Abstract
We used the 16S rRNA gene pyrosequencing approach to investigate the microbial diversity and community composition in several Costa Rican hot springs alongside the latitudinal axis of the country, with a range of temperatures (37–63°C), pH (6–7.5) and other geochemical conditions. A principal component analyses of the physicochemical parameters showed the samples were separated into three geochemically distinct habitats associated with the location (North, Central, and South). Cyanobacteria and Chloroflexi comprised 93% of the classified community, the former being the most abundant phylum in all samples except for Rocas Calientes 1, (63°C, pH 6), where Chloroflexi and Deinococcus‐Thermus represented 84% of the OTUs. Chloroflexi were more abundant as temperature increased. Proteobacteria, Bacteriodetes and Deinococcus‐Thermus comprised 5% of the OTUs represented. Other Phyla were present in very small percentages (<1%). A LINKTREE analysis showed that the community structure of the mats was shaped primarily by pH, separating samples with pH > 6.6 from samples with pH < 6.4. Thus, both pH and temperature were relevant for community composition even within the moderate ranges of variables studied. These results provide a basis for an understanding of the physicochemical influences in moderately thermophilic microbial mats.
Keywords: Chloroflexi, cyanobacteria, hot springs, phototrophic mats, pyrosequencing
In this work we describe the diversity of microbial mats in hot springs at different temperatures and geographical locations in Costa Rica, using high throughput sequencing methods in order to answer questions about the combined potential influence of pH and temperature on bacterial communities.

1. INTRODUCTION
Environmental parameters are known to have a strong impact on the composition of microbial communities. Usually, several variables interact to produce a complex response from the communities. In extreme environments, however, a single factor such as salinity, temperature, pH, or intense radiation usually predominates. Therefore, the effects of such factors on community composition may be easier to study. Hot springs are an example of extreme environments where temperature is usually considered to be the main driving factor (Cole et al., 2013; Sharp, Martínez‐Lorenzo, Brady, Grasby, & Dunfield, 2014). In effect, microorganisms must be adapted to live at high temperatures in order to thrive in such environments and the main groups of Bacteria and Archaea living at different temperature ranges are usually the same in very distant springs. Usually Cyanobacteria, Chloroflexi, Deinococcus‐Thermus, and Aquificae are found as temperature increases in springs in North America, New Zealand, or Tibet (Jiménez et al., 2012; Power et al., 2018; Sharp et al., 2014; Wang et al., 2013). The genera involved are many times the same ones and they show preference for growth at temperatures close to (or slightly below) those in situ (Zeikus & Brock, 1972).
Irrespective of the actual taxa living in such environments, richness, and diversity are considered to decrease with increasing temperature (Pagaling et al., 2012; Ross et al., 2012; Tank, Thiel, Ward, & Bryant, 2017). Accordingly, Sharp et al. (2014) found that temperature was controlling microbial diversity in a large collection of hot springs. However, these authors also found that richness increased with increasing pH, indicating that this variable also had an influence on the diversity. Power et al. (2018) analyzed around 1,000 samples from hot springs in New Zealand and concluded that temperature only had an impact above 70°C, while pH was the main factor determining diversity in the temperature range between 20 and 70 degrees. In both studies samples grouped in two distinct clusters with pH values around 3–4 on the one hand and around 7 on the other. Obviously such a dramatic pH difference must have a strong influence on the microbial community.
Cyanobacteria are important members of the hot springs assemblages. It is known that photosynthetic microbes in general, and cyanobacteria in particular, are sensitive to slight changes in pH due to preference for either bicarbonate or CO2 as a source of carbon. Since we had at our disposal a series of hot springs with a range of pH values not too far from neutrality, we were interested in checking whether pH would still have an influence under these circumstances. Therefore we analyzed the diversity of these hot spring mats and studied the influences of both temperature and pH on their composition.
From north to south, Costa Rica is traversed by four mountain ranges: Guanacaste, Tilarán, Central, and Talamanca (Figure 1). Active volcanoes are found in the three northern ones, where volcanic activity is due to the convergence of the Cocos plate with the American plate (Huene, Ranero, Weinrebe, & Hinz, 2000). The southern Cordillera de Talamanca is not volcanic, but it has substantial hydrothermal activity (Obando, 2004). Along these mountain ranges there are many hot springs (Alvarado & Vargas, 2017; Bundschuch et al., 2007). These have been analyzed mostly in relation to their volcanic activity (Bragado‐Massa et al., 2014), but there are a few studies about the microorganisms in Rincón de la Vieja and in Poás volcanoes thermal springs (Caldwell, Liu, Ferrera, Beveridge, & Reysenbach, 2010; Dai et al., 2016; Hernández, 2012; Sittenfeld et al., 2002; Sittenfeld, Vargas, Sánchez, Mora, & Serrano, 2004) and the microbial assemblages of some of these environments (Hynek, Rogers, Antunovich, Avard, & Alvarado, 2018; Sugimori et al., 2002; Wheeler, 2006). Also, Cyanobacteria isolated from Miravalles volcano hot springs were characterized by Morales (2008) and Finsinger et al. (2008). None of these studies, however, analyzed the bacterial community composition of the microbial mats to determine the effect of geochemical characteristics on that structure. As mentioned, these set of hot springs provided an opportunity to test the effects of temperature and pH at a moderate range of values and we explored the issue using high throughput sequencing to analyze bacterial diversity.
Figure 1.
Geographical location of the sampling sites. 1) Río Negro (RN). 2) Miravalles (MV). 3) Bajo las Peñas (BP). 4) Rocas Calientes (RC). Digital Atlas ITEC, Costa Rica. 2014
2. MATERIALS AND METHODS
2.1. Site characteristics and sample description
The geothermal springs studied are situated in North‐Western, Central, and South‐Eastern Costa Rica (Figure 1). The northern springs sampled were located in Miravalles Volcano (MV) geothermal field, 15 km north of La Fortuna, Guanacaste, and Río Negro (RN), associated with Rincón de la Vieja Volcano, 25 km NE of Liberia, Guanacaste. Two mat samples were taken at each spring, within 50 meters distance from each other. Bajo las Peñas (BP) is a group of springs discharging from Turrialba Volcano, in the province of Cartago. Two springs at 20 meters distance were sampled. The Rocas Calientes (RC) spring is located in the Ujarrás Reserve in Buenos Aires, Puntarenas. This spring consist on hot water emanating from a steep cliff at different points in the rock, with phototrophic microbial growth under the water flowing down to the ground. Samples of these microbial mats were taken at three different zones in the rock at two meters distance from each other and one in the soil.
2.2. Sampling and physicochemical determinations
A total of nine samples were taken in January and July 2012. Temperature, pH and conductivity were measured using an Oakton multiparameter tester. Approximately 1 liter of water was collected in sterile plastic bottles and kept at 4°C for chemical analyses. Chemical analyses of metal ions and S were performed using Inductively Coupled Plasma Optical Emission Spectrometry. Flow Injection analysis was used for N‐NH4 and N‐NO3 determination. Mat samples for diversity were collected with forceps and spatula and transferred to the laboratory in sterile 50 ml polypropylene tubes.
2.3. Nucleic acid extraction
DNA was extracted using several protocols, however, we obtained the best results using Nucleospin Plant II Genomic DNA extraction kit (Macherey‐Nagel) following manufacturer's instructions on 0.5–1 g of mat sample. Integrity of the DNA was examined in 1.0% agarose gels by electrophoresis and quantified with a NanoDrop ND‐1000. Nucleic acids were stored at −70°C.
2.4. Sequencing and processing
DNA samples were sent to Research and Testing Laboratory (Lubbock, Texas, USA) for amplification of the 16S rRNA gene. Tag‐pyrosequencing was done with Roche 454 Titanium platform following manufacturer protocols (454 Life Science). Primers 28F (5′‐GAGTTTGATCNTGGCTCAG) and 519R (5′‐GTNTTACNGCGGCKGCTG) were used for amplification of the hypervariable regions V1, V2, and V3, and approximately 450 bp long tags were obtained. Dowd et al. (2008) described the subsequent PCR and sequencing. A total of 280,907 tags were obtained. The raw tag‐sequences were processed using QIIME (version 1.9.1) (Caporaso et al., 2010). Briefly multiplexed reads were first trimmed, quality‐filtered, and assigned to the corresponding sample. The filtering criteria included eliminating homopolymers, at least 200 bp in length, and a minimal average quality score of 25. Chloroplast sequences were removed. To identify chimeras, the dataset was processed using usearch61. The number of reads per sample was normalized by rarefaction and reads clustered in OTUs at the 97% level of similarity. A representative sequence from each OTU was selected. Then, taxonomy assignment was done with QIIME by searching the representative sequences of each OTU against the SILVA 16S/18S rDNA non‐redundant reference dataset (SSURef 132 NR) (Quast et al., 2013; Yilmaz et al., 2014).
Only OTUs with relative abundance ≥0.00025% across all samples were used for statistical and phylogenetic analyses. All taxonomic assignments of the remaining 126 OTUs were manually checked by comparing them with sequences in the database using a combination of initial BLASTN‐based searches and an extension of the EzTaxon database (Chun et al., 2007), which stores 16S rRNA gene sequences of type strains of validly published names. We used the criteria published by Chun, Kim, Lee, and Choi (2010) for taxonomic assignment of each read (x = similarity): species (x ≥ 97%), genus (97 > x ≥ 94%), family (94 > x ≥ 90%), order (90 > x ≥ 85%), class (85 > x ≥ 80%), and phylum (80 > x ≥ 75%). If the similarity was below the cutoff point, the read was assigned to an "unclassified" group. Sequences from the 126 OTUs using in all the analyses have been submitted to the NCBI GenBank database under accession numbers MK040623‐MK040726 and MK077649‐MK077670.
2.5. Phylogenetic analysis
Phylogenetic analyses were done with MrBayes. The 126 sequences were aligned using ClustalX (Larkin et al., 2007) in MEGA (Tamura, Stecher, Peterson, Filipski, & Kumar, 2013). Evolutionary distances were calculated by Bayesian inference (Huelsenbeck & Ronquist, 2001) and bootstrap was used to evaluate the tree topology by performing 10,000,000 resamplings and is shown for branch nodes supported by more than 50% of the trees. Reference GenBank sequences were used to illustrate the relationship of sequences to representative taxa. Planctopyrus limnophilus X62911 was used as outgroup and the tree was visualized using ITOL (https://itol.embl.de.com). For clarity in the analysis, separate trees were also built for Cyanobacteria, Chloroflexi, Proteobacteria, and “Other” phyla (Deinococcus‐Thermus, Acidobacteria, and Bacteroidetes) using the same methodology.
2.6. Statistical analyses
Principal Components Analyses (PCAs) of environmental values were performed on the Euclidean distance similarity matrix of logarithmic transformed data to determine metadata differences across sites using the “vegan” package in R version 3.4.3 (R Core Team, 2018). For biological data, Bray‐Curtis similarity values were calculated from the normalized and square root transformed OTU table (126 OTUs).
Analysis of similarities (ANOSIM) was used to determine if there were significant differences (p < 0.05) in community structure among thermal spring sites. Interaction effects were tested using a two‐way crossed ANOSIM, where R values (R test statistic) near 0 indicate no difference between groups, whereas those >0 (up to 1) indicate dissimilarities between groups (Clarke & Gorley, 2015). Richness (S) was computed as the total number of OTUs (97% similarity). Estimates of S, Chao1, Shannon diversity (H′), Simpson and rarefaction curves were calculated using QIIME (Caporaso et al., 2010). The RELATE routine as used to test whether the two matrices (biotic and environmental) had correlations, and the BEST procedure of the same software was used to find the best match between the multivariate among‐sample patterns of an assemblage and that from environmental variables associated with those samples. A hierarchical binary divisive cluster analysis in constrained form (LINKTREE), where only divisions which have an “explanation” in terms of a threshold in an environmental variable are permitted, was performed. ANOSIM, RELATE, BEST, and LINKTREE tests were calculated using PRIMER 7/PERMANOVA+ (Clarke & Gorley, 2015). Canonical correspondence analysis (CCA) was performed using PAST software (Hammer, Harper, & Ryan, 2001), to explore relationships of microbial community at the OTU level with physicochemical variables. By considering that predominant species have greater influence within the communities, only 24 major OTUs with relative abundance of >0.001% across all sample data sets were used as a community matrix for CCA. The significance of the CCA models and the explanatory factors were tested using 999 permutations.
3. RESULTS AND DISCUSION
3.1. Physicochemical characteristics of the hot springs
Hot springs in this study showed moderate to high temperatures, pH from slightly acidic to slightly alkaline, and different ion content of the waters (Table 1). A PCA (Figure 2) of the physicochemical parameters grouped the hot springs into three geochemically distinct habitats corresponding to location: North (RN and MV), Central (BP), and South (RC). The first two principal components explained 74% of the total variance. The first axis separated RC (South) from the other sites. This spring had a lower content of magnesium and the highest concentrations of sulfate, calcium, chloride, and sodium. The second axis, in turn, separated BP (Central) from the northern mats. In this case, pH was the most influential variable together with conductivity, ammonia, and sufate. Temperature and pH were negatively correlated (R 2 = 0.548). Sites with more acidic pH had higher concentrations of K, Na, and Cl ions and higher temperature.
Table 1.
Physicochemical parameters for the thermal springs
| Variables | Samples | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| RN2a | RN3 | MV1 | MV2 | BP1 | BP2 | RC1 | RC2 | RC3 | |
| pH | 6.4 | 6.2 | 6.2 | 6.6 | 7.4 | 7.5 | 6.0 | 6.1 | 6.2 |
| Temperature | 55 | 59 | 49 | 42 | 50 | 37 | 63 | 59 | 60 |
| Conductivity (S/cm) | 2,310 | 2,310 | 811 | 713 | 1990 | 1990 | 1,120 | 1,100 | 2,265 |
| N‐NH4 + (mg/L) | ND | ND | ND | 0 | 0.73 | 0.19 | 0.40 | 0.40 | 0.40 |
| N‐NO3 − (mg/L) | ND | ND | 0.10 | 0.26 | 0.05 | 0.40 | ND | ND | ND |
| Ca (mg/L) | 7.32 | 7.32 | 8.61 | 17.94 | 37.02 | 53.32 | 104.10 | 104.10 | 104.10 |
| Mg (mg/L) | 3.56 | 3.56 | 5.81 | 16.47 | 5.84 | 6.61 | ND | ND | ND |
| K (mg/L) | 9.63 | 9.63 | 5.84 | 13.74 | 5.09 | 5.57 | 10.60 | 10.60 | 10.60 |
| Na (mg/L) | 22.85 | 22.85 | 14.80 | 40.85 | 15.08 | 19.11 | 406.20 | 406.20 | 406.20 |
| Cl (mg/L) | 10.0 | 10.0 | 9.1 | 9.3 | 1.1 | 0.8 | 511.9 | 511.9 | 511.9 |
| S (mg/L) | 5.03 | 5.03 | 10.78 | 21.54 | 50.57 | 66.98 | 132.50 | 132.50 | 132.50 |
| Sulphate (mg/L) | 15.0 | 15.0 | 32.4 | 64.5 | 151.8 | 201.0 | 397.5 | 397.5 | 397.5 |
Río Negro (RN), Miravalles (MV), Bajo las Peñas (BP), and Rocas Calientes (RC).
Figure 2.
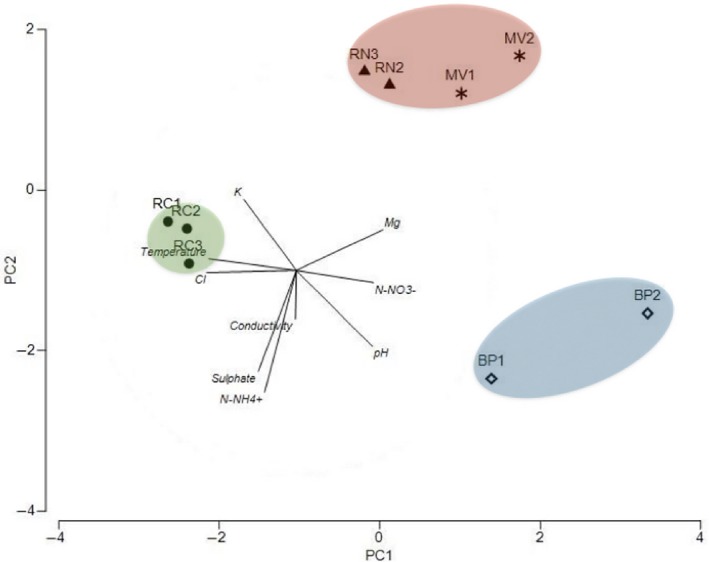
First and second principal component scores and vectors (using metadata) showing separation between three geochemically distinct habitats associated with the location (North enclosed with a dashed line, Central with a dotted line and South with a solid line). Río Negro (RN), Miravalles (MV), Bajo las Peñas (BP), and Rocas Calientes (RC)
3.2. Community composition
We obtained 264,501 clean sequences of the 16S rRNA gene (Table A1). The total number of OTUs was 3,573. Rarefaction curves (Figure A1) indicated that the numbers of OTUs were stabilized after sampling approximately 4,000 sequences, implying a good coverage. Chao estimates ranged between 168 (RC1) and 709 OTUs (RN2). While both the Chao estimate and the Shannon index peaked at an intermediate temperature (55°C), neither one of them followed any clear trend with temperature nor pH (Figure 3).
Figure 3.
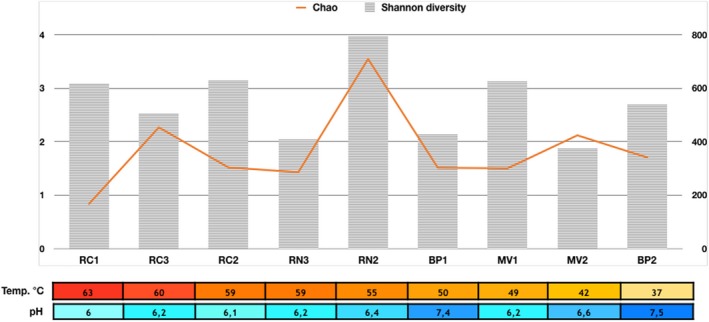
Shannon's diversity index (H′) and Chao index of richness based on 16S rRNA gene amplicon pyrosequencing. Samples have been sorted by temperature, and pH values are also shown. Left axis shows Shannon diversity values and right axis Chao richness. Río Negro (RN), Miravalles (MV), Bajo las Peñas (BP), and Rocas Calientes (RC)
Abundance of the OTUs, their closest BLAST hits, and their accession numbers are listed in Table A2. The relative abundance of each phylum varied among the samples (Figure 4B). Cyanobacteria and Chloroflexi comprised 93% of all the reads. Cyanobacteria were the most abundant phylum in all samples except for RC1 (63°C, pH 6), where Chloroflexi and Deinococcus‐Thermus accounted for 84% of the reads. Chloroflexi were more abundant as temperature increased. Proteobacteria, Bacteroidetes and Deinococcus‐Thermus comprised less than 5%. Other phyla were present in very small quantities (<1%).
Figure 4.
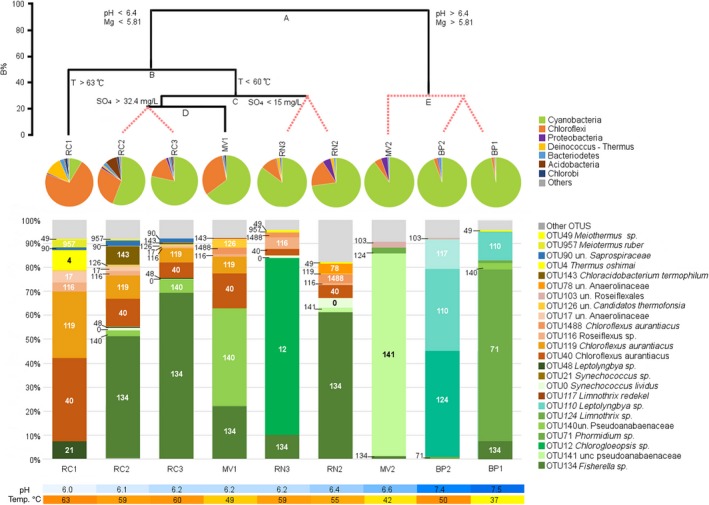
A) Linkage tree analysis (LINKTREE) showing clustering of samples based on the distribution of the 24 most abundant OTUs and environmental variables. Statistically different groups shown by black lines. Red discontinuous lines show nonsignificantly different samples. Note that the split A separates the samples with higher pH and Mg2+ levels, and then B isolates RC1 with the highest spring temperature from the rest of the samples. See text for further details on C and D splits. B%: Bray‐Curtis similarity. B) Major phyla identified in the mats. Only phyla with mean relative abundance greater than 0.01% are shown. The “other” category comprises phyla Armatimonadetes, Planctomycetes, Spirochaetes, Saccharibacteria, and Firmicutes. C) Bar chart showing the 24 most abundant OTUs and their respective abundance in each sample. D) Color scales showing the different pH and temperatures (°C) in each sample
For subsequent analyses we retained only the 126 OTUs accounting for more than 0.00025% of the reads. They are shown in a phylogenetic tree in Figure A2. These 126 OTUs are shown within their respective detailed phylogenetic trees in Figures 5 and 6, Figures A3 and A4. Of these, 33 OTUs were Cyanobacteria, 29 Chloroflexi, 26 Proteobacteria, and 13 Bacteroidetes. The remaining OTUs were Chlorobi and Deinococcus‐Thermus (6 OTUs each), Acidobacteria (4 OTUs), Armatimonadetes and Planctomycetes (3 OTUs each), and Saccharibacteria, Firmicutes, and Spirochaetes with one OTU each. The 24 dominant OTUs represented 92.2% of total sequences and their relative abundance in the different springs is shown in Figure 4C.
Figure 5.
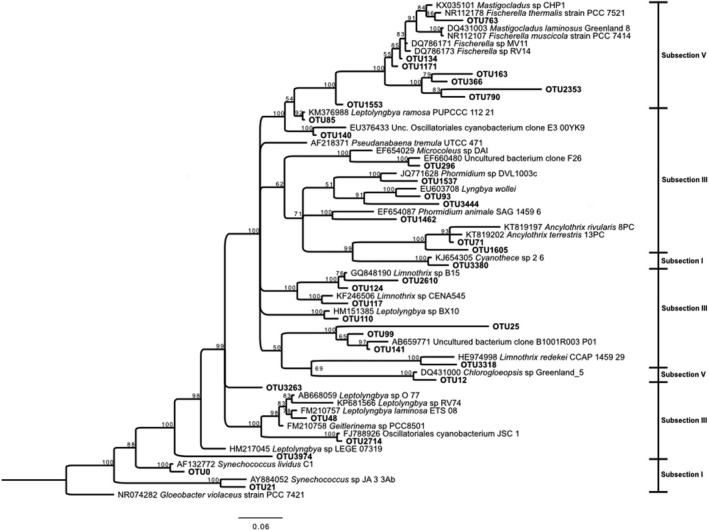
Bayesian tree based on 16S rRNA gene sequences showing the positions of OTUs classified as Cyanobacteria. Bootstrap values based on 10,000,000 replications are shown at branch nodes. Gloeobacter violaceus was used as outgroup. Bar shows 0.06 substitutions per nucleotide
Figure 6.
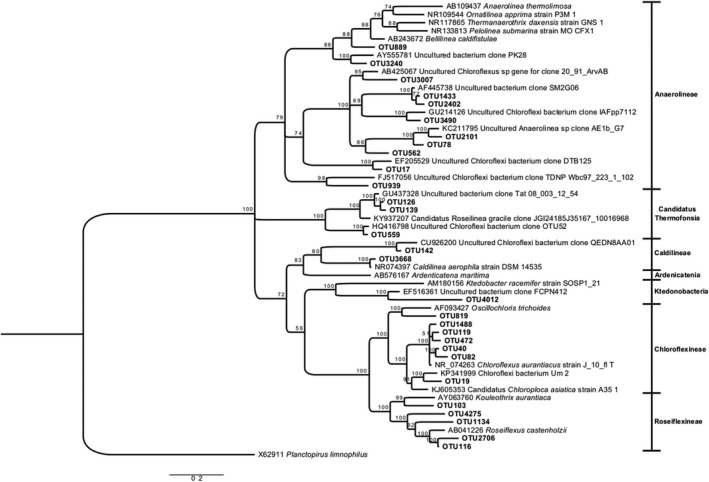
Bayesian tree based on 16S rRNA gene sequences showing the positions of OTUs classified as Chloroflexi. Bootstrap values based on 10,000,000 replications are shown at branch nodes. Planctopirus limnophilus was used as outgroup. Bar shows 0.2 substitutions per nucleotide
3.2.1. Cyanobacteria
The Cyanobacteria tree (Figure 5) included 33 sequences. Branching patterns generally had high levels of support, with lower bootstrap and Bayesian posterior probability values for a few branches, probably reflecting current ambiguities in cyanobacterial taxonomy and sequence length limitations of the analysis (Hongmei et al., 2005). However, this is not different from the patterns found in studies involving a broad range of cyanobacterial genera (Komárek, Kaštovsk, Mareš, & Johansen, 2014; Tomitani, Knoll, Cavanaugh, & Terufumi, 2006). Moreover, many of the genera, families, and orders are polyphyletic. Therefore, here we adopt the pragmatic approach of the classical five subsections, which considers some of the most relevant morphological and ecological traits of Cyanobacteria (Castenholz et al., 2001). The cyanobacteria belonged to subsections V (filamentous, heterocystous, branching cyanobacteria), III (filamentous, non‐heterocystous cyanobacteria), and I (unicellular cyanobacteria). Among those in Section V, Fischerella‐like cyanobacteria are a frequent and major constituent of natural populations at thermal sites, (named either Fischerella or Mastigocladus in different studies, Miller, Castenholz, & Pedersen, 2007). In our samples Fischerella sequences formed two subclusters. One of them included Fischerella OTU134 that was basically identical (99% similar) to cultures MV11 and RV14 isolated from Miravalles thermal spring in a previous study (Finsinger et al., 2008). This OTU was present in almost all the samples and was the most abundant Fischerella OTU (Figure 4C).
Judging from its distribution, its temperature optimum was 60 degrees. This is higher than the optimal temperature found in culture for the two mentioned isolates MV11 and RV14 (35°C), although the isolates grew up to the highest temperature tested (55°C) (Finsinger et al., 2008). Samples MV1 and BP2 had very similar temperatures (49 and 50°C respectively). Yet, OTU134 was very abundant in MV1 and absent from BP2 (with a pH >6.4). In fact this OTU was rare in the three samples with pH values >6.4. Interestingly, these three samples were richer in nitrate and ammonia (Table 1) and, consequently, the ability of Fischerella to fix nitrogen (Alcamán et al., 2017) might not represent an advantage in these springs. The second subcluster included sequences that were phylogenetically distant from Fischerella in databases (OTUs 163, 366, 790, and 2,353) and rare in all samples. These OTUs indicate novelty within the rare biosphere of the mats studied.
The other member of Subsection V was OTU12, present only in RN3 (59°C and pH = 6.2) where it was the dominant cyanobacterium. This OTU had a 98% similarity to a Chlorogloeopsis isolate from an Artic hot spring (Roeselers et al., 2007). Chlorogloeopsis sequences had been found at similar pH but higher temperatures in Iceland (Skirnisdottir et al., 2000). In general, both Fischerella and Chlorogloepsis are N‐fixing (Ward & Castenholz, 2000) and they have been found together at least in stromatolites from the upper Hayden Valley in YNP (Yellowstone National Park) (pH = 5.7, 56°C) (Pepe‐Ranney, Berelson, Corsetti, Treants, & Spear, 2012).
Subsection III was represented by several genera. Taxonomy of this section is confusing and the sequences appeared in different clusters of the tree (Figure 5). The three samples with higher pH values were each dominated by different members of this subsection (Figure 4C). OTU124 (98% similar to Limnothrix sp. B15 from Lake Taihu, China) and OTU110 (98% similar to Leptolyngbya sp. BX10 from the same lake) co‐dominated in BP2, where OTU117 (96% similar to Limnothrix sp. CENA545 from saline‐alkaline lakes in Brazil (Andreote et al., 2014), was also abundant. OTU71 (98% similar to Ancylothrix terrestris 13PC, a new described Oscillatoriaceae from a soil in Brazil (Martins, Rigonato, Taboga, & Branco, 2016), dominated BP1. Finally, OTU141, 97% similar to an uncultured bacterium clone B1001R003_P01 from a rice paddy in Japan (Itoh et al., 2013) was the dominant bacterium in MV2. None of the closest relatives of these OTUs were thermophilic.
Two members of subsection III were found at higher temperatures. OTU140 was 98% similar to a clone from a western USA hot spring (unpublished study) and was also close to a clone from a hot spring in Thailand (Portillo, Sririn, Kanoksilapatham, & Gonzalez, 2009). We found OTU140 in samples with temperatures ranging from 42 to 60°C, but its largest abundance was in sample MV1 with the next to lowest temperature of the samples where it was present. OTU48 in turn, was 97% similar to a thermophilic Leptolyngbya strain O‐77 isolated from a hot spring in Japan (Nakamori, Yatabe, Yoon, & Ogo, 2014). OTU48 was present mostly in the 59–60°C range (samples RC2‐RC3) but not at 63°C, which is consistent with the optimal growth temperature of strain O‐77 (55°C). In addition, there were several more sequences that were always found in low abundance (Figure 5). In particular, a little clade included only sequences without a GenBank close hit (OTUs 25, 99, and 141) as well as other sequences with similarities lower than 91%–92% to their closest relatives, such as OTUs 1,462, 3,444 for example. These sequences indicated phylogenetic novelty among the Cyanobacteria in these hot springs.
Subsection I was represented by Synechococcus sequences in branches apart from the other clades and from each other (Figure 5). This has been reported for Synechoccoccus lividus C1 and Synechococcus sp. JA33Ab (Ferris, Ruff‐Roberts, Kopczynski, Bateson, & Ward, 1996). OTU0 (97.6% similar to Synechococcus C1 from YNP (Ferris et al., 1996; Papke, Ramsing, Bateson, & Ward, 2003; Tank et al., 2017) was present only above 55°C and OTU21 (96% similarity to Synechococcus JA‐3A, (Allewalt, Bateson, Revsbech, Slack, & Ward, 2006) was observed exclusively at the highest temperature (RC1, 63°C). The cyanobacteria most tolerant to high temperatures are unicellular forms (Ionescu, Hindiyeh, Malkawi, & Oren, 2010). Synechococcus JA‐3A belongs to genotype A from Octopus Spring (YNP) and has been reported as a North American endemic (Papke et al., 2003) that tolerates high temperatures (optimum range 50 to 60°C). It has also been found in sites such as Hunter's Hot Springs in Oregon (Miller & Castenholz, 2000) and Mushroom Spring, YNP (Becraft, Frederick, Kühl, Jensen, & Ward, 2011). OTU0 was observed in several springs (Figure 4C), and its abundance increased significantly as temperature rose from 55°C (RN2) to 63°C (RC1). Several studies suggest a co‐occurring distribution of Synechococcus and Chloroflexi due to a metabolic interaction (López‐López, Cerdán, & González‐Siso, 2013; Miller, Strong, Jones, & Ungerer, 2009). Although we can only speculate about this metabolic interaction, we found this co‐occurrence in all our samples above 55°C, except for MV1.
3.2.2. Chloroflexi
Chloroflexi sequences grouped in several clades (Figure 6). The most abundant OTUs were 97% to 99% similar in their 16S rRNA to Chloroflexus aurantiacus J‐10‐fl isolated from Japan (Figure 6), which is the type strain for the species. OTU40 was present in the three RC samples, in MV1 and in both RN samples, all with temperatures above 49°C, and it was the most abundant OTU at the spring with highest temperature (RC1). OTU119 was also present in RC and MV1 but nowhere else and was always less abundant than OTU40. These two OTUs accounted for about 70% of the reads in RC1. A third C. aurantiacus relative, OTU1488, was present in RN. Chloroflexus arauntiacus is a green non sulfur bacterium that is a common member of thermophilic microbial mats around the world (Lau, Aitchison, & Pointing, 2009; Ruff‐Roberts, Kuenen, & Ward, 1994; Urbieta, González‐Toril, Bazán, Giaveno, & Donati, 2015; Wang et al., 2013).
There were also two Roseiflexus OTUs (91–95% similar) that were abundant in the springs with higher temperatures, but they never accounted for more than 5% of the reads. OTU103 was very distantly related (98%) to a soil sequence from China (Chen et al., 2014) while OTU116 was 95% similar to the type strain Roseiflexus castenholzii (T) DSM 13941.
The second clade included 11 sequences similar to environmental Anaerolinea sequences retrieved from thermophilic and aquatic environments. A third clade grouped OTUs 126, 139, and 559 close to Roseilinea gracile, a member of the novel phototrophic class Candidatus Thermofonsia, sister to Anaerolineae (Ward, Hemp, Shih, McGlynn, & Fischer, 2018.). OTUs 17, 78, and 126 were abundant but only present in a few samples: OTU17 in RC samples (60–63°C), OTU78 was most abundant in sample RN2 (55°C, pH 6.4) and OTU126 in MV1 (49°C).
3.3. Other bacteria
There were just a few additional OTUs of any significance (Figure 4C). Three Deinococcus‐Thermus OTUs distributed themselves along the thermal gradient. OTU4, 97% similar to Thermus oshimai (Chen et al., 2014), was slightly abundant at 63°C (Figure A3). OTU957 (99% similar to Meiothermus ruber) was present between 59 and 63°C, and OTU49 (another Meiothermus relative) appeared in two samples at temperatures lower than 59°C. All these Deinococcus‐Thermus have been found in hot springs around the world, such as OTU4 in Sao Pedro do Sul (Portugal) (Williams, Smith, Welch, & Micallef, 1996), or OTU957 in Kamchatka (Russia) (Loginova & Egorova, 1975). Members of the genera Thermus and Meiothermus are generally found in neutral to slightly alkaline natural aquatic environments where temperatures range between 50 and 85°C.
OTU143, an Acidobacteria, was present at RC2. This OTU was 98% similar to Chloroacidobacterium thermophilum isolated from Octopus Spring, YNP (at 44–58°C, pH ~ 8.2) (Bryant & Frigaard, 2006). A Bacteroidetes member was found at the three RC mats in very low abundance. All the remaining OTUs, including several Proteobacteria (Figure A4) were extremely rare and are not discussed.
3.4. Factors determining community composition
As can be gathered both from the PCA (Figure 2) and the relationship of diversity with temperature and pH (Figure 3), both parameters seemed to have an influence on community structure. The two were negatively correlated with each other (R2 = 0.548) and this obscured direct relationships between community composition and the environmental variables separately. Therefore, we carried out a multivariate analysis such as constrained divisive tree (LINKTREE) to see which parameters had a stronger influence on the community (Figure 4A).
First, we compared the biotic and environmental matrices using RELATE analysis. This showed a strong correlation between the community structure and the geochemical characteristics of the samples (R = 0.73). BEST confirmed the importance of two variables, pH and temperature, for microbial mat structure (Rho = 0.85). The LINKTREE analysis (split A in Figure 4A) first separated communities on the basis of pH (samples with pH > 6.6 and pH < 6.4) and Mg2+ content (higher or lower than 5.81 mg/L), with ANOSIM R = 0.9, and a Bray‐Curtis similarity measure (B%) = 96.8. This set apart the BP and MV2 samples that had the highest pH, Mg2+, and NO3 − levels and lower temperatures, from the rest (Figure 4A). These are the only samples were Chloroflexi represented less than 5% of the OTUs while Cyanobacteria accounted for ≥90% of the mat, and were dominated by the filamentous non‐heterocystous cyanobacteria (Figure 4B,C).
We also carried out a CCA (Figure 7) to further clarify the relationships between environmental variables and OTUs. Again, pH and temperature were responsible for the separation of samples, OTUs and environmental variables along the first axis, in accordance with the LINKTREE analysis. Interestingly, the first axis also showed nitrate to be more abundant in BP and MV2. This could be related to the dominance by non‐heterocystous cyanobacteria, as opposed to the other samples. The second axis distinguished between BP and MV2, indicating the importance of chemical composition on the dominant OTUs. K+ and Mg2+ were more abundant in MV2 while ammonia was more abundant in BP.
Figure 7.
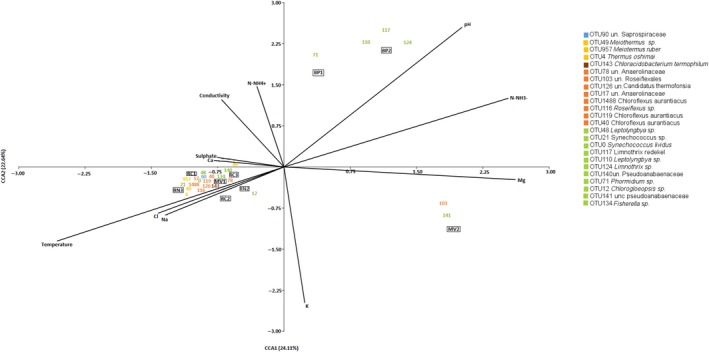
CCA based on the 126 most abundant OTUs and environmental data. The 24 most abundant OTUs are shown as vectors. Río Negro (RN), Miravalles (MV), Bajo las Peñas (BP), Rocas Calientes (RC). CCA, Canonical correspondence analysis
The second split from LINKTREE (B in Figure 4A) was determined by temperature separating RC1 (63°C, pH = 6) from the remaining samples (ANOSIM R = 0.72 and B% = 49.5). RC1 was dominated by Class Chloroflexia, with OTUs 40 and 119 as dominant (near 60% of reads). Deinococcus‐Thermus were also important as explained above.
The third split in LINKTREE (marked C in Figure 4A) divided samples in two clusters according to sulfate concentration and conductivity (ANOSIM R = 0.75 B%: 29.1). Samples from RC2, RC3, and MV1 had sulfate >32.4 mg/L and conductivity <2.27E+03, while those from RN had <15 mg/L of sulfate and conductivity >2.31E+03. The two RN samples clustered together despite the obvious difference in the dominant cyanobacterium, Chlorogloeopsis OTU12 in RN1 and Fischerella OTU134 in RN2 (Figure 4C). Sample RN2 showed the highest diversity (Table A1, Figure 3). This, together with the dominance of Chlorogleopsis OTU12, was likely the reason that it appeared separate from the other samples from the same LINKTREE cluster in the CCA diagram (Figure 7).
The last group of samples was separated by LINKTREE by higher levels of sulfate (>32.4 mg/L SO4 2−) and included RC2, RC3, and MV1. These mats were also dominated by Cyanobacteria (56% RC2 to 78.3% RC3) and Chloroflexi (15.8% RC3 to 32.2% MV1). The cyanobacterial OTUs with higher abundance in these mats were Fischerella OTU134 (samples RC2 and RC3), and OTU140 (MV1). The same Chloroflexi OTUs observed in RC1 (OTU40 and OTU119: Chloroflexus arauntiacus; OTU116: Roseiflexus sp. and Anaerolinaceae OTU17) were present in these samples, but in smaller proportions.
Diversity is assumed to decrease with increasing temperature in hot springs. However, this is only true above 40–45°C. Below this point, diversity may increase with temperature or remain more or less constant. Arroyo et al. (in preparation) found that they could fit a unimodal relationship to data from three hot springs in Southern Chile. Diversity increased with temperature up to 45°C and then decreased as temperature increased further. This breaking point coincides with the inactivation temperature of many proteins and, therefore, reflects a basic fact of biology. In effect, both richness and diversity showed a unimodal relationship with temperature. Similar results were found by Sharp et al. (2014).
Examined with this unimodal relationship in mind, most contradictory results from the literature can be accommodated, although the exact breaking point is not always the same. Thus, Miller et al. (2009) found that richness peaked at 38°C in several YNP hot springs. However, since they only had one spring below this temperature, their figure suggests a monotonous descending relationship. Wang et al. (2013) did not find differences in diversity between samples from Tibet grouped into what they called “low” temperature (20–60°C) and “moderate” temperature (66–75°C). Of course, in this case the samples in the low temperature class would have an average lower than the maximum expected around 45°C and this might obscure the relationship between diversity and temperature. Everroad, Otaki, Matsuura, and Haruta (2012) found a monotonous decreasing relationship in Japanese hot springs, but the lowest temperature examined was 52°C, above the breaking point. In our case, despite a temperature range of 26 degrees, similar for example to that of Miller et al. (2009) of 33 degrees, we did not find a clear relationship with temperature. Therefore, other factors must have influenced the microbial composition. The pH was an obvious candidate. Several studies have analyzed the impact of pH on community composition in hot springs. The most extensive ones are Inskeep, Jay, Tringe, Herrgård, and Rusch (2013) who studied 20 samples from YNP, Sharp et al. (2014) who analyzed 36 samples from the Taupo hydrothermal field in New Zealand and in western Canada, and Power et al. (2018) who analyzed 925 hot springs from the Taupo field. In all these cases springs could be classified as acid (pH = 2–4) or circum‐neutral (pH = 6–8). There were very few alkaline springs with pH above 8 and almost no springs with mildly acidic pH between 5 and 6. Menzel et al. (2015) studied eight springs from different continents with temperatures above 65°C and pH values between 1.8 and 7.0. As discussed above, Sharp et al. (2014) claimed that temperature controls microbial diversity in their springs. However, they also showed a clear relationship between diversity and pH. The acid springs (pH = 2–4) had very low diversity, while the neutral springs (pH = 6–8) had a wide range of diversity values. Thus, temperature only influenced diversity for the neutral springs. At acid pH, this factor was more important.
Inskeep et al. (2013) also used pH as the first factor to classify their springs (pH 2–5 and 5–9), and temperature came next. It is interesting that their classification scheme (their Figure 3) was intuitive, but coincides with our LINKTREE analysis, despite the fact that our ranges of pH and temperature are much narrower than those of Inskeep et al. (2013). It seems than in their case the differences in pH were so large that its importance was obvious, while in our case we had to resort to statistical analysis to show the same effect. Power et al. (2018) had the largest data set ever studied. Once more, their samples fit in two pH clusters, those with acid pH (1–3) and those with neutral or alkaline pH (5–9). They looked at the effects of pH separately for springs with nine different intervals of temperature (10 degrees each). Diversity was significantly related to pH in five intervals between 20 and 70 degrees. There were very few springs below 20°C and the relationship was not significant above 70°C. They concluded that “diversity was primarily influenced by pH at temperaturas <70°C, with temperature only having a significant effect for values >70°C”. When they built an NMDS diagram, the first axis separated samples by pH not by temperature. Again suggesting that this factor was the main driver of microbial diversity. We explored the relative importance of pH and temperature using constrained divisive clustering (LIKNTREE). In this analysis we were comparing the community composition of the different mats in combination with the physic‐chemical parameters. In effect, the first separation was associated with pH and Mg2+ concentrations (Figure 4A). The two groups of samples differed in their dominant cyanobacteria. The high pH group was dominated by nonheterocystous filamentous cyanobacteria belonging to different genera in Subsection III (Pseudoanabaena, Limnothrix, Leptolyngbya), while the low pH group was dominated by filamentous cyanobacteria with heterocysts belonging to Subsection V. A Chlorogloeopsis relative (OTU12) was abundant only in one sample (RN3), while a Fischerella relative (OTU134) dominated all the other mats except the sample with higher temperature (RC1) where Chloroflexi were dominant and a Synechococcus relative (OTU21), was the most abundant cyanobacterium. Interestingly, the three samples with Subsection III cyanobacteria were those with larger concentrations of combined nitrogen (Table 1), either nitrate in MV2 or ammonia in BP1 and BP2. Therefore, the nitrogen fixing abilities of the Subsection V cyanobacteria would not be an advantage. The LINKTREE analysis, however, did not identify nitrogen as a relevant factor. Rather, pH was the most important one.
4. CONCLUSIONS
Cyanobacteria was the most abundant phylum in phototropic microbial mats from hot springs with temperatures ranging 37–60°C and pH 6.1–7.5. Multivariate analysis indicated that pH was the first factor influencing the differences in bacterial community composition of these samples. In summary, high temperature and low pH samples had Fischerella OTU134 as the dominant cyanobacterium, while a series of different Subsection III OTUs were more abundant in the lower temperature and/or higher pH mats. Sample RN3 (59°C, pH = 6.2) was the only one where OTU12, a Chlorogloeopsis relative, was dominant. As mentioned, the importance of pH had already been shown in previous studies. However, the relevance of the present work is that even with moderate ranges of values in both temperature and pH, the two variables combined to produce a mosaic of communities, pH being more important than temperature. Neither factor alone was sufficient to explain the community composition, but the traditional view that temperature is the main driver of diversity in hot springs needs to be revised.
CONFLICT OF INTERESTS
The authors declare that they do not have conflict of interest.
AUTHOR CONTRIBUTIONS
LU‐L, BD, and CPA: conceived the initial study; LU‐L, WH, RM‐A, GG, CRU, BD, and CPA: collected samples; LU‐L, LB, WH and BD: processed samples and data; LU‐L, LB, BD, and CPA: analyzed data; LU‐L, LB, and CPA: wrote the manuscript.
ETHICS STATEMENT
Collection permit VI‐4937‐2011 was granted by the Institutional Commission on Biodiversity of the University of Costa Rica.
ACKNOWLEDGMENTS
Fieldwork was funded by grants CRUSA—CSIC 2010CROO08 and CSIC COOPB20096. Work in Costa Rica was funded by grants from CRUSA 6/1‐(1E)‐10 and University of Costa Rica VI‐801‐B1‐542 to LU‐L and CONICYT grant FONDECYT N°1110696 and 1150171; FONDAP N° 15110009 to BD. Sequencing and work in Spain was supported by grant CTM2016‐80095‐C2‐1‐R from the Spanish Ministry of Science and Innovation to CP‐A. We thank Roy Mackenzie, Bibiana G. Crespo, and Ibrahim Zúñiga, for their help with the processing of sequences and Kaylen González and Alejandro Cedeño for their help with the images.
APPENDIX 1.
Table A1.
Diversity and evenness of bacterial communities calculated based on their 16S rRNA gene sequencing
| Samplec | Raw sequences | Sa | Chao | Shannon index | Simpson index | N b |
|---|---|---|---|---|---|---|
| MV1 | 27,067 | 251 | 300.9 | 3.12 | 0.78 | 25,851 |
| MV2 | 47,181 | 389 | 424.0 | 1.87 | 0.35 | 44,894 |
| RN2 | 27,521 | 619 | 709.3 | 3.96 | 0.71 | 24,055 |
| RN3 | 34,446 | 241 | 286.4 | 2.05 | 0.48 | 33,160 |
| BP1 | 25,101 | 244 | 302.6 | 2.14 | 0.51 | 23,834 |
| BP2 | 24,676 | 293 | 341.4 | 2.69 | 0.71 | 20,953 |
| RC1 | 24,016 | 148 | 168.2 | 3.08 | 0.79 | 23,629 |
| RC2 | 22,912 | 254 | 303.7 | 3.15 | 0.73 | 22,175 |
| RC3 | 47,987 | 396 | 453.5 | 2.53 | 0.55 | 45,950 |
S: total number of OTUs
N: total bacteria 16S rRNA gene sequences after removing chimaeras and chloroplasts.
RN: Río Negro; MV: Miravalles; BP: Bajo las Peñas; RC: Rocas Calientes.
Table A2.
Abundance of OTUs analyzed (>0.00025%) in Costa Rican hot springs, closely related sequence in GenBank database and growth temperature limits
| OTU | Accession N° | % of Total Reads | Closely related sequence (Accession N°)a | Similarity % | Phylum | Abundance | Growth Temp. (°C) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MV1b | MV2 | RN2 | RN3 | BP1 | BP2 | RC1 | RC2 | RC3 | |||||||
| OTU143 | MK040660 | 0.87 | Chloracidobacterium thermophilum B (CP002514) | 98 | Acidobacteria | 98 | 2 | 0 | 0 | 0 | 0 | 0 | 1803 | 533 | 42–60 |
| OTU1483 | MK040665 | 0.03 | Stenotrophobacter roseus strain Ac_15_C4 (NR146022) | 99 | Acidobacteria | 4 | 0 | 71 | 0 | 6 | 2 | 0 | 0 | 0 | 37–55 |
| OTU1423 | MK040659 | 0.05 | Uncultured Acidobacteria bacterium clone YNP_SBC_BP4_B26 (HM448257) | 98 | Acidobacteria | 0 | 0 | 0 | 0 | 0 | 0 | 140 | 0 | 0 | 63 |
| OTU1630 | MK077654 | 0.03 | Uncultured bacterium clone: OK06 (AB559014) | 99 | Acidobacteria | 38 | 2 | 17 | 11 | 0 | 1 | 0 | 0 | 2 | 37–60 |
| OTU1484 | MK040623 | 0.03 | Uncultured bacterium clone BJGMM−3s−108 (JQ800904) | 97 | Armatimonadetes | 17 | 0 | 0 | 39 | 0 | 0 | 0 | 3 | 11 | 59–60 |
| OTU550 | MK040716 | 0.1 | Uncultured bacterium clone HV‐16 (GU233849) | 99 | Armatimonadetes | 0 | 0 | 1 | 123 | 0 | 0 | 157 | 10 | 0 | 55–63 |
| OTU3506 | MK040695 | 0.18 | Uncultured bacterium clone Tat‐08‐003_12_23 ( GU437312) | 97 | Armatimonadetes | 8 | 0 | 3 | 22 | 0 | 0 | 133 | 255 | 76 | 55–63 |
| OTU1741 | MK040672 | 0.04 | Dyadobacter ginsengisoli strain: Gsoil 043(T) (AB245369) | 85 | Bacteroidetes | 0 | 1 | 0 | 0 | 106 | 0 | 0 | 0 | 2 | 42–60 |
| OTU2588 | MK040681 | 0.03 | Flexibacter ruber ATCC 23,103 (M58788) | 89 | Bacteroidetes | 0 | 0 | 0 | 0 | 0 | 97 | 0 | 0 | 0 | 37 |
| OTU2087 | MK077656 | 0.08 | Saprospira grandis (AB088636) | 84 | Bacteroidetes | 0 | 0 | 0 | 0 | 0 | 223 | 0 | 0 | 0 | 37 |
| OTU67 | MK040717 | 0.09 | Uncultured bacterium clone P060905_H09 (HQ385626) | 97 | Bacteroidetes | 0 | 0 | 229 | 7 | 0 | 2 | 0 | 0 | 0 | 37–59 |
| OTU447 | MK040712 | 0.04 | Uncultured bacterium gene for 16S rRNA, partial sequence, clone: BC10‐8 (AB580674) | 94 | Bacteroidetes | 0 | 123 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 42 |
| OTU2031 | MK040674 | 0.03 | Uncultured Bacteroidetes bacterium clone Uvmin2_8 (KJ611546) | 99 | Bacteroidetes | 0 | 78 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 42–55 |
| OTU3175 | MK040688 | 0.04 | Uncultured Bacteroidetes bacterium clone YNP_SBC_FC_B31 (HM448393) | 96 | Bacteroidetes | 0 | 0 | 0 | 0 | 0 | 0 | 98 | 3 | 0 | 59–63 |
| OTU90 | MK040722 | 0.49 | Uncultured Bacteroidetes bacterium clone YNP_SBC_MS3_B18 (HM448177) | 91 | Bacteroidetes | 0 | 0 | 0 | 10 | 0 | 0 | 301 | 400 | 656 | 59–63 |
| OTU29 | MK077660 | 0.07 | Uncultured Bacteroidetes bacterium clone YNP_SBC_MS3_B22 (HM448178) | 94 | Bacteroidetes | 9 | 155 | 18 | 5 | 7 | 0 | 0 | 0 | 8 | 42–60 |
| OTU59 | MK077666 | 0.16 | Uncultured Flavobacteriales bacterium clone ED5‐012 (FJ764420) | 88 | Bacteroidetes | 0 | 0 | 0 | 0 | 0 | 0 | 286 | 133 | 18 | 59–63 |
| OTU932 | MK040724 | 0.03 | Uncultured Sphingobacteriales bacterium clone L2‐2 (JF703526) | 92 | Bacteroidetes | 0 | 68 | 1 | 0 | 1 | 2 | 0 | 0 | 0 | 37–55 |
| OTU34 | MK040637 | 0.03 | Uncultured Sphingobacteriales bacterium clone ST31 (JQ723651) | 95 | Bacteroidetes | 0 | 0 | 2 | 0 | 0 | 81 | 0 | 0 | 0 | 37–55 |
| OTU175 | MK077655 | 0.09 | Uncultured Sphingobacterium sp. clone QLBB088 (AY862023) | 85 | Bacteroidetes | 251 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 42–60 |
| OTU4172 | MK040709 | 0.15 | Ignavibacterium album(T) (CP003418) | 97 | Chlorobi | 158 | 0 | 22 | 77 | 0 | 0 | 0 | 102 | 60 | 55–60 |
| OTU27 | MK077659 | 0.07 | Uncultured bacterium clone: HAuD‐LB4(AB113613) | 86 | Chlorobi | 51 | 0 | 17 | 120 | 0 | 0 | 0 | 0 | 0 | 55–59 |
| OTU3864 | MK040701 | 0.03 | Uncultured Bacteroidetes/Chlorobi group bacterium clone SM1A03 (AF445646) | 89 | Chlorobi | 0 | 70 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 42–59 |
| OTU2226 | MK040677 | 0.05 | Uncultured Chlorobi bacterium clone Aug‐VN130 (JQ795339) | 95 | Chlorobi | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 63 | 80 | 59–60 |
| OTU913 | MK077669 | 0.05 | Uncultured Chlorobi bacterium clone SM2A03 (AF445706) | 98 | Chlorobi | 51 | 0 | 7 | 10 | 0 | 0 | 0 | 5 | 62 | 55–60 |
| OTU102 | MK040646 | 0.24 | Uncultured sludge bacterium A12b (AF234699) | 86 | Chlorobi | 6 | 0 | 1 | 18 | 0 | 0 | 442 | 156 | 51 | 55–63 |
| OTU3668 | MK040697 | 0.03 | Caldilinea aerophila DSM 14535(T) (AP012337) | 97 | Chloroflexi | 0 | 0 | 0 | 17 | 0 | 0 | 50 | 20 | 0 | 59–63 |
| OTU19 | MK040635 | 0.17 | Chloroflexi bacterium Um‐2 (KP341999) | 93 | Chloroflexi | 8 | 2 | 442 | 14 | 0 | 0 | 0 | 0 | 0 | 42–59 |
| OTU119 | MK040632 | 4.76 | Chloroflexus aurantiacus J‐10‐fl(T) (D38365) | 97 | Chloroflexi | 1818 | 0 | 20 | 7 | 2 | 0 | 6,532 | 2,142 | 2,731 | 55–63 |
| OTU1488 | MK040666 | 0.81 | Chloroflexus aurantiacus J‐10‐fl(T) (D38365) | 99 | Chloroflexi | 653 | 1 | 954 | 640 | 0 | 0 | 0 | 0 | 0 | 42–59 |
| OTU40 | MK040707 | 7.13 | Chloroflexus aurantiacus J‐10‐fl(T) (D38365) | 97 | Chloroflexi | 3,809 | 2 | 1,299 | 908 | 2 | 0 | 8,252 | 2,589 | 3,010 | 42–63 |
| OTU472 | MK040714 | 0.09 | Chloroflexus aurantiacus J‐10‐fl(T) (D38365) | 97 | Chloroflexi | 175 | 0 | 0 | 1 | 0 | 0 | 40 | 13 | 24 | 59–63 |
| OTU82 | MK040641 | 0.07 | Chloroflexus aurantiacus J‐10‐fl(T) (D38365) | 95 | Chloroflexi | 200 | 0 | 2 | 1 | 0 | 0 | 0 | 1 | 0 | 55–59 |
| OTU116 | MK040630 | 1.29 | Roseiflexus castenholzii(T) DSM 13941 (CP000804) | 95 | Chloroflexi | 286 | 0 | 168 | 1572 | 0 | 0 | 895 | 408 | 266 | 55–63 |
| OTU2706 | MK040683 | 0.04 | Roseiflexus castenholzii(T) DSM 13941 (CP000804) | 92 | Chloroflexi | 25 | 0 | 1 | 19 | 0 | 0 | 39 | 18 | 20 | 55–63 |
| OTU4275 | MK040710 | 0.03 | Roseiflexus castenholzii(T) DSM 13941 (CP000804) | 91 | Chloroflexi | 8 | 63 | 3 | 0 | 15 | 2 | 0 | 0 | 4 | 37–60 |
| OTU1134 | MK040650 | 0.05 | Roseiflexus sp. RS‐1 (CP000686) | 91 | Chloroflexi | 93 | 0 | 9 | 32 | 0 | 0 | 0 | 0 | 0 | 55–59 |
| OTU2101 | MK040676 | 0.21 | Uncultured Anaerolinea sp. clone AE1b_G7 (KC211795) | 94 | Chloroflexi | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 233 | 354 | 59–60 |
| OTU819 | MK040720 | 0.22 | Uncultured bacterium clone AKIW403 (DQ129386) | 90 | Chloroflexi | 0 | 0 | 0 | 0 | 179 | 424 | 0 | 0 | 0 | 37–50 |
| OTU2402 | MK077657 | 0.06 | Uncultured bacterium clone B25 (AF407718) | 100 | Chloroflexi | 69 | 0 | 96 | 0 | 0 | 0 | 0 | 1 | 0 | 55–59 |
| OTU939 | MK077670 | 0.03 | Uncultured bacterium clone B25r (KJ766177) | 95 | Chloroflexi | 0 | 88 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 42 |
| OTU3240 | MK077662 | 0.03 | Uncultured bacterium clone BBL‐OTU64 (JQ791637) | 88 | Chloroflexi | 3 | 71 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 42–49 |
| OTU4012 | MK040708 | 0.05 | Uncultured bacterium clone FCPN412 (EF516361) | 89 | Chloroflexi | 0 | 0 | 0 | 0 | 148 | 0 | 0 | 0 | 0 | 50 |
| OTU1433 | MK040661 | 0.07 | Uncultured bacterium clone SM2G06 (AF445738) | 98 | Chloroflexi | 24 | 0 | 173 | 4 | 0 | 0 | 0 | 0 | 0 | 55–59 |
| OTU126 | MK040655 | 0.53 | Uncultured bacterium clone Tat‐08‐003_12_54 (GU437328) | 97 | Chloroflexi | 932 | 1 | 10 | 89 | 0 | 0 | 53 | 118 | 260 | 42–63 |
| OTU139 | MK077651 | 0.11 | Uncultured bacterium clone Tat‐08‐003_12_54 (GU437328) | 96 | Chloroflexi | 180 | 0 | 0 | 23 | 0 | 0 | 15 | 29 | 69 | 59–63 |
| OTU17 | MK040624 | 0.62 | Uncultured Chloroflexi bacterium clone DTB125 (EF205529) | 94 | Chloroflexi | 0 | 0 | 0 | 0 | 0 | 1 | 1,155 | 438 | 132 | 37–63 |
| OTU3490 | MK077663 | 0.03 | Uncultured Chloroflexi bacterium clone IAFpp7112 (GU214126) | 93 | Chloroflexi | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 82 | 60 |
| OTU78 | MK077668 | 0.39 | Uncultured Chloroflexi bacterium clone IAFpp722 (GU214145) | 98 | Chloroflexi | 0 | 5 | 1,062 | 31 | 0 | 0 | 0 | 0 | 2 | 42–60 |
| OTU559 | MK077664 | 0.07 | Uncultured Chloroflexi bacterium clone OTU52 (HQ416798) | 97 | Chloroflexi | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 111 | 75 | 59–63 |
| OTU562 | MK077665 | 0.07 | Uncultured Chloroflexi bacterium clone Pink_D09 (GQ483857) | 91 | Chloroflexi | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 15 | 173 | 55–60 |
| OTU142 | MK077652 | 0.07 | Uncultured Chloroflexi bacterium clone QEDN8AA01 (CU926200) | 94 | Chloroflexi | 25 | 3 | 46 | 29 | 7 | 1 | 18 | 46 | 7 | 37–63 |
| OTU3007 | MK040686 | 0.25 | Uncultured Chloroflexus sp. clone: 20‐91‐ArvAB (AB425067) | 91 | Chloroflexi | 5 | 643 | 9 | 4 | 0 | 0 | 0 | 0 | 41 | 42–60 |
| OTU103 | MK077649 | 0.43 | Uncultured Kouleothrix sp. clone M2‐008 (KF183047) | 98 | Chloroflexi | 0 | 1,111 | 3 | 0 | 0 | 89 | 0 | 0 | 0 | 37–55 |
| OTU889 | MK040721 | 0.06 | Uncultured soil bacterium clone 1_D9 (EU589265) | 95 | Chloroflexi | 5 | 126 | 41 | 1 | 0 | 0 | 0 | 0 | 0 | 42–59 |
| OTU1605 | MK040670 | 0.04 | Ancylothrix terrestris 13PC (KT819202) | 95 | Cyanobacteria | 2 | 0 | 0 | 0 | 100 | 4 | 0 | 0 | 0 | 37–50 |
| OTU71 | MK040625 | 6.19 | Ancylothrix terrestris 13PC (KT819202) | 98 | Cyanobacteria | 4 | 4 | 0 | 0 | 17,063 | 153 | 0 | 0 | 10 | 37–60 |
| OTU12 | MK040653 | 8.8 | Chlorogloeopsis sp, Greenland_5 (DQ431000) | 98 | Cyanobacteria | 1 | 0 | 7 | 24,493 | 0 | 0 | 0 | 0 | 0 | 55–59 |
| OTU3380 | MK040693 | 0.04 | Cyanothece sp. 2.6 (KJ654305) | 97 | Cyanobacteria | 0 | 73 | 31 | 0 | 0 | 0 | 0 | 0 | 0 | 42–55 |
| OTU1171 | MK040651 | 0.08 | Fischerella sp. MV11 (DQ786169) | 97 | Cyanobacteria | 60 | 11 | 20 | 8 | 1 | 0 | 0 | 37 | 87 | 42–60 |
| OTU134 | MK040656 | 24.9 | Fischerella sp. MV11 (DQ786169) | 99 | Cyanobacteria | 5,700 | 577 | 14,721 | 3,317 | 1842 | 5 | 0 | 11,323 | 31,859 | 37–60 |
| OTU1553 | MK040669 | 0.04 | Fischerella sp. MV11 (DQ786169) | 95 | Cyanobacteria | 63 | 7 | 0 | 0 | 0 | 0 | 0 | 3 | 27 | 42–60 |
| OTU163 | MK040671 | 0.07 | Fischerella sp. MV11 (DQ786169) | 94 | Cyanobacteria | 74 | 0 | 1 | 1 | 2 | 1 | 0 | 8 | 117 | 37–60 |
| OTU2353 | MK040680 | 0.04 | Fischerella sp. MV11 (DQ786169) | 92 | Cyanobacteria | 16 | 0 | 12 | 10 | 0 | 0 | 0 | 10 | 60 | 55–60 |
| OTU366 | MK040696 | 0.15 | Fischerella sp. MV11 (DQ786169) | 96 | Cyanobacteria | 297 | 21 | 3 | 0 | 0 | 0 | 0 | 14 | 79 | 42–60 |
| OTU763 | MK040718 | 0.05 | Fischerella sp. MV11 (DQ786169) | 95 | Cyanobacteria | 0 | 0 | 129 | 0 | 0 | 0 | 0 | 0 | 0 | 55 |
| OTU790 | MK040719 | 0.12 | Fischerella sp. MV11 (DQ786169) | 95 | Cyanobacteria | 21 | 33 | 63 | 61 | 20 | 0 | 0 | 36 | 99 | 42–60 |
| OTU48 | MK040639 | 0.28 | Leptolyngbya O77 (AP017367) | 97 | Cyanobacteria | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 33 | 736 | 55–60 |
| OTU85 | MK040644 | 0.21 | Leptolyngbya ramosa PUPCCC (KM376988) | 97 | Cyanobacteria | 0 | 0 | 230 | 0 | 0 | 9 | 303 | 41 | 1 | 37–63 |
| OTU110 | MK040649 | 3.61 | Leptolyngbya sp, BX10 (HM151385) | 98 | Cyanobacteria | 2 | 0 | 0 | 0 | 2,854 | 7,186 | 0 | 0 | 4 | 37–60 |
| OTU3263 | MK040689 | 0.04 | Leptolyngbya sp. LEGE 07319 (HM217045) | 94 | Cyanobacteria | 0 | 0 | 5 | 0 | 1 | 0 | 0 | 5 | 105 | 50–60 |
| OTU3974 | MK040704 | 0.05 | Leptolyngbya sp. LEGE 07319 (HM217045) | 91 | Cyanobacteria | 0 | 141 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 42 |
| OTU3318 | MK040691 | 0.09 | Limnothrix redekei CCAP 1459/29 (HE974998) | 96 | Cyanobacteria | 0 | 0 | 0 | 0 | 173 | 65 | 0 | 0 | 0 | 37–50 |
| OTU124 | MK040654 | 3.81 | Limnothrix sp, B15 (GQ848190) | 98 | Cyanobacteria | 3 | 1,037 | 0 | 0 | 266 | 9,295 | 0 | 0 | 0 | 37–50 |
| OTU2610 | MK040682 | 0.05 | Limnothrix sp, B15 (GQ848190) | 96 | Cyanobacteria | 0 | 140 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 37–42 |
| OTU117 | MK040631 | 0.95 | Limnothrix sp, CENA545 (KF246506) | 96 | Cyanobacteria | 0 | 0 | 0 | 0 | 16 | 2,622 | 0 | 0 | 0 | 37–50 |
| OTU93 | MK040642 | 0.07 | Lyngbya wollei (EU603708) | 97 | Cyanobacteria | 0 | 48 | 148 | 0 | 0 | 0 | 0 | 0 | 0 | 42–55 |
| OTU3444 | MK040694 | 0.03 | Lyngbya wollei (EU603709) | 92 | Cyanobacteria | 0 | 32 | 59 | 0 | 0 | 0 | 0 | 0 | 0 | 42–55 |
| OTU296 | MK040685 | 0.09 | Microcoleus sp. PCC 7113 (CP003630) | 94 | Cyanobacteria | 0 | 0 | 260 | 0 | 0 | 0 | 0 | 1 | 0 | 55–59 |
| OTU2714 | MK040684 | 0.11 | Oscillatoriales cyanobacterium JSC‐1 (FJ788926) | 99 | Cyanobacteria | 0 | 0 | 309 | 2 | 0 | 0 | 0 | 0 | 3 | 55–60 |
| OTU1462 | MK040636 | 0.04 | Phormidium animale SAG 1459‐6 (EF654087) | 92 | Cyanobacteria | 0 | 0 | 0 | 0 | 60 | 51 | 0 | 0 | 1 | 37–60 |
| OTU1537 | MK040667 | 0.15 | Phormidium sp, DVL1003c (JQ771628) | 96 | Cyanobacteria | 0 | 0 | 0 | 0 | 49 | 371 | 0 | 0 | 0 | 37–50 |
| OTU0 | MK040626 | 0.67 | Synechococcus lividus C1 (AF132772) | 99 | Cyanobacteria | 0 | 0 | 938 | 380 | 0 | 0 | 2 | 355 | 199 | 55–63 |
| OTU21 | MK040675 | 0.63 | Synechococcus sp, JA‐3‐3Ab genotype A‐NACy05a (AY884052) | 96 | Cyanobacteria | 0 | 0 | 0 | 0 | 0 | 0 | 1737 | 17 | 4 | 59–63 |
| OTU141 | MK040658 | 13.81 | Uncultured bacteriumclone: B1001R003_P01 (AB659771) | 97 | Cyanobacteria | 2 | 37,959 | 503 | 2 | 0 | 0 | 0 | 0 | 1 | 42–60 |
| OTU99 | MK040726 | 0.08 | Uncultured bacteriumclone: B1001R003_P01 (AB659771) | 94 | Cyanobacteria | 0 | 214 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 42–55 |
| OTU140 | MK040634 | 5.16 | Uncultured Oscillatoriales cyanobacterium clone E3‐00YK9 (EU376433) | 98 | Cyanobacteria | 10,539 | 44 | 2 | 10 | 668 | 0 | 0 | 518 | 2,584 | 42–60 |
| OTU25 | MK077658 | 0.03 | Uncultured Oscillatoriales cyanobacterium clone H_10 (FJ490330) | 86 | Cyanobacteria | 0 | 0 | 95 | 0 | 0 | 0 | 0 | 0 | 0 | 55 |
| OTU3924 | MK040702 | 0.03 | Uncultured Firmicutes bacterium clone D2D09 (EU753609) | 91 | Firmicutes | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 90 | 0 | 55–59 |
| OTU127 | MK040628 | 0.05 | Uncultured bacterium clone Drod‐B13 (FJ206764) | 99 | Planctomycetes | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 34 | 95 | 59–60 |
| OTU1271 | MK077650 | 0.04 | Uncultured bacterium clone Drod‐B45 (FJ206785) | 89 | Planctomycetes | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 94 | 59–60 |
| OTU3173 | MK077661 | 0.03 | Uncultured bacterium isolate 1112865250968 (HQ119290) | 85 | Planctomycetes | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 83 | 59–60 |
| OTU1829 | MK040673 | 0.04 | Altererythrobacter dongtanensis JM27(T) (GU166344) | 97 | Proteobacteria | 0 | 11 | 91 | 0 | 1 | 0 | 0 | 0 | 0 | 42–55 |
| OTU118 | MK040652 | 0.1 | Elioraea tepidiphila DSM 17972(T) (KB899943) | 98 | Proteobacteria | 0 | 0 | 127 | 21 | 0 | 0 | 0 | 61 | 58 | 55–60 |
| OTU1452 | MK040663 | 0.04 | Erythrobacter sp. 5IX/A01/140 (AY576736 | 98 | Proteobacteria | 0 | 25 | 74 | 14 | 9 | 1 | 0 | 0 | 1 | 37–60 |
| OTU132 | MK040633 | 0.08 | Haliangium tepidum SMP‐10(T) (AB062751) | 92 | Proteobacteria | 0 | 233 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 42–55 |
| OTU227 | MK040679 | 0.06 | Hydrogenophaga defluvii strain BSB 9.5(T) (NR029024) | 95 | Proteobacteria | 0 | 134 | 23 | 0 | 0 | 0 | 0 | 0 | 0 | 42–55 |
| OTU4289 | MK040711 | 0.05 | KY386562 Polymorphobacter sp. strain R‐68699 (KY386562) | 95 | Proteobacteria | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 11 | 114 | 55–60 |
| OTU31 | MK040687 | 0.14 | Lacibacterium aquatile LTC‐2(T) (HE795994) | 92 | Proteobacteria | 0 | 384 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 42–55 |
| OTU1068 | MK040647 | 0.04 | Leptothrix mobilis strain Feox‐1 DSM10617(T) (NR026333) | 97 | Proteobacteria | 0 | 0 | 1 | 0 | 0 | 122 | 0 | 0 | 0 | 37–55 |
| OTU1471 | MK040664 | 0.03 | Lysobacter thermophilus strain YIM 77875 (JQ746036) | 99 | Proteobacteria | 0 | 1 | 73 | 0 | 0 | 0 | 0 | 0 | 0 | 42–55 |
| OTU108 | MK040629 | 0.07 | Piscinibacter defluvii SH‐1(T) (KU667249) | 98 | Proteobacteria | 0 | 194 | 0 | 0 | 1 | 13 | 0 | 0 | 0 | 37–50 |
| OTU3722 | MK040699 | 0.15 | Polyangium spumosum strain Pl sm5 (GU207881) | 94 | Proteobacteria | 0 | 416 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 42–50 |
| OTU3822 | MK040700 | 0.06 | Porphyrobacter cryptus ALC‐2 (T) (AF465834) | 99 | Proteobacteria | 0 | 47 | 102 | 11 | 1 | 2 | 0 | 0 | 3 | 37–60 |
| OTU3955 | MK040703 | 0.03 | Pseudorhodoplanes sinuspersici strain RIPI 110 (NR145909) | 97 | Proteobacteria | 0 | 15 | 51 | 0 | 6 | 0 | 0 | 1 | 1 | 42–60 |
| OTU1373 | MK040657 | 0.03 | Rubritepida flocculans DSM 14296(T) (AF465832) | 98 | Proteobacteria | 0 | 0 | 59 | 15 | 0 | 0 | 0 | 0 | 0 | 55–59 |
| OTU3321 | MK040692 | 0.14 | Salinarimonas ramus strain SL014B‐41A4 (NR108683) | 95 | Proteobacteria | 0 | 0 | 393 | 0 | 0 | 0 | 0 | 0 | 0 | 55 |
| OTU1 | MK040627 | 0.08 | Tabrizicola aquatica strain RCRI19(T) (HQ392507) | 99 | Proteobacteria | 0 | 167 | 43 | 0 | 0 | 2 | 0 | 0 | 0 | 37–55 |
| OTU1542 | MK040668 | 0.05 | Tepidimonas taiwanensis I1‐1(T) (AY845054) | 98 | Proteobacteria | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 41 | 90 | 59–60 |
| OTU3716 | MK040698 | 0.04 | Thermophilic methanotroph HB (U89299) | 92 | Proteobacteria | 0 | 0 | 0 | 0 | 0 | 0 | 38 | 11 | 60 | 59–63 |
| OTU101 | MK040645 | 0.15 | Uncultured bacterium clone JulG‐B86 (FJ206635) | 96 | Proteobacteria | 0 | 0 | 10 | 134 | 0 | 0 | 97 | 111 | 65 | 55–63 |
| OTU464 | MK040713 | 0.03 | Uncultured bacterium clone kab116 (FJ936833) | 95 | Proteobacteria | 38 | 0 | 0 | 1 | 0 | 0 | 1 | 24 | 17 | 59–63 |
| OTU1092 | MK040648 | 0.03 | Uncultured bacterium clone NC24c1_18286 (JQ368669) | 88 | Proteobacteria | 0 | 0 | 0 | 0 | 78 | 0 | 0 | 0 | 0 | 50 |
| OTU907 | MK040723 | 0.03 | Uncultured bacterium clone: B1001R003_P01.(AB659771) | 94 | Proteobacteria | 0 | 74 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 42 |
| OTU609 | MK077667 | 0.03 | Uncultured bacterium partial clone RNB‐C147 (LN680248) | 92 | Proteobacteria | 0 | 0 | 0 | 0 | 0 | 0 | 42 | 18 | 14 | 59–63 |
| OTU96 | MK040643 | 0.14 | Uncultured beta proteobacterium clone Aug‐CD266 (JQ795254) | 96 | Proteobacteria | 7 | 65 | 22 | 2 | 1 | 2 | 0 | 2 | 290 | 37–60 |
| OTU6 | MK040640 | 0.07 | Uncultured Haliangium sp. clone Pad‐72 J (X505319) | 98 | Proteobacteria | 0 | 0 | 201 | 0 | 0 | 0 | 0 | 0 | 0 | 55 |
| OTU3992 | MK040705 | 0.07 | Uncultured Rhodocyclaceae bacterium clone Elev_16S_555 (EF019343) | 91 | Proteobacteria | 0 | 168 | 1 | 0 | 2 | 15 | 0 | 0 | 0 | 37–55 |
| OTU3272 | MK040690 | 0.04 | Uncultured bacterium clone FFCH13324 (EU135381) | 92 | Saccharibacteria | 0 | 0 | 0 | 0 | 2 | 107 | 0 | 0 | 0 | 37–50 |
| OTU1458 | MK077653 | 0.04 | Leptonema illini DSM 21528 (JH597773) | 82 | Spirochaetes | 0 | 0 | 0 | 120 | 0 | 0 | 0 | 0 | 0 | 59 |
| OTU2227 | MK040678 | 0.05 | Meiothermus hypogaeus AZM34c11(T) (AB586707) | 96 | Deinococcus‐Thermus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 24 | 121 | 60 |
| OTU41 | MK040638 | 0.03 | Meiothermus hypogaeus AZM34c11(T) (AB586707) | 97 | Deinococcus‐Thermus | 1 | 0 | 40 | 4 | 0 | 0 | 0 | 0 | 30 | 55–60 |
| OTU49 | MK040715 | 0.32 | Meiothermus ruber DSM 1,279(T) (CP001743) | 95 | Deinococcus‐Thermus | 0 | 0 | 26 | 609 | 144 | 1 | 41 | 56 | 4 | 37–63 |
| OTU957 | MK040725 | 0.38 | Meiothermus ruber DSM 1279(T) (CP001743) | 99 | Deinococcus‐Thermus | 0 | 0 | 6 | 102 | 0 | 0 | 783 | 151 | 22 | 55–63 |
| OTU1443 | MK040662 | 0.16 | Meiothermus terrae YIM 77755(T) (KF603888) | 98 | Deinococcus‐Thermus | 0 | 0 | 435 | 5 | 0 | 0 | 0 | 0 | 0 | 55–59 |
| OTU4 | MK040706 | 0.71 | Thermus oshimai strain SPS‐17(T) (Y18416) | 97 | Deinococcus‐Thermus | 0 | 0 | 0 | 0 | 0 | 0 | 1978 | 11 | 2 | 59–63 |
When possible, we use only published or type strain reference sequences to compare with OTU sequences from this work.
Río Negro (RN), Miravalles (MV), Bajo las Peñas (BP), Rocas Calientes (RC).
Figure A1.
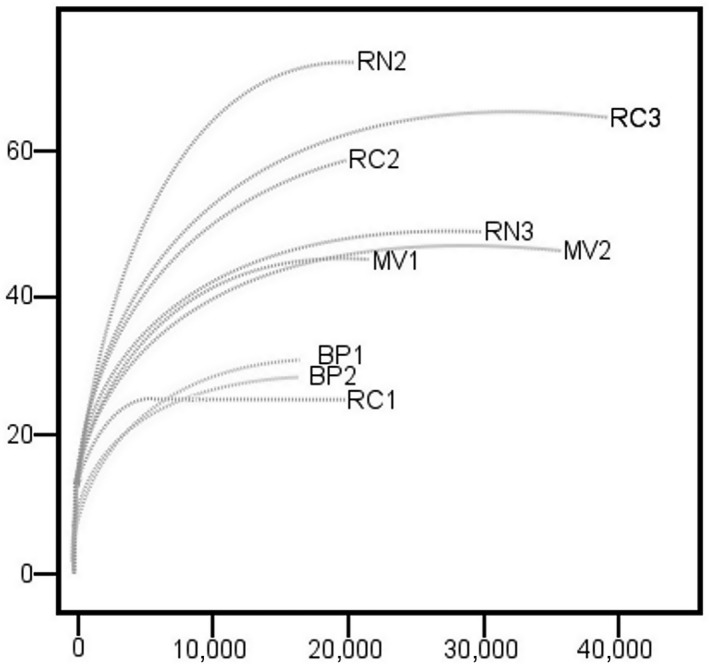
Rarefaction curves for gene sequences from nine hot spring samples. Río Negro (RN), Miravalles (MV), Bajo las Peñas (BP), Rocas Calientes (RC)
Figure A2.
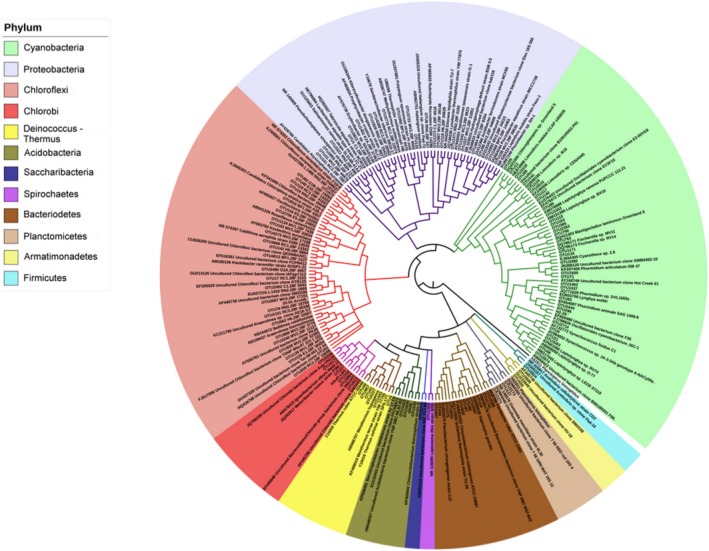
Bayesian tree based on 16S rRNA gene sequences showing the positions of the 126 most abundant OTUs present in samples of hot spring microbial mat communities and their closest sequences in GenBank. Planctopirus limnophilus was used as outgroup. The image was generated using the interactive Tree of Life (ITOL; http://itol.embl.de/)
Figure A3.
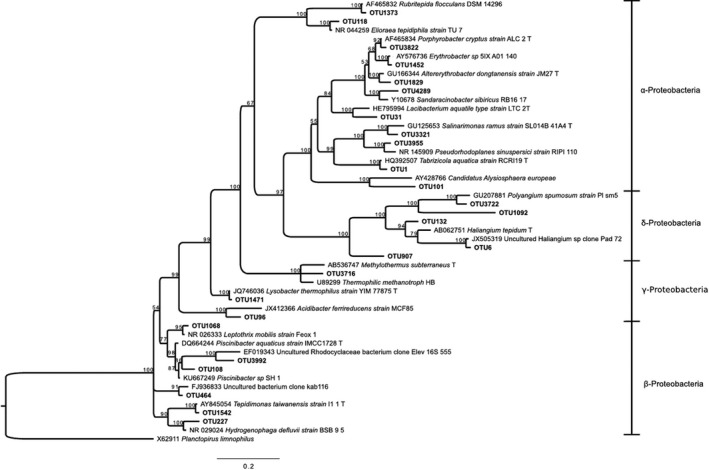
Bayesian tree based on 16S rRNA gene sequences showing the positions of OTUs classified as Proteobacteria. Bootstrap values based on 10,000,000 replications are shown at branch nodes. Planctopirus limnophilus was used as outgroup. Bar shows 0.2 substitutions per nucleotide
Figure A4.
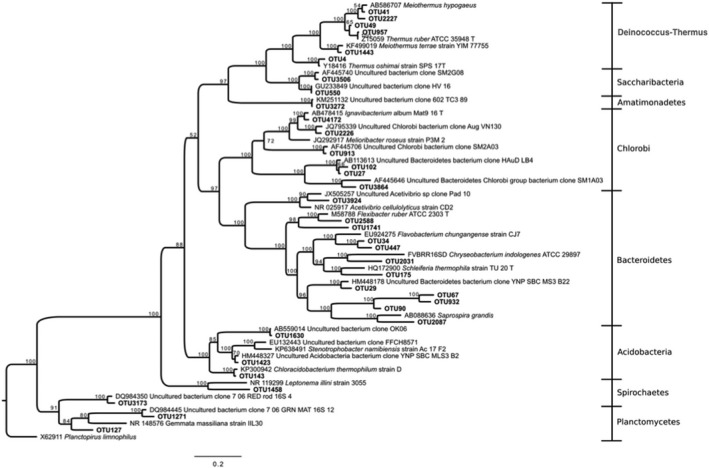
Bayesian tree based on 16S rRNA gene sequences showing the positions of OTUs classified as Deinococcus‐Thermus, Bacteroidetes, and Acidobacteria. Bootstrap values based on 10,000,000 replications are shown at branch nodes. Planctopirus limnophilus was used as outgroup. Bar shows 0.2 substitutions per nucleotide
Uribe‐Lorío L, Brenes L, Hernández‐Ascencio W, et al. The influence of temperature and pH on bacterial community composition of microbial mats in hot springs from Costa Rica. MicrobiologyOpen. 2019;8:e893 10.1002/mbo3.893
DATA AVAILABILITY STATEMENT
Sequences from the 126 OTUs generated and analyzed during this study are available in the NCBI GenBank database under accession numbers MK040623‐MK040726 and MK077649‐MK077670.
REFERENCES
- Alcamán, M. , Alcorta, J. , Bergman, B. , Vásquez, M. , Polz, M. , & Díez, B. (2017). Physiological and gene expression responses to nitrogen regimes and temperatures in Mastigocladus sp. strain CHP1, a predominant thermotolerant cyanobacterium of hot springs. Systematic and Applied Microbiology, 40, 102–113. 10.1016/j.syapm.2016.11.007 [DOI] [PubMed] [Google Scholar]
- Allewalt, J. P. , Bateson, M. M. , Revsbech, N. P. , Slack, K. , & Ward, D. M. (2006). Effect of temperature and light on growth of and photosynthesis by synechococcus isolates typical of those predominating in the octopus spring microbial mat community of Yellowstone National Park. Applied and Environment Microbiology, 72(1), 544–550. 10.1128/AEM.72.1.544-550.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado, G. E. , & Vargas, A. (2017). Historia del descubrimiento y aprovechamiento de las fuentes termales en Costa Rica. Revista Geológica De América Central, 57 10.15517/rgac.v0i57.30148 [DOI] [Google Scholar]
- Andreote, A. P. , Vaz, M. M. , Genuário, D. B. , Barbiero, L. , Rezende‐Filho, A. T. , & Fiore, M. F. (2014). Nonheterocytous cyanobacteria from Brazilian saline‐alkaline lakes. Journal of Phycology, 50(4), 675–684. 10.1111/jpy.12192 [DOI] [PubMed] [Google Scholar]
- Becraft, E. D. , Frederick, M. C. , Kühl, M. , Jensen, S. I. , & Ward, D. M. (2011). Fine‐scale distribution patterns of synechococcus ecological diversity in microbial mats of mushroom spring, Yellowstone National Park. Applied and Environmental Microbiology, 77(21), 7689–7697. 10.1128/AEM.05927-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragado‐Massa, E. , Marchamalo, M. , Rejas, J. G. , Bonatti, J. , Martínez‐Frías, J. , & Martínez, R. (2014). Monitoring hydrothermal alteration in active volcanoes using remote sensing: The case of Turrialba volcano (Costa Rica). Revista Geológica De América Central, 51, 69–82. [Google Scholar]
- Bryant, D. , & Frigaard, N. (2006). Prokaryotic photosynthesis and phototrophy illuminated. Trends in Microbiology, 14(11), 488–496. 10.1016/j.tim.2006.09.001 [DOI] [PubMed] [Google Scholar]
- Bundschuch, J. , Birkle, P. , Finch, R. , Day, M. , Romero, J. , Paniagua, S. , et al. (2007). Geology‐related tourism for sustainable development In Bundschuch J., & Alvarado G. E. (Ed.), Central America: Geology, resources and hazards. London, UK: Taylor Y Francis; Retrieved from https://trove.nla.gov.au/work/32195195?q&sort=holdings+desc&_=1559607701227&versionId=39113965 [Google Scholar]
- Caldwell, S. , Liu, Y. , Ferrera, I. , Beveridge, T. , & Reysenbach, A. (2010). Thermocrinis minervae sp. nov., a hydrogen‐ and sulfur‐oxidizing, thermophilic member of the Aquificales from a Costa Rican terrestrial hot spring. International Journal of Systematic and Evolutionary Microbiology, 60(2), 338–343. 10.1099/ijs.0.010496-0 [DOI] [PubMed] [Google Scholar]
- Caporaso, J. G. , Kuczynski, J. , Stombaugh, J. , Bittinger, K. , Bushman, F. D. , Costello, E. K. , … Knight, R. (2010). QIIME allows analysis of high‐throughput community sequencing data. Nature Methods, 7, 335–336. 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castenholz, R. W. , Wilmotte, A. , Herdman, M. , Rippka, R. , Waterbury, J. B. , Iteman, I. , … Phylum, B. X. (2001). Cyanobacteria In Boone D. R., Castenholz R. W., & Garrity G. M. (Eds.), Bergey’s Manual® of Systematic Bacteriology (pp. 721). Published by John Wiley & Sons, Inc., in association with Bergey's Manual Trust. [Google Scholar]
- Chen, M. , Li, X. , Yang, Q. , Chi, X. , Pan, L. , Chen, N. A. , … Yu, S. (2014). Dynamic succession of soil bacterial community during continuous cropping of peanut (Arachis hypogaea L.). PLoS ONE, 9(7), e101355 10.1371/journal.pone.0101355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun, J. , Kim, K. Y. , Lee, J.‐H. , & Choi, Y. (2010). The analysis of oral microbial communities of wild‐type and toll‐like receptor 2‐deficient mice using a 454 GS FLX Titanium pyrosequencer. BMC Microbiology. 6 De Abril De, 10(1), 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke, K. R. , & Gorley, R. (2015). PRIMER v7: User Manual/Tutorial (pp. 296). Plymouth, UK: PRIMER‐E. [Google Scholar]
- Cole, J. K. , Peacock, J. P. , Dodsworth, J. A. , Williams, A. J. , Thompson, D. B. , Dong, H. , … Hedlund, B. P. (2013). Sediment microbial communities in Great Boiling Spring are controlled by temperature and distinct from water communities. The ISME Journal, 7(4), 718–729. 10.1038/ismej.2012.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai, X. , Wang, H. , Zhang, Z. , Li, K. , Zhang, X. , Mora‐López, M. , … Huang, L. I. (2016). Genome sequencing of sulfolobus sp. A20 from costa rica and comparative analyses of the putative pathways of carbon, nitrogen, and sulfur metabolism in various sulfolobus strains. Frontiers in Microbiology, 7, 1902 10.3389/fmicb.2016.01902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd, S. E. , Callaway, T. R. , Wolcott, R. D. , Sun, Y. , McKeehan, T. , Hagevoort, R. G. , & Edrington, T. S. (2008). Evaluation of the bacterial diversity in the feces of cattle using 16S rDNA bacterial tag‐encoded FLX amplicon pyrosequencing (bTEFAP). BMC Microbiology, 8(1), 125– 10.1186/1471-2180-8-125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everroad, R. , Otaki, H. , Matsuura, K. , & Haruta, S. (2012). Diversification of bacterial community composition along a temperature gradient at a thermal spring. Microbes and Environments, 27(4), 374–381. 10.1264/jsme2.ME11350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris, M. , Ruff‐Roberts, A. , Kopczynski, E. , Bateson, M. , & Ward, D. (1996). Enrichment culture and microscopy conceal diverse thermophilic Synechococcus populations in a single hot spring microbial mat habitat. Applied and Environment Microbiology, 62(3), 1045–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finsinger, K. , Scholz, I. , Serrano, A. , Morales, S. , Uribe‐Lorio, L. , Mora, M. , … Hess, W. R. (2008). Characterization of true‐branching cyanobacteria from geothermal sites and hot springs of Costa Rica. Environmental Microbiology, 10(2), 460–473. 10.1111/j.1462-2920.2007.01467.x [DOI] [PubMed] [Google Scholar]
- Hammer, Ø. , Harper, D. A. T. , & Ryan, P. D. (2001). PAST paleontological statistics software package for education and data analysis. Paleontología Electronica, 4, 9. [Google Scholar]
- Hernández, W. (2012). Characterization of Two Microbial Communities of Extreme Environment of Rincón de la Vieja National Park Volcano, Guanacaste, Costa Rica [Magister Scientiae in Microbiology]. Costa Rica: University of Costa Rica. [Google Scholar]
- Hongmei, J. , Aitchison, J. , Lacap, D. , Peerapornpisal, Y. , Sompong, U. , & Pointing, S. (2005). Community phylogenetic analysis of moderately thermophilic cyanobacterial mats from China, the Philippines and Thailand. Extremophiles, 9(4), 325–332. 10.1007/s00792-005-0456-1 [DOI] [PubMed] [Google Scholar]
- Huelsenbeck, J. P. , & Ronquist, F. (2001). MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics, 17(8), 754–755. 10.1093/bioinformatics/17.8.754 [DOI] [PubMed] [Google Scholar]
- Hynek, B. , Rogers, K. , Antunovich, M. , Avard, G. , & Alvarado, G. (2018). Lack of microbial diversity in an extreme mars analog setting: Poás Volcano, Costa Rica. Astrobiology, 18(7), 923–933. 10.1089/ast.2017.1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inskeep, W. P. , Jay, Z. J. , Tringe, S. G. , Herrgård, M. J. , & Rusch, D. B. (2013). YNP Metagenome Project Steering Committee and Working Group Members. The YNP metagenome project: Environmental parameters responsible for microbial distribution in the Yellowstone geothermal ecosystem. Frontiers in Microbiology, 4(67), 1–15. 10.3389/fmicb.2013.00067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionescu, D. , Hindiyeh, M. , Malkawi, H. , & Oren, A. (2010). Biogeography of thermophilic cyanobacteria: Insights from the Zerka Ma’in hot springs (Jordan). FEMS Microbiology Ecology, 72(1), 103–113. 10.1111/j.1574-6941.2010.00835.x [DOI] [PubMed] [Google Scholar]
- Itoh, H. , Ishii, S. , Shiratori, Y. , Oshima, K. , Otsuka, S. , Hattori, M. , & Senoo, K. (2013). Seasonal transition of active bacterial and archaeal communities in relation to water management in paddy soils. Microbes and Environments, 28(3), 370–380. 10.1264/jsme2.ME13030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez, D. J. , Andreote, F. D. , Chaves, D. , Montaña, J. S. , Osorio‐Forero, C. , Junca, H. , … Baena, S. (2012). Structural and functional insights from the metagenome of an acidic hot spring microbial planktonic community in the Colombian Andes. PLoS ONE, 7(12), e52069 10.1371/journal.pone.0052069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, B. K. , Lim, Y.‐W. , Kim, M. , Kim, S. , Chun, J. , Lee, J.‐H. , & Jung, Y. (2007). EzTaxon: A web‐based tool for the identification of prokaryotes based on 16S ribosomal RNA gene sequences. International Journal of Systematic and Evolutionary Microbiology, 57, 2259–2261. 10.1099/ijs.0.64915-0 [DOI] [PubMed] [Google Scholar]
- Komárek, J. , Kaštovsk, J. , Mareš, J. , & Johansen, J. R. (2014). Taxonomic classification of cyanoprokaryotes (cyanobacterial genera) 2014, using a polyphasic approach. Preslia, 86, 295–335. [Google Scholar]
- Larkin, M. A. , Blackshields, G. , Brown, N. P. , Chenna, R. , McGettigan, P. A. , McWilliam, H., … Higgins, D. G. (2007). Clustal W and Clustal X version 2.0. Bioinformatics, 23(21), 2947–2948. 10.1093/bioinformatics/btm404 [DOI] [PubMed] [Google Scholar]
- Lau, M. , Aitchison, J. , & Pointing, S. (2009). Bacterial community composition in thermophilic microbial mats from five hot springs in central Tibet. Extremophiles, 13(1), 139–149. 10.1007/s00792-008-0205-3 [DOI] [PubMed] [Google Scholar]
- Loginova, L. , & Egorova, L. (1975). Thermus ruber obligate thermophilic bacteria in the thermal springs of Kamchatka. Mikrobiologiia, 44(4), 661–665. [PubMed] [Google Scholar]
- López‐López, O. , Cerdán, M. E. , & González‐Siso, M. I. (2013). Hot spring metagenomics. Life, 3(2), 308–320. 10.3390/life3020308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins, M. , Rigonato, J. , Taboga, S. , & Branco, L. (2016). Proposal of Ancylothrix gen. nov., a new genus of Phormidiaceae (Cyanobacteria, Oscillatoriales) based on a polyphasic approach. International Journal of Systematic and Evolutionary Microbiology, 66(6), 2396–2405. 10.1099/ijsem.0.001044 [DOI] [PubMed] [Google Scholar]
- Menzel, P. , Gudbergsdóttir, S. R. , Rike, A. G. , Lin, L. , Zhang, Q. I. , Contursi, P. , … Peng, X. U. (2015). Comparative metagenomics of eight geographically remote terrestrial hot springs. Microbial Ecology, 70(2), 411–424. 10.1007/s00248-015-0576-9 [DOI] [PubMed] [Google Scholar]
- Miller, S. , & Castenholz, R. (2000). Evolution of thermotolerance in hot spring cyanobacteria of the genus Synechococcus. Applied and Environment Microbiology, 66(10), 4222–4231. 10.1128/AEM.66.10.4222-4229.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, S. , Castenholz, R. , & Pedersen, D. (2007). Phylogeography of the thermophilic cyanobacterium Mastigocladus laminosus. Applied and Environment Microbiology, 73(15), 4751–4760. 10.1128/AEM.02945-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, S. R. , Strong, A. L. , Jones, K. L. , & Ungerer, M. C. (2009). Bar‐coded pyrosequencing reveals shared bacterial community properties along the temperature gradients of two alkaline hot springs in Yellowstone National Park. Applied and Environment Microbiology, 75(13), 4565–4572. 10.1128/AEM.02792-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales, S. (2008). Morphological diversity and phylogenetic position of cyanobacteria found in hot springs and volcanoes of Costa Rica [Magister Scientiae in Microbiology]. Costa Rica: University of Costa Rica. [Google Scholar]
- Nakamori, H. , Yatabe, T. , Yoon, K. , & Ogo, S. (2014). Purification and characterization of an oxygen‐evolving photosystem II from Leptolyngbya sp. strain O‐77. Journal of Bioscience and Bioengineering, 118(2), 119–124. 10.1016/j.jbiosc.2014.01.009 [DOI] [PubMed] [Google Scholar]
- Obando, L. G. (2004). Geología y petrografía del Cerro Buenavista (Cerro de la Muerte) y alrededores, Costa Rica. Revista Geológica De América Central, 30, 31–39. [Google Scholar]
- Pagaling, E. , Grant, W. D. , Cowan, D. A. , Jones, B. E. , Ma, Y. , Ventosa, A. , & Heaphy, S. (2012). Bacterial and archaeal diversity in two hot spring microbial mats from the geothermal region of Tengchong, China. Extremophiles, 16(4), 607–618. 10.1007/s00792-012-0460-1 [DOI] [PubMed] [Google Scholar]
- Papke, R. , Ramsing, N. , Bateson, M. , & Ward, D. (2003). Geographical isolation in hot spring cyanobacteria. Environmental Microbiology, 5(8), 650–659. 10.1046/j.1462-2920.2003.00460.x [DOI] [PubMed] [Google Scholar]
- Pepe‐Ranney, C. , Berelson, W. , Corsetti, F. , Treants, M. , & Spear, J. (2012). Cyanobacterial construction of hot spring siliceous stromatolites in Yellowstone National Park. Environmental Microbiology, 14(5), 1182–1197. 10.1111/j.1462-2920.2012.02698.x [DOI] [PubMed] [Google Scholar]
- Portillo, M. , Sririn, V. , Kanoksilapatham, W. , & Gonzalez, J. (2009). Differential microbial communities in hot spring mats from Western Thailand. Extremophiles, 13(2), 321–331. 10.1007/s00792-008-0219-x [DOI] [PubMed] [Google Scholar]
- Power, J. F. , Carere, C. R. , Lee, C. K. , Wakerley, G. L. , Evans, D. W. , Button, M. , … Stott, M. B. (2018). Microbial biogeography of 1,000 geothermal springs in New Zealand. bioRxiv. 9(1), 1–12. 10.1038/s41467-018-05020-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quast, C. , Pruesse, E. , Yilmaz, P. , Gerken, J. , Schweer, T. , Yarza, P. , … Glöckner, F. O. (2013). The SILVA ribosomal RNA gene database project: Improved data processing and web‐based tools. Nucleic Acids Research, 41(D1), D590–D596. 10.1093/nar/gks1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . (2018) A language and environment for statistical computing R Foundation for Statistical Computing, Vienna, Austria. Retrieved from https://www.R-project.org/
- Roeselers, G. , Norris, T. B. , Castenholz, R. W. , Rysgaard, S. , Glud, R. N. , Kühl, M. , & Muyzer, G. (2007). Diversity of phototrophic bacteria in microbial mats from Arctic hot springs (Greenland). Environmental Microbiology, 9(1), 26–38. 10.1111/j.1462-2920.2006.01103.x [DOI] [PubMed] [Google Scholar]
- Ross, K. A. , Feazel, L. M. , Robertson, C. E. , Fathepure, B. Z. , Wright, K. E. , Turk‐MacLeod, R. M. , … Pace, N. R. (2012). Phototrophic phylotypes dominate mesothermal microbial mats associated with hot springs in Yellowstone National Park. Environmental Microbiology, 64(4), 162–170. 10.1007/s00248-012-0012-3 [DOI] [PubMed] [Google Scholar]
- Ruff‐Roberts, A. , Kuenen, J. , & Ward, D. (1994). Distribution of cultivated and uncultivated cyanobacteria and Chloroflexus‐like bacteria in hot spring microbial mats. Applied and Environment Microbiology, 60(2), 697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp, C. , Martínez‐Lorenzo, A. , Brady, A. , Grasby, S. , & Dunfield, P. (2014). Methanotrophic bacteria in warm geothermal spring sediments identified using stable‐isotope probing. FEMS Microbiology Ecology, 90(1), 92–102. 10.1111/1574-6941.12375 [DOI] [PubMed] [Google Scholar]
- Sittenfeld, A. , Mora, M. , Ortega, J. , Albertazzi, F. , Cordero, A. , Roncel, M. , … Serrano, A. (2002). Characterization of a photosynthetic Euglena strain isolated from an acidic hot mud pool of a volcanic area of Costa Rica. FEMS Microbiology Ecology, 42(1), 151–161. 10.1016/S0168-6496(02)00327-6 [DOI] [PubMed] [Google Scholar]
- Sittenfeld, A. , Vargas, M. , Sánchez, E. , Mora, M. , & Serrano, A. (2004). Una nueva especie de Euglena (Euglenozoa: Euglenales) aislada de ambientes extremófilos en las Pailas de Barro del Volcán Rincón de la Vieja, Costa Rica. Revista De Biología Tropical, 52(1), 27–30. [PubMed] [Google Scholar]
- Skirnisdottir, S. , Hreggvidsson, G. O. , Hjorleifsdottir, S. , Marteinsson, V. T. , Petursdottir, S. K. , Holst, O. , & Kristjansson, J. K. (2000). Influence of sulfide and temperature on species composition and community structure of hot spring microbial mats. Microbial Ecology, 66(7), 2835–3284. 10.1128/AEM.66.7.2835-2841.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimori, K. , Igarashi, H. , Martínez, M. , Duarte, E. , Fernández, E. , Malavassi, E. , … Valdés, J`. (2002). Microbial life in the acid lake and hot springs of Poás Volcano, Costa Rica. Colima Volcano International Meeting; Colima, Mexico.
- Tamura, K. , Stecher, G. , Peterson, D. , Filipski, A. , & Kumar, S. (2013). MEGA6: Molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution, 30(12), 2725–2729. 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tank, M. , Thiel, V. , Ward, D. M. , & Bryant, D. A. (2017). A panoply of phototrophs: An overview of the thermophilic chlorophototrophs of the microbial mats of alkaline siliceous hot springs in Yellowstone National Park, WY, USA In Hallenbeck P. C. (Ed.), Modern topics in the phototrophic prokaryotes. Cham, Switzerland: Springer. [Google Scholar]
- Tomitani, A. , Knoll, A. , Cavanaugh, C. M. , & Terufumi, O. (2006). The evolutionary diversification of cyanobacteria: Molecular–phylogenetic and paleontological perspectives. Proceedings of the National Academy of Sciences, 103(14), 5442–5447. 10.1073/pnas.0600999103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbieta, M. , González‐Toril, E. , Bazán, Á. , Giaveno, M. , & Donati, E. (2015). Comparison of the microbial communities of hot springs waters and the microbial biofilms in the acidic geothermal area of Copahue (Neuquén, Argentina). Extremophiles, 19(2), 437–450. 10.1007/s00792-015-0729-2 [DOI] [PubMed] [Google Scholar]
- von Huene, R. , Ranero, C. , Weinrebe, W. , & Hinz, K. (2000). Quaternary convergent margin tectonics of Costa Rica, segmentation of the Cocos Plate, and Central American volcanism. Tectonics, 19(2), 314–334. 10.1029/1999TC001143 [DOI] [Google Scholar]
- Wang, S. , Hou, W. , Dong, H. , Jiang, H. , Huang, L. , Wu, G. , et al. (2013). Control of temperature on microbial community structure in hot springs of the Tibetan Plateau. PLoS ONE, 9(6), e99751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward, D. , & Castenholz, R. (2000). Cyanobacteria in geothermal habitats In Whitton B., & Potts M. (Eds.), The ecology of cyanobacteria (pp. 669). Dordrecht, The Netherlands: Springer. [Google Scholar]
- Ward, L. M. , Hemp, J. , Shih, P. M. , McGlynn, S. E. , & Fischer, W. W. (2018). Evolution of phototrophy in the Chloroflexi phylum driven by horizontal gene transfer. Frontiers in Microbiology, 9 10.3389/fmicb.2018.00260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler, B. (2006). A biodiversity study of high temperature mud pool microbial communitities: Implications of regional/geographical isolation and endemism [Magister degree]. University of Delaware. [Google Scholar]
- Williams, R. , Smith, K. , Welch, S. , & Micallef, J. (1996). Thermus oshimai sp. nov., isolated from hot springs in Portugal, Iceland, and the Azores, and comment on the concept of a limited geographical distribution of Thermus species. International Journal of Systematic Bacteriology, 46(2), 403–408. 10.1099/00207713-46-2-403 [DOI] [PubMed] [Google Scholar]
- Yilmaz, P. , Parfrey, L. W. , Yarza, P. , Gerken, J. , Pruesse, E. , Quast, C. , … Glöckner, F. O. (2014). The SILVA and “All‐species Living Tree Project (LTP)” taxonomic frameworks. Nucleic Acids Research, 42(D1), D643–D648. 10.1093/nar/gkt1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus, J. , & Brock, T. D. (1972). Effects of thermal additions from the Yellowstone Geyser Basins on the bacteriology of the Firehole River. Ecology, 53(2), 283–290. 10.2307/1934083 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Sequences from the 126 OTUs generated and analyzed during this study are available in the NCBI GenBank database under accession numbers MK040623‐MK040726 and MK077649‐MK077670.


