Abstract
Granulocyte colony-stimulating factor (GM-CSF), produced by CD4+ T cells, has recently been implicated in the pathogenesis of inflammatory diseases, such as multiple sclerosis and juvenile arthritis. However, the role of GM-CSF-producing CD4+ T cells in sepsis remains unknown. This study reports peripheral changes in GM-CSF-producing CD4+ T cells in septic patients and the possible underlying mechanism by which GM-CSF influences the outcome of sepsis. Forty-three septic patients, 20 SIRS patients, and 20 healthy controls were enrolled in this study and followed for 28 days to assess mortality. We measured the peripheral frequency of GM-CSF+CD4+ T cells and recorded their associated relationship with disease progression. Our data demonstrated that peripheral GM-CSF-producing CD4+ T cells were significantly higher in septic patients than in both SIRS patients and healthy controls. These cells exhibit a memory phenotype and impaired IFN-γ-secreting capacity in sepsis patients. Using a receiver operating curve analysis with 8.01% as a cut-off point, the percentage of GM-CSF+CD4+ T cells could predict the outcome of septic patients. Combined with the increase in GM-CSF-producing CD4+ T cells, inflammatory cytokines IL-1β and IL-6 were also upregulated. Using an in vitro neutrophil model, we found that GM-CSF inhibited C3aR expression, while inducing IL-8 production. Furthermore, this effect was transferrable in plasma from sepsis patients and was attenuated by inhibition of GM-CSF using an anti-GM-CSF antibody. These results indicate that GM-CSF-producing CD4+ T cells may serve as a marker of sepsis severity. Thus, targeting GM-CSF overproduction may benefit sepsis patients.
Keywords: GM-CSF, CD4+ T cells, sepsis, SIRS
Introduction
Sepsis is a host response disorder resulting from severe invasive pathogen infection that is life threatening. Sepsis is the leading cause of death among critically ill patients. Although prompt treatment with antibiotics and advances in life-supporting technologies help to some degree, the mortality of sepsis remains high.1 A “cytokine storm” has long been thought to be the culprit of sepsis and has dominated clinical and basic research for many years.2 Despite extensive clinical studies, the pathogenesis of sepsis remains poorly understood, and the condition lacks effective therapies.
Granulocyte colony-stimulating factor (GM-CSF) is a traditional hematopoietic-stimulating factor routinely used in the clinic for treating neutropenia following chemotherapy. GM-CSF is currently considered a potent pro-inflammatory cytokine that plays an important role in many inflammatory responses and is used as a therapeutic target for some inflammatory diseases.3 In an animal model of experimental autoimmune encephalomyelitis (EAE), GM-CSF-producing CD4+ T cells were found to be responsible for inducing EAE. Moreover, CD4+ T cells lacking GM-CSF failed to induce neuroinflammation, despite exhibiting normal production of interleukin-17 (IL-17) and interferon-γ (IFN-γ).4 Therefore, in contrast to other known helper T-cell-derived cytokines, GM-CSF is thought to play an important role in the initiation of some autoimmune diseases, regardless of helper T-cell polarization.5
Interestingly, in a clinical setting, application GM-CSF has been associated with the ability to reverse sepsis-associated immunosuppression and restore monocytic immunocompetence.6 Furthermore, GM-CSF combined with antibiotic therapy exerts a coordinated effect of reducing both the risk of infection and the length of hospitalization for sepsis patients.7 Although such compelling evidence highlights the role of GM-CSF in the pathology of sepsis, the specific dynamics of GM-CSF-producing CD4+ T cells and their correlation with disease outcome in sepsis remain unknown. This study focused on the dynamics of GM-CSF-producing CD4+ T cells in sepsis and its predictive value in the prognosis of septic patients.
Results
Clinical characteristics of the enrolled subjects
Twenty healthy subjects, 20 patients with systemic inflammatory response syndrome (SIRS), and 43 patients with sepsis were enrolled in this study. Both SIRS and sepsis patients were comparable in age and gender to healthy controls (HCs) (P = 0.62 and P = 0.76, respectively). The sepsis-related organ failure assessment (SOFA) score of sepsis patients was significantly higher than that of SIRS patients (P < 0.01). The most common site of infection among sepsis patients was the lung (23 patients, 53.5%), followed by the abdomen (15 patients, 34.9%), blood (3 patients, 6.9%), and urinary system (2 patients, 4.7%). Pathogen cultures included Gram-negative bacteria (24 patients, 55.8%), Gram-positive bacteria (9 patients, 20.9%), fungi (3 patients, 7.0%), and negative cultures (7 patients, 16.3%). The mean length of the intensive care unit (ICU) stay for sepsis patients was 23.5 ± 7.6 days. The 28-day mortality rate of sepsis patients was 34.9%. Clinical characteristics of enrolled subjects are summarized in Table 1.
Table 1.
Clinical characteristics of the enrolled subjects
| HC | SIRS | Sepsis | P-value | |
|---|---|---|---|---|
| Number of subjects | 20 | 20 | 43 | |
| Age (years) | 46.2 ± 9.3 | 45.9 ± 7.4 | 44.9 ± 8.6 | 0.62 |
| Sex (male/female) | 15/5 | 13/7 | 29/14 | 0.76 |
| SOFA score | - | 4.0 ± 1.1 | 9.7 ± 4.1 | < 0.01 |
| WBC (×109/L) | 5.1 ± 2.4 | 17.6 ± 4.2 | 15.9 ± 6.0 | < 0.01 |
| PCT (ng/mL) | - | - | 6.2 ± 3.8 | - |
| Pathogen culture | ||||
| Gram−bacteria | - | - | 24 (55.8%) | - |
| Gram+ bacteria | - | - | 9 (20.9%) | - |
| Fungi | - | - | 3 (7.0%) | - |
| Negative culture | - | - | 7 (16.3%) | - |
| Mechanical ventilation (n) | - | 4 (9.3%) | 17 (39.5%) | 0.21 |
| Mechanical ventilation (days) | - | 1.25 ± 0.4 | 8.1 ± 2.7 | < 0.01 |
| Renal replacement therapy (n) | - | 0 (0%) | 12 (27.9%) | 0.02 |
| ICU stay (days) | - | 2.8 ± 0.7 | 23.5 ± 7.6 | < 0.01 |
| Mortality (nonsurviving/surviving) | - | 0% (0/20) | 34.9% (15/28) | < 0.01 |
Data are shown as number (%) or mean ± SD
SIRS systemic inflammatory response syndrome, SOFA sepsis-related organ failure assessment, WBC white blood cell count, PCT procalcitonin
CD4+ T cells are the primary GM-CSF-producing lymphocytes
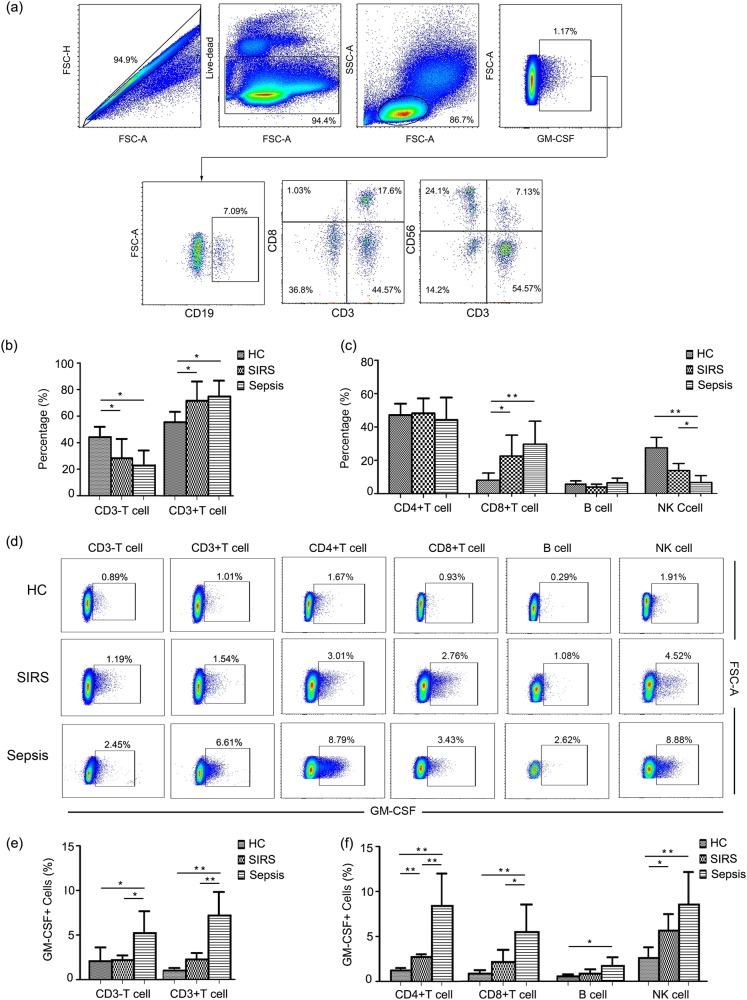
To verify the source of GM-CSF production, components of GM-CSF-producing cells were analyzed separately, comprising CD3+ T cells, CD3− T cells, CD4+ T cells, CD8+ T cells, B cells, and NK cells (Fig. 1a). As shown in Fig. 1b, GM-CSF was primarily secreted by CD3+ T cells in all subjects. In addition, CD4+ T cells accounted for the majority of GM-CSF-producing cells. From the population of GM-CSF-positive cells, the proportion of CD3+ T cells gradually increased, whereas the proportion of CD3− T cells decreased with disease progression. When GM-CSF-producing cells were analyzed separately among CD4+ T cells, CD8+ T cells, B cells, and NK cells, the proportion for GM-CSF-positive lymphocytes increased among CD8+ T cells but decreased for NK cells (Fig. 1c). In addition, we also analyzed expression of GM-CSF among CD3+ T cells, CD3− T cells, CD4+ T cells, CD8+ T cells, B cells, and NK cells. Our results revealed an overall increase of GM-CSF production in all of these subsets (Figs. 1d, e). However, CD4+ T cells sustained the greatest capacity to produce GM-CSF compared with other cell types (Fig. 1f).
Fig. 1.
GM-CSF is primarily produced by CD4+ T cells. a Representative dot plots illustrating the source of GM-CSF in lymphocyte population. b Pooled data showing the proportion of CD3+ and CD3− T cells from GM-CSF-positive cells. c Pooled data showing the proportion of CD4+ T cells, CD8+ T cells, NK cells, and B cells from GM-CSF-positive cells. d Representative dot plots showing expression of GM-CSF in different lymphocyte populations. e Pooled data illustrating expression of GM-CSF among CD3+ T cells and CD3− T cells in different groups. f Pooled data showing expression of GM-CSF in CD4+ T cells, CD8+ T cells, NK cells, and B cells in different groups. Each group included eight subjects for pooled data collection. *P < 0.05; **P < 0.01
Peripheral GM-CSF+CD4+ T cells exhibit a memory phenotype and decreased secretion of IFN-γ in sepsis patients
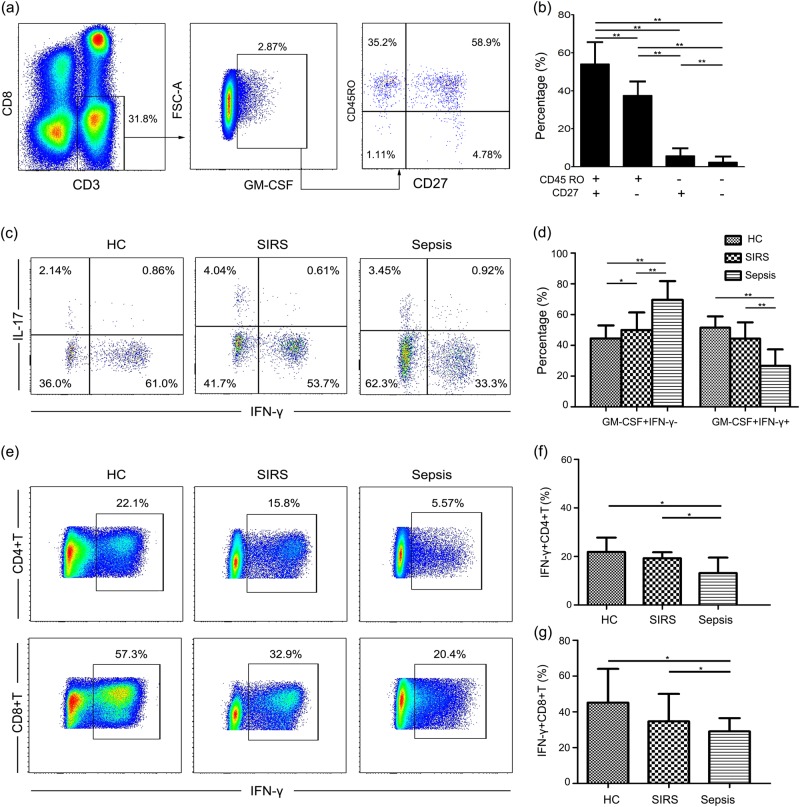
We next examined the phenotype of GM-CSF-secreting CD4+ T cells. We observed that GM-CSF-producing CD4+ T cells exhibit a memory phenotype, and most GM-CSF-secreting CD4+ T cells are central memory T cells, as indicated by the expression of CD45RO and CD27 (Figs. 2a, b). In addition, subsets of GM-CSF-secreting CD4+ T cells did not differ between groups. Furthermore, we also demonstrated that GM-CSF-secreting CD4+ T cells could be divided into different subtypes (Fig. 2c): (1) GM-CSF-secreting CD4+ T cells coexpressing IFN-γ; (2) GM-CSF-secreting CD4+ T cells coexpressing IL-17A; and (3) some CD4+ T cells only producing GM-CSF and no other cytokines. Moreover, GM-CSF-secreting CD4+ T cells coexpressing IFN-γ were decreased in the sepsis patients compared with the SIRS patients and HCs, whereas GM-CSF-secreting CD4+ IFN-γ− T cells were increased (Fig. 2d). GM-CSF-secreting CD4+ IL-17A+ T cells did not differ between the groups. Coinciding with the decrease in GM-CSF+ IFN-γ+ CD4+ T cells, we also found that expression of IFN-γ among CD4+ T cells and CD8+ T cells was decreased in sepsis patients (Figs. 2e, f), which might reflect the dysfunction observed in the bulk T-cell population. These findings further indicate that decreased IFN-γ+ CD4+ T cells are the major cause of reduced IFN-γ expression among GM-CSF+ CD4+ T cells.
Fig. 2.
Phenotype and cytokine production of GM-CSF+CD4+ T cells. a Representative dot plots showing expression of CD45RO and CD27 on GM-CSF+CD4+ T cells. b Pooled data showing expression of CD45RO and CD27 on GM-CSF+CD4+ T cells. c Representative dot plots showing expression of IFN-γ and IL-17A in the population of GM-CSF+CD4+ T cells. d Pooled data showing expression of IFN-γ in GM-CSF+ CD4+ T cells in different groups. e Representative dot plots showing expression of IFN-γ in CD4+ T cells and CD8+ T cells. f Pooled data showing expression of IFN-γ in CD4+ T cells in different groups. g Pooled data showing expression of IFN-γ in CD8+ T cells in different groups. Each group included eight subjects for pooled data collection. *P < 0.05; **P < 0.01
Peripheral GM-CSF+CD4+ T cells are increased in septic patients and positively correlate with sepsis severity
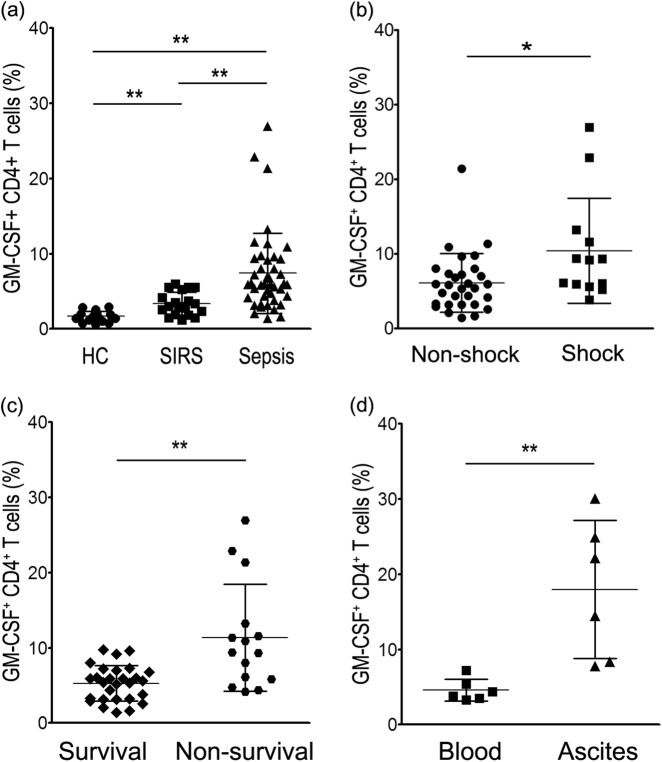
To decipher the significance of GM-CSF-secreting CD4+ T cells in sepsis patients, we analyzed the dynamic of these cells and found that the percentage of GM-CSF-secreting CD4+ T cells was significantly higher in sepsis and SIRS patients compared with the HCs (Fig. 3a). Importantly, when sepsis patients were further divided by disease severity, patients in septic shock displayed exacerbated numbers of GM-CSF-secreting CD4+ T cells compared with nonsepsis shock patients (Fig. 3b). We used expression of GM-CSF-secreting CD4+ T cells to stratify patients into surviving and nonsurviving groups (Fig. 3c). Finally, the number of GM-CSF-secreting CD4+ T cells were higher in ascites fluid than in peripheral blood, possibly reflecting the dynamic of GM-CSF+ CD4+ T cells at the local site of infection (Fig. 3d).
Fig. 3.
Dynamics of GM-CSF+ CD4+ T-cell expression in sepsis patients. a Pooled data showing expression of GM-CSF+ CD4+ T cells in healthy controls and patients with SIRS and sepsis. b, c Increasing GM-CSF+ CD4+ T-cell expression is associated with sepsis severity. d Differential expression of GM-CSF+ CD4+ T cells in blood and ascites from patients with sepsis caused by intra-abdominal infection. *P < 0.05; **P < 0.01
Peripheral GM-CSF+CD4+ T cells serve as a biomarker for predicting sepsis prognosis
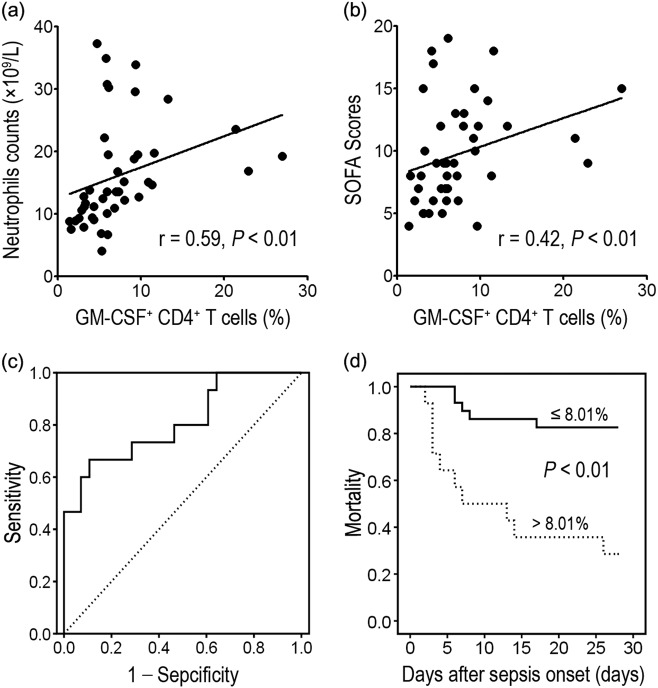
For further exploration into the relationship between GM-CSF-secreting CD4+ T cells and disease severity, we compared expression of GM-CSF-secreting CD4+ T cells with neutrophil counts and SOFA scores. We determined that the number of GM-CSF-secreting CD4+ T cells positively correlates with both peripheral neutrophil counts (r = 0.59, P < 0.01) and SOFA scores (r = 0.42, P < 0.01) on the day of diagnosis (Figs. 4a, b). Receiver operating characteristic (ROC) curve analysis revealed that the area under the curve was 0.81. Using 8.01% as a cut-off point for predicting the survival of sepsis patients, the specificity and sensitivity of the presence of GM-CSF-secreting CD4+ T cells were 89.3% and 66.7%, respectively (Figs. 4d, e).
Fig. 4.
Increase of GM-CSF+CD4+ T-cell expression is positively associated with sepsis severity. a, b GM-CSF+CD4+ T-cell levels are positively correlated with neutrophil counts and SOFA scores in sepsis patients. c Receiving operating curve of GM-CSF+ CD4+ T cells on Day 0 for predicting mortality. d Kaplan–Meier survival curves of septic patients showing that patients with high levels of GM-CSF+CD4+ T cells ( > 8.01%) have increased mortality compared with patients with low levels of GM-CSF+ CD4+ T cells ( ≤ 8.01%). Follow-up period: 28 days. P < 0.01
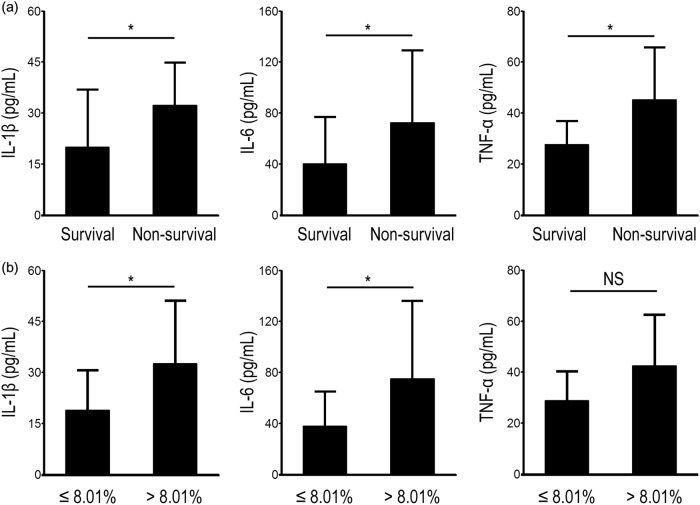
The inflammatory cytokines IL-1β, IL-6, and tumor necrosis factor-α (TNF-α) were simultaneously detected in both surviving and nonsurviving patients, and we found that levels of all three were significantly higher in nonsurviving patients (Fig. 5a). When we used 8.01% as a cut-off value, the concentrations of IL-1β and IL-6 between the two groups were also significantly different (Fig. 5b), whereas levels of TNF-α remained unchanged. Taken together, these results indicate that the number of GM-CSF-secreting CD4+ T cells correlates with pro-inflammatory cytokine production in sepsis patients.
Fig. 5.
Pro-inflammatory cytokine production in sepsis patients is associated with increased GM-CSF+ CD4+ T cells. a Pooled data showing that production of pro-inflammatory cytokines, including IL-1β, IL-6, and TNF-α, is associated with sepsis outcome. b Sepsis patients with high proportions of GM-CSF+CD4+ T cells ( > 8.01%) have increased expression of IL-1β and IL-6 compared with patients with low proportions of GM-CSF+ CD4+ T cells ( ≤ 8.01%). *P < 0.05; NS no significance
GM-CSF mediates neutrophil dysfunction
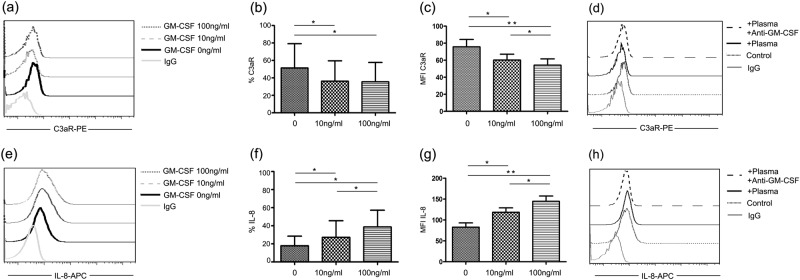
To examine the functional consequences of overproduction of GM-CSF, we evaluated neutrophil response to GM-CSF. When neutrophils isolated from peripheral blood were stimulated with GM-CSF, we observed a significant decrease in C3aR expression (Figs. 6a–c) and increased IL-8 production compared with unstimulated controls (Figs. 6e–g). To verify the critical role of GM-CSF in neutrophil overactivation in sepsis patients, we incubated neutrophils isolated from HCs with plasma of sepsis patients. Neutrophils from HCs displayed decreased C3aR and enhanced IL-8 production when cocultured with plasma from sepsis patients. Furthermore, these effects were attenuated by treatment with an anti-GM-CSF antibody (Figs. 6d, h). These results suggest that GM-CSF produced by CD4+ T cells is an important factor mediating neutrophil function in sepsis.
Fig. 6.
Increased GM-CSF production is associated with decreased C3aR expression and increased IL-8 production in neutrophils. a–c Representative dot plots and pooled data showing expression of C3aR on neutrophils following incubation with different doses of GM-CSF. d C3aR on healthy neutrophils incubated with sepsis patient plasma before and after blocking with an anti-GM-CSF antibody. e–g Representative dot plots and pooled data showing production of IL-8 in neutrophils following incubation with different doses of GM-CSF. h IL-8 in healthy neutrophils incubated with sepsis patient plasma before and after blocking with anti-GM-CSF antibody. *P < 0.05; **P < 0.01
Discussion
It has long been accepted that a “cytokine storm” resulting from host response to severe infection accounts for the onset of sepsis.8 Unfortunately, most clinical trials aiming to block cytokine production have failed to improve patient survival. This suggests that there may be other mechanisms or some unidentified factors that are involved in the pathogenesis of sepsis.
GM-CSF is a traditional hematopoietic-stimulating factor that induces proliferation of myeloid cells from bone-marrow progenitors.9 Recently, GM-CSF has been revealed as a pro-inflammatory cytokine produced by helper T cells that plays a pivotal role in promoting the early stages of immune response.10 In an in vivo study, GM-CSF-secreting CD4+ T cells were determined to be a key factor in the outcome of EAE, an animal model of human multiple sclerosis. In addition, elevated GM-CSF-secreting CD4+ T cells in the cerebrospinal fluid of multiple sclerosis (MS) patients have also been reported to positively correlate with disease severity.11 However, the dynamics of GM-CSF-secreting CD4+ T cells in sepsis and the consequences of such an increase in GM-CSF remain unknown.
In this study, we investigated both the source of GM-CSF-secreting cells and expression of GM-CSF in different lymphocyte populations. Our data indicate that GM-CSF production is primarily driven by CD4+ T cells in all subjects. Although the proportion of CD8+ T cells among GM-CSF+ cells gradually increased in sepsis patients, CD4+ T cells continued to occupy the majority of GM-CSF-producing cells. Moreover, due to the loss of NK cells during disease progression, the contribution of NK cells to GM-CSF+ cells gradually decreased in sepsis patients. In addition, we found an overall increase of GM-CSF-producing CD3+ T cells, CD3− T cells, CD4+ T cells, CD8+ T cells, B cells, and NK cells in sepsis patients. In addition to CD4+ T cells, CD8+ T cells and NK cells are two other important subsets that exhibit high GM-CSF-secreting capacity and warrant further investigation. Overall, considering that CD4+ T cells comprise the majority of GM-CSF-producing cells and display a high level of GM-CSF expression, we focused on the dynamics of GM-CSF-secreting CD4+ T cells in our enrolled patients.
We examined both the phenotype and cytokine profile of GM-CSF-secreting CD4+ T cells in our patients. We found that most GM-CSF-secreting CD4+ T cells exhibited a central memory phenotype, being both CD45RO+ and CD27+, and we observed no difference in T-cell subsets between SIRS and sepsis patients. To assess the cytokine profiles of GM-CSF-secreting CD4+ T cells, we simultaneously determined levels of IFN-γ and IL-17A production in these cells. We found that although there was no difference in GM-CSF and IL-17A coexpression among CD4+ T cells, CD4+ GM-CSF+ cells coexpressing IFN-γ were decreased in sepsis patents. Moreover, levels of CD4+ and CD8+ IFN-γ+ T cells were concordantly reduced in sepsis patients. These findings might reflect a heterogeneous population of GM-CSF-secreting CD4+ T cells, the phenotype and function of which may differ between patients.
Our data provide four pieces of evidence to support the association between increased GM-CSF-secreting CD4+ T cells and disease progression in sepsis. First, the level of GM-CSF-secreting CD4+ T cells is significantly increased in sepsis patients compared with SIRS patients and healthy subjects. Furthermore, septic shock patients have greater levels of GM-CSF-secreting CD4+ T cells compared with nonshock patients. Notably, GM-CSF-secreting CD4+ T cells in ascites fluid are more prevalent than in peripheral blood, which indicates that GM-CSF-secreting CD4+ T cells aggregate at the site of infection. Second, we found significant positive correlations between GM-CSF-secreting CD4+ T cells, neutrophil counts, and SOFA scores. Third, high levels of GM-CSF-secreting CD4+ T cells are associated with higher mortality in sepsis patients. Fourth, the peripheral frequency of GM-CSF-producing CD4+ T cells is predictive of sepsis outcome. When using 8.01% as a cut-off point, the levels of IL-1β and IL-6 were coordinately different between surviving and nonsurviving septic patients. In SIRS patients, although the expression of GM-CSF+ CD4+ T cells was also increased and higher than in HCs, transient immune activation caused by a stress reaction might be the cause. However, the precise mechanism whereby this occurs remains to be investigated.
Inflammasome-derived IL-1β reportedly drives production of GM-CSF by CD4+ T cells.12 As demonstrated in this report, high levels of GM-CSF-secreting CD4+ T cells were associated with high mortality and increased production of IL-1β. In addition, we also identified overproduction of IL-6 in the plasma of sepsis patients. Although these results only reveal that high levels of GM-CSF-secreting CD4+ T cells coexist with high levels of IL-1β and IL-6, whether a feedback loop exists between IL-1β, IL-6, and GM-CSF-producing CD4+ T cells in vivo remains unclear. Furthermore, although levels of TNF-α differed between surviving and nonsurviving sepsis patients, it did not correlate with the number of GM-CSF-secreting CD4+ T cells. Thus, further studies involving larger cohorts are needed to identify the relationship between GM-CSF-producing CD4+ T cells and other pro-inflammatory cytokines, as well as their potential role in the pathogenesis of sepsis.
In addition to the dynamics of GM-CSF-producing CD4+ T cells in sepsis, effects of increased GM-CSF were also investigated. We demonstrated that GM-CSF increased neutrophil activation and inhibited chemokine receptor expression. Moreover, plasma derived from sepsis patients induced the same effects on neutrophils derived from healthy donors, and these effects were inhibited by treatment with anti-GM-CSF antibodies. Thus, these results characterize the impact of GM-CSF on neutrophils. In our previous study, we found that C3aR expression in neutrophils was decreased in sepsis patients, for which the total complement activation products (i.e., C3a and C3b) were regarded to be the main cause.13 Based on the present findings, we propose that GM-CSF might be another important cytokine influencing complement receptor expression. Indeed, C3aR agonism reportedly plays an important role in ameliorating intestinal ischemia–reperfusion injuries by efficiently inhibiting neutrophil mobilization.14 However, the role of C3aR, and whether decreased C3aR in neutrophils is a consequence of complement activation or an inducer of dysfunction in neutrophils remains to be investigated in sepsis patients.
GM-CSF has been described as an immunostimulatory therapeutic, with evidence for neutrophil reprogramming.15 In addition, GM-CSF treatment has been reported to reverse sepsis-associated immunosuppression, reflected by restoration of monocyte pro-inflammatory cytokine release and Human Leukocyte Antigen-DR (HLA-DR) expression.6 Furthermore, administration of recombinant GM-CSF was reported to increase survival in sepsis patients.7,16 In our study, we found that increased GM-CSF-secreting CD4+ T cells are associated with the progression of sepsis and poor outcome, conflicting with previous clinical studies.6 We propose that the presence of GM-CSF-producing CD4+ T cells appears both during the early and late phases of sepsis. In addition, inappropriate activation and positioning of these cells contributes to the pathological manifestation of sepsis and the profound dysfunctional changes in neutrophils associated with the outcome of sepsis. We also observed that GM-CSF-producing CD4+ T cells are a heterogeneous population that coexpress other cytokines, including IFN-γ and IL-17A. The ultimate role played by GM-CSF-producing CD4+ T cells is likely dependent upon which cell subset is prominent in individual sepsis patients.
There are some limitations associated with our study that should be considered. The sample size of sepsis patients in our study was small; therefore, the current findings should be confirmed in a large-scale study or by another clinical center. We did not perform a follow-up study in this report, and future research is required to verify our findings. We do not provide direct evidence to confirm increased levels of GM-CSF in the plasma of sepsis patients. One reason for this is that GM-CSF exists at low levels in the plasma due to interaction with target cells immediately following secretion. Another reason may be due to the lack of a commercial enzyme-linked immunosorbent assay (ELISA) kit with high sensitivity for detecting GM-CSF in humans. In addition to CD4+ T cells, we found that CD8+ T cells and NK cells are additional subsets that displayed high GM-CSF-secreting capacity. Similar to findings from a previous study, NK cells are considered an important source of IFN-γ and GM-CSF, playing a dual role in different bacterial infection models.17 However, the dynamic of GM-CSF-secreting NK cells in our sepsis patients was not clear, and the outcome requires further investigation in future studies. Whether GM-CSF has an impact on other innate immunocytes, such as innate response activator-B cells, considered protective against microbial sepsis, remains unknown.18 In addition to immunocytes, other immune molecules expressed on monocytes have been shown to be important markers for predicting mortality in septic patients, such as programmed cell death ligand 1 (PD-L1) and HLA-DR.19,20 The correlation between GM-CSF-producing CD4+ T cells and PD-L1 or HLA-DR was not investigated in our study. Thus, our results indicate only that increased GM-CSF-secreting CD4+ T cells are related to outcome in sepsis patients.
Conclusions
In summary, our results show that increased GM-CSF-secreting CD4+ T cells are associated with poor prognosis in sepsis patients. These observations enrich our understanding of GM-CSF-secreting CD4+ T cells as a prognostic marker for sepsis patients and may identify new avenues for sepsis treatment.
Methods
Patients and design
HCs, patients with SIRS, and patients with sepsis were consecutively enrolled from July 2016 to April 2017. The diagnostic criteria for sepsis and sepsis shock were defined in accordance with the 2016 International Sepsis Definitions.21 Both SIRS and sepsis patients enrolled in this study were admitted to the ICU of our hospital. SIRS patients accepted elective surgery as mentioned in our previous study.22 Exclusion criteria included patients younger than 18 years old, pregnancy, malignancy, infection with human immunodeficiency virus, presence of organ transplantation, or immunosuppressive treatments. Following patient admission to the ICU, the following data were recorded, including age, sex, reason for admission, principal diagnosis, vital signs, respiratory parameters, blood routine tests, SOFA score, and microbiological culture results. Blood samples from patients with SIRS or sepsis were obtained within 12 h after SIRS or sepsis diagnosis. Septic patients were followed for 28 days to assess mortality. Written informed consent was obtained from all patients or their respective representative. This study was approved by the ethics committee of Beijing 302 Hospital for human research.
Sample collection
Peripheral blood was collected from each subject in tubes and anti-coagulated with heparin. Peripheral blood mononuclear cells (PBMCs) were then isolated by density gradient separation. Ascites caused by intra-abdominal infection were collected from septic patients in 50 mL Corning tubes. Ascitic mononuclear cells were also isolated by density gradient separation. Neutrophils were isolated as described previously.23 PBMCs, ascitic mononuclear cells, and neutrophils were all resuspended in RPMI-1640 media supplemented with 10% fetal calf serum (FCS) and processed for incubation. Plasma was collected and stored at –80 °C for subsequent analysis.
Flow cytometry analysis
In all, 106 PBMCs per well were plated in 48-well plates in 400 μL culture medium (RPMI-1640 supplemented with 10% FCS). Cells were then stimulated for 6 h with phorbol 12-myristate 13-acetate (50 ng/mL; Sigma-Aldrich) and ionomycin (100 ng/mL; Sigma-Aldrich, Louis, MO) in the presence of GolgiStop (BD Pharmingen, San Diego, CA, USA). PBMCs were then harvested for surface immunostaining. Dead cells were excluded by live–dead staining. Surface marker antibodies, including CD4, CD8, CD19, CD56, CD45RO, and CD27, were all obtained from BD Pharmingen. Corresponding intracellular antibodies, including GM-CSF (Biolegend, San Diego CA, USA), IL-17A (eBioscience, San Diego, CA, USA), IFN-γ (BD Pharmingen), and isotype controls were used. Stained cells were analyzed using a FACS Canto and FlowJo software (Tristar, San Carlos, CA) as previously described.24
Freshly isolated neutrophils were resuspended in RPMI-1640 medium containing 10% FCS. In all, 2 × 106 neutrophils per well were plated into 96-well plates in 200 μL culture medium and separately stimulated with GM-CSF (10 ng/mL, 100 ng/mL; Peprotech, Rocky Hill, NJ) for 2 h at 37 °C. Cells were then harvested to evaluate expression of C3aR and IL-8. An anti-CD66b antibody was used to identify neutrophils. All antibodies were purchased from Biolegend (Biolegend, San Diego CA, USA). For anti-GM-CSF antibody blockade, 50 μL of plasma from patients was first cocultured with an anti-GM-CSF antibody (0.2 μm/mL, PeproTech) at room temperature for 1 h. Plasma was added to 50 μL neutrophils derived from HCs at a concentration of 2 × 106/mL. Following 2 or 4 h of coculture at 37 °C, levels of C3aR and IL-8 expression were measured.
Cytokines analysis by ELISA
Concentration of cytokines in the plasma, including IL-1β, IL-6, and TNF-α, were measured using commercial ELISA kits (eBioscience, San Diego CA, USA) in accordance with the manufacturer’s instructions. The optical density (OD) value of each well was read at 450 nm with an ELISA plate reader. Standard curves were established to calculate concentration of the respective cytokines.
Statistics
Data analysis was performed using SPSS version 13.0 software. Quantitative data are expressed as the mean ± standard deviation. Statistical differences between two groups were determined by a Mann–Whitney nonparametric U-test. Statistical difference of the frequency distribution between groups was calculated with a Pearson’s chi-square test. Correlation analysis was evaluated by the Spearman rank correlation test. Kaplan–Meier curves were plotted to evaluate the impact of GM-CSF on survival. Potential differences in overall survival between groups was evaluated with log-rank tests. A ROC curve analysis provided a global and standardized appreciation of the accuracy of a marker for predicting an event during follow-up. Values of P < 0.05 were considered significant.
Acknowledgements
We appreciate the cooperation of all septic individuals, SIRS individuals and healthy volunteers who participated in this study. In addition, we would like to thank the native English-speaking scientists of Elixigen Company (Huntington Beach, California) for editing our manuscript. This work was supported by the National Natural Science Foundation of China [grant number 81400626] and the National Natural Science Foundation for innovation group [grant number 81721002].
Competing interests
The authors declare no competing interests.
Ethical approval:
This study was reviewed and approved by the Ethics Committee of Beijing 302 Hospital, China.
Informed consent
Written informed consent was obtained from each subject before recruitment.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Huihuang Huang, Siyu Wang, Tianjun Jiang
Contributor Information
Fu-Sheng Wang, Email: fswang302@163.com.
Ruonan Xu, Email: xuruonan2004@aliyun.com.
References
- 1.Levy MM, et al. SCCM/ESICM/ACCP/ATS/SI:2001SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit. Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 2.Balk RA. Severe sepsis and septic shock. Crit. Care Clin. 2000;16:179–192. doi: 10.1016/S0749-0704(05)70106-8. [DOI] [PubMed] [Google Scholar]
- 3.Van Nieuwenhuijze A, et al. GM-CSF as a therapeutic target in inflammatory diseases. Mol. Immunol. 2013;56:675–682. doi: 10.1016/j.molimm.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Codarri L, et al. RORγt derives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat. Immunol. 2011;12:560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- 5.EI-Behi M, et al. The encephalitogenicity of TH17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat. Immunol. 2011;12:568–575. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meisel C, et al. Granulocyte–macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression. Am. J. Respir. Crit. Care. Med. 2009;180:640–648. doi: 10.1164/rccm.200903-0363OC. [DOI] [PubMed] [Google Scholar]
- 7.Orozco H, et al. Molgramostim (GM-CSF) associated with antibiotic treatment in nontraumatic abdominal sepsis. Arch. Surg. 2006;141:150–153. doi: 10.1001/archsurg.141.2.150. [DOI] [PubMed] [Google Scholar]
- 8.Xu R, Lin F, Bao C, Wang FS. Mechanism of C5a-induced immunologic derangement in sepsis. Cell. Mol. Immunol. 2017;14:792–793. doi: 10.1038/cmi.2017.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burgess AW, Camakaris J, Metcalf D. Purification and properties of colony-stimulating factor from mouse lung-conditioned medium. J. Biol. Chem. 1977;252:1998–2003. [PubMed] [Google Scholar]
- 10.Zhang J, et al. A novel subset of helper T cells promotes immuneresponses by secreting GM-CSF. Cell Death Differ. 2013;20:1731–1741. doi: 10.1038/cdd.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rasouli J, et al. Expression of GM-CSF in T cells is increased in multiple sclerosis and suppressed by IFN-β therapy. J. Immunol. 2015;194:5085–5093. doi: 10.4049/jimmunol.1403243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luken JR, Barr MJ, Chaplin DD, Chi H, Lanneganti TD. Inflammasome-derived IL-1β regulates the production of GM-CSF by CD4(+) T cells and γδT cells. J. Immunol. 2012;188:3107–3115. doi: 10.4049/jimmunol.1103308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hazelzet JA, et al. Complement activation in relation to capillary leakage in children with septic shock and purpura. Infect. Immun. 1998;66:5350–5356. doi: 10.1128/iai.66.11.5350-5356.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu MC, et al. The receptor for complement component C3a mediates protection from intestinal ischemia-reperfusion injuries by inhibiting neutrophil mobilization. Proc. Natl. Acad. Sci. USA. 2013;110:9439–9444. doi: 10.1073/pnas.1218815110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kovach MA, Standiford TJ. The function of neutrophils in sepsis. Curr. Opin. Infect. Dis. 2012;25:321–327. doi: 10.1097/QCO.0b013e3283528c9b. [DOI] [PubMed] [Google Scholar]
- 16.Nelson S, et al. A randomised controlled trial of filgrastim as an adjunct to antibiotics for treatment of hospitalised patients with community-acquired pneumonia. J. Infect. Dis. 1998;178:1075–1080. doi: 10.1086/515694. [DOI] [PubMed] [Google Scholar]
- 17.Rauch PJ, et al. Innate response activator B cells protect against microbial sepsis. Science. 2012;335:597–601. doi: 10.1126/science.1215173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Souza-Fonseca-Guimaraes F, Cavaillon JM, Adib-Conquy M. Bench-to-bedside review: natural killer cells in sepsis - guilty or not guilty? Crit. Care. 2013;17:235–240. doi: 10.1186/cc12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shao R, et al. Monocyte programmed death ligand-1 expression after 3-4 days of sepsis is associated with risk stratification and mortality in septic patients: a prospective cohort study. Crit. Care. 2016;20:124–134. doi: 10.1186/s13054-016-1301-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drewry AM, et al. Comparison of monocyte human leukocyte antigen-DR expression and stimulated tumor necrosis factor alpha production as outcome predictors in severe sepsis: a prospective observational study. Crit. Care. 2016;20:334–344. doi: 10.1186/s13054-016-1505-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singer M, Deutschman CS. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu R, et al. Complement 5a receptor –mediated neutrophil dysfunction is associated with a poor outcome in sepsis. Cell. Mol. Immunol. 2016;13:103–109. doi: 10.1038/cmi.2014.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu R, et al. Low expression of CXCR1/2 on neutrophils predicts poor survival in patients with hepatitis B virus-related acutr-on-chronic liver failure. Sci. Rep. 2016;6:38714–38723. doi: 10.1038/srep38714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang JY, et al. Interleukin-17–producing CD4(+) T cells increase with severity of liver damage in patients with chronic hepatitis B. Hepatology. 2010;51:81–91. doi: 10.1002/hep.23273. [DOI] [PubMed] [Google Scholar]