Abstract
This article provides an overview of recent advances in understanding the effects of alcohol use disorders (AUD) on the brain from the perspective of Magnetic Resonance Imaging (MRI) research in preclinical models and clinical studies. As a non-invasive investigational tool permitting assessment of morphological, metabolic, and hemodynamic changes over time, MRI offers insight into the dynamic course of alcoholism beginning with initial exposure through periods of binge drinking and escalation, sobriety, and relapse and has been useful in differential diagnosis of neurological diseases associated with AUD. Structural MRI has revealed acute and chronic effects of alcohol on both white and gray matter volumes. MR Spectroscopy, able to quantify brain metabolites in vivo, has shed light on biochemical alterations associated with alcoholism. Diffusion Tensor Imaging permits microstructural characterization of white-matter fiber tracts. Functional MRI has allowed for elucidation of hemodynamic changes relate during tasks or at rest. emission tomography (PET), a non-MRI imaging tool, has led to a deeper understanding of alcohol-induced receptor and neurotransmitter changes during various stages of drinking and abstinence. Together, such in vivo imaging tools have expanded our understanding of the dynamic course of alcoholism including evidence for regional specificity of the effects of AUD, hints at mechanisms underlying the shift from casual to compulsive use of alcohol, and profound recovery with sustained abstinence.
Keywords: Alcohol Use Disorder, alcoholism, alcohol dependence, animal models, preclinical models, Magnetic Resonance Imaging, Diffusion Tensor Imaging, Magnetic Resonance Spectroscopy, functional Magnetic Resonance Imaging, Positron Emission Tomography
Introduction
Alcohol Use Disorder (AUD) is one of the most common psychiatric disorders in first world countries and affects millions around the globe (Rehm et al., 2015). It is estimated that AUD accounts for 4% of the global disease burden (Connor, Haber, & Hall, 2016). In the United States (2012 −2013), 36% of the male and 23% of the female population met Diagnostic and Statistical Manual of Mental Disorders (DSM5) criteria for AUD at least once during their lifetime (Connor et al., 2016; Grant et al., 2015). This most recent version of the DSM conceptualizes alcoholism as a spectrum, ranging in severity from mild to severe, and referred to as AUD. ‘Uncomplicated alcoholism’ is a term used to distinguish AUD with complications such as liver disease from AUD with no obvious clinical features (Zahr & Pfefferbaum, 2017). The previous DSM version (DSM-IV-r) differentiated between alcohol abuse (i.e., a maladaptive pattern of drinking that results in failure to fulfill obligations; conflicts with the law; exposure of individuals to physically hazardous situations) and alcoholism (i.e., a maladaptive pattern of drinking characterized by tolerance, withdrawal, loss of control over intake, and continued consumption despite knowledge of adverse consequences). Throughout this review the terms ‘alcoholism’ (DSM-IV-r) and ‘AUD’ (DSM5) will be used interchangeably.
Prolonged alcohol consumption impacts peripheral organs, including the digestive tract, liver, heart, pancreas, kidneys, and lungs (cf., Zahr & Pfefferbaum, 2017). Alcoholism can furthermore result in severe neurological consequences, including Wernicke’s encephalopathy (WE), which if left untreated can progress to Korsakoff’s syndrome (KS); hepatic encephalopathy (HE); central pontine myelinolysis (CPM); and Marchiafava-Bignami disease (MBD) (cf., Zahr & Pfefferbaum, 2017). Apart from direct effects, high levels of alcohol consumption are associated with an increased risk for seizures, stroke, and traumatic brain injury (Alterman & Tarter, 1985; de los Rios et al., 2012; Eyer et al., 2011).
Of untreated AUD patients, 21% were able to remain abstinent for up to one year after an initial hospital admittance (Moyer & Finney, 2002). With standard psychotherapeutic and pharmacological treatment, abstinence rates may increase by 4–22%, depending on treatment intensity and follow-up duration (Miller, Walters, & Bennett, 2001; Monahan & Finney, 1996; Pettinati & Rabinowitz, 2006). Thus, current AUD treatments are only mildly to moderately effective. A better understanding of the physiological changes and molecular mechanisms underlying AUD may improve treatment options. In vivo imaging techniques, by allowing for longitudinal observations of alcoholism throughout the course of the disease can unravel disease processes and may potentially contribute to the development of improved medications.
The aim of this review is to give an outline of recent findings made with various imaging tools that contribute to a better understanding of AUD. Initial sections describe reports by imaging modality: structural Magnetic Resonance Imaging (MRI), Magnetic Resonance Spectroscopy (MRS), Diffusion Tensor Imaging (DTI), functional Magnetic Resonance Imaging (fMRI), and Positron Emission Tomography (PET). The final section summarizes findings made in preclinical rodent models and discusses the relevance of such models to human studies.
Structural Magnetic Resonance Imaging
Since the 1980s, conventional structural MRI has allowed researchers to visualize the living human brain. Visualization of brain structure is possible because each brain tissue-type (i.e., grey matter, white matter, and cerebrospinal fluid (CSF)) contains different quantities of water (Rumboldt, 2010). Structural MRI allows visualization of the brain on several axes: from bottom to top (axial), from front to back (coronal), and from left to right (sagittal). The 3 dimensional nature of acquired images provides greater accuracy in aligning images with internal landmarks and consequently greater consistency between data from replicate images (Rohlfing, 2006). Structural MRI is currently the most effective tool in identifying and diagnosing neurological disorders caused by long-term alcohol consumption (Antunez et al., 1998; Chung, Kim, Yoo, Lim, & Lee, 2003).
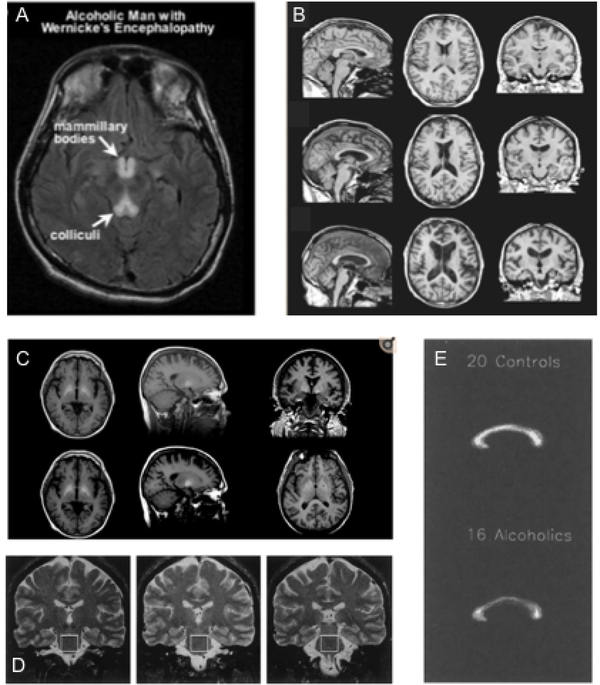
Wernicke’s encephalopathy (WE), which arises from thiamine (vitamin B1) deficiency associated with chronic alcohol consumption (but see, Berger & Singhal, 2014), may, if left untreated, progress to Korsakoff’s syndrome (KS), a severe neurological disorder characterized by anterograde and retrograde amnesia (Kopelman, Thomson, Guerrini, & Marshall, 2009; Zahr, Kaufman, & Harper, 2011). WE symptomatology constitutes visual, gait, and mental disturbances, including memory loss and emotional changes (Thomson et al., 2008). On T2 images, individuals with WE show symmetrical hyperintensities (bright spots) in midbrain gray matter surrounding the cerebral aqueduct, mammillary bodies, and third ventricle (Figure 1A) (Lenz et al., 2002; Sullivan & Pfefferbaum, 2009). Hyperintensities are thought to be caused by altered interstitial fluid mobility and water content, which may indicate demyelination or axonal damage (Wardlaw, Valdes Hernandez, & Munoz-Maniega, 2015). Brain regions affected (e.g., volume loss) by WE include the thalamus, cerebellum (vermis, dentate nuclei), pons, medulla, tectal plates, olivary bodies, and midbrain (red nuclei, substantia nigra) (Ha, Weon, Jang, Kang, & Choi, 2012; Kalidass, Sunnathkal, Rangashamanna, & Paraswani, 2012; Kang, Kang, Choi, & Choi, 2005; Liou, Kuo, & Chen, 2012; Murata et al., 2001; Nishida, Sato, Kobayashi, Morimoto, & Hamaoka, 2009). MRI imaging of the more severe KS reveals substantial volume shrinkage of the mammillary bodies (Sullivan et al., 1999), hippocampus (Sullivan et al., 2003), thalamus, orbitofrontal cortices (Jernigan et al., 1991), cerebellum, and pons (Figure 1B) (Zahr et al., 2009). Together these studies in WE/KS demonstrate dramatic morphological changes particularly targeting limbic and frontocerebellar circuitry.
Figure 1:
(A) Brain scan of an alcoholic man with Wernicke’s encephalopathy showing the typical hypertensities in the mammilary bodies and colliculi (reprint with permission from (Sullivan & Zahr, 2008)). (B) Exemplary MRI pictures highliting morphological changes observed in KE (reprint with permission from (Zahr & Pfefferbaum, 2017)). (C) Representative images from a control subject and a patient with hepatic encephalopathy (reprint with permission from (Zahr & Pfefferbaum, 2017)). (D) MRI scans from healthy controls, uncomplicated alcoholics, and KE patients that also show signs of central pontine myelinolysis (reprint with permission from (Sullivan & Pfefferbaum, 2001)). (E) Thinning of the corpus callosum of older alcoholics indicating Marchiafava-Bignami disease (reprint with permission from (Pfefferbaum et al., 1996)).
Hepatic Encephalopathy (HE) is believed to arise from high levels of ammonia circulating in the blood stream, occurring during acute or chronic liver disease, often as a consequence of alcoholism. Altered ammonia levels in the body directly influence brain metabolism and can lead to glial swelling and neuronal cell death (Kundra, Jain, Banga, Bajaj, & Kar, 2005; Rama Rao & Norenberg, 2014). HE patients may appear confused, disoriented, and have poor coordination (Prakash & Mullen, 2010; Vaquero, Chung, Cahill, & Blei, 2003). MRI images show bilateral, symmetrical high-intensity signals in the basal ganglia, prominent in globus pallidus, and substantia nigra (Figure 1C) (Binesh et al., 2006; Cordoba, Sanpedro, Alonso, & Rovira, 2002; Naegele et al., 2000), as well as along the cortico-spinal tract and white-matter of the cerebral hemispheres (Rovira et al., 2002).
Central Pontine Myelinolysis (CPM), another neurological disorder strongly linked to a history of alcoholism, is likely due to low sodium and other electrolyte disturbances (Messert, Orrison, Hawkins, & Quaglieri, 1979). Patients develop symptoms such as decreased voluntary muscle control and acute changes in consciousness (S. Kumar, Fowler, Gonzalez-Toledo, & Jaffe, 2006; Pfister, Einhaupl, & Brandt, 1985). “Bat-wing” lesions in the pons appearing hypointense (dark) on T1-weighted or hyperintense (bright) on T2-weighted images (Figure 1D) (DeWitt et al., 1984; Kleinschmidt-Demasters, Rojiani, & Filley, 2006; Pfister et al., 1985) likely reflect loss of cellular and axonal integrity.
Marchiafava-Bigami Disease (MBD) is marked by a mildly impaired mental status (e.g. confusion) and sometimes dysarthria (i.e., impairment of the speech)(H. Lee, Holburn, & Price, 2003) or ataxia (i.e., loss of control over bodily movement)(Arbelaez, Pajon, & Castillo, 2003). Although this disease is poorly understood, there exist indications that it may be linked to chronic alcohol consumption and nutritional deficiencies. MBD is traditionally characterized by demyelination and necrosis of the corpus callosum (Figure 1E) (Hillbom et al., 2014).
Each of the syndromes is characterized by extensive structural deviations from a healthy brain; the morphological changes, however, are not exclusively linked to a diagnosable neurological disorder. These changes are also, though to a lesser extent, consistently described in uncomplicated alcoholism. For example, volume deficits in frontal cortex, thalamus, and mammillary bodies of uncomplicated alcoholics compared to healthy controls have been reported (Sullivan et al., 2003; Sullivan et al., 1999; van Holst, de Ruiter, van den Brink, Veltman, & Goudriaan, 2012; Zahr & Pfefferbaum, 2017). Thinning of the corpus callosum (main characteristics of MBD) (Estruch et al., 1997; Pfefferbaum, Lim, Desmond, & Sullivan, 1996) and volume reduction of the pons are also observed in uncomplicated alcoholics compared to healthy controls (Pfefferbaum, Rosenbloom, Serventi, & Sullivan, 2002; Sullivan et al., 2003). Basal ganglia structures are affected (Durazzo et al., 2011; Makris et al., 2008) and include the caudate (Boutte et al., 2012), putamen (Jernigan et al., 1991), amygdala (Fein et al., 2006), and nucleus accumbens (Sullivan, Deshmukh, De Rosa, Rosenbloom, & Pfefferbaum, 2005). Such changes to nodes of the basal ganglia reward circuitry may contribute to the gradual progression of AUD, as these structures are involved in habit formation, reward evaluation, and cue-induced relapse (Koob & Volkow, 2016). Collectively, such findings suggest that neurological disorders such as WE, KS, CPM, and MBD occur on a continuum during the dynamic course of alcoholism before they emerge as clinically apparent syndromes.
Longitudinal structural MRI studies demonstrate that some brain morphological changes are reversible upon discontinuation of alcohol consumption. After periods of abstinence, previously enlarged ventricles decrease in size (Schroth, Naegele, Klose, Mann, & Petersen, 1988; Shear, Jernigan, & Butters, 1994) and a number of brain areas increase in volume including the temporal, insular, and anterior cingulate cortices; amygdala; thalamus; hippocampus; brainstem; and cerebellar cortex (Cardenas, Studholme, Gazdzinski, Durazzo, & Meyerhoff, 2007; Demirakca et al., 2011; Liu, Lemieux, Shorvon, Sisodiya, & Duncan, 2000; van Eijk et al., 2013; Wrase et al., 2008). Whether complete recovery occurs is difficult to conclude because older age contributes to alcoholism-related brain compromise: for example, older relative to younger alcoholics have significantly reduced capacity for recovery (Munro, Saxton, & Butters, 2000). Furthermore, brain differences may predate alcohol exposure: prior to alcohol exposure, children with at least one AUD-diagnosed parent demonstrate increased impulsivity and poor spatial memory and morphological changes to medial and lateral orbital and superior parietal cortices (Henderson et al., 2018). Similarly, sons from high-density AUD families have altered cortical thickness (middle frontal gyrus and inferior parietal lobule), which correlates with perturbed emotional processing and problems with executive functioning (Holla, Bharath, Venkatasubramanian, & Benegal, 2018).
Magnetic Resonance Spectroscopy
Magnetic Resonance Spectroscopy (MRS) allows for the in vivo quantification of neurometabolites based on their molecular structure. The largest signals arise from N-acetylaspartate (NAA), total creatine and phosphocreatine (tCr), and choline-containing compounds (Cho). In addition to the key MRS-visible metabolites NAA, tCr, and Cho, the combined resonance of glutamate (Glu) + glutamine (Gln), often referred to as Glx, has also been reported in the alcoholism literature.
A case study on a Japanese man who had consumed alcohol for 50 years, eaten poorly for several days, and presented with acute WE due to vitamin-B deficiency revealed low levels of NAA/tCr in the thalamus and cerebellum and a typically undetected (i.e., in healthy controls) lactate peak in the cerebellum. Thiamine treatment elevated NAA/tCr in the thalamus, but not in the cerebellum (Murata et al., 2001). A similar study reproduced the lactate findings, but reported no effect on NAA (Rugilo et al., 2003), while another reported reversible changes in NAA but no effect of WE on the lactate peak (Mascalchi et al., 2002).
In a variety of brain regions (e.g., frontal lobe, parietal white matter, anterior cingulate gyrus, basal ganglia, and occipital white/gray matter), MRS results in alcohol-related cirrhosis and HE relative to healthy controls are remarkably consistent and comparable to findings in non-alcoholic HE (e.g., Cordoba et al., 2001; Gupta et al., 1993; Häussinger et al., 1994) showing lower levels of Cho/tCr and myo-inositol (mI)/tCr, and higher levels of Glx/tCr (Ahluwalia et al., 2015; Binesh et al., 2006; Chavarria et al., 2013; Jain et al., 2013; Kreis, Ross, Farrow, & Ackerman, 1992; Laubenberger et al., 1997; Miese et al., 2006; Pujol et al., 1996; Singhal et al., 2010; Taylor-Robinson, Buckley, Changani, Hodgson, & Bell, 1999; Taylor-Robinson, Sargentoni, Marcus, Morgan, & Bryant, 1994; Thomas et al., 1998). Levels of Cho and mI are lowest and Glx highest in patients with HE (Geissler et al., 1997; J. H. Lee et al., 1999; Poveda et al., 2010; Ross et al., 1994; Tarasow et al., 2003). In the only report of MRS conducted on a case of alcoholism-associated CPM, a 53 year old man presented with gait disturbances and hearing loss and a voxel in the pons (that showed a lesion on conventional MRI) revealed elevated Cho/tCr, interpreted as reflecting edema or demyelination (Nomoto, Arasaki, & Tamaki, 2004).
The majority of MRS studies in alcoholism have focused on the 3 major metabolites: NAA, Cho, and tCr. Reduced NAA/tCr and Cho/tCr ratios relative in chronic alcoholics relative to normal controls have been reported in the frontal and medial temporal lobes, cerebellum, and thalamus (Bendszus et al., 2001; Ende et al., 2005; Jagannathan, Desai, & Raghunathan, 1996). Lower NAA levels are typically interpreted as reflecting neuronal compromise (Petroff, Pleban, & Spencer, 1995). Various interpretations for Cho changes in alcoholism have been suggested (e.g., undetected, subclinical pathologies such as thiamine deficiency or liver cirrhosis) (Zahr et al., 2010). Further challenging the interpretation of changes in Cho in alcoholism is that acute alcohol intake in healthy individuals is associated with elevations of Cho (Ende et al., 2006; Tunc-Skarka, Weber-Fahr, & Ende, 2015). Metabolic changes associated with AUD appear to normalize upon discontinuation of alcohol consumption (Bartsch et al., 2007; Bendszus et al., 2001; Durazzo, Gazdzinski, Rothlind, Banys, & Meyerhoff, 2006; Parks et al., 2002).
In animals, a relatively large body of literature demonstrates that acute ethanol, at concentrations associated with intoxication, potentiates GABAA receptor function and GABA release (e.g., Lobo & Harris, 2008; McCool, Frye, Pulido, & Botting, 2003; Olsen, Hanchar, Meera, & Wallner, 2007) while inhibiting glutamate NMDA receptors (Criswell, Ming, Griffith, & Breese, 2003; Dildy & Leslie, 1989; Hoffman, Rabe, Moses, & Tabakoff, 1989; Lovinger, White, & Weight, 1989). Synaptic responses mediated by NMDA receptors, including extracellular glutamate levels (Carboni, Isola, Gessa, & Rossetti, 1993; Moghaddam & Bolinao, 1994) are also reduced by acute EtOH (Lovinger, White, & Weight, 1990; Morrisett & Swartzwelder, 1993; Roberto et al., 2004).
It appears that NMDA receptor function is suppressed during intoxication even after prolonged ethanol exposure resulting in a compensatory and selective upregulation of NMDA receptors (Hu, Follesa, & Ticku, 1996). Thus, ethanol withdrawal following chronic exposure results in increased NMDA receptor activity and elevated extracellular glutamate, especially after repeated cycles of withdrawal (Dahchour & De Witte, 1999; Gonzales, Bungay, Kilanmaa, Samson, & Rosselti, 1996; Rossetti & Carboni, 1995), postulated to contribute to CNS hyperexcitability (Becker, 1998; Littleton, 1998) and excitotoxicity (Chandler, Newsom, Sumners, & Crews, 1993; Iorio, Tabakoff, & Hoffman, 1993). Indeed, a meta-analysis of rodent microdialysis studies confirms elevated extracellular concentrations of glutamate in several brain regions that strongly correlates with alcohol withdrawal severity (Fliegel, Brand, Spanagel, & Noori, 2013).
Early work in human plasma showed elevations in a number of amino acids during withdrawal (Majumdar, Shaw, Thomson, Pratt, & Greenwood, 1983). In patients admitted to emergency rooms for alcohol intoxication, blood measures determined GABA and glutamate levels at admission (T1) and after 24h (T2). Relative to healthy controls, GABA levels were low and glutamate levels were high (but higher at T1 relative to T2) at both time points in the alcoholics (Brousse et al., 2012). CSF from alcohol-withdrawing patients shows elevations in NAAG (a neuropeptide) but not glutamate (Tsai & Coyle, 1998). The human frontal cortex harvested from alcoholics showed moderate upregulation of NMDA receptors relative to controls (Freund & Anderson, 1996).
MRS methods to distinguish Glu from Gln require specialized spectral acquisition and editing (e.g., Choi et al., 2006; Schubert, Gallinat, Seifert, & Rinneberg, 2004). In reviewing the MRS-literature on the in vivo detection of glutamate metabolism changes in alcoholism, whether Glx, Glu, or Gln were measured is indicated. An important consideration is that MRS methods cannot distinguish between intracellular and extracellular pools of glutamate and thus the relevance of changes in glutamate levels can be difficult to interpret.
There is MRS data in keeping with the predications of the “glutamate model of withdrawal”: within 24 hours of the last drink, a trend for higher Glx (Yeo et al., 2013) and higher Glu (relative to tCr)(Hermann et al., 2012; Streit et al., 2018) has been reported in the anterior cingulate cortex of alcohol-dependent relative to control participants. A trend for higher Glu/tCr ratios was observed in the anterior cingulate of alcohol dependent participants scanned at 4 days and 4 weeks abstinence (Umhau et al., 2010). Another study similarly found higher Glu levels in nucleus accumbens (but not cingulate) of alcohol dependent participants (1–10 days into abstinence) compared to controls which was associated with higher craving for alcohol (Bauer et al., 2013). Normalization of acutely elevated Glu has been reported with two weeks of abstinence (Hermann et al., 2012): lower than control levels of Glu have been reported in alcoholdependent subjects during prolonged abstinence (2–50 days since last drink) in anterior cingulate (Mon, Durazzo, & Meyerhoff, 2012), medial frontal cortex (Thoma et al., 2011), and occipital cortex (Glu/Gln, Bagga, Khushu, et al., 2014).
A number of MRS studies, however, report effects on Glu contradictory to the model, regardless of the timing of data acquisition relative to heavy drinking. For example, a trend towards lower Glu in frontal white matter was observed in heavy drinkers compared to light drinkers, where heavier drinking and greater loss of control over drinking were associated with lower Glu levels (Ende et al., 2013). A negative correlation was also reported in the anterior cingulate between basal Glu levels and the number of drinking days in the past 2 week (i.e., more drinking days, less Glu) (Cheng et al., 2018). Similarly, number of heavy drinking days in the 14 days preceding observation was significantly inversely associated with anterior cingulate Glu concentrations (Prisciandaro et al., 2016).
In other MRS studies, there were no significant group differences reported with respect to Glx or Glu levels (cf., Cheng et al., 2018; Ende et al., 2013; Frischknecht et al., 2017; Zahr, Carr, et al., 2016; Zahr, Mayer, Rohlfing, Sullivan, & Pfefferbaum, 2014). GABA levels were unaffected by chronic alcoholism except in smokers (higher GABA at 1 week abstinence which resolved at 1 month) (Mason et al., 2006). With respect to Gln measured directly, it has been reported as higher (Thoma et al., 2011) and lower (Prisciandaro et al., 2016) in the anterior cingulate of AUD relative to control groups, while a yet another study observed no group differences but correlations in AUD between Gln levels in anterior cingulate and nucleus accumbens and craving (Bauer et al., 2013). In summary, additional in vivo research is required to determine the importance of glutamate to the dynamic course of AUD.
Diffusion Tensor imaging
Diffusion Tensor imaging (DTI) is an advanced MRI technique that enables examination of brain microstructure. Commonly reported DTI measures are fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD). FA describes the degree of directional coherence of diffusion, which is believed to reflect axonal diameter, axonal density, and myelination. A decrease in FA is generally associated with an increase in RD (associated with demyelination) or a decrease in AD (reflecting axonal injury) (Beaulieu, 2002; Pfefferbaum & Sullivan, 2005). A key advantage of DTI is its ability to detect white matter changes before they become apparent on conventional MR images (Pfefferbaum & Sullivan, 2005; Pfefferbaum et al., 2000).
KS patients show FA deficits in the fornix (Nahum et al., 2015), Papez and medial limbic circuits compared to alcoholics without KS (Segobin et al., 2015). HE patients have MD elevations in corpus callosum, internal capsule, frontal white matter (Kale et al., 2006), and occipital white matter (R. Kumar et al., 2008). CPM patients show elevated MD in middle cerebellar peduncles (Min, Park, & Hwang, 2012; Nair, Ramli, Rahmat, & Mei-Ling, 2012).
DTI can detect white-matter abnormalities in alcoholics without quantifiable white-matter volume loss (Pfefferbaum et al., 2000). Significant abnormalities (reduced FA and increased MD) have been detected in the corpus callosum, frontal forceps, internal and external capsules, fornix, superior cingulate, and longitudinal fasciculi of alcoholics relative to controls (Fortier et al., 2014; Muller-Oehring, Schulte, Fama, Pfefferbaum, & Sullivan, 2009; Pfefferbaum, Rosenbloom, Adalsteinsson, & Sullivan, 2007; Pfefferbaum et al., 2000). Quantitative DTI investigations have reported microstructural compromise of white matter tracts between midbrain and pons, and in fronto-limbic, fronto-parietal, and fronto-occipital fiber tracts (Bagga, Sharma, et al., 2014; Chanraud et al., 2009; Maksimovskiy et al., 2014; Yeh, Simpson, Durazzo, Gazdzinski, & Meyerhoff, 2009). DTI results suggest microstructural deficits may be more pronounced in alcoholic women (Pfefferbaum, Adalsteinsson, & Sullivan, 2006), despite a lower quantity of lifetime alcohol consumed (Sasaki et al., 2009).
Microstructural alterations in AUD have been associated with functional deficits including memory decline and poor executive functioning (Harris et al., 2008; Konrad et al., 2012; Pfefferbaum, Rosenbloom, Rohlfing, & Sullivan, 2009; Trivedi et al., 2013). For example, alcoholics demonstrate relations between low FA in the splenium and working memory impairment (Pfefferbaum et al., 2000); and low FA in corpus callosum, parietal, occipital, and frontal white matter regions with decision making deficits (Iowa Gambling Test) (Zorlu et al., 2014). Abnormal FA in nine different white matter tracts (i.e. anterior corona radiata, body of corpus callosum, cingulate gyrus, external capsule, fornix, inferior fronto-occipital fasciculus, posterior corona radiata, retro-lenticular limb of internal capsule, and superior longitudinal fasciculus) was directly related to greater BOLD-reactivity in reward processing brain-regions in response to alcohol-taste cues (Monnig et al., 2014).
As with other modalities, DTI studies support brain recovery with abstinence. For example, active alcohol users have abnormal diffusivity in frontal-temporal regions correlated with impulsivity and deficits in attention and memory; by contrast, abstinent alcoholics show attenuated DTI changes specific to parietal regions associated with visuospatial information processing and self-awareness problems (Monnig et al., 2013). Other reports show normalization of frontal white matter microstructure with just one month of abstinence in recovering alcoholics that do not smoke (Gazdzinski, Durazzo, Mon, Yeh, & Meyerhoff, 2010), and reversal of white matter damage in the corpus callosum with one year of abstinence (Alhassoon et al., 2012; Zorlu et al., 2014).
Functional Magnetic Resonance Imaging
Functional Magnetic Resonance Imaging (fMRI) is based on image contrast due to hemodynamic responses indirectly reflecting neuronal activity (Logothetis & Pfeuffer, 2004). Changes in cerebral blood flow are related to increased or decreased energy utilization by brain cells (Logothetis, Pauls, Augath, Trinath, & Oeltermann, 2001). The fMRI blood-oxygen-level-dependent (BOLD) signal has been used in alcoholics to explore functional brain activity related to cue reactivity, craving, impulsivity, self-control, and to determine baseline synchrony between brain areas at rest (i.e., resting-state fMRI).
The incentive salience theory of addiction describes how long-lasting changes in motivational neurocircuitry, induced by drug consumption, renders the brain-reward system sensitized to drug-associated stimuli (cues), essentially leading to cravings and relapse (Berridge & Robinson, 1998). fMRI studies demonstrate an alcohol cue-induced attentional bias in alcoholics compared to healthy controls (Schacht, Anton, & Myrick, 2013; Vollstadt-Klein et al., 2012). Indeed, several fMRI investigations have linked visual alcohol cues to a hemodynamic response in the brain regions related to the reward circuitry including the anterior cingulate cortex, medial prefrontal cortex, ventral striatum, and insula (Ihssen, Cox, Wiggett, Fadardi, & Linden, 2011; Myrick et al., 2008). Others studies have reported BOLD changes in brain areas of alcoholics linked to self-control, memory, and reflective thinking (Krienke et al., 2014), indicating significant changes in circuitry related to decision-making. Individuals who binge consume alcohol and are at risk for developing AUD display a heightened BOLD response in both the left and right nucleus accumbens to monetary reward cues in comparison to healthy controls (Crane et al., 2017). This finding suggests fundamental differences in reward processing that may increase the risk for AUD. A polymorphism in the tachykinin receptor 1 gene, which encodes a receptor for substance P (Koob, 2015), is associated with alcohol-cue induced craving-related increases in BOLD signaling in parts of the frontal cortex, caudate/putamen, insula, and lentiform nucleus in patients with AUD (Blaine, Claus, Harlaar, & Hutchison, 2013).
The stimulus-response habit-formation theory describes a transition from initial drug-consumption to compulsive drug-taking in the absence of conscious awareness (i.e., habit) (Everitt & Robbins, 2005). This is thought to occur via changes to striatonigral pathways, gradually transitioning from encoding the rewarding effects of the drug in the nucleus accumbens to encoding in the dorsal striatum (Belin & Everitt, 2008). When comparing light and heavy drinkers, cue-induced brain activation shifted from the ventral (light drinkers) to the dorsal striatum (heavy drinkers) (Vollstadt-Klein et al., 2010). Furthermore, alcoholics over-rely on habit learning rather than goal-directed actions, as demonstrated by increases in BOLD responses in the posterior putamen and decreases in the ventromedial prefrontal cortex during an instrumental learning task (Sjoerds et al., 2013).
In accordance with DSM-IV-r criteria describing devaluation of natural rewards in alcoholism, alcohol-dependent individuals in comparison with healthy controls show an increase in striatal BOLD responses to visual alcohol cues, together with a decrease to non-drug-related monetary cues (Wrase et al., 2007). Furthermore, reduced nucleus accumbens reactivity to non-drug rewards was shown to correlate with higher impulsivity scores (Beck et al., 2009). However, other types of cues (i.e., non-visual cues) have led to more variable results. For example, olfactory cues increased BOLD signaling in the nucleus accumbens in high-risk drinkers (Kareken et al., 2004). A later study from the same group, however, failed to find this hemodynamic response to the most preferred alcohol odor of non-dependent heavy drinkers with a family history of AUD (Kareken et al., 2010). Exposure to the taste of alcohol, however, elicits strong BOLD responses in the prefrontal cortex, striatum, and the ventral tegmental area/substantia nigra in alcoholics (Filbey et al., 2008).
The resting state default mode network describes a network of connected brain areas that are particularly active when an individual is not performing a task and is comprised of the ventral medial prefrontal cortex, cingulate/retrosplenial cortex, inferior parietal lobule, lateral temporal cortex, dorsal medial prefrontal cortex, and hippocampal formation (Buckner, Andrews-Hanna, & Schacter, 2008). AUD changes the resting state default-mode network from connectivity between posterior cingulate cortex and middle cingulate cortex (MCC) towards increased connectivity between midbrain and MCC. This reflects a shift from higher order structures to more cue-reactive circuits, indicating compromised executive control (Muller-Oehring, Jung, Pfefferbaum, Sullivan, & Schulte, 2015; Schulte, Muller-Oehring, Sullivan, & Pfefferbaum, 2012). Alcoholics relative to healthy controls have higher regional homogeneity (ReHo) values during resting state. ReHo describes the functional connectivity between a given activation cluster and its nearest neighboring activation cluster and thus can be understood as an index of network centrality (for a detailed technical review see, for a detailed technical review see, Jiang & Zuo, 2016). Hence the changes in ReHo of AUD individuals indicate that specific regional neuropopulations are more strongly connected during resting state; these include superior frontal gyrus, medial frontal gyrus, and middle temporal gyrus (Tu, Wang, Liu, & Zheng, 2018). Abstinent alcoholics who completed a detoxification program versus those that withdrew from treatment showed enhanced resting state functional connectivity between striatum and insular cortex, the executive network and the amygdala, and the salience network and the striatum, precuneus, and amygdala. This network hyper-connectivity was directly correlated to the individuals’ craving intensity (Kohno, Dennis, McCready, & Hoffman, 2017). Furthermore, AUD patients that abstained from alcohol for 3 weeks to 10 months had a less efficient connectivity between the left hippocampus and other task-activated networks when viewing emotional faces, while their pallidum was more efficiently connected when viewing alcoholic beverages. Hence longer sobriety may lead to adaptive neural changes shifting connectivity from systems encoding emotional salience towards drug reward, which in turn, may affect the individual’s cognitive control and ability to prevent relapse (Alba-Ferrara, Muller-Oehring, Sullivan, Pfefferbaum, & Schulte, 2016).
Positron Emission Tomography
Positron Emission Tomography (PET) is a non-MRI imaging modality that utilizes specific radiotracers (e.g. [18F]FDG, [11C]Glucose) to investigate brain glucose metabolism, neurotransmitter receptor availability, or neurotransmitter levels measured indirectly via competitive receptor binding-displacement (e.g. D2/D3 receptor ligand [11C]raclopride, μ-opioid receptor ligand [11C]carfentanil). The latter ligands more specifically show the difference in binding of the tracer to its target structure with and without intervention and can be used to estimate relative changes in extracellular levels of a specific neurotransmitter.
Relative to controls, AUD subjects show reduced cerebral glucose metabolism, pronounced in medial frontal and parietal cortical areas (Gilman et al., 1990; Wik et al., 1988). Acute alcohol intoxication also reduces brain glucose metabolism (Volkow et al., 1990; Volkow et al., 2006). It appears that brain energy metabolism during acute alcohol intoxication shifts to alternative sources: in an [11C]acetate PET study of AUD subjects and occasional social drinkers, glial cells preferentially utilized acetate (a byproduct of alcohol breakdown) over glucose (Volkow et al., 2013). In heavy alcohol users, baseline acetate uptake was more pronounced than in occasional social drinkers and correlated with the self-reported quantity of daily alcohol intake (Jiang et al., 2013; Volkow et al., 2013).
Alcohol, like most drugs of abuse, has been shown to profoundly increase dopamine release in the nucleus accumbens of rodents (Tomkins & Sellers, 2001). Yet, human PET studies exploring dopamine signaling in AUD are contradictory. For example, one study reported that intravenous infusions of alcohol caused a significant increase in dopamine (measured by competitive D2/D3 receptor binding via [11C]raclopride) in the right ventral striatum in non-treatment seeking alcoholics, but not in social drinkers (Yoder et al., 2016). By contrast, an alcohol-induced rise in ventral striatal and caudate extracellular dopamine was associated with pleasurable effects of alcohol in non-alcoholics (Aalto et al., 2015). Such differences may be accounted for by genetic polymorphisms: striatal dopamine levels are high in social drinkers who carry the A118G gene variant of the μ opioid receptor. Individuals with this point-mutation display risky alcohol consumption and are more likely to develop AUD (Ramchandani et al., 2011).
Dopaminergic responses alone are unlikely to fully account for the pleasure and reward experienced following acute alcohol consumption. Endogenous opioid levels rapidly increase in the nucleus accumbens and orbitofrontal cortex upon alcohol consumption, as demonstrated using PET imaging of [11C]carfentanil (Mitchell et al., 2012). Moreover, increased μ-opioid receptor availability in the ventral striatum of detoxified alcoholics correlates with self-reports of craving intensity (Heinz et al., 2005). These findings were supported in a separate study using the μ-opioid receptor tracer [11C]diprenorphine (Williams et al., 2009).
One of the most consistently reported findings in long-term alcoholics is a reduction of dopamine D2/D3 receptors throughout the brain (for a detailed review see for a detailed review see Volkow et al., 2017). Loss of inhibitory dopaminergic receptors could explain some of the behavioral features displayed by alcoholics, such as lack of impulse inhibition and compulsive behavioral patterns (Volkow, Koob, & McLellan, 2016). However, it remains controversial if such changes persist after long-term abstinence. Whereas Volkow and colleagues demonstrated a persistent reduction of striatal D2/D3 receptors for 4 months after abstinence (Volkow et al., 2002), Rominger and colleagues showed significant upregulation of D2/D3 receptors in a subpopulation of alcoholics with 1 year of abstinence (Rominger et al., 2012).
From mice to men – translational imaging findings
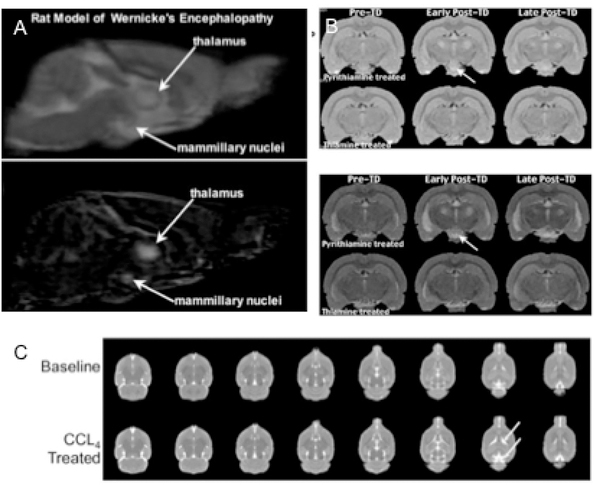
Preclinical models of alcohol-related neurological syndromes include studies on on WE/KS and HE. A thiamine-deficient diet over 3–4 weeks can reduce thiamine, but administration of a thiamine pyrophosphokinase inhibitor such as pyrithiamine is often required to recapitulate human symptoms in animals (Hazell & Butterworth, 2009). Structural MRI findings in thiamine-deficient rats show hyperintense signals on T2-weighted images in thalamus, collicular bodies, mammillary bodies, corpus callosum, and cerebellar peduncles (Figure 2A;B) (Dror et al., 2010; Pfefferbaum, Adalsteinsson, Bell, & Sullivan, 2007; Zahr, Alt, et al., 2014). Current rodent models of HE are deemed unsatisfactory by the International Society for Hepatic Encephalopathy (Butterworth et al., 2009). Nevetheless, a study using carbon tetrachloride (CCl4) as an agent to induce acute liver failure in rodents revealed brain morphological changes in lateral ventricles (top arrow) and cisterns (Figure 2C) (Zahr, Sullivan, et al., 2016).
Figure 2:
(A) Morphological changes in a preclinical rat model of Wernicke’s encephalopathy and (B) Korsakoff syndrome (reprint with permission from (Pfefferbaum, Adalsteinsson, et al., 2007)). (C) MRI scans of a CCL4 treatment based preclinical animal model for hepathic encephalopathy (reprint with permission from (Zahr, Rohlfing, et al., 2016)).
Because the taste of alcohol is aversive to most animals, heterogeneous stock rats for example, consume only modest amounts of ethanol and blood alcohol levels (BALs) achieved are minimal. One of the criteria for a successful animal model of alcoholism is the demonstration of physical dependence per se must be unequivocally attributed to the chronic administration and subsequent removal of ethanol. Common models to induce physical dependence to alcohol (cf., Mello, 1973) include exposure to ethanol via drinking (e.g., Xu et al., 2018), intraperitoneal (i.p.) (e.g., Liew et al., 2016) or intragastric (i.g.) (e.g., Luo, Shen, Chen, Wang, & Yu, 2017; Zahr, Rohlfing, et al., 2016) injections, and vapor chambers (e.g., Frischknecht et al., 2017). Typically i.p. and i.g. methods are used for acute and binge like protocols, while free drinking and vapor chambers are used for more chronic exposure protocols (Bell et al., 2017). While vapor chambers are the preferred method for sustained high BALs, in addition to inhalation, animals are exposed to ethanol via skin absorption. Problems with drinking protocols include a transitory alcohol preference in the absence of withdrawal signs and symptoms upon cessation of drinking. Finally, these methods include an integral stress factor, which, however, some have argued provides face validity (al’Absi, Carr, & Bongard, 2007). Fundamentally, the relative value of a behavioral (self-administration) or pharmacological (forced administration) model is dependent upon the type of questions that are asked.
One of the most consistent, translational findings made with structural MRI in alcohol-exposed rodents is ventricular enlargement, which has been found to be influenced by both timing and method of alcohol exposure. Rats that achieve binge-like BALs via gavage feeding show a larger effect (Zahr et al., 2010; Zahr, Mayer, Rohlfing, Hsu, et al., 2014; Zahr et al., 2013) than rats exposed to ethanol chronically via vapor (Pfefferbaum et al., 2008). The effect on ventricle size is transient in the binge models as ventricular volume recovers rapidly within one week of abstinence, contrary to the findings from human research (Sullivan & Pfefferbaum, 2009; Zahr & Pfefferbaum, 2017; Zahr, Rohlfing, et al., 2016). Still other studies have found that rats exposed to ethanol vapor during adolescence show persistent ventricular increases and deficits in hippocampal volume into adulthood (Ehlers, Liu, Wills, & Crews, 2013; Gass et al., 2014), while mice exposed to alcohol during adolescence (postnatal day 28 to 37) develop persisting brain-volume deficits (as measured on postnatal day 60) in the olfactory bulb and basal forebrain (Coleman, He, Lee, Styner, & Crews, 2011; Coleman, Liu, Oguz, Styner, & Crews, 2014).
MRS has been used to investigate the rodent brain following ethanol exposure via acute binge, repeated binge, and chronic exposure via vapor chamber. In these studies, NAA is lower and Cho is higher in animals following ethanol exposure (Zahr et al., 2010; Zahr, Mayer, Rohlfing, Hsu, et al., 2014; Zahr et al., 2013). Repeated cycles of binge intoxication also resulted in transient decreases of NAA and increases of Cho during intoxication, but recovery during abstinence (Zahr, Rohlfing, et al., 2016). In a chronic alcohol exposure study in rats, NAA levels were lower in the ethanol-exposed than in the control group, but failed to reach significance while Cho levels demonstrated a dose-response curve (i.e., increasing levels with higher and longer alcohol exposure) (Zahr et al., 2009). Normalization of NAA and Cho levels upon discontinuation of alcohol exposure seems to be translatable and indicates that some metabolic changes are directly linked to the toxicity of alcohol (Bartsch et al., 2007).
While alcohol-induced reductions in NAA has been consistently found in both human and rodent studies, Cho results have varied. Typically, below normal levels of Cho are observed in alcoholics in early abstinence (within 5 weeks), with significant increases at 3 and 6 months of sobriety (Ende et al., 2005). In chronic alcoholism, however, Cho levels may be affected by confounding factors such as hepatitis C infection (Zahr, Mayer, Rohlfing, Sullivan, et al., 2014). Social drinkers show significant relations between higher levels of frontal Cho and greater alcohol consumption in the past 2 weeks (Tunc-Skarka et al., 2015) to 3 months (Ende et al., 2006). Similarly, rats show high Cho upon acute intoxication (Zahr et al., 2010; Zahr, Mayer, Rohlfing, Hsu, et al., 2014; Zahr et al., 2013).
The decrease in glucose metabolism reported with [18F]-FDG PET in long-term alcoholics is similar to decreases observed during an acute alcohol challenge in naïve rats. However, these changes do not persist when the animals consume alcohol for 3 to 12 months (Gispert et al., 2017). This finding is an indication that rodents tend to metabolize alcohol in a different manner than do human beings.
DTI investigations of acute alcohol intoxication in rats showed detectable but transient FA changes in the frontal lobe (Chen, Zeng, Shen, Kong, & Zheng, 2017). Indeed, transient FA changes upon acute alcohol intoxication are similar between rodents and human beings (Kong, Zheng, Lian, & Zhang, 2012). While binge alcohol exposure resulted in transient decreases in both FA and MD, chronic exposure to vaporized ethanol did quantifiably affect these measures (Pfefferbaum, Zahr, Mayer, Rohlfing, & Sullivan, 2015). In another chronic study, intermittent alcohol exposure showed no effects on FA, but did show reduced AD (hippocampus, cortex, and cerebellum), reduced RD (hippocampus and cortex), and reduced MD (cerebellum and corpus callosum) (Vetreno, Yaxley, Paniagua, & Crews, 2016). In general, the effects of chronic alcoholism on FA and MD described in the human literature (e.g., e.g., Bagga, Sharma, et al., 2014; Chanraud et al., 2009; Maksimovskiy et al., 2014; Pfefferbaum et al., 2000; Yeh et al., 2009) have not been replicated in the preclinical literature.
Preclinical resting-state and functional MRI investigations are relatively scarce in the context of AUD. One study reports that 30 days of alcohol consumption affects 9 resting-state networks in rats: 8 showed a decrease in functional connectivity, while the striatal-prefrontal circuit became hyper-connected (Perez-Ramirez, Diaz-Parra, Ciccocioppo, Canals, & Moratal, 2017). This finding parallels those in AUD, where circuitry responsible for cue-reactivity becomes dominant (Kohno et al., 2017; Schulte et al., 2012). Rats given alcohol via oral gavage during adolescence showed, in adulthood, decreased baseline resting-state connectivity among prefrontal cortical sub-regions (prelimbic-infralimbic cortex and infralimbic-orbitofrontal cortex) and decreased connectivity between PFC and striatal regions (prelimbic-nucleus accumbens, infralimbic-caudate putamen, infralimbic-nucleus accumbens, orbitofrontal cortex-caudate putamen, and orbitofrontal cortex-nucleus accumbens) (Broadwater et al., 2018).
Collectively, preclinical imaging studies provide insight into specific time points of a complex and cyclical disease. Preclinical imaging has the potential to provide greater understanding of the dynamics of alcohol-related effects on the structure, metabolism, and function of the brain.
Concluding remarks
Studying diseases concomitant with alcoholism has provided insight into mechanisms and brain targets of uncomplicated alcoholism. Diseases such as WE/KS can be approximately modeled in animals, while HE, CPM, and alcoholism per se can be challenging to model in rodents. In reviewing the neuroimaging literature related in AUD, some challenges become immediately apparent. The dynamic nature of the disease makes it difficult to capture individuals with AUD at the same time point of the disease course and humans tend to have complex histories with a variety of confounders that may contribute to brain changes. While longitudinal studies now help tackle some of these limitations, preclinical studies are also critical to help isolate and describe changes at distinct time points of disease progression. Indeed, the study of both humans and animal models is valuable to allow for the a better understanding of AUD. A critical and comprehensive assessment of humans with DSM-5 defined alcoholism is essential to describing the chronic effects of alcohol on the brain. Animal models, although imperfect, allow for a deeper investigation of the mechanism underlying the brain changes characterized in AUD. Alcoholism is known to reduce red blood cell count (Whitehead, Robinson, Allaway, & Hale, 1995); on admission to alcoholism treatment, those with lower red blood cell counts had larger ventricles (Pfefferbaum, Rosenbloom, Serventi, & Sullivan, 2004). Whether this relationship is causal, for example, could be asked in animals by reducing red blood count using hydroxyurea and determining whether there is an associated ventricular enlargement.
The growing number of studies indicating that alcoholism-induced brain changes can be reversed by prolonged abstinence is a hopeful message to those struggling with AUD.
Significance Statement.
Acute alcohol impairs brain function while chronic alcoholism can cause brain damage: evidence for profound brain volume recovery with abstinence speaks against the urban myth that drinking alcohol kills brain cells. Mechanisms of brain injury due to alcoholism may be better understood by studying common disease concomitants of alcoholism. Genetics may determine the type of injury incurred (e.g., nutritional deficiency versus liver damage). Animal models, while imperfect, have contributed to a better understanding of the effects of chronic alcohol consumption on the brain. Recent efforts in the field are focused on interventions that promote sobriety.
Acknowledgments
This manuscript was developed and written with the support of National Institute of Alcohol Abuse and Alcoholism (NIAAA) grants K05 AA017168, R37 AA010723, R01 AA005965, and U01 AA013521.
Grant information: AA010723, AA005965, AA013521
Footnotes
Conflict of Interest
The authors have no competing interests to disclose.
References
- Aalto S, Ingman K, Alakurtti K, Kaasinen V, Virkkala J, Nagren K, … Scheinin H (2015). Intravenous ethanol increases dopamine release in the ventral striatum in humans: PET study using bolus-plus-infusion administration of [(11)C]raclopride. J Cereb Blood Flow Metab, 35(3), 424–431. doi: 10.1038/jcbfm.2014.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahluwalia V, Wade JB, Moeller FG, White MB, Unser AB, Gavis EA, … Bajaj JS (2015). The etiology of cirrhosis is a strong determinant of brain reserve: A multimodal magnetic resonance imaging study. Liver Transpl, 21(9), 1123–1132. doi: 10.1002/lt.24163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- al’Absi M, Carr SB, & Bongard S (2007). Anger and psychobiological changes during smoking abstinence and in response to acute stress: prediction of smoking relapse. Int J Psychophysiol, 66(2), 109–115. doi: 10.1016/j.ijpsycho.2007.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alba-Ferrara L, Muller-Oehring EM, Sullivan EV, Pfefferbaum A, & Schulte T (2016). Brain responses to emotional salience and reward in alcohol use disorder. Brain Imaging Behav, 10(1), 136–146. doi: 10.1007/s11682-015-9374-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhassoon OM, Sorg SF, Taylor MJ, Stephan RA, Schweinsburg BC, Stricker NH, … Grant I (2012). Callosal white matter microstructural recovery in abstinent alcoholics: a longitudinal diffusion tensor imaging study. Alcohol Clin Exp Res, 36(11), 1922–1931. doi: 10.1111/j.15300277.2012.01808.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alterman AI, & Tarter RE (1985). Relationship between familial alcoholism and head injury. J Stud Alcohol, 46(3), 256–258. [DOI] [PubMed] [Google Scholar]
- Antunez E, Estruch R, Cardenal C, Nicolas JM, Fernandez-Sola J, & Urbano-Marquez A (1998). Usefulness of CT and MR imaging in the diagnosis of acute Wernicke’s encephalopathy. AJR Am J Roentgenol, 171(4), 1131–1137. doi: 10.2214/ajr.171.4.9763009 [DOI] [PubMed] [Google Scholar]
- Arbelaez A, Pajon A, & Castillo M (2003). Acute Marchiafava-Bignami disease: MR findings in two patients. AJNR Am J Neuroradiol, 24(10), 1955–1957. [PMC free article] [PubMed] [Google Scholar]
- Bagga D, Khushu S, Modi S, Kaur P, Bhattacharya D, Garg ML, & Singh N (2014). Impaired visual information processing in alcohol-dependent subjects: a proton magnetic resonance spectroscopy study of the primary visual cortex. J Stud Alcohol Drugs, 75(5), 817–826. [DOI] [PubMed] [Google Scholar]
- Bagga D, Sharma A, Kumari A, Kaur P, Bhattacharya D, Garg ML, … Singh N (2014). Decreased white matter integrity in fronto-occipital fasciculus bundles: relation to visual information processing in alcohol-dependent subjects. Alcohol, 48(1), 43–53. doi: 10.1016/j.alcohol.2013.10.009 [DOI] [PubMed] [Google Scholar]
- Bartsch AJ, Homola G, Biller A, Smith SM, Weijers HG, Wiesbeck GA, … Bendszus M (2007). Manifestations of early brain recovery associated with abstinence from alcoholism. Brain, 130(Pt 1), 36–47. doi: 10.1093/brain/awl303 [DOI] [PubMed] [Google Scholar]
- Bauer J, Pedersen A, Scherbaum N, Bening J, Patschke J, Kugel H, … Ohrmann P (2013). Craving in alcohol-dependent patients after detoxification is related to glutamatergic dysfunction in the nucleus accumbens and the anterior cingulate cortex. Neuropsychopharmacology, 38(8), 14011408. doi: 10.1038/npp.2013.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C (2002). The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed, 15(7–8), 435–455. doi: 10.1002/nbm.782 [DOI] [PubMed] [Google Scholar]
- Beck A, Schlagenhauf F, Wustenberg T, Hein J, Kienast T, Kahnt T, … Wrase J (2009). Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biol Psychiatry, 66(8), 734–742. doi: 10.1016/j.biopsych.2009.04.035 [DOI] [PubMed] [Google Scholar]
- Becker HC (1998). Kindling in alcohol withdrawal. Alcohol Health Res World, 22(1), 25–33. [PMC free article] [PubMed] [Google Scholar]
- Belin D, & Everitt BJ (2008). Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron, 57(3), 432–441. doi: 10.1016/j.neuron.2007.12.019 [DOI] [PubMed] [Google Scholar]
- Bell RL, Hauser SR, Liang T, Sari Y, Maldonado-Devincci A, & Rodd ZA (2017). Rat animal models for screening medications to treat alcohol use disorders. Neuropharmacology, 122, 201243. doi: 10.1016/j.neuropharm.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendszus M, Weijers HG, Wiesbeck G, Warmuth-Metz M, Bartsch AJ, Engels S, … Solymosi L (2001). Sequential MR imaging and proton MR spectroscopy in patients who underwent recent detoxification for chronic alcoholism: correlation with clinical and neuropsychological data. AJNR Am J Neuroradiol, 22(10), 1926–1932. [PMC free article] [PubMed] [Google Scholar]
- Berger JR, & Singhal D (2014). The neurologic complications of bariatric surgery. Handb Clin Neurol, 120, 587–594. doi: 10.1016/B978-0-7020-4087-0.00039-5 [DOI] [PubMed] [Google Scholar]
- Berridge KC, & Robinson TE (1998). What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev, 28(3), 309–369. [DOI] [PubMed] [Google Scholar]
- Binesh N, Huda A, Thomas MA, Wyckoff N, Bugbee M, Han S, … Fawzy F (2006). Hepatic encephalopathy: a neurochemical, neuroanatomical, and neuropsychological study. J Appl Clin Med Phys, 7(1), 86–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaine S, Claus E, Harlaar N, & Hutchison K (2013). TACR1 genotypes predict fMRI response to alcohol cues and level of alcohol dependence. Alcohol Clin Exp Res, 37 Suppl 1, E125–130. doi: 10.1111/j.1530-0277.2012.01923.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutte D, Calhoun VD, Chen J, Sabbineni A, Hutchison K, & Liu J (2012). Association of genetic copy number variations at 11 q14.2 with brain regional volume differences in an alcohol use disorder population. Alcohol, 46(6), 519–527. doi: 10.1016/j.alcohol.2012.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwater MA, Lee SH, Yu Y, Zhu H, Crews FT, Robinson DL, & Shih YI (2018). Adolescent alcohol exposure decreases frontostriatal resting-state functional connectivity in adulthood. Addict Biol, 23(2), 810–823. doi: 10.1111/adb.12530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brousse G, Arnaud B, Vorspan F, Richard D, Dissard A, Dubois M, … Schmidt J (2012). Alteration of glutamate/GABA balance during acute alcohol withdrawal in emergency department: a prospective analysis. Alcohol Alcohol, 47(5), 501–508. doi: 10.1093/alcalc/ags078 [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, & Schacter DL (2008). The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci, 1124, 1–38. doi: 10.1196/annals.1440.011 [DOI] [PubMed] [Google Scholar]
- Butterworth RF, Norenberg MD, Felipo V, Ferenci P, Albrecht J, Blei AT, & Members of the, I. C. o. E. M. o. H. E. (2009). Experimental models of hepatic encephalopathy: ISHEN guidelines. Liver Int, 29(6), 783–788. doi: 10.1111/j.1478-3231.2009.02034.x [DOI] [PubMed] [Google Scholar]
- Carboni S, Isola R, Gessa GL, & Rossetti ZL (1993). Ethanol prevents the glutamate release induced by N-methyl-D-aspartate in the rat striatum. Neurosci Lett, 152(1–2), 133–136. [DOI] [PubMed] [Google Scholar]
- Cardenas VA, Studholme C, Gazdzinski S, Durazzo TC, & Meyerhoff DJ (2007). Deformation-based morphometry of brain changes in alcohol dependence and abstinence. Neuroimage, 34(3), 879–887. doi: 10.1016/j.neuroimage.2006.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler LJ, Newsom H, Sumners C, & Crews F (1993). Chronic ethanol exposure potentiates NMDA excitotoxicity in cerebral cortical neurons. J Neurochem, 60(4), 1578–1581. [DOI] [PubMed] [Google Scholar]
- Chanraud S, Reynaud M, Wessa M, Penttila J, Kostogianni N, Cachia A, … Martinot JL (2009). Diffusion tensor tractography in mesencephalic bundles: relation to mental flexibility in detoxified alcohol-dependent subjects. Neuropsychopharmacology, 34(5), 1223–1232. doi: 10.1038/npp.2008.101 [DOI] [PubMed] [Google Scholar]
- Chavarria L, Alonso J, Garcia-Martinez R, Simon-Talero M, Ventura-Cots M, Ramirez C, … Cordoba J (2013). Brain magnetic resonance spectroscopy in episodic hepatic encephalopathy. J Cereb Blood Flow Metab, 33(2), 272–277. doi: 10.1038/jcbfm.2012.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XR, Zeng JY, Shen ZW, Kong LM, & Zheng WB (2017). Diffusion Kurtosis Imaging Detects Microstructural Changes in the Brain after Acute Alcohol Intoxication in Rats. Biomed Res Int, 2017, 4757025. doi: 10.1155/2017/4757025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Kellar D, Lake A, Finn P, Rebec GV, Dharmadhikari S, … Newman S (2018). Effects of Alcohol Cues on MRS Glutamate Levels in the Anterior Cingulate. Alcohol Alcohol, 53(3), 209215. doi: 10.1093/alcalc/agx119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi C, Coupland NJ, Bhardwaj PP, Malykhin N, Gheorghiu D, & Allen PS (2006). Measurement of brain glutamate and glutamine by spectrally-selective refocusing at 3 Tesla. Magn Reson Med, 55(5), 997–1005. doi: 10.1002/mrm.20875 [DOI] [PubMed] [Google Scholar]
- Chung SP, Kim SW, Yoo IS, Lim YS, & Lee G (2003). Magnetic resonance imaging as a diagnostic adjunct to Wernicke encephalopathy in the ED. Am J Emerg Med, 21(6), 497–502. [DOI] [PubMed] [Google Scholar]
- Coleman LG Jr., He J, Lee J, Styner M, & Crews FT (2011). Adolescent binge drinking alters adult brain neurotransmitter gene expression, behavior, brain regional volumes, and neurochemistry in mice. Alcohol Clin Exp Res, 35(4), 671–688. doi: 10.1111/j.1530-0277.2010.01385.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman LG Jr., Liu W, Oguz I, Styner M, & Crews FT (2014). Adolescent binge ethanol treatment alters adult brain regional volumes, cortical extracellular matrix protein and behavioral flexibility. Pharmacol Biochem Behav, 116, 142–151. doi: 10.1016/j.pbb.2013.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor JP, Haber PS, & Hall WD (2016). Alcohol use disorders. Lancet, 387(10022), 988–998. doi: 10.1016/S0140-6736(15)00122-1 [DOI] [PubMed] [Google Scholar]
- Cordoba J, Alonso J, Rovira A, Jacas C, Sanpedro F, Castells L, … Guardia J (2001). The development of low-grade cerebral edema in cirrhosis is supported by the evolution of (1)Hmagnetic resonance abnormalities after liver transplantation. J Hepatol, 35(5), 598–604. [DOI] [PubMed] [Google Scholar]
- Cordoba J, Sanpedro F, Alonso J, & Rovira A (2002). 1H magnetic resonance in the study of hepatic encephalopathy in humans. Metab Brain Dis, 17(4), 415–429. [DOI] [PubMed] [Google Scholar]
- Crane NA, Gorka SM, Weafer J, Langenecker SA, de Wit H, & Phan KL (2017). Preliminary Evidence for Disrupted Nucleus Accumbens Reactivity and Connectivity to Reward in Binge Drinkers. Alcohol Alcohol, 52(6), 647–654. doi: 10.1093/alcalc/agx062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criswell HE, Ming Z, Griffith BL, & Breese GR (2003). Comparison of effect of ethanol on Nmethyl-D-aspartate- and GABA-gated currents from acutely dissociated neurons: absence of regional differences in sensitivity to ethanol. J Pharmacol Exp Ther, 304(1), 192–199. doi: 10.1124/jpet.102.041590 [DOI] [PubMed] [Google Scholar]
- Dahchour A, & De Witte P (1999). Effect of repeated ethanol withdrawal on glutamate microdialysate in the hippocampus. Alcohol Clin Exp Res, 23(10), 1698–1703. [DOI] [PubMed] [Google Scholar]
- de los Rios F, Kleindorfer DO, Khoury J, Broderick JP, Moomaw CJ, Adeoye O, … Kissela BM (2012). Trends in substance abuse preceding stroke among young adults: a population-based study. Stroke, 43(12), 3179–3183. doi: 10.1161/STROKEAHA.112.667808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirakca T, Ende G, Kammerer N, Welzel-Marquez H, Hermann D, Heinz A, & Mann K (2011). Effects of alcoholism and continued abstinence on brain volumes in both genders. Alcohol Clin Exp Res, 35(9), 1678–1685. doi: 10.1111/j.1530-0277.2011.01514.x [DOI] [PubMed] [Google Scholar]
- DeWitt LD, Buonanno FS, Kistler JP, Zeffiro T, DeLaPaz RL, Brady TJ, … Pykett IL (1984). Central pontine myelinolysis: demonstration by nuclear magnetic resonance. Neurology, 34(5), 570–576. [DOI] [PubMed] [Google Scholar]
- Dildy JE, & Leslie SW (1989). Ethanol inhibits NMDA-induced increases in free intracellular Ca2+ in dissociated brain cells. Brain Res, 499(2), 383–387. [DOI] [PubMed] [Google Scholar]
- Dror V, Eliash S, Rehavi M, Assaf Y, Biton IE, & Fattal-Valevski A (2010). Neurodegeneration in thiamine deficient rats-A longitudinal MRI study. Brain Res, 1308, 176–184. doi: 10.1016/j.brainres.2009.10.032 [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Rothlind JC, Banys P, & Meyerhoff DJ (2006). Brain metabolite concentrations and neurocognition during short-term recovery from alcohol dependence: Preliminary evidence of the effects of concurrent chronic cigarette smoking. Alcohol Clin Exp Res, 30(3), 539–551. doi: 10.1111/j.1530-0277.2006.00060.x [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Tosun D, Buckley S, Gazdzinski S, Mon A, Fryer SL, & Meyerhoff DJ (2011). Cortical thickness, surface area, and volume of the brain reward system in alcohol dependence: relationships to relapse and extended abstinence. Alcohol Clin Exp Res, 35(6), 1187–1200. doi: 10.1111/j.1530-0277.2011.01452.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Liu W, Wills DN, & Crews FT (2013). Periadolescent ethanol vapor exposure persistently reduces measures of hippocampal neurogenesis that are associated with behavioral outcomes in adulthood. Neuroscience, 244, 1–15. doi: 10.1016/j.neuroscience.2013.03.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ende G, Hermann D, Demirakca T, Hoerst M, Tunc-Skarka N, Weber-Fahr W, … Vollstadt-Klein S (2013). Loss of control of alcohol use and severity of alcohol dependence in non-treatment-seeking heavy drinkers are related to lower glutamate in frontal white matter. Alcohol Clin Exp Res, 37(10), 1643–1649. doi: 10.1111/acer.12149 [DOI] [PubMed] [Google Scholar]
- Ende G, Walter S, Welzel H, Demirakca T, Wokrina T, Ruf M, … Mann K (2006). Alcohol consumption significantly influences the MR signal of frontal choline-containing compounds. Neuroimage, 32(2), 740–746. doi: 10.1016/j.neuroimage.2006.03.049 [DOI] [PubMed] [Google Scholar]
- Ende G, Welzel H, Walter S, Weber-Fahr W, Diehl A, Hermann D, … Mann K (2005). Monitoring the effects of chronic alcohol consumption and abstinence on brain metabolism: a longitudinal proton magnetic resonance spectroscopy study. Biol Psychiatry, 58(12), 974–980. doi: 10.1016/j.biopsych.2005.05.038 [DOI] [PubMed] [Google Scholar]
- Estruch R, Nicolas JM, Salamero M, Aragon C, Sacanella E, Fernandez-Sola J, & Urbano-Marquez A (1997). Atrophy of the corpus callosum in chronic alcoholism. J Neurol Sci, 146(2), 145–151. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, & Robbins TW (2005). Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci, 8(11), 1481–1489. doi: 10.1038/nn1579 [DOI] [PubMed] [Google Scholar]
- Eyer F, Schuster T, Felgenhauer N, Pfab R, Strubel T, Saugel B, & Zilker T (2011). Risk assessment of moderate to severe alcohol withdrawal--predictors for seizures and delirium tremens in the course of withdrawal. Alcohol Alcohol, 46(4), 427–433. doi: 10.1093/alcalc/agr053 [DOI] [PubMed] [Google Scholar]
- Fein G, Landman B, Tran H, McGillivray S, Finn P, Barakos J, & Moon K (2006). Brain atrophy in long-term abstinent alcoholics who demonstrate impairment on a simulated gambling task. Neuroimage, 32(3), 1465–1471. doi: 10.1016/j.neuroimage.2006.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Ray L, Smolen A, Claus ED, Audette A, & Hutchison KE (2008). Differential neural response to alcohol priming and alcohol taste cues is associated with DRD4 VNTR and OPRM1 genotypes. Alcohol Clin Exp Res, 32(7), 1113–1123. doi: 10.1111/j.1530-0277.2008.00692.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliegel S, Brand I, Spanagel R, & Noori HR (2013). Ethanol-induced alterations of amino acids measured by in vivo microdialysis in rats: a meta-analysis. In Silico Pharmacol, 1, 7. doi: 10.1186/2193-9616-1-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier CB, Leritz EC, Salat DH, Lindemer E, Maksimovskiy AL, Shepel J, … McGlinchey RE (2014). Widespread effects of alcohol on white matter microstructure. Alcohol Clin Exp Res, 38(12), 2925–2933. doi: 10.1111/acer.12568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund G, & Anderson KJ (1996). Glutamate receptors in the frontal cortex of alcoholics. Alcohol Clin Exp Res, 20(7), 1165–1172. [DOI] [PubMed] [Google Scholar]
- Frischknecht U, Hermann D, Tunc-Skarka N, Wang GY, Sack M, van Eijk J, … Weber-Fahr W (2017). Negative Association Between MR-Spectroscopic Glutamate Markers and Gray Matter Volume After Alcohol Withdrawal in the Hippocampus: A Translational Study in Humans and Rats. Alcohol Clin Exp Res, 41(2), 323–333. doi: 10.1111/acer.13308 [DOI] [PubMed] [Google Scholar]
- Gass JT, Glen WB Jr., McGonigal JT, Trantham-Davidson H, Lopez MF, Randall PK, … Chandler LJ (2014). Adolescent alcohol exposure reduces behavioral flexibility, promotes disinhibition, and increases resistance to extinction of ethanol self-administration in adulthood. Neuropsychopharmacology, 39(11), 2570–2583. doi: 10.1038/npp.2014.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Mon A, Yeh PH, & Meyerhoff DJ (2010). Cerebral white matter recovery in abstinent alcoholics--a multimodality magnetic resonance study. Brain, 133(Pt 4), 1043–1053. doi: 10.1093/brain/awp343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissler A, Lock G, Frund R, Held P, Hollerbach S, Andus T, … Holstege A (1997). Cerebral abnormalities in patients with cirrhosis detected by proton magnetic resonance spectroscopy and magnetic resonance imaging. Hepatology, 25(1), 48–54. [DOI] [PubMed] [Google Scholar]
- Gilman S, Adams K, Koeppe RA, Berent S, Kluin KJ, Modell JG, … Brunberg JA (1990). Cerebellar and frontal hypometabolism in alcoholic cerebellar degeneration studied with positron emission tomography. Ann Neurol, 28(6), 775–785. doi: 10.1002/ana.410280608 [DOI] [PubMed] [Google Scholar]
- Gispert JD, Figueiras FP, Vengeliene V, Herance JR, Rojas S, & Spanagel R (2017). Changes in cerebral [(18)F]-FDG uptake induced by acute alcohol administration in a rat model of alcoholism. Behav Brain Res, 327, 29–33. doi: 10.1016/j.bbr.2017.03.038 [DOI] [PubMed] [Google Scholar]
- Gonzales R, Bungay PM, Kilanmaa K, Samson HH, & Rosselti ZL (1996). In vivo links between neurochemistry and behavioral effects of ethanol. Alcohol Clin Exp Res, 20(8 Suppl), 203A–209A. [DOI] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, … Hasin DS (2015). Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry, 72(8), 757–766. doi: 10.1001/jamapsychiatry.2015.0584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RK, Saraswat VA, Poptani H, Dhiman RK, Kohli A, Gujral RB, & Naik SR (1993). Magnetic resonance imaging and localized in vivo proton spectroscopy in patients with fulminant hepatic failure. Am J Gastroenterol, 88(5), 670–674. [PubMed] [Google Scholar]
- Ha ND, Weon YC, Jang JC, Kang BS, & Choi SH (2012). Spectrum of MR imaging findings in Wernicke encephalopathy: are atypical areas of involvement only present in nonalcoholic patients? AJNR Am J Neuroradiol, 33(7), 1398–1402. doi: 10.3174/ajnr.A2979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GJ, Jaffin SK, Hodge SM, Kennedy D, Caviness VS, Marinkovic K, … Oscar-Berman M (2008). Frontal white matter and cingulum diffusion tensor imaging deficits in alcoholism. Alcohol Clin Exp Res, 32(6), 1001–1013. doi: 10.1111/j.1530-0277.2008.00661.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häussinger D, Laubenberger J, Dahl SV, Ernst T, Bayer S, Langer M, … Hennig J (1994). Proton magnetic resonance spectroscopy studies on human brain myo-inositol in hypo-osmolarity and hepatic encephalopathy. Gastroenterology, 107(5), 1475–1480. [DOI] [PubMed] [Google Scholar]
- Hazell AS, & Butterworth RF (2009). Update of cell damage mechanisms in thiamine deficiency: focus on oxidative stress, excitotoxicity and inflammation. Alcohol Alcohol, 44(2), 141–147. [DOI] [PubMed] [Google Scholar]
- Heinz A, Reimold M, Wrase J, Hermann D, Croissant B, Mundle G, … Mann K (2005). Correlation of stable elevations in striatal mu-opioid receptor availability in detoxified alcoholic patients with alcohol craving: a positron emission tomography study using carbon 11-labeled carfentanil. Arch Gen Psychiatry, 62(1), 57–64. doi: 10.1001/archpsyc.62.1.57 [DOI] [PubMed] [Google Scholar]
- Henderson KE, Vaidya JG, Kramer JR, Kuperman S, Langbehn DR, & O’Leary DS (2018). Cortical Thickness in Adolescents with a Family History of Alcohol Use Disorder. Alcohol Clin Exp Res, 42(1), 89–99. doi: 10.1111/acer.13543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann D, Weber-Fahr W, Sartorius A, Hoerst M, Frischknecht U, Tunc-Skarka N, … Sommer WH (2012). Translational magnetic resonance spectroscopy reveals excessive central glutamate levels during alcohol withdrawal in humans and rats. Biol Psychiatry, 71(11), 10151021. doi: 10.1016/j.biopsych.2011.07.034 [DOI] [PubMed] [Google Scholar]
- Hillbom M, Saloheimo P, Fujioka S, Wszolek ZK, Juvela S, & Leone MA (2014). Diagnosis and management of Marchiafava-Bignami disease: a review of CT/MRI confirmed cases. J Neurol Neurosurg Psychiatry, 85(2), 168–173. doi: 10.1136/jnnp-2013-305979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman PL, Rabe CS, Moses F, & Tabakoff B (1989). N-methyl-D-aspartate receptors and ethanol: inhibition of calcium flux and cyclic GMP production. J Neurochem, 52(6), 1937–1940. [DOI] [PubMed] [Google Scholar]
- Holla B, Bharath RD, Venkatasubramanian G, & Benegal V (2018). Altered brain cortical maturation is found in adolescents with a family history of alcoholism. Addict Biol. doi: 10.1111/adb.12662 [DOI] [PubMed] [Google Scholar]
- Hu XJ, Follesa P, & Ticku MK (1996). Chronic ethanol treatment produces a selective upregulation of the NMDA receptor subunit gene expression in mammalian cultured cortical neurons. Brain Res Mol Brain Res, 36(2), 211–218. [DOI] [PubMed] [Google Scholar]
- Ihssen N, Cox WM, Wiggett A, Fadardi JS, & Linden DE (2011). Differentiating heavy from light drinkers by neural responses to visual alcohol cues and other motivational stimuli. Cereb Cortex, 21(6), 1408–1415. doi: 10.1093/cercor/bhq220 [DOI] [PubMed] [Google Scholar]
- Iorio KR, Tabakoff B, & Hoffman PL (1993). Glutamate-induced neurotoxicity is increased in cerebellar granule cells exposed chronically to ethanol. Eur J Pharmacol, 248(2), 209–212. [DOI] [PubMed] [Google Scholar]
- Jagannathan NR, Desai NG, & Raghunathan P (1996). Brain metabolite changes in alcoholism: an in vivo proton magnetic resonance spectroscopy (MRS) study. Magn Reson Imaging, 14(5), 553557. [DOI] [PubMed] [Google Scholar]
- Jain L, Sharma BC, Srivastava S, Puri SK, Sharma P, & Sarin S (2013). Serum endotoxin, inflammatory mediators, and magnetic resonance spectroscopy before and after treatment in patients with minimal hepatic encephalopathy. J Gastroenterol Hepatol, 28(7), 1187–1193. doi: 10.1111/jgh.12160 [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Butters N, DiTraglia G, Schafer K, Smith T, Irwin M, … Cermak LS (1991). Reduced cerebral grey matter observed in alcoholics using magnetic resonance imaging. Alcohol Clin Exp Res, 15(3), 418–427. [DOI] [PubMed] [Google Scholar]
- Jiang L, Gulanski BI, De Feyter HM, Weinzimer SA, Pittman B, Guidone E, … Mason GF (2013). Increased brain uptake and oxidation of acetate in heavy drinkers. J Clin Invest, 123(4), 1605–1614. doi: 10.1172/JCI65153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, & Zuo XN (2016). Regional Homogeneity: A Multimodal, Multiscale Neuroimaging Marker of the Human Connectome. Neuroscientist, 22(5), 486–505. doi: 10.1177/1073858415595004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kale RA, Gupta RK, Saraswat VA, Hasan KM, Trivedi R, Mishra AM, … Narayana PA (2006). Demonstration of interstitial cerebral edema with diffusion tensor MR imaging in type C hepatic encephalopathy. Hepatology, 43(4), 698–706. doi: 10.1002/hep.21114 [DOI] [PubMed] [Google Scholar]
- Kalidass B, Sunnathkal R, Rangashamanna DV, & Paraswani R (2012). Atypical Wernicke’s encephalopathy showing involvement of substantia nigra. J Neuroimaging, 22(2), 204–207. doi: 10.1111/j.1552-6569.2010.00545.x [DOI] [PubMed] [Google Scholar]
- Kang SY, Kang JH, Choi JC, & Choi G (2005). Wernicke’s encephalopathy: unusual manifestation on MRI. J Neurol, 252(12), 1550–1552. doi: 10.1007/s00415-005-0886-9 [DOI] [PubMed] [Google Scholar]
- Kareken DA, Bragulat V, Dzemidzic M, Cox C, Talavage T, Davidson D, & O’Connor SJ (2010). Family history of alcoholism mediates the frontal response to alcoholic drink odors and alcohol in at-risk drinkers. Neuroimage, 50(1), 267–276. doi: 10.1016/j.neuroimage.2009.11.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kareken DA, Claus ED, Sabri M, Dzemidzic M, Kosobud AE, Radnovich AJ, … Li TK (2004). Alcohol-related olfactory cues activate the nucleus accumbens and ventral tegmental area in high-risk drinkers: preliminary findings. Alcohol Clin Exp Res, 28(4), 550–557. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt-Demasters BK, Rojiani AM, & Filley CM (2006). Central and extrapontine myelinolysis: then...and now. J Neuropathol Exp Neurol, 65(1), 1–11. [DOI] [PubMed] [Google Scholar]
- Kohno M, Dennis LE, McCready H, & Hoffman WF (2017). Executive Control and Striatal RestingState Network Interact with Risk Factors to Influence Treatment Outcomes in Alcohol-Use Disorder. Front Psychiatry, 8, 182. doi: 10.3389/fpsyt.2017.00182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong LM, Zheng WB, Lian GP, & Zhang HD (2012). Acute effects of alcohol on the human brain: diffusion tensor imaging study. AJNR Am J Neuroradiol, 33(5), 928–934. doi: 10.3174/ajnr.A2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad A, Vucurevic G, Lorscheider M, Bernow N, Thummel M, Chai C, … Fehr C (2012). Broad disruption of brain white matter microstructure and relationship with neuropsychological performance in male patients with severe alcohol dependence. Alcohol Alcohol, 47(2), 118–126. doi: 10.1093/alcalc/agr157 [DOI] [PubMed] [Google Scholar]
- Koob GF (2015). The dark side of emotion: the addiction perspective. Eur J Pharmacol, 753, 73–87. doi: 10.1016/j.ejphar.2014.11.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, & Volkow ND (2016). Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry, 3(8), 760–773. doi: 10.1016/S2215-0366(16)00104-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopelman MD, Thomson AD, Guerrini I, & Marshall EJ (2009). The Korsakoff syndrome: clinical aspects, psychology and treatment. Alcohol Alcohol, 44(2), 148–154. doi: 10.1093/alcalc/agn118 [DOI] [PubMed] [Google Scholar]
- Kreis R, Ross BD, Farrow NA, & Ackerman Z (1992). Metabolic disorders of the brain in chronic hepatic encephalopathy detected with H-1 MR spectroscopy. Radiology, 182(1), 19–27. [DOI] [PubMed] [Google Scholar]
- Krienke UJ, Nikesch F, Spiegelhalder K, Hennig J, Olbrich HM, & Langosch JM (2014). Impact of alcohol-related video sequences on functional MRI in abstinent alcoholics. Eur Addict Res, 20(1), 33–40. doi: 10.1159/000349909 [DOI] [PubMed] [Google Scholar]
- Kumar R, Gupta RK, Elderkin-Thompson V, Huda A, Sayre J, Kirsch C, … Thomas MA (2008). Voxel-based diffusion tensor magnetic resonance imaging evaluation of low-grade hepatic encephalopathy. J Magn Reson Imaging, 27(5), 1061–1068. doi: 10.1002/jmri.21342 [DOI] [PubMed] [Google Scholar]
- Kumar S, Fowler M, Gonzalez-Toledo E, & Jaffe SL (2006). Central pontine myelinolysis, an update. Neurol Res, 28(3), 360–366. doi: 10.1179/016164106X110346 [DOI] [PubMed] [Google Scholar]
- Kundra A, Jain A, Banga A, Bajaj G, & Kar P (2005). Evaluation of plasma ammonia levels in patients with acute liver failure and chronic liver disease and its correlation with the severity of hepatic encephalopathy and clinical features of raised intracranial tension. Clin Biochem, 38(8), 696–699. doi: 10.1016/j.clinbiochem.2005.04.013 [DOI] [PubMed] [Google Scholar]
- Laubenberger J, Haussinger D, Bayer S, Gufler H, Hennig J, & Langer M (1997). Proton magnetic resonance spectroscopy of the brain in symptomatic and asymptomatic patients with liver cirrhosis. Gastroenterology, 112(5), 1610–1616. [DOI] [PubMed] [Google Scholar]
- Lee H, Holburn GH, & Price RR (2003). Proton MR spectroscopic studies of chronic alcohol exposure on the rat brain. J Magn Reson Imaging, 18(2), 147–151. doi: 10.1002/jmri.10335 [DOI] [PubMed] [Google Scholar]
- Lee JH, Seo DW, Lee YS, Kim ST, Mun CW, Lim TH, … Suh DJ (1999). Proton magnetic resonance spectroscopy (1H-MRS) findings for the brain in patients with liver cirrhosis reflect the hepatic functional reserve. Am J Gastroenterol, 94(8), 2206–2213. [DOI] [PubMed] [Google Scholar]
- Lenz V, Vargas MI, Bin JF, Bogorin A, Grebici-Guessoum M, Jacques C, … Dietemann JL (2002). [Value of MRI findings in Gayet-Wernicke encephalopathy]. J Neuroradiol, 29(3), 153160. [PubMed] [Google Scholar]
- Liew HK, Cheng HY, Huang LC, Li KW, Peng HF, Yang HI, … Pang CY (2016). Acute Alcohol Intoxication Aggravates Brain Injury Caused by Intracerebral Hemorrhage in Rats. J Stroke Cerebrovasc Dis, 25(1), 15–25. doi: 10.1016/j.jstrokecerebrovasdis.2015.08.027 [DOI] [PubMed] [Google Scholar]
- Liou KC, Kuo SF, & Chen LA (2012). Wernicke encephalopathy with atypical magnetic resonance imaging. Am J Emerg Med, 30(9), 2086 e2081–2083. doi: 10.1016/j.ajem.2011.12.013 [DOI] [PubMed] [Google Scholar]
- Littleton J (1998). Neurochemical mechanisms underlying alcohol withdrawal. Alcohol Health Res World, 22(1), 13–24. [PMC free article] [PubMed] [Google Scholar]
- Liu RS, Lemieux L, Shorvon SD, Sisodiya SM, & Duncan JS (2000). Association between brain size and abstinence from alcohol. Lancet, 355(9219), 1969–1970. [DOI] [PubMed] [Google Scholar]
- Lobo IA, & Harris RA (2008). GABA(A) receptors and alcohol. Pharmacol Biochem Behav, 90(1), 90–94. doi: 10.1016/j.pbb.2008.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, & Oeltermann A (2001). Neurophysiological investigation of the basis of the fMRI signal. Nature, 412(6843), 150–157. doi: 10.1038/35084005 [DOI] [PubMed] [Google Scholar]
- Logothetis NK, & Pfeuffer J (2004). On the nature of the BOLD fMRI contrast mechanism. Magn Reson Imaging, 22(10), 1517–1531. doi: 10.1016/j.mri.2004.10.018 [DOI] [PubMed] [Google Scholar]
- Lovinger DM, White G, & Weight FF (1989). Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science, 243(4899), 1721–1724. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, White G, & Weight FF (1990). Ethanol inhibition of neuronal glutamate receptor function. Ann Med, 22(4), 247–252. [DOI] [PubMed] [Google Scholar]
- Luo J, Shen Z, Chen G, Wang D, & Yu X (2017). Pontine Changes in Metabolites and Axonal Fibres of Rats Following Four-week Alcohol Exposure: In Vivo Diffusion Tensor Imaging and 1hmagnetic Resonance Spectroscopy Study at 7.0 T. Alcohol Alcohol, 52(2), 145–150. doi: 10.1093/alcalc/agw087 [DOI] [PubMed] [Google Scholar]
- Majumdar SK, Shaw GK, Thomson AD, Pratt O, & Greenwood J (1983). Changes in plasma amino acid patterns in chronic alcoholic patients during ethanol withdrawal syndrome: their clinical implications. Med Hypotheses, 12(3), 239–251. [DOI] [PubMed] [Google Scholar]
- Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, … Harris GJ (2008). Decreased volume of the brain reward system in alcoholism. Biol Psychiatry, 64(3), 192202. doi: 10.1016/j.biopsych.2008.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksimovskiy AL, McGlinchey RE, Fortier CB, Salat DH, Milberg WP, & Oscar-Berman M (2014). White Matter and Cognitive Changes in Veterans Diagnosed with Alcoholism and PTSD. J Alcohol Drug Depend, 2(1), 144. doi: 10.4172/2329-6488.1000144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascalchi M, Belli G, Guerrini L, Nistri M, Del Seppia I, & Villari N (2002). Proton MR spectroscopy of Wernicke encephalopathy. AJNR Am J Neuroradiol, 23(10), 1803–1806. [PMC free article] [PubMed] [Google Scholar]
- Mason GF, Petrakis IL, de Graaf RA, Gueorguieva R, Guidone E, Coric V, … Krystal JH (2006). Cortical gamma-aminobutyric acid levels and the recovery from ethanol dependence: preliminary evidence of modification by cigarette smoking. Biol Psychiatry, 59(1), 85–93. [DOI] [PubMed] [Google Scholar]
- McCool BA, Frye GD, Pulido MD, & Botting SK (2003). Effects of chronic ethanol consumption on rat GABA(A) and strychnine-sensitive glycine receptors expressed by lateral/basolateral amygdala neurons. Brain Res, 963(1–2), 165–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK (1973). A review of methods to induce alcohol addiction in animals. Pharmacol Biochem Behav, 1(1), 89–101. [DOI] [PubMed] [Google Scholar]
- Messert B, Orrison WW, Hawkins MJ, & Quaglieri CE (1979). Central pontine myelinolysis. Considerations on etiology, diagnosis, and treatment. Neurology, 29(2), 147–160. [DOI] [PubMed] [Google Scholar]
- Miese F, Kircheis G, Wittsack HJ, Wenserski F, Hemker J, Modder U, … Cohnen M (2006). 1HMR spectroscopy, magnetization transfer, and diffusion-weighted imaging in alcoholic and nonalcoholic patients with cirrhosis with hepatic encephalopathy. AJNR Am J Neuroradiol, 27(5), 1019–1026. [PMC free article] [PubMed] [Google Scholar]
- Miller WR, Walters ST, & Bennett ME (2001). How effective is alcoholism treatment in the United States? J Stud Alcohol, 62(2), 211–220. [DOI] [PubMed] [Google Scholar]
- Min Y, Park SH, & Hwang SB (2012). Corticospinal tract and pontocerebellar fiber of central pontine myelinolysis. Ann Rehabil Med, 36(6), 887–892. doi: 10.5535/arm.2012.36.6.887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JM, O’Neil JP, Janabi M, Marks SM, Jagust WJ, & Fields HL (2012). Alcohol consumption induces endogenous opioid release in the human orbitofrontal cortex and nucleus accumbens. Sci Transl Med, 4(116), 116ra116. doi: 10.1126/scitranslmed.3002902 [DOI] [PubMed] [Google Scholar]
- Moghaddam B, & Bolinao ML (1994). Biphasic effect of ethanol on extracellular accumulation of glutamate in the hippocampus and the nucleus accumbens. Neurosci Lett, 178(1), 99–102. [DOI] [PubMed] [Google Scholar]
- Mon A, Durazzo TC, & Meyerhoff DJ (2012). Glutamate, GABA, and other cortical metabolite concentrations during early abstinence from alcohol and their associations with neurocognitive changes. Drug Alcohol Depend, 125(1–2), 27–36. doi: 10.1016/j.drugalcdep.2012.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan SC, & Finney JW (1996). Explaining abstinence rates following treatment for alcohol abuse: a quantitative synthesis of patient, research design and treatment effects. Addiction, 91(6), 787–805. [DOI] [PubMed] [Google Scholar]
- Monnig MA, Caprihan A, Yeo RA, Gasparovic C, Ruhl DA, Lysne P, … Thoma RJ (2013). Diffusion tensor imaging of white matter networks in individuals with current and remitted alcohol use disorders and comorbid conditions. Psychol Addict Behav, 27(2), 455–465. doi: 10.1037/a0027168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnig MA, Thayer RE, Caprihan A, Claus ED, Yeo RA, Calhoun VD, & Hutchison KE (2014). White matter integrity is associated with alcohol cue reactivity in heavy drinkers. Brain Behav, 4(2), 158–170. doi: 10.1002/brb3.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrisett RA, & Swartzwelder HS (1993). Attenuation of hippocampal long-term potentiation by ethanol: a patch-clamp analysis of glutamatergic and GABAergic mechanisms. J Neurosci, 13(5), 2264–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer A, & Finney JW (2002). Outcomes for untreated individuals involved in randomized trials of alcohol treatment. J Subst Abuse Treat, 23(3), 247–252. [DOI] [PubMed] [Google Scholar]
- Muller-Oehring EM, Jung YC, Pfefferbaum A, Sullivan EV, & Schulte T (2015). The Resting Brain of Alcoholics. Cereb Cortex, 25(11), 4155–4168. doi: 10.1093/cercor/bhu134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Oehring EM, Schulte T, Fama R, Pfefferbaum A, & Sullivan EV (2009). Global-local interference is related to callosal compromise in alcoholism: a behavior-DTI association study. Alcohol Clin Exp Res, 33(3), 477–489. doi: 10.1111/j.1530-0277.2008.00858.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro CA, Saxton J, & Butters MA (2000). The neuropsychological consequences of abstinence among older alcoholics: a cross-sectional study. Alcohol Clin Exp Res, 24(10), 1510–1516. [PubMed] [Google Scholar]
- Murata T, Fujito T, Kimura H, Omori M, Itoh H, & Wada Y (2001). Serial MRI and (1)H-MRS of Wernicke’s encephalopathy: report of a case with remarkable cerebellar lesions on MRI. Psychiatry Res, 108(1), 49–55. [DOI] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Randall PK, & Voronin K (2008). Effect of naltrexone and ondansetron on alcohol cue-induced activation of the ventral striatum in alcohol-dependent people. Arch Gen Psychiatry, 65(4), 466–475. doi: 10.1001/archpsyc.65.4.466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naegele T, Grodd W, Viebahn R, Seeger U, Klose U, Seitz D, … Voigt K (2000). MR imaging and (1)H spectroscopy of brain metabolites in hepatic encephalopathy: time-course of renormalization after liver transplantation. Radiology, 216(3), 683–691. doi: 10.1148/radiology.216.3.r00se27683 [DOI] [PubMed] [Google Scholar]
- Nahum L, Pignat JM, Bouzerda-Wahlen A, Gabriel D, Liverani MC, Lazeyras F, … Schnider A (2015). Neural Correlate of Anterograde Amnesia in Wernicke-Korsakoff Syndrome. Brain Topogr, 28(5), 760–770. doi: 10.1007/s10548-014-0391-5 [DOI] [PubMed] [Google Scholar]
- Nair SR, Ramli NM, Rahmat K, & Mei-Ling ST (2012). Central pontine and extrapontine myelinolysis: Diffusion weighted imaging and diffusion tensor imaging on follow-up. Neurol India, 60(4), 426–428. doi: 10.4103/0028-3886.100712 [DOI] [PubMed] [Google Scholar]
- Nishida M, Sato H, Kobayashi N, Morimoto M, & Hamaoka K (2009). Wernicke’s encephalopathy in a patient with nephrotic syndrome. Eur J Pediatr, 168(6), 731–734. doi: 10.1007/s00431-008-0833-8 [DOI] [PubMed] [Google Scholar]
- Nomoto N, Arasaki K, & Tamaki M (2004). Central pontine myelinolysis in chronic alcoholism demonstrated by magnetic resonance imaging and spectroscopy. Eur Neurol, 51(3), 179–180. doi: 10.1159/000077668 [DOI] [PubMed] [Google Scholar]
- Olsen RW, Hanchar HJ, Meera P, & Wallner M (2007). GABAA receptor subtypes: the “one glass of wine” receptors. Alcohol, 41(3), 201–209. doi: 10.1016/j.alcohol.2007.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks MH, Dawant BM, Riddle WR, Hartmann SL, Dietrich MS, Nickel MK, … Martin PR (2002). Longitudinal brain metabolic characterization of chronic alcoholics with proton magnetic resonance spectroscopy. Alcohol Clin Exp Res, 26(9), 1368–1380. doi: 10.1097/01.ALC.0000029598.07833.2D [DOI] [PubMed] [Google Scholar]
- Perez-Ramirez U, Diaz-Parra A, Ciccocioppo R, Canals S, & Moratal D (2017). Brain functional connectivity alterations in a rat model of excessive alcohol drinking: A resting-state network analysis. Conf Proc IEEE Eng Med Biol Soc, 2017, 3016–3019. doi: 10.1109/EMBC.2017.8037492 [DOI] [PubMed] [Google Scholar]
- Petroff OA, Pleban LA, & Spencer DD (1995). Symbiosis between in vivo and in vitro NMR spectroscopy: the creatine, N-acetylaspartate, glutamate, and GABA content of the epileptic human brain. Magn Reson Imaging, 13(8), 1197–1211. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, & Rabinowitz AR (2006). Choosing the right medication for the treatment of alcoholism. Curr Psychiatry Rep, 8(5), 383–388. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Bell RL, & Sullivan EV (2007). Development and resolution of brain lesions caused by pyrithiamine- and dietary-induced thiamine deficiency and alcohol exposure in the alcohol-preferring rat: a longitudinal magnetic resonance imaging and spectroscopy study. Neuropsychopharmacology, 32(5), 1159–1177. doi: 10.1038/sj.npp.1301107 [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, & Sullivan EV (2006). Supratentorial profile of white matter microstructural integrity in recovering alcoholic men and women. Biol Psychiatry, 59(4), 364372. doi: 10.1016/j.biopsych.2005.06.025 [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Desmond JE, & Sullivan EV (1996). Thinning of the corpus callosum in older alcoholic men: a magnetic resonance imaging study. Alcohol Clin Exp Res, 20(4), 752–757. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Rohlfing T, & Sullivan EV (2009). Degradation of association and projection white matter systems in alcoholism detected with quantitative fiber tracking. Biol Psychiatry, 65(8), 680–690. doi: 10.1016/j.biopsych.2008.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Serventi KL, & Sullivan EV (2002). Corpus callosum, pons, and cortical white matter in alcoholic women. Alcohol Clin Exp Res, 26(3), 400–406. [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Adalsteinsson E, & Sullivan EV (2007). Diffusion tensor imaging with quantitative fibre tracking in HIV infection and alcoholism comorbidity: synergistic white matter damage. Brain, 130(Pt 1), 48–64. doi: 10.1093/brain/awl242 [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Serventi KL, & Sullivan EV (2004). Brain volumes, RBC status, and hepatic function in alcoholics after 1 and 4 weeks of sobriety: predictors of outcome. Am J Psychiatry, 161(7), 1190–1196. doi: 10.1176/appi.ajp.161.7.1190 [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, & Sullivan EV (2005). Disruption of brain white matter microstructure by excessive intracellular and extracellular fluid in alcoholism: evidence from diffusion tensor imaging. Neuropsychopharmacology, 30(2), 423–432. doi: 10.1038/sj.npp.1300623 [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Hedehus M, Adalsteinsson E, Lim KO, & Moseley M (2000). In vivo detection and functional correlates of white matter microstructural disruption in chronic alcoholism. Alcohol Clin Exp Res, 24(8), 1214–1221. [PubMed] [Google Scholar]
- Pfefferbaum A, Zahr NM, Mayer D, Rohlfing T, & Sullivan EV (2015). Dynamic responses of selective brain white matter fiber tracts to binge alcohol and recovery in the rat. PLoS One, 10(4), e0124885. doi: 10.1371/journal.pone.0124885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Zahr NM, Mayer D, Vinco S, Orduna J, Rohlfing T, & Sullivan EV (2008). Ventricular expansion in wild-type Wistar rats after alcohol exposure by vapor chamber. Alcohol Clin Exp Res, 32(8), 1459–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister HW, Einhaupl KM, & Brandt T (1985). Mild central pontine myelinolysis: a frequently undetected syndrome. Eur Arch Psychiatry Neurol Sci, 235(3), 134–139. [DOI] [PubMed] [Google Scholar]
- Poveda MJ, Bernabeu A, Concepcion L, Roa E, de Madaria E, Zapater P, … Jover R (2010). Brain edema dynamics in patients with overt hepatic encephalopathy A magnetic resonance imaging study. Neuroimage, 52(2), 481–487. doi: 10.1016/j.neuroimage.2010.04.260 [DOI] [PubMed] [Google Scholar]
- Prakash R, & Mullen KD (2010). Mechanisms, diagnosis and management of hepatic encephalopathy. Nat Rev Gastroenterol Hepatol, 7(9), 515–525. doi: 10.1038/nrgastro.2010.116 [DOI] [PubMed] [Google Scholar]
- Prisciandaro JJ, Schacht JP, Prescot AP, Renshaw PF, Brown TR, & Anton RF (2016). Associations Between Recent Heavy Drinking and Dorsal Anterior Cingulate N-Acetylaspartate and Glutamate Concentrations in Non-Treatment-Seeking Individuals with Alcohol Dependence. Alcohol Clin Exp Res, 40(3), 491–496. doi: 10.1111/acer.12977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J, Kulisevsky J, Moreno A, Deus J, Alonso J, Balanzo J, … Capdevila A (1996). Neurospectroscopic alterations and globus pallidus hyperintensity as related magnetic resonance markers of reversible hepatic encephalopathy. Neurology, 47(6), 1526–1530. [DOI] [PubMed] [Google Scholar]
- Rama Rao KV, & Norenberg MD (2014). Glutamine in the pathogenesis of hepatic encephalopathy: the trojan horse hypothesis revisited. Neurochem Res, 39(3), 593–598. doi: 10.1007/s11064-0120955-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramchandani VA, Umhau J, Pavon FJ, Ruiz-Velasco V, Margas W, Sun H, … Heilig M (2011). A genetic determinant of the striatal dopamine response to alcohol in men. Mol Psychiatry, 16(8), 809–817. doi: 10.1038/mp.2010.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Anderson P, Barry J, Dimitrov P, Elekes Z, Feijao F, … Gmel G (2015). Prevalence of and potential influencing factors for alcohol dependence in Europe. Eur Addict Res, 21(1), 6–18. doi: 10.1159/000365284 [DOI] [PubMed] [Google Scholar]
- Roberto M, Schweitzer P, Madamba SG, Stouffer DG, Parsons LH, & Siggins GR (2004). Acute and chronic ethanol alter glutamatergic transmission in rat central amygdala: an in vitro and in vivo analysis. J Neurosci, 24(7), 1594–1603. doi: 10.1523/JNEUROSCI.5077-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlfing T (2006). Transformation model and constraints cause bias in statistics on deformation fields. Med Image Comput Comput Assist Interv, 9(Pt 1), 207–214. [DOI] [PubMed] [Google Scholar]
- Rominger A, Cumming P, Xiong G, Koller G, Boning G, Wulff M, … Pogarell O (2012). [18F]Fallypride PET measurement of striatal and extrastriatal dopamine D 2/3 receptor availability in recently abstinent alcoholics. Addict Biol, 17(2), 490–503. doi: 10.1111/j.13691600.2011.00355.x [DOI] [PubMed] [Google Scholar]
- Ross BD, Jacobson S, Villamil F, Korula J, Kreis R, Ernst T, … Moats RA (1994). Subclinical hepatic encephalopathy: proton MR spectroscopic abnormalities. Radiology, 193(2), 457–463. [DOI] [PubMed] [Google Scholar]
- Rossetti ZL, & Carboni S (1995). Ethanol withdrawal is associated with increased extracellular glutamate in the rat striatum. Eur J Pharmacol, 283(1–3), 177–183. [DOI] [PubMed] [Google Scholar]
- Rovira A, Cordoba J, Sanpedro F, Grive E, Rovira-Gols A, & Alonso J (2002). Normalization of T2 signal abnormalities in hemispheric white matter with liver transplant. Neurology, 59(3), 335–341. [DOI] [PubMed] [Google Scholar]
- Rugilo CA, Uribe Roca MC, Zurru MC, Capizzano AA, Pontello GA, & Gatto EM (2003). Proton MR spectroscopy in Wernicke encephalopathy. AJNR Am J Neuroradiol, 24(5), 952–955. [PMC free article] [PubMed] [Google Scholar]
- Rumboldt Z (2010). The Holy Grail and the quest for the gold standard. AJNR Am J Neuroradiol, 31(9), 1617–1618. doi: 10.3174/ajnr.A2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki H, Abe O, Yamasue H, Fukuda R, Yamada H, Takei K, … Ohtomo K (2009). Structural and diffusional brain abnormality related to relatively low level alcohol consumption. Neuroimage, 46(2), 505–510. doi: 10.1016/j.neuroimage.2009.02.007 [DOI] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, & Myrick H (2013). Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addict Biol, 18(1), 121–133. doi: 10.1111/j.1369-1600.2012.00464.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroth G, Naegele T, Klose U, Mann K, & Petersen D (1988). Reversible brain shrinkage in abstinent alcoholics, measured by MRI. Neuroradiology, 30(5), 385–389. [DOI] [PubMed] [Google Scholar]
- Schubert F, Gallinat J, Seifert F, & Rinneberg H (2004). Glutamate concentrations in human brain using single voxel proton magnetic resonance spectroscopy at 3 Tesla. Neuroimage, 21(4), 17621771. doi: 10.1016/j.neuroimage.2003.11.014 [DOI] [PubMed] [Google Scholar]
- Schulte T, Muller-Oehring EM, Sullivan EV, & Pfefferbaum A (2012). Synchrony of corticostriatalmidbrain activation enables normal inhibitory control and conflict processing in recovering alcoholic men. Biol Psychiatry, 71(3), 269–278. doi: 10.1016/j.biopsych.2011.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segobin S, Ritz L, Lannuzel C, Boudehent C, Vabret F, Eustache F, … Pitel AL (2015). Integrity of white matter microstructure in alcoholics with and without Korsakoff’s syndrome. Hum Brain Mapp, 36(7), 2795–2808. doi: 10.1002/hbm.22808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shear PK, Jernigan TL, & Butters N (1994). Volumetric magnetic resonance imaging quantification of longitudinal brain changes in abstinent alcoholics. Alcohol Clin Exp Res, 18(1), 172–176. [DOI] [PubMed] [Google Scholar]
- Singhal A, Nagarajan R, Hinkin CH, Kumar R, Sayre J, Elderkin-Thompson V, … Thomas MA (2010). Two-dimensional MR spectroscopy of minimal hepatic encephalopathy and neuropsychological correlates in vivo. J Magn Reson Imaging, 32(1), 35–43. doi: 10.1002/jmri.22216 [DOI] [PubMed] [Google Scholar]
- Sjoerds Z, de Wit S, van den Brink W, Robbins TW, Beekman AT, Penninx BW, & Veltman DJ (2013). Behavioral and neuroimaging evidence for overreliance on habit learning in alcoholdependent patients. Transl Psychiatry, 3, e337. doi: 10.1038/tp.2013.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit F, Treutlein J, Frischknecht U, Hermann D, Mann K, Kiefer F, … Ende G (2018). Glutamate concentration in the anterior cingulate cortex in alcohol dependence: association with alcohol withdrawal and exploration of contribution from glutamatergic candidate genes. Psychiatr Genet, 28(5), 94–95. doi: 10.1097/YPG.0000000000000202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Deshmukh A, De Rosa E, Rosenbloom MJ, & Pfefferbaum A (2005). Striatal and forebrain nuclei volumes: contribution to motor function and working memory deficits in alcoholism. Biol Psychiatry, 57(7), 768–776. doi: 10.1016/j.biopsych.2004.12.012 [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Harding AJ, Pentney R, Dlugos C, Martin PR, Parks MH, … Pfefferbaum A (2003). Disruption of frontocerebellar circuitry and function in alcoholism. Alcohol Clin Exp Res, 27(2), 301–309. doi: 10.1097/01.ALC.0000052584.05305.98 [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Lane B, Deshmukh A, Rosenbloom MJ, Desmond JE, Lim KO, & Pfefferbaum A (1999). In vivo mammillary body volume deficits in amnesic and nonamnesic alcoholics. Alcohol Clin Exp Res, 23(10), 1629–1636. [PubMed] [Google Scholar]
- Sullivan EV, & Pfefferbaum A (2001). Magnetic resonance relaxometry reveals central pontine abnormalities in clinically asymptomatic alcoholic men. Alcohol Clin Exp Res, 25(8), 1206–1212. [PubMed] [Google Scholar]
- Sullivan EV, & Pfefferbaum A (2009). Neuroimaging of the Wernicke-Korsakoff syndrome. Alcohol Alcohol, 44(2), 155–165. doi: 10.1093/alcalc/agn103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, & Zahr NM (2008). Neuroinflammation as a neurotoxic mechanism in alcoholism: commentary on “Increased MCP-1 and microglia in various regions of human alcoholic brain”. Exp Neurol, 213(1), 10–17. doi: 10.1016/j.expneurol.2008.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarasow E, Panasiuk A, Siergiejczyk L, Orzechowska-Bobkiewicz A, Lewszuk A, Walecki J, & Prokopowicz D (2003). MR and 1H MR spectroscopy of the brain in patients with liver cirrhosis and early stages of hepatic encephalopathy. Hepatogastroenterology, 50(54), 2149–2153. [PubMed] [Google Scholar]
- Taylor-Robinson SD, Buckley C, Changani KK, Hodgson HJ, & Bell JD (1999). Cerebral proton and phosphorus-31 magnetic resonance spectroscopy in patients with subclinical hepatic encephalopathy. Liver, 19(5), 389–398. [DOI] [PubMed] [Google Scholar]
- Taylor-Robinson SD, Sargentoni J, Marcus CD, Morgan MY, & Bryant DJ (1994). Regional variations in cerebral proton spectroscopy in patients with chronic hepatic encephalopathy. Metab Brain Dis, 9(4), 347–359. [DOI] [PubMed] [Google Scholar]
- Thoma R, Mullins P, Ruhl D, Monnig M, Yeo RA, Caprihan A, … Gasparovic C (2011). Perturbation of the glutamate-glutamine system in alcohol dependence and remission. Neuropsychopharmacology, 36(7), 1359–1365. doi: 10.1038/npp.2011.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MA, Huda A, Guze B, Curran J, Bugbee M, Fairbanks L, … Fawzy F (1998). Cerebral 1H MR spectroscopy and neuropsychologic status of patients with hepatic encephalopathy. AJR Am J Roentgenol, 171(4), 1123–1130. doi: 10.2214/ajr.171.4.9763008 [DOI] [PubMed] [Google Scholar]
- Thomson AD, Cook CC, Guerrini I, Sheedy D, Harper C, & Marshall EJ (2008). Wernicke’s encephalopathy: ‘Plus ca change, plus c’est la meme chose’. Alcohol Alcohol, 43(2), 180–186. doi: 10.1093/alcalc/agm149 [DOI] [PubMed] [Google Scholar]
- Tomkins DM, & Sellers EM (2001). Addiction and the brain: the role of neurotransmitters in the cause and treatment of drug dependence. CMAJ, 164(6), 817–821. [PMC free article] [PubMed] [Google Scholar]
- Trivedi R, Bagga D, Bhattacharya D, Kaur P, Kumar P, Khushu S, … Singh N (2013). White matter damage is associated with memory decline in chronic alcoholics: a quantitative diffusion tensor tractography study. Behav Brain Res, 250, 192–198. doi: 10.1016/j.bbr.2013.05.001 [DOI] [PubMed] [Google Scholar]
- Tsai G, & Coyle JT (1998). The role of glutamatergic neurotransmission in the pathophysiology of alcoholism. Annu Rev Med, 49, 173–184. doi: 10.1146/annurev.med.49.1.173 [DOI] [PubMed] [Google Scholar]
- Tu X, Wang J, Liu X, & Zheng J (2018). Aberrant regional brain activities in alcohol dependence: a functional magnetic resonance imaging study. Neuropsychiatr Dis Treat, 14, 847–853. doi: 10.2147/NDT.S158221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunc-Skarka N, Weber-Fahr W, & Ende G (2015). Recreational alcohol use induces changes in the concentrations of choline-containing compounds and total creatine in the brain: a (1)H MRS study of healthy subjects. MAGMA, 28(5), 503–510. doi: 10.1007/s10334-015-0486-3 [DOI] [PubMed] [Google Scholar]
- Umhau JC, Momenan R, Schwandt ML, Singley E, Lifshitz M, Doty L, … Heilig M (2010). Effect of acamprosate on magnetic resonance spectroscopy measures of central glutamate in detoxified alcohol-dependent individuals: a randomized controlled experimental medicine study. Arch Gen Psychiatry, 67(10), 1069–1077. doi: 10.1001/archgenpsychiatry.2010.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eijk J, Demirakca T, Frischknecht U, Hermann D, Mann K, & Ende G (2013). Rapid partial regeneration of brain volume during the first 14 days of abstinence from alcohol. Alcohol Clin Exp Res, 37(1), 67–74. doi: 10.1111/j.1530-0277.2012.01853.x [DOI] [PubMed] [Google Scholar]
- van Holst RJ, de Ruiter MB, van den Brink W, Veltman DJ, & Goudriaan AE (2012). A voxelbased morphometry study comparing problem gamblers, alcohol abusers, and healthy controls. Drug Alcohol Depend, 124(1–2), 142–148. doi: 10.1016/j.drugalcdep.2011.12.025 [DOI] [PubMed] [Google Scholar]
- Vaquero J, Chung C, Cahill ME, & Blei AT (2003). Pathogenesis of hepatic encephalopathy in acute liver failure. Semin Liver Dis, 23(3), 259–269. doi: 10.1055/s-2003-42644 [DOI] [PubMed] [Google Scholar]
- Vetreno RP, Yaxley R, Paniagua B, & Crews FT (2016). Diffusion tensor imaging reveals adolescent binge ethanol-induced brain structural integrity alterations in adult rats that correlate with behavioral dysfunction. Addict Biol, 21(4), 939–953. doi: 10.1111/adb.12232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Hitzemann R, Wolf AP, Logan J, Fowler JS, Christman D, … et al. (1990). Acute effects of ethanol on regional brain glucose metabolism and transport. Psychiatry Res, 35(1), 3948. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Kim SW, Wang GJ, Alexoff D, Logan J, Muench L, … Tomasi D (2013). Acute alcohol intoxication decreases glucose metabolism but increases acetate uptake in the human brain. Neuroimage, 64, 277–283. doi: 10.1016/j.neuroimage.2012.08.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Koob GF, & McLellan AT (2016). Neurobiologic Advances from the Brain Disease Model of Addiction. N Engl J Med, 374(4), 363–371. doi: 10.1056/NEJMra1511480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Franceschi D, Fowler JS, Thanos PP, Maynard L, … Kai Li T (2006). Low doses of alcohol substantially decrease glucose metabolism in the human brain. Neuroimage, 29(1), 295–301. doi: 10.1016/j.neuroimage.2005.07.004 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Maynard L, Fowler JS, Jayne B, Telang F, … Pappas N (2002). Effects of alcohol detoxification on dopamine D2 receptors in alcoholics: a preliminary study. Psychiatry Res, 116(3), 163–172. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wiers CE, Shokri-Kojori E, Tomasi D, Wang GJ, & Baler R (2017). Neurochemical and metabolic effects of acute and chronic alcohol in the human brain: Studies with positron emission tomography. Neuropharmacology, 122, 175–188. doi: 10.1016/j.neuropharm.2017.01.012 [DOI] [PubMed] [Google Scholar]
- Vollstadt-Klein S, Loeber S, Richter A, Kirsch M, Bach P, von der Goltz C, … Kiefer F (2012). Validating incentive salience with functional magnetic resonance imaging: association between mesolimbic cue reactivity and attentional bias in alcohol-dependent patients. Addict Biol, 17(4), 807–816. doi: 10.1111/j.1369-1600.2011.00352.x [DOI] [PubMed] [Google Scholar]
- Vollstadt-Klein S, Wichert S, Rabinstein J, Buhler M, Klein O, Ende G, … Mann K (2010). Initial, habitual and compulsive alcohol use is characterized by a shift of cue processing from ventral to dorsal striatum. Addiction, 105(10), 1741–1749. doi: 10.1111/j.1360-0443.2010.03022.x [DOI] [PubMed] [Google Scholar]
- Wardlaw JM, Valdes Hernandez MC, & Munoz-Maniega S (2015). What are white matter hyperintensities made of? Relevance to vascular cognitive impairment. J Am Heart Assoc, 4(6), 001140. doi: 10.1161/JAHA.114.001140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead TP, Robinson D, Allaway SL, & Hale AC (1995). The effects of cigarette smoking and alcohol consumption on blood haemoglobin, erythrocytes and leucocytes: a dose related study on male subjects. Clin Lab Haematol, 17(2), 131–138. [PubMed] [Google Scholar]
- Wik G, Borg S, Sjogren I, Wiesel FA, Blomqvist G, Borg J, … et al. (1988). PET determination of regional cerebral glucose metabolism in alcohol-dependent men and healthy controls using 11Cglucose. Acta Psychiatr Scand, 78(2), 234–241. [DOI] [PubMed] [Google Scholar]
- Williams TM, Davies SJ, Taylor LG, Daglish MR, Hammers A, Brooks DJ, … Lingford-Hughes A (2009). Brain opioid receptor binding in early abstinence from alcohol dependence and relationship to craving: an [11C]diprenorphine PET study. Eur Neuropsychopharmacol, 19(10), 740–748. doi: 10.1016/j.euroneuro.2009.06.007 [DOI] [PubMed] [Google Scholar]
- Wrase J, Makris N, Braus DF, Mann K, Smolka MN, Kennedy DN, … Heinz A (2008). Amygdala volume associated with alcohol abuse relapse and craving. Am J Psychiatry, 165(9), 1179–1184. doi: 10.1176/appi.ajp.2008.07121877 [DOI] [PubMed] [Google Scholar]
- Wrase J, Schlagenhauf F, Kienast T, Wustenberg T, Bermpohl F, Kahnt T, … Heinz A (2007). Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage, 35(2), 787–794. doi: 10.1016/j.neuroimage.2006.11.043 [DOI] [PubMed] [Google Scholar]
- Xu S, Zhu W, Wan Y, Wang J, Chen X, Pi L, … Cao Q (2018). Decreased Taurine and Creatine in the Thalamus May Relate to Behavioral Impairments in Ethanol-Fed Mice: A Pilot Study of Proton Magnetic Resonance Spectroscopy. Mol Imaging, 17, 1536012117749051. doi: 10.1177/1536012117749051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh PH, Simpson K, Durazzo TC, Gazdzinski S, & Meyerhoff DJ (2009). Tract-Based Spatial Statistics (TBSS) of diffusion tensor imaging data in alcohol dependence: abnormalities of the motivational neurocircuitry. Psychiatry Res, 173(1), 22–30. doi: 10.1016/j.pscychresns.2008.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo RA, Thoma RJ, Gasparovic C, Monnig M, Harlaar N, Calhoun VD, … Hutchison KE (2013). Neurometabolite concentration and clinical features of chronic alcohol use: a proton magnetic resonance spectroscopy study. Psychiatry Res, 211(2), 141–147. doi: 10.1016/j.pscychresns.2012.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder KK, Albrecht DS, Dzemidzic M, Normandin MD, Federici LM, Graves T, … Kareken DA (2016). Differences in IV alcohol-induced dopamine release in the ventral striatum of social drinkers and nontreatment-seeking alcoholics. Drug Alcohol Depend, 160, 163–169. doi: 10.1016/j.drugalcdep.2016.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Alt C, Mayer D, Rohlfing T, Manning-Bog A, Luong R, … Pfefferbaum A (2014). Associations between in vivo neuroimaging and postmortem brain cytokine markers in a rodent model of Wernicke’s encephalopathy. Exp Neurol, 261, 109–119. doi: 10.1016/j.expneurol.2014.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Carr RA, Rohlfing T, Mayer D, Sullivan EV, Colrain IM, & Pfefferbaum A (2016). Brain metabolite levels in recently sober individuals with alcohol use disorder: Relation to drinking variables and relapse. Psychiatry Research-Neuroimaging, 250, 42–49. doi: 10.1016/j.pscychresns.2016.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Kaufman KL, & Harper CG (2011). Clinical and pathological features of alcohol-related brain damage. Nat Rev Neurol, 7(5), 284–294. doi: 10.1038/nrneurol.2011.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Mayer D, Rohlfing T, Hasak MP, Hsu O, Vinco S, … Pfefferbaum A (2010). Brain injury and recovery following binge ethanol: evidence from in vivo magnetic resonance spectroscopy. Biol Psychiatry, 67(9), 846–854. doi: 10.1016/j.biopsych.2009.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Mayer D, Rohlfing T, Hsu O, Vinco S, Orduna J, … Pfefferbaum A (2014). Rat strain differences in brain structure and neurochemistry in response to binge alcohol. Psychopharmacology (Berl), 231(2), 429–445. doi: 10.1007/s00213-013-3253-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Mayer D, Rohlfing T, Orduna J, Luong R, Sullivan EV, & Pfefferbaum A (2013). A mechanism of rapidly reversible cerebral ventricular enlargement independent of tissue atrophy. Neuropsychopharmacology, 38(6), 1121–1129. doi: 10.1038/npp.2013.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Mayer D, Rohlfing T, Sullivan EV, & Pfefferbaum A (2014). Imaging neuroinflammation? A perspective from MR spectroscopy. Brain Pathol, 24(6), 654–664. doi: 10.1111/bpa.12197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Mayer D, Vinco S, Orduna J, Luong R, Sullivan EV, & Pfefferbaum A (2009). In vivo evidence for alcohol-induced neurochemical changes in rat brain without protracted withdrawal, pronounced thiamine deficiency, or severe liver damage. Neuropsychopharmacology, 34(6), 1427–1442. doi: 10.1038/npp.2008.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, & Pfefferbaum A (2017). Alcohol’s Effects on the Brain: Neuroimaging Results in Humans and Animal Models. Alcohol Res, 38(2), 183–206. [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Rohlfing T, Mayer D, Luong R, Sullivan EV, & Pfefferbaum A (2016). Transient CNS responses to repeated binge ethanol treatment. Addict Biol, 21(6), 1199–1216. doi: 10.1111/adb.12290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Sullivan EV, Rohlfing T, Mayer D, Collins AM, Luong R, & Pfefferbaum A (2016). Concomitants of alcoholism: differential effects of thiamine deficiency, liver damage, and food deprivation on the rat brain in vivo. Psychopharmacology (Berl), 233(14), 2675–2686. doi: 10.1007/s00213-016-4313-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorlu N, Karavul Ucman T, Gelal F, Colak Kalayci C, Polat S, Saricicek A, … Gulseren S (2014). Abnormal white matter integrity in long-term abstinent alcohol dependent patients. Psychiatry Res, 224(1), 42–48. doi: 10.1016/j.pscychresns.2014.07.006 [DOI] [PubMed] [Google Scholar]