Abstract
Melanins are ancient biological pigments found in all kingdoms of life. In fungi, their role in microbial pathogenesis is well established; however, these complex biomolecules also confer upon fungal microorganisms the faculty to tolerate extreme environments such as the Earth’s poles, the International Space Station and places contaminated by toxic metals and ionizing radiation. A remarkable property of melanin is its capacity to interact with a wide range of electromagnetic radiation frequencies, functioning as a protecting and energy harvesting pigment. Other roles of fungal melanin include scavenging of free radical, thermo-tolerance, metal ion sequestration, cell development, and mechanical-chemical cellular strength. In this review, we explore the various functions ascribed to this biological pigment in fungi and its remarkable physicochemical properties.
Keywords: fungal melanization, thermal melanism, energy transducer, fungal melanin, fungal pigments, black fungi, energy harvest, free radical scavenging
Graphical Abstract
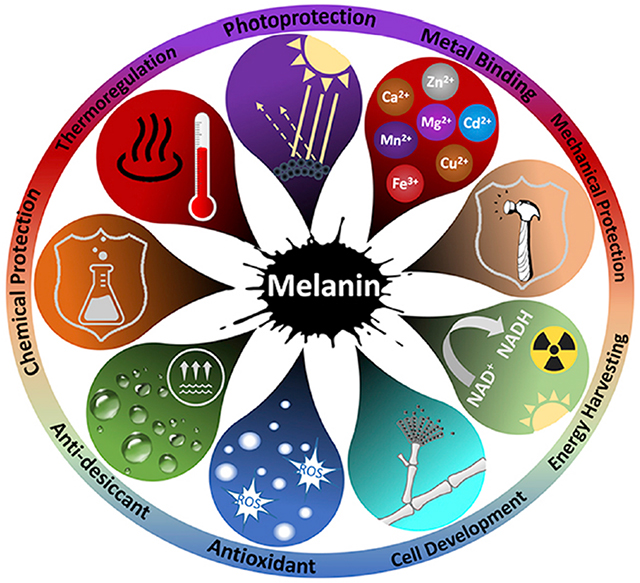
Functions of fungal melanin.
Fungal melanins play multiple biological functions including photoprotection, energy harvest and thermoregulation by readily absorbing and transducing electromagnetic radiation. Fungal melanins also function in free radical and metal binding; protection against dehydration, chemical and mechanical stressors; and fungal development and conidiation.
Introduction
Pigments are produced by most living organisms and give our world a variety of colors through the absorption and refraction of specific wavelengths of light. There are many kinds of biological pigments in nature, ranging from monomeric (i.e. carotenoids, luciferin, flavonoids, and heme/porphyrin-based, such as chlorophylls, bilirubin, hemoglobin, hemocyanin) to polymeric (i.e. melanins, tannins, and humic substances). All pigments contain conjugated moieties (i.e. aromatics rings) that allow electronic resonances and mediate energy transfer reactions in cells. The capture and/or reflected radiation energy by pigments serves multiple biological functions ranging from camouflage or makeup to fundamental roles in the maintenance of life including harnessing solar energy for metabolic use and protection against radiation damage.
Among the biological pigments, melanins represent a unique class. Historically, melanins have been difficult to define and categorize due to their diversity and structural complexity. Melanins can be classified into eumelanins, pheomelanins, neuromelanin and allomellalins (Ambrico, 2016). All are heterogeneous polyphenols that form higher-order structures with unique physicochemical properties, including broadband optical absorption, paramagnetism, charge transport and remarkable structural stability. These properties allow melanins to perform diverse functions in biological systems and melanization represents a general adaptation mechanism to climate changes (Roulin, 2014). The widespread presence of melanins in biology implies a functional importance for this class of biomolecules in the evolution of life on Earth.
In the fungal kingdom, melanization is observed across all phyla. Some fungal species are constitutively melanized while others melanize only under specific developmental phases (i.e. conidia, yeast filamentous growth), in response to environmental queues, and/or in the presence of phenolic melanin precursors (Bell and Wheeler, 1986). Fungal species that are constitutively melanized are referred to as melanotic, black, dematiaceous, microcolonial or meristematic fungi. Those fungal species that only melanized under certain conditions could be termed “facultative melanotic” fungi. Melanotic fungi appear to be phylogenetically diverse (Sterflinger et al., 1999) with a worldwide distribution, typically colonizing harsh environmental niches not suitable to most life forms (Table 1). Such environments are characterized by diverse conditions of drastic temperature fluctuations, elevated radiation exposure, high osmotic pressure, oxidative stresses, low water activity and nutrient availability. Melanization allows these microorganisms to tolerate the various physical and chemical stresses from their surroundings (Dadachova and Casadevall, 2008; Gessler et al., 2014; Robinson, 2001) making them polyextremophiles.
Table1.
Examples of extreme environments occupied by melanotic fungi.
Most fungal melanins are generated from the polymerization of 1,8-dihydroxynaphthalene (DHN), but fungal species can also utilize other pigment precursors such as: tyrosine, gamma-glutaminyl-4-hydroxybenzene (GHB), catechol, homogentisic acid, catecholamines, and (+)-scytalone (Bell et al., 1976; Bell and Wheeler, 1986; Belozerskaya et al., 2016; Solano, 2014; Weijn et al., 2013; Wheeler and Bell, 1988). During melanin synthesis, the phenolic precursors undergo multiple oxidation and reduction steps, which can occur enzymatically or passively by spontaneous polymerizations. In fact, a translucent L-Dopa aqueous solution will eventually precipitate into melanin particles at room temperatures even in the absence of enzymes (Mason, 1955; Nosanchuk et al., 2001; Soares Bronze-Uhle et al., 2015). Melanin biosynthesis involves multiple enzymes including polyphenoloxidases (i.e. tyrosinase, laccase, catechol oxidase)- key enzymes that carry out the rate-limiting initial oxidations of phenolic melanin precursors (Bell et al., 1976; Bell and Wheeler, 1986; d’Ischia et al., 2013; Eisenman and Casadevall, 2012; Ito and Wakamatsu, 2008; Solano, 2014; Wheeler and Bell, 1988). Their activity depends on copper ions at their catalytic site, which help orient the reducing substrate and coordinate molecular oxygen for catalysis (Cowley et al., 2016; Decker and Tuczek, 2000; Gasparetti and tutkimuskeskus, 2012; Goldfeder et al., 2014). Thus, copper homeostasis is key for fungal melanin biosynthesis (Mauch et al., 2013; Upadhyay et al., 2013). The biosynthesis of fungal melanins was previously reviewed and will not be discussed in depth here (Bell and Wheeler, 1986; Bultler, 1987; Eisenman and Casadevall, 2012; Henson et al., 1999; Langfelder et al., 2003; Ma and Sun, 2012; Nosanchuk et al., 2015). It has been suggested that fungal melanins, regardless of their precursor, may share similar functional groups and comparable physicochemical properties (Fogarty and Tobin, 1996). However, such properties have yet to be studied in the various fungal melanins. Given the large number of melanotic species and biosynthetic pathways for melanin production, it is likely that fungal melanins express a variety of structural and chemical characteristics that remain to be defined.
The structure of fungal melanin, or melanin in general, remains poorly understood (Nosanchuk et al., 2015; Solano, 2014, 2016). Like other amorphous substances in nature (i.e. wood), an unambiguous determination of its higher-order structural conformations (analogous to secondary, tertiary, and/or quaternary structures) is beyond our current technological and analytical horizons. Our present understanding of the structure and properties of melanin originate primarily from studies on synthetic melanins and/or non-fungal natural melanins (mainly from squid/cuttlefish), which are not identical, but do share physicochemical characteristics (Abbas et al., 2009; Ambrico, 2016; d’Ischia et al., 2009; Duff et al., 1988; Haywood et al., 2006; Ligonzo et al., 2009; Meredith and Sarna, 2006). One model of melanin structure suggests that indolic and/or phenolic monomers are polymerized into a series of ordered planar arrangements of regularly-interspaced stacked layers similar to graphite, that can cross-link into more heterogeneous and disordered macromolecular configurations (Kim et al., 2016; Meredith and Sarna, 2006; Nosanchuk et al., 2015). This is known as the local-order-global-disorder model of melanin; it involves a combination of π-stacking, hydrogen and ionic bonded nanostructures of unclear dimensions, which then aggregate to form disordered particles with spherical dimensions known as melanin granules (Bridelli, 1998; Chen et al., 2014; Clancy et al., 2000; Kim et al., 2016; Meredith and Sarna, 2006; Nosanchuk et al., 2015; Tran et al., 2006; Watt et al., 2009). High-resolution microscopy of fungal melanins also show granular patterns and X-ray diffraction analysis has produced patterns consistent with a stacked planar sheet structure with inter-sheet distances of approximately 0.4 nanometers (Bayry et al., 2014; Casadevall et al., 2012; Franzen et al., 2008b; Franzen et al., 1999; Gomez et al., 2001; Morris-Jones et al., 2005; Morris-Jones et al., 2003; Nosanchuk et al., 2002; Walker et al., 2010). The mean size, mass, and unit of fungal melanin granules remain unknown.
In fungi, melanin may be contained at the cell surface or released into the extracellular space (Dong and Yao, 2012; Doss et al., 2003; Gadd and de Rome, 1988; Hegnauer et al., 1985; Jalmi et al., 2012). The exact location of melanin granules at the cell surface varies between fungal species. For instance, in Cryptococcus neoformans, melanin granules are deposited between the plasma membrane and the innermost part of the cell wall (Eisenman et al., 2005). In other fungal species, melanin inserts within or at the surface of the cell wall matrix (Caesar-Tonthat et al., 1995; Carzaniga et al., 2002; Ellis and Griffiths, 1974, 1975; Gadd and Griffiths, 1980; Kogej et al., 2007). Melanin deposition in the fungal cell wall involves intimate molecular interactions with chitin structures (Baker et al., 2007; Banks et al., 2005; Bull, 1970; Chatterjee et al., 2015; Tsirilakis et al., 2012; Walker et al., 2010; Walton et al., 2005; Wang et al., 1999). Disruption of chitin metabolism results in a “leaky melanin” phenotype, where the pigment is no longer contained in the cell wall and is release to the extracellular milieu (Baker et al., 2007; Banks et al., 2005) (Baker et al., 2007; Banks et al., 2005; Tsirilakis et al., 2012). Traces of chitin Nuclear Magnetic Resonance (NMR) signatures are always detected in melanin isolates from C. neoformans (Chatterjee et al., 2015), which means these polysaccharides are in close association with the pigment such that they resist the hydrolysis steps during melanin preparations (Wang et al., 1996). Other biomolecules such as lipids, peptides, and carbohydrates are also detected in Cryptococcal melanin purifications, but their identification and relevance in melanogenesis are still unknown (Chatterjee et al., 2015; Tian et al., 2003; Zhong et al., 2008).
The synthesis of fungal melanin is similar to animal melanogenesis in that it occurs inside lipids vesicles or melanosomes (Eisenman et al., 2009; Franzen et al., 2008a; Upadhyay et al., 2016; Walker et al., 2010). This is likely to protect the cell from the highly reactive free radical phenolic intermediates produced during intracellular melanogenesis and vesicular structures may be necessary to contain and provide shape to the products of a free radical reaction. Evidence for fungal melanosomes include: (i) the melanin coat is visibly formed by layers of spherical melanin granules with size dimensions that are comparable to fungal vesicles (Alviano et al., 1991; Eisenman et al., 2005; Hegnauer et al., 1985; Morris-Jones et al., 2005; San-Blas et al., 1996; Walker et al., 2010); (ii) NMR of melanin isolates contain lipid signatures (Alviano et al., 1991; Chatterjee et al., 2015; Chatterjee et al., 2014; Eisenman et al., 2009); (iii) laccase, the enzyme that catalyzes melanin formation, is found within vesicles (Rodrigues et al., 2008), and (iv) isolated vesicles can melanized in the presence of L-Dopa (Eisenman et al., 2009). A recent study showed that melanin synthesis in Aspergillus starts within intracellular endosomes that are secreted unconventionally into the cell wall, where additional melanin biosynthetic enzymes can accumulate (Upadhyay et al., 2016). Laccase localizes at the cell wall in a pH-dependent manner (Panepinto and Williams, 2007; Zhu et al., 2001) consistent with the possibility of in situ melanogenesis at the fungal cell wall.
Functions of melanin in fungal biology
Melanins exhibit physicochemical and structural characteristics not replicated by any other pigment or biomolecule. The inherent complex nature of melanin limits our capacity to elucidate their higher-order structure and therefore understand their functions. Over recent years, physicochemical studies on synthetic and non-fungal natural melanins have provided valuable insights about the properties underlying their multiple biological functions in eukaryotic systems (Abbas et al., 2009; d’Ischia et al., 2009; Meredith and Sarna, 2006). Elucidation of the physiochemical properties of fungal melanins is necessary requirement to better understand the diverse functions of melanin in fungal biology.
The wide geographical distribution of black fungi suggests that melanins provide these organisms with special properties that translate into survival and/or adaptation advantages in extreme environmental conditions. The contribution of melanins to fungal virulence and human pathogenesis has stimulated significant functional characterization studies. Fungal melanin is considered an important virulence factor in a number of fungal species, acting as non-specific armor during infection that protects the fungus against host immune mechanisms (Butler et al., 2001; Nosanchuk and Casadevall, 2003; Perfect et al., 1998). However, fungal melanization clearly serves many biological purposes outside the human host, including photoprotection, energy harvest, free radical quenching, protection against heat and cold stress, metal chelation, cell strength, resistance to desiccation and cell development.
In the following sections, we discuss the various functions of fungal melanins in light of some general physicochemical properties described for melanins of non-fungal origin.
Photoprotection
Electromagnetic radiation is both necessary (e.g. photosynthesis) and potentially harmful for life depending on the frequency and time of exposure. Many deleterious effects are associated with exposure to high-energy electromagnetic wavelengths or ionizing radiation (including gamma, X-rays, and ultraviolet ABC (UVA, UVB, UVC) frequencies). These high frequencies can remove electrons from water and other biomolecules (i.e DNA, proteins) generating free-radicals intermediates known as Reactive Oxygen Species, ROS (i.e. superoxide anions, ⋅ O2−, peroxide, ⋅ O2−2, hydrogen peroxide, H2O2, hydroperoxyl, HO2−, hydroxyl radicals, ⋅OH− and hydroxyl ion, OH−) (Ikehata and Ono, 2011; Riley, 1994). ROS are known to damage the structure and function of sensitive intracellular molecules. As a result, various biological mechanisms have evolved to limit and repair photodamage. Melanization is a conserved protection mechanism against ionizing radiation because of its optical and antioxidant properties (see below).
The role of melanin in photoprotection is widely known in the human skin, functioning as a natural sunscreen by absorbing and dissipating the photons from ionizing radiation within its structure (Wolbarsht et al., 1981). While other biological pigments are only capable of absorbing a narrow range of light frequencies, the complex-disordered structure of melanins results in the absorption of the entire UV-Visible portion of the electromagnetic spectrum (Meredith and Sarna, 2006); a similar characteristic of amorphous solid semiconductors (McGinness, 1972). Synthetic and natural melanin suspensions exhibit a distinctive monotonic optical broad-band absorption curve with maxima near the UV region, decreasing as it reaches near infrared frequencies (Meredith and Sarna, 2006; Solano, 2016; Tran et al., 2006; Watt et al., 2009). Although it is expected that all melanins should exhibit similar broad-band light absorption, the optical properties of individual fungal melanins remain largely unexplored and could differ substantially between species.
Analogous to the tanning of human skin, fungal melanogenesis is also stimulated by exposure to ionizing radiation and resulting in pigmented yeasts which are more resistant to radiotoxicity than their albino counterparts (Bultler, 1987; Durrell and Shields, 1960; Ellis and Griffiths, 1975; Gauslaa and Solhaug, 2001; Selbmann et al., 2011; Wang and Casadevall, 1994; Zhdanova et al., 1978). It is important to note that black fungi are able to survive ionizing radiation levels that are lethal to any other eukaryote (Dighton et al., 2008; Mironenko et al., 2000; Onofri et al., 2012; Selbmann et al., 2015; Zhdanova et al., 1991) which yields its own interesting implications with regards to the limits of life. The mechanism by which fungal melanin protects a cell from radiation damage likely involves a combination of several processes for absorption-dissipation of radiation energy, including: changes in melanin’s chemical composition and structure, inelastic scattering of photons by protons or electrons (or Compton scattering), non-radiative dissipation of absorbed photons (a process that results in energy decay in the form of heat), and antioxidant or free-radical scavenging (Dadachova et al., 2008; Khajo et al., 2011; Revskaya et al., 2012).
The photoprotection capacity may differ among melanin types, radiation frequencies, and types of irradiation exposure. It is possible that photoprotection can turn into photodamage after a certain threshold of irradiation where melanin itself can produce cytotoxic radical species. Thus, under certain conditions the presence of melanin can result in increased sensitivity to radiation. For an excellent review on the photoprotective and photosensitizing functions of skin melanin refer to (Solano, 2016).
Antioxidant
A common property of all biological pigments is their ability to accept and neutralize exogenous free radicals (McGraw, 2005). Melanins are powerful antioxidants. In fungi, they contribute to virulence by interfering with host defense factors, including neutralizing the oxidative burst of phagocytic cells (Cunha et al., 2010; Nosanchuk and Casadevall, 2003; Schnitzler et al., 1999). Fungal melanins can also protect from hypochlorite, permanganate and hydrogen peroxide (Jacobson et al., 1995). In addition to acting as powerful radical quenchers, melanins are themselves stable free radicals substances containing unpaired electrons that can respond to magnetic fields, hence its paramagnetic character. Thus, melanins can receive or donate protons depending on the conditions. The electron exchange that occurs in the melanin molecule can either oxidize or reduce metals, and fungal melanins have been used as platforms for metal nanoparticle synthesis (Apte et al., 2013).
The paramagnetic signature of melanins can be detected using electroparamagnetic spin resonance (ESR); a technique commonly used to differentiate it from other dark pigments. The free radical abundance in the melanin molecule depends on temperature, pH, humidity, and presence of metals (Enochs et al., 1993; Mostert et al., 2012). In addition, this free radical population in the melanin molecule increases upon irradiation with light in a wavelength-dependent manner such that lower wavelengths are better at inducing free radicals (Khajo et al., 2011; Sarna and Sealy, 1984). Importantly, these free electrons generated by light absorption can further be dissipated via metabolic redox chemical reactions with other organic compounds in contact to the charged melanin (Dadachova et al., 2007).
Energy harvesting
Similar to other light-harvesting biological pigments (i.e. chlorophylls, carotenoids) microbial melanins can absorb radiation energy and transduce it onto life-nurturing chemical processes (Dadachova et al., 2007; Robertson et al., 2012; Turick et al., 2003; Turick et al., 2002) (Beatty et al., 2005; Dadachova et al., 2007; Dadachova and Casadevall, 2008; Robertson et al., 2012; Turick et al., 2003; Turick et al., 2011; Turick et al., 2002). The role of fungal melanin as an energy harvesting pigment was suspected from observations in radionuclide-contaminated environments that were colonized by melanotic microorganisms and the fungal growth attraction towards radiation sources in a process termed radiotropism (Mironenko et al., 2000; Tugay et al., 2006). In 2007, Dadachova et al. provided experimental evidence that fungal melanin mediates an energy transduction process from light into useful metabolic energy (Dadachova et al., 2007). Using three genetically distinct fungal species the authors showed that irradiation with sub-lethal doses of gamma rays resulted in (i) an enhanced metabolic activity by 1.4 fold increase in the NADPH levels, and (ii) higher replicative rates resulting in a doubling of CFUs, paralleled by increases in dry mass and metabolic incorporation along with uptake of labeled acetate (Dadachova et al., 2007). These findings were confirmed and extended (Robertson et al., 2012). The fungal growth enhancement of melanotic fungi is dependent on the level of cell melanization and radiation dose rates (Shuryak et al., 2014). A metabolic response is not exclusive to gamma rays since irradiation of melanized fungal cells with UV-Vis radiation also results in changes in cellular ATP levels (Bryan et al., 2011). The antioxidant activity of melanin, as well as, its ability to transduce radiation energy into heat may play complementary roles in promoting fungal growth following irradiation (Bryan et al., 2011; Dadachova et al., 2007; Findly et al., 1983; Jones and Findly, 1986; Lilly et al., 1984). The capacity of melanin to harness energy from radiation is possible due to its paramagnetic and electric properties (Dadachova et al., 2007; Turick et al., 2011).
The electroconductive properties of melanin were first identified more than five decades ago (Longuet-Higgins, 1960). In the 1970’s McGinness first proposed that melanin behaves as an amorphous organic semiconductor, being capable of switching between two resistive states provided enough voltage and/or heat (McGinness et al., 1974; McGinness, 1972). By definition, a semiconductor is matter capable of transporting electrical charge if delivered with sufficient potential or thermal energy (e.g. electric/magnetic field or heat, respectively).
Consistent with the semiconductor model, synthetic melanin shows a negative thermoelectric voltage under vacuum and its conductivity increases with increasing temperature (Jastrzebska et al., 1995; Osak et al., 1989). The ability of melanins to transport electric charge is significantly affected by the level of hydration, demonstrating that water is an essential part of the charge transport mechanism (Mostert et al., 2012). However, recent studies have shown that the thermoelectric effect of melanin at ambient conditions gives a positive thermoelectric voltage and the resistive behavior is not always observed, inconsistent with the classic amorphous semiconductor model (Jastrzebska et al., 1995; Jastrzebska and Wilezok, 1987; Osak et al., 1989; Trukhan et al., 1970). Melanin is now being described by experts as a porous mixed conductor, where the dominant charge it transports can change from electrons to protons as a function of increasing hydration levels (Giacomantonio, 2005). The electroconductive properties of melanin are very attractive for the development of new technologies for bioelectronics and other sustainable electronics (Albano et al., 2016; Young, 1965).
Thermoregulation (protection against heat and cold stress)
A role of melanin in thermoregulation stems from its ability to effectively absorb solar radiation and dissipate it non-radiatively in the form of heat (Meredith and Riesz, 2004). Much like a blackbody, melanin absorbs most light and re-emits or reflects very little of the absorbed light (also known as low radiative yield) (Meredith and Riesz, 2004). The result of this energy absorption depends on a variety of parameters including the type of melanin, light frequency and the level of hydration. Although some of the energy absorbed could result in the accumulation of free radicals (Khajo et al., 2011; Sarna and Sealy, 1984), most of it is dissipated in the form of heat (Forest and Simon, 1998; McGinness and Proctor, 1973; Meredith and Riesz, 2004). This process is thought to occur by a highly efficient electron-phonon coupling; the excited electronic state of the melanin molecule relaxes via resonance with the phonons or vibrational modes in the melanin molecule (Meng and Kaxiras, 2008; Nofsinger et al., 2001; Riesz, 2007).
Melanin-mediated heat gain from sunlight is particularly important for ectothermic (‘cold-blooded’) organisms, which rely on the mechanisms of conduction, convection, and radiation in order to maintain temperature homeostasis. This is different from endothermic (‘warm-blooded’) species that maintain their body temperatures primarily from the metabolism of food. The ability of melanotic organisms to capture electromagnetic radiation and convert it to heat allows them to heat up faster and reach higher equilibrium temperatures than non-pigmented organisms. Therefore, melanization could be beneficial for an ectotherm inhabiting cold environments with low solar radiation levels, but detrimental in hot tropical climates because of the risk of over-heating. These ideas form the basis of the theory of thermal melanism of ectotherms, which can predict their geographical distribution and ecology (Clusella Trullas et al., 2007; Gates, 1980; Jong et al., 1996; Kingsolver, 1987; Watt, 1969). Examples of thermal melanism have been reported in a number of reptiles and insects (Clusella Trullas et al., 2007; Clusella-Trullas et al., 2008; Clusella-Trullas et al., 2009; Jong et al., 1996; Moreno Azocar et al., 2016; Muri et al., 2015; Tanaka, 2005). A recent study showed that turtles (Trachemys scripta elegans) reared in colder temperatures developed darker integument relative to those reared in warmer conditions (Rowe et al., 2016). The relevance of these ideas in maintaining temperature homeostasis in microorganisms and relevance to fungal ecology in unknown.
Although the role of melanin in fungal thermoregulation is scarce, a role in protection against heat stress was observed for Wangiella [Exophiala] dermatitidis (Paolo et al., 2006). Melanin-deficient mutants of Monilinia fructicola produced conidia that were more susceptible to high temperatures and other stressors (Rehnstrom and Free, 1996). In C. neoformans, melanization increased tolerance to heat and cold stress via a still unknown mechanism putatively involving quenching of heat-induce ROS or buffering heat flux (Rosas and Casadevall, 1997). Another example where melanin exhibits a role in thermoregulation is observed in symbiotic scenarios between plants and fungi, where melanized endophytes help the plant adapt to temperature changes possibly by helping dissipate heat and/or absorb ROS (Redman et al., 2002). Melanin synthesis via the tyrosinase/laccase enzymatic pathway is regulated by temperature (Jacobson and Emery, 1991; Kim et al., 2003).
Metal binding
Given the aromatic composition of melanin polymers and the variety of hydroxyl, carboxyl, amine and phenolic functional groups present in the pigment, it is not surprising that melanin can form molecular interactions with many organic and inorganic molecules. In fact, fungal melanin can form covalent, ionic and hydrophobic bonds with proteins, polysaccharides, pesticides, drugs and other pollutants (Fogarty and Tobin, 1996; Larsson, 1993). Melanins exhibit both high binding affinity and capacity to a large number of metal ions (ie. Ca2+, Mg2+, Zn2+, Cu2+, Cd2+, Mn2+, Mn2+ and Pb2+). This binding is dependent on pH, type of melanin and metal ion (Ben-Shachar et al., 1991; Bridelli et al., 1999; Costa et al., 2012; Fogarty and Tobin, 1996; Hong et al., 2004; Hong and Simon, 2006; Liu et al., 2004; Samokhvalov et al., 2004; Szpoganicz et al., 2002). Metal binding involves interactions with carboxyl, amine, and hydroxyl functional groups of the pigment. For instance, Mg2+, Ca2+, and Zn2+, are coordinated preferentially by carboxyl, Cu2+ by hydroxyl groups and iron (Fe3+) by hydroxyl, amine, imine and acetate groups (Costa et al., 2012; Hong et al., 2004; Hong and Simon, 2006; Samokhvalov et al., 2004). Recent electrochemical fingerprinting analysis of natural eumelanin demonstrated that its monomers may oligomerize into tetramers of porphyrin-like protomolecules, capable of coordinating multiple ions (Kim et al., 2016). The affinity of melanin to bind iron is of special interest considering its importance and abundance in biological tissues, as well as, the involvement of iron and neuromelanin in the development of pathologic changes in the human brain, as observed in diseases such as Parkinson’s disease (Double et al., 2003; Efimova et al., 2010; Schroeder and Gerber, 2014).
Metal binding modifies melanin’s physicochemical properties which may alter function. Biosorption of metals changes the paramagnetic state of melanin, increasing or decreasing the free radical populations (Buszman et al., 2006; Zdybel et al., 2015). For instance, binding of diamagnetic Zn2+ ions results in an increase in the free radical population of melanin, but the paramagnetic Cu2+ binding has the opposite effect (Buszman et al., 2006). Metal binding may also change the interaction of melanin with drugs and other compounds (Wrzesniok et al., 2012a; Wrzesniok et al., 2012b; Zdybel et al., 2015), presumably by blocking active centers in the melanin molecule (Wrzesniok et al., 2011).
Fungal melanins are capable of binding multiple metals (ie. Cu2+, Ca2+, Mg2+, and Zn2+) which can induce melanogenesis in some fungal species (Caesar-Tonthat et al., 1995; McDougall and Blanchette, 1996; McLean et al., 1998; Zhan et al., 2011). The metal scavenging activity of fungal melanin allows for the bioabsorption of essential metals from rocks and other environmental niches (Fogarty and Tobin, 1996; Gadd and de Rome, 1988; Rizzo et al., 1992; Zunino and Martin, 1997). Although melanin has also been proposed to protect fungi from heavy metal toxicity (Caesar-Tonthat et al., 1995; Garcia-Rivera and Casadevall, 2001; Hong et al., 2004; McDougall and Blanchette, 1996; McLean et al., 1998; Saiz-Jimenez and Shafizadeh, 1984; Tashirev et al., 2012; Zhan et al., 2011), such protection has not been observed in all cases (Frederick et al., 1999).
The remarkable ability of melanin polymers to bind metals in amounts that range from nano to micromolar amounts per mg of dry fungal mass (Fogarty and Tobin, 1996) and other toxic compounds is being exploited in bioremediation strategies of polluted waters and recovery of valuable metals ions from solution (Gadd and de Rome, 1988; Saini and Melo, 2013; Sono et al., 2012; Yan and Viraraghavan, 2003). Other applications include real-time sensitive detection of metal ions via melanin-modified sensors (Fan et al., 2014; Huang et al., 2007) and iron-melanin ingestible supplements for the treatment of iron deficiency anemia (Wang et al., 2014). The metal binding ability and metal-altered properties of fungal melanins may vary widely between species and may potentially serve in a number of biotechnology applications.
Resistance to mechanical and chemical stress
The ability of black fungi to persist in environments of high osmotic or hydrostatic pressures (i.e. high altitudes, hypersalinity, deep waters) demonstrates the capacity of melanin to protect against various chemical and mechanical pressures. In addition to its remarkable chemical stability, melanin increases cell strengths and rigidity by depositing near the cell wall where it may crosslink with different macromolecules. Cell wall melanization also changes cell permeability and cellular turgor forces (Money et al., 1998). Melanization also protects the cell against chemical degradation such as acid hydrolysis and heavy metal toxicity (i.e. silver nitrate) (Garcia-Rivera and Casadevall, 2001). Moreover, melanization results in higher resistance to hydrolytic enzymes capable of digesting cell wall components (Bloomfield and Alexander, 1967; Kuo and Alexander, 1967; Potgieter and Alexander, 1966) as well as, osmotic stress (Kejzar et al., 2013; Kogej et al., 2007; Rehnstrom and Free, 1996; Singaravelan et al., 2008).
Melanin pigment is so resistant and stable that it can withstand the process of fossilization, whereas most organic macromolecules degrade and disappear (Lindgren et al., 2012b). Such structural stability over long periods of time also highlights the marked function of melanin as an energy transducer and suggests interesting thermodynamic properties. The ability of melanin to absorb different types of energy (i.e. radiation, free radicals) and effectively dissipate this energy could contribute to its chemical stability (Meredith and Riesz, 2004).
Although melanins are remarkably stable macromolecules they are still susceptible to biodegradation. There is evidence for melanosome degradation by hydrolytic enzymes within host cells (Borovansky and Elleder, 2003). A number of fungal species have been reported to produce manganese and/or lignin peroxidases with strong melanolytic activity (Butler and Day, 1998a; Jastrzebska et al., 1995; Liu et al., 1995; Mohorcic et al., 2007; Nagasaki, 2008; Ratto et al., 2001; White, 1958). Aspergillus fumigatus is able to degrade and utilize melanin as a carbon source (Luther and Lipke, 1980).
Protection against desiccation
Fungal melanization was associated with protection and adaptation to dry conditions (Zhdanova and Pokhodenko, 1973). For example, Inhibition of melanin synthesis by the ectomycorrhizal Cenococcum geophilum results in increase susceptibility to osmotic stress and desiccation (Fernandez and Koide, 2013). The dark colored zones in spalted woods are caused by the melanization of certain fungal species (i.e. Armilaria mellea) as a survival mechanism in response to low moisture (Tudor et al., 2012). Fungal melanin may protect against desiccation by increasing the ability of cells to absorb and retain water. Melanin is known for its hygroscopic character and strong association with water which determines its electroconductive properties (Jastrzebska et al., 1995; White, 1958). Studies with synthetic and natural melanin (of non-fungal origin) have shown that melanin is associated with two forms of water: (i) one that is weakly bound and can be easily removed by drying or heating above 60 °C and (ii) another more tightly bound within the melanin structure that requires higher temperatures (above 150 °C) for its removal (Albanese et al., 1984; Bridelli et al., 1981). Since melanization can change the porosity in the cell wall (Eisenman et al., 2005; Howard et al., 1991; Jacobson and Ikeda, 2005; Kogej et al., 2007), melanin could affect the osmolite exchange making the cell more hypertonic and reduce water loss. The effect of melanisation in controlling water balance and desiccation resistance is an important determinant in the ecology of ectothermic insect species (Parkash et al., 2009).
Cell development
Although fungal melanin does not appear to be essential for growth, it is required for normal cell development in multiple fungal species. The importance of melanin in fungal development has been primarily observed in filamentous species that, regardless of hyaline (translucent) hyphae or white mycelium, some produce melanized appressoria, sclerotia, conidia and reproductive structures (Bell and Wheeler, 1986; Butler and Day, 1998b; Henson et al., 1999). Several studies have demonstrated an association between melanin biosynthesis and healthy conidiation and germination. For example, disruption of melanin biosynthesis in the endophytic fungus Pestalotiopsis microspore showed little effect on vegetative growth but caused substantial alterations in the production, morphogenesis, and germination of conidia (Yu et al., 2015). These melanin-deficient conidia also appear to have altered cell wall integrity; a defect also observed with other species (Engh et al., 2007; Tsai et al., 1998; Yu et al., 2015). Altered surface morphology was reported in mutant conidia of Aspergillus fumigatus lacking melanin (Jahn et al., 1997; Langfelder et al., 1998; Tsai et al., 1997). Inhibition of melanin biosynthesis by tricyclazole results in defects in conidia formation and germination in Chaetomium globosum (Hu et al., 2012). Mutants of Alternaria alternata defective in melanin production produce conidia with reduced size, higher susceptibility to UV light and impaired septation of developed hyphae (Kawamura et al., 1999). A role of melanin in fungal cell development is not surprising considering the many biological functions and properties of melanin, previously discussed. The presence of melanin should impart mechanical and chemical resistance to fungal structures that may only be relevant at certain developmental stages, as fungal melanization is in turn regulated by genetic factors controlling cellular development (Engh et al., 2007; Fetzner et al., 2014; Islamovic et al., 2015; Tsai et al., 1998; Tsai et al., 1999; Upadhyay et al., 2013; Wu et al., 2012).
Concluding remarks
This review covers the diverse functions of fungal melanins apart from its role in virulence. The structure of melanin has such complexity that it allows capture and transduction of radiation energy, thus protecting fungi against harmful forms of radiation, as well as, mediating energy use for metabolic processes. This capacity of fungal melanin to transduce radiation into metabolic energy, or radiosynthesis (Dadachova et al., 2014), suggests the presence of autotrophy in a kingdom historically classified as strictly heterotrophic. Given the contribution of fungal species to the total biomass of the Earth, this process may present significant implications for estimations of Earth’s energy balance.
The mechanisms contributing to the protective role of fungal melanin against heat and cold stress are of special interest given the extreme-unique ecological distribution of black fungi (Gostincar et al., 2012). The chelating properties of melanins towards free radicals and metals mediate protection and poses melanotic fungi as powerful tools in bioremediation and other biotechnologies (Apte et al., 2013; Dighton et al., 2008; Mahmoud, 2004; Steiner et al., 2002; Zhdanova et al., 1990).
Understanding fungal melanin function at the structural level remains a challenge given its complex nature and the paucity of techniques to analyze amorphous substances. Most, if not all, the properties of melanin described in this brief review pertain to synthetic or other natural melanins and have been broadly extrapolated to fungal melanins. The study of physicochemical properties of fungal melanin provides a favorable model to study the biological impact of melanin structure and properties in cell physiology of higher eukaryotes. Considering the large biodiversity and polyextremophilic capabilities of black fungi, as well as, the various melanin biosynthetic pathways in these species, fungal melanins may exhibit still unseen physicochemical characteristics. In this regard, X-ray diffraction studies on various fungal melanin isolates exhibit different structural profiles relative to what has been reported from synthetic melanins and neuromelanin (Casadevall et al., 2012).
When considering melanin’s protective role in fungal species against a plethora of environmental stressors (i.e. hydrolytic compounds, osmotic stress, desiccation), as well as, its ancient and widespread presence in biological systems (Lindgren et al., 2012a), it is evident that melanization presents an important adaptation mechanism to Earth’s climate history and eukaryotic evolution. The study of fungal melanins may provide valuable insights intp the origins and limits of Earth’s life, as well as, reveal an extended potential of melanins in numerous biotechnological applications.
Highlights.
Black fungi are polyextremophiles.
Fungal melanins protect against a broad range of physical and chemical environmental stressors.
Fungal melanins function in photoprotection, antioxidation, thermoregulation, metal bioabsorption, anti-desiccation, and cell strength/development.
Melanins play multiple ecological and biochemical functions in living organisms, consistent with a general adaptation mechanism to climate fluctuations.
The physicochemical properties of fungal melanins may differ among species.
Acknowledgements
This work was supported by the National Institutes of Health [5R01AI052733-13]. The authors are grateful to Dr. Joshua D. Nosanchuk, Dr. Laura Granell-Ortiz and Dr. Maggie P. Wear for valuable editing of the manuscript and contributions to the graphical abstract. The graphical abstract was designed and created by Dr. Wear.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest
The authors declare no competing interests.
References
- Abbas M, D’Amico F, Morresi L, Pinto N, Ficcadenti M, Natali R, Ottaviano L, Passacantando M, Cuccioloni M, Angeletti M, Gunnella R, 2009. Structural, electrical, electronic and optical properties of melanin films. Eur Phys J E Soft Matter 28, 285–291. [DOI] [PubMed] [Google Scholar]
- Albanese G, Bridelli MG, Deriu A, 1984. Structural dynamics of melanin investigated by Rayleigh scattering of Mossbauer radiation. Biopolymers 23, 1481–1498. [Google Scholar]
- Albano LGS, Di Mauro E, Kumar P, Cicoira F, Graeff CFO, Santato C, 2016. Novel insights on the physicochemical properties of eumelanins and their DMSO derivatives. Polymer International 65, 1315–1322. [Google Scholar]
- Alviano CS, Farbiarz SR, De Souza W, Angluster J, Travassos LR, 1991. Characterization of Fonsecaea pedrosoi melanin. J Gen Microbiol 137, 837–844. [DOI] [PubMed] [Google Scholar]
- Ambrico M, 2016. SPECIAL ISSUE: Melanin, a long lasting history bridging natural pigments and organic bioelectronics. Polymer International 65, 1249–1250. [Google Scholar]
- Apte M, Girme G, Bankar A, Ravikumar A, Zinjarde S, 2013. 3, 4-dihydroxy-L-phenylalanine-derived melanin from Yarrowia lipolytica mediates the synthesis of silver and gold nanostructures. J Nanobiotechnology 11,2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babic MN, Zalar P, Zenko B, Schroers HJ, Dzeroski S, Gunde-Cimerman N, 2015. Candida and Fusarium species known as opportunistic human pathogens from customer-accessible parts of residential washing machines. Fungal Biol 119, 95–113. [DOI] [PubMed] [Google Scholar]
- Baker BJ, Lutz MA, Dawson SC, Bond PL, Banfield JF, 2004. Metabolically active eukaryotic communities in extremely acidic mine drainage. Appl Environ Microbiol 70, 6264–6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker LG, Specht CA, Donlin MJ, Lodge JK, 2007. Chitosan, the deacetylated form of chitin, is necessary for cell wall integrity in Cryptococcus neoformans. Eukaryot Cell 6, 855–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban Y, Tang M, Chen H, Xu Z, Zhang H, Yang Y, 2012. The response of dark septate endophytes (DSE) to heavy metals in pure culture. PLoS One 7, e47968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks IR, Specht CA, Donlin MJ, Gerik KJ, Levitz SM, Lodge JK, 2005. A chitin synthase and its regulator protein are critical for chitosan production and growth of the fungal pathogen Cryptococcus neoformans. Eukaryot Cell 4, 1902–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayry J, Beaussart A, Dufrene YF, Sharma M, Bansal K, Kniemeyer O, Aimanianda V, Brakhage AA, Kaveri SV, Kwon-Chung KJ, Latge JP, Beauvais A, 2014. Surface structure characterization of Aspergillus fumigatus conidia mutated in the melanin synthesis pathway and their human cellular immune response. Infect Immun 82, 3141–3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty JT, Overmann J, Lince MT, Manske AK, Lang AS, Blankenship RE, Van Dover CL, Martinson TA, Plumley FG, 2005. An obligately photosynthetic bacterial anaerobe from a deep-sea hydrothermal vent. Proc Natl Acad Sci U S A 102, 9306–9310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell AA, Puhalla JE, Tolmsoff WJ, Stipanovic RD, 1976. Use of mutants to establish (+)-scytalone as an intermediate in melanin biosynthesis by Verticillium dahliae. Can J Microbiol 22, 787–799. [DOI] [PubMed] [Google Scholar]
- Bell AA, Wheeler MH, 1986. Biosynthesis and Functions of Fungal Melanins. Annual Review of Phytopathology 24, 411–451. [Google Scholar]
- Belozerskaya TA, Gessler NN, Aver’yanov AA, 2016. Melanin Pigments of Fungi, in: Merillon J-M, Ramawat KG (Eds), Fungal Metabolites. Springer International Publishing, Cham, pp. 1–29. [Google Scholar]
- Ben-Shachar D, Riederer P, Youdim MB, 1991. Iron-melanin interaction and lipid peroxidation: implications for Parkinson’s disease. J Neurochem 57, 1609–1614. [DOI] [PubMed] [Google Scholar]
- Bloomfield BJ, Alexander M, 1967. Melanins and resistance of fungi to lysis. J Bacteriol 93, 1276–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogomolova EV, Minter DW, 2003. A new microcolonial rock-inhabiting fungus from marble in Chersonesos (Crimea, Ukraine). Mycotaxon 86, 195–204. [Google Scholar]
- Borovansky J, Elleder M, 2003. Melanosome degradation: fact or fiction. Pigment Cell Res 16, 280–286. [DOI] [PubMed] [Google Scholar]
- Bridelli M, Capelletti R, Crippa PR, 1981. Electret state and hydrated structure of melanin. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry 128, 555–567. [Google Scholar]
- Bridelli MG, 1998. Self-assembly of melanin studied by laser light scattering. Biophys Chem 73, 227–239. [DOI] [PubMed] [Google Scholar]
- Bridelli MG, Tampellini D, Zecca L, 1999. The structure of neuromelanin and its iron binding site studied by infrared spectroscopy. FEBS Lett 457, 18–22. [DOI] [PubMed] [Google Scholar]
- Bryan R, Jiang Z, Friedman M, Dadachova E, 2011. The effects of gamma radiation, UV and visible light on ATP levels in yeast cells depend on cellular melanization. Fungal Biol 115, 945–949. [DOI] [PubMed] [Google Scholar]
- Bull AT, 1970. Chemical composition of wild-type and mutant Aspergillus nidulans cell walls. The nature of polysaccharide and melanin constituents. J Gen Microbiol 63, 75–94. [DOI] [PubMed] [Google Scholar]
- Bultler MJ, 1987. Melanin production by the black yeast Phaeococcomyces sp. Digitized Theses. [Google Scholar]
- Burford EP, Fomina M, Gadd GM, 2003. Fungal involvement in bioweathering and biotransformation of rocks and minerals. Mineralogical Magazine 67, 1127–1155. [Google Scholar]
- Buszman E, Pilawa B, Zdybel M, Wilczynski S, Gondzik A, Witoszynska T, Wilczok T, 2006. EPR examination of Zn2+ and Cu2+ binding by pigmented soil fungi Cladosporium cladosporioides. Sci Total Environ 363, 195–205. [DOI] [PubMed] [Google Scholar]
- Butler MJ, Day AW, 1998a. Destruction of Fungal Melanins by Ligninases of Phanerochaete chrysosporium and Other White Rot Fungi. International Journal of Plant Sciences 159, 989–995. [Google Scholar]
- Butler MJ, Day AW, 1998b. Fungal melanins: a review. Canadian Journal of Microbiology 44, 1115–1136. [Google Scholar]
- Butler MJ, Day AW, Henson JM, Money NP, 2001. Pathogenic Properties of Fungal Melanins. Mycologia 93, 1–8. [Google Scholar]
- Buzzini P MR, 2013. Cold-adapted yeasts. Springer, New York. [Google Scholar]
- Caesar-Tonthat T, Van Ommen KF, Geesey GG, Henson JM, 1995. Melanin production by a filamentous soil fungus in response to copper and localization of copper sulfide by sulfide-silver staining. Appl Environ Microbiol 61, 1968–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell SA, Dianese JC, Fell J, Gunde-Cimerman N, Zalar P, 2011. Unusual fungal niches. Mycologia 103, 1161–1174. [DOI] [PubMed] [Google Scholar]
- Cappitelli F, Nosanchuk JD, Casadevall A, Toniolo L, Brusetti L, Florio S, Principi P, Borin S, Sorlini C, 2007. Synthetic consolidants attacked by melanin-producing fungi: case study of the biodeterioration of Milan (Italy) cathedral marble treated with acrylics. Appl Environ Microbiol 73, 271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carzaniga R, Fiocco D, Bowyer P, O’Connell RJ, 2002. Localization of melanin in conidia of Alternaria alternata using phage display antibodies. Mol Plant Microbe Interact 15, 216–224. [DOI] [PubMed] [Google Scholar]
- Casadevall A, Nakouzi A, Crippa PR, Eisner M, 2012. Fungal melanins differ in planar stacking distances. PLoS One 7, e30299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Prados-Rosales R, Itin B, Casadevall A, Stark RE, 2015. Solid-state NMR Reveals the Carbon-based Molecular Architecture of Cryptococcus neoformans Fungal Eumelanins in the Cell Wall. J Biol Chem 290, 13779–13790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Prados-Rosales R, Tan S, Itin B, Casadevall A, Stark RE, 2014. Demonstration of a common indole-based aromatic core in natural and synthetic eumelanins by solid-state NMR. Org Biomol Chem 12, 6730–6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-T, Chuang C, Cao J, Ball V, Ruch D, Buehler MJ, 2014. Excitonic effects from geometric order and disorder explain broadband optical absorption in eumelanin. Nat Commun 5. [DOI] [PubMed] [Google Scholar]
- Clancy CMR, Nofsinger JB, Hanks RK, Simon JD, 2000. A Hierarchical Self-Assembly of Eumelanin. The Journal of Physical Chemistry B 104, 7871–7873. [Google Scholar]
- Clusella Trullas S, van Wyk JH, Spotila JR, 2007. Thermal melanism in ectotherms. Journal of Thermal Biology 32, 235–245. [Google Scholar]
- Clusella-Trullas S, Terblanche JS, Blackburn TM, Chown SL, 2008. Testing the thermal melanism hypothesis: a macrophysiological approach. Functional Ecology 22, 232–238. [Google Scholar]
- Clusella-Trullas S, van Wyk JH, Spotila JR, 2009. Thermal benefits of melanism in cordylid lizards: a theoretical and field test. Ecology 90, 2297–2312. [DOI] [PubMed] [Google Scholar]
- Costa TG, Younger R, Poe C, Farmer PJ, Szpoganicz B, 2012. Studies on Synthetic and Natural Melanin and Its Affinity for Fe(III) Ion. Bioinorg Chem Appl 2012, 712840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley RE, Tian L, Solomon EI, 2016. Mechanism of O2 activation and substrate hydroxylation in noncoupled binuclear copper monooxygenases. Proc Natl Acad Sci U S A 113, 12035–12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha MM, Franzen AJ, Seabra SH, Herbst MH, Vugman NV, Borba LP, de Souza W, Rozental S, 2010. Melanin in Fonsecaea pedrosoi: a trap for oxidative radicals. BMC Microbiol 10, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Ischia M, Napolitano A, Pezzella A, Meredith P, Sarna T, 2009. Chemical and structural diversity in eumelanins: unexplored bio-optoelectronic materials. Angew Chem Int Ed Engl 48, 3914–3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Ischia M, Wakamatsu K, Napolitano A, Briganti S, Garcia-Borron JC, Kovacs D, Meredith P, Pezzella A, Picardo M, Sarna T, Simon JD, Ito S, 2013. Melanins and melanogenesis: methods, standards, protocols. Pigment Cell Melanoma Res 26, 616–633. [DOI] [PubMed] [Google Scholar]
- Dadachova E, Bryan R, Casadevall A, 2014. Radiosynthesis as an alternative energy utilization process in melanized organisms and uses thereof. Google Patents.
- Dadachova E, Bryan RA, Howell RC, Schweitzer AD, Aisen P, Nosanchuk JD, Casadevall A, 2008. The radioprotective properties of fungal melanin are a function of its chemical composition, stable radical presence and spatial arrangement. Pigment Cell Melanoma Res 21, 192–199. [DOI] [PubMed] [Google Scholar]
- Dadachova E, Bryan RA, Huang X, Moadel T, Schweitzer AD, Aisen P, Nosanchuk JD, Casadevall A, 2007. Ionizing radiation changes the electronic properties of melanin and enhances the growth of melanized fungi. PLoS One 2, e457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadachova E, Casadevall A, 2008. Ionizing radiation: how fungi cope, adapt, and exploit with the help of melanin. Curr Opin Microbiol 11, 525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leo F, Urzi C, de Hoog GS, 2003. A new meristematic fungus, Pseudotaeniolina globosa. Antonie van Leeuwenhoek 83, 351–360. [DOI] [PubMed] [Google Scholar]
- Decker H, Tuczek F, 2000. Tyrosinase/catecholoxidase activity of hemocyanins: structural basis and molecular mechanism. Trends Biochem Sci 25, 392–397. [DOI] [PubMed] [Google Scholar]
- Diakumaku E, Gorbushina AA, Krumbein WE, Panina L, Soukharjevski S, 1995. Black fungi in marble and limestones - an aesthetical, chemical and physical problem for the conservation of monuments. The Science of the Total Environment 167, 295–304. [Google Scholar]
- Dighton J, Tugay T, Zhdanova N, 2008. Fungi and ionizing radiation from radionuclides. FEMS Microbiol Lett 281, 109–120. [DOI] [PubMed] [Google Scholar]
- Dong C, Yao Y, 2012. Isolation, characterization of melanin derived from Ophiocordyceps sinensis, an entomogenous fungus endemic to the Tibetan Plateau. Journal of Bioscience and Bioengineering 113, 474–479. [DOI] [PubMed] [Google Scholar]
- Doss RP, Deisenhofer J, Krug von Nidda HA, Soeldner AH, McGuire RP, 2003. Melanin in the extracellular matrix of germlings of Botrytis cinerea. Phytochemistry 63, 687–691. [DOI] [PubMed] [Google Scholar]
- Double KL, Gerlach M, Schunemann V, Trautwein AX, Zecca L, Gallorini M, Youdim MB, Riederer P, Ben-Shachar D, 2003. Iron-binding characteristics of neuromelanin of the human substantia nigra. Biochem Pharmacol 66, 489–494. [DOI] [PubMed] [Google Scholar]
- Duff GA, Roberts JE, Foster N, 1988. Analysis of the structure of synthetic and natural melanins by solid-phase NMR. Biochemistry 27, 7112–7116. [DOI] [PubMed] [Google Scholar]
- Durrell LW, Shields LM, 1960. Fungi Isolated in Culture from Soils of the Nevada Test Site. Mycologia 52, 636–641. [Google Scholar]
- Efimova LA, Krylova SG, Zueva EP, Khotimchenko Iu S, Khotimchenko M, 2010. [Experimental investigation of antiinflammatory and anesthetic properties of calcium pectate]. Eksp Klin Farmakol 73, 23–26. [PubMed] [Google Scholar]
- Eisenman HC, Casadevall A, 2012. Synthesis and assembly of fungal melanin. Appl Microbiol Biotechnol 93, 931–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman HC, Frases S, Nicola AM, Rodrigues ML, Casadevall A, 2009. Vesicle-associated melanization in Cryptococcus neoformans. Microbiology 155, 3860–3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman HC, Nosanchuk JD, Webber JB, Emerson RJ, Camesano TA, Casadevall A, 2005. Microstructure of cell wall-associated melanin in the human pathogenic fungus Cryptococcus neoformans. Biochemistry 44, 3683–3693. [DOI] [PubMed] [Google Scholar]
- Ellis DH, Griffiths DA, 1974. The location and analysis of melanins in the cell walls of some soil fungi. Canadian Journal of Microbiology 20, 1379–1386. [Google Scholar]
- Ellis DH, Griffiths DA, 1975. Melanin deposition in the hyphae of a species of Phomopsis. Can J Microbiol 21,442–452. [DOI] [PubMed] [Google Scholar]
- Engh I, Nowrousian M, Kuck U, 2007. Regulation of melanin biosynthesis via the dihydroxynaphthalene pathway is dependent on sexual development in the ascomycete Sordaria macrospora. FEMS Microbiol Lett 275, 62–70. [DOI] [PubMed] [Google Scholar]
- Enochs WS, Nilges MJ, Swartz HM, 1993. A standardized test for the identification and characterization of melanins using electron paramagnetic resonance (EPR) spectroscopy. Pigment Cell Res 6, 91–99. [DOI] [PubMed] [Google Scholar]
- Fan Q, Cheng K, Hu X, Ma X, Zhang R, Yang M, Lu X, Xing L, Huang W, Gambhir SS, Cheng Z, 2014. Transferring biomarker into molecular probe: melanin nanoparticle as a naturally active platform for multimodality imaging. J Am Chem Soc 136, 15185–15194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez CW, Koide RT, 2013. The function of melanin in the ectomycorrhizal fungus Cenococcum geophilum under water stress. Fungal Ecology 6, 479–486. [Google Scholar]
- Fetzner R, Seither K, Wenderoth M, Herr A, Fischer R, 2014. Alternaria alternata transcription factor CmrA controls melanization and spore development. Microbiology 160, 1845–1854. [DOI] [PubMed] [Google Scholar]
- Findly RC, Gillies RJ, Shulman RG, 1983. In vivo phosphorus-31 nuclear magnetic resonance reveals lowered ATP during heat shock of Tetrahymena. Science 219, 1223–1225. [DOI] [PubMed] [Google Scholar]
- Fogarty RV, Tobin JM, 1996. Fungal melanins and their interactions with metals. Enzyme Microb Technol 19, 311–317. [DOI] [PubMed] [Google Scholar]
- Forest SE, Simon JD, 1998. Wavelength-dependent photoacoustic calorimetry study of melanin. Photochem Photobiol 68, 296–298. [PubMed] [Google Scholar]
- Franzen AJ, Cunha MM, Miranda K, Hentschel J, Plattner H, da Silva MB, et al. , 2008a. Ultrastructural characterization of melanosomes of the human pathogenic fungus Fonsecaea pedrosoi. J. Struct. Biol 162, 75–84. [DOI] [PubMed] [Google Scholar]
- Franzen AJ, Cunha MM, Miranda K, Hentschel J, Plattner H, da Silva MB, Salgado CG, de Souza W, Rozental S, 2008b. Ultrastructural characterization of melanosomes of the human pathogenic fungus Fonsecaea pedrosoi. J Struct Biol 162, 75–84. [DOI] [PubMed] [Google Scholar]
- Franzen AJ, de Souza W, Farina M, Alviano CS, Rozental S, 1999. Morphometric and densitometric study of the biogenesis of electron-dense granules in Fonsecaea pedrosoi. FEMS Microbiol Lett 173, 395–402. [DOI] [PubMed] [Google Scholar]
- Frederick BA, Caesar-Tonthat TC, Wheeler MH, Sheehan KB, Edens WA, Henson JM, 1999. Isolation and characterisation of Gaeumannomyces graminis var. graminis melanin mutants. Mycological Research 103, 99–110. [Google Scholar]
- Friedman EI, 1982. Endolithic microorganisms in the Antarctic Cold Desert. Science 215, 1045–1053. [DOI] [PubMed] [Google Scholar]
- Gadd GM, de Rome L, 1988. Biosorption of copper by fungal melanin. Applied Microbiology and Biotechnology 29, 610–617. [Google Scholar]
- Gadd GM, Griffiths AJ, 1980. Effect of copper on morphology of Aureobasidium pullulans. Transactions of the British Mycological Society 74, 387–392. [Google Scholar]
- Garcia-Rivera J, Casadevall A, 2001. Melanization of Cryptococcus neoformans reduces its susceptibility to the antimicrobial effects of silver nitrate. Med Mycol 39, 353–357. [DOI] [PubMed] [Google Scholar]
- Gasparetti C, tutkimuskeskus V.t. 2012. Biochemical and Structural Characterisation of the Copper Containing Oxidoreductases Catechol Oxidase, Tyrosinase, and Laccase from Ascomycete Fungi.
- Gates DM, 1980. Biophysical Ecology. Springer, New York. [Google Scholar]
- Gauslaa Y, Solhaug AK, 2001. Fungal melanins as a sun screen for symbiotic green algae in the lichen Lobaria pulmonaria. Oecologia 126, 462–471. [DOI] [PubMed] [Google Scholar]
- Gessler NN, Egorova AS, Belozerskaia TA, 2014. [Melanin pigments of fungi under extreme environmental conditions (review)]. Prikl Biokhim Mikrobiol 50, 125–134. [DOI] [PubMed] [Google Scholar]
- Giacomantonio C, 2005. Change transport in melanin, a disordered bio-organic conductor. The University of Queensland, Brisbane, Australia: Honors Thesis. [Google Scholar]
- Goldfeder M, Kanteev M, Isaschar-Ovdat S, Adir N, Fishman A, 2014. Determination of tyrosinase substrate-binding modes reveals mechanistic differences between type-3 copper proteins. Nat Commun 5, 4505. [DOI] [PubMed] [Google Scholar]
- Gomez BL, Nosanchuk JD, Diez S, Youngchim S, Aisen P, Cano LE, Restrepo A, Casadevall A, Hamilton AJ, 2001. Detection of melanin-like pigments in the dimorphic fungal pathogen Paracoccidioides brasiliensis in vitro and during infection. Infect Immun 69, 5760–5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbushina AA, Beck A, Schulte A, 2005. Microcolonial rock inhabiting fungi and lichen photobionts: evidence for mutualistic interactions. Mycol Res 109, 1288–1296. [DOI] [PubMed] [Google Scholar]
- Gorbushina AA, Krumbein WE, Panina L, Soukharjevski S.a., Wollenzien U, 1993. On the role of black fungi in colourchange and biodeterioration of antique marbles. Geomicrobiol.J 11,205–221. [Google Scholar]
- Gostincar C, Muggia L, Grube M, 2012. Polyextremotolerant black fungi: oligotrophism, adaptive potential, and a link to lichen symbioses. Front Microbiol 3, 390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunde-Cimerman N, Zalar P, 2014. Extremely Halotolerant and Halophilic Fungi Inhabit Brine in Solar Salterns Around the Globe. Food Technology & Biotechnology 52, 170–179. [Google Scholar]
- Gunde-Cimermana N, Zalarb P, de Hoogc S, Plemenitasd A, 2000. Hypersaline waters in salterns - natural ecological niches for halophilic black yeasts. FEMS Microbiol Ecol 32, 235–240. [DOI] [PubMed] [Google Scholar]
- Haywood RM, Lee M, Linge C, 2006. Synthetic melanin is a model for soluble natural eumelanin in UVA-photosensitised superoxide production. Journal of Photochemistry and Photobiology B: Biology 82, 224–235. [DOI] [PubMed] [Google Scholar]
- Hegnauer H, Nyhle’n LE, Rast DM, 1985. Ultrastructure of native and synthetic Agaricus bisporus melanins—Implications as to the compartmentation of melanogenesis in fungi. Experimental Mycology 9, 1–29. [Google Scholar]
- Henson JM, Butler MJ, Day AW, 1999. THE DARK SIDE OF THE MYCELIUM: Melanins of Phytopathogenic Fungi. Annu Rev Phytopathol 37, 447–471. [DOI] [PubMed] [Google Scholar]
- Hong L, Liu Y, Simon JD, 2004. Binding of metal ions to melanin and their effects on the aerobic reactivity. Photochem Photobiol 80, 477–481. [DOI] [PubMed] [Google Scholar]
- Hong L, Simon JD, 2006. Insight into the binding of divalent cations to Sepia eumelanin from IR absorption spectroscopy. Photochem Photobiol 82, 1265–1269. [DOI] [PubMed] [Google Scholar]
- Howard RJ, Ferrari MA, Roach DH, Money NP, 1991. Penetration of hard substrates by a fungus employing enormous turgor pressures. Proc Natl Acad Sci U S A 88, 11281–11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Hao X, Lou J, Zhang P, Pan J, Zhu X, 2012. A PKS gene, pks-1, is involved in chaetoglobosin biosynthesis, pigmentation and sporulation in Chaetomium globosum. Science China Life Sciences 55, 1100–1108. [DOI] [PubMed] [Google Scholar]
- Huang GS, Wang MT, Su CW, Chen YS, Hong MY, 2007. Picogram detection of metal ions by melanin-sensitized piezoelectric sensor. Biosens Bioelectron 23, 319–325. [DOI] [PubMed] [Google Scholar]
- Ikehata H, Ono T, 2011. The mechanisms of UV mutagenesis. J Radiat Res 52, 115–125. [DOI] [PubMed] [Google Scholar]
- Islamovic E, Garcia-Pedrajas MD, Chacko N, Andrews DL, Covert SF, Gold SE, 2015. Transcriptome Analysis of a Ustilago maydis ust1 Deletion Mutant Uncovers Involvement of Laccase and Polyketide Synthase Genes in Spore Development. Mol Plant Microbe Interact 28, 42–54. [DOI] [PubMed] [Google Scholar]
- Isola D, Selbmann L, de Hoog GS, Fenice M, Onofri S, Prenafeta-Boldu FX, Zucconi L, 2013. Isolation and screening of black fungi as degraders of volatile aromatic hydrocarbons. Mycopathologia 175, 369–379. [DOI] [PubMed] [Google Scholar]
- Ito S, Wakamatsu K, 2008. Chemistry of mixed melanogenesis--pivotal roles of dopaquinone. Photochem Photobiol 84, 582–592. [DOI] [PubMed] [Google Scholar]
- Jacobson ES, Emery HS, 1991. Temperature regulation of the cryptococcal phenoloxidase. J Med Vet Mycol 29, 121–124. [DOI] [PubMed] [Google Scholar]
- Jacobson ES, Hove E, Emery HS, 1995. Antioxidant function of melanin in black fungi. Infect Immun 63, 4944–4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson ES, Ikeda R, 2005. Effect of melanization upon porosity of the cryptococcal cell wall. Med Mycol 43, 327–333. [DOI] [PubMed] [Google Scholar]
- Jahn B, Koch A, Schmidt A, Wanner G, Gehringer H, Bhakdi S, Brakhage AA, 1997. Isolation and characterization of a pigmentless-conidium mutant of Aspergillus fumigatus with altered conidial surface and reduced virulence. Infect Immun 65, 5110–5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalmi P, Bodke P, Wahidullah S, Raghukumar S, 2012. The fungus Gliocephalotrichum simplex as a source of abundant, extracellular melanin for biotechnological applications. World J Microbiol Biotechnol 28, 505–512. [DOI] [PubMed] [Google Scholar]
- Jastrzebska MM, Isotalo H, Paloheimo J, Stubb H, 1995. Electrical conductivity of synthetic DOPA-melanin polymer for different hydration states and temperatures. J Biomater Sci Polym Ed 7, 577–586. [DOI] [PubMed] [Google Scholar]
- Jastrzebska MM, Wilezok T, 1987. Thermoelectric effect in synthetic dopamelanins. Studia Biophys. 122, 46. [Google Scholar]
- Jiang H, Liu N-N, Liu G-L, Chi Z, Wang J-M, Zhang L-L, Chi Z-M, 2016. Melanin production by a yeast strain XJ5–1 of Aureobasidium melanogenum isolated from the Taklimakan desert and its role in the yeast survival in stress environments. Extremophiles 20, 567–577. [DOI] [PubMed] [Google Scholar]
- Jones KA, Findly RC, 1986. Induction of heat shock proteins by canavanine in Tetrahymena. No change in ATP levels measured in vivo by NMR. J Biol Chem 261, 8703–8707. [PubMed] [Google Scholar]
- Jong P, Gussekloo S, Brakefield P, 1996. Differences in thermal balance, body temperature and activity between non-melanic and melanic two-spot ladybird beetles (Adalia bipunctata) under controlled conditions. J Exp Biol 199, 2655–2666. [DOI] [PubMed] [Google Scholar]
- Kawamura C, Tsujimoto T, Tsuge T, 1999. Targeted disruption of a melanin biosynthesis gene affects conidial development and UV tolerance in the Japanese pear pathotype of Alternaria alternata. Mol Plant Microbe Interact 12, 59–63. [DOI] [PubMed] [Google Scholar]
- Kejzar A, Gobec S, Plemenitas A, Lenassi M, 2013. Melanin is crucial for growth of the black yeast Hortaea werneckii in its natural hypersaline environment. Fungal Biol 117, 368–379. [DOI] [PubMed] [Google Scholar]
- Khajo A, Bryan RA, Friedman M, Burger RM, Levitsky Y, Casadevall A, Magliozzo RS, Dadachova E, 2011. Protection of melanized Cryptococcus neoformans from lethal dose gamma irradiation involves changes in melanin’s chemical structure and paramagnetism. PLoS One 6, e25092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DS, Park SH, Kwon SB, Joo YH, Youn SW, Sohn UD, Park KC, 2003. Temperature regulates melanin synthesis in melanocytes. Arch Pharm Res 26, 840–845. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Khetan A, Wu W, Chun SE, Viswanathan V, Whitacre JF, Bettinger CJ, 2016. Evidence of Porphyrin-Like Structures in Natural Melanin Pigments Using Electrochemical Fingerprinting. Adv Mater 28, 3173–3180. [DOI] [PubMed] [Google Scholar]
- Kingsolver JG, 1987. Evolution and Coadaptation of Thermoregulatory Behavior and Wing Pigmentation Pattern in Pierid Butterflies. Evolution 41,472–490. [DOI] [PubMed] [Google Scholar]
- Kogej T, Stein M, Volkmann M, Gorbushina AA, Galinski EA, Gunde-Cimerman N, 2007. Osmotic adaptation of the halophilic fungus Hortaea werneckii: role of osmolytes and melanization. Microbiology 153, 4261–4273. [DOI] [PubMed] [Google Scholar]
- Kuo MJ, Alexander M, 1967. Inhibition of the lysis of fungi by melanins. J Bacteriol 94, 624–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langfelder K, Jahn B, Gehringer H, Schmidt A, Wanner G, Brakhage AA, 1998. Identification of a polyketide synthase gene (pksP) of Aspergillus fumigatus involved in conidial pigment biosynthesis and virulence. Med Microbiol Immunol 187, 79–89. [DOI] [PubMed] [Google Scholar]
- Langfelder K, Streibel M, Jahn B, Haase G, Brakhage AA, 2003. Biosynthesis of fungal melanins and their importance for human pathogenic fungi. Fungal Genet Biol 38, 143–158. [DOI] [PubMed] [Google Scholar]
- Larsson BS, 1993. Interaction between chemicals and melanin. Pigment Cell Res 6, 127–133. [DOI] [PubMed] [Google Scholar]
- Le Calvez T, Burgaud G, Mahe S, Barbier G, Vandenkoornhuyse P, 2009. Fungal diversity in deep-sea hydrothermal ecosystems. Appl Environ Microbiol 75, 6415–6421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligonzo T, Ambrico M, Augelli V, Perna G, Schiavulli L, Tamma MA, Biagi PF, Minafra A, Capozzi V, 2009. Electrical and optical properties of natural and synthetic melanin biopolymer. Journal of Non-Crystalline Solids 355, 1221–1226. [Google Scholar]
- Lilly MB, Ng TC, Evanochko WT, Katholi CR, Kumar NG, Elgavish GA, Durant JR, Hiramoto R, Ghanta V, Glickson JD, 1984. Loss of high-energy phosphate following hyperthermia demonstrated by in vivo 31P-nuclear magnetic resonance spectroscopy. Cancer Res 44, 633–638. [PubMed] [Google Scholar]
- Lindgren J, Uvdal P, Sjovall P, Nilsson DE, Engdahl A, Schultz BP, Thiel V, 2012a. Molecular preservation of the pigment melanin in fossil melanosomes. Nat Commun 3, 824. [DOI] [PubMed] [Google Scholar]
- Lindgren J, Uvdal P, Sjövall P, Nilsson DE, Engdahl A, Schultz BP, Thiel V, 2012b. Molecular preservation of the pigment melanin in fossil melanosomes. Nat Commun 3, 824. [DOI] [PubMed] [Google Scholar]
- Liu Y, Hong L, Kempf VR, Wakamatsu K, Ito S, Simon JD, 2004. Ion-exchange and adsorption of Fe(NI) by Sepia melanin. Pigment Cell Res 17, 262–269. [DOI] [PubMed] [Google Scholar]
- Liu Y-T, Lee S-H, Liao Y-Y, 1995. Isolation of a Melanolytic Fungus and Its Hydrolytic Activity on Melanin. Mycologia 87, 651–654. [Google Scholar]
- Longuet-Higgins HC, 1960. On the origin of the free radical property of melanins. Arch Biochem Biophys 86, 231–232. [DOI] [PubMed] [Google Scholar]
- Luther JP, Lipke H, 1980. Degradation of melanin by Aspergillus fumigatus. Appl Environ Microbiol 40, 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X-P, Sun X-X, 2012. Melanin : biosynthesis, functions, and health effects. Nova Science Publishers, Hauppauge N.Y. [Google Scholar]
- Mahmoud YAG, 2004. Uptake of Radionuclides by Some Fungi. Mycobiology 32, 110–114. [Google Scholar]
- Mason HS, 1955. Comparative biochemistry of the phenolase complex. Advances in Ensymology and Related Areas of Molecular Biology 16, 105–184. [DOI] [PubMed] [Google Scholar]
- Mauch RM, Cunha Vde O, Dias AL, 2013. The copper interference with the melanogenesis OF Cryptococcus neoformans. Rev Inst Med Trop Sao Paulo 55, 117–120. [DOI] [PubMed] [Google Scholar]
- McDougall DN, Blanchette RA, 1996. Metal Ion Adsorption by Pseudosclerotial Plates of Phellinus weirii. Mycologia 88, 98–103. [Google Scholar]
- McGinness J, Corry P, Proctor P, 1974. Amorphous semiconductor switching in melanins. Science 183, 853–855. [DOI] [PubMed] [Google Scholar]
- McGinness J, Proctor P, 1973. The importance of the fact that melanin is black. J Theor Biol 39, 677–678. [DOI] [PubMed] [Google Scholar]
- McGinness JE, 1972. Mobility gaps: a mechanism for band gaps in melanins. Science 177, 896–897. [DOI] [PubMed] [Google Scholar]
- McGraw KJ, 2005. The antioxidant function of many animal pigments: are there consistent health benefits of sexually selected colourants? Animal Behaviour 69, 757–764. [Google Scholar]
- McLean J, Purvis OW, Williamson BJ, Bailey EH, 1998. Role for lichen melanins in uranium remediation. Nature 391,649–650. [Google Scholar]
- Meng S, Kaxiras E, 2008. Mechanisms for ultrafast nonradiative relaxation in electronically excited eumelanin constituents. Biophys J 95, 4396–4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith P, Riesz J, 2004. Radiative relaxation quantum yields for synthetic eumelanin. Photochem Photobiol 79, 211–216. [DOI] [PubMed] [Google Scholar]
- Meredith P, Sarna T, 2006. The physical and chemical properties of eumelanin. Pigment Cell Res 19, 572–594. [DOI] [PubMed] [Google Scholar]
- Mironenko NV, Alekhina IA, Zhdanova NN, Bulat SA, 2000. Intraspecific variation in gamma-radiation resistance and genomic structure in the filamentous fungus Alternaria alternata: a case study of strains inhabiting Chernobyl reactor no. 4. Ecotoxicol Environ Saf 45, 177–187. [DOI] [PubMed] [Google Scholar]
- Mohorcic M, Friedrich J, Renimel I, Andre P, Mandin D, Chaumont J-P, 2007. Production of melanin bleaching enzyme of fungal origin and its application in cosmetics. Biotechnology and Bioprocess Engineering 12, 200–206. [Google Scholar]
- Money NP, Caesar-TonThat TC, Frederick B, Henson JM, 1998. Melanin synthesis is associated with changes in hyphopodial turgor, permeability, and wall rigidity in gaeumannomyces graminis var. graminis. Fungal Genet Biol 24, 240–251. [DOI] [PubMed] [Google Scholar]
- Moreno Azocar DL, Bonino MF, Perotti MG, Schulte JA, 2nd, Abdala C.S., Cruz F.B., 2016. Effect of body mass and melanism on heat balance in Liolaemus lizards of the goetschi clade. J Exp Biol 219, 1162–1171. [DOI] [PubMed] [Google Scholar]
- Morris-Jones R, Gomez BL, Diez S, Uran M, Morris-Jones SD, Casadevall A, Nosanchuk JD, Hamilton AJ, 2005. Synthesis of melanin pigment by Candida albicans in vitro and during infection. Infect Immun 73, 6147–6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris-Jones R, Youngchim S, Gomez BL, Aisen P, Hay RJ, Nosanchuk JD, Casadevall A, Hamilton AJ, 2003. Synthesis of melanin-like pigments by Sporothrix schenckii in vitro and during mammalian infection. Infect Immun 71, 4026–4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostert AB, Powell BJ, Pratt FL, Hanson GR, Sarna T, Gentle IR, Meredith P, 2012. Role of semiconductivity and ion transport in the electrical conduction of melanin. Proc Natl Acad Sci U S A 109, 8943–8947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muri D, Schuerch J, Trim N, Golay J, Baillifard A, El Taher A, Dubey S, 2015. Thermoregulation and microhabitat choice in the polymorphic asp viper (Vipera aspis). J Therm Biol 53, 107–112. [DOI] [PubMed] [Google Scholar]
- Nagasaki KKM; Murakami S; Takenaka S; Koike K; Aoki K, 2008. Purification, characterization, and gene cloning of Ceriporiopsis sp strain MD-1 peroxidases that decolorize human hair melanin. Applied And Environmental Microbiology 16, 5106–5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nofsinger JB, Ye T, Simon JD, 2001. Ultrafast Nonradiative Relaxation Dynamics of Eumelanin. The Journal of Physical Chemistry B 105, 2864–2866. [Google Scholar]
- Nosanchuk JD, Casadevall A, 2003. The contribution of melanin to microbial pathogenesis. Cell Microbiol 5, 203–223. [DOI] [PubMed] [Google Scholar]
- Nosanchuk JD, Gomez BL, Youngchim S, Diez S, Aisen P, Zancope-Oliveira RM, Restrepo A, Casadevall A, Hamilton AJ, 2002. Histoplasma capsulatum synthesizes melanin-like pigments in vitro and during mammalian infection. Infect Immun 70, 5124–5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosanchuk JD, Ovalle R, Casadevall A, 2001. Glyphosate inhibits melanization of Cryptococcus neoformans and prolongs survival of mice after systemic infection. J Infect Dis 183, 1093–1099. [DOI] [PubMed] [Google Scholar]
- Nosanchuk JD, Stark RE, Casadevall A, 2015. Fungal Melanin: What do We Know About Structure? Front Microbiol 6, 1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikova N, De Boever P, Poddubko S, Deshevaya E, Polikarpov N, Rakova N, Coninx I, Mergeay M, 2006. Survey of environmental biocontamination on board the International Space Station. Res Microbiol 157, 5–12. [DOI] [PubMed] [Google Scholar]
- Onofri S, Barreca D, Selbmann L, Isola D, Rabbow E, Horneck G, de Vera JP, Hatton J, Zucconi L, 2008. Resistance of Antarctic black fungi and cryptoendolithic communities to simulated space and Martian conditions. Stud Mycol 61, 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onofri S, de la Torre R, de Vera JP, Ott S, Zucconi L, Selbmann L, Scalzi G, Venkateswaran KJ, Rabbow E, Sanchez Inigo FJ, Horneck G, 2012. Survival of rock-colonizing organisms after 1.5 years in outer space. Astrobiology 12, 508–516. [DOI] [PubMed] [Google Scholar]
- Osak W, Tkacz K, Czternastek H, SłAwiński J, 1989. I – V characteristics and electrical conductivity of synthetic melanin. Biopolymers 28, 1885–1890. [Google Scholar]
- Panepinto JC, Williams PR, 2007. The cell biology of virulence - Lessons from the pathogenic fungus Cryptococcus neoformans. Comunicating Current Research and Educational Topics and Trends in Applied Microbiology. [Google Scholar]
- Paolo WF Jr., Dadachova E, Mandal P, Casadevall A, Szaniszlo PJ, Nosanchuk JD, 2006. Effects of disrupting the polyketide synthase gene WdPKS1 in Wangiella [Exophiala] dermatitidis on melanin production and resistance to killing by antifungal compounds, enzymatic degradation, and extremes in temperature. BMC Microbiol 6, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkash R, Singh S, Ramniwas S, 2009. Seasonal changes in humidity level in the tropics impact body color polymorphism and desiccation resistance in Drosophila jambulina-Evidence for melanism-desiccation hypothesis. J Insect Physiol 55, 358–368. [DOI] [PubMed] [Google Scholar]
- Perfect JR, Wong B, Chang YC, Kwon-Chung KJ, Williamson PR, 1998. Cryptococcus neoformans: virulence and host defences. Med Mycol 36 Suppl 1, 79–86. [PubMed] [Google Scholar]
- Potgieter HJ, Alexander M, 1966. Susceptibility and resistance of several fungi to microbial lysis. J Bacteriol 91, 1526–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragon M, Restoux G, Moreira D, Moller AP, Lopez-Garcia P, 2011. Sunlight-exposed biofilm microbial communities are naturally resistant to chernobyl ionizing-radiation levels. PLoS One 6, e21764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratto M, Chatani M, Ritschkoff AC, Viikari L, 2001. Screening of micro-organisms for decolorization of melanins produced by bluestain fungi. Appl Microbiol Biotechnol 55, 210–213. [DOI] [PubMed] [Google Scholar]
- Redman RS, Sheehan KB, Stout RG, Rodriguez RJ, Henson JM, 2002. Thermotolerance generated by plant/fungal symbiosis. Science 298, 1581. [DOI] [PubMed] [Google Scholar]
- Rehnstrom AL, Free SJ, 1996. The isolation and characterization of melanin-deficient mutants of Monilinia fructicola. Physiological and Molecular Plant Pathology 49, 321–330. [Google Scholar]
- Revskaya E, Chu P, Howell RC, Schweitzer AD, Bryan RA, Harris M, Gerfen G, Jiang Z, Jandl T, Kim K, Ting LM, Sellers RS, Dadachova E, Casadevall A, 2012. Compton scattering by internal shields based on melanin-containing mushrooms provides protection of gastrointestinal tract from ionizing radiation. Cancer Biother Radiopharm 27, 570–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesz JJ, 2007. The Spectroscopic Properties of Melanin. University of Queensland. [Google Scholar]
- Riley PA, 1994. Free radicals in biology: oxidative stress and the effects of ionizing radiation. Int J Radiat Biol 65, 27–33. [DOI] [PubMed] [Google Scholar]
- Rizzo DM, Blanchette RA, Palmer MA, 1992. Biosorption of metal ions by Armillaria rhizomorphs. Canadian Journal of Botany 70, 1515–1520. [Google Scholar]
- Robertson KL, Mostaghim A, Cuomo CA, Soto CM, Lebedev N, Bailey RF, Wang Z, 2012. Adaptation of the black yeast Wangiella dermatitidis to ionizing radiation: molecular and cellular mechanisms. PLoS One 7, e48674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson CH, 2001. Cold adaptation in Arctic and Antarctic fungi. New Phytologist\ 151\, 341–353\. [Google Scholar]
- Rodrigues ML, Nakayasu ES, Oliveira DL, Nimrichter L, Nosanchuk JD, Almeida IC, Casadevall A, 2008. Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryot Cell 7, 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas AL, Casadevall A, 1997. Melanization affects susceptibility of Cryptococcus neoformans to heat and cold. FEMS Microbiol Lett 153, 265–272. [DOI] [PubMed] [Google Scholar]
- Roulin A, 2014. Melanin-based colour polymorphism responding to climate change. Glob Chang Biol 20, 3344–3350. [DOI] [PubMed] [Google Scholar]
- Rowe JW, Clark DL, Mortensen RA, Commissaris CV, Wittle LW, Tucker JK, 2016. Thermal and substrate color-induced melanization in laboratory reared red-eared sliders (Trachemys scripta elegans). Journal of Thermal Biology 61, 125–132. [DOI] [PubMed] [Google Scholar]
- Ruibal C, Gueidan C, Selbmann L, Gorbushina AA, Crous PW, Groenewald JZ, Muggia L, Grube M, Isola D, Schoch CL, Staley JT, Lutzoni F, de Hoog GS, 2009. Phylogeny of rock-inhabiting fungi related to Dothideomycetes. Stud Mycol 64, 123–133S127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruibal C, Platas G, Bills GF, 2005. Isolation and characterization of melanized fungi from limestone formations in Mallorca. Mycological Progress 4, 23–38. [Google Scholar]
- Saini AS, Melo JS, 2013. Biosorption of uranium by melanin: kinetic, equilibrium and thermodynamic studies. Bioresour Technol 149, 155–162. [DOI] [PubMed] [Google Scholar]
- Saiz-Jimenez C, Shafizadeh F, 1984. Iron and copper binding by fungal phenolic polymers: an electron spin resonance study. Current Microbiology 10, 281–285. [Google Scholar]
- Samokhvalov A, Liu Y, Simon JD, 2004. Characterization of the Fe(III)-binding site in Sepia eumelanin by resonance Raman confocal microspectroscopy. Photochem Photobiol 80, 84–88. [DOI] [PubMed] [Google Scholar]
- San-Blas G, Guanipa O, Moreno B, Pekerar S, San-Blas F, 1996. Cladosporium carrionii and Hormoconis resinae (C. resinae): cell wall and melanin studies. Curr Microbiol 32, 11–16. [DOI] [PubMed] [Google Scholar]
- Sarna T, Sealy RC, 1984. Free radicals from eumelanins: quantum yields and wavelength dependence. Arch Biochem Biophys 232, 574–578. [DOI] [PubMed] [Google Scholar]
- Schnitzler N, Peltroche-Llacsahuanga H, Bestier N, Zundorf J, Lutticken R, Haase G, 1999. Effect of melanin and carotenoids of Exophiala (Wangiella) dermatitidis on phagocytosis, oxidative burst, and killing by human neutrophils. Infect Immun 67, 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder RL, Gerber JP, 2014. A reappraisal of Fe(III) adsorption by melanin. J Neural Transm (Vienna) 121, 1483–1491. [DOI] [PubMed] [Google Scholar]
- Selbmann L, de Hoog GS, Zucconi L, Isola D, Ruisi S, van den Ende AH, Ruibal C, De Leo F, Urzi C, Onofri S, 2008. Drought meets acid: three new genera in a dothidealean clade of extremotolerant fungi. Stud Mycol 61, 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selbmann L, Grube M, Onofri S, Isola D, Zucconi L, 2013. Antarctic epilithic lichens as niches for black meristematic fungi. Biology (Basel) 2, 784–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selbmann L, Isola D, Zucconi L, Onofri S, 2011. Resistance to UV-B induced DNA damage in extreme-tolerant cryptoendolithic Antarctic fungi: detection by PCR assays. Fungal Biol 115, 937–944. [DOI] [PubMed] [Google Scholar]
- Selbmann L, Zucconi L, Isola D, Onofri S, 2015. Rock black fungi: excellence in the extremes, from the Antarctic to space. Curr Genet 61, 335–345. [DOI] [PubMed] [Google Scholar]
- Sert HB, Sumbul H, Sterflinger K, 2007. Microcolonial fungi from antique marbles in Perge / side / Termessos (Antalya / Turkey). Antonie van Leeuwenhoek 91,217–227. [DOI] [PubMed] [Google Scholar]
- Shuryak I, Bryan RA, Nosanchuk JD, Dadachova E, 2014. Mathematical modeling predicts enhanced growth of X-ray irradiated pigmented fungi. PLoS One 9, e85561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singaravelan N, Grishkan I, Beharav A, Wakamatsu K, Ito S, Nevo E, 2008. Adaptive melanin response of the soil fungus Aspergillus niger to UV radiation stress at “Evolution Canyon”, Mount Carmel, Israel. PLoS One 3, e2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares Bronze-Uhle E, Piacenti-Silva M, Paulin JV, Battocchio C, de Oliveira Graeff CF, 2015. Synthesis of water-soluble melanin. ArXiv e-prints 1508.07457. [Google Scholar]
- Solano F, 2014. Melanins: Skin Pigments and Much More—Types, Structural Models, Biological Functions, and Formation Routes. New Journal of Science 2014, 28. [Google Scholar]
- Solano F, 2016. Photoprotection versus photodamage: updating an old but still unsolved controversy about melanin. Polymer International 65, 1276–1287. [Google Scholar]
- Sono K, Lye D, Moore CA, Boyd WC, Gorlin TA, Belitsky JM, 2012. Melanin-based coatings as lead-binding agents. Bioinorg Chem Appl 2012, 361803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley JT, Palmer FE, Adams JB, 1982. Microcolonial fungi: common inhabitants on desert rocks? Science 215, 1093–1095. [DOI] [PubMed] [Google Scholar]
- Steiner M, Linkov I, Yoshida S, 2002. The role of fungi in the transfer and cycling of radionuclides in forest ecosystems. Journal of Environmental Radioactivity 58, 217–241. [DOI] [PubMed] [Google Scholar]
- Sterflinger K, de Hoog GS, Haase G, 1999. Phylogeny and exology fo meristematic ascomycetes. Stud Mycol 43, 5–22. [Google Scholar]
- Sterflinger K, Krumbein WE, 1997. Dematiaceous fungi as a major agent for biopitting on Mediterranean marbles and miestones. Geomicrobiol.J 14, 21–230. [Google Scholar]
- Szpoganicz B, Gidanian S, Kong P, Farmer P, 2002. Metal binding by melanins: studies of colloidal dihydroxyindole-melanin, and its complexation by Cu(II) and Zn(II) ions. J Inorg Biochem 89, 45–53. [DOI] [PubMed] [Google Scholar]
- Tanaka K, 2005. Thermal aspects of melanistic and striped morphs of the snake Elaphe quadrivirgata. Zoolog Sci 22, 1173–1179. [DOI] [PubMed] [Google Scholar]
- Tashirev AB, Romanovskaia VA, Rokitko PV, Matveeva NA, Shilin SO, Tashireva AA, 2012. [Synthesis of melanin pigments by Antarctic black yeast]. Mikrobiol Z 74, 2–8. [PubMed] [Google Scholar]
- Tesei D, Marzban G, Zakharova K, Isola D, Selbmann L, Sterflinger K, 2012. Alteration of protein patterns in black rock inhabiting fungi as a response to different temperatures. Fungal Biol 116, 932–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrasher JD, Gray MR, Kilburn KH, Dennis DP, Yu A, 2012. A Water-Damaged Home and Health of Occupants: A Case Study. Journal of Environmental and Public Health 2012, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian S, Garcia-Rivera J, Yan B, Casadevall A, Stark RE, 2003. Unlocking the molecular structure of fungal melanin using 13C biosynthetic labeling and solid-state NMR. Biochemistry 42, 8105–8109. [DOI] [PubMed] [Google Scholar]
- Tran ML, Powell BJ, Meredith P, 2006. Chemical and structural disorder in eumelanins: a possible explanation for broadband absorbance. Biophys J 90, 743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trukhan EM, Perevozchikov NF, Ostrovskii MA, 1970. [Photoconductivity of the pigment epithelium of the eye]. Biofizika 15, 1052–1055. [PubMed] [Google Scholar]
- Tsai HF, Chang YC, Washburn RG, Wheeler MH, Kwon-Chung KJ, 1998. The developmentally regulated alb1 gene of Aspergillus fumigatus: its role in modulation of conidial morphology and virulence. J Bacteriol 180, 3031–3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HF, Washburn RG, Chang YC, Kwon-Chung KJ, 1997. Aspergillus fumigatus arp1 modulates conidial pigmentation and complement deposition. Mol Microbiol 26, 175–183. [DOI] [PubMed] [Google Scholar]
- Tsai HF, Wheeler MH, Chang YC, Kwon-Chung KJ,1999. A developmentally regulated gene cluster involved in conidial pigment biosynthesis in Aspergillus fumigatus. J Bacteriol 181,6469–6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsirilakis K, Kim C, Vicencio AG, Andrade C, Casadevall A, Goldman DL, 2012. Methylxanthine inhibit fungal chitinases and exhibit antifungal activity. Mycopathologia 173, 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudor D, Robinson SC, Cooper PA, 2012. The influence of moisture content variation on fungal pigment formation in spalted wood. AMB Express 2, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugay T, Zhdanova NN, Zheltonozhsky V, Sadovnikov L, Dighton J, 2006. The influence of ionizing radiation on spore germination and emergent hyphal growth response reactions of microfungi. Mycologia 98, 521–527. [DOI] [PubMed] [Google Scholar]
- Turick CE, Caccavo F Jr., Tisa LS, 2003. Electron transfer from Shewanella algae BrY to hydrous ferric oxide is mediated by cell-associated melanin. FEMS Microbiol Lett 220, 99–104. [DOI] [PubMed] [Google Scholar]
- Turick CE, Ekechukwu AA, Milliken CE, Casadevall A, Dadachova E, 2011. Gamma radiation interacts with melanin to alter its oxidation-reduction potential and results in electric current production. Bioelectrochemistry 82, 69–73. [DOI] [PubMed] [Google Scholar]
- Turick CE, Tisa LS, Caccavo F Jr., 2002. Melanin production and use as a soluble electron shuttle for Fe(III) oxide reduction and as a terminal electron acceptor by Shewanella algae BrY. Appl Environ Microbiol 68, 2436–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay S, Torres G, Lin X,2013. Laccases involved in 1,8-dihydroxynaphthalene melanin biosynthesis in Aspergillus fumigatus are regulated by developmental factors and copper homeostasis. Eukaryot Cell 12, 1641–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay S, Xu X, Lowry D, Jackson Jennifer C, Roberson Robert W, Lin X, 2016. Subcellular Compartmentalization and Trafficking of the Biosynthetic Machinery for Fungal Melanin. Cell Reports 14, 2511–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenkoornhuyse P, Baldauf SL, Leyval C, Straczek J, Young JPW, 2002. Extensive Fungal Diversity in Plant Roots. Science 295, 2051. [DOI] [PubMed] [Google Scholar]
- Vember VV, Zhdanova NN, 2001. [Peculiarities of linear growth of the melanin-containing fungi Cladosporium sphaerospermum Penz. and Alternaria alternata (Fr.) Keissler]. Mikrobiol Z 63, 3–12. [PubMed] [Google Scholar]
- Vicente VA, Attili-Angelis D, Pie MR, Queiroz-Telles F, Cruz LM, Najafzadeh MJ, de Hoog GS, Zhao J, Pizzirani-Kleiner A, 2008. Environmental isolation of black yeast-like fungi involved in human infection. Stud Mycol 61, 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CA, Gomez BL, Mora-Montes HM, Mackenzie KS, Munro CA, Brown AJ, Gow NA, Kibbler CC, Odds FC, 2010. Melanin externalization in Candida albicans depends on cell wall chitin structures. Eukaryot Cell 9, 1329–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton FJ, Idnurm A, Heitman J, 2005. Novel gene functions required for melanization of the human pathogen Cryptococcus neoformans. Mol Microbiol 57, 1381–1396. [DOI] [PubMed] [Google Scholar]
- Wang FR, Xie ZG, Ye XQ, Deng SG, Hu YQ, Guo X, Chen SG, 2014. Effectiveness of treatment of iron deficiency anemia in rats with squid ink melanin-Fe. Food Funct 5, 123–128. [DOI] [PubMed] [Google Scholar]
- Wang Y, Aisen P, Casadevall A, 1996. Melanin, melanin “ghosts,” and melanin composition in Cryptococcus neoformans. Infect Immun 64, 2420–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Casadevall A, 1994. Decreased susceptibility of melanized Cryptococcus neoformans to UV light. Appl Environ Microbiol 60, 3864–3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Zheng L, Hauser M, Becker JM, Szaniszlo PJ, 1999. WdChs4p, a homolog of chitin synthase 3 in Saccharomyces cerevisiae, alone cannot support growth of Wangiella (Exophiala) dermatitidis at the temperature of infection. Infect Immun 67, 6619–6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt AAR, Bothma JP, Meredith P, 2009. The supramolecular structure of melanin. Soft Matter 5, 3754–3760. [Google Scholar]
- Watt WB, 1969. ADAPTIVE SIGNIFICANCE OF PIGMENT POLYMORPHISMS IN COLIAS BUTTERFLIES, II. THERMOREGULATION AND PHOTOPERIODICALLY CONTROLLED MELANIN VARIATION IN Colias eurytheme. Proc Natl Acad Sci U S A 63, 767–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weijn A, Bastiaan-Net S, Wichers HJ, Mes JJ, 2013. Melanin biosynthesis pathway in Agaricus bisporus mushrooms. Fungal Genet Biol 55, 42–53. [DOI] [PubMed] [Google Scholar]
- Wheeler MH, Bell AA, 1988. Melanins and their importance in pathogenic fungi. Curr Top Med Mycol 2, 338–387. [DOI] [PubMed] [Google Scholar]
- White LP, 1958. Melanin: a naturally occurring cation exchange material. Nature 182, 1427–1428. [DOI] [PubMed] [Google Scholar]
- Wolbarsht ML, Walsh AW, George G, 1981. Melanin, a unique biological absorber. Appl Opt 20, 2184–2186. [DOI] [PubMed] [Google Scholar]
- Wollenzien U, de Hoog GS, Krumbein WE, Urzi C, 1995. On the isolation of microcolonial fungi occurring on and in marble and other calcareous rocks. Science of The Total Environment 167, 287–294. [Google Scholar]
- Wrzesniok D, Buszman E, Grzegorczyk M, Grzegorczyk A, Hryniewicz T, 2012a. Impact of metal ions on netilmicin-melanin interaction. Acta Pol Pharm 69, 41–45. [PubMed] [Google Scholar]
- Wrzesniok D, Buszman E, Lakota D, 2011. Interaction of amikacin and tobramycin with melanin in the presence of Cu2+ and Zn2+ ions. Acta Pol Pharm 68, 493–498. [PubMed] [Google Scholar]
- Wrzesniok D, Buszman E, Miernik-Biela E, 2012b. Amikacin, kanamycin and tobramycin binding to melanin in the presence of Ca(2+) and Mg(2+) ions. Acta Pol Pharm 69, 1035–1041. [PubMed] [Google Scholar]
- Wu D, Oide S, Zhang N, Choi MY, Turgeon BG, 2012. ChLae1 and ChVel1 regulate T-toxin production, virulence, oxidative stress response, and development of the maize pathogen Cochliobolus heterostrophus. PLoS Pathog 8, e1002542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan G, Viraraghavan T, 2003. Heavy-metal removal from aqueous solution by fungus Mucor rouxii. Water Res 37, 4486–4496. [DOI] [PubMed] [Google Scholar]
- Young RS, 1965. MORPHOLOGY AND CHEMISTRY OF MICROSPHERES FROM PROTEINOID A2 - FOX, SIDNEY W, The Origins of Prebiological Systems and of their Molecular Matrices. Academic Press, pp. 347–357. [Google Scholar]
- Yu X, Huo L, Liu H, Chen L, Wang Y, Zhu X, 2015. Melanin is required for the formation of the multi-cellular conidia in the endophytic fungus Pestalotiopsis microspora. Microbiol Res 179, 1–11. [DOI] [PubMed] [Google Scholar]
- Zalar P, de Hoog GS, Gunde-Cimerman N, 1999. Ecology of halotolerant dothideaceous black yeasts. Stud Mycol 43, 38–48. [Google Scholar]
- Zdybel M, Pilawa B, Chodurek E, 2015. Effect of Cadmium(Ii) on Free Radicals in Dopa-Melanin Tested by Epr Spectroscopy. Acta Pol Pharm 72, 901–907. [PubMed] [Google Scholar]
- Zhan F, He Y, Zu Y, Li T, Zhao Z, 2011. Characterization of melanin isolated from a dark septate endophyte (DSE), Exophiala pisciphila. World Journal of Microbiology and Biotechnology 27, 2483–2489. [Google Scholar]
- Zhang Y, Zhang Y, Liu M, Shi X, Zhao Z, 2008. Dark septate endophyte (DSE) fungi isolated from metal polluted soils: their taxonomic position, tolerance, and accumulation of heavy metals in vitro. J Microbiol 46, 624–632. [DOI] [PubMed] [Google Scholar]
- Zhdanova NN, Melezhik AV, Vasilevskaya AI, Pokhodenko VD, 1978. Formation and disappearance of photo induced paramagnetic centers in melanin-containing fungi. News Acad Sci USSA 4, 576–581. [PubMed] [Google Scholar]
- Zhdanova NN, Pokhodenko VD, 1973. [Possible participation of melanin pigment in protecting the fungal cell from desiccation]. Mikrobiologiia 42, 848–853. [PubMed] [Google Scholar]
- Zhdanova NN, Vasilevskaia AI, Artyshkova LV, Gavriliuk VI, Lashko TN, Sadovnikov lu S, 1991. [Complexes of soil micromycetes in the area of the influence of the Chernobyl Atomic Electric Power Station]. MikrobiolZh 53, 3–9. [PubMed] [Google Scholar]
- Zhdanova NN, Vasilevskaya AA, Sadnovikov Y, Artyshkova LA, 1990. The dynamics of micromycete complexescontaminated with soil radionuclides. Mikologia i Fitopatologiya. [Google Scholar]
- Zhong J, Frases S, Wang H, Casadevall A, Stark RE, 2008. Following fungal melanin biosynthesis with solid-state NMR: biopolymer molecular structures and possible connections to cell-wall polysaccharides. Biochemistry 47, 4701–4710. [DOI] [PubMed] [Google Scholar]
- Zhu X, Gibbons J, Garcia-Rivera J, Casadevall A, Williamson PR, 2001. Laccase of Cryptococcus neoformans is a cell wall-associated virulence factor. Infect Immun 69, 5589–5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zunino H, Martin JP, 1997. Metal binding by organic macromolecules in soil. I. Hypothesis interpreting the role of soil organic matter in the translocation of metal ions from rocks to biological systems. Soil Sci 123, 65–76. [Google Scholar]
- Zupancic J, Novak Babic M, Zalar P, Gunde-Cimerman N, 2016. The Black Yeast Exophiala dermatitidis and Other Selected Opportunistic Human Fungal Pathogens Spread from Dishwashers to Kitchens. PLoS One 11, e0148166. [DOI] [PMC free article] [PubMed] [Google Scholar]


