Abstract
Oxidative modification of cysteine residues has been shown to regulate the activity of several protein-tyrosine kinases. We explored the possibility that Fms-like tyrosine kinase 3 (FLT3), a hematopoietic receptor-tyrosine kinase, is subject to this type of regulation. An underlying rationale was that the FLT3 gene is frequently mutated in Acute Myeloid Leukemia patients, and resulting oncogenic variants of FLT3 with ‘internal tandem duplications (FLT3ITD)’ drive production of reactive oxygen in leukemic cells.
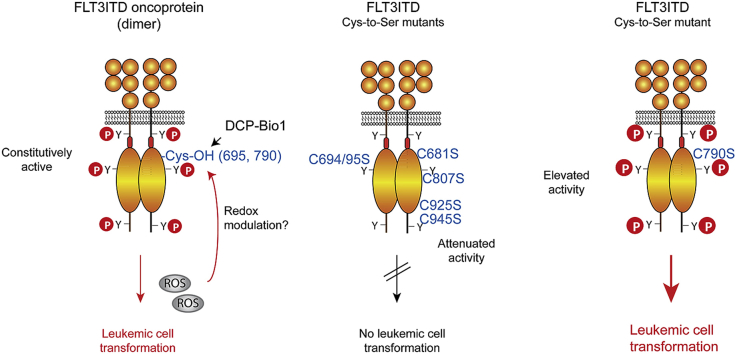
FLT3 was moderately activated by treatment of intact cells with hydrogen peroxide. Conversely, FLT3ITD signaling was attenuated by cell treatments with agents inhibiting formation of reactive oxygen species. FLT3 and FLT3ITD incorporated DCP-Bio1, a reagent specifically reacting with sulfenic acid residues. Mutation of FLT3ITD cysteines 695 and 790 reduced DCP-Bio1 incorporation, suggesting that these sites are subject to oxidative modification. Functional characterization of individual FLT3ITD cysteine-to-serine mutants of all 8 cytoplasmic cysteines revealed phenotypes in kinase activity, signal transduction and cell transformation. Replacement of cysteines 681, 694, 695, 807, 925, and 945 attenuated signaling and blocked FLT3ITD-mediated cell transformation, whereas mutation of cysteine 790 enhanced activity of both FLT3ITD and wild-type FLT3. These effects were not related to altered FLT3ITD dimerization, but likely caused by changed intramolecular interactions.
The findings identify the functional relevance of all cytoplasmic FLT3ITD cysteines, and indicate the potential for redox regulation of this clinically important oncoprotein.
Keywords: Fms-like tyrosine kinase, FLT3, Cysteine residues, Oxidation, Signal transduction, Leukemic cell transformation
Graphical abstract
Highlights
-
•
Fms-like tyrosine kinase 3 (FLT3) can be activated in intact cells by exposure to hydrogen peroxide.
-
•
Signaling of the oncoprotein FLT3ITD (FLT3 with internal tandem duplication) can be attenuated with antioxidants.
-
•
FLT3ITD is oxidized at cytoplasmic cysteine residues 695 and 790.
-
•
The FLT3ITD cytoplasmic cysteine residues 681, 694, 695, 807, 925, and 945 are essential for cell transformation.
-
•
Replacement of cysteine 790 with serine causes FLT3 activation.
-
•
The findings indicate the potential of FLT3/FLT3ITD redox regulation through cytoplasmic cysteine residues.
1. Introduction
Reactive oxygen species (ROS) have been recognized as important mediators in physiological regulation of cell functions and in the context of different pathologies [[1], [2], [3]]. For example, activation of receptor-tyrosine kinases (RTK) by mitogenic growth factors, such as epidermal growth factor (EGF), or platelet-derived growth factor (PDGF) causes elevation of cellular ROS in the form of hydrogen peroxide (H2O2), which is required for conveying a cellular response [4,5]. In cancer cells, constitutively elevated ROS levels occur and may contribute to mitogenic effects of oncoproteins [[6], [7], [8]]. Among the well-established targets of ROS-mediated regulation are transcription factors [9], and protein-tyrosine phosphatases (PTP) [[10], [11], [12]]. Selective, reversible oxidation of PTP in the proximity of RTK occurs as consequence of the local activation of ROS production by NADPH oxidases (NOX) and enables efficient RTK downstream signaling [1,10,13]. More recently, ROS-mediated regulation of protein kinases has emerged as a novel mechanism of post-translational control for this enzyme family (reviewed in Refs. [14,15]). Oxidative modification of kinase cysteines can have both kinase-activating and kinase-inactivating effects. For example, Src tyrosine kinase is activated in the context of cell adhesion to the extracellular matrix, by formation of an intramolecular disulfide between cysteines 245 and 487 (reviewed in Ref. [16]). Src inactivation by intermolecular disulfide formation involving cysteine 277 residues has also been reported [17]. Very recently, sulfenylation of Src cysteines 185 and 277 was shown to activate the kinase by mediating conformational changes [18]. Among RTK, a well investigated case of oxidative regulation is the EGF receptor (EGFR) [[19], [20], [21], [22], [23]]. EGF stimulation leads to generation of ROS through NOX2, and inactivation of several PTP. In addition, EGFR is directly oxidized at the solvent-exposed cysteine 797, leading to formation of a sulfenic acid residue, and in turn assumption of a more active conformation [19,21]. In EGFR-dependent cancer cells, oxidation of Cys797 also causes relative resistance to kinase inhibitors, which exploit Cys797 for irreversible binding [21]. Direct redox-dependent regulation may also play a role in other RTK. For example, in the PDGFβ-receptor (PDGFRβ) two cysteines (Cys822, 940) were identified to mediate inactivation by alkylating agents, suggesting their potential implication in redox-dependent control [24].
Fms-like tyrosine kinase 3 (FLT3) is a member of the PDGFR (class III RTK) family and mainly expressed in hematopoietic cells [25,26]. The encoding FLT3 gene is the most frequently mutated gene in Acute Myeloid Leukemia (AML) [27]. The predominant type of mutations (25–30% of patients), causes internal tandem duplication (ITD) of sequence in the FLT3 juxtamembrane or first kinase subdomain, resulting in constitutively active FLT3ITD kinase proteins [28,29]. FLT3ITD promotes leukemia, confers a particularly unfavorable prognosis and is exploited as a target for treatment with tyrosine kinase inhibitors (TKI) [30,31]. Constitutive activity of FLT3ITD is associated with constitutively elevated ROS, which contribute to cell transformation. One underlying mechanism involves STAT5 activation, enhanced expression of NOX4 and ROS formation, and subsequent ROS-mediated PTP inactivation [8,32,33]. Further mechanisms are likely to also play a role (for review [34,35]). If FLT3ITD can also undergo direct oxidation and if this may have an effect on kinase activity is not known and was addressed in the current study.
We found that exogenous ROS can indeed modulate kinase activity of FLT3 in intact cells, albeit to a moderate extent. Consistently, treatment of cells expressing FLT3ITD with ROS-quenching agents attenuated signal transduction. FLT3ITD oxidation by sulfenic acid formation is suggested by incorporation of a sulfenic acid-specific reagent. The two cytoplasmic cysteine residues of FLT3ITD Cys695 and Cys790 appear preferentially affected. Comprehensive analysis of cysteine-to-serine mutant FLT3ITD proteins revealed critical roles of several cysteine residues for kinase activity and transforming signaling, further supporting cysteine modification as potential mechanism of regulation.
2. Materials and methods
DNA constructs of human FLT3 and FLT3ITD for transient expression were described earlier [36]. A lentiviral expression construct for FLT3ITD containing an EGFP/blasticidine selection cassette (pLV EGFP/Bsd) and a corresponding control vector (pLV mCherry/Bsd) were obtained from VectorBuilder/Cyagen (Santa Clara, CA, USA). C-terminally FLAG-tagged hFLT3 in pCMV2 (FLT3-FLAG, HG10445-CF) was purchased from Sino Biological, Beijing, China. A sequence harboring a common human ITD (see Supplementary Fig. S4B) was transferred from a construct in pcDNA3.1 (−) [36] using restriction with BsgI and EcoRV and standard cloning procedures resulting in FLAG-tagged hFLT3ITD in pCMV2. Mutagenesis was performed with the QuikChange II Site-Directed Mutagenesis Kit (Agilent, Santa Clara, CA, USA). All constructs were verified by DNA sequencing.
To determine effects of H2O2 treatment on FLT3 autophosphorylation in RS4-11 cells, they were starved in RPMI1640 containing 0.5% fetal calf serum (FCS) for 4 h and then sedimented and resuspended at a density of 3–6 x 106 cells/ml in prewarmed phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin (BSA). Treatments were performed with 1 ml suspension (as indicated in the figure legends) at 37 °C under gentle shaking in 1.5 ml microfuge tubes. To terminate treatments, tubes were transferred to ice, cells were sedimented at 500×g at 4 °C for 5 min, washed once with ice-cold PBS and subsequently lysed with 300 μl RIPA lysis buffer containing 50 mM Tris-HCl (pH7.5), 150 mM NaCl, 1% NP40 alternative (492016, Merck-Millipore, Darmstadt, Germany), 0.25% sodium deoxycholate, 1 mM EDTA, and freshly added protease inhibitor cocktail, PhosSTOP, and 1 mM sodium orthovanadate. The cleared lysates were subjected to immunoprecipitation with 2 μg anti-FLT3 antibody (mmc, see Supplementary Information for reagent details) overnight, complexes were collected with protein A/G-agarose beads and analyzed by SDS-PAGE and immunoblotting, which were performed as described previously [36]. Effects of diphenyleneiodonium (DPI) and N-acetylcysteine (NAC) on transforming signaling of FLT3ITD were determined in human MV4-11 cells, which express FLT3ITD endogenously or 32D cells stably expressing FLT3ITD. 2–4 x 106 MV4-11 cells in complete medium or in 2–3 x 106 32D FLT3ITD cells in serum-free medium were treated for 6–12 h, or 4 h, respectively, with the reagents, and lysates were directly analyzed for phosphorylation of FLT3ITD and downstream signaling mediators by immunoblotting.
Transient transfection of HEK293 cells was performed by a standard protocol with a ratio 2.5/1 of polyethyleneimine (PEI) to plasmid DNA in Opti-MEM I (31985–047, Life Technologies GmbH, Darmstadt, Germany) medium. After two days, cells were lysed with lysis buffer containing 50 mM HEPES (pH 7.5), 150 mM NaCl, 1% NP-40 alternative, 1 mM EDTA, and freshly added protease inhibitor cocktail and PhosSTOP, and lysates were directly subjected to SDS-PAGE and immunoblotting for signaling analyses. Alternatively, immunoprecipitation and kinase assays, DCP-Bio1 labeling, or receptor crosslinking with BS3, were performed as described below.
For kinase assays, the transfected cells were treated with the reversible FLT3 inhibitor compound 102 (2 μM) for 3–4 h before lysis, causing a strong reduction in the FLT3 autophosphorylation level. Cells were lysed with lysis buffer as for the signaling analyses. FLT3ITD or corresponding Cys-Ser or Cys-Ala mutant proteins were immunoprecipitated with anti-FLT3 C-20 antibodies (1 μg per lysate of a 6-well dish) for 2 h. The complexes were collected with protein A sepharose CL-4B beads, and the immunoprecipitates were washed two times with 20 mM HEPES (pH 7.5), 150 mM NaCl, 10% glycerol, 0.1% Triton X-100. Kinase assays were performed using aliquots of the immunprecipitated FLT3ITD on beads in 50 mM HEPES, pH7.5. The reaction was started by adding ATP and MnCl2 to 1 mM and 5 mM final concentration, respectively, and incubating at 30 °C for 1 min. The reaction was stopped by addition of SDS-PAGE sample buffer, and kinase activity was evaluated as FLT3ITD phosphorylation by immunoblotting. To test the effect of H2O2 treatment on FLT3 kinase activity, the purification of the FLT3-FLAG protein was accomplished under anaerobic conditions in a hypoxic chamber from GS GLOVEBOX Systemtechnik GmbH (Malsch, Germany). Buffers were deoxygenated by nitrogen bubbling for 1 h immediately before the experiment. For purification, routinely two 10 cm dishes of transfected HEK293 cells, pretreated with compound 102, were processed. Dishes were washed with ice-cold PBS. 600 μl lysis buffer (degassed 50 mM HEPES (pH 7,5), 150 mM NaCl, 1 mM EDTA, supplemented to 1% NP40 with Surfact-Amps™ Detergent Solution (28324, Pierce, Thermo Scientific, Darmstadt, Germany), protease inhibitor cocktail, and PhosSTOP) was used per dish. FLT3-FLAG was immunoprecipitated using 50 μl anti-FLAG M2 beads/dish for 2–3 h. Afterwards the beads were washed once with lysis buffer and twice with kinase buffer (50 mM HEPES, pH7.5). FLT3-FLAG was eluted from the beads with 100 μg/ml FLAG peptide at 4 °C for 30 min and subjected to H2O2 treatments and kinase assays using about 10% of the material from one dish per assay point. To compare kinase activity of wild-type FLT3 and FLT3 Cys790Ser, workup was done in the same way but omitting the anaerobic treatment. Kinase reactions were carried out in 50 mM HEPES, pH7.5 with 5–25 μM ATP and 5 mM MnCl2 or 5 mM MgCl2 at 30 °C for 2–5 min. Reactions were stopped with SDS-PAGE sample buffer and samples were analyzed by immunoblotting.
For DCP-Bio1 labeling, previously published protocols were adapted [37,38]. The respective cells (MV4-11, RS4-11, or transiently transfected HEK293 cells) were subjected to serum starvation and various cell treatments as indicated in the figure legends. They were then washed once with PBS and subsequently lysed in 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.5% Na-deoxycholate, 0.5% NP-40 alternative, 0.5% Triton X-100, freshly supplemented with 100 μM diethylene triamine pentaacetic acid (DTPA), 10 mM N-ethylmaleimide (NEM), 10 mM iodoacetamide (IAA), 200 U/ml catalase, protease inhibitor cocktail, and 1 mM DCP-Bio1 at 4 °C for 2 h, followed by centrifugation (13,000×g, 4 °C, 20 min). Excess DCP-Bio1 reagent was removed from the clear supernatants using Bio-Spin® 6 columns (732–6227, Bio-Rad, Munich, Germany) equilibrated with lysis buffer. For streptavidine pulldown, 20 μl of prewashed NeutrAvidin Agarose (29201 Pierce, Thermo Scientific, Darmstadt, Germany) was then added to the lysates and incubated at 4 °C overnight with end-over-end rotation. On the next day, the beads were washed 4 times with each 1% SDS, 4 M urea in PBS, 1 M NaCl, and ddH2O. Finally, reducing SDS-PAGE sample buffer was added, samples were incubated at 95 °C for 5 min, and subsequently analyzed by immunoblotting. Lysate blots of labeled HEK293 cells, which were pretreated with different reducing agents, were also assessed for effects on FLT3ITD signal transduction by probing with pY100 anti-phophotyrosine antibodies and evaluating FLT3ITD autophosphorylation signals.
Crosslinking assays to detect FLT3ITD dimerization were performed similarly as described earlier, with some modifications [39,40]. Transiently transfected HEK293 cells in 6-well plates were starved with serum-free medium for 3–4 h, followed by treatments indicated in the figure legends. The cell layers were washed once with pre-warmed (37 °C) PBS, followed by addition of 1 ml pre-warmed PBS and 10 μl 150 mM BS3 solution (in DMSO) and incubation in a cell incubator (37 °C, 5% CO2) for 10 min. Crosslinking was stopped by washing the cells with 2 ml ice-cold PBS on ice, and subsequent lysis in 200 μl RIPA lysis buffer (200 μl/6well) supplemented with protease inhibitor cocktail and PhosStop. Clarified cell lysates were subjected to SDS-PAGE in 5.4% gels and immunoblotting.
Stable transduction of 32D cells, assays of viability and proliferation, and colony assays were performed similar as described previously [41]. Details are given in the Supplementary material. Cell lines, antibodies, and other reagents are likewise described in the Supplementary material.
FLT3 structures (PDB 1RJB [42], and PDB 4RT7 [43]) were rendered using the program UCSF Chimera [44], version 1.12 (build 41623), under Mac OS 10.12.6.
3. Results
3.1. Modulation of FLT3 activity by oxidant and antioxidant treatment
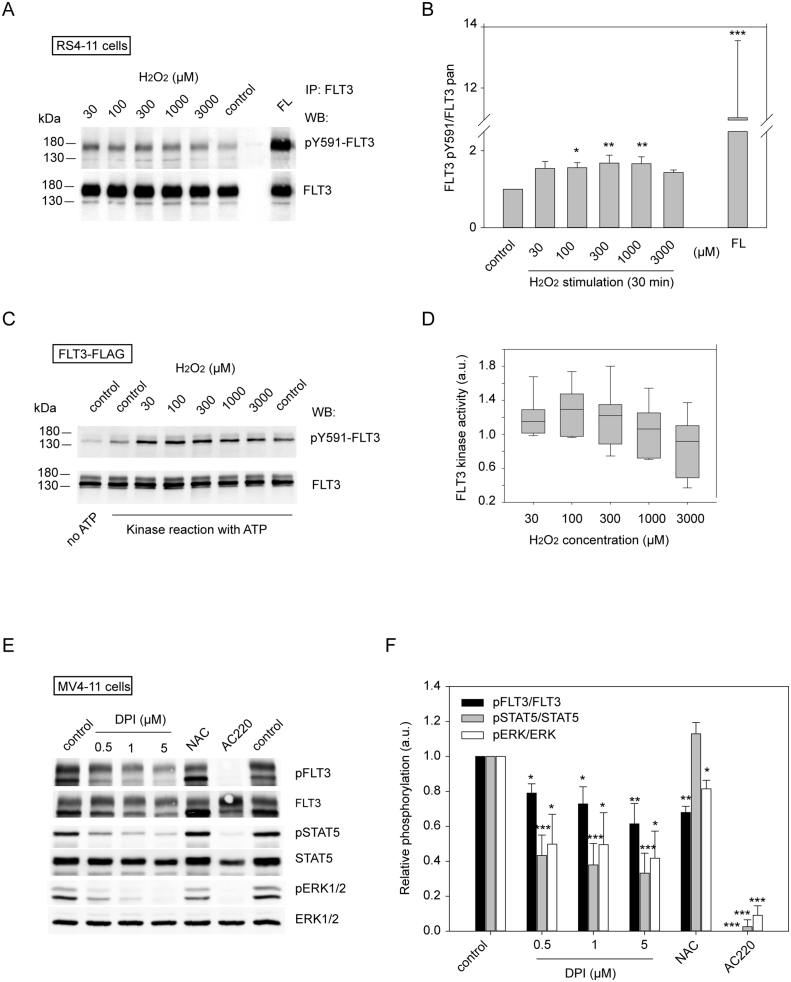
In order to explore the possible regulation of FLT3 by oxidation, we first treated RS4-11 cells, which endogenously express wild-type FLT3, with H2O2 and assessed FLT3 autophosphorylation. Stimulation with FLT3 ligand (FL) was performed for comparison. To detect FLT3 activity, we used an antibody recognizing the phosphorylation site pY591 in the juxtamembrane domain (JMD) of the receptor. Phosphorylation of this motif is a requirement for kinase activation, since it removes a negative constraint of the JMD on signaling activity. Multiple further tyrosine residues (including Tyr589 and 599 in the JMD, Tyr 726 and 768 in the so called kinase-insert domain, Tyr842 in the activation loop, and Tyr955 and 969 in the C-terminal tail) are phosphorylated for full kinase activation and for interaction with signaling molecules (reviewed in Ref. [45]). In FLT3ITD the JMD-mediated regulation is dysfunctional rendering the kinase constitutively active. Tyrosine 591 is therefore constitutively phosphorylated in FLT3ITD and can be used as readout. As shown in Fig. 1A and B, and Supplementary Figs. S1A and B, FLT3 phosphorylation can be stimulated by H2O2. When cells were treated for only short time, stimulation was only seen at high, physiologically irrelevant H2O2 concentrations of 1 or 3 mM (Figs. S1A and B). Upon treatment for 30 min, a moderate but significant stimulation of FLT3 autophosphorylation was already observed with 100 μM H2O2 (Fig. 1A and B). Enhanced FLT3 phosphorylation may be the result of inactivation of relevant PTP, of direct FLT3 activation, or both. To assess direct effects on FLT3, we subsequently tested the effect of H2O2 on FLT3 activity in vitro. We isolated full-length FLT3 from transiently transfected HEK293 cells employing a C-terminal FLAG-tag and elution with FLAG peptide. Kinase activity was measured as autophosphorylation (Fig. 1C and D). At 30–300 μM H2O2 kinase activity tended to be enhanced and at 1–3 mM attenuated. Rather small effects and large assay variation precluded more definitive conclusions. Similar results were obtained using a GST-fusion protein of the FLT3 catalytic domain [46] for kinase assays (data not shown). An important technical difficulty in these experiments is the prevention of spontaneous oxidation in the kinase workup, even if this is performed in an anaerobic hood, as in our experiments. We also assessed the effect of ROS-quenching agents on MV4-11 cells, which endogenously express FLT3ITD, on stably FLT3ITD expressing 32D cells, and on transiently FLT3ITD expressing HEK293 cells. We used diphenyleneiodonium (DPI), a general inhibitor of NADPH-oxidases, and N-acetylcysteine (NAC), which were both previously shown to reduce ROS levels in FLT3ITD-transformed cells [32,47]. FLT3ITD autophosphorylation was significantly attenuated by both agents (Fig. 1E and F). In MV4-11 cells, DPI also caused some reduction of FLT3ITD levels, whereas NAC treatment promoted FLT3ITD levels both by unknown mechanisms. Transforming downstream signaling through signal transducer and activator of transcription (STAT) 5, and extracellular signal regulated kinases 1 and 2 (ERK1/2) was strongly inhibited by DPI, whereas NAC had little effect. Similar results were obtained with FLT3ITD expressing 32D cells (Supplementary Figs. S1C and D). Finally, attenuation of FLT3ITD signal transduction by DPI was also visible in HEK293 cells, however these effects were much weaker, probably because of FLT3ITD overexpression (Supplementary Fig. S1E). The attenuating effects of reducing agents on FLT3ITD phosphorylation are consistent with a possible inhibition by prevention of FLT3 oxidation, or activation of FLT3ITD dephosphorylating PTPs, or both.
Fig. 1.
Effect of H2O2and antioxidant treatment on autophosphorylation of FLT3 in intact cells and in vitro (A, B) RS4-11 cells were serum-starved and treated with H2O2 at the indicated concentrations at 37 °C for 30 min. Positive control was FL stimulation with 100 ng/ml for 10 min. Endogenous FLT3 was immunoprecipitated and autophosphorylation was detected with anti-pY591 FLT3 antibodies (denoted pFLT3); blots were stripped and reprobed with pan-specific anti-FLT3 antibodies (FLT3, C20). (A) Example experiment, (B) quantification of pY-FLT3/FLT3 ratios for multiple experiments (n = 5, significance tested with one-way ANOVA, *p < 0.05, **p < 0.01, ***p < 0.001 for comparison with control) (C, D) FLAG-tagged FLT3 was transiently expressed in HEK293 cells and enriched by anti-FLAG immunoprecipitation and elution with FLAG peptide under anaerobic conditions. Samples were treated with H2O2 for 10 min, and autokinase reactions were performed in absence or presence of 5 μM ATP and 5 mM MnCl2 as indicated. Samples were analyzed by SDS-PAGE and immunoblotting with anti-pY591 FLT3 antibodies, and blots were reprobed with anti-FLT3 (S18). (C) Representative experiment, (D) quantification of pY-FLT3/FLT3 ratios for multiple experiments (n = 7). Box plot illustrating medians within boxes from first quartile (25th percentile) to the third quartile (75th percentile) and whiskers ranging from the 10th to the 90th percentiles. Differences did not reach significance by testing with one-way ANOVA. (E) MV4-11 cells were treated for 8 h with diphenyleneiodonium (DPI, concentrations indicated) or N-acetylcysteine (NAC, 10 mM), or the FLT3 kinase inhibitor AC220 (20 nM). Receptor autophosphorylation, and activation of the downstream mediators of cell transformation STAT5 and ERK1/2 were detected by immunoblotting using phosphospecific antibodies (pFLT3, pSTAT5, pERK1/2) and reblotting with corresponding pan-specific antibodies (FLT3 (S18), STAT5, ERK1/2). (F) Quantification of 6 experiments (treatment time 6–12 h). Values were calculated as arbitrary units (a.u.) relative to untreated cells and are given as means ± SEM. *p < 0.05, **p < 0.01,***p < 0.001 by one-way ANOVA compared to control.
While the data obtained in intact cells suggest that FLT3 may be regulated by oxidation, the in vitro experiments did not reveal a robust and statistically significant direct regulation, possibly because of technical reasons.
3.2. Oxidative cysteine modification can be detected in FLT3 and FLT3ITD
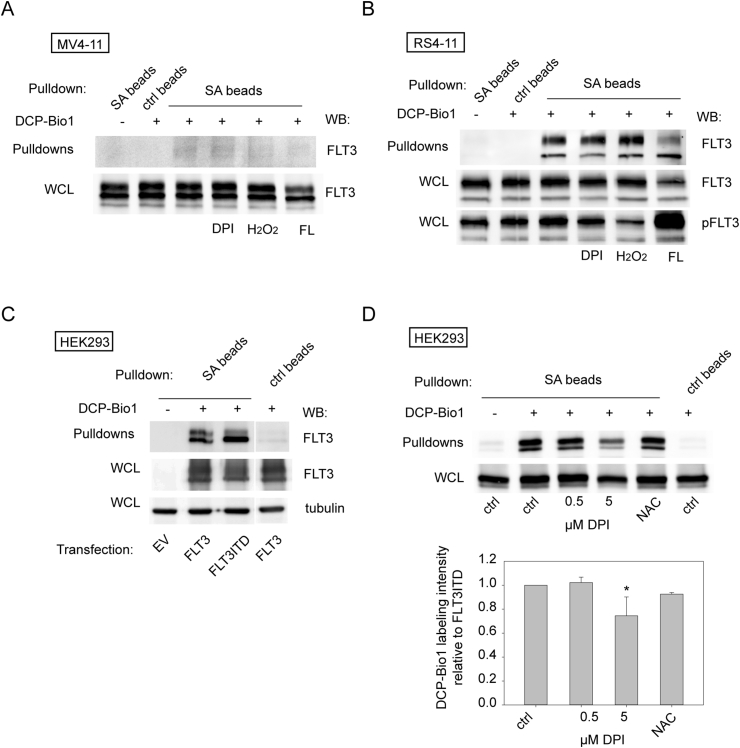
Oxidation can cause formation of cysteine sulfenic acid residues, with several possibilities of further reaction, e.g. leading to glutathionylation or formation of intra- or intermolecular disulfides. Strong oxidant exposure can also promote higher oxidation to sulfinic or sulfonic acid, but this process is less prominent in intact cells and cysteines oxidized to sulfinic or sulfonic acids have hitherto not been found on protein kinases. To further assess the possibility of oxidative modification of FLT3 or FLT3ITD, we therefore employed labeling with DCP-Bio1, a reagent selectively reacting with sulfenic acid residues. This reagent contains the sulfenic-acid specific moiety dimedone, connected to biotin for easy detection. Cells were lysed in the presence of DCP-Bio1 under conditions preventing post-lytic cysteine oxidation [37,38]. Proteins with incorporated DCP-Bio1 were affinity-purified by pulldown with streptavidin beads followed by stringent washings, and FLT3 contained in this fraction was detected by immunoblotting. As shown in Fig. 2, FLT3ITD indeed specifically incorporated DCP-Bio1 under these conditions. This was seen for FLT3ITD at endogenous levels in the human AML cell line MV4-11, albeit rather weakly (Fig. 2A), and readily with wild-type FLT3 in RS4-11 cells (Fig. 2B). Also, FLT3ITD or wild-type FLT3 overexpressed in HEK293 cells (Fig. 2C) were efficiently labeled with DCP-Bio1. In contrast to our expectations, FLT3ITD was, however, not more efficiently labeled than wild-type FLT3, and labeling was only mildly affected by cell treatments with the ROS quencher diphenyleneiodonium (DPI) but not with NAC (Fig. 2B, D), and not with H2O2 treatment, or FL stimulation (Fig. 2A and B). The signal appeared even decreased after ligand stimulation, which was probably caused by less efficient detection of highly phosphorylated FLT3 by the used pan-FLT3 antibody. DPI or NAC treatment of FLT3ITD-expressing HEK293 cells under anaerobic conditions led to reduced DCP-Bio1 incorporation, and further reduction by the ROS quenchers (Supplementary Fig. S1F). However, weak signals and high background in these experiments precluded quantitative assessment. Taken together, the data suggest a certain constitutive level of FLT3 and FLT3ITD oxidation in intact cells, and a mild attenuation by antioxidants.
Fig. 2.
DCP-Bio1 labeling reveals sulfenic acid modification of FLT3ITD cysteine residues. (A, B) MV4-11 cells (A) or RS4-11 cells (B), expressing endogenously FLT3ITD or wild-type FLT3, respectively, were serum-starved and treated as indicated with diphenyleneiodonium (DPI, 10 μM, 4 h), H2O2 (100 μM, 30 min), or FL (100 ng/ml, 10 min). Cells were lysed in presence (or for control absence) of 1 mM DCP-Bio1, and subjected to pulldown with streptavidine (SA) or corresponding control (ctrl) beads. Pulldowns and corresponding lysate aliquots were analyzed for FLT3 (pan-FLT3 S18) by immunoblotting. pFLT3 was also assessed (pY591 phosphorylation) to detect FL stimulation. (C) HEK293 cells were transiently transfected with the indicated plasmids (EV, empty vector). Two days after transfection cells were processed as in A, and B. Note that all parts of the blot in C are from the same membrane and exposure, but have been rearranged for better clarity. Shown results are representative of three experiments with consistent results. (D) DCP-Bio1 labeling of FLT3ITD in HEK293 cells. Cells were pre-treated with DPI at the indicated concentrations or 10 mM N-acetylcysteine (NAC) for 4 h and then processed for DCP-Bio1 labeling as in (A) and (B). Lower panel shows the quantification of 4 independent experiments. *p < 0.05 for comparing with control by t-test.
3.3. Incorporation of DCP-Bio1 is partially mediated by oxidized cysteines 695 and 790
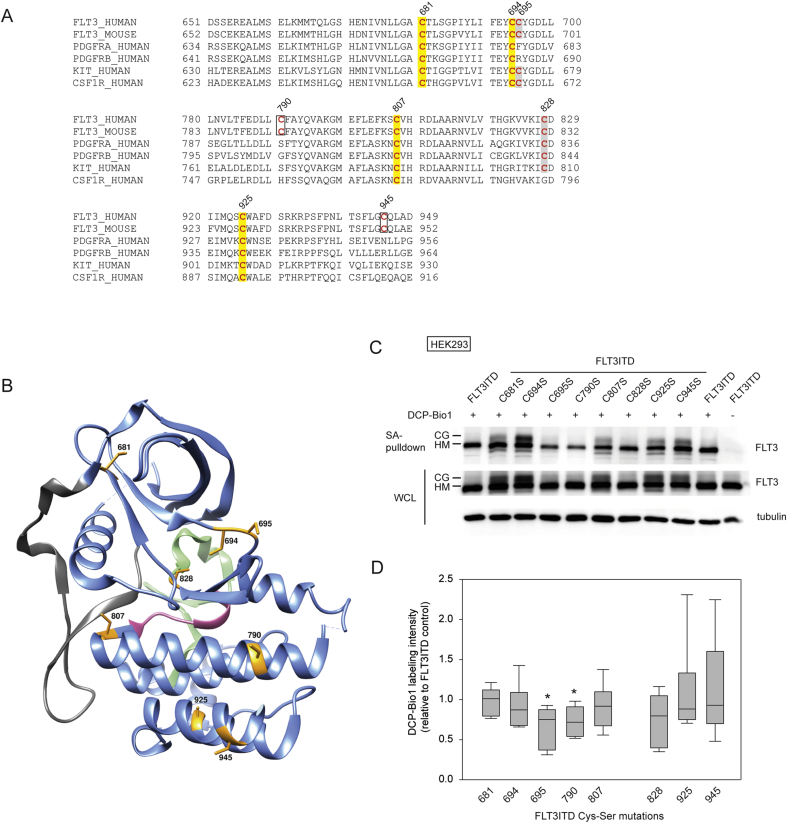
We subsequently considered which FLT3 cysteines may be subject to oxidation. Given the known regulation of other kinases by cysteines in the kinase domain, we focused on the FLT3 cytoplasmic cysteines (Fig. 3A). Several of them are conserved among all or several members of the platelet-derived growth factor receptor (PDGFR) family of receptor-tyrosine kinases, including Cys681, 694, 695, 807, 828, and 925. Cys790 and 945 are unique for FLT3 but conserved among human and mouse FLT3. Inspection of the cysteines in the context of the known crystal structure of the FLT3 cytoplasmic domain [42](Fig. 3B) suggested that, because of distance and orientation, presumably none of these are engaged in intramolecular disulfide bonds. Analysis with the program GETAREA [48] to predict solvent exposure of cysteines revealed putative accessibility of Cys 695, 790, and 945, whereas other cysteines were predicted to be not accessible, at least in the crystal state. We mutated each of these cysteines to serine in the context of a FLT3ITD expression construct. All corresponding FLT3ITD Cys-Ser mutants were expressed in HEK293 cells and subjected to labeling analyses with DCP-Bio1. Loss of those cysteines, which in the parental molecule undergo oxidation and therefore bind label, should result in reduction of the signal in the corresponding Cys-Ser mutant. The results are shown in Fig. 3C and D. The different mutants were generally expressed to similar levels as the parental FLT3ITD protein. The Cys-Ser 828, 925, and 945 mutations did not allow quantitative conclusions with respect to DCP-Bio1 incorporation, due to large assay-to-assay variations. Among the remaining Ser mutations, those of Cys695 and 790 caused a significant reduction in incorporation. It should be noted that sulfenic acid modifications are unstable and that oxidation of several residues may occur. Therefore the significant reduction of the labeling signal in FLT3ITD Cys695Ser and Cys790Ser is remarkable, even though it is moderate. This finding indicates that Cys695 and 790 exist in a partially oxidized state in the context of FLT3ITD.
Fig. 3.
Positions and indirect assessment of oxidation of cysteine residues in the FLT3 cytoplasmic domain. (A) Alignment of class III RTKs reveals conserved (highlighted yellow) and non-conserved unpaired cysteines in the FLT3 cytoplasmic domain. (B) Position of cysteine residues in the cytoplasmic domain of FLT3. The structure of FLT3 (PDB 1RJB) is shown in ribbon representation. The main chain is colored in blue and the Cys residues are highlighted in yellow and their positions in the full-length protein are indicated by black numbers. Several functional motifs of the protein are also indicated: Juxtamembrane domain Tyr572-Trp603 in dark grey, catalytic loop His809-Asn816 in pink, activation loop Asp829-Ala856 in light green. (C) HEK293 cells were transiently transfected with the indicated plasmids expressing FLT3ITD or corresponding Cys-Ser mutants. Two days after transfection, cells were processed as described in Fig. 2 and pulldowns with streptavidine (SA) beads were assessed for presence of FLT3 (S18 antibody). For FLT3ITD, two forms are detectable: The larger (ca. 170 kDa), mature, complex glycosylated (CG) form, and the smaller (ca. 150 kDa) major immature, high-mannose (HM) form. (D) Multiple experiments as in (C) were quantified and the ratio of FLT3 signals in pulldowns and whole cell lysate (WCL) aliquots was calculated. Data are depicted as medians within boxes from first quartile (25th percentile) to the third quartile (75th percentile) and whiskers ranging from the 10th to the 90th percentiles of independent experiments. (n = 6, *p < 0.05 by one-way ANOVA for comparison with control). Results for Cys828Ser, Cys925Ser, and Cys945Ser were very variable and not included in the statistical analysis.
We also attempted to directly detect oxidative modification of FLT3ITD cytoplasmic cysteines by mass spectrometry (MS) using different experimental strategies. While DCP-Bio1 modifications were found in other proteins, modification of FLT3ITD cysteines was not detected with sufficient confidence. Peptides harboring cysteines oxidized to sulfinic or sulfonic acid were not detected either. Labeling of overexpressed FLT3ITD using an indirect labeling approach involving initial alkylation of unmodified cysteines, followed by subsequent mild reduction and alkylation (of initially oxidized and then back-reduced cysteines) using iodoacetyl-PEG2-biotin [49] detected two cysteines, Cys65, and Cys381 in the extracellular domain of FLT3 (Supplementary Fig. S2) both known to be engaged in disulfide-bridges [50]. The failure in identifying oxidatively modified cysteine peptides from the FLT3ITD intracellular domain by mass spectrometry may indicate a very low degree of modification, very rapid turnover of oxidized cysteines, or occurrence of oxidation in only a small fraction of FLT3ITD, perhaps in specific cellular locations. An additional possibility may be a specifically poor recovery of these peptides in the MS workup and ionization process.
3.4. FLT3ITD cytoplasmic cysteine residues are important for kinase signaling activity
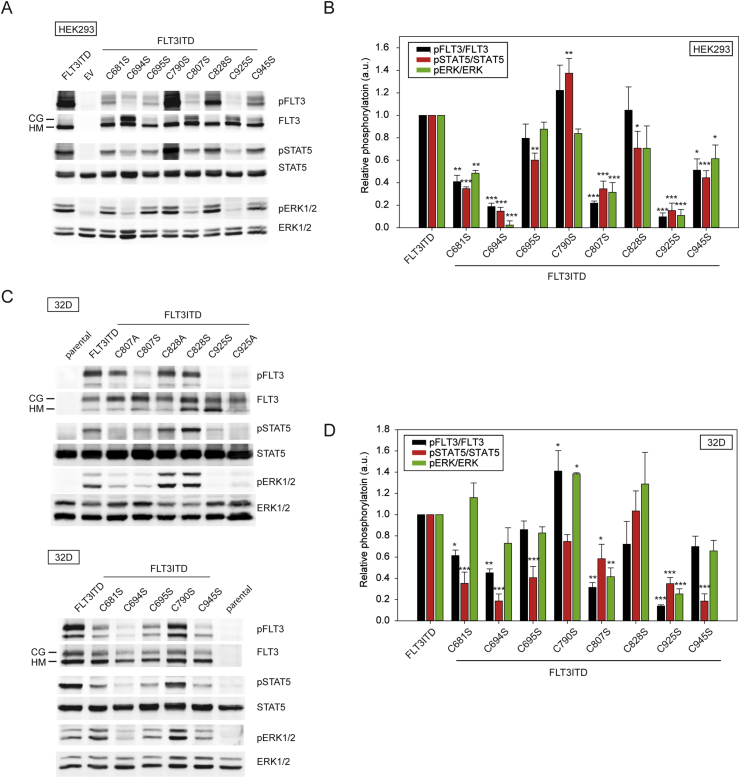
To evaluate the possible functional role of the FLT3ITD cytoplasmic cysteines, we assessed the effect of cysteine-replacement mutations on FLT3ITD autokinase and signaling activity. For a subset of Cys residues (Cys807, 828, and 925), Cys-Ala mutations were analyzed in addition to Cys-Ser mutations. First, autokinase assays were performed in FLT3ITD immunoprecipitates. Mutation of Cys694 and 925 significantly reduced the autokinase activity (Supplementary Figs. S3A and B). The other mutations had no statistically significant effect, but mutations of Cys681, Cys695, Cys807, and Cys945 tended to reduce, while mutation of Cys790 or Cys828 tended to enhance activity. Cys-Ala mutant proteins had similar features as the Cys-Ser proteins (Fig. S3A) and were therefore not further extensively analyzed. Effects of the mutations were much more pronounced when signaling of the different FLT3ITD versions in lysates of intact HEK293 cells were assessed. As readouts we measured autophosphorylation of FLT3ITD at pTyr591, tyrosine phosphorylation of STAT5, and activation of ERK1/2, all parameters correlated with FLT3ITD transforming activity. In these assays, mutations of Cys694, Cys807, and Cys925 strikingly reduced signaling with all readouts (Fig. 4A and B). Consistent with low activity of these FLT3ITD variants, their glycoprotein maturation, indicated by formation of a 170 kDa protein complex glycosylated band (CG) [36,51], was more efficient than that of FLT3ITD, or the active C828S, and C790S versions. Somewhat reduced signaling activity was also observed for Cys681, and 945 mutation. A significant enhancement of STAT5 activation was observed for mutation of Cys790. Notably, significant effects of Cys828 mutations were not detectable in either kinase activity or in signaling analyses. To assess signaling of the FLT3ITD mutants in a more physiological cell background, we generated stable cell pools of 32D cells, a murine myeloid cell line, for all FLT3ITD mutants. Cells were subjected to transduction with lentiviral particles, and subsequent FACS sorting for similar FLT3 surface expression. Signaling analyses revealed similar effects of the cysteine substitutions as in transient transfection experiments. Effects on FLT3ITD autophosphorylation and STAT5 robustly mirrored the previously observed effects, whereas ERK1/2 activity was affected to a lesser extent in the 32D cells (Fig. 4C and D). Consistently, mutation of Cys694, Cys807, and Cys925 reduced signaling strongly, and mutations of cysteines 681, 695, and 945 had some partially attenuating effects. Mutation of Cys828 was without significant effect, and mutation of Cys790 caused a moderate activation. As far as comparatively analyzed, Cys- Ser mutations and Cys- Ala mutations had again similar effects (Fig. 4C).
Fig. 4.
Differential role of FLT3ITD cysteine residues for signal transduction. (A, B) HEK293 cells were transiently transfected with the FLT3ITD constructs as indicated or empty vector (EV). Whole cell lysates (WCL) were subjected to SDS-PAGE and immunoblotting analysis of the indicated signaling proteins using the same antibodies as in Fig. 1 and Fig. S1C. The complex glycosylated (CG) form and the high-mannose (HM) form are indicated. Blots were probed for phosphoproteins first, then stripped and reprobed for total proteins. (A) Representative experiment. (B) Quantification of multiple experiments (n = 4). Values were calculated as arbitrary units (a.u.) relative to not mutated FLT3ITD and are given as means ± SEM. *p < 0.05, **p < 0.01,***p < 0.001 by one-way ANOVA compared to control. (C,D) 32D cells stably transduced with the indicated FLT3ITD expression constructs, or parental 32D cells were washed and starved 4 h from serum and IL-3, then subjected to lysis and immunoblotting analysis as in A, B. (C) Representative experiments. Note that for cysteines 807, 828, and 925, also Cys-Ala mutants were generated and analyzed (left panel). (D) Quantification of multiple experiments (n = 4–7). Only Cys-Ser mutants are included. Values were calculated as arbitrary units (a.u.) relative to not mutated FLT3ITD and are given as means ± SEM. *p < 0.05, **p < 0.01,***p < 0.001 by one-way ANOVA compared to control.
3.5. Effects of cysteine mutations on signaling are not caused by altered dimerization
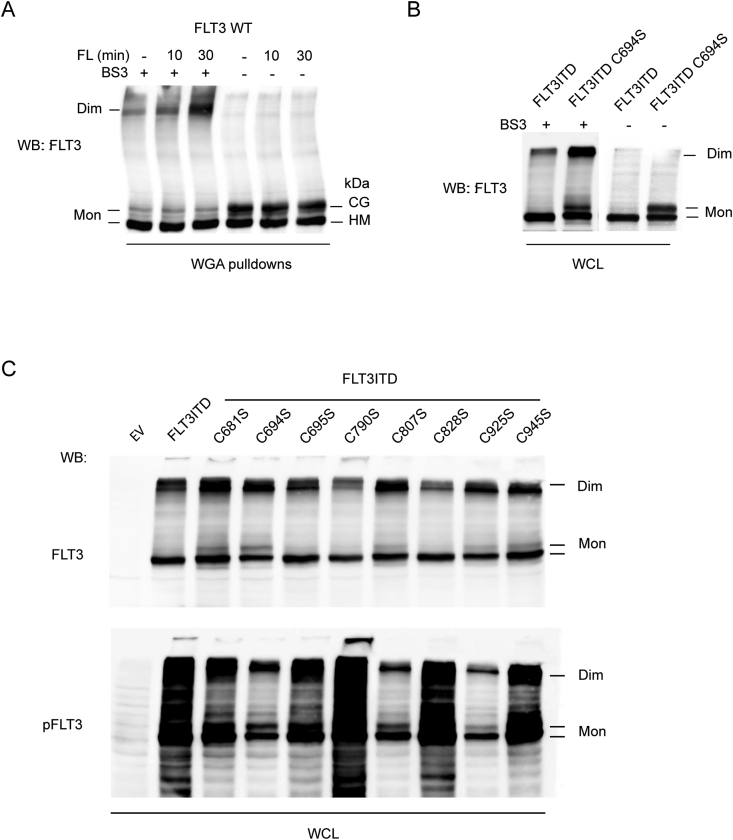
Dimerization or oligomerization is required for ligand-mediated RTK activation. Structural features of FLT3 ligand (FL)-driven dimerization have recently been revealed by resolving the structure of the FLT3 extracellular domain (ECD) in complex with FL [50]. FLT3ITD has been shown to undergo spontaneous dimerization in absence of FL [40], enabling constitutive activity. The FLT3 ECD contains numerous cysteines, but they are all engaged in intramolecular disulfide bridges [50]. We considered that oxidation of cysteines in the cytoplasmic domain may promote formation of intermolecular FLT3ITD dimers. Mutation of such cysteines should then reduce dimerization, and in turn possibly attenuate activity. Dimerization of FLT3ITD and the FLT3ITD Cys- Ser mutants was therefore assessed using a crosslinking-approach. As shown in Fig. 5, FL-induced dimerization (Fig. 5A), and constitutive dimerization of FLT3ITD (Fig. 5B) can be readily detected using this technique. When comparing the Cys- Ser mutants, dimerization efficiency did not correlate with activity observed in the signaling analyses. Mutants with strongly reduced signaling (FLT3ITD Cys694Ser, Cys807Ser or Cys925Ser) were not compromised in their dimerization, rather formed dimers even somewhat more efficiently (Fig. 5C, upper panel). This may have been partially caused by the more efficient surface localization of these catalytically compromised FLT3ITD versions, and thereby better accessibility to the crosslinking agent. FLT3ITD Cys790Ser, which has higher signaling activity than FLT3ITD, did not dimerize more, but rather somewhat less efficiently (Fig. 5C, upper panel). Importantly, the enhanced autophosphorylation activity of FLT3ITD Cys790Ser observed before (Fig. 4) was robustly visible also for the population of dimerized receptor (Fig. 5C, lower panel), suggesting an intrinsic higher kinase activity. Interestingly, enhanced spontaneous kinase activity could also be detected for the Cys790Ser mutant of wild-type FLT3 (Supplementary Fig. S4), further supporting that this residue may be of regulatory importance.
Fig. 5.
Signaling phenotypes of FLT3ITD cysteine mutants are not caused by altered dimerization. HEK293 cells were transiently transfected with the indicated FLT3 expression constructs. Dimers were stabilized by crosslinking with BS3, controls were performed in absence of crosslinker (−). Samples were analyzed by SDS-PAGE with 5.4% gels and immunoblotting. (A) Effect of FLT3 ligand (FL) on dimerization of wild-type (WT) FLT3. Cells were starved from serum 4 h before ligand stimulation. FLT3 was enriched by wheat-germ agglutinin (WGA) pulldown before analysis. The positions of FLT3 monomers (Mon), which exist as immature high-mannose form (HM), or complex glycosylated (CG) mature form, and of FLT3 dimers (Dim) are indicated (detection with pan-FLT3 S18). (B) Spontaneous dimer formation of FLT3ITD, and of the efficiently dimerizing FLT3ITD Cys694S mutant. Analysis of whole cell lysates (WCL) (C) Comparison of dimerization of FLT3ITD and different Cys-Ser mutants as indicated. The blot was also probed with anti-pFLT3 (pY591) to detect the autophosphorylation activity of dimers and monomers.
Molecular dynamics (MD) simulations were performed to explore the possibility of changes in structural dynamics between the wild-type and mutated FLT3 protein. Analysis of the MD runs showed that the single Cys790Ser mutation resulted in a structure with comparable total energy as observed for the wild-type (Supplementary Fig. S5). This might be attributed to the fact that the mutation site is surface-oriented and without close contacts to neighboring residues. Furthermore, the Cys790Ser mutation did not significantly affect the dynamical features of the folded protein core. The trajectory closely resembled the properties observed for the Cys-containing wild-type protein. An increase in the root-mean-square (rms) values (calculated excluding the residues of the unstructured loops) indicated a moderate change in the flexibility of the Cys790Ser mutant as deduced from the respective rms values changing from 2.02 Å (WT) to 2.23 Å (C790S) (Supplementary Fig. S6). Comparison of the MD simulation results for FLT3 wild-type and the FLT3 Cys790Ser mutant revealed also a change in dynamics of cysteines 681, 695, 807, 828, and 925. For example, Cys807 appears less mobile in the Cys790Ser mutant whereas Cys925 exhibits an increased mobility (Supplementary Fig. S7). Thus, modification of specific cysteines may potentially translate into functional alterations through affecting the local conformational dynamics of other cysteines.
3.6. Cytoplasmic cysteine residues are important for FLT3ITD-mediated cell transformation
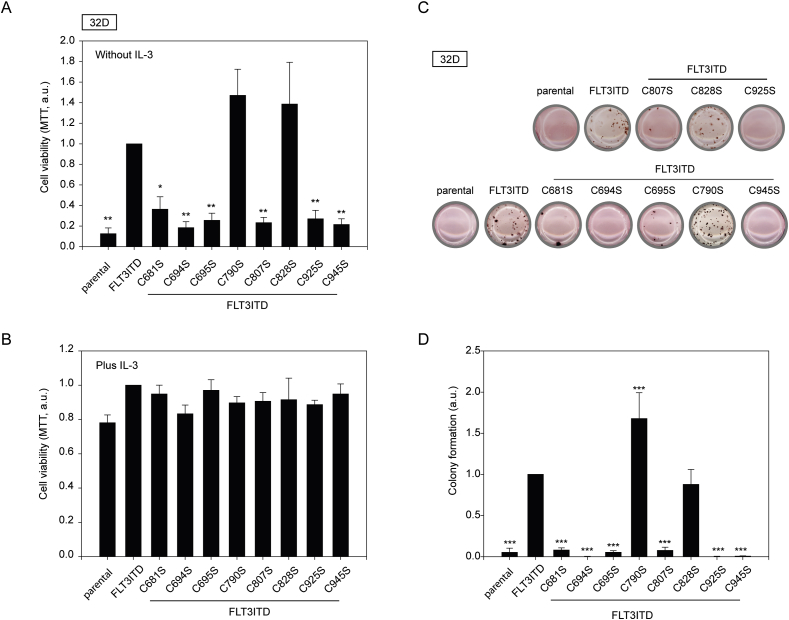
32D cells, which were engineered to express FLT3ITD Cys-Ser mutants or the corresponding parental FLT3ITD were suitable to assess the transforming activity of the different kinases. In absence of IL-3 32D cells do not proliferate and die, unless an active kinase such as FLT3ITD replaces the anti-apoptotic and mitogenic signaling of IL-3. As shown in Fig. 6A, viability and proliferation of cell pools expressing comparable levels of the different FLT3ITD variants correlated with the previously characterized signaling activity. FLT3ITD with mutation of Cys694, 807, and 925 was refractory in sustaining IL-3 independent cell growth. In this assay format, also mutation of Cys681, 695, and 945 nearly abrogated growth. This observation suggests that the detected partial activity of these FLT3ITD variants observed in the signaling assays is insufficient to confer IL-3 independence. FLT3ITD Cys790Ser and Cys828Ser tended to promote growth somewhat better than FLT3ITD, however, these effects were not significant. In presence of IL-3, all derived cell pools exhibited similar growth (Fig. 6B). Interestingly, the Cys828Ser mutation appeared to reduce sensitivity to the kinase inhibitor quizartinib (AC220) detected by either signaling analysis in HEK293 cells (Supplementary Fig. S3C) or by viability assays with 32D cells (Fig. S3D). This notion is consistent with a previously reported interaction of the inhibitor with Cys828 by hydrogen bonding, seen in a co-crystal structure [52].
Fig. 6.
FLT3ITD cysteine residues are essential for transforming activity. 32D cell pools stably transduced with the indicated FLT3ITD versions were subjected to viability/proliferation assays with 3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide (MTT). Assays were performed in absence (A) or presence (B) of IL-3 A. Mean values ± SEM of 3–5 experiments performed with 8 technical replicas are shown. *p < 0.05, **p < 0.01, ***p < 0.001 for comparison with control (FLT3ITD) by one-way ANOVA. Colony assays in methylcellulose (in absence of IL-3) with 32D cell pools stably transduced with the indicated FLT3ITD versions. (C) Representative images. (D) Quantification of independent assays (means ± SEM, n = 8–13, ***p < 0.001 for comparison with control (FLT3ITD) by one-way ANOVA).
We also scored the transforming capability of the FLT3ITD Cys-Ser mutants in colony assays (Fig. 6C and D). The results largely matched the findings in proliferation/viability assays. Mutation of cysteines 681, 694, 695, 807, 925, and 945 abrogated colony growth. The FLT3ITD C828S variant sustained colony growth as efficiently as non-mutated FLT3ITD. Interestingly, cells with FLT3ITD C790S formed colonies more readily than FLT3ITD expressing cells.
Taken together, with the exception of cysteines 790 and 828, all other six tested cytoplasmic cysteine residues appear to be required for FLT3ITD-driven cell transformation.
The effects of mutating the individual cysteines seen in different readouts are summarized in Table 1.
Table 1.
Summary of effects of individual Cys-Ser mutations in different assays. Significant up-or downregulations are indicated by arrows, two arrows symbolize effects of more than 50%. Arrows in parentheses indicate trends or qualitative observations. A lack of significant effects is symbolized with a dash. For DCP-Bio1 labeling, ‘uncertain’ indicates large variation of signals in independent assays, which precluded statistical analysis. For detailed description of the different assays and corresponding conclusions, see the text.
| Assay | Cysteines |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 681 | 694 | 695 | 790 | 807 | 828 | 925 | 945 | ||
| HEK293 FLT3ITD | DCP-Bio1 labeling | – | – | ↓ | ↓ | – | uncertain | uncertain | uncertain |
| Autokinase | (↓) | ↓ | (↓) | (↑) | (↓) | – | ↓ | (↓) | |
| pFLT3ITD | ↓ | ↓↓ | – | – | ↓↓ | – | ↓↓ | ↓ | |
| pSTAT5 | ↓ | ↓↓ | ↓ | ↑ | ↓↓ | ↓ | ↓↓ | ↓ | |
| pERK1/2 | ↓ | ↓↓ | – | – | ↓↓ | – | ↓↓ | ↓ | |
| Dimerization | (↑) | (↑) | – | (↓) | (↑) | (↓) | – | – | |
| 32D FLT3ITD | pFLT3ITD | ↓ | ↓↓ | – | – | ↓↓ | – | ↓↓ | – |
| pSTAT5 | ↓↓ | ↓↓ | ↓↓ | ↑ | ↓ | – | ↓↓ | ↓↓ | |
| pERK1/2 | – | – | – | ↑ | ↓↓ | – | ↓↓ | – | |
| Cell viability (-IL3) | ↓↓ | ↓↓ | ↓↓ | – | ↓↓ | – | ↓↓ | ↓↓ | |
| Colony formation | ↓↓ | ↓↓ | ↓↓ | ↑ | ↓↓ | – | ↓↓ | ↓↓ | |
4. Discussion
In this study we have addressed the question of whether the oncoprotein FLT3ITD may be regulated by oxidative modification of cytoplasmic cysteine residues. Several findings support this possibility. First, the study revealed the importance of several cysteine residues for signaling activity and cell transformation. Cysteines 694, 807 and 925 are essential for FLT3ITD function and their replacement by serine abrogated FLT3ITD signaling, FLT3ITD-driven proliferation and colony formation. Signaling and transformation were also attenuated by mutation of cysteines 681, 695, and 945. Interestingly, mutation of cysteine 790 enhanced FLT3ITD signaling and transforming activity and also increased the kinase activity of wild-type FLT3. These effects are very likely caused by altered intramolecular interactions, because FLT3ITD dimerization is intact for the functionally impaired mutant proteins, and not enhanced by Cys790Ser mutation. Molecular dynamics simulations of FLT3 Cys790Ser are in support of an intramolecular mechanism. Secondly, FLT3ITD readily incorporated DCP-Bio1, a reagent which selectively reacts with cysteine residues in the sulfenic acid state [37]. DCP-Bio1 incorporation was also seen with wild-type FLT3, and was not appreciably affected by cell treatment with H2O2 or ligand stimulation, but mildly by the cellular ROS quencher DPI. Mutation of cysteines 695 and 790 moderately but significantly reduced DCP-Bio1 incorporation. The findings suggest that these two cysteine residues of FLT3/FLT3ITD partially exist in an oxidized state. Third, cell treatments with H2O2 and kinase assays in vitro indicated that FLT3 may be activated by micromolar H2O2 concentrations. Conversely, cell treatments with antioxidants reduced FLT3ITD signal transduction.
The study was prompted by the observation of elevated ROS formation in FLT3ITD transformed cells [8,32]. DCP-Bio1 labeling did not reveal elevated oxidation of FLT3ITD compared with wild-type FLT3 in HEK293 cells. Changes of labeling upon cell treatments known to reduce or enhance cellular ROS levels were mild. Therefore our findings indicate a certain level of constitutive oxidation of both wild-type FLT3 and FLT3ITD, which may be important for signaling function. However, there are known limitations of the DCP-Bio1 labeling technique [38,53], which may have caused the lack of the originally expected quantitative correlations. Some of the DCP-Bio1 reaction may relate to FLT3 oxidation upon cell lysis under aerobic conditions as indicated by lower label incorporation and potentially stronger effect of antioxidants under anaerobic conditions. Analysis of FLT3 oxidation in situ, such as the usage of an appropriate in situ proximity ligation assay [54], will be required to further address the relationship of alterations in cellular ROS formation with the FLT3 oxidation state. Attempts to identify oxidized cysteines in the FLT3ITD cytoplasmic domain directly by mass spectrometry were so far not successful. At this point we cannot conclude stringently which effects of the cysteine-to-serine mutations may indeed reflect effects of oxidation. Oxidation of cysteines 695 and 790, which was indirectly concluded from reduced labeling by DCP-Bio1 of the corresponding Cys-Ser mutants, is consistent with the surface accessibility of these residues in the crystal structure. Moreover, accessibility of Cys695 has recently also been revealed by the reaction of the site with a new irreversible FLT3 inhibitor [55]. Oxidation of Cys695 may potentially reduce reactivity with such an inhibitor. On the other hand, covalent modification of Cys695 with the inhibitor may have similar effects as its mutation, thereby contributing to kinase inhibition. Cys695 is neighbored by Cys694, but their orientation renders formation of disulfide bridges unlikely. The role of the two residues for functional integrity seems also different, since mutating Cys694 has much more drastic effects than mutation of Cys695. We assume that both residues play differential roles for maintaining FLT3 in a functional conformation. Kinase inhibitor reactivity may also be modulated by potential oxidation of Cys828, whose mutation to Ser caused reduced sensitivity to the FLT3 inhibitor quizartinib (AC220).
Cys790, whose mutation to serine further activates FLT3ITD and even activates wild-type FLT3 at least when overexpressed, shows in the FLT3 crystal structure no obvious intramolecular interactions, which would explain activation by mutation. In molecular dynamics simulation, the Cys790Ser variant of FLT3 exhibited a moderately increased flexibility, which may relate to the elevated kinase activity [56,57]. Interestingly, the FLT3 cytoplasmic cysteines appear partially inter-wired with respect to their conformational dynamics. This was concluded from the comparative MD simulations of FLT3 WT and the corresponding Cys790Ser mutant. It is tempting to speculate that oxidative modification of one cysteine may thereby translate into functional alterations through altered conformation of other cysteines.
Dimer formation of FLT3ITD Cys790Ser is not enhanced, but appears rather even reduced. However, the formed dimers are very active, more than those of FLT3ITD. One may speculate that Cys790 can engage in the formation of non-functional FLT3 dimers, i.e. dimers with a non-functional rotational orientation [58]. As consequence of the mutation, the formation of functional dimers may be favored. In intact cells, the mutation may also affect interactions with other regulatory molecules, which potentially depend on Cys790. Interestingly, FLT3 displays many high-molecular weight complexes when analyzed by non-reducing SDS-PAGE (Supplementary Fig. S8). The constitution of these complexes and the role of individual cysteines for their formation remain to be analyzed. Activation of wild-type FLT3 by the Cys790Ser mutation raises the possibility that mutation of Cys790 may be transforming and contribute to leukemia. However, to date no mutations in Cys790 have been reported in AML patient samples (https://cancer.sanger.ac.uk/cosmic), indicating that the phenotype may not be sufficiently penetrant.
Cys807 and 925 are highly conserved among class III RTKs (Fig. 3A). In PDGFRβ, Cys822 (corresponding to Cys807 in FLT3) and Cys940 (corresponding to Cys925 in FLT3) were found as essential for kinase function [24]. PDGFRβ kinase activity was inhibited through alkylation of these cysteines, as well as by mutating them to serine. The authors of that study suggested that Cys822 may participate in the kinase reaction, since it is placed in the catalytic loop, whereas Cys940 was proposed to be important for an active kinase conformation. Our findings for FLT3ITD match these earlier observations for the PDGFβ receptor, in that Cys807 and Cys925 are essential for signaling activity and cell transformation. Notably, mutations of both residues do not impair proper expression, glycoprotein maturation, and FLT3ITD dimerization. Glycoprotein maturation is even enhanced, consistent with our earlier observations that increased kinase activity attenuates maturation [36]. It appears plausible that functional inhibition of FLT3ITD by Cys807 and Cys925 mutation is caused by the same underlying intramolecular mechanisms as previously suggested for PDGFRβ [24]. Moreover, it is very likely that the corresponding cysteine residues in the other RTKs of the family have a similar essential function.
Mutation of Cys945 attenuated signaling activity and abrogated FLT3ITD-driven cell transformation. Interestingly, in co-crystals of FLT3 with the selective kinase inhibitor quizartinib, dimers were reported [43]. In these dimers, the Cys945 residues of two FLT3 molecules are in juxtaposition and in appropriate distance for disulfide-bridge formation (Supplementary Fig. S9). Provided such dimers would exist in solution, they may therefore be stabilized by oxidation of Cys945. Cys945Ser mutation may attenuate formation of such dimers, causing decreased activity of the mutants. However, our dimerization assays did not reveal alterations by the Cys945Ser mutation, rendering such a mechanism unlikely. Again, effects of the Cys945 mutation on the FLT3 kinase conformation may be more likely.
In conclusion, this study suggests that FLT3 may be subject to regulation by oxidative modification. This preposition is based on detection of oxidation by a specific labeling technique and on strong effects of replacing several cytoplasmic cysteine residues with serine. Mass spectrometric identification of oxidized cytoplasmic cysteines is yet elusive and highly warranted to further substantiate this concept. It also remains to be further elucidated to what extent oxidation occurs constitutively, if it is regulated in a temporal and spatial manner, and if alterations of oxidation are part of physiological or pathological FLT3 activity.
Acknowledgement
This work was supported by a grant of the Deutsche Forschungsgemeinschaft (BO 1043/10-2). We are grateful to the Core Facility Flow Cytometry of the FLI – Leibniz Institute on Aging, Jena, for cell sorting.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2019.101325.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Holmström K.M., Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 2014;15(6):411–421. doi: 10.1038/nrm3801. [DOI] [PubMed] [Google Scholar]
- 2.Liou G.Y., Storz P. Reactive oxygen species in cancer. Free Radic. Res. 2010;44(5):479–496. doi: 10.3109/10715761003667554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cadenas S. ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radic. Biol. Med. 2018;117:76–89. doi: 10.1016/j.freeradbiomed.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 4.Sundaresan M., Yu Z.X., Ferrans V.J., Irani K., Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270(5234):296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 5.Rhee S.G. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312(5782):1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 6.Irani K., Xia Y., Zweier J.L., Sollott S.J., Der C.J., Fearon E.R., Sundaresan M., Finkel T., Goldschmidt-Clermont P.J. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science. 1997;275(5306):1649–1652. doi: 10.1126/science.275.5306.1649. [DOI] [PubMed] [Google Scholar]
- 7.Hole P.S., Pearn L., Tonks A.J., James P.E., Burnett A.K., Darley R.L., Tonks A. Ras-induced reactive oxygen species promote growth factor-independent proliferation in human CD34+ hematopoietic progenitor cells. Blood. 2010;115(6):1238–1246. doi: 10.1182/blood-2009-06-222869. [DOI] [PubMed] [Google Scholar]
- 8.Sallmyr A., Fan J., Datta K., Kim K.T., Grosu D., Shapiro P., Small D., Rassool F. Internal tandem duplication of FLT3 (FLT3/ITD) induces increased ROS production, DNA damage, and misrepair: implications for poor prognosis in AML. Blood. 2008;111(6):3173–3182. doi: 10.1182/blood-2007-05-092510. [DOI] [PubMed] [Google Scholar]
- 9.Marinho H.S., Real C., Cyrne L., Soares H., Antunes F. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol. 2014;2:535–562. doi: 10.1016/j.redox.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meng T.C., Buckley D.A., Galic S., Tiganis T., Tonks N.K. Regulation of insulin signaling through reversible oxidation of the protein-tyrosine phosphatases TC45 and PTP1B. J. Biol. Chem. 2004;279(36):37716–37725. doi: 10.1074/jbc.M404606200. [DOI] [PubMed] [Google Scholar]
- 11.Tonks N.K. Protein tyrosine phosphatases: from genes, to function, to disease. Nat. Rev. Mol. Cell Biol. 2006;7(11):833–846. doi: 10.1038/nrm2039. [DOI] [PubMed] [Google Scholar]
- 12.Östman A., Frijhoff J., Sandin A., Böhmer F.D. Regulation of protein tyrosine phosphatases by reversible oxidation. J. Biochem. 2011;150(4):345–356. doi: 10.1093/jb/mvr104. [DOI] [PubMed] [Google Scholar]
- 13.Lee S.R., Kwon K.S., Kim S.R., Rhee S.G. Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor. J. Biol. Chem. 1998;273(25):15366–15372. doi: 10.1074/jbc.273.25.15366. [DOI] [PubMed] [Google Scholar]
- 14.Truong T.H., Carroll K.S. Redox regulation of protein kinases. Crit. Rev. Biochem. Mol. Biol. 2013;48(4):332–356. doi: 10.3109/10409238.2013.790873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corcoran A., Cotter T.G. Redox regulation of protein kinases. FEBS J. 2013;280(9):1944–1965. doi: 10.1111/febs.12224. [DOI] [PubMed] [Google Scholar]
- 16.Giannoni E., Chiarugi P. Redox circuitries driving Src regulation. Antioxidants Redox Signal. 2014;20(13):2011–2025. doi: 10.1089/ars.2013.5525. [DOI] [PubMed] [Google Scholar]
- 17.Kemble D.J., Sun G. Direct and specific inactivation of protein tyrosine kinases in the Src and FGFR families by reversible cysteine oxidation. Proc. Natl. Acad. Sci. U.S.A. 2009;106(13):5070–5075. doi: 10.1073/pnas.0806117106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heppner D.E., Dustin C.M., Liao C., Hristova M., Veith C., Little A.C., Ahlers B.A., White S.L., Deng B., Lam Y.W., Li J., van der Vliet A. Direct cysteine sulfenylation drives activation of the Src kinase. Nat. Commun. 2018;9(1):4522. doi: 10.1038/s41467-018-06790-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paulsen C.E., Truong T.H., Garcia F.J., Homann A., Gupta V., Leonard S.E., Carroll K.S. Peroxide-dependent sulfenylation of the EGFR catalytic site enhances kinase activity. Nat. Chem. Biol. 2011;8(1):57–64. doi: 10.1038/nchembio.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Truong T.H., Carroll K.S. Redox regulation of epidermal growth factor receptor signaling through cysteine oxidation. Biochemistry. 2012;51(50):9954–9965. doi: 10.1021/bi301441e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Truong T.H., Ung P.M., Palde P.B., Paulsen C.E., Schlessinger A., Carroll K.S. Molecular basis for redox activation of epidermal growth factor receptor kinase. Cell Chem. Biol. 2016;23(7):837–848. doi: 10.1016/j.chembiol.2016.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heppner D.E., Hristova M., Dustin C.M., Danyal K., Habibovic A., van der Vliet A. The NADPH oxidases DUOX1 and NOX2 play distinct roles in redox regulation of epidermal growth factor receptor signaling. J. Biol. Chem. 2016;291(44):23282–23293. doi: 10.1074/jbc.M116.749028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heppner D.E., van der Vliet A. Redox-dependent regulation of epidermal growth factor receptor signaling. Redox Biol. 2016;8:24–27. doi: 10.1016/j.redox.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee J.W., Kim J.E., Park E.J., Kim J.H., Lee C.H., Lee S.R., Kwon J. Two conserved cysteine residues are critical for the enzymic function of the human platelet-derived growth factor receptor-beta: evidence for different roles of Cys-822 and Cys-940 in the kinase activity. Biochem. J. 2004;382(Pt 2):631–639. doi: 10.1042/BJ20040624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hjertson M., Sundstrom C., Lyman S.D., Nilsson K., Nilsson G. Stem cell factor, but not flt3 ligand, induces differentiation and activation of human mast cells. Exp. Hematol. 1996;24(6):748–754. [PubMed] [Google Scholar]
- 26.Kikushige Y., Yoshimoto G., Miyamoto T., Iino T., Mori Y., Iwasaki H., Niiro H., Takenaka K., Nagafuji K., Harada M., Ishikawa F., Akashi K. Human Flt3 is expressed at the hematopoietic stem cell and the granulocyte/macrophage progenitor stages to maintain cell survival. J. Immunol. 2008;180(11):7358–7367. doi: 10.4049/jimmunol.180.11.7358. [DOI] [PubMed] [Google Scholar]
- 27.Cancer Genome Atlas Research N. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N. Engl. J. Med. 2013;368(22):2059–2074. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiyoi H., Towatari M., Yokota S., Hamaguchi M., Ohno R., Saito H., Naoe T. Internal tandem duplication of the FLT3 gene is a novel modality of elongation mutation which causes constitutive activation of the product. Leukemia. 1998;12(9):1333–1337. doi: 10.1038/sj.leu.2401130. [DOI] [PubMed] [Google Scholar]
- 29.Kayser S., Schlenk R.F., Londono M.C., Breitenbuecher F., Wittke K., Du J., Groner S., Spath D., Krauter J., Ganser A., Döhner H., Fischer T., Döhner K., German-Austrian A.M.L.S.G. Insertion of FLT3 internal tandem duplication in the tyrosine kinase domain-1 is associated with resistance to chemotherapy and inferior outcome. Blood. 2009;114(12):2386–2392. doi: 10.1182/blood-2009-03-209999. [DOI] [PubMed] [Google Scholar]
- 30.Stirewalt D.L., Radich J.P. The role of FLT3 in haematopoietic malignancies. Nat. Rev. Cancer. 2003;3(9):650–665. doi: 10.1038/nrc1169. [DOI] [PubMed] [Google Scholar]
- 31.Stone R.M., Manley P.W., Larson R.A., Capdeville R. Midostaurin: its odyssey from discovery to approval for treating acute myeloid leukemia and advanced systemic mastocytosis. Blood Adv. 2018;2(4):444–453. doi: 10.1182/bloodadvances.2017011080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Godfrey R., Arora D., Bauer R., Stopp S., Müller J.P., Heinrich T., Böhmer S.A., Dagnell M., Schnetzke U., Scholl S., Östman A., Böhmer F.D. Cell transformation by FLT3 ITD in acute myeloid leukemia involves oxidative inactivation of the tumor suppressor protein-tyrosine phosphatase DEP-1/PTPRJ. Blood. 2012;119(19):4499–4511. doi: 10.1182/blood-2011-02-336446. [DOI] [PubMed] [Google Scholar]
- 33.Jayavelu A.K., Müller J.P., Bauer R., Böhmer S.A., Lässig J., Cerny-Reiterer S., Sperr W.R., Valent P., Maurer B., Moriggl R., Schröder K., Shah A.M., Fischer M., Scholl S., Barth J., Oellerich T., Berg T., Serve H., Frey S., Fischer T., Heidel F.H., Böhmer F.D. NOX4-driven ROS formation mediates PTP inactivation and cell transformation in FLT3ITD-positive AML cells. Leukemia. 2016;30:473–483. doi: 10.1038/leu.2015.234. [DOI] [PubMed] [Google Scholar]
- 34.Jayavelu A.K., Moloney J.N., Böhmer F.D., Cotter T.G. NOX-driven ROS formation in cell transformation of FLT3-ITD-positive AML. Exp. Hematol. 2016;44(12):1113–1122. doi: 10.1016/j.exphem.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 35.Mi T., Wang Z., Bunting K.D. The cooperative relationship between STAT5 and reactive oxygen species in leukemia: mechanism and therapeutic potential. Cancers. 2018;10(10) doi: 10.3390/cancers10100359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmidt-Arras D.E., Böhmer A., Markova B., Choudhary C., Serve H., Böhmer F.D. Tyrosine phosphorylation regulates maturation of receptor tyrosine kinases. Mol. Cell. Biol. 2005;25(9):3690–3703. doi: 10.1128/MCB.25.9.3690-3703.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klomsiri C., Nelson K.J., Bechtold E., Soito L., Johnson L.C., Lowther W.T., Ryu S.E., King S.B., Furdui C.M., Poole L.B. Use of dimedone-based chemical probes for sulfenic acid detection evaluation of conditions affecting probe incorporation into redox-sensitive proteins. Methods Enzymol. 2010;473:77–94. doi: 10.1016/S0076-6879(10)73003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nelson K.J., Klomsiri C., Codreanu S.G., Soito L., Liebler D.C., Rogers L.C., Daniel L.W., Poole L.B. Use of dimedone-based chemical probes for sulfenic acid detection methods to visualize and identify labeled proteins. Methods Enzymol. 2010;473:95–115. doi: 10.1016/S0076-6879(10)73004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gross S., Knebel A., Tenev T., Neininger A., Gaestel M., Herrlich P., Böhmer F.D. Inactivation of protein-tyrosine phosphatases as mechanism of UV-induced signal transduction. J. Biol. Chem. 1999;274(37):26378–26386. doi: 10.1074/jbc.274.37.26378. [DOI] [PubMed] [Google Scholar]
- 40.Kiyoi H., Ohno R., Ueda R., Saito H., Naoe T. Mechanism of constitutive activation of FLT3 with internal tandem duplication in the juxtamembrane domain. Oncogene. 2002;21(16):2555–2563. doi: 10.1038/sj.onc.1205332. [DOI] [PubMed] [Google Scholar]
- 41.Arora D., Stopp S., Böhmer S.A., Schons J., Godfrey R., Masson K., Razumovskaya E., Rönnstrand L., Tänzer S., Bauer R., Böhmer F.D., Müller J.P. Protein-tyrosine phosphatase DEP-1 controls receptor tyrosine kinase FLT3 signaling. J. Biol. Chem. 2011;286(13):10918–10929. doi: 10.1074/jbc.M110.205021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Griffith J., Black J., Faerman C., Swenson L., Wynn M., Lu F., Lippke J., Saxena K. The structural basis for autoinhibition of FLT3 by the juxtamembrane domain. Mol. Cell. 2004;13(2):169–178. doi: 10.1016/s1097-2765(03)00505-7. [DOI] [PubMed] [Google Scholar]
- 43.Smith C.C., Zhang C., Lin K.C., Lasater E.A., Zhang Y., Massi E., Damon L.E., Pendleton M., Bashir A., Sebra R., Perl A., Kasarskis A., Shellooe R., Tsang G., Carias H., Powell B., Burton E.A., Matusow B., Zhang J., Spevak W., Ibrahim P.N., Le M.H., Hsu H.H., Habets G., West B.L., Bollag G., Shah N.P. Characterizing and overriding the structural mechanism of the quizartinib-resistant FLT3 “gatekeeper” F691L mutation with PLX3397. Cancer Discov. 2015;5(6):668–679. doi: 10.1158/2159-8290.CD-15-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25(13):1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 45.Kazi J.U., Rönnstrand L. FMS-like tyrosine kinase 3/FLT3: from basic science to clinical implications. Physiol. Rev. 2019;99(3):1433–1466. doi: 10.1152/physrev.00029.2018. [DOI] [PubMed] [Google Scholar]
- 46.Böhmer F.D., Uecker A. A substrate peptide for the FLT3 receptor tyrosine kinase. Br. J. Hematol. 2009;144(1):127–130. doi: 10.1111/j.1365-2141.2008.07408.x. [DOI] [PubMed] [Google Scholar]
- 47.Tsitsipatis D., Jayavelu A.K., Müller J.P., Bauer R., Schmidt-Arras D., Mahboobi S., Schnöder T.M., Heidel F., Böhmer F.D. Synergistic killing of FLT3ITD-positive AML cells by combined inhibition of tyrosine-kinase activity and N-glycosylation. Oncotarget. 2017;8(16):26613–26624. doi: 10.18632/oncotarget.15772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fraczkiewicz R., Braun W. Exact and efficient analytical calculation of the accessible surface areas and their gradients for macromolecules. J. Comput. Chem. 1998;19(3):319–333. [Google Scholar]
- 49.Boivin B., Yang M., Tonks N.K. Targeting the reversibly oxidized protein tyrosine phosphatase superfamily. Sci. Signal. 2010;3(137):pl2. doi: 10.1126/scisignal.3137pl2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Verstraete K., Vandriessche G., Januar M., Elegheert J., Shkumatov A.V., Desfosses A., Van Craenenbroeck K., Svergun D.I., Gutsche I., Vergauwen B., Savvides S.N. Structural insights into the extracellular assembly of the hematopoietic Flt3 signaling complex. Blood. 2011;118(1):60–68. doi: 10.1182/blood-2011-01-329532. [DOI] [PubMed] [Google Scholar]
- 51.Choudhary C., Olsen J.V., Brandts C., Cox J., Reddy P.N., Böhmer F.D., Gerke V., Schmidt-Arras D.E., Berdel W.E., Müller-Tidow C., Mann M., Serve H. Mislocalized activation of oncogenic RTKs switches downstream signaling outcomes. Mol. Cell. 2009;36(2):326–339. doi: 10.1016/j.molcel.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 52.Zorn J.A., Wang Q., Fujimura E., Barros T., Kuriyan J. Crystal structure of the FLT3 kinase domain bound to the inhibitor Quizartinib (AC220) PLoS One. 2015;10(4) doi: 10.1371/journal.pone.0121177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gupta V., Carroll K.S. Sulfenic acid chemistry, detection and cellular lifetime. Biochim. Biophys. Acta. 2014;1840(2):847–875. doi: 10.1016/j.bbagen.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsutsumi R., Harizanova J., Stockert R., Schröder K., Bastiaens P.I.H., Neel B.G. Assay to visualize specific protein oxidation reveals spatio-temporal regulation of SHP2. Nat. Commun. 2017;8(1):466. doi: 10.1038/s41467-017-00503-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamaura T., Nakatani T., Uda K., Ogura H., Shin W., Kurokawa N., Saito K., Fujikawa N., Date T., Takasaki M., Terada D., Hirai A., Akashi A., Chen F., Adachi Y., Ishikawa Y., Hayakawa F., Hagiwara S., Naoe T., Kiyoi H. A novel irreversible FLT3 inhibitor, FF-10101, shows excellent efficacy against AML cells with FLT3 mutations. Blood. 2018;131(4):426–438. doi: 10.1182/blood-2017-05-786657. [DOI] [PubMed] [Google Scholar]
- 56.Meng Y., Pond M.P., Roux B. Tyrosine kinase activation and conformational flexibility: lessons from Src-family tyrosine kinases. Acc. Chem. Res. 2017;50(5):1193–1201. doi: 10.1021/acs.accounts.7b00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barkho S., Pierce L.C., McGlone M.L., Li S., Woods V.L., Jr., Walker R.C., Adams J.A., Jennings P.A. Distal loop flexibility of a regulatory domain modulates dynamics and activity of C-terminal SRC kinase (csk) PLoS Comput. Biol. 2013;9(9):e1003188. doi: 10.1371/journal.pcbi.1003188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burke C.L., Stern D.F. Activation of Neu (ErbB-2) mediated by disulfide bond-induced dimerization reveals a receptor tyrosine kinase dimer interface. Mol. Cell. Biol. 1998;18(9):5371–5379. doi: 10.1128/mcb.18.9.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.