Abstract
Homocysteine-Methionine (HM) cycle produces universal methyl group donor S-adenosylmethione (SAM), methyltransferase inhibitor S-adenosylhomocysteine (SAH) and homocysteine (Hcy). Hyperhomocysteinemia (HHcy) is established as an independent risk factor for cardiovascular disease (CVD) and other degenerative disease.
We selected 115 genes in the extended HM cycle (31 metabolic enzymes and 84 methyltransferases), examined their protein subcellular location/partner protein, investigated their mRNA levels and mapped their corresponding histone methylation status in 35 disease conditions via mining a set of public databases and intensive literature research. We have 6 major findings. 1) All HM metabolic enzymes are located only in the cytosol except for cystathionine-β-synthase (CBS), which was identified in both cytosol and nucleus. 2) Eight disease conditions encountered only histone hypomethylation on 8 histone residues (H3R2/K4/R8/K9/K27/K36/K79 and H4R3). Nine disease conditions had only histone hypermethylation on 8 histone residues (H3R2/K4/K9/K27/K36/K79 and H4R3/K20). 3) We classified 9 disease types with differential HM cycle expression pattern. Eleven disease conditions presented most 4 HM cycle pathway suppression. 4) Three disease conditions had all 4 HM cycle pathway suppression and only histone hypomethylation on H3R2/K4/R8/K9/K36 and H4R3. 5) Eleven HM cycle metabolic enzymes interact with 955 proteins. 6) Five paired HM cycle proteins interact with each other.
We conclude that HM cycle is a key metabolic sensor system which mediates receptor-independent metabolism-associated danger signal recognition and modulates SAM/SAH-dependent methylation in disease conditions and that hypomethylation on frequently modified histone residues is a key mechanism for metabolic disorders, autoimmune disease and CVD. We propose that HM metabolism takes place in the cytosol, that nuclear methylation equilibration requires a nuclear-cytosol transfer of SAM/SAH/Hcy, and that Hcy clearance is essential for genetic protection.
Keywords: Homocysteine-methionine cycle, Metabolic sensor, SAM/SAH-dependent methylation
Abbreviations
- AD
Alzheimer’s disease
- AOD
Aortic occlusive disease
- Arg (R)
Arginine
- AS
Atherosclerosis
- BA
Breast adenocarcinoma
- βC
β cell
- BC
Bladder cancer
- CAD
Coronary artery disease
- CBS
Cystathionine-β-synthase
- CC
Colon Cancer
- CCRCC
Clear cell renal cell carcinoma
- CKD
Chronic kidney disease
- CTH
Cystathionine-γ-lyase
- CVD
Cardiovascular disease
- Cys
Cysteine
- DAMP
Damage-associated molecular pattern
- DNMT
DNA methyltransferase
- EC
Endothelial cell
- ESC
Embryonic stem cell
- ESCC
Esophageal Squamous Cell Carcinomas
- GA
Gastric Adenocarcinoma
- GEO
Gene Expression Omnibus
- Glu
Glucose
- GSC
Glioblastoma stem cell
- Hcy
Homocysteine
- HFH
Heterozygote family hypercholesterolemia
- HG
Hyperglycemia
- HGPS
Hutchinson-Gilford progeria syndrome
- HHcy
Hyperhomocysteinemia
- HL
Hyperlipidemia
- HM
Homocysteine-methionine
- HMT
Histone methyltransferase
- His (H)
Histone
- HT
Homocysteine thiolactone
- IS
Ischemic stroke
- IC
Intrahepatic Cholangiocarcinoma
- IPA
Ingenuity Pathway Analysis
- Lys (K)
Lysine
- MADS
Metabolism-associated danger signal
- MDS
Myelodysplastic syndrome
- Met
Methionine
- METTL3
Methyltransferase like 3
- METTL4
Methyltransferase like 4
- MT
Methyltransferase
- MTHFR
Methylenetetrahydrofolate reductase
- m6A
N6-methyladenosine
- NSCLC
Non-small cell lung cancer
- OC
Ovarian carcinoma
- OSIMOF
Old sepsis induced multiple organ failure
- Ox-LDL
Oxidized low-density lipoprotein
- Ox-PAPC
Oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine
- PAMP
Pathogen-associated molecular pattern
- PC
Prostate cancer
- PCh
Phosphatidylcholine
- PDA
Pancreatic Ductal Adenocarcinoma
- PD
Parkinson’s disease
- PE
Phosphatidylethanolamine
- PP2A
Protein phosphatase 2A
- PRR
Pathogen recognition receptor
- PRMT
Protein arginine methyltransferases
- RA
Rheumatoid arthritis
- SAH
S-adenosylhomocysteine
- SAM
S-adenosylmethionine
- SLE
Systemic lupus erythematosus
- T2DM
Type 2 diabetes mellitus
- VB12
Vitamin B 12
1. Introduction
Homocysteine-Methionine (HM) cycle is critical for numerous biochemical processes including amino acid metabolism and cellular methylation. The HM cycle comprises eight metabolic pathways and produces the universal methyl donor S-adenosylmethionine (SAM) and competitive methyltransferase inhibitor S-adenosylhomocysteine (SAH), (Fig. 1A). Impaired regulation of the HM cycle results in elevated levels of Hcy, a human disease termed as hyperhomocysteinemia (HHcy), an established independent risk factor for cardiovascular disease (CVD), stroke, type II diabetes, osteoporosis and dementia [[1], [2], [3]].
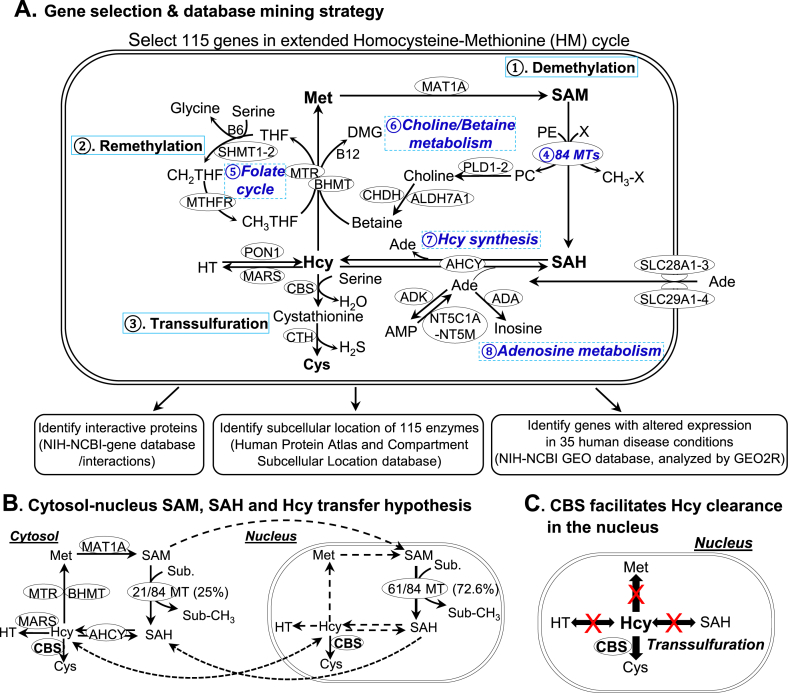
Fig. 1.
HM cycle senses metabolic risk factors and modulates SAM/SAH-dependent methylation and pathogenic signaling. A. Extended HM cycle, gene selection & database mining strategy. We selected 115 genes in this cycle from Human metabolome database (https://www.hmdb.ca/). The HM cycle includes 3 major pathways. ① Demethylation pathway: Met is converted to SAM, which becomes SAH after donating the methyl group for cellular methylation. SAH is then converted to Hcy, a dual directional reaction, interacting with adenosine metabolism. ② Remethylation pathway: Hcy is converted back to Met by regaining the methyl group from folate cycle or choline/betaine metabolism. ③ Transsulfuration pathway: Hcy is converted to Cys. The extended HM cycle includes additional pathways, such as ④ 84 MTs, ⑤ folate cycle, ⑥ choline/betaine metabolism, ⑦ Hcy synthesis and ⑧ adenosine metabolism. These 115 genes’ mRNA levels in human disease conditions were analyzed by mining NIH-NCBI GEO database (https://www.ncbi.nlm.nih.gov/geo/), protein subcellular localization and interacting partner were identified by using information in Human Protein Atlas (https://www.proteinatlas.org/), compartment subcellular location database (https://compartments.jensenlab.org) and NIH-NCBI gene database (https://www.ncbi.nlm.nih.gov/gene), respectively. B. Cytosol-nucleus SAM, SAH and Hcy transfer hypothesis. All essential enzymes in the HM metabolic cycle are located in the cytosol, only 21 out of 84 MTs (25%) in the cytosol but the majority of MTs (72.6%) in the nucleus. Some of the HM cycle metabolic processes are not active in the nucleus because their metabolic enzymes are not identified there and indicated by dash lines. For example, enzymes for SAM synthesis, SAH clearance and Hcy synthesis are missing in the nucleus. C. CBS facilitates Hcy clearance in the nucleus. Since only one Hcy clearance enzyme exists in the nucleus, we propose that CBS facilitates Hcy clearance in the nucleus. Abbreviations: Ade, adenosine; Hcy, homocysteine; SAM, S-Adenosylmethionine; SAH, S-Adenosylhomocysteine; Met, Methionine; MTs, Methyltransferase; CBS, Cystathionine-β-synthase; HT, homocysteine thiolactone. Abbreviations for gene and enzyme names are explained in Supplementary Table 1.
Methylation is an essential chemical modification occurring on DNA, RNA, protein, phospholipid and other small molecules. The HM cycle provides methyl units for most cellular methylation, allowing for the regulation of gene expression, activation, and molecules stabilization [4]. We were the first to define SAH accumulation-based protein/DNA hypomethylation as the essential biochemical mechanism responsible for HHcy-induced endothelial injury and inflammatory monocyte (MC) differentiation in CVD, diabetes, and chronic kidney disease (CKD) [[5], [6], [7], [8], [9]].
Global DNA hypomethylation and differential promoter methylation are major characteristics of tumorigenesis. Global hypomethylation of the entire genome in peripheral blood leukocytes is demonstrated as the risk factor for colorectal and breast cancer [[10], [11], [12], [13]]. DNA hypomethylation on the promoter region was linked to increased expression of cyclin D2 and maspin in gastric carcinoma and elevated carbonic anhydrase family gene in human renal-cell carcinoma [[14], [15], [16]]. DNA hypermethylation on tumor suppressor genes leads to gene silencing and is defined as the mechanistic basis for anti-methylation therapy [4,12]. Promoter hypermethylation of tumor-suppressor retinoblastoma gene is associated with retinoblastoma, osteosarcoma and small-cell lung carcinoma [17]. DNA hypermethylation of tumor-suppressor gene cyclin-dependent kinase inhibitor 2A is correlated with increased risk of breast cancer [18]. DNA methyltransferase (DNMT) inhibitors 5-azacytidine (Vidaza) and 5-aza-2’-deoxycytidine (Dacogen) have been used in myelodysplastic syndrome therapy [19] and produced a 40–50% overall response rate [20].
Global DNA hypomethylation in advanced atherosclerotic lesions and peripheral MC is also identified as a major feature of CVD and coronary artery disease (CAD) [[21], [22], [23], [24]]. We were the first to propose hypomethylation as the basic biochemical mechanism for CVD [25] and CKD [8]. We reported that HHcy selectively inhibits endothelial cell (EC) growth, an onset feature of atherosclerosis, by SAH accumulation-resulted DNMT1 inactivation and by suppression of transcription and DNA hypomethylation on CDE consensus element of cyclin A promoter [26]. We and others discovered that HHcy increased SAH and reduced SAM/SAH ratio, which are correlated with atherosclerosis, inflammatory monocyte (MC)/macrophage (MΦ) differentiation, the severity of CKD and glomerular injury [[27], [28], [29], [30]].
Protein methylation affects protein-protein interactions, membrane properties, structure and function of chromatin, and gene expression. Numerous evidence demonstrated altered histone methylation on histone 3 (H3) and histone 4 (H4) impacts on CVD, cancer and neurodegenerative disease by chromatin remodeling and gene expression changes [29,31]. Twelve methylation sites have been identified on the arginine (R) and lysine (K) residue of histone, including H3R2/K4/R8/K9/R17/K23/R26/K27/K36/K79 and H4R3/K20 [32], which are catalyzed by 55 histone methyltransferases (HMT) [33].
Non-histone protein methylation regulates protein subcellular localization and stability. Methylation on the carboxyl terminal (C-terminal) of some proteins increases its hydrophobicity and ability to associate with the cell membrane [34]. We and others reported that hypomethylation on C-terminal CAAX motif of oncoprotein p21ras [35] leads to reduced p21ras membrane association, suppressed downstream signaling, and selectively inhibited cell growth in Hcy-treated endothelial cells (EC) [36]. Protein phosphatase 2A (PP2A), a serine/threonine phosphatase impacting on cell growth and signaling, can be methylated at C-terminal leucine 309 residues [37,38], which leads to increased phosphatase activity [39]. Protein methylation results in mono- and di-methylation of the guanidine nitrogen atoms of arginine. Arginine can be di-methylated either symmetrically or asymmetrically (SDMA or ADMA). SDMA can be formed by protein arginine methyltransferases (PRMT)-5/7 cartelization, and ADMA is catalyzed by PRMT-1/2/3/4/6. PRMT catalyze arginine methylation of CDK4/6, pRb, E2F1, cyclin D1, p16, p21, p27, and p53 and results in the destabilization of their relevant complex [40]. PRMT also methylate C-terminal glycine-arginine rich (GAR) domain of DNA repair protein MRE11 and regulates its nuclear compartmentalization [41]. Lysine methylation decreases NAD kinase activation [42].
RNA methylation as a reversible post-translational modification affects RNA stability [43]. RNA methyltransferase like 3 (METTL3) and 4 (METTL4) have a tumor-suppressor role and catalyze N6-methyladenosine (m6A) methylation, which accounts for more than 80% of RNA methylation [44]. Depletion of either METTL3 or METTL4 reduces m6A methylation and increases transcript stability in embryonic stem cell (ESC) [43], and enhanced glioblastoma stem cells (GSCs) growth and tumor progression [45]. Hcy induced NOP2/Sun domain family member 2 (NSun2)-mediated mRNA methylation, which increased ICAM‐1 expression in EC [46].
Other molecules such as phospholipid can also be methylated. Phosphatidylethanolamine (PE) is converted to phosphatidylcholine (PCh), a membrane phospholipid, by PE-methyltransferase (PEMT) [47]. PE methylation reduces the ratio of PE:PCh, which is critical for cell membrane integrity and fluidity. Increased PE:PCh ratio is correlated with a decreased membrane fluidity [48]. PE enhances the rigidity and viscosity of cell membrane. Inhibition of PE methylation leads to decreased VLDL secretion [49].
We are the first to propose metabolism-associated danger signal (MADS) recognition as a novel mechanism for metabolic risk factor-induced inflammatory responses [50], which is independent from pattern recognition receptor (PRR)-mediated pathogen-associated molecular pattern (PAMP)/danger-associated molecular pattern (DAMP) recognition [51]. We hypothesize that a metabolic sensor system can respond to metabolic risk factors and mediate pathogenic signaling.
Our current study focuses on examining expression changes of 115 metabolic enzyme genes, which control biochemical process, in extended HM cycle in disease conditions. We tested the hypothesis that HM cycle determines SAH/SAM-dependent methylation and methylation-regulated pathogenic signaling. Via bioinformatics analysis by mining a large set of public databases and extensive literature analysis, we developed novel models and hypotheses for disease mechanism.
2. Materials and methods
Selection of extended Homocysteine-Methionine (HM) cycle genes — we selected 115 genes in extended HM cycle (31 metabolic enzymes and 84 SAM/SAH-dependent MT) from Human metabolome database (https://www.hmdb.ca/) (Supplementary Table 1). We assessed these genes’ mRNA levels in 35 disease conditions, protein-protein interaction, protein subcellular localization from public database as illustrated in Fig. 1A.
Subcellular localization of extended HM cycle enzymes — The subcellular localization of 115 extended HM cycle enzymes were determined utilizing the Human Protein Atlas (https://www.proteinatlas.org/) and compartments subcellular location databases (https://compartments.jensenlab.org/) established by cellular organelle proteomics analysis. Subcellular localization of 21 generally accepted intracellular organelle markers were used as an internal control for the justification of the reliability of these databases as we reported previously [52]. Enzymes localization determined by using the second database are indicated by * in Supplementary Fig. 1A.
Protein-Protein Interaction — We screened protein-protein interaction of 11 key Hcy-related metabolic enzymes and 84 MTs in extended HM cycle using the NCBI Gene database (https://www.ncbi.nlm.nih.gov/gene/) as our previous description [52]. The protein-protein interaction data were established using yeast two-hybrid system, affinity capture-mass spectrometer/Western blot, co-fractionation, proximity label-mass spectrometer, and immunoprecipitation. The identified interactions were denoted as “+”. The paired interactions were circled in Supplementary Fig. 1D.
Expression profiles of 115 genes in extended HM cycle in 35 disease conditions — Microarray datasets were collected from the Array Express of European Bioinformatics Institute (https://www.ebi.ac.uk/arrayexpress), which stores data from high-throughput functional genomics experiments, and imported from the NIH-NCBI Gene Expression Omnibus (GEO) database (Fig. 1A) [53]. These datasets were analyzed utilizing GEO2R from GEO databases as we previously described [52,54]. Differentially expressed genes were defined as p-value ≤ 0.05 and absolute fold change ≥2. We established an association between the HMT gene expression and H3/H4 methylation based on HMT function (Supplementary Table 2, Fig. 3).
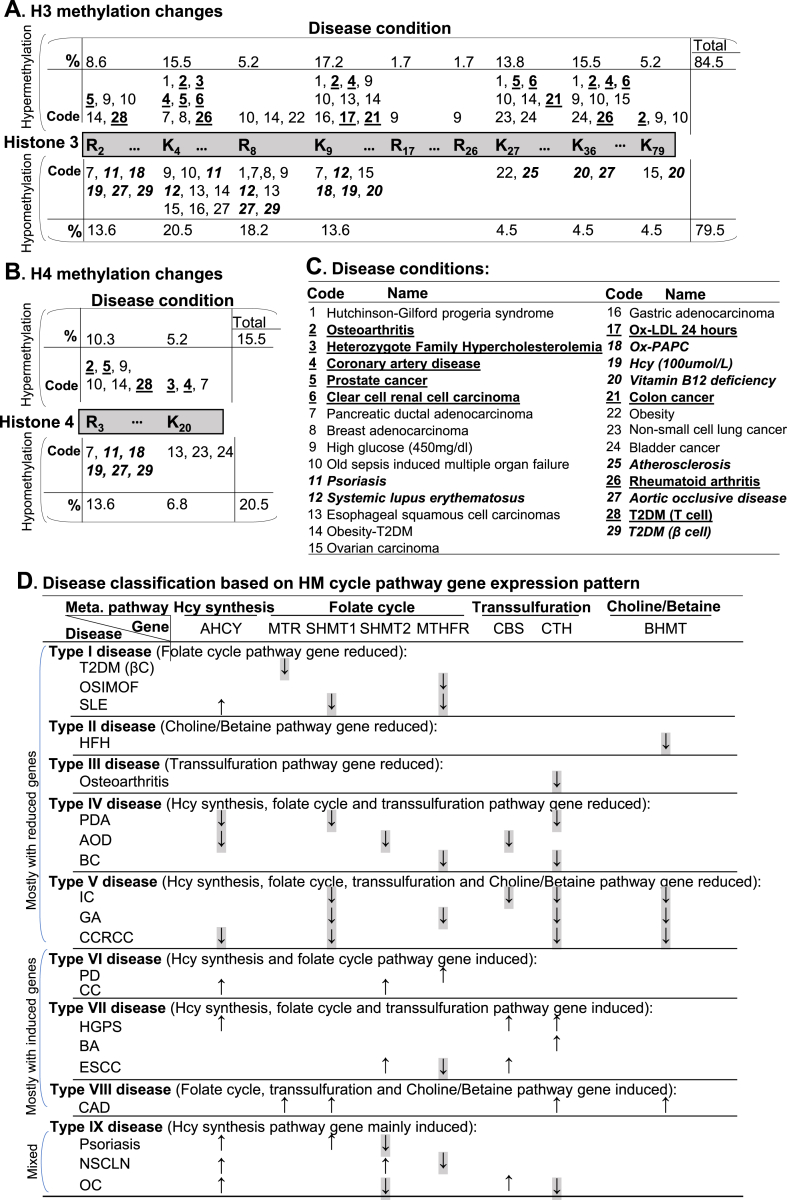
Fig. 3.
HM cycle gene expression-determined SAM/SAH-dependent H3/H4 histone methylations changes in disease, and disease classification. We examined extended HM cycle gene expression of 35 disease conditions microarray data in NIH-NCBI GEO database (https://www.ncbi.nlm.nih.gov/geo/), and determined histone methylations changes based on corresponding MT. We defined induced MT gene as hypermethylation and reduced MT gene as hypomethylation on their corresponding histone methylation site (Supplementary Table 2A). A. H3 methylation changes. H3 histone methylation are changed at H3-R2, K4, R8, K9, R17, R26, K27, K36, K79 in human disease. Disease conditions are presented by code. Percentage indicates the frequency of each modification in total methylation changes modified. Codes for disease with individual hypermethylation changes are placed above the histone bar, whereas for that with individual hypomethylation changes below the histone bar. B. H4 methylation changes. H4 histone methylation are changed at H4-R3 and K20 in human disease. C. Disease conditions. Numeric coded 29 human disease conditions are explained. Numeric codes and disease names are underlined with bold letter (9) for conditions identified as histone hypermethylation only, or highlighted in italic bold letter (8) for conditions identified as histone hypomethylation only. D. Disease classification based on HM cycle pathway gene expression pattern. To examine the association between HM cycle metabolites with human disease, we classified human disease into 9 types based on differential gene expression pattern in HM cycle pathway. Eleven diseases with mostly reduced genes (T2DM-βC, OSIMOF, SLE, HFH, Osteoarthritis, PDA, AOD, BC, IC, GA, CCRCC), 6 with mostly induced genes (PD, CC, HGPS, BA, ESC, CAD), and 3 with mixed gene expression changes (Psoriasis, NSCLN, OC) in HM cycle pathway. Arrows for reduced genes are highlighted with grey shade. Abbreviations: R, Arginine; K, lysine. Abbreviations for diseases: T2DM (βC), Type 2 diabetes mellitus (β cell); OSIMOF, Old sepsis induced multiple organ failure; SLE, systemic lupus erythematosus; HFH, heterozygote family hypercholesterolemia; PDA, pancreatic ductal adenocarcinoma; AOD, aortic occlusive disease; BC, bladder cancer; IC, intrahepatic cholangiocarcinoma; GA, gastric adenocarcinoma; CCRCC, clear cell renal cell carcinoma; PD, Parkinson’s disease; CC, colon cancer; HGPS, Hutchinson-Gilford progeria syndrome; BA, breast adenocarcinoma; ESCC, Esophageal squamous cell carcinomas; CAD, coronary artery disease; NSCLN, non-small cell lung cancer; OC, Ovarian carcinoma. Abbreviations for gene and enzyme names are explained in Supplementary Table 1. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Ingenuity Pathway Analysis — Significant differentially expressed genes were analyzed using Ingenuity Pathway Analysis (IPA, http://www.ingenuity.com/) as our previous publications [52] to characterize the pathophysiological relationship with seven human disease categories and represented as Venn diagrams generated from http://www.interactivenn.net/(Fig. 2).
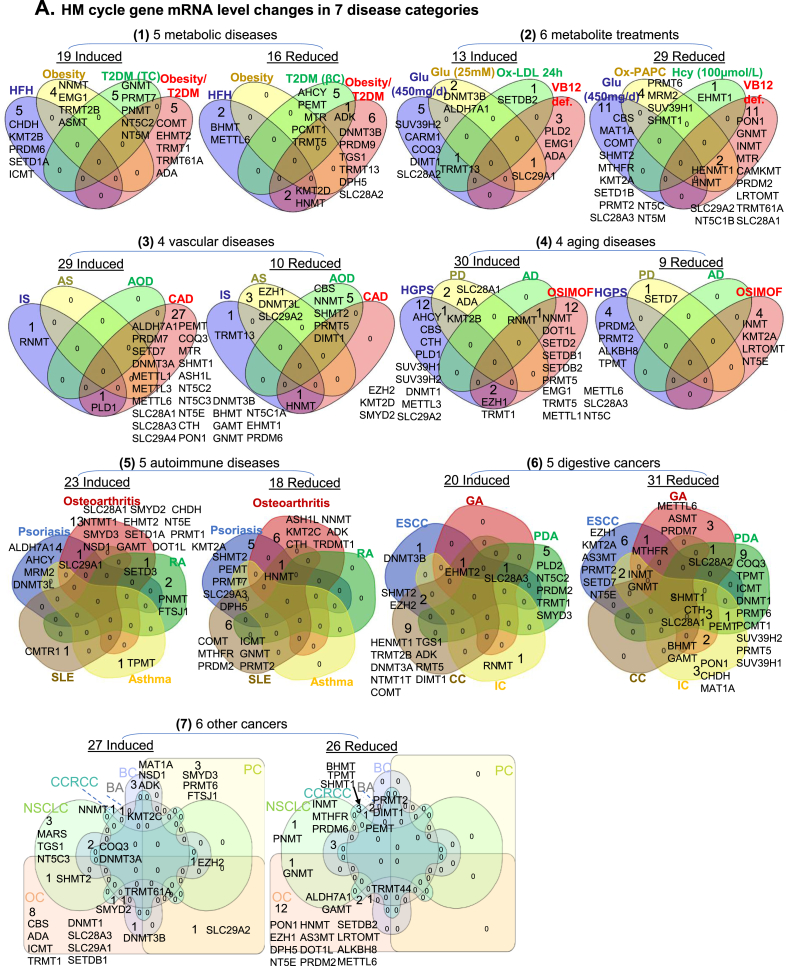
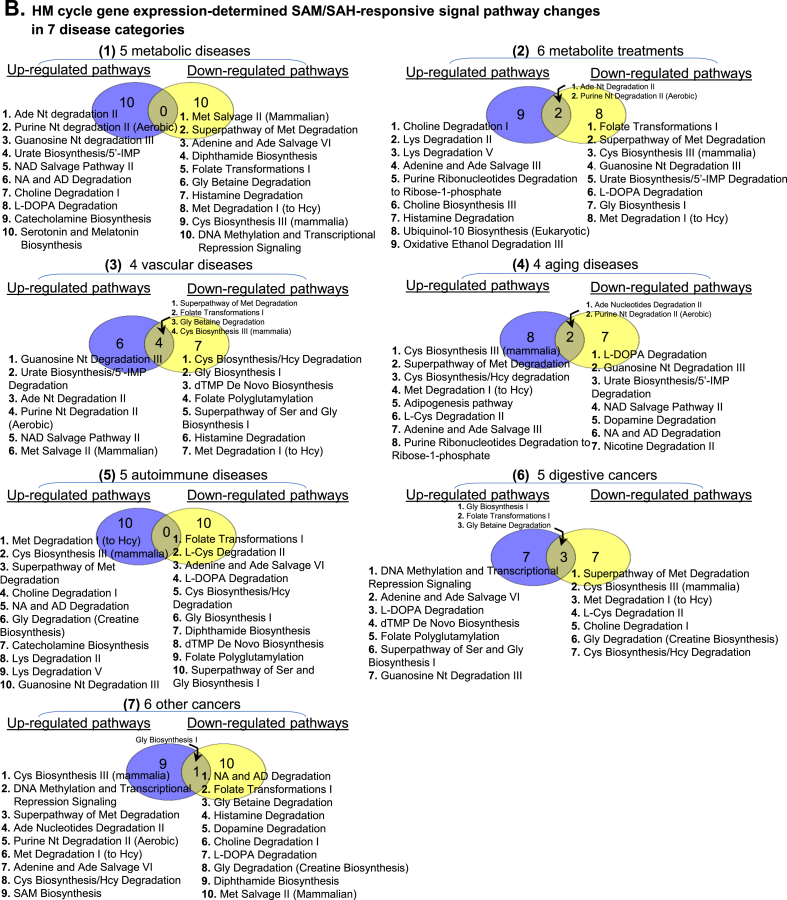
Fig. 2.
Identification of HM cycle gene expression changes and SAM/SAH-responsive signal pathways in 7 disease categories. We examined gene expression of 115 enzymes in extended HM cycle in microarray datasets of 35 disease conditions in NIH-NCBI GEO database (https://www.ncbi.nlm.nih.gov/geo/). These 35 disease conditions were classified in 7 categories. Signal pathways regulated by altered gene expression were analyzed using Ingenuity Pathway Analysis (IPA, http://www.ingenuity.com/), and termed as SAM/SAH-responsive signal pathways. Significantly up- or down-regulated genes and pathways are presented in Venn diagrams and listed. A. HM cycle gene mRNA level changes: 7 disease categories are presented as (1) 5 metabolic diseases; (2) 6 metabolite treatments; (3) 4 vascular diseases; (4) 4 aging diseases; (5) 5 autoimmune diseases; (6) 5 digestive cancers; (7) 6 other cancers. B. HM cycle gene expression-determined SAM/SAH-responsive signal pathway changes: The same 7 disease categories, as in panel A, are presented. Abbreviations for metabolites: Nt, Nucleotides; 5’-IMP, Inosine 5’-phosphate; NA, Noradrenaline; AD, Adrenaline; Met, Methionine; Ade, Adenosine; Gly, Glycine; Cys, Cysteine; Hcy, Homocysteine; Lys, Lysine; Ser, Serine; SAM, S-adenosyl-L-methionine. Abbreviations for diseases: HFH, Heterozygote family hypercholesterolemia; T2DM, Type 2 diabetes mellitus; TC, T cell; βC, β cell; def., deficiency; Glu, glucose; Ox-LDL, Oxidized low-density lipoprotein; Ox-PAPC, Oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine; VB12, Vitamin B 12; IS, Ischemic stroke; AS, Atherosclerosis; AOD, Aortic occlusive disease; CAD, Coronary artery disease; HGPS, Hutchinson-Gilford progeria syndrome; PD, Parkinson’s disease; AD, Alzheimer’s disease; OSIMOF, Old sepsis induced multiple organ failure; RA, Rheumatoid arthritis; SLE, Systemic lupus erythematosus; CC, Colon Cancer; ESCC, Esophageal Squamous Cell Carcinomas; GA, Gastric Adenocarcinoma; IC, Intrahepatic Cholangiocarcinoma; PDA, Pancreatic Ductal Adenocarcinom; OC, Ovarian carcinoma; PC, Prostate cancer; BA, Breast adenocarcinoma, NSCLC, Non-small cell lung cancer; BC, Bladder cancer; CCRCC, Clear cell renal cell carcinoma. Abbreviations for gene and enzyme names are explained in Supplementary Table 1.
3. Results
HM cycle senses metabolic risk factors and modulates SAM/SAH-dependent methylation and pathogenic signaling — We elucidated 8 metabolic pathways in the extended HM cycle which are facilitated by 15 Hcy metabolic enzymes and 84 MT genes (4 DNA MT, 24 RNA MT, 30 histone lysine MT, 6 histone arginine MT, 9 non-histone protein MT and 11 ungrouped small molecular MT), and 16 adenosine metabolism/transporter genes (Fig. 1A, Supplementary Table 1).
The top 3 pathways are the primary pathways. Firstly, the demethylation pathway generates universal methyl donor SAM and methylation inhibitor SAH. Secondly, the remethylation pathway converts Hcy back to Met by receiving a methyl group from folate cycle or choline/betaine metabolism. Thirdly, in the transsulfuration pathway, Hcy is converted to another sulfur-containing amino acid cysteine (Cys) through cystathionine. Pathway 4 to 8 are secondary pathways. Pathway 4 describes all SAM/SAH-dependent methylation reactions catalyzed by 84 MT. In pathway 5, folate cycle facilitates Met production. In pathway 6, choline and betaine also contribute methyl group to Met synthesis. Pathway 7 is the sole source of Hcy synthesis. Hcy can then be reversibly metabolized to homocysteine thiolactone (HT) or cleared by remethylation or transsulfuration pathways. Pathway 8 describes adenosine transport and metabolism. Adenosine is transported into the cell by a family of adenosine transporter proteins, equilibration nucleoside transporter, and produced during Hcy synthesis. Impairment of remethylation and transsufuration pathways, alteration in metabolic enzymes (Met synthase, methylenetetrahydrofolate reductase (MTHFR)), cystathionine-β-synthase (CBS), and cystathionine-γ-lyase (CγL or CTH) or deficiency in cofactors (vitamin B6, B12, folate) are considered as the causes of HHcy, an established independent risk factor for CVD and other degenerative diseases [1,2,55].
Cytosol-nucleus SAM, SAH and Hcy transfer model are proposed based on HM cycle enzymes localization — We described the subcellular localization of the extended 115 HM cycle enzymes in Supplementary Fig. 1A, and have 5 major findings; 1) All essential HM cycle metabolic enzymes are localized only in the cytosol except for Hcy clearance enzyme CBS, which is also in the nucleus (Fig. 1B), suggesting that HM cycle is completely operative only in the cytosol and that Hcy clearance is critical in the nucleus (Fig. 1C). 2) Less (25%, 21/84) SAM/SAH-dependent MTs are located in the cytosol. Most of the non-histone protein MTs (5/9, 55.6%) and ungrouped small molecular MTs (7/11, 63.6%) are located in the cytosol. Some RNA MTs (5/24, 20.8%), histone lysine MTs (2/30, 6.7%) and histone arginine MTs (2/6, 33.3%) are located in the cytosol (Supplementary Fig. 1B). 3) Most (73%, 61/84) SAM/SAH-dependent MTs are located in the nucleus. All DNA MTs (4/4, 100%) and histone arginine MTs (6/6, 100%), and most histone lysine MTs (26/30, 86.7%) and RNA MTs (18/24, 75%) are located in the nucleus. Some non-histone protein MTs (5/9 55.6%) and ungrouped small molecular MTs (2/11, 18.2%) are also located in the nucleus (Supplementary Fig. 1C). 4) SAM, SAH, and Hcy can be transferred between nucleus and cytosol. Most enzymes for SAM synthesis, SAH clearance, and Hcy synthesis are missing in the nucleus (Fig. 1B). 5) CBS facilitates Hcy clearance in the nucleus since CBS is the only Hcy clearance enzyme in the nucleus (Fig. 1C).
Eleven HM cycle metabolic enzymes interact with 955 proteins and five paired with each other — We identified physical interactions between 11 HM cycle metabolic enzymes with 955 proteins and discovered 5 pair protein interactions in the HM cycle (Supplementary Fig. 1D), including CBS:CTH, SHMT1:SHMT2, TRMT61A:MARS, SHMT2:CAMKMT and SHMT2:PRMT1 interactions. This discovery led us to suspect that HM cycle enzymes may form complexes which may determine their functional relationship.
Identification of HM cycle gene expression changes and SAM/SAH-responsive signal pathways in seven disease categories — 35 disease conditions were classified into 7 disease categories. The identified significantly changed genes in these categories and their related SAM/SAH-responsive signal pathways are presented in Fig. 2. Among these 7 disease categories, in (1) metabolic disease, 19 genes were induced and 16 were reduced, which defined 10 up-regulated and 10 down-regulated signal pathways, respectively. In (2) metabolite treatment, 13 genes were induced and 29 were reduced, defining 11 up-regulated and 10 down-regulated signal pathways, respectively, with 2 pathways overlapped. In (3) vascular disease, 29 induced and 10 reduced genes defined 10 up-regulated and 11 down-regulated signal pathways, respectively, with 4 pathways overlapped. In (4) aging diseases, 30 induced and 9 reduced genes defined 10 up-regulated and 9 down-regulated signal pathways, respectively, with 2 pathways overlapped. In (5) autoimmune diseases, 23 induced and 18 reduced genes defined 10 up-regulated and 10 down-regulated signal pathways, respectively. In (6) digestive cancers, 20 induced and 31 reduced genes defined 10 up-regulated and 10 down-regulated signal pathways, respectively, with 3 pathways overlapped. In (7) other cancers, 27 induced and 26 reduced genes defined 10 up-regulated and 11 down-regulated signal pathways, respectively, with 1 pathway overlapped.
Thirty-three HM cycle-regulated and SAM/SAH-dependent H3/H4 histone methylations are changed in 29 human diseases, and 20 diseases are classified into 9 types — We described induced HMT as hypermethylation of their targeted site and reduced HMT as hypomethylation of its corresponding sites (Fig. 3A/B, Supplementary Table 2A). We found that 33 of 36 HMT genes with altered expression in 29 of 35 disease conditions (Fig. 3C, Supplementary Table 3), and summarized 3 major findings. 1) H3 is the major methylation target. Among the identified histone hypermethylation sites, 84.5% occurs on H3 and 15.5% on H4. Whereas, 79.5% of identified histone hypomethylation occurs on H3 and 20.5% on H4. 2) Seven histone residues are frequently changed in methylation status in disease condition (>10% of reported changes). H3K4 (15.5% and 20.5%), H3K9 (17.2% and 13.6%) and H4R3 (10.3% and 13.6%) have frequently reported hypermethylation and hypomethylation changes. H3K27 (13.8%) and H3K36 (15.5%) have only frequently reported hypermethylation. H3R2 (13.6%) and H3R8 (18.2%) have only frequently reported hypomethylation. 3) 8 disease conditions only encountered histone hypomethylation (HHcy, vitamin B12 deficiency, SLE, psoriasis, atherosclerosis, AOD, T2DM, and Ox-PAPC treatment), which occurred on 8 histone residues (H3R2, H3K4, H3R8, H3K9, H3K27, H3K36, H3K79, H4R3). Whereas, 9 disease conditions only encountered histone hypermethylation (osteoarthritis, HFH, CAD, PC, CCRCC, Ox-LDL treatment, CC and RA), which occurred on 8 histone residues (H3R2/K4/K9/K27/K36/K79 and H4R3/K20). We emphasize HHcy (#19 disease)-related hypomethylation on H3R2, H3K9 and H4R3, atherosclerosis (#25 disease)-related hypomethylation on H3K27, and T2DM β cells (#29 disease)-related hypomethylation on H3R2, H3R8 and H4R3. We presented the functional implication of 36 SAM/SAH-dependent HMT and 48 non-HMT in pathophysiological conditions in Supplementary 2A/2B.
We classified 20 diseases into 9 types based on gene expression patterns in 4 metabolic pathways of HM cycle, including Hcy synthesis, folate cycle, transsulfuration, and Choline/Betaine metabolism (Fig. 3D). Type I–V diseases are mostly with reduced HM cycle gene expression. Type I disease (T2DM-βC, OSIMOF, SLE) is featured by a reduced folate cycle pathway gene expression. Type II disease (HFH) only had reduced choline/betaine pathway gene expression. Type III disease (osteoarthritis) only had reduced transsulfuration pathway gene expression. Type IV disease (PDA, AOD, BC) had reduced gene expression in 3 pathways (Hcy synthesis, folate cycle, and transsulfuration). Type V disease (IC, GA, CCRCC) had reduced gene expression in all 4 HM cycle pathways. Type VI-VIII diseases are mostly with induced HM cycle gene expression. Type VI disease (PD, CC) had induced gene expression in Hcy synthesis and folate cycle pathway. Type VII disease (HGPS, BA, ESC) had induced gene expression in 3 pathways (Hcy synthesis, folate cycle and transsulfuration). Type VIII disease (CAD) had induced folate cycle, transsulfuration, and choline/betaine pathway gene expression. Type IX disease (psoriasis, NSCLN, OC) had mixed HM cycle gene expression.
Working model, novel hypotheses and conclusions — We described our working model in Fig. 4A and summarized our major discoveries and novel hypotheses in Fig. 4D/E. Fig. 4B presented the classical model of receptor-dependent pathogenic signaling, which emphasizes ligand-receptor specific molecular recognition. Pathogenic risk factor ligands PAMPs and DAMPs bind to PRR through specific molecular recognition to trigger downstream pathogenic signaling response [56].
Fig. 4.
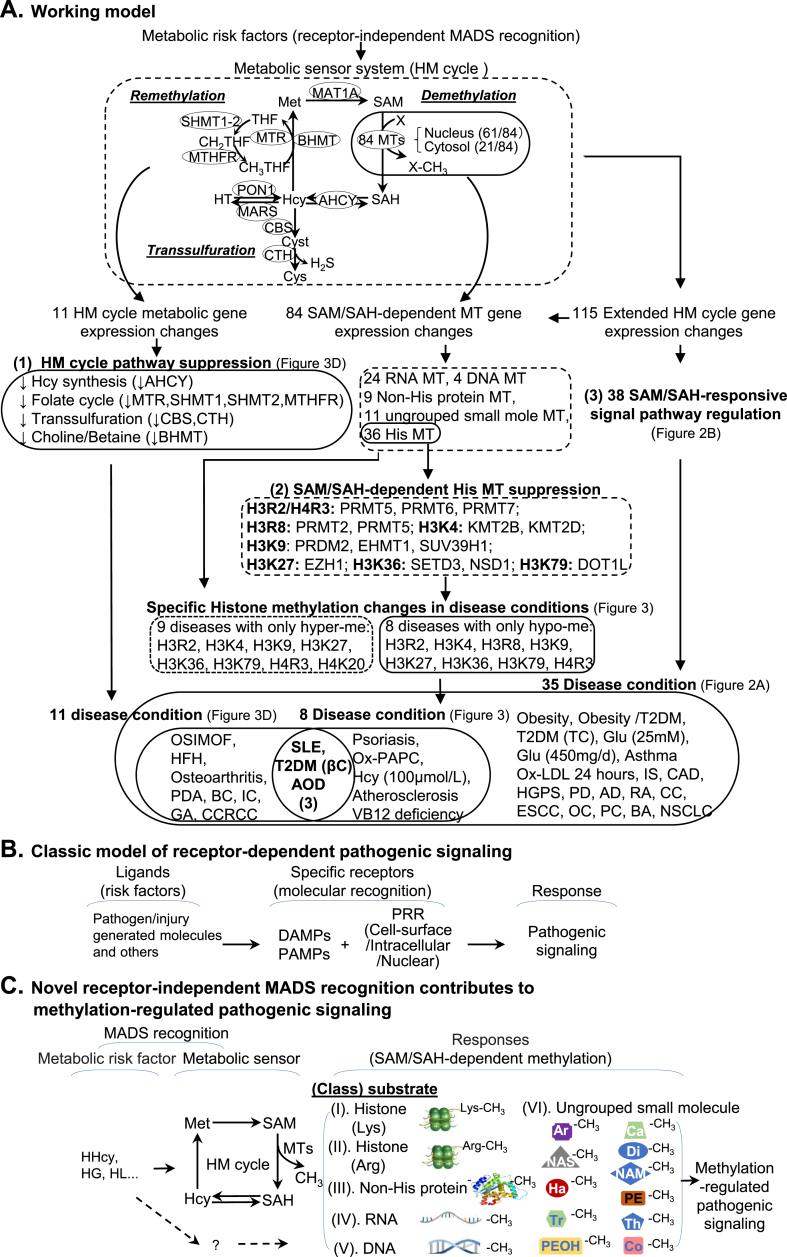
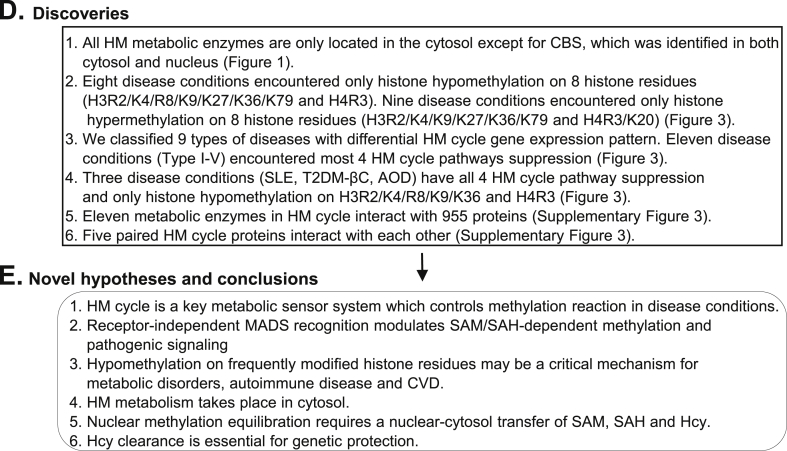
Working model, novel hypotheses, discoveries and conclusions. A. Working model. We identified HM cycle as a metabolic sensor system. Genes in extended HM cycle can be regulated via receptor-independent MADS recognition which leads to three responses: (1) HM cycle pathway suppression, (2) SAM/SAH-dependent histone MTs suppression, and (3) 38 SAM/SAH-responsive signal pathway regulation, which are associated with 11, 8 and 35 disease conditions, respectively. Three human disease conditions, SLE, T2DM (βC) and AOD, overlapped in all three responses and marked by histone hypomethylation at H3R2, H3K4, H3R8, H3K9, H3K36, or H4R3 site. B. Classic model of receptor-dependent pathogenic signaling. This classical model is featured by ligand-receptor specific molecular recognition. C. Novel model of receptor-independent MADS-mediated methylation-regulated pathogenic signaling. This model describes receptor-independent MADS recognition, and emphasizes metabolic risk factor/sensor-mediated pathogenic signaling and HM cycle-regulated SAM/SAH-dependent methylation. D. Discoveries. We listed 6 major discoveries. E. Novel hypotheses and conclusions. Summary of 6 novel hypotheses generated from this study. Abbreviations: HM, homocysteine-methionine; MADS, metabolism-associated danger signal; MT, methyltransferase; CBS, Cystathionine-β-synthase; Arg, Arginine; Lys, Lysine; SLE, Systemic lupus erythematosus; T2DM-βC, Type 2 diabetes mellitus β cell; AOD, Aortic occlusive disease; CVD, Cardiovascular disease; NCBI, National Center of Biotechnology Information; GEO, Gene Expression Omnibus; Hcy, homocysteine; SAH, S-adenosylhomocysteine; SAM, S-adenosylmethionine; H3/H4, Histone 3/Histone 4; his, histone; His-Lys, Histone Lysine; His-Arg, Histone Arginine; hyper-me, hypermethylation; hypo-me, hypomethylation; Glu, glucose. DAMP, damage-associated molecular pattern; PAMP, pathogen-associated molecular pattern; PRR, pathogen recognition receptor; HHcy, hyperhomocysteinemia; HG, hyperglycemia, HL, hyperlipidemia; Ar, arsenite; Ca, catechol; Di, diphthamide; Ha, histamine; NAM, nicotinamide; NAS, N-acetylserotonin; PE, phosphatidylethanolamine; PEOH, phenylethanolamine; Tr, tryptamine; Th, thiopurine; Co, coenzyme. Abbreviations for disease are explained in Fig. 2 legend.
Based on our recent discoveries, we proposed a novel model of “receptor-independent MADS-mediated methylation regulated pathogenic signaling” (Fig. 4C). This model emphasizes metabolic risk factor/sensor-mediated MADS recognition which primarily leads to methylation-dependent pathogenic signaling. We propose that HM cycle is a metabolic sensor system, which determines SAM/SAH-dependent methylation status. SAM/SAH-dependent methylation modify 6 classes of molecules including histone lysine and arginine residues, non-histone proteins, RNA, DNA and ungrouped small molecules (arsenite, catechol, diphthamide, histamine, nicotinamide, N-acetylserotonin, phosphatidylethanolamine, phenylethanolamine, tryptamine, thiopurine and coenzyme).
4. Discussion
The purpose of the current study is to evaluate extended HM cycle gene expression in disease conditions, which is a different strategy compared with our previous database mining studies. In our previous studies, we examined tissue expression profile of 12 core HM cycle genes and 97 genes in the mitochondrial electron transport chain (ETC) complexes in 20 normal human and 19 normal mouse tissues [54,57]. We established the correlation of gene expression with HM cycle metabolites, including Hcy, SAM, SAH and SAM/SAH ratio in mice [54,57] and examined differential gene expression among different cell types and tissues [58].
Our working model in Fig. 4A described the flow of our strategy and discoveries. We summarized 6 major findings in Fig. 4D and emphasized 3 key findings. 1) Eleven disease conditions (Type I–V in Fig. 3D: T2DM-βC, OSIMOF, SLE, HFH, Osteoarthritis, PDA, AOD, BC, IC, GA, CCRCC) encountered all 4 HM cycle pathways suppression. 2) Eight disease conditions (HHcy, vitamin B12 deficiency, SLE, psoriasis, atherosclerosis, AOD, T2DM-βC, and Ox-PAPC treatment) presented only histone hypomethylation on 8 residues (H3R2/K4/R8/K9/K27/K36/K79 and H4R3). 3) Three disease conditions (SLE, T2DM-βC, AOD) encountered all HM cycle pathway suppression and only histone hypomethylation on H3R2/K4/R8/K9/K36 and H4R3. The lack of significant HM cycle metabolic gene expression changes in other 7 disease categories suggested that HM cycle metabolic regulation may not be involved in these disease conditions.
Our discoveries lead us to hypothesize that (1) HM cycle suppression is a general biochemical feature for metabolic disorders, autoimmune disease and CVD. HM cycle is a key metabolic sensor system which controls methylation reaction in disease conditions. (2) We propose a novel receptor-independent MADS recognition which mediates SAM/SAH–dependent methylation changes in these diseases. This hypothesis is supported by the evidence of HM cycle pathway suppression, annotated by the reduced expression of most of the HM cycle metabolic enzymes, and their association with the only hypomethylation on some critical histone residues. (3) Frequently occurred hypomethylation on H3R2/K4/R8/K9/K36 and H4R3 in metabolic disorders, autoimmune disease and CVD may be a critical mechanism for these diseases. (4) HM metabolism takes place in cytosol. (5) Nuclear methylation equilibration requires a nuclear-cytosol transfer of SAM, SAH and Hcy. (6) Hcy clearance is essential for genetic protection.
We found that H3K4 and H3K9 methylation changes are most frequently observed in disease conditions (Fig. 3). It is known that H3K4 can be mono-, di- or tri-methylated (H3K4me1, H3K4me2 or H3K4me3) and all implicated in transcriptional activation [59]. H3K4 methylation is associated with the promoters of actively transcribed genes and impact on transcriptional elongation [60]. The frequently observed H3K4 hypomethylation in metabolic disorders, autoimmune disease and CVD, maybe responsible for reduced expression of genes playing protective roles in pathological processes. H3K9 can also be mono-, di- or tri-methylated (H3K9me1, H3K9me2 or H3K9me3) and mostly correlated with gene suppression [61]. We discovered the connection between HM cycle suppression and H3K9 hypomethylation in metabolic disorders, autoimmune disease, and CVD. This finding suggests that H3K9 regulatory genes may drive pathological phenotype in these diseases and that H3K9 methyltransferase inhibitor maybe a potential therapy for these diseases.
Histone-arginine methylation is not well understood. Interestingly, we observed a frequent hypomethylation phenotype on H3R2/R8 and H4R3 in metabolic disorders, autoimmune disease and CVD. It was reported that H3R2me2a (asymmetric) counteracts the H3K4me activation marker, making it a repressive marker for gene silencing [62,63]. Whereas, H4R3me2a (asymmetric) was mainly associated with transcriptional activation [64], but H4R3me2s (symmetric) repressed promoter regions [64,65]. H3R8 methylation was linked to transcriptional repression of tumor suppressor genes [66,67]. Our finding of frequent H3R2/R8 and H4R3 hypomethylation in metabolic disorders, autoimmune disease and CVD should be further investigated to determine their functional relevance.
Taken together, we proposed that SAH accumulation is the metabolic sensor responsible for SAM/SAH-dependent methylation and that HM cycle is a key metabolic sensor system controlling methylation-regulated pathological signaling. We believe that HM cycle mediates the receptor-independent MADS recognition and modulates SAM/SAH-dependent methylation. We were the first to establish that HHcy-resulted SAH accumulation reduces cellular methylation potential leading to selected DNA and protein hypomethylation, which contributes to impaired vascular repair and inflammatory MC differentiation in metabolic disorders [7,8,25,26,36].
In addition, we discovered that all HM metabolic enzymes are only located in the cytosol with the exception of CBS, which was identified in both cytosol and nucleus. These findings indicate that HM metabolism takes place in the cytosol and that nuclear methylation equilibration requires a nuclear-cytosol transfer of SAM, SAH, and Hcy. The nuclear localization of CBS indicates that Hcy clearance is essential for genetic protection.
Our study is the first to establish the significant role of HM cycle in SAM/SAH-dependent methylation in methylation-regulated pathological signaling, and to address the importance of Hcy clearance in the nucleus. Further exploring on HM cycle regulation and underlying mechanism should lead to important insights into the understanding of human disease and the discovery of novel therapies.
Disclosures
None.
Acknowledgements
This work was supported in part by the National Institutes of Health (NIH) grants HL82774, HL-110764, HL130233, HL131460, DK104114, DK113775 to HW and HL131460 to HW/EC/XFY.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2019.101322.
Contributor Information
Qinghua Wu, Email: ncwqh@163.com.
Hong Wang, Email: hongw@temple.edu.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Jensen M.K. Novel metabolic biomarkers of cardiovascular disease. Nat. Rev. Endocrinol. 2014;10(11):659–672. doi: 10.1038/nrendo.2014.155. [DOI] [PubMed] [Google Scholar]
- 2.Lee H.O. S-adenosylhomocysteine hydrolase over-expression does not alter S-adenosylmethionine or S-adenosylhomocysteine levels in CBS deficient mice. Mol Genet Metab Rep. 2018;15:15–21. doi: 10.1016/j.ymgmr.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinez E., Martorell J., Riambau V. Review of serum biomarkers in carotid atherosclerosis. J. Vasc. Surg. 2019 doi: 10.1016/j.jvs.2019.04.488. [DOI] [PubMed] [Google Scholar]
- 4.Feinberg A.P., Tycko B. The history of cancer epigenetics. Nat. Rev. Cancer. 2004;4(2):143–153. doi: 10.1038/nrc1279. [DOI] [PubMed] [Google Scholar]
- 5.Zhang D. Hyperhomocysteinemia promotes inflammatory monocyte generation and accelerates atherosclerosis in transgenic cystathionine beta-synthase-deficient mice. Circulation. 2009;120(19):1893–1902. doi: 10.1161/CIRCULATIONAHA.109.866889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang D. Severe hyperhomocysteinemia promotes bone marrow-derived and resident inflammatory monocyte differentiation and atherosclerosis in LDLr/CBS-deficient mice. Circ. Res. 2012;111(1):37–49. doi: 10.1161/CIRCRESAHA.112.269472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fang P. Hyperhomocysteinemia potentiates hyperglycemia-induced inflammatory monocyte differentiation and atherosclerosis. Diabetes. 2014;63(12):4275–4290. doi: 10.2337/db14-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang J. Chronic kidney disease induces inflammatory CD40+ monocyte differentiation via homocysteine elevation and DNA hypomethylation. Circ. Res. 2016;119(11):1226–1241. doi: 10.1161/CIRCRESAHA.116.308750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang P. Ly6C(+) inflammatory monocyte differentiation partially mediates hyperhomocysteinemia-induced vascular dysfunction in type 2 diabetic db/db mice. Arterioscler. Thromb. Vasc. Biol. 2019 doi: 10.1161/ATVBAHA.119.313138. ATVBAHA119313138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Subramaniam D. DNA methyltransferases: a novel target for prevention and therapy. Front.Oncol. 2014;4:80. doi: 10.3389/fonc.2014.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuchiba A. Global methylation levels in peripheral blood leukocyte DNA by LUMA and breast cancer: a case-control study in Japanese women. Br. J. Canc. 2014;110(11):2765–2771. doi: 10.1038/bjc.2014.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Traube F.R., Carell T. The chemistries and consequences of DNA and RNA methylation and demethylation. RNA Biol. 2017;14(9):1099–1107. doi: 10.1080/15476286.2017.1318241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kohler F., Rodriguez-Paredes M. DNA methylation in epidermal differentiation, aging, and cancer. J. Investig. Dermatol. 2019 doi: 10.1016/j.jid.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 14.Cho M. Hypomethylation of the MN/CA9 promoter and upregulated MN/CA9 expression in human renal cell carcinoma. Br. J. Canc. 2001;85(4):563–567. doi: 10.1054/bjoc.2001.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oshimo Y. Promoter methylation of cyclin D2 gene in gastric carcinoma. Int. J. Oncol. 2003;23(6):1663–1670. [PubMed] [Google Scholar]
- 16.Akiyama Y. Cell-type-specific repression of the maspin gene is disrupted frequently by demethylation at the promoter region in gastric intestinal metaplasia and cancer cells. Am. J. Pathol. 2003;163(5):1911–1919. doi: 10.1016/S0002-9440(10)63549-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giacinti C., Giordano A. RB and cell cycle progression. Oncogene. 2006;25(38):5220–5227. doi: 10.1038/sj.onc.1209615. [DOI] [PubMed] [Google Scholar]
- 18.Hesari A. Evaluation of the two polymorphisms rs1801133 in MTHFR and rs10811661 in CDKN2A/B in breast cancer. J. Cell. Biochem. 2019;120(2):2090–2097. doi: 10.1002/jcb.27517. [DOI] [PubMed] [Google Scholar]
- 19.Kaminskas E. Approval summary: azacitidine for treatment of myelodysplastic syndrome subtypes. Clin. Cancer Res. : Off. J. Am.Assoc.Cancer Res. 2005;11(10):3604–3608. doi: 10.1158/1078-0432.CCR-04-2135. [DOI] [PubMed] [Google Scholar]
- 20.Kihslinger J.E., Godley L.A. The use of hypomethylating agents in the treatment of hematologic malignancies. Leuk. Lymphoma. 2007;48(9):1676–1695. doi: 10.1080/10428190701493910. [DOI] [PubMed] [Google Scholar]
- 21.Aavik E., Babu M., Yla-Herttuala S. DNA methylation processes in atheosclerotic plaque. Atherosclerosis. 2019;281:168–179. doi: 10.1016/j.atherosclerosis.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Hiltunen M.O. DNA hypomethylation and methyltransferase expression in atherosclerotic lesions. Vasc. Med. 2002;7(1):5–11. doi: 10.1191/1358863x02vm418oa. [DOI] [PubMed] [Google Scholar]
- 23.Aavik E. Global DNA methylation analysis of human atherosclerotic plaques reveals extensive genomic hypomethylation and reactivation at imprinted locus 14q32 involving induction of a miRNA cluster. Eur. Heart J. 2015;36(16):993–1000. doi: 10.1093/eurheartj/ehu437. [DOI] [PubMed] [Google Scholar]
- 24.Deng Q. Genomic 5-mC contents in peripheral blood leukocytes were independent protective factors for coronary artery disease with a specific profile in different leukocyte subtypes. Clin. Epigenet. 2018;10:9. doi: 10.1186/s13148-018-0443-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee M.E., Wang H. Homocysteine and hypomethylation. A novel link to vascular disease. Trends Cardiovasc. Med. 1999;9(1–2):49–54. doi: 10.1016/s1050-1738(99)00002-x. [DOI] [PubMed] [Google Scholar]
- 26.Jamaluddin M.D. Homocysteine inhibits endothelial cell growth via DNA hypomethylation of the cyclin Agene. Blood. 2007;110(10):3648–3655. doi: 10.1182/blood-2007-06-096701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang S. m6A demethylase ALKBH5 maintains tumorigenicity of glioblastoma stem-like cells by sustaining FOXM1 expression and cell proliferation program. Cancer Cell. 2017;31(4):591–606.e6. doi: 10.1016/j.ccell.2017.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J. m6A mRNA methylation regulates AKT activity to promote the proliferation and tumorigenicity of endometrial cancer. Nat. Cell Biol. 2018;20(9):1074–1083. doi: 10.1038/s41556-018-0174-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wierda R.J. Epigenetics in atherosclerosis and inflammation. J. Cell Mol. Med. 2010;14(6A):1225–1240. doi: 10.1111/j.1582-4934.2010.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yi F., Li P.-L. Mechanisms of homocysteine-induced glomerular injury and sclerosis. Am. J. Nephrol. 2008;28(2):254–264. doi: 10.1159/000110876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hyun K. Writing, erasing and reading histone lysine methylations. Exp. Mol. Med. 2017;49(4):e324. doi: 10.1038/emm.2017.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smolle M., Workman J.L. Transcription-associated histone modifications and cryptic transcription. Biochim. Biophys. Acta. 2013;1829(1):84–97. doi: 10.1016/j.bbagrm.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shao Y. Metabolic diseases downregulate the majority of histone modification enzymes, making a few upregulated enzymes novel therapeutic Targets--"Sand out and gold stays. J. Cardiovasc. Transl. Res. 2016;9(1):49–66. doi: 10.1007/s12265-015-9664-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clarke S., Tamanoi F. Fighting cancer by disrupting C-terminal methylation of signaling proteins. J. Clin. Investig. 2004;113(4):513–515. doi: 10.1172/JCI21059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hancock J.F., Paterson H., Marshall C.J. A polybasic domain or palmitoylation is required in addition to the CAAX motif to localize p21ras to the plasma membrane. Cell. 1990;63(1):133–139. doi: 10.1016/0092-8674(90)90294-o. [DOI] [PubMed] [Google Scholar]
- 36.Wang H. Inhibition of growth and p21ras methylation in vascular endothelial cells by homocysteine but not cysteine. J. Biol. Chem. 1997;272(40):25380–25385. doi: 10.1074/jbc.272.40.25380. [DOI] [PubMed] [Google Scholar]
- 37.Xing Y. Structural mechanism of demethylation and inactivation of protein phosphatase 2A. Cell. 2008;133(1):154–163. doi: 10.1016/j.cell.2008.02.041. [DOI] [PubMed] [Google Scholar]
- 38.Yu X.X. Methylation of the protein phosphatase 2A catalytic subunit is essential for association of Balpha regulatory subunit but not SG2NA, striatin, or polyomavirus middle tumor antigen. Mol. Biol. Cell. 2001;12(1):185–199. doi: 10.1091/mbc.12.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DeGrande S.T. Molecular mechanisms underlying cardiac protein phosphatase 2A regulation in heart. J. Biol. Chem. 2013;288(2):1032–1046. doi: 10.1074/jbc.M112.426957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raposo A.E., Piller S.C. Protein arginine methylation: an emerging regulator of the cell cycle. Cell Div. 2018;13:3. doi: 10.1186/s13008-018-0036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boisvert F.-M. Methylation of MRE11 regulates its nuclear compartmentalization. Cell cycle (Georgetown, Tex ) 2005;4(7):981–989. doi: 10.4161/cc.4.7.1830. [DOI] [PubMed] [Google Scholar]
- 42.Roberts D.M. Trimethyllysine and protein function. Effect of methylation and mutagenesis of lysine 115 of calmodulin on NAD kinase activation. J. Biol. Chem. 1986;261(4):1491–1494. [PubMed] [Google Scholar]
- 43.Wang Y. N6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nat. Cell Biol. 2014;16(2):191–198. doi: 10.1038/ncb2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roundtree I.A. Dynamic RNA modifications in gene expression regulation. Cell. 2017;169(7):1187–1200. doi: 10.1016/j.cell.2017.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cui Q. m6A RNA methylation regulates the self-renewal and tumorigenesis of glioblastoma stem cells. Cell Rep. 2017;18(11):2622–2634. doi: 10.1016/j.celrep.2017.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luo Y. NSun2 deficiency protects endothelium from inflammation via mRNA methylation of ICAM-1. Circ. Res. 2016;118(6):944–956. doi: 10.1161/CIRCRESAHA.115.307674. [DOI] [PubMed] [Google Scholar]
- 47.Biochemistry of Lipids, Lipoproteins and Membranes. sixth ed. Elsevier. xxiii; Amsterdam ; Boston: 2016. p. 599. ed. [Google Scholar]
- 48.Dawaliby R. Phosphatidylethanolamine is a key regulator of membrane fluidity in eukaryotic cells. J. Biol. Chem. 2016;291(7):3658–3667. doi: 10.1074/jbc.M115.706523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nishimaki-Mogami T., Suzuki K., Takahashi A. The role of phosphatidylethanolamine methylation in the secretion of very low density lipoproteins by cultured rat hepatocytes: rapid inhibition of phosphatidylethanolamine methylation by bezafibrate increases the density of apolipoprotein B48-containing lipoproteins. Biochim. Biophys. Acta. 1996;1304(1):21–31. doi: 10.1016/s0005-2760(96)00100-2. [DOI] [PubMed] [Google Scholar]
- 50.Dai J. Metabolism-associated danger signal-induced immune response and reverse immune checkpoint-activated CD40(+) monocyte differentiation. J. Hematol. Oncol. 2017;10(1):141. doi: 10.1186/s13045-017-0504-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Venereau E., Ceriotti C., Bianchi M.E. DAMPs from cell death to new life. Front. Immunol. 2015;6:422. doi: 10.3389/fimmu.2015.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang L. Novel extracellular and nuclear caspase-1 and inflammasomes propagate inflammation and regulate gene expression: a comprehensive database mining study. J. Hematol. Oncol. 2016;9(1):122. doi: 10.1186/s13045-016-0351-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu K. GATA3, HDAC6, and BCL6 regulate FOXP3+ treg plasticity and determine treg conversion into either novel antigen-presenting cell-like treg or Th1-treg. Front. Immunol. 2018;9:45. doi: 10.3389/fimmu.2018.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cueto R. Identification of homocysteine-suppressive mitochondrial ETC complex genes and tissue expression profile - novel hypothesis establishment. Redox biology. 2018;17:70–88. doi: 10.1016/j.redox.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bhatia P., Singh N. Homocysteine excess: delineating the possible mechanism of neurotoxicity and depression. Fundam. Clin. Pharmacol. 2015;29(6):522–528. doi: 10.1111/fcp.12145. [DOI] [PubMed] [Google Scholar]
- 56.Huang X. Identification of novel pretranslational regulatory mechanisms for NF-kappaB activation. J. Biol. Chem. 2013;288(22):15628–15640. doi: 10.1074/jbc.M113.460626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen N.C. Regulation of homocysteine metabolism and methylation in human and mouse tissues. FASEB J. : Off. Publ. Fed. Am. Soc. Exp. Biol. 2010;24(8):2804–2817. doi: 10.1096/fj.09-143651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu Y. Endocytosis and membrane receptor internalization: implication of F-BAR protein Carom. Front. Biosci. 2017;22:1439–1457. doi: 10.2741/4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Collins B.E. Histone H3 lysine K4 methylation and its role in learning and memory. Epigenet. Chromatin. 2019;12(1):7. doi: 10.1186/s13072-018-0251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Greer E.L., Shi Y. Histone methylation: a dynamic mark in health, disease and inheritance. Nat. Rev. Genet. 2012;13(5):343–357. doi: 10.1038/nrg3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stewart M.D., Li J., Wong J. Relationship between histone H3 lysine 9 methylation, transcription repression, and heterochromatin protein 1 recruitment. Mol. Cell.Biol. 2005;25(7):2525–2538. doi: 10.1128/MCB.25.7.2525-2538.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Migliori V. Symmetric dimethylation of H3R2 is a newly identified histone mark that supports euchromatin maintenance. Nat. Struct. Mol. Biol. 2012;19(2):136–144. doi: 10.1038/nsmb.2209. [DOI] [PubMed] [Google Scholar]
- 63.Kirmizis A. Arginine methylation at histone H3R2 controls deposition of H3K4 trimethylation. Nature. 2007;449(7164):928–932. doi: 10.1038/nature06160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Di Lorenzo A., Bedford M.T. Histone arginine methylation. FEBS Lett. 2011;585(13):2024–2031. doi: 10.1016/j.febslet.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang L., Pal S., Sif S. Protein arginine methyltransferase 5 suppresses the transcription of the RB family of tumor suppressors in leukemia and lymphoma cells. Mol. Cell.Biol. 2008;28(20):6262–6277. doi: 10.1128/MCB.00923-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pal S. Human SWI/SNF-associated PRMT5 methylates histone H3 arginine 8 and negatively regulates expression of ST7 and NM23 tumor suppressor genes. Mol. Cell.Biol. 2004;24(21):9630–9645. doi: 10.1128/MCB.24.21.9630-9645.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chung J. Protein arginine methyltransferase 5 (PRMT5) inhibition induces lymphoma cell death through reactivation of the retinoblastoma tumor suppressor pathway and polycomb repressor complex 2 (PRC2) silencing. J. Biol. Chem. 2013;288(49):35534–35547. doi: 10.1074/jbc.M113.510669. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.