 191, 203 |
| Bone augmentation | Increase surface area; PMMA carries osteogenic and antibiotic drugs; Tricalcium phosphate and Allograft fibulas act more as a scaffold |
BMD SMI BV/TV BMSi |
DXA QCT |
Femoral neck fractures Spine fractures Comminuted proximal humerus fractures |
Damage surrounding soft tissues or initiate thermal bone necrosis; Difficult to remove | 38, 162– 164, 196, 204, 205 |
| External fixation | Lower fixation failure rates | BMD, TBS |
DXA HR-pQCT |
Comminution of tibial plateau fractures | 190 | |
| Primary arthroplasty | Early mobilization and weight bearing |
BMD, TBS Subchondral bone quality |
DXA HR-pQCT DensiProbe |
Acute acetabular fractures; Displaced intra-articular fractures of the tibial plateau | 193 |
BMD bone mineral density, DXA dual-energy X-ray absorptiometry, QCT quantitative computed tomography, HR-pQCT high-resolution peripheral QCT, μMRI micromagnetic resonance imaging, TBS trabecular bone score, BMSi bone material strength index, PMMA polymethylmethacrylate, SMI structure model index, BV/TV bone volume fraction
Conclusion and perspective
Low bone mass and compromised bone structure in osteoporotic fractures are undesirable for the reduction and rigid fixation, and the decreased regeneration and mechanosensation ability of osteoporotic bone also affect the healing. The initiation of supportive management, including anti-inflammatory drugs and sufficient application of antibiotics, is key for creating the proper environment for bone repair and homeostasis in patients with osteoporotic fractures. The adverse effects of insufficient mechanical loading in bone healing are critical factors that should be considered around the orthopedic procedure. Bench-to-bedside strategies for bone augmentation and hardware selection should be made according to further elucidation of the biomechanics and molecular mechanisms involved in bone repair. Multidisciplinary collaboration will facilitate the improvement of overall osteoporotic care and the reduction of secondary fracture incidence.
Acknowledgements
This study is supported by the National Natural Science Foundation of China (81772369, 81401809, 81373150), CAMS Innovation Fund for Medical Sciences (CIFMS, 2017-I2M-3-001). L.Z. is supported by the Beijing Nova program (Z171100001117110). Y.G. is supported by the Beijing Natural Science Foundation (7192127). The original elements used in the figures are from Servier Medical Art (http://smart.servier.com/).
Competing interests
The authors declare no competing interests.
Contributor Information
Wei Ge, Email: wei.ge@chem.ox.ac.uk.
Peifu Tang, Email: pftang301@163.com.
References
- 1.Brown C. Osteoporosis: staying strong. Nature. 2017;550:S15–s17. doi: 10.1038/550S15a. [DOI] [PubMed] [Google Scholar]
- 2.Sozen T, Ozisik L, Basaran NC. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017;4:46–56. doi: 10.5152/eurjrheum.2016.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yaacobi E, Sanchez D, Maniar H, Horwitz DS. Surgical treatment of osteoporotic fractures: an update on the principles of management. Injury. 2017;48(Suppl. 7):S34–s40. doi: 10.1016/j.injury.2017.08.036. [DOI] [PubMed] [Google Scholar]
- 4.Feron JM, Mauprivez R. Fracture repair: general aspects and influence of osteoporosis and anti-osteoporosis treatment. Injury. 2016;47(Suppl. 1):S10–S14. doi: 10.1016/S0020-1383(16)30003-1. [DOI] [PubMed] [Google Scholar]
- 5.von Ruden C, Augat P. Failure of fracture fixation in osteoporotic bone. Injury. 2016;47(Suppl. 2):S3–S10. doi: 10.1016/S0020-1383(16)47002-6. [DOI] [PubMed] [Google Scholar]
- 6.Smith DM, Khairi MR, Johnston CC., Jr. The loss of bone mineral with aging and its relationship to risk of fracture. J. Clin. Investig. 1975;56:311–318. doi: 10.1172/JCI108095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernatz JT, et al. Osteoporosis is common and undertreated prior to total joint arthroplasty. J. Arthroplast. 2019;34:1347–1353. doi: 10.1016/j.arth.2019.03.044. [DOI] [PubMed] [Google Scholar]
- 8.Singer A, et al. Burden of illness for osteoporotic fractures compared with other serious diseases among postmenopausal women in the United States. Mayo Clin. Proc. 2015;90:53–62. doi: 10.1016/j.mayocp.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 9.Clark D, Nakamura M, Miclau T, Marcucio R. Effects of aging on fracture healing. Curr. Osteoporos. Rep. 2017;15:601–608. doi: 10.1007/s11914-017-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baxter MA, et al. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells. 2004;22:675–682. doi: 10.1634/stemcells.22-5-675. [DOI] [PubMed] [Google Scholar]
- 11.Foulke BA, Kendal AR, Murray DW, Pandit H. Fracture healing in the elderly: a review. Maturitas. 2016;92:49–55. doi: 10.1016/j.maturitas.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 12.Turner CH. Biomechanics of bone: determinants of skeletal fragility and bone quality. Osteoporos. Int.: 2002;13:97–104. doi: 10.1007/s001980200000. [DOI] [PubMed] [Google Scholar]
- 13.Florencio-Silva R, Sasso GR, Sasso-Cerri E, Simoes MJ, Cerri PS. Biology of bone tissue: structure, function, and factors that influence bone cells. BioMed. Res. Int. 2015;2015:421746. doi: 10.1155/2015/421746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwaniec UT, Turner RT. Influence of body weight on bone mass, architecture and turnover. J. Endocrinol. 2016;230:R115–R130. doi: 10.1530/JOE-16-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Linden JC, Weinans H. Effects of microarchitecture on bone strength. Curr. Osteoporos. Rep. 2007;5:56–61. doi: 10.1007/s11914-007-0003-3. [DOI] [PubMed] [Google Scholar]
- 16.Stock SR. The mineral–collagen interface in bone. Calcif. Tissue Int. 2015;97:262–280. doi: 10.1007/s00223-015-9984-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tzaphlidou M. Bone architecture: collagen structure and calcium/phosphorus maps. J. Biol. Phys. 2008;34:39–49. doi: 10.1007/s10867-008-9115-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guerado E, et al. Bone mineral density aspects in the femoral neck of hip fracture patients. Injury. 2016;47(Suppl. 1):S21–S24. doi: 10.1016/S0020-1383(16)30005-5. [DOI] [PubMed] [Google Scholar]
- 19.Qi Z, Liu W, Lu J. The mechanisms underlying the beneficial effects of exercise on bone remodeling: roles of bone-derived cytokines and microRNAs. Prog. Biophys. Mol. Biol. 2016;122:131–139. doi: 10.1016/j.pbiomolbio.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 20.Katsimbri P. The biology of normal bone remodelling. European Journal of Cancer Care. 2017;26(6):e12740. doi: 10.1111/ecc.12740. [DOI] [PubMed] [Google Scholar]
- 21.Boyce BF, Rosenberg E, de Papp AE, Duong LT. The osteoclast, bone remodelling and treatment of metabolic bone disease. Eur. J. Clin. Investig. 2012;42:1332–1341. doi: 10.1111/j.1365-2362.2012.02717.x. [DOI] [PubMed] [Google Scholar]
- 22.Li C, Williams BO, Cao X, Wan M. LRP6 in mesenchymal stem cells is required for bone formation during bone growth and bone remodeling. Bone Res. 2014;2:14006. doi: 10.1038/boneres.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delaisse JM. The reversal phase of the bone-remodeling cycle: cellular prerequisites for coupling resorption and formation. Bone. Rep. 2014;3:561. doi: 10.1038/bonekey.2014.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai X, et al. The dependences of osteocyte network on bone compartment, age, and disease. Bone Res. 2015;3:15009. doi: 10.1038/boneres.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hadjidakis DJ, Androulakis II. Bone remodeling. Ann. New Y. Acad. Sci. 2006;1092:385–396. doi: 10.1196/annals.1365.035. [DOI] [PubMed] [Google Scholar]
- 26.Watson EC, Adams RH. Biology of bone: the vasculature of the skeletal system. Cold Spring Harbor Perspect. Med. 2018;8:a031559. doi: 10.1101/cshperspect.a031559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diab DL, Watts NB. Postmenopausal osteoporosis. Curr. Opin. Endocrinol. Diab. Obes. 2013;20:501–509. doi: 10.1097/01.med.0000436194.10599.94. [DOI] [PubMed] [Google Scholar]
- 28.Duque G, Troen BR. Understanding the mechanisms of senile osteoporosis: new facts for a major geriatric syndrome. J. Am. Geriatr. Soc. 2008;56:935–941. doi: 10.1111/j.1532-5415.2008.01764.x. [DOI] [PubMed] [Google Scholar]
- 29.Marie PJ. Bone cell senescence: mechanisms and perspectives. J. Bone Miner. Res. 2014;29:1311–1321. doi: 10.1002/jbmr.2190. [DOI] [PubMed] [Google Scholar]
- 30.Black DM, Rosen CJ. Clinical practice. Postmenopausal osteoporosis. New Engl. J. Med. 2016;374:254–262. doi: 10.1056/NEJMcp1513724. [DOI] [PubMed] [Google Scholar]
- 31.Yamagishi S. Role of advanced glycation end products (AGEs) in osteoporosis in diabetes. Curr. Drug Targets. 2011;12:2096–2102. doi: 10.2174/138945011798829456. [DOI] [PubMed] [Google Scholar]
- 32.Chen H, Zhou X, Fujita H, Onozuka M, Kubo KY. Age-related changes in trabecular and cortical bone microstructure. Int. J. Endocrinol. 2013;2013:213234. doi: 10.1155/2013/213234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osterhoff G, et al. Bone mechanical properties and changes with osteoporosis. Injury. 2016;47(Suppl. 2):S11–S20. doi: 10.1016/S0020-1383(16)47003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silva MJ. Biomechanics of osteoporotic fractures. Injury. 2007;38(Suppl. 3):S69–S76. doi: 10.1016/j.injury.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 35.Marsell R, Einhorn TA. The biology of fracture healing. Injury. 2011;42:551–555. doi: 10.1016/j.injury.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Einhorn TA, Gerstenfeld LC. Fracture healing: mechanisms and interventions. Nat. Rev. Rheumatol. 2015;11:45–54. doi: 10.1038/nrrheum.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Claes L, Recknagel S, Ignatius A. Fracture healing under healthy and inflammatory conditions. Nat. Rev. Rheumatol. 2012;8:133–143. doi: 10.1038/nrrheum.2012.1. [DOI] [PubMed] [Google Scholar]
- 38.Rothberg DL, Lee MA. Internal fixation of osteoporotic fractures. Curr. Osteoporos. Rep. 2015;13:16–21. doi: 10.1007/s11914-014-0245-9. [DOI] [PubMed] [Google Scholar]
- 39.Lu C, et al. Cellular basis for age-related changes in fracture repair. J. Orthop. Res. 2005;23:1300–1307. doi: 10.1016/j.orthres.2005.04.003.1100230610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ozaki A, Tsunoda M, Kinoshita S, Saura R. Role of fracture hematoma and periosteum during fracture healing in rats: interaction of fracture hematoma and the periosteum in the initial step of the healing process. J. Orthop. Sci. 2000;5:64–70. doi: 10.1007/s007760050010. [DOI] [PubMed] [Google Scholar]
- 41.Chan JK, et al. Low-dose TNF augments fracture healing in normal and osteoporotic bone by up-regulating the innate immune response. EMBO Mol. Med. 2015;7:547–561. doi: 10.15252/emmm.201404487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Timlin M, et al. Fracture hematoma is a potent proinflammatory mediator of neutrophil function. J. Trauma. 2005;58:1223–1229. doi: 10.1097/01.TA.0000169866.88781.F1. [DOI] [PubMed] [Google Scholar]
- 43.Gibon E, Lu L, Goodman SB. Aging, inflammation, stem cells, and bone healing. Stem Cell Res. Ther. 2016;7:44. doi: 10.1186/s13287-016-0300-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Briot K, Geusens P, Em Bultink I, Lems WF, Roux C. Inflammatory diseases and bone fragility. Osteoporos. Int. 2017;28:3301–3314. doi: 10.1007/s00198-017-4189-7. [DOI] [PubMed] [Google Scholar]
- 45.Weng N-p. Aging of the immune system: how much can the adaptive immune system adapt? Immunity. 2006;24:495–499. doi: 10.1016/j.immuni.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McKenna RW, Washington LT, Aquino DB, Picker LJ, Kroft SH. Immunophenotypic analysis of hematogones (B-lymphocyte precursors) in 662 consecutive bone marrow specimens by 4-color flow cytometry. Blood. 2001;98:2498–2507. doi: 10.1182/blood.V98.8.2498. [DOI] [PubMed] [Google Scholar]
- 47.Frasca D, et al. Aging down-regulates the transcription factor E2A, activation-induced cytidine deaminase, and Ig class switch in human B cells. J. Immunol. 2008;180:5283–5290. doi: 10.4049/jimmunol.180.8.5283. [DOI] [PubMed] [Google Scholar]
- 48.Chong Y, et al. CD27+ (memory) B cell decrease and apoptosis-resistant CD27− (naive) B cell increase in aged humans: implications for age-related peripheral B cell developmental disturbances. Int. Immunol. 2005;17:383–390. doi: 10.1093/intimm/dxh218. [DOI] [PubMed] [Google Scholar]
- 49.Weksler ME, Goodhardt M, Szabo P. The effect of age on B cell development and humoral immunity. Springe. Semin. Immunopathol. 2002;24:35–52. doi: 10.1007/s00281-001-0094-3. [DOI] [PubMed] [Google Scholar]
- 50.Swain S, Clise-Dwyer K, Haynes L. Homeostasis and the age-associated defect of CD4 T cells. Semin. Immunol. 2005;17:370–377. doi: 10.1016/j.smim.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kovtun A, et al. The crucial role of neutrophil granulocytes in bone fracture healing. Eur. Cells Mater. 2016;32:152–162. doi: 10.22203/eCM.v032a10. [DOI] [PubMed] [Google Scholar]
- 52.Hearps AC, et al. Aging is associated with chronic innate immune activation and dysregulation of monocyte phenotype and function. Aging Cell. 2012;11:867–875. doi: 10.1111/j.1474-9726.2012.00851.x. [DOI] [PubMed] [Google Scholar]
- 53.Sinder BP, Pettit AR, McCauley LK. Macrophages: their emerging roles in bone. J. Bone Miner. Res. 2015;30:2140–2149. doi: 10.1002/jbmr.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shaw AC, Goldstein DR, Montgomery RR. Age-dependent dysregulation of innate immunity. Nat. Rev. Immunol. 2013;13:875–887. doi: 10.1038/nri3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qian F, et al. Age-associated elevation in TLR5 leads to increased inflammatory responses in the elderly. Aging Cell. 2012;11:104–110. doi: 10.1111/j.1474-9726.2011.00759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cope AP, et al. Chronic exposure to tumor necrosis factor (TNF) in vitro impairs the activation of T cells through the T cell receptor/CD3 complex; reversal in vivo by anti-TNF antibodies in patients with rheumatoid arthritis. J. Clin. Investig. 1994;94:749–760. doi: 10.1172/JCI117394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Frasca D, et al. A molecular mechanism for TNF-α-mediated down-regulation of B cell responses. J. Immunol. (Baltim., MD: 1950) 2012;188:279–286. doi: 10.4049/jimmunol.1003964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Davis LS, Cush JJ, Schulze-Koops H, Lipsky PE. Rheumatoid synovial CD4+ T cells exhibit a reduced capacity to differentiate into IL-4-producing T-helper-2 effector cells. Arthritis Res. 2001;3:54–64. doi: 10.1186/ar140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Isomaki P, et al. Prolonged exposure of T cells to TNF down-regulates TCR zeta and expression of the TCR/CD3 complex at the cell surface. J. Immunol. (Baltim., MD: 1950) 2001;166:5495–5507. doi: 10.4049/jimmunol.166.9.5495. [DOI] [PubMed] [Google Scholar]
- 60.Lim JC, et al. TNFalpha contributes to diabetes impaired angiogenesis in fracture healing. Bone. 2017;99:26–38. doi: 10.1016/j.bone.2017.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oishi Y, Manabe I. Macrophages in age-related chronic inflammatory diseases. Npj Aging Mech. Dis. 2016;2:16018. doi: 10.1038/npjamd.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blanchette KA, Prabhakara R, Shirtliff ME, Wenke JC. Inhibition of fracture healing in the presence of contamination by Staphylococcus aureus: effects of growth state and immune response. J. Orthop. Res. 2017;35:1845–1854. doi: 10.1002/jor.23573. [DOI] [PubMed] [Google Scholar]
- 63.Kumar A, Tassopoulos AM, Li Q, Yu FS. Staphylococcus aureus protein A induced inflammatory response in human corneal epithelial cells. Biochem. Biophys. Res. Commun. 2007;354:955–961. doi: 10.1016/j.bbrc.2007.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Olaru F, Jensen LE. Staphylococcus aureus stimulates neutrophil targeting chemokine expression in keratinocytes through an autocrine IL-1alpha signaling loop. J. Invest. Dermatol. 2010;130:1866–1876. doi: 10.1038/jid.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stenzel W, et al. An essential role for tumor necrosis factor in the formation of experimental murine Staphylococcus aureus-induced brain abscess and clearance. J. Neuropathol. Exp. Neurol. 2005;64:27–36. doi: 10.1093/jnen/64.1.27. [DOI] [PubMed] [Google Scholar]
- 66.Liu H, et al. Staphylococcus aureus epicutaneous exposure drives skin inflammation via IL-36-mediated T cell responses. Cell Host microbe. 2017;22:653–666.e655. doi: 10.1016/j.chom.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hofbauer LC, et al. Interleukin-1beta and tumor necrosis factor-alpha, but not interleukin-6, stimulate osteoprotegerin ligand gene expression in human osteoblastic cells. Bone. 1999;25:255–259. doi: 10.1016/S8756-3282(99)00162-3. [DOI] [PubMed] [Google Scholar]
- 68.Cenci S, et al. Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-alpha. J. Clin. Investig. 2000;106:1229–1237. doi: 10.1172/JCI11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fuller K, Murphy C, Kirstein B, Fox SW, Chambers TJ. TNFalpha potently activates osteoclasts, through a direct action independent of and strongly synergistic with RANKL. Endocrinology. 2002;143:1108–1118. doi: 10.1210/endo.143.3.8701. [DOI] [PubMed] [Google Scholar]
- 70.Scheidt-Nave C, et al. Serum interleukin 6 is a major predictor of bone loss in women specific to the first decade past menopause. J. Clin. Endocrinol. Metab. 2001;86:2032–2042. doi: 10.1210/jcem.86.5.7445. [DOI] [PubMed] [Google Scholar]
- 71.Cuturi MC, et al. Independent regulation of tumor necrosis factor and lymphotoxin production by human peripheral blood lymphocytes. J. Exp. Med. 1987;165:1581–1594. doi: 10.1084/jem.165.6.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Azuma Y, Kaji K, Katogi R, Takeshita S, Kudo A. Tumor necrosis factor-α induces differentiation of and bone resorption by osteoclasts. J. Biol. Chem. 2000;275:4858–4864. doi: 10.1074/jbc.275.7.4858. [DOI] [PubMed] [Google Scholar]
- 73.Gilbert L, et al. Inhibition of osteoblast differentiation by tumor necrosis factor-alpha. Endocrinology. 2000;141:3956–3964. doi: 10.1210/endo.141.11.7739. [DOI] [PubMed] [Google Scholar]
- 74.Kitaura H, et al. Immunological reaction in TNF-α-mediated osteoclast formation and bone resorption in vitro and in vivo. Clin. Dev. Immunol. 2013;2013:8. doi: 10.1155/2013/181849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim JH, et al. The mechanism of osteoclast differentiation induced by IL-1. J. Immunol. (Baltim., MD: 1950) 2009;183:1862–1870. doi: 10.4049/jimmunol.0803007. [DOI] [PubMed] [Google Scholar]
- 76.Udagawa N, et al. Interleukin (IL)-6 induction of osteoclast differentiation depends on IL-6 receptors expressed on osteoblastic cells but not on osteoclast progenitors. J. Exp. Med. 1995;182:1461–1468. doi: 10.1084/jem.182.5.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chakravarti A, Raquil MA, Tessier P, Poubelle PE. Surface RANKL of Toll-like receptor 4-stimulated human neutrophils activates osteoclastic bone resorption. Blood. 2009;114:1633–1644. doi: 10.1182/blood-2008-09-178301. [DOI] [PubMed] [Google Scholar]
- 78.Allaeys I, et al. Osteoblast retraction induced by adherent neutrophils promotes osteoclast bone resorption: implication for altered bone remodeling in chronic gout. Lab. Investig. 2011;91:905–920. doi: 10.1038/labinvest.2011.46. [DOI] [PubMed] [Google Scholar]
- 79.Takayanagi H, et al. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature. 2000;408:600–605. doi: 10.1038/35046102. [DOI] [PubMed] [Google Scholar]
- 80.Schlundt C, et al. Macrophages in bone fracture healing: their essential role in endochondral ossification. Bone. 2018;106:78–89. doi: 10.1016/j.bone.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 81.Horowitz MC, Fretz JA, Lorenzo JA. How B cells influence bone biology in health and disease. Bone. 2010;47:472–479. doi: 10.1016/j.bone.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yonou H, et al. Osteoprotegerin/osteoclastogenesis inhibitory factor decreases human prostate cancer burden in human adult bone implanted into nonobese diabetic/severe combined immunodeficient mice. Cancer Res. 2003;63:2096–2102. [PubMed] [Google Scholar]
- 83.Catalano A, et al. Pain in osteoporosis: from pathophysiology to therapeutic approach. Drugs aging. 2017;34:755–765. doi: 10.1007/s40266-017-0492-4. [DOI] [PubMed] [Google Scholar]
- 84.Zuo Fuxing, Xiong Feng, Wang Xia, Li Xueyuan, Wang Renzhi, Ge Wei, Bao Xinjie. Intrastriatal Transplantation of Human Neural Stem Cells Restores the Impaired Subventricular Zone in Parkinsonian Mice. STEM CELLS. 2017;35(6):1519–1531. doi: 10.1002/stem.2616. [DOI] [PubMed] [Google Scholar]
- 85.Tatsumi S, et al. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 2007;5:464–475. doi: 10.1016/j.cmet.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 86.Glatt V, Evans CH, Tetsworth K. A concert between biology and biomechanics: the influence of the mechanical environment on bone healing. Front. Physiol. 2016;7:678. doi: 10.3389/fphys.2016.00678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Plotkin LI, Bellido T. Osteocytic signalling pathways as therapeutic targets for bone fragility. Nat. Rev. Endocrinol. 2016;12:593–605. doi: 10.1038/nrendo.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Han MKL, de Rooij J. Converging and unique mechanisms of mechanotransduction at adhesion sites. Trends Cell Biol. 2016;26:612–623. doi: 10.1016/j.tcb.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 89.Sikavitsas VI, Temenoff JS, Mikos AG. Biomaterials and bone mechanotransduction. Biomaterials. 2001;22:2581–2593. doi: 10.1016/S0142-9612(01)00002-3. [DOI] [PubMed] [Google Scholar]
- 90.Xiao ZS, Quarles LD. Role of the polycytin-primary cilia complex in bone development and mechanosensing. Ann. New Y. Acad. Sci. 2010;1192:410–421. doi: 10.1111/j.1749-6632.2009.05239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tu X, et al. Osteocytes mediate the anabolic actions of canonical Wnt/beta-catenin signaling in bone. Proc. Natl Acad. Sci. USA. 2015;112:E478–E486. doi: 10.1073/pnas.1409857112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nguyen AM, Jacobs CR. Emerging role of primary cilia as mechanosensors in osteocytes. Bone. 2013;54:196–204. doi: 10.1016/j.bone.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yavropoulou MP, Yovos JG. The molecular basis of bone mechanotransduction. J. Musculoskelet. Neuron. Interact. 2016;16:221–236. [PMC free article] [PubMed] [Google Scholar]
- 94.Ranade SS, Syeda R, Patapoutian A. Mechanically activated ion channels. Neuron. 2015;87:1162–1179. doi: 10.1016/j.neuron.2015.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lewis KJ, et al. Osteocyte calcium signals encode strain magnitude and loading frequency in vivo. Proc. Natl Acad. Sci. USA. 2017;114:11775–11780. doi: 10.1073/pnas.1707863114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xu H, et al. Connexin 43 channels are essential for normal bone structure and osteocyte viability. J. Bone Miner. Res. 2015;30:436–448. doi: 10.1002/jbmr.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Plotkin LI, Speacht TL, Donahue HJ. Cx43 and mechanotransduction in bone. Curr. Osteoporos. Rep. 2015;13:67–72. doi: 10.1007/s11914-015-0255-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lynch ME, Fischbach C. Biomechanical forces in the skeleton and their relevance to bone metastasis: biology and engineering considerations. Adv. Drug Deliv. Rev. 2014;79-80:119–134. doi: 10.1016/j.addr.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fedorchak GR, Kaminski A, Lammerding J. Cellular mechanosensing: getting to the nucleus of it all. Prog. Biophys. Mol. Biol. 2014;115:76–92. doi: 10.1016/j.pbiomolbio.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim CH, You L, Yellowley CE, Jacobs CR. Oscillatory fluid flow-induced shear stress decreases osteoclastogenesis through RANKL and OPG signaling. Bone. 2006;39:1043–1047. doi: 10.1016/j.bone.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 101.Temiyasathit S, Jacobs CR. Osteocyte primary cilium and its role in bone mechanotransduction. Ann. New Y. Acad. Sci. 2010;1192:422–428. doi: 10.1111/j.1749-6632.2009.05243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Goggin PM, Zygalakis KC, Oreffo RO, Schneider P. High-resolution 3D imaging of osteocytes and computational modelling in mechanobiology: insights on bone development, ageing, health and disease. Eur. Cells Mater. 2016;31:264–295. doi: 10.22203/eCM.v031a18. [DOI] [PubMed] [Google Scholar]
- 103.Devlin MJ, Rosen CJ. The bone-fat interface: basic and clinical implications of marrow adiposity. The Lancet. Diab. Endocrinol. 2015;3:141–147. doi: 10.1016/S2213-8587(14)70007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li J, Liu X, Zuo B, Zhang L. The role of bone marrow microenvironment in governing the balance between osteoblastogenesis and adipogenesis. Aging Dis. 2016;7:514–525. doi: 10.14336/AD.2015.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Manolagas SC. The quest for osteoporosis mechanisms and rational therapies: how far we’ve come, how much further we need to go. J. Bone Miner. Res.: 2018;33:371–385. doi: 10.1002/jbmr.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Goodman CA, Hornberger TA, Robling AG. Bone and skeletal muscle: key players in mechanotransduction and potential overlapping mechanisms. Bone. 2015;80:24–36. doi: 10.1016/j.bone.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Boyce BF, Xiu Y, Li J, Xing L, Yao Z. NF-kappaB-mediated regulation of osteoclastogenesis. Endocrinol. Metab. 2015;30:35–44. doi: 10.3803/EnM.2015.30.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tarapore RS, et al. NF-kappaB has a direct role in inhibiting Bmp- and Wnt-induced matrix protein expression. J. Bone Miner. Res. 2016;31:52–64. doi: 10.1002/jbmr.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Thompson WR, Rubin CT, Rubin J. Mechanical regulation of signaling pathways in bone. Gene. 2012;503:179–193. doi: 10.1016/j.gene.2012.04.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Aguirre JI, et al. A novel ligand-independent function of the estrogen receptor is essential for osteocyte and osteoblast mechanotransduction. J. Biol. Chem. 2007;282:25501–25508. doi: 10.1074/jbc.M702231200. [DOI] [PubMed] [Google Scholar]
- 111.Srinivasan S, Gross TS, Bain SD. Bone mechanotransduction may require augmentation in order to strengthen the senescent skeleton. Ageing Res. Rev. 2012;11:353–360. doi: 10.1016/j.arr.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Devlin MJ. Estrogen, exercise, and the skeleton. Evolut. Anthropol. 2011;20:54–61. doi: 10.1002/evan.20299. [DOI] [PubMed] [Google Scholar]
- 113.Loi F, et al. Inflammation, fracture and bone repair. Bone. 2016;86:119–130. doi: 10.1016/j.bone.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lin TH, et al. NF-kappaB as a therapeutic target in inflammatory-associated bone diseases. Adv. protein Chem. Struct. Biol. 2017;107:117–154. doi: 10.1016/bs.apcsb.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Salles MB, et al. Evaluating nuclear factor NF-kappaB activation following bone trauma: a pilot study in a Wistar rats model. PLoS One. 2015;10:e0140630. doi: 10.1371/journal.pone.0140630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu, T., Zhang, L., Joo, D. & Sun, S. C. NF-kappaB signaling in inflammation. Signal Transduct.Target. Ther.2, 10.1038/sigtrans.2017.23 (2017). [DOI] [PMC free article] [PubMed]
- 117.Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011;7:33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 118.Long P, Liu F, Piesco NP, Kapur R, Agarwal S. Signaling by mechanical strain involves transcriptional regulation of proinflammatory genes in human periodontal ligament cells in vitro. Bone. 2002;30:547–552. doi: 10.1016/S8756-3282(02)00673-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Agarwal S, et al. A central role for the nuclear factor-kappaB pathway in anti-inflammatory and proinflammatory actions of mechanical strain. FASEB J. 2003;17:899–901. doi: 10.1096/fj.02-0901fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Novack DV. Role of NF-kappaB in the skeleton. Cell Res. 2011;21:169–182. doi: 10.1038/cr.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yu HS, Kim JJ, Kim HW, Lewis MP, Wall I. Impact of mechanical stretch on the cell behaviors of bone and surrounding tissues. J. Tissue Eng. 2016;7:2041731415618342. doi: 10.1177/2041731415618342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pires Bruno, Silva Rafael, Ferreira Gerson, Abdelhay Eliana. NF-kappaB: Two Sides of the Same Coin. Genes. 2018;9(1):24. doi: 10.3390/genes9010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang L, et al. Involvement of p38MAPK/NF-kappaB signaling pathways in osteoblasts differentiation in response to mechanical stretch. Ann. Biomed. Eng. 2012;40:1884–1894. doi: 10.1007/s10439-012-0548-x. [DOI] [PubMed] [Google Scholar]
- 124.Wang L, et al. Involvement of BMPs/Smad signaling pathway in mechanical response in osteoblasts. Cell. Physiol. Biochem.: Int. J. Exp. Cell Physiol., Biochem. Pharmacol. 2010;26:1093–1102. doi: 10.1159/000323987. [DOI] [PubMed] [Google Scholar]
- 125.Long P, Hu J, Piesco N, Buckley M, Agarwal S. Low magnitude of tensile strain inhibits IL-1beta-dependent induction of pro-inflammatory cytokines and induces synthesis of IL-10 in human periodontal ligament cells in vitro. J. Dent. Res. 2001;80:1416–1420. doi: 10.1177/00220345010800050601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sauerschnig M, et al. Effect of COX-2 inhibition on tendon-to-bone healing and PGE2 concentration after anterior cruciate ligament reconstruction. Eur. J. Med. Res. 2018;23:1. doi: 10.1186/s40001-017-0297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Thorsen K, Kristoffersson AO, Lerner UH, Lorentzon RP. In situ microdialysis in bone tissue. Stimulation of prostaglandin E2 release by weight-bearing mechanical loading. J. Clin. Investig. 1996;98:2446–2449. doi: 10.1172/JCI119061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cheung WH, Miclau T, Chow SK, Yang FF, Alt V. Fracture healing in osteoporotic bone. Injury. 2016;47(Suppl. 2):S21–S26. doi: 10.1016/S0020-1383(16)47004-X. [DOI] [PubMed] [Google Scholar]
- 129.Chow SK, et al. Mechanical stimulation enhanced estrogen receptor expression and callus formation in diaphyseal long bone fracture healing in ovariectomy-induced osteoporotic rats. Osteoporos. Int. 2016;27:2989–3000. doi: 10.1007/s00198-016-3619-2. [DOI] [PubMed] [Google Scholar]
- 130.Thomas M, Puleo D. Infection, inflammation, and bone regeneration: a paradoxical relationship. J. Dent. Res. 2011;90:1052–1061. doi: 10.1177/0022034510393967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Peichl P, Holzer LA, Maier R, Holzer G. Parathyroid hormone 1-84 accelerates fracture-healing in pubic bones of elderly osteoporotic women. J. Bone Jt. Surg. Am. Vol. 2011;93:1583–1587. doi: 10.2106/JBJS.J.01379. [DOI] [PubMed] [Google Scholar]
- 132.Grundnes O, Reikeras O. The role of hematoma and periosteal sealing for fracture healing in rats. Acta Orthop. Scand. 1993;64:47–49. doi: 10.3109/17453679308994527. [DOI] [PubMed] [Google Scholar]
- 133.Yuasa M, et al. Fibrinolysis is essential for fracture repair and prevention of heterotopic ossification. J. Clin. Investig. 2015;125:3723. doi: 10.1172/JCI84059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dohan Ehrenfest DM, et al. Classification of platelet concentrates (Platelet-Rich Plasma-PRP, Platelet-Rich Fibrin-PRF) for topical and infiltrative use in orthopedic and sports medicine: current consensus, clinical implications and perspectives. Muscles Ligaments Tendons J. 2014;4:3–9. doi: 10.32098/mltj.01.2014.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xie H, et al. PDGF-BB secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis. Nat. Med. 2014;20:1270–1278. doi: 10.1038/nm.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wagatsuma A. Effect of aging on expression of angiogenesis-related factors in mouse skeletal muscle. Exp. Gerontol. 2006;41:49–54. doi: 10.1016/j.exger.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 137.Kallala R, et al. In vitro and in vivo effects of antibiotics on bone cell metabolism and fracture healing. Expert Opin. Drug Saf. 2012;11:15–32. doi: 10.1517/14740338.2012.643867. [DOI] [PubMed] [Google Scholar]
- 138.Antoci V, Jr., Adams CS, Hickok NJ, Shapiro IM, Parvizi J. Antibiotics for local delivery systems cause skeletal cell toxicity in vitro. Clin. Orthop. Relat. Res. 2007;462:200–206. doi: 10.1097/BLO.0b013e31811ff866. [DOI] [PubMed] [Google Scholar]
- 139.Cope AP, et al. Chronic tumor necrosis factor alters T cell responses by attenuating T cell receptor signaling. J. Exp. Med. 1997;185:1573–1584. doi: 10.1084/jem.185.9.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chopin F, et al. Long-term effects of infliximab on bone and cartilage turnover markers in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2008;67:353–357. doi: 10.1136/ard.2007.076604. [DOI] [PubMed] [Google Scholar]
- 141.Marotte H, et al. A 1-year case-control study in patients with rheumatoid arthritis indicates prevention of loss of bone mineral density in both responders and nonresponders to infliximab. Arthritis Res. Ther. 2007;9:R61. doi: 10.1186/ar2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Roggia C, et al. Up-regulation of TNF-producing T cells in the bone marrow: a key mechanism by which estrogen deficiency induces bone loss in vivo. Proc. Natl Acad. Sci. USA. 2001;98:13960–13965. doi: 10.1073/pnas.251534698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kimble RB, et al. Simultaneous block of interleukin-1 and tumor necrosis factor is required to completely prevent bone loss in the early postovariectomy period. Endocrinology. 1995;136:3054–3061. doi: 10.1210/endo.136.7.7789332. [DOI] [PubMed] [Google Scholar]
- 144.Gao Y, et al. Estrogen prevents bone loss through transforming growth factor β signaling in T cells. Proc. Natl Acad. Sci. USA. 2004;101:16618–16623. doi: 10.1073/pnas.0404888101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Scherrer CB, Mannion AF, Kyburz D, Vogt M. & Kramers-de Quervain, I. A. Infection risk after orthopedic surgery in patients with inflammatory rheumatic diseases treated with immunosuppressive drugs. Arthritis Care Res. 2013;65:2032–2040. doi: 10.1002/acr.22077. [DOI] [PubMed] [Google Scholar]
- 146.Hlaing TT, Compston JE. Biochemical markers of bone turnover - uses and limitations. Ann. Clin. Biochem. 2014;51:189–202. doi: 10.1177/0004563213515190. [DOI] [PubMed] [Google Scholar]
- 147.Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin. Proc. 2008;83:1032–1045. doi: 10.4065/83.9.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dirschl DR, Rustom H. Practice patterns and performance in U.S. fracture liaison programs: an analysis of > 32,000 patients from the own the bone program. J. Bone Jt. Surg. Am. Vol. 2018;100:680–685. doi: 10.2106/JBJS.17.00665. [DOI] [PubMed] [Google Scholar]
- 149.Hauser M, Siegrist M, Keller I, Hofstetter W. Healing of fractures in osteoporotic bones in mice treated with bisphosphonates—a transcriptome analysis. Bone. 2018;112:107–119. doi: 10.1016/j.bone.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 150.Zaheer S, LeBoff M, Lewiecki EM. Denosumab for the treatment of osteoporosis. Expert Opin. Drug Metab. Toxicol. 2015;11:461–470. doi: 10.1517/17425255.2015.1000860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bone HG, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. The Lancet. Diabetes Endocrinol. 2017;5:513–523. doi: 10.1016/S2213-8587(17)30138-9. [DOI] [PubMed] [Google Scholar]
- 152.Silverman SL, Azria M. The analgesic role of calcitonin following osteoporotic fracture. Osteoporos. Int. 2002;13:858–867. doi: 10.1007/s001980200118. [DOI] [PubMed] [Google Scholar]
- 153.Liu Y, et al. Hexapeptide-conjugated calcitonin for targeted therapy of osteoporosis. J. Control. Release. 2019;304:39–50. doi: 10.1016/j.jconrel.2019.04.042. [DOI] [PubMed] [Google Scholar]
- 154.Ren L, Wang W. Effect of risedronate on femoral periprosthetic bone loss following total hip replacement: a systematic review and meta-analysis. Medicine. 2018;97:e0379. doi: 10.1097/MD.0000000000010379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Redlich K, et al. Repair of local bone erosions and reversal of systemic bone loss upon therapy with anti-tumor necrosis factor in combination with osteoprotegerin or parathyroid hormone in tumor necrosis factor-mediated arthritis. Am. J. Pathol. 2004;164:543–555. doi: 10.1016/S0002-9440(10)63144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Thompson WR, et al. Osteocyte specific responses to soluble and mechanical stimuli in a stem cell derived culture model. Sci. Rep. 2015;5:11049. doi: 10.1038/srep11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cianferotti L, D’Asta F, Brandi ML. A review on strontium ranelate long-term antifracture efficacy in the treatment of postmenopausal osteoporosis. Ther. Adv. Musculoskelet. Dis. 2013;5:127–139. doi: 10.1177/1759720X13483187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Iolascon G, et al. Bone quality and bone strength: benefits of the bone-forming approach. Clin. Cases Miner. Bone Metab. 2014;11:20–24. [PMC free article] [PubMed] [Google Scholar]
- 159.Langdahl BL, et al. Romosozumab (sclerostin monoclonal antibody) versus teriparatide in postmenopausal women with osteoporosis transitioning from oral bisphosphonate therapy: a randomised, open-label, phase 3 trial. Lancet. 2017;390:1585–1594. doi: 10.1016/S0140-6736(17)31613-6. [DOI] [PubMed] [Google Scholar]
- 160.MacFarlane Elena Gallo, Haupt Julia, Dietz Harry C., Shore Eileen M. TGF-β Family Signaling in Connective Tissue and Skeletal Diseases. Cold Spring Harbor Perspectives in Biology. 2017;9(11):a022269. doi: 10.1101/cshperspect.a022269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rys JP, Monteiro DA, Alliston T. Mechanobiology of TGFbeta signaling in the skeleton. Matrix Biol.: J. Int. Soc. Matrix Biol. 2016;52:413–425. doi: 10.1016/j.matbio.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Li M, Liu X, Liu X, Ge B. Calcium phosphate cement with BMP-2-loaded gelatin microspheres enhances bone healing in osteoporosis: a pilot study. Clin. Orthop. Relat. Res. 2010;468:1978–1985. doi: 10.1007/s11999-010-1321-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hu MH, et al. Polymethylmethacrylate augmentation of the pedicle screw: the cement distribution in the vertebral body. Eur. Spine J. 2011;20:1281–1288. doi: 10.1007/s00586-011-1824-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bettencourt A, Almeida AJ. Poly(methyl methacrylate) particulate carriers in drug delivery. J. Microencapsul. 2012;29:353–367. doi: 10.3109/02652048.2011.651500. [DOI] [PubMed] [Google Scholar]
- 165.Webb JC, Spencer RF. The role of polymethylmethacrylate bone cement in modern orthopaedic surgery. J. bone Jt. Surg. Br. Vol. 2007;89:851–857. doi: 10.1302/0301-620X.89B7.19148. [DOI] [PubMed] [Google Scholar]
- 166.Arora M, Chan EK, Gupta S, Diwan AD. Polymethylmethacrylate bone cements and additives: a review of the literature. World J. Orthop. 2013;4:67–74. doi: 10.5312/wjo.v4.i2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Watson JT, Nicolaou DA. Orthobiologics in the augmentation of osteoporotic fractures. Curr. Osteoporos. Rep. 2015;13:22–29. doi: 10.1007/s11914-014-0249-5. [DOI] [PubMed] [Google Scholar]
- 168.Schumaier A, Grawe B. Proximal humerus fractures: evaluation and management in the elderly patient. Geriatr. Orthop. Surg. Rehabilit. 2018;9:2151458517750516. doi: 10.1177/2151458517750516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chan ME, Uzer G, Rubin CT. The potential benefits and inherent risks of vibration as a non-drug therapy for the prevention and treatment of osteoporosis. Curr. Osteoporos. Rep. 2013;11:36–44. doi: 10.1007/s11914-012-0132-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Nagaraja MP, Jo H. The role of mechanical stimulation in recovery of bone loss-high versus low magnitude and frequency of force. Life. 2014;4:117–130. doi: 10.3390/life4020117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Griffin, X. L., Parsons, N., Costa, M. L. & Metcalfe, D. Ultrasound and shockwave therapy for acute fractures in adults. Cochrane Database Syst. Rev. Cd008579, 10.1002/14651858.CD008579.pub3 (2014). [DOI] [PMC free article] [PubMed]
- 172.Miller PD. The history of bone densitometry. Bone. 2017;104:4–6. doi: 10.1016/j.bone.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 173.Seeman E. Pathogenesis of bone fragility in women and men. Lancet. 2002;359:1841–1850. doi: 10.1016/S0140-6736(02)08706-8. [DOI] [PubMed] [Google Scholar]
- 174.de Bakker CMJ, Tseng WJ, Li Y, Zhao H, Liu XS. Clinical evaluation of bone strength and fracture risk. Curr. Osteoporos. Rep. 2017;15:32–42. doi: 10.1007/s11914-017-0346-3. [DOI] [PubMed] [Google Scholar]
- 175.Silva BC, et al. Trabecular bone score (TBS)—a novel method to evaluate bone microarchitectural texture in patients with primary hyperparathyroidism. J. Clin. Endocrinol. Metab. 2013;98:1963–1970. doi: 10.1210/jc.2012-4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Harvey NC, et al. Trabecular bone score (TBS) as a new complementary approach for osteoporosis evaluation in clinical practice. Bone. 2015;78:216–224. doi: 10.1016/j.bone.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Shevroja E, et al. Use of trabecular bone score (TBS) as a complementary approach to dual-energy X-ray absorptiometry (DXA) for fracture risk assessment in clinical practice. J. Clin. Densitom. 2017;20:334–345. doi: 10.1016/j.jocd.2017.06.019. [DOI] [PubMed] [Google Scholar]
- 178.Iki M, et al. Trabecular bone score may improve FRAX(R) prediction accuracy for major osteoporotic fractures in elderly Japanese men: the Fujiwara-kyo Osteoporosis Risk in Men (FORMEN) Cohort Study. Osteoporos. Int. 2015;26:1841–1848. doi: 10.1007/s00198-015-3092-3. [DOI] [PubMed] [Google Scholar]
- 179.Brandi ML. Microarchitecture, the key to bone quality. Rheumatology. 2009;48(Suppl. 4):iv3–iv8. doi: 10.1093/rheumatology/kep273. [DOI] [PubMed] [Google Scholar]
- 180.Eckert JA, Jaeger S, Klotz MC, Schwarze M, Bitsch RG. Can intraoperative measurement of bone quality help in decision making for cementless unicompartmental knee arthroplasty? Knee. 2018;25:609–616. doi: 10.1016/j.knee.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 181.Seebeck J, et al. Effect of cortical thickness and cancellous bone density on the holding strength of internal fixator screws. J. Orthop. Res. 2004;22:1237–1242. doi: 10.1016/j.orthres.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 182.Shea TM, et al. Designs and techniques that improve the pullout strength of pedicle screws in osteoporotic vertebrae: current status. BioMed. Res. Int. 2014;2014:748393. doi: 10.1155/2014/748393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wang T, Boone C, Behn AW, Ledesma JB, Bishop JA. Cancellous screws are biomechanically superior to cortical screws in metaphyseal bone. Orthopedics. 2016;39:e828–e832. doi: 10.3928/01477447-20160509-01. [DOI] [PubMed] [Google Scholar]
- 184.Cornell CN. Internal fracture fixation in patients with osteoporosis. J. Am. Acad. Orthop. Surg. 2003;11:109–119. doi: 10.5435/00124635-200303000-00005. [DOI] [PubMed] [Google Scholar]
- 185.Ab-Lazid R, Perilli E, Ryan MK, Costi JJ, Reynolds KJ. Does cancellous screw insertion torque depend on bone mineral density and/or microarchitecture? J. Biomech. 2014;47:347–353. doi: 10.1016/j.jbiomech.2013.11.030. [DOI] [PubMed] [Google Scholar]
- 186.Karim L, Vashishth D. Role of trabecular microarchitecture in the formation, accumulation, and morphology of microdamage in human cancellous bone. J. Orthop. Res. 2011;29:1739–1744. doi: 10.1002/jor.21448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Greiwe RM, Archdeacon MT. Locking plate technology: current concepts. J. Knee Surg. 2007;20:50–55. doi: 10.1055/s-0030-1248022. [DOI] [PubMed] [Google Scholar]
- 188.Miranda MA. Locking plate technology and its role in osteoporotic fractures. Injury. 2007;38(Suppl. 3):S35–S39. doi: 10.1016/j.injury.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 189.Kralinger F, et al. The influence of local bone density on the outcome of one hundred and fifty proximal humeral fractures treated with a locking plate. J. Bone Jt. Surg. Am. Vol. 2014;96:1026–1032. doi: 10.2106/JBJS.M.00028. [DOI] [PubMed] [Google Scholar]
- 190.Johanson NA, Litrenta J, Zampini JM, Kleinbart F, Goldman HM. Surgical treatment options in patients with impaired bone quality. Clin. Orthop. Relat. Res. 2011;469:2237–2247. doi: 10.1007/s11999-011-1838-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ito K, Hungerbuhler R, Wahl D, Grass R. Improved intramedullary nail interlocking in osteoporotic bone. J. Orthop. Trauma. 2001;15:192–196. doi: 10.1097/00005131-200103000-00008. [DOI] [PubMed] [Google Scholar]
- 192.McKee MD, et al. A multicenter, prospective, randomized, controlled trial of open reduction-internal fixation versus total elbow arthroplasty for displaced intra-articular distal humeral fractures in elderly patients. J. Shoulder Elb. Surg. 2009;18:3–12. doi: 10.1016/j.jse.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 193.Boraiah S, Ragsdale M, Achor T, Zelicof S, Asprinio DE. Open reduction internal fixation and primary total hip arthroplasty of selected acetabular fractures. J. Orthop. Trauma. 2009;23:243–248. doi: 10.1097/BOT.0b013e3181923fb8. [DOI] [PubMed] [Google Scholar]
- 194.Goldhahn J, Suhm N, Goldhahn S, Blauth M, Hanson B. Influence of osteoporosis on fracture fixation-a systematic literature review. Osteoporos. Int. 2008;19:761–772. doi: 10.1007/s00198-007-0515-9. [DOI] [PubMed] [Google Scholar]
- 195.Seebeck J, Goldhahn J, Morlock MM, Schneider E. Mechanical behavior of screws in normal and osteoporotic bone. Osteoporos. Int. 2005;16(Suppl. 2):S107–S111. doi: 10.1007/s00198-004-1777-0. [DOI] [PubMed] [Google Scholar]
- 196.McAndrew CM, et al. Local bone quality measurements correlates with maximum screw torque at the femoral diaphysis. Clin. Biomech. 2018;52:95–99. doi: 10.1016/j.clinbiomech.2018.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Parkinson IH, Fazzalari NL. Whole bone geometry and bone quality in distal forearm fracture. J. Orthop. Trauma. 2008;22:S59–S65. doi: 10.1097/BOT.0b013e318162ab25. [DOI] [PubMed] [Google Scholar]
- 198.Cornell CN, Ayalon O. Evidence for success with locking plates for fragility fractures. HSS J. 2011;7:164–169. doi: 10.1007/s11420-010-9194-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ricci WM, Tornetta P, Borrelli J., Jr. Lag screw fixation of medial malleolar fractures: a biomechanical, radiographic, and clinical comparison of unicortical partially threaded lag screws and bicortical fully threaded lag screws. J. Orthop. trauma. 2012;26:602–606. doi: 10.1097/BOT.0b013e3182404512. [DOI] [PubMed] [Google Scholar]
- 200.Egol KA, Kubiak EN, Fulkerson E, Kummer FJ, Koval KJ. Biomechanics of locked plates and screws. J. Orthop. trauma. 2004;18:488–493. doi: 10.1097/00005131-200409000-00003. [DOI] [PubMed] [Google Scholar]
- 201.Babhulkar S. Unstable trochanteric fractures: Issues and avoiding pitfalls. Injury. 2017;48:803–818. doi: 10.1016/j.injury.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 202.Oheim R, Schinke T, Amling M, Pogoda P. Can we induce osteoporosis in animals comparable to the human situation? Injury. 2016;47(Suppl. 1):S3–S9. doi: 10.1016/S0020-1383(16)30002-X. [DOI] [PubMed] [Google Scholar]
- 203.Sproul RC, Iyengar JJ, Devcic Z, Feeley BT. A systematic review of locking plate fixation of proximal humerus fractures. Injury. 2011;42:408–413. doi: 10.1016/j.injury.2010.11.058. [DOI] [PubMed] [Google Scholar]
- 204.Mellibovsky L, et al. Bone tissue properties measurement by reference point indentation in glucocorticoid-induced osteoporosis. J. Bone Miner. Res. 2015;30:1651–1656. doi: 10.1002/jbmr.2497. [DOI] [PubMed] [Google Scholar]
- 205.Sanchez-Riera L, et al. Osteoporosis and fragility fractures. Best Pract. Res. Clin. Rheumatol. 2010;24:793–810. doi: 10.1016/j.berh.2010.10.003. [DOI] [PubMed] [Google Scholar]