Abstract
Both spore and vegetative forms of Bacillus species have been used as probiotics, and they have high stability to the surrounding atmospheric conditions such as heat, gastric conditions, and moisture. The commercial Bacillus probiotic strains in use are B. cereus, B. clausii, B. coagulans, B. licheniformis, B. polyfermenticus, B. pumilus, and B. subtilis. These strains have antimicrobial, anticancer, antioxidant, and vitamin production properties. However, Bacillus probiotics can also produce toxins and biogenic amines and transfer antibiotic resistance genes; therefore, their safety is a concern. Studies on the microbiome using probiotic Bacillus strains are limited in humans. Most microbiome research has been conducted in chicken, mouse, and pig. Some Bacillus probiotics are used as fermentation starters in plant and soybean and dietary supplement of baking foods as a probiotic carrier. This review summarizes the characterization of Bacillus species as probiotics for human use and their safety, microbiome, and probiotic carrier.
Keywords: Bacillus probiotic, Characterization, Safety, Microbiome, Probiotic carrier
Introduction
Intestinal microbiota plays an important role in the maintenance of host health, as they are involved in nutritional, immunologic, and physiological functions (Hooper and Gordon, 2001). Dysbiosis of the intestinal microbiota can cause many chronic diseases such as inflammatory bowel disease, obesity, cancer, and autism (Zhang et al., 2015). Probiotics are defined as live microorganisms that are administered to hosts in adequate amounts for improving the host health (FAO/WHO, 2006). Probiotics have been used as natural therapeutic agents with health benefits. Probiotic potential has been mainly investigated in the Lactobacillus and Bifidobacterium. Several strains of Saccharomyces cerevisiae, Aspergillus niger, and Bacillus have also been investigated.
The fundamental characteristics of probiotics include decreasing gastrointestinal (GI) complications in hosts. Several studies have shown that orally ingested Bacillus spores can proliferate into the intestine for a certain period (Duc et al., 2004; Park et al., 2003). Probiotic adherence is related to stability, such as survival of strains that have been exposed to the GI tract, autoaggregation, and hydrophobicity (Lee et al., 2015). Fecal analysis revealed that commercial Bacillus probiotics (B. cereus, B. clausii, and B. pumilus) could persist in mouse GI tract for up to 16 days (Duc et al., 2004). Probiotics contain antimicrobial substances, including bacteriocin, short chain fatty acids, and organic acids, and they modulate GI disorders by antimicrobial and antiadhesion effect against pathogen strains (Lee et al., 2015).
Probiotics are also known to have immune-modulatory roles, anticancer effects, and promote the lowering of cholesterol. Their function depends on their metabolites such as bacteriocin, biosurfactant, exopolysaccharide, and siderophore (Kanmani et al., 2013). Recently, the potential effects of psychobiotics on brain cells or emotions have been investigated (Sarkar et al., 2016). These mechanisms are based on modulation of the intestinal microbiome, which mediates its effect through the generation of anti-inflammatory cytokines that influence cognitive function, causing hyperglycemia.
Bacillus strains are widespread in nature and are found in soil, air, fermented foods, and the human gut (Sorokulova, 2013). Bacillus probiotics in the spore form can survive in extreme environmental conditions, enabling long-term survival in conditions that could otherwise kill vegetative bacteria (Nicholson et al., 2000). It has been demonstrated that spores of Bacillus probiotics germinate, grow, and resporulate in the GI tract (Casula and Cutting, 2002; Hoa et al., 2001). Recent studies have investigated various Bacillus strains, including B. clausii, B. pumilus, B. polyfermenticus, and B. subtilis (Green et al., 1999; Hoa et al., 2000). The main disadvantages of using Bacillus stains as probiotics are their ability to transfer genes related to antimicrobial resistance and production of enterotoxins and biogenic amines (BA). Bacillus probiotics have been shown to temporarily reside as symbiotic organisms within the host (Dong et al., 2009).
This review summarizes the use of representative Bacillus species as human probiotics, their safety including antibiotic resistance, toxigenic potential, and BA production, microbiome, and application in food.
Characterization of Bacillus probiotics
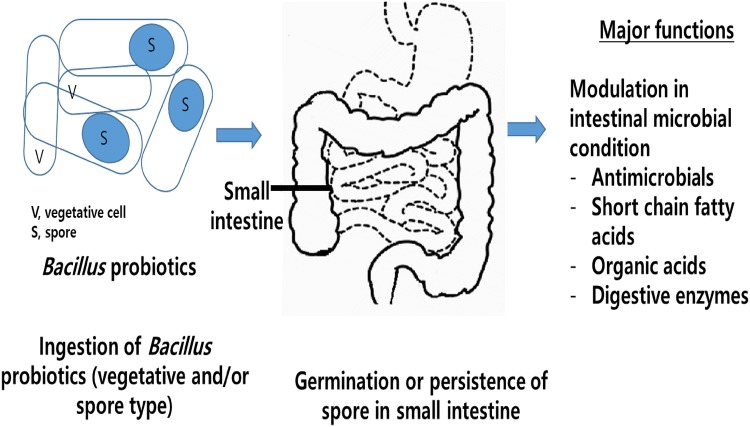
Most commercial Bacillus probiotics consist of B. subtilis, B. polyfermenticus, B. clausii, some B. cereus, B. coagulans, B. pumilus, and B. licheniformis (Table 1). The spores of Bacillus species are considered to be responsible for germination or persistence in the small intestine and modulation of the intestinal conditions (Bernardeau et al., 2017) (Fig. 1). These probiotic characteristics are strain-specific and vary among strains.
Table 1.
List of commercial Bacillus probiotics for human use
| Strain | Products | References |
|---|---|---|
| B. cereus | Bactisubtil® (Marion Merrell Dow Laboratories, France; Hoechst, France; Aventis Pharma, France; Cassella-Med, Germany); Biovicerin® (Geyer Medicamentos S.A. Porto Alegre, Brazil); Subtyl (Mekophar, Vietnam) | Hong et al. (2005) |
| B. clausii |
Domuvar® (BioProgress SpA., Italy); Enterogermina® (Flora-Balance, USA); Lactopure (Pharmed Medicare, India); Lactospore (Sabinsa Corp., USA); Neolactoflorene® (Newpharma S.r.l., Italy); Sustenex® (Ganeden Biotech Inc., USA) |
Cutting (2011), Mandel et al. (2010) |
| B. polyfermenticus SCD | Bispan® (Binex Co. Ltd., South Korea) | Ma et al. (2010), Paik et al. (2005) |
| B. pumilus | Biosubtyl (Biophar Company, Vietnam) | Duc et al. (2004) |
| B. subtilis |
Bio-Kult® (Protexin, UK); Lactipan Plus (Instituto Biochimico Italiano SpA, Italy); Bibactyl (Tendiphar Corporation, Vietnam); Biosubtyl DL (IVAC, Vietnam); Bidisubtilis (Bidiphar Binh Dinh Pharmaceutical and Medical Equipment Company, Vietnam); Pastylbio (Pasteur Institute of Ho Chi Minh City, Vietnam); Medilac (Hanmi Pharmaceutical Co., Ltd., Korea); Nature’s First Food® (Nature’s First Law, USA); Neolactoflorene (Newpharma S.r.l., Italy) Primal Defense™ (Garden of Life® Palm Beach, USA) |
Hoa et al. (2000), Sanders et al. (2003) |
| Mixed type B. licheniformis and B. subtilis | Biosporin® (Biofarma, Ukraine; Garars (Russia); MegaSporeBiotic (Microbiome Labs, USA) | Elshaghabee et al. (2017) |
Fig. 1.
Pictorial representation of Bacillus probiotics for available human use and their functions. The Bacillus probiotics germinate and persist in the small intestine
Bacillus cereus
Bacillus cereus is an important food pathogen and has also been used as a probiotic in humans and livestock (Zhu et al., 2016). Commercial B. cereus strains for human use are available; these include BiosubtylDL (B. cereus), Bactisubtil (B. cereus IP 5832), and Subtyl (B. cereus var. vietnami) (Table 1) (Duc et al., 2004; Hoa et al., 2001). Some studies have investigated their safety including identification, toxin production, and transferable antibiotic resistance (Zhu et al., 2016). However, B. cereus strains can survive longer in the GI tract than can B. clausii and B. subtilis because B. cereus spores can efficiently adhere to human epithelial cells owing to their hydrophobicity (Andersson et al., 1998).
Bacillus clausii
Bacillus clausii strains are used as probiotics mainly because of their immune-modulatory and antimicrobial properties (Ciffo, 1984; Marseglia et al., 2007). Commercial over-the-counter (OTC) B. clausii strains available as probiotics for human use include Tufpro, Ecogro, Enterogermina, Entromax, and Ospor (Table 1) (Abbrescia et al., 2014; Patrone et al., 2016). The effects of B. clausii strains on immune responses include alleviating nasal symptoms during allergic reactions (Marseglia et al., 2007), anti-inflammatory effect against the side effects of antibiotic-based Helicobacter pylori therapy (Nista et al., 2004), and therapeutic effect against urinary tract infection (Fiorini et al., 1985). B. clausii strains demonstrate increased interferon production, mitogenic T cell proliferation, and mitogen-induced lymphokine production in ex vivo and in vivo models (Ciprandi et al., 1986; Muscettola et al., 1992). B. clausii ATCC 9799 has been used in combination with antibiotics (Ciffo, 1984; Mazza, 1994). In addition, B. clausii strains (DSM 8716, ATCC 21536, and ATCC 21537) have been shown to be safe as they are unable to transfer the macrolide resistance gene owing to the inherent presence of the erm gene (Bozdogan et al., 2004).
Bacillus coagulans
Bacillus coagulans have been often mislabeled as Lactobacillus sporogenes because they are lactic acid producing spore-forming bacteria (Hong et al., 2005). This strain can produce coagulin, a bacteriocin, which has antimicrobial effect against a broad spectrum of enteric microbes (Hyronimus et al., 1998). In addition, this strain has been generally recognized as safe (GRAS) by the FDA. Commercial B. coagulans strains include Lactopure/Pharmed Medicare (India), Neolactoflorene®/Newpharm S.r.l. (Italy), and Sustenex®/Ganeden Biotech Inc. (USA) (Table 1) (Mandel et al., 2010). The coagulin produced by B. coagulans I4 is plasmid-linked, heat-stable, and protease-sensitive, and exhibits both bactericidal and bacteriolytic activities (Le Marrec et al., 2000). In humans, B. coagulans reduces the blood lipid concentration (Mohan et al., 1990). Reduction in total cholesterol (330–226 mg%) and LDL (267–173 mg%) has been reported, but no dietary control has been conducted.
Bacillus licheniformis
Bacillus licheniformis is founded mainly in the soil but has been reported on probiotic strains isolated from traditional fermented foods (Lee et al., 2010). Bacillus licheniformis strains have been mainly used as combined-type probiotics (Table 1). The probiotic characteristics of this strain have been mainly investigated in aquaculture of shrimp, fish, and livestock (Swapna et al., 2015). B. licheniformis fermented soybean paste has been shown to have anti-obesity and anti-diabetic properties and also reduced accumulation of β-amyloid in the brain hippocampus (Yang et al., 2015).
Bacillus polyfermenticus
Bacillus polyfermenticus SCD, also known as Bispan® strain (Binex Co., Ltd.), was first isolated from an air sample by Dr. Terakado in 1933 (Table 1) (Paik et al., 2005). It produces various enzymes, which aid digestion in humans. B. polyfermenticus SCD has cholesterol-reducing and antioxidant activities in mice and humans. Recently, B. polyfermenticus strains were isolated from meju (Jung et al., 2012) and kimchi (Lee et al., 2015) and their probiotic application was investigated. Bacillus polyfermenticus produces polyfermenticin SCD, a bacteriocin, which has antimicrobial effect against S. aureus KCCM 32359, Clostridium perfringens ATCC 3624, and H. pylori (Kim et al., 2004; Lee et al., 2001). The cholesterol-lowering effect and antioxidant metabolism of B. polyfermenticus SCD were verified in rats (Paik et al., 2005). The anticarcinogenic effect of B. polyfermenticus SCD was investigated in human colon cancer cells including HT-29, DLD-1, and Caco-2 cells (Ma et al., 2010). Its mechanism of action found to be based on reduction of ErbB2 and ErbB3 protein expression and mRNA levels.
Bacillus subtilis
Bacillus subtilis is a widespread microorganism in nature and a GRAS substance. B. subtilis can grow efficiently in low-cost carbon and nitrogen sources (Olmos and Paniagua-Michel, 2014). Some B. subtilis strains demonstrated their potential as dominant species by isolation in GI tracts, secretion of quorum-sensing pentapeptide, competence, and sporulation factor (Sorokulova, 2013). Commercial B. subtilis strains include Bio-Kult® (Protexin Health Care), Biosporin® (Biofarma, Ukraine); Garars (Russia), Lactipan Plus (Instituto Biochimico Italiano SpA, Italy), Bibactyl (Tendiphar Corporation, Vietnam), Biosubtyl DL (IVAC, Vietnam), Bidisubtilis (Bidiphar Binh Dinh Pharmaceutical and Medical Equipment Company, Vietnam), and Biobaby® (Ildong Pharmaceutical Co. Ltd., Korea) (Table 1) (Hoa et al., 2000; Pinchuk et al., 2001).
Probiotic B. subtilis strains have been investigated for having antimicrobial, antiviral, and anticancer effects. B. subtilis strains have been used as single and mixed type commercial probiotics. B. subtilis P223 inhibits the adhesion of Salmonella enteritidis, Listeria monocytogenes, and E. coli to the HT-29 cells (Jeon et al., 2017). Bacillus subtilis 3 can produce antibiotics including amicoumacin A and nonamicoumacin against H. pylori (Pinchuk et al., 2001). A B. subtilis recombinant strain exhibited antiviral activity against influenza virus, herpes virus, and equine encephalomyelitis virus in in vitro and experimental animal models, respectively (Chudnovskaya et al., 1995). Bacillus subtilis 3 demonstrated an antiviral effect in animal models and against their antiviral substances owing to the expression of peptide P18 (Starosila et al., 2017). The probiotic B. subtilis 3 and peptide P18 together demonstrated their antiviral effect against the influenza virus A/FM/1/47 (H1N1) in Madin-Darby canine kidney (MDCK) cells and mice at a concentration of 106 CFU/well and 107 CFU/mouse, respectively. In mice, the lethal rate decreased to 30% for 14 days while the control, without B. subtilis 3, died on day 8. The probiotic B. subtilis ATCC 6051 was reported to have γ-aminobutyric acid (GABA) producing ability (Wang et al., 2019). GABA has many well-known functions including anxiety inhibition, sleep promotion, reducing blood pressure, and enhancement of immune response (Sarkar et al., 2016).
Safety on Bacillus probiotics
Bacillus strains are regularly consumed inadvertently through fermented foods. Risks of Bacillus probiotics include enterotoxin production, transfer of antibiotic resistance genes, cytotoxicity against normal cells, and production of BA. Specifically, B. cereus has a hazard probability associated with enterotoxin production as human pathogen (Stenfors Arnesen et al., 2008). However, several B. cereus strains have been used as probiotics (Hoa et al., 2000). Therefore, probiotic strains must be investigated to ensure safety of their phenotypes and genotype characteristics.
Toxigenic potential of Bacillus species
Bacillus strains except B. anthracis and B. cereus have a long history of safe use in fermented foods such as natto and doenjang (Sorokulova, 2013). Some Bacillus species are a greater risk to human health, and there are reports of serious local and opportunistic systemic infections and abortions caused by these microorganisms (SCAN, 2000). The use of B. cereus probiotics has posed the risk of enterotoxin production and diarrhea-type disease or emetic-type disease. The following genes further emphasize the need for safe evaluation of these bacteria: enterotoxin genes such as hemolysin (hblD/A), non-hemolytic enterotoxin (nheB), enterotoxin FM (entFM), and emetogenic toxin (ces) (Table 2). Several B. cereus probiotics such as Bactisubtil, BiosubtylDL, and Subtyl, have been reported for their enterotoxin production (Granum and Lund, 1997; Kotiranta et al., 2000; Zhu et al., 2016). In addition, B. cereus for human use, B. licheniformis, and B. subtilis have been reported in cases of food borne diarrheal illness, toxin production, and vomiting (Hong et al., 2005). However, B. coagulans, B. subtilis PY79 and BS3, B. licheniformis BL31, and B. indicus did not show toxicity (Endres et al., 2009; Hong et al., 2008; Sorokulova et al., 2008).
Table 2.
Gene expression related to toxin production by Bacillus probiotics
| Target gene | Sequence (5′ → 3′) | References |
|---|---|---|
|
bceT a (Enterotoxin T) |
TTACATTACCAGGACGTGCTT TGTTTGTGATTGTAATTCAGG |
Matarante et al. (2004) |
|
ces b (cereulide) |
GGTGACACATTATCATATAAGGTG GTTTTCTGGTAACAGCGTTCTAC |
Kim et al. (2015) |
|
cytK a (Cytotoxin K) |
ACAGATATCGG(GT)CAAAATGC TCCAACCCAGTT(AT)(GC)CAGTTC |
Kim et al. (2015) |
|
entFM a (Enterotoxin FM) |
ATGAAAAAAGTAATTTGCAGG TTAGTATGCTTTTGTGTAACC |
Matarante et al., 2004 |
|
hblC a (L2 subunit of HBL) |
GATACTCAATGTGGCAACTGC TTGAGACTGCTCGTCTAGTTG |
Chon et al. (2012) |
|
hblD a (L1 subunit of HBL) |
ACCGGTAACACTATTCATGC GAGTCCATATGCTTAGATGC |
Chon et al. (2012) |
|
hblA a (B subunit of HBL) |
AAGCAATGGAATACAATGGG AGAATCTAAATCATGCCACTGC |
Chon et al. (2012) |
|
hblD/A a (hemolysin BL enterotoxin) |
GGAGCGGTCGTTATTGTTGT GCCGTATCTCCATTGTTCGT |
Matarante et al. (2004) |
|
nheA a (A subunit of NHEb) |
GTTAGGATCACAATCACCGC ACGAATGTAATTTGAGTCGC |
Guinebretiere et al. (2002) |
|
nheB a (B subunit of NHEb) |
CTATCAGCACTTATGGCAG ACTCCTAGCGGTGTTCC |
Matarante et al. (2004) |
|
nheC a (C subunit of NHEb) |
TGGATTCCAAGATGTAACG ATTACGACTTCTGCTTGTGC |
Chon et al. (2012) |
| nprA |
GTATACGGAGATGGTGATGG GGATCACTCATAGAGCGAAG |
Hisieh et al. (1999) |
HBL Hemolysin BL, NHE nonhemolytic enterotoxin
aEnterotoxin genes (hblA, hblC, hblD, nheA, nheB, nheC, cytK, bceT, entFM)
bEmetogenic toxin (ces)
Antibiotic resistance of Bacillus species
Antibiotic resistance is of main interest in view of evaluating the safety of a strain, because the resistance may be conjugatively transferred to other strains. Bacillus subtilis BS3 and B. licheniformis BN31 strains do not carry plasmids (Sorokulova et al., 2008). Antibiotic resistance has been investigated using microbiological cut-off values, quantitative methods of the MIC (minimum inhibitory concentration), or via genetic basis of resistance following the method as per the guideline of Clinical and Laboratory Standards Institute (CLSI) and European Food Safety Authority (EFSA) (Zhu et al., 2016). The antibiotics of LAB strains that have been used are ampicillin, vancomycin, gentamicin, kanamycin, streptomycin, clindamycin, tetracycline, and chloramphenicol. However, Bacillus strains have been listed as resistant/susceptible to all antibiotics except ampicillin (EFSA, 2012). Four probiotic strains of B. clausii are marketed as an OTC medical supplement and have been investigated for antibiotic resistance by antibiotic susceptibility assay, PCR amplification, and DNA sequencing (Abbrescia et al., 2014). However, their antibiotic resistance was considered a useful characteristic when compared to the wild type strain.
Biogenic amine (BA) production
BAs are important compounds for metabolic and physiological functions in all living organisms and they are naturally found in many foods owing to the microbial decarboxylation of precursor amino acids via substrate-specific enzymes (Calzada et al., 2013). BAs are classified into aliphatic (putrescine, cadaverine, spermine, and spermidine), aromatic (tyramine, phenylethylamine, and serotonin), and heterocyclic (histamine and tryptamine) amines. Some BAs have an important function as modulators of hormones and neurotransmitters (Spano et al., 2010). However, histamine and tyramine are chiefly studied owing to their potential physiological actions and toxicological effects. Specifically, histamine and tyramine are toxic to the HT-29 intestinal cell-line at a high concentration (Linares et al., 2016). In the presence of nitrites, BAs can be potential carcinogens when converted to nitrosamines (Naila et al., 2010). When ingested in high concentrations, BAs have considerable toxicological risks such as headache, respiratory distress, heart palpitations, hyper- or hypotension, and several allergic disorders. BA production by probiotic strains is considered to be useful as probiotic or fermentation starter. BA production can be confirmed using glycerol-containing decarboxylase media (Jeon et al., 2018), HPLC analysis of the fermented product, or molecular analysis by PCR (Table 3). In addition, B. licheniformis cy2, B. polyfermenticus CJ9, B. licheniformis KCTC 1918, and B. subtilis KCTC 1028 reported BA production of putrescine, cadaverine, spermidine, phenylethylamine, and spermine (Chang and Chang, 2012). However, B. subtilis P229 did not produced BA (Jeon et al., 2018).
Table 3.
Biogenic amine gene expression in Bacillus probiotics
| Target gene | Sequence (5′ → 3′) | References |
|---|---|---|
|
AGD1 (Agmatine deiminase) |
GAACGACTAGCAGCTAGTTAT CCAATAGCCGATACTACCTTG |
Lucas et al. (2007) |
|
Cad2 (Lysine decarboxylase) |
CAYRTNCCNGGNCAYAA GGDATNCCNGGNGGRTA |
Landete et al. (2005) |
|
Hdc (Histidine decarboxylase) |
GATGGTATTGTTTCKTATGA CAAACACCAGCATCTTC |
Coton and Coton (2005) |
|
Odc (Ornithine decarboxylase) |
TGCA CTTCCATATCCTCCAG GAATTTCTGGAGCAAATC CA |
Granchi et al. (2006) |
|
OD (Putrescine) |
CATCAAGGTGGACAATATTTCCG CCGTTCAACAACTTGTTTGGCA |
Granchi et al. (2006) |
|
TD (Tyrosine decarboxylase) |
ACATAGTCAACCATRTTGAA CAAATGGAAGAAGAAGTAGG |
Coton et al. (2004) |
Microbiome study on Bacillus probiotics
Microbiome can be influenced during the life of the host in response to a variety of factors such as diet, environment, medical interventions, and disease conditions. Therefore, the influence of probiotics has been reported on the GI microbiome of humans and mammals (Thursby and Juge, 2017). The gut microbiota is dominated by two divisions of bacteria that influence the host immune systems: (i) Bacteroidetes and (ii) Firmicutes (Kawai et al., 2018). The symbiotic relationship between the gut microbiota and the host was demonstrated by Guianane and Cotter (2013). The study of gut microbiome in humans focused on the use of probiotics, and main probiotic strains used were lactic acid bacteria such as Lactobacillus casei and Lactobacillus rhamnosus (Kawai et al., 2018; Pirker et al., 2013).
The effect of Bacillus strains on gut microbiome was evaluated in broiler chicken and pig (Jacquier et al., 2019; Ma et al., 2018; Poulsen et al., 2018). The treatment with B. subtilis led to increase in concentration of Ruminococcus, Lachnoclostridium, and Anaerostipes at the cecal level in 500 male broiler chicken (Jacquier et al., 2019). These results suggested that there was immune modulation by butyrate production with the potential change in the microbiome. In another study, B. subtilis influenced the increase of abundance of Christensenellaceae and Caulobacteraceae, and decrease in the concentration of Vampirovibrio, Escherichia/Shigella, and Parabacteroides in broiler chickens (Ma et al., 2018). In suckling and newly weaned piglets, treatment with (1) control (n = 72); (2) gentamicin (n = 71); (3) Bacillus spores (n = 63); (4) gentamicin and Bacillus spores (n = 71), the spore of Bacillus spp. and gentamicin was confirmed diversity of the microbial composition (Poulsen et al., 2018). However, administration of Bacillus spores decreased the diversity of microflora and did not affect dysbiosis.
Probiotic carrier on Bacillus probiotics
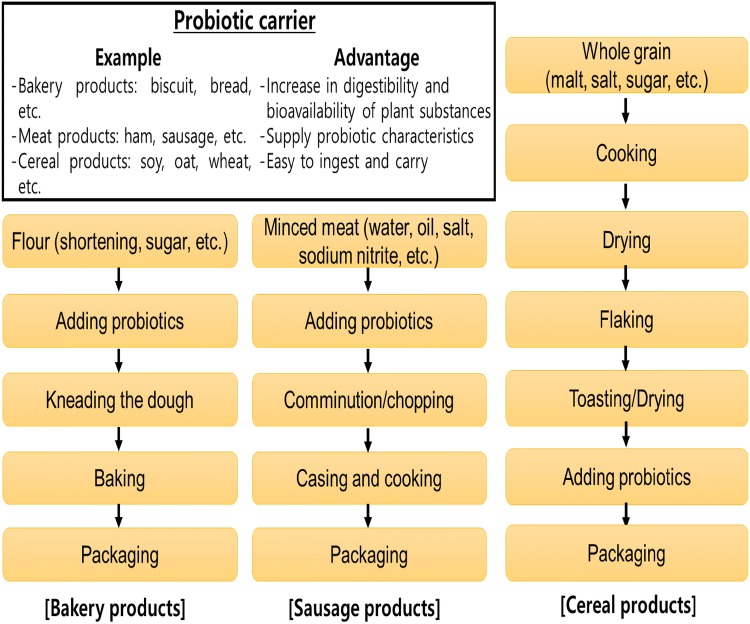
Probiotic foods using lactic acid bacteria have been mainly used as dairy foods such as yogurt, cheese, ice cream, etc. However, Bacillus probiotics applied in vegetable, cereal, and meat products are suggested to undergo processing in some food systems (Fig. 2). LAB and yeast have challenged encapsulation using maltodextrin, skim milk, or alginate for food application (Gul and Athalar, 2019; Suvarna et al., 2018), while Bacillus probiotics have advantage of their high survival due to their spore formation. The application of probiotic strain has lead affinity and receptibility to consumer retaining probiotic characteristics. In addition, the bioavailability of polyphenol substances can increase the β-glucosidase production (Lee and Paik, 2017).
Fig. 2.
Concise schematic of Bacillus probiotics used in the food industry within various products used as probiotic carriers
Fermented soybean foods are an example of probiotic carriers for Bacillus. Fermented soybean foods are diverse such as doenjang (Korea), kanjang (Korea and Japan), cheonggukjang (Korea), natto (Japan), Rabadi (India, Pakistan), Gari (Africa), Tapaiubi (Malaysia), Douchi (China), Soibum (India), Ugba (Nigeria), etc. (Elshaghabee et al., 2017). Natto has been shown to reduce blood clotting by fibrinolysis (Elshaghabee et al., 2017). The use of probiotic Bacillus strain is limited to soybean, bake, cereals, and meat owing to disadvantage of unique smell. Therefore, for their application in the food industry, there is a demand to reduce the unique smell.
In conclusion, this review summarized the use of Bacillus species as probiotics, their function, safety in humans, and modulation of microbiome. Probiotic therapy has been increasingly used as an alternative to antibiotics, immune-modulators, and microbiome regulators. However, the functional probiotic properties are strain specific. Probiotic Bacillus strains have the merit of stability as they can be used in their spore form in storage conditions unlike LAB. Therefore, probiotic Bacillus strains require scientific investigation with regard to their toxin production, antibiotic resistance, and BA production, in order to be labeled safe for use. In addition, the study of gut microbiome using Bacillus probiotics is needed for the development of novel probiotics for changing the microflora.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Na-Kyoung Lee, Email: nakyoung_lee@nate.com.
Won-Suck Kim, Email: wskim@silla.ac.kr.
Hyun-Dong Paik, Phone: +82 2 2049 6011, Email: hdpaik@konkuk.ac.kr.
References
- Abbrescia A, Palese LL, Papa S, Gaballo A, Alifano P, Sardanelli AM. Antibiotic sensitivity of Bacillus clausii strains in commercial preparation. Curr. Med. Chem. 2014;1:102–110. [Google Scholar]
- Andersson A, Granum PE, Ronner U. The adhesion of Bacillus cereus spores to epithelial cells might be an additional virulence mechanism. Int. J. Food Microbiol. 1998;39:93–99. doi: 10.1016/S0168-1605(97)00121-9. [DOI] [PubMed] [Google Scholar]
- Bernardeau M, Lehtinene MJ, Forssten SD, Nurminen P. Importance of the gastrointestinal life cycle of Bacillus for probiotic functionality. J. Food Sci. Technol. 2017;54:2570–2584. doi: 10.1007/s13197-017-2688-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozdogan B, Galopin S, Leclereq R. Characterization of a new erm-related macrolide resistance gene present in probiotic strains of Bacillus clausii. Appl. Environ. Microbiol. 2004;70:280–284. doi: 10.1128/AEM.70.1.280-284.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzada J, del Olmo A, Picon A, Gaya P, Nunez M. Reducing biogenic-amine-producing bacteria, decarboxylase activity, and biogenic amines in raw milk cheese by high-pressure treatments. Appl. Environ. Microbiol. 2013;79:1277–1283. doi: 10.1128/AEM.03368-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casula G, Cutting SM. Bacillus probiotics: spore germination in the gastrointestinal tract. Appl. Environ. Microbiol. 2002;68:2344–2352. doi: 10.1128/AEM.68.5.2344-2352.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M, Chang HC. Development of a screening method for biogenic amine producing Bacillus spp. Int. J. Food Microbiol. 2012;153:269–274. doi: 10.1016/j.ijfoodmicro.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Ciffo F. Determination of the spectrum of antibiotic resistance of the Bacillus subtilis strains of Enterogermina. Chemioterapia. 1984;3:45–52. [PubMed] [Google Scholar]
- Chon JW, Kim JH, Lee SJ, Hyeon JY, Song KY, Park C, Seo KH. Prevalence, phenotypic traits and molecular characterization of emetic toxin-producing Bacillus cereus strains isolated from human stools in Korea. J. Appl. Microbiol. 2012;112:1042–1049. doi: 10.1111/j.1365-2672.2012.05277.x. [DOI] [PubMed] [Google Scholar]
- Chudnovskaya NV, Ribalko SL, Sorokulova IB, Smirnov VV, Belyavskaya VA. Antiviral activity of Bacillus probiotics. Dopovidi. Nac. Acad. Nauk. Ukraini. 124-126 (1995)
- Ciprandi G, Scordamaglia A, Ruffoni S, Pizzorno G, Canonica GW. Effects of an adjunctive treatment with Bacillus subtilis for food allergy. Chemioterapia. 1986;5:408–410. [PubMed] [Google Scholar]
- Coton E, Coton M. Multiplex PCR for colony direct detection of gram-positive histamine-and tyramine-producing bacteria. J. Microbiol. Methods. 2005;63:296–304. doi: 10.1016/j.mimet.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Coton E, Coton M, Lucas P, Lonvaud A. Identification of the gene encoding a putative tyrosine decarboxylase of Carnobacterium divergens 508. Food Microbiol. 2004;21:125–130. doi: 10.1016/j.fm.2003.10.004. [DOI] [Google Scholar]
- Cutting SM. Bacillus probiotics. Food Microbiol. 2011;28:214–220. doi: 10.1016/j.fm.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Dong TC, Van PH, Cutting SM. Bacillus probiotics. Nutra Foods. 2009;8:7–14. [Google Scholar]
- Duc LH, Hong HA, Barbosa TM, Henriques AO, Cutting SM. Characterization of Bacillus probiotics available for human use. Appl. Environ. Microbiol. 2004;70:2161–2171. doi: 10.1128/AEM.70.4.2161-2171.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres JR, Clewell A, Jade KA, Farber T, Hauswirth J, Schauss AG. Safety assessment of a proprietary preparation of a novel probiotic, Bacillus coagulans, as a food ingredient. Food Chem. Toxicol. 2009;47:1231–1238. doi: 10.1016/j.fct.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshaghabee FM, Rokana N, Gulhane RD, Sharma C, Panwar H. Bacillus as potential probiotics: status, concerns, and future perspectives. Front. Microbiol. 2017;8:1490. doi: 10.3389/fmicb.2017.01490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority). Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA J. 10: 2740 (2012)
- FAO/WHO. Probiotics in Food: Health and Nutritional Properties and Guidelines for Evaluation. FAO Food and Nutrition Paper World Health Organization and Food Agriculture Organization of the United Nations (2006)
- Fiorini G, Cimminiello C, Chianese R., II B. subtilis come stimolatore selettivo delle IgA linfocitarie di membrana. Farmaci. 1985;9:331–334. [Google Scholar]
- Granchi L, Talini D, Rigacci S, Guerrini A, Berti A, Vincenzini M. Detection of putrescine-producer Oenococcus oeni strains by PCR. 8th Symposium on Lactic Acid Bacteria, The Netherlands (2006)
- Granum PE, Lund T. Bacillus cereus and its food poisoning toxins. FEMS Microbiol. Lett. 1997;157:223–228. doi: 10.1111/j.1574-6968.1997.tb12776.x. [DOI] [PubMed] [Google Scholar]
- Green DH, Wakeley PR, Page A, Barnes A, Baccigalupi L, Ricca E, Cutting SM. Characterization of two Bacillus probiotics. Appl. Environ. Microbiol. 1999;65:4288–4291. doi: 10.1128/aem.65.9.4288-4291.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guianane CM, Cotter PD. Role of the gut microbiota in health and chronic gastrointestinal disease: understanding a hidden metabolic organ. Therap. Adv. Gastroenterol. 2013;6:295–308. doi: 10.1177/1756283X13482996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinebretiere MH, Broussolle V, Nguyen-The C. Enterotoxigenic profiles of food-poisoning and food-borne Bacillus cereus strains. J. Clin. Microbiol. 2002;40:3053–3056. doi: 10.1128/JCM.40.8.3053-3056.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gul O, Athalar I. Different stress tolerance of spray and freeze dried Lactobacillus casei Shirota microcapsules with different encapsulating agents. Food Sci. Biotechnol. 2019;28:807–816. doi: 10.1007/s10068-018-0507-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisieh YM, Sheu SJ, Chen YL, Tsen HY. Enterotoxigenic profiles and polymerase chain reaction detection of Bacillus cereus group cells and B. cereus strains from food-borne outbreaks. J. Appl. Microbiol. 87: 481-490 (1999) [DOI] [PubMed]
- Hoa NT, Baccigalupi L, Huxham A, Smertenko A, Van PH, Ammendola S, Ricca E, Cutting AS. Characterization of Bacillus species used for oral bacteriotherapy and bacterioprophylaxis of gastrointestinal disorders. Appl. Environ. Microbiol. 2000;66:5241–5247. doi: 10.1128/AEM.66.12.5241-5247.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoa NT, Duc LH, Isticato R, Baccigalupi L, Ricca E, Van PH, Cutting AS. Fate and dissemination of Bacillus subtilis spores in a murine model. Appl. Environ. Microbiol. 2001;67:3819–3823. doi: 10.1128/AEM.67.9.3819-3823.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong HA, Duc LH, Cutting SM. The use of bacterial spore formers as probiotics. FEMS Microbiol. Lett. 2005;29:813–835. doi: 10.1016/j.femsre.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Hong HA, Huang JM, Khaneka R, Hiep LV, Urdaci MC, Urdaci MC, Cutting SM. The safety of Bacillus subtilis and Bacillus indicus as food probiotics. J. Appl. Microbiol. 2008;105:510–520. doi: 10.1111/j.1365-2672.2008.03773.x. [DOI] [PubMed] [Google Scholar]
- Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–1118. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- Hyronimus B, Le Marrec C, Urdaci MC. Coagulin, a bacteriocin-like inhibitory substance produced by Bacillus coagulans I4. J. Appl. Microbiol. 1998;85:42–50. doi: 10.1046/j.1365-2672.1998.00466.x. [DOI] [PubMed] [Google Scholar]
- Jacquier V, Nelson A, Jlali M, Rhayat L, Brinch KS, Devillard E. Bacillus subtilis 29784 induces a shift in broiler gut microbiome toward butyrate-producing bacteria and improves intestinal histomorphology and animal performance. Poult. Sci. 2019;98:2548–2548. doi: 10.3382/ps/pey602. [DOI] [PubMed] [Google Scholar]
- Jeon HL, Lee NK, Yang SJ, Kim WS, Paik HD. Probiotic characterization of Bacillus subtilis P223 isolated from kimchi. Food Sci. Biotechnol. 2017;26:1641–1648. doi: 10.1007/s10068-017-0148-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon HL, Yang SJ, Son SH, Kim WS, Lee NK, Paik HD. Evaluation of probiotic Bacillus subtilis P229 isolated from cheonggukjang and its application in soybean fermentation. LWT-Food Sci. Technol. 2018;97:94–99. doi: 10.1016/j.lwt.2018.06.054. [DOI] [Google Scholar]
- Jung JH, Lee MY, Chang HC. Evaluation of the probiotic potential of Bacillus polyfermenticus CJ6 isolated from Meju, a Korean soybean fermentation starter. J. Microbiol. Biotechn. 2012;22:1510–1517. doi: 10.4014/jmb.1205.05049. [DOI] [PubMed] [Google Scholar]
- Kanmani P, Satish Kumar R, Yuvaraja N, Paaria KA, Pattukumara V, Arula V. Probiotics and its functionally valuable products—a Review. Crit. Rev. Food Sci. Nutr. 2013;53:641–658. doi: 10.1080/10408398.2011.553752. [DOI] [PubMed] [Google Scholar]
- Kawai K, Kamochi R, Oiki S, Murata K, Hashimoto W. Probiotics in human gut microbiota can degrade host glycosaminoglycans. Sci. Rep. 2018;8:10674. doi: 10.1038/s41598-018-28886-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Han JK, Park JS, Lee JS, Lee SH, Cho JI, Kim KS. Various enterotoxin and other virulence factor genes widespread among Bacillus cereus and Bacillus thuringiensis strains. J. Microbiol. Biotechn. 2015;25:872–879. doi: 10.4014/jmb.1502.02003. [DOI] [PubMed] [Google Scholar]
- Kim SM, Lee KH, Lee NK, Kim CJ, Kim CH, Paik HD. Antagonistic activity of polyfermenticin SCD against Helicobacter pylori KCTC 2948. J. Microbiol. Biotechn. 2004;14:148–152. [Google Scholar]
- Kotiranta A, Lounatmaa K, Haapasalo M. Epidemiology and pathogenesis of Bacillus cereus infections. Microbes Infect. 2000;2:189–198. doi: 10.1016/S1286-4579(00)00269-0. [DOI] [PubMed] [Google Scholar]
- Landete JM, Ferrer S, Polo L, Pardo I. Biogenic amines in wines from three Spanish regions. J. Agric. Food Chem. 2005;53:1119–1124. doi: 10.1021/jf049340k. [DOI] [PubMed] [Google Scholar]
- Lee KH, Jun KD, Kim WS, Paik HD. Partial characterization of polyfermenticin SCD, a newly identified bacteriocin of Bacillus polyfermenticus. Lett. Appl. Microbiol. 2001;32:146–151. doi: 10.1046/j.1472-765x.2001.00876.x. [DOI] [PubMed] [Google Scholar]
- Lee MS, Lee NK, Chang KH, Choi SY, Song CK, Paik HD. Isolation and characterization of a protease-producing bacterium, Bacillus amyloliquefaciens P27 from meju as a probiotic starter for fermented meat products. Korean J. Food Sci. An. (2010)
- Lee NK, Paik HD. Bioconversion using lactic acid bacteria: ginsenosides, GABA, and phenolic compounds. J. Microbiol. Biotechn. 2017;27:869–877. doi: 10.4014/jmb.1612.12005. [DOI] [PubMed] [Google Scholar]
- Lee NK, Son SH, Jeon EB, Jung GH, Lee JY, Paik HD. The prophylactic effect of probiotic Bacillus polyfermenticus KU3 against cancer cells. J. Funct. Foods. 2015;14:513–518. doi: 10.1016/j.jff.2015.02.019. [DOI] [Google Scholar]
- Le Marrec C, Hyronimus B, Bressollier P, Verneuil B, Urdaci MC. Biochemical and genetic characterization of coagulin, a new antilisterial bacteriocin in the pediocin family of bacteriocins, produced by Bacillus coagulans I4. Appl. Environ. Microbiol. 2000;66:5213–5220. doi: 10.1128/AEM.66.12.5213-5220.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linares DM, del Rio B, Redruello B, Ladero V, Martin MC, Fernandez M, Ruas-Madiedo P, Alvarez MA. Comparative analysis of the in vitro cytotoxicity of the dietary biogenic amines tyramine and histamine. Food Chem. 2016;197:658–663. doi: 10.1016/j.foodchem.2015.11.013. [DOI] [PubMed] [Google Scholar]
- Lucas PM, Blancato VS, Claisse O, Magni C, Lolkema JS, Lonvaud-Funel A. Agmatine deiminase pathway genes in Lactobacillus brevis are linked to the tyrosine decarboxylation operon in a putative acid resistance locus. Microbiology. 2007;153:2221–2230. doi: 10.1099/mic.0.2007/006320-0. [DOI] [PubMed] [Google Scholar]
- Ma EL, Choi YJ, Choi J, Pothoulakis C, Rhee SH, Im EE. The anti-cancer effect of probiotic Bacillus polyfermenticus on human colon cancer cells is mediated through ErbB2 and ErbB3 inhibition. Int. J. Cancer. 2010;127:780–790. doi: 10.1002/ijc.25011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Wang W, Zhang H, Wang J, Zhang W, Gao J, Wu S, Qi G. Supplemental Bacillus subtilis DSM 32315 manipulates intestinal structure and microbial composition in broiler chickens. Sci. Rep. 2018;8:15358. doi: 10.1038/s41598-018-33762-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel DR, Eichas K, Holmes J. Bacillus coagulans: a viable adjunct therapy for relieving symptoms of rheumatoid arthritis according to a randomized, controlled trial. BMC Complement. Altern. Med. 2010;10:1. doi: 10.1186/1472-6882-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marseglia GL, Tosca M, Cirillo I, Licari A, Leone M, Marseglia A, Castellazzi AM, Ciprandi G. Efficacy of Bacillus clausii spores in the prevention of recurrent respiratory infections in children: a pilot study. Ther. Clin. Risk Manag. 2007;3:13–17. doi: 10.2147/tcrm.2007.3.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matarante A, Baruzzi F, Cocconcelli PS, Morea M. Genotyping and toxigenic potential of Bacillus subtilis and Bacillus pumilus strains occurring in industrial and artisanal cured sausages. Appl. Environ. Microbiol. 2004;70:5168–5176. doi: 10.1128/AEM.70.9.5168-5176.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza P. The use of Bacillus subtilis as an antidiarrhoeal microorganism. Boll. Chim. Farm. 1994;133:3–18. [PubMed] [Google Scholar]
- Muscettola M, Grasso G, Blach-Olszewska Z, Migliaccio P, Borghesi-Nicoletti C, Giarratana M, Gallo VC. Effects of Bacillus subtilis spores on interferon production. Pharmacol Res. 1992;2:176–177. doi: 10.1016/1043-6618(92)90652-R. [DOI] [PubMed] [Google Scholar]
- Naila A, Flint S, Feltcher G, Bremer P, Meerdink G. Control of biogenic amines in food—existing and emerging approaches. J. Food Sci. 2010;75:R139–R150. doi: 10.1111/j.1750-3841.2010.01774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan C, Arora R, Khalilullah M. Preliminary observation on effect of Lactobacillus sporogenes on serum lipid levels in hypercholesterolemic patients. Indian J. Med. Res. 1990;92:431–432. [PubMed] [Google Scholar]
- Nicholson WL, Munakata N, Horneck G, Melosh HJ, Setlow P. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol. Mol. Biol. Rev. 2000;64:548–572. doi: 10.1128/MMBR.64.3.548-572.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nista EC, Candelli M, Cremonini F, Cazzato IA, Zocco MA, Franceschi F, Cammarota G, Gasbarrini G, Gasbarrini A. Bacillus clausii therapy to reduce side-effects of anti-Helicobacter pylori treatment: randomized, double-blind, placebo controlled trial. Aliment. Pharmacol. Ther. 2004;20:1181–1188. doi: 10.1111/j.1365-2036.2004.02274.x. [DOI] [PubMed] [Google Scholar]
- Olmos J, Paniagua-Michel J. Bacillus subtilis a potential probiotic bacterium to formulate functional feeds for aquaculture. J. Microb. Biochem. Technol. 2014;6:361–365. doi: 10.4172/1948-5948.1000169. [DOI] [Google Scholar]
- Paik HD, Park JS, Park E. Effects of Bacillus polyfermenticus SCD on lipid and metabolisms in rats fed a high-fat and high-cholesterol diet. Biol. Pharm. Bull. 2005;28:1270–1274. doi: 10.1248/bpb.28.1270. [DOI] [PubMed] [Google Scholar]
- Park KY, Jung HY, Woo KL, Jun KD, Kang JS, Paik HD. Effects of Bacillus polyfermenticus SCD administration on fecal microflora and putrefactive metabolites in healthy adults. J. Microbiol. Biotechn. 2003;12:657–663. [Google Scholar]
- Patrone V, Molinari P, Morelli L. Microbiological and molecular characterization of commercially available probiotics containing Bacillus clausii from India and Pakistan. Int. J. Food Microbiol. 2016;237:92–97. doi: 10.1016/j.ijfoodmicro.2016.08.012. [DOI] [PubMed] [Google Scholar]
- Pinchuk IV, Bressollier P, Verneuil B, Fenet B, Sorokulova IB, Mégraud F, Urdaci MC. In vitro anti-Helicobacter pylori activity of the probiotic strain Bacillus subtilis 3 is due to secretion of antibiotics. Antimicrob. Agents Chemother. 2001;45:3156–3161. doi: 10.1128/AAC.45.11.3156-3161.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirker A, Stockenhuber A, Remely M, Harrant A, Hippe B, Kamhuber C, Adelmann K, Stockenhuber F, Haslberger AG. Effects of antibiotic therapy on the gastrointestinal microbiota and the influence of Lactobacillus casei. Food Agric. Immunol. 2013;24:315–330. doi: 10.1080/09540105.2012.689816. [DOI] [Google Scholar]
- Poulsen ASR, de Jonge N, Nielsen JL, Højberg O, Lauridsen C, Lauridsen S, Cutting SM, Canibe N. Impact of Bacillus spp. spores and gentamicin on the gastrointestinal microbiota of suckling and newly weaned piglets. PLOS ONE 13: e0207382 (2018) [DOI] [PMC free article] [PubMed]
- Sarkar A, Lehto SM, Harty S, Dinan TG, Cryan JF, Burnel PWJ. Psychobiotics and the manipulation of bacteria-gut-brain signals. Trends Neurosci. 2016;39:763–871. doi: 10.1016/j.tins.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders ME, Morelli L, Tompkins TA. Sporeformers as human probiotics: Bacillus, Sporolactobacillus, and Brevibacillus. Compr. Rev. Food Sci. Food Saf. 2003;2:101–110. doi: 10.1111/j.1541-4337.2003.tb00017.x. [DOI] [PubMed] [Google Scholar]
- SCAN. Opinion of the scientific committee on animal nutrition on the safety of use of Bacillus species in animal nutrition. European commission, health and consumer protection directorate-general. Scientific Committee on Animal Nutrition (SCAN) (2000)
- Sorokulova I. Modern status and perspectives of Bacillus bacteria as probiotics. J. Prob. Health. 2013;1:4. doi: 10.4172/2329-8901.1000e106. [DOI] [Google Scholar]
- Sorokulova IB, Pinchuk IV, Denayrolles M, Osipova IG, Huang JM, Cutting SM, Urdaci MC. The safety of two Bacillus probiotic strains for human use. Dig. Dis. Sci. 2008;53:954–963. doi: 10.1007/s10620-007-9959-1. [DOI] [PubMed] [Google Scholar]
- Spano G, Russo P, Lonvaud-Funel A, Lucas P, Alexandre H, Grandvalet C, Coton E, Coton E, Barnavon L, Bach B, Rattray F, Bunte A, Magni C, Ladero V, Alvarez M, Fernńndez M, Lopez P, de Palencia PF, Corbi A, Trip H, Lolkema JS. Biogenic amines in fermented foods. Eur. J. Clin. Nutr. 2010;64:S95–S100. doi: 10.1038/ejcn.2010.218. [DOI] [PubMed] [Google Scholar]
- Starosila D, Rybalko S, Varbanetz L, Ivanskaya N, Sorokulova I. Anti-influenza activity of Bacillus subtilis probiotic strain. Antimicrob. Agents Chemother. 2017;61:1–11. doi: 10.1128/AAC.00539-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenfors Arnesen LP, Fagerlude A, Granum PE. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol. Rev. 2008;32:579–606. doi: 10.1111/j.1574-6976.2008.00112.x. [DOI] [PubMed] [Google Scholar]
- Suvarna S, Dsouza J, Ragavan ML, Das N. Potential probiotic characterization and effect of encapsulation of probiotic yeast strains on survival in simulated gastrointestinal tract condition. Food Sci. Biotechnol. 2018;27:745–753. doi: 10.1007/s10068-018-0310-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swapna B, Venkatrayulu Ch, Swathi AV. Effect of probiotic bacteria Bacillus licheniformis and Lactobacillus rhamnosus on growth of the Pacific white shrimp Litopenaeus vannamei (Boone, 1931) Euro. J. Exp. Bio. 2015;5:31–36. [Google Scholar]
- Thursby E, Juge N. Introduction to the human gut microbiota. Biochem. J. 2017;474:1832–1836. doi: 10.1042/BCJ20160510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Huang J, Sun L, Xu F, Zhang W, Zhan J. An efficient process for co-production of γ-aminobutyric acid and probiotic Bacillus subtilis cells. Food Sci. Biotechnol. 2019;28:155–163. doi: 10.1007/s10068-018-0461-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HJ, Kwon DY, Kim HJ, Kim MJ, Jung DY, Kang HJ, Kim DS, Kang S, Moon NR, Shin BK, Park S. Fermenting soybeans with Bacillus licheniformis potentiates their capacity to improve cognitive function and glucose homeostaisis in diabetic rats with experimental Alzheimer’s type dementia. Eur. J. Nutr. 2015;51:77–88. doi: 10.1007/s00394-014-0687-y. [DOI] [PubMed] [Google Scholar]
- Zhang YJ, Li S, Gan RY, Gan RY, Zhou T, Xu DP, Li HB. Impacts of gut bacteria on human health and diseases. Int. J. Mol. Sci. 2015;16:7493–7519. doi: 10.3390/ijms16047493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu K, Hölzel CS, Cui Y, Mayer R, Wang Y, Dietrich R, Didier A, Bassitta R, Märtlbauer E, Ding S. Probiotic Bacillus cereus strains, a potential risk for public health in China. Front. Microbiol. 2016;7:718. doi: 10.3389/fmicb.2016.00718. [DOI] [PMC free article] [PubMed] [Google Scholar]