The suboptimal effectiveness of β-lactam antibiotics against Mycobacterium tuberculosis has hindered the utility of this compound class for tuberculosis treatment. However, the results of treatment with a second-line regimen containing meropenem plus a β-lactamase inhibitor were found to be encouraging in a case study of extensively drug-resistant tuberculosis (M. C. Payen, S. De Wit, C. Martin, R.
KEYWORDS: high-throughput screen, β-lactams, synthetic lethality, tuberculosis
ABSTRACT
The suboptimal effectiveness of β-lactam antibiotics against Mycobacterium tuberculosis has hindered the utility of this compound class for tuberculosis treatment. However, the results of treatment with a second-line regimen containing meropenem plus a β-lactamase inhibitor were found to be encouraging in a case study of extensively drug-resistant tuberculosis (M. C. Payen, S. De Wit, C. Martin, R. Sergysels, et al., Int J Tuberc Lung Dis 16:558–560, 2012, https://doi.org/10.5588/ijtld.11.0414). We hypothesized that the innate resistance of M. tuberculosis to β-lactams is mediated in part by noncanonical accessory proteins that are not considered the classic targets of β-lactams and that small-molecule inhibitors of those accessory targets might sensitize M. tuberculosis to β-lactams. In this study, we screened an NIH small-molecule library for the ability to sensitize M. tuberculosis to meropenem. We identified six hit compounds, belonging to either the N-arylindole or benzothiophene chemotype. Verification studies confirmed the synthetic lethality phenotype for three of the N-arylindoles and one benzothiophene derivative. The latter was demonstrated to be partially bioavailable via oral administration in mice. Structure-activity relationship studies of both structural classes identified analogs with potent antitubercular activity, alone or in combination with meropenem. Transcriptional profiling revealed that oxidoreductases, MmpL family proteins, and a 27-kDa benzoquinone methyltransferase could be the targets of the N-arylindole potentiator. In conclusion, our compound-compound synthetic lethality screening revealed novel small molecules that were capable of potentiating the action of meropenem, presumably via inhibition of the innate resistance conferred by β-lactam accessory proteins. β-Lactam compound-compound synthetic lethality may be an alternative approach for drug-resistant tuberculosis.
INTRODUCTION
Drug-resistant tuberculosis (TB) continues to be a major threat to global public health, accounting for 3.5% of new TB cases and 18% of previously treated TB cases (1). New drug development for TB is a time- and resource-consuming process. Over the past five decades, only two new TB drugs have been licensed for clinical use as part of combination therapy to address drug-resistant TB, and those drugs were associated with serious limitations (2). As a potential parallel approach to new drug development, repurposing existing antibiotics, such as clofazimine (3, 4), the trimethoprim-sulfamethoxazole pair (5), linezolid (6), and β-lactams (7–17), for drug-susceptible and drug-resistant TB therapy has shown some promise. Administration of amoxicillin-clavulanic acid, when added to a second-line TB regimen, successfully treated two patients with a drug-resistant form of TB (18). A 2-day early bactericidal activity (EBA) study showed minimal effects of this pair when used alone (19), but a 7-day EBA study showed results comparable to those of ofloxacin, although inferior to those of isoniazid (20). In particular, multidrug-resistant (MDR) TB patients treated with imipenem, a member of the carbapenem class of β-lactams, responded well when the drug was combined with other first-line or second-line TB drugs (21). In a clinical study aimed at evaluating the therapeutic contribution of imipenem-clavulanate (IC), results showed that all 12 patients (4 with MDR TB and 8 with extensively drug-resistant [XDR] TB) who were treated with IC converted to sputum culture negativity and 7 of them were cured (22). In a large clinical study, however, addition of IC to an optimized background regimen (OBR) did not yield a significant difference from the OBR control (23). It is interesting that the combination of meropenem and clavulanic acid, in the presence of one or two active second-line TB drugs, was active against human XDR TB (24, 25) and was more effective than IC in a treatment study of patients with MDR/XDR TB (26). These data suggest that β-lactam antibiotics, when incorporated into an optimized regimen, may serve as useful additions to known, effective, first-line and second-line TB drugs.
The β-lactam antibiotics represent a large family of broad-spectrum antibiotics. Carbapenems are a subclass of β-lactam antibiotics that possess higher intrinsic resistance to the most prevalent β-lactamases (27). Representative members of this structural class include imipenem, meropenem, ertapenem, doripenem, biapenem, and the more recent, orally available faropenem and tebipenem (10, 28–30). The molecular targets of β-lactams are generally accepted to be penicillin-binding proteins (PBPs) such as the transpeptidases, a class of enzymes that catalyze the cross-linkages of transpeptides and thus the biosynthesis of the essential peptidoglycan of bacteria (31). Mycobacterium tuberculosis produces two types of transpeptidases, namely, the traditional d,d-(3,4)-transpeptidases and the l,d-(3)-transpeptidases (32–34). In the stationary growth phase, the peptidoglycan of M. tuberculosis predominantly contains linkages generated by l,d-transpeptidases (35). Meropenem is able to inhibit both d,d-transpeptidases and l,d-transpeptidases (33, 36, 37), suggesting its potential utility in TB chemotherapy. Meropenem, when combined with the β-lactamase inhibitor clavulanic acid, is effective against XDR TB in vitro (7). This finding implies that meropenem could be a drug candidate to be used in combination chemotherapy for drug-resistant TB.
Although β-lactams specifically target the PBPs, the molecular mechanisms that confer resistance are complex. In addition to mutations in the target PBPs (31, 38, 39), efflux pumps (including multidrug efflux pumps) have been identified to play a role in β-lactam resistance (38–42). In our recent gene-compound synthetic lethality studies, we found that ABC transporters, the putative lipoprotein LprQ, a putative acyl transferase (Rv1565c), transcriptional regulators (including the two-component system RegX3), and a polyketide synthase (PpsC) also played roles in β-lactam resistance (38). Bearing in mind the complex nature of resistance mechanisms for β-lactams, in the current study we screened a small-molecule library using a compound-compound synthetic lethality approach, in the presence of a subinhibitory concentration of meropenem. Here we report the in vitro and in vivo characterization of this compound-compound synergy, and we characterize the bacterial transcription response network upon treatment with a representative compound.
RESULTS
High-throughput synthetic lethality screen.
Our high-throughput screen (HTS) was set up and validated using the concentration-response curves for meropenem and clavulanic acid alone and in combination (see Fig. S1 in the supplemental material). The 50% inhibitory concentration (IC50) value for meropenem alone was 8 μg/ml, whereas clavulanic acid alone was inactive even up to 100 μg/ml. However, 50% inhibition of bacterial growth was achieved with the combination of 0.2 μg/ml of clavulanic acid and 0.2 μg/ml of meropenem. Based on these data, we used a meropenem concentration of 2 μg/ml in the synthetic lethality screen, which barely inhibited M. tuberculosis growth, allowing evaluation of synergistic effects with small-molecule potentiators. Initial screening for compounds that were synthetically lethal in the presence of meropenem covered 318,012 small molecules of the NIH Molecular Libraries Small Molecule Repository (MLSMR) at Southern Research Institute (Birmingham, AL). Up to 876 molecules demonstrated preferential inhibition of M. tuberculosis growth, and rescreening in concentration-response analyses resulted in 363 noncytotoxic (72-h growth of Vero E6 cells) confirmed hits. Hit verification and confirmation were followed up, which resulted in six validated compound hits. Of the five indole derivatives, four have aryl substitutions on the indole nitrogen, while the benzothiophene has a urea moiety at the 3-position. (Fig. 1).
FIG 1.
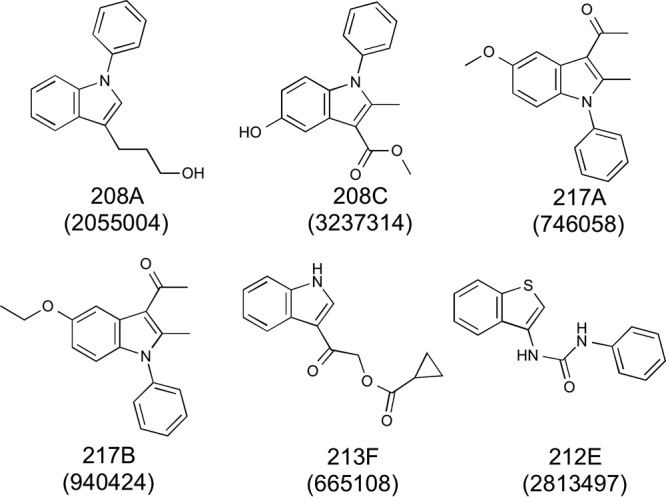
Structures of hit compounds. PubChem CID numbers are indicated in parentheses.
In vitro verification.
Meropenem MICs, with or without the presence of 2 μg/ml of each of the compounds, were determined for M. tuberculosis H37Rv. The MIC of meropenem alone was 16 μg/ml. Addition of 2 μg/ml of three N-arylindoles and the benzothiophene lowered the MIC of meropenem from 16 μg/ml to 8 μg/ml (217A and 217B), 4 μg/ml (213F), or 0.75 μg/ml (212E) (Fig. 2A). No lowering of the MIC values was seen for the remaining two indoles, 208A and 208C. There was no observable inhibitory effect at 2 μg/ml for each compound alone. In a reverse configuration, the MICs of each of the six compounds, with or without 2 μg/ml of meropenem, were also analyzed. While two compounds had no (208C) or minimal (208A) synergistic effect with meropenem, addition of 2 μg/ml of meropenem to the compounds significantly decreased their MICs as much as 16-fold (217B and 213F) or 32-fold (217A and 212E) (Fig. 2B). Again, no inhibitory effect was observed for meropenem alone at 2 μg/ml.
FIG 2.
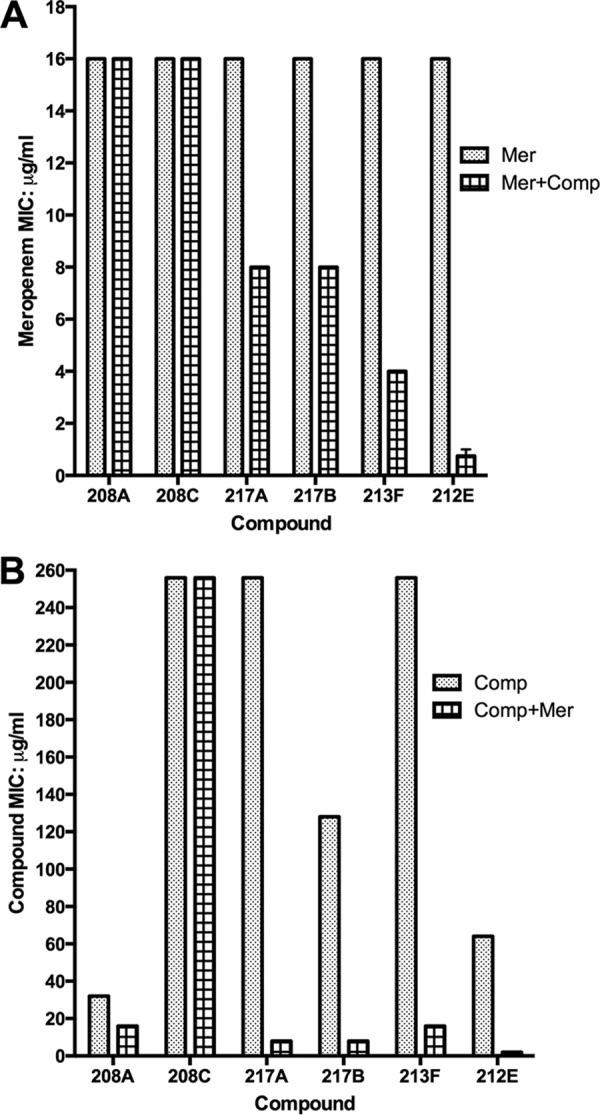
Synthetic lethality. (A) MICs of meropenem (Mer) in the presence or absence of 2 μg/ml of each of the six hit compounds (Comp). (B) MICs of hit compounds in the presence or absence of 2 μg/ml of meropenem. Data are shown as averages of three independent experiments.
Structure-activity relationship studies.
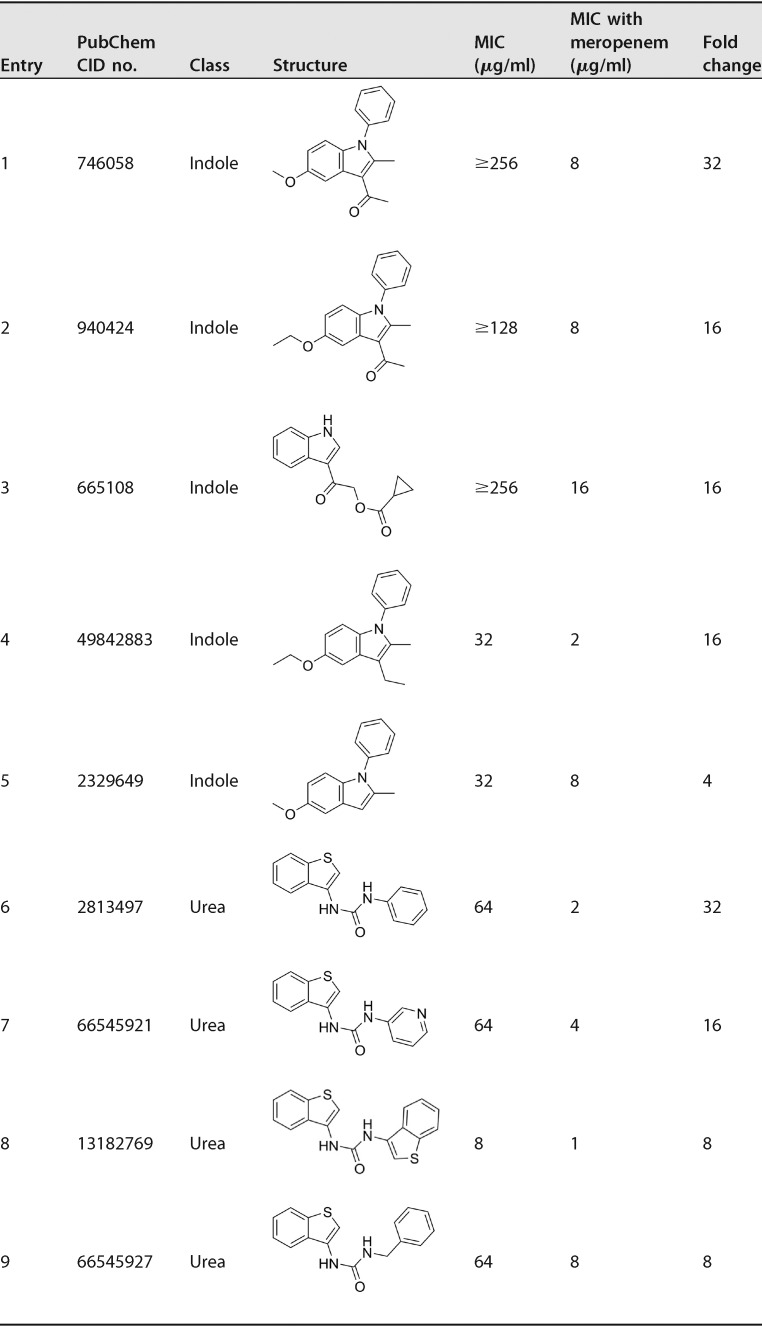
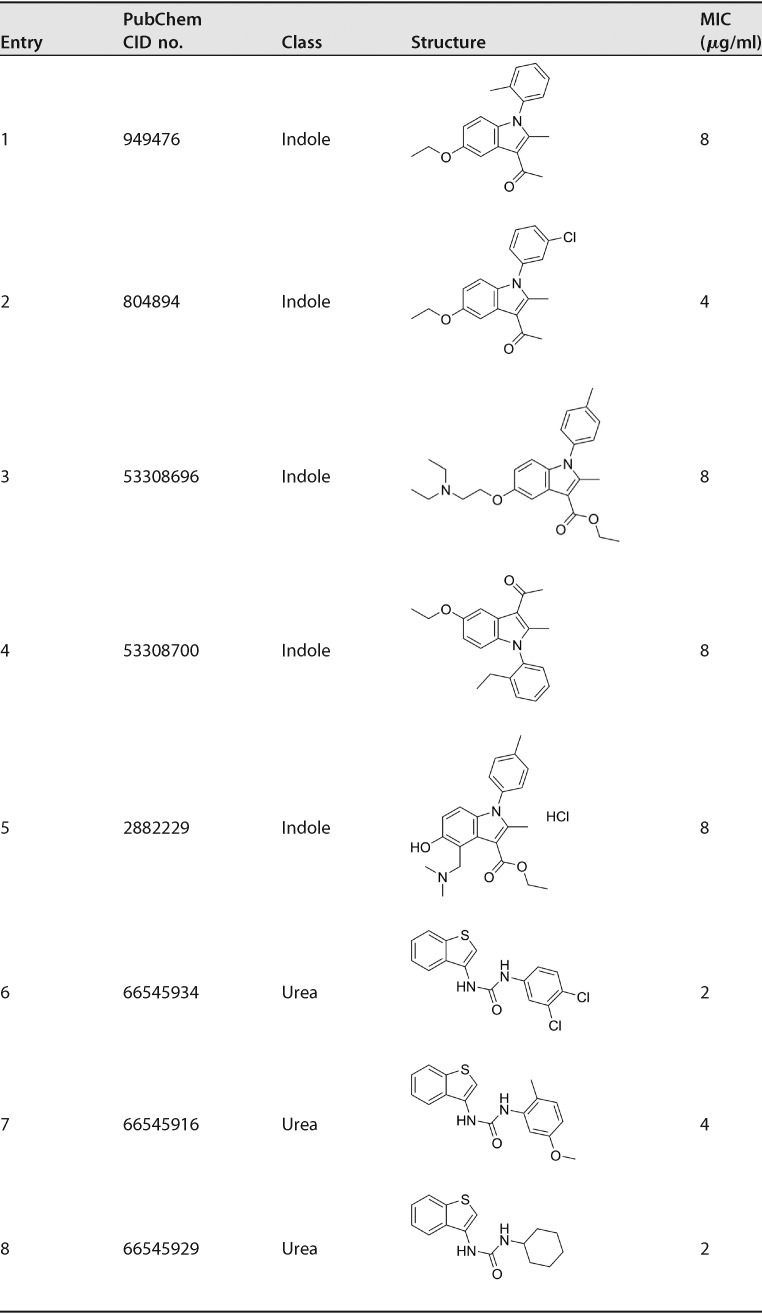
Encouraged by the ability of some N-arylindoles (217A, 217B, and 213F) and the benzothiophene 212E as meropenem sensitizers, we synthesized 68 indole analogs and screened them in the presence of a subinhibitory concentration of meropenem (Table S1). All 68 compounds have been assigned PubChem compound identifier (CID) numbers and respective BioAssay assay identifier (AID) numbers (PubChem BioAssay AID no. 434955, 485340, 504702, 504703, and 504713). The majority of the 68 indoles are 5-substituted with a hydroxyl or alkoxy group and 3-substituted with a methylketone or ethyl ester. The N-arylation on the indole generally did not result in more potent anti-TB compounds. In addition to the three hit indoles, 217A, 217B, and 213F, two more indoles (entries 4 and 5 in Table 1) were identified to be synthetically lethal for M. tuberculosis, showing 16- or 4-fold MIC reduction in the presence of 4 μg/ml of meropenem (Table 1). As controls, rifampin and isoniazid were included in the screen, and no synergy was observed when they were combined with 4 μg/ml of meropenem (Table S1). Interestingly, we also discovered that five indole compounds (entries 1 to 5 in Table 2) were moderately active against M. tuberculosis in the absence of meropenem, showing MIC values of 4 or 8 μg/ml (Table 2). Similarly, we screened 39 benzothiophene and/or urea derivatives (Table S2) and found that four compounds showed synthetic lethality in the presence of 4 μg/ml of meropenem, with MIC reductions of 8- to 32-fold (Table 1). We also found that three urea compounds were active against M. tuberculosis in the absence of meropenem, showing MICs of 2 μg/ml (Table 2). Structurally, the urea derivatives resemble AU1235, an adamantyl urea derivative previously reported to be an MmpL3 inhibitor (43).
TABLE 1.
Compounds showing synthetic lethality with meropenem against M. tuberculosis
TABLE 2.
Compounds active against M. tuberculosis without showing synthetic lethality with meropenem
Serum inhibition titration assay for bioavailability.
The mouse serum inhibition titration assay is a simple method to determine functional oral bioavailability of a compound together with any potential active metabolites. We administered 300 mg/kg of compound 217A to mice and tested the serum for M. tuberculosis growth inhibition in the presence of 2 μg/ml of meropenem. Compound 217A was orally bioavailable in mice and showed partial inhibition at 4- to 16-fold serum dilutions at the 15- and 30-minute postdosage time points (Fig. S2). Compound 217B did not show bioavailability at any time point or at either dosing level. At the given dosing level (300 mg/kg) and time point (30 min), the benzothiophene compound 212E showed meropenem-concentration-dependent bioavailability. No bioavailability was observed when meropenem was at 0 or 2 μg/ml, slight inhibition at 8-fold dilution was observed when meropenem was at 4 μg/ml, and complete inhibition at 8-fold dilution and partial inhibition at 16-fold dilution and thereafter was observed when meropenem was at 8 μg/ml (Fig. S2).
N-Arylindole treatment response networks assessed by RNA-Seq.
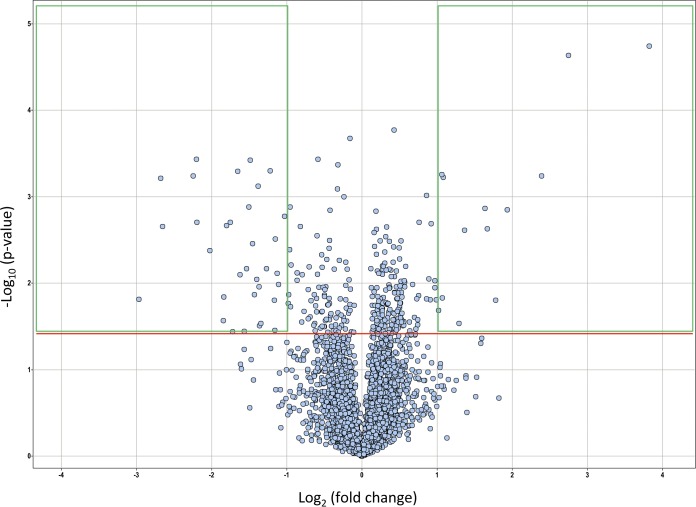
To seek the molecular target of the N-arylindole, we characterized the M. tuberculosis gene transcriptional responses on exposure to compound 217A, using whole-transcriptome shotgun sequencing (RNA-Seq) (Table S3). Differential expression was analyzed, and results are shown in Fig. 3 and Table S4. Thirty-three genes were upregulated at least 2-fold, and 15 showed statistical significance (P < 0.05). Fifty genes were downregulated at least 2-fold, with 34 showing significance (P < 0.05) (Table S4). Data analysis revealed that four of the resistance-nodulation-cell-division (RND) superfamily genes (mmpS4, mmpS5, mmpL5, and mmpL6) were significantly upregulated on exposure to compound 217A. Interestingly, the efflux pump regulator Rv0678 was significantly upregulated along with its substrates (mmpS5 and mmpL5) (Table S4), suggesting a possible mechanism for β-lactam removal or, alternatively, delivery of peptidoglycan substrates that are depleted due to β-lactam action. The organic substance responder Rv0077c (probable oxidoreductase), along with the immediately downstream membrane protein Rv0076c, was highly upregulated; the 27-kDa putative benzoquinone methyltransferase Rv0560c and Mce3C, which plays a role in virulence, detoxification, and adaptation, were also significantly upregulated. Other upregulated genes included those for a transcriptional regulator (Rv0452), an exported protein (Rv0320), and conserved hypothetical proteins (Rv1434, Rv2003c, and Rv2722).
FIG 3.
Volcano plot of differentially expressed genes after N-arylindole (compound 217A) treatment. The horizontal red line indicates a P value of 0.05. Green-framed boxes indicate fold changes of at least 2, either downregulated (left) or upregulated (right).
Multiple categories of genes were repressed or downregulated by N-arylindole treatment. A clear pattern shows that four proline-rich proteins (Rv3413c, Rv1435c, Mtc28, and Rv0312) were significantly downregulated and, interestingly, three invasion-associated genes (Rv1478, Rv1477, and Rv1566c) were also significantly downregulated. A few enzymes of metabolic pathways, such as a nitrite reductase (NirD), an oxidoreductase (Rv0063), an integrase/recombinase (XerC), an esterase (LipL), an acyl-coenzyme A dehydrogenase (FadE5), an acid phosphatase (SapM), a l-lactate dehydrogenase (LldD1), a possible chitinase (Rv1987), and a lipase (LipU), were also downregulated. Additionally, transcription regulator WhiB2, the alternative RNA polymerase sigma factor SigD, probable cation transporter P-type ATPase D (CtpD), and the antigen 85C (FbpC) were downregulated, as were some conserved hypothetical proteins (Table S4). Apparently, treatment of M. tuberculosis bacilli with the β-lactam synergetic partner 217A would be stressful for the bacilli, thus promoting the expression of stress response genes, including those encoding efflux pumps, and reducing the expression of energy transport and metabolism genes, such as that encoding the alternative sigma factor SigD.
In silico analysis of differentially expressed genes, using either KOBAS (44) or PANTHER (45) tools, did not reveal significantly enriched metabolic pathways. However, gene ontology enrichment analysis of those genes indicated significant changes in external encapsulating structure (cell wall) components (Fig. S3), showing spatial proximity to the site of action of the synthetic lethality partner meropenem. In addition, biological processes such as responses to hypoxic nitrosative stress and host immune responses and cell growth were significantly altered.
DISCUSSION
Our previous HTS of the NIH MLSMR for activity on M. tuberculosis growth in vitro identified several novel chemotypes, including the candidate probe (CID no. 2792221), which showed a submicromolar MIC and nondetectable toxicity (46). However, the bifurcated molecular metabolic pathways of M. tuberculosis justify the search for synthetic lethality partners of known targets for approved cognate antibiotics, such as l,d- or d,d-transpeptidases and β-lactams (38). β-Lactams are not considered first-line TB drugs because of their suboptimal activity against M. tuberculosis. However, when combined with the β-lactamase inhibitor clavulanic acid, meropenem is active not only on susceptible M. tuberculosis but also on drug-resistant M. tuberculosis (7). Indeed, Payen and colleagues treated six patients with XDR TB using meropenem and clavulanate as a salvage regimen and successfully cured one patient; five of the six patients achieved sputum culture conversion (24, 47). Although meropenem inhibits both classic d,d-transpeptidases and nonclassic l,d-transpeptidases of M. tuberculosis (33, 36, 37, 48) and meropenem in combination with clavulanic acid was bactericidal to XDR TB in vitro (7), the combination of meropenem and clavulanic acid had no effect in a chronic mouse infection model and had marginal effects in an acute mouse infection model (9). Recent studies have shown that new, orally bioavailable penems (tebipenem and faropenem) (10, 30) and cephalosporins (49, 50) are also active on M. tuberculosis, suggesting a potential role for this class of drugs in outpatient treatment of TB, and the compounds in the current study may serve as potentiators for this class of drugs.
We found that RND family proteins were actively involved in “detoxification” of the N-arylindole compound 217A, as reflected by significant upregulation of the four genes in the family (mmpS4, mmpS5, mmpL5, and mmpL6) (see Table S4 in the supplemental material). Two more mmpL genes (mmpL4 and mmpL11) were upregulated significantly, based on P values, but fell short of the 2.0-fold change cutoff value (Table S3). Interestingly, two mmp genes (mmpS3 and mmpL3) were significantly downregulated, based on P values (Table S3). The RND family proteins of M. tuberculosis have not been well characterized. The mmpS5-mmpL5 operon appears to be active in M. tuberculosis and plays a role as an efflux pump in azole resistance (51), probable meropenem resistance (38), and the probable N-arylindole resistance in this study (Table S4). This operon is regulated by the downstream MarR-like regulator Rv0678 (52), which was also significantly upregulated in this study (Table S4). Proteins in this class generally show weak substrate specificity and thus are less likely to be the true targets of the compound in question.
The most upregulated gene after treatment with the N-arylindole 217A was Rv0077c, encoding a probable oxidoreductase. Its immediate downstream gene, Rv0076c, encoding a membrane protein, was also significantly upregulated (Table S4). While it is possible that Rv0077c is the target for 217A and plays a required role in peptidoglycan biosynthesis, it is probable that Rv0077c participates in detoxification of 217A. In either case, these processes may take place on the cell wall, with Rv0077c and Rv0076c working cooperatively. The upregulation of the 27-kDa methylase with 217A treatment was interesting, because previous studies showed that this gene was induced by exposure to salicylate (53) or rifampin (54). Structurally, 217A, salicylate, and rifampin are unrelated, implying the 27-kDa methylase could be a universal chemical stress responder. Structural similarity between benzothiophene and AU1235 (43) implies that they may share a molecular target; however, we did not explore the molecular target for this chemotype. Further work will be required to detail the molecular mechanism for the synthetic lethality effect of N-arylindole or benzothiophene with meropenem. We note that the N-arylindoles and the benzothiophene urea classes are not β-lactams and thus are unlikely to be direct, orthosteric, β-lactamase inhibitors. This was reflected by the fact that expression of the β-lactamase-encoding blaC gene was not significantly changed after 217A treatment (Table S3). Similarly, the N-arylindole compound was likely not targeting LdtMT2, the major l,d-transpeptidase that generates the 3-3 cross-links of the peptidoglycan synthetic pathway (32), as the gene expression did not change significantly after treatment (P = 0.168) (Table S3). Minor changes (fold change of <2.0) were seen for analogs of LdtMT2, such as Rv0116c (fold change of 1.365), Rv0483 (fold change of −1.194), and Rv1433 (fold change of −1.966). Similarly, no significant changes were seen for the two annotated d,d-transpeptidases (Rv2911 and Rv3330), which is noteworthy because meropenem works on both d,d- and l,d-transpeptidases (33). This finding implies that a new mechanism of synergy between N-arylindoles and meropenem exists, which warrants further studies.
The proof of concept for compound-compound synthetic lethality in M. tuberculosis was clearly shown in vitro in this study. The nonoptimal in vivo bioavailability could be improved with medicinal chemistry approaches. The individually active N-arylindole and benzothiophene compounds identified in the structure-activity relationship (SAR) assay are interesting and should be further investigated for their drug development potentials.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
M. tuberculosis H37Rv was used in this study. BD Difco Middlebrook 7H9 broth with 10% oleic acid-albumin-dextrose-catalase (OADC), 0.2% glycerol, and 0.05% Tween 80 was used for initial bacterial culturing. Synthetic lethality assays, including the HTS, verification, and the SAR screen were carried out in the same medium but without Tween 80.
High-throughput screen.
The HTS was carried out in the presence or absence of 2 μg/ml of meropenem. Compounds were screened at a concentration of 25 μM, in a total volume of 50 μl and in a 384-well plate format. The dimethyl sulfoxide (DMSO) content was 1%, and the inoculum was 2.5 × 103 to 5.0 × 103 CFU per well. Clavulanic acid (β-lactamase inhibitor) and amikacin at 2.5 μg/ml were included on each microtiter plate as positive controls. Plates were incubated for 7 days. Detection was achieved by addition of 9 μl of alamarBlue, and plates were read 24 h later with a plate reader (excitation wavelength, 530 nm; emission wavelength, 590 nm). A summary of the screening campaign can be found at PubChem AID no. 434999 (http://pubchem.ncbi.nlm.nih.gov/assay/assay.cgi?aid=434999).
Verification screen and MIC assay.
Meropenem was prepared fresh daily. The assay was conducted in a 96-well plate format. The DMSO content was 1%, and the inoculum was 1.0 × 104 to 2.0 × 104 CFU per well. After 7 days of incubation, the alamarBlue assay was performed and plates were read with excitation and emission wavelengths of 544 and 590 nm, respectively. Amikacin at 2.5 μg/ml was used as a positive control, and bacteria alone was used as a negative control. Medium was used as a blank control. System validation was reported previously (38).
MIC determination using the alamarBlue assay.
The MIC was defined as the minimum concentration that inhibited at least 90% of the bacterial growth. Compound MICs were determined using the alamarBlue assay, in the presence or absence of 2 μg/ml of meropenem. Meropenem MICs were determined similarly, in the presence or absence of 2 μg/ml of each of the six hit compounds.
Serum inhibition titration assay for bioavailability.
To test oral bioavailability, 6-week-old female BALB/c mice (Charles River Laboratories, Inc.) were used. For N-arylindole compounds 217A and 217B, a single dose at 100 or 300 mg/kg was delivered orally. At 15, 30, and 60 min after administration, the mice were sacrificed under isoflurane anesthesia and cardiac blood was collected. For benzothiophene compound 212E, a single dose at 300 mg/kg was administered orally and blood was collected 30 min after gavage. The vehicle (0.5% carboxymethyl cellulose) was used as a negative control, and the first-line TB drug isoniazid at 10 mg/kg was used as a positive control. Both positive- and negative-control blood samples were collected 15 min after administration. One mouse per dose level per time point was used. Blood was left undisturbed for 30 min at room temperature, to allow clot formation, and then was centrifuged at 1,000 × g for 15 min. Serum was collected and processed for the alamarBlue assay. The serum was serially 2-fold diluted using 96-well microplates, and 104 CFU of M. tuberculosis H37Rv was added to each well. After incubation for 7 days at 37°C, 32.5 μl of alamarBlue working reagent (1 part 10% alamarBlue and 0.625 part 20% Tween 80) was added to each well; plates were read at excitation and emission wavelengths of 544 and 590 nm, respectively, after 16 to 18 h of incubation. For the 217A and 217B assays, final meropenem concentrations of 2 μg/ml were added to all wells. For the 212E assays, final meropenem concentrations of 0, 2, 4, and 8 μg/ml were added to wells. The Institutional Animal Care and Use Committee of the Johns Hopkins University School of Medicine approved all animal procedures performed in this study (protocol no. MO19M98).
Structure-activity relationship assay.
We synthesized 68 N-arylindole analogs and 39 benzothiophene analogs for SAR studies. All compounds have PubChem CID numbers (Tables 1 and 2; also see Tables S1 and S2 in the supplemental material). Purities of all final compounds were >95% (area percentage at 214 nm), as established by analytical high-performance liquid chromatography (HPLC) carried out with a Shimadzu HPLC system using a Waters C18 column. Inhibitory activities of analogs were characterized in the presence of 0 and 4 μg/ml (for N-arylindole derivatives) or 0, 2, and 4 μg/ml (for benzothiophene derivatives) of meropenem. Active compounds were identified as those showing at least 4-fold MIC decreases in the presence of meropenem. For detailed method descriptions, see the supplemental material.
RNA-Seq analysis.
Laboratory strain H37Rv was grown to an optical density at 600 nm of 0.5. Compound 3 (KSC-09-217A) was added to the culture at a final concentration of 64 μg/ml. DMSO at an equal concentration was added as a control. Cells were treated for 6 h at 37°C with shaking. Three biological replicates were carried out. Total RNA isolation, purification, quantification, and sequencing were performed as described previously (38). Fragments per kilobase of transcript per million mapped reads (FPKMs) were generated using an in-house script, which internally uses BEDTools (55). FPKMs from 217A-treated and control cells were first subjected to quantile normalization and then transformed into log2 notation with the Partek Genomics Suite platform (Partek Inc., St. Louis, MO, USA). The distribution of the resulting values was examined across all cell samples, and genes for which the minimum log2 value was at least 2.0 were compared between 217A-treated and control cells, to determine the relative mRNA expression of these two classes. Since the relative gene expression values, expressed as log2(ratio) or log2(fold change), showed normal distribution, they were binned according to standard deviation from their median to provide cutoff thresholds for upregulation or downregulation. We carried out functional enrichment analyses, such as gene ontology and metabolic pathway analyses, by using the Term Enrichment Tool in AmiGO 2 (http://amigo.geneontology.org/amigo), KOBAS 2.0 (kobas.cbi.pku.edu.cn), and PANTHER (pantherdb.org). The parameter cutoff values were set for each of the tools used to obtain the significantly enriched gene ontology terms or pathways. A P value cutoff of 0.05 and a minimum number of gene products of 2 were used for the Term Enrichment Tool. A false discovery rate-corrected P value of 0.05 was used for KOBAS gene ontology and pathway enrichment analyses. A Bonferroni-corrected P value of 0.05 was used for the PANTHER statistical overrepresentation test.
Supplementary Material
ACKNOWLEDGMENTS
We thank the staff of the High-Throughput Screening Center at the Southern Research Specialized Biocontainment Screening Center, and we thank the team of the Specialized Chemistry Center, Molecular Libraries Initiative, at the University of Kansas. We thank Conover Talbot for RNA-Seq data analysis. We thank Chunjing Liu for synthesis of a sample of the compound with PubChem CID no. 53308696 and Ben Neuenswander for analysis of the analytical data.
This work was supported by NIH grants R03 MH084877-01 (to W.R.B.), U54 HG005031 (to J.A.), and U54 HG005034-01 (to E.L.W.) and by federal funds from the National Institute of Allergy and Infectious Diseases under contract HHSN272200900040C.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01319-19.
REFERENCES
- 1.World Health Organization. 2018. Global tuberculosis report. World Health Organization, Geneva, Switzerland: https://apps.who.int/iris/bitstream/handle/10665/274453/9789241565646-eng.pdf?ua=1. [Google Scholar]
- 2.Centers for Disease Control and Prevention. 2013. Provisional CDC guidelines for the use and safety monitoring of bedaquiline fumarate (Sirturo) for the treatment of multidrug-resistant tuberculosis. MMWR Recomm Rep 62(RR-09):1–12. [PubMed] [Google Scholar]
- 3.Grosset JH, Tyagi S, Almeida DV, Converse PJ, Li S-Y, Ammerman NC, Bishai WR, Enarson D, Trébucq A. 2013. Assessment of clofazimine activity in a second-line regimen for tuberculosis in mice. Am J Respir Crit Care Med 188:608–612. doi: 10.1164/rccm.201304-0753OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tyagi S, Ammerman NC, Li S-Y, Adamson J, Converse PJ, Swanson RV, Almeida DV, Grosset JH. 2015. Clofazimine shortens the duration of the first-line treatment regimen for experimental chemotherapy of tuberculosis. Proc Natl Acad Sci U S A 112:869–874. doi: 10.1073/pnas.1416951112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies Forsman L, Schön T, Simonsson USH, Bruchfeld J, Larsson M, Juréen P, Sturegård E, Giske CG, Ängeby K. 2014. Intra- and extracellular activities of trimethoprim-sulfamethoxazole against susceptible and multidrug-resistant Mycobacterium tuberculosis. Antimicrob Agents Chemother 58:7557–7559. doi: 10.1128/AAC.02995-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agyeman AA, Ofori-Asenso R. 2016. Efficacy and safety profile of linezolid in the treatment of multidrug-resistant (MDR) and extensively drug-resistant (XDR) tuberculosis: a systematic review and meta-analysis. Ann Clin Microbiol Antimicrob 15:41. doi: 10.1186/s12941-016-0156-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hugonnet J-E, Tremblay LW, Boshoff HI, Barry CE, Blanchard JS. 2009. Meropenem-clavulanate is effective against extensively drug-resistant Mycobacterium tuberculosis. Science 323:1215–1218. doi: 10.1126/science.1167498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veziris N, Truffot C, Mainardi J-L, Jarlier V. 2011. Activity of carbapenems combined with clavulanate against murine tuberculosis. Antimicrob Agents Chemother 55:2597–2600. doi: 10.1128/AAC.01824-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solapure S, Dinesh N, Shandil R, Ramachandran V, Sharma S, Bhattacharjee D, Ganguly S, Reddy J, Ahuja V, Panduga V, Parab M, Vishwas KG, Kumar N, Balganesh M, Balasubramanian V. 2013. In vitro and in vivo efficacy of β-lactams against replicating and slowly growing/nonreplicating Mycobacterium tuberculosis. Antimicrob Agents Chemother 57:2506–2510. doi: 10.1128/AAC.00023-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horita Y, Maeda S, Kazumi Y, Doi N. 2014. In vitro susceptibility of Mycobacterium tuberculosis isolates to an oral carbapenem alone or in combination with β-lactamase inhibitors. Antimicrob Agents Chemother 58:7010–7014. doi: 10.1128/AAC.03539-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaushik A, Ammerman NC, Tasneen R, Story-Roller E, Dooley KE, Dorman SE, Nuermberger EL, Lamichhane G. 2017. In vitro and in vivo activity of biapenem against drug-susceptible and rifampicin-resistant Mycobacterium tuberculosis. J Antimicrob Chemother 72:2320–2325. doi: 10.1093/jac/dkx152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gurumurthy M, Verma R, Naftalin CM, Hee KH, Lu Q, Tan KH, Issac S, Lin W, Tan A, Seng KY, Lee LS, Paton NI. 2017. Activity of faropenem with and without rifampicin against Mycobacterium tuberculosis: evaluation in a whole-blood bactericidal activity trial. J Antimicrob Chemother 72:2012–2019. doi: 10.1093/jac/dkx081. [DOI] [PubMed] [Google Scholar]
- 13.Cohen KA, El-Hay T, Wyres KL, Weissbrod O, Munsamy V, Yanover C, Aharonov R, Shaham O, Conway TC, Goldschmidt Y, Bishai WR, Pym AS. 2016. Paradoxical hypersusceptibility of drug-resistant Mycobacterium tuberculosis to β-lactam antibiotics. EBioMedicine 9:170–179. doi: 10.1016/j.ebiom.2016.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang D, Wang Y, Lu J, Pang Y. 2016. In vitro activity of β-lactams in combination with β-lactamase inhibitors against multidrug-resistant Mycobacterium tuberculosis isolates. Antimicrob Agents Chemother 60:393–399. doi: 10.1128/AAC.01035-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pagliotto AD, Caleffi-Ferracioli KR, Lopes MA, Baldin VP, Leite CQ, Pavan FR, Scodro RB, Siqueira VL, Cardoso RF. 2016. Anti-Mycobacterium tuberculosis activity of antituberculosis drugs and amoxicillin/clavulanate combination. J Microbiol Immunol Infect 49:980–983. doi: 10.1016/j.jmii.2015.08.025. [DOI] [PubMed] [Google Scholar]
- 16.Kaushik A, Makkar N, Pandey P, Parrish N, Singh U, Lamichhane G. 2015. Carbapenems and rifampin exhibit synergy against Mycobacterium tuberculosis and Mycobacterium abscessus. Antimicrob Agents Chemother 59:6561–6567. doi: 10.1128/AAC.01158-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rullas J, Dhar N, McKinney JD, García-Pérez A, Lelievre J, Diacon AH, Hugonnet JE, Arthur M, Angulo-Barturen I, Barros-Aguirre D, Ballell L. 2015. Combinations of β-lactam antibiotics currently in clinical trials are efficacious in a DHP-I-deficient mouse model of tuberculosis infection. Antimicrob Agents Chemother 59:4997–4999. doi: 10.1128/AAC.01063-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nadler JP, Berger J, Nord JA, Cofsky R, Saxena M. 1991. Amoxicillin-clavulanic acid for treating drug-resistant Mycobacterium tuberculosis. Chest 99:1025–1026. doi: 10.1378/chest.99.4.1025. [DOI] [PubMed] [Google Scholar]
- 19.Donald PR, Sirgel FA, Venter A, Parkin DP, Van de Wal BW, Barendse A, Smit E, Carman D, Talent J, Maritz J. 2001. Early bactericidal activity of amoxicillin in combination with clavulanic acid in patients with sputum smear-positive pulmonary tuberculosis. Scand J Infect Dis 33:466–469. [DOI] [PubMed] [Google Scholar]
- 20.Chambers HF, Kocagöz T, Sipit T, Turner J, Hopewell PC. 1998. Activity of amoxicillin/clavulanate in patients with tuberculosis. Clin Infect Dis 26:874–877. doi: 10.1086/513945. [DOI] [PubMed] [Google Scholar]
- 21.Chambers HF, Turner J, Schecter GF, Kawamura M, Hopewell PC. 2005. Imipenem for treatment of tuberculosis in mice and humans. Antimicrob Agents Chemother 49:2816–2821. doi: 10.1128/AAC.49.7.2816-2821.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arbex MA, Bonini EH, Kawakame Pirolla G, D’Ambrosio L, Centis R, Migliori GB. 2016. Effectiveness and safety of imipenem/clavulanate and linezolid to treat multidrug and extensively drug-resistant tuberculosis at a referral hospital in Brazil. Rev Port Pneumol 22:337–341. doi: 10.1016/j.rppnen.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Tiberi S, Sotgiu G, D'Ambrosio L, Centis R, Arbex MA, Alarcon Arrascue E, Alffenaar JW, Caminero JA, Gaga M, Gualano G, Skrahina A, Solovic I, Sulis G, Tadolini M, Alarcon Guizado V, De Lorenzo S, Roby Arias AJ, Scardigli A, Akkerman OW, Aleksa A, Artsukevich J, Avchinko V, Bonini EH, Chong Marín FA, Collahuazo López L, de Vries G, Dore S, Kunst H, Matteelli A, Moschos C, Palmieri F, Papavasileiou A, Payen M-C, Piana A, Spanevello A, Vargas Vasquez D, Viggiani P, White V, Zumla A, Migliori GB. 2016. Effectiveness and safety of imipenem-clavulanate added to an optimized background regimen (OBR) versus OBR control regimens in the treatment of multidrug-resistant and extensively drug-resistant tuberculosis. Clin Infect Dis 62:1188–1190. doi: 10.1093/cid/ciw088. [DOI] [PubMed] [Google Scholar]
- 24.Payen MC, De Wit S, Martin C, Sergysels R, Muylle I, Van Laethem Y, Clumeck N. 2012. Clinical use of the meropenem-clavulanate combination for extensively drug-resistant tuberculosis. Int J Tuber Lung Dis 16:558–560. doi: 10.5588/ijtld.11.0414. [DOI] [PubMed] [Google Scholar]
- 25.Diacon AH, van der Merwe L, Barnard M, von Groote-Bidlingmaier F, Lange C, García-Basteiro AL, Sevene E, Ballell L, Barros-Aguirre D. 2016. β-Lactams against tuberculosis: new trick for an old dog? N Engl J Med 375:393–394. doi: 10.1056/NEJMc1513236. [DOI] [PubMed] [Google Scholar]
- 26.Tiberi S, Sotgiu G, D'Ambrosio L, Centis R, Abdo Arbex M, Alarcon Arrascue E, Alffenaar JW, Caminero JA, Gaga M, Gualano G, Skrahina A, Solovic I, Sulis G, Tadolini M, Alarcon Guizado V, De Lorenzo S, Roby Arias AJ, Scardigli A, Akkerman OW, Aleksa A, Artsukevich J, Auchynka V, Bonini EH, Chong Marín FA, Collahuazo López L, de Vries G, Dore S, Kunst H, Matteelli A, Moschos C, Palmieri F, Papavasileiou A, Payen M-C, Piana A, Spanevello A, Vargas Vasquez D, Viggiani P, White V, Zumla A, Migliori GB. 2016. Comparison of effectiveness and safety of imipenem/clavulanate- versus meropenem/clavulanate-containing regimens in the treatment of MDR- and XDR-TB. Eur Respir J 47:1758–1766. doi: 10.1183/13993003.00214-2016. [DOI] [PubMed] [Google Scholar]
- 27.Livermore DM, Woodford N. 2000. Carbapenemases: a problem in waiting? Curr Opin Microbiol 3:489–495. doi: 10.1016/S1369-5274(00)00128-4. [DOI] [PubMed] [Google Scholar]
- 28.Kato K, Shirasaka Y, Kuraoka E, Kikuchi A, Iguchi M, Suzuki H, Shibasaki S, Kurosawa T, Tamai I. 2010. Intestinal absorption mechanism of tebipenem pivoxil, a novel oral carbapenem: involvement of human OATP family in apical membrane transport. Mol Pharm 7:1747–1756. doi: 10.1021/mp100130b. [DOI] [PubMed] [Google Scholar]
- 29.Hazra S, Xu H, Blanchard JS. 2014. Tebipenem, a new carbapenem antibiotic, is a slow substrate that inhibits the β-lactamase from Mycobacterium tuberculosis. Biochemistry 53:3671–3678. doi: 10.1021/bi500339j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dhar N, Dubée V, Ballell L, Cuinet G, Hugonnet J-E, Signorino-Gelo F, Barros D, Arthur M, McKinney JD. 2015. Rapid cytolysis of Mycobacterium tuberculosis by faropenem, an orally bioavailable β-lactam antibiotic. Antimicrob Agents Chemother 59:1308–1319. doi: 10.1128/AAC.03461-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wivagg CN, Bhattacharyya RP, Hung DT. 2014. Mechanisms of β-lactam killing and resistance in the context of Mycobacterium tuberculosis. J Antibiot (Tokyo) 67:645–654. doi: 10.1038/ja.2014.94. [DOI] [PubMed] [Google Scholar]
- 32.Gupta R, Lavollay M, Mainardi J-L, Arthur M, Bishai WR, Lamichhane G. 2010. The Mycobacterium tuberculosis protein LdtMt2 is a nonclassical transpeptidase required for virulence and resistance to amoxicillin. Nat Med 16:466–469. doi: 10.1038/nm.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar P, Arora K, Lloyd JR, Lee IY, Nair V, Fischer E, Boshoff HIM, Barry CE. 2012. Meropenem inhibits D,D-carboxypeptidase activity in Mycobacterium tuberculosis. Mol Microbiol 86:367–381. doi: 10.1111/j.1365-2958.2012.08199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cordillot M, Dubée V, Triboulet S, Dubost L, Marie A, Hugonnet J-E, Arthur M, Mainardi J-L. 2013. In vitro cross-linking of Mycobacterium tuberculosis peptidoglycan by l,d-transpeptidases and inactivation of these enzymes by carbapenems. Antimicrob Agents Chemother 57:5940–5945. doi: 10.1128/AAC.01663-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lavollay M, Arthur M, Fourgeaud M, Dubost L, Marie A, Veziris N, Blanot D, Gutmann L, Mainardi J-L. 2008. The peptidoglycan of stationary-phase Mycobacterium tuberculosis predominantly contains cross-links generated by l,d-transpeptidation. J Bacteriol 190:4360–4366. doi: 10.1128/JB.00239-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim HS, Kim J, Im HN, Yoon JY, An DR, Yoon HJ, Kim JY, Min HK, Kim S-J, Lee JY, Han BW, Suh SW. 2013. Structural basis for the inhibition of Mycobacterium tuberculosis l,d-transpeptidase by meropenem, a drug effective against extensively drug-resistant strains. Acta Crystallogr D Biol Crystallogr 69:420–431. doi: 10.1107/S0907444912048998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li W-J, Li D-F, Hu Y-L, Zhang X-E, Bi L-J, Wang D-C. 2013. Crystal structure of L,D-transpeptidase LdtMt2 in complex with meropenem reveals the mechanism of carbapenem against Mycobacterium tuberculosis. Cell Res 23:728–731. doi: 10.1038/cr.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lun S, Miranda D, Kubler A, Guo H, Maiga MC, Winglee K, Pelly S, Bishai WR. 2014. Synthetic lethality reveals mechanisms of Mycobacterium tuberculosis resistance to β-lactams. mBio 5:e01767-14. doi: 10.1128/mBio.01767-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chatterjee SS, Chen L, Gilbert A, da Costa TM, Nair V, Datta SK, Kreiswirth BN, Chambers HF. 2017. PBP4 mediates β-lactam resistance by altered function. Antimicrob Agents Chemother 61:e00932-17. doi: 10.1128/AAC.00932-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Danilchanka O, Mailaender C, Niederweis M. 2008. Identification of a novel multidrug efflux pump of Mycobacterium tuberculosis. Antimicrob Agents Chemother 52:2503–2511. doi: 10.1128/AAC.00298-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balganesh M, Dinesh N, Sharma S, Kuruppath S, Nair AV, Sharma U. 2012. Efflux pumps of Mycobacterium tuberculosis play a significant role in antituberculosis activity of potential drug candidates. Antimicrob Agents Chemother 56:2643–2651. doi: 10.1128/AAC.06003-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dinesh N, Sharma S, Balganesh M. 2013. Involvement of efflux pumps in the resistance to peptidoglycan synthesis inhibitors in Mycobacterium tuberculosis. Antimicrob Agents Chemother 57:1941–1943. doi: 10.1128/AAC.01957-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grzegorzewicz AE, Pham H, Gundi VA, Scherman MS, North EJ, Hess T, Jones V, Gruppo V, Born SE, Korduláková J, Chavadi SS, Morisseau C, Lenaerts AJ, Lee RE, McNeil MR, Jackson M. 2012. Inhibition of mycolic acid transport across the Mycobacterium tuberculosis plasma membrane. Nat Chem Biol 8:334–341. doi: 10.1038/nchembio.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu J, Mao X, Cai T, Luo J, Wei L. 2006. KOBAS server: a web-based platform for automated annotation and pathway identification. Nucleic Acids Res 34:W720–W724. doi: 10.1093/nar/gkl167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mi H, Muruganujan A, Thomas PD. 2013. PANTHER in 2013: modeling the evolution of gene function, and other gene attributes, in the context of phylogenetic trees. Nucleic Acids Res 41:D377–D386. doi: 10.1093/nar/gks1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.White EL, Maddox C, Nebane NM, Tower NA, McKellip S, Manuvakhova A, Reddy L, Sosa M, Rasmussen L, Whig K, Ananthan S, Lun S, Bishai W, Weiner WS, Schoenen F, Dutta A, Aubé J. 2013. Discovery and development of highly potent inhibitors of Mycobacterium tuberculosis growth in vitro In Probe reports from the NIH Molecular Libraries Program. National Center for Biotechnology Information, Bethesda, MD: https://www.ncbi.nlm.nih.gov/books/NBK143189. [PubMed] [Google Scholar]
- 47.Keener AB. 2014. Oldie but goodie: repurposing penicillin for tuberculosis. Nat Med 20:976–978. doi: 10.1038/nm0914-976. [DOI] [PubMed] [Google Scholar]
- 48.Dubée V, Triboulet S, Mainardi J-L, Ethève-Quelquejeu M, Gutmann L, Marie A, Dubost L, Hugonnet J-E, Arthur M. 2012. Inactivation of Mycobacterium tuberculosis l,d-transpeptidase LdtMt1 by carbapenems and cephalosporins. Antimicrob Agents Chemother 56:4189–4195. doi: 10.1128/AAC.00665-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gold B, Smith R, Nguyen Q, Roberts J, Ling Y, Lopez Quezada L, Somersan S, Warrier T, Little D, Pingle M, Zhang D, Ballinger E, Zimmerman M, Dartois V, Hanson P, Mitscher LA, Porubsky P, Rogers S, Schoenen FJ, Nathan C, Aubé J. 2016. Novel cephalosporins selectively active on nonreplicating Mycobacterium tuberculosis. J Med Chem 59:6027–6044. doi: 10.1021/acs.jmedchem.5b01833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lopez Quezada L, Li K, McDonald SL, Nguyen Q, Perkowski AJ, Pharr CW, Gold B, Roberts J, McAulay K, Saito K, Somersan Karakaya S, Javidnia PE, Porras de Francisco E, Amieva MM, Díaz SP, Mendoza Losana A, Zimmerman M, Liang H-PH, Zhang J, Dartois V, Sans S, Lagrange S, Goullieux L, Roubert C, Nathan C, Aubé J. 2019. Dual-pharmacophore pyrithione-containing cephalosporins kill both replicating and nonreplicating Mycobacterium tuberculosis. ACS Infect Dis 5:1433. doi: 10.1021/acsinfecdis.9b00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Milano A, Pasca MR, Provvedi R, Lucarelli AP, Manina G, Ribeiro ALDJL, Manganelli R, Riccardi G. 2009. Azole resistance in Mycobacterium tuberculosis is mediated by the MmpS5-MmpL5 efflux system. Tuberculosis (Edinb) 89:84–90. doi: 10.1016/j.tube.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 52.Radhakrishnan A, Kumar N, Wright CC, Chou T-H, Tringides ML, Bolla JR, Lei H-T, Rajashankar KR, Su C-C, Purdy GE, Yu EW. 2014. Crystal structure of the transcriptional regulator Rv0678 of Mycobacterium tuberculosis. J Biol Chem 289:16526–16540. doi: 10.1074/jbc.M113.538959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun Z, Cheng SJ, Zhang H, Zhang Y. 2001. Salicylate uniquely induces a 27-kDa protein in tubercle bacillus. FEMS Microbiol Lett 203:211–216. doi: 10.1111/j.1574-6968.2001.tb10843.x. [DOI] [PubMed] [Google Scholar]
- 54.De Knegt GJ, Bruning O, ten Kate MT, de Jong M, van Belkum A, Endtz HP, Breit TM, Bakker-Woudenberg I, de Steenwinkel J. 2013. Rifampicin-induced transcriptome response in rifampicin-resistant Mycobacterium tuberculosis. Tuberculosis (Edinb) 93:96–101. doi: 10.1016/j.tube.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 55.Quinlan AR, Hall IM. 2010. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26:841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.