Abstract
Objective
To determine the frequency and relative importance of the most meaningful symptoms in facioscapulohumeral muscular dystrophy (FSHD) and to identify the demographic and clinical features that are associated with the greatest disease burden in this population.
Methods
We performed a cross-sectional study involving 328 participants with FSHD. Collectively, participants reported the prevalence and relative importance of 274 symptoms and 15 symptomatic themes. We assessed the association between symptomatic theme prevalence and participants' age, sex, disease duration, pain level, employment status, and education.
Results
Participants answered >48,000 questions regarding their disease burden. The symptomatic themes with the highest prevalence in our sample were problems with shoulders or arms (96.9%), limitations with activities (94.7%), core weakness (93.8%), fatigue (93.8%), limitations with mobility and walking (93.6%), changed body image due to the disease (91.6%), and pain (87.7%). Problems with shoulders and arms and limitations with mobility and walking had the greatest effect on participants' lives. Employment status and the report of pain had the most extensive association with the prevalence of symptoms, with employment being associated with 8 of 15 of the symptomatic themes and pain being associated with 7 of 15 of the symptomatic themes. Men and women with FSHD experienced a similar prevalence of all symptomatic themes.
Conclusions
Adults with FSHD experience a variety of symptoms that play an important role in their disease burden. These symptoms have a variable prevalence and importance in the FSHD population and are associated with disease duration, employment status, and pain level.
Facioscapulohumeral muscular dystrophy (FSHD) is the third most common muscular dystrophy, with an estimated prevalence of 1:15,000 to 1:20,000.1,2 In the majority of people with FSHD, the disease is caused by a partial loss of a repeated sequence on chromosome 4q35 (FSHD1). Five percent of people with FSHD have a different genetic defect with similar molecular downstream effects and phenotypical expression (FSHD2).3,4 Although phenotypic variability exists, in general, both forms of FSHD present with slowly progressive weakness affecting the face and shoulders with involvement of the lower extremities later in the disease course. Extramuscular involvement is less common and can include retinal vasculopathy, hearing loss, restrictive lung disease, and possibly cardiac arrhythmias.4 Prior studies have linked clinical disease severity and pain in FSHD to a reduced quality of life.5,6
In preparation for clinical trials and to improve clinical care, it is important to understand what symptoms are most frequently experienced and most important to patients with FSHD.7,8 The Patient-Reported Impact of Symptoms in Facioscapulohumeral Muscular Dystrophy (PRISM-FSHD) study is a national cross-sectional study with the goal of defining disease burden from the patient's perspective and identifying factors associated with disease burden. Previously, we conducted qualitative interviews with individuals with FSHD to identify the themes and symptoms that are potentially important to this population.9 Here, we use a large sample of participants with FSHD to determine the importance and occurrence of these previously identified symptoms and themes in FSHD.
Methods
Standard protocol approvals, registrations, and patient consents
The study protocol was reviewed by the University of Rochester Institutional Review Board and qualified for exemption. Before participation, participants reviewed a detailed information letter describing the research.
Study participants
Inclusion criteria were age of ≥21 years, a clinical or genetic diagnosis of FSHD1 or FSHD2, and enrollment in the National Registry of Myotonic Dystrophy and Facioscapulohumeral Muscular Dystrophy Patients and Family Members (urmc.rochester.edu/neurology/national-registry.aspx).10 For participation in this registry, individuals with FSHD sign a consent form and have their clinical record fully reviewed by a neuromuscular specialist to ensure the accuracy of their clinical or genetic diagnosis. While genetic confirmation is not required to enroll in the registry, clinical diagnoses of FSHD are carefully assessed and confirmed by the neuromuscular specialists (R.T.).10 We developed a survey for participants with FSHD that ascertained demographic information, including sex, age, age at symptom onset, level of education, and employment status. The survey also inquired about the presence of 15 symptomatic themes and 274 symptoms, which were previously identified through interviews with individuals with FSHD and others with neuromuscular disease.9,11,12 Symptoms represented singular concepts of disease burden (e.g., difficulty with stairs), while symptomatic themes represented like symptoms and more general concepts of FSHD disease burden (e.g., limitations with mobility or walking).9 Participants used a Likert scale to rate the severity of each potential symptom and theme. Two versions of the survey were generated. The demographic and symptomatic theme questions were identical on both surveys, but the remaining 274 symptom questions were divided between the 2 surveys. Eligible participants were randomly assigned to receive one of the surveys with a recruitment letter and instructions on how to complete the survey. Surveys were collected between September 2010 and November 2011. The survey was written at an eighth grade reading level to optimize its completion in our study population. Surveys of similar length take ≈15 minutes to complete in neuromuscular populations. Participants were provided the opportunity to answer the survey questions over the phone if they preferred. For each theme or symptom, participants were asked, “How much does the following impact your life?” and given the following choices: (1) I don't experience this; (2) I experience this but it does not affect my life; (3) It affects my life a little; (4) It affects my life moderately; (5) It affects my life very much; and (6) It affects my life severely. Lastly, participants were given a postage-paid envelope to anonymously return their survey. These methods have been previously used and described for other neuromuscular populations.11–14
Statistical analysis
We calculated the prevalence of each symptom and theme. To determine how much these symptoms and themes affect participants' lives, we determined the life impact score by allocating a number (numeric scale 0–4) to each affected participant's response: the patient experiences the issue but it does not affect the patient's life = 0; the issue affects the patient's life a little = 1; the issue affects the patient's life moderately = 2; the issue affects the patient's life very much = 3; the issue affects the patient's life severely = 4. The average life impact scores were determined by calculating the mean of all scores of participants who experienced the symptom or theme. A population impact score was calculated for each symptom or theme by multiplying the percentage of participants with the symptom or theme by its average impact score. Composite impact scores were determined by averaging population impact scores across all themes.
Participant responses were further summarized by (1) age (21–30, 31–40, 41–50, 51–60, ≥61 years); (2) sex (male, female); (3) duration of symptoms (<11, 11–20, 21–30, 31–40, ≥41 years); (4) employment status (employed, not employed, excluding participants with age >65 years because retired was not a response option); (5) education (college graduate, not a college graduate); and (6) pain (no or minimal pain [pain impact score ≤1], significant pain [pain impact score >1]). Cutoffs for categorizations were determined before analysis. Descriptive statistics were computed for the prevalence of each theme for the complete sample and for each subgroup; subgroup comparisons were performed with the Fisher exact test. The distributions of impact scores for each theme and composite impact scores were compared between the different subgroups with the Kruskal-Wallis test.
Because of the large number of statistical tests performed, we used the Benjamini-Hochberg procedure15 with a defined false discovery rate of 5%. We applied the procedure separately for 2 groups of analyses: for all subgroup analyses comparing prevalence of each symptomatic theme and for all subgroup analyses comparing the impact scores of each symptomatic theme. Results with values of p ≤0.05 that were determined nonsignificant by this procedure were adjusted.
Data availability
Anonymized data will be shared by request from any qualified investigator.
Results
Surveys were sent to 514 adults with FSHD; 328 participants from 47 different states responded to the survey and answered >48,000 individual questions. Response rates per symptomatic theme question ranged from 96.34% to 99.39%, with an average response rate of 98.47% for each of these questions. Demographic data for the sample are summarized in table 1.
Table 1.
Clinical and demographic information of respondents with FSHD from the national registry
Prevalence of themes and symptoms
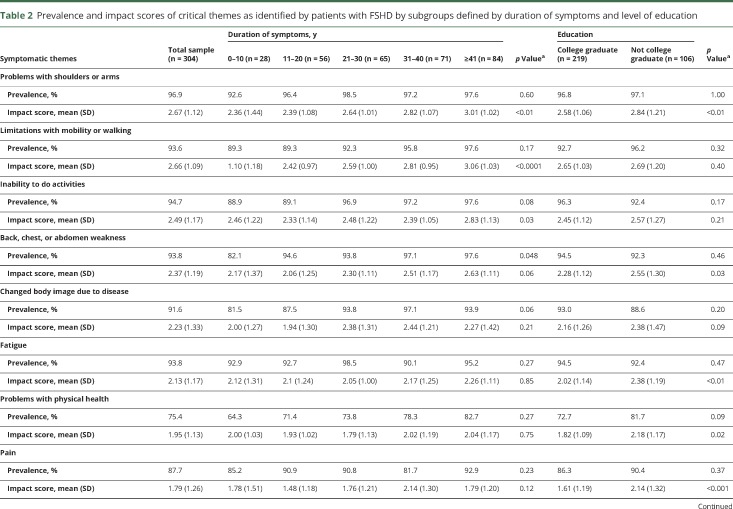
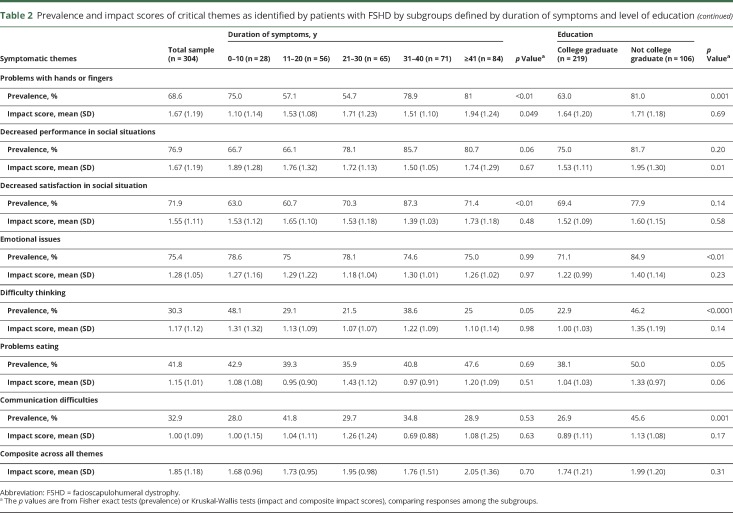
The symptomatic themes that occurred with the highest frequency were problems with shoulders or arms; inability to do activities; back, chest, and abdomen weakness; fatigue; limitations with mobility and walking; and changed body image due to disease (table 2). Of the 274 symptoms, the most prevalent were limitations physically on what the participant can do (96.1%), shoulder weakness (96%), difficulty reaching objects overhead (96%), difficulty lifting objects (96%), reduced arm and shoulder range of motion (95.3%), inability to do the things previously done (95.3%), decreased leg energy (stamina) (95.4%), tired muscles (94.7%), impaired ability to exercise (94.5%), difficulty carrying a load (94.3%), leg weakness (94.2%), awareness of the disease getting worse (94.1%), fear of progression of disease (93.5%), physical fatigue (92.8%), difficulty putting dishes away overhead (92.5%), difficulty with stairs (92.5%), and an inability to run (92.4%) (table 5 available from Dryad, doi.org/10.5061/dryad.sh54526).
Table 2.
Prevalence and impact scores of critical themes as identified by patients with FSHD by subgroups defined by duration of symptoms and level of education
The prevalence of symptomatic themes differed among some subgroups (table 2 and figures 1A and 2A).
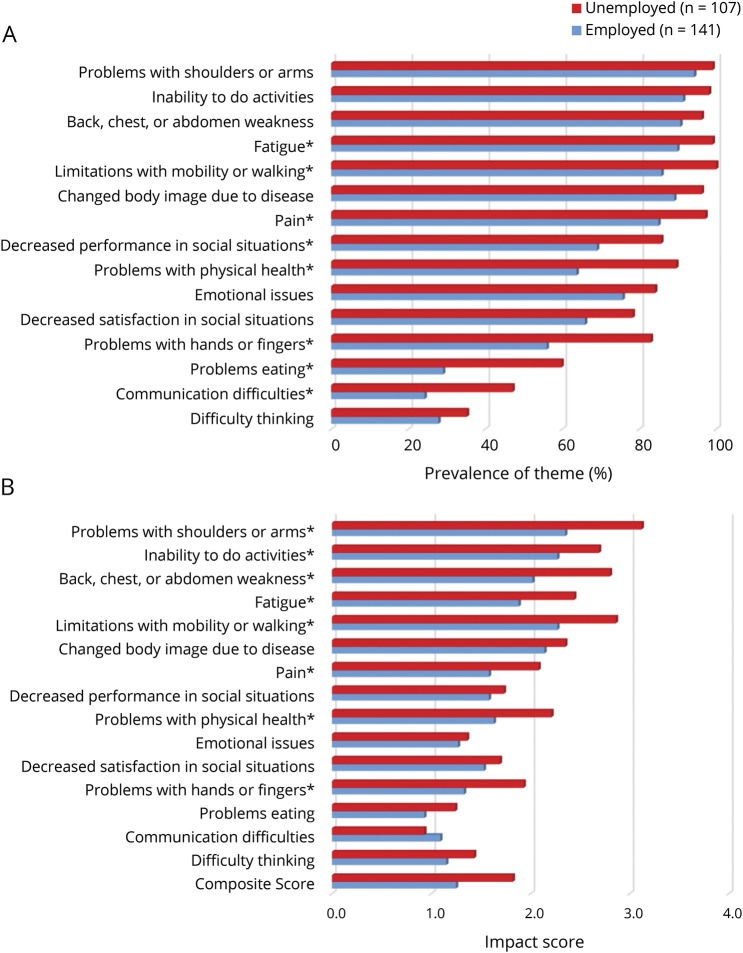
Figure 1. Symptomatic themes by employment status.
(A) Prevalence of symptomatic themes by employment status. *Significant p values ≤0.05 from the Fisher exact test after the Benjamini-Hochberg procedure was applied. (B) Impact of symptomatic themes by employment status. The possible range of impact on lives is 0.0 to 4.0, with higher values representing greater impact on patient's lives. *Significant p value ≤0.05 from the Kruskal-Wallis test after the Benjamini-Hochberg procedure was applied.
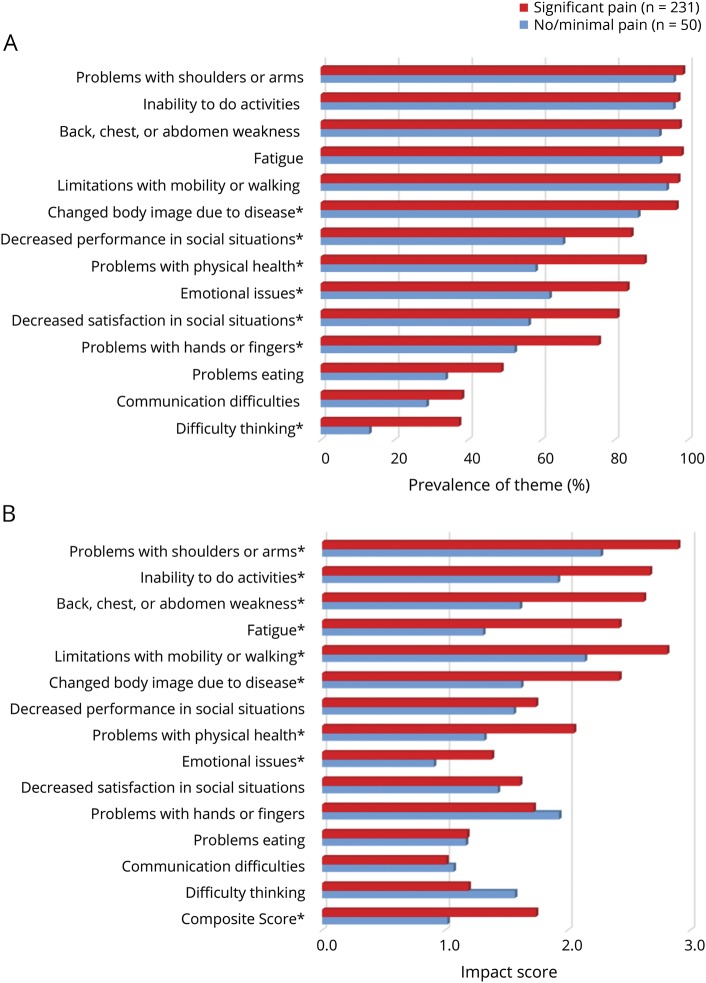
Figure 2. Symptomatic themes by pain level.
(A) Prevalence of symptomatic themes by pain level. *Significant p values ≤0.05 from the Fisher exact test after the Benjamini-Hochberg procedure was applied. (B) Impact of symptomatic themes by pain level. The possible range of impact on lives is 0.0 to 4.0, with higher values representing greater impact on patient's lives. *Significant p value ≤0.05 from the Kruskal-Wallis test after the Benjamini-Hochberg procedure was applied.
The largest subgroup differences in symptomatic theme prevalence occurred between employed and unemployed participants (figure 1A). Problems eating were experienced by 60% of unemployed participants compared to only 29% of employed participants (p < 0.0001). Also more common in unemployed participants with FSHD were problems with hands or fingers (83% vs 56%, p < 0.0001), problems with physical health (90% vs 64%, p < 0.0001), communication difficulties (47% vs 24%, p < 0.01), decreased performance in social situations (86% vs 69%, p = 0.002), limitations with mobility and walking (100% vs 86%, p < 0.0001), pain (97% vs 85%, p = 0.001), and fatigue (99% vs 90%, p = 0.002).
Participants with a longer duration of disease had a higher prevalence of problems with hands and fingers and decreased satisfaction in social situations (table 2).
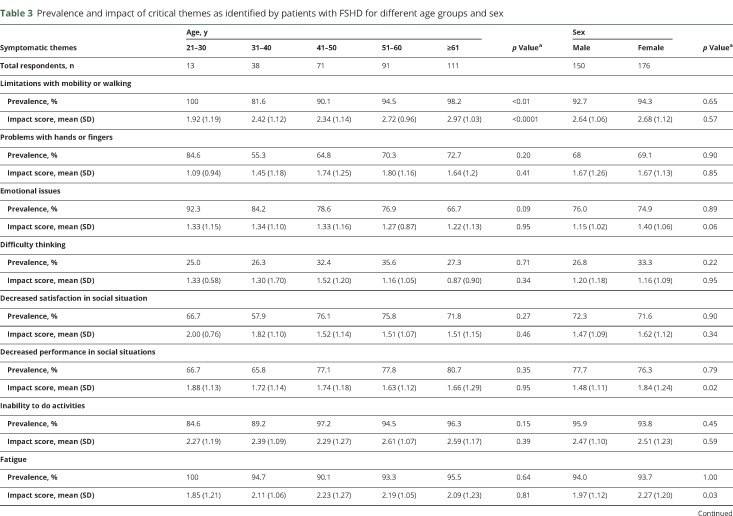
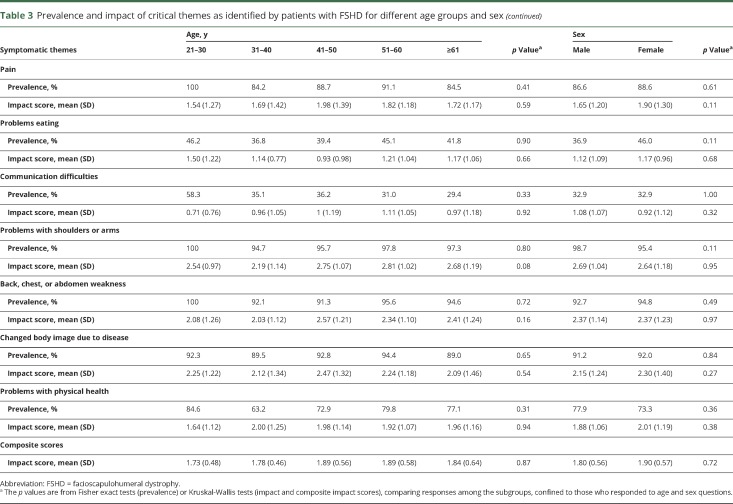
Age was associated with the prevalence of limitations with mobility or walking (table 3).
Table 3.
Prevalence and impact of critical themes as identified by patients with FSHD for different age groups and sex
Participants who experienced moderate or severe pain were more likely to report a changed body image due to disease (p < 0.01), problems with physical health (p < 0.0001), problems with hands or fingers (p < 0.01), decreased performance (p < 0.01) and satisfaction (p < 0.001) in social situations, emotional issues (p = 0.001), and difficulty thinking (p < 0.001) (figure 2A). Participants with a college degree were less likely to report problems with hands or fingers, emotional issues, difficulty thinking, and communication difficulties (table 2).
There were no differences in theme prevalence between women and men (table 3).
Life impact scores of themes and symptoms
The symptomatic themes with the highest effect on participants' lives were problems with shoulders and arms; limitations with mobility or walking; inability to do activities; back, chest, or abdomen weakness; and changed body image due to disease (table 2). Of the 274 symptoms, those that had the greatest effect on the lives of participants with FSHD were difficulty playing sports (3.23), difficulty walking long distances (3.10), inability to hold a job (3.00), difficulty with stairs (2.99), difficulty walking on ice (2.95), inability to run (2.94), lack of job because of disability (2.9), worsening golf game (2.88), and trouble riding a bike (2.88) (table 5 available from Dryad, doi.org/10.5061/dryad.sh54526).
Subgroup analysis of symptomatic themes showed that women reported a higher life impact score compared to men in relation to decreased performance in social situations and fatigue (table 3). Older age was associated with a higher life impact score related to limitations with mobility and walking (table 3). Participants with a longer duration of disease reported a greater effect on their lives secondary to limitations with mobility and walking and problems with shoulders and arms (table 2).
Participants who were unemployed reported a greater effect on their lives secondary to problems with shoulder and arms (p < 0.0001); limitations with mobility and walking (p < 0.0001); inability to do activities (p < 0.01); back, chest, or abdomen weakness (p < 0.0001); fatigue (p < 0.0001); problems with physical health (p < 0.001); pain (p < 0.01); and problems with hands or fingers (p < 0.01) (figure 1B).
Participants with minimal or no pain reported a lower effect on their lives secondary to emotional issues (p = 0.02); problems with physical health (p = 0.001); fatigue (p < 0.0001); changed body image due to disease (p < 0.0001); back, chest, or abdomen weakness (p < 0.0001); inability to do activities (p = 0.0001); limitations with mobility and walking (p < 0.001); and problems with shoulders or arms (p = 0.001) (figure 2B).
Participants with a higher level of education were less affected by problems with shoulders or arms, fatigue, problems with physical health, pain, and decreased performance in social situations (table 2).
The composite impact score across all themes was not associated with education level, employment status, disease duration, sex, or age but was associated with pain level (p = 0.02) (table 2 and figures 1B and 2B).
Population impact scores
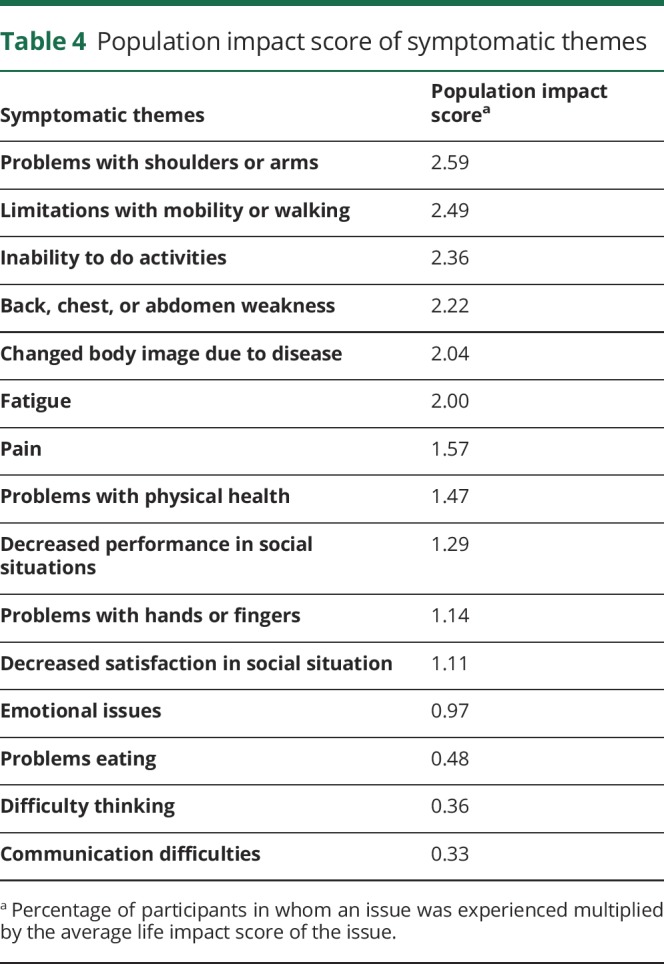
The symptomatic themes with the greatest population impact scores were problems with shoulders and arms; limitations with mobility and walking; inability to do activities; back, chest, or abdomen weakness; and changed body image due to disease (table 4). The symptoms with the highest population impact scores were difficulty playing sports (2.94), difficulty walking long distances (2.82), difficulty with stairs (2.77), inability to run (2.72), inability to do things previously done (2.63), and difficulty reaching objects overhead (2.60) (table 5 available from Dryad, doi.org/10.5061/dryad.sh54526).
Table 4.
Population impact score of symptomatic themes
Discussion
Our study describes the extent and degree of symptomatic disease burden in a national sample of individuals with FSHD. This study demonstrates that people with FSHD are significantly affected by many symptoms that represent physical, emotional, and social health.
This study is one of the largest in FSHD that uses direct patient input to characterize and quantify the diverse symptomatic burden experienced by people with this disease. Not surprisingly, our participants reported a very high prevalence of limitations related to physical function. Of the 274 symptoms, the top 5 most prevalent issues all had to do with physical limitations. Of these, 4 of 5 related to upper extremity function. This is expected because FSHD is a disease affecting the face, shoulders, and upper extremity muscles first, and proximal lower extremity function is typically impaired later in the disease course. However, when we ranked the 274 symptoms by their population impact score, which also takes into account the effect of the symptom on participants' lives, 3 of the top 5 highest-ranked issues related to lower extremity function (difficulty walking long distances, difficulty with stairs, and an inability to run).
We found that the prevalences of many symptomatic themes were associated with participant employment status. This has also been reported in other neuromuscular populations, including myotonic dystrophy type 2 (DM2)12 and spinal muscular atrophy (SMA),14 with similar methodology. In SMA, employment status is associated with the prevalence of 4 of 20 symptomatic themes (choking or swallowing issues; breathing difficulties; pain; and hip, thigh, or knee weakness),14 whereas in DM2, employment status is associated with the prevalence of 9 of 19 symptomatic themes.12 In contrast, the association between the prevalence of symptomatic themes and education status was higher in FSHD (9 of 15 themes) compared to both SMA (0 of 20 themes) and DM2 (1 of 19 themes). While the ability to hold employment and to obtain higher education is likely tied to disease burden, it is also possible that unemployment and education have a role in worsening disease burden. Furthermore, tertiary factors (e.g., socioeconomic or genetic factors) may separately affect symptom prevalence, education, and employment. Type of occupation, which is often related to level of education, also likely has effects on employment status.16 Additional research is needed to better understand how employment and education affect neuromuscular diseases and the specific factors that allow these populations to thrive even in the face of significant disease burden.
Sex effects have been reported in FSHD, with women being less affected than men.17–19 The men and woman in our sample had an identical prevalence of each of the symptomatic themes. However, for some symptomatic themes, the average effect of the theme on participants' lives differed on the basis of sex. For instance, women reported a greater effect of decreased performance in social situations and fatigue compared to men. Similarly, in an Italian study, women with FSHD were found to have higher levels of impairment in social function and emotional aspects compared to men.5 Previously, chronic pain in FSHD has been reported to be more frequent in female than in male patients.6 Another study showed an equal prevalence but a higher intensity of pain in women.20 Our study did not show a sex effect on pain.
Pain is an important, common, and troubling symptom in FSHD that has been reported in other studies.5,21–25 Eighty-two percent of our FSHD study sample reported significant pain, which is concordant with prior reports.6,20 In previous studies assessing types and location of pain, the lower back and shoulders were most often affected.6,20 Previous data have also shown that chronic pain has a major negative effect on the quality of life of patients with FSHD.6 Similarly, we found that significant pain was associated with a higher prevalence of many other symptomatic themes. The occurrence of pain was not dependent on age or disease duration, highlighting that this important symptom affects both young and old participants with FSHD.
Fatigue was also highly prevalent in our study across all subgroups. The prevalence of fatigue in our participants with FSHD is higher than what has been previously reported.20,26 From a clinical and research standpoint, we find it meaningful that there are patient-reported symptoms (e.g., fatigue) that occur in nearly all unemployed patients with FSHD. What is not known is whether the fatigue causes the unemployment, whether employment limits fatigue, or whether these factors are instead related to a separate genetic or environmental exposure. The occurrence of pain and fatigue in FSHD and its relationship to a patient's overall disease burden are important. While many of the symptomatic themes that we identified are currently difficult to treat in FSHD, pain and fatigue represent symptoms that could potentially be modified in the clinical setting if properly identified and addressed through safe and appropriate interventions.
Sixty-four percent of our eligible FSHD-confirmed registry population participated with this study. While this is a solid response rate, we recognize that our participants with FSHD might not be a perfect representative of the greater population of patients with FSHD worldwide. Specifically, our participants enrolled voluntarily and were all active members of the National Registry of Myotonic Dystrophy and Facioscapulohumeral Muscular Dystrophy Patients and Family Members. Thus, our participants' input may favor the views of patients who actively seek to participate in registries and clinical studies. However, we expect a similar sample of people with FSHD to volunteer for participation in future therapeutic trials. We included participants with clinical diagnosis of FSHD without genetic confirmation, which entails a risk of misclassification. However, all registry participants have had their medical record carefully reviewed to ensure the accuracy of their clinical diagnosis, and we believe this risk to be low. In general, our participants were well educated, with ≈67% having a college degree. This percentage is significantly higher than what is reported in the general US population. To this end, our participant responses must be interpreted and viewed in the context of this overall level of academic achievement. While we took steps to keep the survey simple to read and relatively short, we understand that both the language and the survey length could have generated selection bias in our sample. Our study also did not differentiate between the different types (periodic or constant) or locations of pain and did not assess medications, treatment approaches, or comorbid conditions, which could influence participants' responses. Formal assessment of strength and functional status was not performed as part of this study. Future studies may be useful for examining the relationships between strength and functional measures and patient perceptions of their disease burden.
Overall, this study provides extensive direct input into how disease burden is perceived by people with FSHD. While this methodology has been implemented in several neuromuscular diseases with a broad overlap, there are specific items that differ and are unique to each population. Our methodology assesses prevalence and the effect of a symptom on a participant's life. Moving forward, we expect that these patient-reported data will assist researchers in identifying relevant areas of potential therapeutic research, aid in the development and selection of relevant outcome measures, and better educate the governmental agencies dedicated to improving the lives of people with FSHD. In addition, these results may help clinicians to better address the symptomatic areas that are most important to patients while providing people with FSHD additional information on common symptoms that other people with their disease experience.
Glossary
- FSHD
facioscapulohumeral muscular dystrophy
- MD
myotonic dystrophy
- PRISM-FSHD
Patient-Reported Impact of Symptoms in Facioscapulohumeral Muscular Dystrophy
- SMA
spinal muscular atrophy
Appendix. Authors
Study funding
Support for this study was provided by the National Institute of Arthritis and Musculoskeletal and Skin Disorders (1K23-AR055947, 1UO1-AR065119), the Muscular Dystrophy Association, the New York Empire Clinical Research Investigator Program, The Goldberg Nathan Foundation, and the University of Rochester Clinical Translational Science Institute.
Disclosure
J. Hamel has received research funding from the American Academy of Neurology, American Brain Foundation, and Muscular Dystrophy Association. N. Johnson has received grant funding from National Institute of Neurological Disorders and Stroke, and the Food and Drug Administration. He receives royalties for the Congenital and Childhood Myotonic Dystrophy Health Index and Charcot-Marie-Tooth Health Index. He receives research funds from Biogen Idec, Cytokinetics, AMO Pharma, FLEX Pharma, and Acceleron Pharma. He has provided consultation for AMO and FLEX Pharma. R. Tawil has received research funding from the NIH, National Institute of Neurological Disorders and Stroke, The FSH Society, Friends of FSH Research, and Acceleron. He serves on the Advisory Board of Fulcrum Therapeutics. W. Martens, M. McDermott, and N. Dilek report no disclosures relevant to the manuscript. C. Heatwole has received grant funding from the NIH, Food and Drug Administration, and Cure SMA foundation. He is the founder and CEO of the Neuromuscular Quality of Life Institute. He receives royalties for the Myotonic Dystrophy Health Index, FSHD Health Index, Charcot-Marie-Tooth Health Index, Congenital and Childhood Myotonic Dystrophy Health Index, and SMA Health Index. He has provided expert testimony for neuromuscular cases unrelated to this research. He has provided consultation to Biogen, Ionis, aTyr, ExpansionRX, Cytokinetics, Regeneron, AMO, the Christopher Project, and Acceleron Pharma. Go to Neurology.org/N for full disclosures.
References
- 1.Flanigan KM, Coffeen CM, Sexton L, et al. Genetic characterization of a large, historically significant Utah kindred with facioscapulohumeral dystrophy. Neuromuscul Disord 2001;11:525–529. [DOI] [PubMed] [Google Scholar]
- 2.Mostacciuolo ML, Pastorello E, Vazza G, et al. Facioscapulohumeral muscular dystrophy: epidemiological and molecular study in a north-east Italian population sample. Clin Genet 2009;75:550–555. [DOI] [PubMed] [Google Scholar]
- 3.Statland J, Tawil R. Facioscapulohumeral muscular dystrophy. Neurol Clin 2014;32:721–728, ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tawil R, Kissel JT, Heatwole C, et al. Evidence-based guideline summary: evaluation, diagnosis, and management of facioscapulohumeral muscular dystrophy: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology and the Practice Issues Review Panel of the American Association of Neuromuscular & Electrodiagnostic Medicine. Neurology 2015;85:357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Padua L, Aprile I, Frusciante R, et al. Quality of life and pain in patients with facioscapulohumeral muscular dystrophy. Muscle Nerve 2009;40:200–205. [DOI] [PubMed] [Google Scholar]
- 6.Moris G, Wood L, Fernandez-Torron R, et al. Chronic pain has a strong impact on quality of life in facioscapulohumeral muscular dystrophy. Muscle Nerve 2018;57:380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bakker M, Schipper K, Geurts AC, et al. It's not just physical: a qualitative study regarding the illness experiences of people with facioscapulohumeral muscular dystrophy. Disabil Rehabil 2017;39:978–986. [DOI] [PubMed] [Google Scholar]
- 8.Tawil R, Padberg GW, Shaw DW, et al. Clinical trial preparedness in facioscapulohumeral muscular dystrophy: clinical, tissue, and imaging outcome measures 29–30 May 2015, Rochester, New York. Neuromuscul Disord 2016;26:181–186. [DOI] [PubMed] [Google Scholar]
- 9.Johnson NE, Quinn C, Eastwood E, et al. Patient-identified disease burden in facioscapulohumeral muscular dystrophy. Muscle Nerve 2012;46:951–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hilbert JE, Kissel JT, Luebbe EA, et al. If you build a rare disease registry, will they enroll and will they use it? Methods and data from the National Registry of Myotonic Dystrophy (DM) and Facioscapulohumeral Muscular Dystrophy (FSHD). Contemp Clin Trials 2012;33:302–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heatwole C, Bode R, Johnson N, et al. Patient-Reported Impact of Symptoms in Myotonic Dystrophy Type 1 (PRISM-1). Neurology 2012;79:348–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heatwole C, Johnson N, Bode R, et al. Patient-Reported Impact of Symptoms in Myotonic Dystrophy Type 2 (PRISM-2). Neurology 2015;85:2136–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson NE, Heatwole CR, Dilek N, et al. Quality-of-life in Charcot-Marie-Tooth disease: the patient's perspective. Neuromuscul Disord 2014;24:1018–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mongiovi P, Dilek N, Garland C, et al. Patient Reported Impact of Symptoms in Spinal Muscular Atrophy (PRISM-SMA). Neurology 2018;91:e1206–e1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B (Methodol) 1995;57:289–300. [Google Scholar]
- 16.Fowler WM Jr, Abresch RT, Koch TR, et al. Employment profiles in neuromuscular diseases. Am J Phys Med Rehabil 1997;76:26–37. [DOI] [PubMed] [Google Scholar]
- 17.Tonini MM, Passos-Bueno MR, Cerqueira A, et al. Asymptomatic carriers and gender differences in facioscapulohumeral muscular dystrophy (FSHD). Neuromuscul Disord 2004;14:33–38. [DOI] [PubMed] [Google Scholar]
- 18.Zatz M, Marie SK, Cerqueira A, et al. The facioscapulohumeral muscular dystrophy (FSHD1) gene affects males more severely and more frequently than females. Am J Med Genet 1998;77:155–161. [PubMed] [Google Scholar]
- 19.Teveroni E, Pellegrino M, Sacconi S, et al. Estrogens enhance myoblast differentiation in facioscapulohumeral muscular dystrophy by antagonizing DUX4 activity. J Clin Invest 2017;127:1531–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Kooi EL, Kalkman JS, Lindeman E, et al. Effects of training and albuterol on pain and fatigue in facioscapulohumeral muscular dystrophy. J Neurol 2007;254:931–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miro J, Gertz KJ, Carter GT, et al. Pain location and intensity impacts function in persons with myotonic dystrophy type 1 and facioscapulohumeral dystrophy with chronic pain. Muscle Nerve 2014;49:900–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nieto R, Raichle KA, Jensen MP, et al. Changes in pain-related beliefs, coping, and catastrophizing predict changes in pain intensity, pain interference, and psychological functioning in individuals with myotonic muscular dystrophy and facioscapulohumeral dystrophy. Clin J Pain 2012;28:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen MP, Hoffman AJ, Stoelb BL, et al. Chronic pain in persons with myotonic dystrophy and facioscapulohumeral dystrophy. Arch Phys Med Rehabil 2008;89:320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miro J, Raichle KA, Carter GT, et al. Impact of biopsychosocial factors on chronic pain in persons with myotonic and facioscapulohumeral muscular dystrophy. Am J Hosp Palliat Care 2009;26:308–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jensen MP, Abresch RT, Carter GT, et al. Chronic pain in persons with neuromuscular disease. Arch Phys Med Rehabil 2005;86:1155–1163. [DOI] [PubMed] [Google Scholar]
- 26.Kalkman JS, Schillings ML, van der Werf SP, et al. Experienced fatigue in facioscapulohumeral dystrophy, myotonic dystrophy, and HMSN-I. J Neurol Neurosurg Psychiatry 2005;76:1406–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared by request from any qualified investigator.