Abstract
The therapeutic efficacy of a lentiviral vector (LV) expressing the herpes simplex virus thymidine kinase (HSV-TK) was studied in an immunocompetent rat glioblastoma model. Intraperitoneal ganciclovir injections (50 mg/kg/day) were administered for 14 consecutive days, resulting in reduced tumor volumes as monitored by MRI. Survival analyses revealed a significant improvement among the LV-expressing HSV-TK (LV-TK)/ganciclovir-treated animals when compared to non-treated control rats. However, a limiting factor in the use of LV has been the suboptimal small-scale production in flasks. Our aim during the translation phase, prior to entering the final pre-clinical and early clinical phases, was to develop a scalable, robust, and disposable manufacturing process for LV-TKs. We also aimed to minimize future process changes and enable production upscaling to make the process suitable for larger patient populations. The upstream process relies on fixed-bed iCELLis technology and transient plasmid transfection. This is the first time iCELLis 500 commercial-scale bioreactor was used for LV production. A testing strategy to determine the pharmacological activity of LV-TK drug product by measuring cell viability was developed, and the specificity of the potency assay was also proven. In this paper we focus on upstream process development while showing analytical development and the proof-of-concept of LV-TK functionality.
Keywords: lentivirus, bioreactor, transfection, production, scale up, glioma
Introduction
Glioblastoma (GBM) is the most common and aggressive form of brain cancer among adults, constituting approximately 60%–70% of all primary brain tumors. Concomitant radiotherapy and temozolomide (TMZ) chemotherapy has improved the 2-year survival of GBM patients; however, the prognosis remains poor.1 The rapid, invasive growth and tumor heterogeneity of the aggressive forms of GBM enable tumor progression despite traditional therapeutic methods. Thus, multiple gene therapy approaches such as suicide gene therapy,2 oncolytic viruses,3 and immunomodulatory3 and tumor suppression gene therapies4 have been studied in GBM. The combination of herpes simplex virus thymidine kinase (HSV-TK) and a prodrug ganciclovir (GCV) is among the most commonly applied suicide gene therapy approaches. This therapy is known to target the dividing residual tumor cells without affecting the non-dividing neuronal cells. Due to the bystander effect, the neighboring tumor cells that may not contain the transgene are also destroyed.5
Initially, adeno- and retroviral vectors were used to deliver the HSV-TK into patients.6, 7 Adenoviruses were considered to be relatively safe, achieving both high titer production and high transient expression of transgenes in both dividing and non-dividing cells. In clinical trials, adenoviral vectors have exhibited an excellent safety profile. They have been shown to increase survival, as well as time to tumor recurrence. However, GCV treatment needed to be performed within a 14-day window due to the transient nature of adenoviral transgene expression. Ultimately, the survival benefit was not statistically significant.2 It is possible that part of the tumor cells was in quiescent phase during the treatment period and thus not killed by a method affecting only dividing cells. Complete tumor eradication would require additional applications of the vector and/or treatment cycles with GCV. However, recurring doses could increase the already high risk of developing an immune response against adenoviruses, leading to impaired efficacy. Alternatively, other vectors could be used, allowing a longer HSV-TK expression. Similarly to adenoviruses, lentiviral vectors (LVs) enable transduction of both dividing and non-dividing cells. As an advantage of using LVs, long-term transgene expression can be achieved by integrating viral genome to the host cell genome, eliminating the need for further vector administrations. Almost 300 clinical trials with LVs have been conducted but only few have reached later phase. Kymriah and Zynteglo, advanced cell therapy products in which cells are modified ex vivo by LV, have achieved marketing approval.8, 9
The use and efficacy of LV-expressing HSV-TK (LV-TK) with subsequent administration of GCV was found promising in our in vivo experiments. For this, LV was produced in T-flasks, which are not a feasible option for commercial manufacturing. LV production in cell factories (CFs) could be enough for ex vivo therapies, but would still be insufficient for the production of LVs for in vivo applications.10 Large-scale manufacturing of LVs has been the bottleneck in transitions from clinical trials to commercial use, because there are only few scalable production and purification methods for LVs. Here we present the first ever (according to PALL Life Sciences) large-scale iCELLis 500 bioreactor run in which LV-expressing GFP (LV-GFP) was produced based on the optimized small-scale runs.11 Subsequently, we were able to further optimize production, especially with regard to residual DNA removal. The second large-scale run (run 2) in the iCELLis 500/333 was performed in order to produce LV-TK, yielding almost 5 × 1015 viral particles and over 2 × 1011 TUs (transductive units).
In order to enter the preclinical and clinical phases, it is important to have an understanding of product quality during process development. To this end, we developed analytical assays, including an infectivity and a potency assay. Previously, we had used a flow-cytometry-based assay for infective titer analysis of LV-GFP.11 However, this was not possible due to the absence of fluorescent marker gene in the LV-TK product of run 2, so we developed a qPCR-based assay. Viral particle titer, often based on p24 ELISA, is relatively easy to analyze and can be used to directly compare the titers between laboratories. However, infective titer of the same sample can vary even by multiple logs depending on the method and cells used for analysis.12 To be more precise, we compared the infective titers obtained by flow cytometry and qPCR-based methods in different cell lines.
Results
LV-TK Proof-of-Concept
LV-TK under a constitutively active human phosphoglycerate kinase promoter13 was produced by traditional calcium phosphate based plasmid transfection method in T-flasks. The product was purified by ultracentrifugation14 for early studies or by chromatography (Mustang-Q Acrodisc)15 for the in vivo study. After the small-scale production and chromatographic purification, p24 ELISA titer of the LV-TK lot used for proof-of-concept studies was 2.87 × 108 pg/mL.
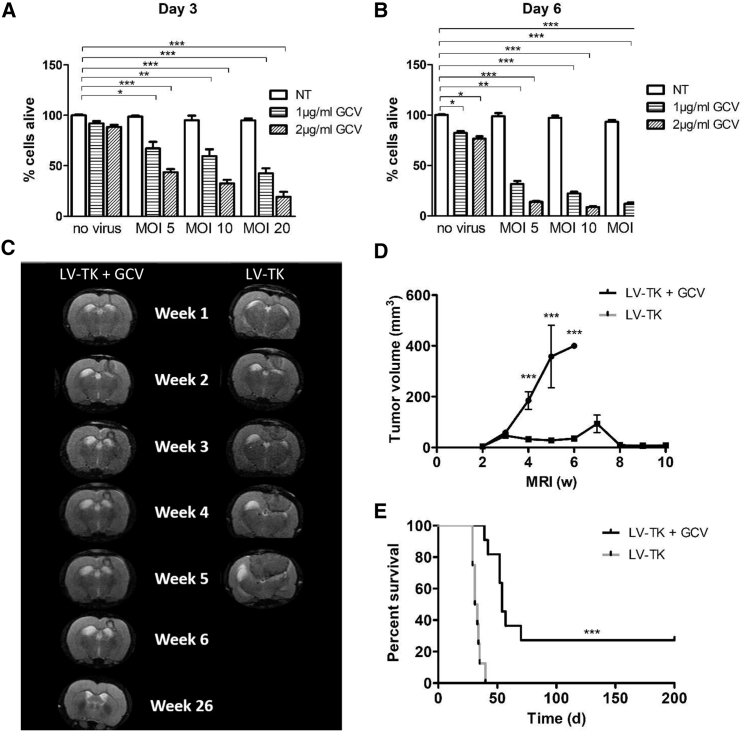
The functionality of LV-TK was tested using a CellTiter 96 AQueous Non-Radioactive Cell Proliferation Assay (MTS) assay in BT4C cells. There was a clear dose response in cell viability to GCV treatment in LV-TK-transduced cells in both time points (measured at days 3 and 6 after transduction, Figures 1A and 1B). The results revealed that LV-TK/GCV gene therapy significantly reduces the viability of BT4C cells over a range of MOIs (5, 10, and 20) up to 6 days after transduction.
Figure 1.
Cell Viability of BT4C Rat Glioma Cells after GCV Treatment Measured at Days 3 and 6 after LV-TK Transduction, and the Efficacy of LV-TK/GCV in a Rat Malignant Glioma Model
(A and B) BT4C cells were either non-transduced or transduced with treatment vector. Cell viability was analyzed with MTS assay 3 days (A) and 6 days (B) post-transduction. Experiments were carried out with three or more replicates. (C) BT4C tumor growth was monitored by MRI. Images represent the average tumor size of the specific time point. (D) Group average tumor volumes in pixels as measured from the MRI images. (E) Survival of the LV-TK treated rats bearing malignant gliomas. NT, non-transduced; MOI, multiplicity of infection; LV-TK, lentivirus vector expressing herpes simplex virus thymidine kinase; GCV, ganciclovir. Results are expressed as mean ± SEM, *p > 0.05, **p > 0.01, ***p > 0.001.
The proof-of-concept study was also done in vivo, using an immunocompetent, orthotopic, syngeneic rat malignant glioma model.16 A total of 10 μL of LV-TK (2.87 × 108 pg/mL) was injected to BT4C-derived malignant gliomas in BDIX rats (n = 17) on 2 consecutive days. Intraperitoneal (i.p.) GCV treatment (50 mg/kg/day) was administered twice a day starting 5 days after the first gene transfer (17 days after tumor implantation) and continued for 14 days. Weekly MRI monitoring showed a rapid tumor growth in non-treated controls and restricted tumor growth (Figures 1C and 1D) with related improved survival (Figure 1E) in the GCV-treated animals. These data proved that LV-TK together with GCV shows potency and efficacy in both in vitro and in vivo experiments.
Process Development in iCELLis
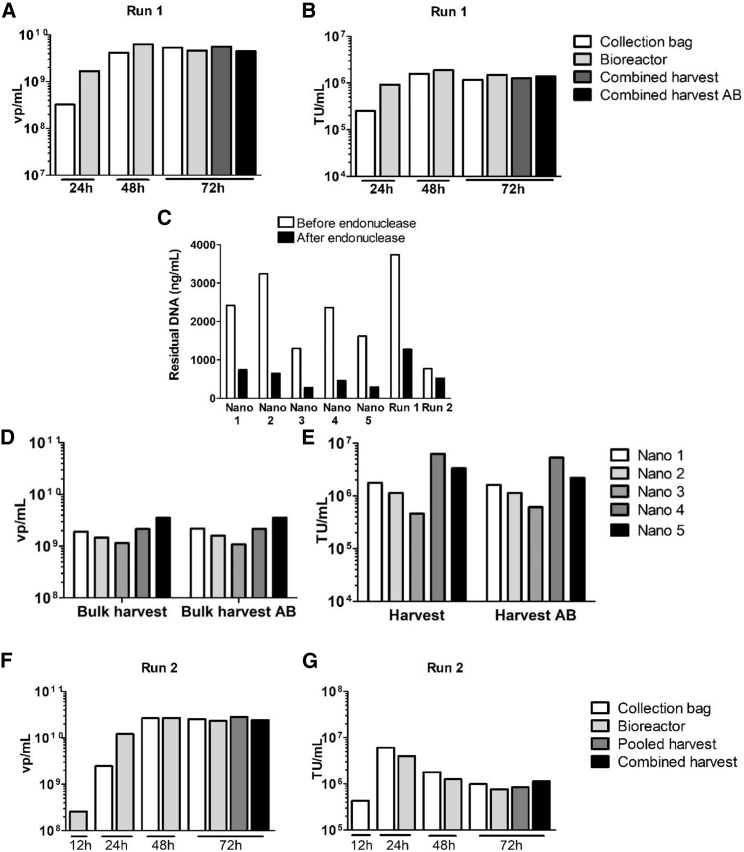
LVs used for proof-of-concept studies were produced in cell culture flasks. Prior to entering the preclinical phase, we developed a scalable and controlled manufacturing process for LV production based on fixed-bed single-use equipment and performed the process scale up (Table 1). To enable easy functional titering with a flow-cytometry-based assay, we produced LV-GFP11 in the optimization runs. Parallel to performing process parameter optimization in iCELLis Nano bioreactors,11 the first large-scale run (run 1, Table 1) for LV-GFP production was performed in iCELLis 500 using 100 m2 high compaction fixed bed in order to test LV productivity and process scalability in large scale. This batch became the internal reference standard for analytical development and assay comparison. Perfusion (i.e., continuously providing fresh media from media container and removing used media out from the bioreactor into a separate waste container) was started on day 1 (i.e., 1 day after the inoculation) and continued until the end of the run with 0.5 g/L glucose target (Figure S1A) by adjusting perfusion rate accordingly. Transfection was performed on day 4 with PEIpro mediated transfection using 1:1 DNA:PEI ratio and 300 ng/cm2 of plasmids required for 3rd generation LV production. Harvest volume of run 1 was 165 L (i.e., 0.165 mL/cm2), and productivity was 9.27 × 108 viral particle (vp)/cm2 (9.27 × 1014 total vp) and 2.3 × 105 TU/cm2 (2.3 × 1011 total TU; Figures 2A and 2B). Despite the endonuclease treatment performed for the harvest material, there was still too much residual DNA (34%, 1,275 ng/mL, Figure 2C).
Table 1.
Comparison of Different iCELLis Runs
| Run Number | Fixed-Bed | Working Volume | Media before Transfection | Media PT | Type of Media Supply | DNA/cm2 | Virus Produced | Endonuclease Treatment |
|---|---|---|---|---|---|---|---|---|
| Run 1 | HC, 100 m2 | 65 L | DMEM, 10% FBS, L-glut. | DMEM, L-glut. | perfusion aiming 0.5 g/L glucose | 300 ng | LV-GFP | after harvest |
| Nano 1 | LC, 2.67 m2 | 800 mL | DMEM, 10% FBS, L-glut., P/S | DMEM, L-glut., P/S | perfusion aiming 0.5 g/L glucose | 300 ng | LV-GFP | after harvest |
| Nano 2 | LC, 2.67 m2 | 800 mL | DMEM, 10% FBS, L-glut., P/S | DMEM, L-glut., P/S | recirculation | 300 ng | LV-GFP | after harvest |
| Nano 3 | LC, 2.67 m2 | 800 mL | DMEM, 10% FBS, L-glut., P/S | DMEM, L-glut., P/S | perfusion aiming 0.5 g/L glucose | 300 ng | LV-GFP | after harvest |
| Nano 4 | LC, 2.67 m2 | 800 mL | DMEM, 10% FBS, L-glut., P/S | DMEM, L-glut., P/S, extras | perfusion aiming 0.5 g/L glucose | 200 ng | LV-GFP | after harvest |
| Nano 5 | LC, 2.67 m2 | 800 mL | DMEM, 10% FBS, L-glut., P/S | DMEM, L-glut., P/S, extras | perfusion aiming 0.5 g/L glucose | 200 ng | LV-TK | after harvest |
| Run 2 | LC, 333 m2 | 65 L | DMEM, 10% FBS, L-glut. | DMEM, L-glut., extras | perfusion aiming 0.5 g/L glucose | 200 ng | LV-TK | during production in bioreactor and after harvest |
In all runs, 7,000 cells/cm2 were inoculated and pH was set to 7.2 before transfections and 7.0 after transfection. Abbreviations are as follows: HC, high compaction; LC, low compaction; PT, post-transfection; FBS, fetal bovine serum; L-glut., L-glutamine (4 mM); P/S, Penicillin-Streptomycin (50 μg/mL-50 U/mL); extras, NEAA + Na-pyruvate + CD-lipid supplement; LV, lentiviral vector; TK, thymidine kinase.
Figure 2.
LV Productivity and Residual DNA in iCELLis Nano Runs and Large Scale Runs
(A and B) vp/mL (A) and TU/mL (B) in the first iCELLis large scale run (run 1). (C) Residual DNA concentration in iCELLis Nano and large-scale runs (runs 1 and 2) before and after benzonase treatment. (D–G) vp/mL (D) and TU/mL (E) in iCELLis Nano runs 1–5 before and after the benzonase treatment, and (F) vp/mL and (G) TU/mL in the second large-scale run (run 2). AB, after benzonase; TU, transductive units; vp, viral particle.
After the first scale-up run, further optimization in iCELLis Nano bioreactors was performed. The five most important runs (Table 1) are described here. Based on previous experience11 in productivity and perfusion control, optimization runs were performed using a low compaction fixed bed. In Nano runs 1–4, LV-GFP was produced, and in Nano run 5, LV-TK. Perfusion targeting at 0.5 g/L of glucose was used (Figure S1A) in all runs except Nano 2, where recirculation (i.e., one media container from which media is circulated into the bioreactor and back to the container) was used instead. Recirculation was found to increase media consumption without increase in LV yields (Figures 2D and 2E).
Because endonuclease treatment performed only in the end of the run did not provide sufficient DNA clearance in run 1, further optimization was needed to reduce residual DNA in harvest material. A complete media change performed post-transfection (PT) before starting virus collection (Nano 3), and use of smaller plasmid quantity during transfection (Nano 4) were found to decrease residual DNA concentration (Figure 2C). Although a complete media change first decreased the productivity (Nano 3, Figures 2D and 2E), it was decided to be included in the protocol as a lower DNA concentration would be beneficial for the downstream (DS) processing. Eventually, addition of sodium pyruvate, chemically defined (CD) lipid supplement, and non-essential amino acids (NEAAs) to PT media (Nano 4) was found to compensate for the loss of productivity resulting from PT media exchange. When LV-TK was produced similarly to Nano 4, productivity level was the same (Nano 5, Figures 2D and 2E). In iCELLis Nano runs, harvest volumes varied between 3 and 4.5 L equaling to 0.11–0.16 mL/cm2, and productivity varied between 1.4 × x108–4.3 × 108 vp/cm2 and 5.4 × 104-6.7 × 105 TU/cm2.
The Second Large-Scale Run with Further Optimized Parameters
Finally, the second large-scale run (run 2) was performed using the further optimized parameters (Table 1), and instead of LV-GFP produced in run 1, LV-TK was produced. Based on iCELLis Nano runs, run 2 was performed in iCELLis 500/333 m2, the largest low compaction fixed bed available, using less DNA/cm2 in transfection compared to run 1. A complete media change was performed before starting harvest at 24 h PT, and PT media was supplemented with sodium pyruvate, NEAAs, and CD lipids. Interestingly, although perfusion rate was adjusted based on glucose concentration as in run 1 and Nano runs (Figure S1A), in run 2 PT media consumption/cm2 was lower and lactate accumulation slower than in run 1 and Nano runs (Figure S1B). This was also reflected by the harvest volume (178 L i.e., 0.05 mL/cm2) being only ∼10 L more than in run 1 even though a 3.3-fold larger fixed-bed size was used.
Although in run 2 almost one log more vp was produced than estimated based on iCELLis Nano runs (1.31 × 109/cm2 and 4.35 × 1015 total vp), TU/cm2 produced was in a similar level to Nano runs (6.1 × 104/cm2 and 2.03 × 1011 total TU; Figures 2F and 2G). Possibly due to the short half-life of LVs and relatively long cooling time of a large volume of LVs containing media, part of the produced LV was likely inactivated during overnight (o/n) storage of first part of the collected media in +4°C. However, similar to Nanos 3–5, use of less DNA/cm2 in transfection and a complete media change PT before virus collection, and moreover addition of endonuclease already into the bioreactor during production, were found to be important for decreasing the residual DNA concentration (Figure 2C). Thus, despite the larger bed size and 2- to 3-fold smaller media consumption/cm2 compared to run 1, residual DNA concentration was 2.5-fold lower, being only 520 ng/mL in the final harvest material.
Analytics
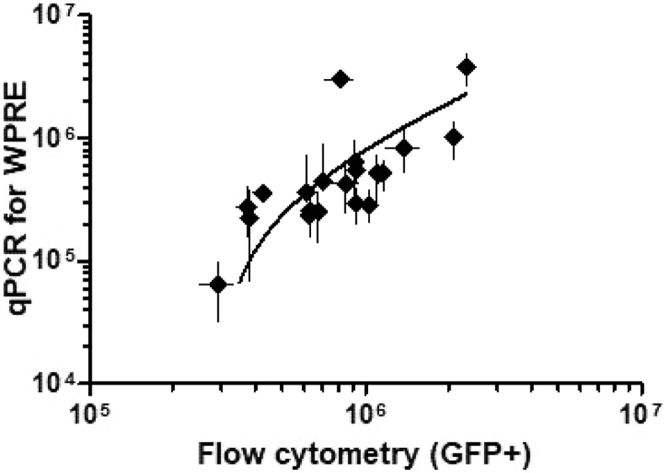
The first steps of process development were performed using LV-GFP, enabling GFP-based titer determination. Despite the straightforward procedure of the GFP flow cytometry titering, the source of cells can affect the results. HeLa cell lines from two sources were tested with the same flow-cytometry-based titering method (Table 2). On average, the lines showed 2.82-fold difference (n = 3, SD = 0.25) between the titers. The comparability between the GFP titering results and those obtained with a qPCR assay (Figure 3) was found acceptable for process development with correlation coefficient of 0.62. The flow cytometry results were on average 2.03-fold higher than qPCR results (n = 21, SD = 0.93).
Table 2.
Comparison of Flow Cytometry Titers Based on GFP Transgene Expression Using Two Different Sources for HeLa Cells
| Processing Step | TU/mL, HeLa Cells Grown in Own Laboratory | TU/mL, Collaborator’s HeLa Cells | Titer Ratio, Collaborator’s Own HeLa Cells |
|---|---|---|---|
| Combined harvest | 6.1 × 105 | 1.86 × 106 | 3.02 |
| Processing intermediate | 6.17 × 105 | 1.52 × 106 | 2.46 |
| Clarified product | 4.59 × 105 | 1.37 × 106 | 2.97 |
| Average titer ratio | 2.87 | ||
| SD | 0.25 |
Figure 3.
Comparison between Flow Cytometry and qPCR-Based Titers Shows Correlation between the Results (Correlation Coefficient 0.62), but the GFP Titers Are Generally Increased Compared to the qPCR Values
Potency Assay Development
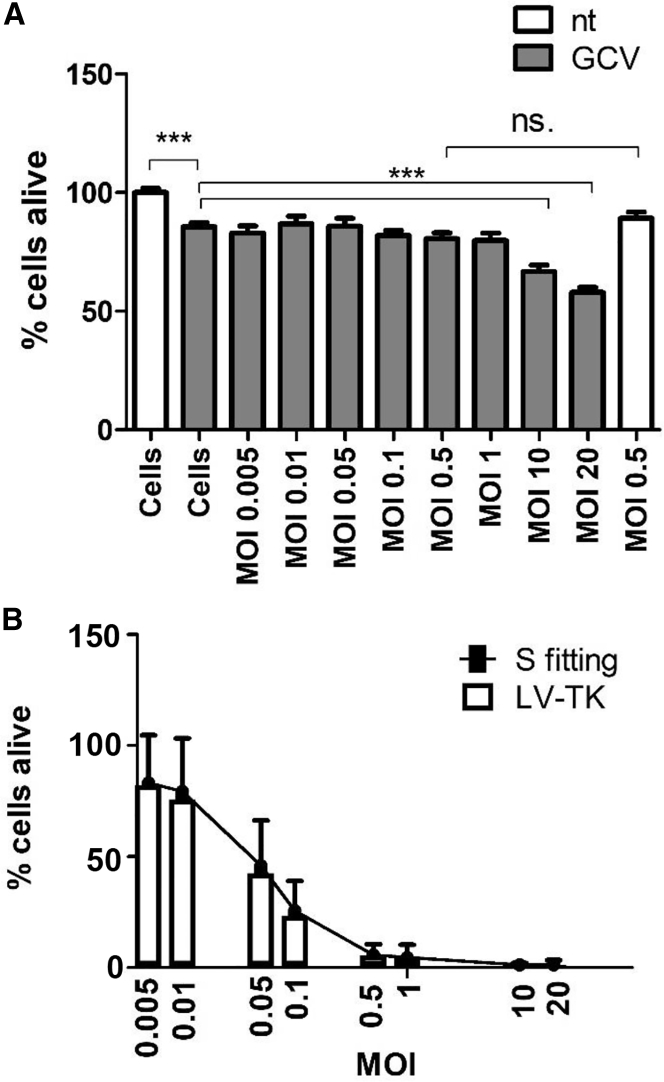
To determine whether the product stays biologically active throughout the process, we tested a potency assay for HSV-TK transgene. The assay is based on LV-TK transduction and GCV treatment of BT4C-cells, with consequent measurement of cell viability by Cell Counting Kit-8 (CCK-8). Control cells were transduced with LV-GFP. The test was repeated four times with three operators (operator 1 twice, operators 2 and 3 once). The assay proved to be highly specific for TK activity, because no effect between non-transduced cells and control vector (LV-GFP) at MOI 0.005–1 supplemented with GCV was seen (Figure 4A). Moreover, there was no difference between LV-GFP MOI 0.5 supplemented with GCV or medium, further showing specificity. At MOIs 10 and 20, cell viability started to decline, but this can be due to the toxic effects of excess GFP product17 or lentiviral vesicular stomatitis virus G protein (VSV-G).18 Significant difference between untreated and GCV-treated cells shows that treatment itself has an impact on cells, and thus proper controls are crucial for correct interpretation of results using this assay.
Figure 4.
Potency Assay Testing
(A) Effect of LV-GFP with or without GCV. Data are presented as mean ± SEM, n = 64/128. Statistical analysis is one-way ANOVA, ***p > 0.001. (B) Effect of LV-TK with GCV. Bar plot presents mean values, line plot S-curve fitting presented as mean ± SD, n = 59–60.
The TK transgene activity was successfully measured with CCK-8, with average IC50 from all tests being MOI 0.06 (with 1 μg/mL GCV; Figure 4B). The test is rather sensitive because the effect of MOIs even below 1 can be measured. On the other hand, when considering assay reproducibility and stability, there is a big deviation of ±0.4 with percent of coefficient of variation (CV%) 67.71 (Table S1). This is due to differences between runs (average MOI 0.06 ± 0.03) and between operators (average MOI 0.05 ± 0.03), which are not uncommon for biological assays.
Discussion
LV-TK showed promising results in in vivo syngeneic immunocompetent rat malignant glioma model. However, the production of LV-TK for the animal study was sub-optimal and not scalable. Before a promising product can enter into the clinical phase, translational development takes place. The product needs a scalable production method and appropriate analytical development. We focused on developing a scalable upstream (US) process utilizing disposable, controlled, and scalable iCELLis technology for adherent cells and showed the first-ever LV scale-up production in iCELLis 500 (according to PALL Life Sciences). In addition, analytical methods were developed to prove infectivity and potency of the produced vector.
Currently, iCELLis fixed-bed technology provides cell growth surface up to 500 m2 that equals to ∼790 Cell Factory 10 (CF10) and enables relatively straightforward scale-up of traditional processes. Production parameters were relatively easy to scale up from flasks to iCELLis Nanos11 and further up to iCELLis 500 scale. The largest challenges were related to the preparation of the transfection mixtures, so that transfection would occur similarly to small scale. In addition, temperature control, e.g., rapid cooling down of harvest material, is challenging when large volumes are handled. Although cell line and media used were the same, media consumption, harvest volume, and virus productivity were not directly proportional to the bed size of iCELLis bioreactors. Thus, harvest volume was almost the same in the two large-scale runs despite the 3.3-fold difference in bed size. On the other hand, a decrease in harvest volume resulting in more concentrated product is beneficial for DS because there is less volume to process. Because of the fragile nature of LV, shorter process time is better for maintaining activity of the vector. LV productivity has been better in low compaction bed.11 Currently the largest low compaction bed size available is 333 m2, which means that with this method, LV harvest volume likely remains below 200 L.
Often 105–107 TU/mL titers have been reported in large-scale LV production.19, 20, 21, 22, 23 Also, the harvest titer is dependent on the medium volume. Although direct comparisons cannot be made between laboratories, according to the infective titer analyzed in our HeLa cells, productivity (>106 TU/mL) in run 2 was comparable to others. Unfortunately, total TUs were similar in both large-scale runs despite the 3.3-fold difference in bed sizes.
In these runs we had two different constructs (different transgenes) and thus different titering methods were used (flow cytometry in run 1, qPCR in run 2). Assuming that vp/TU ratio between run 1 and run 2 were the same, there would have been two logs more TU in run 2. The large variation between infective titers measured in different laboratories using different methods makes it difficult to compare and evaluate the dose of LVs given to patients. This creates a demand for a reference standard for infective titer determination of LV.24 Some estimations can be made based on the potency assay, but clinical trials are required to determine the correct dose. One batch of LV produced in iCELLis 500 (333 m2 bed) would be enough to treat over 40 patients in the treatment of glioma in clinical trials, if the dose for one patient was ∼5 × 109 TU.25 If used ex vivo, a batch would be enough to treat thousands of patients.10 However, the recovery after DS processing is often ∼30%12 and part of the final product is required for the various analyses required for current Good Manufacturing Practice (cGMP) batches.
Fetal bovine serum (FBS) was not used in perfusion after starting harvest to reduce contaminating FBS in final product, also considering the costs and the possibly future availability of FBS. This may result in lower titers than in traditional LV production. It is also possible that lack of FBS (and thus lipids) makes LVs even more fragile, causing challenges for DS processing. To partially compensate the lack of FBS, we used animal-component-free CD lipid in media PT. It is also important to reduce the amount of residual DNA to as low as possible already in upstream (US) phase, because a large amount of contaminating DNA can create problems during DS purification. During US process development we were able to decrease residual DNA concentration in harvest material by optimizing plasmid DNA concentration in transfection, performing a PT media change, and adding endonuclease into the bioreactor already during production.
Viral vectors as a final GMP product are thoroughly analyzed.26 The characterization, biological activity, purity, impurity, and contaminant measurements follow the regulatory guidelines. Analytics is also needed during the process development stage to deepen the understanding, robustness, and quality control of the process. Safety and purity assays are applicable to many vectors but identity, activity, and potency typically require product-specific assays. Those assays are also used to compare the product between its life cycle stages, and thus the earlier those assays are in place, the better the comparability of the product in different stages can be ensured. Therefore, we developed and tested analytical assays already in the process development phase.
LV particle titer is often based on p24 protein analysis by ELISA. It is relatively easy to perform and has low inter-laboratory variation, whereas the infective titer of a sample can vary even by orders of magnitude depending on the method. The experimental details, such as the source and history of the cells used in titering, and technique by which infectivity of cells is analyzed (e.g., flow cytometry versus qPCR) can have a large impact. An example of how the cell line used affects was shown here with the samples titered using both our own ATCC-derived HeLa cell line and another, originally also ATCC-derived HeLa cell line from a collaborating laboratory. Because there is no fluorescent marker gene in LV-TK, infective titer analysis by simple GFP-expression-based flow cytometry, similar to LV-GFP produced in the iCELLis Nano optimization runs,11 was not possible and a qPCR-based method was used. Interestingly, by titering LV-GFP both with flow cytometry and qPCR-based assays, flow cytometry resulted in 2-fold higher titers for most samples. One could suggest that qPCR results in higher titers because it analyzes viral copies/cell, while flow cytometry measures the percentage of GFP-positive cells and a cell can host multiple viruses. However, in qPCR, part of the target copies can be lost during the DNA extraction step, resulting in lower titers when compared to the non-invasive fluorescence-based method.
The infective qPCR titering used measures the number of reverse-transcribed viral genomes inside target cells, which is a straightforward way to analyze the vector infectivity. When performed a few days post-transduction, it does not measure integration efficacy, but it should be noted that the same applies to the traditional GFP-expression assays. Both aforementioned techniques can be used to quantify the long-term expression (≈vector integration) by increasing the cell culture phase to >2 weeks. Usually process development cannot wait for answers, and the fragile nature of LVs requires fast analytics in order to proceed. Thus shorter assays are favored. To determine infectious titer, transduction of cells is necessary and often requires 2–4 days incubation period, which makes these methods sometimes too slow for all the aspects of process control. When process parameters are determined and confirmed, unfortunately, sometimes the p24 ELISA is the only fast titering method available.
In addition, the biological activity of the transgene is a key parameter with gene therapy products, which should be determined by a product-specific potency assay. For TK therapy combined with GCV, treatment potency is measured by its ability to kill cells. Here we tested a quantitative in vitro potency assay for the LV-TK. CCK-8 kit proved to be specific and sensitive to cell killing induced by HSV-TK/GCV treatment, and thus this assay could be used as a potency assay for LV-TK product. Still, deviation was high due to differences between runs and operators. Also, GCV itself had an effect on the cells likely due to native TK expression in mammalian cells. Thus, when choosing a potency assay, development of a stability assay, and setting correct assay, criteria are critical, because large-scale production tests need to be run often and operators change. This requires optimization of the selected assay, as well as selecting appropriate controls and reference material for correct analysis of results.
The next step is the development of a scalable, single-use, and closed DS process for LV-TK, in which harvested LVs are concentrated and purified to meet the titer and purity demands for clinical use. A typical DS process for LVs consists of preliminary clarification, concentration, purification, and formulation by chromatography and tangential flow filtration (TFF).12 DS process scale-up is necessary for large production volumes; however, it requires a finalized US protocol to provide feedstock for DS optimization. Changes in the US protocol generally have an impact on the DS efficiency. Thus, it is beneficial to produce non-optimized large-scale harvest material already for DS small-scale development because from iCELLis 500, more uniform and concentrated harvest material can be got compared to Nanos.
As a conclusion, we showed a proof-of-concept of LV-TK functionality, scaled up the LV production to commercial scale with iCELLis 500 bioreactor, and performed analytical development. Here, we reported the first ever iCELLis 500 run in which LV was produced.
Materials and Methods
Cell Lines
293T cells (ATCC, Manassas, VA, USA) cultivated in high or low glucose DMEM (GIBCO, Paisley, UK and Sigma-Aldrich, Irvine, UK) supplemented with 10% (v/v) FBS (GIBCO) and 50 U/mL penicillin, 50 μg/mL streptomycin (GIBCO), and 4 mM L-glutamine (GIBCO) were used for LV production. No antibiotics were used in iCELLis 500 runs. In iCELLis 500 run 2 media was supplemented also with 1× NEAA (GIBCO), 1 mM sodium pyruvate (GIBCO), and 1:500 CD lipid supplement (GIBCO). 7,000 cells/cm2 were used in inoculation of iCELLis bioreactors. Cells for inoculation were expanded in T-flasks and hyperflasks.
BT4C cells (ATCC) used in in vitro testing were cultured in DMEM, 10% FBS (GIBCO), 4 mM L-glutamine (GIBCO), 50 U/mL penicillin, and 50 μg/mL streptomycin (GIBCO).
HeLa cells (ATCC) used for titering were cultured in DMEM, 10% FBS (GIBCO), 50 U/mL penicillin, and 50 μg/mL streptomycin (GIBCO). FBS was not used during transductions.
All cells were cultivated at +37°C and 5% CO2.
Initial Testing of LV-TK
For initial in vivo testing, 3rd generation VSV-G pseudotyped LV-TK13 vector was produced by traditional calcium-phosphate-based plasmid transfection method in 293T cells27 and purified chromatographically (Mustang Q15). Viruses were titered by HIVp24ELISA (PerkinElmer, Waltham, MA, USA).
The potency of the vector was tested by MTS (Promega, Madison, WI, USA) by seeding 2,500 BT4C cells16 per 96 well-plate, transducing cells later on the same day with LV-TK, starting GCV (1 μg/mL) treatment on the following day, and analyzing the cell proliferation according to manufacturer’s instructions 3 and 6 days after transductions. Absorbance was detected at 490 nm with a Microplate reader using Ascent Software (Thermolab Systems, Waltham, MA, USA). The quantity of MTS formazan product as measured by the absorbance at 490 nm is directly proportional to the number of living cells in culture.
In Vivo Testing of LV-TK
Implantations of tumors derived from BT4C cells were performed to BDIX rats on day 1. Briefly, the male BDIX rats (17 in total) were anesthetized and placed on the stereotactic apparatus where a drill was used to penetrate the skull at the entry point (1 mm posterior to the bregma and 2 mm to the right of the sagittal suture, Figure S2). Injections of BT4C cells (10,000 cells in 5 μL) were made at a depth of 2.5 mm on the right corpus callosum. To avoid back flow of the cells, we performed the injections over 1 min, and the needle was left in place for 10 min.
T2-weighed anatomical MRI imaging (4.7 T MRI system, Magnex, Abington, UK) was used for monitoring of the tumor volumes. The baseline MRI images were taken 2 weeks later (on day 11), and animals were grouped according to the tumor volume. Following this, the LV-TK gene transfers were performed intratumorally with a total of 10 μL of LV-TK (2.87 × 108 pg/mL) injected to three separate sites in a vertical manner (depths of 1.5, 2.0, and 2.5 mm) on 2 subsequent days (days 12 and 13). The GCV treatment (50 mg/kg/day) was administered twice a day intraperitoneally (i.p.) starting 5 days after the first gene transfer (on day 17) and continued for 14 days.
Body weight monitoring was performed roughly twice a week during the experiment. It was measured as a guide for the human endpoints and was not used for any additional analysis. MRI was performed once a week, and tumor volumes were recorded from each animal.
Humane endpoints were used as the criteria for sacrificing animals (body weight loss > 20%, significant deviation from normal behavior). In these circumstances, animals were sacrificed with CO2 inhalation.
All animals were housed in the National Laboratory Animal Centre (Kuopio, Finland), and experimental procedures were approved by the National Animal Experiment Board of Finland.
LV Production in iCELLis Bioreactors
All small-scale iCELLis Nano runs (Nano 1–5) were performed using 2.67 m2 low compaction fixed bed (PALL Life Sciences, Hoegaarden, Belgium). In the first large scale run (run 1) a 100 m2 high compaction bed and in the second large scale run (run 2) 333 m2, the largest low compaction bed available, were used. Working volume in iCELLis Nano runs was 800 mL and in iCELLis 500 runs 65 L. Media volume was increased during inoculation and transfection up to 900 mL (Nano)/70 L (iCELLis 500).
In all runs, pH was set to 7.2 before and to 7.0 after transfections. pH was maintained in target with CO2 and 7.5% sodium bicarbonate (GIBCO) and was monitored both online and offline daily. Process data was collected by BioXpert program. Stirring was set to 1–2.65 cm/s medium linear speed, with higher stirring during inoculation and transfection. Dissolved oxygen was maintained at 50% with air and oxygen supply, and temperature at 37°C. From iCELLis Nano runs, cells were counted at least on day 4 before transfections aiming at 150,000–200,000 cells/cm2 during transfections. Glucose and lactate concentrations were measured at least once a day with a reflectometer (RQflex 10, Merck Millipore, Darmstadt, Germany, Figure S1).
Cells were inoculated at 7,000 cells/cm2 in low glucose DMEM, 10% FBS, and 4 mM L-glutamine (50 U/mL penicillin, 50 μg/mL streptomycin) on day 0. Perfusion with high glucose DMEM – 10% FBS – 4 mM L-glutamine (50 U/mL penicillin, 50 μg/mL streptomycin) was initiated on day 1 aiming at 0.5 g/L of glucose. In Nano 2, recirculation was used instead of perfusion. Cells were transfected on day 4.
In transfection, LV-GFP11 (Nano 1–4, iCELLis 500 run 1) or TK13 (Nano 5, iCELLis 500 run 2) were produced using a four-plasmid system (pVSV-G, pGag-Pol, pRev, and LV plasmid expressing GFP or HSV-TK27). Plasmids were manufactured by PlasmidFactory (Bielefeld, Germany). 200 ng/cm2 (Nano 4 and 5, run 2) or 300 ng/cm2 (Nano 1–3, run 1) of plasmids were transfected with PEIpro (Polyplus-transfection, Illkirch, France) mediated transfection with 1:1 DNA:PEI ratio. DNA and PEI separately mixed with serum-free media were combined and incubated in room temperature (RT) according to the manufacturer’s instructions. Volume corresponding to the transfection mixture was removed from the bioreactor and the mixture was added into the bioreactor after incubation. Perfusion/recirculation was stopped during transfection and was restarted 4–6 h PT with the same media as before transfections (Nano 1–3, run 1) or with DMEM, 10% FBS, 4 mM L-glutamine, 4 mM Na-pyruvate, 1× NEAA, and 1× CD-lipid supplement (Nano 4, 5, and run 2). In Nano 2, recirculation media was renewed before restarting recirculation.
Before starting the virus collection, a complete media change with the same media as used in perfusion PT until media change was performed in Nano 3–5, and in iCELLis 500 run 2. At 24 h PT, harvest by collecting the perfused media (not in Nano 2) was started. After starting the collection, perfusion continued without FBS. In Nano 2, recirculation media (4.5 L) was changed when virus collection was started. 72 h PT runs were ended and bioreactors were drained and combined with the perfused/recirculated media. After draining, bulk harvest was endonuclease treated with 30 U/mL benzonase (Merck, Darmstadt, Germany) in the presence of 2 mM MgCl2 incubating 2 h in +37°C. In addition, in run 2, benzonase (50 U/mL) and 2 mM MgCl2 were added into the bioreactor 24 h and 48 h PT.
Virus Titration
For LV-GFP flow cytometry11, 27 and for LV-TK, qPCR-based methods were used for analysis of the infective titers in HeLa cells (ATCC). Two HeLa cell lines grown in separate laboratories were used when the effect of titering method on the viral titers was tested. In qPCR tittering, the following primers and probe were used: WPRE Forw 5′-GGCACTGACAATTCCGTGGT-3′; WPRE Rev 5′-AGGGACGTAGCAGAAGGACG-3′ and WPRE Probe FAM 5′-CGTCCTTTCCATGGCTGCTCGC-OQA-3′ (Merck). For normalization Taqman Copy Number Reference Assay, human, RNaseP (Thermo Fischer/Life Technologies Bleiswijk, Netherlands) was used. Viral particle titer was determined by measuring p24 concentration (pg/mL) using p24 ELISA (PerkinElmer Life Sciences) and converting the results to vp/mL by assuming 12,500 LV particles per 1 pg of p24.28, 29
Potency Assay
BT4C rat glioma cells were seeded on a 96-well plate, 2,000 cells per well (day 0). After cells had been allowed to adhere for 4 h, they were transduced with LV-GFP or LV-TK using MOIs 0.005, 0.01, 0.05, 0.1, 0.5, 1, 5, 10, 20, or 50, or left non-transduced (cells only). 24 h post-transduction cells were treated with 1 μg/mL GCV (Cymevene 500 mg, Roche, Espoo, Finland). Cell viability was measured 3 days later with CCK-8 (Dojindo, Kumamoto, Japan) according to the manufacturer’s instructions, and absorbance was detected at 450 nm with Varioskan LUX (Thermo Fischer Scientific, Vantaa, Finland). When developing potency assay, measurements were performed six independent times by three operators, with four plates per operator per case. In data analysis, medium background was subtracted from absorbances and values normalized to average of cells only-wells on each plate. Any negative values (below medium background) were interpreted as zero. To obtain IC50-values for LV-TK, MOIs on the x axis were to transformed to logarithmic scale and log(inhibitor) versus response - variable slope (four parameters)-fit in GraphPad Prims 5.04 was used.
Statistics
For statistical analyses, one-way ANOVA with Bonferroni multiple comparison was used. All statistical analyses were performed using GraphPad Prism Versions 5.03 and 5.04 (GraphPad Software, USA).
Author Contributions
A.-M.M. and H.P.L. cloned the LV-TK construct; J.K. and H.S. conducted the initial in vivo testing; V.O. performed initial MTT assay; H.M.L. and A.J.V. performed the iCELLis Nano runs and H.M.L. and E.M.L. performed the iCELLis 500 runs; H.M.L., H.H., V.T., and I.O. took part in analytics; N.P., H.P.L., and S.Y.-H. supervised the process; and H.M.L., E.M.L., H.P.L., H.H., V.T., and V.O. wrote the manuscript. The figures are by H.M.L.
Conflicts of Interest
H.M.L., E.M.L., H.P.L., V.T., I.O., and H.H. are employees of Kuopio Center for Gene and Cell Therapy and FinVector. A.J.V. is employee of University of Eastern Finland, Kuopio Center for Gene and Cell Therapy and FinVector. No conflicts of interest exist for A.-M.M, J.K., H.S., V.O., N.R.P., and S.Y.-H.
Acknowledgments
We would like to thank the following people for help and support: Iina Laaksonen, Joonas Malinen, Riikka Kärna, and Sonja Kotoneva. We also would like to thank Marjut Köylijärvi and Aubrey Bailey for the critical review of the manuscript. In addition, we would like to thank PALL Life Sciences.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omtm.2019.08.006.
Supplemental Information
References
- 1.Kazda T., Dziacky A., Burkon P., Pospisil P., Slavik M., Rehak Z., Jancalek R., Slampa P., Slaby O., Lakomy R. Radiotherapy of Glioblastoma 15 Years after the Landmark Stupp’s Trial: More Controversies than Standards? Radiol. Oncol. 2018;52:121–128. doi: 10.2478/raon-2018-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Westphal M., Ylä-Herttuala S., Martin J., Warnke P., Menei P., Eckland D., Kinley J., Kay R., Ram Z., ASPECT Study Group Adenovirus-mediated gene therapy with sitimagene ceradenovec followed by intravenous ganciclovir for patients with operable high-grade glioma (ASPECT): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14:823–833. doi: 10.1016/S1470-2045(13)70274-2. [DOI] [PubMed] [Google Scholar]
- 3.Stepanenko A.A., Chekhonin V.P. Recent Advances in Oncolytic Virotherapy and Immunotherapy for Glioblastoma: A Glimmer of Hope in the Search for an Effective Therapy? Cancers (Basel) 2018;10:1–24. doi: 10.3390/cancers10120492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bai Y.H., Zhan Y.B., Yu B., Wang W.-W., Wang L., Zhou J.Q., Chen R.K., Zhang F.J., Zhao X.W., Duan W.C. A Novel Tumor-Suppressor, CDH18, Inhibits Glioma Cell Invasiveness Via UQCRC2 and Correlates with the Prognosis of Glioma Patients. Cell. Physiol. Biochem. 2018;48:1755–1770. doi: 10.1159/000492317. [DOI] [PubMed] [Google Scholar]
- 5.Moolten F.L. Tumor chemosensitivity conferred by inserted herpes thymidine kinase genes: paradigm for a prospective cancer control strategy. Cancer Res. 1986;46:5276–5281. [PubMed] [Google Scholar]
- 6.Immonen A., Vapalahti M., Tyynelä K., Hurskainen H., Sandmair A., Vanninen R., Langford G., Murray N., Ylä-Herttuala S. AdvHSV-tk gene therapy with intravenous ganciclovir improves survival in human malignant glioma: a randomised, controlled study. Mol. Ther. 2004;10:967–972. doi: 10.1016/j.ymthe.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Sandmair A.-M., Loimas S., Puranen P., Immonen A., Kossila M., Puranen M., Hurskainen H., Tyynelä K., Turunen M., Vanninen R. Thymidine kinase gene therapy for human malignant glioma, using replication-deficient retroviruses or adenoviruses. Hum. Gene Ther. 2000;11:2197–2205. doi: 10.1089/104303400750035726. [DOI] [PubMed] [Google Scholar]
- 8.US Food and Drug Administration . 2017. FDA approval brings first gene therapy to the United States. August 30, 2017.https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm574058.htm [Google Scholar]
- 9.US Food and Drug Administration . 2017. FDA approves CAR-T cell therapy to treat adults with certain types of large B-cell lymphoma. October 18, 2017.https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm581216.htm [Google Scholar]
- 10.Milone M.C., Fish J.D., Carpenito C., Carroll R.G., Binder G.K., Teachey D., Samanta M., Lakhal M., Gloss B., Danet-Desnoyers G. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol. Ther. 2009;17:1453–1464. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valkama A.J., Leinonen H.M., Lipponen E.M., Turkki V., Malinen J., Heikura T., Ylä-Herttuala S., Lesch H.P. Optimization of lentiviral vector production for scale-up in fixed-bed bioreactor. Gene Ther. 2018;25:39–46. doi: 10.1038/gt.2017.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merten O.-W., Hebben M., Bovolenta C. Production of lentiviral vectors. Mol. Ther. Methods Clin. Dev. 2016;3:16017. doi: 10.1038/mtm.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leinonen H.M., Ruotsalainen A.-K., Määttä A.-M., Laitinen H.M., Kuosmanen S.M., Kansanen E., Pikkarainen J.T., Lappalainen J.P., Samaranayake H., Lesch H.P. Oxidative stress-regulated lentiviral TK/GCV gene therapy for lung cancer treatment. Cancer Res. 2012;72:6227–6235. doi: 10.1158/0008-5472.CAN-12-1166. [DOI] [PubMed] [Google Scholar]
- 14.Tiscornia G., Singer O., Verma I.M. Production and purification of lentiviral vectors. Nat. Protoc. 2006;1:241–245. doi: 10.1038/nprot.2006.37. [DOI] [PubMed] [Google Scholar]
- 15.Kutner R.H., Puthli S., Marino M.P., Reiser J. Simplified production and concentration of HIV-1-based lentiviral vectors using HYPERFlask vessels and anion exchange membrane chromatography. BMC Biotechnol. 2009;9:10. doi: 10.1186/1472-6750-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tyynelä K., Sandmair A.-M., Turunen M., Vanninen R., Vainio P., Kauppinen R., Johansson R., Vapalahti M., Ylä-Herttuala S. Adenovirus-mediated herpes simplex virus thymidine kinase gene therapy in BT4C rat glioma model. Cancer Gene Ther. 2002;9:917–924. doi: 10.1038/sj.cgt.7700515. [DOI] [PubMed] [Google Scholar]
- 17.Ansari A.M., Ahmed A.K., Matsangos A.E., Lay F., Born L.J., Marti G., Harmon J.W., Sun Z. Cellular GFP Toxicity and Immunogenicity: Potential Confounders in in Vivo Cell Tracking Experiments. Stem Cell Rev Rep. 2016;12:553–559. doi: 10.1007/s12015-016-9670-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyanohara A. Preparation of vesicular stomatitis virus-G (VSV-G) conjugate and its use in gene transfer. Cold Spring Harb. Protoc. 2012;2012:453–456. doi: 10.1101/pdb.prot068528. [DOI] [PubMed] [Google Scholar]
- 19.Ausubel L.J., Hall C., Sharma A., Shakeley R., Lopez P., Quezada V., Couture S., Laderman K., McMahon R., Huang P. Production of CGMP-Grade Lentiviral Vectors. Bioprocess Int. 2012;10:32–43. [PMC free article] [PubMed] [Google Scholar]
- 20.Segura M.M., Garnier A., Durocher Y., Coelho H., Kamen A. Production of lentiviral vectors by large-scale transient transfection of suspension cultures and affinity chromatography purification. Biotechnol. Bioeng. 2007;98:789–799. doi: 10.1002/bit.21467. [DOI] [PubMed] [Google Scholar]
- 21.Greene M.R., Lockey T., Mehta P.K., Kim Y.-S., Eldridge P.W., Gray J.T., Sorrentino B.P. Transduction of human CD34+ repopulating cells with a self-inactivating lentiviral vector for SCID-X1 produced at clinical scale by a stable cell line. Hum. Gene Ther. Methods. 2012;23:297–308. doi: 10.1089/hgtb.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheu J., Beltzer J., Fury B., Wilczek K., Tobin S., Falconer D., Nolta J., Bauer G. Large-scale production of lentiviral vector in a closed system hollow fiber bioreactor. Mol. Ther. Methods Clin. Dev. 2015;2:15020. doi: 10.1038/mtm.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ansorge S., Lanthier S., Transfiguracion J., Durocher Y., Henry O., Kamen A. Development of a scalable process for high-yield lentiviral vector production by transient transfection of HEK293 suspension cultures. J. Gene Med. 2009;11:868–876. doi: 10.1002/jgm.1370. [DOI] [PubMed] [Google Scholar]
- 24.ISBioTech Lentivirus Project. https://isbiotech.org/ReferenceMaterials/lentivirus-home.html
- 25.Cloughesy T.F., Landolfi J., Hogan D.J., Bloomfield S., Carter B., Chen C.C., Elder J.B., Kalkanis S.N., Kesari S., Lai A. Phase 1 trial of vocimagene amiretrorepvec and 5-fluorocytosine for recurrent high-grade glioma. Sci. Transl. Med. 2016;8:341ra75. doi: 10.1126/scitranslmed.aad9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White M., Whittaker R., Gándara C., Stoll E.A. A Guide to Approaching Regulatory Considerations for Lentiviral-Mediated Gene Therapies. Hum. Gene Ther. Methods. 2017;28:163–176. doi: 10.1089/hgtb.2017.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Follenzi A., Naldini L. HIV-based vectors. Preparation and use. Methods Mol. Med. 2002;69:259–274. [PubMed] [Google Scholar]
- 28.Piatak M., Jr., Yang L.C., Luk K.C., Lifson J.D., Saag M.S., Clark S.J., Kappes J.C., Hahn B.H., Shaw G.M. Viral dynamics in primary HIV-1 infection. Lancet. 1993;341:1099. doi: 10.1016/0140-6736(93)92463-4. [DOI] [PubMed] [Google Scholar]
- 29.Vogt V.M. Cold Spring Harbor Laboratory Press; 1997. Retroviral Virions and Genomes; pp. 27–69. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.