Abstract
STUDY QUESTION
Is adherence to an a priori defined diet quality indices [Alternate Healthy Index 2010 (AHEI-2010), relative Mediterranean diet score (rMED) or dietary approaches to stop hypertension (DASH)] associated with semen quality and reproductive hormone levels in young men?
SUMMARY ANSWER
Greater adherence to the DASH diet is related to higher sperm counts.
WHAT IS KNOWN ALREADY
Studies assessing the relationship between dietary intake and male reproductive function have mainly been focused on specific nutrients, food groups or data-driven dietary patterns, but the evidence on a priori defined dietary indices is still scarce.
STUDY DESIGN, SIZE, DURATION
Cross-sectional study of 209 male university students recruited from October 2010 to November 2011 in Murcia Region (Southern Spain).
PARTICIPANTS/MATERIALS, SETTING, METHODS
Healthy young men aged 18–23 years were included in this study. Diet was assessed using a validated food frequency questionnaire and three a priori-defined dietary indices (AHEI-2010, rMED and DASH) were calculated. Linear regression was used to analyze the relation between the three dietary indices and semen quality parameters and reproductive hormone levels accounting for potential confounders and covariates.
MAIN RESULTS AND THE ROLE OF CHANCE
We found statistically significant positive associations between the DASH index and sperm concentration (P, trend = 0.04), total sperm count (P, trend = 0.04) and total motile sperm count (P, trend = 0.02). No associations were observed for other semen parameters or male reproductive hormones.
LIMITATIONS, REASONS FOR CAUTION
Even though we adjusted for several known and suspected confounders we cannot exclude the possibility of residual or unmeasured confounding or chance findings. Subjects were blinded to the study outcomes thus reducing the potential influence on their report of diet. Our sample size may be too small to rule out associations with other semen parameters or reproductive hormones. Causal inference is limited, as usual with all observational studies.
WIDER IMPLICATIONS OF THE FINDINGS
The results suggest that greater adherence to the DASH may help improve sperm counts. This study was carried out on young men from the general population. However, results may differ among other populations (e.g. infertile men). Therefore, further research is needed to confirm these findings and extend these results to other populations.
STUDY FUNDING/COMPETING INTEREST(S)
This work was supported by Fundación Séneca, grants No 08808/PI/08 and No 19443/PI/14; Ministerio de Ciencia e Innovación, Instituto de Salud Carlos III (AES), grants No PI10/00985 and No PI13/01237; and grant P30DK046200 from the National Institutes of Health. Authors have no competing interests to declare.
Keywords: AHEI-2010, DASH, dietary indices, reproductive hormones, rMED, semen quality
Introduction
Declining semen quality is an ongoing controversy in male reproductive health (Swan et al., 2000; Jørgensen et al., 2002; Carlsen et al., 2005; Axelsson et al., 2011; Rolland et al., 2013; Levine et al. 2017). Several potential culprits have been suggested as explanations for reported trends in semen quality or altered reproductive hormone levels, including concurrent trends in decreased physical activity and increase in sedentary behavior (Gaskins et al., 2012; Priskorn et al., 2016), obesity (Sermondade et al., 2013), exposure to endocrine-disrupting chemicals (Adoamnei et al., 2018a,b) or change in diet quality (Attaman et al., 2012; Salas-Huetos et al., 2017). The impact of diet on semen quality is, however, still poorly resolved (Mínguez-Alarcón et al., 2018).
Several studies have reported associations between intake of isolated micro or macronutrients, food groups or a posteriori dietary patterns and semen quality and reproductive hormone levels (Mendiola et al., 2009; Vujkovic et al., 2009; Mendiola et al., 2010; Gaskins et al., 2012; Mínguez-Alarcón et al., 2012; Afeiche et al., 2013; Zareba et al., 2013; Afeiche et al., 2014; Chavarro et al., 2014; Chiu et al., 2015; Cutillas-Tolín et al., 2015; Liu et al., 2015; Abbasihormozi et al., 2017; Mínguez-Alarcón et al., 2017; Oostingh et al., 2017; Tiseo et al., 2017; Jurewicz et al., 2018; Adoamnei et al., 2019). A posteriori dietary pattern approach processes the gathered dietary information through multivariate statistical methods, such as principal component analysis (PCA) (Gaskins et al., 2012; Cutillas-Tolín et al., 2015; Liu et al., 2015; Jurewicz et al., 2018; Oostingh et al., 2017).
Nonetheless, the interpretation of the components derived by PCA can be challenging and subjective (Jolliffe and Cadima, 2016). Conversely, an a priori defined dietary indices approach is based on pre-defined healthy patterns and has advantages including relying on current scientific data regarding nutrition, health and disease (Chiuve et al., 2012; Harmon et al., 2015). Several a priori-defined indices have been related to well-known health benefits, showing anti-oxidative and anti-inflammatory actions (Trichopoulou et al., 2003; Fung et al., 2005) and protective factors for chronic diseases or mortality (Fung et al., 2010; Mursu et al., 2013; Harmon et al., 2015; Jacobs et al., 2015, 2016; Mattei et al., 2017), but the reproductive benefits of these dietary indices are still largely unknown. In general, the overwhelming majority of the literature on diet and male reproductive outcomes has been focused on individual nutrients whereas individual and public health recommendations about diet and health are generally made in terms of diet patterns, particularly in more recent years—food or pattern-based as opposed to nutrient-based—(Willett & Stampfer, 2013; American Heart Association, 2015; U.S. Department of Health and Human Services and U.S. Department of Agriculture, 2015; American Institute for Cancer Research, 2019). Moreover, most diet recommendations for health promotion are based on their impact on the prevention of cardiovascular disease and other major chronic diseases but it is unclear to what extent they may also provide male reproductive benefits. To date, only two studies have examined the relationship between the adherence to dietary indices and semen quality parameters in male partners of couples seeking for fertility treatment (Karayiannis et al., 2017; Efrat et al., 2018). Karayiannis et al. (2017) observed a positive relationship between sperm concentration, motility and morphology and the Mediterranean diet score (MDS). Efrat et al. (2018) also reported a positive association between the MDS, healthy eating index (HEI), alternate healthy index (AHEI), alternate MDS (aMED) and dietary approaches to stop hypertension (DASH) and several semen parameters in a comparable population.
However, to our knowledge, no study has examined the relationships between dietary indices and male reproductive parameters in unselected men unaware of their fertility status or testis function. Therefore, we sought to evaluate the associations between these three a priori-defined dietary indices and semen quality and reproductive hormone levels in young men.
Materials and Methods
Study population
The Murcia Young Men’s Study is a cross-sectional study of university students 18–23 years old in the Murcia Region (Southern Spain). Study details are described elsewhere (Mendiola et al., 2013). Briefly, a total of 215 students agreed to participate and completed the study visit between October 2010–November 2011. Six men who reported implausible total caloric intake (>5000 kcal/day) were excluded, leaving 209 men for the current analysis. At the study visit men underwent an andrological examination, provided semen and blood samples and completed questionnaires on lifestyle and food frequency. The Research Ethics Committee of the University of Murcia approved this study and written informed consent was obtained from all subjects.
Dietary assessment and a priori-defined dietary indices
We used a validated 101-food item semi-quantitative food frequency questionnaire (FFQ) to assess the usual diet (available at: http://bibliodieta.umh.es/files/2011/07/CFA101.pdf) (Vioque et al., 2007, 2013). Subjects were asked to report how often, on average, they had consumed each food item over the past year. The questionnaire offered nine options for frequency of consumption for each food, ranging from never or less than once a month to six or more times a day. Nutrient values for each food were obtained from the US Department of Agriculture and supplemented with Spanish sources (Palma et al., 2008; U.S. Department of Health and Human Services, 2018). The reproducibility and validity of this FFQ are comparable with other widely used FFQs (Willett, 2012). The mean correlation coefficients between nutrient intakes estimated using prospectively collected diet records and those estimated with the FFQ were 0.44 for validity and 0.44 for reproducibility (Vioque, 1995). This FFQ also showed satisfactory biochemical validity when compared with the plasma levels of carotenoids and vitamin C in further validation studies with other adult populations (Vioque et al., 2007, 2013). The FFQ dietary information was used to calculate the following three a priori-defined dietary indices AHEI-2010, relative MDS (rMED) and DASH.
Briefly, AHEI-2010 was the updated version of AHEI designed in 2012 by Chiuve and colleagues based on a comprehensive review of the relevant literature and discussions with other nutrition researchers to identify foods and nutrients that have been associated consistently with lower risk of chronic disease in clinical and epidemiologic investigations. The rMED is a variation of the original MDS (Trichopoulou et al., 2003) created by Buckland et al. in 2009 to evaluate the adherence to Mediterranean diet and risk of coronary heart disease in Spain. The DASH index was constructed based on the DASH clinical trial in which dietary pattern rich in fruit, vegetables and low-fat dairy products can substantially lower blood pressure (U.S. Department of Health and Human Services, 2018). This a priori defined index was described by Fung et al. in 2008. Definition of the scoring system, number of components, total score and components of AHEI-2010, rMED, and DASH scores can be seen on Supplementary Table SI.
Semen analysis and physical examination
Semen analyses were carried out as described in detail elsewhere (Mendiola et al., 2013). Briefly, men were asked to abstain from ejaculation for at least 48 hours before sample collection. Abstinence time was recorded as the time between current and previous ejaculation as reported by the study subject. Ejaculate volumes were estimated by specimen weight, assuming a semen density of 1.0 g/ml. Sperm concentration was evaluated by hemocytometer (Improved Neubauer; Hauser Scientific, Inc., Horsham, PA, USA). The spermatozoa were classified as either motile or immotile (World Health Organization, 2010) to report the percentage of motile spermatozoa [progressive (PR) and non-progressive (NP)]. Total sperm count (TSC; volume × sperm concentration) and total motile sperm count [TMSC; volume × sperm concentration × % motile sperm (PR + NP)] were also calculated. Smears for morphology were made, air-dried, fixed, Papanicolaou stained and assessed using strict criteria (Menkveld et al., 1990). The same specialized biologist carried out all the semen analyses. An external quality control on semen samples throughout the study period was carried out in collaboration with the University of Copenhagen’s Department of Growth and Reproduction. Body weight and height were measured using a digital scale (Tanita SC 330-S, London, UK). All participants were measured standing barefoot and wearing underwear at the study visit. BMI was calculated as weight in kilograms divided by squared height in meters. The presence of varicocele or other scrotal abnormalities was evaluated and recorded.
Reproductive hormones measurement
Hormone analysis methods have been described previously (Asklund et al., 2007; Cutillas-Tolín et al., 2015). Briefly, blood samples were drawn from participants’ cubital veins on the same time of the day of semen sample collection and were stored and frozen. Serum levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH) and sex hormone-binding globulin (SHBG) were determined using time-resolved immunofluorometric assays (DELFIA; PerkinElmer, Skovlund, Denmark). Intra- and interassay variations were <5% in each of the three assays. Serum testosterone (T) levels were determined using a time-resolved fluoroimmunoassay (DELFIA; PerkinElmer) with intra- and interassay variation of <8%. Estradiol (E2) was measured by radioimmunoassay (Pantex, Santa Monica, CA) with an intraassay variation of <8% and an interassay variation of <13%. Inhibin B levels were determined by a specific two-sided enzyme immunometric assay (Oxford Bio-Innovation Ltd, Bicester, UK) with intra- and interassay variation of 13% and 18%, respectively. Free testosterone (cFT) was calculated using the equation of Vermeulen et al. (1999) assuming a fixed albumin of 43.8 g/L.
Statistical analyses
Descriptive statistics are shown using crude data. Continuous variables were summarized by median and interquartile range (IQR: 25th–75th) and categorical variables given as number (n) and percentage (%). To test for associations of baseline characteristics across quartiles of the three a priori-defined dietary indices, ANOVA and χ2 tests were used for continuous and categorical variables, respectively. Linear regression was used to examine the associations between the three dietary indices and semen parameters and reproductive hormones. Semen volume, sperm concentration, TSC, TMSC, percentage of morphologically normal sperm, serum FSH and estradiol levels, showed non-normal distributions and were transformed using the natural log (ln) before analysis. Sperm motility and other hormones were used untransformed. The three dietary indices were categorized in quartiles and the lowest quartile of each dietary index was considered as the reference group.
The potential effect of several important or potential covariates or confounders [e.g. age (years), BMI (kg/m2), presence of varicocele (yes/no), smoking (yes/no), ejaculation abstinence time (hours), moderate-vigorous exercise (hours/week), total caloric intake (kcal/day)] were assessed. When inclusion of a potential covariate resulted in a change in the β-coefficient of more than 10%, the variable was retained in the final models. These variables included factors previously related to serum reproductive hormones or semen quality in this or other studies, regardless of whether they had been previously described as predictors of male reproductive health. The final covariates included in the models were: total caloric intake, moderate-vigorous exercise, presence of varicocele, ejaculation abstinence time and time to start of analysis (for sperm motility only). For reproductive hormones, similar models were calculated adjusting for time to blood sampling (minutes).
We examined linearity of associations between the three a priori-defined dietary indices and semen parameters and reproductive hormone levels by regressing reproductive parameters on quartiles of dietary indices and testing for linearity of trend using median measure in each quartile as a continuous variable in the linear regression models (P, trend across quartiles). We considered that a relationship was present when we found a statistically significant linear trend in semen parameters or reproductive hormones across dietary indices quartiles. All tests were two-tailed and the level of statistical significance was set at 0.05. Statistical analyses were performed with the statistical package IBM SPSS 25.0 (IBM Corporation, Armonk, New York, USA.
Results
Demographic characteristics, semen parameters and reproductive hormone levels among a priori-defined dietary indices quartiles are summarized in Table I. Study subjects were healthy young university students [median (IQR): 20.4 [19.6–21.4] years] with BMI of 23.6 (IQR: 21.8–25.5) kg/m2. Almost one-third of the subjects smoked (31.9%) and varicocele was detected in 15% of the participants. Median abstinence time was 71.0 hours (IQR: 59.0–92.0 hours), median sperm concentration 43.4 mill/mL (IQR: 22.0–72.3 mill/mL), morphologically normal sperm 9.0% (IQR: 6.0%–14.0%), progressive motility 48.3% (IQR: 41.4%–55.3%) and semen volume 3.0 mL (IQR: 2.0–4.0 mL). In general, all hormones showed serum levels within normal ranges. Median and IQR values for AHEI-2010, rMED and DASH indices were 56 (IQR: 50–62.5), 9 (IQR: 7–11) and 22 (IQR: 19–27), respectively. Diet indices were modestly to highly correlated with each other, having a Spearman’s rho correlation coefficients between 0.19 and 0.59 with P-values <0.01 (Supplementary Table SII).
Table I.
Demographic characteristics of men in the Murcia Young Men’s Study according to quartiles of adherence to dietary quality indices (n = 209).
| AHEI-2010 | rMED | DASH | ||||
|---|---|---|---|---|---|---|
| Median value for Q1 and Q4 | Q1 (45.0) (n = 55) | Q4 (66.5) (n = 52) | Q1 (6.0) (n = 76) | Q4 (13.0) (n = 37) | Q1 (17.0) (n = 69) | Q4 (30.0) (n = 42) |
| Age (years) | 20.7(18.0–22.7) | 20.2 (18.1–22.3) | 20.4 (18.1–22.7) | 20.4 (18.3–23.0) | 20.7 (18.3–22.9) | 20.2 (18.1–23.1) |
| BMI (kg/m2) | 23.1 (19.0–34.6) | 24.0 (19.9–29.9) | 23.6 (19.4–30.6) | 23.8 (20.4–30.9) | 23.7 (19.2–30.8) | 23.8 (20.5–26.5) |
| Calories intake (kcal/day) | 2470 (1458–3682) | 2337 (1230–3992) | 2271 (1249–3634) | 2496 (1391–3873) | 1775 (1127–2411) | 3277* (2399–4499) |
| Current smoker, n (%) | 22 (40.7) | 14 (26.9) | 21 (28.0) | 14 (38.9) | 20 (29.4) | 14 (33.3) |
| Alcohol intake (g/day) | 6.2 (0.0–30.5) | 6.7 (0.6–26.5) | 5.9 (0.0–39.3) | 14.0 (0.47–38.0) | 5.4 (0.0–25.7) | 10.6* (1.4–32.5) |
| Physical activity (h/wk) | 6.0 (0.0–19.2) | 5.0 (0.0–17.0) | 6.0 0.0–17.5) | 5.7 (0.0–14.5) | 5.0 (0.0–13.5) | 7.0* (0.3–19.9) |
| Abstinence time (hours)a | 83.0 (39.8–144) | 76.5* (40.6–145) | 71.5 (39.9–71.5) | 69.0 (39.6–177) | 72.0 (39.0–119) | 69.0 (39.6–249) |
| Presence of Varicocele, n (%) | 13 (23.6) | 4 (9.6) | 16 (21.1) | 3* (8.1) | 10 (14.5) | 6 (14.3) |
| Taken any medicationb, n (%) | 10 (18.2) | 12 (23.1) | 16 (21.1) | 10 (27.0) | 16 (23.2) | 10 (23.8) |
| Suffered Prolonged diseasec, n (%) | 2 (3.6) | 2 (3.8) | 7 (9.2) | 1 (3.0) | 7 (10.1) | 5 (7.7) |
Continuous variables are shown as median and interquartile range (IQR: 25th–75th) unless otherwise indicated. Kruskal–Wallis test for continuous variables and the χ2 test for categorical variables.
* P-value < 0.05 among quartiles within each dietary index.
aAbstinence time: period calculated as difference between time of current ejaculation and self-reported time of previous ejaculation;
bTaken any medication during 3 months prior to participation in study (mostly antibiotics or medication against allergy);
cLong-lasting disease (including diabetes/thyroid disease), sexually transmitted diseases (diagnosed with epididymitis, chlamydia or gonorrhea). AHEI-2010, Alternate Healthy Index 2010; rMED, Relative Mediterranean Diet Score; DASH, Dietary Approaches to Stop Hypertension.
Men with higher adherence to DASH had greater total energy and alcohol intake than men with low adherence (P < 0.05). Physical activity was greater in men with high adherence to DASH compared to low adherence men (P = 0.02), and abstinence time was significantly lower in men with high adherence to AHEI-2010 compared to low ones (P = 0.03). Lastly, men with high adherence to rMED index compared to low ones presented significantly lower number of varicoceles (P = 0.04).
Greater adherence to the DASH diet was positively associated to TSC (P, trend = 0.04) and sperm concentration (P, trend = 0.04) in multivariate adjusted models (Table II). These differences were further magnified when examining TMSC (Fig. 1). Relative to men in the lowest quartile (Q1) of adherence to DASH index, the adjusted difference (95%CI) of TMSC (millions) for men in the second, third and fourth quartile were 21.1% (−18.5; 60.6), 42.2% (1.6; 82.7) and 73.8% (19.2; 128), respectively (P, trend = 0.02). Similar results were obtained when further adjustment by AHEI-2010 and rMED was carried out (P, trends for sperm counts ≤0.04). Moreover, when examining which specific components of DASH were related to TMSCs, fruits was the most important one (β = 0.11, P = 0.059; Supplementary Table SIII). Adherence to the other diet indices examined was not related to semen quality parameters (Table II). Moreover, none of the diet indices was related to reproductive hormone levels (Table III).
Table II.
Multivariate adjusted1 associations of dietary indices and semen quality parameters (n = 209) reported as percentage (%) difference or untransformed model coefficients (only for motility), with 95%CI.
| Range for each quartile of index | Sperm concentration (mill/mL) | Total Sperm Count (Mill.) | Total Motile Sperm Count (Mill) | Motility (PR + NP) (%) | Morphologically normal sperm (%) |
|---|---|---|---|---|---|
| AHEI-2010 | |||||
| Q1 (34–50) | Ref. | Ref. | Ref. | Ref. | Ref. |
| Q2 (51–56) | 11.5% (−26.2; 49.2%) | 30.1% (−5.2; 65.4%) | 34.7% (−2.9; 72.3%) | −0.64 (−4.8; 3.6) | 16.6% (−8.4; 41.5%) |
| Q3 (57–62) | −12.4% (−49.8; 24.9%) | 0.50% (−35.2; 34.2%) | 2.6% (−34.4; 39.6%) | −0.50 (−4.6; 3.6) | −5.0% (−29.6; 19.5%) |
| Q4 (63–80) | −6.2% (−43.3; 30.9%) | 13.3% (−21.3; 48.0%) | 26.7% (−10.5; 63.8%) | 1.0 (−3.1; 5.1) | −9.7% (−34.1; 14.7%) |
| Ptrend | 0.48 | 0.85 | 0.42 | 0.62 | 0.19 |
| rMED | |||||
| Q1 (1–7) | Ref. | Ref. | Ref. | Ref. | Ref. |
| Q2 (8–9) | −2.7% (−38.5; 33.0%) | 12.4% (−20.9; 45.8%) | 7.6% (−28.1; 43.2%) | −0.89 (−4.9; 3.1) | −0.60% (−24.5; 23.3%) |
| Q3 (10–11) | 0.50% (−34.5; 35.6%) | 27.9% (−4.7; 60.6%) | 25.4% (−9.6; 60.3%) | −1.6 (−5.4; 2.4) | −5.6% (−28.8; 17.6%) |
| Q4 (12–15) | −18.3% (−57.2; 20.6%) | 21.9% (−14.5; 58.3%) | 28.9% (−9.4; 69.1%) | 0.10 (−4.2; 4.3) | 5.5% (−20.2; 31.3%) |
| Ptrend | 0.47 | 0.11 | 0.08 | 0.79 | 0.88 |
| DASH | |||||
| Q1 (10–19) | Ref. | Ref. | Ref. | Ref. | Ref. |
| Q2 (20–22) | 13.3% (−26.5; 53.0%) | 24.5% (−12.6; 61.7%) | 21.1% (−18.5; 60.6%) | −1.1 (−5.6; 3.3) | 23.2% (−3.3; 49.7%) |
| Q3 (23–27) | 32.7% (−7.7; 73.0%) | 31.7% (−6.2; 69.7%) | 42.2% (1.6; 82.7%) | −0.10 (−4.7; 4.6) | 27.5% (0.4; 54.6%) |
| Q4 (28–37) | 47.3% (−7.5; 102%) | 64.7% (13.5; 72.0%) | 73.8% (19.2; 128%) | −1.5 (−8.0; 5.0) | 31.4% (−6.4; 69.2%) |
| Ptrend | 0.04 | 0.04 | 0.02 | 0.80 | 0.13 |
1Adjusted for calories intake (kcal/day), physical activity (h/week), presence of varicocele (yes/no), abstinence time (hours) and time to start of analysis (min; for sperm motility only). Percentage (%) change shows natural logarithm values back-transformed to improve interpretability, and untransformed model coefficients shows the mean difference in percentage points between the percentage of motile sperm (PR + NP) in a given dietary index quartile (second, third, or fourth, Q2, Q3, Q4) and the reference group (first quartile, Q1). AHEI-2010, Alternate Healthy Index 2010; rMED, Relative Mediterranean Diet Score; DASH, Dietary Approaches to Stop Hypertension.
Figure 1. Adjusted relative difference (%) and 95% confidence intervals in TMSC for men in increasing quartiles of adherence to the DASH diet.
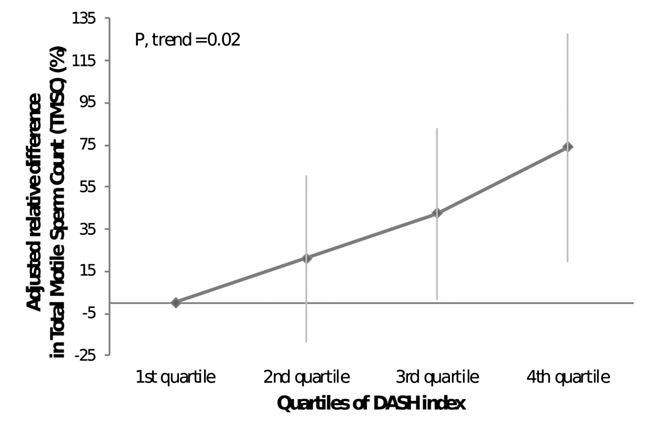
Table III.
Multivariate adjusted1 associations of dietary indices and serum reproductive hormone concentrations (n = 209) reported as percentage (%) difference (only for FSH and estradiol) or untransformed model coefficients, with 95%CI.
| Range for each quartile of index | LH (IU/L) | FSH (IU/L) | Estradiol (pmol/L) | Calculated Free testosterone (pmol/L) | Total testosterone (nmol/L) | Inhibin B (pg/mL) | SHBG (nmol/L) |
|---|---|---|---|---|---|---|---|
| AHEI-2010 | |||||||
| Q1 (34–50) | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Q2 (51–56) | −0.03 -(0.69; 0.64) | −18.7% (−40.3; 2.8%) | 2.0% (−9.3; 13.3%) | −6.2 (−74.7; 62.2) | −0.61 (−3.3; 2.0) | 15.4 (−14.8; 45.6) | −1.2 (−5.4; 3.0) |
| Q3 (57–62) | 0.15 (−0.50; 0.80) | −0.2% (−20.9; 21.3%) | 4.9% (−6.2; 15.9%) | −18.6 (−86.3; 49.1) | 0.02 (−2.6; 2.6) | 11.1 (−18.4; 40.7) | 1.2 (−2.9; 5.3) |
| Q4 (63–80) | 0.11 (−0.55; 0.76) | 2.0% (−19.2; 23.1%) | −0.5% (−11.6; 10.5%) | −4.1 (−71.3; 63.1) | 0.06 (−2.6; 2.5) | 7.4 (−22.2; 37.0) | 0.34 (−3.8; 4.5) |
| Ptrend | 0.64 | 0.48 | 0.94 | 0.82 | 0.88 | 0.70 | 0.61 |
| rMED | |||||||
| Q1 (1–7) | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Q2 (8–9) | 0.28 (−0.35; 0.90) | 10.1% (−10.0; 30.2%) | −6.1% (−16.6; 4.4%) | −39.1 (−103; 24.9) | −1.7 (−4.2; 0.8) | −7.2 (−35.6; 21.1) | −1,3 (−5.2; 2.7) |
| Q3 (10–11) | −0.22 (−0.83; 0.39) | −3.2% (−22.9; 16.5%) | −9.5% (−19.8; 0.80%) | −47.7 (−110; 14.6) | −1.2 (−3.6; 1.3) | 0.35 (−27.4; 28.1) | 1.5 (−2.3; 5.4) |
| Q4 (12–15) | 0.43 (−0.25; 1.1) | 29.1% (7.2; 51.0%) | 4.3% (−7.1; 15.7%) | 39.4 (−29.7; 109) | 0.93 (−1.8; 3.6) | −26.1 (−56.9; 4.7) | −1.3 (−5.6; 3.0) |
| Ptrend | 0.54 | 0.07 | 0.97 | 0.69 | 0.77 | 0.20 | 0.93 |
| DASH | |||||||
| Q1 (10–19) | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Q2 (20–22) | −0.24 (−0.95; 0.47) | 5.4% (−18.0; 28.8%) | −12.3% (−24.3; −0.30%) | −59.4 (−133; 13.8) | −2.2 (−5.0; 0.63) | −6.0 (−38.2; 26.4) | 0.68 (−3.8; 5.2) |
| Q3 (23–27) | 0.15 (−0.58; 0.88) | 3.0% (−21.0; 26.9%) | −0.70% (−13.0; 11.5%) | −30.4(−105; 44.6) | −1.1 (−3.6; 1.8) | −19.0 (−52.1; 14.1) | 0.34 (−4.2; 5.0) |
| Q4 (28–37) | −0.06 (−1.1; 0.95) | 0.5% (−32.7; 33.8%) | −5.4% (−22.4; 11.7%) | −13.5 (−117; 90.4) | −0.56 (−4.6; 3.5) | −30.0 (−76.0; 16.0) | 0.12 (−6.3; 6.6) |
| Ptrend | 0.82 | 0.94 | 0.89 | 0.77 | 0.78 | 0.17 | 0.96 |
1Adjusted for calories intake (kcal/day), physical activity (h/week) and time to blood sampling (min). Percentage (%) change shows natural logarithm values back-transformed to improve interpretability, and untransformed model coefficients shows the mean difference in percentage points between the hormone values in a given dietary index quartile (second, third, or fourth) and the reference group (first quartile). SHBG, sex hormone binding globulin; AHEI-2010, Alternate Healthy Index 2010; rMED, Relative Mediterranean Diet Score; DASH, Dietary Approaches to Stop Hypertension.
Discussion
We found that higher adherence to DASH diet was associated with higher sperm counts. To our knowledge this is the first study evaluating this matter on unselected young men as well as the relationships with reproductive hormone levels. Previously, only two studies have evaluated associations between a priori-defined dietary indices and semen quality (Karayiannis et al., 2017; Efrat et al., 2018). Our results are partially consistent with Efrat et al. (2018) as higher adherence to DASH was associated with higher sperm concentration among men attending a Israeli fertility centre. However, the authors also found that AHEI was positively related to sperm concentration; DASH and AHEI were positively associated with normal sperm morphology; and only AHEI had a significant association with TSC (Efrat et al., 2018). However, our results are not consistent with Karayiannis et al. (2017), as they reported that men from couples attending a Greek fertility clinic in the lowest tertile of the MDS had around 2.6 times higher likelihood of having abnormal sperm concentration, sperm count and motility (using WHO reference values), compared to men in the highest tertile of the score. In our study, a relatively low percentage of men presented sperm parameters below the WHO cut-offs, so a similar comparison would not be appropriate.
The DASH eating plan emphasizes increased consumption of fruits and vegetables, whole grains, nuts and low-fat dairy (Rifai and Silver, 2016). These dietary recommendations are nutritionally translated into lower simple carbohydrate, cholesterol, saturated and trans fats intakes and higher protein, fiber, calcium, magnesium and potassium intakes. DASH diet has been associated with a decrease in blood pressure (Rifai and Silver, 2016) as well as improving insulin resistance and diabetes (Shirani et al., 2013) or metabolic syndrome (Calton et al., 2014; Soares et al., 2016; Welty et al., 2016). Some of the pathophysiologic changes occurring in these conditions are related to oxidative stress, and the components of the DASH diet may enhance antioxidant capacity (Lopes et al., 2003; Shirani et al., 2013; Asemi et al., 2014). That may be one of the reasons we observed an association between DASH and sperm counts, as it is known that oxidative stress impairs semen quality (Agarwal et al., 2014).
Similarly, it is recognized that semen quality can be affected by the presence of an inflammatory microenvironment [e.g. interleukins, tumor necrosis factor-α (TNFα)] (La Vignera et al., 2011). The role of diet in reducing inflammation and thereby modulating the risk of non-communicable diseases is supported by numerous studies (Smidowicz and Regula, 2015; Neale, et al., 2016). Actually, DASH diet may be comparable to an anti-inflammatory a posteriori dietary pattern known as prudent dietary pattern (characterized by high intake of fish, chicken, fruit, vegetables, legumes and whole grains) that has been significantly associated with higher sperm motility in young US men (Gaskins et al., 2012) or concentration (Jurewicz et al., 2018) in men who attended a Polish infertility clinic. Our research group also previously found that a Mediterranean pattern was positively associated with TSC in the same population of young men we are reporting on (Cutillas-Tolín et al., 2015).
Moreover, some food groups included in the DASH index may also have a positive influence on semen quality. Fruits was the most important component related to sperm counts in our study population. Indeed, total fruit and vegetable intake has been associated with a lower risk of asthenoteratospermia (Eslamian et al., 2012) or oligoasthenoteratospermia (Mendiola et al., 2009) in men attending assisted reproduction clinics in Iran or Spain, respectively.
No association was found between the indices: AHEI-2010, rMED and DASH and reproductive hormone concentrations in our study population. The literature about nutrition intake and hormone levels in humans is very scarce. Cutillas-Tolín et al. (2015) found that neither Mediterranean nor Western dietary pattern was related to hormone levels in the same study population employed here. Similarly, in a young US male population, weak or no association between dairy food (Afeiche et al., 2013), meat (Afeiche et al., 2014) or sugar-sweetened beverage intake (Chiu et al., 2014) and reproductive hormone levels have been reported. However, recently, Mínguez-Alarcón et al. (2017) showed that trans, monounsaturated and polyunsaturated fatty acids intake were associated with serum blood levels of several reproductive hormones (testosterone, inhibin or LH) in young Mediterranean men, showing that they may influence testicular function.
This study has some limitations. As with all observational studies, causal inference is restricted, therefore prevents causal interpretations of the observed associations. Adjustment for known and potential confounders and covariates was carried out. However, there is always the possibility of residual or unmeasured confounding, and chance findings must always be contemplated. Moreover, it cannot be precluded that type II error might explain the negative results regarding other sperm parameters or reproductive hormones. There is no perfect method to assess diet, so bias due to measurement errors may also occur, and therefore calculating the prior dietary indices from a FFQ. Nonetheless, the FFQ employed in this work was previously validated in an adult Spanish population of the same area and it has been utilized in other populations (Guxens et al., 2012; Castelló et al., 2014). Many foods, particularly those that are plant-based, have nutrient contents that change substantially depending on the growing environments and preparation methods. However, any bias in assessing diet should not be differential which should strengthen our findings. Only one semen sample was obtained per subject, nevertheless, several studies have reported that one sample is adequate to evaluate semen quality in epidemiological studies (Stokes-Riner et al., 2007; Chiu et al., 2017). Equally, a single sample can be employed to categorize male reproductive hormones (Vermeulen and Verdonck, 1992). It is also worth mentioning the limitation that dietary assessment and time from previous ejaculation were both self-reported. Our population may have been potentially exposed to pesticides in fruit and vegetables and this may have an impact on the observed associations as shown elsewhere (Chiu et al., 2015, 2016). Unfortunately, there is not pesticide residues surveillance data in Spain to construct and validate these equivalent indices previously reported (Chiu et al., 2015, 2016). Consequently, we were not able to explore this potential issue in our study population. Finally, multiple comparisons might be of concern in statistical tests. However, in our study, this might be ameliorated since specific objectives about specific dietary indices and male reproductive outcomes were defined a priori. This study was carried out on healthy young men with unknown fertility; therefore, it is difficult to predict the external validity or generalizability of the obtained results to other male populations. Consequently, future studies in more diverse populations (e.g. fertile or other young men populations), as well as other epidemiological designs providing more evidence of causality are recommended.
In conclusion, higher adherence to DASH index was associated with higher TSC and TMSC. On balance, our data support the hypothesis that a potential diet with high intake of low-fat dairy products, vegetables, fruits and fish and low intake of sugar-sweetened beverages, processed meat and salt, such as DASH diet, might have positive effects on sperm counts. Further research is warranted to confirm these findings and extend these results to other male populations.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge Dr M. Roca, C. Ruiz, E. Belmonte and all the ‘Quirónsalud Dexeus Murcia’ clinic staff for their assistance in data collection; and the young men of the study for their participation. We also thank Dr L. Sarabia-Cos for semen analyses.
Authors’ roles
AMTC, NJ and JM were involved in study conception and study design. JM, NJ and AMTC were involved in study execution and acquisition of data. EA, ACT, JM, EMNM, JV, MMG, NJ, JEC and AMTC contributed to data analysis and interpretation. ACT, EA, MMG, EMNM, JV and JM drafted the manuscript. All authors provided substantial intellectual contributions and approved the final version of the manuscript.
Funding
Fundación Séneca (grants no. 08808/PI/08 and no. 19443/PI/14); Ministerio de Ciencia e Innovación, Instituto de Salud Carlos III (AES) (grants no. PI10/00985 and no. PI13/01237); National Institutes of Health (grant P30DK046200),
Conflict of interest
Authors have no competing interests to declare.
References
- Abbasihormozi S, Kouhkan A, Alizadeh AR, Shahverdi AH, Nasr-Esfahani MH, Sadighi Gilani MA, Salman Yazdi R, Matinibehzad A, Zolfaghari Z. Association of vitamin D status with semen quality and reproductive hormones in Iranian subfertile men. Andrology 2017;5:113–118. [DOI] [PubMed] [Google Scholar]
- Adoamnei E, Mendiola J, Vela-Soria F, Fernández MF, Olea N, Jørgensen N, Swan SH, Torres-Cantero AM. Urinary bisphenol A concentrations are associated with reproductive parameters in young men. Environ Res 2018a;161:122–128. [DOI] [PubMed] [Google Scholar]
- Adoamnei E, Mendiola J, Moñino-García M, Vela-Soria F, Iribarne-Durán LM, Fernández MF, Olea N, Jørgensen N, Swan SH, Torres-Cantero AM. Urinary concentrations of benzophenone-type ultra violet light filters and reproductive parameters in young men. Int J Hyg Environ Health 2018b;221:531–540. [DOI] [PubMed] [Google Scholar]
- Adoamnei E, Mendiola J, Moñino-García M, López-Espín JJ, Navarrete-Muñoz EM, Torres-Cantero AM. Dietary intake of trace elements and semen quality and reproductive hormone levels in young men: relationship with fertility. Rev Int Androl 2019;17:46–54 [Spanish]. [DOI] [PubMed] [Google Scholar]
- Afeiche M, Williams PL, Mendiola J, Gaskins AJ, Jørgensen N, Swan SH, Chavarro JE. Dairy food intake in relation to semen quality and reproductive hormone levels among physically active young men. Hum Reprod 2013;28:2265–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afeiche MC, Williams PL, Gaskins AJ, Mendiola J, Jørgensen N, Swan SH, Chavarro JE. Meat intake and reproductive parameters among young men. Epidemiology 2014;25:323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A, Virk G, Ong C, du Plessis SS. Effect of oxidative stress on male reproduction. World J Mens Health 2014;32:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Heart Association The American Heart Association Diet and Lifestyle Recommendations. 2015. Available at https://www.heart.org/en/healthy-living/healthy-eating/eat-smart/nutrition-basics/aha-diet-and-lifestyle-recommendations (9 June 2019, date last accessed).
- American Institute for Cancer Research Diet-What to Eat for Lower Cancer Risk. 2019. Available at https://www.aicr.org/reduce-your-cancer-risk/diet/ (9 June 2019, date last accessed).
- Asemi Z, Samimi M, Tabassi Z, Shakeri H, Sabihi SS, Esmaillzadeh A. Effects of DASH diet on lipid profiles and biomarkers of oxidative stress in overweight and obese women with polycystic ovary syndrome: a randomized clinical trial. Nutrition 2014;30:1287–1293. [DOI] [PubMed] [Google Scholar]
- Asklund C, Jørgensen N, Skakkebaek NE, Jensen TK. Increased frequency of reproductive health problems among fathers of boys with hypospadias. Hum Reprod 2007;22:2639–2646. [DOI] [PubMed] [Google Scholar]
- Attaman JA, Toth TL, Furtado J, Campos H, Hauser R, Chavarro JE. Dietary fat and semen quality among men attending a fertility clinic. Hum Reprod 2012;27:1466–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsson J, Rylander L, Rignell-Hydbom A, Giwercman A. No secular trend over the last decade in sperm counts among Swedish men from the general population. Hum Reprod 2011;26:1012–1016. [DOI] [PubMed] [Google Scholar]
- Buckland G, González CA, Agudo A, Vilardell M, Berenguer A, Amiano P, Ardanaz E, Arriola L, Barricarte A, Basterretxea M et al. Adherence to the Mediterranean diet and risk of coronary heart disease in the Spanish EPIC cohort Study. Am J Epidemiol 2009;170:1518–1529. [DOI] [PubMed] [Google Scholar]
- Calton EK, James AP, Pannu PK, Soares MJ. Certain dietary patterns are beneficial for the metabolic syndrome: reviewing the evidence. Nutr Res 2014;34:559–568. [DOI] [PubMed] [Google Scholar]
- Carlsen E, Swan SH, Petersen JH, Skakkebæk NE. Longitudinal changes in semen parameters in young Danish men from the Copenhagen area. Hum Reprod 2005;20:942–949. [DOI] [PubMed] [Google Scholar]
- Castelló A, Pollan M, Buijsse B, Ruiz A, Casas A, Baena-Canada J, Lope Carvajal V, Antolín A, Ramos M, Muñoz M et al. Spanish Mediterranean diet and other dietary patterns and breast cancer risk: case-control EpiGEICAM study. Br J Cancer 2014;111:1454–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavarro JE, Mínguez-Alarcón L, Mendiola J, Cutillas-Tolín A, López-Espín JJ, Torres-Cantero AM. Trans fatty acid intake is inversely related to total sperm count in young healthy men. Hum Reprod 2014;29:429–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YH, Afeiche MC, Gaskins AJ, Williams PL, Mendiola J, Jørgensen N, Swan SH, Chavarro JE. Sugar-sweetened beverage intake in relation to semen quality and reproductive hormone levels in young men. Hum Reprod 2014;29:1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YH, Afeiche MC, Gaskins AJ, Williams PL, Petrozza JC, Tanrikut C, Hauser R, Chavarro JE. Fruit and vegetable intake and their pesticide residues in relation to semen quality among men from a fertility clinic. Hum Reprod 2015;30:1342–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YH, Gaskins AJ, Williams PL, Mendiola J, Jørgensen N, Levine H, Hauser R, Swan SH, Chavarro JE. Intake of fruits and vegetables with low-to-moderate pesticide residues is positively associated with semen-quality parameters among young healthy men. J Nutr 2016;146:1084–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YH, Edifor R, Rosner BA, Nassan FL, Gaskins AJ, Mínguez-Alarcón L, Williams PL, Tanrikut C, Hauser R, Chavarro JE et al. What does a single semen sample tell you? Implications for male factor infertility research. Am J Epidemiol 2017;186:918–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 2012;142:1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutillas-Tolín A, Mínguez-Alarcón L, Mendiola J, López-Espín JJ, Jørgensen N, Navarrete-Muñoz EM, Torres-Cantero AM, Chavarro JE. Mediterranean and western dietary patterns are related to markers of testicular function among healthy men. Hum Reprod 2015;30:2945–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efrat M, Stein A, Pinkas H, Unger R, Birk R. Dietary patterns are positively associated with semen quality. Fertil Steril 2018;109:809–816. [DOI] [PubMed] [Google Scholar]
- Eslamian G, Amirjannati N, Rashidkhani B, Sadeghi MR, Hekmatdoost A. Intake of food groups and idiopathic asthenozoospermia: a case-control study. Hum Reprod 2012;27:3328–3336. [DOI] [PubMed] [Google Scholar]
- Fung TT, McCullough ML, Newby PK, Manson JE, Meigs JB, Rifai N, Willett WC, Hu FB. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr 2005;82:163–173. [DOI] [PubMed] [Google Scholar]
- Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med 2008;168:713–720. [DOI] [PubMed] [Google Scholar]
- Fung TT, Hu FB, Wu K, Chiuve SE, Fuchs CS, Giovannucci E. The Mediterranean and dietary approaches to stop hypertension (DASH) diets and colorectal cancer. Am J Clin Nutr 2010;92:1429–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskins AJ, Colaci DS, Mendiola J, Swan SH, Chavarro JE. Dietary patterns and semen quality in young men. Hum Reprod 2012;27:2899–2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guxens M, Ballester F, Espada M, Fernández MF, Grimalt JO, Ibarluzea J, Olea N, Rebagliato M, Tardón A, Torrent M et al. Cohort profile: the INMA—INfancia y Medio Ambiente—(environment and childhood) project. Int J Epidemiol 2012;41:930–940. [DOI] [PubMed] [Google Scholar]
- Harmon BE, Boushey CJ, Shvetsov YB, Ettienne R, Reedy J, Wilkens LR, Le Marchand L, Henderson BE, Kolonel LN. Associations of key diet-quality indexes with mortality in the multiethnic cohort: the dietary patterns methods project. Am J Clin Nutr 2015;101:587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S, Harmon BE, Boushey CJ, Morimoto Y, Wilkens LR, Le Marchand L, Kröger J, Schulze MB, Kolonel LN, Maskarinec G. A priori-defined diet quality indexes and risk of type 2 diabetes: the multiethnic cohort. Diabetologia 2015;58:98–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S, Harmon BE, Ollberding NJ, Wilkens LR, Monroe KR, Kolonel LN, Le Marchand L, Boushey CJ, Maskarinec G. Among 4 diet quality indexes, only the alternate Mediterranean diet score is associated with better colorectal cancer survival and only in African American women in the multiethnic cohort. J Nutr 2016;146:1746–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolliffe IT, Cadima J. Principal component analysis: a review and recent developments. Philos Trans A Math Phys Eng Sci 2016;374:20150202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen N, Carlsen E, Nermoen I, Punab M, Suominen J, Andersen AG, Andersson AM, Haugen TB, Horte A, Jensen TK et al. East-west gradient in semen quality in the Nordic-Baltic area: a study of men from the general population in Denmark, Norway, Estonia and Finland. Hum Reprod 2002;17:2199–2208. [DOI] [PubMed] [Google Scholar]
- Jurewicz J, Radwan M, Sobala W, Radwan P, Bochenek M, Hanke W. Dietary patterns and their relationship with semen quality. Am J Mens Health 2018;12:575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayiannis D, Kontogianni MD, Mendorou C, Douka L, Mastrominas M, Yiannakouris N. Association between adherence to the Mediterranean diet and semen quality parameters in male partners of couples attempting fertility. Hum Reprod 2017;32:215–222. [DOI] [PubMed] [Google Scholar]
- La Vignera S, Vicari E, Condorelli RA, D'Agata R, Calogero AE. Male accessory gland infection and sperm parameters (review). Int J Androl 2011;34:e330–e347. [DOI] [PubMed] [Google Scholar]
- Levine H, Jørgensen N, Martino-Andrade A, Mendiola J, Weksler-Derri D, Mindlis I, Pinotti R, Swan SH. Temporal trends in sperm count: a systematic review and meta-regression analysis. Hum Reprod Update 2017;23:646–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CY, Chou YC, Chao JC, Hsu CY, Cha TL, Tsao CW. The association between dietary patterns and semen quality in a general Asian population of 7282 males. PLoS One 2015;10:e0134224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes HF, Martin KL, Nashar K, Morrow JD, Goodfriend TL, Egan BM. DASH diet lowers blood pressure and lipid-induced oxidative stress in obesity. Hypertension 2003;41:422–430. [DOI] [PubMed] [Google Scholar]
- Mattei J, Sotos-Prieto M, Bigornia SJ, Noel SE, Tucker KL. The Mediterranean diet score is more strongly associated with favorable cardiometabolic risk factors over 2 years than other diet quality indexes in Puerto Rican adults. J Nutr 2017;147:661–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendiola J, Torres-Cantero AM, Moreno-Grau JM, Ten J, Roca M, Moreno-Grau S, Bernabeu R. Food intake and its relationship with semen quality: a case-control study. Fertil Steril 2009;91:812–818. [DOI] [PubMed] [Google Scholar]
- Mendiola J, Torres-Cantero AM, Vioque J, Moreno-Grau JM, Ten J, Roca M, Moreno-Grau S, Bernabeu R. A low intake of antioxidant nutrients is associated with poor semen quality in patients attending fertility clinics. Fertil Steril 2010;93:1128–1133. [DOI] [PubMed] [Google Scholar]
- Mendiola J, Jørgensen N, Mínguez-Alarcón L, Sarabia-Cos L, López-Espín JJ, Vivero-Salmerón G, Ruiz-Ruiz KJ, Fernández MF, Olea N, Swan SH et al. Sperm counts may have declined in young university students in southern Spain. Andrology 2013;1:408–413. [DOI] [PubMed] [Google Scholar]
- Menkveld R, Stander FS, Kotze TJ, Kruger TF, van Zyl JA. The evaluation of morphological characteristics of human spermatozoa according to stricter criteria. Hum Reprod 1990;5:586–592. [DOI] [PubMed] [Google Scholar]
- Mínguez-Alarcón L, Mendiola J, López-Espín JJ, Sarabia-Cos L, Vivero-Salmerón G, Vioque J, Navarrete-Muñoz EM, Torres-Cantero AM. Dietary intake of antioxidant nutrients is associated with semen quality in young university students. Hum Reprod 2012;27:2807–2814. [DOI] [PubMed] [Google Scholar]
- Mínguez-Alarcón L, Chavarro JE, Mendiola J, Roca M, Tanrikut C, Vioque J, Jørgensen N, Torres-Cantero AM. Fatty acid intake in relation to reproductive hormones and testicular volume among young healthy men. Asian J Androl 2017;19:184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mínguez-Alarcón L, Williams PL, Chiu YH, Gaskins AJ, Nassan FL, Dadd R, Petrozza J, Hauser R. Chavarro JE; Earth Study Team. Secular trends in semen parameters among men attending a fertility center between 2000 and 2017: identifying potential predictors. Environ Int 2018;121:1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mursu J, Steffen LM, Meyer KA, Duprez D, Jacobs DR Jr. Diet quality indexes and mortality in postmenopausal women: the Iowa Women's Health Study. Am J Clin Nutr 2013;98:444–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale EP, Batterham MJ, Tapsell LC. Consumption of a healthy dietary pattern results in significant reductions in C-reactive protein levels in adults: a meta-analysis. Nutr Res 2016;36:391–401. [DOI] [PubMed] [Google Scholar]
- Oostingh EC, Steegers-Theunissen RP, de Vries JH, Laven JS, Koster MP. Strong adherence to a healthy dietary pattern is associated with better semen quality, especially in men with poor semen quality. Fertil Steril 2017;107:916–923.e2. [DOI] [PubMed] [Google Scholar]
- Palma I, Farran P, Cervera P. Tablas de composición de alimentos por medidas caseras de consumo habitual en España: CESNID [in Spanish], 4th edn. Barcelona, Spain: McGraw Hill, 2008 [Google Scholar]
- Priskorn L, Jensen TK, Bang AK, Nordkap L, Joensen UN, Lassen TH, Olesen IA, Swan SH, Skakkebaek NE, Jørgensen N. Is sedentary lifestyle associated with testicular function? A cross-sectional Study of 1,210 men. Am J Epidemiol 2016;184:284–294. [DOI] [PubMed] [Google Scholar]
- Rifai L, Silver MA. A review of the DASH diet as an optimal dietary plan for symptomatic heart failure. Prog Cardiovasc Dis 2016;58:548–554. [DOI] [PubMed] [Google Scholar]
- Rolland M, Le Moal J, Wagner V, Royère D, De Mouzon J. Decline in semen concentration and morphology in a sample of 26,609 men close to general population between 1989 and 2005 in France. Hum Reprod 2013;28:462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas-Huetos A, Bulló M, Salas-Salvadó J. Dietary patterns, foods and nutrients in male fertility parameters and fecundability: a systematic review of observational studies. Hum Reprod Update 2017;23:371–389. [DOI] [PubMed] [Google Scholar]
- Sermondade N, Faure C, Fezeu L, Shayeb AG, Bonde JP, Jensen TK, Van Wely M, Cao J, Martini AC, Eskandar M et al. BMI in relation to sperm count: an updated systematic review and collaborative meta-analysis. Hum Reprod Update 2013;19:221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirani F, Salehi-Abargouei A, Azadbakht L. Effects of dietary approaches to stop hypertension (DASH) diet on some risk for developing type 2 diabetes: a systematic review and meta-analysis on controlled clinical trials. Nutrition 2013;29:939–947. [DOI] [PubMed] [Google Scholar]
- Smidowicz A, Regula J. Effect of nutritional status and dietary patterns on human serum C-reactive protein and interleukin-6 concentrations. Adv Nutr 2015;6:738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares NP, Santos AC, Costa EC, Azevedo GD, Damasceno DC, Fayh AP, Lemos TM. Diet-induced weight loss reduces DNA damage and cardiometabolic risk factors in overweight/obese women with polycystic ovary syndrome. Ann Nutr Metab 2016;68:220–227. [DOI] [PubMed] [Google Scholar]
- Stokes-Riner A, Thurston SW, Brazil C, Guzick D, Liu F, Overstreet JW, Wang C, Sparks A, Redmon JB, Swan SH. One semen sample or 2? Insights from a study of fertile men. J Androl 2007;28:638–643. [DOI] [PubMed] [Google Scholar]
- Swan SH, Elkin EP, Fenster L. The question of declining sperm density revisited: an analysis of 101 studies published 1934–1996. Environ Health Perspect 2000;108:961–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiseo BC, Gaskins AJ, Hauser R, Chavarro JE, Tanrikut C, Study Team EARTH. Coenzyme Q10 intake from food and semen parameters in a subfertile population. Urology 2017;102:100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med 2003;348:2599–2608. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services , National Institutes of Health, National Heart Lung, and Blood Institute, Your Guide to Lowering Your Blood Pressure With DASH. https://www.nhlbi.nih.gov/health-topics/dash-eating-plan (7 November 2018, date last accessed).
- U.S. Department of Health and Human Services and U.S. Department of Agriculture 2015–2020 Dietary Guidelines for Americans. 8th Edition. 2015. Available at https://health.gov/dietaryguidelines/2015/guidelines/ (9 June 2019, date last accessed).
- Vermeulen A, Verdonck G. Representativeness of a single point plasma testosterone level for the long term hormonal milieu in men. J Clin Endocrinol Metab 1992;74:939–942. [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999;84:3666–3672. [DOI] [PubMed] [Google Scholar]
- Vioque J. Validez de la evaluación de la ingesta dietética [in Spanish] In: Serra-Majem L, Aranceta J, Mataix J (eds). Nutrición Y Salud Pública. Métodos, Bases Científicas Y Aplicaciones, 1st edn. Barcelona, Spain: Masson, 1995 [Google Scholar]
- Vioque J, Weinbrenner T, Asensio L, Castelló A, Young IS, Fletcher A. Plasma concentrations of carotenoids and vitamin C are better correlated with dietary intake in normal weight than overweight and obese elderly subjects. Br J Nutr 2007;97:977–986. [DOI] [PubMed] [Google Scholar]
- Vioque J, Navarrete-Muñoz EM, Gimenez-Monzó D, García-de-la-Hera M, Granado F, Young IS, Ramón R, Ballester F, Murcia M, Rebagliato M et al. Reproducibility and validity of a food frequency questionnaire among pregnant women in a Mediterranean area, Nutr J 2013;12:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vujkovic M, de Vries JH, Dohle GR, Bonsel GJ, Lindemans J, Macklon NS, van der Spek PJ, Steegers EP, Steegers-Theunissen RPM. Associations between dietary patterns and semen quality in men undergoing IVF/ICSI treatment. Hum Reprod 2009;24:1304–1312. [DOI] [PubMed] [Google Scholar]
- Welty FK, Alfaddagh A, Elajami TK. Targeting inflammation in metabolic syndrome. Transl Res 2016;167:257–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett WC. Nutritional Epidemiology, 3rd edn. Oxford, New York: Oxford University Press, 2012 [Google Scholar]
- Willett WC, Stampfer MJ. Current evidence on healthy eating. Annu Rev Public Health 201334;1:77–95. [DOI] [PubMed] [Google Scholar]
- World Health Organization WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th edn. Geneva, Switzerland: WHO Press, 2010 [Google Scholar]
- Zareba P, Colaci DS, Afeiche M, Gaskins AJ, Jørgensen N, Mendiola J, Swan SH, Chavarro JE. Semen quality in relation to antioxidant intake in a healthy male population. Fertil Steril 2013;100:1572–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


