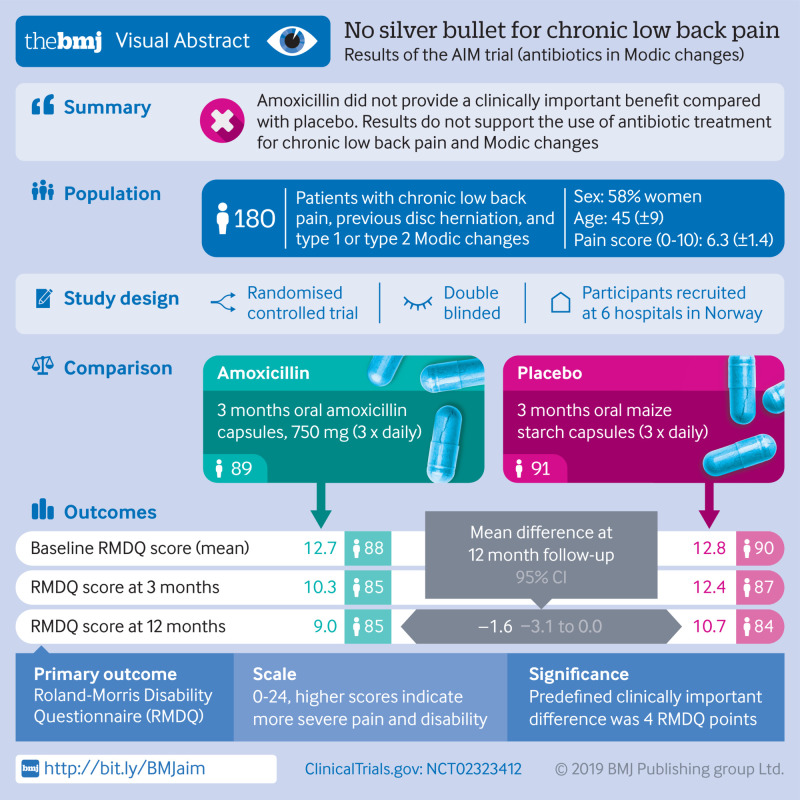
Abstract
Objective
To assess the efficacy of three months of antibiotic treatment compared with placebo in patients with chronic low back pain, previous disc herniation, and vertebral endplate changes (Modic changes).
Design
Double blind, parallel group, placebo controlled, multicentre trial.
Setting
Hospital outpatient clinics at six hospitals in Norway.
Participants
180 patients with chronic low back pain, previous disc herniation, and type 1 (n=118) or type 2 (n=62) Modic changes enrolled from June 2015 to September 2017.
Interventions
Patients were randomised to three months of oral treatment with either 750 mg amoxicillin or placebo three times daily. The allocation sequence was concealed by using a computer generated number on the prescription.
Main outcome measures
The primary outcome was the Roland-Morris Disability Questionnaire (RMDQ) score (range 0-24) at one year follow-up in the intention to treat population. The minimal clinically important between group difference in mean RMDQ score was predefined as 4.
Results
In the primary analysis of the total cohort at one year, the difference in the mean RMDQ score between the amoxicillin group and the placebo group was −1.6 (95% confidence interval −3.1 to 0.0, P=0.04). In the secondary analysis, the difference in the mean RMDQ score between the groups was −2.3 (−4.2 to−0.4, P=0.02) for patients with type 1 Modic changes and −0.1 (−2.7 to 2.6, P=0.95) for patients with type 2 Modic changes. Fifty patients (56%) in the amoxicillin group experienced at least one drug related adverse event compared with 31 (34%) in the placebo group.
Conclusions
In this study on patients with chronic low back pain and Modic changes at the level of a previous disc herniation, three months of treatment with amoxicillin did not provide a clinically important benefit compared with placebo. Secondary analyses and sensitivity analyses supported this finding. Therefore, our results do not support the use of antibiotic treatment for chronic low back pain and Modic changes.
Trial registration
ClinicalTrials.gov NCT02323412.
Introduction
The current management of low back pain offers low to moderate improvement in pain and disability.1 Researchers therefore attempt to identify subgroups of patients who would benefit from specific treatment. A suggested subgroup of patients with chronic low back pain have signal changes in the vertebral bone marrow that extends from the endplate (Modic changes) on magnetic resonance imaging.2 Modic changes are classified into type 1 (oedema type), type 2 (fatty type), and type 3 (sclerotic type, less common).3 The pathogenesis of Modic changes is unclear. One hypothesis is that Modic changes and low back pain are caused by low grade bacterial discitis caused by Cutibacterium acnes (formerly known as Propionibacterium acnes), an aerotolerant Gram positive anaerobe bacteria and a common skin commensal.4 The proposed port of entry into the disc is through the bloodstream, which is made possible during vascularisation related to inflammation caused by a disc herniation.5 Animal models show that C acnes could grow in degenerated discs and cause Modic changes.6 7 However, several microbiological studies of disc biopsies have conflicting results.4
A systematic review found only one randomised trial that assessed the efficacy of antibiotic treatment in patients with low back pain.4 The trial reported a substantial effect of three months of antibiotic treatment compared with placebo (between group difference of 8.3 points on the Roland-Morris Disability Questionnaire (RMDQ) at one year follow-up) in patients with chronic low back pain, prior disc herniation, and type 1 Modic changes.8 The trial conclusion has been questioned based on almost no improvement in the control group,9 no evaluation of blinding efficacy, and a high proportion of participants with previous disc surgery and thus a potential risk of bacterial contamination.10 Current guidelines do not include recommendations (for or against) antibiotic treatment in patients with persistent low back pain and Modic changes.11 About 40-50% of patients with non-specific low back pain have Modic changes, and antibiotic treatment in subgroups of this large patient population could increase antibiotic resistance.12 13 14
We aimed to replicate the findings in the former randomised trial. This trial evaluated the efficacy and harm of three months of oral treatment with amoxicillin at one year follow-up in patients with chronic low back pain and type 1 or 2 Modic changes at the level of a previous lumbar disc herniation.
Methods
Study design
This was a multicentre, randomised, double blind, placebo controlled, parallel group trial with a treatment phase (three months) and a follow-up phase (nine months). The trial was performed in accordance with the Helsinki Declaration and the International Conference on Harmonisation of Good Clinical Practice, reported according to the CONSORT guidelines,15 and was registered at ClinicalTrials.gov in December 2014 under the identifier: NCT02323412. The Regional Committees for Medical Research Ethics South East Norway (2014/158/REK sør-øst) and the Norwegian Medicines Agency (SLV; reference No 14/01368-11; EudraCT No 2013-004505-14) approved the trial before it started. The Clinical Trial Unit at Oslo University Hospital monitored the trial. All participants gave written informed consent. Methods were unchanged after trial commencement. Funding was granted by governmental organisations (Helse Sør-Øst and Helse Vest), which had no part in the planning, performing, or reporting of the trial. The trial protocol is available at ClinicalTrials.gov.
Participants
We recruited patients from outpatient clinics at six hospitals in Norway from June 2015 to September 2017. Inclusion criteria were age 18-65 years; low back pain for more than six months with intensity of at least 5 on a 0-10 numerical rating scale (mean of current, worst within the preceding two weeks, and usual/mean within the preceding two weeks); lumbar disc herniation on magnetic resonance imaging in the preceding two years; and type 1 or type 2 Modic changes (with height ≥10% of vertebral height and diameter >5 mm) at the herniated disc level. In contrast to the trial we were reassessing, we chose to include patients with type 2 Modic changes because differentiating between type 1 and type 2 Modic changes is of uncertain relevance and might depend on the magnetic field strength of the MRI scanner used.16 17 We excluded patients who had surgery for disc herniation in the past year or antibiotic treatment in the past month. Figure S1 in the supplementary appendix contains all eligibility criteria.18
Randomisation, masking, and procedures
Patients were randomised at a median of 13 days after inclusion into either three months of oral treatment with amoxicillin 750 mg three times daily or placebo (maize starch). The tablets were encapsulated (Kragerø Tablettproduksjon AS), with identical capsules, containers, and labels for both treatment groups. A third party statistician created randomisation lists by using Stata 13 (StataCorp, College Station, TX, USA). Lists were stratified by Modic change type (1 or 2) and previous disc herniation surgery or not, with a 1:1:1:1 allocation and random block sizes of four and six; this approach ensured that a similar number of patients received antibiotics or placebo within each stratum. The allocation sequence was concealed. Care providers gave each patient a prescription with a computer generated allocation number to be used at the dedicated hospital pharmacies. All care providers, research staff, statisticians, and patients were unaware of the assignment group during the data collection. Care providers, research staff, and statisticians were also blinded during primary and secondary analyses and first draft of this manuscript. We recommended that participants should not start additional treatments for back pain, but they were allowed to continue ongoing treatment. We encouraged participants not to use non-steroidal anti-inflammatory drugs during the intervention period.
To confirm Modic changes seen on a clinical MRI scan available at screening, baseline MRI was performed at a median of 22 days (interquartile range 15-30) before treatment initiation by using identical 1.5 T protocols at each study centre. We allocated patients with primary (most extensive) or secondary type 1 Modic changes (hypointense on T1 images, hyperintense on T2 images) to the type 1 Modic change group. Patients with type 2 Modic changes (hyperintense on T1, isointense or hyperintense on T2) but not type 1 Modic changes were included in the type 2 Modic change group. We rated borderline type 1 versus type 2 Modic changes (near isointense on T1) as type 2. Two experienced radiologists independently evaluated Modic changes on the baseline MRI, disc herniation on MRI scans from the previous two years, and eligibility for the trial in the type 1 versus type 2 Modic change group versus being ineligible (κ=0.62). The radiologists discussed and solved all disagreements on eligibility.
Outcomes and data collection
The primary outcome was the score on the validated Norwegian version of the RMDQ at one year follow-up. RMDQ scores range from 0 to 24. Higher scores indicate more severe pain and disability. Secondary outcomes included pain related disability (Oswestry Disability Index 2.0), low back pain intensity (0-10 numerical rating scale), and health related quality of life (EuroQol’s health related quality of life (EQ5D-5L), version 2.0). Trial measurements were unchanged after trial commencement and were reported at several time points (table S1, supplementary appendix) by using a web based data capture system (Viedoc) or, in a few cases, using a paper version. Trial care providers (physicians or physiotherapists) performed active surveillance of side effects and adverse events (clinical and biochemical) from baseline to one year using Common Terminology of Clinical Adverse Events version 4.0 in accordance with Medical Dictionary for Regulatory Activities (medDRA) coding.19 Adverse events were monitored and cross checked against clinical patient notes, and we report all of them in this study. We monitored compliance by using weekly patient reported questionnaires and by counting the returned study drug capsules at the end of treatment visit (table S1)
Statistical analysis
We specified all main statistical analyses in a statistical analysis plan in advance of database locking.20 Retrospective analyses not described in the statistical analysis plan are highlighted in the paper and the tables.
In the primary analysis we compared mean RMDQ scores between the two treatment groups in the whole intention to treat population at one year by using analysis of covariance and adjusting for baseline RMDQ score and the stratification variables. Missing RMDQ values were imputed with a multiple imputation model.20 In the secondary analyses, we repeated this comparison in each Modic change type group. We also performed analyses of key supportive objectives (secondary outcomes: Oswestry Disability Index, low back pain intensity, and EQ5D-5L); per protocol analysis and other sensitivity analyses; responder analyses (>30%, >50%, and >75% improvement from baseline, excluding patients with >30% missing RMDQ items); and linear mixed effects models as described in the statistical analysis plan.20 An exploratory objective was to report the incidence of adverse events that occurred from randomisation to one year follow-up. Adverse events were presented descriptively for the safety population according to intervention group, without statistical testing.
We predefined a clinically important between group difference in mean RMDQ score as 4. We did not consider smaller differences to be clinically relevant in this trial (although another study regarded a difference of 2.5 to be relevant21) because we were reassessing the potential curative effect of antibiotic treatment; additionally, a much higher difference (8.3) was reported in the former trial.8 In comparison, a change of 2-3 RMDQ points in individual patients over time could represent measurement error,22 and the minimal detectable change of the RMDQ was 4 in a Norwegian study of chronic low back pain patients.23 In each Modic change type group, we needed 66 analysed patients to detect (β=0.1, α=0.05) a difference of 4 (standard deviation 5) in mean RMDQ score between the two treatment groups. In the total sample, 132 analysed patients allowed detection (β=0.1, α=0.05) of a difference of 2.8 (standard deviation 5) in mean RMDQ score. We added 20% to allow for dropouts and planned to include 80 participants in each group.
When half of the study participants had completed their one year assessment, an independent statistician blinded to treatment groups performed a prespecified interim analysis to ensure that continuation of the study was ethical. If mean RMDQ score differed by more than 7 points between the two treatment groups, an independent data monitoring committee could recommend the study should be stopped. No results from the interim analysis were communicated to the trial staff. We did not adjust the significance level due to the interim analysis because the trial did not have a group sequential design and only had one primary analysis.
We calculated the Bang blinding index in each treatment group based on patients’ reports at one year follow-up about which treatment they thought they had received (antibiotics, placebo, or unsure). The Bang blinding index ranges from −1 (all patients report incorrect treatment) to 1 (all patients report correct treatment); 0 indicates random reporting of treatment group.24
A senior statistician independently carried out primary, secondary, and key supportive objectives analyses by using software package R (version 3.4.4); a PhD student also performed these analyses with software package Stata (version 15). Both were blinded to treatment group.
Patient and public involvement
A patient representative was a member of the scientific board of the study, which made all the major decisions from planning and design of the study, to the dissemination of the study results. The patient representative assessed the burden of the study medication and the time and effort required to participate in the trial, and took part in writing this manuscript. We plan to disseminate the results to study participants and the patient organisation (Norwegian Back Pain Association) before publication.
Results
Patients
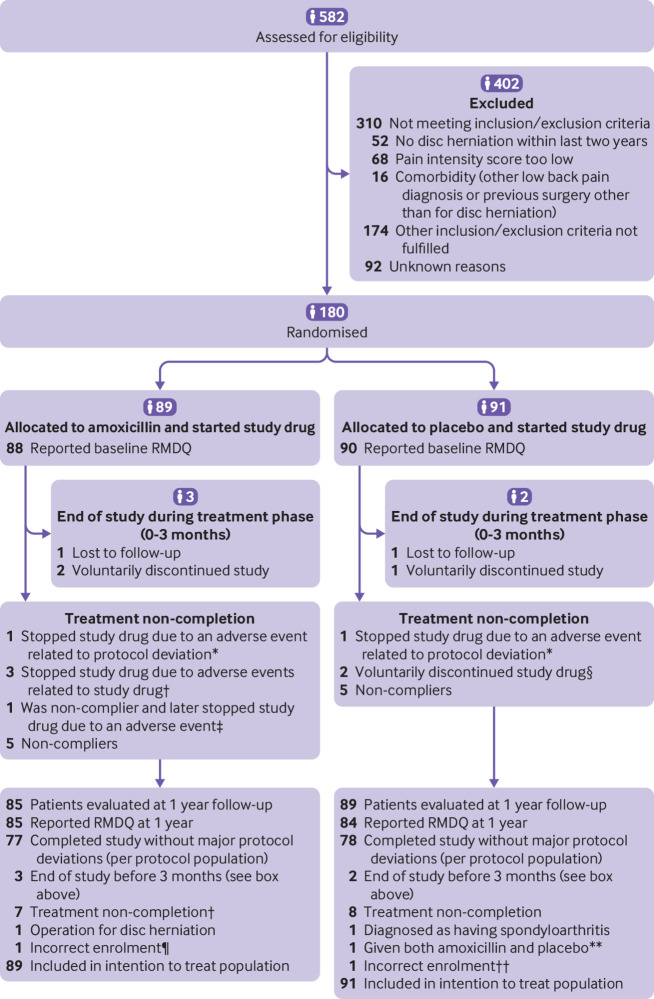
Figure 1 summarises the trial group assignments, loss to follow-up, treatment completion, and protocol deviations. Of the 582 patients assessed for eligibility, 180 underwent randomisation; 118 with type 1 Modic changes and 62 with type 2 Modic changes. Because inclusion of patients with type 1 Modic changes was faster, enrolment was closed before the goal for recruiting patients with type 2 Modic changes had been met (in September 2017). We prospectively presumed at the time of enrolment closure that a power of 88% (using the same specifications as given above for 62 patients) for the type 2 Modic change group was sufficient.
Fig 1.
Flowchart showing trial group assignments, loss to follow-up, treatment completion, and protocol deviations. RMDQ=Roland-Morris Disability Questionnaire. *One patient in amoxicillin group and one patient in placebo group became pregnant (protocol deviation because all patients were instructed to use contraception), not included in per protocol population. †Three patients in amoxicillin group stopped study drug because of adverse events and were included in per protocol population. ‡One patient in amoxicillin group stopped study drug because of adverse events but was not included in per protocol population owing to poor compliance before stopping study drug. §Two patients in placebo group discontinued because they started three month treatment with amoxicillin plus clavulanic acid. ¶Treated with apocillin seven days before randomisation. **Because of a mistake at pharmacy, patient was given a mix of bottles containing amoxicillin and placebo. ††Treated with cephalexin seven days before randomisation.
Table 1 shows baseline characteristics of participants and table 2 presents baseline values of the outcomes. The placebo group had a higher percentage of participants with jobs that required physical workload. We did not consider any other differences between the treatment groups to be relevant. In total, 38 patients (21%) had previous surgery for disc herniation. All 180 randomised patients started on the study drug and were included in the intention to treat analysis. Of these, 155 patients were included in the per protocol analysis.
Table 1.
Baseline characteristics of participants in treatment groups. Values are No/total No (%) unless stated otherwise
| Amoxicillin | Placebo | |
|---|---|---|
| Age (mean (SD)) | 44.7 (9.0), n=89 | 45.2 (9.0), n=91 |
| Women | 53/89 (60) | 52/91 (57) |
| Body mass index (mean (SD)) | 26.1 (4.1), n=89 | 25.9 (4.0), n=90 |
| Smoking, yes | 25/89 (28) | 21/89 (24) |
| Educational level: | ||
| Primary school (9 years) | 10/88 (11) | 9/89 (10) |
| High school (12 years) | 36/88 (41) | 42/89 (47) |
| College or university (<4 years) | 27/88 (31) | 18/89 (20) |
| University (≥4 years) | 15/88 (17) | 20/89 (22) |
| Comorbidity*: | ||
| Score 1 (back pain only) | 62/89 (70) | 60/91 (66) |
| Score 2 | 21/89 (24) | 27/91 (30) |
| Score >2 | 6/89 (7) | 4/91 (4) |
| Previous disc surgery | 18/89 (20) | 20/91 (22) |
| Emotional distress (HSCL-25≥1.75)† | 24/88 (27) | 23/91 (25) |
| FABQ physical activity (0-24)‡ (mean (SD)) | 11.2 (5.9), n=89 | 12.6 (5.8), n=90 |
| FABQ work (0-42)‡ (mean (SD)) | 17.0 (11.7), n=87 | 18.9 (12.0), n=89 |
| Duration of back pain (years) (median (IQR)) | 3.0 (1.5-5.6), n=89 | 3.4 (1.7-7), n=90 |
| Physical workload: | ||
| Mostly sitting | 37/77 (48) | 26/74 (35) |
| Job requires a lot of walking | 20/77 (26) | 20/74 (27) |
| Job requires a lot of walking and lifting | 17/77 (22) | 24/74 (32) |
| Job requires physically heavy work | 3/77 (4) | 4/74 (5) |
| Employment status: | ||
| Working full time | 46/89 (52) | 43/91 (47) |
| Partial sick leave | 14/89 (16) | 20/91 (22) |
| Complete sick leave | 22/89 (25) | 16/91 (18) |
| Disability pension | 3/89 (3) | 7/91 (8) |
| Unemployed | 2/89 (2) | 3/91 (3) |
| Student/other/unknown | 2/89 (2) | 2/91 (2) |
| Modic type 1 group | 58/89 (65) | 60/91 (66) |
| Level of Modic change and previous disc herniation: | ||
| L1/L2 | 0/89 | 0/91 |
| L2/L3 | 2/89 (2) | 2/91 (2) |
| L3/L4 | 7/89 (8) | 5/91 (5) |
| L4/L5 | 48/89 (54) | 29/91 (32) |
| L5/S1 | 58/89 (65) | 74/91 (81) |
SD=standard deviation; IQR=interquartile range.
Functional Comorbidity Index25; score increased by 1 for each of 18 diagnoses associated with decreased physical function.
Emotional distress (Hopkins Symptom Checklist 25); values ≥1.75 associated with psychiatric diagnosis.26
Fear avoidance beliefs questionnaire27; higher values indicate more fear avoidance beliefs.
Table 2.
Primary and secondary outcomes for treatment groups and treatment comparisons (adjusted mean difference) for all time periods
| Outcome | Amoxicillin | Placebo | ANCOVA | Linear mixed effects‡ | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No* | Mean (SD) | No | Mean (SD) | No† | Mean (95% CI) | P value | Mean (95% CI) | P value | |||||
| RMDQ: | |||||||||||||
| Baseline | 88 | 12.7 (4.7) | 90 | 12.8 (3.7) | — | — | — | — | — | ||||
| 3 months | 85 | 10.3 (5.8) | 87 | 12.4 (4.4) | 180 | −1.9 (−3.2 to −0.7) | 0.003 | −1.9 (−3.3 to −0.5) | 0.006 | ||||
| 6 months | 83 | 9.9 (5.9) | 78 | 11.7 (5.2) | 179§ | −1.5 (−2.9 to 0.0)¶ | 0.047 | −1.6 (−3.2 to −0.1) | 0.04 | ||||
| 9 months | 77 | 9.7 (6.4) | 79 | 11.1 (5.4) | 179§ | −1.6 (−3.1 to 0.0)¶ | 0.049 | −1.7 (−3.3 to −0.1) | 0.04 | ||||
| 12 months | 85 | 9.0 (6.2) | 84 | 10.7 (5.6) | 180 | −1.6 (−3.1 to 0.0) | 0.04 | −1.7 (−3.3 to −0.1) | 0.04 | ||||
| ODI: | |||||||||||||
| Baseline | 88 | 31.9 (11.4) | 89 | 31.8 (10.3) | — | — | — | — | — | ||||
| 3 months | 86 | 26.6 (14.7) | 85 | 30.4 (10.7) | 179§ | −3.8 (−6.7 to −0.9)** | 0.01 | −4.1 (−7.0 to −1.2) | 0.006 | ||||
| 12 months | 85 | 24.4 (15.0) | 84 | 28.9 (14.0) | 179§ | −4.8 (−8.3 to −1.4)** | 0.007 | −5.1 (−8.5 to 1.6) | 0.004 | ||||
| Back pain intensity††: | |||||||||||||
| Baseline | 88 | 6.4 (1.2) | 90 | 6.3 (1.5) | — | — | — | — | — | ||||
| 3 months | 85 | 5.2 (2.3) | 85 | 5.4 (1.9) | 179§ | −0.3 (−0.8 to 0.3)** | 0.33 | −0.4 (−0.8 to 0.1) | 0.13 | ||||
| 6 months | 83 | 5.1 (2.2) | 77 | 5.5 (2.2) | 178§ | −0.5 (−1.1 to 0.1)¶** | 0.11 | −0.5 (−1.0 to 0.0) | 0.03 | ||||
| 9 months | 78 | 5.2 (2.4) | 77 | 5.0 (2.3) | 178§ | 0.0 (−0.7 to 0.6)¶** | 0.97 | 0.0 (−0.5 to 0.5) | 0.88 | ||||
| 12 months | 85 | 4.7 (2.3) | 84 | 5.2 (2.3) | 179§ | −0.6 (−1.3 to 0.0)** | 0.06 | −0.7 (−1.2 to −0.2) | 0.005 | ||||
| EQ5D-5L: | |||||||||||||
| Baseline | 89 | 0.55 (0.19) | 91 | 0.54 (0.18) | — | — | — | — | — | ||||
| 3 months | 85 | 0.60 (0.22) | 83 | 0.54 (0.21) | 180 | 0.06 (0.00 to 0.11)** | 0.04 | 0.06 (0.00 to 0.11) | 0.05 | ||||
| 12 months | 84 | 0.65 (0.22) | 83 | 0.58 (0.22) | 180 | 0.07 (0.02 to 0.12)** | 0.01 | 0.07 (0.01 to 0.13) | 0.015 | ||||
| Leg pain intensity (0-10): | |||||||||||||
| Baseline | 89 | 3.2 (2.6) | 90 | 3.2 (2.6) | — | — | — | — | — | ||||
| 3 months | 85 | 3.1 (2.8) | 84 | 3.4 (2.6) | 168 | −0.3 (−0.9 to 0.4)¶** | 0.42 | — | — | ||||
| 12 months | 85 | 2.8 (2.7) | 82 | 3.5 (2.8) | 166 | −0.8 (−1.4 to −0.1)¶** | 0.03 | — | — | ||||
ANCOVA=analysis of covariance; EQ5D-5L=EuroQol’s health related quality of life (score from −0.59 to 1; higher scores indicate better quality of life); ODI=Oswestry Disability Index (score from 0 to 100; higher scores indicate more severe pain and disability); RMDQ=Roland-Morris Disability Questionnaire (score from 0 to 24; higher scores indicate more severe pain and disability).
Number of answered questionnaires of outcome.
Number of cases included in the analyses after multiple imputations.
Interaction between time and treatment at 12 months with simple contrasts.
Some patients excluded from analysis because the imputation model did not manage to impute all missing variables.
Retrospective analyses not described in the registry.
Estimates smaller than recommended thresholds for clinical important change within groups (ODI 13-20; low back pain intensity numerical rating scale 2-3; leg pain intensity numerical rating scale 2-3.5; EQ5D-5L 0.11-0.30).
Linear mixed effects model includes all time points (week 0-13).
Outcomes
At one year, the mean RMDQ score had reduced since baseline in both treatment groups (−3.7 points in the amoxicillin group and −2.1 points in the placebo group) (table 2 and fig 2). The adjusted mean difference in RMDQ score between the amoxicillin group and the placebo group at one year was −1.6 points (95% confidence interval −3.1 to 0.0, P=0.04) (table 2). The adjusted between group difference of the mean RMDQ score was −2.3 (95% confidence interval −4.2 to −0.4, P=0.02) for patients with type 1 Modic changes and −0.1 (−2.7 to 2.6, P=0.95) for patients with type 2 Modic changes. Table 2 and table 3 summarise analyses of the secondary outcomes. The responder analyses (table S2) and sensitivity analyses (table S5) are summarised in the supplementary appendix.
Fig 2.
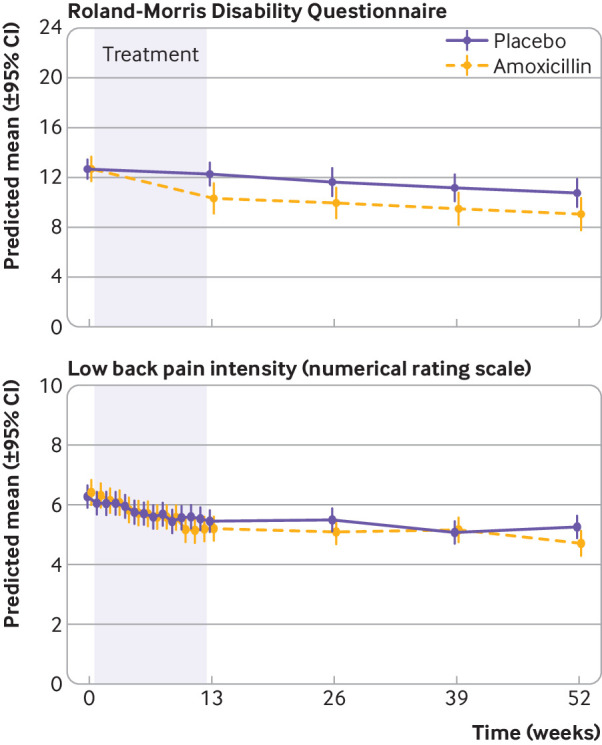
Roland-Morris Disability Questionnaire score and low back pain intensity (numerical rating scale) from baseline to one year
Table 3.
Work, global perceived effect, patient satisfaction, and drug use. Values are No/total No (%) unless stated otherwise
| Outcome | Treatment group | Treatment comparison, P value¶ | |
|---|---|---|---|
| Amoxicillin | Placebo | ||
| Working (including part time sick leave): | |||
| Baseline | 60/89 (67) | 63/91 (69) | — |
| 1 year | 56/85 (63) | 53/84 (58) | 0.62 |
| Global perceived effect at 1 year*: | |||
| Improved | 24/85 (28) | 18/84 (21) | 0.39 |
| No change | 58/85 (68) | 60/84 (71) | |
| Worse | 3/85 (4) | 6/84 (7) | |
| Patient satisfaction at 1 year: | |||
| Satisfied | 35/85 (41) | 28/84 (33) | 0.52 |
| Somewhat satisfied | 8/85 (9) | 9/84 (11) | |
| Neither satisfied nor dissatisfied | 34/85 (40) | 35/84 (42) | |
| Somewhat dissatisfied | 5/85 (6) | 4/84 (5) | |
| Dissatisfied | 3/85 (4) | 8/84 (10) | |
| Concomitant drug treatment use: | |||
| Analgesics, any: | |||
| Baseline† | 62/89 (70) | 61/91 (67) | — |
| 1 year | 61/89 (69) | 67/91 (74) | 0.45 |
| NSAIDs: | |||
| Baseline‡ | 38/89 (43) | 36/91 (40) | — |
| 1 year | 39/89 (44) | 40/91 (44) | 0.99 |
| Opioids: | |||
| Baseline§ | 28/89 (31) | 27/91 (30) | — |
| 1 year | 28/89 (31) | 35/91 (38) | 0.33 |
NSAID=non-steroidal anti-inflammatory drug.
Seven point Likert scale recoded to three categories (improved=completely recovered, much better; no change=somewhat better, no change, somewhat worse; worse=much worse, worse than ever).
Five patients in the amoxicillin group and four patients in the placebo group reported taking analgesics for reasons other than low back pain only.
Two patients in the amoxicillin group reported taking NSAIDs for reasons other than low back pain only.
No patient took strong opioids. One patient in the placebo group reported taking opioids for reasons other than low back pain only.
Comparison of data only at one year follow-up.
Adverse events
In the amoxicillin group, 50 patients (56%) had at least one drug related adverse event (possibly/probably/definitely related to study drug) compared with 31 patients (34%) in the placebo group. One or more serious adverse events occurred in six patients (7%) in the amoxicillin group and two patients (2%) in the placebo group; none was related to the study drug (table 4). In the amoxicillin group, 11 patients (12%) discontinued or paused the study drug because of adverse events compared with two patients (2%) in the placebo group. No deaths occurred during the trial.
Table 4.
Adverse events in treatment groups. Values are No (%)
| Adverse event | Amoxicillin (n=89) | Placebo (n=91) |
|---|---|---|
| Any adverse event: | 75 (84) | 76 (84) |
| Unrelated/unlikely only | 25 (28) | 45 (49) |
| Drug related, any* | 50 (56) | 31 (34) |
| Severity grade ≥2: | 39 (44) | 37 (41) |
| Unrelated/unlikely only | 17 (19) | 32 (35) |
| Drug related, any* | 22 (25) | 5 (5) |
| Any serious adverse event: | 6 (7) | 2 (2) |
| Unrelated/unlikely only | 6 (7) | 2 (2) |
| Drug related, any* | 0 | 0 |
| Reported symptoms or events†: | ||
| Abdominal pain | 11 (12) | 3 (3) |
| Diarrhoea | 17 (19) | 10 (11) |
| Rash | 19 (21) | 5 (5) |
| Vaginal Candida infection‡ | 11 (21) | 0 |
| Oral Candida infection | 1 (1) | 0 |
Defined as possible/probable/definite attribution to study drug by care providers. Any patient with both “unrelated/unlikely” and “drug related” adverse events is counted in the group “drug related”. “Unrelated/unlikely” and “drug related” are summed up in their respective title lines above.
Terms as defined by care givers (not necessarily identical to Common Terminology Criteria for Adverse Events).
Analysed with only women as denominator (n=52 in each treatment group).
Blinding
A total of 167 patients responded to the blinding question at one year. The Bang blinding index was −0.16 (95% confidence interval −0.31 to −0.01, P=0.96) in the amoxicillin group and 0.52 (0.40 to 0.64, P<0.001) in the placebo group (table S3 and fig S4, supplementary appendix).
Discussion
Principal findings
In this study on patients with chronic low back pain and Modic changes at the level of a previous disc herniation, three months of treatment with amoxicillin did not provide a clinically important benefit compared with placebo. Secondary analyses and sensitivity analyses supported this finding.
The analyses of secondary outcomes also found considerably smaller differences than the recommended thresholds for a clinically important change within groups (ranges of recommended thresholds: Oswestry Disability Index 13-20; low back pain intensity numerical rating scale 2-3; EQ5D-5L 0.11-0.30).28 29 30 Fifty patients (56%) in the amoxicillin group compared with 31 (34%) in the placebo group experienced at least one drug related adverse event.
Comparison with the previous study
Our results are not consistent with the findings of the trial we were reassessing, which showed a substantial effect (between group difference of 8.3 points on the RMDQ score) in patients who received a combination treatment of amoxicillin and clavulanic acid for three months.8 In our study, amoxicillin was used without clavulanic acid. Because there is little or no resistance to penicillin among C acnes in vitro,31 and it is known that penetration of discs by clavulanic acid is poor,32 33 we consider the difference in treatment regimens to be an unlikely explanation for the inconsistent trial results. The trial we were reassessing reported a non-significant difference in effect between amoxicillin plus clavulanic acid 500/125 mg and 1000/250 mg three times daily.8 However, the pharmacodynamic property that predicts treatment success with amoxicillin is time above the minimum inhibitory concentration. Further increases in amoxicillin concentration do not increase bactericidal activity, which suggests there should be a minimal dose–response relation. Thus, we consider the dosage of amoxicillin used in the present trial, 750 mg three times daily, to be sufficient.
The former trial included a higher percentage of patients with previous disc surgery (52% in the antibiotic group) compared with our study (20% in the antibiotic group) and had relatively fewer patients at follow-up in the antibiotic treatment group (86% v 96% in our trial). We cannot rule out that this might have influenced the differences in results. The improvement in RMDQ score from baseline to one year follow-up in the placebo group was smaller in the previous trial (from 15.0 to 14.0 v 12.8 to 10.7 in our trial). It is difficult to evaluate whether this could have been owing to poor blinding in the previous study because data on blinding efficacy were lacking.
Clinical relevance
Our trial’s predefined minimal clinically important between group difference of 4 points on the RMDQ is larger than that used in some other randomised trials of patients with low back pain. However, it can be considered conservative given the results of the trial we were reassessing and the proposed rationale for the treatment that an infection leads to Modic changes and low back pain. If the symptoms were mainly because of an infection with C acnes, we would expect a large symptom improvement with effective antibiotic treatment. Perhaps also the nature of the intervention, with its associated risk of complications and contribution to antibiotic resistance, suggest we should demand a larger effect size than for many other suggested treatment options for low back pain. Patients require a 30% extra improvement when taking non-steroidal anti-inflammatory drugs compared with no intervention,34 which equates approximately to our predefined level of difference. We observed a small, statistically significant treatment effect for the primary outcome and two secondary outcomes (Oswestry Disability Index and EQ5D-5L), and among patients with type 1 Modic changes, all in favour of the amoxicillin group. However, the study was overpowered for all these analyses. The statistically significant effect sizes are small and most likely not clinically relevant for the whole study population.
In addition, patients’ expectations of treatment effect might partly explain the effect (table S5, supplementary appendix). We cannot exclude a clinically relevant effect for some patients with type 1 Modic changes because the 95% confidence interval for RMDQ (−4.2 to −0.4) only just included the difference of 4 that the study was designed to detect. Nevertheless, the true difference is probably less than 4. The results for the secondary outcomes in the type 1 Modic change group were similar and indicated no clinically relevant between group differences.
Limitations and generalisability
This study has some limitations. The main limitation was the initiation of antibiotic treatment without first documenting infection in tissue samples taken from individual patients. Such microbiological investigations were not performed owing to risk of complications and suspected low diagnostic yield from samples with low grade infection obtained with needle biopsies.35 A second, related limitation is the heterogeneity of the treated sample. We cannot exclude the possibility of treatment effect in subgroups. Thirdly, a Bang blinding index of 0.52 (95% confidence interval 0.40 to 0.64) in the placebo group indicates there was some degree of unblinding in this group, which potentially reduced the placebo effect in the placebo group, and caused a falsely high between group treatment effect. However, blinding seemed to be maintained in the amoxicillin group, which reduced the impact of possible biases. The main determinants of reporting antibiotic treatment were improvement of symptoms and adverse events (table S4, supplementary appendix). Finally, we cannot rule out the possibility that some patients in the placebo group took amoxicillin without telling us, which could have influenced the difference in outcome between the treatment groups. To reduce this problem we meticulously registered all drugs. External monitors cross checked all registered drugs with patients’ notes.
The inclusion and exclusion criteria of this study, for example relating to herniation, pain score, age, and other diseases, could reduce its generalisability. Particularly, adverse events are more likely to occur in older patients.36 We are not aware of any biological mechanism that could make a treatment effect of amoxicillin more likely in the excluded groups.
Balancing the trial findings
The proportion of patients with drug related adverse events in this study was higher than reported in the literature for long term treatment with penicillins,37 and was also high in the placebo group (34%). Possible explanations for this finding are that common complaints in the general population mimic adverse events, the meticulous registration of adverse events in our trial, and some nocebo effects. Our findings that 12% in the amoxicillin group versus 2% in the placebo group discontinued or paused the study drug because of adverse events, and that no patients experienced drug related serious adverse events, are consistent with previous studies.37
The responder analyses suggest that between five and 18 patients need to be treated for one to improve; numbers and statistical significance vary depending on cut-off values for definition of improvement. The nature of our intervention suggests that we should require high cut-off values. It is our opinion that the numbers do not justify three months of treatment with antibiotics when we consider the increase in adverse events and the context of increasing antibiotic resistance worldwide. In addition, differences between the treatment groups were not consistent across all patient reported outcomes, which further questions the relevance of the small between group difference in the primary analysis.
Conclusions and policy implications
In conclusion, we were not able to replicate the findings of the previous randomised trial. Our study did not show any clinically important effect of three months of oral antibiotic treatment in patients with chronic low back pain, Modic changes, and a former herniated disc. Our results do not support the use of antibiotic treatment for chronic low back pain and Modic changes.
What is already known on this topic
A systematic review from 2015 found only one randomised controlled trial that assessed the efficacy of antibiotic treatment in patients with chronic low back pain and Modic changes
This former trial reported a substantial effect of three months of antibiotic treatment over placebo (difference between groups of 8.3 points on the Roland-Morris Disability Questionnaire (RMDQ) at one year follow-up)
What this study adds
This study did not replicate the findings of the previous randomised controlled trial
Three months of treatment with amoxicillin did not provide a clinically important benefit compared with placebo in patients with chronic low back and Modic changes
The largest observed mean difference between the treatment groups (2.3 points on the RMDQ for patients with type 1 Modic changes) was substantially smaller and below the predefined clinically important between group difference
Acknowledgments
We thank Helse Sør-Øst (grant No 2015090) and Helse Vest (grant No 911938 and 911891) for funding the AIM study, all patients who participated in the study, and Eira Kathleen Ebbs for proofreading the manuscript.
Web extra.
Extra material supplied by authors
Web appendix: Supplementary appendix
Contributors: All authors critically revised the manuscript for important intellectual content. Trial care providers: MPR, AF, AJH, GHM, SR, MWil, BSW, TIK, AA, JSS, and LCHB. AE, PMK, and NV evaluated MRI scans. MWig was the national coordinator of the study and collected biological material with MDV. JA and LCHB did the statistical analyses. AF, AJH, ØPN, AA, JSS, LG, and JIB were principal investigators and took part in planning the study with OL, TEH, MG, BA, CH, EIS, AE, JAZ, and KS. TEH was the patient representative. JAZ was the coordinating investigator, and KS was the project manager. LCHB wrote the first draft with input from KS, and is guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
The AIM study group: University Hospital North Norway, Tromsø (four patients): Terese Fors, Guro Kjos, Ida Beate Østhus (Department of Rehabilitation); Trondheim University Hospital, Trondheim (21 patients): Britt Elin Lurud, Fredrik Granvigen (Department of Physical Medicine and Rehabilitation); Hege Andersen (National Advisory Unit of Spinal Surgery), Vidar Rao (Department of Neurosurgery); Haukeland University Hospital, Bergen (37 patients): Siv Krüger Claussen, Erling Andersen (Department of Clinical Engineering); Vestre Viken Hospital, Drammen (38 patients): Hilde Presberg (Department of Neurology); Oslo University Hospital, Oslo (50 patients): Linda Margareth Pedersen, Karianne Wiger Gammelsrud (Department of Microbiology), Siri Tennebø Flåm, Magnus Dehli Vigeland (Department of Medical Genetics); Østfold Hospital Trust (30 patients): Marianne Thorsø, Knut Morten Huneide, Veronica Sørensen (Department of Physical Medicine and Rehabilitation).
Funding: Funding was granted by governmental organisations Helse Sør-Øst (grant No 2015090) and Helse Vest (grant No 911938 and 911891), which had no part in the planning, performing, or reporting of the trial. The trial protocol is available atClinicalTrials.gov.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from governmental organisations Helse Sør-Øst and Helse Vest for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: The trial was approved by the Regional Committees for Medical Research Ethics South East Norway (2014/158/REK sør-øst) and the Norwegian Medicines Agency (SLV; reference No 14/01368-11; EudraCT No 2013-004505-14) before it started, and was monitored by the Clinical Trial Unit at Oslo University Hospital. All participants gave written informed consent.
Data sharing: Requests to access data should be addressed to kjersti.storheim@medisin.uio.no. Deidentified individual participant data (including data dictionary) will be available to medical researchers by request in accordance with local registration and ethical approval when the article has been published until 1 July 2029. All proposals requesting data access will need to specify an analysis plan and will need approval of the scientific board before any data can be released.
The lead author (LCB) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned and registered have been explained.
References
- 1. Maher C, Underwood M, Buchbinder R. Non-specific low back pain. Lancet 2017;389:736-47. 10.1016/S0140-6736(16)30970-9 [DOI] [PubMed] [Google Scholar]
- 2. Dudli S, Fields AJ, Samartzis D, Karppinen J, Lotz JC. Pathobiology of Modic changes. Eur Spine J 2016;25:3723-34. 10.1007/s00586-016-4459-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Modic MT, Steinberg PM, Ross JS, Masaryk TJ, Carter JR. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology 1988;166:193-9. 10.1148/radiology.166.1.3336678 [DOI] [PubMed] [Google Scholar]
- 4. Urquhart DM, Zheng Y, Cheng AC, et al. Could low grade bacterial infection contribute to low back pain? A systematic review. BMC Med 2015;13:13. 10.1186/s12916-015-0267-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stirling A, Worthington T, Rafiq M, Lambert PA, Elliott TSJ. Association between sciatica and Propionibacterium acnes. Lancet 2001;357:2024-5. 10.1016/S0140-6736(00)05109-6 [DOI] [PubMed] [Google Scholar]
- 6. Chen Z, Zheng Y, Yuan Y, et al. Modic changes and disc degeneration caused by inoculation of Propionibacterium acnes inside intervertebral discs of rabbits: a pilot study. Biomed Res Int 2016;2016:9612437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li B, Dong Z, Wu Y, et al. Association between lumbar disc degeneration and Propionibacterium acnes infection: clinical research and preliminary exploration of animal experiment. Spine (Phila Pa 1976) 2016;41:E764-9. 10.1097/BRS.0000000000001383 [DOI] [PubMed] [Google Scholar]
- 8. Albert HB, Sorensen JS, Christensen BS, Manniche C. Antibiotic treatment in patients with chronic low back pain and vertebral bone edema (Modic type 1 changes): a double-blind randomized clinical controlled trial of efficacy. Eur Spine J 2013;22:697-707. 10.1007/s00586-013-2675-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sotto A, Dupeyron A. Letter to the editor concerning: “Antibiotic treatment in patients with chronic low back pain and vertebral bone edema (Modic type 1 changes): a double-blind randomized controlled trial of efficacy” by Albert HB et al. Eur Spine J (2013) 22:697-707. Eur Spine J 2013;22:1704-5. 10.1007/s00586-013-2898-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dean BJF. Do these results apply to the ‘intervention naive’ patient? Eur Spine J 2013;22:1702. 10.1007/s00586-013-2900-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Low back pain and sciatica in over 16s: assessment and management. NICE guideline [NG59]. November 2016. Accessed 9 April 2019. https://www.nice.org.uk/guidance/ng59/resources.
- 12. Blaser MJ. The microbiome revolution. J Clin Invest 2014;124:4162-5. 10.1172/JCI78366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Herlin C, Kjaer P, Espeland A, et al. Modic changes-Their associations with low back pain and activity limitation: A systematic literature review and meta-analysis. PLoS One 2018;13:e0200677. 10.1371/journal.pone.0200677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jensen TS, Karppinen J, Sorensen JS, Niinimäki J, Leboeuf-Yde C. Vertebral endplate signal changes (Modic change): a systematic literature review of prevalence and association with non-specific low back pain. Eur Spine J 2008;17:1407-22. 10.1007/s00586-008-0770-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c869. 10.1136/bmj.c869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rahme R, Moussa R. The modic vertebral endplate and marrow changes: pathologic significance and relation to low back pain and segmental instability of the lumbar spine. AJNR Am J Neuroradiol 2008;29:838-42. 10.3174/ajnr.A0925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bendix T, Sorensen JS, Henriksson GAC, Bolstad JE, Narvestad EK, Jensen TS. Lumbar modic changes-a comparison between findings at low- and high-field magnetic resonance imaging. Spine (Phila Pa 1976) 2012;37:1756-62. 10.1097/BRS.0b013e318257ffce [DOI] [PubMed] [Google Scholar]
- 18. Storheim K, Espeland A, Grøvle L, et al. Antibiotic treatment In patients with chronic low back pain and Modic changes (the AIM study): study protocol for a randomised controlled trial. Trials 2017;18:596. 10.1186/s13063-017-2306-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Common Terminology Criteria for Adverse Events (CTCAE) v4.0. 2010. (Accessed April 9,2019, at https://evs.nci.nih.gov/ftp1/CTCAE/About.html.)
- 20.Bråten LC RM, Espeland A, Storheim K, Zwart JA, Hellum C, Aßmus J. Statistical analysis plan (SAP) for clinical outcomes in the AIM-study: a randomized trial of antibiotic treatment in patients with chronic low back pain and Modic changes (the AIM-study). ClinicalTrials.gov2018. https://clinicaltrials.gov/ct2/show/NCT02323412?cond=modic+change&rank=1
- 21. UK BEAM Trial Team United Kingdom back pain exercise and manipulation (UK BEAM) randomised trial: effectiveness of physical treatments for back pain in primary care. BMJ 2004;329:1377. 10.1136/bmj.38282.669225.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brouwer S, Kuijer W, Dijkstra PU, Göeken LNH, Groothoff JW, Geertzen JHB. Reliability and stability of the Roland Morris Disability Questionnaire: intra class correlation and limits of agreement. Disabil Rehabil 2004;26:162-5. 10.1080/09638280310001639713 [DOI] [PubMed] [Google Scholar]
- 23. Grotle M, Brox JI, Vøllestad NK. Cross-cultural adaptation of the Norwegian versions of the Roland-Morris Disability Questionnaire and the Oswestry Disability Index. J Rehabil Med 2003;35:241-7. 10.1080/16501970306094 [DOI] [PubMed] [Google Scholar]
- 24. Bang H, Ni L, Davis CE. Assessment of blinding in clinical trials. Control Clin Trials 2004;25:143-56. 10.1016/j.cct.2003.10.016 [DOI] [PubMed] [Google Scholar]
- 25. Groll DL, To T, Bombardier C, Wright JG. The development of a comorbidity index with physical function as the outcome. J Clin Epidemiol 2005;58:595-602. 10.1016/j.jclinepi.2004.10.018 [DOI] [PubMed] [Google Scholar]
- 26. Sandanger I, Moum T, Ingebrigtsen G, Dalgard OS, Sørensen T, Bruusgaard D. Concordance between symptom screening and diagnostic procedure: the Hopkins Symptom Checklist-25 and the Composite International Diagnostic Interview I. Soc Psychiatry Psychiatr Epidemiol 1998;33:345-54. 10.1007/s001270050064 [DOI] [PubMed] [Google Scholar]
- 27. Waddell G, Newton M, Henderson I, Somerville D, Main CJ. A Fear-Avoidance Beliefs Questionnaire (FABQ) and the role of fear-avoidance beliefs in chronic low back pain and disability. Pain 1993;52:157-68. 10.1016/0304-3959(93)90127-B [DOI] [PubMed] [Google Scholar]
- 28. Ostelo RWJG, Deyo RA, Stratford P, et al. Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine (Phila Pa 1976) 2008;33:90-4. 10.1097/BRS.0b013e31815e3a10 [DOI] [PubMed] [Google Scholar]
- 29. Solberg T, Johnsen LG, Nygaard OP, Grotle M. Can we define success criteria for lumbar disc surgery?: estimates for a substantial amount of improvement in core outcome measures. Acta Orthop 2013;84:196-201. 10.3109/17453674.2013.786634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Austevoll IM, Gjestad R, Grotle M, et al. Follow-up score, change score or percentage change score for determining clinical important outcome following surgery? An observational study from the Norwegian registry for Spine surgery evaluating patient reported outcome measures in lumbar spinal stenosis and lumbar degenerative spondylolisthesis. BMC Musculoskelet Disord 2019;20:31. 10.1186/s12891-018-2386-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nord CE, Oprica C. Antibiotic resistance in Propionibacterium acnes. Microbiological and clinical aspects. Anaerobe 2006;12:207-10. 10.1016/j.anaerobe.2006.08.001 [DOI] [PubMed] [Google Scholar]
- 32. Thomas RdeW, Batten JJ, Want S, McCarthy ID, Brown M, Hughes SP. A new in-vitro model to investigate antibiotic penetration of the intervertebral disc. J Bone Joint Surg Br 1995;77:967-70. 10.1302/0301-620X.77B6.7593116 [DOI] [PubMed] [Google Scholar]
- 33. Housden PL, Sullivan MF. Do augmentin or cefuroxime reach effective levels in lumbar vertebral discs when used prophylactically for discectomy? A preliminary report. Eur Spine J 1993;2:145-8. 10.1007/BF00301412 [DOI] [PubMed] [Google Scholar]
- 34. Ferreira ML, Herbert RD, Ferreira PH, et al. The smallest worthwhile effect of nonsteroidal anti-inflammatory drugs and physiotherapy for chronic low back pain: a benefit-harm trade-off study. J Clin Epidemiol 2013;66:1397-404. 10.1016/j.jclinepi.2013.02.018 [DOI] [PubMed] [Google Scholar]
- 35. McNamara AL, Dickerson EC, Gomez-Hassan DM, Cinti SK, Srinivasan A. Yield of image-guided needle biopsy for infectious discitis: a systematic review and meta-analysis. AJNR Am J Neuroradiol 2017;38:2021-7. 10.3174/ajnr.A5337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Giarratano A, Green SE, Nicolau DP. Review of antimicrobial use and considerations in the elderly population. Clin Interv Aging 2018;13:657-67. 10.2147/CIA.S133640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. US Food and Drug Administration. Safety of Long Term Therapy with Penicillin and Penicillin Derivatives 2001 https://www.fda.gov/drugs/bioterrorism-and-drug-preparedness/safety-long-term-therapy-penicillin-and-penicillin-derivatives
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplementary appendix