Abstract
Chronic obstructive pulmonary disease (COPD) is the third leading cause of hospital readmissions in the United States. The quality of care delivered to patients with COPD is known to be lacking across the care continuum, and may contribute to high rates of readmission. As part of the response to these issues, the Centers for Medicare and Medicaid instituted a penalty for 30-day readmissions as part of their Hospital Readmission Reduction Program in October 2014. At the time the penalty was instated, there was little published evidence on effective hospital-based programs to reduce readmissions after acute exacerbations of COPD. Even now, several years later, few published programs exist, and we continue to lack consistent approaches that lead to improved readmission rates. In addition, there was concern that the penalty would widen health disparities. Despite the dearth of published evidence to reduce readmissions beyond available COPD guidelines, many hospitals across the United States began to develop and implement programs, based on little evidence, due to the financial penalty. We, therefore, assembled a diverse group of clinicians, researchers, payers, and program leaders from across the country to present and discuss approaches that had the greatest potential for success. We drew on expertise from ongoing readmission reduction programs, implementation methodologies, and stakeholder perspectives to develop this Workshop Report on current best practices and models for addressing COPD hospital readmissions.
Keywords: COPD, readmissions, quality of care, value-based care, evidence-based care
Contents
Overview
Results
Introduction
Methods
Workshop Objectives
Methods Overview
Results: Summaries and Findings
Stakeholder Perspectives
Patient Perspective
Patient Advocate Perspective
Payer’s Perspective
International Perspective
Case Presentations
One Health System: A Tale of Two Hospitals
Use of Interprofessional Teams
Value-based Care: A Center for Medicare and Medicaid Service–bundled Payments for a Care-Improvement Initiative
The Role of PR
The Role of Patient Navigators
Specialty Care Integration into Primary Care
Quality Improvement and Implementation Framework to Address COPD Readmissions
Summary
Overview
Chronic obstructive pulmonary disease (COPD) is currently the third leading cause of hospital readmissions in the United States (1). Because the quality of care for hospitalized patients with COPD is often inconsistent and does not always follow guideline-recommended care, there is potential to reduce excessive readmissions after hospitalization for COPD exacerbations (2, 3). To address the need for improved care quality and reduced readmissions, the Centers for Medicare and Medicaid (CMS) began penalizing hospitals with excess readmissions after acute exacerbations of COPD (AECOPD) as of October 2014 (start of Fiscal Year 2015) (4). At the time of penalty implementation, there was a lack of published evidence that pointed to effective hospital-based programs to reduce AECOPD admissions and readmissions (5). In addition, there was concern that the CMS Hospital Readmission Reduction Program (HRRP) would widen health disparities, because readmissions occur more commonly among economically disadvantaged communities, and CMS penalties for other diseases are greatest in hospitals with the highest proportion of dually eligible patients (6–9). To avoid a potential penalty, many hospitals across the United States began to develop and implement programs despite the dearth of published evidence. Because details of quality improvement programs are not always published, and many years may pass before the publication of outcomes (10), we sought to synthesize current evidence (both published and unpublished) and describe best practices and models for addressing and reducing AECOPD readmissions across the United States.
These proceedings reflect the results of an official American Thoracic Society (ATS) workshop at the 2016 ATS International Conference.
Results
This workshop provided an opportunity for experts to review and analyze the literature, hear from key stakeholders, including the patient, clinician, and payer perspectives, and review existing readmission reduction programs to summarize the state of practice and identify key barriers and facilitators for success. The following key themes arose:
1. Communication is critical. Our patient and patient advocate stakeholders identified that poor communication at the time of diagnosis, care transitions, and clinical deterioration leads to a worsened patient experience and poor outcomes.
2. Readmissions may be a proxy for other important health factors or outcomes, such as quality of life, social determinants of health (11), adherence deficit, or multimorbidity. Interventions to reduce readmissions may need to expand beyond this single focus regarding COPD-specific treatments to also include improvements in patient education, behavior modification through health coaching, and facilitation of prompt access to outpatient healthcare expertise when needed to impact overall health and quality of life.
3. Implementing COPD guidelines is a necessary, but insufficient, step in reducing readmissions and/or reducing health costs (12). Most previous programs have been successful in improving process measures related to decreasing COPD care variation and increasing the provision of guideline-recommended care for COPD (13). However, due to the readmission penalty targeting all-cause, not just COPD-related, readmissions, efforts to address multimorbidity and social determinants of health are also needed for increased success.
4. The success of readmission reduction programs is difficult to evaluate, due to lack of rigorous study design, such as valid comparators (e.g., randomized parallel studies), and complicated cost frameworks, including variations in diagnostic coding leading to variation in the specific population of interest (13, 14). Programs should embrace randomized schemas or other high-quality program evaluation designs.
5. It is important that programs address quality of care, not just quantity of readmissions. The 30-day readmission metric may not be the most salient measure; the timeframe may need to be adjusted and additional metrics needed to show whether hospital-based interventions improve COPD care and impact patient-centered outcomes, such as mortality, patient satisfaction, adherence, self-efficacy, symptoms, and exercise tolerance. This is particularly important, given the recent association between increased mortality and reduced 30-day readmissions in programs addressing patients with heart failure (15).
6. Improvements in identifying risk factors for readmission and/or “high-risk” patients are needed. Currently, there is no 30-day, COPD-specific risk-prediction tool to identify patients at high risk of 30-day readmission that specifically addresses the CMS HRRP penalty (16). To date, there has been one published tool for 90-day readmissions—the PEARL (Previous admissions, eMRCD score, Age, Right-sided and Left-sided heart failure) score; however, its c-statistic was only around 0.7 (17). Therefore, there is significant room for improvement with regard to developing and validating tools to identify at-risk patients and aid in triaging appropriate care. In the meantime, there are patient characteristics that have been identified as increasing risk, including comorbid anxiety, multimorbidity, and delays to follow-up with primary care physicians (PCPs) that are not addressed by this tool (18–20).
Introduction
COPD is the third leading cause of hospital readmissions in the United States (1). Excess morbidity and mortality associated with acute exacerbations (AECOPD) represents a major public health challenge with a high degree of burden on patients, their families, and society (21–23). Patients with frequent and/or severe AECOPD experience decreased quality of life (21–25), depression (21, 26, 27), and even death up to 1 year after hospitalization (28–30). Direct COPD-related costs are more than $15.5 billion (31). AECOPD and associated hospitalizations account for over half of direct costs, with hospitalizations alone accounting for up to 70% of all costs (22, 31). Therefore, efforts to reduce index and recurrent AECOPD hospitalizations are imperative to improve patient outcomes and reduce societal burden (30).
The quality of care delivered to patients with COPD is known to be lacking across the care continuum, and may contribute to high rates of readmission (2). For example, a minority of patients receive spirometry to confirm a COPD diagnosis (32, 33), despite evidence supporting the usefulness of confirmatory spirometry to reduce admissions and even death (34). In addition, among patients who undergo diagnostic testing, clinicians frequently do not incorporate the results of these tests into care decisions, and may continue COPD-directed therapies, even after pulmonary function tests refute the diagnosis (3). Patients are both under- and overdiagnosed, both with respect to their COPD diagnosis and with respect to exacerbations of COPD (35, 36). Patients hospitalized for AECOPD may not receive all recommended treatments (2). Furthermore, despite the fact that the vast majority of hospitalized patients misuse their respiratory inhalers, evidence-based education during hospitalization to correct this misuse is rarely delivered (37–39). In addition, lack of affordability of medications likely impacts readmissions, because most respiratory inhalers are not tier one on insurance formularies (40). These gaps in care quality may be important targets for interventions designed to reduce readmissions after a hospitalization for COPD.
CMS instituted a penalty for 30-day readmissions as part of their HRRP in October 2014 (4, 41). At the time the penalty was instated, there was little published evidence on effective hospital-based programs to reduce readmissions after AECOPD (5, 42). Even now, only a handful of published programs exist (5, 14, 43–45). In an attempt to avoid the potential for a financial penalty, many hospitals across the United States began to develop and implement programs based on little evidence.
In addition to the challenges posed to all hospitals due to the lack of known effective interventions, concerns existed that safety net hospitals providing care to underserved populations may be at risk for facing excessive penalization (6–9, 46, 47). Published data support these concerns; individuals with lower socioeconomic status are more likely to have COPD, be hospitalized for COPD, be readmitted after a COPD-related hospitalization, and have higher mortality (48, 49).
In this setting, we organized a workshop to identify current best practices and understand unique challenges faced across diverse hospitals and health systems.
Methods
This Workshop Report was prepared according to the standards of the ATS.
Workshop Objectives
To describe best practices and models for addressing and reducing AECOPD readmissions across diverse hospitals and health systems informed by critical stakeholders.
Methods Overview
We assembled a diverse group of stakeholders, including patients, clinicians, researchers, payers, and program leaders, to present and discuss approaches to reducing readmissions. We drew on existing programs, implementation methodologies, and published evidence across COPD and other disease-related readmission reduction programs to develop a workshop program (see Table E1 in the online supplement). This Workshop Report highlights evidence-based best practices to reduce COPD readmissions of significant benefit to clinicians, researchers, hospital administrators, and policymakers. The full methods, including the preworkshop literature review (Table E2), are available in the online supplement.
Results: Summaries and Findings
Stakeholder Perspectives
A primary objective of the workshop was to elicit input on reducing COPD-related readmissions from diverse workshop participants, including patients, patient advocates, purchasers, and members of the international community.
Patient perspective
Although each patient experience is unique, our patient representative identified several common themes that resonated with workshop participants. First, patients with COPD may experience skepticism and disdain from clinicians regarding their diagnosis and outcomes; they have also been blamed for their tobacco use. This negative experience with the healthcare system can complicate care for patients with already high rates of comorbid depression and anxiety, and can compound feelings of guilt that patients have regarding their disease. Second, poor care coordination and clinicians’ lack of knowledge regarding best practices can prevent patients from receiving quality care. For example, our patient workshop member coordinated and activated his own multidisciplinary care team, including a primary care clinician, pulmonologist, and cardiologist. He sought a referral for pulmonary rehabilitation (PR) from his primary care clinician who had not previously informed him of the program or of its benefits for patients with COPD. Other patients might have much more difficulty navigating the healthcare system when faced with multiple barriers, as in the presented example, particularly during the vulnerable period after an AECOPD hospitalization. Our patient representative identified peer support as a valuable tool for patients living with COPD. He provided examples of providing support to others by sharing his own experiences in quitting smoking and attending PR, and he encouraged others to be active partners and to advocate for their own care.
Supporting the perspective provided by our workshop patient participant, the COPD Foundation’s Chronic Obstructive Pulmonary Experience (COPD) Survey found that nearly two-thirds of patients did not have adequate knowledge about COPD exacerbations, and 16% did not know what an exacerbation was at all, highlighting the fundamental inadequacy of current patient education (50).
Patient advocate perspective
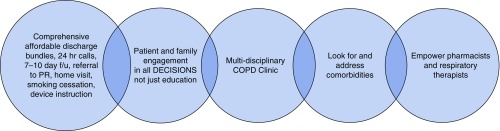
The COPD Foundation is a nonprofit organization, the mission of which includes advocating for the COPD community. The Foundation held summits in 2013 and 2015, which were focused on patient-centered approaches to understanding issues related to COPD readmissions, including barriers to receipt of quality care and identification of best practices (51). Barriers identified by the COPD foundation included: 1) issues with transitions from hospital to home; 2) financial obstacles; 3) a lack of availability within COPD programs, such as PR and peer support; and 4) underutilized tools, such as the electronic health record and dissemination of existing resources (Table 1). In addition, some patients reported a lack of caregiver support at home, which made recovery difficult during posthospitalization periods due to increased emotional and physical stress. Best practices identified by summit participants addressed these barriers, and are summarized in Figure 1.
Table 1.
Barriers to optimal care (breakout sessions, Second Chronic Obstructive Pulmonary Disease Summit)
| Transitions | Finances | Tools/Resources Needed |
|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
||
|
||
|
Definition of abbreviations: EHR = electronic health record; ER = emergency room.
Data from Reference 51.
Figure 1.
Chronic obstructive pulmonary disease (COPD) Foundation Second COPD Readmission Summit: “A few ‘best practices’.” f/u = follow-up; PR = pulmonary rehabilitation.
Payer’s perspective
Payers experience tension between a desire to encourage the best evidence-based practices or interventions, and the expediency with which care models need to move forward without first waiting for a “perfect solution” to the readmission problem. Dr. Daniel Lessler, Chief Medical Officer for the Washington State Health Care Authority (WSHCA), provided a payer’s perspective to workshop participants. The WSHCA is the largest healthcare purchaser in Washington, representing 1.8 million Medicaid enrollees and 350,000 public employees and their dependents (52). Hospital care constitutes a significant proportion of the $10 billion in healthcare costs incurred by WSHCA enrollees annually, with more than $100 million dollars spent on readmissions alone in 2011. From a purchaser perspective, variability in readmissions across hospitals after adjusting for case mix indicates that reducing average readmission rates is likely achievable. Moreover, a system redesign that includes value-based, rather than volume-based, payment is key to sustaining expanded access to healthcare made possible through recent policy changes, such as the Affordable Care Act (53). Strategies employed by the WSHCA to move toward a value-based, and eventual population-based, payment system emphasize the importance of connecting existing community resources to the healthcare system to facilitate care transitions as patients move from hospital to home. For public employee beneficiaries, the WSHCA has partnered with accountable care organizations in a new “total cost of care model” that is designed to reward improvement in healthcare quality and achievement of targeted core performance measures. The program goals include: 1) improving patient experience; 2) integrating physical and mental health programs for comprehensive care; and 3) financial accountability for organizations. By moving away from encounter-based reimbursement to financing “total cost of care,” purchasers hope to encourage innovative care pathways, such as telemedicine, that allow providers to have more flexibility in where and how they care for patients, while improving overall value of care. Whether these types of care models lead to reductions in readmissions is not yet known.
International perspective
Readmission rates for COPD are high across other countries. Across the international community, a handful of efforts have been reported to address the problem of COPD readmissions and improve COPD quality of care. In Canada, the Ontario Ministry of Health and Long-Term Care established the Health System Funding Reform in 2012 (54). Patients with COPD were among the first to be targeted (54). Under the reform, provider funding is based on the types and quantities of patients treated, the services delivered, the quality of care delivered, and patient experiences and outcomes (54). The goal is to incentivize providers to become more efficient and effective by adopting best practices and ensuring that patients in Ontario get “the right care, at the right time, and in the right place.” (54). Initial anecdotal reports indicate that hospitals showed early interest in this initiative and responded by implementing programs to improve COPD care and outcomes. However, costs, process measures, and patient outcomes had not been published at the time of the workshop. Australia and New Zealand have produced a set of guidelines called the COPD-X Plan (55), and Australia has a program that provides training, support, and incentives to primary care providers, surveillance efforts, and medication subsidies (56). These resources are similar to those available in the United States, including a review of COPD care by Han and colleagues (3) and a COPD toolkit developed by a Society of Hospital Medicine Task Force (57).
In summary, both within the United States and across countries around the world, efforts are being made to reduce readmissions after exacerbations of COPD. However, the literature is lacking published data and program descriptions. Most programs identified appear to address overall quality of care, not solely readmissions, and, for the most part, do not address disparities. More work is needed to access information about program experiences in non-U.S. countries, as most of the work is in early stages.
Case Presentations
Five case presentations of COPD readmission reduction programs were presented to illustrate the state of current programs and to address the breadth and depth of COPD readmission reduction programs across the United States. Programs were based on expert opinion and were to be inclusive of diverse geography and type of hospital/health system. The hospitals and health systems included urban academic and community teaching institutions. The interventions discussed varied in scope (multisite vs. single center) and design (ranging from standard quality improvement [QI] frameworks to value-based care models). Two of the programs improved identification of patients with AECOPD upon admission, a critical step in being able to provide program components. Two of the programs developed and used an order set/pathway to deliver their program components. Three of the programs reported reductions in readmission rates. Key lessons, best practices, and comparisons across the programs are described in Table 2.
Table 2.
Hospital Readmission Reduction Programs
| Hospital Type | Health System |
|||
|---|---|---|---|---|
| U.S. Northeast |
U.S. Midwest Academic | U.S. South Academic | ||
| Community Teaching | Academic | |||
| Characteristics | 200+ beds, 15,000 admissions | 800+ beds, 32,000 admissions | 811 beds, 30,000 admissions | 1,150 beds; 49,000 admissions |
| Service | Single pulmonary service | Fellow based; multiple attendings | APN led | One NP |
| Care manager(s) | COPD dedicated CM inpatient/outpatient with close ties to pulmonary practice | Inpatient-specific general CMs | Multiple | Two RNs |
| Physician role | Standard pulmonary consult on all COPD admissions | Pulmonary champions care path development, but not routinely involved in individual patient care | Three physician champions (pulmonologist, hospitalist, pulmonary fellow) | Four COPD leads |
| Program type | QI | QI | QI | BPCI |
| Program elements | CM-led documentation of care plan, education assessment, PR, home visit | Care pathway–led program | APN-led inpatient consult, pharmacy-led medication reconciliation and inhaler education, RN 48 h phone call, APN follow-up visit, APN/MD 24/7 pager, EHR alert for ED visits | RN/NP inpatient consult |
| Real time score for General Health Readmission Risk tool | Medication reconciliation | |||
| Follow-up pulmonary visit | ||||
| Automated and in person post-D/C calls | ||||
| Referral to PR, palliative care, home health, electronic order set | ||||
| System to identify inpatients with AECOPD | N | N | Y | Y |
| Inpatient consult | Single pulmonary service; all seen | Fellow-based | Y—APN | Y—RN or NP |
| Care plan documentation | Y—CM | Y—Routine Hospital D/C | Y—APN | Y—powerplan |
| Education assessment/teaching | Y—CM | Y—Routine Hospital D/C | Y—APN and pharmacists | Y RN or NP |
| RH assessment/referral | Y—CM | N | Y—APN | Y |
| Medication reconciliation | Y—Routine Hospital D/C | Y—Routine Hospital D/C | Y—pharmacists | Y—pharmacists |
| Post-D/C home visit | Y—CM | N—except those qualifying for home VNA | N | N |
| Post-D/C phone call | Y | N—not routine | Y—RN | Y—automated and person–person |
| Post-D/C clinic visit | Y—1–2 wk | Y—pathway recommended 1–2 wk | Y—APN +/− pharmacists 1–2 wk | Y—COPD Clinic, 1–2 wk |
| EHR alert | Y—ED | Y | ||
| Risk score | N | Y | N | Y |
| Direct patient call line/number | Y | Y—health plan based | Y—APN/MD pager | Y |
| Order set/pathway | Y | Y | ||
| Process measures | ALL | <20% utilization of pathway | Improved identification of patients with AECOPD 64–84% | Improved identification of patients with AECOPD 45–85%; improved PR from 5 to <20%; 0–100% phone calls |
| Readmissions | 37% reduction | 27% reduction | 46% reduction | NS |
| Patient feedback | Patients liked program, did not want to be “discharged” from program | |||
| Other info | Site created after D/C trajectory tool being tested in patient subset | Asthma DRG included in BPCI | ||
Definition of abbreviations: AECOPD = acute exacerbations of chronic obstructive pulmonary disease; APN = advanced practice nurse; BPCI = bundled payments for care improvement; CM = case/care manager; COPD = chronic obstructive pulmonary disease; D/C = discharge; DRG = diagnosis-related group; ED = emergency department; EHR = electronic health record; MD = medical doctor; N = no; NP = nurse practitioner; NS = nonsignificant; PR = pulmonary rehabilitation; QI = quality improvement; RN = registered nurse; VNA = visiting Nurse Association; Y = yes.
One health system: a tale of two hospitals
The importance of tailoring programs to specific practice settings was highlighted by the review of a readmissions reduction program implemented at two sites within a single health system (Table 2). The first site, a 200-bed community teaching hospital with a dedicated pulmonary service for patients with COPD with strong ties to primary care, instituted a COPD community care manager to help patients navigate between inpatient and outpatient settings. The care manager interacts directly with the patient and family, documents the care plan, including a customized education assessment, facilitates referrals to PR care, and makes home visits to the patients 2–3 days after discharge. The introduction of the care manager was associated with a 1-year decline in readmissions after a COPD exacerbation from 12% to 6.7%. The health system’s other site was a much larger 800-bed academic university hospital with a fellow-run academic pulmonary service with numerous attendings. In this large hospital, a dedicated COPD care manager was not feasible. Rather, electronic health record–based tools were implemented, including a COPD treatment pathway/order set and a real-time calculation of risk for readmission using a published tool to identify patients at high risk for readmission with the intent of motivating vigilance to identify modifiable factors during admission and after transition (58). Although readmissions still decreased over the course of a year, the magnitude of change was more modest. Specific issues that were identified included low utilization of the pathway/order set and limited variation and predictive ability of the general disease readmission tool when applied to a COPD-specific population. This highlights the need for better, disease-specific tools that use all aspects of the patient past and current admission data to calculate real-time risk predictors.
Use of interprofessional teams
A program at an academic hospital serving a primarily African American, underserved population aimed to address the CMS HRRP by developing an interprofessional, evidence-based approach to reducing COPD readmissions. Using COPD guidelines and readmission programs for other chronic diseases, such as congestive heart failure, as a guide, they developed a systematic approach to a pulmonary consult program with the goal of reaching all patients admitted to the hospital with AECOPD. To ensure that the needs of a large volume of patients could be met, the program included a dedicated, advanced-practice nurse to provide specialized pulmonary care in the hospital and follow-up appointments with patients 1 week after discharge. There were similarities between this program and the programs in the health system example described previously here (Table 2). On the one hand, this hospital was larger in size than the community hospital in the prior example; however, this program incorporated the concept of a single lead practitioner providing care across inpatient and outpatient settings. In addition, the lead practitioner worked with an interprofessional team to ensure that all care elements were completed. The program reduced readmissions by just under 50% in the second half of the first program year compared with the first half. Although these results were promising, this was a quality improvement program and, as such, there was not a control group. Therefore, these results could be due to a secular trend.
Value-based care: a center for Medicare and Medicaid service–bundled payments for a care-improvement initiative
The Center for Medicare and Medicaid Services (CMS) offered bundled payments for care improvement (BPCI) as an optional real-risk care model to provide single payments based on historical data with case mix adjustments and discount the payments up to 3% less than what was paid for the 3 years before for hospital care and extending for care up to 90 days post-discharge (49, 59). If predefined quality metrics are achieved and the participating hospital demonstrates cost savings beyond the negotiated discount, the stakeholders (hospital/physicians/home-health agency) are rewarded with additional payments. The incentive for hospitals to join was to obtain experience with this type of real-risk payment model. The hospital in this example implemented this CMS optional BPCI initiative for COPD, and their program included many similar elements, as described previously here, including an interprofessional team consisting of a single nurse practitioner (NP) and two registered nurses (RNs), with four M.D. COPD leads. The patients received inpatient consults from the lead NP or RN, a postdischarge pulmonology visit within 2 weeks, automated and in-person RN follow-up phone calls and disease education, and referral to home health, PR, and palliative care, as appropriate (Table 2). A standardized electronic order set for AECOPD was also developed and used across the facility. The program found several improvements in process measures, including increased PR referrals and improved phone call rates, but no difference in all-cause readmission rates at 30 days
The role of PR
In a recent systematic review conducted for the ATS/European Respiratory Society statement on “Key Concepts and Advances in Pulmonary Rehabilitation,” PR was found to be associated with an approximate 50% reduction in all-cause readmission following AECOPD (60). When specifically examining the role of PR in 30-day readmissions, the results were mixed, demonstrating that it is difficult to impact short-term outcomes, as the program is traditionally conducted over weeks or months.
The role of patient navigators
Studies are also being conducted to evaluate innovative and patient-centered interventions, such as patient navigators (43, 61). The PCORI (Patient-Centered Outcomes Research Institute)-funded PArTNER (Patient Navigator to Reduce Readmissions) study is a pragmatic trial testing the role of community health workers serving as patient navigators to reduce anxiety and improve social support (coprimary outcomes); the study examines readmissions as a secondary outcome. The community health workers intervention begins in the hospital, then continues with home visits at 2–3 days after discharge plus patient-to-patient peer coaching by phone for another 8 weeks. An innovative feature of PArTNER is the use of patient organizations to deliver the peer-to-peer coaching (e.g., COPD Foundation). Data are not yet published, but, if successful, this could be a model for programs moving forward.
Specialty care integration into primary care
In the previous care models discussed, efforts were made to improve care transitions, including access to existing outpatient services for primary and specialty care. Although pulmonary specialists might provide more consistent guideline-recommended care, specialty care access can be particularly problematic (62). Our current specialty care systems tend to be reactive and referral dependent, requiring PCPs to first recognize an issue and then ask for help from a specialty care system. This is not an efficient approach. Therefore, a system redesign that leverages existing healthcare resources to improve specialty care access for patients and their clinicians is being tested in an ongoing trial within the Department of Veterans Affairs. The program uses existing electronic health records to provide proactive, pulmonologist-facilitated, electronic consults (E-consults) for patients discharged after a hospitalization for an exacerbation of COPD. E-consult recommendations are recorded in chart notes and orders written for PCPs to sign, discontinue, or change as needed; these are timed to occur just before the patient’s follow-up visit when changes in care can be discussed. PCP autonomy, therefore, is maintained and the intervention minimally disrupts clinic workflow. If effective, E-consults could provide a template for interventions within other healthcare systems.
Quality improvement and implementation framework to address COPD readmissions
Several general frameworks have been proposed to improve care quality and reduce readmissions that focus on hospital-to-home transitions. Identified (non–COPD-specific) examples include Project RED (Reengineered Discharge) (63), Project BOOST (Better Outcomes for Older Adults through Safe Transitions) (64), and the IDEAL (Include, Discuss, Educate, Assess, and Listen) Transition in Care Model programs (Table E3) (65). To date, no specific intervention has been designed that reduces COPD readmissions. It may be that a broader framework, which incorporates care for patients even before their first hospital admission, is what is needed to increase high-value care and improve population health for patients with COPD.
Summary
This workshop provided an opportunity for experts to review and analyze the literature, hear from key stakeholders, and review existing readmission reduction programs to summarize the state of practice and identify key barriers and facilitators for a successful reduction of readmissions. The key themes include the following:
1. Communication is critical. Our patient and patient advocate stakeholders identified that poor communication at the time of diagnosis, care transitions, and clinical deterioration leads to a worsened patient experience and poor outcomes.
2. Readmissions may be a proxy for other important health factors or outcomes, such as quality of life, social determinants of health (11), adherence deficit, or multimorbidity. Interventions to reduce readmissions may need to expand beyond this single focus regarding COPD-specific treatments to also include improvements in patient education, behavior modification through health coaching, and facilitation of prompt access to outpatient healthcare expertise when needed to impact overall health and quality of life.
3. Implementing COPD guidelines is a necessary, but insufficient, step in reducing readmissions and/or reducing health costs (12). Most previous programs have been successful in improving process measures related to decreasing COPD care variation and increasing the provision of guideline-recommended care for COPD (13). However, due to the readmission penalty targeting all-cause, not just COPD-related, readmissions, efforts to address multimorbidity and social determinants of health are also needed for increased success.
4. The success of readmission reduction programs is difficult to evaluate, due to lack of rigorous study design, such as valid comparators (e.g., as randomized parallel studies), and complicated cost frameworks, including variations in diagnostic coding, leading to variation in the specific population of interest (13, 14). Programs should embrace randomized schemas or other high-quality program evaluation designs.
5. It is important that programs address quality of care, not just quantity of readmissions. The 30-day readmission metric may not be the most salient measure; the timeframe may need to be adjusted and additional metrics needed to show whether hospital-based interventions improve COPD care and impact patient-centered outcomes, such as mortality, patient satisfaction, adherence, self-efficacy, symptoms, and exercise tolerance. This is particularly important given the recent association between increased mortality and reduced 30-day readmissions in programs addressing patients with heart failure (15).
6. Improvements in identifying risk factors for readmission and/or “high-risk” patients are needed. Currently, there is no 30-day, COPD-specific risk prediction tool to identify patients at high risk of 30-day readmission that specifically addresses the CMS HRRP penalty (16). To date, there has been one published tool for 90-day readmissions—the PEARL score; however, its c-statistic was only around 0.7 (17). Therefore, there is significant room for improvement with regard to developing and validating tools to identify at-risk patients and aid in triaging appropriate care. In the meantime, there are patient characteristics that have been identified as increasing risk, including comorbid anxiety, multimorbidity, and delays to follow-up with PCPs that are not addressed by this tool (18–20).
Supplementary Material
Acknowledgments
This Workshop Report was prepared by an ad hoc subcommittee of the ATS Behavioral Science and Health Services Research, Clinical Problems, and Nursing Assemblies.
Members of the subcommittee are as follows:
Valerie G. Press, M.D., M.P.H.1(Co-Chair)
David H. Au, M.D., M.S.2,3,4‡ (Co-Chair)
Laura C. Feemster, M.D., M.S.2,3 (Co-Chair)
Jean Bourbeau, M.D., M.Sc.5
Mark T. Dransfield, M.D.6
Andrea S. Gershon, M.D., M.Sc.7
Lana Hilling, R.C.P., M.A.A.C.V.P.R.8*
Jerry A. Krishnan, M.D., Ph.D.9
Daniel Lessler, M.D., M.H.A.10*
Steven Meyers*
Richard A. Mularski, M.D., M.S.H.S., M.C.R.11
Frank C. Sciurba, M.D.12
Jamie Sullivan, M.P.H.13
1University of Chicago, Chicago, Illinois; 2Veterans Affairs Puget Sound Health Services Research and Development, Seattle, Washington; 3University of Washington, Seattle, Washington; 4Deputy Editor, AnnalsATS; 5McGill University, Montreal, Quebec, Canada; 6University of Alabama at Birmingham, Birmingham, Alabama; 7Sunnybrook Health Sciences Center, University of Toronto, Toronto, Ontario, Canada; 8John Muir Health, Walnut Creek, California; 9University of Illinois at Chicago, Chicago, Illinois; 10Medicaid, Seattle, Washington; 11Kaiser Permanente, Seattle, Washington; 12University of Pittsburgh, Pittsburgh, Pennsylvania; and 13COPD Foundation, Washington, D.C.
*Member of the subcommittee who was not part of the writing committee (S.M. has no affiliation, as he is a patient, not a doctor/professor).
‡Participation complies with American Thoracic Society requirements for recusal from review and decisions for authored works.
Workshop participants’ content expertise and/or role: V.G.P.—hospital-based interventions, quality improvement, and health disparities; L.C.F.—health behavior, clinical outcomes, and quality of care; D.H.A.—comparative effectiveness research and health inverventions; J.B.—pulmonary rehabilitation and disease management; M.T.D.—Center for Medicare and Medicaid Services bundled payments for care improvement initiative; A.S.G.—population health, policy diversity, and international perspectives; L.H.—coordinator for lung rehabilitation programs; J.A.K.—health services research and administration; D.L.—payer perspective; S.M.—patient perspective; R.A.M.—quality measurement and improvement; F.C.S.—COPD readmissions reduction program; and J.S.—patient/family/community advocacy.
Acknowledgment
The authors thank Kevin Wilson, M.D., American Thoracic Society (ATS) documents editor, for his editorial assistance, and Kimberly Lawrence, ATS, for her administrative assistance and support, and acknowledge additional workshop members who contributed content to the workshop, including Steven Meyers, Lana Hilling, and Daniel Lessler. They also thank Jennifer Park, Nicole Twu, and Mary Akel for their help with this project.
Footnotes
This Official Workshop Report of the American Thoracic Society (ATS) Was Approved by the ATS Board of Directors October 2018
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author Disclosures: V.G.P. received research support from Novartis; served as a consultant for Roundglass. D.H.A. served on a data and safety monitoring board for Novartis; as a consultant for Gilead. J.B. served as a consultant for AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Grifols, and Novartis; received research support from Aerocrine, AstraZeneca, Boehringer Ingelheim, the Canadian Institutes of Health Research, the Canadian Respiratory Research Network, GlaxoSmithKline, and Grifols. M.T.D. served as a consultant and received research support from AstraZeneca, Boehringer Ingelheim, Boston Scientific, GlaxoSmithKline, and PneumRx/BTG; as a consultant for Genentech and Quark Pharmaceuticals; on a data safety and monitoring board for GlaxoSmithKline; received research support from Novartis, Pulmonx, and Yungjin. J.A.K. served as a consultant for CVS/Caremark, eMAX Health, Phillips Respironics, and UpToDate; on a data and safety monitoring board for Sanofi. F.C.S. served on an advisory committee for Boehringer Ingelheim, Circassia, and PneumRx; as a consultant for BTG International; received research support from Boehringer Ingelheim, BTG International, Circassia, PneumRx, Pulmonx, and Spiration. A.S.G., R.A.M., J.S., and L.C.F. reported no relationships with relevant commercial interests.
Contributor Information
Collaborators: on behalf of the American Thoracic Society Behavioral Science and Health Services Research, Clinical Problems, and Nursing Assemblies
References
- 1.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 2.Lindenauer PK, Pekow P, Gao S, Crawford AS, Gutierrez B, Benjamin EM. Quality of care for patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 2006;144:894–903. doi: 10.7326/0003-4819-144-12-200606200-00006. [DOI] [PubMed] [Google Scholar]
- 3.Han MK, Martinez CH, Au DH, Bourbeau J, Boyd CM, Branson R, et al. Meeting the challenge of COPD care delivery in the USA: a multiprovider perspective. Lancet Respir Med. 2016;4:473–526. doi: 10.1016/S2213-2600(16)00094-1. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Medicare & Medicaid ServicesMedicare C for Baltimore. MS 7500 SB, USA. Readmissions-Reduction-Program. 2016. Apr 18 [accessed 2017 Mar 12]. Available from: https://www.cms.gov/medicare/medicare-fee-for-service-payment/acuteinpatientpps/readmissions-reduction-program.html
- 5.Shah T, Press VG, Huisingh-Scheetz M, White SR. COPD readmissions: addressing COPD in the era of value-based health care. Chest. 2016;150:916–926. doi: 10.1016/j.chest.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joynt KE, Jha AK. Characteristics of hospitals receiving penalties under the Hospital Readmissions Reduction Program. JAMA. 2013;309:342–343. doi: 10.1001/jama.2012.94856. [DOI] [PubMed] [Google Scholar]
- 7.Joynt KE, Jha AK. A path forward on Medicare readmissions. N Engl J Med. 2013;368:1175–1177. doi: 10.1056/NEJMp1300122. [DOI] [PubMed] [Google Scholar]
- 8.Joynt KE, Figueroa JE, Oray J, Jha AK. Opinions on the Hospital Readmission Reduction Program: results of a national survey of hospital leaders. Am J Manag Care. 2016;22:e287–e294. [PMC free article] [PubMed] [Google Scholar]
- 9.Carey K, Lin M-Y. Hospital Readmissions Reduction Program: safety-net hospitals show improvement, modifications to penalty formula still needed. Health Aff (Millwood) 2016;35:1918–1923. doi: 10.1377/hlthaff.2016.0537. [DOI] [PubMed] [Google Scholar]
- 10.Stern JM, Simes RJ. Publication bias: evidence of delayed publication in a cohort study of clinical research projects. BMJ. 1997;315:640–645. doi: 10.1136/bmj.315.7109.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ODPHP. Healthy People 2020. Social determinants of health. Washington, DC: Office of Disease Prevention and Health Promotion; 2014 [accessed 2018 Sep 19]. Available from: https://www.healthypeople.gov/2020/topics-objectives/14 topic/social-determinants-of-health
- 12.Global Initiative for Chronic Obstructive Lung Disease (GOLD): Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2018 Report). Global Initiative for Chronic Obstructive Lung Disease. Final revised 2017 Nov 20 [accessed 2018 Jun 3]. Available from: https://goldcopd.org/wp-content/uploads/2017/11/GOLD-2018-v6.0-FINAL-revised-20-Nov_WMS.pdf
- 13.Bhatt SP, Wells JM, Iyer AS, Kirkpatrick DP, Parekh TM, Leach LT, et al. Results of a Medicare bundled payments for care improvement initiative for chronic obstructive pulmonary disease readmissions. Ann Am Thorac Soc. 2017;14:643–648. doi: 10.1513/AnnalsATS.201610-775BC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Press VG, Konetzka RT, White SR. Insights about the economic impact of chronic obstructive pulmonary disease readmissions post implementation of the Hospital Readmission Reduction Program. Curr Opin Pulm Med. 2018;24:138–146. doi: 10.1097/MCP.0000000000000454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta A, Allen LA, Bhatt DL, Cox M, DeVore AD, Heidenreich PA, et al. Association of the Hospital Readmissions Reduction Program Implementation With Readmission and Mortality Outcomes in Heart Failure. JAMA Cardiol. 2018;3:44–53. doi: 10.1001/jamacardio.2017.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Press VG. Is it time to move on from identifying risk factors for 30-day chronic obstructive pulmonary disease readmission? A call for risk prediction tools. Ann Am Thorac Soc. 2018;15:801–803. doi: 10.1513/AnnalsATS.201804-246ED. [DOI] [PubMed] [Google Scholar]
- 17.Echevarria C, Steer J, Heslop-Marshall K, Stenton SC, Hickey PM, Hughes R, et al. The PEARL score predicts 90-day readmission or death after hospitalisation for acute exacerbation of COPD. Thorax. 2017;72:686–693. doi: 10.1136/thoraxjnl-2016-209298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia-Aymerich J, Farrero E, Félez MA, Izquierdo J, Marrades RM, Antó JM Estudi del Factors de Risc d’Agudització de la MPOC investigators. Risk factors of readmission to hospital for a COPD exacerbation: a prospective study. Thorax. 2003;58:100–105. doi: 10.1136/thorax.58.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amalakuhan B, Kiljanek L, Parvathaneni A, Hester M, Cheriyath P, Fischman D.A prediction model for COPD readmissions: catching up, catching our breath, and improving a national problem J Community Hosp Intern Med Perspect 20122DOI: 10.3402/jchimp.v2i1.9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baker CL, Zou KH, Su J. Risk assessment of readmissions following an initial COPD-related hospitalization. Int J Chron Obstruct Pulmon Dis. 2013;8:551–559. doi: 10.2147/COPD.S51507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chenna PR, Mannino DM. Outcomes of severe COPD exacerbations requiring hospitalization. Semin Respir Crit Care Med. 2010;31:286–294. doi: 10.1055/s-0030-1254069. [DOI] [PubMed] [Google Scholar]
- 22.Halpin DMG, Miravitlles M. Chronic obstructive pulmonary disease: the disease and its burden to society. Proc Am Thorac Soc. 2006;3:619–623. doi: 10.1513/pats.200603-093SS. [DOI] [PubMed] [Google Scholar]
- 23.Kanervisto M, Paavilainen E, Heikkilä J. Family dynamics in families of severe COPD patients. J Clin Nurs. 2007;16:1498–1505. doi: 10.1111/j.1365-2702.2006.01804.x. [DOI] [PubMed] [Google Scholar]
- 24.Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:1418–1422. doi: 10.1164/ajrccm.157.5.9709032. [DOI] [PubMed] [Google Scholar]
- 25.Esteban C, Quintana JM, Moraza J, Aburto M, Egurrola M, España PP, et al. Impact of hospitalisations for exacerbations of COPD on health-related quality of life. Respir Med. 2009;103:1201–1208. doi: 10.1016/j.rmed.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Aimonino N, Tibaldi V, Barale S, Bardelli B, Pilon S, Marchetto C, et al. Depressive symptoms and quality of life in elderly patients with exacerbation of chronic obstructive pulmonary disease or cardiac heart failure: preliminary data of a randomized controlled trial. Arch Gerontol Geriatr. 2007;44:7–12. doi: 10.1016/j.archger.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Chow L, Parulekar AD, Hanania NA. Hospital management of acute exacerbations of chronic obstructive pulmonary disease. J Hosp Med. 2015;10:328–339. doi: 10.1002/jhm.2334. [DOI] [PubMed] [Google Scholar]
- 28.Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60:925–931. doi: 10.1136/thx.2005.040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fruchter O, Yigla M. Predictors of long-term survival in elderly patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. Respirology. 2008;13:851–855. doi: 10.1111/j.1440-1843.2008.01367.x. [DOI] [PubMed] [Google Scholar]
- 30.Lindenauer PK, Dharmarajan K, Qin L, Lin Z, Gershon AS, Krumholz HM. Risk trajectories of readmission and death in the first year after hospitalization for chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;197:1009–1017. doi: 10.1164/rccm.201709-1852OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sullivan SD, Ramsey SD, Lee TA. The economic burden of COPD. Chest. 2000;117(2 s) uppl:5S–9S. doi: 10.1378/chest.117.2_suppl.5s. [DOI] [PubMed] [Google Scholar]
- 32.Prieto Centurion V, Huang F, Naureckas ET, Camargo CA, Jr, Charbeneau J, Joo MJ, et al. Confirmatory spirometry for adults hospitalized with a diagnosis of asthma or chronic obstructive pulmonary disease exacerbation. BMC Pulm Med. 2012;12:73. doi: 10.1186/1471-2466-12-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gershon AS, Hwee J, Croxford R, Aaron SD, To T. Patient and physician factors associated with pulmonary function testing for COPD: a population study. Chest. 2014;145:272–281. doi: 10.1378/chest.13-0790. [DOI] [PubMed] [Google Scholar]
- 34.Gershon A, Mecredy G, Croxford R, To T, Stanbrook MB, Aaron SD Canadian Respiratory Research Network. Outcomes of patients with chronic obstructive pulmonary disease diagnosed with or without pulmonary function testing. CMAJ. 2017;189:E530–E538. doi: 10.1503/cmaj.151420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gershon AS, Thiruchelvam D, Chapman KR, Aaron SD, Stanbrook MB, Bourbeau J, et al. Canadian Respiratory Research Network. Health services burden of undiagnosed and overdiagnosed COPD. Chest. 2018;153:1336–1346. doi: 10.1016/j.chest.2018.01.038. [DOI] [PubMed] [Google Scholar]
- 36.Wu H, Wise RA, Medinger AE. Do patients hospitalized with COPD have airflow obstruction? Chest. 2017;151:1263–1271. doi: 10.1016/j.chest.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 37.Press VG, Arora VM, Shah LM, Lewis SL, Ivy K, Charbeneau J, et al. Misuse of respiratory inhalers in hospitalized patients with asthma or COPD J Gen Intern Med 201126635–642.[Published erratum appears in J Gen Intern Med 26:458.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Press VG, Arora VM, Shah LM, Lewis SL, Charbeneau J, Naureckas ET, et al. Teaching the use of respiratory inhalers to hospitalized patients with asthma or COPD: a randomized trial. J Gen Intern Med. 2012;27:1317–1325. doi: 10.1007/s11606-012-2090-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Press VG, Arora VM, Trela KC, Adhikari R, Zadravecz FJ, Liao C, et al. Effectiveness of interventions to teach metered-dose and diskus inhaler techniques: a randomized trial. Ann Am Thorac Soc. 2016;13:816–824. doi: 10.1513/AnnalsATS.201509-603OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patel MR, Press VG, Gerald LB, Barnes T, Blake K, Brown LK, et al. Assembly on Behavioral Sciences and Health Services Research. Improving the affordability of prescription medications for people with chronic respiratory disease: an official American Thoracic Society policy statement. Am J Respir Crit Care Med. 2018;198:1367–1374. doi: 10.1164/rccm.201810-1865ST. [DOI] [PubMed] [Google Scholar]
- 41.Goldfield NI, McCullough EC, Hughes JS, Tang AM, Eastman B, Rawlins LK, et al. Identifying potentially preventable readmissions. Health Care Financ Rev. 2008;30:75–91. [PMC free article] [PubMed] [Google Scholar]
- 42.Prieto-Centurion V, Markos MA, Ramey NI, Gussin HA, Nyenhuis SM, Joo MJ, et al. Interventions to reduce rehospitalizations after chronic obstructive pulmonary disease exacerbations: a systematic review. Ann Am Thorac Soc. 2014;11:417–424. doi: 10.1513/AnnalsATS.201308-254OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krishnan JA, Gussin HA, Prieto-Centurion V, Sullivan JL, Zaidi F, Thomashow BM. Integrating COPD into patient-centered hospital readmissions reduction programs. Chronic Obstr Pulm Dis (Miami) 2015;2:70–80. doi: 10.15326/jcopdf.2.1.2014.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Celli BR, MacNee W ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 45.Global Initiative for Chronic Obstructive Lung Disease. GOLD 2017: Global Strategy for the Diagnosis, Management and Prevention of COPD. Final revised 2017 Nov 20 [accessed 2017 Mar 12]. Available from: http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd/
- 46.Feemster LC, Au DH. Penalizing hospitals for chronic obstructive pulmonary disease readmissions. Am J Respir Crit Care Med. 2014;189:634–639. doi: 10.1164/rccm.201308-1541PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sjoding MW, Cooke CR. Readmission penalties for chronic obstructive pulmonary disease will further stress hospitals caring for vulnerable patient populations. Am J Respir Crit Care Med. 2014;190:1072–1074. doi: 10.1164/rccm.201407-1345LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prescott E, Vestbo J. Socioeconomic status and chronic obstructive pulmonary disease. Thorax. 1999;54:737–741. doi: 10.1136/thx.54.8.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gershon AS, Dolmage TE, Stephenson A, Jackson B. Chronic obstructive pulmonary disease and socioeconomic status: a systematic review. COPD. 2012;9:216–226. doi: 10.3109/15412555.2011.648030. [DOI] [PubMed] [Google Scholar]
- 50.COPD Foundation releases groundbreaking COPE survey results: low patient awareness about COPD exacerbations poses barrier to effective management [new release]. Washington, DC: COPD Foundation. 2014 Jun 17 [accessed 2018 Jul 3]. Available from: http://www.copdfoundation.org/About-Us/Press-Room/Press-Releases/ID/256/COPDFoundation-Releases-Groundbreaking-COPE-Survey-ResultsLow-Patient-Awareness-About-COPD-Exacerbations-PosesBarrier-to-Effective-Management.aspx
- 51.Willard KS, Sullivan JB, Thomashow BM, Jones CS, Fromer L, Yawn BP, et al. The 2nd National COPD Readmissions Summit and beyond: from theory to implementation. Chronic Obstr Pulm Dis. 2016;3:778–790. doi: 10.15326/jcopdf.3.4.2016.0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Washington State Health Care Authority. Data and reports. 2018 [accessed 2018 Jul 17]. Available from: https://www.hca.wa.gov/about-hca/data-reports
- 53.Congress.gov. Text of H.R. 3590 (111th): Patient Protection and Affordable Care Act (Passed Congress version). GovTrack.us. 2010 Mar 23 [accessed 2018 Apr 7]. Available from: https://www.congress.gov/bill/111th-congress/house-bill/3590/text
- 54.Government of Ontario M of H and L-TC. Excellent Care for all—health care professionals—MOHLTC. 2013 Dec 30 [accessed 2018 Jul 3]. Available from: http://www.health.gov.on.ca/en/pro/programs/ecfa/funding/hs_funding_qa.aspx
- 55.Lung Foundation Australia. COPD Guidelines: The COPD-X Plan. 2018 Aug [accessed 2018 Jul 3]. Available from: https://copdx.org.au/
- 56.Lung Foundation Australia. C.O.P.E—COPD Online Patient Education Program. Lung Foundation Australia. 2019 [accessed 2018 Jul 3]. Available from: https://lungfoundation.com.au/patient-support/living-with-a-lung-condition/pulmonary-rehabilitation-2/c-o-p-e-copd-online-patient-education-program/
- 57.Lindenauer P, Au D, Chang W, LaBrin J, Mularski R, Press V.COPD Implementation Toolkit. 2019 [accessed 2019 Jan 7]. Available from: http://www.hospitalmedicine.org/web/quality___innovation/implementation_toolkit/copd/copd_home.aspx%20
- 58.Donzé JD, Williams MV, Robinson EJ, Zimlichman E, Aujesky D, Vasilevskis EE, et al. International Validity of the HOSPITAL Score to Predict 30-Day Potentially Avoidable Hospital Readmissions. JAMA Intern Med. 2016;176:496–502. doi: 10.1001/jamainternmed.2015.8462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Centers for Medicare & Medicaid Services. Bundled Payments for Care Improvement (BPCI) Initiative: general information. Center for Medicare & Medicaid Innovation. 2018 Dec 11 [accessed 2017 Mar 12]. Available from: https://innovation.cms.gov/initiatives/bundled-payments/
- 60.Spruit MA, Singh SJ, Garvey C, ZuWallack R, Nici L, Rochester C, et al. ATS/ERS Task Force on Pulmonary Rehabilitation. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:e13–e64. doi: 10.1164/rccm.201309-1634ST. [DOI] [PubMed] [Google Scholar]
- 61.Ursan ID, Krishnan JA, Pickard AS, Calhoun E, DiDomenico R, Prieto-Centurion V, et al. Engaging patients and caregivers to design transitional care management services at a minority serving institution. J Health Care Poor Underserved. 2016;27:352–365. doi: 10.1353/hpu.2016.0026. [DOI] [PubMed] [Google Scholar]
- 62.Gershon AS, Macdonald EM, Luo J, Austin PC, Gupta S, Sivjee K, et al. Concomitant pulmonologist and primary care for chronic obstructive pulmonary disease: a population study. Fam Pract. 2017;34:708–716. doi: 10.1093/fampra/cmx058. [DOI] [PubMed] [Google Scholar]
- 63.Berkowitz RE, Fang Z, Helfand BKI, Jones RN, Schreiber R, Paasche-Orlow MK. Project ReEngineered Discharge (RED) lowers hospital readmissions of patients discharged from a skilled nursing facility. J Am Med Dir Assoc. 2013;14:736–740. doi: 10.1016/j.jamda.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 64.Maynard GA, Budnitz TL, Nickel WK, Greenwald JL, Kerr KM, Miller JA, et al. 2011 John M. Eisenberg Patient Safety and Quality Awards. Mentored implementation: building leaders and achieving results through a collaborative improvement model: innovation in patient safety and quality at the national level. Jt Comm J Qual Patient Saf. 2012;38:301–310. doi: 10.1016/s1553-7250(12)38040-9. [DOI] [PubMed] [Google Scholar]
- 65.Agency for Healthcare Research and Quality. Guide to Patient and Family Engagement in Hospital Quality and Safety: Strategy 4: care transitions from hospital to home: IDEAL discharge planning. 2017 Dec [accessed 2018 Mar 12]. Available from: https://www.ahrq.gov/professionals/systems/hospital/engagingfamilies/strategy4/index.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.