Abstract
Interferon (IFN) responses to viral infection is necessary to establish intrinsic antiviral state, but, if unchecked can lead to heightened inflammation. Recently, we showed that TLR2 activation contributes to limitation of rhinovirus (RV)-induced IFN response in the airway epithelial cells. We also demonstrated that, compared to normal, airway epithelial cells from patients with chronic obstructive pulmonary disease (COPD) show higher IFN responses to RV, but the underlying mechanisms are not known. Initially, RV-induced IFN responses depends on double stranded RNA receptor activation and then amplified via IFN-stimulated activation of JAK/STAT signaling. Here we show that in normal cells, TLR2 limits RV-induced IFN responses by attenuating STAT1 and STAT2 phosphorylation and this was associated with. TLR2-dependent SIRT-1 expression. Further, inhibition of SIRT-1 enhanced RV-induced IFN responses and this was accompanied by increased STAT1/STAT2 phosphorylation indicating that TLR2 may limit RV-induced IFN responses via SIRT-1. COPD airway epithelial cells showed attenuated IL-8 responses to TLR2 agonist despite expressing TLR2 similar to normal, indicating dysregulation in TLR2 signaling pathway. Unlike normal, COPD cells failed to show RV-induced TLR2-dependent SIRT-1 expression. Pretreatment with quercetin, which increases SIRT-1 expression, normalized RV-induced IFN levels in COPD airway epithelial cells. Inhibition of SIRT-1 in quercetin pretreated COPD cells abolished the normalizing effects of quercetin on RV-induced IFN expression in these cells, confirming that quercetin exerts its effect via SIRT-1. In summary, we show that TLR2 is required for limiting RV-induced IFNs, and this pathway is dysregulated in COPD airway epithelial cells leading to exaggerated IFN production.
Keywords: innate immunity, SOCS, respiratory virus, inflammation, antiviral responses, COPD
Introduction
Rhinovirus (RV), which is responsible for majority of common colds, causes exacerbations in patients with chronic obstructive pulmonary disease (COPD) (1). COPD patients experimentally infected with RV showed more severe and prolonged lung inflammation than normal subjects (2), but the underlying mechanisms are not completely understood.
Epithelial cells lining the tracheobronchial mucosa are the primary target for rhinovirus (RV) and express type I and type III interferons (IFNs) in addition to pro-inflammatory cytokines and chemokines in response to RV infection (3, 4). Both these responses should be tightly regulated to prevent subsequent excessive airway inflammation. Previously, we demonstrated that COPD airway epithelial cells show exaggerated type I and type III IFN expression in response to RV infection (3) and this was not associated with augmented viral clearance. Similarly, mice displaying COPD-like lung disease including mild to moderate emphysema, lung inflammation and airway remodeling, also showed higher IFN response to RV than normal and this was associated with heightened lung inflammation (5). We also showed that RV interaction with TLR2 is required for limiting replication-dependent antiviral type I and type III IFNs expression in normal airway epithelial cells (6). However, the role of TLR2 in COPD bronchial epithelial cells that show exaggerated type I and type III IFNs is yet to be determined. Additionally, mechanisms by which RV induced TLR2 activation limits RV-induced INF expression are not understood.
In airway epithelial cells, RV-induced first wave of type I and III IFN expression depends on the interaction of double stranded RNA (dsRNA) receptors MDA5, RIG-I and TLR3 with dsRNA generated during viral replication (4, 7). The second wave of Type I and type III IFNs expression depends on interaction of these IFNs with their respective receptors. This interaction activates JAK/STAT signaling in a paracrine/autocrine fashion to amplify IFN expression. In addition, interaction of type I and II IFNs with their receptor also upregulates various IFN-stimulated genes (ISG) with broad antiviral activities to establish antiviral state (8, 9). Without this IFN/IFN receptor positive feedback loop, the expression of ISGs is compromised and this could eventually promote viral replication.
In addition to establishing antiviral state, Type I and III IFNs may also contribute to initiation of lung inflammation by promoting recruitment and activation of immune cells. Given the potential of type I and type III IFNs to drive and amplify inflammatory responses, the exaggerated or prolonged expression of type I and type III IFNs may be detrimental following viral infections (10, 11). Therefore, type I and type III IFN responses to viral infection are tightly-regulated by various cell-intrinsic negative regulators to prevent overt lung inflammation following viral infections (reviewed in (12, 13)).
IFN responses to viral infections may be regulated at the initial phase by downregulation of dsRNA receptor expression or downstream signaling. IFNs may also be regulated during amplification phase, which involves STAT activation by various intrinsic factors including suppressor of cytokine signaling (SOCS). Previously, respiratory syncytial virus was shown to induce SOCS1 and 3 via TLR activation, thus reducing the type I IFN expression (14). Gielen et al demonstrated that increased SOCS1 expression contributes to suppression of RV-induced IFNs in airway epithelial cells from subjects with asthma (15). However, whether TLR2 regulates RV-induced IFN responses in airway epithelial cells via enhancing SOCS expression is yet to be determined.
Sirtuin (SIRT)-1 is a type III nicotinamide adenine dinucleotide (NAD)-dependent histone deacetylase and plays an important role in various biological processes including immunity, aging, stress response and metabolism. SIRT-1 regulates expression of various genes not only by deacetylating histones, but also by deacetylating transcription factors including FOXO proteins, NF-ĸB, P53, STAT3 and STAT1 (16-20). For example, in monocytes, SIRT-1 deacetylates P65/RelB at later stages of TLR4 activation by endotoxin (that is after 10 h) to terminate endotoxin-induced NF-ĸB-dependent gene transcription (16). SIRT-1 binds and represses activity of p53 and promotes cell survival under stress (17). In cells under oxidative stress, SIRT-1 forms a complex with FOXO3A and deacetylates FOXO3A to promote resistance to oxidative stress and cell survival (18). SIRT-1 inhibits growth hormone-stimulated STAT3 activation in mouse embryonic cells and this was associated with deacetylation of STAT3 (20). Further, it was demonstrated that acetylation of STAT3 is necessary for its activation. In another study, treatment with SIRT-1 agonists deacetylated STAT3, thus impeding STAT3 translocation to nucleus, and inhibiting T cell differentiation into Th7 and Th17 cells and thus limiting tumor growth in mice (21). SIRT-1 was also shown to inhibit LPS-induced high mobility group box 1 via STAT-1 inactivation in macrophages (19). Since TLR activation enhances SIRT-1 expression and accumulation of NAD+, and SIRT-1 negatively regulates JAK-STAT signaling, it is plausible that TLR2 may limit RV-induced IFNs via SIRT-1.
In this study, we show that RV increases SIRT-1 expression via TLR2, and SIRT-1 limits RV-induced IFN expression by inhibiting JAK/STAT signaling pathway in normal airway epithelial cells. We also demonstrate that COPD airway epithelial cells are not only defective in the expression of SIRT-1, but also display dysregulation in TLR2 activation despite expressing TLR2 and this may contribute to exaggerated IFN responses to RV in these cells.
Materials and Methods
Cell cultures
Airway epithelial cell line, BEAS-2B cells were purchased from American Type Culture Collection (Manassas, VA) and cultured in serum free airway epithelial cell growth medium (BEGM) (22). Basal cells were isolated from the tracheobronchial segments of normal donor lungs and explanted lungs from COPD patients at the time of lung transplantation as described previously (3, 23, 24). Collection of the tissue was approved by Institutional Review Board of University Michigan, Ann Arbor, MI and Temple University, Philadelphia, PA. Patient characteristics are provided in Table 1. The basal cells were cultured in 12 mm transwells as described previously (6, 23). Briefly, basal cells at passage one were cultured in Bronchial Life medium (LifeLine Cell Technologies, Frederick, MD) under submerged conditions until confluent. The cells were then lifted to air/liquid interface and cultured in differentiation medium for another 3 weeks to promote mucociliary differentiation.
Table 1:
Characteristics of patients with COPD and healthy non-smokers
| No | Age (Yr) | Gender | FEV1 (%) | Smoking history (pack years) |
Emphysema |
|---|---|---|---|---|---|
| COPD | |||||
| 1* | 68 | Male | 42 | 84 | Moderate |
| 2* | 75 | Male | 43 | 50 | Severe |
| 3 | 55 | Female | 56 | 27 | Mild |
| 4* | 73 | Male | 34 | 50 | Severe |
| 5 | 58 | Female | 25 | 70 | Moderate |
| 6 | 59 | Male | 22 | 70 | Severe |
| Healthy non-smokers | |||||
| 1* | 67 | Male | |||
| 2* | 70 | Male | |||
| 3 | 47 | Male | |||
| 4* | 75 | Male | |||
| 5* | 67 | Female | |||
| 6 | 50 | Female |
Obtained from Temple Lung Center, Temple University, Philadelphia and rest of the tissue samples were obtained from the University of Michigan.
Immunofluorescence
Different cell types in the mucociliary-differentiated cultures were quantified as previously described with some modifications (23, 25). Briefly, apical surface of the cultures was washed with PBS containing 0.15% sodium bicarbonate, rinsed with PBS, fixed in 4% paraformaldehyde and blocked with 1% BSA/5% normal donkey serum in PBS for 1h. Cultures were then incubated overnight at 4° C with polyclonal antibody to human tracheobronchial mucins (26), acetylated tubulin (MilliporeSigma, St Louis, MO), cytokeratin 5 (abcam, Cambridge, MA), or CC10 (ThermoFisher Scientific, Waltham, MA) to detect goblet cells, cilia, basal cells or club cells respectively. Unbound antibody was removed by washing and bound antibody was detected with appropriate second antibody labeled with AlexaFluor 598 or AlexaFluor 488. The cultures were counter stained with DAPI to detect nuclei. The cultures were mounted, imaged with confocal microscopy and the number of different cell types per 100 nuclei were counted in at least 10 random fields and averaged.
Transient and stable knockout of genes in airway epithelial cells
BEAS-2B cells were transfected with 10 picomoles of non-targeting (NT), or ON-TARGETplus SMART pool siRNA specific to TLR2, (Dharmacon, Chicago, IL) and incubated for 48h. To knockdown TLR2 in normal primary airway epithelial cells, basal cells were transduced with GIPZ Lentiviral vector expressing either TLR2 shRNA or non-targeting shRNA (both purchased from Dharmacon) and cultured at air/liquid interface to promote mucociliary differentiation as described previously (23). Knockdown of gene expression was confirmed by qPCR, or flow cytometry.
To obtain stable SIRT-1 knockout cell line, BEAS-2B cells were transduced with GIPZ Lentiviral vector (Dharmacon) expressing either SIRT-1 shRNA or non-targeting shRNA and cultured in the presence of 2 μg/ml puromycin to select for transduced cells. The cell line showing SIRT-1 knockdown as determined by protein expression was expanded and stored. The SIRT-1 knockout cells were maintained in BEGM containing 2 μg/ml puromycin in all the experiments.
RV and infection
RV16, a major group RV was purchased from ATCC, propagated in H1 HeLa cells, partially purified by ultrafiltration using 100 kDa cut off membrane, and viral titer was determined by plaque assay as previously described (27). Less than 100kDa fraction from purified RV preparation was used as sham control.
BEAS-2B cells were infected with sham or RV at multiplicity of infection (MOI) of 2 or equal volume of sham and incubated for 90 min at 33°C. Infection medium was replaced with fresh medium and incubation was continued at 33°C for another 24 h. Apical surface of mucociliary-differentiated normal or COPD cell cultures was washed with PBS, transwells were transferred to a new receiver plate, and the apical surface was infected with 30 μl of PBS containing RV at MOI of 2, or equivalent volume of sham and incubated at 33°C for 24 h. In selected experiments, cells were incubated at 37°C instead of 33°C after RV infection. In some experiments, cultures were pretreated with either EX-527, a specific inhibitor of SIRT-1 (Cayman Chemicals, Ann Arbor, MI) or 1 μM quercetin (Quercegen Pharmaceuticals, Sudbury, MA), a polyphenol which positively regulates SIRT-1 for 24 h and then the cell cultures were infected with RV and incubated in the presence or absence of EX-527. Cell cultures treated with DMSO (vehicle) served as controls.
Treatment of mucociliary-differentiated airway epithelial cell cultures with Pam3CSK4
Transwells containing mucociliary-differentiated airway epithelial cell cultures were transferred to new receiver plates containing fresh complete medium. Apical surface of the cell cultures was washed and treated with 1 μg/ml Pam3CSK4 (Invivogen, San Diego, CA) or 10 ng/ml FSL-1 at both apical and basolateral sides and incubated for 24 h. Cells treated with medium served as control. Medium from the basolateral chamber was collected for IL-8 determination by ELISA.
Flow cytometry
Mucociliary-differentiated airway epithelial cell cultures were dissociated using Accutase™ (STEMCELL Technologies Inc., Cambridge, MA), neutralized with serum, washed and incubated with blocking buffer. Cells were then incubated with FITC labeled-anti-human TLR2 antibody or FITC-labeled isotype control (BioLegend, San Diego, CA), washed, fixed and analyzed by flow cytometry.
To determine the endocytosis of the virus, COPD cell cultures pretreated with quercetin or vehicle were washed, infected apically with RV, and incubated for 2 h. The cells were washed to remove extracellular virus, dissociated with accutase (ThermoFisher Scientific) and endocytosed virus was detected by using monoclonal antibody to RV (MAb R16–7, kindly provided by Weiming Li, University of Wisconsin, Madison, WI) and the cells were analyzed by flow cytometry as described previously (28).
Western blot analysis
After relevant treatment, cells were washed with cold PBS and lysed in RIPA buffer containing protease and phosphatase inhibitors. Equal amount of protein was subjected to Western blot analysis with antibodies to MDA5 (Santa Cruz Biotechnology, Santa Cruz, CA), SOCS1, SOCS3 total and phospho IRF3, STAT1 or STAT2 (Cell Signaling, Danvers, MA), or β-actin (Sigma Aldrich, St. Louis, MO). Specific bands were quantified by densitometry using NIH imageJ, and the results expressed as fold change over β-actin or respective total protein.
Real time PCR
After relevant treatment, total RNA was isolated from airway epithelial cells and the expression of IFN-β, IFN-λ1, IFN-λ2, and TLR2 was determined by using gene specific primers and normalized to house-keeping gene, glyceraldehyde 3-phosphate dehydrogenase (G3PDH) (29) . Viral load was determined by quantitative qPCR and expressed as number of copies/μg of RNA as previously described (27).
ELISA
After relevant treatment, basolateral medium from mucociliary differentiated cultures and culture supernatants from submerged BEAS-2B cell cultures were collected and protein levels of IL-8 (R & D systems, Minneapolis, MN), IFN-λ1 and IFN-λ2 (MyBiosource Inc., San Diego, CA) was determined by ELISA.
Statistical analysis
The data was analyzed by using SigmaStat (Systat Software Inc., San Jose, CA), which has the capacity to determine whether the data is normally distributed (Gaussian distribution) using probability density theory. Statistical significance for normally distributed data was assessed by unpaired student T test (for comparisons between 2 groups) or by analysis of variance (ANOVA) with Student-Newman-Keuls post-hoc test (for comparisons between 3 or more groups). If the data was not normally distributed non-parametric tests such as Wilcoxon Rank Sum test to compare between 2 groups, Sign rank test for paired comparison and ANOVA on ranks with Dunn’s post-hoc test for comparing 3 or more groups. For comparison of differences between normal and COPD cultures, we used non-parametric tests because of the small sample size. A p value of ≤0.05 was considered as statistically significant.
Results
Mucociliary-differentiated COPD airway epithelial cells show attenuated responses to TLR2 agonist
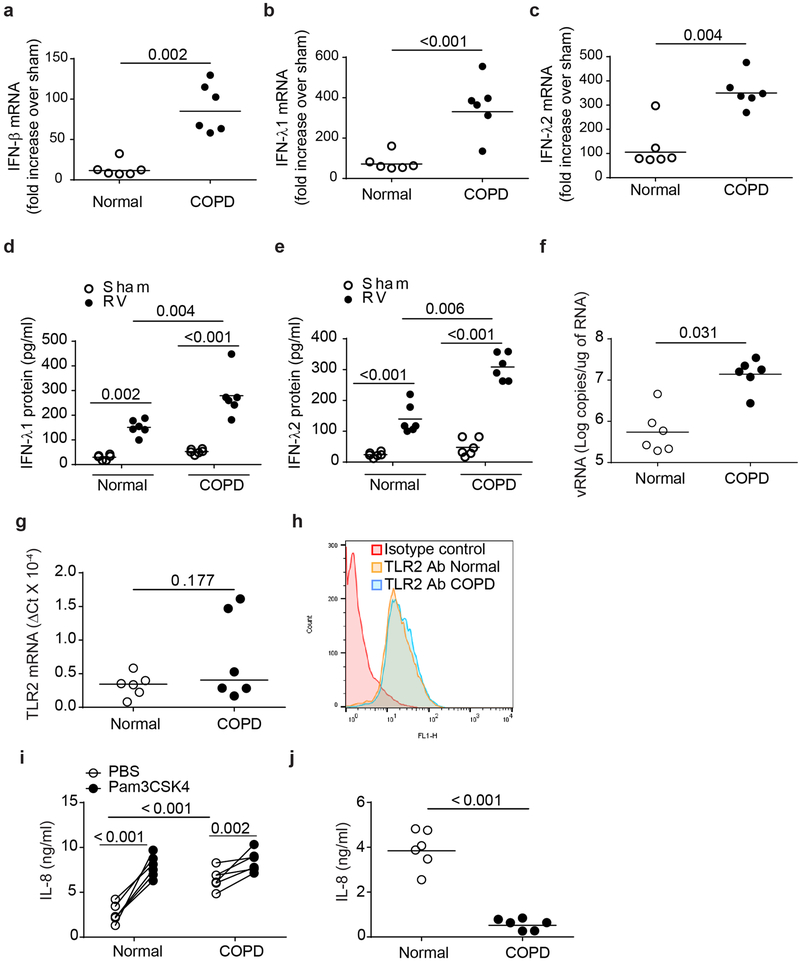
Previously, we demonstrated that COPD cultures differ from normal cultures in showing goblet cell hyperplasia and pro-inflammatory phenotype (3, 23). In this study, we determined the phenotype of mucociliary cultures by quantifying the major cell types present in the cultures by immunofluorescence microscopy. COPD cell cultures showed relatively more basal and goblet cells and less ciliated cells compared to normal cell cultures indicating goblet cell and basal cell hyperplasia (Table 2) as observed previously (23, 30). To determine the antiviral IFN responses of these cultures, we infected the COPD and normal cell cultures with sham or RV and examined for mRNA levels of IFN-β, IFN-λ1 and IFN-λ2 and protein levels of IFN-λ1 and IFN-λ2 at 24 h post-infection. As previously observed, RV-infected COPD airway epithelial cells showed higher mRNA expression of all three IFNs (Figure 1a - 1c) and proteins levels of IFN-λ1 and IFN-λ2 (Figure 1d and 1e) than similarly-infected normal cells (3). Incubation at 37° C instead of 33° C following RV infection further enhanced RV-stimulated mRNA expression of all three IFNs in both normal and COPD airway epithelial cells, but again COPD showed much higher expression of all three IFNs (Supplemental Figure 1). Despite expressing excessive levels of IFNs, COPD cells showed significantly higher viral load than normal cells (Figure 1f).
Table 2:
Quantification of major cell types in mucociliary-differentiated normal and COPD cultures
| Cell type | Basal cells | Goblet cells | Club cells | Ciliated cells |
|---|---|---|---|---|
| Healthy non-smokers | 25 ± 5.7 | 15 ± 4.1 | 8 ± 3.8 | 41 ± 8.4 |
| COPD | 38 ± 6.1* | 25 ± 2.4* | 9 ± 4.2 | 22 ± 6.4* |
Mucociliary-differentiated airway epithelial cell cultures were fixed and probed with antibodies specific to basal, goblet, Club or ciliated cells. Different cell types were counted by immunofluorescence and expressed as number of cells per 100 cells. The data represent average ± S.D. calculated from cultures established from 6 healthy non-smokers and 6 COPD subjects (*p ≤ 0.05, unpaired t test).
Figure 1.
COPD airway epithelial cells show attenuated IL-8 response to Pam3CSK4. Normal and COPD mucociliary-differentiated airway epithelial cell cultures established from airway basal cells isolated from 6 normal and 6 COPD donors were infected with sham or RV and after 24 h, IFN expression was determined by qPCR (a – c). Data was normalized to house-keeping gene, G3PDH, and expressed as fold increase over respective sham controls. Protein levels of IFNs was determined in the basolateral medium by ELISA (d and e). Viral load was determined by quantitative PCR using total RNA (f). In a parallel experiment, untreated normal and COPD airway epithelial cell cultures were used for determination of TLR2 expression at mRNA level by qPCR (g) or at protein level by flow cytometry (h). mRNA expression of TLR2 was normalized to G3PDH. Histograms in (h) are representative of 6 normal and 6 COPD cell cultures. Normal and COPD cell cultures were treated with Pam3CSK4 both apically and basolaterally, incubated for 24 hours and IL-8 was measured in the basolateral medium by ELISA (i). IL-8 protein was also determined by intracomparison to detect the attenuated responses to Pam3CSK4 in COPD cells (j). Data in a – g, i and j represent median with range and statistical significance was determined by using either Wilcoxon rank-sum test, (a, b, c, f, g, j), ANOVA on ranks (d and e) or sign rank test, paired comparison (i).
Since interaction of RV with TLR2 limits RV-induced type I and type III IFN responses in normal airway epithelial cells (6), we examined whether reduced expression or dysregulated activation of TLR2 contribute to RV-induced exaggerated IFN expression in COPD airway epithelial cells. First, we examined the expression of TLR2 in COPD airway epithelial cell cultures at both mRNA and protein level by qPCR and flow cytometry respectively. There was no difference in the expression of TLR2 between normal and COPD mucociliary-differentiated airway epithelial cell cultures at either mRNA or protein level (Figure 1g and 1h). To determine if COPD cells are defective in TLR2 activation, we examined the IL-8 responses to Pam3CSK4, a TLR2 agonist that is recognized by TLR2/TLR1 heterodimer. As previously observed COPD airway epithelial cells showed higher levels of IL-8 than normal cells under unstimulated conditions (Figure 1i) (3, 24). However, in response to Pam3CSK4 treatment, COPD cells showed attenuated IL-8 responses than similarly treated normal airway epithelial cells (Figure 1i and 1j). Similarly attenuated IL-8 response was observed in COPD cells when challenged with another TLR2 ligand FSL-1, an agonist that signals through TLR2/TLR6 heterodimer (Supplemental Figure 2). These results indicate that COPD airway epithelial cells show defect in activation rather than expression of TLR2. Since TLR2 interaction with RV limits RV-induced IFN responses, we postulated that dysregulated TLR2 signaling may contribute to exaggerated IFN responses to RV infection in COPD airway epithelial cells. Therefore, we focused on determining the mechanisms by which TLR2 limits RV-induced IFN responses using TLR2 agonist PAM3CSK4 and incubating cells at 33°C when infected with RV.
RV-induced TLR2 activation does not suppress dsRNA receptor signaling.
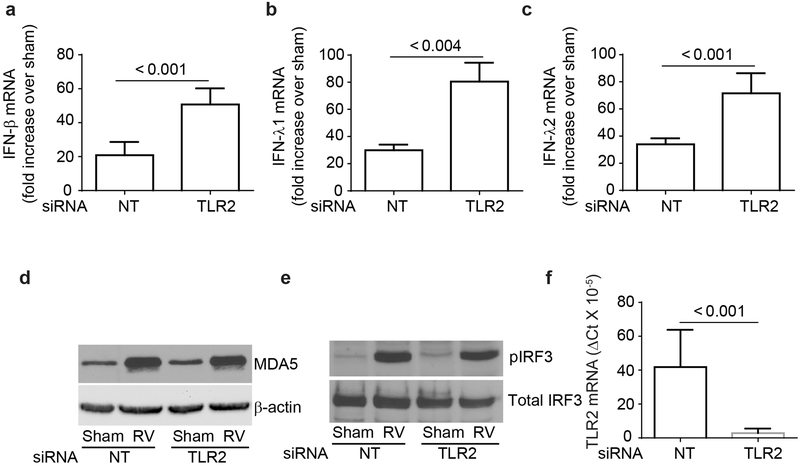
RV has been shown to induce IFN responses through activation of dsRNA receptor, MDA5 and subsequent phosphorylation of IRF3 (4). To determine whether TLR2 limits RV-induced IFN response by downregulating MDA5 signaling, BEAS-2B cells transfected with NT or TLR2 siRNA were infected with RV or sham and the expression of IFNs, MDA5, and phosphorylation of IRF3 were determined. As previously observed, RV-stimulated IFN expression was significantly higher in cells transfected with TLR2 siRNA than in cells transfected with NT siRNA (Figure 2a-2c). Expression of MDA5 increased in both NT and TLR2 siRNA-transfected cells equally following RV infection (Figure 2d). Further, there was no difference in the RV-induced phosphorylation of IRF3 between NT and TLR2 siRNA-transfected cells (Figure 2e). These results indicate that TLR2 may not affect the first wave RV-induced IFN responses that depends on dsRNA receptor signaling. Knockdown of TLR2 was confirmed by qPCR (Figure 2f).
Figure 2.
TLR2-regulated RV-induced IFNs is not due to dysregulation in MDA5 signaling pathway. BEAS-2B cells were either transfected with TLR2 or non-targeting (NT) siRNA, infected with sham or RV and incubated for 24 h and mRNA expression of IFNs was assessed by RT-qPCR (a – c). Data was normalized to G3PDH and expressed as fold change over sham-infected cells. Similarly infected cultures were lysed in RIPA buffer after 4h incubation, and cell lysates containing equal amounts of total protein were subjected to Western blot analysis with antibodies to MDA5 and β-actin (d) or phospho- and total IRF3 (e). Images are representative of 3 independent experiments. Total RNA from NT or TLR2 siRNA-transfected cells was subjected to RT-qPCR to determine the expression of TLR2 and the data normalized to G3PDH (f). Data in a – c, and f represent average ± SEM calculated from three independent experiments (t test).
TLR2 limits STAT activation following RV infection
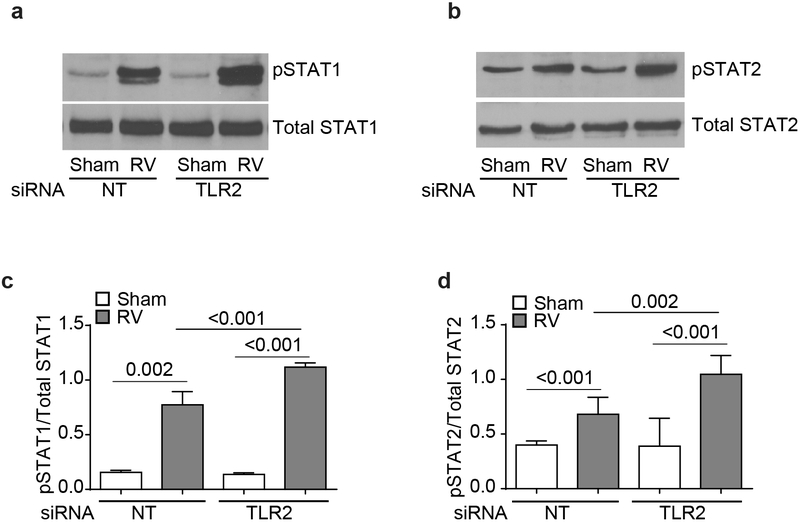
RV-induced type I and type III interferons activates JAK/STAT signaling pathway to amplify the expression of IFNs. Therefore, we examined whether TLR2 signaling affects STAT1/2 activation following RV infection. Interestingly, RV-infected TLR2 siRNA-transfected cells showed significantly higher phosphorylation of both STAT1 and STAT2 than similarly-infected NT siRNA-transfected cells (Figure 3a - 3d). These results imply that TLR2 may limit RV-induced IFN responses by attenuating activation of JAK/STAT signaling pathway stimulated by first wave of IFNs expressed via dsRNA receptor activation.
Figure 3.
TLR2 reduces RV-induced STAT phosphorylation. NT- or TLR2 siRNA-transfected cells were infected with RV or sham, and incubated for 24 h. Cell lysates containing equal amount of protein were subjected to Western blot analysis with antibodies to total or phospho-STAT1 or STAT2 antibodies. Images are representative of 3 to 4 independent experiments (a and b). Band intensities of total and phospho-STAT1 and STAT2 were determined by using NIH imageJ and expressed as ratio of phospho-STAT/total STAT (c and d). Data represents average ± SEM calculated from 3 to 4 independent experiments (ANOVA).
TLR2 inactivates STAT1 and STAT2 via SIRT-1 and not SOCS proteins.
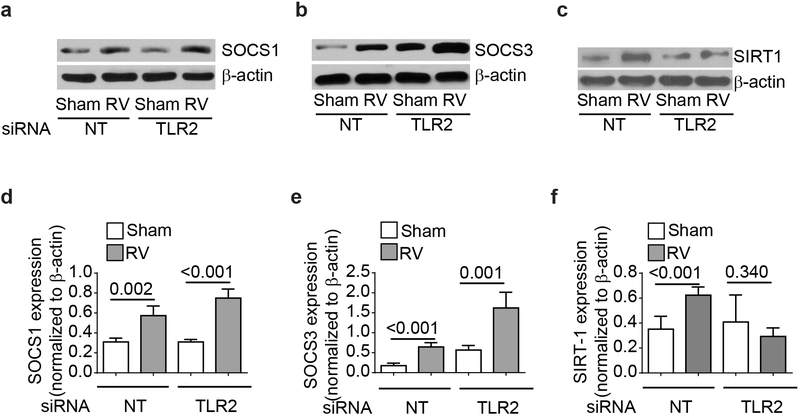
RV has been shown to upregulate SOCS1 and SOCS3 in airway epithelial cells at 24 hours post-infection (15). Since SOCS proteins inhibit STAT activation, we examined whether TLR2 limits STAT activation via enhancing SOCS expression following RV infection. We found that RV increases expression of SOCS1 in BEAS-2B cells and there was no difference between NT and TLR2 siRNA-transfected cells (Figure 4a, and 4c). Interestingly, sham-infected TLR2 siRNA-transfected cells showed higher SOCS3 expression than NT siRNA-transfected cells, which further increased following RV infection with net increase similar to NT siRNA-transfected cells (Figure 4b, and 4e). These results indicate that TLR2 may reduce STAT1 activation through inhibitory molecules other than SOCS proteins.
Figure 4.
TLR2 contributes to expression of RV-induced SIRT-1 but not SOCS protein. NT- or TLR2 siRNA-transfected cells were infected with RV or sham, incubated for 24 h and cells were lysed in RIPA buffer. Cell lysates containing equal amounts of protein were subjected to Western blot analysis with antibodies to SOCS1, SOCS3, SIRT-1 and β-actin (a – c). Images are representative of 3 to 4 independent experiments. Intensity of the bands was determined by using imageJ and normalized to β-actin (d - f). Data represents average ± SEM calculated from 3 to 4 independent experiments (T test).
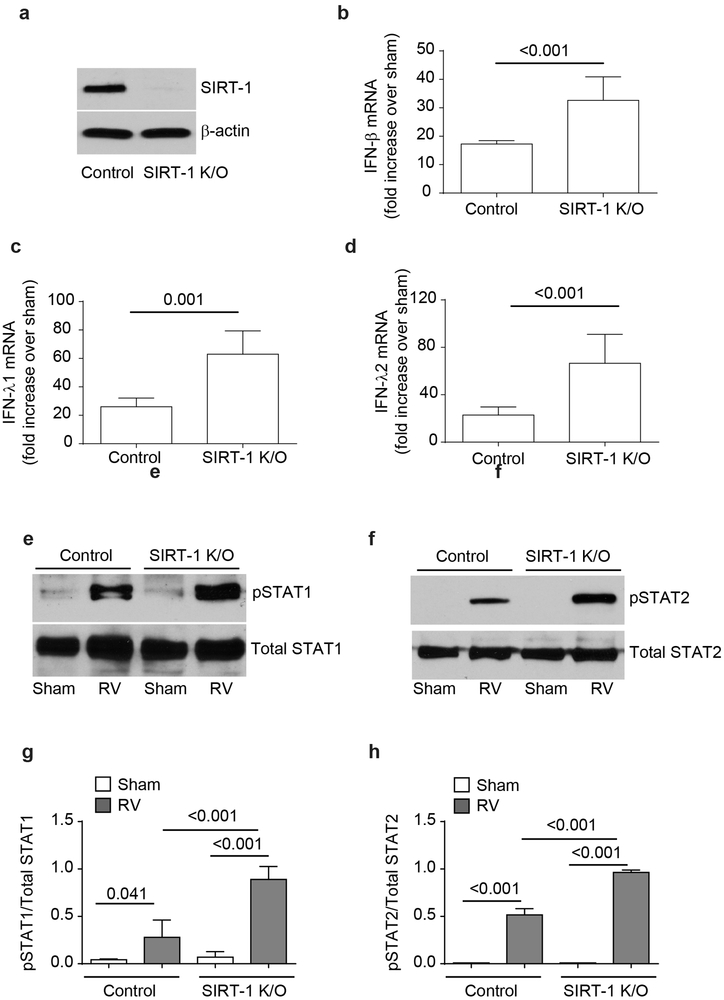
Since SIRT-1 plays a role in STAT1 and STAT3 inactivation (19-21), we next assessed the expression of SIRT-1 following RV infection. We found that RV significantly enhanced the expression of SIRT-1 protein in NT siRNA-transfected cells (Figure 4c and 4f), but not in TLR2 siRNA-transfected cells. Such increase in SIRT-1 was not observed when cells were infected with UV-RV (data not shown). To examine the contribution of SIRT-1 in limiting RV-induced IFN responses, we generated stable SIRT-1 knockout BEAS-2B cell line (Figure. 5a) and assessed the RV-induced IFN expression. Compared to control, SIRT-1 knockout cells showed higher mRNA expression of IFN-β, IFN-λ1 and IFN-λ2 in response to RV infection at 24 h post-infection (Figure 5b - 5d). Additionally following RV infection, SIRT-1 knock out cells also showed enhanced STAT1 and STAT2 phosphorylation compared to control cells (Figure 5e - 5h). These results imply that TLR2 may limit RV-induced IFN expression via upregulation of SIRT-1 expression and subsequent reduction in STAT1 and STAT2 activation.
Figure 5.
SIRT-1 knockout cells show exaggerated IFN responses to RV infection. Cell lysates from control or SIRT-1 knockout cells were subjected to Western blot analysis with SIRT-1 to confirm knockdown of SIRT-1 (a). SIRT-1 knockout or control cells were infected with RV and after 24h, mRNA expression of IFN-β, IFN-λ1 and IFN-λ2 was assessed by qPCR, IFN expression was normalized to G3PDH and then expressed as fold increase over sham (b – d). In some experiments, total proteins from Sham or RV-infected control and SIRT-1 knockout cells was subjected to Western blot analysis with total and phopho-STAT1 and STAT2 antibodies (e and f) and band intensities were quantified and expressed as ratio of phopho-STAT/total STAT (g and h). Images are representative of 4 independent experiments. Data represents average ± SEM calculated from 4 independent experiments (b – d, g and h). Data was analyzed by unpaired T test (b – d) or by ANOVA (g and h).
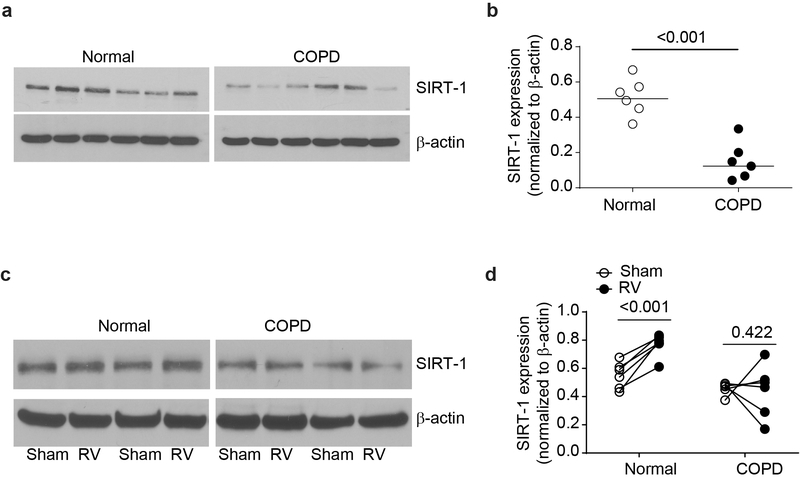
RV does not induce SIRT-1 expression in COPD airway epithelial cell cultures
Previously, we demonstrated that compared to normal, COPD airway epithelial cell cultures show reduced expression of SIRT-1 mRNA (3). In this study, we found that compared to normal, mucociliary-differentiated COPD airway epithelial cell cultures also show reduced protein levels of SIRT-1 (Figure 6a and 6b). Following RV infection, SIRT-1 levels increased significantly in the normal but not in COPD epithelial cell cultures (Figure 6c and 6d).
Figure 6.
COPD airway epithelial cell cultures show defect in the expression of SIRT-1. Total protein was isolated from COPD or normal mucociliary-differentiated airway epithelial cell cultures and subjected to Western blot analysis with SIRT-1 antibody (a). Density of the SIRT-1 band was normalized to β-actin and presented as range with median (b). In some experiments, COPD and normal airway epithelial cell cultures were infected with sham or RV and incubated at 33°C for 24 h and the SIRT-1 protein expression was determined by Western blot analysis (c and d). Density of the SIRT-1 bands was normalized to β-actin and data represent intracomparison of SIRT-1 expression between sham and RV-infected cells for each culture (d). Images in a is representative of cultures established from 6 independent donors from each group. The data in b represent range with median and statistical significance was determined by using Wilcoxon rank-sum test. Data in d was analyzed by paired analysis using sign rank test.
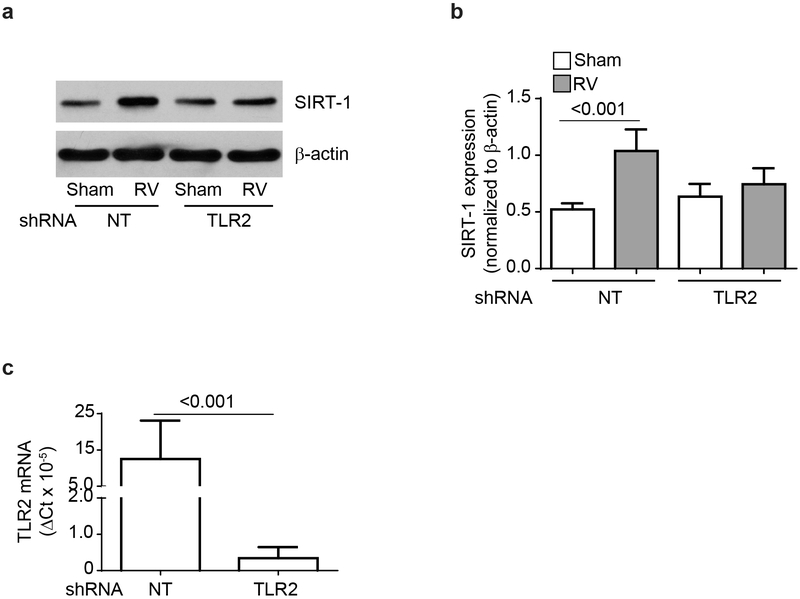
Since, COPD cells show dysregulated TLR2 signaling, and fail to show increase in the SIRT-1 expression following RV infection, we examined whether RV induces SIRT-1 via TLR2 activation in normal airway epithelial cells. Genetic inhibition of TLR2 in the mucociliary-differentiated normal airway epithelial cell cultures attenuated RV-induced SIRT-1 levels (Figure 7a and 7b). Genetic inhibition of TLR2 by shRNA was confirmed by qPCR (Figure 7c). These results confirm that TLR2 regulates RV-induced SIRT-1 not only in airway epithelial cell line, but also in normal primary mucociliary-differentiated airway epithelial cells.
Figure 7.
TLR2 is required for RV-induced SIRT-1 expression in primary airway epithelial cell cultures. Normal basal airway epithelial cells were transduced with non-targeting (NT) or TLR2 shRNA expressing lentivector and cultured at air/liquid interface to promote mucociliary differentiation. Cell cultures were then apically infected with RV or sham and SIRT-1 expression was determined at 24 h post infection by Western blot analysis (a). Image is representative of 3 independent experiments). Data was normalized to β-actin and data presented represent mean ± SEM calculated from 3 independent experiments and statistical significance was determined by T test within each group (b). From a parallel experiments, total RNA was isolated and subjected to qPCR to determine the expression of TLR2 (c). Data represent mean ± SEM calculated from 3 independent experiments and data was analyzed by T test.
SIRT-1 regulates RV-induced IFN expression in primary airway epithelial cells
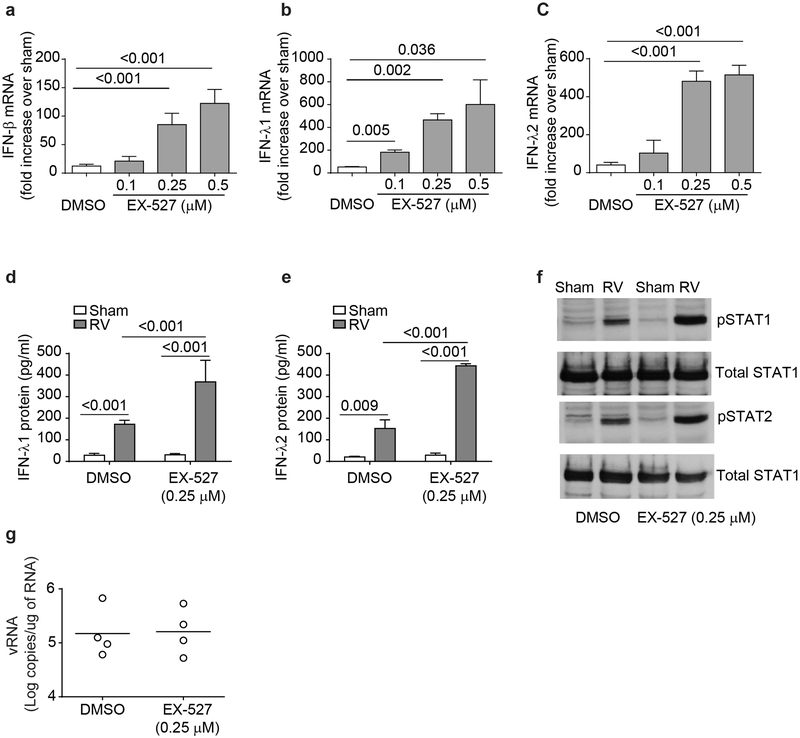
To determine the role of SIRT-1 in limiting RV-induced IFN responses in primary normal mucociliary-differentiated airway epithelial cells, normal cell cultures were treated with EX-527, a specific chemical inhibitor of SIRT-1, infected with sham or RV, and examined for IFN expression. Airway epithelial cells treated with EX-527 and infected with RV showed significantly higher mRNA levels of IFN-β, IFN-λ1 and IFN-λ2 and protein levels of IFN-λ1 and IFN-λ2 than similarly-infected vehicle-treated cells (Figure 8a - 8e). This was associated with increase in the phosphorylation of both STAT1 and STAT2 (Figure 8f). However, despite increased IFN expression, RV load remained unchanged in cells treated with EX-527 (Figure 8g).
Figure 8.
Inhibition of SIRT-1 enhances RV-induced IFN responses in normal airway epithelial cells. Normal mucociliary-differentiated cell cultures were pretreated with EX-527 for 24 h, infected with sham or RV and then incubated in the presence or absence of EX-527 for another 24 h. Total RNA was isolated and subjected to qPCR to determine mRNA expression of IFNs (a - c). Protein levels of IFNs in the basolateral medium was assessed by ELISA (d and e). From some experiments, total protein was isolated and subjected to Western blot analysis with total and phospho-STAT1 and STAT2 antibodies (f). Image is representative of 4 independent experiments. Viral load was determined by quantitative RT-qPCR and data represent range with median from 4 experiments (g). Data in a - e represent mean ± SEM calculated from 4 independent experiments and statistical significance was determined by ANOVA.
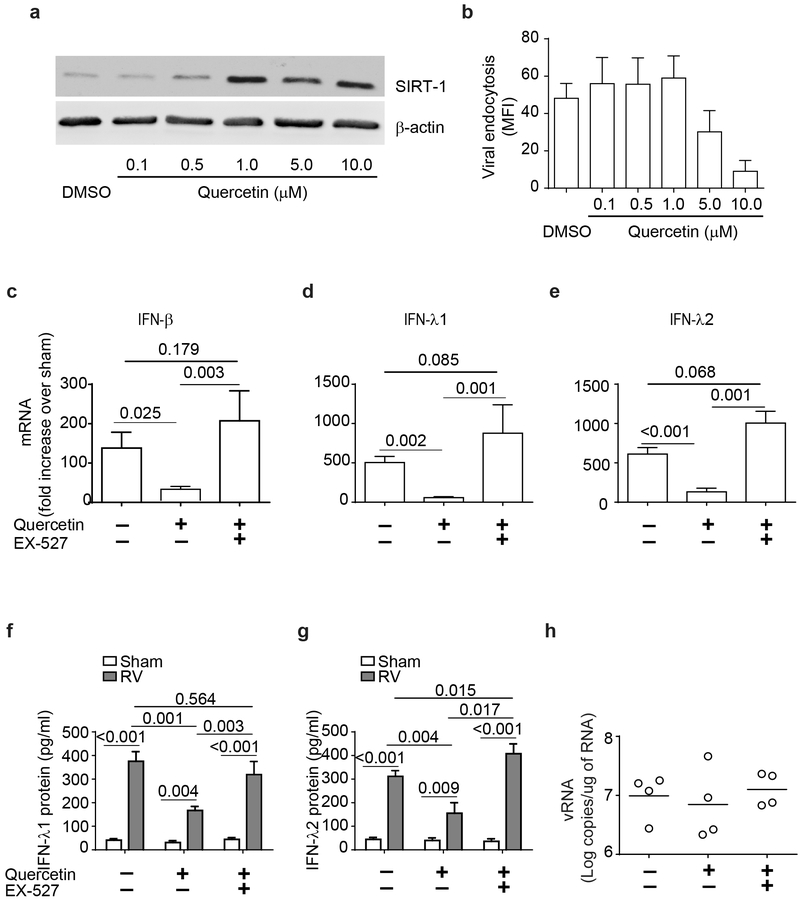
Previously, we have demonstrated that quercetin treatment enhances SIRT-1 levels in a mouse model of COPD (31). Therefore, we used quercetin to increase SIRT-1 levels in COPD airway epithelial cells to examine whether SIRT-1 also regulates RV-induced IFN responses in these cells. Initial experiment was conducted to optimize quercetin concentration that is required for maximal expression of SIRT-1 protein in COPD cells without affecting the viral endocytosis. We found that quercetin at as low as 1 μM significantly increased SIRT-1 levels in COPD cells without affecting endocytosis of virus (Figure 9a and 9b). Therefore, in subsequent experiments, mucociliary-differentiated COPD cells were pretreated with 1 μM quercetin for 24 h and then examined for IFN responses to RV in the presence or absence of EX-527. Quercetin pretreatment significantly attenuated RV-induced IFN responses in COPD airway epithelial cells (Figure 9c - 9g) and the IFN levels were similar to that observed in RV-infected normal cells (Table 3). Treatment with EX-527, a SIRT-1 inhibitor restored the levels of RV-induced IFN expression in quercetin-pretreated cells indicating that quercetin exerts its effect through SIRT-1. Interestingly there was no significant difference in the viral load between the untreated and cell treated with quercetin alone or with quercetin and EX-527 (Figure 9h).
Figure 9.
Quercetin treatment reduces RV-induced IFN expression via SIRT-1. Mucociliary-differentiated COPD airway epithelial cell cultures were treated with varying concentrations of quercetin for 24 h and SIRT-1 expression and viral endocytosis was determined by Western blot analysis and Flow cytometry respectively (a and b). COPD airway epithelial cell cultures were pretreated with 1 μM quercetin for 24 h, infected with sham or RV, incubated for 24 h in the presence or absence of EX-527and mRNA expression of IFNs was determined. Data was normalized to G3PDH and then expressed as fold increase over sham (c - e). Basolateral medium was analyzed by ELISA to determine the protein levels of IFNs (f and g). Viral load was determined by quantitative RT-qPCR and data represent range with median (h). Data represents mean ± SEM calculated from 4 independent experiments (a – e) and statistical significance analyzed by ANOVA.
Table 3:
Quercetin pretreatment normalizes RV-induced type I and type III IFN responses in COPD airway epithelial cells
| IFNs mRNA | Normal | COPD | |
|---|---|---|---|
| DMSO | Quercetin | ||
| IFN-β | 13.43 ± 3.91 | 138.10 ± 20.11* | 34.58 ± 6.71*$ |
| IFN-λ1 | 78.54 ± 17.02 | 504.04 ± 39.17* | 58.03 ± 6.07* |
| IFN-λ2 | 122.3 ± 35.72 | 611.81 ± 41.16* | 131.40 ± 23.67* |
COPD airway epithelial cells pretreated with 1 μM quercetin or vehicle (DMSO) for 16h and normal airway epithelial cells were infected with sham or RV and IFN mRNA levels were determined at 24 post-infection. Data was normalized to G3PDH and expressed as fold increase over sham. Data represent mean ± SEM calculated from 4 independent experiments (*p ≤ 0.05, different from normal; $ different from DMSO treated COPD, ANOVA).
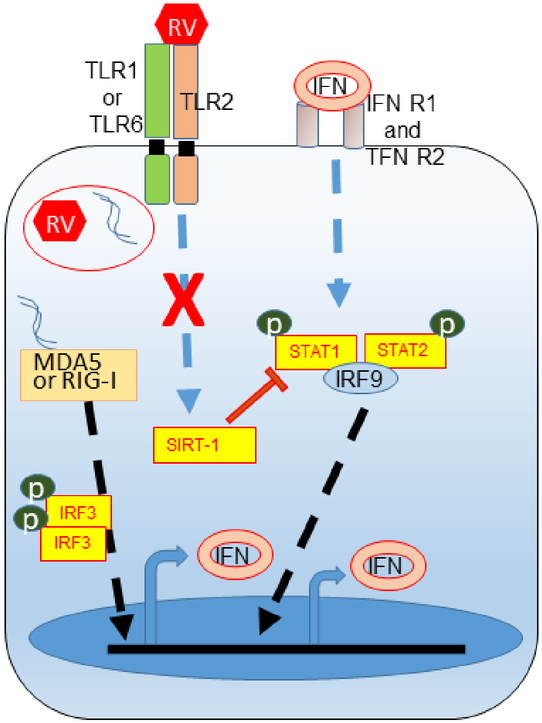
Taken together, these results indicate that TLR2 regulates RV-induced expression of IFNs via SIRT-1 in airway epithelial cells. Both SIRT-1 expression and TLR2 signaling pathway are attenuated in COPD airway epithelial cells and this may lead to exaggerated IFN expression in response to RV infection in these cells (Figure 10).
Figure 10.
Illustration showing defective mechanisms in COPD airway epithelial cells that leads to exaggerated IFN responses to RV infection
In normal cells RV-induced TLR2 signaling induces SIRT-1 activation, which in turn limits amplification of RV-stimulated IFN expression by inhibiting JAK/STAT pathway. COPD cells are defective in both TLR2 signaling and expression of SIRT-1 which leads to excessive amplification of RV-induced IFN expression.
Discussion
This study highlights one of the mechanisms that regulates RV-induced IFN expression in normal airway epithelial cells, thus preventing the exaggerated IFN expression. Our previous study indicated that TLR2 is necessary for limiting RV-induced IFN expression in normal airway epithelial cells, but the underlying molecular mechanisms were not elucidated (6). Here we demonstrate for the first time that RV induces TLR2-dependent SIRT-1 expression, which reduces amplification of IFNs via inhibition of JAK-STAT signaling pathway. COPD cells, which show exaggerated IFN expression following RV infection were found to be defective not only in activating TLR2 signaling, but also in the expression of SIRT-1. Finally, we show that quercetin, a polyphenol, normalizes RV-induced expression of IFNs in COPD cells via SIRT-1.
Activation of TLR2 or EGFR signaling has been shown to attenuate IFN responses to virus infection (6, 32). EGFR activation induced by RV was shown to attenuate subsequent type III IFN response in BEAS-2B cells and this was associated with increased persistence of virus (32). Activation of TLR2 signaling by TLR2 agonist was shown to deplete interleukin receptor associated kinase −1 (IRAK-1), and this in turn inhibited IFN expression stimulated by single strand (ss) RNA, which signals through MyD88-dependent TLR7/TLR9 signaling pathway (33). IRAK-1 is essential downstream adapter protein in MyD88-dependent TLR7/TLR9 signaling pathway. Subsequently we showed that RV, which interacts with TLR2 and also depletes IRAK-1 (34). However, inhibition of TLR2 or IRAK-1 in BEAS-2B cells enhanced, instead of attenuating RV-induced IFN expression (6). This is not surprising, because, RV primarily induces IFN responses via dsRNA generated during replication (4, 7), but not by ssRNA. In this study, we confirmed that TLR2 also limits RV-induced IFN responses in primary airway epithelial cells from normal subjects. COPD airway epithelial cells, which show exaggerated IFN responses to RV infection were found to be defective in activating TLR2 signaling pathway despite expressing TLR2 similar to normal cells. We are currently investing the mechanisms underlying dysregulated TLR2 signaling in COPD airway epithelial cells and is a topic for future publication.
Accumulating evidences indicate that some DNA viruses induce IFN-β expression via TLR2 signaling which subsequently activates IRF1 and IRF7 in inflammatory monocytes (35, 36). In another study, prior activation of TLR2 was shown to enhance type I IFN production to subsequent challenge with agonists of TLR and dsRNA receptors through increased activation of IRF3 in monocytes (37). In contrast, we found that activation of TLR2 by RV attenuates subsequent type I and type III IFN responses in airway epithelial cells. Additionally, knockdown of TLR2 had no effect on either RV-induced expression of dsRNA receptor, MDA5 or activation of IRF3 in airway epithelial cells. The observed discrepancy may be due to different cell types used, that is monocyte versus epithelial cells. These observations also indicate that TLR2 activation by RV may not affect dsRNA mediated initial wave of IFN responses in RV-infected cells.
Activation of JAK/STAT signaling amplifies IFN expression in virus-infected cells (38-40). Interestingly, we found that TLR2 reduces RV-induced STAT1 and STAT2 activation indicating TLR2 may inhibit amplification of IFNs that involves JAK/STAT signaling pathway. Activation of TLR4 or TLR2 signaling has been shown to increase SOCS1 and SOCS3 expression via p38 MAP kinase to regulate pathogen activated inflammatory responses (41, 42). RV infection activates p38 in airway epithelial cells (43), and this may induce SOCS expression. Although the mechanisms were not delineated, RV was reported to increase expression of both SOCS1 and SOCS3 in airway epithelial cells (15). Further, enhanced SOCS1expression was thought to contribute to attenuated IFN production in response to RV in airway epithelial cells from asthmatic patients. SOCS proteins inhibit JAK/STAT signaling pathway and therefore it is conceivable that TLR2 may limit RV- induced IFN expression via SOCS1/SOCS3. However, although RV induced expression of both SOCS1 and SOCS3 as previously observed (15), knockdown of TLR2 had no effect on RV-induced SOCS proteins indicating that TLR2 may not attenuate JAK/STAT signaling via SOCS1/SOCS3.
In recent years, SIRT-1 has been shown to negatively regulate STAT activation by deacetylating STAT1 and STAT3 (19-21, 44). Moreover, SIRT-1 expression is reduced in the lungs of COPD patients, and in COPD airway epithelial cells cultured in vitro (3, 45, 46). In the present study, we found that RV significantly increases SIRT-1 expression in normal, but not in COPD airway epithelial cells. Additionally, while knockdown of TLR2 inhibited RV-induced SIRT-1 and increased IFN expression in normal cells, restoring SIRT-1 levels by treatment with quercetin normalized RV-induced IFN expression in COPD airway epithelial cells. Therefore, it is plausible that TLR2-dependent RV-induced SIRT-1 may contribute to exaggerated IFN responses RV infection. We recognize that, quercetin which inhibits PI-3 kinase activity may inhibit viral endocytosis, which is an essential first step in viral replication-dependent IFN expression (47-49). However, this is unlikely, because quercetin was used at much lower concentration, that is at 1μM, and at this concentration viral endocytosis was not affected. Secondly, quercetin was removed prior to infecting the cells with RV and previously we have shown that RV replication was inhibited only in the presence of quercetin (47).
COPD cell cultures show different cellular composition including goblet and basal cell hyperplasia, and reduction in the number of ciliated cells compared to normal cell cultures. Such differences in cellular composition can potentially affect the distribution of TLR2 receptor (cell surface versus intracellular expression), which influences availability of TLR2 receptors for RV interaction, or expression of downstream molecules in the TLR2 signaling pathway that leads to SIRT-1 expression. Therefore, the change in cellular composition may potentially influence RV induced IFN responses in COPD cells.
IFNs have been demonstrated to play an important role in viral clearance by stimulating ISGs, which has broad spectrum antiviral activity (8, 9). COPD cells despite expressing exaggerated levels of IFNs in response to RV infection, show higher viral load than normal cells. Additionally increasing IFNs expression by inhibiting either TLR2 or SIRT-1 did not enhance clearance of virus in normal cells. These observations imply that exaggerated expression of IFNs may not always translate to augmented viral clearance, but it may rather contribute to inflammation. SIRT-1, which negatively regulates IFNs expression at amplification phase may therefore prevent exuberant inflammation, following infection.
RV has been shown to interact with TLR2 via capsid protein (50). Consistent with this finding, previously we demonstrated that UV- RV, which has intact capsid protein interacts with TLR2 and depletes IRAK-1 similar to replication sufficient RV (34). However in this study, we found that although UV-RV interacts with airway epithelial cells, it does not increase SIRT-1 expression implying that effector factors downstream of TLR2 signaling stimulated by intact RV may be necessary for SIRT-1 regulation.
One of the limitations of the present study is the absence of control ex-smoker group. This is due to unavailability of cells from healthy ex-smokers with comparable smoking history to our COPD cohort. Therefore, it is not possible to specify whether the observed RV-induced exaggerated IFN responses is the consequence of COPD or of smoking.
In summary, as far as we know this is the first report to demonstrate a role for TLR2 in limiting RV-induced IFN responses via SIRT-1 in airway epithelial cells. Further, we show that COPD cells have dysregulated TLR2 signaling axis, despite expressing TLR2 and this may contribute to exaggerated IFN responses to RV infection in these cells. Finally, we show that quercetin modulates IFN responses to RV by upregulating the expression of SIRT-1. SIRT-1 being a deacetylase, it also negatively regulates pro-inflammatory responses. Based on these results, we speculate that increasing SIRT-1 levels may prevent RV-induced exacerbations in COPD.
Supplementary Material
Key points.
Interaction of RV with TLR2 regulates RV-induced IFN expression via SIRT-1
SIRT-1 regulates RV-induced IFN expression via inhibition of JAK-STAT pathway
RV-induced excessive IFNs in COPD is associated with defective TLR2 signaling
Acknowledgements
We thank Dr. F Martinez (Weill Cornell School of Medicine, New York) and Temple Lung Center Biobank for providing bronchial segments from normal and COPD subjects at University of Michigan, Ann Arbor and Temple University, Philadelphia respectively.
Grant Support: This work was supported by NIH grant HL897720, AT007620 to US.
Abbreviations used:
- dsRNA
double stranded RNA
- COPD
chronic obstructive pulmonary disease
- IFN
interferon
- IRF
interferon regulatory factor
- MDA5
melanoma differentiation-associated gene 5
- NT siRNA
non-targeting siRNA
- RIG-I
retinoic acid-inducible gene I
- TLR
toll-like receptor
- MOI
multiplicity of infection
- RV
rhinovirus
References
- 1.Wilkinson TMA, Aris E, Bourne S, Clarke SC, Peeters M, Pascal TG, Schoonbroodt S, Tuck AC, Kim V, Ostridge K, Staples KJ, Williams N, Williams A, Wootton S, Devaster JM, and Group AS. 2017. A prospective, observational cohort study of the seasonal dynamics of airway pathogens in the aetiology of exacerbations in COPD. Thorax 72: 919–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mallia P, Message SD, Gielen V, Contoli M, Gray K, Kebadze T, Aniscenko J, Laza-Stanca V, Edwards MR, Slater L, Papi A, Stanciu LA, Kon OM, Johnson M, and Johnston SL. 2011. Experimental rhinovirus infection as a human model of chronic obstructive pulmonary disease exacerbation. Am J Respir Crit Care Med 183: 734–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schneider D, Ganesan S, Comstock AT, Meldrum CA, Mahidhara R, Goldsmith AM, Curtis JL, Martinez FJ, Hershenson MB, and Sajjan U. 2010. Increased cytokine response of rhinovirus-infected airway epithelial cells in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 182: 332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Q, Nagarkar DR, Bowman ER, Schneider D, Gosangi B, Lei J, Zhao Y, McHenry CL, Burgens RV, Miller DJ, Sajjan U, and Hershenson MB. 2009. Role of double-stranded RNA pattern recognition receptors in rhinovirus-induced airway epithelial cell responses. J Immunol 183: 6989–6997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farazuddin M, Mishra R, Jing Y, Srivastava V, Comstock AT, and Sajjan US. 2018. Quercetin prevents rhinovirus-induced progression of lung disease in mice with COPD phenotype. PLoS One 13: e0199612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ganesan S, Pham D, Jing Y, Farazuddin M, Hudy MH, Unger B, Comstock AT, Proud D, Lauring AS, and Sajjan US. 2016. TLR2 Activation Limits Rhinovirus-Stimulated CXCL-10 by Attenuating IRAK-1-Dependent IL-33 Receptor Signaling in Human Bronchial Epithelial Cells. J Immunol 197: 2409–2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slater L, Bartlett NW, Haas JJ, Zhu J, Message SD, Walton RP, Sykes A, Dahdaleh S, Clarke DL, Belvisi MG, Kon OM, Fujita T, Jeffery PK, Johnston SL, and Edwards MR. 2010. Co-ordinated role of TLR3, RIG-I and MDA5 in the innate response to rhinovirus in bronchial epithelium. PLoS Pathog 6: e1001178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaitin DA, and Schreiber G. 2007. Upregulation of a small subset of genes drives type I interferon-induced antiviral memory. J Interferon Cytokine Res 27: 653–664. [DOI] [PubMed] [Google Scholar]
- 9.Haller O, Kochs G, and Weber F. 2006. The interferon response circuit: induction and suppression by pathogenic viruses. Virology 344: 119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davidson S, Crotta S, McCabe TM, and Wack A. 2014. Pathogenic potential of interferon alphabeta in acute influenza infection. Nat Commun 5: 3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altman MC, Reeves SR, Parker AR, Whalen E, Misura KM, Barrow KA, James RG, Hallstrand TS, Ziegler SF, and Debley JS. 2018. Interferon response to respiratory syncytial virus by bronchial epithelium from children with asthma is inversely correlated with pulmonary function. J Allergy Clin Immunol 142: 451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arimoto KI, Miyauchi S, Stoner SA, Fan JB, and Zhang DE. 2018. Negative regulation of type I IFN signaling. J Leukoc Biol. [DOI] [PubMed] [Google Scholar]
- 13.Makris S, Paulsen M, and Johansson C. 2017. Type I Interferons as Regulators of Lung Inflammation. Frontiers in immunology 8: 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oshansky CM, Krunkosky TM, Barber J, Jones LP, and Tripp RA. 2009. Respiratory syncytial virus proteins modulate suppressors of cytokine signaling 1 and 3 and the type I interferon response to infection by a toll-like receptor pathway. Viral Immunol 22: 147–161. [DOI] [PubMed] [Google Scholar]
- 15.Gielen V, Sykes A, Zhu J, Chan B, Macintyre J, Regamey N, Kieninger E, Gupta A, Shoemark A, Bossley C, Davies J, Saglani S, Walker P, Nicholson SE, Dalpke AH, Kon OM, Bush A, Johnston SL, and Edwards MR. 2015. Increased nuclear suppressor of cytokine signaling 1 in asthmatic bronchial epithelium suppresses rhinovirus induction of innate interferons. J Allergy Clin Immunol 136: 177–188 e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu TF, Yoza BK, El Gazzar M, Vachharajani VT, and McCall CE. 2011. NAD+-dependent SIRT1 deacetylase participates in epigenetic reprogramming during endotoxin tolerance. J Biol Chem 286: 9856–9864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, Guarente L, and Gu W. 2001. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell 107: 137–148. [DOI] [PubMed] [Google Scholar]
- 18.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, and Greenberg ME. 2004. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303: 2011–2015. [DOI] [PubMed] [Google Scholar]
- 19.Kim YM, Park EJ, Kim JH, Park SW, Kim HJ, and Chang KC. 2016. Ethyl pyruvate inhibits the acetylation and release of HMGB1 via effects on SIRT1/STAT signaling in LPS-activated RAW264.7 cells and peritoneal macrophages. Int Immunopharmacol 41: 98–105. [DOI] [PubMed] [Google Scholar]
- 20.Nie Y, Erion DM, Yuan Z, Dietrich M, Shulman GI, Horvath TL, and Gao Q. 2009. STAT3 inhibition of gluconeogenesis is downregulated by SirT1. Nat Cell Biol 11: 492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Limagne E, Thibaudin M, Euvrard R, Berger H, Chalons P, Vegan F, Humblin E, Boidot R, Rebe C, Derangere V, Ladoire S, Apetoh L, Delmas D, and Ghiringhelli F. 2017. Sirtuin-1 Activation Controls Tumor Growth by Impeding Th17 Differentiation via STAT3 Deacetylation. Cell reports 19: 746–759. [DOI] [PubMed] [Google Scholar]
- 22.Fulcher ML, Gabriel S, Burns KA, Yankaskas JR, and Randell SH. 2005. Well-differentiated human airway epithelial cell cultures. Methods in molecular medicine 107: 183–206. [DOI] [PubMed] [Google Scholar]
- 23.Jing Y, Gimenes J, Sharma R, Pham D, Comstock A, Yu D, and Sajjan SU. 2018. NOTCH3 contributes to rhinovirus-induced goblet cell hyperplasia in COPD airway epithelial cells. Thorax 74: 18–32. [DOI] [PubMed] [Google Scholar]
- 24.Ganesan S, Unger BL, Comstock AT, Angel KA, Mancuso P, Martinez FJ, and Sajjan US. 2013. Aberrantly activated EGFR contributes to enhanced IL-8 expression in COPD airways epithelial cells via regulation of nuclear FoxO3A. Thorax 68: 131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faris AN, Ganesan S, Chattoraj A, Chattoraj SS, Comstock AT, Unger BL, Hershenson MB, and Sajjan US. 2016. Rhinovirus Delays Cell Repolarization in a Model of Injured/Regenerating Human Airway Epithelium. Am J Respir Cell Mol Biol 55: 487–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sajjan U, Moreira J, Liu M, Humar A, Chaparro C, Forstner J, and Keshavjee S. 2004. A novel model to study bacterial adherence to the transplanted airway: inhibition of Burkholderia cepacia adherence to human airway by dextran and xylitol. J Heart Lung Transplant 23: 1382–1391. [DOI] [PubMed] [Google Scholar]
- 27.Newcomb DC, Sajjan US, Nagarkar DR, Wang Q, Nanua S, Zhou Y, McHenry CL, Hennrick KT, Tsai WC, Bentley JK, Lukacs NW, Johnston SL, and Hershenson MB. 2008. Human rhinovirus 1B exposure induces phosphatidylinositol 3-kinase-dependent airway inflammation in mice. Am J Respir Crit Care Med 177: 1111–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chattoraj SS, Ganesan S, Faris A, Comstock A, Lee WM, and Sajjan US. 2011. Pseudomonas aeruginosa suppresses interferon response to rhinovirus infection in cystic fibrosis but not in normal bronchial epithelial cells. Infect Immun 79: 4131–4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Comstock AT, Ganesan S, Chattoraj A, Faris AN, Margolis BL, Hershenson MB, and Sajjan US. 2011. Rhinovirus-induced barrier dysfunction in polarized airway epithelial cells is mediated by NADPH oxidase 1. J Virol 85: 6795–6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghosh M, Miller YE, Nakachi I, Kwon JB, Baron AE, Brantley AE, Merrick DT, Franklin WA, Keith RL, and Vandivier RW. 2018. Exhaustion of Airway Basal Progenitor Cells in Early and Established Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 197: 885–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ganesan S, Faris AN, Comstock AT, Chattoraj SS, Chattoraj A, Burgess JR, Curtis JL, Martinez FJ, Zick S, Hershenson MB, and Sajjan US. 2010. Quercetin prevents progression of disease in elastase/LPS-exposed mice by negatively regulating MMP expression. Respir Res 11: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ueki IF, Min-Oo G, Kalinowski A, Ballon-Landa E, Lanier LL, Nadel JA, and Koff JL. 2013. Respiratory virus-induced EGFR activation suppresses IRF1-dependent interferon lambda and antiviral defense in airway epithelium. J Exp Med 210: 1929–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu YC, Simmons DP, Li X, Abbott DW, Boom WH, and Harding CV. 2012. TLR2 signaling depletes IRAK1 and inhibits induction of type I IFN by TLR7/9. J Immunol 188: 1019–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Unger BL, Faris AN, Ganesan S, Comstock AT, Hershenson MB, and Sajjan US. 2012. Rhinovirus Attenuates Non-typeable Hemophilus influenzae-stimulated IL-8 Responses via TLR2-dependent Degradation of IRAK-1. PLoS Pathog 8: e1002969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barbalat R, Lau L, Locksley RM, and Barton GM. 2009. Toll-like receptor 2 on inflammatory monocytes induces type I interferon in response to viral but not bacterial ligands. Nat Immunol 10: 1200–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dietrich N, Lienenklaus S, Weiss S, and Gekara NO. 2010. Murine toll-like receptor 2 activation induces type I interferon responses from endolysosomal compartments. PLoS One 5: e10250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perkins DJ, Polumuri SK, Pennini ME, Lai W, Xie P, and Vogel SN. 2013. Reprogramming of murine macrophages through TLR2 confers viral resistance via TRAF3-mediated, enhanced interferon production. PLoS Pathog 9: e1003479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gulraiz F, Bellinghausen C, Dentener MA, Reynaert NL, Gaajetaan GR, Beuken EV, Rohde GG, Bruggeman CA, and Stassen FR. 2014. Efficacy of IFN-lambda1 to protect human airway epithelial cells against human rhinovirus 1B infection. PLoS One 9: e95134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Proud D, and Leigh R. 2011. Epithelial cells and airway diseases. Immunol Rev 242: 186–204. [DOI] [PubMed] [Google Scholar]
- 40.Durbin RK, Kotenko SV, and Durbin JE. 2013. Interferon induction and function at the mucosal surface. Immunol Rev 255: 25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chi H, Barry SP, Roth RJ, Wu JJ, Jones EA, Bennett AM, and Flavell RA. 2006. Dynamic regulation of pro- and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses. Proc Natl Acad Sci U S A 103: 2274–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bode JG, Ludwig S, Freitas CA, Schaper F, Ruhl M, Melmed S, Heinrich PC, and Haussinger D. 2001. The MKK6/p38 mitogen-activated protein kinase pathway is capable of inducing SOCS3 gene expression and inhibits IL-6-induced transcription. Biological chemistry 382: 1447–1453. [DOI] [PubMed] [Google Scholar]
- 43.Dumitru CA, Dreschers S, and Gulbins E. 2006. Rhinoviral infections activate p38MAP-kinases via membrane rafts and RhoA. Cell Physiol Biochem 17: 159–166. [DOI] [PubMed] [Google Scholar]
- 44.Bernier M, Paul RK, Martin-Montalvo A, Scheibye-Knudsen M, Song S, He HJ, Armour SM, Hubbard BP, Bohr VA, Wang L, Zong Y, Sinclair DA, and de Cabo R. 2011. Negative regulation of STAT3 protein-mediated cellular respiration by SIRT1 protein. J Biol Chem 286: 19270–19279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakamaru Y, Vuppusetty C, Wada H, Milne JC, Ito M, Rossios C, Elliot M, Hogg J, Kharitonov S, Goto H, Bemis JE, Elliott P, Barnes PJ, and Ito K. 2009. A protein deacetylase SIRT1 is a negative regulator of metalloproteinase-9. FASEB J 23: 2810–2819. [DOI] [PubMed] [Google Scholar]
- 46.Rajendrasozhan S, Yang SR, Kinnula VL, and Rahman I. 2008. SIRT1, an antiinflammatory and antiaging protein, is decreased in lungs of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 177: 861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ganesan S, Faris AN, Comstock AT, Wang Q, Nanua S, Hershenson MB, and Sajjan US. 2012. Quercetin inhibits rhinovirus replication in vitro and in vivo. Antiviral Res 94: 258–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim Y, Narayanan S, and Chang KO. 2010. Inhibition of influenza virus replication by plant-derived isoquercetin. Antiviral Res 88: 227–235. [DOI] [PubMed] [Google Scholar]
- 49.Yao C, Xi C, Hu K, Gao W, Cai X, Qin J, Lv S, Du C, and Wei Y. 2018. Inhibition of enterovirus 71 replication and viral 3C protease by quercetin. Virology journal 15: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Triantafilou K, Vakakis E, Richer EA, Evans GL, Villiers JP, and Triantafilou M. 2011. Human rhinovirus recognition in non-immune cells is mediated by Toll-like receptors and MDA-5, which trigger a synergetic pro-inflammatory immune response. Virulence 2: 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.