Abstract
Coffee consumption is associated with reduced risk of metabolic syndrome, obesity and diabetes, which may be related to the effects of coffee and its bioactive components on lipid metabolism. Coffee contains caffeine, a known neuromodulator that acts as an adenosine receptor antagonist, as well as other components, such as chlorogenic acids, trigonelline, cafestol and kahweol. Thus, this review discusses the up-to-date knowledge of mechanisms of action of coffee and its bioactive compounds on lipid metabolism. Although there is evidence that coffee and/or its bioactive compounds regulate transcription factors (e.g. peroxisome proliferator-activated receptors and sterol regulatory element binding proteins) and enzymes (e.g. AMP-activated protein kinase) involved in lipogenesis, lipid uptake, transport, fatty acid β-oxidation and/or lipolysis, needs for the understanding of coffee and its effects on lipid metabolism in humans remain to be answered.
Keywords: Alkaloid, Phenolic acid, Cholesterol, Obesity, Fat
Introduction
Coffee is an ancient drink that is increasingly popular around the world. People who drink coffee are not only attracted to its flavor, but also to its potential health benefits, including lower risk of metabolic syndrome, obesity and diabetes (Farah, 2012; Grosso et al., 2017; Santos and Lima, 2016). As altered lipid metabolism is common to these conditions, the effects of coffee bioactives on lipid metabolism have been suggested as underlying mechanisms of the health benefits of coffee (Grosso et al., 2017; Santos and Lima, 2016). Therefore, this review primarily discusses the current knowledge of coffee and its bioactive components on lipid metabolism.
Coffee composition
The composition of regular coffee varies mostly according to type of beans, roasting and brewing methods (Cruz et al., 2018; Vignoli et al., 2014). The most popular coffee beans are from Coffeea arabica (Arabica) or C. canephora (Robusta) with significant differences in their composition, including caffeine content; e.g. drinks from Robusta beans had higher caffeine levels than Arabica (Vignoli et al., 2014). Roasting coffee beans degrades heat unstable compounds (e.g. phenolic acids and trigonelline) and changes their sensory profile (Farah, 2012). For instance, light or medium roast coffee beans are used to make coffee drinks with more chlorogenic acids (CGA) than dark roast coffee beans (Vignoli et al., 2014). Brewing methods influence the coffee drink composition as well; Turkish-style coffee drink had higher concentrations of diterpenes (cafestol and kahweol) than filtered coffee drink (Rendon et al., 2018). These differences in composition have shown to influence the potential biological properties of coffee (Cruz et al., 2018).
The most common coffee extraction is performed by hot water from beans, but other plant parts or solvents are used to develop other coffee products. Water extraction from the coffee fruit (pulp) or silver skin (bean testa), usually discarded in the regular coffee production, retained some of the coffee bioactive compounds, containing about 1% CGA and 1–3% caffeine (Ameca et al., 2018; Martinez-Saez et al., 2014; Ontawong et al., 2019b). Different solvent extraction methods change the coffee extract composition. Decaffeinated coffee, which has 2–15 mg caffeine per serving, can be produced by organic solvents or supercritical CO2 methods (de Azevedo et al., 2008; Farah, 2012). Ethanol extraction is used to make the commercially available green coffee bean extracts (GCBE), which contain 27–50% CGA and 2–10% caffeine (Choi et al., 2016; Kim et al., 2014; Shimoda et al., 2006). Taken together, coffee processing methods have a great impact on its composition and related biological properties.
Coffee bioactive compounds
Caffeine (Fig. 1), an alkaloid that has a variety of potential biological effects, is found at concentration between 50 and 380 mg/100 mL in regular coffee drink (Farah, 2012; Grosso et al., 2017). Caffeine is an adenosine receptor antagonist, related to its mostly known function as a neuromodulator, that boosts energy expenditure (Harpaz et al., 2017; Wu et al., 2017). Although caffeine has potential beneficial effects against Parkinson’s disease and type-2 diabetes, some need to control caffeine intake due to its effect on increased blood pressure (Grosso et al., 2017).
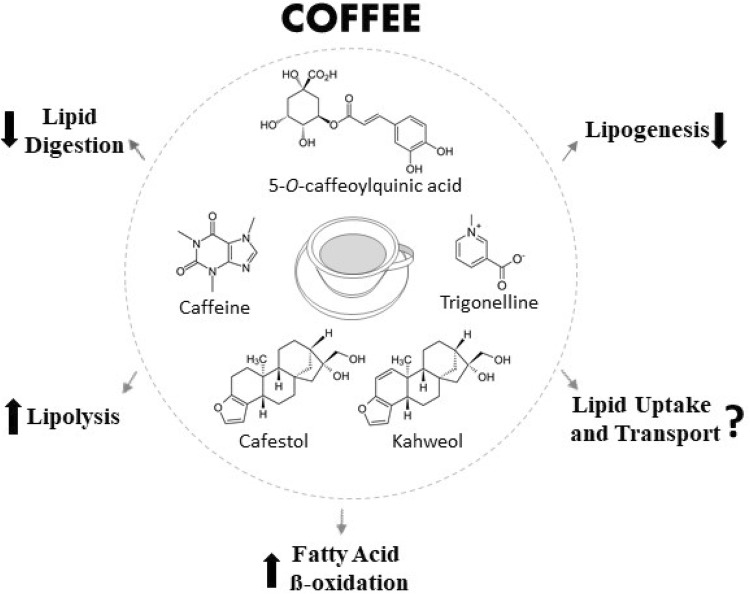
Fig. 1.
Illustrative summary of the overall effects of coffee and its bioactive compounds on lipid metabolism. Molecular structures of chlorogenic acids (phenolic acids) representative: 5-O-caffeoylquinic acid; alkaloids: caffeine and trigonelline; diterpenes: cafestol and kahweol. ↓, decrease;?, inconclusive; ↑, increase
There are different CGA esters in coffee and their concentrations combined range from 35 to 500 mg/100 mL in the regular coffee drink (Farah, 2012). CGA esters are formed between cinnamic acids (caffeic acid, ferulic acid and p-coumaric acid) and quinic acid; here, any CGA ester will be referred as CGA (Clifford et al., 2017). Among them, 5-O-caffeyolquinic acid (Fig. 1) is the most studied CGA ester and is linked to the GCBE’s fat-lowering effects (Farias-Pereira et al., 2018). In addition, the CGA precursors (i.e. cinnamic acids) or its degraded products were related to antioxidant properties (Jeszka-Skowron et al., 2016; Kamiyama et al., 2015; Yue et al., 2019).
Trigonelline (Fig. 1), an alkaloid derivative of niacin (vitamin B3), is present at 40–50 mg/100 mL in regular coffee drink (Farah, 2012). Although there is limited evidence of the physiological effects of trigonelline, trigonelline has shown to have antioxidant and anti-inflammatory effects (Mohamadi et al., 2018). Additionally, trigonelline has shown to be potential anti-diabetes and anti-obesity agent, which may also be linked to niacin’s effects on lipid metabolism (Riedel et al., 2014; Sharma et al., 2018; Yoshinari et al., 2009).
Cafestol (Fig. 1) is one of the coffee diterpenes found at 0.25–0.3 mg/100 mL in the regular coffee drink, and up to 4 mg/100 mL in unfiltered coffee drink (Rendon et al., 2018). High amounts of cafestol intake increased blood cholesterol levels; daily intake of 60 mg cafestol increased about 30 mg/dL total cholesterol levels in humans after 28 days (Urgert et al., 1997). This is due to the fact that cafestol is an agonist of farnesoid X receptors (FXR), responsible for the increase of blood cholesterol levels by inhibiting bile acid synthesis (Post et al., 1997; Ricketts et al., 2007). On the other hand, cafestol has shown beneficial biological effects, such as anti-obesity, anti-diabetes, anticancer and anti-inflammatory properties (Lima et al., 2017; Mellbye et al., 2015, 2017; Shokouh et al., 2018; van Cruchten 2010).
Kahweol (Fig. 1), present at range of 0.14–0.2 mg/100 mL in the regular coffee drink, is another diterpene mostly found in Arabica coffee beans (Farah, 2012; Rendon et al., 2018). In vitro studies have shown that kahweol is a potential antioxidant, anti-obesity and anticancer agent (Baek et al., 2017; Lee and Jeong, 2007; Oh et al., 2018). Although kahweol and cafestol are structurally similar, their effects on lipid metabolism have been shown to be different; cafestol was more effective as cholesterol-raising factor, while kahweol was more effective as an adipogenesis inhibitor (Baek et al., 2017; Urgert et al., 1997).
Coffee regulates lipid metabolism
Coffee and human health
Human studies have shown that moderate consumption of coffee (2–3 cups/day) is associated with reduced risk of metabolic syndrome, obesity and type 2 diabetes (Grosso et al., 2017; Santos and Lima, 2016). Daily consumption of coffee (510 mg CGA and 120 mg caffeine) or GCBE (372 mg CGA and 14.48 mg caffeine) ameliorated some parameters for metabolic syndrome after 8 weeks, including reduced body fat and insulin resistance (Roshan et al., 2018; Sarria et al., 2018). Consistently, daily intake of 600 mg CGA increased fat oxidation in healthy male subjects after 5 days (Park et al., 2017).
The effects of coffee are influenced by genetic differences in the population; i.e., rate of caffeine metabolism contributed significantly to physiological responses to coffee (Palatini et al., 2015; Robertson et al., 2018). Daily intake of coffee (174.4 mg CGA and 175.2 mg caffeine) reduced postprandial glucose levels in people who metabolizes caffeine slowly, but increased postprandial glucose levels in people who metabolize caffeine quickly after 12 weeks (Robertson et al., 2018). However, a follow-up study reported that hypertensive patients who metabolize caffeine slowly had higher risk of impaired fasting glucose, compared to whom metabolize caffeine quickly or non-coffee drinkers (Palatini et al., 2015).
A systematic review of clinical trials has discussed inconsistent results of different types of coffee on glucose metabolism and suggested that CGA and other compounds than caffeine within coffee contribute to the coffee’s effects on human health (Reis et al., 2019). For instance, a cross-over study showed that decaffeinated coffee (equivalent to 17–24 mg caffeine or 0.24–0.33 mg caffeine/kg body weight), but not caffeinated coffee (equivalent to 101–144 mg caffeine or 1.4–2.0 mg caffeine/kg body weight), improved insulin sensitivity in healthy men (Reis et al., 2018). Therefore, many of the inconsistent effects of coffee on human health may be due to variation of coffee composition.
Although some epidemiological studies show that moderate coffee consumption is associated with reduced risk of cardiovascular diseases, whether coffee has adverse or beneficial effects on blood lipids profile and its mechanisms is still being investigated (Godos et al., 2014; Poole et al., 2017; Saeed et al., 2019). A meta-analysis showed that coffee consumption (2.4–8 cups/day) increased total cholesterol, low-density lipoproteins (LDL) and triglycerides levels after 2–11 weeks (Grosso et al., 2017). Others have shown that coffee has a null or beneficial effect on lipid profile; coffee or GCBE did not have an impact on lipid profile in healthy subjects (Robertson et al., 2018; Roshan et al., 2018), while coffee reduced blood triglycerides levels in subjects with high cholesterol levels after 8 weeks (Sarria et al., 2018). In addition, unfiltered coffee was strongly associated with the undesirable changes in lipid profile, probably due to inhibitory effects of cafestol on bile acid synthesis (Saeed et al., 2019; Urgert et al., 1997). Overall, these human trials provide limited evidence that coffee and its bioactive compounds regulate lipid metabolism. This review will summarize the current proposed mechanisms of action of coffee and its bioactive compounds on lipid metabolism (Tables 1 and 2).
Table 1.
Summary of mechanistic studies of coffee extracts on lipid metabolism
| Material | Dosesa | Time (day) | Model | Effectsb | Targetsc | References |
|---|---|---|---|---|---|---|
| Green coffee bean extracts | 5 mg/mL (50% CGA and 2% caffeine) | 3 | Caenorhabditis elegans | ↓TG | ↓ACC; ↑ACS2; ↑ECH4; ↑FOXO; ↓FAR4; ↑HSL; ↓SREBP | Farias-Pereira et al. (2018) |
| 1% diet (27% CGA and 10% caffeine) | 14 | ddY mice (♂) | ↓b.w.; ↓TG | ↑CPT | Shimoda et al. (2006) | |
| 330 mg/kg b.w. (p.o.) (28% CGA and 9% caffeine) | 70 | High fat diet-fed ICR and C57BL/6 mice (♂) | ↓b.w.; ↓Chol; ↓HDL; ↓Leptin; ↓TG | ↓ACRP30; ↓PPARγ | Kim et al. (2014) | |
| 200 mg/kg b.w. (p.o.) (50% CGA) | 42 | High fat diet-fed C57BL/6 J mice (♂) | ↑Adiponectin; ↓b.w.; ↓Chol; ↓FFA; ↓Glucose; ↓LDL; ↓Leptin; ↓TG | ↑AMPK; ↑ATGL; ↓C/EBPα; ↑CPT1; ↓FAS; ↑HSL; ↑PPARα; ↓PPARγ; ↓SREBP1c; ↓SREBP2 | Choi et al. (2016) | |
| Water extracts | 1% diet (77% CGA) | 14–105 | High fat diet-fed C57BL/6 J mice (♂) | ↓b.w.; ↓Chol; ↑Energy expenditure; ↓Glucose; ↓Inflammation; ↓Insulin; ↓Leptin; ↓TG | ↓ACC1; ↓ACC2; ↓FAS; ↑miR-122; ↓SREBP1c; ↓SCD1; ↑UCP2 | Murase et al. (2010) |
| 0.5% water | 70 | High fat diet -fed Swiss mice (♂) | ↑Adiponectin; ↓Glucose; ↓Inflammation; ↓Insulin; ↑Leptin | ↑PKB | Caria et al. (2014) | |
| 2% diet | 63 | High fat diet-fed C57BL/6 mice (♂) | ↓b.w.; ↓Inflammation; ↓Insulin; ↓TG | ↓SCD1 | Jia et al. (2014) | |
| 0.1% water | 119 | Aged C57BL/6 NCr mice (♂) | ↑ATP; ↓FFA; ↑Locomotor activity; ↓TG | ↓mTOR; ↑PPARα | Takahashi et al. (2017) | |
| 1 g/kg b.w. (p.o.) (1.2% CGA and 0.4% caffeine) | 84 | High fat diet-fed Wistar rats (♂) | ↓b.w.; ↓Chol; ↓Insulin; ↓LDL; ↓TG | ↑LXRα; ↓NPC1L1; ↑PPARα; ↓PPARγ | Ontawong et al. (2019a, b) |
ab.w., body weight; p.o., per os
b↓, decrease; ↑, increase; b.w., body weight; Chol, cholesterol; FFA, free fatty acids; TG, triglycerides
cACC, acetyl-CoA carboxylase; ACRP, adipocyte complement-related protein; AMPK, AMP-activated protein kinase; ATGL, adipose triglyceride lipase; C/EBP, CCAAT/enhancer binding protein; CPT, carnitine palmitoyl transferase; ECH, enoyl-CoA hydratase; FAR, fatty acid- and retinoid-binding protein; FAS, fatty acid synthase; FOXO, forkhead box O; HSL, hormone sensitive lipase; LXR, liver X receptor; miR, microRNA; mTOR, mammalian target of rapamycin; NPC1L1, NPC1-like intracellular cholesterol transporter 1; PKB, protein kinase B; PPAR, peroxisome proliferator activated receptor; SCD, stearoyl-CoA desaturase; SREBP, sterol regulatory element-binding protein; UCP, uncoupling protein
Table 2.
Summary of mechanistic studies of coffee bioactive compounds on lipid metabolism
| Material | Dosesa | Time (day) | Model | Effectsb | Targetsc | References |
|---|---|---|---|---|---|---|
| Cafestol | 0.05% diet | 56 | High fat diet-fed C57BL6/J mice (♂) | ↓Bile acids; ↓b.w.; ↑FFA ↑Glycerol; ↓Inflammation; ↓Insulin; ↓Leptin; ↓TG | ↑ATGL; ↑CPT1; ↓FAS; ↑HSL; ↓SREBP1c; ↑UCP1 | van Cruchten (2010) |
| Caffeine | 5% | 20 | Zebrafish | ↓b.w.; ↓TG | ↓ACC1; ↑ACOX; ↓ATG12; ↓BECN1; ↓CD36; ↓SREBP1; ↓UCP2 | Zheng et al. (2015) |
| 60 mg/kg b.w. (s.c.) | 4 h | Obese yellow KK mice (♀) | ↑Adrenaline; ↑FFA | ↑UCP1; ↑UCP2; ↑UCP3 | Kogure et al. (2002) | |
| 30 mg/kg b.w (i.p.); 0.05% water | 3–28 | C57BL/6 mice (♂) | ↓b.w.; ↓TG | ↑ACC; ↓CPT1α; ↑LC3; ↓mTOR; ↓SQSTM1 | Sinha et al. (2014) | |
| 20 mg/kg b.w. (p.o.) | 70 | High energy diet-fed C57BL/6 mice (♂) | ↓b.w.; ↓Chol ↓FFA; ↓TG | ↓ACC; ↑AMPK; ↑cAMP | Zhang et al. (2015) | |
| 20 mg/kg b.w. (p.o.) | 42 | High fat diet-fed Sprague–Dawley rats (♂) | ↓b.w.; ↓TG | ↓ACC; ↓FAS; ↑IRS1; ↑PPARα; ↓SREBP1c | Liu et al. (2017) | |
| 60 mg/kg b.w. (p.o.) | 11–14 | High fat diet-fed C57BL/6; ob/ob mice (♂) | ↓b.w.; ↓Glucose; ↓TG | ↓A1R; ↑UCP1 | Wu et al. (2017) | |
| Chlorogenic acids | 2.65 mg/mL | 3 | Caenorhabditis elegans | ↓TG | ↓ACC; ↓ACS2; ↑ECH4; ↓FAR4; ↑FOXO; ↓C/EBP; ↓SREBP | Farias-Pereira et al. (2018) |
| 80 mg/kg b.w. (i.p.) | 56 | High fat diet -fed hamsters (♂) | ↓b.w.; ↓Chol; ↓FFA; ↓Glucose; ↓HDL; ↓Insulin; ↓LDL; ↓TG | ↑HL; ↓LPL; ↑PPARα | Li et al. (2009) | |
| 0.02% diet | 56 | High fat diet-fed ICR mice (♂) | ↑Adiponectin; ↓b.w.; ↓Chol; ↓FFA; ↓Insulin; ↓Leptin; ↓TG | ↓ACAT; ↓FAS; ↓HMGR; ↑PPARα | Cho et al. (2010) | |
| 250 mg/kg b.w. (i.p) | 14 | Leprdb/db mice (♂) | ↑Adiponectin; ↓b.w.; ↓Chol; ↓FFA; ↓Glucose; ↓Insulin; ↓TG | ↓ACC; ↑AMPK; ↑CaMKK | Ong et al. (2013) | |
| 90 mg/kg b.w. (p.o.) | 84 | High fat diet-fed Sprague–Dawley rats (♂) | ↓b.w.; ↓Chol; ↓FFA; ↓Glucose; ↓Insulin; ↓HDL; ↓LDL; ↓TG | ↓ACC; ↓CD36; ↑CPT2; ↓FABP4; ↓FAS; ↓LPL; ↓LXRα; ↑PPARα; ↑RXRα | Huang et al. (2015) | |
| 100 mg/kg b.w. (i.p.) | 42–105 | High fat diet-fed C57BL/6 mice (♂) | ↓b.w.; ↓Chol; ↓FFA; ↓Glucose; ↓Insulin; ↓TG | ↑ACOX1; ↓CD36; ↑CPT1; ↓FABP4; ↓MGAT; ↑PPARα; ↓PPARγ | Ma et al. (2015) | |
| 150 mg/kg b.w (p.o.) | 42 | High fat diet-fed Sprague–Dawley rats (♂) | ↓b.w.; ↓Chol; ↓FFA; ↓TG | ↓ACC; ↑AMPK; ↑CPT1 | Sudeep et al. (2016) | |
| 150 mg/kg b.w. (p.o.) | 42 | High fat diet-fed ICR mice (♂) | ↑Adiponectin; ↓b.w.; ↓Chol; ↑HDL; ↓LDL; ↓TG | ↓C/EBPα; ↓FABP4; ↓FAS; ↓LPL; ↓SREBP1c; ↑PPARα; ↓PPARγ | Wang et al. (2019) | |
| Trigonelline | 0.056% diet | 43 | Goko rats (♂) | ↓Bile acid; ↓Chol; ↓FFA; ↓Glucose; ↓Insulin; ↓TG | ↓FAS | Yoshinari et al. (2009) |
| 50 mg/kg b.w. (p.o.) | 30 | Alloxan-induced diabetes Wistar rats (♂) | ↑b.w.; ↓Chol; ↓Glucose; ↑HDL; ↓LDL; ↓TG | ↓Intestinal Lipase | Hamden et al. (2013) | |
| 50 mg/kg b.w (p.o.) | 112 | High fat (or cholesterol) diet-fed C57BL/6 J mice (♂) | ↓b.w.; ↓Chol; ↓Glucose ↓Insulin; ↓TG | ↑AMPK; ↑BECN1; ↓CD36; ↓mTOR; ↓PPARγ; ↓SREBP1 | Sharma et al. (2018) |
ab.w., body weight; i.p, intraperitoneal; p.o., per os; s.c., subcutaneous
b↓, decrease; ↑, increase; b.w., body weight; Chol, cholesterol; FFA, free fatty acids; TG, triglycerides
cA1R, adenosine 1 receptor; ACAT, acyl-CoA cholesterol acyltransferase; ACC, acetyl-CoA carboxylase; ACOX, acyl-CoA oxidase; AMPK, AMP-activated protein kinase; ATG, autophagy related; ATGL, adipose triglyceride lipase; BECN, beclin; C/EBP, CCAAT/enhancer binding protein; cAMP, cyclic AMP; CaMKK, calcium/calmodulin-dependent protein kinase kinase; CD, cluster of differentiation; CPT, carnitine palmitoyl transferase; ECH, enoyl-CoA hydratase; FABP, fatty acid-binding protein; FAR, fatty acid- and retinoid-binding protein; FAS, fatty acid synthase; FOXO, forkhead box O; HL, hepatic lipase; HMGR, HMG-CoA reductase; HSL, hormone sensitive lipase; IRS, insulin receptor substrate; LC, microtubule-associated protein 1A/1B-light chain; LPL, lipoprotein lipase; LXR, liver X receptor; MGAT, monoacylglycerol acyltransferase; mTOR, mammalian target of rapamycin; PPAR, peroxisome proliferator activated receptor; RXR, retinoid X receptor; SREBP, sterol regulatory element-binding protein; UCP, uncoupling protein
Coffee reduces lipogenesis
Coffee has shown to have fat-lowering effects in humans, which was associated with reduced lipogenesis (Santos and Lima, 2016). Coffee extracts (Choi et al., 2016; Farias-Pereira et al., 2018; Jia et al., 2014; Murase et al., 2010), CGA (Cho et al., 2010; Farias-Pereira et al., 2018; Huang et al., 2015; Ong et al., 2013; Sudeep et al., 2016; Zheng et al., 2014), caffeine (Liu et al., 2017; Sinha et al., 2014; Zheng et al., 2014, 2015), trigonelline (Yoshinari et al., 2009) and cafestol (van Cruchten 2010) have shown to reduce activity of key enzymes for lipogenesis: acetyl-CoA carboxylase (ACC), fatty acid synthase (FAS) and/or stearoyl-CoA desaturase (SCD). ACC and FAS are responsible for the first two steps of de novo lipogenesis, while SCD for the synthesis of monounsaturated fatty acids for fat storage (Proenca et al., 2014). The enzymatic inhibitory effects of coffee and/or its bioactive compounds were in part via regulation of upstream transcription factors for lipogenesis: CCAAT/enhancer-binding proteins (C/EBP), peroxisome proliferator-activated receptors (PPAR, especially PPARγ), and/or sterol regulatory element-binding proteins (SREBP) (Choi et al., 2016; Farias-Pereira et al., 2018; Kim et al., 2014; Liu et al., 2017; Ma et al., 2015; Murase et al., 2010; Ontawong et al., 2019a; Sharma et al., 2018; van Cruchten 2010; Wang et al., 2019; Zheng et al., 2014; Zheng et al., 2015). These transcription factors are well-known to regulate adipogenesis, including lipogenesis (Chung et al., 2016; Proenca et al., 2014).
In addition, coffee reduces lipogenesis by regulating another metabolic pathway, AMP-activated protein kinase (AMPK), which inhibits ACC and FAS (Ong et al., 2013; Proenca et al., 2014). In fact, coffee, CGA, caffeine and trigonelline were able to activate AMPK (Egawa et al., 2011; Mathew et al., 2014; Ong et al., 2013; Sharma et al., 2018; Sudeep et al., 2016; Wei Ong et al., 2012; Zhang et al., 2015). Many factors regulate AMPK activity, including the second messenger cyclic AMP (cAMP), which is increased by caffeine (Zhang et al., 2015). Along with increased cAMP, caffeine and CGA activated Ca2+/calmodulin-dependent protein kinase (CaMK), which can subsequently regulate the AMPK activation (Egawa et al., 2011; Mathew et al., 2014; Ong et al., 2013).
Coffee can also activate the forkhead box O (FOXO), involved in the insulin-signaling pathway known to regulate lipogenesis. GCBE and CGA reduced body fat dependent to increased FOXO nuclear translocation, leading to an decreased lipogenesis in Caenorhabditis elegans (Farias-Pereira et al., 2018). These suggest that the fat-lowering effects of coffee by inhibition of lipogenesis are potentially from its effects on insulin-mediated pathway via FOXO.
Epigenetic modifications by coffee might contribute to its effects on lipogenesis as well; coffee and CGA upregulated miR-122, a microRNA abundant in the liver with the inhibition of SREBP, ACC and FAS in murine hepatocytes (Murase et al., 2010). Similarly, others reported that coffee increased miR-96, a microRNA involved in SREBP expression in human intestinal epithelial Caco-2 cells (Jeon et al., 2013; Nakayama et al., 2017). Therefore, coffee and its bioactive compounds may inhibit lipogenesis via epigenetic changes.
Coffee compounds regulate lipid uptake and transport
Coffee can regulate the fatty acid translocase (FAT/CD36/SR-B2), a key transmembrane protein for lipid uptake and transport (Marechal et al., 2018). Caffeine, CGA and trigonelline have shown to decrease the diet-induced hepatic CD36 overexpression (Huang et al., 2015; Ma et al., 2015; Sharma et al., 2018; Zheng et al., 2015). CD36 is not only important for the uptake of dietary fatty acids, but also its ability to bind lipoproteins in the liver (Calvo et al., 1998; Ramasamy, 2014). Thus, the decreased expression of CD36 by coffee compounds is probably related to changes in blood lipid profile, including reduced triglycerides, cholesterol and LDL levels (Huang et al., 2015; Ma et al., 2015; Sharma et al., 2018; Zheng et al., 2015). It was further suggested that caffeine, CGA and trigonelline regulated CD36 via AMPK- and PPARγ -dependent pathways (Huang et al., 2015; Ma et al., 2015; Marechal et al., 2018; Quan et al., 2013; Sharma et al., 2018).
Other lipid-binding proteins involved in lipid uptake and transport, such as fatty acid-binding proteins (FABP) and fatty acid transporters proteins (FATP), were regulated by coffee bioactive components (Baek et al., 2017; Farias-Pereira et al., 2018; Lally et al., 2012; Su et al., 2013). It is suggested that the decreased lipid uptake and transport is related to lipogenesis inhibition; FABP4 (also called aP2), a target for PPARγ, was downregulated by caffeine and kahweol in adipocytes (Baek et al., 2017; Su et al., 2013). Consistently, a fatty acid- and retinoid-binding protein, FAR-4, was required for GCBE and CGA to reduce fat accumulation in C. elegans (Farias-Pereira et al., 2018). However, coffee compounds can increase lipid uptake and transport in the muscle driven by fatty acid β-oxidation; caffeine increased lipid uptake and transport in muscle tissue by regulating FABP, FATP1 and FATP4, partially dependent on mitochondrial CD36 (Lally et al., 2012). Taken together, coffee bioactive compounds regulate tissue-specific lipid uptake and transport via CD36 and other lipid-binding proteins.
Coffee increases fatty acid β-oxidation
There are many reports of coffee and its bioactive compounds on regulating fatty acid β-oxidation (Cho et al., 2010; Choi et al., 2016; Farias-Pereira et al., 2018; Huang et al., 2015; Li et al., 2009; Liu et al., 2017; Ma et al., 2015; Shimoda et al., 2006; Sinha et al., 2014; Sudeep et al., 2016; van Cruchten 2010; Wang et al., 2019; Zheng et al., 2014, 2015). Coffee, CGA, caffeine and cafestol have shown to increase the rate-limiting enzyme for mitochondrial fatty acid β-oxidation, carnitine palmitoyl transferase (CPT), which transports acyl-CoA from cytosol into mitochondria (Choi et al., 2016; Huang et al., 2015; Ma et al., 2015; Shimoda et al., 2006; Sinha et al., 2014; Sudeep et al., 2016; van Cruchten 2010). In addition, peroxisomal fatty acid β-oxidation was increased by CGA and/or caffeine via regulation of acyl-CoA oxidases (ACOX), the first step of the peroxisomal fatty acid β-oxidation (Ma et al., 2015; Reddy and Hashimoto, 2001; Zheng et al., 2014, 2015).
It is suggested that coffee regulates enzymes of fatty acid β-oxidation by activating PPARα in the liver and adipose tissues (Cho et al., 2010; Choi et al., 2016; Huang et al., 2015; Li et al., 2009; Liu et al., 2017; Ma et al., 2015; Ontawong et al., 2019a; Wang et al., 2019). Moreover, PPARβ/δ, involved in the fatty acid β-oxidation in muscle tissue, may play a role in the coffee’s effects; caffeine upregulated PPARβ/δ in muscle cells (Chung et al., 2016; Schnuck et al., 2018). However, it was reported that a coffee extract and CGA did not act as PPAR agonists in kidney CV-1 cells (Murase et al., 2010). Therefore, the mechanism in which coffee and its bioactive compounds activate PPAR is yet to be clear.
Other nuclear hormone receptors are reported to be involved in the coffee bioactive components’ effects on fatty acid β-oxidation. For instance, CGA has shown to increase expression of retinoid X receptor (RXR) and decrease liver X receptor (LXR) (Huang et al., 2015), which share similarities with PPARα (Boergesen et al., 2012). There is evidence that cafestol acts as a FXR agonist (Ricketts et al., 2007); FXR is not only involved in cholesterol metabolism, but involved in fatty acid β-oxidation (Massafra and van Mil, 2018; Yang et al., 2019). Thus, it is possible that cafestol and CGA regulate fatty acid β-oxidation via FXR, RXR and LXR. Therefore, it can be considered that coffee has pleiotropic effects by regulating transcription factors that potentially impact fatty acid β-oxidation.
Coffee regulates lipolysis
Coffee and caffeine consumption increased lipolysis, measured by free fatty acids and/or glycerol, peaking after 2–4 h in humans (Flanagan et al., 2014; Mougios et al., 2003; Vandenberghe et al., 2016). It was suggested that caffeine increases lipolysis in adipose tissue by inhibiting adenosine receptor and increasing catecholamine levels via the sympathetic nervous system (Carrageta et al., 2018; Kogure et al., 2002; Wu et al., 2017). The lipolytic effects of caffeine are mediated by the increased cAMP levels that activate enzymes for lipolysis, especially hormone-sensitive lipases (HSL) (Carrageta et al., 2018; Proenca et al., 2014; Zhang et al., 2015). Consistently, GCBE, CGA and cafestol upregulated HSL and adipose triglyceride lipases, both responsible for lipolysis in adipose tissue (Choi et al., 2016; Peng et al., 2018; van Cruchten 2010). However, GCBE, not CGA, upregulated HSL expression in C. elegans (Farias-Pereira et al., 2018). Since post-transcriptional regulation of these enzymes is important (Liu et al., 2018), the effects of coffee and its compounds on lipase’s activities will need to be determined to confirm the activities of coffee on lipolysis.
The lipolytic effects of coffee compounds are regulated by an additional pathway, the mammalian target of rapamycin (mTOR) (Caron et al., 2015). mTOR was inhibited by coffee, caffeine, trigonelline and kahweol in vivo or in vitro (Oh et al., 2018; Sharma et al., 2018; Sinha et al., 2014; Takahashi et al., 2017). Consistently, the lipolytic effects of caffeine were related to autophagy-lysosomal pathway dependent on AMPK and CaMK, known to cross-talk with mTOR (Mathew et al., 2014; Sinha et al., 2014). Therefore, the effects of coffee and its compounds on the nutrient-sensing pathways mTOR, AMPK and CaMK may contribute to the effects of coffee on lipolysis.
Coffee reduces lipid digestion
Coffee and its bioactive compounds may reduce dietary lipid digestion, partially due to inhibition of digestive lipase (Cha et al., 2012; Noh et al., 2006; Ontawong et al., 2019b). GCBE inhibits pancreatic lipase activity, in which half-maximal inhibitory concentration (IC50) was estimated to be 1.98 mg/mL in in vitro digestive simulation (Cha et al., 2012; Narita et al., 2012). The inhibitory effects of lipase by coffee is more likely due to CGA than caffeine; IC50 for CGA was 13–287 µM and IC50 for caffeine was > 500 µM (Cha et al., 2012). Trigonelline was also found to inhibit lipase and other digestive enzymes in rats (Hamden et al., 2013). GCBE inhibited pancreatic lipase by decreasing surface area of lipid emulsion and increasing lipid droplet size in vitro (Narita et al., 2012).
Coffee bioactive compounds can also affect lipid digestion by reducing the function or synthesis of bile acids, emulsifying agents that enhance lipid digestion (Ontawong et al., 2019b; Post et al., 1997). CGA was able to bind bile acids in vitro, suggesting that it reduces the function of bile acid on lipid digestion (Ontawong et al., 2019b). Moreover, cafestol was found to inhibit bile acid synthesis in rodents, which potentially changes lipid digestion (Post et al., 1997; Ricketts et al., 2007). Therefore, the inhibition of bile acid synthesis and lipase activity by coffee and its bioactive compounds may reduce dietary lipid digestion.
In conclusion, coffee and its bioactive components have shown to regulate lipid metabolism. Although there is more evidence for coffee extracts, especially GCBE, CGA and caffeine, other, less studied compounds (trigonelline, cafestol and kahweol) have shown potential to act on lipid metabolism in vivo and/or in vitro studies. Many questions about their mechanisms on lipid metabolism remain to be answered, and perhaps with the use of ‘omics’ technologies in humans, we will be able to understand and validate the effects of coffee on human health in future.
Acknowledgements
This work was financially supported by the Brazilian National Counsel of Technological and Scientific Development [CNPq, Conselho Nacional de Desenvolvimento Científico e Tecnológico]. The authors thank Mr. Joshua Barsczewski for his assistance editing this manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ameca GM, Cerrilla MEO, Córdoba PZ, Cruz AD, Hernández MS, Haro JH. Chemical composition and antioxidant capacity of coffee pulp. Ciênc. agrotec. 2018;42:307–313. doi: 10.1590/1413-70542018423000818. [DOI] [Google Scholar]
- Baek J-H, Kim N-J, Song J-K, Chun K-H. Kahweol inhibits lipid accumulation and induces glucose-uptake through activation of AMP-activated protein kinase (AMPK) BMB Rep. 2017;50:566–571. doi: 10.5483/BMBRep.2017.50.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boergesen M, Pedersen TA, Gross B, van Heeringen SJ, Hagenbeek D, Bindesboll C, Caron S, Lalloyer F, Steffensen KR, Nebb HI, Gustafsson J-A, Stunnenberg HG, Staels B, Mandrup S. Genome-wide profiling of liver X receptor, retinoid X receptor, and peroxisome proliferator-activated receptor alpha in mouse liver reveals extensive sharing of binding sites. Mol. Cell. Biol. 2012;32:852–867. doi: 10.1128/MCB.06175-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo D, Gomez-Coronado D, Suarez Y, Lasuncion MA, Vega MA. Human CD36 is a high affinity receptor for the native lipoproteins HDL, LDL, and VLDL. J. Lipid Res. 1998;39:777–788. [PubMed] [Google Scholar]
- Caria CRP, de Oliveira CC, Gotardo ÉFM, de Souza VT, Rocha T, Macedo JA, Carvalho P, Ribeiro ML, Gambero A. Caffeinated and decaffeinated instant coffee consumption partially reverses high-fat diet-induced metabolic alterations in mice. Food Res. Int. 2014;61:120–126. doi: 10.1016/j.foodres.2014.02.025. [DOI] [Google Scholar]
- Caron A, Richard D, Laplante M. The Roles of mTOR Complexes in Lipid Metabolism. Annu. Rev. Nutr. 2015;35:321–348. doi: 10.1146/annurev-nutr-071714-034355. [DOI] [PubMed] [Google Scholar]
- Carrageta DF, Dias TR, Alves MG, Oliveira PF, Monteiro MP, Silva BM. Anti-obesity potential of natural methylxanthines. J. Funct. Foods. 2018;43:84–94. doi: 10.1016/j.jff.2018.02.001. [DOI] [Google Scholar]
- Cha KH, Song D-G, Kim SM, Pan C-H. Inhibition of gastrointestinal lipolysis by green tea, coffee, and gomchui (Ligularia fischeri) tea polyphenols during simulated digestion. J. Agric. Food Chem. 2012;60:7152–7157. doi: 10.1021/jf301047f. [DOI] [PubMed] [Google Scholar]
- Cho A-S, Jeon S-M, Kim M-J, Yeo J, Seo K-I, Choi M-S, Lee M-K. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem. Toxicol. 2010;48:937–943. doi: 10.1016/j.fct.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Choi B-K, Park S-B, Lee D-R, Lee HJ, Jin Y-Y, Yang SH, Suh J-W. Green coffee bean extract improves obesity by decreasing body fat in high-fat diet-induced obese mice. Asian Pac. J. Trop. Med. 2016;9:635–643. doi: 10.1016/j.apjtm.2016.05.017. [DOI] [PubMed] [Google Scholar]
- Chung S, Kim YJ, Yang SJ, Lee Y, Lee M. Nutrigenomic functions of PPARs in obesogenic environments. PPAR Res. 2016;2016:4794576. doi: 10.1155/2016/4794576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford MN, Jaganath IB, Ludwig IA, Crozier A. Chlorogenic acids and the acyl-quinic acids: discovery, biosynthesis, bioavailability and bioactivity. Nat. Prod. Rep. 2017;34:1391–1421. doi: 10.1039/C7NP00030H. [DOI] [PubMed] [Google Scholar]
- Cruz RG, Vieira TMFS, Lira SP. Potential antioxidant of brazilian coffee from the region of Cerrado. Food Sci. Technol. 2018;38:447–453. doi: 10.1590/1678-457x.08017. [DOI] [Google Scholar]
- de Azevedo ABA, Mazzafera P, Mohamed RS, Demelo SABV, Kieckbusch TG. Extraction of caffeine, chlorogenic acids and lipids from green coffee beans using supercritical carbon dioxide and co-solvents. Brazilian J. Chem. Eng. 2008;25:543–552. doi: 10.1590/S0104-66322008000300012. [DOI] [Google Scholar]
- Egawa T, Hamada T, Ma X, Karaike K, Kameda N, Masuda S, Iwanaka N, Hayashi T. Caffeine activates preferentially α1-isoform of 5′AMP-activated protein kinase in rat skeletal muscle. Acta Physiol. 2011;201:227–238. doi: 10.1111/j.1748-1716.2010.02169.x. [DOI] [PubMed] [Google Scholar]
- Farah A. Coffee constituents. In: Chu Y-F, editor. Coffee: emerging health effects and disease prevention. New York: John Wiley & Sons Ltd; 2012. pp. 21–58. [Google Scholar]
- Farias-Pereira R, Oshiro J, Kim K-H, Park Y. Green coffee bean extract and 5-O-caffeoylquinic acid regulate fat metabolism in Caenorhabditis elegans. J. Funct. Foods. 2018;48:586–593. doi: 10.1016/j.jff.2018.07.049. [DOI] [Google Scholar]
- Flanagan J, Bily A, Rolland Y, Roller M. Lipolytic activity of Svetol(R), a decaffeinated green coffee bean extract. Phytother. Res. 2014;28:946–948. doi: 10.1002/ptr.5085. [DOI] [PubMed] [Google Scholar]
- Godos J, Pluchinotta FR, Marventano S, Buscemi S, Li Volti G, Galvano F, Grosso G. Coffee components and cardiovascular risk: beneficial and detrimental effects. Int. J. Food Sci. Nutr. 2014;65:925–936. doi: 10.3109/09637486.2014.940287. [DOI] [PubMed] [Google Scholar]
- Grosso G, Godos J, Galvano F, Giovannucci EL. Coffee, caffeine, and health outcomes: an umbrella review. Annu. Rev. Nutr. 2017;37:131–156. doi: 10.1146/annurev-nutr-071816-064941. [DOI] [PubMed] [Google Scholar]
- Hamden K, Mnafgui K, Amri Z, Aloulou A, Elfeki A. Inhibition of key digestive enzymes related to diabetes and hyperlipidemia and protection of liver-kidney functions by trigonelline in diabetic rats. Sci. Pharm. 2013;81:233–246. doi: 10.3797/scipharm.1211-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpaz E, Tamir S, Weinstein A, Weinstein Y. The effect of caffeine on energy balance. J. Basic Clin. Physiol. Pharmacol. 2017;28(1):1–10. doi: 10.1515/jbcpp-2016-0090. [DOI] [PubMed] [Google Scholar]
- Huang K, Liang X, Zhong Y, He W, Wang Z. 5-Caffeoylquinic acid decreases diet-induced obesity in rats by modulating PPARα and LXRα transcription. J. Sci. Food Agric. 2015;95:1903–1910. doi: 10.1002/jsfa.6896. [DOI] [PubMed] [Google Scholar]
- Jeon T-I, Esquejo RM, Roqueta-Rivera M, Phelan PE, Moon Y-A, Govindarajan SS, Esau CC, Osborne TF. An SREBP-responsive microRNA operon contributes to a regulatory loop for intracellular lipid homeostasis. Cell Metab. 2013;18:51–61. doi: 10.1016/j.cmet.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeszka-Skowron M, Sentkowska A, Pyrzyńska K, De Peña MP. Chlorogenic acids, caffeine content and antioxidant properties of green coffee extracts: influence of green coffee bean preparation. Eur. Food Res. Technol. 2016;242:1403–1409. doi: 10.1007/s00217-016-2643-y. [DOI] [Google Scholar]
- Jia H, Aw W, Egashira K, Takahashi S, Aoyama S, Saito K, Kishimoto Y, Kato H. Coffee intake mitigated inflammation and obesity-induced insulin resistance in skeletal muscle of high-fat diet-induced obese mice. Genes Nutr. 2014;9:389. doi: 10.1007/s12263-014-0389-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiyama M, Moon J-K, Jang HW, Shibamoto T. Role of degradation products of chlorogenic acid in the antioxidant activity of roasted coffee. J. Agric. Food Chem. 2015;63:1996–2005. doi: 10.1021/jf5060563. [DOI] [PubMed] [Google Scholar]
- Kim J, Jang JY, Cai J, Kim Y, Shin K, Choi E-K, Lee S-P, Kim J-C, Kim T-S, Jeong H-S, Kim Y-B. Ethanol extracts of unroasted Coffea canephora robusta beans suppress adipogenesis in preadipocytes and fat accumulation in rats fed a high-fat diet. Food Sci. Biotechnol. 2014;23:2029–2035. doi: 10.1007/s10068-014-0276-0. [DOI] [Google Scholar]
- Kogure A, Sakane N, Takakura Y, Umekawa T, Yoshioka K, Nishino H, Yamamoto T, Kawada T, Yoshikawa T, Yoshida T. Effects of caffeine on the uncoupling protein family in obese yellow KK mice. Clin. Exp. Pharmacol. Physiol. 2002;29:391–394. doi: 10.1046/j.1440-1681.2002.03675.x. [DOI] [PubMed] [Google Scholar]
- Lally JSV, Jain SS, Han XX, Snook LA, Glatz JFC, Luiken JJFP, McFarlan J, Holloway GP, Bonen A. Caffeine-stimulated fatty acid oxidation is blunted in CD36 null mice. Acta Physiol. 2012;205:71–81. doi: 10.1111/j.1748-1716.2012.02396.x. [DOI] [PubMed] [Google Scholar]
- Lee KJ, Jeong HG. Protective effects of kahweol and cafestol against hydrogen peroxide-induced oxidative stress and DNA damage. Toxicol. Lett. 2007;173:80–87. doi: 10.1016/j.toxlet.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Li S-Y, Chang C-Q, Ma F-Y, Yu C-L. Modulating effects of chlorogenic acid on lipids and glucose metabolism and expression of hepatic peroxisome proliferator-activated receptor-α in golden hamsters fed on high fat diet. Biomed. Environ. Sci. 2009;22:122–129. doi: 10.1016/S0895-3988(09)60034-9. [DOI] [PubMed] [Google Scholar]
- Lima CS, Spindola DG, Bechara A, Garcia DM, Palmeira-Dos-Santos C, Peixoto-da-Silva J, Erustes AG, Michelin LFG, Pereira GJS, Smaili SS, Paredes-Gamero E, Calgarotto AK, Oliveira CR, Bincoletto C. Cafestol, a diterpene molecule found in coffee, induces leukemia cell death. Biomed. Pharmacother. 2017;92:1045–1054. doi: 10.1016/j.biopha.2017.05.109. [DOI] [PubMed] [Google Scholar]
- Liu C-W, Tsai H-C, Huang C-C, Tsai C-Y, Su Y-B, Lin M-W, Lee K-C, Hsieh Y-C, Li T-H, Huang S-F, Yang Y-Y, Hou M-C, Lin H-C, Lee F-Y, Lee S-D. Effects and mechanisms of caffeine to improve immunological and metabolic abnormalities in diet-induced obese rats. Am. J. Physiol. Metab. 2017;314:E433–E447. doi: 10.1152/ajpendo.00094.2017. [DOI] [PubMed] [Google Scholar]
- Liu J, Peng Y, Yue Y, Shen P, Park Y. Epigallocatechin-3-gallate reduces fat accumulation in Caenorhabditis elegans. Prev. Nutr. food Sci. 2018;23:214–219. doi: 10.3746/pnf.2018.23.3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Gao M, Liu D. Chlorogenic acid improves high fat diet-induced hepatic steatosis and insulin resistance in mice. Pharm. Res. 2015;32:1200–1209. doi: 10.1007/s11095-014-1526-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marechal L, Laviolette M, Rodrigue-Way A, Sow B, Brochu M, Caron V, Tremblay A. The CD36-PPARgamma pathway in metabolic disorders. Int. J. Mol. Sci. 2018;19:E1529. doi: 10.3390/ijms19051529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Saez N, Ullate M, Martin-Cabrejas MA, Martorell P, Genovés S, Ramon D, del Castillo MD. A novel antioxidant beverage for body weight control based on coffee silverskin. Food Chem. 2014;150:227–234. doi: 10.1016/j.foodchem.2013.10.100. [DOI] [PubMed] [Google Scholar]
- Massafra V, van Mil SWC. Farnesoid X receptor: A “homeostat” for hepatic nutrient metabolism. Biochim. Biophys. acta. Mol. basis Dis. 2018;1864:45–59. doi: 10.1016/j.bbadis.2017.10.003. [DOI] [PubMed] [Google Scholar]
- Mathew TS, Ferris RK, Downs RM, Kinsey ST, Baumgarner BL. Caffeine promotes autophagy in skeletal muscle cells by increasing the calcium-dependent activation of AMP-activated protein kinase. Biochem. Biophys. Res. Commun. 2014;453:411–418. doi: 10.1016/j.bbrc.2014.09.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellbye FB, Jeppesen PB, Hermansen K, Gregersen S. Cafestol, a bioactive substance in coffee, stimulates insulin secretion and increases glucose uptake in muscle cells: studies in vitro. J. Nat. Prod. 2015;78:2447–2451. doi: 10.1021/acs.jnatprod.5b00481. [DOI] [PubMed] [Google Scholar]
- Mellbye FB, Jeppesen PB, Shokouh P, Laustsen C, Hermansen K, Gregersen S. Cafestol, a bioactive substance in coffee, has antidiabetic properties in KKAy mice. J. Nat. Prod. 2017;80:2353–2359. doi: 10.1021/acs.jnatprod.7b00395. [DOI] [PubMed] [Google Scholar]
- Mohamadi N, Sharififar F, Pournamdari M, Ansari M. A review on biosynthesis, analytical techniques, and pharmacological activities of trigonelline as a plant alkaloid. J. Diet. Suppl. 2018;15:207–222. doi: 10.1080/19390211.2017.1329244. [DOI] [PubMed] [Google Scholar]
- Mougios V, Ring S, Petridou A, Nikolaidis MG. Duration of coffee- and exercise-induced changes in the fatty acid profile of human serum. J. Appl. Physiol. 2003;94:476–484. doi: 10.1152/japplphysiol.00624.2002. [DOI] [PubMed] [Google Scholar]
- Murase T, Misawa K, Minegishi Y, Aoki M, Ominami H, Suzuki Y, Shibuya Y, Hase T. Coffee polyphenols suppress diet-induced body fat accumulation by downregulating SREBP-1c and related molecules in C57BL/6 J mice. Am. J. Physiol. - Endocrinol. Metab. 2010;300:E122–E133. doi: 10.1152/ajpendo.00441.2010. [DOI] [PubMed] [Google Scholar]
- Nakayama T, Funakoshi-Tago M, Tamura H. Coffee reduces KRAS expression in Caco-2 human colon carcinoma cells via regulation of miRNAs. Oncol. Lett. 2017;14:1109–1114. doi: 10.3892/ol.2017.6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita Y, Iwai K, Fukunaga T, Nakagiri O. Inhibitory activity of chlorogenic acids in decaffeinated green coffee beans against porcine pancreas lipase and effect of a decaffeinated green coffee bean extract on an emulsion of olive oil. Biosci. Biotechnol. Biochem. 2012;76:2329–2331. doi: 10.1271/bbb.120518. [DOI] [PubMed] [Google Scholar]
- Noh SK, Koo SI, Wang S. Epigallocatechin gallate and caffeine differentially inhibit the intestinal absorption of cholesterol and fat in ovariectomized rats. J. Nutr. 2006;136:2791–2796. doi: 10.1093/jn/136.11.2791. [DOI] [PubMed] [Google Scholar]
- Oh SH, Hwang YP, Choi JH, Jin SW, Lee GH, Han EH, Chung YH, Chung YC, Jeong HG. Kahweol inhibits proliferation and induces apoptosis by suppressing fatty acid synthase in HER2-overexpressing cancer cells. Food Chem. Toxicol. 2018;121:326–335. doi: 10.1016/j.fct.2018.09.008. [DOI] [PubMed] [Google Scholar]
- Ong KW, Hsu A, Tan BKH. Anti-diabetic and anti-lipidemic effects of chlorogenic acid are mediated by ampk activation. Biochem. Pharmacol. 2013;85:1341–1351. doi: 10.1016/j.bcp.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Ontawong A, Boonphang O, Pasachan T, Duangjai A, Pongchaidecha A, Phatsara M, Jinakote M, Amornlerdpison D, Srimaroeng C. Hepatoprotective effect of coffee pulp aqueous extract combined with simvastatin against hepatic steatosis in high-fat diet-induced obese rats. J. Funct. Foods. 2019;54:568–577. doi: 10.1016/j.jff.2019.02.011. [DOI] [Google Scholar]
- Ontawong A, Duangjai A, Muanprasat C, Pasachan T, Pongchaidecha A, Amornlerdpison D, Srimaroeng C. Lipid-lowering effects of Coffea arabica pulp aqueous extract in Caco-2 cells and hypercholesterolemic rats. Phytomedicine. 2019;52:187–197. doi: 10.1016/j.phymed.2018.06.021. [DOI] [PubMed] [Google Scholar]
- Palatini P, Benetti E, Mos L, Garavelli G, Mazzer A, Cozzio S, Fania C, Casiglia E. Association of coffee consumption and CYP1A2 polymorphism with risk of impaired fasting glucose in hypertensive patients. Eur. J. Epidemiol. 2015;30:209–217. doi: 10.1007/s10654-015-9990-z. [DOI] [PubMed] [Google Scholar]
- Park I, Ochiai R, Ogata H, Kayaba M, Hari S, Hibi M, Katsuragi Y, Satoh M, Tokuyama K. Effects of subacute ingestion of chlorogenic acids on sleep architecture and energy metabolism through activity of the autonomic nervous system: a randomised, placebo-controlled, double-blinded cross-over trial. Br. J. Nutr. 2017;117:979–984. doi: 10.1017/S0007114517000587. [DOI] [PubMed] [Google Scholar]
- Peng S-G, Pang Y-L, Zhu Q, Kang J-H, Liu M-X, Wang Z. Chlorogenic acid functions as a novel agonist of PPARγ2 during the differentiation of mouse 3T3-L1 preadipocytes. Biomed Res. Int. 2018;2018:8594767. doi: 10.1155/2018/8594767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole R, Kennedy OJ, Roderick P, Fallowfield JA, Hayes PC, Parkes J. Coffee consumption and health: umbrella review of meta-analyses of multiple health outcomes. BMJ. 2017;359:j5024. doi: 10.1136/bmj.j5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post SM, de Wit EC, Princen HM. Cafestol, the cholesterol-raising factor in boiled coffee, suppresses bile acid synthesis by downregulation of cholesterol 7 alpha-hydroxylase and sterol 27-hydroxylase in rat hepatocytes. Arterioscler. Thromb. Vasc. Biol. 1997;17:3064–3070. doi: 10.1161/01.ATV.17.11.3064. [DOI] [PubMed] [Google Scholar]
- Proenca ARG, Sertie RAL, Oliveira AC, Campana AB, Caminhotto RO, Chimin P, Lima FB. New concepts in white adipose tissue physiology. Braz J. Med. Biol. Res. 2014;47:192–205. doi: 10.1590/1414-431X20132911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan HY, Kim DY, Chung SH. Caffeine attenuates lipid accumulation via activation of AMP-activated protein kinase signaling pathway in HepG2 cells. BMB Rep. 2013;46:207–212. doi: 10.5483/BMBRep.2013.46.4.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy I. Recent advances in physiological lipoprotein metabolism. Clin. Chem. Lab. Med. 2014;52:1695–1727. doi: 10.1515/cclm-2013-0358. [DOI] [PubMed] [Google Scholar]
- Reddy JK, Hashimoto T. Peroxisomal beta-oxidation and peroxisome proliferator-activated receptor alpha: an adaptive metabolic system. Annu. Rev. Nutr. 2001;21:193–230. doi: 10.1146/annurev.nutr.21.1.193. [DOI] [PubMed] [Google Scholar]
- Reis CEG, Dórea JG, da Costa THM. Effects of coffee consumption on glucose metabolism: a systematic review of clinical trials. J. Tradit. Complement. Med. 2019;9:184–191. doi: 10.1016/j.jtcme.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis CEG, Paiva CLRDS, Amato AA, Lofrano-Porto A, Wassell S, Bluck LJC, Dorea JG, da Costa THM. Decaffeinated coffee improves insulin sensitivity in healthy men. Br. J. Nutr. 2018;119:1029–1038. doi: 10.1017/S000711451800034X. [DOI] [PubMed] [Google Scholar]
- Rendon MY, Dos Santos Scholz MB, Bragagnolo N. Physical characteristics of the paper filter and low cafestol content filter coffee brews. Food Res. Int. 2018;108:280–285. doi: 10.1016/j.foodres.2018.03.041. [DOI] [PubMed] [Google Scholar]
- Ricketts M-L, Boekschoten MV, Kreeft AJ, Hooiveld GJEJ, Moen CJA, Muller M, Frants RR, Kasanmoentalib S, Post SM, Princen HMG, Porter JG, Katan MB, Hofker MH, Moore DD. The cholesterol-raising factor from coffee beans, cafestol, as an agonist ligand for the farnesoid and pregnane X receptors. Mol. Endocrinol. 2007;21:1603–1616. doi: 10.1210/me.2007-0133. [DOI] [PubMed] [Google Scholar]
- Riedel A, Lang R, Rohm B, Rubach M, Hofmann T, Somoza V. Structure-dependent effects of pyridine derivatives on mechanisms of intestinal fatty acid uptake: regulation of nicotinic acid receptor and fatty acid transporter expression. J. Nutr. Biochem. 2014;25:750–757. doi: 10.1016/j.jnutbio.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Robertson TM, Clifford MN, Penson S, Williams P, Robertson MD. Postprandial glycaemic and lipaemic responses to chronic coffee consumption may be modulated by CYP1A2 polymorphisms. Br. J. Nutr. 2018;119:792–800. doi: 10.1017/S0007114518000260. [DOI] [PubMed] [Google Scholar]
- Roshan H, Nikpayam O, Sedaghat M, Sohrab G. Effects of green coffee extract supplementation on anthropometric indices, glycaemic control, blood pressure, lipid profile, insulin resistance and appetite in patients with the metabolic syndrome: a randomised clinical trial. Br. J. Nutr. 2018;119:250–258. doi: 10.1017/S0007114517003439. [DOI] [PubMed] [Google Scholar]
- Saeed M, Naveed M, BiBi J, Ali Kamboh A, Phil L, Chao S. Potential nutraceutical and food additive properties and risks of coffee: a comprehensive overview. Crit. Rev. Food Sci. Nutr. 2019 doi: 10.1080/10408398.2018.1489368. [DOI] [PubMed] [Google Scholar]
- Santos RMM, Lima DRA. Coffee consumption, obesity and type 2 diabetes: a mini-review. Eur. J. Nutr. 2016;55:1345–1358. doi: 10.1007/s00394-016-1206-0. [DOI] [PubMed] [Google Scholar]
- Sarria B, Martinez-Lopez S, Sierra-Cinos JL, Garcia-Diz L, Mateos R, Bravo-Clemente L. Regularly consuming a green/roasted coffee blend reduces the risk of metabolic syndrome. Eur. J. Nutr. 2018;57:269–278. doi: 10.1007/s00394-016-1316-8. [DOI] [PubMed] [Google Scholar]
- Schnuck JK, Gould LM, Parry HA, Johnson MA, Gannon NP, Sunderland KL, Vaughan RA. Metabolic effects of physiological levels of caffeine in myotubes. J. Physiol. Biochem. 2018;74:35–45. doi: 10.1007/s13105-017-0601-1. [DOI] [PubMed] [Google Scholar]
- Sharma L, Lone NA, Knott RM, Hassan A, Abdullah T. Trigonelline prevents high cholesterol and high fat diet induced hepatic lipid accumulation and lipo-toxicity in C57BL/6 J mice, via restoration of hepatic autophagy. Food Chem. Toxicol. 2018;121:283–296. doi: 10.1016/j.fct.2018.09.011. [DOI] [PubMed] [Google Scholar]
- Shimoda H, Seki E, Aitani M. Inhibitory effect of green coffee bean extract on fat accumulation and body weight gain in mice. BMC Complement. Altern. Med. 2006;6:9. doi: 10.1186/1472-6882-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokouh P, Jeppesen PB, Hermansen K, Nørskov NP, Laustsen C, Jacques Hamilton-Dutoit S, Qi H, Stødkilde-Jørgensen H, Gregersen S. A combination of coffee compounds shows insulin-sensitizing and hepatoprotective effects in a rat model of diet-induced metabolic syndrome. Nutrients. 2018;10:6. doi: 10.3390/nu10010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha RA, Farah BL, Singh BK, Siddique MM, Li Y, Wu Y, Ilkayeva OR, Gooding J, Ching J, Zhou J, Martinez L, Xie S, Bay B-H, Summers SA, Newgard CB, Yen PM. Caffeine stimulates hepatic lipid metabolism by the autophagy-lysosomal pathway in mice. Hepatology. 2014;59:1366–1380. doi: 10.1002/hep.26667. [DOI] [PubMed] [Google Scholar]
- Su S-H, Shyu H-W, Yeh Y-T, Chen K-M, Yeh H, Su S-J. Caffeine inhibits adipogenic differentiation of primary adipose-derived stem cells and bone marrow stromal cells. Toxicol. In Vitro. 2013;27:1830–1837. doi: 10.1016/j.tiv.2013.05.011. [DOI] [PubMed] [Google Scholar]
- Sudeep HV, Venkatakrishna K, Patel D, Shyamprasad K. Biomechanism of chlorogenic acid complex mediated plasma free fatty acid metabolism in rat liver. BMC Complement. Altern. Med. 2016;16:274. doi: 10.1186/s12906-016-1258-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yanai S, Shimokado K, Ishigami A. Coffee consumption in aged mice increases energy production and decreases hepatic mTOR levels. Nutrition. 2017;38:1–8. doi: 10.1016/j.nut.2016.12.021. [DOI] [PubMed] [Google Scholar]
- Urgert R, Essed N, van der Weg G, Kosmeijer-Schuil TG, Katan MB. Separate effects of the coffee diterpenes cafestol and kahweol on serum lipids and liver aminotransferases. Am. J. Clin. Nutr. 1997;65:519–524. doi: 10.1093/ajcn/65.2.519. [DOI] [PubMed] [Google Scholar]
- van Cruchten STJ. Cafestol: a multi-faced compound kinetics and metabolic effects of cafestol in mice. PhD thesis, Wageningen University, Wageningen, NL. (2010)
- Vandenberghe C, St-Pierre V, Courchesne-Loyer A, Hennebelle M, Castellano C-A, Cunnane SC. Caffeine intake increases plasma ketones: an acute metabolic study in humans. Can. J. Physiol. Pharmacol. 2016;95:455–458. doi: 10.1139/cjpp-2016-0338. [DOI] [PubMed] [Google Scholar]
- Vignoli JA, Viegas MC, Bassoli DG, Benassi MT. Roasting process affects differently the bioactive compounds and the antioxidant activity of arabica and robusta coffees. Food Res. Int. 2014;61:279–285. doi: 10.1016/j.foodres.2013.06.006. [DOI] [Google Scholar]
- Wang Z, Lam K-L, Hu J, Ge S, Zhou A, Zheng B, Zeng S, Lin S. Chlorogenic acid alleviates obesity and modulates gut microbiota in high-fat-fed mice. Food Sci. Nutr. 2019;7:579–588. doi: 10.1002/fsn3.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Ong K, Hsu A, Tan BKH, Calbet JA. Chlorogenic acid stimulates glucose transport in skeletal muscle via AMPK activation: a contributor to the beneficial effects of coffee on diabetes. PLoS One. 2012;7:e32718. doi: 10.1371/journal.pone.0032718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Meng J, Shen Q, Zhang Y, Pan S, Chen Z, Zhu L-Q, Lu Y, Huang Y, Zhang G. Caffeine inhibits hypothalamic A1R to excite oxytocin neuron and ameliorate dietary obesity in mice. Nat. Commun. 2017;8:15904. doi: 10.1038/ncomms15904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JS, Qi W, Farias-Pereira R, Choi S, Clark JM, Kim D, Park Y. Permethrin and ivermectin modulate lipid metabolism in steatosis-induced HepG2 hepatocyte. Food Chem. Toxicol. 2019;125:595–604. doi: 10.1016/j.fct.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinari O, Sato H, Igarashi K. Anti-diabetic effects of pumpkin and its components, trigonelline and nicotinic acid, on Goto-Kakizaki rats. Biosci. Biotechnol. Biochem. 2009;73:1033–1041. doi: 10.1271/bbb.80805. [DOI] [PubMed] [Google Scholar]
- Yue Y, Shen P, Xu Y, Park Y. p-Coumaric acid improves oxidative and osmosis stress responses in Caenorhabditis elegans. J. Sci. Food Agric. 2019;99:1190–1197. doi: 10.1002/jsfa.9288. [DOI] [PubMed] [Google Scholar]
- Zhang S-J, Li Y-F, Wang G-E, Tan R-R, Tsoi B, Mao G-W, Zhai Y-J, Cao L-F, Chen M, Kurihara H, Wang Q, He R-R. Caffeine ameliorates high energy diet-induced hepatic steatosis: sirtuin 3 acts as a bridge in the lipid metabolism pathway. Food Funct. 2015;6:2578–2587. doi: 10.1039/C5FO00247H. [DOI] [PubMed] [Google Scholar]
- Zheng G, Qiu Y, Zhang Q-F, Li D. Chlorogenic acid and caffeine in combination inhibit fat accumulation by regulating hepatic lipid metabolism-related enzymes in mice. Br. J. Nutr. 2014;112:1034–1040. doi: 10.1017/S0007114514001652. [DOI] [PubMed] [Google Scholar]
- Zheng X, Dai W, Chen X, Wang K, Zhang W, Liu L, Hou J. Caffeine reduces hepatic lipid accumulation through regulation of lipogenesis and ER stress in zebrafish larvae. J. Biomed. Sci. 2015;22:105. doi: 10.1186/s12929-015-0206-3. [DOI] [PMC free article] [PubMed] [Google Scholar]