Receipt of parenteral nutrition (PN) remains an independent risk factor for developing catheter-related bloodstream infections (CR-BSI) caused by fungi, including by the polymorphic fungus Candida albicans, which is notoriously adept at forming drug-resistant biofilm structures. Among a variety of macronutrients, PN solutions contain lipid emulsions to supply daily essential fats and are often delivered via central venous catheters (CVCs).
KEYWORDS: biofilm, parenteral nutrition, infection, Candida albicans, catheter, lipid emulsions, Candida, fungi, infection control, lipids
ABSTRACT
Receipt of parenteral nutrition (PN) remains an independent risk factor for developing catheter-related bloodstream infections (CR-BSI) caused by fungi, including by the polymorphic fungus Candida albicans, which is notoriously adept at forming drug-resistant biofilm structures. Among a variety of macronutrients, PN solutions contain lipid emulsions to supply daily essential fats and are often delivered via central venous catheters (CVCs). Therefore, using an in vitro biofilm model system, we sought to determine whether various clinical lipid emulsions differentially impacted biofilm growth in C. albicans. We observed that the lipid emulsions Intralipid and Omegaven both stimulated C. albicans biofilm formation during growth in minimal medium or a macronutrient PN solution. Conversely, Smoflipid inhibited C. albicans biofilm formation by approximately 50%. Follow-up studies revealed that while Smoflipid did not impair C. albicans growth, it did significantly inhibit hypha formation and hyphal elongation. Moreover, growth inhibition could be recapitulated in Intralipid when supplemented with capric acid—a fatty acid present in Smoflipid but absent in Intralipid. Capric acid was also found to dose dependently inhibit C. albicans biofilm formation in PN solutions. This is the first study to directly compare different clinical lipid emulsions for their capacity to affect C. albicans biofilm growth. Results derived from this study necessitate further research regarding different lipid emulsions and rates of fungus-associated CR-BSIs.
INTRODUCTION
Some patients (e.g., preterm neonates, patients with bowel resection, and the critically ill) are dependent on parenteral nutrition (PN) for essential daily caloric needs (1, 2). PN solutions contain electrolytes, micronutrients, and the macronutrients dextrose, amino acids, and lipid emulsions. A central venous catheter (CVC) is typically used for administration due to the high risk of phlebitis associated with the highly osmolality in PN solutions and/or the need for a venous access point for long-term (e.g., over 30 days) PN administration (3). Catheter-related bloodstream infections (CR-BSIs) are a serious complication of CVCs used for the administration of PN and are considered a health hazard (4). Estimates from 2009 suggest that there are approximately 23,000 CR-BSIs among patients in inpatient wards (5). Several clinical and meta-analyses estimate that CR-BSI develops in patients receiving PN at rates ranging from 0.5 to 10 infections per 1,000 catheter days (6–9). Despite recent decreases in CR-BSIs due to various prevention measures, receipt of PN remains an independent risk factor for a CR-BSI in pediatric, adult, intensive care unit, and home infusion therapy patients (10–12). It is unclear which component of PN increases the infectious risk, but macronutrient composition is likely an important factor, as increased parenteral calories can increase the risk of CR-BSIs while receiving PN (13). While risk for CR-BSIs caused by any pathogen is elevated while on PN, multiple clinical guidelines illustrate a link between PN administration and Candida infections in particular (14, 15).
Candida albicans is a cause of CR-BSIs associated with high morbidity (e.g., central line replacement) and mortality, as fungus-related sepsis has one of the highest fatality rates (up to 45% in some populations) compared to those of other pathogens (16–19). C. albicans is able to morphogenetically switch between a yeast and a filamentous hyphal form, an attribute key to its pathogenicity. The yeast form is important for dissemination, and the hyphal form crucial for tissue invasion and biofilm formation (20, 21). Biofilms are highly structured communities of cells that adhere to biotic and abiotic surfaces, including implanted medical devices like CVCs (22, 23). C. albicans biofilms typically form as initially adherent yeasts begin to proliferate, establishing a basal layer of attached cells. After early adherence, this complex layer of yeast cells is followed by robust hyphal extension that constructs a dense cellular meshwork. As the biofilm matures, it secretes a polysaccharide-rich extracellular matrix. The mature biofilm can then disperse yeast cells to potentially seed other sites (24). C. albicans biofilms exhibit resistance to antifungals at concentrations that are otherwise effective against planktonic C. albicans (25). Failure to achieve device sterilization by standard antifungal therapy often necessitates device removal. However, in the case of patients receiving long-term PN and young neonates/children, vascular sites for catheter reinsertion may be limited. Prevention of catheter-related infections and avoidance of catheter removal is a common goal for patients requiring PN.
It is debated which macronutrient in PN solutions increases the chance of fungal infections, although in vitro models suggest that biofilm formation may be independently increased with the addition of lipids (26–28). The main goal of lipid therapy in PN is to prevent essential fatty acid deficiency, and Intralipid (based widely on ω-6 fatty acids extracted from soybean oil) has been the most widely used lipid emulsion for the past 20 years (29). Long-term Intralipid administration can result in an altered ω-6/ω-3 fatty acid ratio and is linked to morbidities such as cholestasis (30). In order to overcome the risks of prolonged lipid administration and reduce bowel inflammation associated with an abnormal ω-6/ω-3 serum ratio, newer lipid emulsions, such as Smoflipid and Omegaven, have been developed (29). Smoflipid is composed of roughly equivalent levels of soybean oil (ω-6), medium-chain triglycerides (e.g., coconut oil), olive oil (ω-9), and fish oil (ω-3) and approximately resembles the lipid profile common to breastmilk (31–34). Omegaven is composed mainly of ω-3 fatty acids derived from fish oil (29).
There is limited information available as to whether newer lipid emulsions may affect CR-BSIs or impact the biology of C. albicans. Therefore, in this study we aimed to determine whether C. albicans biofilm formation was significantly altered by cultivation in PN macronutrient solutions containing Intralipid, Smoflipid, or Omegaven. In addition, we further define the role of individual fatty acids present in newer lipid emulsions in Candida biomass and biofilm formation.
RESULTS
Determination of minimal medium to support cell growth but not impact biofilm formation.
TrophAmine (a mix of essential and nonessential amino acids used in pediatric patients) and dextrose are common components of PN formulations. Therefore, we first wanted to assess which concentrations would be able to support moderate C. albicans growth so that lipid supplementation may show a demonstrable effect. Biofilms were grown for 24 h with serial dilutions of 2% TrophAmine and 17.5% dextrose in 1× yeast nitrogen base (YNB) medium (without ammonium sulfate or amino acids), as these starting concentrations are commonly found in typical PN solutions. Because C. albicans needs both a carbon and nitrogen source for robust growth, wells contained minimal amounts of either glucose (0.1%) or amino acids (0.02%) of the nontitrated macronutrient. Biofilm growth in these media was compared to growth in RPMI 1640, a widely used cell culture medium for the cultivation of C. albicans biofilms (35–38). At concentrations above 0.25%, TrophAmine significantly enhanced biofilm growth over that in the RPMI control (Fig. 1A). Similarly, a nonsignificant trend was observed for glucose, in which higher concentrations supported increased biofilm growth (Fig. 1B). We choose the lowest concentrations of TrophAmine (0.02%) and dextrose (0.1%) in 1× YNB (without amino acids or ammonium sulfate) to define the minimal medium used throughout this study.
FIG 1.
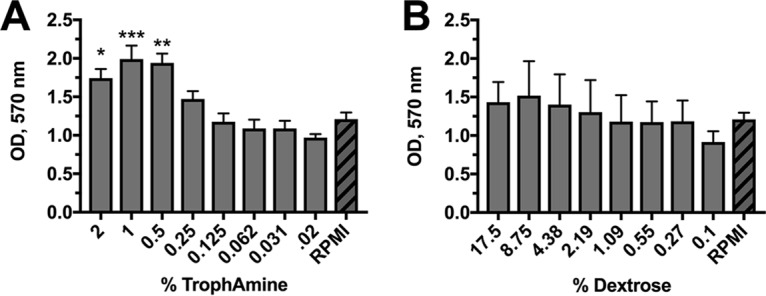
Optimized dosing of TrophAmine and dextrose to identify a minimal growth medium for C. albicans biofilm formation. C. albicans biofilms were grown for 24 h in 1× YNB medium (without amino acids or ammonium sulfate) containing serial dilutions of (A) TrophAmine or (B) dextrose. Biomass was quantified by the crystal violet method. Data are expressed as the mean (n = 3) raw optical density at 570 nm (OD570) and compared to growth in RPMI 1640 medium (hashed bars), which is routinely used for fungal biofilm studies. Error bars represent standard error of the mean (SEM). *, P < 0.05; **, P < 0.01; ***, P < 0.001, using one-way analysis of variance (ANOVA) with Dunnett’s posttest.
Smoflipid represses biofilm growth.
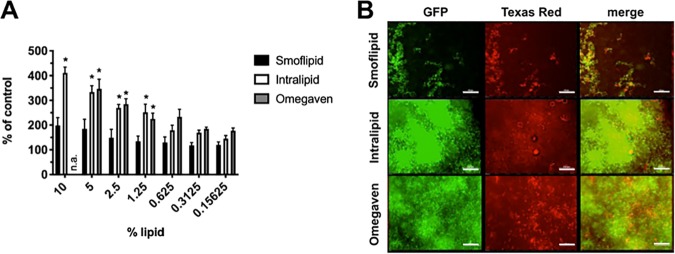
We next wanted to determine whether various clinical lipid emulsions differentially impact C. albicans biofilm growth. In order to answer this, C. albicans biofilms were cultivated in the clinical lipid emulsions Smoflipid, Intralipid, or Omegaven and serially diluted in minimal medium. We chose to use minimal medium initially to observe maximal impact that lipids may play in modulating fungal biofilm formation. Both Intralipid and Omegaven significantly stimulated biofilm growth compared to that in the no-lipid control. However, growth in Smoflipid was only marginally and insignificantly increased (Fig. 2A). Qualitative fluorescence microscopy images echoed the quantitative results demonstrating that comparatively little biofilm formed in Smoflipid (Fig. 2B).
FIG 2.
C. albicans biofilm formation is differentially affected by growth in clinical lipid emulsions. (A) Biofilms were grown in various concentrations of lipid emulsions (Smoflipid, Intralipid, and Omegaven) in minimal medium (1× YNB, 0.02% TrophAmine, and 0.1% dextrose) for 24 h. Biomass was quantified by the crystal violet method. Data are expressed as the mean (n = 3) percentage of the lipid-free controls. Error bars represent SEM. *, P < 0.05 using one-way ANOVA with Dunnett’s posttest. n.a., not assessed due to lower starting lipid percentage. (B) Green fluorescent protein (GFP)-expressing C. albicans biofilms were cultivated for 24 h on chamber slides using a similar medium and stained with Texas red-conjugated concanavalin A. Representative images (n = 3) were captured by fluorescence microscopy. Bar, 100 μm.
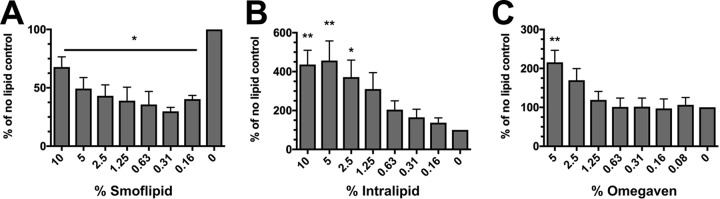
Subsequently, we wanted to determine whether similar growth effects could also be observed under more clinically relevant conditions. Therefore, a macronutrient PN solution was made containing 17.5% dextrose and 2% TrophAmine and a range of lipid emulsion concentrations. Similarly to growth in lipid-supplemented minimal medium, Intralipid and Omegaven both stimulated biofilm growth (Fig. 3A and C). Surprisingly, biofilm formation was not only reduced during growth in Smoflipid, but it was actually repressed (Fig. 3B). Together, these results suggest that Smoflipid fails to augment C. albicans biofilm to the same extent as Intralipid or Omegaven under nutrient-deplete and -replete conditions.
FIG 3.
Biofilm formation is modulated by lipid emulsions in a macronutrient PN solution. C. albicans biofilms were grown in macronutrient PN solution (1× YNB, 2% TrophAmine, and 17.5% dextrose) containing various concentrations of the lipid emulsions (A) Smoflipid, (B) Intralipid, or (C) Omegaven. Data are expressed as the mean (n = 3) percentage of the lipid-free controls. Error bars represent SEM. *, P < 0.05; **, P < 0.01 using one-way ANOVA with Dunnett’s posttest.
Smoflipid does not impair planktonic cell growth.
We next wanted to determine whether biofilm repression in Smoflipid was due to reduced cell growth or inhibition of the biofilm process. Thus, we grew C. albicans planktonically in 10% (Smoflipid or Intralipid) or 5% (Omegaven) lipid emulsions in biofilm minimal medium or minimal medium alone in planktonic culture. Enumeration of CFU revealed that growth under each lipid condition was similar, suggesting that the biofilm phenotypes observed during growth in lipid emulsions was not linked to growth modulation (Fig. 4).
FIG 4.
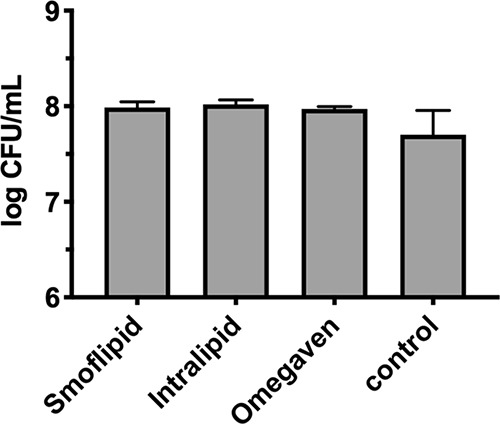
Lipid emulsions do not alter C. albicans growth. Planktonic cultures of C. albicans were grown in minimal medium supplemented with 10% Smoflipid, 10% Intralipid, or 5% Omegaven. A lipid-free control was also utilized. Data are representative of the mean (n = 3) CFU count following serial dilution and growth on yeast-peptone-dextrose (YPD) agar. Error bars represent standard deviation (SD). A one-way ANOVA with Dunnett’s posttest was used to assess significance.
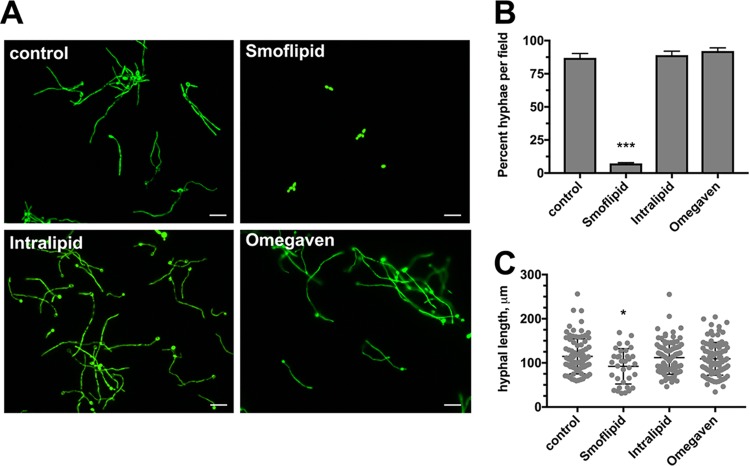
Smoflipid targets hyphal growth and reduces hyphal length.
We next wished to determine whether C. albicans hyphal growth is impacted by these lipid emulsions. We used the fluorescent C. albicans strain CAI4+pKE4-GFP to better visualize hyphae in the opaque lipid emulsions. Fluorescence microscopy revealed that growth in Omegaven yielded hyphal growth similar to that in the control. Growth in Intralipid yielded seemingly robust hyphal growth, and large hyphal aggregates were often observed. Interestingly, Smoflipid appeared to drastically attenuate the capacity of C. albicans to undergo the yeast-to-hypha switch (Fig. 5A). Moreover, quantitative assessment of hyphal growth by enumerating the percentage of hyphae per field confirmed this phenotype (Fig. 5B). Further analysis demonstrated that not only was the yeast-to-hypha switch reduced during growth in Smoflipid but that when hyphae do form they are significantly shorter (Fig. 5C). As hypha formation is crucial for robust biofilm formation by C. albicans, these results largely explain the observed biofilm growth defect in Smoflipid.
FIG 5.
Smoflipid inhibits the yeast-to-hypha transition. (A) C. albicans strain CAI4+pKE4-GFP was grown in minimal medium alone (control) or minimal medium supplemented with 10% Smoflipid, 10% Intralipid, or 5% Omegaven for 4 h. Wet mounts (20 μl) were prepared, and images of 5 nonadjacent fields were captured by fluorescence microscopy. A representative (n = 3) of each is depicted. Bar, 30 μm. (B) Hyphal cells, as well as total cells, per field were enumerated for 10 random images. Data are representative of the mean (n = 3) percent hyphae per field. Error bars represent SEM. ***, P < 0.001 using a one-way ANOVA and Dunnett’s posttest. (C) Using similar images above, hyphal lengths were quantitatively assessed using ImageJ. Data represent the median (n = 3) hyphal length ± SD. *, P < 0.05 using a one-way ANOVA and Dunnett’s posttest.
Lipid emulsion fatty acid composition affects hyphal growth.
When we compared the composition of the different lipid emulsions, it was observed that Smoflipid, unlike Intralipid and Omegaven, contains caprylic and capric acid (Table S1). In addition, both Smoflipid and Omegaven contain docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), whereas Intralipid lacks these fatty acids. Thus, we determined whether either one of these fatty acids might be responsible for the inhibitory effects on C. albicans hyphal growth and biofilm formation observed during growth in Smoflipid. Therefore, we grew C. albicans CAI4+pKE4-GFP planktonically in 10% Intralipid (which lacks all of these 4 fatty acids) supplemented with caprylic acid (128.3 mM), capric acid (55.1 mM), DHA (7.4 mM), or EPA (6.9 mM) at concentrations found in 10% Smoflipid used in our assays. Aliquots were taken after allowing sufficient time for hyphal growth and were examined by fluorescence microscopy. DHA and EPA had no effect on hyphal growth, appearing similar to the no-lipid control. However, capric acid completely prevented C. albicans hypha formation (Fig. 6A). Like growth in Smoflipid, this effect was not dependent on fungal toxicity, as quantitative CFU counts revealed similar levels of growth in Intralipid supplemented with capric acid to that in Intralipid alone (Fig. 6B). As pH can influence filamentation in C. albicans, we wanted to rule out the possibility that addition of capric acid was blocking filamentation simply via a pH-dependent mechanism (39, 40). Therefore, we performed hyphal growth assays in 10% Intralipid solution (pH 5.9 ± 0.1) and 10% Intralipid solutions set to the pH of 10% Smoflipid (pH 5.7 ± 0.1) or 10% Intralipid containing 55.1 mM capric acid (pH 5.3 ± 0.1) to mimic the pH of these conditions. Fluorescence microscopy images and quantitative counts demonstrated no reduction in hyphal growth at these lower pH values, suggesting that pH alone cannot explain reduced hyphal growth phenotypes observed during growth in Smoflipid or capric acid (Fig. S1). Interestingly, while supplementation with caprylic acid demonstrated significant antifungal effects, it also appeared to completely solubilize the lipid emulsion, which would likely limit its use as a potential lipid supplement in the clinic (Fig. S2).
FIG 6.
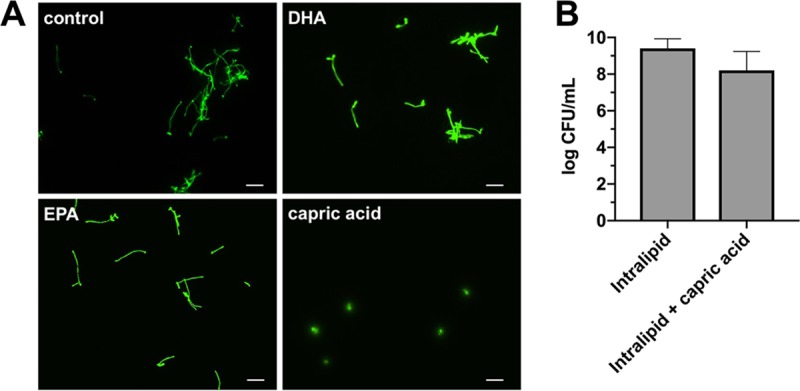
Capric acid impairs C. albicans filamentation without inhibiting growth. (A) C. albicans strain CAI4+pKE4-GFP was grown for 4 h in minimal medium containing 10% Intralipid supplemented with capric acid (55.14 mM), DHA (6.85 mM), or EPA (7.44 mM). Aliquots (20 μl) were used to make wet mounts, and five nonadjacent fields were captured by fluorescence microscopy. A representative of each is depicted. Bar, 30 μm. (B) Cells were grown planktonically overnight in minimal medium containing 10% Intralipid alone or supplemented with capric acid (55.14 mM). Growth was assessed by CFU counts following serial dilution plating onto YPD agar. Data are representative of the mean (n = 3) count. Error bars represent standard deviation (SD). Significance was assessed using a Mann-Whitney U test.
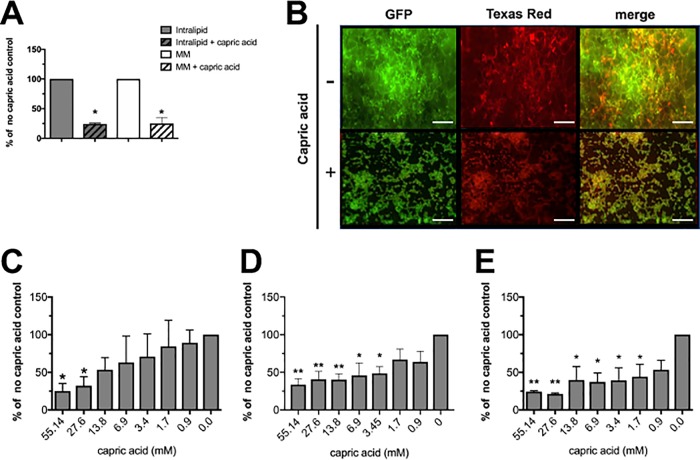
Capric acid inhibits C. albicans biofilm formation.
We next wanted to determine whether capric acid supplementation could prevent C. albicans biofilm growth. Biofilms in minimal medium both with and without 10% Intralipid were cultivated in the presence of absence of capric acid (55.1 mM). Similarly to hyphal repression, biofilm formation was significantly inhibited compared to the no-capric-acid control (Fig. 7A and B). Qualitative images of biofilms grown in Intralipid with or without capric acid reflected this phenotype. Lastly, we performed dose-response assays to determine the relative potency of capric acid to inhibit biofilm formation in minimal medium, minimal medium containing Intralipid, or macronutrient PN solution containing Intralipid and capric acid. Capric acid up to 13.8 mM was effective at significantly reducing biofilm growth in minimal medium (Fig. 7C). Similarly, capric acid demonstrated efficacy above 3.45 mM in minimal medium containing Intralipid (Fig. 7D). Importantly, capric acid demonstrated significant reduction of biofilm growth in macronutrient PN containing Intralipid at concentrations greater than 1.7 mM (Fig. 7E).
FIG 7.
Capric acid represses C. albicans biofilm formation. (A) Biofilms (gray bars) were cultivated in minimal medium supplemented with 10% Intralipid (open) or 10% Intralipid containing 55.14 mM capric acid (hashed). Similarly, biofilms were grown in these same conditions (open, no capric acid; hashed, 55.14 mM capric acid) in the absence of Intralipid (white bars). Data represent the mean (n = 3) percentage of the capric acid-free control. *, P < 0.05 using Student’s t test. (B) Representative images of GFP-expressing biofilms grown in minimal medium containing 10% Intralipid with or without capric acid (55.14 mM). Biofilms were stained with Texas red-conjugated concanavalin A and imaged by fluorescence microscopy. Bar, 100 μm. Dose-response assays for capric acid were performed in (C) minimal medium, (D) minimal medium containing 10% Intralipid, or (E) macronutrient PN solution containing 10% Intralipid. Data represent the mean (n = 3) percentages of the lipid-free controls. *, P < 0.05; **, P < 0.01 using a one-way ANOVA and Dunnett’s posttest.
DISCUSSION
Our experiments uniquely illustrated differences between lipid products regarding their effect on C. albicans biofilm formation. The repression of biofilm growth noted in Smoflipid was due to inhibition of hypha formation and length. These data have the potential to impact the rate and/or treatment of CR-BSIs in patients receiving PN.
The newer lipid emulsions were introduced mainly to decrease PN-related morbidities like parenteral nutrition-associated cholestasis (PNAC) (2). While much literature exists that correlates PN administration and CR-BSIs, the studies analyzing PN with infection rates or biofilm formation have focused primarily on Intralipid (10, 27, 41). A paucity of information exists on how newer lipid emulsions impact Candida biofilm formation and CR-BSI. The differential capacity of lipid emulsions to stimulate fungal growth and biofilm formation may carry important clinical implications for determining which lipid emulsion to use based on individual patient scenarios.
Biofilm formation is believed to be a significant contributor to the pathogenesis of CR-BSIs like those caused by C. albicans. Biofilm presence can make infections highly recalcitrant to common antifungal and catheter lock therapies (25, 42). PN solutions typically contain relatively large amounts of glucose (e.g., 10 to 25%) and amino acids (e.g., 2%) (43, 44). Residual solutions remaining in the catheter line, even if further diluted, could provide rich nutritional substrates for microbial biofilm growth. Indeed, a study by Herek et al. demonstrated that glucose concentration in PN solutions stimulated Candida parapsilosis biofilm formation but did not analyze the impact of protein or lipids (45). Interestingly, Shin et al. showed that high protein and glucose concentrations resembling those found in PN solutions did not promote C. albicans biofilm formation, suggesting that factors other than high glucose and protein concentrations in PN emulsions may play a role in exacerbated C. albicans pathogenicity or biofilm growth during PN therapy (46). Our data support this finding, as moderately high glucose concentrations (17.5%) had only marginal impact on biofilm growth over that of minimal medium containing only 0.1% of this carbon source. Together, these studies highlight the interspecies heterogeneity in response to growth in PN solutions, and such differences likely span the spectrum of the Candida species.
Although the role of glucose and protein content of PN solutions in promoting fungal biofilm formation is unclear, several studies have determined varied effects of lipid emulsion or fatty acid components on C. albicans biology. Swindell et al. demonstrated that Intralipid increased C. albicans biofilm formation compared to that in non-lipid-supplemented medium (27). Our data are in agreement, given that Intralipid (and also Omegaven) increased biofilm growth in minimal medium and concomitantly with clinically relevant concentrations of glucose and protein. The detailed mechanisms driving augmented hyphal growth in lipid emulsions remain elusive. However, it is clear that lipid and fatty acid composition (e.g., medium-chain varieties) plays a significant role in the process, as Smoflipid uniquely repressed biofilm growth in the presence of protein and dextrose concentrations commonly seen in PN formulations.
There exists a substantial body of literature describing antimicrobial effects of medium-chain fatty acids against a plethora of bacteria and fungi. C. albicans appears to be uniquely sensitive to fatty acids with carbon chain lengths of C8, C10, and C12, represented by caprylic, capric, and lauric acid, respectively (47). As early as the 1960s, Tsukahara demonstrated that caprylic acid was a robust, fast-acting fungicide (48). Treatment of C. albicans with caprylic acid at even relatively low concentrations (e.g., 10−5 M) led to total killing in as little as 10 min of contact time. A study by Bergsson et al. further supported these early observations by demonstrating that capric and lauric acid also exhibited fungicidal effects (49). Closer inspection of C. albicans morphology following treatment with these fatty acids by transmission electron microscopy revealed no overall defects in cell size or plasma membrane alternations. However, cellular cytoplasm appeared to be disorganized, which the authors hypothesized could be due to turgor pressure changes induced by fatty acid treatment. Our results are somewhat at odds with those of this study, given we observed no growth inhibitory effect observed during culture in nutrient broth supplemented with lipid emulsions or capric acid. Instead, our results more closely mirror those by several other groups, which also did not report growth modulatory effects of lipid emulsions or capric acid on C. albicans (50–53). Importantly, several of these studies did demonstrate reduced hyphal growth and hypha-associated gene expression in the presence of capric acid, similar to our findings (47, 54). Given that hypha formation is required for robust biofilm architecture in this fungal species, fatty acids would also be predicted to indirectly affect this growth modality.
Although the role of capric acid in C. albicans growth and hypha formation has been previously established, the link between the presence of capric acid in Smoflipid and reduction of Candida CR-BSIs or Candida biofilm formation on CVCs used for PN administration has not yet been clearly defined or statistically vetted. That said, an analysis focused on incidence of fungal CR-BSIs using Smoflipid versus other lipid emulsions would be warranted to validate our findings on a broadly relevant scale, where patient status, medication coadministration, and infection control measures could vastly impact outcomes. Regarding this point, various lipid emulsions not only differentially affect microbial agents but also affect host physiology, including modulation of the immune system (55–60). After all, newer lipid products were designed and marketed to be less inflammatory. Interestingly, a small crossover study of 8 healthy volunteers receiving lipid emulsions composed of either long-chain fatty acids (LCFA) only or of a mixture of long-chain and medium-chain fatty acids (MCFA) followed by a placebo period revealed that MCFA-LCFA mixtures altered cytokine expression following C. albicans challenge of isolated peripheral blood mononuclear cells compared to that with LCFA and placebo controls (61). Specifically, levels of interleukin 10 (IL-10; anti-inflammatory) were increased and gamma interferon (lFN-ɣ; proinflammatory) were decreased resulting in a shift of the Th1/Th2 cytokine balance to a less inflammatory state. This could have potential consequences for protective antifungal responses in localized tissue. However, other clinical evidence, including a randomized clinical trial, suggests that overall infection rate is decreased with the use of lipid products containing fish oil or combinations of fish oil and MCFAs (62–64). Unfortunately, studies assessing infection rates associated with use of different lipid products have not clearly differentiated causative pathogens and were not specific to central line infections. These conflicting data highlight the multifactorial nature of PN and lipid therapy. Thus, the data presented here should be interpreted with caution until further studies are completed if extrapolating to the clinical setting or other microbes.
A current approach to preventing and sometimes treating CR-BSIs involves the use of antimicrobial catheter lock solutions when a line is not in use (e.g., a cycled PN patient). These solutions can sterilize the lumenal catheter surface and prevent biofilm growth (42, 65). The use of these solutions in the lumen of catheters for extended periods of time to prevent infections is commonly referred to as antimicrobial lock therapy (ALT). Prevention of catheter contamination could improve sepsis rates associated with catheter use (66). Our results show that capric acid may be useful for prevention of biofilm development either as a standalone lock solution, when administered within Smoflipid, or as an additive to lipid emulsions lacking this fatty acid (e.g., Intralipid). Caprylic acid has been studied using both in vitro and animal models and in conjunction with other agents such as glyceryl trinitrate as a potential lock therapy (67). However, capric acid-based lock solutions have been minimally studied and their efficacy following PN exposure remains unclear. The capacity of capric acid to diminish biofilm production in a common PN macronutrient solution illustrates the relevance of this agent in patients receiving PN via a CVC. A lipid product that both provides essential lipid calories and contains an agent that prevents biofilm formation would have a significant impact on CR-BSIs in patients receiving PN. Collectively, these data suggest another method of CR-BSI prevention and possible treatment. Whether these data may also apply to limit the growth of other prevalent CR-BSIs pathogens (e.g., staphylococci) is among the important questions to be addressed in future studies.
In summary, our results show that there are major differences between PN lipid emulsions and their ability to stimulate biofilm formation. We have demonstrated that Smoflipid significantly inhibits Candida biofilm formation in vitro by inhibiting hyphal growth, most likely due to capric acid activity. Importantly, we showed that Intralipid and Omegaven both stimulate biofilm formation, which might have important clinical implications for the use of these PN emulsions in patients requiring PN. In addition, use of Smoflipid may help prevent CR-BSIs in this patient population. Moreover, PN-specific or general-use catheter lock solutions could include capric acid to aid in the prevention of C. albicans biofilm formation. Future in vitro, in vivo, and clinical studies are warranted to further evaluate the utility of these findings.
MATERIALS AND METHODS
Strains and growth conditions.
Biofilm-forming C. albicans prototypical isolate SC5314 and C. albicans CAI4+pKE4-GFPγ (single IRO1-URA3 locus restored) were used in this study and are as described previously (68, 69). Both organisms were maintained as frozen stocks at −80°C. Prior to use, C. albicans was subcultured onto yeast-peptone-dextrose (YPD) agar (Difco) at 30°C. A single colony was cultured in 1× yeast nitrogen base (YNB) liquid medium containing 0.5% ammonium sulfate and 2% glucose (Difco) at 30°C for 24 h. Following growth, C. albicans was washed in phosphate-buffered saline (PBS) by centrifugation, counted on a hemocytometer, and adjusted to 1 × 107 CFU/ml in 1× YNB.
Biofilm growth.
For biofilm formation, 100 μl of adjusted C. albicans culture in 1× YNB was added to each well of a 96-well cell culture-treated polystyrene microtiter plate (1 × 106 CFU per well). Plates were incubated for 2 h at 37°C in a humidified chamber to allow C. albicans to adhere to the surface. After 2 h, nonadherent cells were carefully removed by pipetting and the wells were washed 3× with sterile cell-culture grade distilled water (dH2O) to completely remove all adherent cells. Various concentrations of Intralipid (Baxter Healthcare Corporation, Deerfield, IL, USA), Smoflipid (Fresenius Kabi, Bad Homburg, Germany) at 10%, 5%, 2.5%, 1.25%, 0.625%, 0.3125%, and 0.15625%, or Omegaven (Fresenius Kabi) at 5%, 2.5%, 1.25%, 0.625%, 0.3125%, 0.15625%, and 0.078125% were added to macronutrient PN solutions consisting of 2% TrophAmine (Braun Medical, Bethlehem, PA), 17.5% dextrose (Baxter Healthcare Corporation), and 1× YNB (without ammonium sulfate or amino acids). In some experiments, 1× YNB (without ammonium sulfate, amino acids, or glucose) was supplemented with TrophAmine (2%, 1%, 0.5%, 0.25%, 0.125%, 0.0625%, 0.03125%, or 0.02%) or dextrose (17.5%, 8.75%, 4.375%, 2.1875%, 1.09375%, 0.546875%, 0.273438%, or 0.1%) in order to define a minimal medium (1× YNB, 0.02% TrophAmine, and 0.1% dextrose) that supported growth but did not enhance biofilm formation. As a control, biofilms were prepared similarly using 1× RPMI 1640 cell culture medium. Plates were incubated for 24 h at 37°C to induce biofilm formation.
Crystal violet assay for biofilm quantitation.
In order to quantify biomass during growth, biofilms were grown as described above and processed for crystal violet staining as previously described (70). Briefly, wells were extensively washed in dH2O to remove nonadherent cells, stained with 0.1% crystal violet, and repeatedly washed in dH2O. Bound crystal violet was resolubilized in 95% ethanol, and the absorbance was read at 570 nm on a microplate reader. Wells containing medium alone were similarly stained and used to blank subtract from experimental wells. Wells containing neat or diluted lipid solutions alone did not show significant staining above that of medium-only controls (data not shown). Each condition was conducted using technical triplicates and the data were averaged. Experiments were independently repeated (n = 3) and data are represented as the experimental mean + standard error of the mean (SEM). Results were reported as either raw optical density at 570 nm (OD570) nm readings or as percentage of the relative control.
Planktonic growth and quantitative growth assay.
C. albicans culture was prepared as described above and adjusted to a final concentration of 1 × 106 cells/ml in 2× minimal medium (described above) supplemented with final concentrations of 10% Smoflipid, 10% Intralipid, or 5% Omegaven. Cells were grown at 30°C in a shaking incubator for 20 h at 225 rpm. The following day, cultures were serially diluted 10-fold and plated onto YPD agar by the drop plate method (71). The plates were incubated for 24 h at 37°C, colonies were enumerated, and numbers of CFU/ml were reported as the mean + SEM (n = 3).
Quantitative hyphal growth assays.
A C. albicans strain engineered to constitutively express green fluorescent protein (GFP), CAI4+pKE4-GFP, was grown overnight in 1× YNB, washed in PBS, diluted to 1 × 106 cells/ml as described above in minimal medium containing 10% Smoflipid, 10% Intralipid, or 5% Omegaven, and cultured in a 37°C shaking incubator for 4 h to allow for hyphal growth. Aliquots (20 μl) were removed, and wet mounts were prepared and then imaged with a Nikon Ni-U fluorescence microscope using a 488-nm laser and GFP filter set. Percent hyphae per field were enumerated using a total of 10 random images per condition. Hyphal length was assessed using ImageJ by calibrating distance to Ni-U scalebars. At least 10 cells per field were enumerated from 10 random fields per condition. Results for qualitative and quantitative microscopy were representative of experimental replicates (n = 3).
Hyphal growth assay with fatty acids.
CAI4+pKE4-GFP was prepared as described above and diluted to 1 × 106 cells/ml in minimal medium containing 10% Intralipid spiked with a final concentration of 55.1 mM capric acid, 128.3 mM caprylic acid, 7.4 mM EPA, or 6.8 mM DHA. Cultures were grown in a 37°C shaking incubator for 4 h. Aliquots (20 μl) were removed and imaged as described above.
Measurement and modulation of PN lipid emulsion pH.
A standard pH meter was used to assess the pH of solutions of 10% Smoflipid, 10% Intralipid, or 10% Intralipid containing 55.1 mM capric acid prepared in minimal medium. In some cases, the pH of Intralipid was altered by adjusting with 1N hydrochloric acid.
Imaging of C. albicans biofilm architecture in lipid emulsions.
C. albicans strain CAI4+pKE4-GFP was cultured as described above, and 200 μl of adjusted C. albicans culture was added to each chamber of a Permanox-coated 8-well chamber slide (1 × 106 CFU per well). Plates were incubated for 2 h at 37°C in a humidified chamber to allow for cell attachment. Nonadherent cells were carefully removed by washing 3× with sterile dH2O. Smoflipid, Intralipid (10%), Omegaven (5%), YNB minimal medium, or Intralipid supplemented with capric acid (55.1 mM) was added to the wells. Biofilms were allowed to grow for 48 h in total at 37°C in a humidified chamber with the medium replaced after 24 h of growth. After incubation, the supernatant was aspirated, and nonadherent cells were removed by washing with sterile dH2O. Biofilms were fixed in 4% formaldehyde and stained with concanavalin A-Texas red (50 μg/ml) for 30 min. Images were captured by fluorescence microscopy (Nikon Ni-U) using GFP and tetramethylrhodamine (TRITC) filter sets.
Image construction and capture.
Images were constructed in Microsoft PowerPoint and converted to appropriate format and resolution using Adobe Photoshop. In some cases, images were captured by digital camera.
Statistics and image construction.
All experiments were performed in biological triplicate and repeated a minimum of three times. Biomasses from crystal violet staining, CFU counts, and hyphal growth were compared using one-way analysis of variance (ANOVA) and Dunnett’s posttest. Differences with a P value of <0.05 were considered significant. All statistical analyses were performed and graphs were composed with GraphPad Prism.
Supplementary Material
ACKNOWLEDGMENTS
The present work was funded by the College of Pharmacy Dean’s Enhancement Program–Collaborative Seed Grant of the University of Tennessee Health Science Center.
We kindly thank Jarrod Fortwendel (UTHSC) for providing expertise with the fluorescence microscope and Glen Palmer for providing C. albicans strain CAI4+pKE4-GFP.
B.M.P. and J.S.S. designed the study; H.M.E.W., J.P.F., and M.E.C. collected the data; B.M.P., J.S.S., and H.M.E.W. performed the data analysis and interpreted study results; and B.M.P., J.S.S., and H.M.E.W. wrote the paper. All authors gave approval of the final version to be submitted.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01394-19.
REFERENCES
- 1.Dudrick SJ, Wilmore DW, Vars HM, Rhoads JE. 2001. Long-term total parenteral nutrition with growth, development, and positive nitrogen balance. 1968. Nutr Hosp 16:286–292. [PubMed] [Google Scholar]
- 2.Kasirer Y, Bin-Nun A, Raveh A, Schorrs I, Mimouni FB, Hammerman C. 2019. SMOFlipid protects preterm neonates against perinatal nutrition-associated cholestasis. Am J Perinatol doi: 10.1055/s-0038-1676977. [DOI] [PubMed] [Google Scholar]
- 3.Kuwahara T, Asanami S, Tamura T, Kaneda S. 1998. Effects of pH and osmolality on phlebitic potential of infusion solutions for peripheral parenteral nutrition. J Toxicol Sci 23:77–85. doi: 10.2131/jts.23.77. [DOI] [PubMed] [Google Scholar]
- 4.Walshe CM, Boner KS, Bourke J, Hone R, Phelan D. 2010. Diagnosis of catheter-related bloodstream infection in a total parenteral nutrition population: inclusion of sepsis defervescence after removal of culture-positive central venous catheter. J Hosp Infect 76:119–123. doi: 10.1016/j.jhin.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Liang SY, Marschall J. 2011. Vital signs: central line-associated blood stream infections—United States, 2001, 2008, and 2009. Ann Emerg Med 58:447–450. doi: 10.1016/j.annemergmed.2011.07.035. [DOI] [PubMed] [Google Scholar]
- 6.Dreesen M, Foulon V, Spriet I, Goossens GA, Hiele M, De Pourcq L, Willems L. 2013. Epidemiology of catheter-related infections in adult patients receiving home parenteral nutrition: a systematic review. Clin Nutr 32:16–26. doi: 10.1016/j.clnu.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Hoang V, Sills J, Chandler M, Busalani E, Clifton-Koeppel R, Modanlou HD. 2008. Percutaneously inserted central catheter for total parenteral nutrition in neonates: complications rates related to upper versus lower extremity insertion. Pediatrics 121:e1152–e1159. doi: 10.1542/peds.2007-1962. [DOI] [PubMed] [Google Scholar]
- 8.O’Connor A, Hanly AM, Francis E, Keane N, McNamara DA. 2013. Catheter associated blood stream infections in patients receiving parenteral nutrition: a prospective study of 850 patients. J Clin Med Res 5:18–21. doi: 10.4021/jocmr1032w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walshe C, Bourke J, Lynch M, McGovern M, Delaney L, Phelan D. 2012. Culture positivity of CVCs used for TPN: investigation of an association with catheter-related infection and comparison of causative organisms between ICU and non-ICU CVCs. J Nutr Metab 2012:257959. doi: 10.1155/2012/257959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fonseca G, Burgermaster M, Larson E, Seres DS. 2018. The relationship between parenteral nutrition and central line-associated bloodstream infections: 2009–2014. JPEN J Parenter Enteral Nutr 42:171–175. doi: 10.1177/0148607116688437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Netto R, Mondini M, Pezzella C, Romani L, Lucignano B, Pansani L, D’Argenio P, Cogo P. 2017. Parenteral nutrition is one of the most significant risk factors for nosocomial infections in a pediatric cardiac intensive care unit. JPEN J Parenter Enteral Nutr 41:612–618. doi: 10.1177/0148607115619416. [DOI] [PubMed] [Google Scholar]
- 12.Tokars JI, Cookson ST, McArthur MA, Boyer CL, McGeer AJ, Jarvis WR. 1999. Prospective evaluation of risk factors for bloodstream infection in patients receiving home infusion therapy. Ann Intern Med 131:340–347. doi: 10.7326/0003-4819-131-5-199909070-00004. [DOI] [PubMed] [Google Scholar]
- 13.Dissanaike S, Shelton M, Warner K, O’Keefe GE. 2007. The risk for bloodstream infections is associated with increased parenteral caloric intake in patients receiving parenteral nutrition. Crit Care 11:R114. doi: 10.1186/cc6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O’Grady NP, Raad II, Rijnders BJ, Sherertz RJ, Warren DK. 2009. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 49:1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, Zaoutis TE, Sobel JD. 2016. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 62:e1–e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kett DH, Azoulay E, Echeverria PM, Vincent JL. 2011. Candida bloodstream infections in intensive care units: analysis of the extended prevalence of infection in intensive care unit study. Crit Care Med 39:665–670. doi: 10.1097/CCM.0b013e318206c1ca. [DOI] [PubMed] [Google Scholar]
- 17.Leroy O, Gangneux JP, Montravers P, Mira JP, Gouin F, Sollet JP, Carlet J, Reynes J, Rosenheim M, Regnier B, Lortholary O. 2009. Epidemiology, management, and risk factors for death of invasive Candida infections in critical care: a multicenter, prospective, observational study in France (2005–2006). Crit Care Med 37:1612–1618. doi: 10.1097/CCM.0b013e31819efac0. [DOI] [PubMed] [Google Scholar]
- 18.Saliba P, Hornero A, Cuervo G, Grau I, Jimenez E, García D, Tubau F, Martínez-Sánchez JM, Carratalà J, Pujol M. 2018. Mortality risk factors among non-ICU patients with nosocomial vascular catheter-related bloodstream infections: a prospective cohort study. J Hosp Infect 99:48–54. doi: 10.1016/j.jhin.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Perlroth J, Choi B, Spellberg B. 2007. Nosocomial fungal infections: epidemiology, diagnosis, and treatment. Med Mycol 45:321–346. doi: 10.1080/13693780701218689. [DOI] [PubMed] [Google Scholar]
- 20.Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, Ghannoum MA. 2001. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J Bacteriol 183:5385–5394. doi: 10.1128/jb.183.18.5385-5394.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sudbery PE. 2011. Growth of Candida albicans hyphae. Nat Rev Microbiol 9:737–748. doi: 10.1038/nrmicro2636. [DOI] [PubMed] [Google Scholar]
- 22.Davey ME, O'toole GA. 2000. Microbial biofilms: from ecology to molecular genetics. Microbiol Mol Biol Rev 64:847–867. doi: 10.1128/MMBR.64.4.847-867.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nobile CJ, Johnson AD. 2015. Candida albicans biofilms and human disease. Annu Rev Microbiol 69:71–92. doi: 10.1146/annurev-micro-091014-104330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gulati M, Nobile CJ. 2016. Candida albicans biofilms: development, regulation, and molecular mechanisms. Microbes Infect 18:310–321. doi: 10.1016/j.micinf.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taff HT, Mitchell KF, Edward JA, Andes DR. 2013. Mechanisms of Candida biofilm drug resistance. Future Microbiol 8:1325–1337. doi: 10.2217/fmb.13.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sriram K, Meguid MM. 2015. Addition of lipids to parenteral nutrition does not cause fungal infections. Nutrition 31:1443–1446. doi: 10.1016/j.nut.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 27.Swindell K, Lattif AA, Chandra J, Mukherjee PK, Ghannoum MA. 2009. Parenteral lipid emulsion induces germination of Candida albicans and increases biofilm formation on medical catheter surfaces. J Infect Dis 200:473–480. doi: 10.1086/600106. [DOI] [PubMed] [Google Scholar]
- 28.Tumbarello M, Fiori B, Trecarichi EM, Posteraro P, Losito AR, De Luca A, Sanguinetti M, Fadda G, Cauda R, Posteraro B. 2012. Risk factors and outcomes of candidemia caused by biofilm-forming isolates in a tertiary care hospital. PLoS One 7:e33705. doi: 10.1371/journal.pone.0033705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anez-Bustillos L, Dao DT, Baker MA, Fell GL, Puder M, Gura KM. 2016. Intravenous fat emulsion formulations for the adult and pediatric patient: understanding the differences. Nutr Clin Pract 31:596–609. doi: 10.1177/0884533616662996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin CR, Dasilva DA, Cluette-Brown JE, Dimonda C, Hamill A, Bhutta AQ, Coronel E, Wilschanski M, Stephens AJ, Driscoll DF, Bistrian BR, Ware JH, Zaman MM, Freedman SD. 2011. Decreased postnatal docosahexaenoic and arachidonic acid blood levels in premature infants are associated with neonatal morbidities. J Pediatr 159:743–749.e2. doi: 10.1016/j.jpeds.2011.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diamond IR, Sterescu A, Pencharz PB, Kim JH, Wales PW. 2009. Changing the paradigm: Omegaven for the treatment of liver failure in pediatric short bowel syndrome. J Pediatr Gastroenterol Nutr 48:209–215. doi: 10.1097/MPG.0b013e318182c8f6. [DOI] [PubMed] [Google Scholar]
- 32.Fallon EM, Le HD, Puder M. 2010. Prevention of parenteral nutrition-associated liver disease: role of omega-3 fish oil. Curr Opin Organ Transplant 15:334–340. doi: 10.1097/MOT.0b013e3283394879. [DOI] [PubMed] [Google Scholar]
- 33.Gura KM, Lee S, Valim C, Zhou J, Kim S, Modi BP, Arsenault DA, Strijbosch RA, Lopes S, Duggan C, Puder M. 2008. Safety and efficacy of a fish-oil-based fat emulsion in the treatment of parenteral nutrition-associated liver disease. Pediatrics 121:e678–e786. doi: 10.1542/peds.2007-2248. [DOI] [PubMed] [Google Scholar]
- 34.Puder M, Valim C, Meisel JA, Le HD, de Meijer VE, Robinson EM, Zhou J, Duggan C, Gura KM. 2009. Parenteral fish oil improves outcomes in patients with parenteral nutrition-associated liver injury. Ann Surg 250:395–402. doi: 10.1097/SLA.0b013e3181b36657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daniels KJ, Park YN, Srikantha T, Pujol C, Soll DR. 2013. Impact of environmental conditions on the form and function of Candida albicans biofilms. Eukaryot Cell 12:1389–1402. doi: 10.1128/EC.00127-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lohse MB, Gulati M, Valle Arevalo A, Fishburn A, Johnson AD, Nobile CJ. 2017. Assessment and optimizations of Candida albicans in vitro biofilm assays. Antimicrob Agents Chemother 61: e02749-16. doi: 10.1128/AAC.02749-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Montelongo-Jauregui D, Srinivasan A, Ramasubramanian AK, Lopez-Ribot JL. 2016. An in vitro model for oral mixed biofilms of Candida albicans and Streptococcus gordonii in synthetic saliva. Front Microbiol 7:686. doi: 10.3389/fmicb.2016.00686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nett JE, Cain MT, Crawford K, Andes DR. 2011. Optimizing a Candida biofilm microtiter plate model for measurement of antifungal susceptibility by tetrazolium salt assay. J Clin Microbiol 49:1426–1433. doi: 10.1128/JCM.02273-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verran J, Shakespeare AP, Willcox DP, Knox KW. 1991. The effect of pH on adhesion and hyphal formation by strains of Candida albicans. Microb Ecol Health Dis 4:73–80. doi: 10.3109/08910609109140266. [DOI] [Google Scholar]
- 40.Vylkova S. 2017. Environmental pH modulation by pathogenic fungi as a strategy to conquer the host. PLoS Pathog 13:e1006149. doi: 10.1371/journal.ppat.1006149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reitzel RA, Rosenblatt J, Chaftari AM, Raad II. 2019. Epidemiology of infectious and noninfectious catheter complications in patients receiving home parenteral nutrition: a systematic review and meta-analysis. JPEN J Parenter Enteral Nutr doi: 10.1002/jpen.1609. [DOI] [PubMed] [Google Scholar]
- 42.Walraven CJ, Lee SA. 2013. Antifungal lock therapy. Antimicrob Agents Chemother 57:1–8. doi: 10.1128/AAC.01351-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bolder U, Ebener C, Hauner H, Jauch KW, Kreymann G, Ockenga J, Traeger K, Working group for developing the guidelines for parenteral nutrition of The German Association for Nutritional Medicine. 2009. Carbohydrates—guidelines on parenteral nutrition, chapter 5. Ger Med Sci 7:Doc23. doi: 10.3205/000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yarandi SS, Zhao VM, Hebbar G, Ziegler TR. 2011. Amino acid composition in parenteral nutrition: what is the evidence? Curr Opin Clin Nutr Metab Care 14:75–82. doi: 10.1097/MCO.0b013e328341235a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herek TC, Menegazzo VR, Ogaki MB, Perini HF, Maia LF, Furlaneto MC. 2019. Biofilm formation by blood isolates of Candida parapsilosis sensu stricto in the presence of a hyperglycidic solution at comparable concentrations of total parenteral nutrition. Rev Soc Bras Med Trop 52:e20180182. doi: 10.1590/0037-8682-0182-2018. [DOI] [PubMed] [Google Scholar]
- 46.Shin JH, Kee SJ, Shin MG, Kim SH, Shin DH, Lee SK, Suh SP, Ryang DW. 2002. Biofilm production by isolates of Candida species recovered from nonneutropenic patients: comparison of bloodstream isolates with isolates from other sources. J Clin Microbiol 40:1244–1248. doi: 10.1128/jcm.40.4.1244-1248.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang CB, Alimova Y, Myers TM, Ebersole JL. 2011. Short- and medium-chain fatty acids exhibit antimicrobial activity for oral microorganisms. Arch Oral Biol 56:650–654. doi: 10.1016/j.archoralbio.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsukahara T. 1961. Fungicial action of caprylic acid for Candida albicans. Japan J Microb 5:383–394. doi: 10.1111/j.1348-0421.1961.tb00217.x. [DOI] [Google Scholar]
- 49.Bergsson G, Arnfinnsson J, Steingrimsson O, Thormar H. 2001. In vitro killing of Candida albicans by fatty acids and monoglycerides. Antimicrob Agents Chemother 45:3209–3212. doi: 10.1128/AAC.45.11.3209-3212.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Austin PD, Hand KS, Elia M. 2014. Factors influencing Candida albicans growth in parenteral nutrition with and without lipid emulsion: using an established framework to inform maximum duration of infusion policy decisions. Clin Nutr 33:489–494. doi: 10.1016/j.clnu.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 51.Jadhav A, Mortale S, Halbandge S, Jangid P, Patil R, Gade W, Kharat K, Karuppayil SM. 2017. The dietary food components capric acid and caprylic acid inhibit virulence factors in Candida albicans through multitargeting. J Med Food 20:1083–1090. doi: 10.1089/jmf.2017.3971. [DOI] [PubMed] [Google Scholar]
- 52.Kuwahara T, Shimono K, Kaneda S, Tamura T, Ichihara M, Nakashima Y. 2010. Growth of microorganisms in total parenteral nutrition solutions containing lipid. Int J Med Sci 7:101–109. doi: 10.7150/ijms.7.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murzyn A, Krasowska A, Stefanowicz P, Dziadkowiec D, Łukaszewicz M. 2010. Capric acid secreted by S. boulardii inhibits C. albicans filamentous growth, adhesion and biofilm formation. PLoS One 5:e12050. doi: 10.1371/journal.pone.0012050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takahashi M, Inoue S, Hayama K, Ninomiya K, Abe S. 2012. Inhibition of Candida mycelia growth by a medium chain fatty acids, capric acid in vitro and its therapeutic efficacy in murine oral candidiasis. Med Mycol J 53:255–261. doi: 10.3314/mmj.53.255. [DOI] [PubMed] [Google Scholar]
- 55.Calder PC, Bond JA, Newsholme EA. 1990. Fatty acid inhibition of lipopolysaccharide-stimulated B lymphocyte proliferation. Biochem Soc Trans 18:904–905. doi: 10.1042/bst0180904. [DOI] [PubMed] [Google Scholar]
- 56.Calder PC, Yaqoob P, Thies F, Wallace FA, Miles EA. 2002. Fatty acids and lymphocyte functions. Br J Nutr 87 Suppl 1:S31–S48. doi: 10.1079/bjn2001455. [DOI] [PubMed] [Google Scholar]
- 57.Costabile M, Hii CS, Melino M, Easton C, Ferrante A. 2005. The immunomodulatory effects of novel beta-oxa, beta-thia, and gamma-thia polyunsaturated fatty acids on human T lymphocyte proliferation, cytokine production, and activation of protein kinase C and MAPKs. J Immunol 174:233–243. doi: 10.4049/jimmunol.174.1.233. [DOI] [PubMed] [Google Scholar]
- 58.Hughes DA, Southon S, Pinder AC. 1996. (n-3) Polyunsaturated fatty acids modulate the expression of functionally associated molecules on human monocytes in vitro. J Nutr 126:603–610. doi: 10.1093/jn/126.3.603. [DOI] [PubMed] [Google Scholar]
- 59.Kew S, Wells S, Thies F, McNeill GP, Quinlan PT, Clark GT, Dombrowsky H, Postle AD, Calder PC. 2003. The effect of eicosapentaenoic acid on rat lymphocyte proliferation depends upon its position in dietary triacylglycerols. J Nutr 133:4230–4238. doi: 10.1093/jn/133.12.4230. [DOI] [PubMed] [Google Scholar]
- 60.Lokesh BR, Sayers TJ, Kinsella JE. 1990. Interleukin-1 and tumor necrosis factor synthesis by mouse peritoneal macrophages is enhanced by dietary n-3 polyunsaturated fatty acids. Immunol Lett 23:281–285. doi: 10.1016/0165-2478(90)90073-Y. [DOI] [PubMed] [Google Scholar]
- 61.Wanten GJ, Netea MG, Naber TH, Curfs JH, Jacobs LE, Verver-Jansen TJ, Kullberg BJ. 2002. Parenteral administration of medium- but not long-chain lipid emulsions may increase the risk for infections by Candida albicans. Infect Immun 70:6471–6474. doi: 10.1128/IAI.70.11.6471-6474.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grau-Carmona T, Bonet-Saris A, García-de-Lorenzo A, Sánchez-Alvarez C, Rodríguez-Pozo A, Acosta-Escribano J, Miñambres E, Herrero-Meseguer JI, Mesejo A. 2015. Influence of n-3 polyunsaturated fatty acids enriched lipid emulsions on nosocomial infections and clinical outcomes in critically ill patients: ICU lipids study. Crit Care Med 43:31–39. doi: 10.1097/CCM.0000000000000612. [DOI] [PubMed] [Google Scholar]
- 63.Manzanares W, Langlois PL, Dhaliwal R, Lemieux M, Heyland DK. 2015. Intravenous fish oil lipid emulsions in critically ill patients: an updated systematic review and meta-analysis. Crit Care 19:167. doi: 10.1186/s13054-015-0888-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pradelli L, Mayer K, Muscaritoli M, Heller AR. 2012. n-3 fatty acid-enriched parenteral nutrition regimens in elective surgical and ICU patients: a meta-analysis. Crit Care 16:R184. doi: 10.1186/cc11668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Segarra-Newnham M, Martin-Cooper EM. 2005. Antibiotic lock technique: a review of the literature. Ann Pharmacother 39:311–318. doi: 10.1345/aph.1E316. [DOI] [PubMed] [Google Scholar]
- 66.Toltzis P. 2006. Antibiotic lock technique to reduce central venous catheter-related bacteremia. Pediatr Infect Dis J 25:449–450. doi: 10.1097/01.inf.0000217264.11288.5a. [DOI] [PubMed] [Google Scholar]
- 67.Rosenblatt J, Reitzel RA, Raad I. 2015. Caprylic acid and glyceryl trinitrate combination for eradication of biofilm. Antimicrob Agents Chemother 59:1786–1788. doi: 10.1128/AAC.04561-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Butts A, DeJarnette C, Peters TL, Parker JE, Kerns ME, Eberle KE, Kelly SL, Palmer GE. 2017. Target abundance-based fitness screening (TAFiS) facilitates rapid identification of target-specific and physiologically active chemical probes. mSphere 2:e00379-17. doi: 10.1128/mSphere.00379-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gillum AM, Tsay EY, Kirsch DR. 1984. Isolation of the Candida albicans gene for orotidine-5’-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet 198:179–182. doi: 10.1007/bf00328721. [DOI] [PubMed] [Google Scholar]
- 70.Peters BM, Ward RM, Rane HS, Lee SA, Noverr MC. 2013. Efficacy of ethanol against Candida albicans and Staphylococcus aureus polymicrobial biofilms. Antimicrob Agents Chemother 57:74–82. doi: 10.1128/AAC.01599-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sieuwerts S, de Bok FA, Mols E, de Vos WM, Vlieg JE. 2008. A simple and fast method for determining colony forming units. Lett Appl Microbiol 47:275–278. doi: 10.1111/j.1472-765X.2008.02417.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.