Abstract
The objective of this randomized clinical trial was to compare performance of cow-calf pairs in southern Ontario treated with fenbendazole or ivermectin, or not treated, for gastrointestinal nematode infections. Treatments were administered to 128 cow-calf pairs over 2 years. Weights, body condition score, and fecal egg counts (FEC) were collected at treatment and at 28-day intervals. Treating calves with an anthelmintic was significantly advantageous compared with not treating, and there was no significant difference between treatment with fenbendazole or ivermectin. Neither treatment nor calf FEC had a significant effect on calf weaning weight. This could be the result of time of treatment, low initial FEC, or lack of power. Treatment affected cow FEC (P = 0.003). Cows in the ivermectin groups had the lowest FEC (P < 0.05), but because FEC were all low, biological significance is questionable. Additional work is needed to provide recommendations on when an anthelmintic should be used.
Résumé
Efficacité du fenbendazole et de l’ivermectin pour traiter les infections à nématodes gastrointestinaux dans un troupeau de vaches-veaux en Ontario. L’objectif de cet essai clinique randomisé était de comparer les performances de paires de vaches-veaux dans le sud de l’Ontario traitées avec du fenbendazole ou de l’ivermectin, ou non-traitées, pour des infections à nématodes gastro-intestinaux. Les traitements furent administrés à 128 paires de vaches-veaux sur une période de 2 ans. Le poids, le pointage de l’état corporel, et le dénombrement des oeufs dans les fèces (FEC) furent colligés au moment du traitement et à des intervalles de 28 jours. Traiter des veaux avec un anthelmintique était significativement avantageux comparativement à ne pas les traiter, et il n’y avait pas de différence significative entre un traitement au fenbendazole ou à l’ivermectin. Ni l’un ou l’autre des traitements ou les FEC n’avaient un effet significatif sur le poids au sevrage des veaux. Ceci pourrait être dû au moment du traitement, un FEC initial peu élevé, ou un manque de puissance. Les traitements ont affecté les FEC des vaches (P = 0,003). Les vaches dans le groupe ivermectin avaient les plus bas FEC (P < 0,05), mais étant donné que tous les FEC étaient bas, la signification biologique est questionnable. Du travail supplémentaire est requis pour fournir des recommandations sur le moment où un anthelmintique devrait être utilisé.
(Traduit par Dr Serge Messier)
Introduction
Anthelmintics are commonly used on beef operations for control of internal parasites (1). Gastrointestinal nematodes (GIN) are one of the most important classes of internal parasites to the beef industry in terms of negative biologic and economic impact (2–4). Currently, 2 anthelmintic drug classes are commonly used to treat these infections: fenbendazole (benzimidazole) and ivermectin (macrocyclic lactone). Subclinical GIN infections are relatively common in northern temperate regions (5). These infections can result in decreased weight gain, decreased carcass quality, reduced nitrogen balance, negatively affected protein metabolism, and suppressed immune response (6,7). Studies have shown that metaphylactic treatment of calves with an anthelmintic can help diminish these effects (8,9). Additional studies demonstrated the benefit of routinely treating calves with fenbendazole or ivermectin products, on improved weaning weight (WW) (10,11). These studies were conducted in regions with different grazing conditions and weather patterns; therefore, it might not be appropriate to extrapolate the results to Ontario herds. There has been no research comparing the efficacy of these 2 drug classes in the same cow-calf herd in Ontario. The objective of this trial was to determine the effect of fenbendazole or ivermectin treatment compared with a negative control on GIN fecal egg count (FEC) in cows and calves and on production performance of the nursing calves.
Methods and materials
Study design
The trial was conducted at the University of Guelph Elora Beef Research Centre in Centre Wellington, Ontario. At this facility, cross-bred cows are overwintered in groups in a barn. Cows are fed a total mixed ration formulated to meet nutrient requirements for beef cows (12). Previous anthelmintic use on the farm consisted of a single application of a generic ivermectin product (Noromectin; Norbrook, Newry, Northern Ireland), 0.5 mg/kg body weight (BW), for calves at weaning that coincides with housing in the fall. Cattle were not treated after they were calves. Breeding was done late June to early August, using artificial insemination. The care of all animals enrolled in this trial met the guidelines laid out by the Canadian Council on Animal Care (13) and the trial was approved by the University of Guelph Animal Care Committee (AUP No. 2678).
Animals in this trial grazed fields that have been dedicated pasture since 1985 with sporadic reseeding. The pasture was composed of a mixture of common pasture legumes and grasses. Each field was approximately 1.62 ha and subdivided into 8 paddocks using electric fence for rotational grazing. Stocking density of 4 cow-calf pairs per field was used (0.41 ha per cow-calf pair). Animals were moved to a new paddock every 2 to 11 d, depending on pasture conditions. Animal fence-line contact was eliminated with the rotation pattern.
The trial was conducted from 21 May to 29 October in 2014 and 13 May to 13 October in 2015. Animals were eligible for inclusion if they had a single, healthy calf, did not have difficulty calving, and did not experience disease (e.g., metritis, mastitis, or pneumonia) around the time of calving. Animals that had twins or were the recipient of a cross-fostered calf were not eligible for enrollment.
Eligible dams were randomly assigned to a treatment group. In the second year, animals that were in the trial the previous year were reassigned to the same treatment. Primiparous animals (11, 3, and 7, in control, fenbendazole, and ivermectin, respectively) replaced animals that were culled or did not calve within the calving window of the second year of the trial. They were randomly assigned to a treatment by calving date using a random number generator. A total of 128 cow-calf pairs over the 2 y were randomly assigned to 1 of 3 treatment groups: fenbendazole (Safe-Guard Suspension 10%; Merck Animal Health, Intervet Canada, Kirkland, Quebec), administered orally at 5 mg/kg BW, ivermectin (Noromectin Pour-On; Norbrook), administered topically at 0.5 mg/kg BW, or negative control (i.e., no treatment). Both cows and calves in the groups were treated on the day of turnout (i.e., 13 to 21 May). Sample size was calculated with 95% confidence interval (CI), 80% power, and expected weight difference between treated and control groups of 9 ± 5.9 kg. Clustering within fields was accounted for.
Once assigned to a treatment, all enrolled animals were combined into treatment groups and randomly assigned to field groups of 4 cow-calf pairs, with 1 treatment per field. Five fields were randomly allocated to each of the fenbendazole and ivermectin groups and 6 to the negative control group in 2014 and the treatment allocated to a field remained consistent between years.
Sample collection and laboratory analysis
Cows and calves were weighed (accurate to 2 kg), fecal samples collected rectally, and body condition scores (BCS, via visual assessment on scale of 1 to 5) of the multiparous and primiparous animals were recorded at turnout (Table 1) and at sample collection times throughout the season. In 2014, all animals were sampled at turnout (day 0), days 41 to 44 and every 28 d thereafter. The large gap between the first and second sample number was due to unanticipated complications with the weigh scale. In 2015, animals were sampled at day 0, day 14, day 28, and every 28 d thereafter. All animals were sampled in October before removal from pasture. Researchers did not administer treatments and did not examine what animals were assigned to what treatment until the completion of the study.
Table 1.
Baseline data of naturally gastrointestinal nematode-infected cows and calves included in trial investigating effects of anthelmintic treatment on spring born calves and cow pastured in Ontario. Arithmetic means and standard deviations (SD) of initial entry weight and fecal egg count for 2014 and 2015. Baseline data for cows
| Treatment | N | Initial weight (kg) ± SD | Initial fecal egg count (epg) ± SD | |
|---|---|---|---|---|
|
| ||||
| Primiparous | Multiparous | |||
| Negative control | 16 | 32 | 635.04 ± 13.07 | 8.05 ± 13.07 |
| Fenbendazole | 8 | 32 | 641.40 ± 105.77 | 20.34 ± 42.54 |
| Ivermectin | 11 | 29 | 642.78 ± 70.41 | 6.03 ± 9.75 |
|
| ||||
| Baseline data for calves | ||||
|
| ||||
| Treatment | Na | Initial weight (kg) ± SD | Initial fecal egg count (epg) ± SD | |
|
| ||||
| Females | Males | |||
|
| ||||
| Negative control | 27 | 21 | 79.10 ± 17.79 | 0 ± 0 |
| Fenbendazole | 26 | 14 | 69.38 ± 29.11 | 0 ± 0 |
| Ivermectin | 17 | 23 | 74.93 ± 19.75 | 0 ± 0 |
N — Number of animals enrolled in trial; total of 48, 40, 40 cow-calf pairs in control, fenbendazole and ivermectin treatment groups, respectively.
epg — eggs per gram; SD — standard deviation.
Rectal feces was collected between 1000 and 1300 h, placed directly into sterile specimen containers in a cooler and transferred to a refrigerator (4°C) within 3 h of collection. Fecal egg counts were performed using the Modified Wisconsin Flotation Technique, following the protocol of the Animal Health Laboratory, University of Guelph, Guelph, Ontario (M. Lake, personal communication). Specific gravity of the sucrose solution was between 1.30 to 1.33 units. The double centrifuge method was used for egg recovery (264 × g for 5 min). Since a recovery rate of 62.5% is expected for this technique (14), a correction factor of 1.6 was used to correct for egg loss. Slides were examined under 10× magnification and all nematode (family Trichostrongylidae) eggs were counted. The minimum detection limit for the Wisconsin method is 1 to 2 eggs per gram (epg) of feces (Animal Health Laboratory).
Meteorological data
Climatic data were obtained from the Agricultural and Forest Meteorology Group — Elora Research Station/Guelph Turfgrass Institute, Ontario Agricultural College, University of Guelph. Missing data were sourced from Environment Canada. Total rainfall (mm), the mean average relative humidity (%), and the mean average temperature (°C) were calculated for each month. These data were compared to the almost 30-year average (1981 to 2010) for Waterloo-Wellington, Ontario from Canadian Climate Normals station data.
Data analysis
All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, North Carolina, USA). A significance level of P ≤ 0.05 was set. Summary and descriptive statistics were performed to provide an initial overview of the data.
The variable cow age was dichotomized into primi- and multi-parous. The natural logarithm of cow and calf FEC was applied with a bias correction term of 0.25, [ln(FEC + 0.25)]. This logarithmic transformation was performed to improve the normality of the FEC data. Mean ± standard deviation (SD) of calf and cow FEC were calculated. Days on pasture (DOP) equalled the number of days elapsed from the date the animal was put to pasture and an observation date. Observation times were categorized into 7 “sample numbers,” as animals were repeatedly sampled 6 and 7 times throughout the 2014 and 2015 pasture seasons (Table 2). Due to differences in turnout times between years there was overlap in date ranges (i.e., DOP were not equivalent in the calendar). A dummy variable for early and late turnout was created to test for difference in turnout time within 2015. Half of the animals in 2015 were turned out 13 to 15 May and were categorized as early turnout; the second half was turned out 2 wk later (i.e., late turnout). Days on pasture was not consistent for all animals at each sampling time as initial processing for the study and placement on pasture was more time consuming and fewer animals could be enrolled per day. For later sample numbers, more animals could be sampled on 1 day. Thus, animals may vary by 2 to 3 DOP at a sample number. Additionally, due to the malfunctions in the scale in 2014, the time frame between sample 1 and sample 2 was 44 d, 16 d longer than originally planned, and animals were sampled every 28 d after sample 2. Thus, in comparing DOP at each sample number between 2014 and 2015, 2014 animals would have been on pasture approximately 16 d longer. Precipitation, relative humidity, and temperature were averaged for all days within a sample number and were included in the FEC statistical models. All predictor variables underwent univariable screening to determine which were offered to the models.
Table 2.
Categorization of sampling intervals, dates, and days on pasture for the 2014 and 2015 pasture seasons.
| Sample number | Days on pasture range | Date range | Median days on pasture | Difference (in days) |
|---|---|---|---|---|
| 1 | 1 | 13 May to 29 May | 1 | — |
| 2a | 13 to 15 | 27 May to 11 June | 14 | 14 |
| 3 | 27 to 44 | 10 June to 9 July | 35.5 | 21.5 |
| 4 | 55 to 72 | 8 July to 6 Aug. | 63.5 | 28 |
| 5 | 83 to 100 | 5 Aug. to 3 Sept. | 91.5 | 28 |
| 6 | 110 to 128 | 1 Sept. to 1 Oct. | 119 | 27.5 |
| 7 | 140 to 156 | 1 Oct. to 29 Oct. | 148 | 29 |
Observations for sample number 2 were only collected in 2015.
PROC MIXED was used to construct the first 3 mixed models, modeling calf FEC, cow FEC and WW, manually using backwards stepwise elimination. PROC GLIMMIX was used to model pregnancy rates. Collinearity between predictor variables that questioned the assumption of independence (Pearson correlation coefficient ≥ 0.8) was assessed by testing pair-wise correlations, using PROC CORR.
The outcomes of interest were the natural logarithm of calf FEC (LCAFEC) and cow FEC (LCOFEC), unadjusted WW and pregnancy rate in cows. Causal diagrams were constructed and assessed for all models, looking for possible confounders and intervening variables. For the first model, LCAFEC, the following variables were included in the initial multivariable model: treatment, year, sample number, sex, parity, calf average daily gain, turnout, LCOFEC, weather variables, calf weight, and cow weight. Since initial calf egg counts were all zero, they were removed from the LCAFEC model as the analysis of variance assumption could not be met. A zero-inflated model was investigated but could not be fit. The LCOFEC model included year, treatment, sample number, parity, turnout, weather variables, and cow weight in the initial multivariate model. Since multiple observations were collected from the same animals over the pasture season a REPEATED statement was investigated in the first 2 FEC models. This was to account for within calf/cow and field auto-correlation. All possible error structures were investigated (AR, ARH, TOEP, TOEP 2 to 6, TOPEH, TOPEH 2 to 6, UN, and UN 2 to 6). There was less within calf correlation (i.e., correlations between the repeated measures of individual animals) than expected so the REPEATED statement was not used in the LCAFEC model. Instead, a statement was included to allow for variation in time among sample numbers. This made the most improvement to the model [lowest Akaike’s information criterion (AIC)]. In the LCOFEC model, a REPEATED statement was included with an autoregressive (AR) error structure.
For the unadjusted calf WW model, since outcome was measured once, explanatory variables could not be repeated measurements. The mean and SD of LCAFEC and LCOFEC were calculated and included, in this model, as explanatory variables. The initial models included treatment, year, sex, parity, birth weight, turnout, calf age, mean, and SD of LCAFEC, mean and SD of LCOFEC, and cow weight at weaning. The PROC GLIMMIX pregnancy rate model included treatment, year, mean, and SD of LCOFEC, parity, mean BCS, mean cow weight. A RANDOM statement was included in all models to account for clustering within fields within treatments.
All possible explanatory variables, 2-way interactions, and quadratic terms (continuous variables) were included in the models. A liberal P-value of P ≥ 0.2 was used to select variables to be removed from the model. Different error structures were tested to look for possible improvement in the model (lowest AIC value) and variables with P ≥ 0.05 were removed. All main effects and interactions of interest that were not significant (i.e., P ≥ 0.05) at the beginning, were placed back in the model at the end to check for a change in significance.
Residual analyses were completed for all models. Residuals were plotted against the predicted outcomes and explanatory variability, to look for homoscedasticity, non-linearity, and outliers. Histograms of residuals and normal probability plots, as well as 4 tests offered by SAS 9.4 (Shapiro-Wilk, Kolmogorov-Smirnov, Cramer-von Mises, and Anderson-Darling) were used to assess normality.
The models were repeated with the influential observations removed and differences in coefficients were noted. Major changes would suggest additional analysis to give reason for observation removal. The models were also checked for confounding. Variables were tested for changes in the coefficients of ≥ 20%, and changes to the P-value. Outliers or influential points were compared to the original data collection records to explain behavior.
Results
No influential points, outliers, or confounders were removed from any of the models. Treatment was kept in models even when not statistically significant, as it was the main variable of interest. Age of calf at sampling, DOP, and sample number (a proxy DOP) had significant Pearson pairwise correlation coefficients. Age of calf at sampling and DOP were, therefore, not included in the models.
Meteorological data
There were no significant differences between monthly average temperatures, relative humidity, and total precipitation and the 30-year averages for this region. None of the data was significant in the LCAFEC or LCOFEC models (i.e., P ≥ 0.05).
Fecal egg counts
Eggs found were presumed to be those of Cooperia, Ostertagia, Trichostrongylus, Haemonchus, and Oesophagostomum genera. No animals in the trial had clinical signs of parasitism (e.g., diarrhea, weight loss, submandibular edema). Over both years only subclinical levels of GIN infection were detected. The FEC ranged from 0 to 816 epg of fecal matter in calves (mean: 21.2 ± 63.8 epg). Egg counts were zero for all calves at turnout; this was likely a result of absence of exposure to the infective stage of the parasite. The number of GIN eggs found in cows was often low, ranging from 0 to 202 epg (mean: 6.0 ± 16.9 epg). Over the 2 y there was a total of 704 FEC observations for calves, with 15.4% (128/832) missing and 789 FEC observations for cows, with 5% (43/832) missing. Most (93%) of these missing FEC were a result of feces collection (i.e., no fecal matter present in the rectum at time of collection or inadequate sample size). The other 7% were a result of laboratory processing errors.
Final calf fecal egg count model
The model for LCAFEC included treatment, sample number, and a significant interaction between treatment and sample number (P = 0.014). The random effect of field within treatment had a conservative P = 0.068.
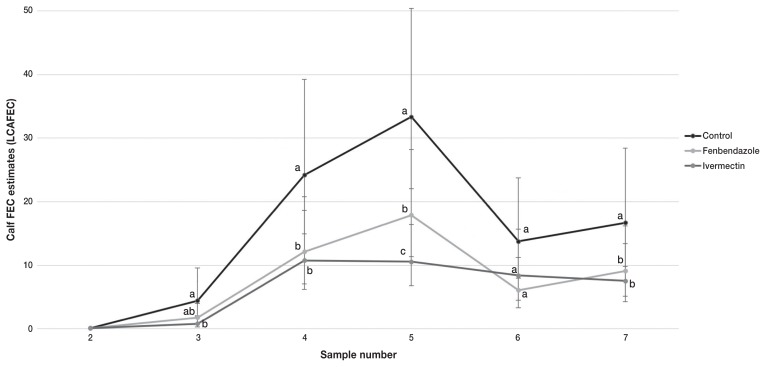
Within all treatment groups LCAFEC statistically peaked at sample number 4 (55 to 72 DOP) (Figure 1). After sample number 2 (13 to 15 DOP) egg counts rose slightly and were not significantly different between the 3 treatment groups. The LCAFEC estimates were significantly higher for negative control calves at samples number 3 (27 to 44 DOP) and 5 (83 to 100 DOP). There was no statistically significant difference in LCAFEC between treatment groups from sample number 6 (110 to 128 DOP) until the end of the trial.
Figure 1.
Natural logarithm of calf fecal egg counts (LCAFEC) estimates ± standard error (SE) with bias correction term of 0.25, for control, fenbendazole, and ivermectin treatment at turnout in an Ontario cow-calf herd.
The interaction term treatment and sample number was significant in the LCAFEC model. There was a significant difference in LCAFEC between calves that were not treated with an anthelmintic and those that were treated with fenbendazole or ivermectin (P < 0.01). However, there was no significant difference between fenbendazole- and ivermectin-treated calves in terms of LCAFEC (P = 0.42).
Final cow fecal egg count model
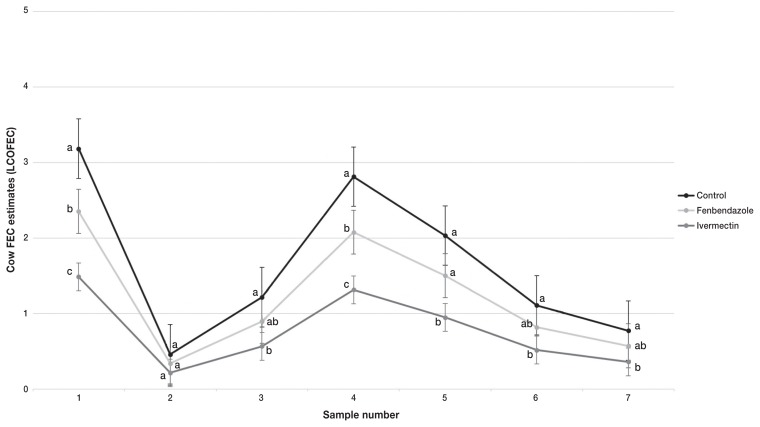
Sample number had a significant effect on LCOFEC (P < 0.0001). In all 3 treatment groups LCOFEC estimates were the highest in the spring at initial sampling (Figure 2). Egg counts dropped by sample number 2 (13 to 15 DOP) and rose again to peak at sample number 4 (55 to 72 DOP). From then on, cow FEC decreased steadily until the end of the study. Counts stayed low in all treatment groups throughout the grazing season. Treatment also had a significant effect on LCOFEC (P = 0.003). Negative control cows had 1.35 (95% CI: 0.92, 1.99; P = 0.11) and 2.14 (95% CI: 1.46, 3.14; P = 0.0009) times more epg than the fenbendazole and ivermectin treatment groups, respectively. Fenbendazole-treated cows had 1.58 (95% CI: 1.06, 2.36; P = 0.028) times more epg than ivermectin treated cows. Therefore, the cows in the ivermectin group had the lowest FEC.
Figure 2.
Natural logarithm of cow fecal egg counts (LCOFEC) estimates ± standard error (SE) with bias correction term of 0.025, for negative control, fenbendazole, and ivermectin treatment at turnout in an Ontario cow-calf herd. Cow weight was set to the mean in the model. The LCOFEC estimate with letters that differ are statistically different between treatments, P ≤ 0.05.
Parity and cow weight were also significant in the LCOFEC model. Primiparous cows had 1.45 times more epg than multiparous cows (P = 0.019). In addition, a heavier cow had a lower FEC, as cow weight increased 1 kg, FEC decreased by 0.99 epg (P < 0.0001).
Final calf weaning weight model
Arithmetic means of calf weights at turnout were 80 ± 17.79 kg, 70 ± 29.11 kg, and 74 ± 19.75 kg for negative control, fenbendazole, and ivermectin groups, respectively. Neither mean nor SD LCAFEC had a significant effect on unadjusted WW of calves (P = 0.34). Treatment did not have a significant effect on WW (P = 0.35).
Pregnancy rates
Over the 2 y, the pregnancy rates from artificial insemination breeding were 72%, 87%, and 87% for control, fenbendazole, and ivermectin treatments, respectively. Treatment did not have a statistically significant effect on pregnancy rates (P = 0.18).
Discussion
Typically, cow-calf producers treat their cows with an anthelmintic in the fall to control for internal and external parasites prior to housing. Due to trial design animals were not treated before housing. Cows and calves were treated in the spring before turnout. Trial animals were all housed together over the winter. Treatment of cows in the fall would have allowed for possible cross contamination of treatments between cattle (15). In addition to timing of treatment, trial design did not include the administration of a placebo and animal handlers and researchers were not technically blinded to treatment. To attempt to minimize bias, researchers did not administer treatments and did not review treatment records until all measurements were completed. Farm personnel were not reminded of treatment and had no access to treatment records after enrollment. Additionally, most measurements collected were objective (e.g., FEC, weight) to decrease any risk of bias of more subjective measurements, should someone manage to remember the treatments. Despite these attempts to minimize bias, there is still a chance that some bias is possible.
The FEC patterns in all treatment groups are relatively typical of GIN in this type of climate. In the spring, there is resumption of development of hypobiotic L4 larvae in cows with the highest FEC seen at sample number 1. This results in additional pasture contamination, along with over-wintered refugia (16). Calves become infected and start expelling GIN eggs in the feces as early as 13 DOP. With continuing opportunity for new infections, there is a rise in egg counts in the spring and into the summer. In northern temperate regions, there are 4 well-defined seasons. It is typical to see egg counts drop later in the fall as L4 larvae enter a state of hypobiosis. In addition, during the winter housing period there is little to no transmission of GIN infection (17).
In the months of September and October, calf FEC were not statistically lower than during July and August. Research in Quebec has shown egg counts peak later in the fall and then drop off in calves (18). Similar work shows a constant increase in calf FEC in autumn in both North and South America following weaning (19–21). If the trial was to be continued into the winter months, one would expect to see calf FEC drop.
Ivermectin-treated cows had statistically lower FEC than those treated with fenbendazole or no anthelmintic. However, natural logarithm FEC estimates in the model were extremely low in all treatment groups. The effects of the significant interaction between treatment and sample number in the LCAFEC model varied over time. There was a significant difference between treatment groups at some sample numbers, but not others; the rank order for mean LCAFEC varied by treatment and time (i.e., sample number). Due to the high degrees of freedom in this model (575) and the unlikely biological significance, it is likely that this interaction constitutes a Type I error. At most sample numbers there was a significant advantage between treating versus not treating with an anthelmintic in terms of FEC. There was, however, no significant difference between the 2 treatments (fenbendazole versus ivermectin; P = 0.42). No difference between these 2 drug class has been seen previously (22). Although there is an advantage to treating with either anthelmintic in calves, and treating with ivermectin in cows, this does not indicate that there is a biological advantage.
Little work has been done to detect treatment thresholds. Egg count ranges have been reported, but there is variation amongst different regions (5,16,18). Work is needed to determine what constitutes a high, medium, or low GIN infection.
In this study, differences in pregnancy rates between cows treated and those not treated with an anthelmintic were not significant (P = 0.18). There have been studies done previously with similar results (4,23). The cows in this herd were well-conditioned and artificial insemination was used for breeding. This could have affected the outcome in comparison to a naturally bred herd. The sample size in this trial was low and thus may not have been large enough to detect a significant difference in pregnancy rates between the treatment groups, even though the numeric differences in the raw pregnancy rates are rather large.
The study results show minimal difference between the 2 commonly used anthelmintics, fenbendazole and ivermectin. The study suggests that there is need for additional research in this area. One limitation of this study was the low sample size. By enrolling more animals, the power to detect smaller differences would increase as the population variance is quite large. In addition, this trial was limited to a single farm. A study in North Dakota looking at the effects of treatment with fenbendazole on calf performance, revealed significant differences in response to treatments amongst the 4 herds included (24). To further extrapolate results, it would be beneficial to conduct similar trials on various farms and in different herds across the province. This would provide a clearer picture of the prevalence of GIN in cattle in Ontario and the effectiveness of these 2 anthelmintics in different geographical regions and populations.
Another limitation of this study was that the genera of GIN that contributed to the FEC were unknown. This information is important as prepatent periods and pathogenicity vary among genera. In this trial, it was assumed that the common GIN genera made up the infection in this pastured cow-calf herd both years. This might not be the case. In future studies, it would be beneficial to perform coproculture to distinguish what nematodes are involved. Pasture sample to distinguish larvae burdens on pasture would also be beneficial.
It would have been beneficial to have the treatments occur later in the grazing period, when the infection pressure could be determined. There is a risk that the differing persistence in activity between the products could affect the FEC seen. Ivermectin has a longer duration of activity and thus may be effective against some level of reinfection from parasites overwintering on pastures. Fenbendazole does not have any residual activity and it may appear that fenbendazole is not as effective simply due to this difference.
Calf FEC did not have a significant effect on unadjusted WW in this study. Most of the literature suggests that increase in egg counts results in drop in calf performance, manifested as decreased average daily gain, weight gain, and/or WW (3,25,26). It is possible that this study lacked sufficient sample size to detect a correlation between calf FEC and growth performance. It is also possible that the level of GIN parasitism found in the calves was not high enough to hinder the performance of the calves while nursing. Since there was no impact of FEC on animal performance, it is possible that these cattle do not require initial treatment. It has been suggested that grazing calves might not require anthelmintic treatment while they are nursing (27). Gasbarre (27) suggests treatment of cows 1 mo into the grazing season and treatment of calves at weaning.
Future research should be targeted towards determining threshold levels where anthelmintic treatment is necessary to control subclinical GIN infection and parasitism in cows and calves in Ontario. Fecal egg counts are a useful tool for veterinarians and producers; however, there is no work published to differentiate what is a low-, medium-, or high-level GIN infection in this region and when anthelmintic intervention is necessary.
Acknowledgments
This project was supported by the Beef Farmers of Ontario through the Agriculture Adaptation Council. The authors acknowledge help provided by the staff at the Elora Beef Research Centre, support from the University of Guelph for summer student positions, and William Sears for statistical advice. CVJ
Footnotes
This manuscript represents a portion of a thesis submitted by Kaley Mackie to the University of Guelph, Department of Population Medicine as partial fulfillment of the requirements for a Master of Science degree.
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Murray CF, Fick LJ, Pajor EA, Barkema HW, Jelinski MD, Windeyer MC. Calf management practices and associations with herd-level morbidity and mortality on beef cow-calf operations. Animal. 2016;10:468–477. doi: 10.1017/S1751731115002062. [DOI] [PubMed] [Google Scholar]
- 2.Stromberg BE, Gasbarre LC. Gastrointestinal nematode control programs with an emphasis on cattle. Vet Clin North Am Food Anim Pract. 2006;22:543–565. doi: 10.1016/j.cvfa.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Ward JK, Ferguson DL, Parkhurst AM. Internal parasite levels and response to anthelmintic treatment by beef cows and calves. J Anim Sci. 1991;69:917–922. doi: 10.2527/1991.693917x. [DOI] [PubMed] [Google Scholar]
- 4.Corwin RM. Economics of gastrointestinal parasitism of cattle. Vet Parasitol. 1997;72:451–460. doi: 10.1016/s0304-4017(97)00110-6. [DOI] [PubMed] [Google Scholar]
- 5.Stromberg BE, Gasbarre LC, Ballweber LR, et al. Prevalence of internal parasites in beef cows in the United States: Results of the National Animal Health Monitoring Systems (NAHMS) beef study, 2007–2008. Can J Vet Res. 2015;79:290–295. [PMC free article] [PubMed] [Google Scholar]
- 6.Randall RW, Gibbs HC. Studies on the effects of two levels of gastrointestinal helminthiasis on digestion and energy metabolism in calves. Am J Vet Res. 1981;42:1730–1734. [PubMed] [Google Scholar]
- 7.Wiggin CJ, Gibbs HC. Adverse immune reactions and the pathogenesis of Ostertagia ostertagi infections in calves. Am J Vet Res. 1990;51:825–832. [PubMed] [Google Scholar]
- 8.Hersom MJ, Myer RO, Carter JN. Influence on weaning weights of nursing beef cattle calves de-wormed 90 days prior to weaning. Livest Sci. 2011;136:270–272. [Google Scholar]
- 9.Guichon PT, Jim GK, Booker CW, Schunicht OC, Wildman BK, Brown JR. Relative cost-effectiveness of treatment of feedlot calves with ivermectin versus treatment with a combination of fenbendazole, permethrin, and fenthion. J Am Vet Med Assoc. 2000;216:1965–1969. doi: 10.2460/javma.2000.216.1965. [DOI] [PubMed] [Google Scholar]
- 10.Borgsteede FHM, Burg WP. Worm burdens in cows II. An analysis of the population of nematodes in the abomasa of adult dairy cows. Vet Parasitol. 1982;10:323–330. doi: 10.1016/0304-4017(82)90084-x. [DOI] [PubMed] [Google Scholar]
- 11.Reinhardt CD, Hutcheson JP, Nichols WT. A fenbendazole oral drench in addition to an ivermectin pour-on reduces parasite burden and improves feedlot and carcass performance of finishing heifers compared with endectocides alone. J Anim Sci. 2006;84:2243–2250. doi: 10.2527/jas.2005-598. [DOI] [PubMed] [Google Scholar]
- 12.National Research Councin (NRC) Nutrient requirments of beef cattle. 7th revised ed. Washington, DC, USA: National Academy Press; 2000. [Google Scholar]
- 13.Canadian Council on Animal Care (CCAC) CCAC guidelines on: The care and use of farm animals in research, teaching and testing. Ottawa, Ontario: CCAC; 2009. [Google Scholar]
- 14.Egwang TG, Slocombe JO. Evaluation of the Cornell-Wisconsin centrifugal flotation technique for recovering trichostrongylid eggs from bovine feces. Can J Comp Med. 1982;46:133–137. [PMC free article] [PubMed] [Google Scholar]
- 15.Laffont CM, Alvinerie M, Bousquet-Mélou A, Toutain PL. Licking behaviour and environmental contamination arising from pour-on ivermectin for cattle. Int J Parasitol. 2001;31:1687–1692. doi: 10.1016/s0020-7519(01)00285-5. [DOI] [PubMed] [Google Scholar]
- 16.Smith HJ, Archibald RM. On the survival of overwintering bovine gastrointestinal nematode larvae during the subsequent grazing season. Can J Comp Med. 1969;33:44–47. [PMC free article] [PubMed] [Google Scholar]
- 17.Gibbs HC. The epidemiology of bovine ostertagiasis in the north temperate regions of North America. Vet Parasitol. 1988;27:39–47. doi: 10.1016/0304-4017(88)90059-3. [DOI] [PubMed] [Google Scholar]
- 18.Ranjan S, Trudeau C, Prichard RK, Piché C, Bauck S. Epidemiological study of parasite infection in a cow-calf beef herd in Quebec. Vet Parasitol. 1992;42:281–293. doi: 10.1016/0304-4017(92)90070-p. [DOI] [PubMed] [Google Scholar]
- 19.Entrocasso CM. Epidemiology and control of bovine ostertagiasis in South America. Vet Parasitol. 1988;27:59–65. doi: 10.1016/0304-4017(88)90061-1. [DOI] [PubMed] [Google Scholar]
- 20.Slocombe JO, Curtis RA. Aspects of the epidemiology of nematode infections in a cow-calf herd in Ontario. Can J Vet Res. 1989;53:336–339. [PMC free article] [PubMed] [Google Scholar]
- 21.Snyder DE. Epidemiology of Ostertagia ostertagi in cow-calf herds in the southeastern USA. Vet Parasitol. 1993;46:277–288. doi: 10.1016/0304-4017(93)90065-u. [DOI] [PubMed] [Google Scholar]
- 22.Miller JE, Morrison DG. Effect of fenbendazole and ivermectin on development of strongylate nematode eggs and larvae in calf feces. Vet Parasitol. 1992;43:265–270. doi: 10.1016/0304-4017(92)90168-9. [DOI] [PubMed] [Google Scholar]
- 23.Stuedemann J, Ciordia H, Myers G, McCampbell HC. Effect of a single strategically timed dose of fenbendazole on cow and calf performance. Vet Parasitol. 1989;34:77–86. doi: 10.1016/0304-4017(89)90167-2. [DOI] [PubMed] [Google Scholar]
- 24.Wohlgemuth K, Biondini M, Misek A, Anderson L. Deworming beef cows and calves with fenbendazole: Effect on weaning weight of calves. Bov Pr. 1990;25:27–30. [Google Scholar]
- 25.Sutherland I, Scott I. Gastrointestinal Nematodes of Sheep and Cattle: Biology and Control. Oxford, UK: Wiley-Blackwell; 2010. [Google Scholar]
- 26.Walker RS, Miller JE, Monlezun CJ, LeMay D, Navarre C, Ensley D. Gastrointestinal nematode infection and performance of weaned stocker calves in response to anthelmintic control strategies. Vet Parasitol. 2013;197:152–159. doi: 10.1016/j.vetpar.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 27.Gasbarre LC. Anthelmintic resistance in cattle nematodes in the US. Vet Parasitol. 2014;204:3–11. doi: 10.1016/j.vetpar.2014.03.017. [DOI] [PubMed] [Google Scholar]