Abstract
Purpose
Treatment with pembrolizumab, an anti–programmed death-1 antibody, at 10 mg/kg administered once every 2 weeks, displayed durable antitumor activity in programmed death-ligand 1 (PD-L1) –positive recurrent and/or metastatic (R/M) head and neck squamous cell carcinoma (HNSCC) in the KEYNOTE-012 trial. Results from the expansion cohort, in which patients with HNSCC, irrespective of biomarker status, received a fixed dose of pembrolizumab at a less frequent dosing schedule, are reported.
Patients and Methods
Patients with R/M HNSCC, irrespective of PD-L1 or human papillomavirus status, received pembrolizumab 200 mg intravenously once every 3 weeks. Imaging was performed every 8 weeks. Primary end points were overall response rate (ORR) per central imaging vendor (Response Evaluation Criteria in Solid Tumors v1.1) and safety. Secondary end points included progression-free survival, overall survival, and association of response and PD-L1 expression. Patients who received one or more doses of pembrolizumab were included in analyses.
Results
Of 132 patients enrolled, median age was 60 years (range, 25 to 84 years), 83% were male, and 57% received two or more lines of therapy for R/M disease. ORR was 18% (95% CI, 12 to 26) by central imaging vendor and 20% (95% CI, 13 to 28) by investigator review. Median duration of response was not reached (range, ≥ 2 to ≥ 11 months). Six-month progression-free survival and overall survival rates were 23% and 59%, respectively. By using tumor and immune cells, a statistically significant increase in ORR was observed for PD-L1–positive versus –negative patients (22% v 4%; P = .021). Treatment-related adverse events of any grade and grade ≥ 3 events occurred in 62% and 9% of patients, respectively.
Conclusion
Fixed-dose pembrolizumab 200 mg administered once every 3 weeks was well tolerated and yielded a clinically meaningful ORR with evidence of durable responses, which supports further development of this regimen in patients with advanced HNSCC.
INTRODUCTION
Head and neck squamous cell carcinoma (HNSCC) is the seventh most common cancer worldwide.1 In the United States, it is estimated there will be approximately 61,760 new cases of HNSCC and 13,190 deaths in 2016.2 Primary risk factors include tobacco smoking and alcohol consumption.3 Another risk factor for a growing subset of head and neck cancers is human papillomavirus (HPV) infection.4 Several differences among smoking- and alcohol-related versus HPV-associated HNSCC have been reported, including different patient characteristics, genomic profiles, and prognoses; for example, patients with HPV-associated HNSCC often have better outcomes than those with HPV-unrelated disease.5
A common first-line treatment regimen for recurrent and/or metastatic (R/M) HNSCC is the combination of cetuximab, platinum, and fluorouracil (EXTREME regimen), which has demonstrated a median overall survival (OS) of 10 months.6 Although this regimen has shown promising efficacy, patients who experience disease progression on first-line therapy or who are platinum refractory are seldom responsive to treatment, with response rates to second-line therapies—typically methotrexate, cetuximab, or taxanes—that range from 3% to 13%.4,7
HNSCC is generally associated with deficiencies of the immune system8,9; patients exhibit impaired natural killer cell activity, poor antigen-presenting function, low absolute lymphocyte counts,10 and mutations in genes that regulate inflammation.9,11 In the healthy immune system, programmed death-1 (PD-1) receptor functions as an immune checkpoint and is expressed primarily on the surface of activated CD4+ and CD8+ T cells.12,13 Engagement of PD-1 by either of its ligands, programmed death-ligand 1 or 2 (PD-L1 or PD-L2) results in inhibition of T-cell activation and limits the response to inflammation.14,15 In HNSCC and other solid tumors, tumor-infiltrating lymphocytes, and especially T helper 1 cells, activate interferon-mediated signaling and induce expression of PD-L1 on cells in the tumor environment, which protects tumor cells from tumor-directed immunity.16,17 Moreover, HNSCC tumor cells are known to exhibit high levels of PD-L1 expression.17,18 Preclinical studies indicate that blockade of the PD-1 and PD-L1 interaction enhances T-cell activation and inhibits tumor growth.16,17,19
Pembrolizumab, a highly selective humanized monoclonal immunoglobulin G4 antibody that blocks the interaction between PD-1 and its ligands, has demonstrated antitumor activity in multiple tumor types.20-23 Support for pembrolizumab in the treatment of R/M HNSCC has been described in the initial HNSCC cohort of the KEYNOTE-012 trial, which demonstrated clinical activity of pembrolizumab 10 mg/kg administered intravenously once every 2 weeks in patients with PD-L1–positive R/M HNSCC.24 Overall response rate (ORR) to this body weight–based dosing regimen was 18% (central imaging vendor review), with responses in 25% of patients who were HPV positive and 14% in those who were HPV negative. In that study, responses were durable (median, 53 weeks); median (95% CI) progression-free survival (PFS) and OS were 2 months (2 to 4 months) and 13 months (5 months to not-reached), respectively.
Recent studies that have used population pharmacokinetics and exposure-response models suggest that a lower, fixed dose of pembrolizumab (200 mg) and a less frequent administration schedule (once every 3 weeks) may be sufficient for target engagement and clinical activity.25,26 A fixed-dose regimen confers several advantages over body weight–based dosing, including safety, convenience, reduction of waste, and adherence. The aim of the current study was to report the safety and efficacy of a fixed-dose regimen in an all-comer population of patients with R/M HNSCC, regardless of PD-L1 or HPV status, from a larger HNSCC expansion cohort of the KEYNOTE-012 trial.
PATIENTS AND METHODS
Patients
The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines, and was approved by relevant regulatory and independent ethics committees. All patients provided written informed consent before study entry.
The KEYNOTE-012 trial was a phase Ib, multicenter, nonrandomized, multicohort study of pembrolizumab in patients with advanced solid tumors. Patients age ≥ 18 years with histologically or cytologically confirmed R/M HNSCC, measurable disease per RECIST (v1.1; Response Evaluation Criteria in Solid Tumors), Eastern Cooperative Oncology Group performance status of 0 or 1, and adequate organ function were eligible for enrollment in the expansion cohort. Patients had to provide an archival tumor sample at screening and newly obtained tumor biopsies before starting treatment and after 9 weeks of treatment; there were no eligibility criteria on the basis of biomarker expression—patients could be PD-L1 positive or negative and have HPV-associated or non–HPV-associated disease. HPV-associated disease refers to patients with a primary tumor location of the oropharynx who were considered by the site investigator to be HPV positive. Non–HPV-associated disease refers to patients with a non–HPV-associated oropharyngeal cancer and all patients with a primary tumor location outside of the oropharynx. There was no limit to the number of prior therapies; patients who were treatment naive were allowed. Patients who received previous treatments that specifically targeted T-cell costimulation or checkpoint pathways were excluded. Prior systemic immunosuppressive therapy had to be concluded within 7 days, chemotherapy or targeted small-molecule therapy within 2 weeks, and anticancer monoclonal antibody therapy within 4 weeks from the start of pembrolizumab. Patients with additional progressing malignancies, psychiatric or substance abuse disorders, HIV or active infection, CNS metastases, hepatitis B or C, or autoimmune disease were excluded.
Study Design
Patients in this cohort received pembrolizumab 200 mg administered intravenously once every 3 weeks. Treatment continued for 24 months or until progressive disease (PD), intolerable toxicity, or investigator and/or patient decision to withdraw. Clinically stable patients with PD could remain on therapy until PD confirmation by a follow-up scan. Treatment response was assessed every 8 weeks by using computed tomography or magnetic resonance imaging. Patients who achieved complete response (CR) had the option of discontinuing therapy.
Adverse events (AEs) were monitored and graded according to National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. Treatment was interrupted for grade 3 AEs or severe drug-related AEs until toxicity resolved to grade 0 to 1. Treatment was discontinued for grade 4 treatment-related AEs or if grade 3 AEs did not resolve within 12 weeks of the last treatment dose. Laboratory safety evaluations were performed within 10 days of the first study treatment and up to 72 hours before each dose.
The coprimary end points were safety and ORR per RECIST v1.1 by central imaging vendor review. The proportion of patients who achieved a CR, partial response (PR), and stable disease was determined. Secondary end points included ORR (RECIST v1.1 per investigator), PFS, OS, duration of response (DOR), and the correlation of response and PD-L1 expression.
The all-patients-as-treated population, which was defined as all patients who received one or more doses of pembrolizumab, was used for safety and efficacy analyses. An additional analysis of the primary efficacy end point was also conducted in all patients who received one or more doses of pembrolizumab, had measurable disease at baseline, and had one or more postbaseline scan or who discontinued treatment because of PD or treatment-related AE (full analysis set population).
Biomarker Analysis
PD-L1 expression was retrospectively evaluated by using an immunohistochemistry (IHC) assay, an investigational version of the PD-L1 IHC 22C3 pharmDx assay (Dako, Carpinteria, CA) that uses the 22C3 (Merck, Kenilworth, NJ) anti–PD-L1 antibody. This assay was recently approved as the first PD-L1 IHC companion diagnostic for use in non–small-cell lung cancer (NSCLC) in the United States. The staining protocol used in this study was as described in the instructions for the approved commercial assay. The assay was used to determine PD-L1 expression on newly obtained or archival tumor samples. Two scoring methods were used to assess expression prevalence. One scoring method determined the percentage of tumor cells with membranous PD-L1 expression; details are provided in the scoring instructions for the approved commercial assay for NSCLC. A second scoring method took into account membranous PD-L1 staining on both tumor and mononuclear inflammatory cells. Both scores were measured on a scale from 0% to 100%; PD-L1 positivity was predefined as ≥ 1% using either of the two scoring methods.
Statistical Analysis
With 100 evaluable patients, the study provided > 99% power to detect a difference of 15 percentage points in ORR under the null hypothesis of ORR = 5% with a type I error rate of 2.5% if the true ORR was 20%. Success for this hypothesis required at least 11 of 100 responses. Per protocol, the actual number of patients enrolled exceeded the target to ensure at least 100 evaluable patients.
Exact methods for binomial parameters were used to determine ORR per RECIST v1.1. Kaplan-Meier statistics were used to estimate PFS, OS, and DOR. Follow-up duration, which was defined as the time from enrollment to death or database cutoff, was calculated for all patients.
Logistic (ORR) or Cox (PFS and OS) proportional hazards regression one-sided testing was performed to assess the relationship between efficacy and PD-L1 expression measured by using tumor cells alone and a combination of tumor and immune cells. These analyses used centrally reviewed ORR, PFS, and OS.
RESULTS
Patients
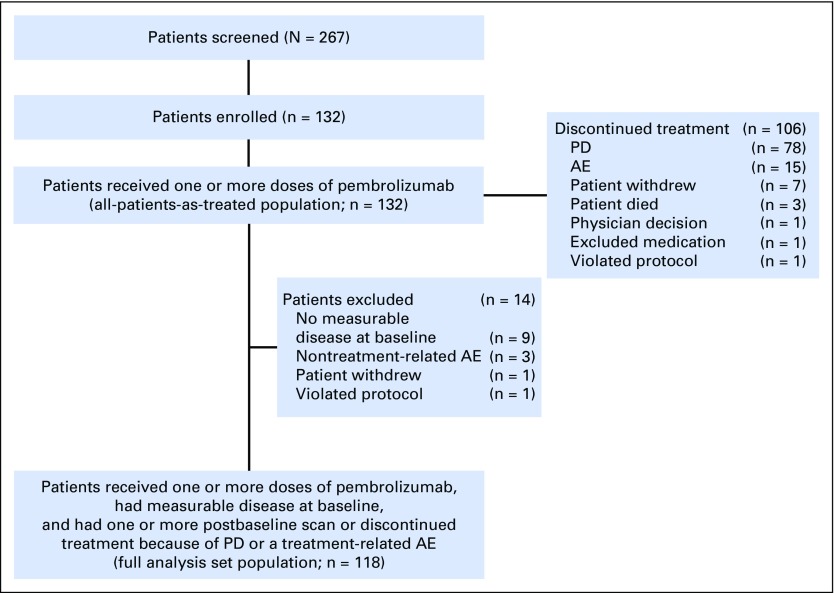
In total, 132 patients were enrolled between June 12, 2014, and October 8, 2014; the cutoff for data accrual was September 1, 2015. All enrolled patients received one or more doses of pembrolizumab—an all-patients-as-treated population—and were included in safety and efficacy analyses. The full analysis set population used for the primary efficacy end point included 118 patients (Fig 1). Twenty-eight patients (21%) had HPV-associated HNSCC and 104 (79%) had non–HPV-associated disease. Patients were a median of age 60 years (range, 25 to 84 years), and 110 (83%) were male (Table 1). Patients were heavily pretreated: 57% received two or more prior therapies for R/M disease. During the study, therapy was discontinued in 106 patients (80%) because of PD (n = 78), an AE (n = 15), physician or patient decision (n = 8), death (n = 3), need for medication exclusion (n = 1), and protocol violation (n = 1; Fig 1).
Fig 1.
Consort diagram. Patient disposition. AE, adverse event; PD, progressive disease.
Table 1.
Baseline Demographic and Patient Characteristics (all-patients-as-treated population)

Safety
Median number of days on pembrolizumab was 88 (range, 1 to 411 days). Treatment-related AEs occurred in 82 patients (62%; Table 2). The most frequently occurring treatment-related AEs of any grade included fatigue (n = 28), hypothyroidism (n = 14), and decreased appetite (n = 11). Twelve patients (9%) experienced a grade 3 or 4 treatment-related AE (Table 2), most commonly decreased appetite, facial swelling, and pneumonitis, which occurred in 2 patients each. There were no treatment-related deaths. Eight patients (6%) discontinued treatment because of treatment-related AEs, and 29 patients (22%) had one or more dose interruptions as a result of an AE. AEs of special interest because of immune-related etiology, regardless of causality, occurred in 26 patients (20%); most events were of grade 1 or 2 severity. Grade 3 immune-related AEs included pneumonitis (n = 2), diabetes mellitus (n = 1), decubitus ulcer (n = 1), colitis (n = 1), and drug-induced liver injury (n = 1); one grade 4 immune-related AE (diabetic ketoacidosis) occurred. Of these, two patients who experienced grade 3 pneumonitis, one patient with grade 3 colitis, and one patient with grade 2 interstitial lung disease had to discontinue study treatment.
Table 2.
Treatment-Related Adverse Events by Grade Severity (all-patients-as-treated population; N = 132)

Efficacy
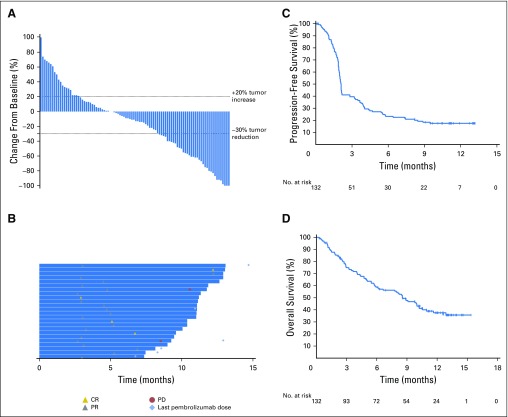
After a median follow-up duration of 9 months (interquartile range, 3 to 11 months), ORR in the all-patients-as-treated population was 18% (95% CI, 12% to 26%) by central imaging vendor review and 20% (95% CI, 13% to 28%) by investigator review (Table 3). An additional analysis of the primary end point using the full analysis set population yielded ORRs of 20% (95% CI, 13% to 29%) and 21% (95% CI, 14% to 29%) by central imaging vendor review and investigator review, respectively. In the all-patients-as-treated population by central review, 4 (3%) of 132 patients achieved a CR, 20 (15%) achieved a PR, 26 (20%) had stable disease, and 61 (46%) experienced disease progression. The total disease control rate, which was defined as the sum of CR, PR, and stable disease for ≥ 6 months, was 20%. No cases of pseudoprogression were observed in this cohort. The ORR was 32% (9 of 28 patients) and 14% (15 of 104 patients) among those with HPV-associated and non–HPV-associated disease, respectively (Table 3). When patients with non–HPV-associated disease were further evaluated by their tumor location in the oropharynx, oral cavity, hypopharynx, and larynx, ORR was 15% (11 of 76 patients). Patients with non–HPV-associated disease in other locations had an ORR of 14% (4 of 28 patients). A reduction in target lesion size of any amount was found in 61% of all patients (Fig 2A).
Table 3.
Antitumor Activity of Pembrolizumab (confirmed responses per RECIST v1.1, central imaging vendor review)
Fig 2.
Efficacy of pembrolizumab on the basis of RECIST (v1.1; Response Evaluation Criteria in Solid Tumors) by central imaging vendor review. (A) Maximum percentage change from baseline in target lesions. Includes patients who had a comparable number of lesions between baseline and postbaseline scans (n = 96). (B) Treatment exposure and response duration in responders (all responders, n = 24). (C and D) Kaplan-Meier estimates of (C) progression-free survival and (D) overall survival (all-patients-as-treated population, N = 132). CR, complete response; PD, progressive disease; PR, partial response.
Median time to response was 2 months (range, 2 to 11 months). Observed responses were durable and persisted over several assessments, with a median DOR that was not reached (range, ≥ 2 to ≥ 11 months) at the data cutoff. At the time of writing, 26 patients remained on therapy, and 20 (83%) of 24 radiologic responses were ongoing (Fig 2B).
Median PFS was 2 months (95% CI, 2.0 to 2.2 months) and the PFS rate at 6 months was 23% (Fig 2C). The 6-month PFS rate in patients with HPV-associated and non–HPV-associated HNSCC was 37% and 20%, respectively. Median OS was 8 months (95% CI, 6 to 10 months). The 6-month OS rate was 59% in all patients (Fig 2D) and 70% and 56% in patients with HPV-associated and non–HPV-associated disease, respectively.
Biomarker Analysis
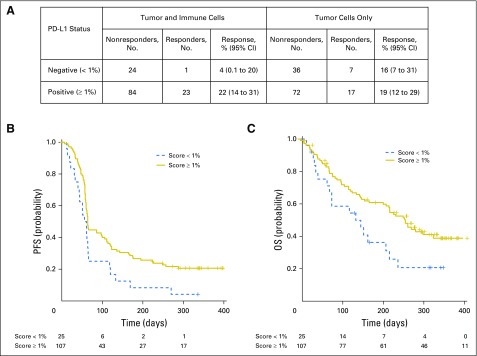
When PD-L1 expression analyses were restricted to only tumor cells, there was no statistically significant increase in the probability of response for patients with positive (≥ 1%) versus negative (< 1%) tumors (P = .348, one-sided test). Conversely, when immune cells were included in the scoring system, a statistically significant increase in the probability of response for positive (≥ 1%) versus negative (< 1%) patients was observed (P = .021, one-sided test). ORR in patients who were PD-L1 positive by tumor and immune cell scoring was 22%, whereas those who were negative had an ORR of 4% (Fig 3). Similarly, statistically significant differences for PFS and OS were observed when scoring took into account staining in both tumor and immune cells (PFS: P = .008; OS: P = .008, one-sided tests) but not tumor cells alone (PFS: P = .195; OS: P = .132, one-sided tests). Median OS times for patients who were PD-L1 positive versus negative by tumor and immune cell scoring were 303 days and 151 days, respectively (Fig 3).
Fig 3.
(A) Association of efficacy and programmed death-ligand 1 (PD-L1) expression. Overall response by PD-L1 expression in tumor and immune cells and tumor cells alone. (B and C) Kaplan-Meier estimates of (B) progression-free survival (PFS) and (C) overall survival (OS) on the basis of a positive expression cutoff of ≥ 1% in tumor and immune cells (all-patients-as-treated population, N = 132).
DISCUSSION
This study establishes the efficacy and safety of a fixed dose of pembrolizumab (200 mg) administered once every 3 weeks in patients with HNSCC. With an ORR of 18% by central imaging vendor review, an ORR of 20% by investigator review, an OS of 8 months, and a DOR that was not reached, the clinical benefit of pembrolizumab in biomarker-unselected patients with R/M HNSCC was similar to that seen in the initial cohort of patients who were PD-L1 positive (ORR, 18% by central imaging vendor review; median OS, 13 months; median DOR, 53 weeks).24 The response in this heavily pretreated population compares favorably with that observed with single-agent cetuximab (ORR, 13%; DOR, 4 months).27 In addition, median OS was on par with the longest median survival reported in patients with treatment-naive R/M HNSCC (10 months), which was achieved with a combination of platinum, cetuximab, and fluorouracil (EXTREME regimen),6 and similar to that observed with afatinib in patients with previously treated R/M disease (7 months).28 In contrast to the significant toxicity observed with the EXTREME regimen, pembrolizumab was well tolerated in this study. The safety profile in the expansion cohort was consistent with the safety profiles of pembrolizumab that were reported in the initial HNSCC cohort and in studies in melanoma and NSCLC.21,22,25
Approximately one half of all patients in this study with HNSCC of the oropharynx had HPV-associated disease, which has been associated with longer survival than non–HPV-associated HNSCC.5 Similar to the initial HNSCC cohort of the KEYNOTE-012 trial, a higher response to pembrolizumab was observed in patients with HPV-associated versus non–HPV-associated HNSCC.24 Of note, an ORR of 14% was consistently observed in patients with HPV-negative disease in both KEYNOTE-012 HNSCC cohorts, which suggests that those with non–HPV-associated R/M HNSCC—a group with particularly poor prognosis—may also benefit from pembrolizumab. This is not surprising given the emerging evidence that supports the use of PD-1–targeted therapies to treat both HPV-associated and non–HPV-associated HNSCC.18,29 Although HPV-associated and non–HPV-associated HNSCC differ in several ways, and the mechanisms that lead to PD-L1 expression may differ between the two, PD-L1 has been shown to be overexpressed in both settings. Whether there is a difference between PD-L1 expression levels in HNSCC on the basis of HPV association remains unclear given the current literature.
A limitation of this study is the lack of a consistent method used to determine HPV status. HPV association was determined by the site investigator by using the method of their choice; p16 IHC was used by the majority of sites. Whereas p16 IHC is a useful surrogate for HPV infection in oropharyngeal HNSCC, it has limited utility outside of the oropharynx where HPV is less prevalent.30,31 For that reason, patients with nonoropharyngeal HNSCC were considered to be HPV-negative regardless of p16 status. In addition, the number of patients with HPV-associated disease in this study was substantially less than the number with non–HPV-associated HNSCC. Response by HPV association should be interpreted with these limitations in mind.
On the basis of an evolving body of evidence in HNSCC and other solid tumors that suggest PD-L1 expression on tumor-infiltrating immune cells may contribute to clinical outcome,32,33 two scoring systems were used to determine PD-L1 expression: one that analyzed expression on only tumor cells and another that included both tumor and immune cells. Although we did not independently determine the contribution of each cell type, we have demonstrated that PD-L1 expression on immune cells significantly contributes to the predictability of response in HNSCC, as PD-L1 expression on tumor cells alone was not significantly correlated with response.
Results from the current study indicate that the less frequent, fixed dose of pembrolizumab is tolerable and provides comparable antitumor activity with pembrolizumab administered in a body weight–based dosing regimen. Patients responded to pembrolizumab regardless of HPV status, and PD-L1 expression on tumor and immune cells was found to be associated with response. These findings support ongoing phase II (KEYNOTE-055) and phase III (KEYNOTE-040 and KEYNOTE-048) studies of the fixed-dose regimen in patients with advanced HNSCC.
ACKNOWLEDGMENT
We thank Q2 solutions (Edinburgh, United Kingdom) for PD-L1 analysis, Dako (Carpinteria, CA) for ongoing PD-L1 support, Amy Blum, Karl Heath, Ken Emancipator, and Archana Ray (employees of Merck) for their contributions, and Jared Lunceford (employee of Merck) for his contributions to the biomarker analyses. We also thank Matthew Grzywacz, Dana Francis, and the ApotheCom Merck oncology team (Yardley, PA) for assistance with manuscript editing (funded by Merck).
Footnotes
Processed as a Rapid Communication manuscript.
Supported by Merck.
Presented in part at the 2015 Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 29-June 2, 2015.
This study was designed by academic advisors and representatives of the sponsor. Investigators and representatives of the sponsor collected the data. Data were analyzed by authors and representatives of the sponsor and interpreted by all authors. All authors had full access to the data, vouch for their accuracy, and attest that the study conformed to the protocol.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT01848834.
AUTHOR CONTRIBUTIONS
Conception and design: Laura Q.M. Chow, Barbara Burtness, Jared Weiss, Kumudu Pathiraja, Jonathan D. Cheng, Tanguy Y. Seiwert
Provision of study materials or patients: Laura Q.M. Chow, Robert Haddad, Joseph Paul Eder, Hyunseok Kang, Kei Muro, Jared Weiss, Tanguy Y. Seiwert
Collection and assembly of data: Laura Q.M. Chow, Robert Haddad, Shilpa Gupta, Amit Mahipal, Ranee Mehra, Makoto Tahara, Raanan Berger, Joseph Paul Eder, Barbara Burtness, Bhumsuk Keam, Hyunseok Kang, Kei Muro, Jared Weiss, Ravit Geva, Chia-Chi Lin, Hyun Cheol Chung, Amy Meister, Marisa Dolled-Filhart, Kumudu Pathiraja, Tanguy Y. Seiwert
Data analysis and interpretation: Laura Q.M. Chow, Robert Haddad, Shilpa Gupta, Amit Mahipal, Ranee Mehra, Makoto Tahara, Se-Hoon Lee, Bhumsuk Keam, Hyunseok Kang, Jared Weiss, Hyun Cheol Chung, Marisa Dolled-Filhart, Kumudu Pathiraja, Jonathan D. Cheng, Tanguy Y. Seiwert
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Antitumor Activity of Pembrolizumab in Biomarker-Unselected Patients With Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma: Results From the Phase Ib KEYNOTE-012 Expansion Cohort
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Laura Q.M. Chow
Consulting or Advisory Role: Novartis, Merck, Pfizer, Sanofi/Genzyme, Seattle Genetics, Bristol-Myers Squibb, Amgen
Research Funding: Novartis (Inst), Merck (Inst), Pfizer (Inst), Bristol-Myers Squibb (Inst), Genentech (Inst), Incyte (Inst), AstraZeneca/Medimmune (Inst), VentiRx (Inst), Eli Lily/Imclone (Inst)
Honoraria: Amgen, Emergent BioSolutions
Robert Haddad
Consulting or Advisory Role: Celgene, Bayer, Merck, Eisai, Bristol-Myers Squibb
Research Funding: Boehringer Ingelheim (Inst), Merck (Inst), Bristol-Myers Squibb (Inst), Celgene (Inst), AstraZeneca (Inst)
Shilpa Gupta
Honoraria: Pfizer, Genentech, Seattle Genetics
Consulting or Advisory Role: Pfizer, Seattle Genetics, Genentech
Speakers' Bureau: Genentech
Research Funding: Astellas Medivation (Inst), Innocrin Pharma (Inst), Pfizer (Inst), MedImmune (Inst), Merck (Inst)
Amit Mahipal
No relationship to disclose
Ranee Mehra
Employment: GlaxoSmithKline (I)
Stock or Other Ownership: GlaxoSmithKline (I)
Consulting or Advisory Role: Genentech, Bayer, Novartis, Bristol-Myers Squibb
Research Funding: Genentech
Travel, Accommodations, Expenses: Mirati Therapeutics
Makoto Tahara
Honoraria: Merck Serono, Bristol-Myers Squibb, Eisai, Otsuka, Bayer
Consulting or Advisory Role: Ono Pharmaceutical, MSD, Bayer, AstraZeneca, Pfizer
Research Funding: Eisai (Inst), Merck Sharp & Dohme (Inst), Boehringer Ingelheim (Inst), AstraZeneca (Inst), Ono Pharmaceutical (Inst), Novartis (Inst), Bayer (Inst), NanoCarrier (Inst), Pfizer (Inst)
Raanan Berger
No relationship to disclose
Joseph Paul Eder
Honoraria: Merck
Other Relationship: Merck
Barbara Burtness
Consulting or Advisory Role: VentiRx, Merck, MedImmune, Boehringer Ingelheim, Amgen
Research Funding: Genentech, Merck
Expert Testimony: Johnson & Johnson
Se-Hoon Lee
Honoraria: Roche, AstraZeneca, MSD Oncology
Consulting or Advisory Role: AstraZeneca, Pfizer, Novartis, Bristol-Myers Squibb
Bhumsuk Keam
No relationship to disclose
Hyunseok Kang
Honoraria: AstraZeneca
Research Funding: Merck, AstraZeneca, Immunogen, VentiRx, Plexxikon
Kei Muro
Honoraria: Takeda Pharmaceuticals, Chugai Pharma, Yakult Honsha, Merck Serono, Taiho Pharmaceutical
Jared Weiss
Consulting or Advisory Role: Biodesix, Clovis Oncology, AstraZeneca, OncoPlex Diagnostics, Eli Lilly, EMD Serono
Research Funding: Astellas Pharma (Inst), Celgene (Inst), MedImmune (Inst), Pfizer (Inst), Novartis (Inst), Merck (Inst)
Ravit Geva
Honoraria: Merck Sharp & Dohme, Pfizer, Bristol-Myers Squibb, Eli Lilly, Roche, Novartis
Chia-Chi Lin
No relationship to disclose
Hyun Cheol Chung
Consulting or Advisory Role: Celltrione, Taiho Pharmaceutical, MSD, Eli Lilly, Quintiles, Bristol-Myers Squibb
Speakers' Bureau: Merck Serono, Eli Lilly
Research Funding: Eli Lilly, GlaxoSmithKline, Merck Sharp & Dohme
Amy Meister
Employment: Merck
Stock or Other Ownership: Merck
Marisa Dolled-Filhart
Employment: Merck
Stock or Other Ownership: Merck, Merck (I)
Kumudu Pathiraja
Employment: Merck
Jonathan D. Cheng
Employment: Merck
Stock or Other Ownership: Merck
Tanguy Y. Seiwert
Honoraria: Bayer, Onyx Pharmaceuticals, Merck, Amgen, Bristol-Myers Squibb, Merck Serono, AstraZeneca
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Cohen EE, LaMonte SJ, Erb NL, et al. American Cancer Society head and neck cancer survivorship care guideline. CA Cancer J Clin. 2016;66:203–239. doi: 10.3322/caac.21343. [DOI] [PubMed] [Google Scholar]
- 3.Pelucchi C, Gallus S, Garavello W, et al. Cancer risk associated with alcohol and tobacco use: Focus on upper aero-digestive tract and liver. Alcohol Res Health. 2006;29:193–198. [PMC free article] [PubMed] [Google Scholar]
- 4.Denaro N, Russi EG, Adamo V, et al. State-of-the-art and emerging treatment options in the management of head and neck cancer: News from 2013. Oncology. 2014;86:212–229. doi: 10.1159/000357712. [DOI] [PubMed] [Google Scholar]
- 5.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 7.Patel AN, Mehnert JM, Kim S. Treatment of recurrent metastatic head and neck cancer: Focus on cetuximab. Clin Med Insights Ear Nose Throat. 2012;5:1–16. doi: 10.4137/CMENT.S5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Q, Prince ME, Moyer JS. Immunotherapy for head and neck squamous cell carcinoma. Oral Oncol. 2015;51:299–304. doi: 10.1016/j.oraloncology.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Seiwert TY, Zuo Z, Keck MK, et al. Integrative and comparative genomic analysis of HPV-positive and HPV-negative head and neck squamous cell carcinomas. Clin Cancer Res. 2015;21:632–641. doi: 10.1158/1078-0432.CCR-13-3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gildener-Leapman N, Ferris RL, Bauman JE. Promising systemic immunotherapies in head and neck squamous cell carcinoma. Oral Oncol. 2013;49:1089–1096. doi: 10.1016/j.oraloncology.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cancer Genome Atlas Network Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517:576–582. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Latchman Y, Wood CR, Chernova T, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 15.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwai Y, Ishida M, Tanaka Y, et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strome SE, Dong H, Tamura H, et al. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res. 2003;63:6501–6505. [PubMed] [Google Scholar]
- 18.Lyford-Pike S, Peng S, Young GD, et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013;73:1733–1741. doi: 10.1158/0008-5472.CAN-12-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown JA, Dorfman DM, Ma FR, et al. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170:1257–1266. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- 20.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 21.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 22.Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): An open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17:956–965. doi: 10.1016/S1470-2045(16)30066-3. [DOI] [PubMed] [Google Scholar]
- 25.Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: A randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109–1117. doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 26.Freshwater T, Stone J, de Greef R, et al. Assessment of pembrolizumab (MK-3475) dosing strategy based on population pharmacokinetics and exposure-response models. J Pharmacomet Pharmacodynam. 2015;42:S15. [Google Scholar]
- 27.Vermorken JB, Trigo J, Hitt R, et al. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J Clin Oncol. 2007;25:2171–2177. doi: 10.1200/JCO.2006.06.7447. [DOI] [PubMed] [Google Scholar]
- 28.Machiels JP, Haddad RI, Fayette J, et al. Afatinib versus methotrexate as second-line treatment in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck progressing on or after platinum-based therapy (LUX-Head & Neck 1): An open-label, randomised phase 3 trial. Lancet Oncol. 2015;16:583–594. doi: 10.1016/S1470-2045(15)70124-5. [DOI] [PubMed] [Google Scholar]
- 29.Yu GT, Bu LL, Huang CF, et al. PD-1 blockade attenuates immunosuppressive myeloid cells due to inhibition of CD47/SIRPα axis in HPV negative head and neck squamous cell carcinoma. Oncotarget. 2015;6:42067–42080. doi: 10.18632/oncotarget.5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schlecht NF, Brandwein-Gensler M, Nuovo GJ, et al. A comparison of clinically utilized human papillomavirus detection methods in head and neck cancer. Mod Pathol. 2011;24:1295–1305. doi: 10.1038/modpathol.2011.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stephen JK, Divine G, Chen KM, et al. Significance of p16 in site-specific HPV positive and HPV negative head and neck squamous cell carcinoma. Cancer Clin Oncol. 2013;2:51–61. doi: 10.5539/cco.v2n1p51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang CY, Lin MW, Chang YL, et al. Programmed cell death-ligand 1 expression in surgically resected stage I pulmonary adenocarcinoma and its correlation with driver mutations and clinical outcomes. Eur J Cancer. 2014;50:1361–1369. doi: 10.1016/j.ejca.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 33.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]