Abstract
Aims: Salivary duct carcinoma (SDC) is an aggressive salivary malignancy that has high mortality rates and is often resistant to chemotherapy. Anti-PD1/PD-L1 checkpoint inhibitors have led to dramatic improvement in various cancers. Other immunotherapeutic approaches e.g. cancer vaccines have shown promising results. Cancer testis antigens (CTAs), e.g. PRAME, are regarded as promising vaccine targets due to their tumor-specific expression pattern. Methods and results: We analyzed the immunoexpression of PD-L1, PD-1, major histocompatibility complex class I (MHC I) and PRAME in 53 SDCs. The immunoexpression of PD-L1 in tumor cells (TC) and immune cells (IC), PD-1 in IC, PRAME in TC, and MHC I in TC were analyzed, and were correlated with outcome. PRAME expression was observed in 83% of SDCs. No PRAME staining was present in normal salivary gland tissue. Using the three established diagnostic algorithms proposed for head and neck squamous cell carcinoma, being CPS≥1, TC%≥1%, and TC%≥25%: 35 (66%), 17 (32%) and 3 cases (6%) were deemed positive for PD-L1, respectively. PD-1-positive IC was noted in 35 (66%) of cases. MHC I downregulation was seen in 82% SDCs. There was a significant correlation among PD-L1 expression in IC, PD-1 in IC and PRAME in TC. PD-L1 expression in TC and lack of PD-1-expression in IC were associated with decreased disease specific survival in SDC. Conclusions: Alterations of tumor immune microenvironment is common in SDCs, including expression of PD-1/PD-L1 and PRAME, which opens the doors to potential novel immune therapy, e.g. cancer vaccination and PD-1/PD-L1 blockade in these tumors.
Keywords: PRAME, CTA, PD-L1, PD-1, MHC I, salivary duct carcinoma
INTRODUCTION
Using checkpoint inhibitors in the management of patients with head and neck squamous cell carcinoma (HNSCC) has become increasingly prevalent following successful early clinical trials. Additionally, it has been shown that blockade of programmed death-1 (PD-1) and/or programmed death ligand-1 (PD-L1) has positive therapeutic benefits in patients with HNSCC1–7. Moreover, PD-1 and PD-L1 have emerged as the key checkpoints that can be manipulated using inhibitory monoclonal antibodies. In this context, the expression profile of PD-L1 is typically evaluated to identify patients who can clinically benefit from such inhibitors8, 9. PD-1 is usually expressed on adaptive immune cells as well as natural killer cells while PD-L1, in addition to being expressed by lymphocytes, antigen-presenting cells and endothelial cells, is also expressed by tumor cells (TC)10. The normal physiologic interaction between PD-L1 and PD-1 inhibits immune response and promotes self-tolerance10. In many human cancers, including head and neck carcinoma, the PD-1/PD-L1 pathway is used by TC to evade recognition by the host immune system3–5, 11, 12.
Besides immune checkpoint blockade, other immunotherapeutic approaches such as vaccine strategies against tumor-associated antigens have shown promising results in clinical trials13. Cancer vaccination targeting tumor antigens are designed to eradicate cancer cells through expansion and reactivation of tumor-specific T cells that are reactive to specific cancer antigens and potentiating host immune responses14, 15. Cancer testis antigens (CTAs), a family of antigens whose expression is normal adult tissues is restricted to testicular germ cells but which are present in a wide range of cancer, are regarded promising vaccine targets due to their tumor-restricted expression pattern16. PRAME (Preferentially Expressed Antigen in Melanoma) is a highly immunogenic CTA. Preliminary analyses indicate that PRAME shows a higher incidence and more homogeneous expression in cancer than other classical CTAs such as MAGE, and NY-ESO-116.
Major histocompatibility complex class I proteins (MHC I) is a crucial component for immunodetection by CD8(+) cytotoxic T cells. The expression of MHC I and tumor-specific antigens is the key of a properly functioning antigen processing machinery. MHC I defect on the tumor cell surface, a common feature observed in many cancer types, including HNSCC, has been proposed as one of the underlying mechanisms for tumor immune escape, resistance to immune therapy, decreased sensitivity to cancer vaccination, tumor progression and adverse clinical outcomes13, 15, 17–21.
Salivary duct carcinoma (SDC) is a high-grade salivary gland malignancy that is resistant to chemotherapy and often follows an aggressive clinical course with frequent local recurrence and metastasis22. To date, little is known about the immune milieu, in particular the expression of PD1, PD-L1, PRAME and MHC I in salivary gland malignancies in general and in SDC in particular4, 23–25. In order to address this need, we sought to assess the immunoexpression of PD-1, PD-L1, MHC I and PRAME in a retrospective cohort of 53 SDCs.
MATERIALS AND METHODS
Case selection and clinicopathologic review
This work was approved by the research ethics board of Memorial Sloan Kettering Cancer Center (MSKCC, New York, NY, US) and Sunnybrook Health Sciences Centre (SHSC, Toronto, Ontario, Canada). A total of 53 SDCs were retrieved from pathology archive (MSKCC n=40, from 2000 to 2013; SHSC: n=13, from 2004 to 2018). The histologic slides were reviewed and the diagnosis of SDC was confirmed by at least one head and neck pathologist (BX or NK). Clinicopathologic features and follow up data were gathered, including the following: site of the primary tumor, the presence of pleomorphic adenoma (PA) component (i.e. SDC de novo vs. SDC ex PA), AJCC pT stage, pN stage, recurrence free survival (RFS), and disease specific survival (DSS).
Immunohistochemistry and scoring algorithms
The details of antibodies and the scoring algorithms for each antibody used in this study were summarized in Table 1. In brief, for PD-L1, the percentage of positive TC was calculated as the surface area of TC showing partial or complete membranous staining of any intensity divided by the surface area of the tumor. The percentage of PD-L1 positive IC was calculated as the surface area of IC demonstrating cytoplasmic and/or membranous staining of any intensity divided by the surface area of the tumor. These percentages were then further divided into several categories: <1%, 1–4%, 5–24%, 25%−49%, and ≥50%. As the threshold for PD-L1 immunopositivity in SDCs has not yet been established, the PD-L1 immunopositivity was determined using the criteria established in KEYNOTE 012, Checkmate 141, and MEDI4736 trials for HNSCC3, 6, 7. These criteria are: 1) a combined positive score (CPS, calculated as the sum of positive TCs and positive ICs divided by the total TCs multiplied by 100) of at least 1, 2) ≥1% TC stained, and 3) ≥25% TC stained3, 6, 7. For PD-1, the number of PD-1-positive IC per high power fields (HPF, 400X) was counted at hotspot and categorized into three categories: negative (0/HPF), low expression (<25 PD-1-positive IC per HPF), and high expression (≥25 PD-1-positive IC per HPF). The median value (25/HPF) was used as cut-off value to separate high and low PD-1 expression group. The following two parameters were documented for PRAME IHC: 1) staining pattern in TCs (cytoplasmic and/or nuclear); and 2) the percentage of positive TC (number of PRAME-positive TC/total number of TC). IHC analysis of PRAME was performed as described previously26. PRAME immunopositivity was defined as any immunolabeling within tumor. Lastly, the percentage of TC (number of positive-TC/total number of TC) demonstrating membranous immunopositivity of MHC I was recorded and divided into three categories: positive (≥75% TC), heterogenous (25–74% TC), and negative (<25% TC).
Table 1.
Primary antibodies and scoring algorithm
| Primary antibodies | Scoring scheme | |
|---|---|---|
| PD-L1 | SP263 (Ventana, Tucson, AZ. US) E1L3N (Cell signaling Technology, Danvers, MA, US) |
Three criteria for positivity: 1. Positive: Combined positive score (CPS) ≥1 2. Positive: ≥1% tumor cells (TC) 3. Positive: ≥25% TC |
| PD-1 | NAT 105 (Abcam, Cambridge, MA, US) | Number of positive IC per high power fields (HPF, 400X) at hotspot Negative: 0/HPF Low: 1–25/HPF High: ≥25/HPF |
| PRAME | EPR20330 (Abcam, Cambridge, MA, US) | Positive: any cytoplasmic or nuclear immunostain in TC |
| MHC I | A4 (ThermoFisher Scientific, Waltham, MA) | Positive: >75% TC Heterogenous: 25–75% TC Negative: < 25% TC |
Statistics
All statistical analyses were performed using the SPSS software 24.0 (IBM Corporation, New York, NY, USA). The correlation of PD-L1, PD-1, PRAME, and MHC I with clinicopathologic and outcome data were assessed using appropriate statistical analysis, i.e. chi-square testing or Fisher’s exact test for categorical variants and Log rank test with Kaplan Meier analysis for RFS and DSS. Hazard ratio and 95% confidence interval were calculated using Cox proportional regression model. The Pearson correlation coefficient was computed among the expression of each antibody, namely PD-L1 in TC, PD-L1 in IC, PD-1 in IC, PRAME in TC, and MHC I in TC. P values less than 0.05 were considered to be statistically significant.
RESULTS
The expression of PD-L1, PD-1, PRAME and MHC I in SDC
The expression of PD-L1, PD-1, MHC I and PRAME in TC and PD-L1 and PD-1 in IC is illustrated in Table 2 and Figure 1. Using the three established diagnostic algorithms proposed for HNSCC, being CPS≥1, TC%≥1%, and TC%≥25% respectively, 35 (66%), 17 (32%) and 3 cases (6%) were deemed positive for PD-L1. The frequency of PD-L1 immunopositivity was not significantly different between patients with SDC exPA and those with SDC de novo when using a threshold of TC%≥1% or TC%≥25% (Fisher’s exact test, p=1.000 and p=0.543 respectively). On the other hand, PD-L1 immunopositivity appeared to be more frequent in SDC ex-PA group (15 of 17 cases, 88%) compared with SDC de novo group (20/36, 56%) when using a cutoff of CPS≥1 (Fisher’s exact test, p=0.029).
Table 2.
Expression of PD-L1, PD-l, PRAME and MHC I in salivary duct carcinoma
| N | % | ||
|---|---|---|---|
| PD-L1 positivity (n=53) | CPS ≥1 | 35 | 66% |
| ≥1% TC | 17 | 32% | |
| ≥25% TC | 3 | 6% | |
| Percentage of PD-L1 positive tumor cells (n=53) | <1% | 36 | 68% |
| 1–4% | 9 | 17% | |
| 5–24% | 5 | 9% | |
| 25–49% | 1 | 2% | |
| ≥50% | 2 | 4% | |
| Percentage of PD-L1 positive immune cells (n=53) | <1% | 18 | 34% |
| 1–4% | 12 | 23% | |
| 5–24% | 15 | 28% | |
| 25–49% | 6 | 11% | |
| ≥50% | 2 | 4% | |
| PD-1 positive immune cells (n=53) | Negative (0/HPF) | 18 | 34% |
| Low (<25 PD1(+)-IC/HPF | 16 | 30% | |
| High (≥25 PD1(+)-IC/HPF | 19 | 36% | |
| PRAME in tumor cells (n=40) | Negative | 7 | 17.5% |
| Positive | 33 | 82.5% | |
| 1–24% TC | 10 | 25% | |
| 25–49% TC | 6 | 15% | |
| 50–74% TC | 9 | 23% | |
| 75–100% TC | 8 | 20% | |
| Pattern: cytoplasmic and nuclear | 7 | 21% | |
| Pattern: cytoplasmic only | 26 | 79% | |
| MHC I in tumor cells (n=40) | <25% TC | 25 | 63% |
| 25–75% TC | 8 | 20% | |
| >75% TC | 7 | 18% | |
| Clinico-pathologic characteristics | |||
| Tumor type (n=53) | De novo | 36 | 68% |
| Ex PA | 17 | 32% | |
| AJCC pT stage (n=52) | T1/T2 | 14 | 27% |
| T3/T4 | 38 | 73% | |
| AJCC pN stage (n=53) | Nx | 4 | 8% |
| N0 | 14 | 26% | |
| N+ | 35 | 66% | |
| Status at last follow up (n=46) | No evidence of disease | 20 | 43% |
| Alive with disease | 7 | 15% | |
| Dead of disease | 19 | 41% | |
| Recurrence (n=46) | No | 20 | 44% |
| Yes | 26 | 57% | |
| Distant metastasis | 16 | 35% | |
| Locoregional | 3 | 7% | |
| Both distant and locoregional | 7 | 15% | |
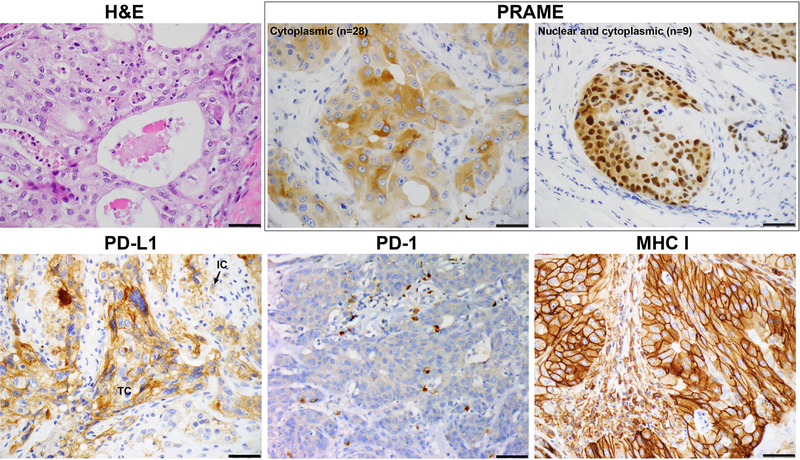
Figure 1. The expression of PRAME, PD-L1, PD-1, and MHC I in salivary duct carcinoma.
PRAME is expressed only in tumor cells (TC) with a cytoplasmic only or cytoplasmic/nuclear staining pattern. PD-L1 can be expressed in a membranous pattern in TC or in the cytoplasm or membrane of immune cells (IC). PD-1 expression in IC, and MHC I in TC.
PD-1 expression was detected in IC in 35 (66%) SDCs, including 19 cases with high number of PD-1-positive IC within the tumor and 16 with low number of PD-1-positive IC.
Among the 40 tested SDCs, PRAME was completely absent in background normal tissue, e.g. acini and ducts of salivary gland, inflammatory cells, and stromal component. In contrast, expression of PRAME in TC was noted in 33 (82.5%) SDCs, including 10 (25%) cases showing PRAME expression in 1–25% of TC, 6 (15%) in 25–49% of TCs, 9 (23%) in 50–74% of TC, and 8 (20%) in 75–100% of TC. In the majority of cases (n=26, 79%), PRAME expression was limited to the cytoplasm in TC, while only 7 (21%) cases had both nuclear and cytoplasmic immunolabeling.
MHC class I was universally present on the cell surface in the background normal tissue, e.g. acini and ducts of salivary gland and stromal cells. Decreased MHC I expression in TC was observed in 82% of SDCs including 8 (20%) cases showing heterogenous MHC I immunostain in 25 to 75% of TC, and 25 (63%) SDC exhibiting markedly attenuated to absent MHC I labeling in less than 25% of TC.
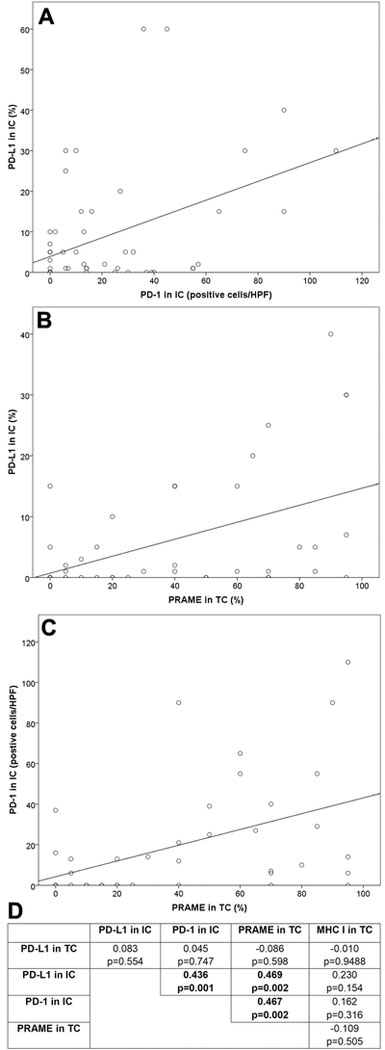
Pearson correlation analysis (Figure 2) demonstrated a significant correlation 1) between PD-L1 in IC and PD-1 in IC (Pearson correlation coefficient=0.436, p=0.001); 2) between PD-L1 in IC and PRAME in TC (Pearson correlation coefficient=0.469, p=0.002); and 3) between PD-1 in IC and PRAME in TC (Pearson correlation coefficient=0.467, p=0.002). No significant correlation was detected in any other pair of parameters.
Figure 2. Correlation among PD-L1, PD-1, PRAME, and MHC I in salivary duct carcinoma.
A significant correlation is detected between 1) between the expression of PD-L1 in IC and PD-1 in IC (panel A); 2) between PD-1 in IC and PRAME in TC (panel B), and 3) between PD-L1 in IC and PRAME in TC (panel C). Pearson correlation coefficients and p values are obtained using Pearson Correlation analysis (panel D).
Prognostic significance of PD-L1, PD-1, PRAME, and MHC I in SDC
Among the 53 patients with SDC, 35 (66%) had nodal metastases at the time of initial surgery. Forty-six patients had available follow-up with a median follow up of 34 months (range: 1–129). Among these 46 patients, 26 (57%) developed recurrences, including 16 distant metastases, 3 locoregional recurrences, and 7 both distant and locoregional recurrences. Nineteen patients (41%) suffered disease-specific death.
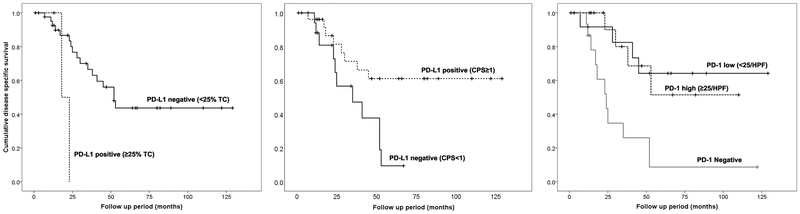
The expression of PD-L1, PD-1, PRAME, and MHC I did not predict the risk of lymph node metastases (Fisher’s exact test, p=0.981 for PD-L1, p=0.076 for PD-1, p=0.214 for PRAME, and p=0.083 for MHC I). PD-L1 immunopositivity in at least 25% of TC was associated with a decreased DSS (Table 3 and Figure 3, hazard ratio=5.343, 95% confidence interval=1.068–26.737, p=0.022), whereas PD-L1 immunopositivity determined using a CPS ≥1 was associated with improved DSS and DFS (hazard ratio=0.342 for DSS and 0.341 for DSS, p=0.022 and 0.010 respectively). The presence of PD-1-positive IC, was associated with improved survival regardless of the expression level (hazard ratio=0.407, 95% confidence interval=0.214–0.776, p=0.001). None of the other studied immunomarkers predicted DSS or RFS.
Table 3. Prognostic significance of PD-L1, PD-1, PRAME and MHC I.
TC: tumor cells, IC: immune cells, HR: hazard ratio, CI: confidence interval. P values were obtained using Log rank test.
| Disease specific Survival | Recurrence fee survival | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| PD-L1 in TC (TC 25%) | 5.343 | 1.068–26.737 | 0.022 | 3.534 | 0.768–16.260 | 0.105 |
| PD-L1 positivity (CPS 1%) | 0.342 | 0.136–0.857 | 0.022 | 0.341 | 0.150–0.775 | 0.010 |
| PD-1 in IC | 0.407 | 0.214–0.776 | 0.001 | 0.793 | 0.495–1.271 | 0.336 |
| PRAME in TC | 0.363 | 0.110–1.192 | 0.082 | 0.494 | 0.176–1.392 | 0.182 |
| MHC I in TC | 1.245 | 0.681–2.276 | 0.068 | 1.101 | 0.624–1.940 | 0.740 |
Figure 3. Prognostic significance of PD-L1 and PD-1.
(Left) The expression of PD-L1 in at least 25% of tumor cells (TC) was associated with worse disease specific survival. (Middle) PD-L1 positivity defined as CPS ≥1 was associated with improved disease specific survival. (Right) The presence of PD-1-positive immune cells (IC) was associated with improved survival.
DISCUSSION
One hallmark of cancer cells is the accumulation of various genotypic and phenotypic alterations that differentiate them from normal cells. High mutation burden and disruption of DNA methylation of promoter CpG islands in cancer cells lead to aberrant tumor antigen expression. Tumor antigens, including CTAs, are presented by the MHC I complex on tumor cell surface which can be recognized by the CD8(+) cytotoxic T lymphocytes through T-cell receptor, leading to adaptive host immune response and tumor killing27, 28. However, cancer cells can evade immune recognition through multiple immune tolerance mechanisms, including 1) defective antigen presentation through downregulation or complete loss of MHC I complex on tumor cells leading to defective binding by the T cell receptor, and 2) excessive expression of immune checkpoint inhibitors, e.g. PD-L1 in TCs and PD-1 in tumor infiltrated lymphocytes (TILs), resulting in anergic state of TILs29–32. In the present study, we investigated the immune environment in SDC an aggressive high-grade salivary malignancy, focusing on the immunoexpression of PD-L1, PD-1, cancer testis antigen PRAME and MHC I.
PD-L1 expression in TCs impairs T-cell regulation, disrupts antitumoral immune response and contributes to an immunosuppressive microenvironment allowing tumor immune escape1, 33–36. In several cancer types such as HNSCC12, oropharyngeal SCC37, pulmonary adenocarcinoma38, esophageal SCC39 and urothelial carcinoma40, PD-L1 immunoexpression has been reported to be associated with advanced tumor stage, poor clinical outcome and/or decreased overall survival. Similarly, in a cohort of 219 salivary gland carcinomas of various types including 31 SDCs, Mukaigawa et al. reported that high level of PD-L1 expression in TC was associated with poor overall survival, DSS and DFS25. Using staining in 1% TC as the cutoff for PD-L1 positivity, Nakano et al. reported that PD-L1 positivity was associated with decreased DSS in a cohort of 30 patients with salivary carcinoma, including 8 SDCs41. The results of our study confirmed such findings with PD-L1 positivity in at least 25% of TC found to be associated with decreased DSS. However, the study cohort is relatively small, and only 3 tumors had PD-L1 positivity in ≥ 25% of TC. Adding to the complexity is the fact that PD-L1 immunopositivity defined by a CPS of ≥1was associated with improved DSS and DFS. A possible explanation of this discrepancy that these PD-L1-positive TC represent the cells which are creating the immunosuppressive environment whereas the PD-L1 positive cells in CPS represent more infiltration by the less active exhausted IC. Nevertheless, the prognostic value of PD-L1 in SDCs should be further explored with future large-scale studies.
To date, only four studies have reported PD-L1 positivity in salivary gland malignancies and none of them focused on SDC. In the first study, Cohen et al. reported a frequency of 18% PD-L1 positivity, using 22C3 antibody clone, in 26 of 142 salivary gland carcinomas with various histologic types, applying a positivity cutoff of ≥ 1% in TC or IC4. One of the positive cases was SDC. The frequency of PD-L1 positivity in SDC could not be derived as the cancer classification in the PD-L1-negative cases was not provided in this study. In the second study, Mukaigawa et al. utilized tissue micro array of 218 salivary gland carcinomas, anti-PD-L1 antibody E1L3N, and a threshold of 1% TC. In their study, PD-L1 was positive in 23% (50 of 218) of all tested salivary gland carcinomas and 48% (15 of 31) of SDCs. In the third study, Harada et al. reported 51% PD-L1 positivity in TC and 43% PD-L1 positivity in IC in 47 different types of salivary malignancies using a laboratory developed PD-L1 clone (Abcam, Cambridge, UK) and a threshold of 5% positivity24. This study did not include any SDC. Lastly, Nakano et al. reported an overall PD-L1 positivity of 37% in 30 cases of salivary carcinoma using SP263 clone and a cutoff of 1% TC41. Among the 8 SDCs included in the study, 4 (50%) were positive for PD-L1. In the present study, we used the SP263 clone(the FDA-approved companion test for durvalumab anti-PD-L1/anti-PD1 treatment) and two thresholds which were previously established in clinical trials to predict maximal therapy benefits, namely ≥ 25% TC (the criterion for non-small cell carcinoma and HNSCC) or ≥ 25% TC or IC (the criterion for urothelial carcinoma)11, 29, 42. In our cohort, the reported rate of PD-L1 immunopositivity in this pure cohort of SDCs was 6%, 32%, and 66%, using the three thresholds proposed in various clinical trials for HNSCC. The 1% TC threshold yielded results close to the PD-L1 positive rate reported by Mukaigawa et al. (48%) and Nakano et al. (37% overall, and 50% in SDC). Based on current evidence, it is clear that the threshold used can significantly affect the rate of PD-L1 immunopositivity in SDC. Clinical trials of anti-PD1/anti-PD-L1 therapy targeting specifically to SDC are needed to determine the relevant threshold to predict therapy efficacy and to interpret PD-L1 immunopositivity in this group of tumors. Additionally, we identified that the frequency of PD-L1 immunopositivity was significantly higher in SDCex-PA compared with SDC de novo using a cutoff of CPS≥1, but not a cutoff of ≥ 25% TC or ≥ 1% TC. However, given the small size of this cohort, larger-scale studies are needed to determine if the frequency of PD-L1 differs between subtypes of SDC.
In tumor microenvironment, the engagement of PD-L1 in TC and PD-1 in various IC, including activated T cells, leads to T cell dysfunction, inappropriate host immune response to actionable tumor antigens, immune tolerance and tumor progression43–45. PD-1 expression can be upregulated in various types of active IC, e.g. T cells, B cells, NK cells, dendritic cells, monocytes and macrophages, following persistent exposure to antigens46–48. In this study, we have shown that PD-1-positive IC can be detected in 66% of SDCs, including 36% with high number of positive IC. There was a significant correlation between PD-1-positive IC and percentage of PRAME-positive TC, further supports the hypothesis that PD-1 upregulation in IC can be a result of tumor antigen exposure. There was also a significant correlation between PD-L1 in IC and PD-1 in IC in our study, a phenomenon that has been previously reported in non-small cell lung carcinoma49. Interestingly, PD-1 expression in IC was associated with an improved DSS in our cohort on univariate analyses. Several previous studies have reported conflicting results on the prognostic significance of PD-1 expression. For example, Fiedler et al. have showed that low PD-1 was associated with advanced tumor stage and local recurrence in HNSCC, while it appeared that PD-1 expression in urothelial carcinoma was an adverse prognostic factor for decreased survival40, 50. Additional future studies preferably with larger cohort size and/or meta-analysis are needed to clarify the prognostic significant of PD-1 in SDC and other cancer types.
Cancer testis antigens are common tumor antigens that are specifically expressed in multiple solid cancer types, including HNSCC and salivary gland carcinoma23, 51–56. The expression of CTAs in adult human organs is limited to immune-privileged sites, e.g. testis and for some CTAs occasionally placenta and brain21, 51. In this study, we showed that PRAME, a highly immunogenic CTA, is expressed in 83% of SDCs, but none of the background tissues, e.g. salivary glands, IC, and stromal cells. The high frequency of PRAME expression and its selective expression pattern solely in TC render PRAME a possible diagnostic tool to detect malignancy and a promising target for cancer vaccination in SDC. Besides the current study, Harada et al.23 have also studied the frequency of CTAs MAGE-A, NY-ESO-1, CT6 and GAGE7 in a cohort of 95 cases of salivary gland carcinoma including 12 patients with SDC. In their cohort, 50% of SDCs expressed NY-ESO-1 and 68% were positive for CT7. Interesting, the authors have reported MAGE-A as an independent risk factor for locoregional recurrence in 46 cases of adenoid cystic carcinoma. In our study, PRAME expression was not associated with adverse clinical outcomes, e.g. risk of lymph node metastasis at the initial presentation, RFS and DSS. In addition, multiple previous studies have investigated the frequency and prognostic significance of CTAs in HNSCC and showed a high frequency of CTA expression in these tumors but inconsistent prognostic significance52–57. Taken together, these data have indicated that CTAs, e.g. PRAME, are highly prevalent in head and neck cancer, including SDC and may serve as a tumor antigen target for the development of cancer vaccination.
The presence of MHC class I-tumor antigen complex on the tumor cell surface to tumor antigen-specific cytotoxic T cells is an important step of T cell-mediated adaptive immune response28. Downregulation of MHC class I is a frequent phenomenon observed in many cancer types and is a key mechanism of cancer immune evasion, contributing to the disappointing clinical results of T-cell based immunotherapy and tumor antigen-specific cancer vaccination28, 51. Complete loss or heterogenous expression of MHC I has been reported in 19 to 66% of HNSCC17–20, 28 and has been associated with adverse clinical outcomes17, 19, 58. The expression of MHC I in SDC and its prognostic significance have not yet been well-studied. In this study, we have reported downregulation of MHC I on tumor cell surface in 82% of SDCs, including 63% showing (near) absence of MHC I. We failed to demonstrate an association between MHC I downregulation and clinical outcome (e.g. DFS and DSS), possibly due to the small number of studied cases. Future studies may be needed to clarify the prognostic significance of MHC I in SDC.
CONCLUSIONS
A significant proportion of SDC shows alterations of tumor immune microenvironment, including PD-L1 expression in 6% to 66%, upregulation of PD-1 in IC in 66%, expression of PRAME in 82.5%, and downregulation of MHC class I in 82%. The expression of tumor specific antigen and the overexpression of immune checkpoint inhibitors PD-1/PD-L1 indicate that patients with SDC may benefit from novel immune therapy approaches, e.g. cancer vaccination and anti-PD1/PDL1 therapy.
Conflicts of Interest and Source of Funding:
• The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
• Research reported in this publication was supported in part by the Cancer Center Support Grant of the National Institutes of Health/National Cancer Institute under award number P30CA008748. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
• The SP263 antibody was purchased using a departmental Educational Grant courtesy of Astra Zeneca.
References
- 1.De Meulenaere A, Vermassen T, Aspeslagh S et al. Turning the tide: Clinical utility of pd-l1 expression in squamous cell carcinoma of the head and neck. Oral Oncology 2017;70;34–42. [DOI] [PubMed] [Google Scholar]
- 2.Economopoulou P, Kotsantis I, Psyrri A. The promise of immunotherapy in head and neck squamous cell carcinoma: Combinatorial immunotherapy approaches. ESMO Open 2017;1;e000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chow LQM, Haddad R, Gupta S et al. Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: Results from the phase ib keynote-012 expansion cohort. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2016;34;3838–3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen RB, Delord JP, Doi T et al. Pembrolizumab for the treatment of advanced salivary gland carcinoma: Findings of the phase 1b keynote-028 study. American journal of clinical oncology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferris RL, Blumenschein G Jr., Fayette J et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 2016;375;1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferris RL, Blumenschein G Jr., Fayette J et al. Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of checkmate 141 with analyses by tumor pd-l1 expression. Oral Oncol 2018;81;45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levy A, Massard C, Soria JC, Deutsch E. Concurrent irradiation with the anti-programmed cell death ligand-1 immune checkpoint blocker durvalumab: Single centre subset analysis from a phase 1/2 trial. Eur J Cancer 2016;68;156–162. [DOI] [PubMed] [Google Scholar]
- 8.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. Journal of Clinical Oncology 2015;33;1974–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature Reviews Cancer 2012;12;252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tumeh PC, Harview CL, Yearley JH et al. Pd-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515;568–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rebelatto MC, Midha A, Mistry A et al. Development of a programmed cell death ligand-1 immunohistochemical assay validated for analysis of non-small cell lung cancer and head and neck squamous cell carcinoma. Diagnostic pathology 2016;11;95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Müller T, Braun M, Dietrich D et al. Pd-l1: A novel prognostic biomarker in head and neck squamous cell carcinoma. Oncotarget 2017;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melero I, Gaudernack G, Gerritsen W et al. Therapeutic vaccines for cancer: An overview of clinical trials. Nature Reviews Clinical Oncology 2014;11;509–524. [DOI] [PubMed] [Google Scholar]
- 14.Guo C, Manjili MH, Subjeck JR, Sarkar D, Fisher PB, Wang X-Y. Therapeutic cancer vaccines. Advances in Cancer Research: Elsevier, 2013;421–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Burg SH, Arens R, Ossendorp F, van Hall T, Melief CJM. Vaccines for established cancer: Overcoming the challenges posed by immune evasion. Nature Reviews Cancer 2016;16;219–233. [DOI] [PubMed] [Google Scholar]
- 16.Whitehurst AW. Cause and consequence of cancer/testis antigen activation in cancer. Annual Review of Pharmacology and Toxicology 2014;54;251–272. [DOI] [PubMed] [Google Scholar]
- 17.Nasman A, Andersson E, Nordfors C et al. Mhc class i expression in hpv positive and negative tonsillar squamous cell carcinoma in correlation to clinical outcome. Int J Cancer 2013;132;72–81. [DOI] [PubMed] [Google Scholar]
- 18.Bandoh N, Ogino T, Katayama A et al. Hla class i antigen and transporter associated with antigen processing downregulation in metastatic lesions of head and neck squamous cell carcinoma as a marker of poor prognosis. Oncol Rep 2010;23;933–939. [DOI] [PubMed] [Google Scholar]
- 19.Ogino T, Shigyo H, Ishii H et al. Hla class i antigen down-regulation in primary laryngeal squamous cell carcinoma lesions as a poor prognostic marker. Cancer Research 2006;66;9281–9289. [DOI] [PubMed] [Google Scholar]
- 20.Bandoh N, Ogino T, Hayashi T et al. Hla class i antigen defects in maxillary sinus squamous cell carcinoma: Potential prognostic significance. International Congress Series 2003;1240;487–488. [Google Scholar]
- 21.Tagliamonte M, Petrizzo A, Tornesello ML, Buonaguro FM, Buonaguro L. Antigen-specific vaccines for cancer treatment. Hum Vaccin Immunother 2014;10;3332–3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ. World health organization classification of tumours: Pathology and genetics of head and neck tumours (4th edition). Lyon: International Agency for Research on Cancer (IARC), 2017. [Google Scholar]
- 23.Beppu S, Ito Y, Fujii K et al. Expression of cancer/testis antigens in salivary gland carcinomas with reference to mage-a and ny-eso-1 expression in adenoid cystic carcinoma. Histopathology 2017;71;305–315. [DOI] [PubMed] [Google Scholar]
- 24.Harada K, Ferdous T, Ueyama Y. Pd-l1 expression in malignant salivary gland tumors. BMC Cancer 2018;18;156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mukaigawa T, Hayashi R, Hashimoto K, Ugumori T, Hato N, Fujii S. Programmed death ligand-1 expression is associated with poor disease free survival in salivary gland carcinomas. Journal of surgical oncology 2016;114;36–43. [DOI] [PubMed] [Google Scholar]
- 26.Lezcano C, Jungbluth AA, Nehal KS, Hollmann TJ, Busam KJ. Prame expression in melanocytic tumors. Am J Surg Pathol 2018;42;1456–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Durgeau A, Virk Y, Corgnac S, Mami-Chouaib F. Recent advances in targeting cd8 t-cell immunity for more effective cancer immunotherapy. Frontiers in immunology 2018;9;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferris RL, Hunt JL, Ferrone S. Human leukocyte antigen (hla) class i defects in head and neck cancer: Molecular mechanisms and clinical significance. Immunologic Research 2005;33;113–134. [DOI] [PubMed] [Google Scholar]
- 29.Tsao MS, Kerr KM, Dacic S, Yatabe Y, Hirsch FR. Iaslc atlas of pd-l1 immunohistochemistry testing in lung cancer. 1 ed. Aurora, Colorado, 80011, USA: International Association for the Study of Lung Cancer (IASLC), 2017;128. [Google Scholar]
- 30.Thibodeau J, Bourgeois-Daigneault MC, Lapointe R. Targeting the mhc class ii antigen presentation pathway in cancer immunotherapy. Oncoimmunology 2012;1;908–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salmaninejad A, Zamani MR, Pourvahedi M, Golchehre Z, Hosseini Bereshneh A, Rezaei N. Cancer/testis antigens: Expression, regulation, tumor invasion, and use in immunotherapy of cancers. Immunol Invest 2016;45;619–640. [DOI] [PubMed] [Google Scholar]
- 32.Brahmer JR, Tykodi SS, Chow LQM et al. Safety and activity of anti–pd-l1 antibody in patients with advanced cancer. New England Journal of Medicine 2012;366;2455–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heineman TE, Widman A, Kuan EC, St John M. The genetic landscape of programmed death ligand-1 (pd-l1) alterations in head and neck cancer. Laryngoscope Investig Otolaryngol 2017;2;99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel SP, Kurzrock R. Pd-l1 expression as a predictive biomarker in cancer immunotherapy. Molecular cancer therapeutics 2015;14;847–856. [DOI] [PubMed] [Google Scholar]
- 35.Zandberg DP, Strome SE. The role of the pd-l1:Pd-1 pathway in squamous cell carcinoma of the head and neck. Oral Oncol 2014;50;627–632. [DOI] [PubMed] [Google Scholar]
- 36.Lyford-Pike S, Peng S, Young GD et al. Evidence for a role of the pd-1:Pd-l1 pathway in immune resistance of hpv-associated head and neck squamous cell carcinoma. Cancer Res 2013;73;1733–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steuer CE, Griffith CC, Nannapaneni S et al. A correlative analysis of pd-l1, pd-1, pd-l2, egfr, her2, and her3 expression in oropharyngeal squamous cell carcinoma. Molecular cancer therapeutics 2018;17;710–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chan AWH, Tong JHM, Kwan JSH et al. Assessment of programmed cell death ligand-1 expression by 4 diagnostic assays and its clinicopathological correlation in a large cohort of surgical resected non-small cell lung carcinoma. Mod Pathol 2018. [DOI] [PubMed] [Google Scholar]
- 39.Guo W, Wang P, Li N et al. Prognostic value of pd-l1 in esophageal squamous cell carcinoma: A meta-analysis. Oncotarget 2018;9;13920–13933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krabbe LM, Heitplatz B, Preuss S et al. Prognostic value of pd-1 and pd-l1 expression in patients with high grade upper tract urothelial carcinoma. J Urol 2017;198;1253–1262. [DOI] [PubMed] [Google Scholar]
- 41.Nakano T, Takizawa K, Uezato A, Taguchi K, Toh S, Masuda M. Prognostic value of programed death ligand-1 and ligand-2 co-expression in salivary gland carcinomas. Oral Oncol 2019;90;30–37. [DOI] [PubMed] [Google Scholar]
- 42.Faiena I, Cummings AL, Crosetti AM, Pantuck AJ, Chamie K, Drakaki A. Durvalumab: An investigational anti-pd-l1 monoclonal antibody for the treatment of urothelial carcinoma. Drug Des Devel Ther 2018;12;209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bellmunt J, Powles T, Vogelzang NJ. A review on the evolution of pd-1/pd-l1 immunotherapy for bladder cancer: The future is now. Cancer Treatment Reviews 2017;54;58–67. [DOI] [PubMed] [Google Scholar]
- 44.Berger KN, Pu JJ. Pd-1 pathway and its clinical application: A 20year journey after discovery of the complete human pd-1 gene. Gene 2018;638;20–25. [DOI] [PubMed] [Google Scholar]
- 45.Dong Y, Sun Q, Zhang X. Pd-1 and its ligands are important immune checkpoints in cancer. Oncotarget 2017;8;2171–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahmadzadeh M, Johnson LA, Heemskerk B et al. Tumor antigen-specific cd8 t cells infiltrating the tumor express high levels of pd-1 and are functionally impaired. Blood 2009;114;1537–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P et al. Tumor-infiltrating ny-eso-1–specific cd8+t cells are negatively regulated by lag-3 and pd-1 in human ovarian cancer. Proceedings of the National Academy of Sciences 2010;107;7875–7880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zou W, Chen L. Inhibitory b7-family molecules in the tumour microenvironment. Nature Reviews Immunology 2008;8;467–477. [DOI] [PubMed] [Google Scholar]
- 49.Del CM-BP, Driver B, Morales-Rosado JA et al. Correlation between programmed death receptor-1 expression in tumor-infiltrating lymphocytes and programmed death ligand-1 expression in non-small cell lung carcinoma. Archives of pathology & laboratory medicine 2018. [DOI] [PubMed] [Google Scholar]
- 50.Hayakawa N, Kikuchi E, Mikami S, Fukumoto K, Oya M. The role of pd-1 positivity in the tumour nest on clinical outcome in upper tract urothelial carcinoma patients treated with radical nephroureterectomy. Clinical oncology (Royal College of Radiologists (Great Britain)) 2018;30;e1–e8. [DOI] [PubMed] [Google Scholar]
- 51.Aitken AS, Roy DG, Bourgeois-Daigneault MC. Taking a stab at cancer; oncolytic virus-mediated anti-cancer vaccination strategies. Biomedicines 2017;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cuffel C, Rivals JP, Zaugg Y et al. Pattern and clinical significance of cancer-testis gene expression in head and neck squamous cell carcinoma. Int J Cancer 2011;128;2625–2634. [DOI] [PubMed] [Google Scholar]
- 53.Figueiredo DL, Mamede RC, Spagnoli GC et al. High expression of cancer testis antigens mage-a, mage-c1/ct7, mage-c2/ct10, ny-eso-1, and gage in advanced squamous cell carcinoma of the larynx. Head Neck 2011;33;702–707. [DOI] [PubMed] [Google Scholar]
- 54.Atanackovic D, Blum I, Cao Y et al. Expression of cancer-testis antigens as possible targets for antigen-specific immunotherapy in head and neck squamous cell carcinoma. Cancer biology & therapy 2006;5;1218–1225. [DOI] [PubMed] [Google Scholar]
- 55.Han L, Jiang B, Wu H, Zhang S, Lu X. Expression and prognostic value of mage-a9 in laryngeal squamous cell carcinoma. Int J Clin Exp Pathol 2014;7;6734–6742. [PMC free article] [PubMed] [Google Scholar]
- 56.Laban S, Giebel G, Klumper N et al. Mage expression in head and neck squamous cell carcinoma primary tumors, lymph node metastases and respective recurrences-implications for immunotherapy. Oncotarget 2017;8;14719–14735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Piotti KC, Scognamiglio T, Chiu R, Chen YT. Expression of cancer/testis (ct) antigens in squamous cell carcinoma of the head and neck: Evaluation as markers of squamous dysplasia. Pathol Res Pract 2013;209;721–726. [DOI] [PubMed] [Google Scholar]
- 58.Meissner M Defects in the human leukocyte antigen class i antigen processing machinery in head and neck squamous cell carcinoma: Association with clinical outcome. Clinical Cancer Research 2005;11;2552–2560. [DOI] [PubMed] [Google Scholar]