Abstract
Objective:
U.S. Food and Drug Administration (FDA)–approved medications exist for the treatment of alcohol use disorders. However, their effectiveness depends on proper adherence to the prescribed regimen. Differences in adherence across medications may have implications for clinical outcomes and may provide helpful information in considering treatment options. This study aims to identify significant differences in adherence if present.
Method:
A retrospective chart review was conducted in the Veterans Integrated Service Networks (VISN)-7 region of Veterans Affairs hospital and community-based outpatient clinics within South Carolina and Georgia. Prescriptions of FDA-approved alcohol use disorder medications from 2010 through 2015 were reviewed. Adherence was determined by the proportion of days the veteran had oral or injectable medication available over a 6-month period as noted by medication fills (reported as 0%–100% medication availability). We compared adherence for specific medications using chi-square, t test, logistic regression for dichotomous outcomes, and linear regression for continuous outcomes.
Results:
A total of 715 subjects and 807 medication trials were included. Mean adherence (percentage of days that medication was available) was 41.3% for disulfiram, 44.7% for acamprosate, 49.8% for oral naltrexone, and 54.6% for extended-release injectable naltrexone. The mean adherence was significantly different between disulfiram and oral naltrexone (p = .002) as well as disulfiram and extended-release injectable naltrexone (p = .004). Adherence of 80% was achieved in 11.9%, 19.4%, 22.7%, and 24.4% of treatment courses with disulfiram, acamprosate, naltrexone, and extended-release injectable naltrexone, respectively. These differences were significant for disulfiram versus oral naltrexone (p = .004) and disulfiram versus extended-release injectable naltrexone (p = .05).
Conclusions:
These results demonstrate that overall adherence to medication-assisted treatment for alcohol use disorder is low across all medications. When directly compared, disulfiram had significantly lower adherence than both oral and extended-release injectable naltrexone.
Alcohol use disorders (AUDs) are common in the United States, with an estimated 12-month prevalence in 2015 of 6.2% among Americans age 18 years and older (Substance Abuse and Mental Health Services Administration, 2015). In fiscal year 2012, 440,000 veterans treated through the Veterans Health Administration had a documented AUD diagnosis, and it is thought that military members may have a higher rate of AUD compared with civilians (Hagedorn et al., 2016; Teeters et al., 2017). In 2010, it was estimated that the economic cost of excessive drinking was U.S. $249 billion (Centers for Disease Control and Prevention, 2016).
Multiple medications exist that have demonstrated efficacy to treat AUDs. There are four U.S. Food and Drug Administration (FDA)–approved medications available for the treatment of AUDs: acamprosate, disulfiram, oral naltrexone, and extended-release injectable naltrexone. Despite the availability of these pharmacological options, AUD remains prevalent, suggesting that barriers to effective treatment exist. Although an optimal duration of medication treatment has not been established, the National Institute on Alcohol Abuse and Alcoholism (NIAAA) recommends a minimum of an initial 3 months of pharmacotherapy, with the caveat that it is reasonable to continue for a year or longer if the patient finds the medication beneficial (NIAAA, 2005). Additional studies reference the need for further supportive or booster treatment, such as a relapse-prevention medication, during the first 6–12 months (Hunt et al., 1971; Maisto et al., 1998, 2002).
The majority of individuals with an AUD never present for treatment. In one study, less than 15% of individuals who met lifetime criteria for an AUD reported ever receiving alcohol treatment (Cohen et al., 2007). When individuals do seek treatment, available medications are often underutilized. For example, in fiscal year 2012, receipt of medications (specifically the four FDA-approved medications discussed above and topiramate) among the Veterans Health Administration population diagnosed with an AUD ranged from 6.8% to 11.1% (Rubinsky et al., 2015). Although low, this prescribing rate may be an improvement from fiscal year 2009, in which the estimate among the same population was 3.4%, although topiramate was not included in this analysis (Harris et al., 2012).
The World Health Organization (WHO) defines adherence as “the extent to which the person’s behavior (including medication taking) corresponds with agreed recommendations from a healthcare provider” (Sabate, 2003). It has been estimated that as many as half of all patients do not adhere to prescribed medication regimens (Osterberg & Blaschke, 2005), and adherence has been shown to be of particular importance in regard to outcomes in chronic disease states (DiMatteo et al., 2002). Patients with substance use disorders have been shown to have high rates of nonadherence, and even higher nonadherence rates have been observed among those with comorbid psychiatric and substance-related illnesses (Magura et al., 2002; McLellan et al., 2000).
Multiple dynamics factor into suboptimal adherence, including but not limited to uncertainty of medication efficacy, concern for or actual side effects, severity of illness, complexity of regimen, drug interactions with alcohol or other substances, and stigma (Weiss, 2004). In addition, younger age and emotional factors have been noted as barriers to adherence in pharmacotherapy for AUD (Lohit et al., 2016). Several studies have attempted interventions to increase adherence, with methods ranging from contingency management to mobile phone text reminders, with mixed success (Preston et al., 1999; Stoner et al., 2015). Other recommendations for increased adherence include medication reminders, extended-release formulations, and patient education (Peterson, 2007).
Several methodologies for estimated adherence to medication exist. Direct measures include levels of the drug or its metabolites in bodily fluids such as blood or urine, detection of a biological marker such as riboflavin given with the medication, and observed drug-taking (Farmer, 1999). These direct measures are expensive and often impractical in clinical settings. Analysis of secondary databases such as electronic pharmacy records or pharmacy insurance claims can be used to estimate adherence (Lam & Fresco, 2015). These methods assume that refilling prescriptions corresponds with the patient taking the medication. Previous research has considered this to be an acceptable assumption. By examining refill records, medication availability can be used to estimate consumption over a specific period. However, partial adherence when a patient takes the medication only part of the time cannot be detected with these measures.
Although there is evidence that general adherence to medications is suboptimal, there are few studies directly comparing the adherence rates between multiple medications for AUDs. The primary aim of this study was to determine if significant differences in adherence rates exist across FDA-approved medications for AUDs in a Veterans Health Administration population. It was hypothesized that extended-release injectable naltrexone would have the highest adherence, primarily related to its every-28-day dosing formulation. Previous research has shown a reduction in medication adherence as the dosing frequency increases (Coleman et al., 2012). As a result, among the oral medications, naltrexone as once-a-day administration was hypothesized to have a higher adherence rate than acamprosate, generally prescribed three times a day. Disulfiram was hypothesized to have the worst adherence because of the negative physiological consequences of drinking while taking this medicine, as individuals are likely to discontinue or not start the medication if they have strong urges to drink. Clarification of adherence rates in these medications may inform best practices around prescriptions, counseling, and follow-up during treatment for AUDs.
Method
A retrospective chart review was conducted of the computerized patient record system in the Veterans Integrated Service Networks (VISN)-7 region of Veterans Affairs (VA) hospital and community-based outpatient clinics that includes the cities of Charleston, Myrtle Beach, and Beaufort, SC, and Savannah and Hinesville, GA. Coordinating with the pharmacy staff, a list was obtained of all prescriptions filled for the four FDA-approved AUD medications from January 1, 2010, through December 31, 2015. Similar to previous research, a continuous, multiple-interval measure of medication acquisition (CMA) was calculated to estimate adherence (Hess et al., 2006). CMA approximation of adherence was determined by the proportion of days the veteran had oral and injectable medication available over a 6-month period as noted by medication fills (reported as 0%–100% medication availability). As adherence has not been specifically defined for pharmacotherapy for AUD, 80% medication availability by pharmacy refill data was considered adherent, as defined in cardiovascular research (Ho et al., 2009). Using the same definition, a dichotomous measure of 80% adherence versus less than 80% was obtained. For injectable medication, adherence was reported similarly as for oral medication; however, subjects were given credit for 28 days of medication availability for each injection received (all injections included were documented as given by the lab nurse in the computerized patient record system at the time of injection). General baseline characteristics of subjects were collected, including age at onset of each medication trial, gender, race, and ethnicity.
Adherence measures were compared between the four medications: acamprosate, disulfiram, oral naltrexone, and extended-release injectable naltrexone. We used t tests to make pairwise comparisons of mean adherence between medications. Chi-square tests were used for overall and pairwise comparisons of the proportion with 80% adherence between medications. To evaluate and control for possible confounding by sociodemographic differences between groups, generalized linear models were fit, controlling for possible confounders. For continuous outcomes such as percentage of adherence, we used linear regression models, and for categorical outcomes such as “above 80% adherence,” we used logistic regression models. In these multivariate models, we evaluated possible confounding factors including patient age and race/ethnicity.
Each subject’s chart was reviewed, and exclusion criteria were as follows: transfer out of the VA system during the 6-month period, death, or if the medication was prescribed for an indication other than an AUD (e.g., naltrexone prescribed for opioid use disorder). Some subjects were prescribed multiple trials of the same medication within the period of the study; in these cases, only the first trial was included in the analyses. In addition, some subjects were prescribed more than one of the above-listed medications during the time of the study. As each medication has a different mechanism of action, some individuals may respond better to one medication than another. As such, if the anticipated clinical response did not occur, a trial of another medication would be a reasonable clinical action. The reasons for the different trials varied and included the following: adverse effects, ease of administration (e.g., once-a-day medication or once-a-month injection compared with multiple times a day), patient request, clinical goal of enhancing adherence, discontinuing naltrexone due to the need for opioids, patient’s report of not experiencing intended reaction (e.g., lack of disulfiram–alcohol reaction), etc. In these cases, only the first trial of each medication prescribed for a subject was included in the study. However, combination trials (e.g., naltrexone and acamprosate prescribed simultaneously) were excluded to minimize bias.
Results
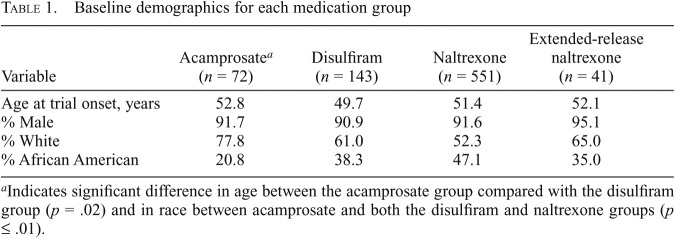
We included 715 subjects in the study, of whom 82 had trial records for more than one medication trial (acamprosate, disulfiram, oral naltrexone, and/or extended-release injectable naltrexone). Across all subjects, we included 807 medication trials (acamprosate = 72; disulfiram = 143; oral naltrexone = 551; extended-release injectable naltrexone = 41), reflecting the first trial of that medication for each patient. General baseline characteristics of subjects included in this study are listed in Table 1. Age at onset was significantly higher for acamprosate when compared with disulfiram (p = .02). A significant difference in the racial composition was found between the acamprosate group and both the disulfiram and naltrexone groups (p ≤ 0.01); there were no other significant baseline differences in age or gender (Table 1).
Table 1.
Baseline demographics for each medication group
| Variable | Acamprosatea (n = 72) | Disulfiram (n = 143) | Naltrexone (n = 551) | Extended-release naltrexone (n = 41) |
| Age at trial onset, years | 52.8 | 49.7 | 51.4 | 52.1 |
| % Male | 91.7 | 90.9 | 91.6 | 95.1 |
| % White | 77.8 | 61.0 | 52.3 | 65.0 |
| % African American | 20.8 | 38.3 | 47.1 | 35.0 |
Indicates significant difference in age between the acamprosate group compared with the disulfiram group (p = .02) and in race between acamprosate and both the disulfiram and naltrexone groups (p ≤ .01).
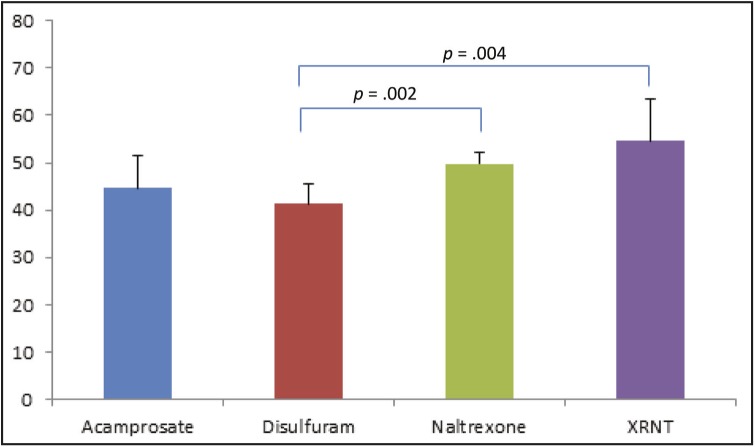
Mean adherence was 41.3% (SD = 25.5) for disulfiram, 44.7% (SD = 29.7) for acamprosate, 49.8% (SD = 29.1) for oral naltrexone, and 54.6% (SD = 29.1) for extended-release injectable naltrexone (Figure 1). Mean adherence was significantly different between disulfiram and oral naltrexone (p = .002) and between disulfiram and extended-release injectable naltrexone (p = .004). A significant difference was not observed between acamprosate and extended-release injectable naltrexone (p = .08) or between oral naltrexone and extended-release injectable naltrexone (p = .3). Linear regression models controlling for possible confounders showed similar results.
Figure 1.
Mean medication adherence as defined by the proportion of days the veteran had oral medication available over a 6-month period as noted by medication fills
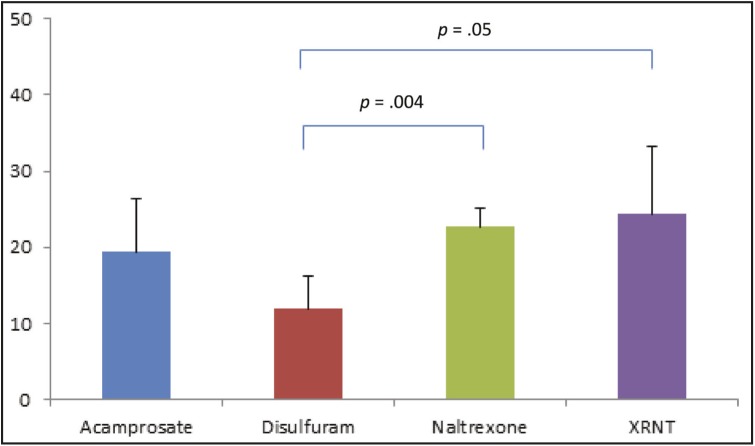
Adherence of 80% was achieved in 11.9%, 19.4%, 22.7%, and 24.4% of treatment courses with disulfiram, acamprosate, oral naltrexone, and extended-release injectable naltrexone, respectively (Figure 2). These differences were significant for disulfiram versus oral naltrexone (p = .004) and disulfiram versus extended-release injectable naltrexone (p = .05). A significant difference was not observed for disulfiram versus acamprosate (p = 0.1) or acamprosate versus oral naltrexone (p = .5), acamprosate versus extended-release injectable naltrexone (p = .5), or oral naltrexone versus extended-release injectable naltrexone (p = .8). Logistic regression models controlling for possible confounders showed similar results.
Figure 2.
Percentage of subjects among each of the four medications who achieved adherence, as defined by having medication availability at least 80% of days over a 6-month period (oral) or administered medication covering at least 80% of days (injectable)
Discussion
These results demonstrate that overall adherence to medication-assisted treatment for AUD is low across all medications. When directly compared, disulfiram had significantly lower adherence than both oral and extended-release naltrexone. These differences remained significant when controlling for baseline demographics.
Adherence to disulfiram was significantly lower than both forms of naltrexone but not acamprosate. The sample size of acamprosate may have been too small to detect a significant difference. Previous research has found that adherence to disulfiram is problematic, with use optimized in supervised settings or in highly motivated individuals with the expectancy of an adverse pharmacological effect of a disulfiram–alcohol reaction accounting for disulfiram’s effectiveness (Jørgensen et al., 2011). Acamprosate adherence and oral naltrexone adherence were not significantly different in this study, although patients using these medications had some degree of higher adherence than those using disulfiram, given that acamprosate and naltrexone are generally better tolerated, particularly when concurrently consuming alcohol. Given that naltrexone is a once-a-day medication, whereas acamprosate is scheduled to be taken three times a day with two pills per dose, it was hypothesized that oral naltrexone would have greater adherence. A significant difference was not observed in this study and may be due to the limitation of only measuring oral medication prescribed versus the amount ingested.
Given that this study calculated adherence based on days of oral medicine availability based on refills, adherence to oral medications in this study is likely overestimated (Arnet et al., 2014). Although current VA treatment guidelines do not recommend the “as-needed” use of these medications, patients may choose to take them only when they feel it is necessary (Department of Veterans Affairs & Department of Defense, 2015). Also, prescribers may verbally instruct a patient to use as needed, in contrast to the written instructions. This is one of the limitations of the study and points to the challenge of measuring adherence via a retrospective pharmacy prescription refill review. Methods for assessing adherence to extended-release injectable naltrexone, however, likely lead to a more accurate assessment of adherence, as it is known that subjects received the full 28-day dose when administration is documented. This suggests that extended-release injectable naltrexone may be significantly more adhered to than all oral medications in this study.
Another potential limitation of our analysis is that a small percentage of our population was treated with more than one type of medication during the study period. We chose in our analysis to take a straightforward and inclusive approach by examining the first trial of each separate medication for each patient. Accordingly, patients with more than one medication recorded during the study period were included in more than one of our medication groups. To the extent that an individual’s adherence on one medication course may be similar to adherence on a different medication, the observations are not truly independent of each other. However, this is a conservative bias in our analysis, because the lack of independence would be expected to reduce observed differences between groups. Therefore, after considering this possible bias, we would argue that any differences in adherence that we observed between groups are more likely to be due to the medications under study.
Other limitations exist in this study. Adherence defined as 80% medication availability was based on cardiovascular studies, as the percentage in AUDs is yet to be defined. Additional research in this area is needed. There was a relatively small sample of medications other than oral naltrexone included in this study, and this may have led to insufficient power to detect other significant differences. The results were reflective solely of a veteran population, and thus the generalizability of this study to broader populations may be difficult. This was not a randomized controlled trial; therefore, other confounders may exist that could explain the results. An inherent limitation of pharmacy prescription refill data is the inability to ascertain the intended duration or spacing of treatment. The selected period of 6 months was chosen to assess the minimum 3 months of initial pharmacotherapy recommended by the NIAAA and to capture a period of high vulnerability to relapse. Other time intervals should be considered as the optimal duration of treatment is defined in future research. Given the methodology of this study using medication fills, nearly all individuals had at least one 30-day prescription filled, and thus if the duration of adherence had been for only 1 month, results would have been of little value. However, if adherence had been assessed over a period of a year, adherence results would have been increasingly low. Therefore, measuring adherence over a 6-month period was chosen as a reasonable alternative.
As mentioned previously, multiple dynamics factor into suboptimal adherence, including but not limited to uncertainty of medication efficacy, concern for or actual side effects, severity of illness, complexity of regimen, drug interactions with alcohol or other substances, stigma, younger age, and emotional factors. To more comprehensively understand how each of these factors contributes to adherence rates, further studies should review specific reasons for patient discontinuation of or decreased adherence to a medication.
As noted, controlling for differences in the demographics did not affect the results; the racial differences between acamprosate and both disulfiram and naltrexone were significant. In a recent review of the electronic medical records of almost 300,000 VA patients with AUDs, the use of medications was low across all groups, with African Americans less likely than European Americans to receive pharmacotherapy (Williams et al., 2017). It is unclear if the difference in acamprosate in this study represents a racial healthcare disparity or undertreatment of more severe AUDs in African Americans within the VA. Further research is needed specifically addressing racial/ethnic differences between individual medications in a large nationally representative sample.
In conclusion, AUDs are prevalent throughout the United States, including the veteran population. Some barriers include engaging more individuals in treatment as well as maximizing appropriate use of available medication treatments for treatment seekers. Yet these results suggest that when patients are started on a medication, adherence as defined in this study is low across all medications, with disulfiram being significantly lower than the other options. Further, results of adherence to oral medications are likely overestimated and suggest that extended-release injectable naltrexone may have higher adherence than any oral medication in the study. Further research using other methods is warranted to confirm these findings.
Acknowledgments
We thank the Ralph H. Johnson VA Medical Center and its military veterans as well as Sudie Back, Ph.D., Kelly Barth, M.D., Nicola Thornley, M.P.H., Sarah Book, M.D., and Kathleen Brady, M.D., Ph.D.
Footnotes
This research was supported by National Institute on Drug Abuse Grants R25 DA020537 and K24 DA038240.
References
- Arnet I., Abraham I., Messerli M., Hersberger K. E. A method for calculating adherence to polypharmacy from dispensing data records. International Journal of Clinical Pharmacy. 2014;36:192–201. doi: 10.1007/s11096-013-9891-8. doi:10.1007/s11096-013-9891-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Excessive drinking is draining the U.S. economy. 2016. Retrieved from https://www.cdc.gov/features/costsofdrinking. [Google Scholar]
- Cohen E., Feinn R., Arias A., Kranzler H. R. Alcohol treatment utilization: Findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug and Alcohol Dependence. 2007;86:214–221. doi: 10.1016/j.drugalcdep.2006.06.008. doi:10.1016/j.drugalcdep.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Coleman C. I., Limone B., Sobieraj D. M., Lee S., Roberts M. S., Kaur R., Alam T. Dosing frequency and medication adherence in chronic disease. Journal of Managed Care Pharmacy. 2012;18:527–539. doi: 10.18553/jmcp.2012.18.7.527. doi:10.18553/jmcp.2012.18.7.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Veterans Affairs & Department of Defense. VA/DoD clinical practice guidelines for the management of substance use disorders. 2015 https://www.healthquality.va.gov/guidelines/MH/sud/VADODSUDCPGRevised22216.pdf Retrieved from.
- DiMatteo M. R., Giordani P. J., Lepper H. S., Croghan T. W. Patient adherence and medical treatment outcomes: A meta-analysis. Medical Care. 2002;40:794–811. doi: 10.1097/00005650-200209000-00009. doi:10.1097/00005650-200209000-00009. [DOI] [PubMed] [Google Scholar]
- Farmer K. C. Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clinical Therapeutics. 1999;21:1074–1090. doi: 10.1016/S0149-2918(99)80026-5. doi:10.1016/S0149-2918(99)80026-5. [DOI] [PubMed] [Google Scholar]
- Hagedorn H. J., Brown R., Dawes M., Dieperink E., Myrick D. H., Oliva E. M., Harris A. H. S. Enhancing access to alcohol use disorder pharmacotherapy and treatment in primary care settings: ADaPT-PC. Implementation Science. 2016;11:64. doi: 10.1186/s13012-016-0431-5. doi:10.1186/s13012-016-0431-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A. H., Oliva E., Bowe T., Humphreys K. N., Kivlahan D. R., Trafton J. A. Pharmacotherapy of alcohol use disorders by the Veterans Health Administration: Patterns of receipt and persistence. Psychiatric Services. 2012;63:679–685. doi: 10.1176/appi.ps.201000553. doi:10.1176/appi.ps.201000553. [DOI] [PubMed] [Google Scholar]
- Hess L. M., Raebel M. A., Conner D. A., Malone D. C. Measurement of adherence in pharmacy administrative databases: A proposal for standard definitions and preferred measures. Annals of Pharmacotherapy. 2006;40:1280–1288. doi: 10.1345/aph.1H018. doi:10.1345/aph.1H018. [DOI] [PubMed] [Google Scholar]
- Ho P. M., Bryson C. L., Rumsfeld J. S. Medication adherence: Its importance in cardiovascular outcomes. Circulation. 2009;119:3028–3035. doi: 10.1161/CIRCULATIONAHA.108.768986. doi:10.1161/CIRCULATIONAHA.108.768986. [DOI] [PubMed] [Google Scholar]
- Hunt W. A., Barnett L. W., Branch L. G. Relapse rates in addiction programs. Journal of Clinical Psychology. 1971;27:455–456. doi: 10.1002/1097-4679(197110)27:4<455::aid-jclp2270270412>3.0.co;2-r. doi:10.1002/1097-4679(197110)27:4<455::AID-JCLP2270270412>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Jørgensen C. H., Pedersen B., Tønnesen H. The efficacy of disulfiram for the treatment of alcohol use disorder. Alcoholism: Clinical and Experimental Research. 2011;35:1749–1758. doi: 10.1111/j.1530-0277.2011.01523.x. doi:10.1111/j.1530-0277.2011.01523.x. [DOI] [PubMed] [Google Scholar]
- Lam W. Y., Fresco P. Medication adherence measures: An overview. BioMed Research International, 2015. 2015 doi: 10.1155/2015/217047. Article ID 217047. doi:10.1155/2015/217047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohit K., Kulkarni C., Galgali R. B. Factors influencing adherence to anti-craving medications and drinking outcomes in patients with alcohol dependence: A hospital-based study. Journal of Pharmacology & Pharmacotherapeutics. 2016;7:72–79. doi: 10.4103/0976-500X.184770. doi:10.4103/0976-500X.184770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magura S., Laudet A. B., Mahmood D., Rosenblum A., Knight E. Adherence to medication regimens and participation in dual-focus self-help groups. Psychiatric Services. 2002;53:310–316. doi: 10.1176/appi.ps.53.3.310. doi:10.1176/appi.ps.53.3.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisto S. A., Clifford P. R., Longabaugh R., Beattie M. The relationship between abstinence for one year following pretreatment assessment and alcohol use and other functioning at two years in individuals presenting for alcohol treatment. Journal of Studies on Alcohol. 2002;63:397–403. doi: 10.15288/jsa.2002.63.397. doi:10.15288/jsa.2002.63.397. [DOI] [PubMed] [Google Scholar]
- Maisto S. A., McKay J. R., O’Farrell T. J. Twelve-month abstinence from alcohol and long-term drinking and marital outcomes in men with severe alcohol problems. Journal of Studies on Alcohol. 1998;59:591–598. doi: 10.15288/jsa.1998.59.591. doi:10.15288/jsa.1998.59.591. [DOI] [PubMed] [Google Scholar]
- McLellan A. T., Lewis D. C., O’Brien C. P., Kleber H. D. Drug dependence, a chronic medical illness: Implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689–1695. doi: 10.1001/jama.284.13.1689. doi:10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. Helping patients who drink too much: A clinician’s guide. 2005. Retrieved from https://pubs.niaaa.nih.gov/publications/practitioner/cliniciansguide2005/guide.pdf. [Google Scholar]
- Osterberg L., Blaschke T. Adherence to medication. The New England Journal of Medicine. 2005;353:487–497. doi: 10.1056/NEJMra050100. doi:10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- Peterson A. M. Improving adherence in patients with alcohol dependence: A new role for pharmacists. American Journal of Health-System Pharmacy. 2007;64(Supplement 3):S23–S29. doi: 10.2146/ajhp060648. doi:10.2146/ajhp060648. [DOI] [PubMed] [Google Scholar]
- Preston K. L., Silverman K., Umbricht A., DeJesus A., Montoya I. D., Schuster C. R. Improvement in naltrexone treatment compliance with contingency management. Drug and Alcohol Dependence. 1999;54:127–135. doi: 10.1016/s0376-8716(98)00152-5. doi:10.1016/S0376-8716(98)00152-5. [DOI] [PubMed] [Google Scholar]
- Rubinsky A. D., Chen C., Batki S. L., Williams E. C., Harris A. H. Comparative utilization of pharmacotherapy for alcohol use disorder and other psychiatric disorders among U.S. Veterans Health Administration patients with dual diagnoses. Journal of Psychiatric Research. 2015;69:150–157. doi: 10.1016/j.jpsychires.2015.07.016. doi:10.1016/j.jpsychires.2015.07.016. [DOI] [PubMed] [Google Scholar]
- Sabate E., editor. Adherence to long-term therapies: Evidence for action. Geneva, Switzerland: World Health Organization; 2003. Retrieved from https://www.who.int/chp/knowledge/publications/adherence_report/en. [Google Scholar]
- Stoner S. A., Arenella P. B., Hendershot C. S. Randomized controlled trial of a mobile phone intervention for improving adherence to naltrexone for alcohol use disorders. PLoS One. 2015;10:e0124613. doi: 10.1371/journal.pone.0124613. doi:10.1371/journal.pone.0124613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2015 National Survey on Drug Use and Health: Detailed tables. Table 5.2B—Substance use disorder for specific substances in past year, by age group: Percentages, 2014 and 2015. 2015. Retrieved from https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.htm#tab5-2b. [Google Scholar]
- Teeters J. B., Lancaster C. L., Brown D. G., Back S. E. Substance use disorders in military veterans: Prevalence and treatment challenges. Substance Abuse and Rehabilitation. 2017;8:69–77. doi: 10.2147/SAR.S116720. doi:10.2147/SAR.S116720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. D. Adherence to pharmacotherapy in patients with alcohol and opioid dependence. Addiction. 2004;99:1382–1392. doi: 10.1111/j.1360-0443.2004.00884.x. doi:10.1111/j.1360-0443.2004.00884.x. [DOI] [PubMed] [Google Scholar]
- Williams E. C., Gupta S., Rubinsky A. D., Glass J. E., Jones-Webb R., Bensley K. M., Harris A. H. S. Variation in receipt of pharmacotherapy for alcohol use disorders across racial/ethnic groups: A national study in the U.S. Veterans Health Administration. Drug and Alcohol Dependence. 2017;178:527–533. doi: 10.1016/j.drugalcdep.2017.06.011. doi:10.1016/j.drugalcdep.2017.06.011. [DOI] [PubMed] [Google Scholar]