Abstract
The sirtuin family of NAD+-dependent protein deacetylases promotes longevity and counteract age-related diseases. One of the major targets of Sirtuins are the FoxO family of transcription factors. FoxOs play a major role in the adaptation of cells to a variety of stressors such as oxidative stress and growth factor deprivation. Studies with murine models of cell-specific loss- or gain-of-function of Sirtuins or FoxOs and with Sirtuin1 stimulators have provided novel insights into the function and signaling of these proteins on the skeleton. These studies have revealed that both Sirtuins and FoxOs acting directly in cartilage and bone cells are critical for normal skeletal development, homeostasis and that their dysregulation might contribute to skeletal disease. Deacetylation of FoxOs by Sirt1 in osteoblasts and osteoclasts stimulates bone formation and inhibits bone resorption, making Sirt1 ligands promising therapeutic agents for diseases of low bone mass. While a similar link has not been established in chondrocytes, Sirt1 and FoxOs both have chondroprotective actions, suggesting that Sirt1 activators may have similar efficacy in preventing cartilage degeneration due to aging or injury. In this article we summarize these advances and discuss their implications for the pathogenesis of age-related osteoporosis and osteoarthritis.
Keywords: aging, autophagy, chondrocytes, osteoblasts, osteoclasts, osteocytes, oxidative stress, senescence, ROS, Wnt signaling
1. Sirtuins in skeletal health and disease
The sirtuin family of NAD+-dependent protein deacetylase/mono-ADP-ribosyltransferase enzymes is conserved from bacteria to humans, controls a variety of cellular processes such as DNA repair and apoptosis, mitochondrial biogenesis, cell stress responses, response to hypoxia and circadian rhythms [1, 2]. Seven mammalian sirtuins (SIRT1-7) are known. SIRT1, SIRT6, and SIRT7 are predominantly located in the nucleus, where they regulate the expression of specific genes by deacylation/deacetylation of histones and non-histone proteins while SIRT3, SIRT4, and SIRT5 localize to mitochondria. SIRT1 deacetylates histones H3, H4 and H1 but also modifies more than 50 non-histone proteins[3], including transcription factors such as p53, nuclear factor-κB (NF-κB), and FoxOs. Besides their major role as lysine deacetylases, Sirts can also catalyze other acyl-lysine modifications, including depropionylation, demalonylation, desuccinylation, decrotonylation, delipoamidation, other long-chain fatty acid deacylations and mono-ADP-ribosylation. These are commonly referred to as deacylation reactions [4, 5].
Sirtuins promote longevity in diverse species and mediate many of the beneficial effects of caloric restriction, such as a reduced incidence of cancer, cardiovascular disease and diabetes [6]. Like the case in every other tissue, Sirt1 is the best studied sirtuin in bone and cartilage. Sirt1 actions in chondrocytes and bone cells are critical for normal skeletal development and homeostasis. Nonetheless, recent studies indicate that Sirt3, Sirt6 and Sirt7 also contribute to skeletal homeostasis. Despite these recent advances, much less is known about the role of sirtuins in skeletal aging. In view of the beneficial effects of sirtuins, there has been considerable effort to find small molecules to stimulate their activity for therapeutic purposes [7]. The first natural Sirt1 activator to be discovered was resveratrol (3,4′,5-trihydroxystilbene) [8]. Currently, a multitude of synthetic Sirt1 activators have been developed and shown to prolong lifespan and delay innumerous diseases of aging in model organisms [7], including osteoporosis and osteoarthritis. These compounds also show promise to improve cardiovascular and metabolic disease in human clinical trials [9].
1.1. SIRT1
Initial attempts at examining the role of Sirt1 on the skeleton were performed in Sirt1 KO mice. These mice are small, sterile and display high rates of perinatal lethality [10, 11]. Sirt1 KO also exhibit delayed mineralization of the skull, vertebrae and digits and defects in the development and closure of craniofacial sutures [12]. In contrast, Sirt1 haplo-insufficient (Sirt1+/−) mice develop normally and have no overt phenotype. Nevertheless, female mice exhibit a significant reduction in trabecular and cortical bone mass in long bones, characterized by decreased bone formation while male mice had no bone phenotype [13]. Likewise, deletion of Sirt1 in adult mice decreases cortical bone mass indicating that the skeletal effects of Sirt1 are not restricted to development and growth [14].
The effects of Sirt1 on the skeleton are mediated, at least in part, via cells of the osteoblast lineage (Table 1). Murine models of Sirt1 deletion in cell of the mesenchymal lineage have elucidated that Sirt1 in osteoblast and osteocytes increases trabecular bone mass, while Sirt1 in osteoblast progenitors increases cortical bone by stimulating bone formation at the endocortical surface [15-18]. Several lines of evidence indicate that Sirt1 promotes bone formation by stimulating Wnt signaling. Specifically, deacetylation of FoxOs by Sirt1 prevents FoxO association with β-catenin and potentiates Wnt signaling, leading to increased osteoblast proliferation[17]. The stimulatory actions of Sirt1 on osteoblastogenesis might also be mediated by direct effects on β-catenin and Runx2 [15, 19]. In addition, Sirt1 may promote bone formation by decreasing the expression of the Wnt signaling-antagonist Sost [13, 20].
Table 1.
Summary of the effects of sirtuins on the skeleton as determined from studies using whole body or conditional KO mice.
| Sirtuins | Cell type | Cellular effects | Bone structure | References |
|---|---|---|---|---|
| Sirt1 | Osteoclast | Osteoclast number ⬇ | Trabecular bone mass ⬆ | 16, 18 |
| Osteoblast progenitor | Osteoblast number ⬆ | Cortical bone mass ⬆ | 15, 17 | |
| Osteoblast | Osteoblast number ⬆ | Trabecular bone mass⬆ | 16, 18 | |
| Chondrocyte | Apoptosis ⬇ | Chondroprotective | 36 | |
| Sirt3 | All | Trabecular bone during growth⬇ | 24 | |
| No change in young adults | 25 | |||
| All* | No changes in young adults; trabecular and cortical bone mass at 13 months ⬇ | 25 | ||
| Osteoclast number ⬆ | ||||
| MAR ⬇ | ||||
| Chondroprotective | 49 | |||
| Sirt6 | All | Bone formation ⬇ | Failure to accrue a normal size skeleton | 27-29 |
| Sirt7 | Osteoblast | Bone formation ⬆ | Trabecular and cortical bone mass ⬆ | 33 |
| Bone resorption ⬇ | ||||
| All | Promotes osteoarthritis | 54 | ||
Effects of Sirt3 were also determined from work with a model of whole body overexpression of Sirt3.
In contrast to its stimulatory actions on osteoblastogenesis, Sirt1 in myeloid lineage cells inhibits osteoclastogenesis and bone resorption [16, 18]. Sirt1 deacetylates and, thereby, stimulates FoxO-mediated transcription in osteoclasts. These effects inhibit mitochondrial ATP production and ROS accumulation.
As the case with Sirt1+/− mice, the skeletal effects of targeted Sirt1 deletion in bone cells were readily seen in females but not in males [16, 17]. However, the reasons for the sex specific effects of Sirt1 on the skeleton remain unknown.
1.2. SIRT3
SIRT3 is a major mitochondrial deacetylase [21] and influences most key aspect of mitochondrial biology including nutrient oxidation, ATP and ROS generation, mitochondrial dynamics, and the mitochondrial unfolded protein response. Despite these influence in mitochondria, SIRT3-deficient mice are metabolically normal at a young age, with no changes in body composition including BMD, as determined by DXA [21]. Nonetheless, deficiency of SIRT3 leads to accelerated development of diseases of aging including the metabolic syndrome, cancer, cardiovascular and neurodegenerative diseases [22, 23].
Sirt3−/− mice have low trabecular bone mass in long bones during growth [24], but no changes were detected in adult mice [25]. On the other hand, mice with global Sirt3 overexpression have unaltered bone mass at a young age but exhibit low bone mass associated with increased osteoclastogenesis and decreased mineral apposition rate at 13 months of age [25]. Thus, SIRT3 might exert age-dependent effects on bone, but further studies are needed to elucidate the role of this mitochondrial sirtuin on the skeleton.
1.3. SIRT6
Studies attempting to elucidate the role of SIRT6 in bone have used SIRT6−/− mice which display a progeroid degenerative syndrome including reduced size, acute loss of subcutaneous fat, lordokyphosis, colitis, and severe lymphopenia [26]. These mice also have low circulating insulin-like growth factor (IGF-I) and glucose levels and die between 3 and 4 weeks of age. Not surprisingly, bone mineral density and cancellous and cortical bone volume are much reduced in SIRT6−/− compared to control mice [27, 28]. Histomorphometric analysis performed in 3-week-old mice revealed impaired bone formation, while effects on resorption remain controversial [27, 29]. Ex-vivo osteoblast and osteoclast cell cultures from SIRT6−/− mice suggest that Sirt6 contributes to osteoblast and osteoclast formation. Nevertheless, the extremely small size and overall poor health condition caused by Sirt6 deletion, makes interpretation of the data extremely difficult. Thus, elucidation of the role of Sirt6 in skeletal homeostasis awaits studies with conditional knock-out mice.
1.4. SIRT7
SIRT7 acts as a histone desuccinylase and contributes to the maintenance of genome stability by participating in the repair of DNA double-strand breaks [30]. SirT7−/− mice have elevated perinatal lethality and those that survive to adulthood have a short lifespan and show signs of accelerated aging including kyphosis, reduced weight and fat content, compromised hematopoietic stem cell function, increased p16INK4 expression, and reduced circulating IGF-1 protein [31, 32]. Deletion of SIRT7 in osteoblasts and osteocytes leads to low cortical and trabecular bone mass secondary to decreased bone formation and increased bone resorption [33]. The stimulatory effects of Sirt7 on osteoblasts might be due to deacylation of lysine (K) 368 in the C-terminal region of Osterix1 which increases its transactivation activity.
2. Sirtuins in cartilage homeostasis and osteoarthritis
2.1. SIRT1
As in bone, the majority of studies investigating sirtuin roles in cartilage homeostasis, aging, and osteoarthritis (OA) pathogenesis have focused on SIRT1. In mice, Sirt1 haploinsufficiency results in delayed growth and increased spontaneous OA by 9 months of age, which is associated with increased chondrocyte apoptosis [34]. Similar changes were observed in transgenic mice homozygous for a Sirt1 inactivating mutation [35]. Chondrocyte-specific deletion of Sirt1 resulted in normal development but increased severity of OA with aging and following joint injury [36] (Table 1). These findings in mice are consistent with reduced levels of SIRT1 measured in human OA cartilage [37, 38] and suggest a chondroprotective role for the sirtuin. SIRT1 inhibition increases apoptosis and pro-catabolic gene expression by human articular chondrocytes, particularly under challenge with pro-inflammatory cytokines [39-41] or nitric oxide [38]. In contrast, SIRT1 activation not only reduces these catabolic responses [38, 39, 41] but also enhances chondrogenic gene expression [42], in part through the deacetylation and increased nuclear localization of SOX9 [43]. SIRT1 exerts survival and other chondroprotective effects through regulation of mitochondrial biogenesis, oxidative stress, autophagy, and ER stress responses – pathways that are known to drive OA progression [44]. In human OA chondrocytes, for example, reduced SIRT1 activity was associated with reduced mitochondrial biogenesis; pharmacological activation of the energy sensor AMP-activated protein kinase (AMPK) activated proliferator-activated receptor gamma coactivator 1α (PGC-1α), a master regulator of mitochondrial biogenesis, through SIRT1 deacetylation to enhance chondrocyte ATP production [44].
Despite abundant evidence of a chondroprotective role for SIRT1, its activity may not be exclusively beneficial to joint homeostasis. Monteagudo et al. provide evidence that the loss of SIRT1 modulation due to inhibition of Disruptor of telomeric silencing 1-like (DOT1L), a histone methyltransferase, increased chondrocyte Wnt signaling and led to OA in mice [45]. These results seem consistent with decreased SIRT1 levels in osteoblasts from human OA subchondral bone, which increased SOST expression and reduced canonical Wnt signaling [46]. Yet the findings of Monteagudo et al. also seem to conflict with another recent study showing upregulation of Wnt signaling mediator lymphoid enhancer factor (LEF)-1 and matrix metalloproteinase (MMP)-13 levels in Sirt1−/− mice, as well as inhibition of LEF-1-mediated MMP-13 expression by SIRT1 overexpression in human OA chondrocytes [47]. More studies on the interconnection between SIRT1 and Wnt signaling in the context of OA pathogenesis are required. In addition to cartilage and peri-articular bone, joint homeostasis is also determined by contributions from the synovium. SIRT1 levels are increased in synovium from patients with rheumatoid arthritis (RA), and SIRT1 can enhance pro-catabolic gene expression by synovial fibroblasts while inhibiting their apoptosis; of note, SIRT1 levels were reported lower in OA synoviocytes [48]. As the precise activities of the sirtuins continue to be unveiled, preclinical evaluation of sirtuin modulators for OA therapy should consider their effects on multiple joint tissues.
2.2. SIRT2-7
To date, less is known about the roles of the other sirtuins in cartilage homeostasis and OA. Levels of SIRT3 decrease with age in rat and mouse cartilage as well as in human OA cartilage, which has been associated with increased acetylation and reduced activity of mitochondrial antioxidant enzyme superoxide dismutase 2 (SOD2); moreover, Sirt3−/− mice displayed accelerated OA [49]. Reduced SIRT3 levels and increased SOD2 acetylation has since been confirmed in human OA chondrocytes by a separate group, who further demonstrated that these changes were associated with increased mitochondrial (mt)ROS and mtDNA damage. Pharmacological AMPK activation improved mtDNA integrity and organelle function through increased SIRT3 activity [50]. SIRT6 levels are also decreased in cartilage from patients with OA as well as in aged mice [51], although its levels may be enhanced within proliferating cell nuclear antigen (PCNA)-positive chondrocyte clusters within OA tissue [52]. Consistent with both observations, SIRT6 RNA inhibition enhanced markers of DNA damage, telomere dysfunction, and senescence within human cultured OA chondrocytes [52], while SIRT6 overexpression reduced expression of senescence markers in a similar population [51]. In contrast to the chondroprotective functions demonstrated for SIRT1, SIRT3 and SIRT6, SIRT2 and SIRT4 both increase stability of HIF-2α in articular chondrocytes, stimulating pro-catabolic gene expression in these cells [53]. However, their direct catabolic function during aging or osteoarthritis requires further study. SIRT7 may also have negative actions in cartilage, as Sirt7−/− mice displayed resistance to age-related and exercise-induced OA and Sirt7 inhibition increased Sox9 activity in the chondrogenic ATDC5 cell line [54].
3. Actions of Sirt stimulators on skeletal aging
Resveratrol is a polyphenol found in nuts, grapes and other plant sources that affords protection against inflammation, oxidative stress and cancer [55, 56]. Resveratrol can stimulate Sirt1 and innumerous human and rodent studies have elucidated effects for resveratrol in ameliorating disorders such as cardiovascular disease, diabetes and inflammation (reviewed in detail by Novelle et al [57]). Although resveratrol can also activate the estrogen receptor, AMPK and MAPK, among others [58], acute deletion of SIRT1 in adult mice prevents many of the physiological effects of resveratrol and other sirtuin-activating compounds (STACs) [59, 60]. Resveratrol administration increases bone mass in young mice due to an increase in osteoblast number [61]. Similarly, the small molecule Sirt1 activator SRT1720 increases bone mass in growing mice due to stimulation of bone formation and inhibition of resorption [20]. These effects are associated with a decrease in sclerostin levels. In cultured cells, resveratrol and other Sirt1 activators, such as SRT1720 or SRT2104, promote osteoblast differentiation and reduce osteoclasts formation [62-67].
Notably, administration of resveratrol, SRT1720 or SRT2104 to mice attenuates the loss of bone mass with aging [14, 18, 68]. In line with these findings, old mice overexpressing Sirt1 have high bone mass [69]. Sirt1 stimulators also cause a significant increase in bone mass in the estrogen deficiency or unloading models of osteoporosis [14, 18, 70-72]. Perhaps more important, resveratrol promotes a significant increase in bone mass in elderly obese men [73]. These findings provide compelling evidence to suggest that Sirt1 may serve as a therapeutic target for combating age-related bone loss. Nevertheless, it remains unknown whether a decrease in Sirt1 activity contributes to natural skeletal aging.
Accumulating evidence demonstrates that natural and synthetic activators of SIRT1 have chondroprotective actions and, therefore, promise as OA therapeutics. Resveratrol inhibits chondrocyte apoptosis induced by pro-inflammatory cytokines [74, 75], in part through SIRT1-mediated deacetylation of p65 and inhibition of canonical NF-κB signaling [39, 76]. Chondroprotective efficacy has been reported following resveratrol delivery in murine and rabbit models of osteoarthritis [77, 78]. In human chondrocytes cultured in vitro, olive oil-derived hydroxytyrosol (4-(2-Hydroxyethyl)-1,2-benzenediol) inhibited H2O2-induced DNA damage and cell death through SIRT1-mediated autophagy [79, 80]. As for synthetic activators, systemic delivery of SRT1720 transiently decreased histological OA scores and osteophyte volumes in mice that had received medial meniscus destabilization, which coincided decreased catabolic marker expression within the cartilage [81]. Further preclinical evaluation of these SIRT1 activators is needed using models of both injury-induced and age-associated OA.
4. FoxO transcription factors
In mammals, FoxO1 (or FKHR), FoxO3 (or FKHRL1), FoxO4 (also called AFX) and FoxO6 [82] represent a subclass of a large family of forkhead proteins characterized by the presence of a winged-helix DNA binding domain called Forkhead box. FoxOs are major targets of the insulin-IGF1 signaling pathway which inhibits FoxO activity via Akt-mediated phosphorylation. Another post-translational modification that alters FoxO activity is acetylation/deacetylation. Deacetylation of FoxOs by Sirt1 promotes or inhibits FoxO-mediated transcription depending on the cellular context and the target genes [83]. FoxOs play a major role in the adaptation of cells to a variety of stressors such as oxidative stress and growth factor deprivation [84], by promoting cell cycle arrest [85, 86], DNA damage repair, autophagy, and scavenging of free radicals [87-89] [83, 90]. FoxO1, 3, and 4 have broad and overlapping patterns of expression in many mammalian tissues, including bone [82, 91] and cartilage [92]. Even though they all recognize the same DNA target sequence [93], studies with models of individual or combined FoxO deletion have elucidated that FoxO1, 3, and 4 exert both redundant and non-redundant functions [94-98].
5. FoxOs control bone resorption and formation
Mouse models of loss and gain-of-function of FoxOs have elucidated that FoxOs are important regulators of osteoclast differentiation and bone resorption. Specifically, combined loss of FoxO1, 3 and 4 in the myeloid lineage promotes cell proliferation, osteoclast formation and bone resorption leading to reduced trabecular and cortical bone mass [99]. Conversely, overexpression of FoxO3 attenuates osteoclastogenesis and bone resorption, and increases bone mass. RANKL, via Akt-mediated phosphorylation, decreases FoxO protein levels and transcriptional activity. This leads to a decrease in catalase and an increase in ROS, which in turn potentiates osteoclast formation and bone resorption [99-101] ((Fig. 1). FoxOs also stimulate the expression of hemeoxygenase-1 (HO-1) in osteoclast progenitors [99]. HO-1 catabolizes heme and attenuates mitochondrial oxidative phosphorylation and ATP production in macrophages. Notably, the increase in ROS due to loss of FoxO function in myeloid progenitors not only decreases bone mass, but also promotes atherogenesis in mice [98, 99]. Deacetylation of FoxOs by Sirt1 stimulates FoxO transcriptional activity and inhibits osteoclast formation [65]. Thus, the antiosteoclastogenic effects of FoxOs can be harnessed by Sirt1 stimulators (Fig. 1).
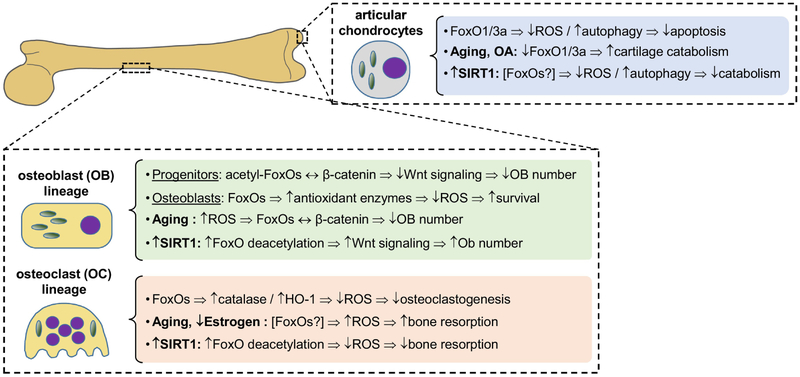
Figure 1: FoxO actions in osteoblasts, osteoclasts, and chondrocytes and their regulation by SIRT1 activators.
In cells of the osteoblastic lineage, acetylation of FoxOs dictates their sequestion of β-catenin and consequent modulation of Wnt signaling. In osteoclast progenitors, FoxOs suppress ROS levels, an important driver of osteoclastogenesis. Activators of SIRT1, through deacetylation of FoxOs, increase bone formation and decrease bone resorption, suggesting their use in preventing bone loss with aging. In chondrocytes, reduced levels of FoxO1 and 3a with age and OA contribute to increased oxidative stress and reduced autophagy, leading to increased chondrocyte apoptosis or catabolic gene expression. As in bone, FoxOs may mediate the chondroprotective actions of SIRT1 activators, but this has not yet been established.
Mice with combined deletion of FoxO1, FoxO3 and FoxO4 in osteoprogenitors exhibit high bone mass due to increased β-catenin/TCF transcription and cell proliferation [102] . These findings indicate that FoxOs act on osteoblast progenitors to attenuate cell cycling, most probably in order to restrain proliferation in situations of increased stress. Acetylation of FoxOs promotes the interaction between FoxO and β-catenin while Sirt1-mediated FoxOs deacetylation prevents this interaction and potentiates Wnt signaling, leading to increased osteoblast proliferation [17]. In contrast to the effects in osteoprogenitors, FoxOs in osteoblasts and osteocytes stimulate bone formation by attenuating ROS and promoting cell survival. These actions of FoxOs are due to increased expression of antioxidant enzymes like catalase and superoxide dismutase and prevention of oxidative stress [91, 103]. In addition, FoxO1 promotes the accumulation of glutathione, a peptide with redox-active sulfhydryl moieties which reduces ROS. The increase in glutathione is due to stimulation of protein synthesis caused by FoxO1 interaction with ATF4, a transcription factor that promotes amino acid import [103]. Actions of FoxOs in osteoblasts also decrease bone resorption via paracrine mechanisms, most likely, due to stimulation of osteoprotegerin (OPG) [91, 102-105].
6. FoxOs in cartilage homeostasis and osteoarthritis
Chondrocytes within human and mouse articular cartilage predominantly express FoxO1 and FoxO3 compared to FoxO4. FoxO1 and FoxO3 levels both decrease with age and with OA, although abundant phosphorylated FoxO1 and FoxO3 were observed within chondrocyte clusters in OA cartilage [92]. These findings are consistent with a recent RNA-sequencing analysis identifying the FoxO signaling pathway as among the most dysregulated in human OA cartilage compared to normal tissue [106]. In mice, both total and phosphorylated FoxO1 and FoxO3 levels decrease in articular cartilage with age and after surgical joint injury [92]. The cartilage-specific deletion of FoxO1, FoxO3, FoxO4 or all three isoforms in mice was recently reported: combined FoxO deletion produced OA-like changes by 6 months of age, which was similar to deletion of FoxO1 alone; in contrast, FoxO3 deletion did not result in more severe OA than controls until 18 months [107]. Taken together, these studies suggest important roles for FoxO1 and FoxO3 in maintaining articular cartilage homeostasis (Fig. 1).
Both expression and phosphorylation of FoxO1, FoxO3, and FoxO4 were all detected in the cell populations of OA synovium, though FoxO4 phosphorylation was not as intense as in RA synovium. Pro-inflammatory cytokine challenge can increase FoxO1 phosphorylation in fibroblast-like synoviocytes isolated from OA tissue and FoxO4 phosphorylation in peripheral blood-derived macrophages [108]. FoxO3a has also been implicated in synovial T-cell survival during RA [109]. Considering these synovium-specific FoxO activities, additional studies using animal models of joint aging and injury are required to demonstrate whether FoxOs are a sufficiently specific target for intervention into OA progression.
7. FoxOs in skeletal aging
Several common mechanisms have been proposed to drive the natural aging process and, at least, some of these mechanisms also contribute to skeletal fragility [110, 111]. FoxOs are homologous to the C. elegans transcription factor DAF-16 (abnormal DAuer Formation-16). Loss of function mutations of the insulin-IGF1 receptor in C. elegans increase lifespan, an effect that is completely dependent on DAF-16 [112-114]. The role of FoxOs on longevity might be evolutionary conserved as multiple studies in humans have consistently revealed FoxOs, in particular FoxO3, as “longevity genes” [115]. Besides the insulin-IGF1 pathway, FoxOs modulate several other mechanisms of aging including oxidative stress, senescence and loss of proteostasis and, thereby, can influence the loss of bone mass with age and the development of osteoarthritis.
7.1. Oxidative Stress
Mitochondrial dysfunction and a consequent increase in ROS production have for long been considered a driver of aging [116]. In mice, ROS accumulates in bone with old age or with sex steroid deficiency [117, 118]. Loss of bone mass with aging is due to a decrease in the number of osteoblasts and this decrease is caused, at least in part, by an increase in mitochondrial ROS in cells of the osteoblast lineage, while mitochondrial ROS in osteoclasts contributes to the loss of bone mass with estrogen deficiency [119].
ROS activate FoxOs via several post-translational modifications namely JNK- and Mst1-mediated phosphorylation and p300/CBP-mediated acetylation [120-122]. ROS also promote the association of FoxOs to β-catenin and, thereby, a reduction in the β-catenin required for Wnt signaling and cell proliferation [123-127]. Accordingly, glucose-induced oxidative stress decreases proliferation of embryonic stem cells via a FoxO3/β-catenin complex-induced expression of the cyclin inhibitor p21Cip1 [128]. The interaction between β-catenin and FoxOs is evolutionary conserved as evidenced by the fact that in C. elegans the β-catenin orthologue, BAR-1, is required for DAF-16 mediated resistance to oxidative damage [129]. The findings that oxidative stress inhibit Wnt signaling via FoxOs and that mice lacking FoxOs in osteoprogenitors exhibit high bone mass throughout life supports the contention that FoxOs contribute to the deleterious effects of ROS on the skeleton.
In human articular chondrocyte cultures, inhibition of FoxO1 alone or both FoxO1 and FoxO3 increases cell death in response to oxidative stress, in part through reduced expression of antioxidant proteins and of SIRT1 [130]. Conversely, FoxO3 overexpression increases antioxidant enzyme levels [130], and FoxO3 mediates these same effects when induced by a pharmacological activator of AMPK [131].
7.2. Autophagy
The integrity of proteins is maintained by folding mechanisms, as well as by degradation processes executed by the ubiquitin-proteasome and the autophagy-lysosome systems both of which decrease in old age [132, 133]. Autophagy is the process of degradation and recycling of cytoplasmic proteins and organelles in response to starvation. Autophagy also degrades protein aggregates to prevent cytotoxicity. Various diseases of aging are associated with decreased autophagy and impaired protein homeostasis (proteostasis) [134]. Several autophagy-related genes (atg genes) encode proteins that are responsible for the recruitment of cargo, formation of autophagosomes, fusion with the lysosome, and release of degradation products [135]. Expression of autophagy-related genes declines in muscle tissue from aged humans and several cell types from aged rodents, including osteoarthritic bone chondrocytes [136-138].
Inactivation of autophagy in osteoblasts and osteocytes in young mice decreases bone mass and mimics the effects of aging on the skeleton [139-142]. Likewise, suppression of autophagy in neurons, muscle and beta cells, has been associated with premature aging and age-related disorders [137, 143-145]. FoxOs promotes the expression of several autophagy genes in muscle, neurons, cardiomyocytes and hematopoietic stem cells [146-148]. Thus, maintenance of proteostasis appears to be critical for the pro-longevity effects of FoxO [149, 150]. While the contribution of FoxOs to osteoblast or osteocyte autophagy remain unknown, both FoxO1 and FoxO3 are known to stimulate autophagy in human and murine articular chondrocytes [107, 130]. FoxO3 inhibition decreases autophagy and enhances ROS levels in response to corticosteroid challenge [151]. Moreover, the chondroprotective compound glucosamine increases autophagy in murine and human chondrocytes by dephosphorylation and activation of FoxO3 [152].
7.3. Cellular senescence
Another well-established mechanism of aging is cellular senescence, a process in which damaged cells are withdrawn from the cell cycle, avoid apoptosis, and alter their secretory activity a process known as the senescence associated secretory phenotype [153]. Accumulation of senescent cells contributes to several age-related diseases [154]. In bone, the number of senescent osteoprogenitors, osteocytes and chondrocytes increases with age and contribute to osteoporosis and osteoarthritis [155-157]. In some tissues FoxO4 is critical for senescent cell viability by binding to active p53 and, thereby, preventing p53-mediated apoptosis and promoting p21 expression and cell cycle arrest [158]. Administration of an interfering peptide that precludes the FoxO4/p53 interaction promotes apoptosis of senescent cells and attenuates the loss of hair, renal function, and activity in aged mice. Senescent osteoprogenitors from old mice exhibit activation of p53 and increased p21 and, most probably, contribute to the decrease in bone formation with old age [155]. However, it remains unknown whether FoxOs mediate any of effects of senescence in bone cells and, thereby, contribute to osteoporosis or osteoarthritis.
8. Summary
Recent research in animal models has revealed that the rate of physiological aging can be ameliorated by a variety of behavioral, genetic, and pharmacological means. Most importantly, decreased rate of aging in animal models is often accompanied by a delay (and decreased severity) of a number of age-associated diseases. Sirtuins and FoxOs are well-established players in longevity in nematodes, flies, and mammals and represent a critical node for several degenerative diseases of aging including osteoporosis and osteoarthritis. Activation of SIRT1 in mice is associated with a delay in the onset of many other aging-related diseases and can promote longevity. There are great expectations that this can also be accomplished in humans. Deacetylation of FoxOs by Sirt1 in the brain, pancreas and muscle counteract the development of neurodegenerative diseases, metabolic syndrome, sarcopenia, and cardiovascular disease [7, 159, 160]. In bone, deacetylation of FoxOs by Sirt1 decreases osteoclast and increases osteoblast number, making this signaling axis an ideal therapeutic target to counteract the loss of bone. This premise is further substantiated by findings that Sirt1 stimulators attenuate osteoporosis and osteoarthritis in different disease models. Exciting recent discoveries have elucidated that common mechanisms of aging such as oxidative stress and cellular senescence contribute to skeletal involution and osteoarthritis [111, 157]. It is, therefore, critical to continue the search for mechanisms of skeletal aging so that both osteoporosis and osteoarthritis solidify their position on the list of degenerative disease that are amenable to treatment with anti-aging drugs.
Acknowledgements
The research of the authors is supported by the National Institutes of Health (R01 AR56679, P20 GM125503) and the UAMS College of Medicine Bone and Joint Initiative.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Guarente L, Sirtuins in aging and disease, Cold Spring Harb. Symp. Quant. Biol 72 (2007) 483–488. [DOI] [PubMed] [Google Scholar]
- [2].Haigis MC, Sinclair DA, Mammalian sirtuins: biological insights and disease relevance, Annu. Rev Pathol 5 (2010) 253–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Nakagawa T, Guarente L, SnapShot: sirtuins, NAD, and aging, Cell Metab 20(1) (2014) 192–192 e1. [DOI] [PubMed] [Google Scholar]
- [4].Choudhary C, Weinert BT, Nishida Y, Verdin E, Mann M, The growing landscape of lysine acetylation links metabolism and cell signalling, Nat Rev Mol Cell Biol 15(8) (2014) 536–550. [DOI] [PubMed] [Google Scholar]
- [5].Wagner GR, Hirschey MD, Nonenzymatic protein acylation as a carbon stress regulated by sirtuin deacylases, Mol Cell 54(1) (2014) 5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Haigis MC, Guarente LP, Mammalian sirtuins--emerging roles in physiology, aging, and calorie restriction, Genes Dev 20(21) (2006) 2913–21. [DOI] [PubMed] [Google Scholar]
- [7].Bonkowski MS, Sinclair DA, Slowing ageing by design: the rise of NAD+ and sirtuin-activating compounds, Nat Rev Mol Cell Biol 17(11) (2016) 679–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA, Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan, Nature 425(6954) (2003) 191–6. [DOI] [PubMed] [Google Scholar]
- [9].Kane AE, Sinclair DA, Sirtuins and NAD(+) in the Development and Treatment of Metabolic and Cardiovascular Diseases, Circ Res 123(7) (2018) 868–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].McBurney MW, Yang X, Jardine K, Hixon M, Boekelheide K, Webb JR, Lansdorp PM, Lemieux M, The mammalian SIR2alpha protein has a role in embryogenesis and gametogenesis, Mol Cell Biol 23(1) (2003) 38–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cheng HL, Mostoslavsky R, Saito S, Manis JP, Gu Y, Patel P, Bronson R, Appella E, Alt FW, Chua KF, Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice, Proc Natl Acad Sci U S A 100(19) (2003) 10794–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lemieux ME, Yang X, Jardine K, He X, Jacobsen KX, Staines WA, Harper ME, McBurney MW, The Sirt1 deacetylase modulates the insulin-like growth factor signaling pathway in mammals, Mech Ageing Dev 126(10) (2005) 1097–105. [DOI] [PubMed] [Google Scholar]
- [13].Cohen-Kfir E, Artsi H, Levin A, Abramowitz E, Bajayo A, Gurt I, Zhong L, D'Urso A, Toiber D, Mostoslavsky R, Dresner-Pollak R, Sirt1 is a regulator of bone mass and a repressor of Sost encoding for sclerostin, a bone formation inhibitor, Endocrinology 152(12) (2011) 4514–4524. [DOI] [PubMed] [Google Scholar]
- [14].Merken EM, Mitchell SJ, Martin-Montalvo A, Minor RK, Almeida M, Gomes AP, Scheibye-Knudsen M, Palacios HH, Licata JJ, Zhang Y, Becker KG, Khaiwesh H, Gonzalez-Reyes JA, Villalba JM, Baur JA, Vlasuk GP, Ellis JL, Sinclair DA, Bernier M, de Cabo R, SRT2104 extends survival of male mice on a standard diet and preserves bone and muscle mass, Aging Cell 13(5) (2014) 787–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Simic P, Zainabadi K, Bell E, Sykes DB, Saez B, Lotinun S, Baron R, Scadden D, Schipani E, Guarente L, SIRT1 regulates differentiation of mesenchymal stem cells by deacetylating beta-catenin, EMBO Mol Med 5(3) (2013) 430–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Edwards JR, Perrien DS, Fleming N, Nyman JS, Ono K, Connelly L, Moore MM, Lwin ST, Yuli FE, Mundy GR, Elefteriou F, Silent information regulator (Sir)T1 inhibits NF-kappaB signaling to maintain normal skeletal remodeling, J Bone Miner Res 28(4) (2013) 960–969. [DOI] [PubMed] [Google Scholar]
- [17].Iyer S, Han L, Bartel SM, Kim HN, Gubrij I, de Cabo R, O'Brien CA, Manolagas SC, Almeida M, Sirtuin 1 (Sirt1) Promotes Cortical Bone Formation by Preventing beta (β)-Catenin Sequestration by FoxO Transcription Factors in Osteoblast Progenitors, J Biol Chem 289(35) (2014) 24069–24078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zainabadi K, Liu CJ, Caldwell ALM, Guarente L, SIRT1 is a positive regulator of in vivo bone mass and a therapeutic target for osteoporosis, PLoS One 12(9) (2017) e0185236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zainabadi K, Liu CJ, Guarente L, SIRT1 is a positive regulator of the master osteoblast transcription factor, RUNX2, PLoS One 12(5) (2017) e0178520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Stegen S, Stockmans I, Moermans K, Thienpont B, Maxwell PH, Carmeliet P, Carmeliet G, Osteocytic oxygen sensing controls bone mass through epigenetic regulation of sclerostin, Nat Commun 9(1) (2018) 2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, Kim J, Yancopoulos G, Valenzuela D, Murphy A, Yang Y, Chen Y, Hirschey MD, Bronson RT, Haigis M, Guarente LP, Farese RV Jr., Weissman S, Verdin E, Schwer B, Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation, Mol Cell Biol 27(24) (2007) 8807–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].McDonnell E, Peterson BS, Bomze HM, Hirschey MD, SIRT3 regulates progression and development of diseases of aging, Trends Endocrinol Metab 26(9) (2015) 486–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].van de Ven RAH, Santos D, Haigis MC, Mitochondrial Sirtuins and Molecular Mechanisms of Aging, Trends Mol Med 23(4) (2017) 320–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Huh JE, Shin JH, Jang ES, Park SJ, Park DR, Ko R, Seo DH, Kim HS, Lee SH, Choi Y, Kim HS, Lee SY, Sirtuin 3 (SIRT3) maintains bone homeostasis by regulating AMPK-PGC-1beta axis in mice, Sci Rep 6 (2016) 22511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ho L, Wang L, Roth TM, Pan Y, Verdin EM, Hsiao EC, Nissenson RA, Sirtuin-3 Promotes Adipogenesis, Osteoclastogenesis, and Bone Loss in Aging Male Mice, Endocrinology 158(9) (2017) 2741–2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mostoslavsky R, Chua KF, Lombard DB, Pang WW, Fischer MR, Gellon L, Liu P, Mostoslavsky G, Franco S, Murphy MM, Mills KD, Patel P, Hsu JT, Hong AL, Ford E, Cheng HL, Kennedy C, Nunez N, Bronson R, Frendewey D, Auerbach W, Valenzuela D, Karow M, Hottiger MO, Hursting S, Barrett JC, Guarente L, Mulligan R, Demple B, Yancopoulos GD, Alt FW, Genomic instability and aging-like phenotype in the absence of mammalian SIRT6, Cell 124(2) (2006) 315–29. [DOI] [PubMed] [Google Scholar]
- [27].Sugatani T, Agapova O, Malluche HH, Hruska KA, SIRT6 deficiency culminates in low-turnover osteopenia, Bone 81 (2015) 168–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zhang DM, Cui DX, Xu RS, Zhou YC, Zheng LW, Liu P, Zhou XD, Phenotypic research on senile osteoporosis caused by SIRT6 deficiency, Int J Oral Sci 8(2) (2016) 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhang D, Jing J, Lou F, Li R, Ping Y, Yu F, Wu F, Yang X, Xu R, Li F, Wang K, Bai M, Pi C, Xie J, Zheng L, Ye L, Zhou X, Evidence for excessive osteoclast activation in SIRT6 null mice, Sci Rep 8(1) (2018) 10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Li L, Shi L, Yang S, Yan R, Zhang D, Yang J, He L, Li W, Yi X, Sun L, Liang J, Cheng Z, Shi L, Shang Y, Yu W, SIRT7 is a histone desuccinylase that functionally links to chromatin compaction and genome stability, Nat Commun 7 (2016) 12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Vazquez BN, Thackray JK, Simonet NG, Kane-Goldsmith N, Martinez-Redondo P, Nguyen T, Bunting S, Vaquero A, Tischfield JA, Serrano L, SIRT7 promotes genome integrity and modulates non-homologous end joining DNA repair, EMBO J 35(14) (2016) 1488–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Vakhrusheva O, Smolka C, Gajawada P, Kostin S, Boettger T, Kubin T, Braun T, Bober E, Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice, Circ Res 102(6) (2008) 703–10. [DOI] [PubMed] [Google Scholar]
- [33].Fukuda M, Yoshizawa T, Karim MF, Sobuz SU, Korogi W, Kobayasi D, Okanishi H, Tasaki M, Ono K, Sawa T, Sato Y, Chirifu M, Masuda T, Nakamura T, Tanoue H, Nakashima K, Kobashigawa Y, Morioka H, Bober E, Ohtsuki S, Yamagata Y, Ando Y, Oike Y, Araki N, Takeda S, Mizuta H, Yamagata K, SIRT7 has a critical role in bone formation by regulating lysine acylation of SP7/Osterix, Nat Commun 9(1) (2018) 2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gabay O, Oppenhiemer H, Meir H, Zaal K, Sanchez C, Dvir-Ginzberg M, Increased apoptotic chondrocytes in articular cartilage from adult heterozygous SirT1 mice, Ann Rheum Dis 71(4) (2012) 613–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gabay O, Zaal KJ, Sanchez C, Dvir-Ginzberg M, Gagarina V, Song Y, He XH, McBurney MW, Sirt1-deficient mice exhibit an altered cartilage phenotype, Joint Bone Spine 80(6) (2013) 613–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Matsuzaki T, Matsushita T, Takayama K, Matsumoto T, Nishida K, Kuroda R, Kurosaka M, Disruption of Sirt1 in chondrocytes causes accelerated progression of osteoarthritis under mechanical stress and during ageing in mice, Ann Rheum Dis 73(7) (2014) 1397–404. [DOI] [PubMed] [Google Scholar]
- [37].Fujita N, Matsushita T, Ishida K, Kubo S, Matsumoto T, Takayama K, Kurosaka M, Kuroda R, Potential involvement of SIRT1 in the pathogenesis of osteoarthritis through the modulation of chondrocyte gene expressions, J Orthop Res 29(4) (2011) 511–5. [DOI] [PubMed] [Google Scholar]
- [38].Takayama K, Ishida K, Matsushita T, Fujita N, Hayashi S, Sasaki K, Tei K, Kubo S, Matsumoto T, Fujioka H, Kurosaka M, Kuroda R, SIRT1 regulation of apoptosis of human chondrocytes, Arthritis Rheum 60(9) (2009) 2731–40. [DOI] [PubMed] [Google Scholar]
- [39].Moon MH, Jeong JK, Lee YJ, Seol JW, Jackson CJ, Park SY, SIRT1, a class III histone deacetylase, regulates TNF-alpha-induced inflammation in human chondrocytes, Osteoarthritis Cartilage 21(3) (2013) 470–80. [DOI] [PubMed] [Google Scholar]
- [40].Gagarina V, Gabay O, Dvir-Ginzberg M, Lee EJ, Brady JK, Quon MJ, Hall DJ, SirT1 enhances survival of human osteoarthritic chondrocytes by repressing protein tyrosine phosphatase 1B and activating the insulin-like growth factor receptor pathway, Arthritis Rheum 62(5) (2010) 1383–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Matsushita T, Sasaki H, Takayama K, Ishida K, Matsumoto T, Kubo S, Matsuzaki T, Nishida K, Kurosaka M, Kuroda R, The overexpression of SIRT1 inhibited osteoarthritic gene expression changes induced by interleukin-1beta in human chondrocytes, J Orthop Res 31(4) (2013) 531–7. [DOI] [PubMed] [Google Scholar]
- [42].Dvir-Ginzberg M, Gagarina V, Lee EJ, Hall DJ, Regulation of cartilage-specific gene expression in human chondrocytes by SirT1 and nicotinamide phosphoribosyltransferase, J Biol Chem 283(52) (2008) 36300–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Bar Oz M, Kumar A, Elayyan J, Reich E, Binyamin M, Kandel L, Liebergall M, Steinmeyer J, Lefebvre V, Dvir-Ginzberg M, Acetylation reduces SOX9 nuclear entry and ACAN gene transactivation in human chondrocytes, Aging Cell 15(3) (2016) 499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wang Y, Zhao X, Lotz M, Terkeltaub R, Liu-Bryan R, Mitochondrial biogenesis is impaired in osteoarthritis chondrocytes but reversible via peroxisome proliferator-activated receptor gamma coactivator 1alpha, Arthritis Rheumatol 67(8) (2015) 2141–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Monteagudo S, Cornelis FMF, Aznar-Lopez C, Yibmantasiri P, Guns LA, Carmeliet P, Cailotto F, Lories RJ, DOT1L safeguards cartilage homeostasis and protects against osteoarthritis, Nat Commun 8 (2017) 15889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Abed E, Couchourel D, Delalandre A, Duval N, Pelletier JP, Martel-Pelletier J, Lajeunesse D, Low sirtuin 1 levels in human osteoarthritis subchondral osteoblasts lead to abnormal sclerostin expression which decreases Wnt/beta-catenin activity, Bone 59 (2014) 28–36. [DOI] [PubMed] [Google Scholar]
- [47].Elayyan J, Lee EJ, Gabay O, Smith CA, Qiq O, Reich E, Mobasheri A, Henrotin Y, Kimber SJ, Dvir-Ginzberg M, LEF1-mediated MMP13 gene expression is repressed by SIRT1 in human chondrocytes, FASEB J 31(7) (2017) 3116–3125. [DOI] [PubMed] [Google Scholar]
- [48].Niederer F, Ospelt C, Brentano F, Hottiger MO, Gay RE, Gay S, Detmar M, Kyburz D, SIRT1 overexpression in the rheumatoid arthritis synovium contributes to proinflammatory cytokine production and apoptosis resistance, Ann Rheum Dis 70(10) (2011) 1866–73. [DOI] [PubMed] [Google Scholar]
- [49].Fu Y, Kinter M, Hudson J, Humphries KM, Lane RS, White JR, Hakim M, Pan Y, Verdin E, Griffin TM, Aging Promotes Sirtuin 3-Dependent Cartilage Superoxide Dismutase 2 Acetylation and Osteoarthritis, Arthritis Rheumatol 68(8) (2016) 1887–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Chen LY, Wang Y, Terkeltaub R, Liu-Bryan R, Activation of AMPK-SIRT3 signaling is chondroprotective by preserving mitochondrial DNA integrity and function, Osteoarthritis Cartilage 26(11) (2018) 1539–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wu Y, Chen L, Wang Y, Li W, Lin Y, Yu D, Zhang L, Li F, Pan Z, Overexpression of Sirtuin 6 suppresses cellular senescence and NF-kappaB mediated inflammatory responses in osteoarthritis development, Sci Rep 5 (2015) 17602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Nagai K, Matsushita T, Matsuzaki T, Takayama K, Matsumoto T, Kuroda R, Kurosaka M, Depletion of SIRT6 causes cellular senescence, DNA damage, and telomere dysfunction in human chondrocytes, Osteoarthritis Cartilage 23(8) (2015) 1412–20. [DOI] [PubMed] [Google Scholar]
- [53].Oh H, Kwak JS, Yang S, Gong MK, Kim JH, Rhee J, Kim SK, Kim HE, Ryu JH, Chun JS, Reciprocal regulation by hypoxia-inducible factor-2alpha and the NAMPT-NAD(+)-SIRT axis in articular chondrocytes is involved in osteoarthritis, Osteoarthritis Cartilage 23(12) (2015) 2288–2296. [DOI] [PubMed] [Google Scholar]
- [54].Korogi W, Yoshizawa T, Karim MF, Tanoue H, Yugami M, Sobuz SU, Hinoi E, Sato Y, Oike Y, Mizuta H, Yamagata K, SIRT7 is an important regulator of cartilage homeostasis and osteoarthritis development, Biochem Biophys Res Commun (2018). [DOI] [PubMed] [Google Scholar]
- [55].Baur JA, Sinclair DA, Therapeutic potential of resveratrol: the in vivo evidence, Nat Rev Drug Discov 5(6) (2006) 493–506. [DOI] [PubMed] [Google Scholar]
- [56].Baur JA, Ungvari Z, Minor RK, Le Couteur DG, de CR, Are sirtuins viable targets for improving healthspan and lifespan?, Nat Rev Drug Discov 11(6) (2012) 443–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Novelle MG, Wahl D, Dieguez C, Bernier M, de Cabo R, Resveratrol supplementation: Where are we now and where should we go?, Ageing Res Rev 21 (2015) 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Kulkarni SS, Canto C, The molecular targets of resveratrol, Biochim Biophys Acta 1852(6) (2015) 1114–23. [DOI] [PubMed] [Google Scholar]
- [59].Knight CM, Gutierrez-Juarez R, Lam TK, Arrieta-Cruz I, Huang L, Schwartz G, Barzilai N, Rossetti L, Mediobasal hypothalamic SIRT1 is essential for resveratrol's effects on insulin action in rats, Diabetes 60(11) (2011) 2691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Price NL, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro JS, Hubbard BP, Varela AT, Davis JG, Varamini B, Hafner A, Moaddel R, Rolo AP, Coppari R, Palmeira CM, de CR, Baur JA, Sinclair DA, SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function, Cell Metab 15(5) (2012) 675–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Zhao M, Ko SY, Garrett IR, Mundy GR, Gutierrez GE, Edwards JR, The polyphenol resveratrol promotes skeletal growth in mice through a sirtuin 1-bone morphogenic protein 2 longevity axis, Br J Pharmacol 175(21) (2018) 4183–4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Backesjo CM, Li Y, Lindgren U, Haldosen LA, Activation of Sirt1 decreases adipocyte formation during osteoblast differentiation of mesenchymal stem cells, J Bone Miner Res 21(7) (2006) 993–1002. [DOI] [PubMed] [Google Scholar]
- [63].Zhou H, Shang L, Li X, Zhang X, Gao G, Guo C, Chen B, Liu Q, Gong Y, Shao C, Resveratrol augments the canonical Wnt signaling pathway in promoting osteoblastic differentiation of multipotent mesenchymal cells, Exp Cell Res 315(17) (2009) 2953–62. [DOI] [PubMed] [Google Scholar]
- [64].Shakibaei M, Buhrmann C, Mobasheri A, Resveratrol-mediated SIRT-1 Interactions with p300 Modulate Receptor Activator of NF-{kappa}B Ligand (RANKL) Activation of NF-{kappa}B Signaling and Inhibit Osteoclastogenesis in Bone-derived Cells, J Biol. Chem 286(13) (2011) 11492–11505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kim HN, Han L, Iyer S, de Cabo R, Zhao H, O'Brien CA, Manolagas SC, Almeida M, Sirtuin1 Suppresses Osteoclastogenesis by Deacetylating FoxOs, Mol Endocrinol 29(10) (2015) 1498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].He X, Andersson G, Lindgren U, Li Y, Resveratrol prevents RANKL-induced osteoclast differentiation of murine osteoclast progenitor RAW 264.7 cells through inhibition of ROS production, Biochem. Biophys. Res Commun 401(3) (2010) 356–362. [DOI] [PubMed] [Google Scholar]
- [67].Gurt I, Artsi H, Cohen-Kfir E, Hamdani G, Ben-Shalom G, Feinstein B, El-Haj M, Dresner-Pollak R, The Sirt1 Activators SRT2183 and SRT3025 Inhibit RANKL-Induced Osteoclastogenesis in Bone Marrow-Derived Macrophages and Down-Regulate Sirt3 in Sirt1 Null Cells, PLoS One 10(7) (2015) e0134391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R, Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span, Cell Metab 8(2) (2008) 157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Herranz D, Munoz-Martin M, Canamero M, Mulero F, Martinez-Pastor B, Fernandez-Capetillo O, Serrano M, Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer, Nat Commun 1 (2010) 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Momken I, Stevens L, Bergouignan A, Desplanches D, Rudwill F, Chery I, Zahariev A, Zahn S, Stein TP, Sebedio JL, Pujos-Guillot E, Falempin M, Simon C, Coxam V, Andrianjafiniony T, Gauquelin-Koch G, Picquet F, Blanc S, Resveratrol prevents the wasting disorders of mechanical unloading by acting as a physical exercise mimetic in the rat, FASEB J 25(10) (2011) 3646–3660. [DOI] [PubMed] [Google Scholar]
- [71].Su JL, Yang CY, Zhao M, Kuo ML, Yen ML, Forkhead proteins are critical for bone morphogenetic protein-2 regulation and anti-tumor activity of resveratrol, J Biol. Chem 282(27) (2007) 19385–19398. [DOI] [PubMed] [Google Scholar]
- [72].Artsi H, Cohen-Kfir E, Gurt I, Shahar R, Bajayo A, Kalish N, Bellido TM, Gabet Y, Dresner-Pollak R, The Sirtuin1 activator SRT3025 down-regulates sclerostin and rescues ovariectomy-induced bone loss and biomechanical deterioration in female mice, Endocrinology 155(9) (2014) 3508–3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Ornstrup MJ, Harslof T, Kjaer TN, Langdahl BL, Pedersen SB, Resveratrol increases bone mineral density and bone alkaline phosphatase in obese men: a randomized placebo-controlled trial, J Clin Endocrinol Metab 99(12) (2014) 4720–9. [DOI] [PubMed] [Google Scholar]
- [74].Shakibaei M, John T, Seifarth C, Mobasheri A, Resveratrol inhibits IL-1 beta-induced stimulation of caspase-3 and cleavage of PARP in human articular chondrocytes in vitro, Ann N Y Acad Sci 1095 (2007) 554–63. [DOI] [PubMed] [Google Scholar]
- [75].Dave M, Attur M, Palmer G, Al-Mussawir HE, Kennish L, Patel J, Abramson SB, The antioxidant resveratrol protects against chondrocyte apoptosis via effects on mitochondrial polarization and ATP production, Arthritis Rheum 58(9) (2008) 2786–97. [DOI] [PubMed] [Google Scholar]
- [76].Lei M, Wang JG, Xiao DM, Fan M, Wang DP, Xiong JY, Chen Y, Ding Y, Liu SL, Resveratrol inhibits interleukin 1beta-mediated inducible nitric oxide synthase expression in articular chondrocytes by activating SIRT1 and thereby suppressing nuclear factor-kappaB activity, Eur J Pharmacol 674(2–3) (2012) 73–9. [DOI] [PubMed] [Google Scholar]
- [77].Wang J, Gao JS, Chen JW, Li F, Tian J, Effect of resveratrol on cartilage protection and apoptosis inhibition in experimental osteoarthritis of rabbit, Rheumatol Int 32(6) (2012) 1541–8. [DOI] [PubMed] [Google Scholar]
- [78].Li W, Cai L, Zhang Y, Cui L, Shen G, Intra-articular resveratrol injection prevents osteoarthritis progression in a mouse model by activating SIRT1 and thereby silencing HIF-2alpha, J Orthop Res 33(7) (2015) 1061–70. [DOI] [PubMed] [Google Scholar]
- [79].Cetrullo S, D'Adamo S, Guidotti S, Borzi RM, Flamigni F, Hydroxytyrosol prevents chondrocyte death under oxidative stress by inducing autophagy through sirtuin 1-dependent and -independent mechanisms, Biochim Biophys Acta 1860(6) (2016) 1181–91. [DOI] [PubMed] [Google Scholar]
- [80].D'Adamo S, Cetrullo S, Guidotti S, Borzi RM, Flamigni F, Hydroxytyrosol modulates the levels of microRNA-9 and its target sirtuin-1 thereby counteracting oxidative stress-induced chondrocyte death, Osteoarthritis Cartilage 25(4) (2017) 600–610. [DOI] [PubMed] [Google Scholar]
- [81].Nishida K, Matsushita T, Takayama K, Tanaka T, Miyaji N, Ibaraki K, Araki D, Kanzaki N, Matsumoto T, Kuroda R, Intraperitoneal injection of the SIRT1 activator SRT1720 attenuates the progression of experimental osteoarthritis in mice, Bone Joint Res 7(3) (2018) 252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Greer EL, Brunet A, FOXO transcription factors at the interface between longevity and tumor suppression, Oncogene 24(50) (2005) 7410–7425. [DOI] [PubMed] [Google Scholar]
- [83].van der Horst A, Burgering BM, Stressing the role of FoxO proteins in lifespan and disease, Nat. Rev. Mol. Cell Biol 8(6) (2007) 440–450. [DOI] [PubMed] [Google Scholar]
- [84].Eijkelenboom A, Burgering BM, FOXOs: signalling integrators for homeostasis maintenance, Nat Rev Mol Cell Biol 14(2) (2013) 83–97. [DOI] [PubMed] [Google Scholar]
- [85].Medema RH, Kops GJ, Bos JL, Burgering BM, AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1, Nature 404(6779) (2000) 782–787. [DOI] [PubMed] [Google Scholar]
- [86].Tran H, Brunet A, Grenier JM, Datta SR, Fornace AJ Jr., DiStefano PS, Chiang LW, Greenberg ME, DNA repair pathway stimulated by the forkhead transcription factor FOXO3a through the Gadd45 protein, Science 296(5567) (2002) 530–534. [DOI] [PubMed] [Google Scholar]
- [87].Ramaswamy S, Nakamura N, Sansal I, Bergeron L, Sellers WR, A novel mechanism of gene regulation and tumor suppression by the transcription factor FKHR, Cancer Cell 2(1) (2002) 81–91. [DOI] [PubMed] [Google Scholar]
- [88].Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, Huang TT, Bos JL, Medema RH, Burgering BM, Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress, Nature 419(6904) (2002) 316–321. [DOI] [PubMed] [Google Scholar]
- [89].Nemoto S, Finkel T, Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway, Science 295(5564) (2002) 2450–2452. [DOI] [PubMed] [Google Scholar]
- [90].Calnan DR, Brunet A, The FoxO code, Oncogene 27(16) (2008) 2276–2288. [DOI] [PubMed] [Google Scholar]
- [91].Ambrogini E, Almeida M, Martin-Millan M, Paik J, dePinho R, Han L, Goellner J, Weinstein R, Jilka R, O'Brien C, Manolagas S, FoxO-mediated defense against oxidative stress in osteoblasts is indispensable for skeletal homeostasis in mice, Cell Metab 11(2) (2010) 136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Akasaki Y, Hasegawa A, Saito M, Asahara H, Iwamoto Y, Lotz MK, Dysregulated FOXO transcription factors in articular cartilage in aging and osteoarthritis, Osteoarthritis Cartilage 22(1) (2014) 162–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Obsil T, Obsilova V, Structure/function relationships underlying regulation of FOXO transcription factors, Oncogene 27(16) (2008) 2263–2275. [DOI] [PubMed] [Google Scholar]
- [94].Paik JH, Kollipara R, Chu G, Ji H, Xiao Y, Ding Z, Miao L, Tothova Z, Horner JW, Carrasco DR, Jiang S, Gilliland DG, Chin L, Wong WH, Castrillon DH, DePinho RA, FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis, Cell 128(2) (2007) 309–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams IR, Sears C, Armstrong SA, Passegue E, DePinho RA, Gilliland DG, FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress, Cell 128(2) (2007) 325–339. [DOI] [PubMed] [Google Scholar]
- [96].Paik JH, Ding Z, Narurkar R, Ramkissoon S, Muller F, Kamoun WS, Chae SS, Zheng H, Ying H, Mahoney J, Hiller D, Jiang S, Protopopov A, Wong WH, Chin L, Ligon KL, DePinho RA, FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis, Cell Stem Cell 5(5) (2009) 540–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Renault VM, Rafalski VA, Morgan AA, Salih DA, Brett JO, Webb AE, Villeda SA, Thekkat PU, Guillerey C, Denko NC, Palmer TD, Butte AJ, Brunet A, FoxO3 regulates neural stem cell homeostasis, Cell Stem Cell 5(5) (2009) 527–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Tsuchiya K, Westerterp M, Murphy AJ, Subramanian V, Ferrante AW Jr., Tall AR, Accili D, Expanded Granulocyte/Monocyte Compartment in Myeloid-Specific Triple FoxO Knockout Increases Oxidative Stress and Accelerates Atherosclerosis in Mice, Circ. Res 112(7) (2013) 992–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Bartell SM, Kim HN, Ambrogini E, Han L, Iyer S, Serra US, Rabinovitch P, Jilka RL, Weinstein RS, Zhao H, O'Brien CA, Manolagas SC, Almeida M, FoxO proteins restrain osteoclastogenesis and bone resorption by attenuating H2O2 accumulation, Nat Commun 5 (2014) 3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Tan P, Guan H, Xie L, Mi B, Fang Z, Li J, Li F, FOXO1 inhibits osteoclastogenesis partially by antagnozing MYC, Sci Rep 5 (2015) 16835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Liu W, Wang S, Wei S, Sun L, Feng X, Receptor activator of NF-kappaB (RANK) cytoplasmic motif, 369PFQEP373, plays a predominant role in osteoclast survival in part by activating Akt/PKB and its downstream effector AFX/FOXO4, J Biol Chem 280(52) (2005) 43064–72. [DOI] [PubMed] [Google Scholar]
- [102].Iyer S, Ambrogini E, Bartell SM, Han L, Roberson PK, Cabo R, Jilka RL, Weinstein RS, O'Brien CA, Manolagas SC, Almeida M, FoxOs attenuate bone formation by suppressing Wnt signaling, J Clin Invest 123(8) (2013) 3404–3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Rached MT, Kode A, Xu L, Yoshikawa Y, Paik JH, DePinho RA, Kousteni S, FoxO1 is a positive regulator of bone formation by favoring protein synthesis and resistance to oxidative stress in osteoblasts, Cell Metab 11(2) (2010) 147–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Ferron M, Wei J, Yoshizawa T, Del Fattore A, DePinho RA, Teti A, Ducy P, Karsenty G, Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism, Cell 142(2) (2010) 296–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Iyer S, Han L, Ambrogini E, Yavropoulou M, Fowlkes J, Manolagas SC, Almeida M, Deletion of FoxO1, 3, and 4 in Osteoblast Progenitors Attenuates the Loss of Cancellous Bone Mass in a Mouse Model of Type 1 Diabetes, J Bone Miner Res 32(1) (2017) 60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Fisch KM, Gamini R, Alvarez-Garcia O, Akagi R, Saito M, Muramatsu Y, Sasho T, Koziol JA, Su AI, Lotz MK, Identification of transcription factors responsible for dysregulated networks in human osteoarthritis cartilage by global gene expression analysis, Osteoarthritis Cartilage 26(11) (2018) 1531–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Matsuzaki T, Alvarez-Garcia O, Mokuda S, Nagira K, Olmer M, Gamini R, Miyata K, Akasaki Y, Su AI, Asahara H, Lotz MK, FoxO transcription factors modulate autophagy and proteoglycan 4 in cartilage homeostasis and osteoarthritis, Sci Transl Med 10(428) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Ludikhuize J, de Launay D, Groot D, Smeets TJ, Vinkenoog M, Sanders ME, Tas SW, Tak PP, Reedquist KA, Inhibition of forkhead box class O family member transcription factors in rheumatoid synovial tissue, Arthritis Rheum 56(7) (2007) 2180–91. [DOI] [PubMed] [Google Scholar]
- [109].Turrel-Davin F, Tournadre A, Pachot A, Arnaud B, Cazalis MA, Mougin B, Miossec P, FoxO3a involved in neutrophil and T cell survival is overexpressed in rheumatoid blood and synovial tissue, Ann Rheum Dis 69(4) (2010) 755–60. [DOI] [PubMed] [Google Scholar]
- [110].Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G, The hallmarks of aging, Cell 153(6) (2013) 1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Farr JN, Almeida M, The Spectrum of Fundamental Basic Science Discoveries Contributing to Organismal Aging, J Bone Miner Res 33(9) (2018) 1568–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R, A C. elegans mutant that lives twice as long as wild type, Nature 366(6454) (1993) 461–464. [DOI] [PubMed] [Google Scholar]
- [113].Lin K, Dorman JB, Rodan A, Kenyon C, daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans, Science 278(5341) (1997) 1319–1322. [DOI] [PubMed] [Google Scholar]
- [114].Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA, Ruvkun G, The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans, Nature 389(6654) (1997) 994–999. [DOI] [PubMed] [Google Scholar]
- [115].Davy PMC, Allsopp RC, Donlon TA, Morris BJ, Willcox DC, Willcox BJ, FOXO3 and Exceptional Longevity: Insights From Hydra to Humans, Curr Top Dev Biol 127 (2018) 193–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].HARMAN D, Aging: a theory based on free radical and radiation chemistry, J Gerontol 11(3) (1956) 298–300. [DOI] [PubMed] [Google Scholar]
- [117].Lean JM, Davies JT, Fuller K, Jagger CJ, Kirstein B, Partington GA, Urry ZL, Chambers TJ, A crucial role for thiol antioxidants in estrogen-deficiency bone loss, J Clin Invest 112(6) (2003) 915–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK, Kousteni S, O'Brien CA, Bellido T, Parfitt AM, Weinstein RS, Jilka RL, Manolagas SC, Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids, J Biol. Chem 282(37) (2007) 27285–27297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Ucer S, Iyer S, Kim HN, Han L, Rutlen C, Allison K, Thostenson JD, de CR, Jilka RL, O'Brien C, Almeida M, Manolagas SC, The Effects of Aging and Sex Steroid Deficiency on the Murine Skeleton Are Independent and Mechanistically Distinct, J Bone Miner Res 32(3) (2017) 560–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME, Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase, Science 303(5666) (2004) 2011–2015. [DOI] [PubMed] [Google Scholar]
- [121].Frescas D, Valenti L, Accili D, Nuclear trapping of the forkhead transcription factor FoxO1 via Sirt-dependent deacetylation promotes expression of glucogenetic genes, J Biol. Chem 280(21) (2005) 20589–20595. [DOI] [PubMed] [Google Scholar]
- [122].Kitamura YI, Kitamura T, Kruse JP, Raum JC, Stein R, Gu W, Accili D, FoxO1 protects against pancreatic beta cell failure through NeuroD and MafA induction, Cell Metab 2(3) (2005) 153–163. [DOI] [PubMed] [Google Scholar]
- [123].Almeida M, Han L, Martin-Millan M, O'Brien CA, Manolagas SC, Oxidative stress antagonizes Wnt signaling in osteoblast precursors by diverting beta-catenin from T cell factor-to forkhead box O-mediated transcription, J Biol. Chem 282(37) (2007) 27298–27305. [DOI] [PubMed] [Google Scholar]
- [124].Hoogeboom D, Essers MA, Polderman PE, Voets E, Smits LM, Burgering BM, Interaction of FOXO with beta-catenin inhibits beta-catenin/T cell factor activity, J Biol. Chem 283(14) (2008) 9224–9230. [DOI] [PubMed] [Google Scholar]
- [125].Almeida M, Ambrogini E, Han L, Manolagas SC, Jilka RL, Increased lipid oxidation causes oxidative stress, increased PPAR{gamma} expression and diminished pro-osteogenic Wnt signaling in the skeleton, J Biol. Chem 284(40) (2009) 27438–27448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Almeida M, Han L, Ambrogini E, Weinstein RS, Manolagas SC, Glucocorticoids and tumor necrosis factor (TNF) alpha increase oxidative stress and suppress WNT signaling in osteoblasts, J Biol Chem 286 (2011) 44326–44335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Weinstein RS, Wan C, Liu Q, Wang Y, Almeida M, O'Brien CA, Thostenson J, Roberson PK, Boskey AL, Clemens TL, Manolagas SC, Endogenous glucocorticoids decrease skeletal angiogenesis, vascularity, hydration, and strength in aged mice, Aging Cell 9(2) (2010) 147–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].McClelland Descalzo DL, Satoorian TS, Walker LM, Sparks NR, Pulyanina PY, Zur Nieden NI, Glucose-Induced Oxidative Stress Reduces Proliferation in Embryonic Stem Cells via FOXO3A/beta-Catenin-Dependent Transcription of p21(cip1), Stem Cell Reports 7(1) (2016) 55–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Essers MA, Vries-Smits LM, Barker N, Polderman PE, Burgering BM, Korswagen HC, Functional interaction between beta-catenin and FOXO in oxidative stress signaling, Science 308(5725) (2005) 1181–1184. [DOI] [PubMed] [Google Scholar]
- [130].Akasaki Y, Alvarez-Garcia O, Saito M, Carames B, Iwamoto Y, Lotz MK, FoxO transcription factors support oxidative stress resistance in human chondrocytes, Arthritis Rheumatol 66(12) (2014) 3349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Zhao X, Petursson F, Viollet B, Lotz M, Terkeltaub R, Liu-Bryan R, Peroxisome proliferator-activated receptor gamma coactivator 1alpha and FoxO3A mediate chondroprotection by AMP-activated protein kinase, Arthritis Rheumatol 66(11) (2014) 3073–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Cuervo AM, Autophagy and aging: keeping that old broom working, Trends Genet 24(12) (2008) 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Morimoto RI, Cuervo AM, Proteostasis and the aging proteome in health and disease, J Gerontol A Biol Sci Med Sci 69 Suppl 1 (2014) S33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Labbadia J, Morimoto RI, The biology of proteostasis in aging and disease, Annu Rev Biochem 84 (2015) 435–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Mizushima N, Yoshimori T, Ohsumi Y, The role of Atg proteins in autophagosome formation, Annu Rev Cell Dev Biol 27 (2011) 107–32. [DOI] [PubMed] [Google Scholar]
- [136].Carames B, Olmer M, Kiosses WB, Lotz MK, The relationship of autophagy defects to cartilage damage during joint aging in a mouse model, Arthritis Rheumatol 67(6) (2015) 1568–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Carnio S, LoVerso F, Baraibar MA, Longa E, Khan MM, Maffei M, Reischl M, Canepari M, Loefler S, Kern H, Blaauw B, Friguet B, Bottinelli R, Rudolf R, Sandri M, Autophagy impairment in muscle induces neuromuscular junction degeneration and precocious aging, Cell Rep 8(5) (2014) 1509–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Cuervo AM, Dice JF, Age-related decline in chaperone-mediated autophagy, J Biol Chem 275(40) (2000) 31505–13. [DOI] [PubMed] [Google Scholar]
- [139].Onal M, Piemontese M, Xiong J, Wang Y, Han L, Ye S, Komatsu M, Selig M, Weinstein RS, Zhao H, Jilka RL, Almeida M, Manolagas SC, O'Brien CA, Suppression of autophagy in osteocytes mimics skeletal aging, J Biol Chem 288(24) (2013) 17432–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Piemontese M, Onal M, Xiong J, Han L, Thostenson JD, Almeida M, O'Brien CA, Low bone mass and changes in the osteocyte network in mice lacking autophagy in the osteoblast lineage, Sci Rep 6 (2016) 24262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Nollet M, Santucci-Darmanin S, Breuil V, Al-Sahlanee R, Cros C, Topi M, Momier D, Samson M, Pagnotta S, Cailleteau L, Battaglia S, Farlay D, Dacquin R, Barois N, Jurdic P, Boivin G, Heymann D, Lafont F, Lu SS, Dempster DW, Carle GF, Pierrefite-Carle V, Autophagy in osteoblasts is involved in mineralization and bone homeostasis, Autophagy 10(11) (2014) 1965–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Liu F, Fang F, Yuan H, Yang D, Chen Y, Williams L, Goldstein SA, Krebsbach PH, Guan JL, Suppression of autophagy by FIP200 deletion leads to osteopenia in mice through the inhibition of osteoblast terminal differentiation, J Bone Miner Res 28(11) (2013) 2414–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Komatsu M, Wang QJ, Holstein GR, Friedrich VL Jr., Iwata J, Kominami E, Chait BT, Tanaka K, Yue Z, Essential role for autophagy protein Atg7 in the maintenance of axonal homeostasis and the prevention of axonal degeneration, Proc Natl Acad Sci U S A 104(36) (2007) 14489–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Abdellatif M, Sedej S, Carmona-Gutierrez D, Madeo F, Kroemer G, Autophagy in Cardiovascular Aging, Circ Res 123(7) (2018) 803–824. [DOI] [PubMed] [Google Scholar]
- [145].Jung HS, Chung KW, Won Kim J, Kim J, Komatsu M, Tanaka K, Nguyen YH, Kang TM, Yoon KH, Kim JW, Jeong YT, Han MS, Lee MK, Kim KW, Shin J, Lee MS, Loss of autophagy diminishes pancreatic beta cell mass and function with resultant hyperglycemia, Cell Metab 8(4) (2008) 318–24. [DOI] [PubMed] [Google Scholar]
- [146].Zhao J, Brault JJ, Schild A, Goldberg AL, Coordinate activation of autophagy and the proteasome pathway by FoxO transcription factor, Autophagy 4(3) (2008) 378–80. [DOI] [PubMed] [Google Scholar]
- [147].Sengupta A, Molkentin JD, Yutzey KE, FoxO transcription factors promote autophagy in cardiomyocytes, J Biol Chem 284(41) (2009) 28319–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Schaffner I, Minakaki G, Khan MA, Balta EA, Schlotzer-Schrehardt U, Schwarz TJ, Beckervordersandforth R, Winner B, Webb AE, DePinho RA, Paik J, Wurst W, Klucken J, Lie DC, FoxO Function Is Essential for Maintenance of Autophagic Flux and Neuronal Morphogenesis in Adult Neurogenesis, Neuron 99(6) (2018) 1188–1203 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Webb AE, Brunet A, FOXO transcription factors: key regulators of cellular quality control, Trends Biochem Sci 39(4) (2014) 159–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Lapierre LR, Kumsta C, Sandri M, Ballabio A, Hansen M, Transcriptional and epigenetic regulation of autophagy in aging, Autophagy 11(6) (2015) 867–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Shen C, Cai GQ, Peng JP, Chen XD, Autophagy protects chondrocytes from glucocorticoids-induced apoptosis via ROS/Akt/FOXO3 signaling, Osteoarthritis Cartilage 23(12) (2015) 2279–87. [DOI] [PubMed] [Google Scholar]
- [152].Carames B, Kiosses WB, Akasaki Y, Brinson DC, Eap W, Koziol J, Lotz MK, Glucosamine activates autophagy in vitro and in vivo, Arthritis Rheum 65(7) (2013) 1843–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Campisi J, d'Adda di FF, Cellular senescence: when bad things happen to good cells, Nat Rev Mol Cell Biol 8(9) (2007) 729–740. [DOI] [PubMed] [Google Scholar]
- [154].Childs BG, Gluscevic M, Baker DJ, Laberge RM, Marquess D, Dananberg J, van Deursen JM, Senescent cells: an emerging target for diseases of ageing, Nat Rev Drug Discov 16(10) (2017) 718–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Kim HN, Chang J, Shao L, Han L, Iyer S, Manolagas SC, O'Brien CA, Jilka RL, Zhou D, Almeida M, DNA damage and senescence in osteoprogenitors expressing Osx1 may cause their decrease with age, Aging Cell (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Farr JN, Xu M, Weivoda MM, Monroe DG, Fraser DG, Onken JL, Negley BA, Sfeir JG, Ogrodnik MB, Hachfeld CM, LeBrasseur NK, Drake MT, Pignolo RJ, Pirtskhalava T, Tchkonia T, Oursler MJ, Kirkland JL, Khosla S, Targeting cellular senescence prevents age-related bone loss in mice, Nat Med 23(9) (2017) 1072–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Jeon OH, Kim C, Laberge RM, Demaria M, Rathod S, Vasserot AP, Chung JW, Kim DH, Poon Y, David N, Baker DJ, van Deursen JM, Campisi J, Elisseeff JH, Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment, Nat Med 23(6) (2017) 775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Baar MP, Brandt RM, Putavet DA, Klein JD, Derks KW, Bourgeois BR, Stryeck S, Rijksen Y, van Willigenburg H, Feijtel DA, van der Pluijm I, Essers J, van Cappellen WA, van IWF, Houtsmuller AB, Pothof J, de Bruin RW, Madl T, Hoeijmakers JH, Campisi J, de Keizer PL, Targeted Apoptosis of Senescent Cells Restores Tissue Homeostasis in Response to Chemotoxicity and Aging, Cell 169(1) (2017) 132–147 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Imai S, Guarente L, NAD+ and sirtuins in aging and disease, Trends Cell Biol 24(8) (2014) 464–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Kim H-N, Iyer S, Ring R, Almeida M, The Role of FoxOs in Bone Health and Disease, Current Topics in Developmental Biology 127 (2018) 15. [DOI] [PubMed] [Google Scholar]