Direct evidence of lignin–carbohydrate linkages was found in lignin substrates isolated and synthetized mimicking their native state and overcoming any kind of harsh extraction and chemical pre-treatments.
Keywords: Benzyl ester, benzyl ether, dehydrogenation polymer, xtracellular lignin, gamma (γ)-ester, lignin–carbohydrate complex, phenyl glycoside
Abstract
The question of whether lignin is covalently linked to carbohydrates in native wood, forming what is referred to as lignin–carbohydrate complexes (LCCs), still lacks unequivocal proof. This is mainly due to the need to isolate lignin from woody materials prior to analysis, under conditions leading to partial chemical modification of the native wood polymers. Thus, the correlation between the structure of the isolated LCCs and LCCs in situ remains open. As a way to circumvent the problematic isolation, biomimicking lignin polymerization in vivo and in vitro is an interesting option. Herein, we report the detection of lignin–carbohydrate bonds in the extracellular lignin formed by tissue-cultured Norway spruce cells, and in modified biomimetic lignin synthesis (dehydrogenation polymers). Semi-quantitative 2D heteronuclear singular quantum coherence (HSQC)-, 31P -, and 13C-NMR spectroscopy were applied as analytical tools. Combining results from these systems, four types of lignin–carbohydrate bonds were detected; benzyl ether, benzyl ester, γ-ester, and phenyl glycoside linkages, providing direct evidence of lignin–carbohydrate bond formation in biomimicked lignin polymerization. Based on our findings, we propose a sequence for lignin–carbohydrate bond formation in plant cell walls.
Introduction
Lignocellulosic biomass consists mainly of cellulose, hemicelluloses, and lignin, in addition to minor polymers which include a class of acidic polysaccharides called pectins. The polymers interact with each other through physical and chemical forces, which are not yet fully investigated. For instance, a covalent connectivity between lignin and hemicelluloses in native wood cell walls forming so-called lignin–carbohydrate complexes (LCCs) has been proposed in the literature. There is a lot of indirect evidence related to covalent linkages between lignin and carbohydrates in various lignocellulosics (Balakshin et al., 2014), but still no unequivocal proof exists of their presence in the substrates in situ. Current criticism regarding the existence of LCCs in native plant cell walls is attributed to characterization techniques and mechanistic pathways for their formation. On the characterization front, the challenge is to understand possible modifications that occur under mechanical and/or chemical treatments required to extract lignin from the woody matrix prior to analyses. Mechanical treatment is conventionally ball milling performed following Wiley milling as described by Bjorkman (1956).
In the past, several solvent systems with varying degrees of mildness have been used to dissolve the ball-milled wood partially or completely (Björkman, 1956). The initial ball milling step is a drawback since some modifications occur to native cell wall polymers. The extent of such modifications on polymer structure remains insufficiently understood. Another criticism is whether the ensuing dissolution with some harsh solvents imparts modifications, for example LCC formation. Several solvent classes have been used including acidic, neutral, and basic systems (Koshijima et al., 1976; Eriksson and Lindgren, 1977; Lawoko et al., 2005; Du et al., 2013). The use of neutral solvents under common conditions (low temperature, <80 °C) should not result in any modifications. Moreover, the use of acetic acid for isolation of specific LCC preparations has not been shown to result in (additional) LCC formation (Balakshin et al., 2007). However, other fractionation solvents, under the conditions used, may result in LCC modification and/or formation (Del Rio et al., 2016).
Four different types of native lignin–carbohydrate bonds are proposed in the literature, namely benzyl ethers, benzyl esters, γ-esters, and phenyl glycosides (Freudenberg and Neish, 1968; Yaku et al., 1976; Fengel and Wegener, 1989; Balakshin et al., 2001, 2007). Among various methods for LCC analysis, 2D NMR methods have been the most revealing techniques (Balakshin et al., 2001, 2014; Nishimura et al., 2018, Rencoret et al., 2019). Although the first LCC assignments (Balakshin et al., 2001) were rather tentative, further experiments have confirmed them. In addition to new model compound studies (Miyagawa et al., 2014), they were confirmed with an HMBC (heteronuclear multiple bond correlation) technique (Balakshin et al., 2007), selective 13C enrichment (Evtuguin et al., 2005), and wet chemistry pre-treatments followed by NMR studies (Balakshin et al., 2007, 2011). Moreover, rigorous NMR studies for LCC have very recently been published, showing unequivocal evidence for ether linkages between glucomannan and the lignin fraction obtained from milled wood of Japanese red pine (Pinus densifora) (Nishimura et al., 2018) and incorporation of hydroxystilbene glucosides into Norway spruce (Picea abies) bark lignin (Rencoret et al., 2019).
The mechanism of formation of phenyl glycosides is barely discussed in the literature. One speculative explanation relies on the acid-catalyzed addition of a phenolic hydroxyl to the reducing end of a carbohydrate moiety, by the well-known chemistry of hemi-acetal formation. If this is the case, in the acidic pH (~5–6) present in the plant cell wall (Felle, 2001), the linkage is likely to be formed. Such a scenario could exist locally in the proximity of acidic polysaccharides such as glucuronoxylan and pectins. Another possibility is transglycosylation of a phenolic end group to the reducing end of a polysaccharide catalyzed by one of the multiple types of transglycosylating enzymes. These are known to cleave and religate hemicellulose and also cellulose chains for cell wall modifications during plant development (Yaku et al, 1976; Fry, 1995; Simmons et al., 2015). A very recent study conducted on Norway spruce (P. abies) bark lignin demonstrated unequivocally the incorporation of natural phenyl glycoside monomers into lignin’s hydroxystilbene, supporting, for the first time, the formation of phenyl glycoside-type linkages in native wood substrates (Rencoret et al., 2019). Formation of benzyl ethers and benzyl esters is based on simple nucleophile addition chemistry on an electrophilic site; Cα of a quinone methide. This intermediate is created during lignin polymerization whenever a β-radical couples to form dimers (Ralph et al., 2004), and chain growth proceeds by coupling of pre-formed oligomers (Ralph et al., 2004) or endwise coupling of monomers to the growing polymer (Sarkanen, 1971; Ralph et al., 2004; Tobimatsu and Schuetz, 2019). Not surprisingly, when the coupling involves a β-5 or β-β bond, a favorable intramolecular addition reaction with available nucleophiles (phenol-OH and -OH in Cγ, respectively) occurs to form ring structures such as phenyl coumaran and pinoresinol structures. This favorability is due to internal trapping of the quinone methide, and is well documented by NMR studies showing the presence of such structures in lignin (Ralph et al., 2004). In the case of β-O-4, the addition becomes intermolecular.
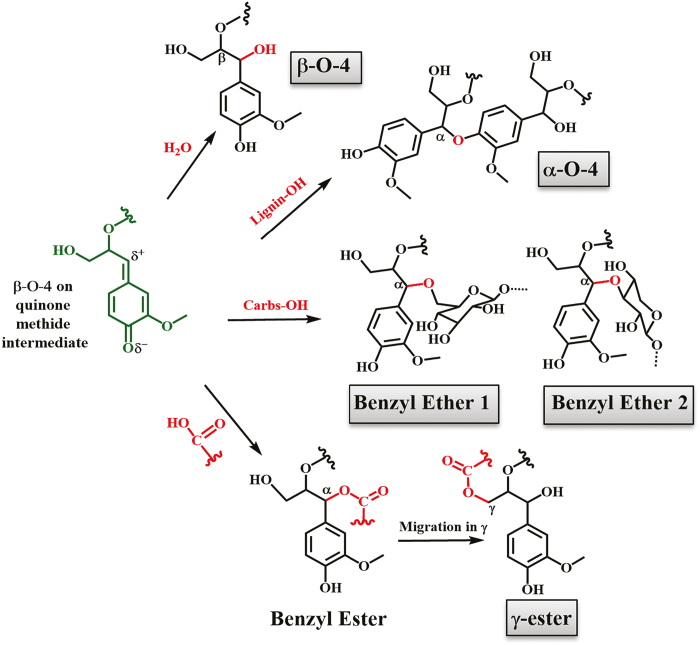
The formation of benzyl ethers or benzyl esters is not straightforward due to the presence of water, a nucleophile abundant in the plant cell walls especially at the beginning of lignification, very capable of performing nucleophilic attack on quinone methides. Accordingly, the product of water addition is α-hydroxylated β-O-4 structures in lignin (Ralph et al., 2004). However, detection of LCCs of benzyl ether- and γ-ester types in wood isolates by 2D NMR was first reported a while ago (Balakshin et al., 2001, 2007; Evtuguin et al., 2005) (γ-esters; Fig. 1), with the γ-ester suggested to result from migration of the ester from the benzylic position (Balakshin et al., 2001, 2007; Evtuguin et al., 2005). The question remains of whether this indeed occurs in the presence of water.
Fig. 1.
Suggested formation mechanisms of α-hydroxylated β-O-4, benzyl ether, and ester bonds in native LCCs due to nucleophilic attacks in the electrophilic site (α) of β-O-4 subunits. Carbs-OH, carbohydrate -OH. (This figure is available in color at JXB online.)
The formation of lignin–carbohydrate linkages in the aqueous environment of the plant cell wall is the focus of our studies. The local concentration of water near the growing lignin chain may be low due to lignin hydrophobicity. Indeed, the literature suggests that hemicellulose or pectin is in close contact with lignin during polymerization in aqueous systems (Cathala and Monties, 2001; Cathala et al., 2001; Barakat et al., 2007; Li et al., 2015). Such studies present a plausible explanation for how lignin–carbohydrate bonds may form in aqueous systems, and require further attention.
In this work, we investigated these questions by biomimicking LCC formation in vivo by using an extracellular-lignin-forming cell culture of Norway spruce (Simola et al., 1992; Kärkönen et al., 2002; Laitinen et al., 2017) as a source of lignin, and in vitro by producing dehydration polymers (DHPs) in the presence of hemicellulose (xylan; Barakat et al., 2007; Li et al., 2015) and galacturonic acid, a constituent of pectin. Our focus was to specifically demonstrate whether certain types of LCC linkages could occur in vivo and/or in vitro or just be created exclusively during extraction/isolation procedures, such as ball milling.
Materials and methods
All chemicals used were of analytical grade and purchased from Sigma-Aldrich.
Tissue-culturing and treatment of extracellular lignin
Norway spruce (Picea abies L. Karst.) cells were maintained as a callus culture on solid nutrient medium and transferred to liquid cultures for lignin formation (Kärkönen et al., 2002; Koutaniemi et al., 2005). The cultures were incubated at 20 °C on a platform shaker (100 rpm, in 16 h light/8 h dark, Osram warm white, 20–50 µmol m−2 s−1). The culture medium was collected when extracellular lignin (ECL) was clearly visible in the culture medium (~5–15 d after transfer of cells into liquid conditions). Cells were removed by filtering through Miracloth, and ECL was pelleted by centrifugation. ECL was washed with water to remove soluble compounds. Lignin-bound proteins were removed by extracting the ECL with buffered 1 M NaCl as described in Warinowski et al. (2016), after which the ECL was washed with water and lyophilized.
Synthetic lignin (DHP) synthesis
Synthesis of lignin (DHP) was performed as reported elsewhere (Warinowski et al., 2016), with horseradish peroxidase (HRP) type VI [1 mg, 4.16×10−6–5.5×10−6 kat coniferyl alcohol (CA)-oxidizing activity]. Both hydrogen peroxide (H2O2; 34 mM, 20 ml in water) and CA (prepared according to Lu and Ralph, 1998; Kim and Ralph, 2005) (34 mM, 20 ml in 50% acetone) were simultaneously injected, at a constant rate (250 μl h−1) using an NE-1800 eight channel programmable syringe pump, into 50% aqueous acetone solution of beech glucuronoxylan [10% (w/v), Sigma-Aldrich] or galacturonic acid (0.2 M, Sigma-Aldrich). The pH (between 5.5 and 6) was adjusted, in the latter case, to 6.5–7 before addition of HRP to increase the concentration of carboxylate anion (pKa ~4). As a control, DHP without any carbohydrate supplementation was produced (CA-DHP). Polymerization was carried for 20 h under slow stirring (150 rpm) at room temperature. After injection, the reaction was allowed to proceed for an additional 4 h. Synthetic lignins (DHPs) were collected by centrifugation (2000 rpm, 30 min) after removal of acetone under reduced pressure, washed twice with water, and lyophilized.
Carbohydrate and lignin compositional analyses
The sugar composition of the ECL was determined after acidic methanolysis (Bertaud et al., 2002; Appeldoorn et al., 2010) incubating 1 mg of lyophilized ECL with 1 ml of 2 M HCl in dry methanol for 5 h at 100 °C. Samples were then neutralized with pyridine, dried under a stream of air, and further hydrolyzed with 2 M trifluoroacetic acid (TFA) at 120 °C for 1 h. The samples were again dried under a stream of air and dissolved in water. The monosaccharides were analyzed using high-performance anion exchange chromatography with pulsed amperometric detection (HPAEC-PAD) with the ICS-3000 system (Dionex) equipped with a CarboPac PA1 column (4×250 mm, Dionex), as previously reported (McKee et al., 2016).
The lignin content in the ECL preparation was analyzed by Klason lignin analysis as described by Effland (1977), together with acid-soluble lignin (TAPPI, 1991). The former was determined gravimetrically after acid hydrolysis of the ECL, whereas the latter was characterized by UV spectroscopy of the hydrolysate at 205 nm using an absorptivity of 128 l cm−1 g−1. All experiments were carried out in duplicate.
Size exclusion chromatography
Molecular weight distributions and polydispersity of the lignin samples were investigated by size exclusion chromatography (SEC) dissolving ~5 mg of lyophilized samples in 2 ml of DMSO+0.5% LiBr (w/w) solution. After filtration of the samples through 0.45 µm PTFE filters, analysis was performed with SEC 1260 Infinity (Polymer Standard Services, Germany). The equipment consisted of an isocratic pump (G1310B), a micro degasser (G1379B), and a standard autosampler (G1329B). The detection system included a UV detector (G1314B) in series with a refractive index detector (G1362A). The mobile phase was DMSO+0.5% LiBr set to a constant flow rate of 0.5 ml min–1 for a total run time of 65 min. The injection volume was 100 μl. The separation system consisted of a PSS GRAM Precolumn, and PSS GRAM 100 Å and PSS GRAM 10 000 Å analytical columns thermostated at 60 °C and connected in series. For standard calibration, pullulan standards with nominal masses of 708, 337, 194, 47.1, 21.1, 9.6, 6.1, 1.08, and 0.342 kDa were used.
NMR analysis
Quantitative 31P-NMR analysis was performed as reported before (Granata and Argyropoulos, 1995). In brief, an accurately weighed amount of lignin (~30 mg) was phosphorylated using 2-chloro-4,4,5,5-tetramethyl-1,3,2-dioxaphospholane (Cl-TMDP). All chemical shifts reported are relative to the reaction product of water with Cl-TMDP, which gives a sharp signal in dimethylformamide/pyridine/CDCl3 (vratio: 1/1/4) at 132.2 ppm.
For HSQC-NMR, a 10–15% sample solution was prepared in deuterated DMSO-d6. The experiments were carried out with a Bruker pulse program ‘hsqcetgpsi’, and spectra were acquired with the following parameters: size of flame ionization detector 1024, pulse 9.2 mm, number of dummy scans 16, spectral width 13 ppm, and a relaxation delay of 1.5 s. The number of scans was set to 120 which led to a run time of 14 h. The unsubstituted carbon 2 of aromatic groups was used as an internal standard for quantifications as described elsewhere (Sette et al., 2011). The central DMSO (δ C/δ H=39.5/2.5 ppm) was used as an internal reference.
On CA-DHP, the original method by Zhang and Gellerstedt (2007) adopted by Balakshin et al. (2011) for LCC studies, which applies to both 13C and 2D HSQC analyses, was used. The 13C-NMR experiments were carried out with the Bruker pulse program ‘zgig’ with 90° pulse width using an acquisition time of 1.4 s and a relaxation delay of 1.7 s. Chromium(III) acetyl acetonate (2 mg) was added to the DHP solution to provide complete relaxation of all nuclei. A total of 24 000 scans were collected.
A spectrum of the ECL was acquired at 25 °C using a Shigemi NMR microtube. Quantitative 13C-NMR spectra were acquired at a sample concentration of 30% in DMSO-d6 on a Bruker AVANCE 500 MHz spectrometer equipped with a 5 mm QNP probe using an inverse-gated proton-decoupling sequence under the conditions described earlier (Capanema et al., 2004). Chromium(III) acetyl acetonate (0.016 M) was added to the NMR tube prior to quantitative 13C-NMR acquisition to provide complete relaxation of all nuclei. Acquisition parameters included a 90° pulse width, a relaxation delay of 1.7 s, and an acquisition time of 1.2 s. A total of 20 000 scans were collected.
2D HSQC-NMR spectra were acquired at a sample concentration of ~10% on a Bruker Avance III 950 MHz spectrometer equipped with a cryo-platform and a Bruker 5 mm ID CPTCI (1H/13C/15N/D) cryo-probe with an Z-Axis Gradient spectrometer. The acquisition parameters were: 24 transient scans per block were acquired using 2000 data points in the F2 (1H) dimension for an acquisition time of 72 ms and 512 data points in the F1 (13C) dimension for an acquisition time of 5.36 ms and for a total experiment time of 4 h 20 min. The 2D data set was processed with 2000×2000 data points using Qsine function in both dimensions.
Results and discussion
We aimed to circumvent the relatively harsh conditions used to isolate LCCs from plant materials in order to determine if they could possibly form during lignin biosynthesis at cell wall proximate conditions. The first strategy we adopted was to analyze ECL formed by tissue-cultured Norway spruce cells in vivo. The second approach was a modified version of the classical production of synthetic lignin in vitro, also referred to commonly as DHPs. In the modification, the cell wall polysaccharide glucuronoxylan was included in the synthesis. An additional experiment was done with inclusion of galacturonic acid monomers, building blocks of pectin, for reasons discussed later. The aim was to mimic the mechanisms of formation of native LCCs in the cell wall. Both of the adopted strategies produced lignins which were easy to isolate. These were recovered through simple centrifugation, washed with water, and freeze-dried.
In terms of structural analysis, synthetic lignins, so-called DHPs, differ from native or isolated lignins, such as milled wood lignin (MWL), by a lower content of β-O-4 bonds. In DHP lignins, dehydrodimerization reactions of CA, resulting from coupling of at least one of the monolignols at its β-position, are over-represented due to the fact that monolignol radicals preferentially couple with each other rather than cross-couple with dimers or oligomers (Ralph, 2004). The resulting synthetic lignin is, therefore, a condensed polymer with low β-O-4 content.
Previous studies on lignin interunit linkage of ECL have shown that the relative composition of interunit linkages is intermediate to that in MWL and in DHPs (Koutaniemi et al., 2005; Warinowski et al., 2016). The main contributors making ECL, rather than DHP, more similar to MWL were both the relatively high content of β-O-4 and the noticeable presence of dibenzodioxocin structures (Warinowski et al., 2016) which cannot arise from monolignol dehydrodimerization reactions (Ralph et al., 2004).
However, DHPs have been used for fundamental studies aimed at understanding mechanisms of lignification (Terashima et al., 1996; Cathala and Monties, 2001; Cathala et al., 2001; Kärkönen et al., 2002; Ralph et al., 2004; Koutaniemi et al., 2005; Li et al., 2015; Warinoski et al., 2016; Tobimatsu and Schuetz, 2019).
Mechanisms for the formation of the lignin–carbohydrate ethers and esters have been proposed (Fig. 1) but, to our knowledge, not experimentally shown.
Investigating if LCCs form at cell wall proximate conditions
Analysis of ECL
Unlike extracted wood lignins, the ECL released by the suspension-cultured cells of Norway spruce (Simola et al., 1992; Kärkönen et al., 2002; Koutaniemi et al., 2005; Warinowski et al., 2016) can be isolated in an unchanged state. The obvious benefit is overcoming any kind of extraction under harsh conditions, since ECL can be isolated from the culture medium just by centrifugation, and washed with water. The spruce cell culture, originating from 1985, has been used to study the structure and biosynthesis of lignin (Simola et al., 1992; Brunow et al., 1993, 1998; Kärkönen et al., 2002, 2014; Koutaniemi et al., 2005, 2007, 2015; Warinowski et al., 2016; Laitinen et al., 2017). The NMR analyses conducted revealed that the structure of ECL, with relatively higher levels of β-O-4 and dibenzodioxocin than in DHPs, is quite similar to MWL as a sign that polymerization occurred by the coupling of monolignol radicals to a growing polymer by so-called ‘end-wise’ polymerization (Ämmälahti and Brunow, 2000; Koutaniemi et al., 2015; Warinowski et al., 2016). In the present analysis, molecular weight distribution showed that the degree of polymerization of the soluble fraction of ECL in DMSO was 7–8 (mol. wt=1500 Da) with quite a narrow distribution (Đ M=1.4) as shown in Supplementary Fig. S1 at JXB online).
ECL was shown to contain carbohydrates (~17% w/w), especially pectic sugars (Warinowski et al., 2016), making this material an excellent substrate to evaluate lignin–carbohydrate bonds. We have investigated the presence of LCCs in the ECL. Carbohydrate analysis indicated the presence of various sugars being bound to ECL, with arabinose, galactose, and galacturonic acid especially abundant (Table 1), giving an indirect indication of interactions between lignin and pectin, which contains all the named sugars. Xylose, mannose, and glucose were also present, although in small amounts (Table 1). The dominance of arabinose, galactose, and galacturonic acid is consistent with studies that showed that these sugars were associated with lignin formed at early stages of plant development (Balakshin et al., 2007; Rencoret et al., 2011). ECL therefore seems to mimic the early-deposited lignins in the cell wall.
Table 1.
Sugar unit composition and lignin content of ECL
| Monosaccharide content (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Fuc | Ara | Rha | Gal | Glc | Xyl | Man | GalA | GlcA |
| 0.7±0.1 | 46.6±1.1 | 1.5±0.2 | 31.1±0.9 | 4.7±0.1 | 0.9±0.1 | 2.1±0.1 | 10.2±0.5 | 2.2±0.3 |
| Lignin content (%) | ||||||||
| Klason | ASL | |||||||
| 68.7±0.8 | 1.8±0.2 |
The values are expressed as relative % (w/w). Sugar composition was analyzed by HPAEC-PAD after acid methanolysis followed by TFA hydrolysis. ASL, acid-soluble lignin.
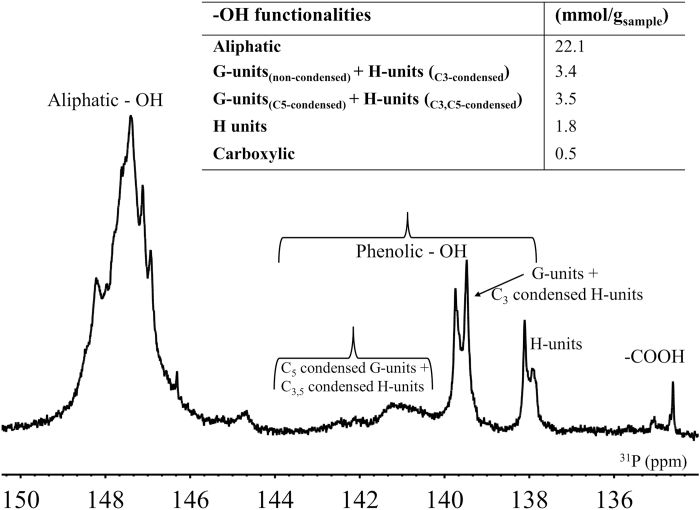
Another unique feature for the ECL, unveiled by both HSQC- and 31P-NMR, was the relatively high (20%) content of para-hydroxyphenyl hydroxyphenyl (H) units (Fig. 2), which has also been noted before (Brunow et al., 1993; Viljamaa et al., 2018). H units were considerably more abundant than in MWL preparations, which consist predominantly of guaiacyl units (G units) with trace amounts of H units (Capanema et al., 2004). An increased proportion of H units has also been reported for lignin formed at early stages of lignification (Whiting and Goring, 1982; Terashima and Fukushima, 1988; Rencoret et al., 2011), and in lignins formed under stress, such as in compression wood (Timell, 1982), further reinforcing the argument for ECL mimicking the early-deposited lignin in plant cell walls. In addition to the Norway spruce cell culture lignin used in the present experiment, other conifer cell culture lignins have been observed to have elevated H unit contents (Eberhardt et al., 1993; Lange et al., 1995).
Fig. 2.
31P NMR spectra of ECL and quantification of hydroxyl functionalities.
For the HSQC studies, solubility of ECL in DMSO-d6 was ~70%. HSQC analysis of interunit linkages showed the expected structural outcome with the dominance of β-O-4 structures (35% of C9 units; Table 2) and condensed structures with resinol (β-β) and phenylcoumaran (β-5) accounting together for 40% of all interunit linkages. Dibenzodioxin (DBDO) structures were also present.
Table 2.
Percentage of lignin interunit linkages and LCCs detected by NMR in the extracellular lignin (ECL), in the synthetic lignin produced with horseradish peroxidase (CA-DHP), and in DHPs produced with HRP in the presence of galacturonic acid (GalA-DHP) or methylglucuronoxylan (10%, Xylan10-DHP)
| Lignin interunit linkages (relative % of C9 units) | ECL | CA- DHP | CA-DHPa | GalA- DHP | Xylan10- DHP | Water phase |
|---|---|---|---|---|---|---|
| β-O-4 | 35 | 32 | 30 | 11 | 15 | 9 |
| β-5 | 21 | 24 | 20 | 35 | 19 | 10 |
| β-β | 20 | 18 | 16.4 | 25 | 20 | 7 |
| DBDO | 4 | 2 | 2.6 | – | 4 | – |
| SD | 2 | 2 | 2 | – | – | – |
| CA b | 15 | 20 | 26 | 27 | 20 | 50 |
| LCC | ||||||
| BE 1 | (4.5) | – | – | – | – | – |
| BE 2 | 2 | – | – | – | – | 1 |
| Best | – | – | – | 3 | – | 1 |
| GE b | – | – | – | – | – | 3 |
| PG | D | – | – | – | – | – |
Water phase: supernatant of the Xylan10-DHP production mixture. Abbreviations used are given in Fig. 3
–, not detected; D, detected but not quantified, (), γ-O-4 structures.
a Quantified with 13C NMR-HSQC.
b Very relative values due to the difference in response factor for γ-CH2 versus aromatic 13C/1H,
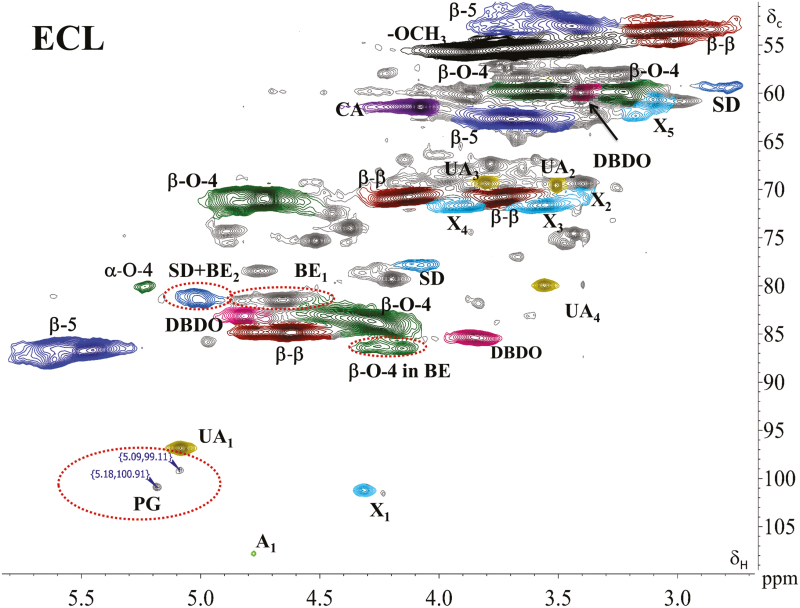
When focused to lignin–carbohydrate linkages in the ECL, the spectra showed, in addition to typical lignin substructures (Fig. 3), the existence of small sugar signals assigned to arabinoglucuronoxylan (Fig. 4). The carbohydrate signals have also been detected in studies on MWL (Lundquist et al., 1977; Björkman, 1956, 1957; Balakshin et al., 2011). Interestingly, absence of the signals assigned to galactose and galacturonic acid, sugars abundant in pectins, was striking, since these were among the most abundant sugar units in the ECL (Table 1). Furthermore, primary alcohol-based benzyl ethers (BE1) (δ C/δ H=80.1–81.2/4.21–4.68 ppm) were detected in line with previous works (Toikka et al., 1998; Toikka and Brunow, 1999). These seemed, however, to be mainly lignin–lignin linkages (αOAlk), since the amount of C6 sugars that would contribute to these was small, or were absent. A small part of this signal could, however, be due to benzyl ether linkage to C5 in arabinose, since weak signals from arabinose were observed. Benzyl ethers to a secondary alcohol (BE2), presumably due to C2 or C3 hydroxyls of xylose in arabinoglucuronoxylan, unfortunately overlapped with spirodienone structures in lignin, if present. The amount of spirodienone structures only can be quantified from the signal of CH-β at 79.4/4.11 ppm (Capanema et al., 2005; Zhang et al., 2006; Balakshin et al., 2011); the former was a factor of two higher, strongly suggesting the presence of the benzyl ether (BE2) linkage with xylan. In addition, phenyl glycosides were detected, consistent with recent studies (Miyagawa et al., 2014; Rencoret et al., 2019) (δ C/δ H=99–101/5.1–5.2; Fig. 4), suggesting a phenyl glycoside linkage towards glucose.
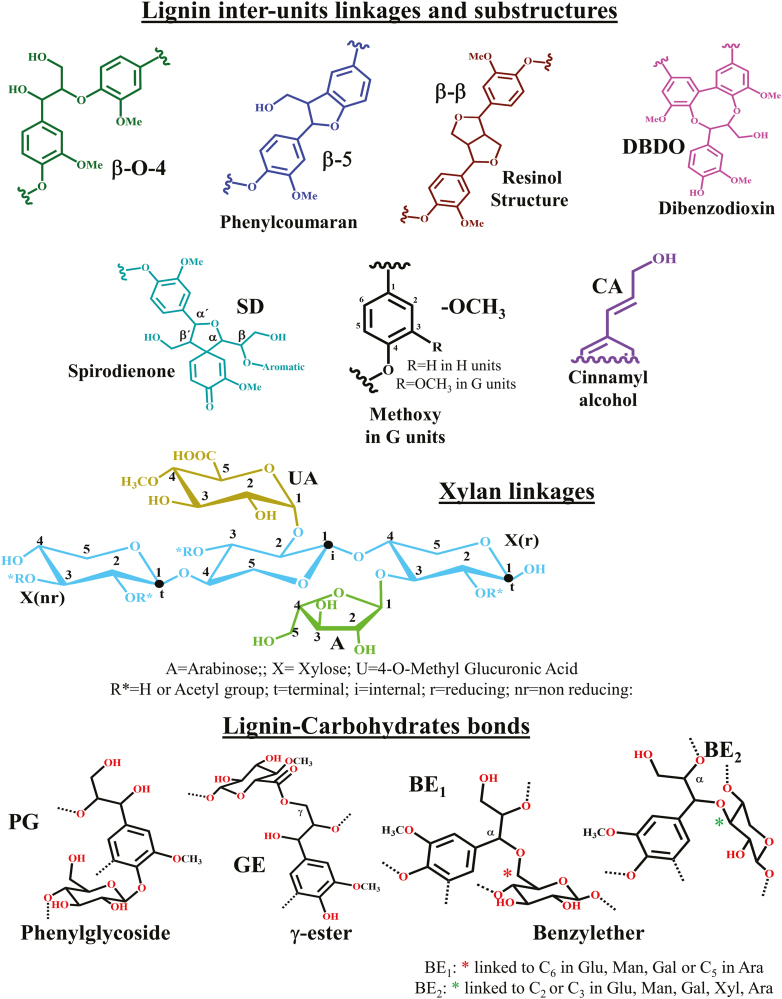
Fig. 3.
Main interunit linkages and end groups in lignin, xylan linkages, and lignin–carbohydrate bonds identified in the 2D HSQC-NMR spectra shown in Figs 5 and 6.
Fig. 4.
HSQC spectrum in DMSO-d6 of ECL. Color and abbreviations used are given in Fig. 3.
From the ECL studies conducted here, therefore, the presence of LCC benzyl ethers and phenyl glycosides was corroborated by the presence of signals in the spectra from xylose and arabinose. Interestingly, the carbohydrate composition of the original ECL sample (Table 1) was not reflected in the spectra (Fig. 4). The spectra suggest the presence of xylose with the bulk of the ECL that was soluble in the NMR solvents used. These data also suggest that the insoluble fraction, which accounted for ~30% of the ECL, contained pectic substances detected in the methanolysis analysis (Table 1). As pectins are largely constituted of galacturonic acid, it is likely that lignin-carbohydrate esters, if present, were enriched in the fraction that could not be analyzed due to the solubility issues.
Interestingly, the signals from 4-O-methylglucuronic acid groups were relatively stronger in ECL (Fig. 4) than those observed in wood-extracted xylans (Fig. 5c, d; Supplementary Fig. S2). However, the quantification of various carbohydrate signals from the HSQC spectra is inaccurate due to different response factors of different 13C/1H carbohydrates and, therefore, their quantitative comparison is very challenging. In the context of lignin–carbohydrate bond types, it could be argued that the detected phenyl glycosides are due to non-LCC metabolites naturally present in the cell cultures. However, coniferin β-glucosidase activity has been detected in the culture medium of spruce cells, whereas no coniferin was observed to be present (Kärkönen et al., 2002). More recently, the same group detected several types of glycosylated oligolignols in the culture medium of both lignin-forming and non-lignin-forming cultures (Laitinen et al., 2017). According to the earlier analysis, coniferin was not detected in the medium, but was detected intracellularly in cells that did not produce extracellular lignin due to H2O2 scavenging. Thus, although the extracellular lignin had been thoroughly washed with water before the present NMR analysis, it is possible that small signals assigned to phenyl glycoside could have originated from trace amounts of the low molecular weight metabolites still present in the samples. Alternatively, incorporation of such oligolignol glucosides into ECL may occur through nucleophilic attack to quinone methide intermediates during ECL polymerization. Sugar glycosides, however, normally resonate at a higher proton field (<4.6 ppm) which is not in the area we have assigned to the phenyl glycosides. Glycosides to uronic acids, on the other hand, appear in a lower field on proton (>4.8 ppm) but a higher field on carbon (<100 ppm), which also lie outside our assignment. Furthermore, the absence of any signals related to pectin rules out the possibility that the cross-peak assigned to the anomeric peak in phenyl glycoside overlaps with, for instance, the internal anomeric signals between galacturonic acid and rhamnose appearing at 99.8/5.02 ppm (Balakshin et al., 2007, 2011).
Fig. 5.
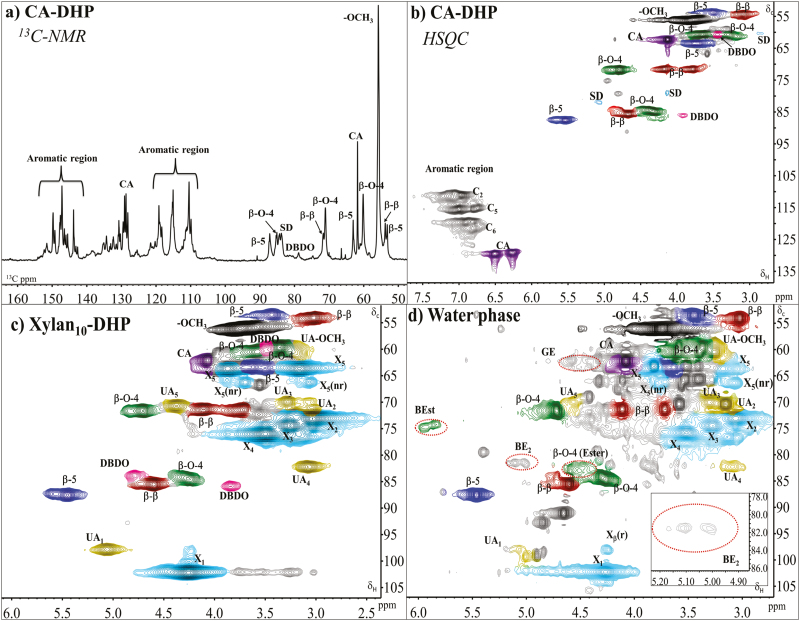
Expanded (a) 13C spectrum and (b) HSQC spectrum of the CA-DHP. HSQC spectrum of (c) DHP produced in the presence of 10% methylglucuronoxylan (Xylan10-DHP), and (d) supernatant of the Xylan10-DHP production mixture. All spectra were run in DMSO-d6. Presence and absence of characteristic LCCs are marked by red, dotted circles. Colors and the abbreviations used are given in Fig. 3.
The chemical shift values for phenyl glycosides have very recently obtained additional support by a comprehensive study of Rencoret et al. (2019) which has confirmed the HSQC assignments with an HMBC technique. The authors reported incorporation of hydroxystilbene glucosides into lignin of Norway spruce bark.
Modified dehydrogenation polymer (DHP) synthesis
Due to the lack of detection of lignin–carbohydrate esters in the ECL studies described above, further investigations were required in order to determine if they could form under other conditions. The working hypothesis was that if the esters were present in the ECL, they possibly escaped detection due to their low concentration in the NMR solvent-soluble polymer. Considering the low concentration of xylan in the DMSO-soluble ECL, synthesizing lignin (DHPs) in the presence of galacturonic acid or glucuronoxylan to increase possible formation of ester linkages to galacturonic acid/glucuronic acid was worth investigating.
First, DHP was synthesized from CA in 50% acetone using commercial HRP and H2O2 (CA-DHP; Fig. 5a, b). For the analysis, two NMR quantification methods for the common lignin interunit bonds were applied (Table 2). In the 13C NMR-HSQC method described by Zhang and Gellerstedt (2007), a cluster quantification of signals with similar T2-relaxation 13C-NMR spectra is performed using the aromatic carbon signals as an internal reference. The quantitative values are then applied to HSQC, where the peaks are better resolved, to allow more specific interunit quantitation. The second method utilizes only HSQC using the C2 of aromatics as internal references based on an earlier observation that the signal of G-2 CH in softwood native lignins (or G2+S2,6/2 in hardwood ones) is close to 100/Ar (Zhang and Gellerstedt, 2007; Balakshin and Capanema, 2015). Although the second method is less precise and allows only relative comparison for CH2 moieties (such as CA and γ-esters), it was used for the other DHP samples because of the shorter NMR running time.
We next investigated the formation of esters by including a conjugate base of a simple acid—the sodium salt of galacturonic acid—to the DHP production mixture (GalA-DHP). This strategy, adopted to use the conjugate base instead of the acid, was to increase the concentration of the carboxylate nucleophile, so that esters, if formed, would be detectable. In the presence of galacturonic acid, formation of a benzyl ester was observed (Supplementary Fig. S3), evidenced in CαHα correlation in α-esterified β-O-4 appearing at 74.3/5.96 ppm and corresponding CβHβ at 82.5/4.6 ppm [β-O-4(Ester) peak]. No γ-ester was detected, indicating that uronosyl migration (see Fig. 1) did not occur.
From the analyses of ECL (Fig. 4) and DHP produced in the presence of galacturonic acid (GalA-DHP; Supplementary Fig. S3), clear evidence of benzyl ethers was still lacking, since the BE2 signals, if present, were overlapping with the signal of CH-α' at 81.7/5.09 in spirodienone and BE1 with lignin–lignin ethers (Balakshin et al., 2011). One way to solve the first inconvenience was to synthesize lignin at a high concentration of xylan, in order to increase the probability of BE2 formation, and then compare the volume integrals of the two signals of spirodienone (Cα'/Hα' and Cβ/Hβ at 81.7/5.09 and at 79.4/4.11 ppm, respectively). Thus, DHP was produced in a 10% solution of polymeric beech methylglucuronoxylan. Both the precipitate (Xylan10-DHP) and the supernatant were collected, analyzed by HSQC, and the signals compared with those in the references, namely CA-DHP (Fig. 5b) and commercial methylglucuronoxylan (Supplementary Fig. S2).
In the Xylan10-DHP (Fig. 5c), no evidence of LCC was found, suggesting either that the high molar mass fraction of the xylan and the DHP co-precipitated due to physical interactions, or that lignin–carbohydrate bonds were below the detection limit. Interestingly, the amount of DBDO interunit linkages detected in the Xylan10-DHP produced with high (~5 µkat) CA-oxidizing activity of HRP were at the same level as those in the ECL (Table 2). These interunit linkages were considered to form with a high oxidation rate of the polymer (Warinowski et al., 2016). The observation that DHP produced in the presence of xylans contains elevated levels of DBDO is of interest, since the reference DHPs produced with the same enzyme activity, but without carbohydrate supplementation, contained fewer of these units (Table 2). Due to water solubility of the xylan, ~90–95% of the xylan ended up in the water phase (supernatant; Fig. 5d), together with the unreacted CA (giving a relative value for 50% of all aromatic signals; Table 2). HSQC analysis of this fraction also revealed the presence of some dimeric/oligomeric lignin fractions (mol. wt ~300 Da, by SEC analysis), with interunit linkages consisting of phenylcoumaran, resinol, and β-O-4 structures, all present at roughly the same level (~10%).
Interestingly, both benzyl esters (δ C/δ H=74.3/5.96 ppm) and γ-esters (δ C/δ H=62.3/4.58 ppm) (Li and Helm, 1995) were detected in the water phase, with the latter markedly more abundant, at 3% relative to lignin interunit linkages (Table 2).
Native acyl groups in lignin (e.g. ferulate, p-coumarate, and benzoate) (Ralph et al., 2004) as well as acetyl on the carbohydrate backbone could overlap in the same region of γ-esters (δ C/δ H: 62–65/4.0–4.5 ppm) (Balakshin et al., 2011). However, this was not the case for the analyzed substrate (supernatant of Xylan10-DHP) where only CA and deacetylated beech methylglucuronoxylan (Supplementary Fig. S2) were adopted. Furthermore, the presence of Cβ/Hβ cross-signal at δ C/δ H: 82.5/4.6 ppm [β-O-4(Ester); Fig. 5d] which is a diagnostic peak of the presence of ester in the γ-position of the β-O-4 lignin structure, is further evidence of the presence of γ-ester. In addition, in a similar work where the DHP approach was adopted as a tool to selectively functionalize lignin, the cross-peak assignment of benzyl ester (δ C/δ H=74.3/5.96 ppm; Fig. 5d) has been thoroughly demonstrated by HMBC and HSQC-TOCSY, showing multiple bond correlations both within the same spin system and between aromatic and 13C/1H carbohydrates (Giummarella et al., 2018).
In addition, BE2s to xylan were detected (Fig. 5d, and the expanded region in the bottom right corner). This conclusion was reached due to the absence of signals assigned to spirodienone from Cβ'/Hβ' at 59.6/2.80 ppm and the signal of CβHβ at 79.4/4.11 ppm (see Fig. 1 for difference between β and β' in the spirodieneone structure) meaning that the signal at 81.6/5.02 ppm where Cα/Hα should be is actually the BE2 in xylan. In softwood arabinoglucuronoxylan, formation of BE1 via primary alcohol in arabinose has been detected (Balakshin et al., 2011). However, in the case of beech xylan where arabinose is absent, the only possibilities are the BE2 type.
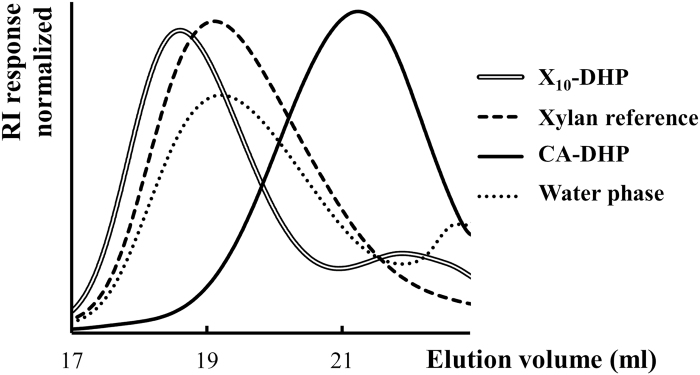
A molecular weight analysis by SEC (Fig. 6) showed a dramatic decrease in the elution volume, corresponding to a higher hydrodynamic volume of both DHP produced in the presence of 10% methylglucuronoxylan and the supernatant of the Xylan10-DHP experiment as compared with the DHP produced without hemicellulose supplementation (CA-DHP; Fig. 6). The results support that xylan was attached to DHP, resulting in a higher molar mass when compared with DHP produced without any hemicellulose supplementation.
Fig. 6.
SEC chromatograms in DMSO+0.5% LiBr of Xylan10-DHP and its water phase (supernatant) fractions compared with both DHP produced without hemicellulose supplementation (CA-DHP) and beech xylan used as a reference.
Conclusion
In the present work, we investigated the possibilities of lignin–carbohydrate bond formation at the proximate cell wall conditions. ECL lignin from the Norway spruce cell culture was used as one research material. In addition, synthetic lignins, classically referred to as DHPs, were modified by inclusion of galacturonic acid or water-soluble xylan in the synthesis step, to form derivatives of synthetic lignins (GalA-DHP and Xylan10-DHP, respectively). Altogether, four types of lignin–carbohydrate bonds, namely benzyl ethers, benzyl esters, γ-esters, and phenyl glycosides, were detected. Thus, experimental evidence through direct linkage studies by HSQC-NMR showed possibilities of lignin–carbohydrate bonds being formed in the plant cell walls rather than formed during the isolation procedure. Results from SEC studies further support the above conclusion.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Analysis by size exclusion chromatography (SEC) in DMSO+0.5% LiBr of Norway spruce extracellular lignin (ECL).
Fig. S2. Expanded HSQC spectrum of beech methylglucuronoxylan.
Fig. S3. Expanded HSQC spectrum of DHP produced with HRP in the presence of galacturonate (GalA-DHP).
Acknowledgements
This work was supported by the Knut and Alice Wallenberg’s research foundation within the Wallenberg Wood Science Centre. AK was funded by the Academy of Finland (grant 251390).
References
- Ämmälahti E, Brunow G. 2000. Use of β-13 C labelled coniferyl alcohol to detect ‘End-Wise’ polymerization in the formation of DHPs. Holzforschung 54, 604–608. [Google Scholar]
- Appeldoorn MM, Kabel MA, Van Eylen D, Gruppen H, Schols HA. 2010. Characterization of oligomeric xylan structures from corn fiber resistant to pretreatment and simultaneous saccharification and fermentation. Journal of Agricultural and Food Chemistry 58, 11294–11301. [DOI] [PubMed] [Google Scholar]
- Balakshin MY, Capanema EA. 2015. Comprehensive structural analysis of biorefinery lignins with a quantitative 13 C NMR approach. RSC Advances 5, 87187–87199. [Google Scholar]
- Balakshin MY, Capanema E, Berlin A. 2014. Isolation and analysis of lignin–carbohydrate complexes preparations with traditional and advanced methods: a review. In: Atta-ur-Rahman, ed. Studies in natural products chemistry, Vol. 42 Amsterdam: Elsevier, 83–115. [Google Scholar]
- Balakshin MY, Capanema EA, Chang HM. 2007. MWL fraction with a high concentration of lignin–carbohydrate linkages: isolation and 2D NMR spectroscopic analysis. Holzforschung 61, 1–7. [Google Scholar]
- Balakshin M, Capanema E, Gracz H, Chang HM, Jameel H. 2011. Quantification of lignin–carbohydrate linkages with high-resolution NMR spectroscopy. Planta 233, 1097–1110. [DOI] [PubMed] [Google Scholar]
- Balakshin MY, Evtuguin DV, Pascoal Neto C, Silva AMS, Domingues PM, Amado FML. 2001. Studies on lignin and lignin–carbohydrate complex by application of advanced spectroscopic techniques. In: Proceedings of the 11th ISWP conference. Nice, 11–14. [Google Scholar]
- Barakat A, Winter H, Rondeau-Mouro C, Saake B, Chabbert B, Cathala B. 2007. Studies of xylan interactions and cross-linking to synthetic lignins formed by bulk and end-wise polymerization: a model study of lignin carbohydrate complex formation. Planta 226, 267–281. [DOI] [PubMed] [Google Scholar]
- Bertaud F, Sundberg A, Holmbom B. 2002. Evaluation of acid methanolysis for analysis of wood hemicelluloses and pectins. Carbohydrate Polymers 48, 319–324. [Google Scholar]
- Björkman A. 1956. Studies on finely divided wood. Part 1. Extraction of lignin with neutral solvents. Svensk Papperstidning 59, 477–485. [Google Scholar]
- Björkman A. 1957. Studies on finely divided wood. Part 3. Extraction of lignin–carbohydrate complexes with neutral solvents. Svensk Papperstidning 60, 243–251. [Google Scholar]
- Brunow G, Ämmälahti E, Niemi T, Sipilä J, Simola LK, Kilpeläinen I. 1998. Labelling of a lignin from suspension cultures of Picea abies. Phytochemistry 47, 1495–1500. [Google Scholar]
- Brunow G, Kilpeläinen I, Lapierre C, Lundquist K, Simola LK, Lemmetyinen J. 1993. The chemical structure of extracellular lignin released by cultures of Picea abies. Phytochemistry 32, 845–850. [Google Scholar]
- Capanema EA, Balakshin MY, Kadla JF. 2004. A comprehensive approach for quantitative lignin characterization by NMR spectroscopy. Journal of Agricultural and Food Chemistry 52, 1850–1860. [DOI] [PubMed] [Google Scholar]
- Capanema EA, Balakshin MY, Kadla JF. 2005. Quantitative characterization of a hardwood milled wood lignin by nuclear magnetic resonance spectroscopy. Journal of Agricultural and Food Chemistry 53, 9639–9649. [DOI] [PubMed] [Google Scholar]
- Cathala B, Chabbert B, Joly C, Dole P, Monties B. 2001. Synthesis, characterisation and water sorption properties of pectin-dehydrogenation polymer (lignin model compound) complex. Phytochemistry 56, 195–202. [DOI] [PubMed] [Google Scholar]
- Cathala B, Monties B. 2001. Influence of pectins on the solubility and the molar mass distribution of dehydrogenative polymers (DHPs, lignin model compounds). International Journal of Biological Macromolecules 29, 45–51. [DOI] [PubMed] [Google Scholar]
- Del Río JC, Prinsen P, Cadena EM, Martínez ÁT, Gutiérrez A, Rencoret J. 2016. Lignin–carbohydrate complexes from sisal (Agave sisalana) and abaca (Musa textilis): chemical composition and structural modifications during the isolation process. Planta 243, 1143–1158. [DOI] [PubMed] [Google Scholar]
- Du X, Gellerstedt G, Li J. 2013. Universal fractionation of lignin–carbohydrate complexes (LCCs) from lignocellulosic biomass: an example using spruce wood. The Plant Journal 74, 328–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhardt TL, Bernards MA, He L, Davin LB, Wooten JB, Lewis NG. 1993. Lignification in cell suspension cultures of Pinus taeda. In situ characterization of a gymnosperm lignin. Journal of Biological Chemistry 268, 21088–21096. [PubMed] [Google Scholar]
- Effland MJ. 1977. Modified procedure to determine acid-insoluble lignin in wood and pulp. Tappi 60, 143–144. [Google Scholar]
- Eriksson Ö, Lindgren BO. 1977. About the linkage between lignin and hemicelluloses in wood. Svensk Papperstidning 80, 59–63. [Google Scholar]
- Evtuguin DV, Goodfellow BJ, Pascole Neto C, Terashima N. 2005. Characterization of lignin–carbohydrate linkages in Eucalyptus globulus by 2D/3D NMR spectroscopy using specific carbon-13 labelling technique. In: Proceedings of 59th Appita Annual Conference and Exhibition: Incorporating the 13th ISWFPC. Auckland, 439–444. [Google Scholar]
- Felle HH. 2001. pH: signal and messenger in plant cells. Plant Biology 3, 577–591. [Google Scholar]
- Fengel D, Wegener G. 1989. Wood: chemistry, ultrastructure, reactions. Berlin: Walter de Gruyter, 167–174. [Google Scholar]
- Freudenberg K, Neish AC. 1968. Constitution and biosynthesis of lignin. Berlin-Heidelberg: Springer-Verlag, 116–122. [Google Scholar]
- Fry SC. 1995. Polysaccharide-modifying enzymes in the plant cell wall. Annual Review of Plant Biology 46, 497–520. [Google Scholar]
- Giummarella N, Gioia C, Lawoko M. 2018. A one-pot biomimetic synthesis of selectively functionalized lignins from monomers: a green functionalization platform. Green Chemistry 20, 2651–2662. [Google Scholar]
- Granata A, Argyropoulos DS. 1995. 2-Chloro-4,4,5,5-tetramethyl-1,3,2-dioxaphospholane, a reagent for the accurate determination of the uncondensed and condensed phenolic moieties in lignins. Journal of Agricultural and Food Chemistry 43, 1538–1544. [Google Scholar]
- Kärkönen A, Koutaniemi S, Mustonen M, Syrjänen K, Brunow G, Kilpeläinen I, Teeri TH, Simola LK. 2002. Lignification related enzymes in Picea abies suspension cultures. Physiologia Plantarum 114, 343–353. [DOI] [PubMed] [Google Scholar]
- Kärkönen A, Meisrimler CN, Takahashi J, et al. 2014. Isolation of cellular membranes from lignin-producing tissues of Norway spruce and analysis of redox enzymes. Physiologia Plantarum 152, 599–616. [DOI] [PubMed] [Google Scholar]
- Kim H, Ralph J. 2005. Simplified preparation of coniferyl and sinapyl alcohols. Journal of Agricultural and Food Chemistry 53, 3693–3695. [DOI] [PubMed] [Google Scholar]
- Koshijima T, Yaku F, Tanaka R. 1976. Fractionation of Björkman LCC from Pinus densiflora. Applied Polymer Symposia 28, 1025–1039. [Google Scholar]
- Koutaniemi S, Malmberg HA, Simola LK, Teeri TH, Kärkönen A. 2015. Norway spruce (Picea abies) laccases: characterization of a laccase in a lignin-forming tissue culture. Journal of Integrative Plant Biology 57, 341–348. [DOI] [PubMed] [Google Scholar]
- Koutaniemi S, Toikka MM, Kärkönen A, Mustonen M, Lundell T, Simola LK, Kilpeläinen IA, Teeri TH. 2005. Characterization of basic p-coumaryl and coniferyl alcohol oxidizing peroxidases from a lignin-forming Picea abies suspension culture. Plant Molecular Biology 58, 141–157. [DOI] [PubMed] [Google Scholar]
- Koutaniemi S, Warinowski T, Kärkönen A, et al. 2007. Expression profiling of the lignin biosynthetic pathway in Norway spruce using EST sequencing and real-time RT–PCR. Plant Molecular Biology 65, 311–328. [DOI] [PubMed] [Google Scholar]
- Laitinen T, Morreel K, Delhomme N, et al. 2017. A key role for apoplastic H2O2 in Norway spruce phenolic metabolism. Plant Physiology 174, 1449–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange BM, Lapierre C, Sandermann H Jr. 1995. Elicitor-induced spruce stress lignin (structural similarity to early developmental lignins). Plant Physiology 108, 1277–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawoko M, Henriksson G, Gellerstedt G. 2005. Structural differences between the lignin–carbohydrate complexes present in wood and in chemical pulps. Biomacromolecules 6, 3467–3473. [DOI] [PubMed] [Google Scholar]
- Li K, Helm RF. 1995. Synthesis and rearrangement reactions of ester-linked lignin–carbohydrate model compounds. Journal of Agricultural and Food Chemistry 43, 2098–2103. [Google Scholar]
- Li Q, Koda K, Yoshinaga A, Takabe K, Shimomura M, Hirai Y, Tamai Y, Uraki Y. 2015. Dehydrogenative polymerization of coniferyl alcohol in artificial polysaccharides matrices: effects of xylan on the polymerization. Journal of Agricultural and Food Chemistry 63, 4613–4620. [DOI] [PubMed] [Google Scholar]
- Lu F, Ralph J. 1998. Highly selective syntheses of coniferyl and sinapyl alcohols. Journal of Agricultural and Food Chemistry 46, 1794–1796. [DOI] [PubMed] [Google Scholar]
- Lundquist K, Ohlsson B, Simonson R. 1977. Isolation of lignin by means of liquid–liquid extraction. Svensk Papperstidning 80, 143–144. [Google Scholar]
- McKee LS, Sunner H, Anasontzis GE, Toriz G, Gatenholm P, Bulone V, Vilaplana F, Olsson L. 2016. A GH115 α-glucuronidase from Schizophyllum commune contributes to the synergistic enzymatic deconstruction of softwood glucuronoarabinoxylan. Biotechnology for Biofuels 9, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagawa Y, Mizukami T, Kamitakahara H, Takano T. 2014. Synthesis and fundamental HSQC NMR data of monolignol β-glycosides, dihydromonolignol β-glycosides and p-hydroxybenzaldehyde derivative β-glycosides for the analysis of phenyl glycoside type lignin–carbohydrate complexes (LCCs). Holzforschung, 68, 747–760. [Google Scholar]
- Nishimura H, Kamiya A, Nagata T, Katahira M, Watanabe T. 2018. Direct evidence for α ether linkage between lignin and carbohydrates in wood cell walls. Scientific Reports 8, 6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph J, Lundquist K, Brunow G, et al. 2004. Lignins: natural polymers from oxidative coupling of 4-hydroxyphenyl-propanoids. Phytochemistry Reviews 3, 29–60. [Google Scholar]
- Rencoret J, Gutiérrez A, Nieto L, Jiménez-Barbero J, Faulds CB, Kim H, Ralph J, Martínez AT, Del Río JC. 2011. Lignin composition and structure in young versus adult Eucalyptus globulus plants. Plant Physiology 155, 667–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rencoret J, Neiva D, Marques G, Gutiérrez A, Kim H, Gominho J, Pereira H, Ralph J, Del Río JC. 2019. Hydroxystilbene glucosides are incorporated into Norway spruce bark lignin. Plant Physiology 180, 1310–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkanen KV. 1971. Precursors and their polymerization. In: Sarkanen KV, Ludwing CH, eds. Lignins: occurrence, formation, structure, and reactions. New York: Wiley-Interscience, 95–163. [Google Scholar]
- Sette M, Wechselberger R, Crestini C. 2011. Elucidation of lignin structure by quantitative 2D NMR. Chemistry 17, 9529–9535. [DOI] [PubMed] [Google Scholar]
- Simmons TJ, Mohler KE, Holland C, Goubet F, Franková L, Houston DR, Hudson AD, Meulewaeter F, Fry SC. 2015. Hetero-trans-β-glucanase, an enzyme unique to Equisetum plants, functionalizes cellulose. The Plant Journal 83, 753–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simola LK, Lemmetyinen J, Santanen A. 1992. Lignin release and photomixotrophism in suspension cultures of Picea abies. Physiologia Plantarum 84, 374–379. [Google Scholar]
- TAPPI 1991. Acid-soluble lignin in wood and pulp, TAPPI UM 250. https://www.tappi.org/content/SARG/T222.pdf. [Google Scholar]
- Terashima N, Atalla RH, Ralph SA, Landucci LL, Lapierre C, Monties B. 1996. New preparations of lignin polymer models under conditions that approximate cell wall lignification. II. Structural characterization of the models by thioacidolysis. Holzforschung 50, 9–14. [Google Scholar]
- Terashima N, Fukushima K. 1988. Heterogeneity in formation of lignin—XI: an autoradiographic study of the heterogeneous formation and structure of pine lignin. Wood Science and Technology 22, 259–270. [Google Scholar]
- Timell TE. 1982. Recent progress in the chemistry and topochemistry of compression wood. Wood Science and Technology 16, 83–122. [Google Scholar]
- Tobimatsu Y, Schuetz M. 2019. Lignin polymerization: how do plants manage the chemistry so well? Current Opinion in Biotechnology 56, 75–81. [DOI] [PubMed] [Google Scholar]
- Toikka M, Brunow G. 1999. Lignin–carbohydrate model compounds. Reactivity of methyl 3-O-(α-l-arabinofuranosyl)-β-d-xylopyranoside and methyl β-d-xylopyranoside towards a β-O-4-quinone methide. Journal of the Chemical Society Perkin Transactions 1, 1877–1883. [Google Scholar]
- Toikka M, Sipilä J, Teleman A, Brunow G. 1998. Lignin–carbohydrate model compounds. Formation of lignin–methyl arabinoside and lignin–methyl galactoside benzyl ethers via quinone methide intermediates. Journal of the Chemical Society Perkin Transactions 1, 3813–3818. [Google Scholar]
- Viljamaa S, Dikareva E, Tolonen J, et al. 2018. Cryopreservation of the Norway spruce tissue culture line able to produce extracellular lignin. Plant Cell, Tissue and Organ Culture 133, 225–235. [Google Scholar]
- Warinowski T, Koutaniemi S, Kärkönen A, Sundberg I, Toikka M, Simola LK, Kilpeläinen I, Teeri TH. 2016. Peroxidases bound to the growing lignin polymer produce natural like extracellular lignin in a cell culture of Norway spruce. Frontiers in Plant Science 7, 1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting P, Goring DAI. 1982. Chemical characterization of tissue fractions from the middle lamella and secondary wall of black spruce tracheids. Wood Science and Technology 16, 261–267. [Google Scholar]
- Yaku F, Yamada Y, Koshijima T. 1976. Lignin carbohydrate complex Pt. II. Enzymic degradation of acidic polysaccharide in Björkman LCC. Holzforschung 30, 148–156. [Google Scholar]
- Zhang L, Gellerstedt G. 2007. Quantitative 2D HSQC NMR determination of polymer structures by selecting suitable internal standard references. Magnetic Resonance in Chemistry 45, 37–45. [DOI] [PubMed] [Google Scholar]
- Zhang L, Gellerstedt G, Ralph J, Lu F. 2006. NMR studies on the occurrence of spirodienone structures in lignins. Journal of Wood Chemistry and Technology 26, 65–79. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.