Abstract
Objective:
To compare the predictive ability of estimated fetal weight (EFW) percentiles, according to seven growth standards, to detect fetuses at risk for adverse perinatal outcomes.
Methods:
This retrospective cohort study included 3,437 African-American women. Population-based (Hadlock, INTERGROWTH-21st, WHO, FMF), ethnic specific (NICHD), customized (GROW) and African American customized (PRB/NICHD) growth standards were applied to the last available scan prior to delivery. Prediction performance indices and relative risk (RR), carried by an EFW<10th and EFW>90th percentile according to each standard, were calculated for individual and composite adverse perinatal outcomes. The sensitivity at a fixed (10%) false-positive rate (FPR), as well as the partial (FPR<10%) and full area under the ROC curves (AUC), were compared among the standards.
Results:
1) Ten percent (341/3437) of the neonates were classified as small-for-gestational-age (SGA) at birth, and of these, 16.4% (56/341) had at least one adverse perinatal outcome. SGA neonates were at a 1.5-fold increased risk of any adverse outcome (p<0.05); 2) the screen-positive rate (EFW<10th percentile) of growth standards varied from 6.8% (NICHD) to 24.4 % (FMF); 3) EFW<10th percentile, according to all standards, was associated with an increased risk for all adverse perinatal outcomes considered (all, p<0.05); 4) the highest RRs carried by an EFW<10th percentile were: 5.1 for perinatal mortality (WHO); 5.0 for perinatal hypoglycemia (NICHD); 3.4 for mechanical ventilation (NICHD); 2.9 for Apgar score <7 at 5 minutes (GROW); 2.7 for Neonatal Intensive Care Unit (NICU) admission (NICHD); and 2.5 for the composite adverse perinatal outcome (NICHD). Although confidence intervals overlapped among all standards for each individual outcome, the RR for NICHD [2.46(1.9–3.1)] was higher than the one for FMF 1.47(1.2–1.8) for the composite outcome. 5) the sensitivity for the composite adverse perinatal outcome varied substantially among standards (15% for NICHD to 32% for FMF) given mostly to differences in the FPR, and they subsided when the FPR was set to the same value (10%); 6) the comparison of the AUC revealed a significant improvement for the PRB/NICHD (AUC=0.70) compared to Hadlock (AUC=0.66) and FMF (AUC=0.64) standards for the prediction of perinatal mortality; complementarily, the evaluation of the partial AUC (FPR<10%) revealed that the INTERGROWTH-21 standard had an advantage over the Hadlock standard for NICU admissions and mechanical ventilation (all, p<0.05). 7) Although large-for-gestational-age (LGA) fetuses (EFW>90th) were also at risk of adverse perinatal outcomes, according to INTERGROWTH-21 (RR=1.4) and Hadlock (RR=1.7) standards, much fewer cases (2–5 fold) were detected by an LGA compared to an SGA screening by the same standards.
Conclusions:
Fetuses with an EFW<10th as well as those with EFW>90th percentile were at increased risk of adverse perinatal outcomes according to all, or some of the seven standards, respectively. The relative risk carried by an EFW<10th percentile for the composite adverse perinatal outcome was higher for the most stringent (NICHD) than the least stringent (FMF) standard for SGA screening. The complementary analysis based on the AUC suggests slightly improved detection of adverse perinatal outcomes by more recent population-based (INTERGROWTH-21st) and customized (PRB/NICHD) standards compared to the Hadlock and FMF standards.
Keywords: estimated fetal weight, growth restriction, customized fetal growth standards, perinatal morbidity, perinatal mortality, Neonatal Intensive Care Unit admission, mechanical ventilation
Plain Language Summary:
Several fetal growth standards were recently introduced, and herein we assess their utility to predict perinatal morbidity and mortality.
INTRODUCTION
Low and high birthweight is associated with increased perinatal morbidity and mortality.1–17 Therefore, antenatal surveillance of fetal growth is essential to promote close monitoring and to suggest potential measures to reduce the risk (e.g., induction of labor).18–27 Indeed, antenatal detection of high-risk fetuses is associated with a significant reduction in stillbirth and perinatal morbidity rates.28–32
Antenatal screening for growth restriction using ultrasound relies on the estimation of fetal weight (EFW) and comparison to a reference, also known as a growth chart or growth standard. The 10th/90th percentile cut-offs, first suggested by Battaglia and Lubchenco33 for neonatal birth weight, and later adopted by Hadlock et al.34 for EFW, are used to identify fetuses at risk for adverse outcomes.35–37
After Hadlock’s “one-size-fits-all” growth chart was introduced, Gardosi et al.38 proposed an adjustable fetal growth chart in which percentile curves are shifted up or down to account for non-pathologic factors such as maternal height, weight, parity, race/ethnicity, and fetal sex.39–45 The effects of these factors were assumed to be proportionally constant during gestation, and adjustment coefficients were estimated from birthweight data in specific populations.46–52 More recent customized standards do not rely on the proportionality assumption and allow these effects to vary among the specific centile curves.53
The potential of customized birthweight standards to improve identification of neonates at risk for adverse perinatal morbidity and mortality has been well established.54–67 Nevertheless, recent initiatives to develop growth standards did not perform customization of growth charts or customized only for a subset of non-pathologic factors known to affect fetal growth. For example, the World Health Organization (WHO) growth standard only customizes by fetal sex, 68–70 while the National Institute of Child Health and Human Development (NICHD) developed ethnic-specific charts without adjusting for other factors.71 In addition, the INTERGROWTH-21st project proposed a “one-size-fits-all” standard, without customization, yet the decision not to adjust for fetal sex was based on ethical grounds.72–76 Similarly, the Fetal Medicine Foundation (FMF) proposed a non-customized fetal growth standard by reconciling fetal weight and birthweight data in a multi-ethnic population that included a large majority (69%) of white women 77.
Given the plethora of fetal growth standards available, with their intrinsic differences in the design and in the characteristics of the populations from which they are derived, it is important to determine how these differences impact their utility. Therefore, we conducted a retrospective study, comparing the ability of an EFW<10th and EFW>90th percentile to identify fetuses at risk of perinatal morbidity and mortality, according to the seven aforementioned growth standards.
METHODS
Study Design
A retrospective cohort study was conducted at the Center for Advanced Obstetrical Care and Research of the Perinatology Research Branch, Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health, U.S. Department of Health and Human Services. All patients included in this study were enrolled in research protocols approved by the Human Investigation Committee of Wayne State University and the Institutional Review Board of NICHD.
The study population consisted of pregnant women who had at least one ultrasound evaluation prior to delivery and for whom perinatal information was available. Women with multiple gestations, those with known fetal anomalies or chromosomal aberrations, and those who were lost to follow-up or delivered elsewhere were excluded from the study. Detailed demographic data, medical history, and pregnancy outcomes were extracted from the patients’ electronic medical records.
Outcomes
The adverse perinatal outcomes considered in the study were as follows: 1) perinatal mortality; 2) Neonatal Intensive Care Unit (NICU) admission; 3) Apgar scores <7 at 5 minutes after delivery; 4) neonatal hypoglycemia; 5) the need for mechanical ventilation; 6) neonatal hypothermia; 7) meconium aspiration syndrome; and 8) composite adverse perinatal outcome, involving one or more of the outcomes above. Only outcomes affecting 20 or more of the 3437 patients were analyzed individually; otherwise, they contributed only to the analysis of the composite adverse perinatal outcome.
Perinatal mortality was defined as stillbirth or neonatal death within 7 days of birth.78 Stillbirth was defined as death of the fetus diagnosed after 20 weeks of gestation confirmed by ultrasound examination prior to delivery. NICU admission was defined as documented newborn admission to the NICU at any time during the hospitalization. Apgar scores <7 at 5 minutes after delivery were calculated according to an accepted method for reporting the status of the newborn immediately after birth.79, 80 Neonatal hypoglycemia was defined as a glucose level <45 mg/dL.81 Mechanical ventilation was defined when a ventilation machine was used to improve the exchange of air between the lungs and the atmosphere. Neonatal hypothermia defined as a neonatal axillary temperature less than 36.5°C.78, 82 Meconium aspiration syndrome was diagnosed in infants who had dyspnea, tachycardia, and need for supplemental oxygen by hours of life, and diffuse irregular patchy infiltrates on chest radiographs.83 Of note, infants with meconium below the vocal cords but with no clinical or radiographic evidence of disease were not diagnosed with aspiration syndrome.
Fetal growth screening
Screen-positive for small-(SGA) and large (LGA) -for-gestational-age was based on an EFW<10th and EFW>90th percentile, respectively, for each standard. The observed EFW at last scan prior to delivery was derived using the formula published for each individual standard based on biometrical parameters [i.e., abdominal circumference (AC), femur length (FL), head circumference (HC), and biparietal diameter (BPD)], as follows:
Hadlock 1:
EFW was calculated with a three-parameter Hadlock equation (HC, AC, and FL) 84, used by other recent growth standards (NICHD, WHO, PRB/NICHD, FMF), and compared to the same centile curves reported by Hadlock et al. in 199134 using a four-parameter equation.
Hadlock 2:
EFW was calculated using a four-parameter formula (AC, FL, HC, and BPD) originally reported by Hadlock et al.,84 and the observed value was compared to the centile curves derived for this EFW formula.34 This fetal weight assessment was the one used clinically for the detection of SGA in the study population.
INTERGROWTH-21st:
EFW was calculated from AC and HC using the equation proposed by the authors, and observed values were compared to the centile curves reported.75, 85
The World Health Organization (WHO) fetal growth standard:
EFW was calculated based on a three-parameter Hadlock formula (HC, AC, FL) 84 and compared to the reference centile without customization for fetal sex.68–70
The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD):
EFW was calculated using the three-parameter Hadlock formula (HC, AC, FL) 84 and compared to the centile curves derived for the African-American population.71
Gestational Related Optimal Weight (GROW):
EFW was calculated using the three-parameter Hadlock formula (HC, AC, FL)84 and a corresponding customized percentile was obtained using the GROW software (V8.0.1).86 Customization was made for ethnic origin, maternal height, weight and parity, and fetal sex.
Perinatology Research Branch / Eunice Kennedy Shriver National Institute of Child Health and Human Development (PRB/NICHD):
EFW was calculated using the three-parameter Hadlock formula (HC, AC, FL),84 and corresponding customized centiles were calculated using the R package available at http://bioinformaticsprb.med.wayne.edu/software/prb-nichd-fetal-growth-standard/. Customization of the growth centiles was made for maternal height, weight and parity, and fetal sex.53
Fetal Medicine Foundation (FMF):
EFW was calculated based on a three-parameter Hadlock formula (HC, AC, FL) 84 and compared to the reference centiles described in Nicolaides et al. 77.
Statistical analysis
We evaluated the sensitivity and specificity of the screening test as well as the relative risk (RR) carried by an EFW<10th and EFW>90th percentile according to each standard for each outcome. When screening for SGA, for standards providing an exact percentile for any given observed EFW value (GROW, Hadlock, INTERGROWTH-21st, PRB/NICHD, FMF), Receiver Operating Characteristic (ROC) curves were constructed and the full and partial (FPR<10%) areas under the ROC curves (AUC) were calculated, and compared to Hadlock 1, considered as a reference using the pROC package 87. For these standards, the sensitivity at a 10% false-positive rate was also determined for each outcome for SGA screening.
RESULTS
Characteristics of the study population
The study population included 3,437 African-American women. The characteristics of the study population are summarized in Table 1. Of those, 478 women delivered preterm (<37 weeks of gestation) and 2,959 women delivered at term. The mean gestational age at delivery was 38.5 ± 2.4 weeks, and the mean interval from sonographic EFW measurement to delivery was 3.6 weeks ± 3.51 weeks.
Table 1: Characteristics of the study population (n=3,437).
Data are given as median [interquartile range] or number (%). Maternal height and weight were recorded in inches and pounds and then converted into cm and kg, respectively, prior to analysis.
| Characteristic | Statistic |
|---|---|
| Maternal age (y) | 23 [20–27] |
| Parity | |
| Nulliparous | 1259 (36.6%) |
| Multiparous | 2178 (63.4%) |
| Body mass index | 27.5 [22.9–33.7] |
| Height (cm) | 162.6(157.5–167.6) |
| Weight (kg) | 72.6(60.8–90.3) |
| Smoking status | |
| Smoker | 634 (18.5%) |
| Non-smoker | 2803 (81.5%) |
| Gestational age at delivery (weeks) | 39.0 [38.0–39.9] |
| Interval from scan to delivery (weeks) | 2.6 [1.0–5.3] |
| Preterm delivery | 478 (13.9 %) |
| Mode of delivery | |
| Vaginal | 2475 (72.0%) |
| Caesarean section | 962 (28.0%) |
| Sex | |
| Male | 1755 (51.1%) |
| Female | 1682 (48.9%) |
| Birthweight (g) | 3145 [2790–3465] |
| SGA by Alexander | 341 (9.9%) |
The median maternal body mass index of the population was 27.5 [interquartile range (IQR) 22.9–33.7], and 18.5% of women (634/3437) were smokers. About 10% (341/3,437) of the neonates were classified as SGA and 7.2% (250/3,437) as LGA according to the United States national reference for birthweight standards reported by Alexander et al.88 The cohort included 11.7% (403/3,437) neonates diagnosed with at least one adverse perinatal outcome, with 219 of the 403 neonates being delivered preterm. The group of 20 cases with perinatal mortality included 11 cases of stillbirth and 9 cases of neonatal death.
Of all the neonates with at least one adverse perinatal outcome, 13.9% of neonates (56/403) were SGA (birthweight <10th centile). The RR of adverse perinatal outcomes carried by a birthweight <10th centile is shown using a forest plot in Figure S1. The RR of the composite adverse perinatal outcome associated with an SGA delivery was 1.5 [95% CI 1.15–1.94], and the highest RR for an individual outcome reached 3.49 [95% CI 2.23–5.46] for neonatal hypoglycemia.
Association between EFW<10th and adverse perinatal outcomes
Screen positive rates:
There was large variability in the screen-positive rate (EFW<10th percentile) across the different standards: 6.8% for NICHD, 9.4% for GROW, 11.6% for WHO, 13.2% for INTERGROWTH-21st, 13.5% for PRB/NICHD, 16.2% for Hadlock 2, 16.5% for Hadlock 1 and 24.4% for FMF.
Relative risk:
An EFW<10th centile at the last scan before delivery (screen positive) was associated with an increased risk in individual and composite adverse neonatal outcomes for all standards (Figure 1, Table 2, and Table S1). The RR for the composite adverse perinatal outcome was significantly lower for the least stringent standard (FMF) RR=1.47 [95% confidence interval (CI) 1.2–1.8] compared to the most stringent standard (NICHD) RR=2.46 [95% CI 1.9–3.1]. The highest RRs of an individual adverse outcome were for perinatal mortality 5.05 [95% CI 2.08–12.29] (WHO); neonatal hypoglycemia 5.0 [95% CI 3.27–7.83] (NICHD); mechanical ventilation 3.39 [95% CI 2.43–4.74] (NICHD); Apgar score <7 at 5 minutes 2.88 [95% CI 1.80–4.63] (GROW); and NICU admission 2.68 [95% CI 2.01–3.57] (NICHD). Of note, for all individual outcomes, the CIs of the RR overlapped among standards. Nonetheless, there were notable differences in RR estimates among standards for specific outcomes. For instance, for perinatal mortality, the lowest RR was 2.18 (Hadlock 1), and the highest RR was 5.05 (WHO).
Figure 1:
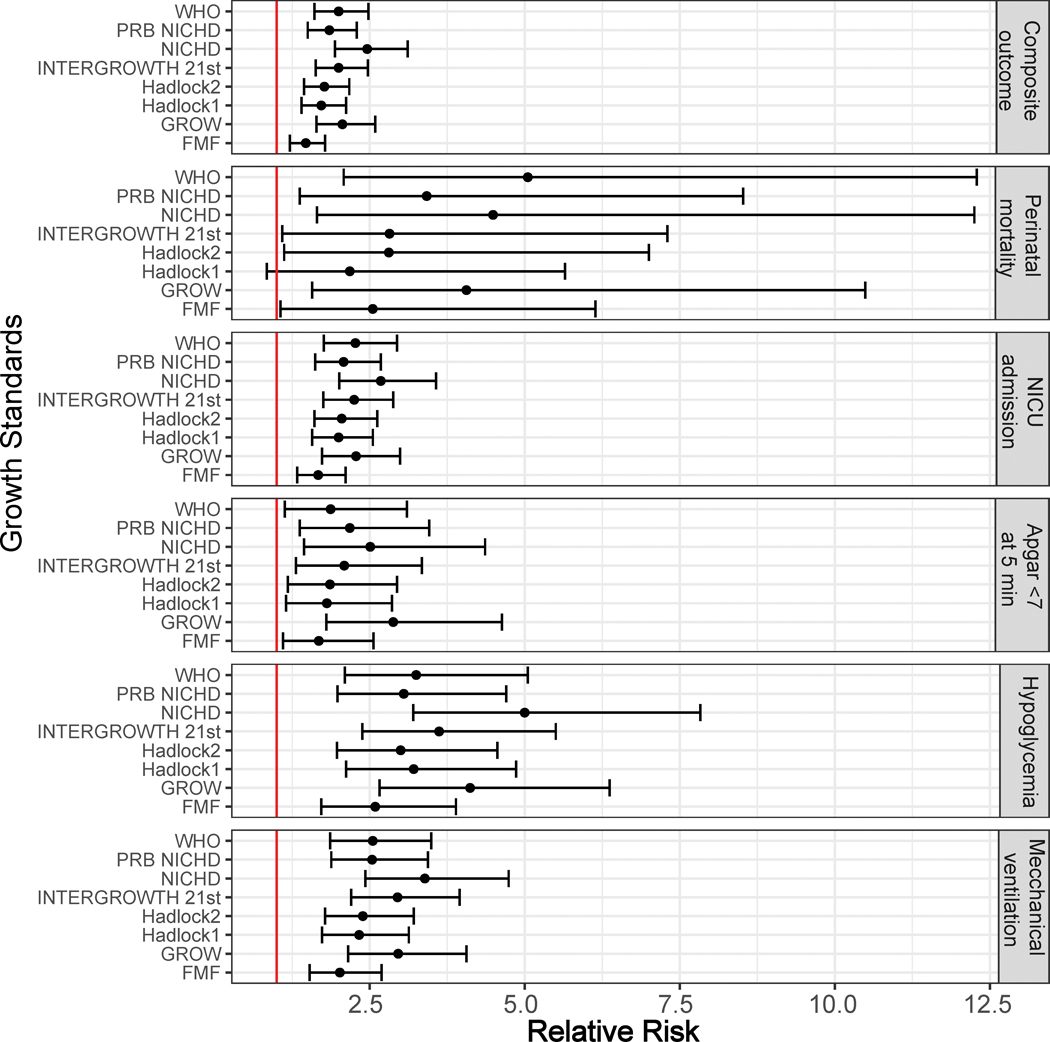
Association between an EFW<10th percentile and adverse perinatal outcomes. Relative risk and 95% confidence intervals are shown using a forest plot. Estimated fetal weight (EFW) and percentile values are calculated as described in the Methods section.
Table 2: Relative risk carried by an EFW<10th percentile for adverse perinatal outcome.
Data are shown as relative risk estimates and 95% confidence intervals.
| Hadlock 1 | Hadlock 2 | PRB/NICHD | NICHD | WHO | INTERGROWTH- 21st |
GROW | FMF | |
|---|---|---|---|---|---|---|---|---|
| Composite adverse perinatal outcome (n=403) [219*] | 1.72 (1.4–2.12) | 1.77 (1.44–2.17) | 1.85 (1.5–2.29) | 2.46 (1.94–3.11) | 2.0 (1.61–2.48) | 2.0 (1.63–2.47) | 2.06 (1.64–2.59) | 1.47(1.21–1.78) |
| Perinatal mortality (n=20) [17*] | 2.18 (0.84–5.65) | 2.81 (1.12–7) | 3.42 (1.37–8.52) | 4.49 (1.65–12.25) | 5.05 (2.08–12.29) | 2.82 (1.09–7.3) | 4.06 (1.57–10.49) | 2.55(1.06–6.14) |
| NICU admission (n=282) [176*] | 2.0 (1.57–2.55) | 2.05 (1.61–2.62) | 2.08 (1.62–2.68) | 2.68 (2.01–3.57) | 2.27 (1.76–2.94) | 2.25 (1.75–2.88) | 2.28 (1.73–2.99) | 1.67(1.33–2.11) |
| Apgar <7 at 5 min (n=91) [48*] | 1.81 (1.15–2.86) | 1.86 (1.18–2.94) | 2.18 (1.37–3.46) | 2.51 (1.44–4.36) | 1.87 (1.13–3.1) | 2.09 (1.31–3.34) | 2.88 (1.8–4.63) | 1.68(1.1–2.56) |
| Hypoglycemia (n=90) [58*] | 3.21 (2.12–4.86) | 3.0 (1.97–4.56) | 3.05 (1.98–4.7) | 5.0 (3.2–7.83) | 3.25 (2.1–5.05) | 3.62 (2.38–5.5) | 4.12 (2.66–6.37) | 4.56(2.95–7.05) |
| Mechanical Ventilation (n=187) [148*] | 2.33 (1.73–3.13) | 2.39 (1.78–3.21) | 2.54 (1.88–3.44) | 3.39 (2.43–4.74) | 2.55 (1.86–3.49) | 2.95 (2.2–3.95) | 2.96 (2.15–4.06) | 2.02(1.53–2.69) |
number of cases delivered preterm (<37 weeks).
Sensitivity and specificity
The sensitivity of EFW<10th centile for the composite adverse perinatal outcome ranged between 15% (NICHD) and 32% (FMF) with these two standards having the highest (27%) and lowest (16%) positive predictive values, respectively (see Table S1). The highest sensitivities for individual outcomes at the 10th percentile cut-off were obtained using the FMF standard: neonatal hypoglycemia 46%; perinatal mortality 45%; mechanical ventilation 40%; NICU admission 35%; and Apgar score <7 at 5 minutes 35%. The higher sensitivities for FMF standard were typically accompanied by lower specificities. The specificity for the composite adverse perinatal outcome ranged between 77% (FMF) and 94% (NICHD). The highest specificities for individual outcomes were as follows: neonatal hypoglycemia 94% (NICHD); perinatal mortality 91% (GROW); mechanical ventilation 94% (NICHD); NICU admission 94% (NICHD); and Apgar score <7 at 5 minutes 93% (NICHD) (Table S1).
Of note, while the sensitivity values discussed above were obtained using a 10th percentile cut-off for each standard to define screen-positive, the full spectrum of sensitivities can be seen in Figure 2.
Figure 2:
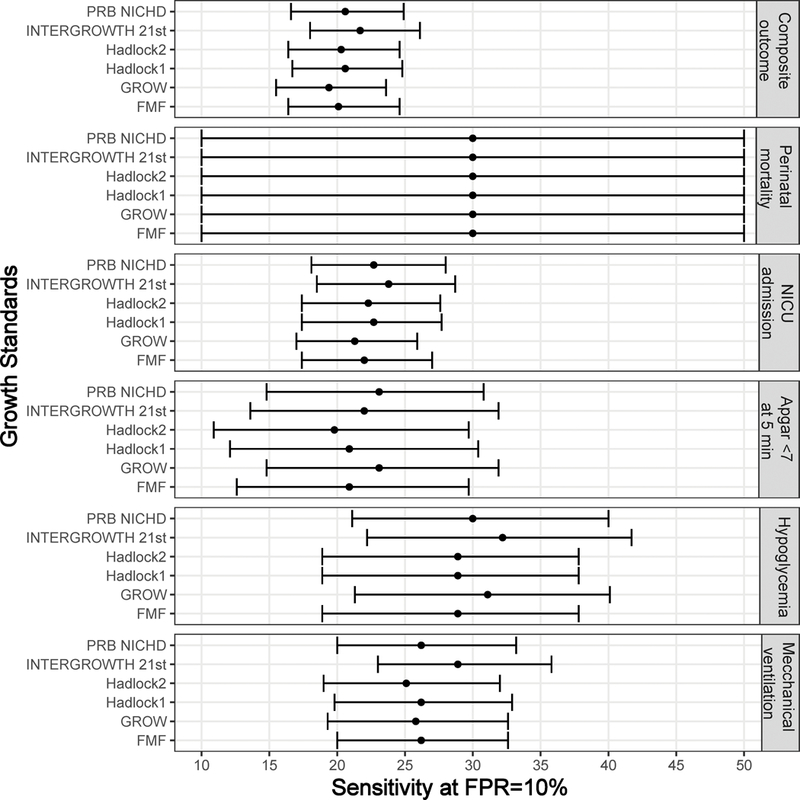
Sensitivity at fixed false-positive rate. For standards providing an exact percentile value, the test positive is based on a cut-off chosen so that the false-positive rate is 10% for each outcome considered. Sensitivity and 95% confidence intervals are shown using a forest plot.
Sensitivity at a fixed false-positive rate
To determine how much of the differences in sensitivities among standards described above are due to different stringency levels of the different standards (hence, specificity), we also determined the sensitivity at a fixed (10%) false-positive rate for standards providing exact percentiles. Indeed, there was a high similarity in sensitivity among standards when the false-positive rate was set to the same value (10%) (Figure 2). For instance, the sensitivity (at 10% FPR) for the composite adverse outcome varied only from 19.4% (GROW) to 21.7% (INTERGROWTH-21st) across the five standards shown in Figure 2, while for perinatal mortality, it was the same (30%) for all six standards shown in Figure 2. To reach the same false-positive rate (10%) for the composite adverse outcome, it was required to use a 6.6 percentile cut-off for the Hadlock-1 standard, 8.0 for both PRB/NICHD and INTERGROWTH-21st, 11.2 for GROW, and 2.0 percentile cut-off for FMF standard.
ROC curve analysis
The AUC statistics for individual and composite outcomes were overall either failed (0.5–0.6) or poor (0.6–0.7) and rather similar among the different growth standards (Figure 3 and Table 3). However, the PRB/NICHD standard had a higher AUC (0.70) for the prediction of perinatal mortality compared to Hadlock 1 (0.66) and FMF (0.64) (p<0.05). The AUC was also slightly higher for Hadlock 2 standard (AUC=0.67) compared to FMF (AUC=0.64) for perinatal mortality (Table S3), and for INTERGROWTH-21st standard (AUC=0.58) compared to FMF (AUC=0.56) for Apgar <7 at 5 min.
Figure 3:
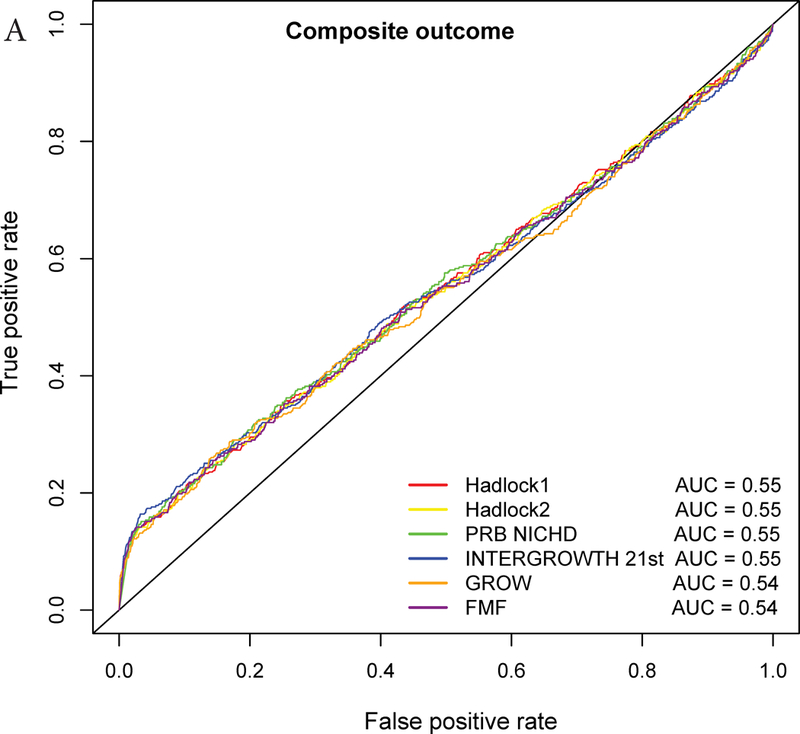
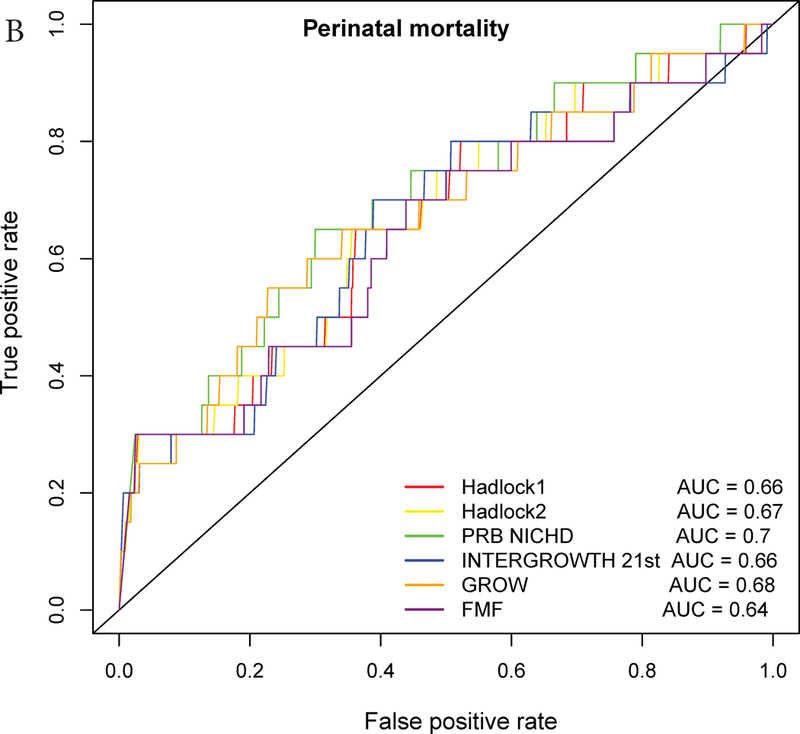
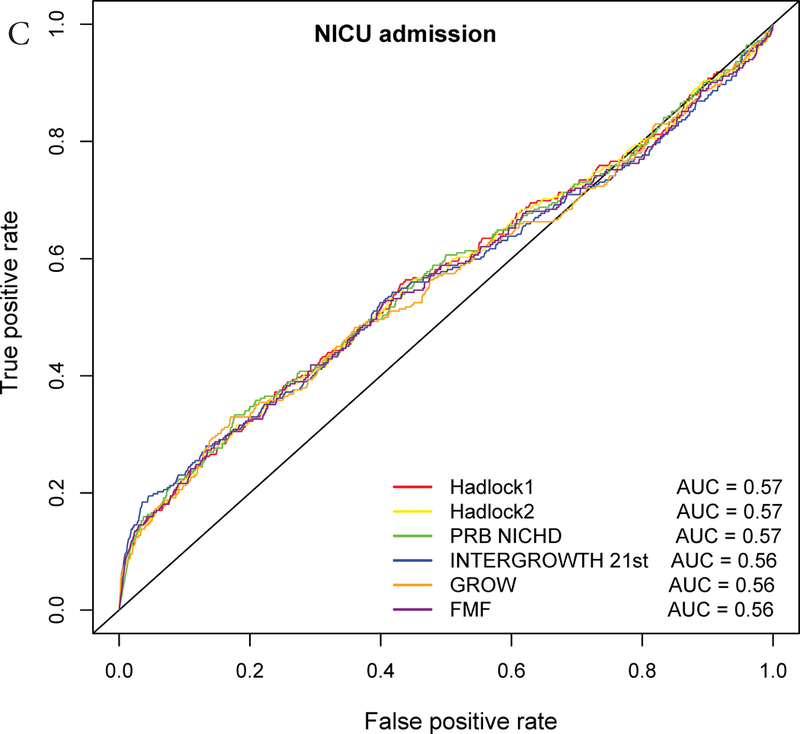
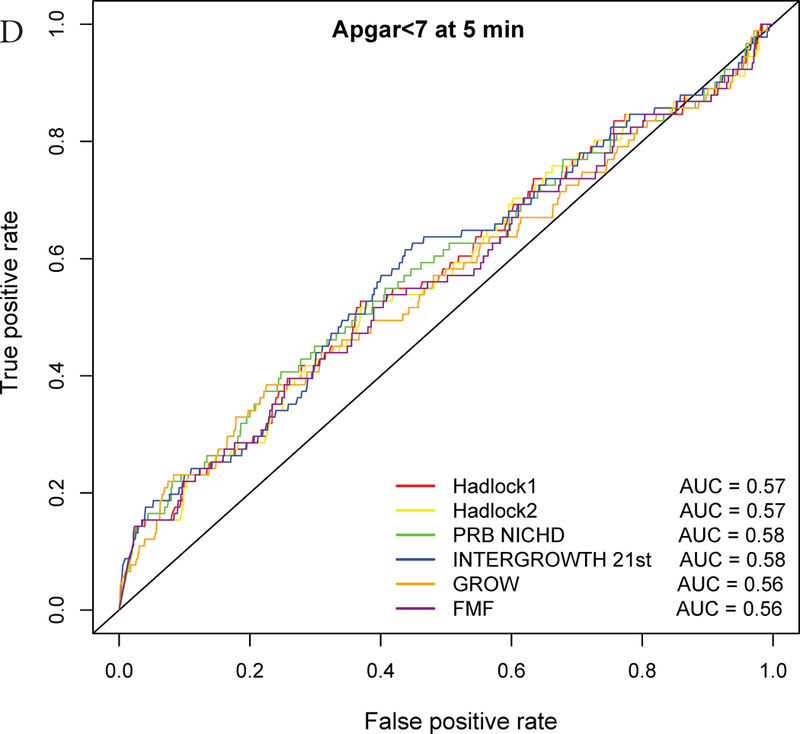
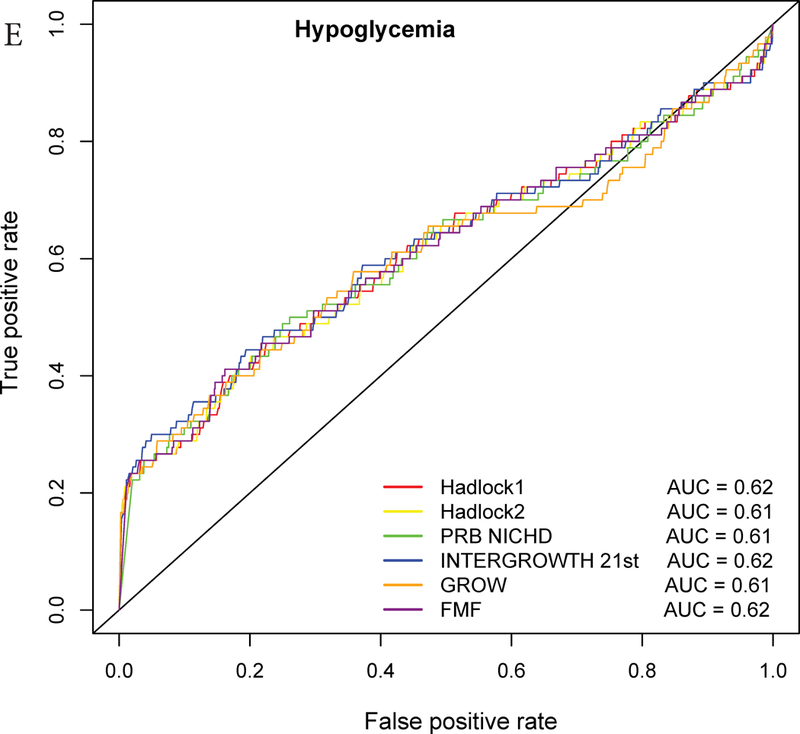
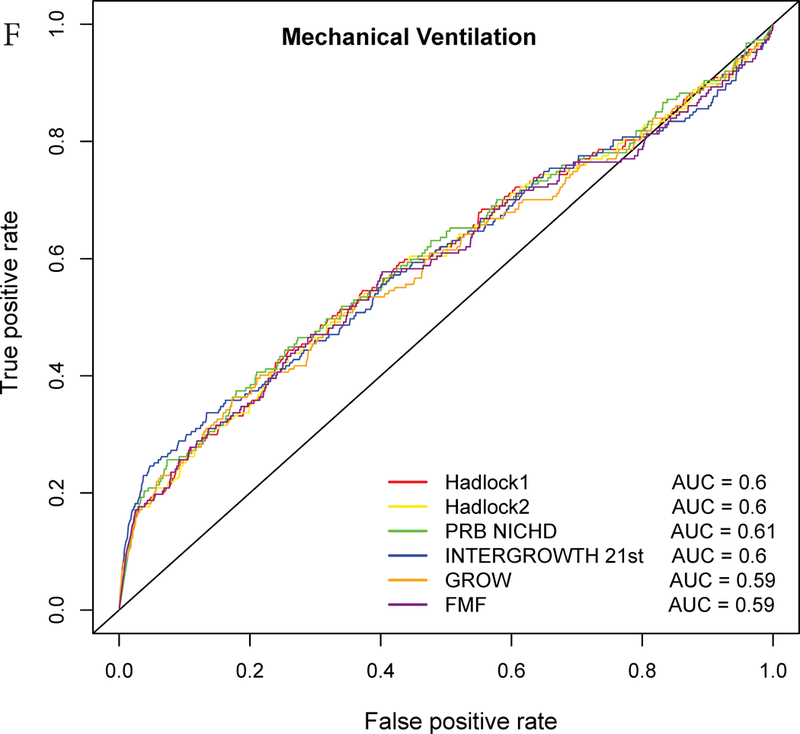
Receiver Operating Characteristic (ROC) curves for prediction of adverse neonatal outcomes. The ROC curves are constructed from the percentile values derived from each standard, and the area under the curves (AUC) is shown in the legend. The following outcomes are considered: A – Composite adverse perinatal outcomes; B – Perinatal mortality; C – NICU admission; D – Apgar score <7 at 5 minutes; E – Hypoglycemia; F – Mechanical ventilation.
Table 3: Area under the ROC curves for prediction of adverse perinatal outcomes by a low EFW percentile compared to Hadlock 1 standard.
Area under the ROC curve (AUC) for each standard (%) was compared against the Hadlock 1 standard. AUC values in bold are significantly (p<0.05) higher compared to Hadlock 1 standard by at least 2%. Partial AUC values in bold are significantly (p<0.05) higher compared to Hadlock 1 standard by at least 0.2%.
| AUC (%) | partial AUC (%) (FPR<10%) | ||||||
|---|---|---|---|---|---|---|---|
| Perinatal Outcome | Standard | Reference Hadlock1 |
p | Standard | Reference Hadlock1 |
p | |
| Composite adverse perinatal outcome | Hadlock2 | 54.7 | 54.9 | 0.082 | 1.5 | 1.5 | 0.517 |
| PRB/NICHD | 55 | 0.781 | 1.5 | 0.541 | |||
| INTERGROWTH-21st | 54.7 | 0.675 | 1.6 | 0.036 | |||
| GROW | 54.1 | 0.107 | 1.4 | 0.405 | |||
| FMF | 54.4 | <0.001* | 1.5 | 0.313 | |||
| Perinatal mortality | Hadlock2 | 66.8 | 66.2 | 0.157 | 2.6 | 2.6 | 0.406 |
| PRB/NICHD | 69.9 | 0.011* | 2.6 | 0.803 | |||
| INTERGROWTH-21st | 65.7 | 0.827 | 2.4 | 0.495 | |||
| GROW | 67.5 | 0.554 | 2.3 | 0.256 | |||
| FMF | 64 | 0.001* | 2.6 | 0.991 | |||
| NICU admission | Hadlock2 | 56.6 | 56.8 | 0.200 | 1.5 | 1.5 | 0.627 |
| PRB/NICHD | 56.9 | 0.856 | 1.6 | 0.520 | |||
| INTERGROWTH-21st | 56.2 | 0.285 | 1.7 | 0.017 | |||
| GROW | 55.9 | 0.148 | 1.5 | 0.576 | |||
| FMF | 56.2 | <0.001* | 1.5 | 0.440 | |||
| Apgar <7 at 5 min | Hadlock2 | 57.2 | 57.4 | 0.263 | 1.3 | 1.4 | 0.105 |
| PRB/NICHD | 58.1 | 0.287 | 1.5 | 0.101 | |||
| INTERGROWTH-21st | 58.4 | 0.272 | 1.6 | 0.179 | |||
| GROW | 56.3 | 0.246 | 1.4 | 0.708 | |||
| FMF | 56.3 | 0.001* | 1.4 | 0.827 | |||
| Mechanical Ventilation | Hadlock2 | 59.6 | 60 | 0.039* | 1.8 | 1.8 | 0.558 |
| PRB/NICHD | 60.6 | 0.194 | 1.9 | 0.064 | |||
| INTERGROWTH-21st | 59.8 | 0.843 | 2.2 | 0.003 | |||
| GROW | 59.1 | 0.295 | 1.8 | 0.656 | |||
| FMF | 59.1 | <0.001* | 1.8 | 0.550 | |||
| Hypoglycemia | Hadlock2 | 61.4 | 61.7 | 0.151 | 2.5 | 2.5 | 0.828 |
| PRB/NICHD | 61.4 | 0.557 | 2.3 | 0.198 | |||
| INTERGROWTH-21st | 62.2 | 0.656 | 2.7 | 0.072 | |||
| GROW | 60.8 | 0.423 | 2.5 | 0.546 | |||
| FMF | 61.5 | 0.425 | 2.4 | 0.362 | |||
Nevertheless, when considering only the part of the ROC curve for which the FPR<10% and computing the partial area under the curve, we noted a slightly higher values for the INTERGROWTH-21st standard compared to the Hadlock 1 and FMF standards for the prediction of NICU admission (all, p<0.05) (Figure 3, Table 3, Table S3). Similarly, for the partial AUC was slightly higher for INTERGROWTH-21st standard compared to FMF standard for hypoglycemia (Figure 3, Table S3). The use of the partial AUC is motivated by the fact that it is more important for the different standards to have higher sensitivity at a low and, hence, more clinically relevant false-positive rate.
Association between EFW>90th and adverse perinatal outcomes
The screening rates for LGA were overall smaller than those for SGA, and they also varied greatly among standards: Hadlock 1 and 2 (2.9%), GROW (6.4%), INTERGROWTH-21st (7%), PRB/NICHD (9%), FMF (9.6%), WHO (10.2%), and NICHD (12.5%). Among the seven standards considered, LGA screening by INTERGROWTH-21st (RR=1.4) and Hadlock (RR=1.7) standards led to a significant association with the composite adverse perinatal outcome, yet sensitivity was 2-to 5-fold lower (5% for Hadlock and 10% for INTERGROWTH-21st) compared to SGA screening with the same standards (see Table S2). LGA fetuses were also at risk of hypoglycemia according to the Hadlock standard (RR=2.9) with only 8% (sensitivity) of cases being detected.
DISCUSSION
Customized vs. non-customized standards
More than 100 fetal growth standards were proposed for fetal growth assessment41. Several studies suggested that customized fetal growth 38, 45, 89, 90 and birthweight 54–67 assessment better predicts morbidity, while other studies found the opposite or were inconclusive. 39, 40, 55, 57, 66, 91–105 Sovio at al.66 reported that customized third trimester growth assessment did not improve the association with neonatal morbidity compared to non-customized standards, while Blue et al. 103 reported superior performance of non-customized standards than ethnic-specific standards. We therefore compared seven fetal growth standards for prediction of adverse perinatal outcomes and evaluated the extent to which differences in sensitivity are due to different overall stringencies of the standards (how low or high the 10th centile curve is and, hence, the screen-positive rate) as opposed to 1) differences in the shape of the 10th percentile curve and/or 2) factors considered in the customization that lead to different percentiles across standards for the same observed EFW.
Comparison of screen-positive rates
The screen-positive rate for SGA and LGA varied considerably with NICHD African American standard identifing only 6.8% as SGA and 12.5% as LGA; hence, this standard can be considered overall too low for our population. By contrast, Hadlock’s chart identified 16.5% of fetuses as SGA and only 2.9% as LGA; hence, this standard can be considered too high. Although the 10th centile of the FMF standard was the highest compared to all standards, resulting in the largest screen positive rate for SGA (24.4%), the 90th centile of this chart was similar to the one of the other standards and classified 9.6% of fetuses as LGA, based on the last availble scan.
While a previous study65 in a U.S. population identified a large difference in screen-positive rates of birthweight <10th percentile between the INTERGROWTH-21st (3.5%) and GROW (11.1%) standards, the assessment of EFW presented herein resulted in less discrepancy (11.6%, GROW; 13.5%, INTERGROWTH-21st) likely due to differences in the populations.
Comparison of relative risks
Sovio at al.23 reported that a third-trimester EFW<10th percentile was associated with a 1.6-fold increase in the risk of neonatal morbidity, which is similar to the 1.7 estimate derived herein with Hadlock’s standard. Moreover, we showed that fetuses with EFW<10th percentile were at increased risk of individual adverse perinatal outcomes according to all standards, with the highest risk estimate being for perinatal mortality (WHO standard, RR=5.05). Overall, the most stringent standard for SGA screening (NICHD) resulted in consistently higher relative risk estimates for adverse perinatal outcomes, while the least stringent standard (FMF) had the lowest relative risk estimates. The differences in relative risk among these most extreme standards were significant for the composite adverse perinatal outcome, yet the overlapping confidence intervals among all other standards impeded drawing conclusions regarding the superiority of one standard over another for individual adverse perinatal outcomes.
Comparison of area under the ROC curve
To complement the typical assessment based on relative risk and sensitivity for adverse neonatal outcomes,65 we also compared the full and partial AUC of low EFW percentiles. While sensitivity may vary due to differences in screen-positive rates, the AUC analysis considers all possible cut-offs and compares standards in terms of their ability to rank fetuses from the most (lowest percentile) to the least (highest percentile) at risk of sub-optimal growth. Even for non-customized standards, such differences in the reordering of the fetuses with respect to their risk are expected due to the shape of the 10th centile curve, which, for the same screen-positive rate, alters the balance of preterm and term fetuses being screened positive in a given cohort. Differences in performance of growth standards are also expected due differences in pregnancy characteristics considered in customization (if any) and analytical approaches and populations used to establish the standards 106.
The AUC for prediction of perinatal mortality with the PRB/NICHD standard was higher than for Hadlock 1 and FMF standards, yet the improvement emerged at FPR>15%; hence, a difference was not detected when comparing the partial area under the curve (FPR<10%). Of note, using a 20th percentile cut-off on the PRB/NICHD growth standard identifies one-half of fetuses at risk of perinatal mortality and one-third of those at risk of any adverse perinatal outcome considered herein (Figure 3).
Based on the partial AUC, the INTERGROWTH-21st standard showed superiority over Hadlock and FMF standards for individual perinatal outcomes. This can be understood since fetuses at risk for these outcomes had lower percentiles according to INTERGROWTH-21st compared to the Hadlock and FMF standards, resulting in higher sensitivity at low FPR (Figure 3, Table 3, Table S3). Therefore, the ROC curve-based analyses provided a perspective not attainable by simply comparing relative risk at the 10% EFW cut-off.
Strengths and limitations
This is the first study to compare seven fetal growth standards used worldwide, for prediction of adverse perinatal outcomes in the same population. The limitations are: 1) the population comprised only African-American women, and future studies are required to determine whether findings extrapolate to other populations; 2) the population included a wide range of gestational ages at the last ultrasound scan that was related to the actual distribution of gestational age at delivery; 3) the current study evaluated several but not all adverse perinatal outcomes due to their low frequencies; 4) the cohort included in this study was derived from a larger set of 4,001 pregnancies used to develop the PRB/NICHD standard; hence, prediction performance estimates for this particular standard may be biased.
Conclusion
This study demonstrated that differences in stringency (and hence FPR) among standards explain the variability in sensitivity and relative risk of adverse perinatal outcomes. When considering a wider range of FPR by ROC curve analysis, the recent international (INTERGROWTH-21st) and customized (PRB/NICHD) standards seem to improve detection of fetuses at risk of some adverse perinatal outcomes compared to Hadlock and FMF standards in a African-American population. Although LGA fetuses were also at risk of adverse perinatal outcomes, much fewer cases will be detected by LGA than SGA screening.
Supplementary Material
Acknowledgments
Funding: This research was supported, in part, by the Perinatology Research Branch, Division of Obstetrics and Maternal-Fetal Medicine, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS); and, in part, with Federal funds from NICHD/NIH/DHHS under Contract No. HHSN275201300006C. ALT was also supported by the Wayne State School of Medicine Perinatal Initiative.
Footnotes
Disclosure Statement: The authors report no conflicts of interest.
REFERENCES
- 1.Schoendorf KC, Hogue CJ, Kleinman JC, Rowley D. Mortality among infants of black as compared with white college-educated parents. The New England journal of medicine 1992; 326: 1522–1526. [DOI] [PubMed] [Google Scholar]
- 2.Gardosi J, Mul T, Mongelli M, Fagan D. Analysis of birthweight and gestational age in antepartum stillbirths. British journal of obstetrics and gynaecology 1998; 105: 524–530. [DOI] [PubMed] [Google Scholar]
- 3.McIntire DD, Bloom SL, Casey BM, Leveno KJ. Birth weight in relation to morbidity and mortality among newborn infants. The New England journal of medicine 1999; 340: 1234–1238. [DOI] [PubMed] [Google Scholar]
- 4.Alexander GR, Kogan MD, Himes JH. 1994–1996 U.S. singleton birth weight percentiles for gestational age by race, Hispanic origin, and gender. Maternal and child health journal 1999; 3: 225–231. [DOI] [PubMed] [Google Scholar]
- 5.Ozanne SE, Fernandez-Twinn D, Hales CN. Fetal growth and adult diseases. Seminars in perinatology 2004; 28: 81–87. [DOI] [PubMed] [Google Scholar]
- 6.Kajantie E, Osmond C, Barker DJ, Forsen T, Phillips DI, Eriksson JG. Size at birth as a predictor of mortality in adulthood: a follow-up of 350 000 person-years. International journal of epidemiology 2005; 34: 655–663. [DOI] [PubMed] [Google Scholar]
- 7.Vashevnik S, Walker S, Permezel M. Stillbirths and neonatal deaths in appropriate, small and large birthweight for gestational age fetuses. The Australian & New Zealand journal of obstetrics & gynaecology 2007; 47: 302–306. [DOI] [PubMed] [Google Scholar]
- 8.Savchev S, Sanz-Cortes M, Cruz-Martinez R, Arranz A, Botet F, Gratacos E, Figueras F. Neurodevelopmental outcome of full-term small-for-gestational-age infants with normal placental function. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology 2013; 42: 201–206. [DOI] [PubMed] [Google Scholar]
- 9.Blair EM, Nelson KB. Fetal growth restriction and risk of cerebral palsy in singletons born after at least 35 weeks’ gestation. American journal of obstetrics and gynecology 2015; 212: 520.e521-527. [DOI] [PubMed] [Google Scholar]
- 10.Mendez-Figueroa H, Truong VT, Pedroza C, Khan AM, Chauhan SP. Small-for-gestational-age infants among uncomplicated pregnancies at term: a secondary analysis of 9 Maternal-Fetal Medicine Units Network studies. American journal of obstetrics and gynecology 2016; 215: 628.e621–628.e627. [DOI] [PubMed] [Google Scholar]
- 11.Iliodromiti S, Mackay DF, Smith GC, Pell JP, Sattar N, Lawlor DA, Nelson SM. Customised and Noncustomised Birth Weight Centiles and Prediction of Stillbirth and Infant Mortality and Morbidity: A Cohort Study of 979,912 Term Singleton Pregnancies in Scotland. PLoS medicine 2017; 14: e1002228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chauhan SP, Rice MM, Grobman WA, Bailit J, Reddy UM, Wapner RJ, Varner MW, Thorp JM Jr., Leveno KJ, Caritis SN, Prasad M, Tita ATN, Saade G, Sorokin Y, Rouse DJ, Tolosa JE. Neonatal Morbidity of Small-and Large-for-Gestational-Age Neonates Born at Term in Uncomplicated Pregnancies. Obstetrics and gynecology 2017; 130: 511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McEwen EC, Guthridge SL, He VY, McKenzie JW, Boulton TJ, Smith R. What birthweight percentile is associated with optimal perinatal mortality and childhood education outcomes? American journal of obstetrics and gynecology 2018; 218: S712–s724. [DOI] [PubMed] [Google Scholar]
- 14.Madden JV, Flatley CJ, Kumar S. Term small-for-gestational-age infants from low-risk women are at significantly greater risk of adverse neonatal outcomes. American journal of obstetrics and gynecology 2018; 218: 525.e521–525.e529. [DOI] [PubMed] [Google Scholar]
- 15.Esakoff TF, Cheng YW, Sparks TN, Caughey AB. The association between birthweight 4000 g or greater and perinatal outcomes in patients with and without gestational diabetes mellitus. American journal of obstetrics and gynecology 2009; 200: 672 e671–674. [DOI] [PubMed] [Google Scholar]
- 16.Oral E, Cagdas A, Gezer A, Kaleli S, Aydinli K, Ocer F. Perinatal and maternal outcomes of fetal macrosomia. European journal of obstetrics, gynecology, and reproductive biology 2001; 99: 167–171. [DOI] [PubMed] [Google Scholar]
- 17.Sinclair BA, Rowan JA, Hainsworth OT. Macrosomic infants are not all equal. The Australian & New Zealand journal of obstetrics & gynaecology 2007; 47: 101–105. [DOI] [PubMed] [Google Scholar]
- 18.McKenna D, Tharmaratnam S, Mahsud S, Bailie C, Harper A, Dornan J. A randomized trial using ultrasound to identify the high-risk fetus in a low-risk population. Obstetrics and gynecology 2003; 101: 626–632. [DOI] [PubMed] [Google Scholar]
- 19.Savchev S, Figueras F, Cruz-Martinez R, Illa M, Botet F, Gratacos E. Estimated weight centile as a predictor of perinatal outcome in small-for-gestational-age pregnancies with normal fetal and maternal Doppler indices. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology 2012; 39: 299–303. [DOI] [PubMed] [Google Scholar]
- 20.Roex A, Nikpoor P, van Eerd E, Hodyl N, Dekker G. Serial plotting on customised fundal height charts results in doubling of the antenatal detection of small for gestational age fetuses in nulliparous women. The Australian & New Zealand journal of obstetrics & gynaecology 2012; 52: 78–82. [DOI] [PubMed] [Google Scholar]
- 21.Trudell AS, Cahill AG, Tuuli MG, Macones GA, Odibo AO. Risk of stillbirth after 37 weeks in pregnancies complicated by small-for-gestational-age fetuses. American journal of obstetrics and gynecology 2013; 208: 376.e371–377. [DOI] [PubMed] [Google Scholar]
- 22.DeVore GR. The importance of the cerebroplacental ratio in the evaluation of fetal well-being in SGA and AGA fetuses. American journal of obstetrics and gynecology 2015; 213: 5–15. [DOI] [PubMed] [Google Scholar]
- 23.Sovio U, White IR, Dacey A, Pasupathy D, Smith GCS. Screening for fetal growth restriction with universal third trimester ultrasonography in nulliparous women in the Pregnancy Outcome Prediction (POP) study: a prospective cohort study. Lancet (London, England) 2015; 386: 2089–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lees CC, Marlow N, van Wassenaer-Leemhuis A, Arabin B, Bilardo CM, Brezinka C, Calvert S, Derks JB, Diemert A, Duvekot JJ, Ferrazzi E, Frusca T, Ganzevoort W, Hecher K, Martinelli P, Ostermayer E, Papageorghiou AT, Schlembach D, Schneider KT, Thilaganathan B, Todros T, Valcamonico A, Visser GH, Wolf H. 2 year neurodevelopmental and intermediate perinatal outcomes in infants with very preterm fetal growth restriction (TRUFFLE): a randomised trial. Lancet (London, England) 2015; 385: 2162–2172. [DOI] [PubMed] [Google Scholar]
- 25.Temming LA, Dicke JM, Stout MJ, Rampersad RM, Macones GA, Tuuli MG, Cahill AG. Early Second-Trimester Fetal Growth Restriction and Adverse Perinatal Outcomes. Obstetrics and gynecology 2017; 130: 865–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams M, Turner S, Butler E, Gardosi J. Fetal growth surveillance -Current guidelines, practices and challenges. Ultrasound (Leeds, England) 2018; 26: 69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCowan LM, Figueras F, Anderson NH. Evidence-based national guidelines for the management of suspected fetal growth restriction: comparison, consensus, and controversy. American journal of obstetrics and gynecology 2018; 218: S855–s868. [DOI] [PubMed] [Google Scholar]
- 28.Lindqvist PG, Molin J. Does antenatal identification of small-for-gestational age fetuses significantly improve their outcome? Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology 2005; 25: 258–264. [DOI] [PubMed] [Google Scholar]
- 29.Gardosi J, Madurasinghe V, Williams M, Malik A, Francis A. Maternal and fetal risk factors for stillbirth: population based study. BMJ (Clinical research ed) 2013; 346: f108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alfirevic Z, Stampalija T, Dowswell T. Fetal and umbilical Doppler ultrasound in high-risk pregnancies. The Cochrane database of systematic reviews 2017; 6: Cd007529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Figueras F, Gratacos E. An integrated approach to fetal growth restriction. Best practice & research Clinical obstetrics & gynaecology 2017; 38: 48–58. [DOI] [PubMed] [Google Scholar]
- 32.Smith GCS. Universal screening for foetal growth restriction. Best practice & research Clinical obstetrics & gynaecology 2018; 49: 16–28. [DOI] [PubMed] [Google Scholar]
- 33.Battaglia FC, Lubchenco LO. A practical classification of newborn infants by weight and gestational age. The Journal of pediatrics 1967; 71: 159–163. [DOI] [PubMed] [Google Scholar]
- 34.Hadlock FP, Harrist RB, Martinez-Poyer J. In utero analysis of fetal growth: a sonographic weight standard. Radiology 1991; 181: 129–133. [DOI] [PubMed] [Google Scholar]
- 35.Gardosi JO. Prematurity and fetal growth restriction. Early human development 2005; 81: 43–49. [DOI] [PubMed] [Google Scholar]
- 36.Pedersen NG, Figueras F, Wojdemann KR, Tabor A, Gardosi J. Early fetal size and growth as predictors of adverse outcome. Obstetrics and gynecology 2008; 112: 765–771. [DOI] [PubMed] [Google Scholar]
- 37.Simic M, Stephansson O, Petersson G, Cnattingius S, Wikstrom AK. Slow fetal growth between first and early second trimester ultrasound scans and risk of small for gestational age (SGA) birth. PloS one 2017; 12: e0184853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gardosi J, Chang A, Kalyan B, Sahota D, Symonds EM. Customised antenatal growth charts. Lancet (London, England) 1992; 339: 283–287. [DOI] [PubMed] [Google Scholar]
- 39.Clausson B, Gardosi J, Francis A, Cnattingius S. Perinatal outcome in SGA births defined by customised versus population-based birthweight standards. BJOG : an international journal of obstetrics and gynaecology 2001; 108: 830–834. [DOI] [PubMed] [Google Scholar]
- 40.Ego A, Subtil D, Grange G, Thiebaugeorges O, Senat MV, Vayssiere C, Zeitlin J. Customized versus population-based birth weight standards for identifying growth restricted infants: a French multicenter study. American journal of obstetrics and gynecology 2006; 194: 1042–1049. [DOI] [PubMed] [Google Scholar]
- 41.Gardosi J, Francis A, Turner S, Williams M. Customized growth charts: rationale, validation and clinical benefits. American journal of obstetrics and gynecology 2018; 218: S609–s618. [DOI] [PubMed] [Google Scholar]
- 42.Gardosi J, Mongelli M, Wilcox M, Chang A. An adjustable fetal weight standard. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology 1995; 6: 168–174. [DOI] [PubMed] [Google Scholar]
- 43.Hutcheon JA, Zhang X, Cnattingius S, Kramer MS, Platt RW. Customised birthweight percentiles: does adjusting for maternal characteristics matter? BJOG : an international journal of obstetrics and gynaecology 2008; 115: 1397–1404. [DOI] [PubMed] [Google Scholar]
- 44.Kierans WJ, Joseph KS, Luo ZC, Platt R, Wilkins R, Kramer MS. Does one size fit all? The case for ethnic-specific standards of fetal growth. BMC pregnancy and childbirth 2008; 8: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hanley GE, Janssen PA. Ethnicity-specific birthweight distributions improve identification of term newborns at risk for short-term morbidity. American journal of obstetrics and gynecology 2013; 209: 428.e421–426. [DOI] [PubMed] [Google Scholar]
- 46.Pang MW, Leung TN, Sahota DS, Lau TK, Chang AM. Customizing fetal biometric charts. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology 2003; 22: 271–276. [DOI] [PubMed] [Google Scholar]
- 47.McCowan L, Stewart AW, Francis A, Gardosi J. A customised birthweight centile calculator developed for a New Zealand population. The Australian & New Zealand journal of obstetrics & gynaecology 2004; 44: 428–431. [DOI] [PubMed] [Google Scholar]
- 48.Mongelli M, Figueras F, Francis A, Gardosi J. A customized birthweight centile calculator developed for an Australian population. The Australian & New Zealand journal of obstetrics & gynaecology 2007; 47: 128–131. [DOI] [PubMed] [Google Scholar]
- 49.Figueras F, Meler E, Iraola A, Eixarch E, Coll O, Figueras J, Francis A, Gratacos E, Gardosi J. Customized birthweight standards for a Spanish population. European journal of obstetrics, gynecology, and reproductive biology 2008; 136: 20–24. [DOI] [PubMed] [Google Scholar]
- 50.Gardosi J, Francis A. A customized standard to assess fetal growth in a US population. American journal of obstetrics and gynecology 2009; 201: 25.e21–27. [DOI] [PubMed] [Google Scholar]
- 51.Unterscheider J, Geary MP, Daly S, McAuliffe FM, Kennelly MM, Dornan J, Morrison JJ, Burke G, Francis A, Gardosi J, Malone FD. The customized fetal growth potential: a standard for Ireland. European journal of obstetrics, gynecology, and reproductive biology 2013; 166: 14–17. [DOI] [PubMed] [Google Scholar]
- 52.Ghi T, Cariello L, Rizzo L, Ferrazzi E, Periti E, Prefumo F, Stampalija T, Viora E, Verrotti C, Rizzo G. Customized Fetal Growth Charts for Parents’ Characteristics, Race, and Parity by Quantile Regression Analysis: A Cross-sectional Multicenter Italian Study. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine 2016; 35: 83–92. [DOI] [PubMed] [Google Scholar]
- 53.Tarca AL, Romero R, Gudicha DW, Erez O, Hernandez-Andrade E, Yeo L, Bhatti G, Pacora P, Maymon E, Hassan SS. A new customized fetal growth standard for African American women: the PRB/NICHD Detroit study. American journal of obstetrics and gynecology 2018; 218: S679-S691.e674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mongelli M, Gardosi J. Reduction of false-positive diagnosis of fetal growth restriction by application of customized fetal growth standards. Obstetrics and gynecology 1996; 88: 844–848. [DOI] [PubMed] [Google Scholar]
- 55.McCowan LM, Harding JE, Stewart AW. Customized birthweight centiles predict SGA pregnancies with perinatal morbidity. BJOG : an international journal of obstetrics and gynaecology 2005; 112: 1026–1033. [DOI] [PubMed] [Google Scholar]
- 56.Figueras F, Figueras J, Meler E, Eixarch E, Coll O, Gratacos E, Gardosi J, Carbonell X. Customised birthweight standards accurately predict perinatal morbidity. Archives of disease in childhood Fetal and neonatal edition 2007; 92: F277–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Groom KM, Poppe KK, North RA, McCowan LM. Small-for-gestational-age infants classified by customized or population birthweight centiles: impact of gestational age at delivery. American journal of obstetrics and gynecology 2007; 197: 239.e231–235. [DOI] [PubMed] [Google Scholar]
- 58.Gardosi J, Clausson B, Francis A. The value of customised centiles in assessing perinatal mortality risk associated with parity and maternal size. BJOG : an international journal of obstetrics and gynaecology 2009; 116: 1356–1363. [DOI] [PubMed] [Google Scholar]
- 59.Mikolajczyk RT, Zhang J, Betran AP, Souza JP, Mori R, Gulmezoglu AM, Merialdi M. A global reference for fetal-weight and birthweight percentiles. Lancet (London, England) 2011; 377: 1855–1861. [DOI] [PubMed] [Google Scholar]
- 60.Odibo AO, Cahill AG, Odibo L, Roehl K, Macones GA. Prediction of intrauterine fetal death in small-for-gestational-age fetuses: impact of including ultrasound biometry in customized models. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology 2012; 39: 288–292. [DOI] [PubMed] [Google Scholar]
- 61.Kase BA, Carreno CA, Blackwell SC. Customized estimated fetal weight: a novel antenatal tool to diagnose abnormal fetal growth. American journal of obstetrics and gynecology 2012; 207: 218.e211–215. [DOI] [PubMed] [Google Scholar]
- 62.Gardosi J, Giddings S, Clifford S, Wood L, Francis A. Association between reduced stillbirth rates in England and regional uptake of accreditation training in customised fetal growth assessment. BMJ open 2013; 3: e003942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smith NA, Bukowski R, Thomas AM, Cantonwine D, Zera C, Robinson JN. Identification of pathologically small fetuses using customized, ultrasound and population-based growth norms. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology 2014; 44: 595–599. [DOI] [PubMed] [Google Scholar]
- 64.Khandaker S Assessment of Antepartum Fetal Growth by Customized “GROW” Curves Versus Noncustomized Growth Curves in Correlation with Neonatal Growth Pattern. Journal of obstetrics and gynaecology of India 2014; 64: 189–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Francis A, Hugh O, Gardosi J. Customized vs INTERGROWTH-21(st) standards for the assessment of birthweight and stillbirth risk at term. American journal of obstetrics and gynecology 2018; 218: S692–s699. [DOI] [PubMed] [Google Scholar]
- 66.Sovio U, Smith GCS. The effect of customization and use of a fetal growth standard on the association between birthweight percentile and adverse perinatal outcome. American journal of obstetrics and gynecology 2018; 218: S738–s744. [DOI] [PubMed] [Google Scholar]
- 67.Romero R, Tarca AL. Fetal size standards to diagnose a small-or a large-for-gestational-age fetus. American journal of obstetrics and gynecology 2018; 218: S605–s607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Merialdi M, Widmer M, Gulmezoglu AM, Abdel-Aleem H, Bega G, Benachi A, Carroli G, Cecatti JG, Diemert A, Gonzalez R, Hecher K, Jensen LN, Johnsen SL, Kiserud T, Kriplani A, Lumbiganon P, Tabor A, Talegawkar SA, Tshefu A, Wojdyla D, Platt L. WHO multicentre study for the development of growth standards from fetal life to childhood: the fetal component. BMC pregnancy and childbirth 2014; 14: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kiserud T, Piaggio G, Carroli G, Widmer M, Carvalho J, Neerup Jensen L, Giordano D, Cecatti JG, Abdel Aleem H, Talegawkar SA, Benachi A, Diemert A, Tshefu Kitoto A, Thinkhamrop J, Lumbiganon P, Tabor A, Kriplani A, Gonzalez Perez R, Hecher K, Hanson MA, Gulmezoglu AM, Platt LD. The World Health Organization Fetal Growth Charts: A Multinational Longitudinal Study of Ultrasound Biometric Measurements and Estimated Fetal Weight. PLoS medicine 2017; 14: e1002220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kiserud T, Benachi A, Hecher K, Perez RG, Carvalho J, Piaggio G, Platt LD. The World Health Organization fetal growth charts: concept, findings, interpretation, and application. American journal of obstetrics and gynecology 2018; 218: S619–s629. [DOI] [PubMed] [Google Scholar]
- 71.Buck Louis GM, Grewal J, Albert PS, Sciscione A, Wing DA, Grobman WA, Newman RB, Wapner R, D’Alton ME, Skupski D, Nageotte MP, Ranzini AC, Owen J, Chien EK, Craigo S, Hediger ML, Kim S, Zhang C, Grantz KL . Racial/ethnic standards for fetal growth: the NICHD Fetal Growth Studies. American journal of obstetrics and gynecology 2015; 213: 449.e441–449.e441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Altman DG, Ohuma EO. Statistical considerations for the development of prescriptive fetal and newborn growth standards in the INTERGROWTH-21st Project. BJOG : an international journal of obstetrics and gynaecology 2013; 120 Suppl 2: 71–76, v. [DOI] [PubMed] [Google Scholar]
- 73.Cheikh Ismail L, Knight HE, Ohuma EO, Hoch L, Chumlea WC. Anthropometric standardisation and quality control protocols for the construction of new, international, fetal and newborn growth standards: the INTERGROWTH-21st Project. BJOG : an international journal of obstetrics and gynaecology 2013; 120 Suppl 2: 48–55, v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Villar J, Altman DG, Purwar M, Noble JA, Knight HE, Ruyan P, Cheikh Ismail L, Barros FC, Lambert A, Papageorghiou AT, Carvalho M, Jaffer YA, Bertino E, Gravett MG, Bhutta ZA, Kennedy SH. The objectives, design and implementation of the INTERGROWTH-21st Project. BJOG : an international journal of obstetrics and gynaecology 2013; 120 Suppl 2: 9–26, v. [DOI] [PubMed] [Google Scholar]
- 75.Papageorghiou AT, Ohuma EO, Altman DG, Todros T, Cheikh Ismail L, Lambert A, Jaffer YA, Bertino E, Gravett MG, Purwar M, Noble JA, Pang R, Victora CG, Barros FC, Carvalho M, Salomon LJ, Bhutta ZA, Kennedy SH, Villar J, International F, Newborn Growth Consortium for the 21st C. International standards for fetal growth based on serial ultrasound measurements: the Fetal Growth Longitudinal Study of the INTERGROWTH-21st Project. Lancet 2014; 384: 869–879. [DOI] [PubMed] [Google Scholar]
- 76.Papageorghiou AT, Kennedy SH, Salomon LJ, Altman DG, Ohuma EO, Stones W, Gravett MG, Barros FC, Victora C, Purwar M, Jaffer Y, Noble JA, Bertino E, Pang R, Cheikh Ismail L, Lambert A, Bhutta ZA, Villar J. The INTERGROWTH-21(st) fetal growth standards: toward the global integration of pregnancy and pediatric care. American journal of obstetrics and gynecology 2018; 218: S630–s640. [DOI] [PubMed] [Google Scholar]
- 77.Nicolaides KH, Wright D, Syngelaki A, Wright A, Akolekar R. Fetal Medicine Foundation fetal and neonatal population weight charts. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology 2018; 52: 44–51. [DOI] [PubMed] [Google Scholar]
- 78.Organization WH. Neonatal and perinatal mortality: country, regional and global estimates. 2006. [Google Scholar]
- 79.The Apgar Score. Pediatrics 2015; 136: 819–822. [DOI] [PubMed] [Google Scholar]
- 80.Simon LV, Bragg BN. APGAR Score In StatPearls.StatPearls Publishing LLC: Treasure Island (FL), 2018. [PubMed] [Google Scholar]
- 81.Nuntnarumit P, Chittamma A, Pongmee P, Tangnoo A, Goonthon S. Clinical performance of the new glucometer in the nursery and neonatal intensive care unit. Pediatrics international : official journal of the Japan Pediatric Society 2011; 53: 218–223. [DOI] [PubMed] [Google Scholar]
- 82.Organization WH. Thermal protection of the newborn: a practical guide. 1997. [Google Scholar]
- 83.Lee J, Romero R, Lee KA, Kim EN, Korzeniewski SJ, Chaemsaithong P, Yoon BH. Meconium aspiration syndrome: a role for fetal systemic inflammation. American journal of obstetrics and gynecology 2016; 214: 366.e361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hadlock FP, Harrist RB, Sharman RS, Deter RL, Park SK. Estimation of fetal weight with the use of head, body, and femur measurements--a prospective study. American journal of obstetrics and gynecology 1985; 151: 333–337. [DOI] [PubMed] [Google Scholar]
- 85.Stirnemann J, Villar J, Salomon LJ, Ohuma E, Ruyan P, Altman DG, Nosten F, Craik R, Munim S, Cheikh Ismail L, Barros FC, Lambert A, Norris S, Carvalho M, Jaffer YA, Noble JA, Bertino E, Gravett MG, Purwar M, Victora CG, Uauy R, Bhutta Z, Kennedy S, Papageorghiou AT. International estimated fetal weight standards of the INTERGROWTH-21(st) Project. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology 2017; 49: 478–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gardosi J A. F. Customised Centile Calculator. GROW version 8.0.1. Gestation Network 2018. www.gestation.net. [Google Scholar]
- 87.Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, Muller M. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC bioinformatics 2011; 12: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstetrics and gynecology 1996; 87: 163–168. [DOI] [PubMed] [Google Scholar]
- 89.Gardosi J, Francis A. Controlled trial of fundal height measurement plotted on customised antenatal growth charts. British journal of obstetrics and gynaecology 1999; 106: 309–317. [DOI] [PubMed] [Google Scholar]
- 90.Anderson NH, Sadler LC, McKinlay CJD, McCowan LME. INTERGROWTH-21st vs customized birthweight standards for identification of perinatal mortality and morbidity. American journal of obstetrics and gynecology 2016; 214: 509.e501–509.e507. [DOI] [PubMed] [Google Scholar]
- 91.Zaw W, Gagnon R, da Silva O. The risks of adverse neonatal outcome among preterm small for gestational age infants according to neonatal versus fetal growth standards. Pediatrics 2003; 111: 1273–1277. [DOI] [PubMed] [Google Scholar]
- 92.Gardosi J, Clausson B, Francis A. The use of customised versus population-based birthweight standards in predicting perinatal mortality. BJOG : an international journal of obstetrics and gynaecology 2007; 114: 1301–1302; author reply 1303. [DOI] [PubMed] [Google Scholar]
- 93.Odibo AO, Francis A, Cahill AG, Macones GA, Crane JP, Gardosi J. Association between pregnancy complications and small-for-gestational-age birth weight defined by customized fetal growth standard versus a population-based standard. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet 2011; 24: 411–417. [DOI] [PubMed] [Google Scholar]
- 94.Larkin JC, Hill LM, Speer PD, Simhan HN. Risk of morbid perinatal outcomes in small-for-gestational-age pregnancies: customized compared with conventional standards of fetal growth. Obstetrics and gynecology 2012; 119: 21–27. [DOI] [PubMed] [Google Scholar]
- 95.Landres IV, Clark A, Chasen ST. Improving antenatal prediction of small-for-gestational-age neonates by using customized versus population-based reference standards. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine 2013; 32: 1581–1586. [DOI] [PubMed] [Google Scholar]
- 96.Carberry AE, Raynes-Greenow CH, Turner RM, Jeffery HE. Customized versus population-based birth weight charts for the detection of neonatal growth and perinatal morbidity in a cross-sectional study of term neonates. American journal of epidemiology 2013; 178: 1301–1308. [DOI] [PubMed] [Google Scholar]
- 97.Costantine MM, Mele L, Landon MB, Spong CY, Ramin SM, Casey B, Wapner RJ, Varner MW, Rouse DJ, Thorp JM Jr., Sciscione A, Catalano P, Caritis SN, Sorokin Y, Peaceman AM, Tolosa JE, Anderson GD. Customized versus population approach for evaluation of fetal overgrowth. American journal of perinatology 2013; 30: 565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Costantine MM, Lai Y, Bloom SL, Spong CY, Varner MW, Rouse DJ, Ramin SM, Caritis SN, Peaceman AM, Sorokin Y, Sciscione A, Mercer BM, Thorp JM, Malone FD, Harper M, Iams JD. Population versus customized fetal growth norms and adverse outcomes in an intrapartum cohort. American journal of perinatology 2013; 30: 335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Moussa HN, Wu ZH, Han Y, Pacheco LD, Blackwell SC, Sibai BM, Saade G, Costantine MM. Customized versus Population Fetal Growth Norms and Adverse Outcomes Associated with Small for Gestational Age Infants in a High-Risk Cohort. American journal of perinatology 2015; 32: 621–626. [DOI] [PubMed] [Google Scholar]
- 100.Moon M, Baek MJ, Ahn E, Odibo AO. Association between small for gestational age and intrauterine fetal death: comparing a customized South Korean growth standard versus a population-based fetal growth chart. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet 2016; 29: 872–874. [DOI] [PubMed] [Google Scholar]
- 101.Chiossi G, Pedroza C, Costantine MM, Truong VTT, Gargano G, Saade GR. Customized vs population-based growth charts to identify neonates at risk of adverse outcome: systematic review and Bayesian meta-analysis of observational studies. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology 2017; 50: 156–166. [DOI] [PubMed] [Google Scholar]
- 102.Blue NR, Savabi M, Beddow ME, Katukuri VR, Fritts CM, Izquierdo LA, Chao CR. The Hadlock Method Is Superior to Newer Methods for the Prediction of the Birth Weight Percentile. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine 2018. DOI: 10.1002/jum.14725. [DOI] [PubMed] [Google Scholar]
- 103.Blue NR, Beddow ME, Savabi M, Katukuri VR, Chao CR. Comparing the Hadlock fetal growth standard to the NICHD racial/ethnic standard for the prediction of neonatal morbidity and small for gestational age. American journal of obstetrics and gynecology 2018. DOI: 10.1016/j.ajog.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 104.Mendez-Figueroa H, Chauhan SP, Barrett T, Truong VTT, Pedroza C, Blackwell SC. Population versus Customized Growth Curves: Prediction of Composite Neonatal Morbidity. American journal of perinatology 2018. DOI: 10.1055/s-0038-1675161. [DOI] [PubMed] [Google Scholar]
- 105.Monier I, Ego A, Benachi A, Ancel PY, Goffinet F, Zeitlin J. Comparison of the Hadlock and INTERGROWTH formulas for calculating estimated fetal weight in a preterm population in France. American journal of obstetrics and gynecology 2018; 219: 476.e471–476.e412. [DOI] [PubMed] [Google Scholar]
- 106.Grantz KL, Hediger ML, Liu D, Buck Louis GM. Fetal growth standards: the NICHD fetal growth study approach in context with INTERGROWTH-21st and the World Health Organization Multicentre Growth Reference Study. American journal of obstetrics and gynecology 2018; 218: S641-S655.e628. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


