Abstract
Objectives
High fructose feeding changes fibroblast growth factor 21 (FGF21) regulation. Lactobacillus rhamnosus GG (LGG) supplementation reduces fructose-induced non-alcoholic fatty liver disease (NAFLD). The aim of this study was to determine the role of FGF21 and underlying mechanisms in the protective effects of LGG.
Methods
FGF21 knockout (KO) mice and C57BL/6 wild type (WT) mice were fed 30% fructose for 12 weeks. LGG was administered to the mice in the last 4 weeks during fructose feeding. FGF21-adiponectin (ADPN)-mediated hepatic lipogenesis and inflammation were investigated.
Results
FGF21 expression was robustly increased after 5-weeks of feeding and significantly decreased after 12-weeks of feeding in fructose-induced NAFLD mice. LGG administration reversed the depressed FGF21 expression, increased adipose production of ADPN, and reduced hepatic fat accumulation and inflammation in the WT mice but not in the KO mice. Hepatic nuclear carbohydrate responsive-element binding protein (ChREBP) was increased by fructose and reduced by LGG, resulting in a reduction in the expression of lipogenic genes. The methylated form of protein phosphatase 2A (PP2A) C, which dephosphorylates and activates ChREBP, was upregulated by fructose and normalized by LGG. Leucine carboxyl methyltransferase-1, which methylates PP2AC, was also increased by fructose and decreased by LGG. However, those beneficial effects of LGG were blunted in the KO mice. Hepatic dihydrosphingosine-1-phosphate, which inhibits PP2A, was markedly increased by LGG in the WT mice but attenuated in the KO mice. LGG decreased adipose hypertrophy and increased serum levels of ADPN, which regulates sphingosine metabolism. This beneficial effect was decreased in the KO mice.
Conclusion
LGG administration increases hepatic FGF21 expression and serum ADPN concentration, resulting in a reduced ChREBP activation through dihydrosphingosine-1-phosphate-mediated PP2A deactivation, and subsequently reversed fructose-induced NAFLD. Thus, our data suggest that FGF21 is required for the beneficial effects of LGG in reversal of fructose-induced NAFLD.
Keywords: Fibroblast growth factor 21, Fructose, NAFLD, Probiotics, Lactobacillus rhamnosus GG
Highlights
-
•
Lactobacillus rhamnosus GG (LGG) attenuates fructose-induced NAFLD.
-
•
LGG increases FGF21 and adiponectin expression.
-
•
LGG inhibits fructose-activated ChREBP and reduces hepatic lipogenesis.
-
•
FGF21 is required for the therapeutic effects of LGG against fructose-induced NAFLD.
1. Intruduction
Non-alcoholic fatty liver disease (NAFLD) represents a broad spectrum of liver diseases ranging from steatosis to steatohepatitis, fibrosis, cirrhosis, and even liver cancer [1]. Although a variety of factors contribute to the development of NAFLD, fructose consumption, largely in the form of high-fructose corn syrup, is particularly associated with this metabolic disorder [2], [3], [4]. Fructose consumption has increased dramatically in US [5], [6], [7]. Over-consumption of fructose has been associated with increased visceral adiposity, insulin resistance, and increased hepatic de novo lipogenesis (DNL) [8], [9], [10], [11], [12], [13] that lead to the development of steatosis and NAFLD and progression to cirrhosis/fibrosis [14], while fructose restriction results in attenuation of NAFLD [15]. However, effective therapeutic strategies are still lacking.
Increasing evidence from both preclinical and clinical studies suggests that probiotics are beneficial in the treatment of a variety of gastrointestinal diseases such as inflammatory bowel disease (IBD) [16], NAFLD [17], and alcoholic liver disease (ALD) [18]. Lactobacillus rhamnosus GG (LGG), the best-characterized probiotic strain, has been consistently shown to be beneficial in the metabolic syndrome. Recently, studies showed that LGG supplementation reduced high fructose-induced liver steatosis and inflammation [19]. Inhibition of gut microbiota dysbiosis and bacterial translocation is postulated beneficial to the probiotic effects in NAFLD [20], [21]. However, the precise mechanism by which probiotics prevent or reverse fatty liver development in fructose-associated NAFLD is not well defined.
Previous studies showed that fructose consumption robustly activated hepatic carbohydrate responsive-element binding protein (ChREBP) [22]. ChREBP is a transcription factor that senses carbohydrate and regulates a variety of genes in metabolic pathways, including DNL [23]; a key event that leads to hepatic steatosis in NAFLD. ChREBP knockout mice have reduced DNL rates leading to a decrease in hepatic triglyceride contents [23], [24], suggesting that ChREBP plays a critical role in the regulation of lipogenic program activation in fructose-induced NAFLD. Recent studies indicate that sugar-mediated activation of ChREBP regulates hepatic production of FGF21 [25], [26]. FGF21 is produced predominantly in the liver and, to a lesser extent, in adipose and other tissues. FGF21 binds to FGF receptors and the co-receptor β-Klotho to exert its endocrine effects. FGF21 functions in the adipose tissue to induce adiponectin (ADPN) expression, which, in turn, reduces hepatic fat accumulation.
In the current study, we found that prolonged fructose feeding decreased upregulation of FGF21, Moreover, LGG supplementation could rescue the FGF21 expression as well as increase the blood ADPN concentration. The goal of this study was to determine how LGG supplementation is able to decrease hepatic ChREBP activation and steatosis induced by fructose. Using FGF21 KO mice, we found that FGF21 is required for the beneficial effects of LGG in fructose-induced NALFD through FGF21-adiponectin-ChREBP signaling pathways.
2. Material and methods
2.1. Animals
Six-to 8-week-old FGF21 knockout (KO) and C57BL/6 wild type (WT) female mice were housed in a conventional animal room. All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of University of Louisville. FGF21 KO mice were provided by Dr. Steve Kliewer [27]. The mice were subjected to a 12:12 h light/dark cycle in low-stress conditions with free access to food and water ad libitum. The mice were divided into a control group (water), a fructose group (30% fructose-containing water), and a fructose + LGG group. Five to eight mice were used in each group. Control mice were gavaged daily with an equal volume of vehicle (cultural media) or LGG at a dose of 109 CFU/day. Serum and tissue samples were collected at the end for assays.
2.2. Food consumption
Food and water intake of mice was assessed twice weekly, when cages were changed. At each cage-changing, a known amount of food was placed, and the fresh water bottles were weighed. The weights of the previously used water bottles as well as the weight of the food left were then measured to assess the amount of water and food consumed since the last cage change.
2.3. Blood collection and biochemical assays
Mouse serum was collected at time of euthanasia. Serum triglyceride (TG), low-density lipoprotein (LDL), high-density lipoprotein (HDL), very low-density lipoprotein (VLDL), glucose, alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were analyzed by Piccolo Xpress system (ABAXIS, Union City, CA). Quantitative measurements of FGF21 (R&D, Minneapolis, MN), Adiponectin (EMD Millipore Corporation, Billerica, MA) and insulin (EMD Millipore Corp., Billerica, MA) in mouse serum samples were performed using ELISA kits, respectively.
2.4. Measurement of fat mass by dual-energy X-ray absorptiometry (DXA)
Mouse fat mass was measured by DXA (GE Lunar Co, Madison, WI), and data were analyzed using Lunar PIXImus mouse software. Prior to scanning, mice were anesthetized by an intramuscular injection of 1.5% pentobarbitone at a dose of 40 mg/kg. During measurements, the animals were laid in prone position, and the duration of each scan was 3–5 min. Body fat mass was recorded.
2.5. Oral glucose tolerance test (OGTT)
The day before the mice were sacrificed, the mice underwent an OGTT. OGTT was performed by giving a bonus of glucose solution (2 g/kg) by gavage after overnight fasting. Glycemia was measured through the tail tip blood before and after glucose load at the times indicated.
2.6. Liver triglyceride and cholesterol assay
For the liver triglyceride and cholesterol assay, 70–100 mg of liver tissue was homogenized in 1 ml of 50 mM NaCl. Homogenate (500 μl) was mixed with 4 ml of the extraction reagent (methanol:chloroform = 1:2) and incubated overnight at 4 °C before being centrifuged at 1800g for 20 min at room temperature. The lower chloroform phase was carefully collected and dried using a Speed Vac, and the pellets were used for triglyceride assay using the triglyceride and cholesterol Kit (Thermo Fisher Scientific Inc., Waltham, MA).
2.7. TUNEL assay
Formalin-fixed paraffin liver sections were sectioned at 5 μm. The sections were stained for TUNEL with the ApopTag Peroxidase in situ Apoptosis Detection Kit (Chemicon, Temecula, CA) as described previously [28]. In brief, the slides were deparaffinized and rehydrated, then treated with proteinase K. Slides were treated with 3% hydrogen peroxide to quench endogenous peroxidases, and then incubated with terminal deoxynucleotidyl transferase (TdT) and anti-digoxigenin-peroxidase respectively. Diaminobenzidine (DAB) was then applied. Hematoxylin was used as counterstaining. Under the microscope, apoptotic cells exhibited a brown nuclear stain as the TUNEL positive and were counted manually.
2.8. Liver histology and fat analyses
The liver sections were fixed in formalin and embedded in paraffin. The sliced liver sections were then stained with H&E as described previously [29]. For hepatic fat visualization, frozen liver sections were processed for staining with Oil red O and then studied by light microscopy [30] .
2.9. Cell culture and treatment
HepG2 and H4IIE cells were purchased from ATCC and maintained in 10% FBS (Gibco, Grand Island, NY) in Dulbecco's Modified Eagle's Medium (ATCC, Manassas, VA) containing 100 Unit/ml penicillin and 10 μg/ml streptomycin at 37 °C under a 5% CO2 atmosphere. HepG2 cells were exposed to fructose (5 mM) for 2, 4, 8, 12 and 24 h and FGF21 mRNA level was detected by RT-PCR. H4IIE cells were treated with LGG cultural supernatant (LGGs) at a dose equivalent to 109 CFU/ml for 8 h. Cellular LKB1 and pAMPK proteins were detected by Western blot.
2.10. Nuclear protein extracts and western blot analysis
Nuclear proteins were extracted from livers with Nuclear Extraction Kit (Abcam, Cambridge, MA). Protein concentration was determined using a Pierce BCA Protein Assay kit (Thermo Fisher Scientific, Inc., Waltham, MA). Western blot was performed as described previously [29] to detect PPARα (peroxisome proliferator-activated receptor-α), CPT1 (Carnitine palmitoyltransferase I), SREBP (Sterol-regulatory element binding protein), ADPN receptor 1, ADPN receptor 2, methyl-PP2A-C(Santa Cruz Biotechnologies, Santa Cruz, CA), FAS (fatty acid synthetase), SCD1 (Stearoyl-CoA desaturase-1), PP2A (protein phosphatase 2A) A, PP2A B, PP2A C, pAMPK, LKB1 (Cell Signaling Technologies, Beverly, MA), and ChREBP (Novus Biologicals, Littleton, CO). Blots were scanned using a Bio-Rad Imaging System (Image Lab™ Upgrade for ChemiDoc™ XRS + System #170-8299). All specific bands were quantified with the Automated Digitizing System (Image Lab 4.1). Results are representative of three independent experiments.
2.11. Sphingolipids: ceramide analysis by mass spectrometry
Ceramide species were quantified in the liver as previously described [31]. Briefly, an aliquot of powdered liver tissue (25–35 mg) was suspended in ice-cold saline (500 μl, 1 M NaCl) and spiked with C17:0 ceramide internal standard. Lipids were extracted and ceramide subspecies analyzed by HPLC coupled with on-line electrospray ionization tandem mass spectrometry. Total ceramide level was calculated from the sum of analyzed ceramide species.
2.12. Quantitative real time RT-PCR
The mRNA levels were assessed by real-time RT-PCR. In brief, total RNA was isolated with Trizol according to manufacturer's protocol (Invitrogen, Carlsbad, CA) and reverse-transcribed using GenAmp RNA PCR kit (Applied Biosystems, Foster City, CA). The cDNA was amplified in 96-well reaction plates with a SYBR green PCR Master Mix (Applied Biosystems, Forster, CA) on an ABI 7500 real-time PCR thermocycler. The sequences of forward and reverse primers are listed in Supplementary Table 1. For the animals' tissues, 18s rRNA was used to normalize. β-actin was used for data normalization in HepG2 cell line. Dissociation curve analysis was performed after PCR amplification to confirm the specificity of the primers. Relative mRNA expression was calculated using the ΔΔCt method.
2.13. Statistical analysis
Statistical analyses were performed using the statistical computer package GraphPad Prism version 6 (GraphPad Software Inc., San Diego, CA, USA). Results are expressed as means ± SEM. Statistical comparisons were made using two-way analysis of variance (ANOVA) with Bonferroni post-hoc test or one-way ANOVA with Tukey's post-hoc test or Student's t-test where it was appropriate. Differences were considered to be significant at *P < 0.05, **P < 0.01, ***P < 0.001.
3. Results
3.1. Fructose feeding increases FGF21 expression
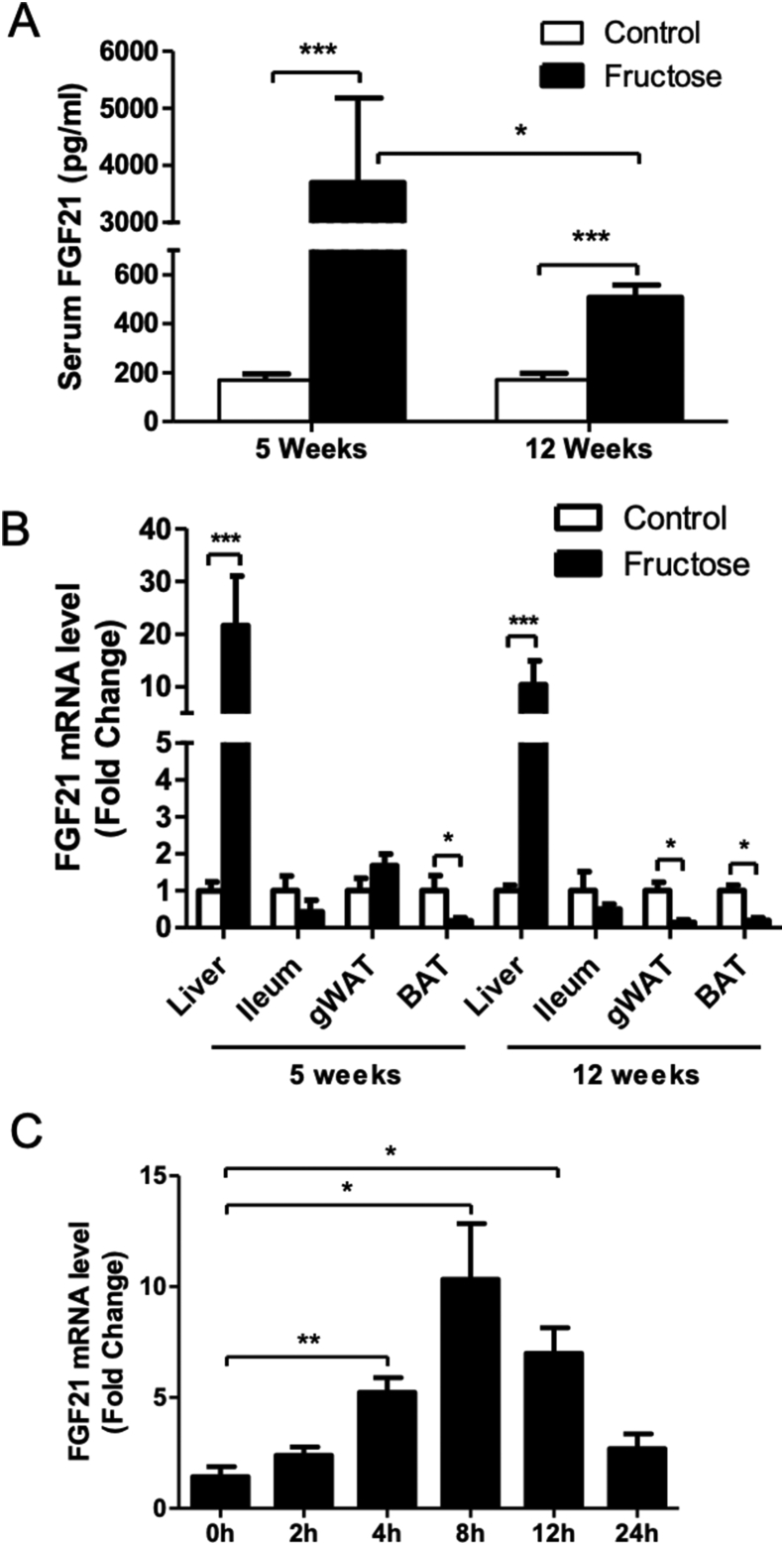
Previous studies demonstrated a rapid elevation of FGF21 following fructose challenge in humans and in animals [25], [32]. To investigate the effect of prolonged fructose feeding on FGF21 expression, mice were fed fructose-containing water (30% w/v) for 5 and 12 weeks. Circulating FGF21 levels were determined. Five weeks of fructose feeding robustly increased plasma FGF21 protein levels about 22-fold compared to control diet feeding. However, this increase was markedly reduced after 12 weeks of fructose feeding (Figure 1A). Fructose feeding significantly increased liver FGF21 mRNA expression at both 5 and 12 weeks, while FGF21 expression was significantly decreased in gonadal white adipose tissue (gWAT) and brown adipose tissue (BAT) at 12 weeks and in BAT at 5 weeks. Ileum expression of FGF21 was changed at both 5 and 12 weeks (Figure 1B). Thus, the liver is likely the major source for the circulating FGF21 in fructose-fed mice. It has to be noted that the small difference in mRNA levels may not explain the large change in serum FGF21 level. This is likely due to the defect in FGF21 protein translation or secretion by prolong fructose treatment. To examine the effect of fructose on FGF21 expression in hepatocytes, HepG2 cells were incubated with fructose. As shown in Figure 1C, 5 mM fructose treatment increased FGF21 mRNA expression in a time dependent manner.
Figure 1.
Fructose feeding increases FGF21 expression. C57BL/6 WT mice were fed 30% fructose containing water (w/v) as described in the Material and Methods. (A) Serum levels of FGF21 in WT mice fed fructose for 5 weeks or 12 weeks. (B) FGF21 mRNA expression in the liver, ileum, gWAT and brown adipose tissue (BAT). (C) mRNA levels of FGF21 in HepG2 cells after 5 mM fructose treatment for 2, 4, 8, 12, and 24 h.
3.2. Metabolic effects of fructose feeding in WT and FGF21 KO mice
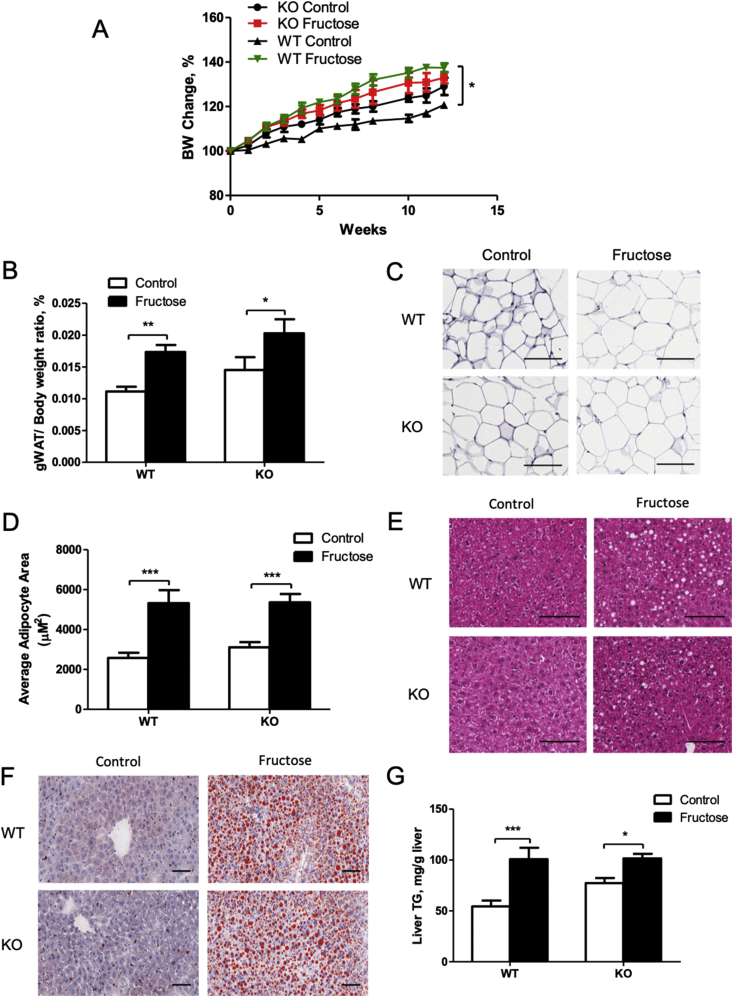
To determine whether FGF21 deficiency exacerbates fructose-induced metabolic disorders, age-matched female WT and FGF21 KO mice were fed water or fructose-containing water for 5 or 12 weeks. Total calorie intake was not changed by fructose feeding (Fig. S1A), but solid normal chow diet consumption was decreased in both types of mice (Fig. S1B and Fig. S1C) due to fructose taking. KO mice on the control diet had an increased body weight gain compared to WT mice. In contrast, fructose feeding resulted in a significant body weight gain in WT mice, which was attenuated in the KO mice (Figure 2A). Twelve-week fructose feeding significantly increase body fat mass in the WT mice. Interestingly, increase of the body fat due to fructose feeding was attenuated in the KO mice (Fig. S1D). Fructose-feeding significantly increased gWAT weight in both fructose-fed WT and KO mice compared to the control mice (Figure 2B), which was confirmed by enlarged adipocyte, as shown in Figure 2C,D. Basal serum insulin level was higher in the KO mice compared to the WT mice and increased (not statistically significant) in both WT and KO mice after fructose feeding (Fig. S1E). Basal serum glucose levels were indistinguishable between WT and KO mice. Fructose feeding significantly increased serum glucose levels in the WT mice, but not in the KO mice (Fig. S1F). Interestingly, glucose tolerance tests suggest a slightly better glucose tolerance in the KO mice compared to the WT mice fed control diet. However, fructose feeding impaired glucose tolerance in both WT and the KO mice with no significant difference (Fig. S1G). Serum triglyceride, LDL, VLDL, and HDL were all marginally increased in fructose-fed WT and KO mice (Fig. S2).
Figure 2.
Effects of fructose on body weight, adipose, hepatic fat accumulation in the WT and FGF21 KO mice. WT and FGF21 KO mice were treated as described in the Material and Methods. (A) Body weight change in response to 12-week fructose feeding. (B) gWAT/body weight ratio. (C) Hematoxylin and eosin staining of gWAT depots (×20). (D) Quantification of average adipocyte size. (E) Hematoxylin and eosin (×20) and (F) Oil red O staining of the liver sections (×10). (G) Liver triglyceride (TG) concentrations. Scale bars: 100 μm.
Liver lipid accumulation is often described as a hallmark of prolonged fructose feeding. Histology analysis of liver sections showed that fructose feeding for 12 weeks increased hepatic fat accumulation (Figure 2E,F). However, we did not observe significant changes between the WT and the KO mice. As shown with H&E and Oil red O staining, hepatic triglyceride level was higher in the fructose-treated mice than in the control mice (Figure 2G). Interestingly, data at 5 weeks also showed increased hepatic fat in fructose-fed mice, with the KO mice having higher hepatic fat compared to WT mice (Fig. S3).
The results presented above indicate that fructose feeding increased serum FGF21 concentration, impaired the insulin sensitivity, and increased adipocytes and hepatic fat accumulation.
3.3. LGG supplementation reverses fructose-induced NAFLD in WT mice but not in FGF21 KO mice
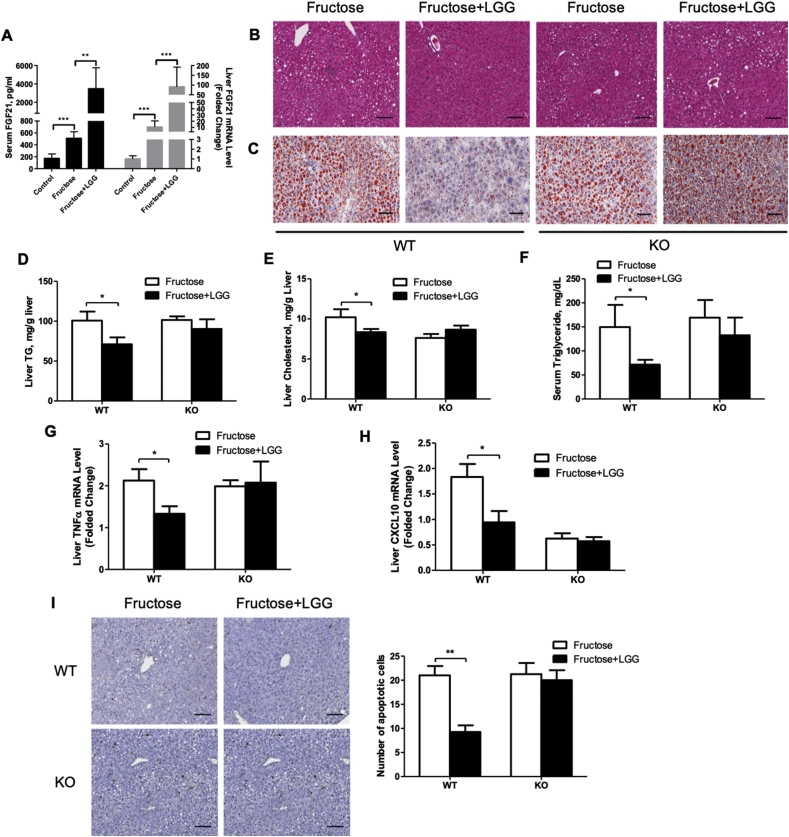
Our previous study demonstrated that LGG supplementation reduced alcohol-induced hepatic fat accumulation [33]. To further evaluate the effects of probiotic LGG on fructose-induced liver injury and to analyze the role of FGF21, we supplemented LGG in last 4 weeks of a 12-week fructose-feeding period in both WT and the KO mice. Interestingly, LGG supplementation markedly elevated circulating FGF21 levels and liver FGF21 mRNA expression in the WT mice (Figure 3A) to a comparable level of the 5-week fructose feeding (Figure 1A). Histological analysis of liver sections showed significantly increased numbers of fat droplets in fructose-fed WT mice, and this effect was reversed by LGG supplementation (Figure 3B,C). Consistent with this result, hepatic triglyceride and cholesterol levels were significantly decreased by LGG supplementation in WT mice (Figure 3D,E). However, this hepatic lipid lowering effect of LGG was eliminated in the FGF21 KO mice (Figure 3B–E). Whole body fat mass was slightly decreased by LGG in WT mice (Fig. S4A). Although serum VLDL level was decreased slightly in WT mice, levels of LDL and HDL were not affected by LGG supplementation in both WT and KO mice (Figs. S4B–D), while serum levels of triglycerides and glucose were significantly improved in the WT mice but not in the KO mice (Figures. 3F and S4E).
Figure 3.
LGG supplementation reverses fructose-induced NAFLD in WT mice but not in FGF21 KO mice. WT and FGF21 KO mice were treated as described in the Material and Methods. (A) Serum FGF21 concentrations (left y axis) and FGF21 mRNA expression in the liver (right y axis). (B) Hematoxylin and eosin staining and (C) Oil red O staining of the liver sections. Original magnification, ×10. Liver TG (D) and cholesterol (E) concentrations. (F) Serum TG levels. Relative liver mRNA levels of TNFα (G), and CXCL10 (H). (I) Representative images of terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining of liver sections (×10, left panel). The number of apoptotic cells was determined by counting the cells in at least 10 randomly selected high-power fields (right panel). Scale bars: 100 μm.
3.4. Effect of LGG on liver inflammation and injury
Inflammation is often associated with NAFLD. Although both serum ALT and AST levels were not significantly different (Fig. S5), LGG treatment significantly reduced liver mRNA levels of pro-inflammatory cytokines TNFα and CXCL10 in the fructose-fed WT mice but not in the KO mice (Figure 3G,H). Compared to the WT mice, KO mice had comparable mRNA expressions of basal TNFα but reduced mRNA levels of basal CXCL10 (Figure 3G,H). The reason for this reduction is currently unknown. However, it is clear that the KO mice were resistant to the treatment by LGG. TUNEL assay revealed that LGG supplementation significantly reduced the number of apoptotic cells in the livers of fructose-fed WT mice. Again, this effect was not seen in the KO mice (Figure 3I).
3.5. Effects of LGG on liver lipogenesis and fatty acid β-oxidation
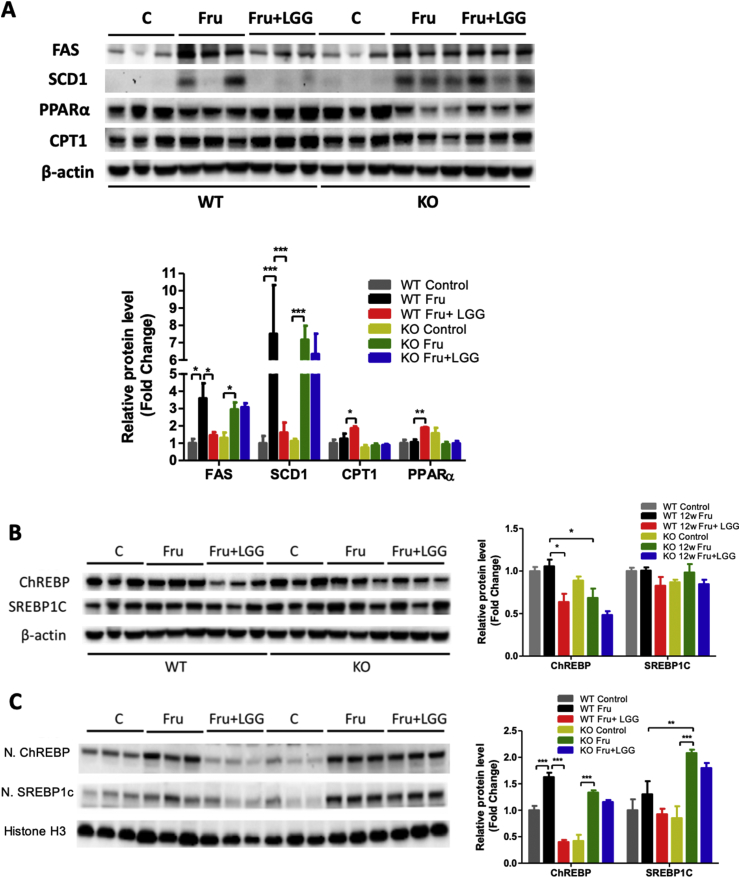
In both WT and KO mice, fructose consumption markedly increased hepatic protein levels of FAS and SCD1, critical enzymes in DNL. LGG treatment significantly inhibited this increment in the WT mice but not in the KO mice (Figure 4A). Fructose consumption did not alter the protein level of CPT1, a critical gene responsible for fatty acid β-oxidation, in either WT or KO mice (Figure 4A). However, LGG treatment significantly increased CPT1 levels only in WT mice. PPARα protein level was not changed by fructose in WT, but decreased in the KO mice. LGG treatment significantly elevated hepatic PPARα only in the WT mice (Figure 4A).
Figure 4.
Effects of LGG on liver lipogenesis and fatty acid β-oxidation. WT and FGF21 KO mice were treated as described in the Material and Methods. (A, B) Liver proteins were analyzed by western blotting. Protein bands intensity was quantified by densitometry analysis. β-actin levels served as loading controls. (C) Liver nuclear proteins were analyzed by western blotting. The quantification of protein bands by densitometry analysis. Histone H3 levels served as loading controls.
We further analyzed two major transcription factors responsible for DNL. Total liver ChREBP and SREBP1c protein levels were unchanged by 12-week fructose feeding in either WT or KO mice. However, KO mice had less hepatic ChREBP compared to WT mice fed fructose. LGG supplementation significantly decreased total hepatic ChREBP protein only in the WT mice. Total hepatic SREBP1c protein were unchanged by LGG in either WT or KO mice (Figure 4B). Interestingly, nuclear levels of ChREBP were increased by fructose in two types of mice, and LGG prevented the nuclear translocation only in WT mice (Figure 4C). Nuclear SREBP1c protein level was not altered by fructose in WT mice but significantly increased in the KO liver. LGG supplementation did not change the nuclear SREBP1c protein level in either WT or KO mice (Figure 4C). These results clearly demonstrate that FGF21 is required in order for LGG to attenuate ChREBP activation.
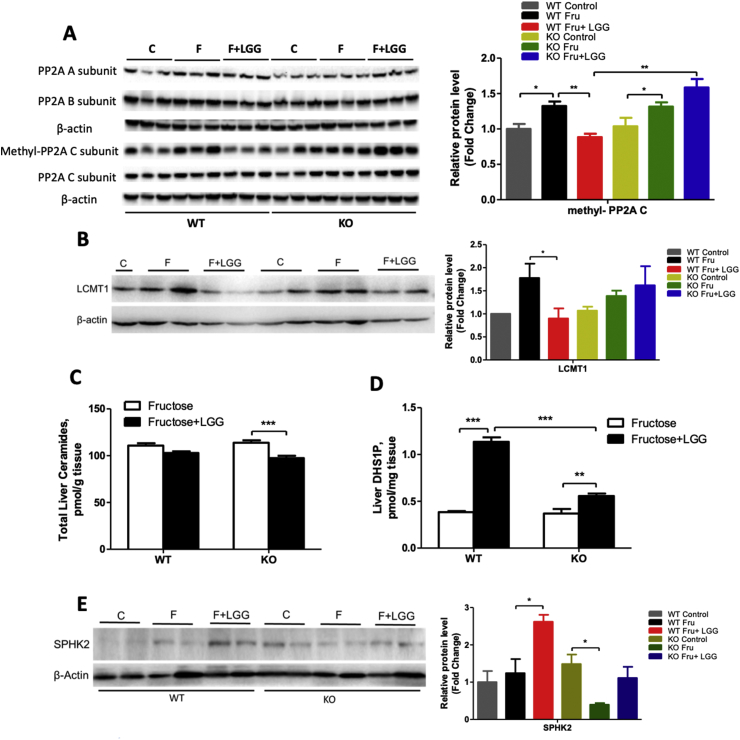
3.6. LGG supplementation decreases hepatic ceramide concentration and PP2A activity
Previous studies showed that ChREBP nuclear localization is promoted by dephosphorylation mediated by protein phosphatase 2A (PP2A) activation [34], [35]. Thus, we measured protein levels of all PP2A subunits. All hepatic A, B, and C subunits of PP2A were not changed by fructose and LGG, either in the WT or in the KO mice (Figure 5A). However, methylated PP2AC (m-PP2AC), the active form of PP2AC, was upregulated by fructose feeding in the hepatic tissues of both WT and KO mice (Figure 5A). Interestingly, LGG supplementation significantly reduced m-PP2AC levels, but only in the WT mice (Figure 5A).
Figure 5.
Effects of LGG on PP2A proteins and ceramide metabolism. WT and FGF21 KO mice were treated as described in the Material and Methods. (A) Liver proteins of PP2A isoforms. The quantification of protein bands by densitometry analysis. (B) Protein level of LCMT1. Liver ceramides (C) and DHS1P (D) concentrations. (E) Protein level of SphK2. β-actin levels served as loading controls.
The alteration of m-PP2AC by LGG led us to measure the level of leucine carboxyl methyltransferase-1 (LCMT1), which has been shown to methylate PP2AC [36]. As shown in Figure 5B, fructose feeding increased LCMT1 protein expression in the livers of the WT mice, and it was attenuated by LGG supplementation. However, in the KO mice, the inhibitory effect of LGG was blunted.
Previous studies showed that PP2A activation was inhibited by (dehydro) sphingosine 1 phosphate (S1P/DHS1P) [37], a metabolite of ceramide regulated by sphingosine kinases (Sphks). Liver total ceramide concentration slightly decreased in WT mice but significantly decreased by LGG treatment in KO mice (Figure 5C). The mechanism by which LGG lowers more liver ceramide in the KO than in the WT mice is currently unknown. Importantly, liver DHS1P was markedly increased by LGG in the fructose-fed WT mice. This upregulating effect was significantly blunted in the KO mice (Figure 5D). Protein level of SphK2, the major form of SphKs in the liver, was slightly increased by fructose feeding but significantly increased by LGG in the WT mice. Again, this effect was blunted in the KO mice (Figure 5E). Interestingly, basal level of liver SphK2 was increased in the KO mice compared to the WT mice (Figure 5E). A recent study showed that FGF21 is a negative regulator of bile acid synthesis [38] and administration of FGF21 significantly decreased hepatic level of bile acid, which has been shown to upregulated SphK2 [39]. It is likely that absence of FGF21 may result in an increase of bile acid and thus a basal upregulation of SphK2. Nevertheless, fructose feeding decreased SphK2 levels in the KO mice. Additional studies are needed to elucidate this phenomenon.
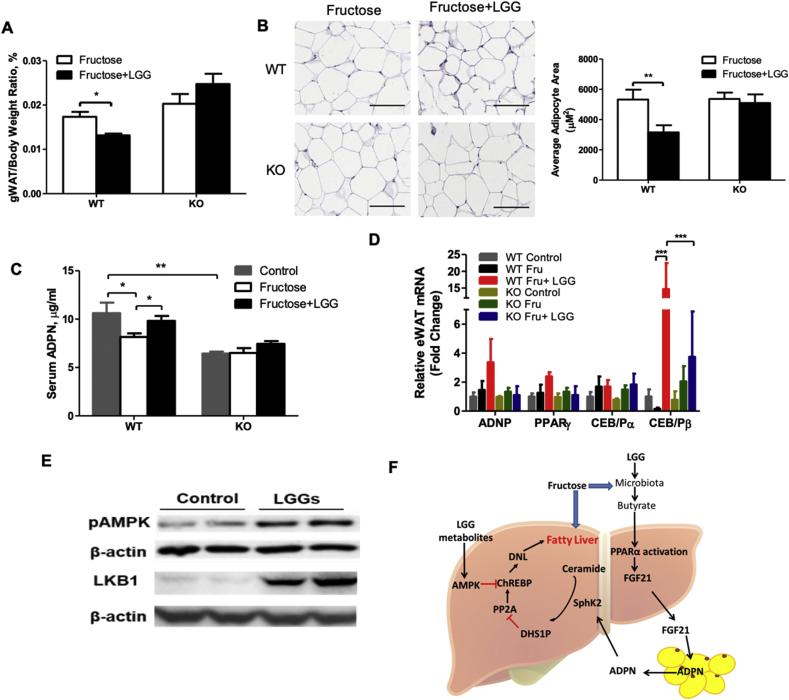
3.7. LGG supplementation decreases adipose tissue size and increased serum ADPN production
SphK2 is regulated by ADPN signaling in the liver. Hepatic ADPN receptor 1 and 2 (AdipoR1 and -2) sense ADPN to activate SphK2. Fructose and LGG did not change the protein levels of AdipoR1 and -2 in either WT or KO mice (Fig. S6). Since ADPN is mainly produced in the adipose tissue, we analyzed ADPN regulation in adipose tissue. Fructose feeding significantly increased gWAT weight in either WT or KO mice (Figure 2B), which was significantly attenuated by LGG supplementation only in the WT mice (Figure 6A). Adipose tissue staining revealed that adipocyte size was enlarged significantly by fructose feeding (Figure 2C) and reversed by LGG supplementation in the WT mice (Figure 6B). However, this reduction was not observed in the FGF21 KO mice (Figure 6B). To determine the effect of LGG on the adipose function, we measured serum level of ADPN and mRNA levels ADPN production related genes in adipose tissues. As shown in Figure 6C, in the WT mice, serum ADPN concentration was reduced by fructose and elevated by LGG supplementation. The basal level of serum ADPN in the KO mice was significantly lower than that of the WT mice. Fructose feeding and LGG supplementation did not alter the serum ADPN concentrations in the KO mice. Adipose tissue ADPN gene expression was not changed in fructose-treated mice but was numerically increased by LGG supplementation in the WT mice (Figure 6D). The mRNA levels of transcription factors that regulate ADPN expression were examined. PPARγ and CCAAT-enhancer binding protein α (CEB/Pα) mRNA levels were not significantly changed by fructose and LGG in either WT or KO adipose tissue (Figure 6D). However, CEB/Pβ mRNA levels were decreased by fructose and markedly elevated by LGG supplementation in the WT mice, while no significant changes occurred in the KO mice (Figure 6D). These results indicate a role of LGG in adipose remodeling favoring ADPN production.
Figure 6.
LGG decreases adipocytes and increases serum ADPN production and the effects of LGGs on AMPK activation in rat hepatocytes. WT and FGF21 KO mice were treated as described in the Material and Methods. (A) gWAT/body weight ratio. (B) Hematoxylin and eosin staining of gWAT (×20). Quantification of adipocyte size (right panel). (C) Serum ADPN concentrations. (D) Relative liver mRNA levels of ADPN, PPARγ, CEB/Pα, and CEB/Pβ. (E) Protein levels of LKB1 and pAMPK in hepatocytes. (F) Schematic illustration of hypothesized mechanisms. Scale bars: 100 μm.
3.8. LGG supernatant increases pAMPK in hepatocytes
AMP-activated protein kinase (AMPK) phosphorylates ChREBP and decreases ChREBP activation [40]. To determine the role of LGG on AMPK signaling, rat hepatic H4IIE cells were incubated with LGG culture supernatant (LGGs). Eight-hour treatment significantly increased liver kinase B1 (LKB1) protein level, which is one of the major kinases to phosphorylate AMPK. As a result, pAMPK was significantly increased (Figure 6E).
4. Discussion
Probiotics have been used in animal models and currently in many clinical trials for NAFLD treatment. While there are an increasing number of studies, the precise mechanisms underlying probiotic actions are still lacking. In the present study, we provide evidence that FGF21 is required for many beneficial effects of probiotic LGG in the reversal of fructose-induced NAFLD in mice. Specifically, we showed that LGG increased hepatic FGF21 and adipose tissue ADPN expression, resulting in an upregulation of SphK2 and an inactivation of PP2AC that leads to the reduced ChREBP activity and reversal of fructose-induced NAFLD.
The rise in prevalence of NAFLD strongly suggests that environmental factors play an important role in the disease pathogenesis. High saturated fat consumption has been considered as the major risk factor for NAFLD [41]. However, restriction of saturated fat while increasing refined sugar intake does not reduce the prevalence of metabolic diseases, including NAFLD [42]. An important, but not well-studied change is the dietary fructose consumption, which has increased dramatically in US in the past decades, especially in the pediatric population [5], [6], [7]. More importantly, consumption of fructose, but not glucose, has been associated with increased visceral adiposity, insulin resistance, and increased hepatic DNL [8], [9], [10], [11], [12], [13], which is the dominant fat accumulation mechanism in NAFLD compared to fatty acid β-oxidation [43]. Moreover, fructose restriction results in a reduction in NAFLD. Unfortunately, there is no effective treatment for fructose-associated NAFLD other than life style modification.
Increasing evidence from preclinical and clinical studies suggests that probiotics are beneficial in the treatment of NAFLD [17]. Lactobacillus rhamnosus GG (LGG), the best-characterized probiotic strain, has been consistently shown to be beneficial in the metabolic syndrome. Recent studies showed that LGG supplementation prevented high fructose-induced liver steatosis and inflammation [19]. Whether LGG can reverse established fructose-induced NAFLD was unknown until our current study. We showed that 12-week fructose feeding significantly increased liver fat accumulation, and this was reversed by 4-week LGG supplementation, suggesting a therapeutic effect of LGG in fructose-associated NAFLD.
A previous study showed that LGG supplementation increased beneficial gut bacteria abundance, favoring butyrate production in mice fed fructose [19]. Butyrate is known to regulate intestinal barrier function and activate PPARα in the liver [44], [45]. FGF21 is a hepatokine that is induced in the fasting state or by consumption of a ketogenic diet through activation of PPARα [46], [47], [48]. FGF21 also senses over-nutrition through ChREBP activation [49], [50]. The dynamic regulation of FGF21 serves as an energy sensor to regulate lipid metabolism. Previous studies have shown that FGF21 is increased in the patients with obesity and NAFLD [51], [52]. Acute fructose challenge dramatically increased blood FGF21 in humans [32]. An FGF21-resistant state has been proposed in diabetic and obese subjects [53], [54]. However, some other studies showed that overt FGF21 resistance was not evident [55]. Although FGF21 levels are high in NAFLD, pharmacological administration of FGF21 reduced body weight and fatty liver and improved glycemia [56]. Upregulation of FGF21 is likely an adaptive response to energy disturbance in metabolic syndrome. We observed that 5-week fructose feeding dramatically increased circulating FGF21, but it was significantly reduced by the end of a 12-week fructose feeding. These results suggest that prolonged fructose exposure reduces the adaptive upregulation of FGF21. Although the mechanisms underlying this reduction by prolonged fructose feeding remains to be explored, several potential mechanisms have been indicated. Fructose consumption causes intestinal barrier dysfunction leading to an increase of bacterial products such as lipopolysaccharides [19], which has been shown to decrease FGF21 [57]. PPARα is an important transcription factor for FGF21 induction. Although the protein level of PPARα was not changed by fructose, but the activity might be decreased. Indeed, fructose feeding decreased intestinal butyrate concentration, which is a known ligand for PPARα activation [58], [59]. Most importantly, supplementation of LGG in last 4 weeks of a 12-week fructose feeding regimen increased PPARα expression, which may contribute to the restored circulating FGF21 to the level comparable to 5-week fructose feeding.
DNL is the dominant event in obesity-associated hepatic fat accumulation, which is mediated in part by the transcription factors ChREBP and SREBP [60]. ChREBP senses carbohydrate metabolites and regulates gene expression in multiple programs including DNL [23]. Although the role of ChREBP remains controversial in hypertriglyceridemia and insulin resistance, it is known that fructose-activation of ChREBP contributes to hepatic steatosis [24]. Inhibition of ChREBP in obese and ob/ob mice leads to reversal of hepatic steatosis [24]. Our significant finding is that LGG supplementation decreased ChREBP nuclear localization only in fructose-fed WT mice, and this was accompanied by decreased hepatic expression of lipogenic enzymes and steatosis. FGF21 deficiency diminished this inhibitory effect of LGG, suggesting a requirement of FGF21.
The specific action of FGF21 is determined by the tissue expression of FGF receptors and the single transmembrane co-receptor, β-Klotho [61]. Adipocytes have high expression levels of both FGFR1 and β-Klotho and act as a major FGF21 target cell type [62]. Consistent with previous studies, high fructose feeding increased body weight gain, serum glucose, and fat mass in WT mice. Those increases were attenuated in the KO mice. However, gWAT weight in the KO mice tended to be increased by fructose feeding compared to WT mice. This adipocyte enlargement and inflammation impairs the expression and secretion of one of the major adipokines, ADPN. We showed that fructose feeding significantly decreased serum ADPN concentration in WT mice. FGF21 KO mice have significantly lower basal ADPN level in the blood, which was not changed by fructose feeding. Importantly, treatment by LGG reversed the fructose-suppressed ADPN levels in WT mice but not in the KO mice. The increased serum levels of ADPN are associated with increased level of C/EBPβ, which regulates ADPN gene expression [63], [64]. Therefore, the beneficial effect of LGG on ADPN production is FGF21-dependent. This finding is important because therapeutically targeting endogenous ADPN expression has been proposed as an effective and promising treatment in metabolic diseases [65].
A critical question is how does LGG exert its function to regulate ChREBP activity through FGF21 and ADPN. Activation of ChREBP is mediated by de-phosphorylation leading to nuclear translocation and dimerization of ChREBPα and ChREBPβ. Carbohydrate metabolites, such as X5P and glucose-6-phosphate (G6P), upregulate PP2A resulting in a de-phosphorylation and nuclear translocation of ChREBP [35], [66]. Previous studies have shown that methylation of the C subunit of PP2A (m-PP2AC) activates the enzyme [67]. Our data clearly showed that fructose feeding elevated hepatic m-PP2AC levels in both WT and KO mice, while LGG treatment decreased m-PP2AC only in the WT mice, suggesting a requirement of FGF21 for the inhibitory effects on PP2A by LGG. Further analysis of LCMT1, a methyltransferase responsible for PP2A methylation [68], showed a decrease in protein level by LGG, further supporting this notion. In addition, ceramide activates PP2A leading to a dephosphorylation, and consequently an activation of ChREBP, while sphingosine-1-phosphate (S1P), a ceramide metabolite, inhibits PP2A activity [69]. S1P is converted from ceramide through Sphk1/2, which is mediated by ADPN activation [70]. Increases in hepatic Sphk2 protein and DHS1P suggest that the de-activation of ChREBP by LGG is mediated, at least partially, by ADPN-regulated ceramide metabolism. In contrast, AMPK phosphorylates ChREBP at Ser568, which decreases ChREBP activation [40]. Our data showed that LGG supernatant increased LKB1 and pAMPK protein level, suggesting that LGG may also exert its action through AMPK to regulate ChREBP activity in fructose-associated NAFLD.
ADPN has an anti-inflammatory effect. Loss of this anti-inflammatory signal contributes to the development of inflammation in adipose tissues and other target tissues such as liver [65]. A recent study showed that FGF21 might have immunosuppressive effects through inhibition of NFκB [71]. Our data demonstrate that LGG treatment reduced the upregulation of TNFα and CXCL10 in WT mice but not in the FGF21 KO mice, suggesting that the anti-inflammatory effect of LGG is mediated by ADPN-FGF21 signaling. Interestingly, FGF21 KO mice had decreased CXCL10 upon fructose exposure and did not respond to LGG treatment. CXCL10 recruits immune cells to the site of tissue injury in the initial phase of inflammation, and excess CXCL10 expression is deleterious due to the induction of pro-inflammatory cytokines such as TNFα [72]. Our data suggest that anti-inflammatory effects of LGG require FGF21-ADPN signaling to suppress fructose-induced liver inflammation.
Although our results clearly showed a requirement of FGF21 in the protective effect of LGG against fructose-induced NAFLD, how LGG increases hepatic FGF21 expression in the setting of fructose exposure remains unknown. It is known that LGG administration profoundly altered the gut microbiome in a variety of pathophysiological conditions [73], [74], [75]. Analyzing the contribution of gut bacteria to the effects of LGG on FGF21 regulation will help to investigate the underlying mechanisms. Studies showed that LGG administration increased butyrate-producing bacteria abundance [76]. Increasing fecal butyrate concentrations may counteract the decrease in hepatic PPARα caused by prolonged fructose feeding and thus enhance FGF21 expression. It is also possible that LGG metabolites suppress certain hepatic bile acids that are strong antagonists of farnesoid X receptor (FXR), which has been shown to regulate FGF21 expression. Indeed, our data showed that LGG significantly decreased tauro-β-muricholic acid (TβMCA), a potent FXR inhibitor [77], [78], in a bile-duct ligation-induced liver fibrosis mouse model (data not shown). A dynamic and balanced regulation of FGF21 by LGG through PPARα, ChREBP and FXR warrants further investigation in fructose-induced NAFLD. In addition, identifying the specific active regulating molecules that have FGF21 inducing activity will further clarify the mechanisms by which LGG protects against fructose-associated NAFLD.
In conclusion, the present study clearly demonstrated that probiotic LGG supplementation could reverse fructose-induced experimental NAFLD. Mechanistically, LGG restores the prolonged fructose exposure-decreased hepatic expression of FGF21, which, in turn, increases adipose ADPN production. Elevated ADPN increases hepatic sphingosine 1 phosphate and inhibits PP2A activity. Together with activated AMPK, the upregulated FGF21-ADPN signaling reduces ChREBP activation that decreases fatty liver. Our results suggest that FGF21 is required for the beneficial effects of LGG, and LGG may function to regulate the gut-adipose-liver axis to reduce fructose-associated NAFLD (Figure 6F).
Contributors
CZ and LL designed and performed the experiments, analyzed the data and wrote the manuscript. QL, FL, LZ, FZ, and TS performed partial experiments and provided technical support. SB, XL, and YC contributed to the conceptual review of the study. CJM provided critical conceptual review of the study and wrote the manuscript. WF conceived, designed and supervised the study and wrote the manuscript.
Acknowledgment
The current study was supported by NIH grants U01AA021901, U01AA021893-01, U01AA022489-01A1, R01AA023681, P20GM113226, P50AA024337 (C.J.M.); R21AA020848 and R01AA023190 (W.F.). Support was also provided by the VA (1I01BX002996, C.J.M), National Natural Science Foundation of China (81600670, C. Z.), Zhejiang Provincial Natural Science Foundation (LGF19H070002, LY19H030003, LGF19H030012), and Jilin Agricultural Science and Technology University Startup Foundation for Talent (20190329). The authors thank Ms. Marion McClain for the editorial support for this manuscript.
Conflict of interest
All authors declare that there is no conflict of interest regarding to the publication of this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2019.08.020.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Nalbantoglu I.L., Brunt E.M. Role of liver biopsy in nonalcoholic fatty liver disease. World Journal of Gastroenterology. 2014;20:9026–9037. doi: 10.3748/wjg.v20.i27.9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung M., Ma J., Patel K., Berger S., Lau J., Lichtenstein A.H. Fructose, high-fructose corn syrup, sucrose, and nonalcoholic fatty liver disease or indexes of liver health: a systematic review and meta-analysis. American Journal of Clinical Nutrition. 2014;100:833–849. doi: 10.3945/ajcn.114.086314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neuschwander-Tetri B.A. Carbohydrate intake and nonalcoholic fatty liver disease. Current Opinion in Clinical Nutrition and Metabolic Care. 2013;16:446–452. doi: 10.1097/MCO.0b013e328361c4d1. [DOI] [PubMed] [Google Scholar]
- 4.Vos M.B., Lavine J.E. Dietary fructose in nonalcoholic fatty liver disease. Hepatology. 2013;57:2525–2531. doi: 10.1002/hep.26299. [DOI] [PubMed] [Google Scholar]
- 5.DeChristopher L.R., Uribarri J., Tucker K.L. Intake of high-fructose corn syrup sweetened soft drinks, fruit drinks and apple juice is associated with prevalent arthritis in US adults, aged 20–30 years. Nutrition & Diabetes. 2016;6:e199. doi: 10.1038/nutd.2016.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vos M.B., Kimmons J.E., Gillespie C., Welsh J., Blanck H.M. Dietary fructose consumption among US children and adults: the Third National Health and Nutrition Examination Survey. Medscape Journal of Medicine. 2008;10:160. [PMC free article] [PubMed] [Google Scholar]
- 7.Douard V., Ferraris R.P. The role of fructose transporters in diseases linked to excessive fructose intake. Journal of Physiology. 2013;591:401–414. doi: 10.1113/jphysiol.2011.215731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cox C.L., Stanhope K.L., Schwarz J.M., Graham J.L., Hatcher B., Griffen S.C. Consumption of fructose-sweetened beverages for 10 weeks reduces net fat oxidation and energy expenditure in overweight/obese men and women. European Journal of Clinical Nutrition. 2012;66:201–208. doi: 10.1038/ejcn.2011.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox C.L., Stanhope K.L., Schwarz J.M., Graham J.L., Hatcher B., Griffen S.C. Consumption of fructose- but not glucose-sweetened beverages for 10 weeks increases circulating concentrations of uric acid, retinol binding protein-4, and gamma-glutamyl transferase activity in overweight/obese humans. Nutrition & Metabolism (London) 2012;9:68. doi: 10.1186/1743-7075-9-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silbernagel G., Machann J., Unmuth S., Schick F., Stefan N., Haring H.U. Effects of 4-week very-high-fructose/glucose diets on insulin sensitivity, visceral fat and intrahepatic lipids: an exploratory trial. British Journal of Nutrition. 2011;106:79–86. doi: 10.1017/S000711451000574X. [DOI] [PubMed] [Google Scholar]
- 11.Stanhope K.L., Schwarz J.M., Keim N.L., Griffen S.C., Bremer A.A., Graham J.L. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. Journal of Clinical Investigation. 2009;119:1322–1334. doi: 10.1172/JCI37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teff K.L., Grudziak J., Townsend R.R., Dunn T.N., Grant R.W., Adams S.H. Endocrine and metabolic effects of consuming fructose- and glucose-sweetened beverages with meals in obese men and women: influence of insulin resistance on plasma triglyceride responses. Journal of Clinical Endocrinology and Metabolism. 2009;94:1562–1569. doi: 10.1210/jc.2008-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin R., Vos M.B. Fructose and liver function – is this behind nonalcoholic liver disease? Current Opinion in Clinical Nutrition and Metabolic Care. 2015;18:490–495. doi: 10.1097/MCO.0000000000000203. [DOI] [PubMed] [Google Scholar]
- 14.Abdelmalek M.F., Suzuki A., Guy C., Unalp-Arida A., Colvin R., Johnson R.J. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology. 2010;51:1961–1971. doi: 10.1002/hep.23535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ibarra-Reynoso L.D.R., Lopez-Lemus H.L., Garay-Sevilla M.E., Malacara J.M. Effect of restriction of foods with high fructose corn syrup content on metabolic indices and fatty liver in obese children. Obesity Facts. 2017;10:332–340. doi: 10.1159/000476069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plaza-Diaz J., Ruiz-Ojeda F.J., Vilchez-Padial L.M., Gil A. Evidence of the anti-inflammatory effects of probiotics and synbiotics in intestinal chronic diseases. Nutrients. 2017;9 doi: 10.3390/nu9060555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xue L., He J., Gao N., Lu X., Li M., Wu X. Probiotics may delay the progression of nonalcoholic fatty liver disease by restoring the gut microbiota structure and improving intestinal endotoxemia. Scientific Reports. 2017;7:45176. doi: 10.1038/srep45176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh S., Osna N.A., Kharbanda K.K. Treatment options for alcoholic and non-alcoholic fatty liver disease: a review. World Journal of Gastroenterology. 2017;23:6549–6570. doi: 10.3748/wjg.v23.i36.6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ritze Y., Bardos G., Claus A., Ehrmann V., Bergheim I., Schwiertz A. Lactobacillus rhamnosus GG protects against non-alcoholic fatty liver disease in mice. PLoS One. 2014;9:e80169. doi: 10.1371/journal.pone.0080169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eslamparast T., Eghtesad S., Hekmatdoost A., Poustchi H. Probiotics and nonalcoholic fatty liver disease. Middle East Journal of Digestive Diseases. 2013;5:129–136. [PMC free article] [PubMed] [Google Scholar]
- 21.Doulberis M., Kotronis G., Gialamprinou D., Kountouras J., Katsinelos P. Non-alcoholic fatty liver disease: an update with special focus on the role of gut microbiota. Metabolism: Clinical and Experimental. 2017;71:182–197. doi: 10.1016/j.metabol.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 22.Koo H.Y., Miyashita M., Cho B.H., Nakamura M.T. Replacing dietary glucose with fructose increases ChREBP activity and SREBP-1 protein in rat liver nucleus. Biochemical and Biophysical Research Communications. 2009;390:285–289. doi: 10.1016/j.bbrc.2009.09.109. [DOI] [PubMed] [Google Scholar]
- 23.Iizuka K., Horikawa Y. ChREBP: a glucose-activated transcription factor involved in the development of metabolic syndrome. Endocrine Journal. 2008;55:617–624. doi: 10.1507/endocrj.k07e-110. [DOI] [PubMed] [Google Scholar]
- 24.Dentin R., Benhamed F., Hainault I., Fauveau V., Foufelle F., Dyck J.R. Liver-specific inhibition of ChREBP improves hepatic steatosis and insulin resistance in ob/ob mice. Diabetes. 2006;55:2159–2170. doi: 10.2337/db06-0200. [DOI] [PubMed] [Google Scholar]
- 25.Fisher F.M., Kim M., Doridot L., Cunniff J.C., Parker T.S., Levine D.M. A critical role for ChREBP-mediated FGF21 secretion in hepatic fructose metabolism. Molecular Metabolism. 2017;6:14–21. doi: 10.1016/j.molmet.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iizuka K., Takeda J., Horikawa Y. Glucose induces FGF21 mRNA expression through ChREBP activation in rat hepatocytes. FEBS Letters. 2009;583:2882–2886. doi: 10.1016/j.febslet.2009.07.053. [DOI] [PubMed] [Google Scholar]
- 27.Potthoff M.J., Inagaki T., Satapati S., Ding X., He T., Goetz R. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:10853–10858. doi: 10.1073/pnas.0904187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y., Zhao C., Xiao J., Liu L., Zhang M., Wang C. Fibroblast growth factor 21 deficiency exacerbates chronic alcohol-induced hepatic steatosis and injury. Scientific Reports. 2016;6:31026. doi: 10.1038/srep31026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y., Kirpich I., Liu Y., Ma Z., Barve S., McClain C.J. Lactobacillus rhamnosus GG treatment potentiates intestinal hypoxia-inducible factor, promotes intestinal integrity and ameliorates alcohol-induced liver injury. American Journal of Pathology. 2011;179:2866–2875. doi: 10.1016/j.ajpath.2011.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y., Liu Y., Sidhu A., Ma Z., McClain C., Feng W. Lactobacillus rhamnosus GG culture supernatant ameliorates acute alcohol-induced intestinal permeability and liver injury. American Journal of Physiology – Gastrointestinal and Liver Physiology. 2012;303:G32–G41. doi: 10.1152/ajpgi.00024.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kasumov T., Huang H., Chung Y.M., Zhang R., McCullough A.J., Kirwan J.P. Quantification of ceramide species in biological samples by liquid chromatography electrospray ionization tandem mass spectrometry. Analytical Biochemistry. 2010;401:154–161. doi: 10.1016/j.ab.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dushay J.R., Toschi E., Mitten E.K., Fisher F.M., Herman M.A., Maratos-Flier E. Fructose ingestion acutely stimulates circulating FGF21 levels in humans. Molecular Metabolism. 2015;4:51–57. doi: 10.1016/j.molmet.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang M., Wang C., Wang C., Zhao H., Zhao C., Chen Y. Enhanced AMPK phosphorylation contributes to the beneficial effects of Lactobacillus rhamnosus GG supernatant on chronic-alcohol-induced fatty liver disease. Journal of Nutritional Biochemistry. 2015;26:337–344. doi: 10.1016/j.jnutbio.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakiyama H., Wynn R.M., Lee W.R., Fukasawa M., Mizuguchi H., Gardner K.H. Regulation of nuclear import/export of carbohydrate response element-binding protein (ChREBP): interaction of an alpha-helix of ChREBP with the 14-3-3 proteins and regulation by phosphorylation. Journal of Biological Chemistry. 2008;283:24899–24908. doi: 10.1074/jbc.M804308200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kabashima T., Kawaguchi T., Wadzinski B.E., Uyeda K. Xylulose 5-phosphate mediates glucose-induced lipogenesis by xylulose 5-phosphate-activated protein phosphatase in rat liver. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:5107–5112. doi: 10.1073/pnas.0730817100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Baere I., Derua R., Janssens V., Van Hoof C., Waelkens E., Merlevede W. Purification of porcine brain protein phosphatase 2A leucine carboxyl methyltransferase and cloning of the human homologue. Biochemistry. 1999;38:16539–16547. doi: 10.1021/bi991646a. [DOI] [PubMed] [Google Scholar]
- 37.Salas A., Ponnusamy S., Senkal C.E., Meyers-Needham M., Selvam S.P., Saddoughi S.A. Sphingosine kinase-1 and sphingosine 1-phosphate receptor 2 mediate Bcr-Abl1 stability and drug resistance by modulation of protein phosphatase 2A. Blood. 2011;117:5941–5952. doi: 10.1182/blood-2010-08-300772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen M.M., Hale C., Stanislaus S., Xu J., Veniant M.M. FGF21 acts as a negative regulator of bile acid synthesis. Journal of Endocrinology. 2018;237:139–152. doi: 10.1530/JOE-17-0727. [DOI] [PubMed] [Google Scholar]
- 39.Nagahashi M., Takabe K., Liu R., Peng K., Wang X., Wang Y. Conjugated bile acid-activated S1P receptor 2 is a key regulator of sphingosine kinase 2 and hepatic gene expression. Hepatology. 2015;61:1216–1226. doi: 10.1002/hep.27592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawaguchi T., Osatomi K., Yamashita H., Kabashima T., Uyeda K. Mechanism for fatty acid “sparing” effect on glucose-induced transcription: regulation of carbohydrate-responsive element-binding protein by AMP-activated protein kinase. Journal of Biological Chemistry. 2002;277:3829–3835. doi: 10.1074/jbc.M107895200. [DOI] [PubMed] [Google Scholar]
- 41.Leamy A.K., Egnatchik R.A., Young J.D. Molecular mechanisms and the role of saturated fatty acids in the progression of non-alcoholic fatty liver disease. Progress in Lipid Research. 2013;52:165–174. doi: 10.1016/j.plipres.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kearns C.E., Schmidt L.A., Glantz S.A. Sugar industry and coronary heart disease research: a historical analysis of internal industry documents. JAMA Internal Medicine. 2016;176:1680–1685. doi: 10.1001/jamainternmed.2016.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Softic S., Cohen D.E., Kahn C.R. Role of dietary fructose and hepatic de novo lipogenesis in fatty liver disease. Digestive Diseases and Sciences. 2016;61:1282–1293. doi: 10.1007/s10620-016-4054-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang H.B., Wang P.Y., Wang X., Wan Y.L., Liu Y.C. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Digestive Diseases and Sciences. 2012;57:3126–3135. doi: 10.1007/s10620-012-2259-4. [DOI] [PubMed] [Google Scholar]
- 45.Harten S.K., Shukla D., Barod R., Hergovich A., Balda M.S., Matter K. Regulation of renal epithelial tight junctions by the von Hippel-Lindau tumor suppressor gene involves occludin and claudin 1 and is independent of E-cadherin. Molecular Biology of the Cell. 2009;20:1089–1101. doi: 10.1091/mbc.E08-06-0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Galman C., Lundasen T., Kharitonenkov A., Bina H.A., Eriksson M., Hafstrom I. The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPARalpha activation in man. Cell Metabolism. 2008;8:169–174. doi: 10.1016/j.cmet.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 47.Badman M.K., Pissios P., Kennedy A.R., Koukos G., Flier J.S., Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metabolism. 2007;5:426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 48.Li H., Gao Z., Zhang J., Ye X., Xu A., Ye J. Sodium butyrate stimulates expression of fibroblast growth factor 21 in liver by inhibition of histone deacetylase 3. Diabetes. 2012;61:797–806. doi: 10.2337/db11-0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.von Holstein-Rathlou S., BonDurant L.D., Peltekian L., Naber M.C., Yin T.C., Claflin K.E. FGF21 mediates endocrine control of simple sugar intake and sweet taste preference by the liver. Cell Metabolism. 2016;23:335–343. doi: 10.1016/j.cmet.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maekawa R., Seino Y., Ogata H., Murase M., Iida A., Hosokawa K. Chronic high-sucrose diet increases fibroblast growth factor 21 production and energy expenditure in mice. Journal of Nutritional Biochemistry. 2017;49:71–79. doi: 10.1016/j.jnutbio.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 51.Dushay J., Chui P.C., Gopalakrishnan G.S., Varela-Rey M., Crawley M., Fisher F.M. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology. 2010;139:456–463. doi: 10.1053/j.gastro.2010.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yilmaz Y., Eren F., Yonal O., Kurt R., Aktas B., Celikel C.A. Increased serum FGF21 levels in patients with nonalcoholic fatty liver disease. European Journal of Clinical Investigation. 2010;40:887–892. doi: 10.1111/j.1365-2362.2010.02338.x. [DOI] [PubMed] [Google Scholar]
- 53.Fisher F.M., Chui P.C., Antonellis P.J., Bina H.A., Kharitonenkov A., Flier J.S. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes. 2010;59:2781–2789. doi: 10.2337/db10-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nonogaki K., Yamazaki T., Murakami M., Kaji T. Ingestion of eicosapentaenoic acid in the early stage of social isolation reduces a fibroblast growth factor 21 resistant state independently of body weight in KKA(y) mice. Biochemical and Biophysical Research Communications. 2015;464:674–677. doi: 10.1016/j.bbrc.2015.07.058. [DOI] [PubMed] [Google Scholar]
- 55.Hale C., Chen M.M., Stanislaus S., Chinookoswong N., Hager T., Wang M. Lack of overt FGF21 resistance in two mouse models of obesity and insulin resistance. Endocrinology. 2012;153:69–80. doi: 10.1210/en.2010-1262. [DOI] [PubMed] [Google Scholar]
- 56.Kharitonenkov A., Shiyanova T.L., Koester A., Ford A.M., Micanovic R., Galbreath E.J. FGF-21 as a novel metabolic regulator. Journal of Clinical Investigation. 2005;115:1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lauritzen E.S., Rittig N., Bach E., Moller N., Bjerre M. LPS infusion suppresses serum FGF21 levels in healthy adult volunteers. Endocrine Connections. 2017;6:39–43. doi: 10.1530/EC-16-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wagnerberger S., Spruss A., Kanuri G., Stahl C., Schroder M., Vetter W. Lactobacillus casei Shirota protects from fructose-induced liver steatosis: a mouse model. Journal of Nutritional Biochemistry. 2013;24:531–538. doi: 10.1016/j.jnutbio.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 59.Weng H., Endo K., Li J., Kito N., Iwai N. Induction of peroxisomes by butyrate-producing probiotics. PLoS One. 2015;10:e0117851. doi: 10.1371/journal.pone.0117851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Strable M.S., Ntambi J.M. Genetic control of de novo lipogenesis: role in diet-induced obesity. Critical Reviews in Biochemistry and Molecular Biology. 2010;45:199–214. doi: 10.3109/10409231003667500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ogawa Y., Kurosu H., Yamamoto M., Nandi A., Rosenblatt K.P., Goetz R. BetaKlotho is required for metabolic activity of fibroblast growth factor 21. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:7432–7437. doi: 10.1073/pnas.0701600104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kurosu H., Choi M., Ogawa Y., Dickson A.S., Goetz R., Eliseenkova A.V. Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. Journal of Biological Chemistry. 2007;282:26687–26695. doi: 10.1074/jbc.M704165200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Park B.H., Qiang L., Farmer S.R. Phosphorylation of C/EBPbeta at a consensus extracellular signal-regulated kinase/glycogen synthase kinase 3 site is required for the induction of adiponectin gene expression during the differentiation of mouse fibroblasts into adipocytes. Molecular and Cellular Biology. 2004;24:8671–8680. doi: 10.1128/MCB.24.19.8671-8680.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Adachi T., Inoue M., Hara H., Suzuki S. Effects of PPARgamma ligands and C/EBPbeta enhancer on expression of extracellular-superoxide dismutase. Redox Report: Communications in Free Radical Research. 2004;9:207–212. doi: 10.1179/135100004225005985. [DOI] [PubMed] [Google Scholar]
- 65.Kadowaki T., Yamauchi T., Kubota N., Hara K., Ueki K., Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. Journal of Clinical Investigation. 2006;116:1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Uyeda K., Repa J.J. Carbohydrate response element binding protein, ChREBP, a transcription factor coupling hepatic glucose utilization and lipid synthesis. Cell Metabolism. 2006;4:107–110. doi: 10.1016/j.cmet.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 67.Lechward K., Awotunde O.S., Swiatek W., Muszynska G. Protein phosphatase 2A: variety of forms and diversity of functions. Acta Biochimica Polonica. 2001;48:921–933. [PubMed] [Google Scholar]
- 68.Hwang J., Lee J.A., Pallas D.C. Leucine carboxyl methyltransferase 1 (LCMT-1) methylates protein phosphatase 4 (PP4) and protein phosphatase 6 (PP6) and differentially regulates the stable formation of different PP4 holoenzymes. Journal of Biological Chemistry. 2016;291:21008–21019. doi: 10.1074/jbc.M116.739920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dobrowsky R.T., Hannun Y.A. Ceramide stimulates a cytosolic protein phosphatase. Journal of Biological Chemistry. 1992;267:5048–5051. [PubMed] [Google Scholar]
- 70.Holland W.L., Miller R.A., Wang Z.V., Sun K., Barth B.M., Bui H.H. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nature Medicine. 2011;17:55–63. doi: 10.1038/nm.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yu Y.H., Li S.M., Liu Y.N., Tian G.Y., Yuan Q.Y., Bai F.L. Fibroblast growth factor 21 (FGF21) ameliorates collagen-induced arthritis through modulating oxidative stress and suppressing nuclear factor-kappa B pathway. International Immunopharmacology. 2015;25:74–82. doi: 10.1016/j.intimp.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 72.Zhang X., Shen J.Y., Man K., Chu E.S.H., Yau T.O., Sung J.C.Y. CXCL10 plays a key role as an inflammatory mediator and a non-invasive biomarker of non-alcoholic steatohepatitis. Journal of Hepatology. 2014;61:1365–1375. doi: 10.1016/j.jhep.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 73.Bajaj J.S., Heuman D.M., Hylemon P.B., Sanyal A.J., Puri P., Sterling R.K. Randomised clinical trial: Lactobacillus GG modulates gut microbiome, metabolome and endotoxemia in patients with cirrhosis. Alimentary Pharmacology & Therapeutics. 2014;39:1113–1125. doi: 10.1111/apt.12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bull-Otterson L., Feng W., Kirpich I., Wang Y., Qin X., Liu Y. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PLoS One. 2013;8:e53028. doi: 10.1371/journal.pone.0053028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim B., Park K.Y., Ji Y., Park S., Holzapfel W., Hyun C.K. Protective effects of Lactobacillus rhamnosus GG against dyslipidemia in high-fat diet-induced obese mice. Biochemical and Biophysical Research Communications. 2016;473:530–536. doi: 10.1016/j.bbrc.2016.03.107. [DOI] [PubMed] [Google Scholar]
- 76.Ferrario C., Taverniti V., Milani C., Fiore W., Laureati M., De Noni I. Modulation of fecal Clostridiales bacteria and butyrate by probiotic intervention with Lactobacillus paracasei DG varies among healthy adults. Journal of Nutrition. 2014;144:1787–1796. doi: 10.3945/jn.114.197723. [DOI] [PubMed] [Google Scholar]
- 77.Li F., Jiang C., Krausz K.W., Li Y., Albert I., Hao H. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nature Communications. 2013;4:2384. doi: 10.1038/ncomms3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sayin S.I., Wahlstrom A., Felin J., Jantti S., Marschall H.U., Bamberg K. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metabolism. 2013;17:225–235. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.