Abstract
Inspired by self-assembling peptides found in native proteins, deliberately designed engineered peptides have shown outstanding biocompatibility, biodegradability, and extracellular matrix-mimicking microenvironments. Assembly of the peptides can be triggered by external stimuli, such as electrolytes, temperature, and pH. The formation of nanostructures and subsequent nanocomposite materials often occur under physiological conditions. The respective properties of side chains in each amino acids provide numerous sites for chemical modification and conjugation choices of the peptides, enabling various resulting supramolecular nanostructures and hydrogels with adjustable mechanical and physicochemical properties. Moreover, additional functionalities can be easily induced into the hydrogels, including shear-thinning, bioactivity, self-healing, and shape memory. It further broaden the scope of application of self-assemble peptide materials. This review outlines designs of self-assembly peptide (β-sheet, α-helix, collagen-like peptides, elastin-like polypeptides, and peptide amphiphiles) with potential additional functionalities and their biomedical applications in bioprinting, tissue engineering, and drug delivery.
Highlights
-
•
The review article summarizes the general strategies to design self-assemble peptides and discusses their mechanisms.
-
•
Self-assemble peptide hydrogels with advanced functionalities are discussed.
-
•
Biomedical applications in tissue engineering, drug delivery, and potential bioprinting are summarized.
-
•
Perspective development and current challenges of self-assemble peptide materials are discussed.
1. Introduction
Amino acids are the building blocks of protein which perform a number of functions including catalyzing, supporting cells in ECM, DNA replication, transporting desired molecules, and so on [1]. Since late 1980s, short peptides extracted from native proteins with self-assembling properties began to attract increasing interest [2]. Subsequently, numerous engineered peptides mimicking the conformation of self-assembling ones derived from protein molecules were developed. The secondary structure such as β-sheet and α-helix were utilized to be responsible for assembly triggered by external stimuli. Ionic strength, pH, and temperature are the mostly used triggers to initiate the formation of supramolecular nanostructures [3]. The bottom-up strategy for nanoclusters and nanocomposite hydrogels based on stimuli-responsiveness behavior exhibits potency for biomedical applications due to its biocompatibility, biodegradability, ECM-like microenvironments. Moreover, the various properties (negative or positive charge, hydrophobicity, hydrophilicity, and polarity) of the side chains in amino acids provide possibilities for chemical modification with infinite sequence combinations. Additionally, the primary structure of amino acids provide sites for attachments to polymeric substrates. Morever, various properties other than self-assembly can be facilely induced, including self-healing, shear-thinning, shape memory, and so on.
The designed peptides can be fabricated on the automated solid phase peptide synthesizer efficiently and economically. Formation of nanostructures and subsequent networks constructed by self-assemble peptides often occurs under physiological conditions. For some instances, the electrolytes in DMEM medium can trigger the assembly of the peptides, enabling entrapment of cells within the scaffolds. With adjustable mechanical strength and capability to incorporate bioactive peptide motifs, improved efficiency of self-assemble peptide materials has been demonstrated in tissue engineering and regeneration, drug delivery, biosensors, and immunotherapy [[4], [5], [6], [7]]. This review article starts with a summarization of the general strategies to design self-assemble peptides and their mechanisms in Section 2. Then, it outlines advanced functionalities which are capable to be incorporated into these materials in Section 3 and their biomedical applications in Section 4, respectively. Finally, this review is closed with a brief discussion of perspective development and current challenges in this area.
2. Design principles of self-assembling peptides and mechanism of self-assembly
Several strategies have been developed for design of self-assemble peptides over the past decades. Different supramolecular nanostructures constructed by engineered peptides with beta sheet, alpha helix, triple helix, ELP-like, or amphiphile structures were designed to self-assemble with specific properties and functionalities.
2.1. β-sheets peptides
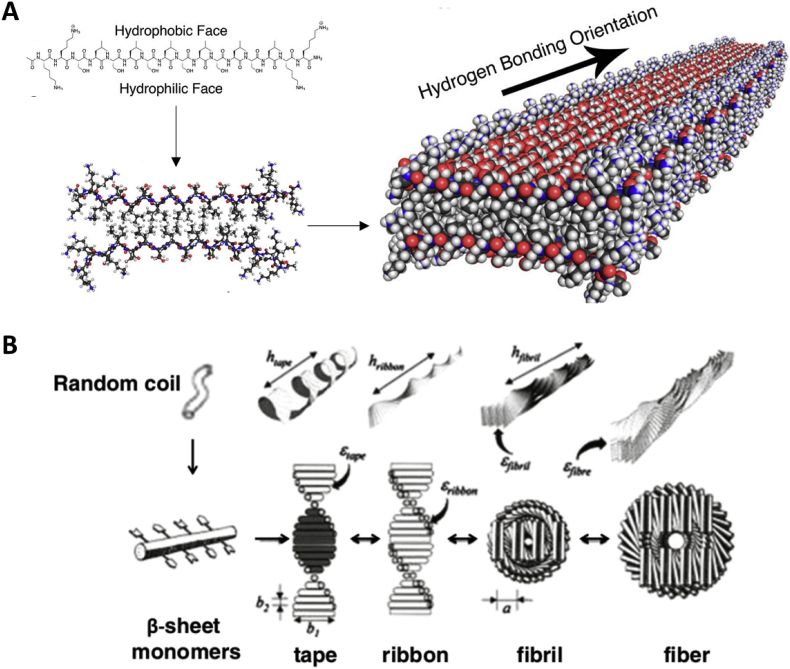
Inspired by the self-assembling peptide amino acid sequence (Ac-(AEAEAKAK)2-CONH2) found in the yeast protein zuotin [8], numerous designs of peptides that form β-sheets and the subsequent self-assembled structures emerged in the past decades. These peptides are composed of 12–16 alternating hydrophilic (R or K as positive residues, and E or D as negative residues) and hydrophobic residues (A or L residues) that drive the formation of β-sheet structures with a hydrophobic face on one side and a hydrophilic face on the other in aqueous media. The charge arrangements on the hydrophilic side differ in different designs and can be classified into four mostly used moduli: “- + - + - + -+” is modulus I; “--++--++” is modulus II; “---+++” is modulus III; and “----++++” is modulus IV [9]. As shown in Fig. 1, self-assembling of peptides is initiated by adding electrolytes to reduce the electrostatic repulsions of the peptide monomers. Then hydrophobic interactions play an important role by excluding surrounding aqueous media between hydrophobic faces of neighboring peptides. β-sheet bilayers that results from parallel or anti-parallel alignment of peptides form fibrils via intra and intermolecular hydrogen bonds and electrostatic interactions [[10], [11], [12], [13]]. Despite the first generation of self-assembling peptides which associate into nanofibers, other hierarchical structural arrays including ribbons, tapes, fibrils can also be designed [3,[14], [15], [16]].
Fig. 1.
(A) β-Sheet forming short peptides with alternating ionic complementary properties [17]. (Copyright © 2017 American Chemical Society) (B) Short amphiphilic β-sheet peptides that self-assemble into anti-parallel nanotapes and further aggregate into ribbons and higher order structures. In a recent paper, shorter sequences (P9-6 and P7-6) with aliphatic hydrophobic resides (in green) were demonstrated to form fibrillar structures [18]. (Copyright (2001) National Academy of Sciences.)
Moreover, several self-assembling protein-derived peptides have been identified to form extended beta strands. The alanine glycine-rich peptide was obtained via hydrolysis of Bormbyx mori silk fibroin and forms cylindrical fibers attributed to extended beta sheets formation [19]. Peptide sequences derived from bone marrow homing peptides were biotinylated by the Gelain lab, which exhibits enhanced assembly performance and scaffold biomechanics [20]. Furthermore, functional motifs were linked to the peptides to promote cell adhesion, proliferation, and differentiation with physiologically comparable mechanical properties and nanofiber morphology [21,22].
2.2. α-helix
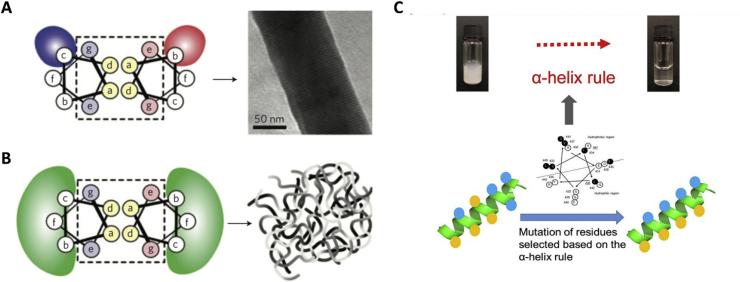
Alpha helix, which is a basic folding pattern found in proteins, have been used to develop a number of fibrous biomaterials. These α-helix coiled coils are also called hydrogelating self-assembling fibres (hSAF) [23,24]. A seven residues (abcdefg, shown in Fig. 2) sequence serves as the basic heptad unit for hSAF. The a and d positions (hydrophobic residues) as well as the e and g positions (charged residues) are responsible for directing the dimer interface. The residues at b, c, and f positions are exposed on surface of the assemblies and vary in different designs. These positions are often occupied by hydrophobic alanine residue or glutamine with propensity to hydrogen bonds. The configuration of the coiled coil motifs can be manipulated by changing environmental conditions such as temperature, pH and ionic strength [25]. Decoration of RGD to hSAF peptides can enhance biocompatibility for potential biomedical applications such as tissue engineering and drug delivery [[26], [27], [28], [29]]. Dual-peptide hSAFs systems were also developed for better control of assembly [30].
Fig. 2.
Hydrogelating SAF design principles. A, Thick SAF designs, specific charged interactions between certain b and c positions lead to peptide alignment and fibre thickening. B, Thin hSAFs, specific interactions at all b, c and f sites were replaced with weaker, more-general interactions, to result in smaller, more flexible, bundles of thinner fibres [31]. (Reproduced and adapted with permission from Nature) C, Hypothesis of self-assembly from peptide monomers to supramolecular networks of Schematic model based on the α-helix rule [32]. (Reproduced and adapted with permission from Nature).
2.3. Collagen-like peptides
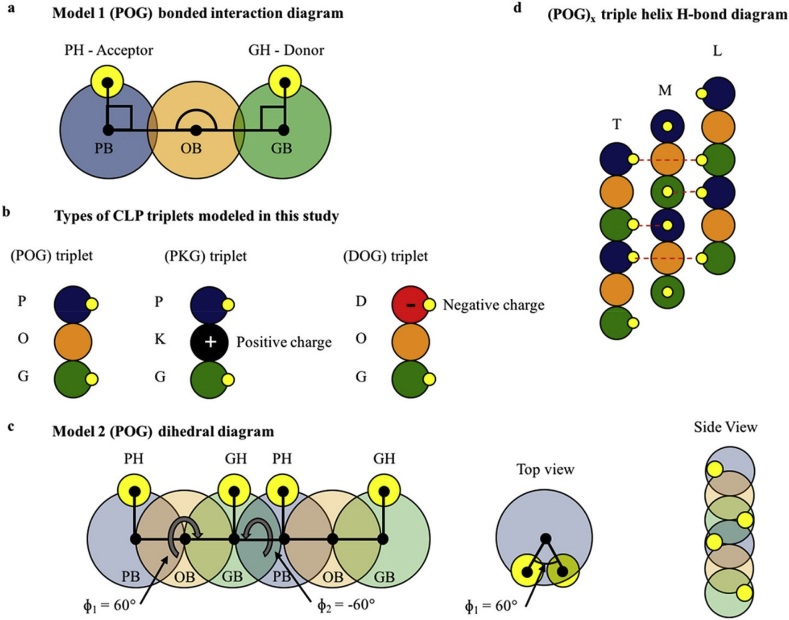
Collagen is the main component of the extracellular matrix in humans. Although the collagen family of proteins contains 28 different types with widely varied architectures and functionalities, they all possess triple helix bundles [34]. Periodic interchain hydrogen bonding between three polyproline-II type helices make up the collagen triple helix (Fig. 3) [35]. Each strand are made of “G-X-Y” tripeptide repeats, where the X and Y positions are often occupied by proline and (4R)-hydroxyproline in native collagens. Due to the limitation of using animal-derived collagens, collagen-like peptides (CLPs) were used as the alternative to eliminate thermal instability and contamination with pathogenic substance [[36], [37], [38]]. Various CLPs have been developed not only to mimic the collagen fibril formation, but also to assemble into higher order hierarchical structures via π-π stacking interactions, lateral electrostatic interactions, and metal-triggered assembly to achieve precise chemical compositions and molecular structures [35,[39], [40], [41], [42], [43], [44], [45]].
Fig. 3.
(a) Diagram of model 1 coarse-grained (POG) triplet with bonded interactions shown by lines and angles connecting specific bead types. (b) Types of CLP triplets used in the simulations studied in this work. (c) Diagram of model 2 coarse-grained CLP strand with the dihedral angles listed. (d) (POG)x triple helical H-bond diagram highlighting the donor–acceptor interaction and the offset of the individual strands where T represents the trailing strand, M represents the middle strand, and L represents the leading strand [33]. (Copyright © 2018 American Chemical Society.).
2.4. Elastin-like polypeptides
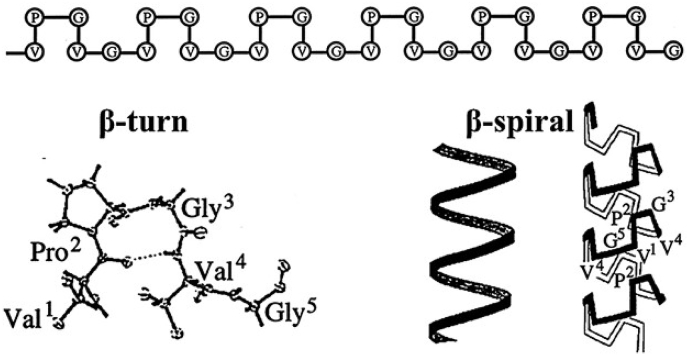
Elastin-like polypeptides (ELPs) are a class of genetically engineered peptides derived from tropoelastin which self-assemble under certain physiological conditions [46,47]. ELPs are composed of repeat “VPGXG” sequences, where the polarity of X position (a variable amino acid except proline) determines the transition temperature of the peptides [48]. These biopolymers display transition from random coils to cylindrical micelles formed by beta sheet when the temperature is raised above the transition temperature (Fig. 4) [14,49]. ELPs polypeptides have been used in a number of designs for multifunctional biomaterials due to their rubber-like elasticity, large extensibility, flexible deformation, high resilience upon stretching, thermos-responsiveness, and biodegradability [[50], [51], [52], [53]]. ELPs matrix have been reported using as injectable hydrogel via network formation at body temperature (above the transition temperature) [48]. The temperature dependent phase behavior of the polypeptides were also applied with both soluble and insoluble phase as carriers for drug delivery [54,55].
Fig. 4.
Poly(VPGVG) adopts a β‐spiral structure at temperatures above Tt. The dedeVPGVG repeats form β‐turns, stabilized by intramolecular hydrogen bonds between the backbones of the first and fourth residues of the pentapeptide. These β‐turns arrange into helical β‐spirals, which are represented with and without displaying the structure of the β‐turns in the turns of the helix. Reprinted with permission from Urry D. W., Physical chemistry of biological free energy transduction as demonstrated by elastic protein‐based polymers [56]. (Copyright © 1997 American Chemical Society.).
2.5. Peptide amphiphile
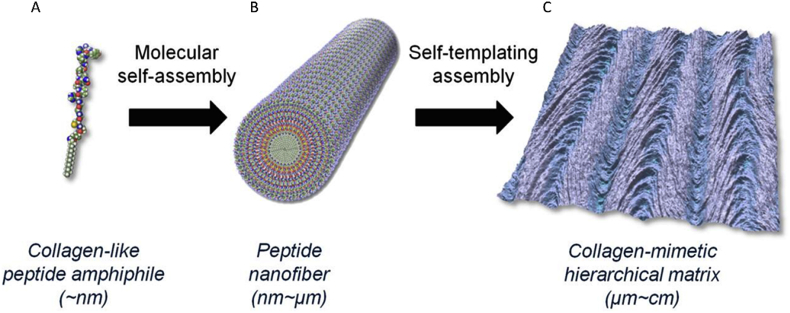
Peptide amphipiles are hybrids that incorporated a peptide head with single or multiple alkyl chains. Attaching peptides with self-assembly capability to amphiphilic surfactants provides a strategy to control the nanostructure assembling into cylindrical or fibril geometries instead of micelles [57,58]. Both negative charged and hydrophobic amino acid residues were incorporated into the engineered peptide amphipiles to balance the charge in cell culture media and facilitate self-assembly [59]. Hydrogen bonding and beta sheets drive the peptide amphiphiles to assemble into nanofibers, of which the diameter and length can be adjusted by the lipid tail length, number of charged sequences, and self-assembly conditions [50]. This structure also forces the attached bioactive motifs to present on the surface for better interaction with protein receptors and cells. The self-assembly of β-sheets peptides attached amphipiles can also be triggered by adding electrolyte solution, culture media washing, and pH balancing to reduce the electrostatic repulsion between monomers like beta sheet forming peptides [60,61]. In addition, collagen-like peptide amphiphiles were developed by coupling phage display-identified collagen-like peptides to long-chain to assemble into diverse, hierarchically organized nanofibrous structures [45] (Fig. 5).
Fig. 5.
Design of peptide amphiphile into biomimetic structures: Molecular model of a peptide amphiphile (A), nanofiber: a 12-mer peptide identified through phage display library screening is coupled to a hydrophobic fatty acid chain (C16), which undergoes self-assembly into nanofibers (B), and Resulting materials can serve as soft- and hard-tissue guiding scaffolds (C) [45]. (Copyright © 2015 American Chemical Society.).
3. Functionalities of self-assembling peptides hydrogels
The good biocompatibility and mechanical properties enable self-assembling peptides hydrogels to be applied in various biomedical applications. Furthermore, bioactive properties, sol-gel transition, self-healing, and shape memory functionalities were incorporated into designs of them to further broaden the scope of applications of self-assembling peptides.
3.1. Bioactive properties
Even though bare self-assemble peptide hydrogels exhibit excellent biocompatibility and biodegradability both in vitro and in vivo, enhancing cell adhesion and integrin binding is necessary for their applications in biomedical fields. Bioactive functional motifs have been extracted from extracellular matrix (ECM) proteins due to their capability to bind to an integrin. Small size of these ligands are preferred due to little influence to the assembled nanostructure. Intracellular signaling pathways including calcium channels, kinases, and phosphatases can be mediated via choosing specific bioactive ligands. For example, cell-cell and extracellular matrix interaction can be mediated by collagen derived RGD and DGEA ligand to promote cellular interaction through transmembrane receptors [50,[62], [63], [64]]. Glycoproteins such as laminin and fibronectin are also important components in ECM. They are responsible for cell attachment, migration, adhesion, and differentiation [[65], [66], [67]]. A number of ligands, including LGTIPG, PDGSR, LRE, LRCDN, IKLLI, KQAGDV, and REDV, were extracted from ECM glycoproteins to promote cell adhesion [62,64,68,69].
Among these sequences, IKVAV, derived from laminin, is one of the most studied ligands which can promote cell adhesion, differentiation, and neurite outgrowth [[70], [71], [72]]. IKVAV sequence has been attached to RADA16-I peptides or amphiphiles for neural tissue engineering and wound healing. Promotion of nerve regeneration with elongated axonal and attenuated astrogliosis as well as reduced glia scarring were obtained [73,74]. Another lamini-derived peptide motif —YIGSR was demonstrated possess capability to promote not only neurite outgrowth but also endothelial cell adhesion. Incorporation of this bioactive ligands to beta sheet forming peptides by Cui successfully improve spatial learning and memory recovery in Alzheimer's Disease rats by implantation [75].
Osteoinductive functional peptide sequences were extracted from BMPs which interact with the target cells through heterotetramers of serine/threonine kinase to activate the Smad intracellular pathway [76,77]. A peptide sequence comprising “NSVNSKIPKACCVPTELSAI” residues was developed and demonstrated with osteogenic activity [[78], [79], [80]]. Bone forming peptides (BFP, such as GQGFSYPYKAVFSTQ, KGGQGFSYPYKAVFSTQ) were extracted from bone morphogenetic protein-7 (BMP-7), and osteogenic conversions of BFP treated stem cells to osteoblastic phenotype were observed [[81], [82], [83]]. Angiogenic bioactive ligands were also explored to facilitate formation of new blood vessels. VEGF-derived QK peptides was designed to mimic the 17–25 helix region of the growth factor [84]. It has been found that the peptide sequence play a similar role as VEGF to induce endothelial cell proliferation, migration, and invasion [85].
3.2. Sol-gel transition
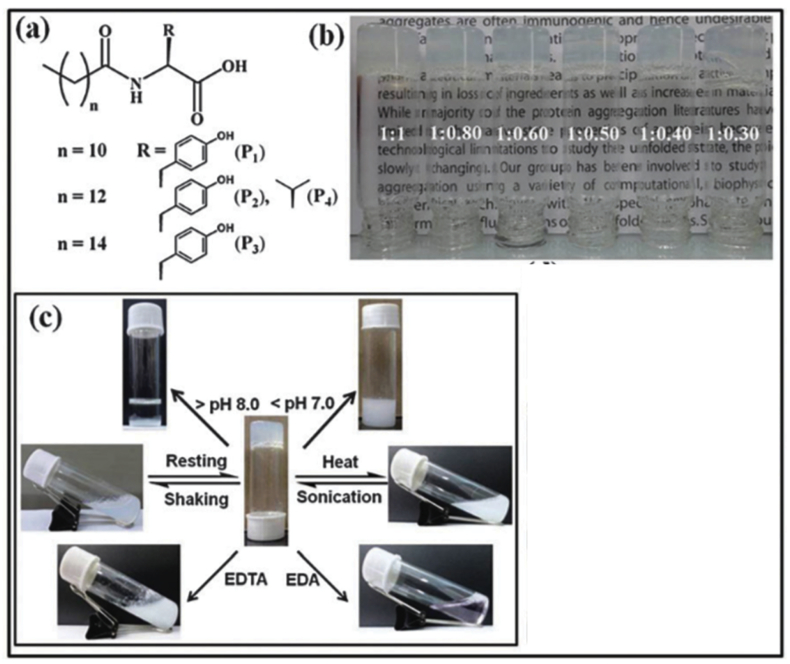
Injectable hydrogels as delivery vehicles for drug, cells, and other bioactive molecules attracts increasing interest for their hydrophilic property, non-invasive procedure, and biocompatibility. Sol-gel transition property is desirable for designing injectable hydrogels. For some peptide hydrogels, the transition from liquid to a solid-like state (gel) can be triggered by altering ionic strength, temperature, or pH of the medium [86]. The beta sheet formed nanofibers can undergo a gel-sol transition upon sonication, and this process is reversible by re-assembly of fragmented fibrils [13,87]. The reversible sol-gel transition property efficiently eliminates the syringe-clogging during injection. A series of peptide amphiphile based multi-stimuli responsive sol-gel transition have also been developed [88,89]. As an example, tyrosine based amphiphiles were synthesized, and hydrogels were formed in appearance of Ni2+ ions. The hydrogel exhibits thixotropic property with various stimuli including pH, temperature, mechanical forces and external chemicals (Fig. 6) [88]. This property not only enable the hydrogels to be used as injectable vehicles, but also provide a strategy to uniformly encapsulate cells within the hydrogels. ELP-like peptide, for its temperature dependent transition, has also been used to enhance the mechanical property of the network after injection. An engineered ELP polypeptides with RGD cell adhesion ligands were designed by Heilshorn group which utilize the ELP aggregates at body temperature to enhance the hydrogel network formation [48].
Fig. 6.
(a) Chemical structures of various amphiphiles (P1—P4). (b) Pictures of glass vials containing metallo-hydrogels obtained from different proportions of the P3 and nickel salt (NiCl2). (c) Multi-stimuli responsiveness shown by the hydrogel obtained from P3 [88]. (Copyright ©The Royal Society of Chemistry 2014.).
3.3. Self-healing
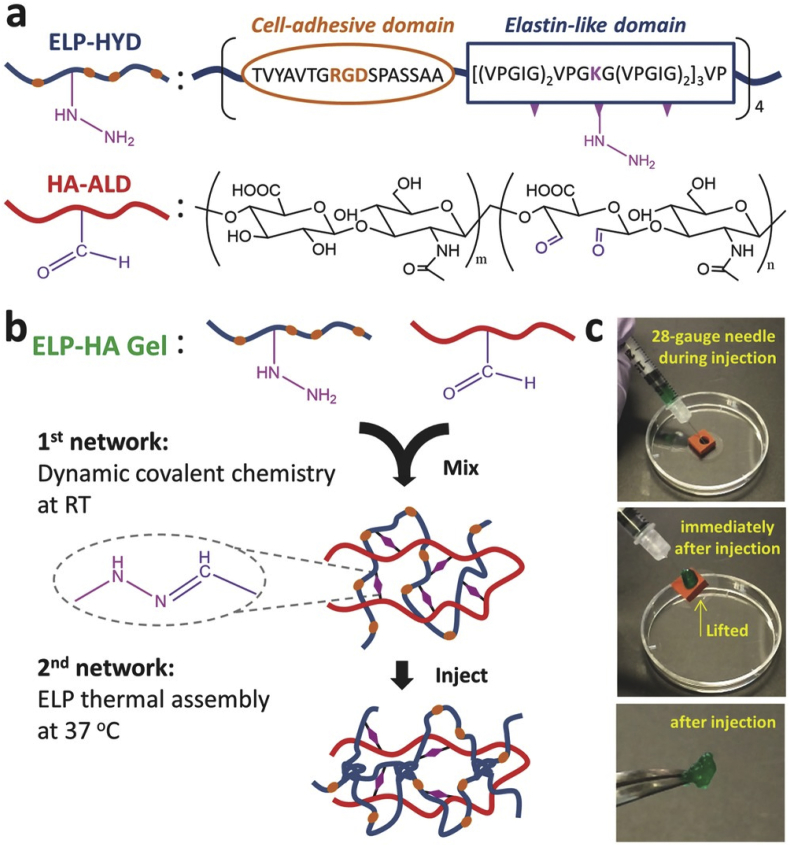
Inspired by the spontaneous healing after injury in most biological systems, designs of smart synthetic materials with similar self-healing property presented in last decades. Self-healing materials can be classified into two groups: extrinsic and intrinsic self-healing materials. Recovery of extrinsic self-healing materials upon damage highly relies on the release of healing agents which are sequestered in capsules or vessels and spread uniformly within the material [90,91]. Spontaneous recovery in these self-healing materials is finite, as self-healing event cannot occur anymore after depletion of the local healing agents. Instead of using healing agents, inherent reversibility of chemical bonds or physical interactions are utilized to be responsible for recovery in intrinsic self-healing materials. Single or combination of multiple reversible physical interactions, including hydrogen bonds, hydrophobic interactions, ionic interactions, π-π stacking and host-guest interactions, have been reported successfully healing the damaged region of materials [[92], [93], [94], [95]]. For self-assemble peptide hydrogels, the networks are formed due to combination of intra and intermolecular hydrogen bonds, hydrophobic, electrostatic interactions, the reversibility of which enables potential self-recovery of the materials. In some designs, the peptide hydrogels exhibit mechano-responsive self-healing property, which is also called thixotropic property. The self-healing of these hydrogels is often coupled with reversible sol-gel transition. Upon mechanical shaking or shear stress, a gel-sol transition is induced and the networks are quickly reformed after removing the mechanical load. Reversible sol-gel transition with self-healing property provide an optimized model for injectable vehicle, in which the drug and/or cells can be delivered to the target site with neither hydrogel loss nor syringe clogging. A peptide hydrogel constructed by 20 amino acids and responsible to DMEM was developed by Schneider. The shear-thinning property of the hydrogel allow delivery of cells to the target sites with homogenous distribution and unaffected viability [96]. Dynamic covalent bonds were also induced into peptide sequence to enforce mechanical strength of the self-healing hydrogels. As an example, a dual-crosslink system comprising hydrazone bonds and ELP aggregates induced by temperature stimuli were reported (Fig. 7) [48]. The ELP peptide transition upon temperature change to 37 °C greatly enhance the gelation rate and mechanical property of the hydrogel after injection.
Fig. 7.
Injectable ELP–HA hydrogels. a) ELP–HA is composed of hydrazine-modified elastin-like protein (ELP-HYD) and aldehyde-modified hyaluronic acid (HA-ALD). b) Schematic of ELP–HA hydrogel formation. c) Photographs demonstrating the injectability and rapid self-healing of ELP–HA hydrogels [48]. (Copyright © 2017, John Wiley and Sons).
3.4. Shape memory
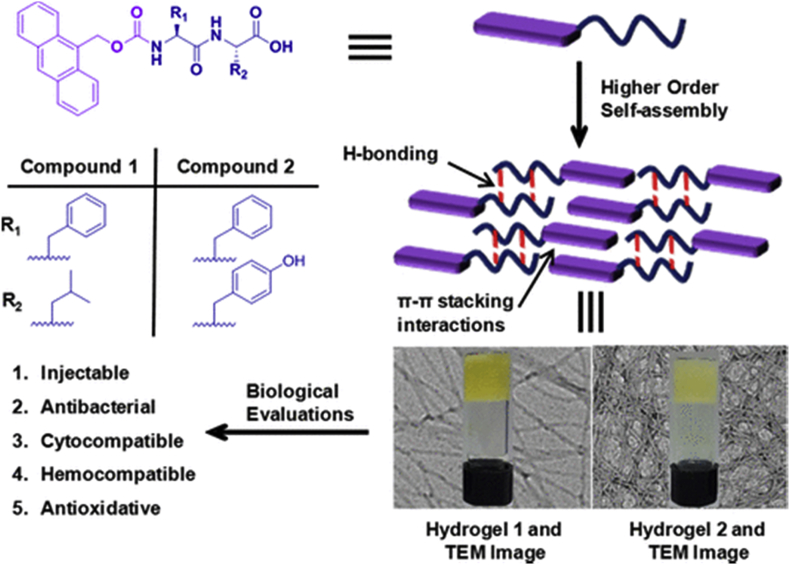
Besides good biocompatibility, non-cytotoxicity, and biodegradability, the size and shape of hydrogels for biomedical application is a key factor in design. To keep the shape during biomedical applications requires physiological stress-bearing of the hydrogels. However, complete restoration of the mechanical performance for the self-assemble peptide hydrogels is still a challenge as most of them are crosslinked via relatively weak non-covalent interactions. To improve the property of the hydrogels, shape memory has been incorporated into designs of self-assemble peptide hydrogels. The design of shape-memory hydrogel requires macroporous structure to allow water flowing freely; high polarity for water absorption; and resilience for shape recovery [97,98]. ELP polypeptides were used to design shape memory hydrogels due to its high elasticity and resilience. For example, a multifunctional elastin hybrid hydrogel was developed by Liu with a fixed shape, excellent flexibility, and injectable property [97]. Additionally, the stress dependent conductivity enables its potential application as pressure sensitive sensor for in vivo use. Shape memory property can also be induced by attaching certain functional groups to peptide amphiphiles. As an example, a shape-memory and self-healable hydrogel was prepared by attaching Amoc (9-anthracenemethoxycarbonyl) cap to dipeptides [99]. Tough and shape-memory hydrogel was formed at a concentration of 20 mM via π-π stacking and hydrophobic interactions with potential anti-bacterial property (Fig. 8).
Fig. 8.
Chemical structures of amoc-capped peptides [99]. (Copyright © 2018 american chemical society).
4. Biomedical applications of self-assembling hydrogels
4.1. Bio-printing
In vitro two-dimensional culture was carried out as the most widely used method for drug screening and disease modelling before animal tests. However, cells grown on flat and hard plastic surfaces cannot reflect the essential features due to lacking the complex and dynamic cell-cell communications as well as cell-matrix interactions [100]. Instead, three-dimensional (3D) culture were developed to recapitulate the features in in vivo microenvironment. The biomimetic 3D models provide spatial depth as well as cell connectivity as a better in vivo physiology for drug screening and disease modelling. 3D bioprinting is a promising technology for its ability to rapidly and reproducibly fabricate a 3D construct comprising multiple cell types and bioactive moieties with precise distribution. However, lack of suitable bioinks constrains the development of bioprinting. As mentioned in Section 3, the amino acid residues of self-assemble peptides provide the primary structure and sites for chemical modification to incorporate self-healing, shear-thinning, and bioactive properties into the hydrogel. It makes the self-assemble peptides as an ideal candidate for bioink to form the designed shape quickly with uniformly distributed bioactive motifs and cells. The stimuli-responsive gelation and adjustable mechanical properties facilitate extrusion of self-assembling peptides hydrogel during printing. The shear-thinning performance of hydrogel can not only prevent syringe clogging but also protect cells from apoptosis. Rapid solidification of self-assemble peptides triggered by external stimuli (such as ionic strength, pH, and temperature) can maintain the shape fidelity upon deposition. Even though the use of self-assemble peptides as bioink remains unexplored now, they show great potency for gathering excellent biocompatibility, biodegradability, self-healing, stimuli-responsive gelation, and shape-memory properties together.
4.2. Tissue engineering
Self-assemble peptide hydrogels are widely used in tissue engineering as it can provide supportive 3D microenvironment for cells to adhere, infiltrate, migrate, and proliferate [[101], [102], [103]]. Furthermore, the biological activities and functions of cells can be dictated by embedded bioactive motifs in the matrix. It was reported that the human normal dermal fibroblasts cultured in RADA16-I nanofiber hydrogels show chondrogenic potential with significant upregulation of proteoglycan aggrecan expression [104]. In addition, RADA hydrogels were used to promote neutrite growth and murine pluripotent stem cells’ differentiation by providing the ECM-like support [105,106]. FKFE scaffolds were also studied for its application in tissue engineering. The FKFE-II hydrogel was used to carry cells with optimized mechanical properties to match the native nucleus pulposus. After 3D culture of nucleus pulposus cells embedded peptide scaffolds, upregulation of KRT8, KRT18, FOXF1 indicate successful regeneration with high throughout cell viability [107]. Increased deposition of nucleus pulposus ECM components (aggrecan and type II collagen) was also observed. Moreover, KLDL12 self-assemble peptides were used to fill polylactic acid/beta-tricalcium phosphate scaffolds to enhance bone regeneration without cell transplantation [108]. Interestingly, comparison of human umbilical vein endothelial cells attachment to four beta sheet forming peptide scaffolds were studied by Sieminski [109]. The results show that the cells favor to expande into RADA scaffolds comparing with KLDL and FKFE hydrogels due to preferred stiffness of the materials. Besides beta sheet forming peptides, alpha helix peptide hydrogels were also studied. RGD functionalized peptide scaffold was used to increase the proliferative activity of murine embryonic neural stem cells [26] (Fig. 9). Mature neuron-like behavior was observed by electrophysiological measurements, implying possible capability of functional peptides to overcome the challenging conundrum in nerve-tissue repair. The application potency of self-assemble peptide amphiphiles were also explored. Motamed developed a beta sheet peptides attached amphiphile which forms hydrogel at physiological conditions [101]. It promotes adhesion and proliferation of neural cells by providing a suitable microenvironment.
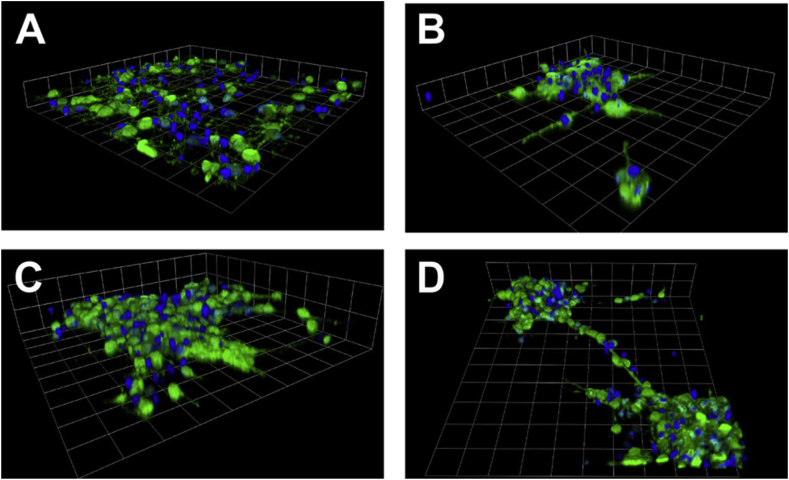
Fig. 9.
3D neurosphere formation by NSCs in hSAF gels. 3D reconstruction of z-stack fluorescent images on (A) laminin, (B) undecorated hSAF, and (C) RGDS-decorated hSAF gels. (D) 3D reconstruction of z-stack fluorescent images of cells on RGDS-decorated gels showing neurosphere connections. DAPI-stained cell nuclei (blue) and nestin expression (green). (A–D) Grid scales: 24.75 μm [26].
(Copyright © 2015 American Chemical Society).
As mentioned in the last section, functional peptide sequences can be used to decorate the self-assembling peptides to mediate the biological activities of cells. LLVFGAKMLPHHGA sequence were used to mimic the mineralization functional motif of hydroxyapatite for bone tissue engineering [110]. The LLVFGAD sequence is responsible for self-assembly, while the rest residues promotes mineralization by providing numerous functional groups like hydroxyapatite. SNV, KPS, and KAI bioactive motifs of BMP-7 were used to decorate RADA16-I peptides for nucleus pulposus regeneration [111]. The results show that the SNV and KPS functionalized nanofiber hydrogels enhance proliferation, migration, and ECM secretion of human degenerated nucleus pulposus cells comparing with non and KAI decorated RADA hydrogels. A multi-layered peptide amphiphile hydrogel was designed by Cakmak to culture human articular chondrocytes and bone marrow mesenchymal stem cells in a osteochondral cocktail medium (Fig. 10) [112]. Regeneration of both cartilage and bone tissue was obtained without use of any growth factor.
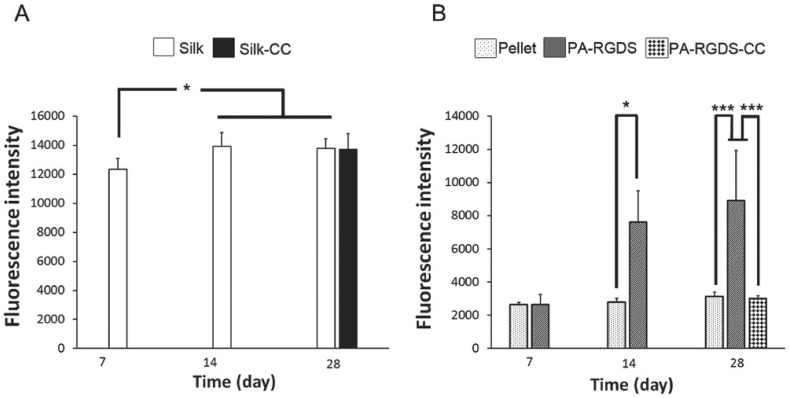
Fig. 10.
Cell viability and proliferation analysis of A) hBMSCs and B) hACs in their respective medium and biomaterials by Alamar Blue assay. The results are represented as the mean ± standard deviation for n = 3. Statistically significant differences are denoted by symbols; *p < 0.05, **p < 0.01 and ***p < 0.001 (Silk‐CC and PA‐RGDS‐CC indicate the scaffolds which were transferred to the CC medium) [112]. (Copyright © 2018, John Wiley and Sons).
4.3. Drug delivery
The deliberately engineered self-assemble peptides can constitute various supramolecular nanostructures to deliver drugs to the targeted sites. The bottom-up strategy is capable to carry amphiphilic drugs with high loading, low leakage, and high permeability through bio-membranes into the target cells [50]. Moreover, the stimuli-responsive property of self-assemble scaffolds enable programmable release of therapeutics. The feature of peptide hydrogels can be facilely modified via attachment of chemical or bioactive motifs to immobilize drug molecules through physical interactions or covalent bonds. The release of therapeutics can also be adjusted by mesh size and degradation of the network. D- and L-RADA16 hydrogels were designed to deliver TGF-beta 1 with a release plateau of 72 h by Zhou [113]. This system was used to 3D culture bone mesenchymal stem cells. By providing slow and sustained release of growth factor, favorable influence on cell proliferation was observed [113]. Multidomain peptides (MDPs) were designed with beta sheet motifs to form nanofibrous architectures. Small molecules, proteins, and cells can be easily traped within the matrices or within the hydrophobic core [17]. Cytokine delivery of self-assemble peptide was reported by Kumar with spatio-temporal activation of THP-1 monocytes and macrophages [114]. The injectable hydrogel promotes macrophage infiltration and polarization through macrophage-material interactions without proinflammatory environment generated [114].
The self-assemble peptide hydrogels were utilized to carry cardiovascular, bone, brain, and anticancer drugs in last decades [50,[115], [116], [117], [118]]. Mazza and coworkers developed a peptide amphiphile with dalargin derivative to assemble into nanofibers against degradation in the plasma (Fig. 11) [115]. Reduced clearance from the brain parenchyma was obtained by enhancing the transport across the blood-brain barrier due to the amphiphilic nature of peptides [115]. A heparin-modified RADA peptide hydrogel self-assembled under physiological conditions were used to deliver VEGF, and improvement of cardiac function after myocardial infarction was obtained [116]. In addition, a EAKA peptide based self-assemble material was fabricated by inducing protein A/G and antibody, and show effective cytokine promotion and tumor retention [5].
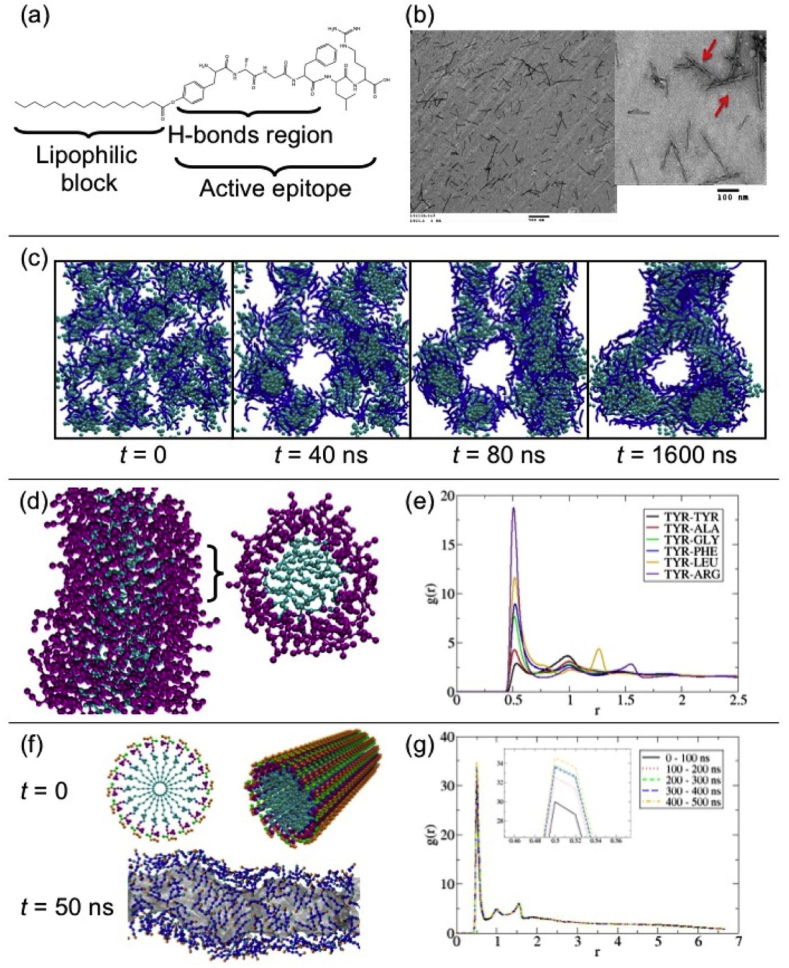
Fig. 11.
(a) pDal molecular structure. (b) cylindrical and twisted nanofibers as seen using TEM (red arrows indicate twisted nanofibers). (c) snapshots from molecular simulations of the self-assembly of pDal nanofibers starting from a random configuration. (d) 2 nm length cross section of the nanofiber formed during simulation, revealing the wrapping around of the peptide moiety (shown in purple) around the fiber axis; the hydrophobic core shown in cyan. (e) pairwise radial distribution function for the TYR backbone bead with respect to all other amino acid backbone beads in a nanofiber. (f) Idealized nanofiber structure with the pDal molecules arranged. (g) Pairwise radial distribution function g(r) between the ARG backbone bead and the TYR backbone bead, calculated over five different time periods of the simulation [115]. (Copyright © 2013 American Chemical Society).
5. Conclusion
Self-assemble peptides have been deliberately engineered to construct supramolecular nanostructures with specific properties. Not only the biocompatibility, biodegradability, and low toxicity from the amino acid build-blocks, but also possible induction of self-healing, shape-memory, shear-thinning properties by chemical modification let them attract increasing interest in the past decades. The stimuli-responsive gelation or transition of the peptides enable their applications in programmable delivery of drugs to optimize the therapeutic treatment with minimized the side effect. In addition, the combination of self-healing and shear-thinning provide an optimal candidate for injectable hydrogels and bioinks. Cells and bioactive motifs embedded in the carriers can be protected by the native or modified functionalities of self-assembled network. Even though application of self-assemble peptide scaffolds in bioprinting remain barely explored now, it is promising that it can serve as desired building blocks to construct complex 3D microenvironments encapsulate multiple cell types and bioactive molecules with precise distribution. The possible incorporated shape-memory functionality also enable its application in minimal invasive way - the prepared scaffolds can be folded to implant through a small incision and recovered the designed shape at the target site. Furthermore, functional peptide motifs derived from ECM molecules and bioactive growth factors can be easily attached to self-assemble peptides due to their native amino acids constituents. The induced functional peptide sequence can serve in a similar way by mediating intracellular signaling pathways with reduced immune response and less cost. Proof-of-concept demonstrations of effectively enhanced performance of self-assemble peptide materials in 3D cell culture, tissue engineering, and drug delivery have been established.
However, there are still several challenges preventing self-assemble peptides from real clinical applications. The stability and consistency of functionalized peptide-constructed nanostructures need to be further improved. The impact of self-assemble peptide sequences on immune system varies from non-immunogenic to self-adjuvanting in each design. The immunogenic mechanism of peptides in different type should be studied with more details. Besides, more accurate illustration of how external stimuli, such as pH, temperature, ionic strength, and so on, should be achieved step-by-step to optimized the stability of nanostructures formations in different batch. The mechanism of nanocomposite hydrogels should also be further explored. Despite these challenges remained, self-assemble peptides show great potential to incorporate more functionalities and properties into one system, and more biomedical application of them can be expected.
Acknowledgements
The present work was financially supported by the National Natural Science Foundation of China (No. 31430030, U1601220), Natural Science Foundation of Guangdong Province (2017A030308004) and Natural Science Foundation of Guangzhou City (201804020011).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Gutteridge A., Thornton J.M. Understanding nature's catalytic toolkit. Trends Biochem. Sci. 2005;30(11):622–629. doi: 10.1016/j.tibs.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Koutsopoulos S. Self-assembling peptide nanofiber hydrogels in tissue engineering and regenerative medicine: progress, design guidelines, and applications. J. Biomed. Mater. Res. 2016;104(4):1002–1016. doi: 10.1002/jbm.a.35638. [DOI] [PubMed] [Google Scholar]
- 3.Mandal D., Nasrolahi Shirazi A., Parang K. Self-assembly of peptides to nanostructures. Org. Biomol. Chem. 2014;12(22):3544–3561. doi: 10.1039/c4ob00447g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang H. Cellular membrane enrichment of self-assembling d-peptides for cell surface engineering. ACS Appl. Mater. Interfaces. 2014;6(12):9815–9821. doi: 10.1021/am502250r. [DOI] [PubMed] [Google Scholar]
- 5.Wen Y. Retaining antibodies in tumors with a self-assembling injectable system. Mol. Pharm. 2013;10(3):1035–1044. doi: 10.1021/mp300504z. [DOI] [PubMed] [Google Scholar]
- 6.Saunders M.J. Engineering fluorogen activating proteins into self-assembling materials. Bioconjug. Chem. 2013;24(5):803–810. doi: 10.1021/bc300613h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wen Y. Antibody-functionalized peptidic membranes for neutralization of allogeneic skin antigen-presenting cells. Acta Biomater. 2014;10(11):4759–4767. doi: 10.1016/j.actbio.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang S. Spontaneous assembly of a self-complementary oligopeptide to form a stable macroscopic membrane. Proc. Natl. Acad. Sci. 1993;90(8):3334–3338. doi: 10.1073/pnas.90.8.3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang S. Fabrication of novel biomaterials through molecular self-assembly. Nat. Biotechnol. 2003;21:1171. doi: 10.1038/nbt874. [DOI] [PubMed] [Google Scholar]
- 10.Caplan M.R. Control of self-assembling oligopeptide matrix formation through systematic variation of amino acid sequence. Biomaterials. 2002;23(1):219–227. doi: 10.1016/s0142-9612(01)00099-0. [DOI] [PubMed] [Google Scholar]
- 11.Zhang S. Unusually stable β-sheet formation in an ionic self-complementary oligopeptide. Biopolymers. 1994;34(5):663–672. doi: 10.1002/bip.360340508. [DOI] [PubMed] [Google Scholar]
- 12.Hauser C.A.E., Zhang S. Designer self-assembling peptide materials for diverse applications. Macromol. Symp. 2010;295(1):30–48. [Google Scholar]
- 13.Yokoi H., Kinoshita T., Zhang S. Dynamic reassembly of peptide RADA16 nanofiber scaffold. Proc. Natl. Acad. Sci. U.S.A. 2005;102(24):8414–8419. doi: 10.1073/pnas.0407843102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pradeep K. Self-assembling peptides: implications for patenting in drug delivery and tissue engineering. Recent Pat. Drug Deliv. Formulation. 2011;5(1):24–51. doi: 10.2174/187221111794109510. [DOI] [PubMed] [Google Scholar]
- 15.Nagy K.J. Enhanced mechanical rigidity of hydrogels formed from enantiomeric peptide assemblies. J. Am. Chem. Soc. 2011;133(38):14975–14977. doi: 10.1021/ja206742m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J. D-amino acids boost the selectivity and confer supramolecular hydrogels of a nonsteroidal anti-inflammatory drug (NSAID) J. Am. Chem. Soc. 2013;135(2):542–545. doi: 10.1021/ja310019x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore A.N., Hartgerink J.D. Self-assembling multidomain peptide nanofibers for delivery of bioactive molecules and tissue regeneration. Accounts Chem. Res. 2017;50(4):714–722. doi: 10.1021/acs.accounts.6b00553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aggeli A. Hierarchical self-assembly of chiral rod-like molecules as a model for peptide β-sheet tapes, ribbons, fibrils, and fibers. Proc. Natl. Acad. Sci. 2001;98(21):11857–11862. doi: 10.1073/pnas.191250198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J. Self-assembly of a peptide amphiphile based on hydrolysed Bombyx mori silk fibroin. Chem. Commun. 2011;47(37):10296–10298. doi: 10.1039/c1cc12633d. [DOI] [PubMed] [Google Scholar]
- 20.Saracino G.A.A., Gelain F. Modelling and analysis of early aggregation events of BMHP1-derived self-assembling peptides. J. Biomol. Struct. Dyn. 2014;32(5):759–775. doi: 10.1080/07391102.2013.790848. [DOI] [PubMed] [Google Scholar]
- 21.Gelain F. BMHP1-Derived self-assembling peptides: hierarchically assembled structures with self-healing propensity and potential for tissue engineering applications. ACS Nano. 2011;5(3):1845–1859. doi: 10.1021/nn102663a. [DOI] [PubMed] [Google Scholar]
- 22.Silva D. Synthesis and characterization of designed BMHP1-derived self-assembling peptides for tissue engineering applications. Nanoscale. 2013;5(2):704–718. doi: 10.1039/c2nr32656f. [DOI] [PubMed] [Google Scholar]
- 23.Ryadnov M.G., Woolfson D.N. Engineering the morphology of a self-assembling protein fibre. Nat. Mater. 2003;2:329. doi: 10.1038/nmat885. [DOI] [PubMed] [Google Scholar]
- 24.Papapostolou D. Engineering nanoscale order into a designed protein fiber. Proc. Natl. Acad. Sci. 2007;104(26):10853–10858. doi: 10.1073/pnas.0700801104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu Y.B. Coiled-coils: stability, specificity, and drug delivery potential. Adv. Drug Deliv. Rev. 2002;54(8):1113–1129. doi: 10.1016/s0169-409x(02)00058-3. [DOI] [PubMed] [Google Scholar]
- 26.Mehrban N. Functionalized α-helical peptide hydrogels for neural tissue engineering. ACS Biomater. Sci. Eng. 2015;1(6):431–439. doi: 10.1021/acsbiomaterials.5b00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lomander A., Hwang W., Zhang S. Hierarchical self-assembly of a coiled-coil peptide into fractal structure. Nano Lett. 2005;5(7):1255–1260. doi: 10.1021/nl050203r. [DOI] [PubMed] [Google Scholar]
- 28.Holowka E.P., Pochan D.J., Deming T.J. Charged polypeptide vesicles with controllable diameter. J. Am. Chem. Soc. 2005;127(35):12423–12428. doi: 10.1021/ja053557t. [DOI] [PubMed] [Google Scholar]
- 29.Xu C., Breedveld V., Kope Soci J. Reversible hydrogels from self-assembling genetically engineered protein block copolymers. Biomacromolecules. 2005;6(3):1739–1749. doi: 10.1021/bm050017f. [DOI] [PubMed] [Google Scholar]
- 30.Hirst A.R., Smith D.K. Two-component gel-phase materials—highly tunable self-assembling systems. Chem. Eur J. 2005;11(19):5496–5508. doi: 10.1002/chem.200500241. [DOI] [PubMed] [Google Scholar]
- 31.Banwell E.F. Rational design and application of responsive α-helical peptide hydrogels. Nat. Mater. 2009;8:596. doi: 10.1038/nmat2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsui D. Rational identification of aggregation hotspots based on secondary structure and amino acid hydrophobicity. Sci. Rep. 2017;7(1):9558. doi: 10.1038/s41598-017-09749-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Condon J.E., Jayaraman A. Development of a coarse-grained model of collagen-like peptide (CLP) for studies of CLP triple helix melting. J. Phys. Chem. B. 2018;122(6):1929–1939. doi: 10.1021/acs.jpcb.7b10916. [DOI] [PubMed] [Google Scholar]
- 34.Ramachandran G.N., Kartha G. Structure of collagen. Nature. 1955;176:593. doi: 10.1038/176593a0. [DOI] [PubMed] [Google Scholar]
- 35.Luo T., Kiick K.L. Collagen-like peptides and peptide–polymer conjugates in the design of assembled materials. Eur. Polym. J. 2013;49(10):2998–3009. doi: 10.1016/j.eurpolymj.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fallas J.A. Structural insights into charge pair interactions in triple helical collagen-like proteins. J. Biol. Chem. 2012;287(11):8039–8047. doi: 10.1074/jbc.M111.296574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Persikov A.V., Ramshaw J.A.M., Brodsky B. Prediction of collagen stability from amino acid sequence. J. Biol. Chem. 2005;280(19):19343–19349. doi: 10.1074/jbc.M501657200. [DOI] [PubMed] [Google Scholar]
- 38.Boudko S.P., Engel J. Structure formation in the C terminus of type III collagen guides disulfide cross-linking. J. Mol. Biol. 2004;335(5):1289–1297. doi: 10.1016/j.jmb.2003.11.054. [DOI] [PubMed] [Google Scholar]
- 39.Cejas M.A. Thrombogenic collagen-mimetic peptides: self-assembly of triple helix-based fibrils driven by hydrophobic interactions. Proc. Natl. Acad. Sci. 2008;105(25):8513–8518. doi: 10.1073/pnas.0800291105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cejas M.A. Collagen-related Peptides: self-assembly of short, single strands into a functional biomaterial of micrometer scale. J. Am. Chem. Soc. 2007;129(8):2202–2203. doi: 10.1021/ja066986f. [DOI] [PubMed] [Google Scholar]
- 41.KADLER K.E. Collagen fibril formation. Biochem. J. 1996;316(1):1–11. doi: 10.1042/bj3160001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rele S. D-periodic collagen-mimetic microfibers. J. Am. Chem. Soc. 2007;129(47):14780–14787. doi: 10.1021/ja0758990. [DOI] [PubMed] [Google Scholar]
- 43.Pires M.M. Controlling the morphology of metal-promoted higher ordered assemblies of collagen peptides with varied core lengths. Langmuir. 2012;28(4):1993–1997. doi: 10.1021/la203848r. [DOI] [PubMed] [Google Scholar]
- 44.Pires M.M. Metal-mediated tandem coassembly of collagen peptides into banded microstructures. J. Am. Chem. Soc. 2011;133(37):14469–14471. doi: 10.1021/ja2042645. [DOI] [PubMed] [Google Scholar]
- 45.Jin H.-E. Biomimetic self-templated hierarchical structures of collagen-like peptide amphiphiles. Nano Lett. 2015;15(10):7138–7145. doi: 10.1021/acs.nanolett.5b03313. [DOI] [PubMed] [Google Scholar]
- 46.Betre H. Characterization of a genetically engineered elastin-like polypeptide for cartilaginous tissue repair. Biomacromolecules. 2002;3(5):910–916. doi: 10.1021/bm0255037. [DOI] [PubMed] [Google Scholar]
- 47.Deming T. vol. 310. Springer Science & Business Media; 2012. (Peptide-Based Materials). [Google Scholar]
- 48.Wang H. Covalently adaptable elastin-like protein–hyaluronic acid (ELP–HA) hybrid hydrogels with secondary thermoresponsive crosslinking for injectable stem cell delivery. Adv. Funct. Mater. 2017;27(28):1605609. doi: 10.1002/adfm.201605609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi P., Gustafson J.A., MacKay J.A. Genetically engineered nanocarriers for drug delivery. Int. J. Nanomed. 2014;9:1617–1626. doi: 10.2147/IJN.S53886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eskandari S. Recent advances in self-assembled peptides: implications for targeted drug delivery and vaccine engineering. Adv. Drug Deliv. Rev. 2017;110–111:169–187. doi: 10.1016/j.addr.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 51.Wang Q. High throughput screening of dynamic silk-elastin-like protein biomaterials. Adv. Funct. Mater. 2014;24(27):4303–4310. doi: 10.1002/adfm.201304106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raucher D., Massodi I., Bidwell G.L. Thermally targeted delivery of chemotherapeutics and anti-cancer peptides by elastin-like polypeptide. Expert Opin. Drug Deliv. 2008;5(3):353–369. doi: 10.1517/17425247.5.3.353. [DOI] [PubMed] [Google Scholar]
- 53.Huang W. Design of multistimuli responsive hydrogels using integrated modeling and genetically engineered silk–elastin-like proteins. Adv. Funct. Mater. 2016;26(23):4113–4123. doi: 10.1002/adfm.201600236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herrero-Vanrell R. Self-assembled particles of an elastin-like polymer as vehicles for controlled drug release. J. Contr. Release. 2005;102(1):113–122. doi: 10.1016/j.jconrel.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 55.Zhang S. Design of nanostructured biological materials through self-assembly of peptides and proteins. Curr. Opin. Chem. Biol. 2002;6(6):865–871. doi: 10.1016/s1367-5931(02)00391-5. [DOI] [PubMed] [Google Scholar]
- 56.Urry D.W. Physical chemistry of biological free energy transduction as demonstrated by elastic protein-based polymers. J. Phys. Chem. B. 1997;101(51):11007–11028. [Google Scholar]
- 57.Hamley I.W. Self-assembly of amphiphilic peptides. Soft Matter. 2011;7(9):4122–4138. [Google Scholar]
- 58.Cui H., Webber M.J., Stupp S.I. Self-assembly of peptide amphiphiles: from molecules to nanostructures to biomaterials. Peptide Sci. 2010;94(1):1–18. doi: 10.1002/bip.21328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koss K.M., Unsworth L.D. Neural tissue engineering: bioresponsive nanoscaffolds using engineered self-assembling peptides. Acta Biomater. 2016;44:2–15. doi: 10.1016/j.actbio.2016.08.026. [DOI] [PubMed] [Google Scholar]
- 60.Niece K.L. Self-assembly combining two bioactive peptide-amphiphile molecules into nanofibers by electrostatic attraction. J. Am. Chem. Soc. 2003;125(24):7146–7147. doi: 10.1021/ja028215r. [DOI] [PubMed] [Google Scholar]
- 61.Beniash E. Self-assembling peptide amphiphile nanofiber matrices for cell entrapment. Acta Biomater. 2005;1(4):387–397. doi: 10.1016/j.actbio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 62.Harrington D.A. Branched peptide-amphiphiles as self-assembling coatings for tissue engineering scaffolds. J. Biomed. Mater. Res. 2006;78A(1):157–167. doi: 10.1002/jbm.a.30718. [DOI] [PubMed] [Google Scholar]
- 63.Barnard A., Smith D.K. Self-assembled multivalency: dynamic ligand arrays for high-affinity binding. Angew. Chem. Int. Ed. 2012;51(27):6572–6581. doi: 10.1002/anie.201200076. [DOI] [PubMed] [Google Scholar]
- 64.Ian W. Nanoscale tissue engineering: spatial control over cell-materials interactions. Nanotechnology. 2011;22(21):212001. doi: 10.1088/0957-4484/22/21/212001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peter D.Y., Bruce L.P. Developmental and pathogenic mechanisms of basement membrane assembly. Curr. Pharmaceut. Des. 2009;15(12):1277–1294. doi: 10.2174/138161209787846766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Durbeej M. Laminins. Cell Tissue Res. 2009;339(1):259. doi: 10.1007/s00441-009-0838-2. [DOI] [PubMed] [Google Scholar]
- 67.Williams C.M. Fibronectin expression modulates mammary epithelial cell proliferation during acinar differentiation. Cancer Res. 2008;68(9):3185–3192. doi: 10.1158/0008-5472.CAN-07-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhu J., Hu J., Marchant R.E. 9 - biomimetic hydrogels as scaffolds for tissue-engineering applications. In: Ruys A.J., editor. Biomimetic Biomaterials. Woodhead Publishing; 2013. pp. 238–275. [Google Scholar]
- 69.Rahmany M.B., Van Dyke M. Biomimetic approaches to modulate cellular adhesion in biomaterials: a review. Acta Biomater. 2013;9(3):5431–5437. doi: 10.1016/j.actbio.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 70.Li Q., Chau Y. Neural differentiation directed by self-assembling peptide scaffolds presenting laminin-derived epitopes. J. Biomed. Mater. Res. 2010;94A(3):688–699. doi: 10.1002/jbm.a.32707. [DOI] [PubMed] [Google Scholar]
- 71.Powell S.K. Neural cell response to multiple novel sites on laminin-1. J. Neurosci. Res. 2000;61(3):302–312. doi: 10.1002/1097-4547(20000801)61:3<302::AID-JNR8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 72.Sur S. Tuning supramolecular mechanics to guide neuron development. Biomaterials. 2013;34(20):4749–4757. doi: 10.1016/j.biomaterials.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Silva G.A. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science. 2004;303(5662):1352–1355. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- 74.Cigognini D. Evaluation of early and late effects into the acute spinal cord injury of an injectable functionalized self-assembling scaffold. PLoS One. 2011;6(5):e19782. doi: 10.1371/journal.pone.0019782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cheng T.-Y. Neural stem cells encapsulated in a functionalized self-assembling peptide hydrogel for brain tissue engineering. Biomaterials. 2013;34(8):2005–2016. doi: 10.1016/j.biomaterials.2012.11.043. [DOI] [PubMed] [Google Scholar]
- 76.Senta H. Cell responses to bone morphogenetic proteins and peptides derived from them: biomedical applications and limitations. Cytokine Growth Factor Rev. 2009;20(3):213–222. doi: 10.1016/j.cytogfr.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 77.Visser R. Peptides for bone tissue engineering. J. Contr. Release. 2016;244:122–135. doi: 10.1016/j.jconrel.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 78.Suzuki Y. Alginate hydrogel linked with synthetic oligopeptide derived from BMP-2 allows ectopic osteoinduction in vivo. J. Biomed. Mater. Res. 2000;50(3):405–409. doi: 10.1002/(sici)1097-4636(20000605)50:3<405::aid-jbm15>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 79.Esmaiel J. Osteogenic peptides in bone regeneration. Curr. Pharmaceut. Des. 2013;19(19):3391–3402. doi: 10.2174/1381612811319190006. [DOI] [PubMed] [Google Scholar]
- 80.He X., Yang X., Jabbari E. Combined effect of osteopontin and BMP-2 derived peptides grafted to an adhesive hydrogel on osteogenic and vasculogenic differentiation of marrow stromal cells. Langmuir. 2012;28(12):5387–5397. doi: 10.1021/la205005h. [DOI] [PubMed] [Google Scholar]
- 81.Deng Y. A novel hydrogel surface grafted with dual functional peptides for sustaining long-term self-renewal of human induced pluripotent stem cells and manipulating their osteoblastic maturation. Adv. Funct. Mater. 2018;28(11):1705546. [Google Scholar]
- 82.Wang M. In vitro culture and directed osteogenic differentiation of human pluripotent stem cells on peptides-decorated two-dimensional microenvironment. ACS Appl. Mater. Interfaces. 2015;7(8):4560–4572. doi: 10.1021/acsami.5b00188. [DOI] [PubMed] [Google Scholar]
- 83.Kim H.K. Osteogenesis induced by a bone forming peptide from the prodomain region of BMP-7. Biomaterials. 2012;33(29):7057–7063. doi: 10.1016/j.biomaterials.2012.06.036. [DOI] [PubMed] [Google Scholar]
- 84.D'Andrea L.D. Targeting angiogenesis: structural characterization and biological properties of a <em>de novo</em> engineered VEGF mimicking peptide. Proc. Natl. Acad. Sci. U.S.A. 2005;102(40):14215–14220. doi: 10.1073/pnas.0505047102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Finetti F. Functional and pharmacological characterization of a VEGF mimetic peptide on reparative angiogenesis. Biochem. Pharmacol. 2012;84(3):303–311. doi: 10.1016/j.bcp.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 86.Huang R. Self-assembling peptide–polysaccharide hybrid hydrogel as a potential carrier for drug delivery. Soft Matter. 2011;7(13):6222–6230. [Google Scholar]
- 87.Wen Y. Coassembly of amphiphilic peptide EAK16-II with histidinylated analogues and implications for functionalization of β-sheet fibrils in vivo. Biomaterials. 2014;35(19):5196–5205. doi: 10.1016/j.biomaterials.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 88.Basak S., Nanda J., Banerjee A. Multi-stimuli responsive self-healing metallo-hydrogels: tuning of the gel recovery property. Chem. Commun. 2014;50(18):2356–2359. doi: 10.1039/c3cc48896a. [DOI] [PubMed] [Google Scholar]
- 89.Saha S. Amino acid-based multiresponsive low-molecular weight metallohydrogels with load-bearing and rapid self-healing abilities. Chem. Commun. 2014;50(23):3004–3006. doi: 10.1039/c3cc49869g. [DOI] [PubMed] [Google Scholar]
- 90.Chen J. Functional self-healing materials and their potential applications in biomedical engineering. Adv. Compos. Hybrid Mater. 2018;1(1):94–113. [Google Scholar]
- 91.Diesendruck C.E. Biomimetic self-healing. Angew. Chem. Int. Ed. 2015;54(36):10428–10447. doi: 10.1002/anie.201500484. [DOI] [PubMed] [Google Scholar]
- 92.Herbst F. Self-healing polymers via supramolecular forces. Macromol. Rapid Commun. 2013;34(3):203–220. doi: 10.1002/marc.201200675. [DOI] [PubMed] [Google Scholar]
- 93.Cao J. Multiple hydrogen bonding enables the self-healing of sensors for human–machine interactions. Angew. Chem. 2017;129(30):8921–8926. doi: 10.1002/anie.201704217. [DOI] [PubMed] [Google Scholar]
- 94.Chen J. Repetitive biomimetic self-healing of Ca2+-induced nanocomposite protein hydrogels. Sci. Rep. 2016;6:30804. doi: 10.1038/srep30804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen J. Self-healing of thermally-induced, biocompatible and biodegradable protein hydrogel. RSC Adv. 2016;6(61):56183–56192. [Google Scholar]
- 96.Haines-Butterick L. Controlling hydrogelation kinetics by peptide design for three-dimensional encapsulation and injectable delivery of cells. Proc. Natl. Acad. Sci. 2007;104(19):7791–7796. doi: 10.1073/pnas.0701980104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu Y. Highly flexible and resilient elastin hybrid cryogels with shape memory, injectability, conductivity, and magnetic responsive properties. Adv. Mater. 2016;28(35):7758–7767. doi: 10.1002/adma.201601066. [DOI] [PubMed] [Google Scholar]
- 98.Fan J.-B. Directly coating hydrogel on filter paper for effective oil–water separation in highly acidic, alkaline, and salty environment. Adv. Funct. Mater. 2015;25(33):5368–5375. [Google Scholar]
- 99.Gavel P.K. Investigations of peptide-based biocompatible injectable shape-memory hydrogels: differential biological effects on bacterial and human blood cells. ACS Appl. Mater. Interfaces. 2018;10(13):10729–10740. doi: 10.1021/acsami.8b00501. [DOI] [PubMed] [Google Scholar]
- 100.Xu X., Farach-Carson M.C., Jia X. Three-dimensional in vitro tumor models for cancer research and drug evaluation. Biotechnol. Adv. 2014;32(7):1256–1268. doi: 10.1016/j.biotechadv.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Motamed S. A self-assembling β-peptide hydrogel for neural tissue engineering. Soft Matter. 2016;12(8):2243–2246. doi: 10.1039/c5sm02902c. [DOI] [PubMed] [Google Scholar]
- 102.Annabi N. 25th anniversary article: rational design and applications of hydrogels in regenerative medicine. Adv. Mater. 2014;26(1):85–124. doi: 10.1002/adma.201303233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Peak C.W. Elastomeric cell-laden nanocomposite microfibers for engineering complex tissues. Cell. Mol. Bioeng. 2015;8(3):404–415. [Google Scholar]
- 104.Bussmann B.M. Chondrogenic potential of human dermal fibroblasts in a contractile, soft, self-assembling, peptide hydrogel. J. Tissue Eng. Regenerat. Med. 2016;10(2):E54–E62. doi: 10.1002/term.1766. [DOI] [PubMed] [Google Scholar]
- 105.Ni N. Self-assembling peptide nanofiber scaffolds enhance dopaminergic differentiation of mouse pluripotent stem cells in 3-dimensional culture. PLoS One. 2013;8(12):e84504. doi: 10.1371/journal.pone.0084504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Holmes T.C. Extensive neurite outgrowth and active synapse formation on self-assembling peptide scaffolds. Proc. Natl. Acad. Sci. 2000;97(12):6728–6733. doi: 10.1073/pnas.97.12.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wan S. Self-assembling peptide hydrogel for intervertebral disc tissue engineering. Acta Biomater. 2016;46:29–40. doi: 10.1016/j.actbio.2016.09.033. [DOI] [PubMed] [Google Scholar]
- 108.Hee K.S. Self-assembling peptide nanofibers coupled with neuropeptide substance P for bone tissue engineering. Tissue Eng. 2015;21(7–8):1237–1246. doi: 10.1089/ten.tea.2014.0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sieminski A.L. Primary sequence of ionic self-assembling peptide gels affects endothelial cell adhesion and capillary morphogenesis. J. Biomed. Mater. Res. 2008;87A(2):494–504. doi: 10.1002/jbm.a.31785. [DOI] [PubMed] [Google Scholar]
- 110.Li K. Biomimetic ultralight, highly porous, shape-adjustable, and biocompatible 3D graphene minerals via incorporation of self-assembled peptide nanosheets. Adv. Funct. Mater. 2018;28(29):1801056. [Google Scholar]
- 111.Tao H. BMP7-Based functionalized self-assembling peptides for nucleus pulposus tissue engineering. ACS Appl. Mater. Interfaces. 2015;7(31):17076–17087. doi: 10.1021/acsami.5b03605. [DOI] [PubMed] [Google Scholar]
- 112.Çakmak S. A silk fibroin and peptide amphiphile-based Co-culture model for osteochondral tissue engineering. Macromol. Biosci. 2016;16(8):1212–1226. doi: 10.1002/mabi.201600013. [DOI] [PubMed] [Google Scholar]
- 113.Zhou A. Controlled release of TGF-beta 1 from RADA self-assembling peptide hydrogel scaffolds. Drug Des. Dev. Ther. 2016;10:3043–3051. doi: 10.2147/DDDT.S109545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kumar V.A. Self-assembling multidomain peptides tailor biological responses through biphasic release. Biomaterials. 2015;52:71–78. doi: 10.1016/j.biomaterials.2015.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mazza M. Nanofiber-based delivery of therapeutic peptides to the brain. ACS Nano. 2013;7(2):1016–1026. doi: 10.1021/nn305193d. [DOI] [PubMed] [Google Scholar]
- 116.Guo H.-d. Sustained delivery of VEGF from designer self-assembling peptides improves cardiac function after myocardial infarction. Biochem. Biophys. Res. Commun. 2012;424(1):105–111. doi: 10.1016/j.bbrc.2012.06.080. [DOI] [PubMed] [Google Scholar]
- 117.Polo-Corrales L., Latorre-Esteves M., Ramirez-Vick J.E. Scaffold design for bone regeneration. J. Nanosci. Nanotechnol. 2014;14(1):15–56. doi: 10.1166/jnn.2014.9127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Alam S., Panda J.J., Chauhan V.S. Novel dipeptide nanoparticles for effective curcumin delivery. Int. J. Nanomed. 2012;7:4207–4222. doi: 10.2147/IJN.S33015. [DOI] [PMC free article] [PubMed] [Google Scholar]