Abstract
The increasing prevalence of infections involving intracellular apicomplexan parasites such as Plasmodium, Toxoplasma, and Cryptosporidium (the causative agents of malaria, toxoplasmosis and cryptosporidiosis respectively) represent a significant global healthcare burden. Despite their significance, few treatments are available; a situation that is likely to deteriorate with the emergence of new resistant strains of parasites. To lay the foundation for programs of drug discovery and vaccine development, genome sequences for many of these organisms have been generated, together with large scale expression and proteomic datasets. Comparative analyses of these datasets are beginning to identify the molecular innovations supporting both conserved processes mediating fundamental roles in parasite survival and persistence, as well as lineage- specific adaptations associated with divergent life cycle strategies. The challenge is how best to exploit these data to derive insights into parasite virulence and identify those genes representing the most amenable targets.
In this review, we outline genomic datasets currently available for apicomplexans and discuss biological insights that have emerged as a consequence of their analysis. Of particular interest are systems-based resources, focusing on areas of metabolism and host invasion that are opening up opportunities for discovering new therapeutic targets.
Keywords: apicomplexan genomics, parasite genomics, systems-based approaches, metabolism, host-invasion, host cell modulation
Introduction
Apicomplexans are a group of single celled obligate eukaryotic intracellular parasites (Cavalier-Smith, 1993, Adl et al., 2007), that form a major clade of the superphylum, Alveolata. The prevalence of infections involving parasites such as Plasmodium, Cryptosporidium and Toxoplasma (the causative agents of malaria, cryptosporidiosis and toxoplasmosis) represents a huge burden on child health worldwide: malaria impacts over 200 million individuals and kills over 300000 children (WHO, 2016); Cryptosporidium infections are the 2nd leading cause of the 800,000 infant diarrheal associated fatalities (Kotloff et al., 2013, Checkley et al., 2015); while Toxoplasma, which is estimated to infect about 30% of the worldwide population (Flegr et al., 2014), is the leading cause of infectious retinitis in children and is life-threatening in pregnancy and to the immunocompromised (Figure 1) (Garza-Leon and Garcia, 2012, Torgerson and Mastroiacovo, 2013, Schluter et al., 2014). Other species, such as members of Eimeria and Neospora, have a significant impact on agriculture, causing huge losses in the poultry (McDonald and Shirley, 2009) and cattle (Goodswen et al., 2013) industries, respectively. Despite their significance, treatment options are limited and the emergence of strains resistant to current drugs highlights an urgent need to develop new therapeutics (Hyde, 2007, Petersen et al., 2011a, Doliwa et al., 2013, Miotto et al., 2013, Seeber and Steinfelder, 2016). To help drive such programs, technological advances in sequencing and other high-throughput approaches has enabled researchers to assemble extensive genomic, transcriptomic, and proteomic datasets for many of these organisms. The current challenge lies in integrating these complementary datasets to better understand the biological processes by which these parasites are able to infect their hosts and cause disease, with emphasis on identifying which proteins play essential roles and may be targeted for intervention strategies.
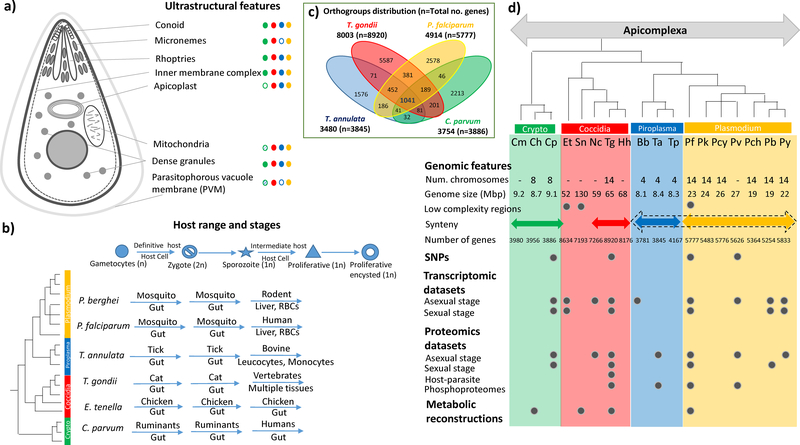
Figure 1: Ultrastructure, Life Cycle, and Genome Structure of Apicomplexa –
The figure highlights details of ultrastructural details unique to apicomplexa, along with their distribution in major clades (Green – Cryptosporidia, Red – Coccidia, Blue – Piroplasmida, Yellow – Plasmodium). Unusual organelles are shown as shaded ellipses – extracytoplasmic PVM (1) and reduced mitochondria-like organelles (2) in Cryptosporidium species. The various life cycle stages are listed along with the host and tissue range common for specific members of each clade in the quadrant below. A venn diagram representing the distribution of orthologous groups among four representative apicomplexans of the major clades is shown. On the right hand side, various genome sequence features as well as details of available functional datasets for sequenced genomes are listed. The species tree for the apicomplexan organisms used in this analysis is shown (based on http://tolweb.org, (3), (4)). The apicomplexan species listed are: Cm – Cryptosporidum muris, Ch – Cryptosporidium hominis, Cp – Cryptosporidium parvum, Et – Eimeria tenella, Sn – Sarcocystis neurona, Nc – Neospora caninum, Tg – Toxoplasma gondii, Hh – Hammodia hammondi, Bb – Babesia bovis, Ta – Theileria annulata, Tp – Theileria parva, Pf – Plasmodium falciparum, Pk – Plasmodium knowlesi, Pcy – Plasmodium cynomolgi, Pv – Plasmodium vivax, Pch – Plasmodium chabaudi, Pb – Plasmodium berghei, Py – Plasmodium yoelii.
Apicomplexan parasites inhabit a wide range of environments, from marine to terrestrial, and also exhibit variation in host specificity. They possess complex life cycles, often involving multiple hosts and developmental stages (Figure 1b). These stages include: sporogony (invasive stage, with a single round of asexual reproduction), merogony (invasive stage, with multiple rounds of asexual reproduction) and gametogony (sexual reproduction). These stages may occur in the same organism and same tissue (monoxenic lifestyle) or in different organisms and different tissues (heteroxenic lifestyle).
Apicomplexans can be classified into three major clades based on their phylogenetic relationships and host specificities: Aconoidasida, Coccidia and a third lineage featuring Gregarina and Cryptosporidium species (Barta and Thompson, 2006, Adl et al., 2007, Wasmuth et al., 2009). The Aconoidasida include the Haemosporida (Plasmodium) and the Piroplasmida (Babesia and Theileria), and lead heteroxenous life cycles, alternating between an arthropod vector, in which the parasite undergoes sexual reproduction, and a vertebrate host supporting asexual propagation, typically in the circulatory system. On the other hand, Cryptosporidium species are restricted to the gastro-intestinal (GI) tract of animals while Coccidia feature members that are either fully, or partially restricted to the GI tract. For example, the coccidian Eimeria tenella, which causes coccidiosis in poultry, undergoes a monoxenic lifestyle, restricted to a single host in which it colonizes epithelial cells of the intestine. Other, so called ‘tissue-cyst forming’ coccidians such as Sarcocystis hominis and Toxoplasma gondii feature heteroxenous life cycles where gametogony occurs in the intestinal epithelium of the definitive host (e.g. cat for T. gondii and human for S. hominis) and merogony occurs in the tissues of intermediate hosts. Although individual lineages share distinctive life cycle strategies, each species has its own specialisation with respect to host range, and even the tissue type that it infects (Cowper et al., 2012, Woo et al., 2015). For example T. gondii is thought to be capable of exploiting all warm blooded animals as intermediate hosts, while S. hominis relies on bovines as its intermediate host. Likewise, although all ~200 Plasmodium species infect erythrocytes, they show specialised adaptations; P. vivax only infects smaller (or) newer reticulocytes (Malleret et al., 2015), whereas P. falciparum can infect all reticulocytes (White, 2009).
All apicomplexans are characterised by several unique ultrastructural features and organelles that provide essential functions for the parasite to complete its life cycle (Figure 1a). The inner membrane complex (IMC) is a highly specialised endomembrane system found directly beneath plasma membrane in all alveolates, made up of several flattened sacs called alveoli, providing shape and stability to the cell as well as enabling replication, motility and invasion (Gubbels and Duraisingh, 2012, Harding and Meissner, 2014). The unique secretory organelles found at the apical end of the parasite – the bar shaped micronemes, club shaped rhoptries (consisting of two subcompartments – rhoptry neck region and rhoptry bulb region), and dense granules – are instrumental for carrying out motility, invasion, and host modulation processes (Kemp et al., 2013). Dense granules are known to be present in Coccidia and Cryptosporidium (Bonnin et al., 1995). Although dense granule-like structures have been identified in Theileria (Shaw et al., 1991), Babesia (Gohil et al., 2010) and Plasmodium (Culvenor et al., 1991), it is not clear whether they perform equivalent roles (Mercier et al., 2005). Another unique aspect of apicomplexans is the apicoplast, which is a four-membraned organelle hosting several important metabolic pathways and essential for the survival of the parasite (Lim and McFadden, 2010, McFadden and Yeh, 2016). This organelle is absent in Cryptosporidium and Gregarina.
In 2002, the first genome of an apicomplexan, P. falciparum, was sequenced (Gardner et al., 2002). Since then, the advent of next generation sequencing (NGS) technologies have resulted in increasingly impressive genomic and transcriptomic datasets (Goodwin et al., 2016). Over the last decade, genomes from all major apicomplexan clades have been generated, along with their close relatives, the free living, non-parasitic chromerids (Abrahamsen et al., 2004, Xu et al., 2004, Gardner et al., 2005, Pain et al., 2005, Carlton et al., 2008, Reid et al., 2012, Walzer et al., 2013, Reid et al., 2014, Blazejewski et al., 2015, Woo et al., 2015, Lorenzi et al., 2016b) (Figure 1d). Further, falling costs in sequencing is ushering in a new era of population genomics (Ellegren, 2014), in which tens or even hundreds of isolates may be sequenced to gain insights into evolutionary dynamics and epidemiology (Miotto et al., 2013, Miotto et al., 2015, Lorenzi et al., 2016a).
Analysis of apicomplexan genomes shows that like other parasites, the genome sizes of apicomplexans are reduced in comparison to those of free-living eukaryotes (Kissinger and DeBarry, 2011). The largest apicomplexan genomes belong to the Coccidia clade, which vary from 50–130 million base pairs (Mbp), while those from other clades are much smaller (Haemosporidia - 20–25 Mbp; Piroplasmida - 8 Mbp; and Cryptosporidium spp. - 9 Mbp). Although the factors governing genome sizes in apicomplexans is unknown, it is likely that they are partially influenced by the lineage-specific losses (Woo et al., 2015) observed during their evolution as well as occurrence of repetitive elements, which are prevalent in the genomes of Eimeria spp., P. falciparum and S. neurona (Battistuzzi et al., 2016). Genome size is reflected in terms of the number of genes encoded, with B. bovis possessing the smallest complement (3781 genes) and T. gondii the largest (8920 genes) (Figure 1d). The reduced genomes of Cryptosporidium spp. appears to be related to their ability to salvage many nutrients from their host, reducing the need to support many complex biochemical pathways. Interestingly, the number of chromosomes associated with each group appears conserved: 4 in the Piroplasmids (Jones et al., 1997, Pain et al., 2005, Katzer et al., 2011), 8 in Cryptosporidium spp. (Blunt et al., 1997, Xu et al., 2004), and 14 in Haemosporidia (Janse et al., 1994, Gardner et al., 2002, Pain et al., 2008, Tachibana et al., 2012). In Coccidia, genetic linkage maps are available only for T. gondii, identifying 14 chromosomes (Khan et al., 2005).
Genomic resources available for apicomplexan parasites
As of October 2016, genomes of 18 apicomplexan species have been sequenced, with some species featuring genomes from many additional strains. Genome sequences have been deposited in major repositories such as those available at the Wellcome Trust Sanger Institute, National Centre for Biotechnology Information, and GeneDB. However, the most comprehensive resource for apicomplexan genomic datasets is EupathDB (Aurrecoechea et al., 2013) - a central repository for storing information about eukaryotic pathogens. The database is subdivided according to apicomplexan parasite clades – CryptoDB (Cryptosporidum, Gregarina), ToxoDB (Coccidians), PlasmoDB, PiroplasmaDB (Babesia, Theileria), with data for chromerids also deposited in CryptoDB. This portal summarizes the available genomic resources for the organisms and serves as a single-point access for various datasets including: genomic (including nuclear and organellar sequences and population genomic data), transcriptomic (expressed sequence tags, microarrays, RNA-Seq and SAGE tags), proteomic and epigenomic (ChIP-Seq). Two other noteworthy resources for transcriptomic data include the Database of Apicomplexa Transcriptomics, an up-to-date portal hosting transcriptomic datasets of various kinds, including single-cell and isolate-based data (Jakalski et al., 2015) and PartiGeneDB, which hosts EST datasets (Parkinson et al., 2004). Proteomic datasets specific to T. gondii and C. parvum are also available in EpicDB (Madrid-Aliste et al., 2009).
Aside from EupathDB, several apicomplexan-specific repositories dedicated to specialised aspects of apicomplexan biology are also available. The Liverpool Library of Apicomplexan Metabolic Pathways (LLAMP) is a web resource that provides draft metabolic reconstructions for eight species based on annotations provided by EuPathDB supplemented with additional literature evidence as well as sequence homology based predictions (Shanmugasundram et al., 2013). The Malaria Parasite Metabolic Pathways (MPMP) is a manually curated web repository for functional genomics of P. falciparum (Ginsburg and Abdel-Haleem, 2016). Serving as a gold standard for metabolic annotations for P. falciparum, the MPMP database also provides information regarding gene expression data, protein localization, and drug-related data (e.g. links to the PubChem small molecule database (Kim et al., 2016) and literature sources) for the parasite. Additional tools for predicting protein localization to various sub-cellular organelles have been developed, such as ApicoAP (Cilingir et al., 2012) and HECTAR (Gschloessl et al., 2008) for predicting apicoplast-targeting motifs, as well as the more generic iPSORT (Bannai et al., 2002) for recognising signal peptide, mitochondrial target peptides, and chloroplast target peptides, and SignalP (Petersen et al., 2011b) for predicting secreted proteins.
Gene complements provide insights into apicomplexan biology
Comparisons between gene complements across apicomplexans, highlight both core conserved functionality as well as lineage-specific innovations. Focusing on genes involved in housekeeping functions, such as translation, protein folding and cell cycle, each species features roughly equivalent sets (Table 1), with the higher numbers associated with P. falciparum and T. gondii likely associated with more extensive curation efforts. Surprisingly, some of the proteins involved in housekeeping function are reported to be significantly diverged enough from other eukaryotes to not be related by sequence similarity (Wasmuth et al., 2009). In terms of metabolism and transport, gene numbers reflect lineage-specific distribution, with higher losses in Cryptosporidium spp. and Piroplasmids. Most significantly, almost 50% of the proteins within a species are of unknown function (Table 1), with 77% unknown for T. parva and 68% unknown for E. tenella, emphasising the pressing need to improve functional annotation efforts. Comprehensive sequence based comparisons suggest that most apicomplexan sequences are specific to the phylum with only 25% of apicomplexan sequences sharing significant similarity with sequences outside the phylum (Wasmuth et al., 2009). Furthermore many genera-specific innovations within the Apicomplexa are associated with specialised pathways involved in host cell invasion and modification of host processes (Wasmuth et al., 2009). More recently, a gene family analysis based on the proteomes of 26 alveolates, revealed that members of the Apicomplexa share ~2200 orthologous groups of genes with all other alveolates, while only 81 groups are associated with the emergence of parasitism. This suggests that the genome of free-living ancestor of apicomplexans already encoded much of the machinery that may have been adapted to support a parasitic life style. As with the earlier study, numerous lineage-specific gene gains and losses were identified, many associated with host-parasite interactions. For example, the divergence of the coccidian lineage was associated with 537 losses and 414 gains (Woo et al., 2015). In a later section we discuss some of the functions associated with these changes.
Table 1:
Distribution of apicomplexan proteins in various biological processes
| Category | GOSlim Term / Source | Crypto | Coccidia | Piroplasmida | Plasmodium | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cmur | Cpar | Eten | Ncan | Tgon | Hham | Bbov | Tpar | Tann | Pfal | Pber | Pviv | ||
| Proteome size | 3980 | 3886 | 8634 | 7266 | 8920 | 8176 | 3781 | 3845 | 4167 | 5777 | 5254 | 5626 | |
| Housekeeping | |||||||||||||
| cellular protein modification process | GO:0006464 | 163 | 144 | 193 | 236 | 245 | 185 | 88 | 102 | 87 | 265 | 149 | 228 |
| translation | GO:0006412 | 120 | 118 | 163 | 195 | 207 | 164 | 197 | 237 | 175 | 315 | 233 | 266 |
| biosynthetic process | GO:0009058 | 202 | 183 | 248 | 216 | 327 | 265 | 178 | 240 | 172 | 495 | 274 | 356 |
| catabolic process | GO:0009056 | 66 | 60 | 77 | 89 | 94 | 71 | 54 | 60 | 55 | 148 | 72 | 111 |
| protein folding | GO:0006457 | 38 | 34 | 36 | 52 | 50 | 36 | 37 | 47 | 35 | 100 | 60 | 65 |
| nucleobase-containing compound catabolic process | GO:0034655 | 35 | 32 | 41 | 53 | 56 | 32 | 31 | 31 | 30 | 49 | 42 | 45 |
| cell cycle | GO:0007049 | 18 | 16 | 10 | 15 | 17 | 12 | 8 | 13 | 9 | 72 | 12 | 32 |
| response to stress | GO:0006950 | 43 | 39 | 48 | 69 | 94 | 64 | 31 | 46 | 34 | 111 | 47 | 89 |
| homeostatic process | GO:0042592 | 25 | 20 | 20 | 35 | 34 | 30 | 18 | 16 | 15 | 47 | 29 | 38 |
| Metabolism | |||||||||||||
| cellular nitrogen compound metabolic process | GO:0034641 | 209 | 161 | 236 | 183 | 298 | 234 | 192 | 233 | 177 | 492 | 290 | 399 |
| DNA metabolic process | GO:0006259 | 92 | 87 | 100 | 108 | 128 | 94 | 77 | 96 | 83 | 155 | 98 | 134 |
| small molecule metabolic process | GO:0044281 | 93 | 80 | 120 | 148 | 155 | 105 | 88 | 100 | 81 | 245 | 145 | 193 |
| lipid metabolic process | GO:0006629 | 43 | 33 | 50 | 74 | 84 | 63 | 28 | 34 | 24 | 138 | 53 | 77 |
| carbohydrate metabolic process | GO:0005975 | 41 | 43 | 78 | 82 | 91 | 78 | 27 | 29 | 25 | 64 | 39 | 60 |
| cellular amino acid metabolic process | GO:0006520 | 35 | 38 | 64 | 88 | 93 | 75 | 46 | 52 | 47 | 92 | 69 | 80 |
| tRNA metabolic process | GO:0006399 | 49 | 45 | 55 | 62 | 65 | 57 | 52 | 55 | 54 | 84 | 59 | 68 |
| Signaling | |||||||||||||
| signal transduction | GO:0007165 | 70 | 55 | 79 | 88 | 98 | 81 | 45 | 77 | 40 | 97 | 64 | 77 |
| Transport | |||||||||||||
| transport | GO:0006810 | 154 | 125 | 145 | 190 | 210 | 130 | 102 | 149 | 111 | 344 | 168 | 200 |
| transmembrane transport | GO:0055085 | 68 | 62 | 80 | 98 | 111 | 98 | 49 | 66 | 70 | 78 | 62 | 64 |
| vesicle-mediated transport | GO:0016192 | 47 | 41 | 48 | 58 | 59 | 44 | 38 | 37 | 40 | 95 | 58 | 69 |
| Invasion | |||||||||||||
| Inner membrane complex | (1)*, (2)#, (3)@, EupathDB | 11* | 12* | 9* | 11* | 41*# | 22 | - | 14* | 12* | 28@ | 21 | 20 |
| Rhoptry neck proteins | (4)*, EupathDB | 1* | 1* | 3* | 7* | 11 | 10 | 4* | 4* | 4* | 7 | 7 | 7 |
| Micronemal proteins | (5)*, (6)#, EupathDB | - | 3* | 8* | 5 | 25 | 25 | 0 | 0 | 1 | 17# | 10* | 2 |
| Host associated processes | |||||||||||||
| ROPK | (7) | 0 | 0 | 27 | 44 | 55 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Non-ROPK rhoptry proteins | (6)*, EupathDB | 0 | 0 | 2 | - | 23* | 23 | 2 | 0 | 3 | 22* | 11 | 10 |
| FIKK | EupathDB | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 21 | 1 | 1 |
| SVSP | (8) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 85 | 51 | 0 | 0 | 0 |
| SRS | (8)*, EupathDB | 0 | 0 | 0 | 227* | 111 | 95 | 0 | 0 | 0 | 0 | 0 | 0 |
| SAG | EupathDB | 0 | 0 | 87 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dense granule proteins | (9)*, EupathDB | 1* | 1* | 1 | 0 | 16 | 15 | 0 | 0 | 0 | 0 | 0 | 0 |
| Var | (8) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 60 | 0 | 0 |
| |Rifin | EupathDB | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 185 | 0 | 0 |
| stevor | EupathDB | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 42 | 0 | 0 |
| Pir | (8) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 346 |
| PHIST | (8)*, EupathDB | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 79 | 3 | 39* |
| ves | (8) | 0 | 0 | 0 | 0 | 0 | 0 | 72 | 0 | 0 | 0 | 0 | 0 |
| tpr/tar | (8) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 39 | 93 | 0 | 0 | 0 |
| Transcription factors | |||||||||||||
| AP2 | (6) | - | 9 | 15 | 53 | 53 | - | 20 | 19 | 18 | 26 | 25 | 27 |
| Unknown function | |||||||||||||
| Hypothetical proteins | EupathDB | 2148 | 1478 | 5850 | 4096 | 4285 | 3966 | 1846 | 2983 | 2113 | 2112 | 1905 | 2320 |
The table shows the distribution of number of proteins for various species according to different categories. The top 20 GOSlim terms were grouped into functionally related categories, such as Housekeeping, Metabolism, Transport, and Signaling. The numbers for P. falciparum may have some annotation bias associated with them. The details of proteins involved in apicomplexan-specific processes such as invasion and host-parasite interactions were sourced from specific literature sources wherever available (indicated as *), and from EupathDB otherwise. An estimate of number of proteins of currently unknown function was also obtained from EupathDB.
Jumbled genomes - missing synteny, abundance of low complexity regions
Comparisons of gene orderings reveal blocks of synteny between homologous genes in closely related apicomplexans. Notably, such relationships do not extend across the entire phylum (DeBarry and Kissinger, 2011) although one study did find that gene composition (not ordering) was significantly conserved between Cryptosporidium and Plasmodium species (Mazurie et al., 2013). Further, syntenic relationships are typically restricted within a lineage, one exception occurring within the Aconoidasida where ~1,300 orthologs shared between Plasmodium and Theileria parasites, are distributed across 435 microsyntenic regions (Pain et al., 2005). Within a lineage, different clades show varying degrees of synteny (i.e. blocks of collinear genes). For example, T. gondii shares synteny with other tissue-cyst forming coccidians (Reid et al., 2012, Walzer et al., 2013), albeit more restricted with Sarcocystis neurona (Blazejewski et al., 2015), but not with the non tissue-cyst forming coccidians Eimeria spp. (Lorenzi et al., 2016b). Within a genus, species show significant synteny, in proportion to the phylogenetic separation between the species (Carlton et al., 2008, Reid et al., 2014). Interestingly sites of syntenic breaks within a genus, both at telomeres and other regions, appear enriched for species-specific genes and multi-copy gene families and depleted for core genes (DeBarry and Kissinger, 2011). Many of the families found at these sites are implicated in host-parasite interactions, which is elaborated below (Reid, 2015).
Contributing to this lack of synteny, as well as the large variations in genome sizes, is the presence of repetitive sequence, both within and between coding regions (Battistuzzi et al., 2016). For S. neurona, the presence of repetitive sequence has resulted in the doubling of its genome size relative to other coccidians (Reid et al., 2014). Repetitive elements include simple repeats (duplication of simple sets of nucleotides), tandem repeats (duplications of complex sets of nucleotides – typically found at telomeres and centromeres of chromosomes) and transposable elements (TEs, sequences which can change position within a genome) (Kapitonov and Jurka, 2008). TEs are classed into two types: Class I (retrotransposons), which operate via a copy-and-paste mechanism and include Long- and Short-Interspersed Nuclear Elements (LINEs and SINEs respectively), and Class II (DNA transposons), which operate via a cut-and-paste mechanism. The abundance and distribution of each class of repeat varies between genomes. For example, in P. falciparum, simple repeats predominate, representing ~14% of its genome, while for E. tenella, simple repeats and LINEs make up ~13% and 11.5% of its genome respectively and for S. neurona, its ~130 Mbp genome features a high proportion of DNA transposons (11.5%), LINEs (7.7%) and simple repeats (6%) (Blazejewski et al., 2015). In terms of distribution, for Eimeria. spp., around 50% of the genes are found in repeat-rich regions, resulting in proteins enriched in homopolymeric amino acid repeats (Reid et al., 2014). In S. neurona, only Class I transposons are distributed in exons and Class II transposons are almost exclusively located in introns and intergenic regions. Intriguingly, although evolutionary analyses suggests these transposons are no longer active, their continued maintenance within the S. neurona genome, relative to other coccidians, suggests they may play some functional role (Blazejewski et al., 2015). In P. falciparum, 35% of the genes encode homopolymeric repeats, most of which are asparagine-rich, spread across most protein families (Singh et al., 2004). Although the functional role of these repeats is unclear, studies in Eimeria and P. falciparum suggest they are located mostly in loop regions and away from sites involved in domain-domain interactions and active sites and are therefore unlikely to interfere with protein structure or function.
Invasion machinery: Apicomplexan-specific conserved mechanism of host invasion
Host cell invasion is a rapid and complex process that relies on an orchestrated cascade of interactions between invading parasite and host cell. To orchestrate these processes, apicomplexans have evolved hundreds of specialised invasion proteins, many transported into exocytic organelles (micronemes, rhoptries and dense granules) that occupy up to one third of the cell volume (Figure 1a). The discharge of these apical organelles marks the beginning of host cell penetration and occurs in a tightly coordinated program (Sibley, 2011, Sharma and Chitnis, 2013). First, parasite surface receptors (e.g. SAG1-related sequences (SRS) in Toxoplasma / 6-Cysteine s48/45 family of proteins in Plasmodium (Arredondo et al., 2012)) initiate host cell recognition and attachment. This is followed by secretion of the microneme proteins that strengthen host cell attachment and play a major role in the formation of the so-called “moving junction” which forms a specific interface to facilitate invasion. Formation of this junction relies on a set of conserved parasite proteins – rhoptry neck protein 2 (RON2) and apical membrane antigen 1 (AMA1) (Lamarque et al., 2011, Tonkin et al., 2011). In Plasmodium, only the canonical RON2-AMA1 interaction has been characterised (Srinivasan et al., 2011) whereas three additional paralogs of AMA1 and two paralogs of RON2 are reported in Toxoplasma, expressed in a stage-specific manner (Poukchanski et al., 2013, Lamarque et al., 2014). Structural analysis of the different RON2-AMA1 pairs identify a pair with substantially divergent structure and an atypical mechanism, revealing molecular diversity at parasite host-cell interface, likely relevant for stage-specific changes (Parker et al., 2016). Next, the parasite discharges the contents of the rhoptries, which are released into the host cell (Kemp et al., 2013). A subset of rhoptry neck proteins (RONs) are critical for forming the moving junction (Proellocks et al., 2010), while other rhoptry proteins (ROPs) (Counihan et al., 2013), together with dense granule (GRA) proteins (Mercier et al., 2005, Mercier and Cesbron-Delauw, 2015), interact with host cell targets, manipulating pathways to protect the (now) intracellular parasite against clearance (Toxoplasma), or extensively remodelling the host cell by altering its mechanical properties (Plasmodium in RBCs) (Tiburcio et al., 2015). Many rhoptry proteins are secreted into the host cell during the process of invasion (Lim et al., 2012) whereas several dense granule proteins are secreted after PVM formation. Providing the parasite with the motile force to gain entry to the host cell is the inner-membrane complex (IMC). The IMC acts as a scaffold through which the glideosome complex (GAP40/GAP45/GAP50) exploits actomyosin (actin/MyoA/MLC) to provide the driving force for anterograde movement (Kono et al., 2013, Bargieri et al., 2014, Harding and Meissner, 2014, Tardieux and Baum, 2016). However, recent studies show that host invasion can occur even when gliding motility is blocked, suggesting a relook at the existing mechanism (or) the presence of alternate mechanisms (Andenmatten et al., 2013, Meissner et al., 2013). Although the host cell invasion process is largely conserved across apicomplexans, details vary among the different species and different stages. Theileria has a distinct mechanism and can enter the host cell in any orientation (unlike the others which enter via the apical complex), independent of parasite and host cell actin, using a zippering mechanism (Shaw, 2003). Plasmodium and Toxoplasma modulate the host cell cytoskeleton, whereas Cryptosporidium recruits host cell sugar transporters in addition to modulating the actin cytoskeleton (Chen et al., 2005, O’Hara and Chen, 2011). Further, after the parasite enters host cell, virulent strains of Toxoplasma use proteins secreted from rhoptries for modulating the host cell (mice) IRG resistance GTPases to protect them from clearance (Khaminets et al., 2010), whereas these factors do not protect the mice against Plasmodium (Liesenfeld et al., 2011).
Genome comparisons reveal complex evolutionary patterns associated with the emergence of these invasion related proteins. For example, amongst the 30 IMC proteins identified to date is a common core of highly conserved proteins, representing the recruitment and subsequent diversification of ancient eukaryotic proteins, supplemented with many lineage-specific proteins (Table 1). These latter genes, which include the Plasmodium specific PF3D7_1345600 (MAL13P1.228), may provide the IMC with additional lineage-specific roles such as scaffolding during gametocytogenesis (Kono et al., 2012). Similarly, several attachment proteins secreted by the micronemes and rhoptries are also well conserved across the phylum, including the microneme protein, AMA1, and its interaction complex (RON2/4/5), responsible for binding the parasite to the host cell surface (Boucher and Bosch, 2015), while others such as the Plasmodium-specific – Duffy binding proteins which bind to Duffy antigen on erythrocyte surface (eg. PvDBP1 and PvDBP2 from P. vivax) and reticulocyte binding proteins which enable selective invasion of reticulocytes (RBP; eg. PvRBP1, PvRBP2 from P. vivax), are restricted to distinct lineages. Even when conserved, microneme proteins can exhibit distinct ligand binding specificities even between closely related species (Carruthers and Tomley, 2008). For example, the T. gondii and N. caninum orthologs of MIC1 (TgMIC1 and NcMIC1) bind sialic acid and glycosaminoglycans respectively; while, unlike TgMIC4, NcMIC4 can bind lactose.
In contrast to proteins mediating cell attachment and host cell entry, those proteins involved in manipulating host pathways tend to be less well conserved, with many families of proteins specific to individual lineages, reflecting divergent life cycle strategies (Kemp et al., 2013). For example, as the parasite enters the cell, the release of rhoptry and dense granule proteins results in the formation and subsequent modification of the parasitophorous vacuole. Babesia and Theileria, which lack this compartment, also lack many of these genes (Lingelbach and Joiner, 1998). Cryptosporidium is enclosed in a unique parasitophorous vacuole –like structure, thought to derive through extension of the host cell microvillus membrane (Clode et al., 2015). This separates the parasite from both the host cell cytoplasm and gut environment, which may explain the relatively low number of host associated genes. In the next section we provide further details on these genes and pathways.
Host cell modulation: Lineage- and species- specific gene families modulating host-specific adaptations
Once inside the host cell, further suites of protein effectors are secreted into the host cytosol to interact with host cell targets, manipulating pathways and optimizing nutrient acquisition (Gubbels and Duraisingh, 2012). In Plasmodium, secreted proteins can be divided into 2 categories: a) those characterised by a PEXEL (plasmodium export element) or HTS (host-targeting signal) motif (Hiller et al., 2004, Marti et al., 2004) at the N-terminus, cleaved by plasmepsin V, for dense granule targeting (Russo et al., 2010) b) PEXEL negative exported proteins, which share an internal transmembrane segment (Marti and Spielmann, 2013, de Koning-Ward et al., 2016). The exported proteins are translocated across PVM into the host cell via the Protein Translocon of Exported Proteins (PTEX) complex (de Koning-Ward et al., 2009, Elsworth et al., 2014) in P. falciparum, exporting hundreds of proteins across PVM into the host cell (Bullen et al., 2012). Although a PEXEL-like sequence motif has been characterised for dense granule targeting in Toxoplasma, it is not involved in protein export into the host cell (Hsiao et al., 2013). In Toxoplasma, dense granule proteins GRA17 and GRA23 have been identified to mediate movement of small molecules across PVM (Gold et al., 2015). An in-silico comparative analysis of protein secretion and effectors has identified PEXEL-like motifs in Babesia and Cryptosporidium and plasmepsin V orthologues in all lineages, whereas translocon components are restricted to Plasmodium (Pelle et al., 2015), supporting the experimental results. Clustering the set of predicted secreted proteins using TribeMCL identifies 331 families (Pelle et al., 2015). These effectors tend to be associated with large, lineage-specific, variant gene families, which are sequentially diverse, rapidly evolving, and responsible for species-specific lifestyles (Table 1; (Reid, 2015)). Aside from the presence of signal peptides, little homology is observed between these families. Amongst these families are those involved in sequestration and antigenic variation (e.g. var - Plasmodium and ves - Babesia), others are involved in modulating the host immune response (e.g. Sag1-related sequences (SRS) – tissue cyst forming coccidia), and several others in modulating the host cell (ROP kinases - Coccidia, FIKK - Plasmodium and SVSP - Theileria) (Ward et al., 2004, Schneider and Mercereau-Puijalon, 2005, Schmuckli-Maurer et al., 2009, Sibley et al., 2009, Lim et al., 2012, Wei et al., 2013), in order to protect the parasite and enable its growth. For example, ROP5 and ROP18 modulate host immunity related GTPases (Behnke et al., 2012), while ROP16 modifies host cell signalling and ROP38 can inhibit host cell transcription (Kemp et al., 2013). Even where families may be conserved, their complements can vary dramatically across species and even strains, for example the coccidians, N. caninum and S. neurona feature 227 and 23 members of the SRS proteins respectively, while T. gondii strains Me49 and GT1 feature 109 and 90 SRS proteins respectively (Wasmuth et al., 2012). Similarly, the genomes of T. parva and T. annulata encode 85 and 51 SVSP proteins respectively.
Differences in the complements of these proteins relate to distinct life cycle requirements. For example, SRS proteins are composed of signature 6-cysteine domains, previously classified into one of 8 families (Wasmuth et al., 2012). S. neurona which features a reduced complement of SRS proteins relative to other tissue-cyst forming coccidians, nonetheless feature an expansion of SRS proteins with family 2 domains, previously associated with cyst wall integrity (Tomita et al., 2013). This raises the possibility that the emergence of this family was critical for the formation of cysts enabling the transition from a monoxenic to a heteroxenic life cycle. The subsequent expansion of SRS proteins through tandem duplication, particularly those composed of two domains featuring family 7 and family 8 and implicated in host immune modulation, may have subsequently allowed the parasite to broaden its host range. Coccidians also display significant variation in ROP kinases (E. tenella – 27; S. neurona - 15; N. caninum - 44; and T. gondii - 55; (Talevich and Kannan, 2013, Blazejewski et al., 2015)). Further, while the tissue-cyst forming coccidians share many ROP kinase orthologs, most members in E. tenella lack orthologs in the other species, suggesting unique functions, perhaps associated with its purely enteric lifestyle (Blazejewski et al., 2015).
The mechanisms underlying the expansions of many of these families vary across lineages and can be related to their genomic context. By promoting recombination, the localization of var, rif and stevor multigene families to subtelomeric regions is thought to be responsible for the generation of the genetic diversity driving antigenic variation in Plasmodium (Scherf et al., 1998). Interestingly, for P. knowlesi, whereas multigene families are not associated with subtelomeric regions, the pir and SICAvar gene families, involved in antigenic variation, are nevertheless associated with telomere-like repeats suggested to play a role in recombination (Pain et al., 2008). On the other hand, the coccidian families of surface antigens, srs and sag are scattered throughout the genome, mostly within tandem arrays that likely arose through gene duplication and subsequent gene conversion (Reid et al., 2012, Wasmuth et al., 2012). Notably the genomes of Cryptosporidium spp. do not encode any large gene families, consistent with studies indicating a lack of antigenic variation (Widmer and Sullivan, 2012).
Transcriptional regulation in apicomplexa: the AP2 gene family
Although apicomplexans possess RNA-polymerase associated factors and basal transcription factors, they lack many specific transcription factors (TFs) observed in other eukaryotes (Coulson et al., 2004, Callebaut et al., 2005). Instead, to support complex developmental cycles, potentially featuring multiple hosts, apicomplexans rely on a phylum-specific expansion of novel TFs that feature a version of the AP2 (Apetala2)-integrase DNA binding domain (Balaji et al., 2005). Although AP2 TFs are abundant in all apicomplexans, only 4 are shared across most apicomplexan clades, with the rest representing lineage-specific expansions (Table 1; (Woo et al., 2015)). Tissue-cyst coccidians have the largest complement with 53 AP2 TFs, followed by Plasmodium species (~25) and piroplasmids (~20); monoxenic species feature smaller complements (Cryptosporidium spp. – 9; Eimeria tenella – 15). The lower incidence of AP2 TFs in Cryptosporidium spp. has been suggested to be offset by the presence of other TFs (Oberstaller et al., 2014). Studies in P. falciparum have revealed that AP2 TFs have unique binding preferences; possessing high affinity primary binding motifs as well as secondary binding motifs (De Silva et al., 2008, Campbell et al., 2010). The ability to bind multiple, distinct motifs significantly increases the potential complexity of the transcriptional regulatory networks governed by this family. Several studies are unravelling the roles of AP2 TFs different in aspects of their life cycle and development (Painter et al., 2011), including chromosome biology (Flueck et al., 2010), commitment to gametocytogenesis (Kafsack et al., 2014, Sinha et al., 2014), normal morphogenesis inside mosquito (Kaneko et al., 2015) in Plasmodium, and parasite virulence, host invasion (Walker et al., 2013), and tissue cyst development (Radke et al., 2013) in Toxoplasma.
Apicomplexan metabolism: Lineage-specific adaptations to host environment
It is well-established that variations in metabolic potential help govern a pathogen’s ability to colonize, persist and establish virulence within infected hosts (McKinney et al., 2000, Song et al., 2013, Xia et al., 2013). With the availability of genome sequences, there is mounting interest in the use of genome-scale metabolic reconstructions to identify critical pathways required for growth. Such reconstructions rely on accurately annotating enzymes from sequence data alone, for which several tools are available (Claudel-Renard et al., 2003, Arakaki et al., 2006, Moriya et al., 2007, Hung et al., 2010). However, initial reconstructions based on these methods alone are typically incomplete, with many reactions, even in essential pathways, missing, due to an inability to detect the gene responsible. For apicomplexans, these ‘pathway holes’ constitute a significant component; in some reconstructions accounting for 40% of the reactions (Pinney et al., 2007). Consequently, methods have been developed to fill these gaps through experimental- and literature-based evidence (Schomburg et al., 2013, Shanmugasundram et al., 2013), comparative genomics approaches (Lee and Sonnhammer, 2003, Chen and Vitkup, 2006, Suhre, 2007, Zhou et al., 2008), as well as integrative approaches (Dale et al., 2010, Hung and Parkinson, 2011, Benedict et al., 2014).
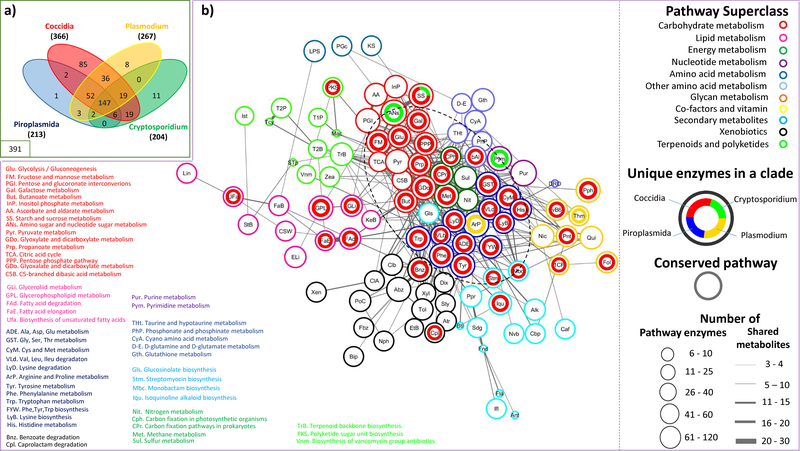
A comparative picture of the metabolic potential of different apicomplexan species is provided in Figure 2. Of the 391 enzyme categories (ECs) predicted from genomes of 18 apicomplexan species using an integrative annotation strategy (Hung and Parkinson, 2011), only 147 are shared across all clades (Figure 2a). Among the major clades, Cryptosporidium spp. and Piroplasmida have the most streamlined metabolism, with 204 and 213 enzymes (as defined by distinct enzyme commission (EC) numbers) respectively, whereas Plasmodium and Coccidia have a much larger metabolic repertoire, comprising 267 and 366 enzymes respectively, with Coccidia featuring 85 unique enzymes. Cryptosporidium and Piroplasmida appear to have lost the most enzymes relative to other apicomplexans (52 and 19 respectively), reflective of streamlined metabolisms with many nutrients scavenged from their hosts (Mogi and Kita, 2010). Species within a lineage largely share the same metabolic repertoire; for example, C. muris (199 enzymes), C. parvum (181 enzymes), C. hominis (175 enzymes) share 170 common enzymes; B. bovis (204 enzymes), T. parva (203 enzymes), T. annulata (203 enzymes) share 200 common enzymes; P. knowlesi and P. vivax share an identical metabolic complement (265 enzymes), with minor variations from other Plasmodium species (261 to 267 enzymes); the tissue-cyst coccidians S. neurona (305 enzymes), N. caninum (356 enzymes), T. gondii (354 enzymes) and H. hammondi (354 enzymes) share a common complement of 300 enzymes (254 with E. tenella). Most of the clade-specific enzymes are associated with core pathways (Figure 2b). For example, the 8 Plasmodium-specific enzymes are associated with arginine-proline metabolism and thiamine biosynthesis, while the 11 Cryptosporidium-specific enzymes are found in pyrimidine, amino sugar, and starch and sucrose metabolism, reflecting a previous analysis that showed different lineages may have acquired different sets of enzymes to perform similar core metabolic functions (Hung and Parkinson, 2011). As noted above, coccidians have the largest complement of metabolic enzymes, many of which are clade-specific and associated with core pathways dominated by carbohydrate and amino acid metabolism. This suggests that coccidians have evolved a diverse metabolic repertoire to adapt to multiple environments including, for example, enzymes driving gluconeogenesis that provides carbohydrate reserves to allow oocysts to sporulate outside the host (Ginger, 2006).
Figure 2: Metabolic potential in apicomplexan clades –
The venn diagram represents the distribution of enzymes common to all apicomplexans, shared between various clades, and unique to each clade. The distribution of conserved pathways and pathways with clade-specific enzymes (≥2 in a clade) is represented as a network. The network represents KEGG pathways (nodes) connected by number of shared metabolites, with pathways belonging to a superclass (same border colours) grouped together wherever possible. Each node is represented as a circos chart depicting the number of unique enzymes present in each major clade. Conserved pathways are indicated as empty circles. The core of the network, enclosed in a dashed circle, mainly encompasses pathways from amino acid, carbohydrate, energy, and nucleotide metabolism, with quite a few conserved pathways, as well as several clade-specific pathways, especially from coccidia. The abbreviated pathway names are expanded for those in the core, and the pathways with unique enzymes, in the periphery.
While most metabolic functionality occurs in the cytoplasm, several pathways are partitioned to mitochondria and the apicoplast, a plastid of secondary endosymbiotic origin (Sheiner et al., 2013, McFadden and Yeh, 2016). Amongst the enzymes in the apicoplast are those involved in type II fatty acid biosynthesis (Shears et al., 2015), isoprenoid synthesis (providing cofactors for electron transport chain and glycoprotein synthesis), heme biosynthesis and the formation of iron-sulfur clusters. Recently, isoprenoid precursor biosynthesis by apicoplast has been shown to be essential for normal gametocytogenesis in P. falciparum (Wiley et al., 2015). Further, this has also been identified to be the only essential role during Plasmodium erythrocyte stages, with type II fatty acid biosynthesis pathway being dispensable. However, this pathway appears to be essential during Plasmodium liver stages and in Toxoplasma (Sheiner et al., 2013), suggesting that species-specific and host cell-type specific differences exist. While less than 50 genes (mainly involved in transcription and translation), are encoded by the plastid’s own genome (Lim and McFadden, 2010), the remaining derive from nuclear encoded genes (predictions of apicoplast targeted nuclear-encoded genes is ~8–10% for Plasmodium and Theilera and only ~1% for Babesia (Sato, 2011)) and are targeted to the organelle through specific signal peptides. The apicoplast forms a tight physical association with mitochondria, attributed to metabolic dependencies; isoprenoid precursors generated by the apicoplast form the basis of ubiquinones driving the electron transport chain in mitochondria, while acetyl CoA, generated by mitochondria, is exploited as a major carbon source for fatty acid synthesis in the apicoplast (Sheiner et al., 2013). Furthermore, the apicoplast and mitochondrion share components of the haem biosynthetic pathway, which commences in the mitochondrion and proceeds in the apicoplast, before completing in the mitochondrion (Koreny et al., 2013). With highly reduced mitochondria (mitosomes), C. parvum and C. hominis lack a working tricarboxylic acid (TCA) cycle and rely instead on glycolysis for energy production (Henriquez et al., 2005). Interestingly, the mitosome of the related parasite C. muris appears less reduced and features a functional TCA cycle (Henriquez et al., 2005, Mogi and Kita, 2010). Further, lacking an apicoplast, Cryptosporidium spp. also rely on type I fatty acid metabolic pathways, present in species forming oocysts shed into the environment (Cryptosporidium, Toxoplasma, Eimeria), for de novo biosynthesis. Theileria spp. also show a reduced dependence on apicoplast and higher dependence of host for many substrates, again lacking the enzymes required for type II fatty acid biosynthesis (Gardner et al., 2005).
Due to their essential role in growth and survival, many metabolic pathways have been targeted for drug development, including shikimate pathway (McConkey et al., 2004), fatty acid metabolism (Goodman and McFadden, 2008, Shears et al., 2015), and isoprenoid biosynthesis (Moreno and Li, 2008). However, key to these strategies is identifying those enzymes that mediate the most critical roles in these pathways. In the next section we describe how modeling has contributed to an improved understanding of metabolic function in the Apicomplexa.
Modeling metabolism in the apicomplexa
With the increasing availability of high quality metabolic reconstructions, a variety of modeling approaches have been developed to gain insights into the roles of individual enzymes and pathways in parasite growth. Among the more robust approaches are constraint based models such as flux balance analysis (FBA) which rely on stoichiometric relationships in reactions rather than explicit kinetic data (Figure 3b; (Lee et al., 2006, Raman and Chandra, 2009)). FBA operates by calculating sets of fluxes across a metabolic network that optimizes a specific objective (e.g. maximizing growth rate of the parasite; (Hung and Parkinson, 2011)). FBA has so far been applied to four apicomplexans: C. hominis, P. falciparum, T. gondii, and S. neurona. The first was for C. hominis and comprised an analysis of 231 genes involved in 540 reactions (Vanee et al., 2010). The model was integrated with proteomics data from the sporozoite and oocyst stages, to predict the importance of the differential expression of high- and low affinity hexokinases in oocysts (associated with glucose-limited environments outside the host), and sporozoites (associated with the glucose-rich environment within the host) respectively. For P. falciparum, three models have been generated (Huthmacher et al., 2010, Plata et al., 2010, Fang et al., 2014). The first featured a compartmentalized network of 366 genes and 1001 reactions; FBA predictions showed high correlation with gene knockout data and drug inhibition assays, and predicted 40 novel drug targets that lacked significant sequence similarity with human sequences (Plata et al., 2010). Four of these are associated with shikimate biosynthesis, three with ubiquinone metabolism, and one with nicotinamide metabolism (nicotinate nucleotide adenylyltransferase). The last enzyme was experimentally validated to be a potentially effective and druggable target using drug inhibition experiments. In a separate model of 376 genes and 1019 reactions, Fang and colleagues used time-dependent gene expression, to explore stage-specific growth across the intraerythrocytic development cycle (IDC) of the parasite, helping link specific metabolites to corresponding physiological functions, such as the likely role of coenzyme A in late-IDC DNA replication and cell division (Fang et al., 2014). The model also captures the contribution of different energy producing pathways to ATP production in the IDC, with the bulk generated from glycolysis.
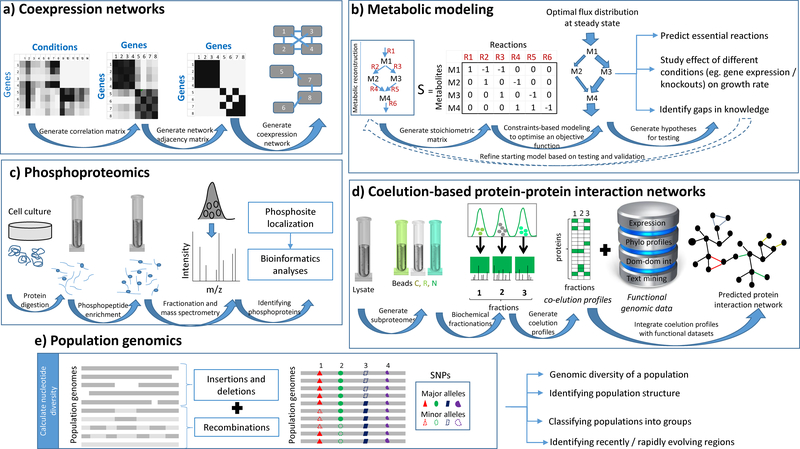
Figure 3: High-throughput post-genomic approaches –
The figure highlights the conceptual framework behind five post-genomic approaches utilised to study protein function in a systems-based context – a) Coexpression networks b) Metabolic modeling c) Phosphoproteomics d) Coelution-based protein-protein interaction networks e) Population genomics
The first metabolic model for T. gondii was a curated version consisting of 382 genes involving 571 reactions, separated across 5 subcellular compartments (Song et al., 2013). Applying reaction constraints based on gene expression data, FBA revealed that strain-specific differences in growth rates are driven by differing capacities for energy production, highlighting the potential of metabolism to impact the parasite’s virulence. Further, the model predicted strain-specific differences in drug susceptibilities, which were subsequently validated through drug inhibition studies. A later study, involving the semi-automated curation of 527 genes involved in 867 reactions, predicted two enzymes involved in acetyl CoA biosynthesis, ATP-citrate lyase and acetyl-CoA synthase, to be functionally redundant, with their simultaneous knockout to be lethal; a finding that was also confirmed experimentally (Tymoshenko et al., 2015). Lastly, a model of S. neurona comprising 330 genes and 536 reactions, predicted the parasite to be more sensitive to in silico knockouts of enzymes from glycolysis and TCA cycle (Blazejewski et al., 2015). However, the presence of alpha-glucosidase, suggested S. neurona may exploit fructose and sucrose as alternate energy sources to glucose, offering the potential for the parasite to rapidly adapt to new hosts. These studies demonstrate the potential of modeling to capture the metabolic complexity of apicomplexan life cycles and drive the generation of new testable hypotheses.
Systems biology for the apicomplexa: Beyond metabolism
Moving beyond genomic analyses, with the recognition that genes and their protein products do not operate in isolation but form parts of complex biological systems, there has been increasing interest in applying systems-based approaches to the study of parasite processes (Figure 3). With well characterized pathway relationships, metabolism has naturally been at the forefront of such studies. However advances in transcriptomics and proteomics are opening up new opportunities for systems analyses based on co-expression, phosphorylation and co-elution profiles.
Co-expression networks
Extensive transcriptional datasets are now available for several apicomplexans (Figure 1c) and provide unique opportunities to gain functional insights on the basis of co-expression profiles (Figure 3; (Le Roch et al., 2003)). In brief, for each gene a profile of expression is created from the multiple transcriptome datasets that have been generated. Pairwise comparisons between these profiles for each pair of genes then yields a matrix of correlation scores (e.g. Pearson correlation coefficient; (Stuart et al., 2003)). This matrix can then be represented as a network in which nodes (genes) are connected by edges if they exhibit a correlation score above a specified threshold (Figure 3a). The nodes in the resulting network can then be clustered, on the basis of shared patterns of interactions (Zhang and Horvath, 2005, Horvath and Dong, 2008), to define groups or modules of co-expressed genes representing functionally related genes (e.g. members of the same pathway). Applied to datasets examining the impact of 20 chemical compounds on gene expression in P. falciparum (Hu et al., 2010), a co-expression network approach was used to identify many functionally related genes sharing similar expression profiles, suggesting shared regulatory mechanisms. For example, 31 of 42 proteins predicted to be part of the invasion process were experimentally observed to be localized to apical organelles (20 proteins), parasite periphery (4 proteins) or IMC (7 proteins). Further, on the basis of the function of their neighbours within the co-expression network, three proteins: PF3D7_1345600 (MAL13P1.228), PF3D7_1460600 (PF14_0578) and PF3D7_0522600 (PFE1130w) were identified as novel members of the IMC. More recently, the analysis of 2 expression datasets associated with T. gondii encompassing 22 time points revealed two distinct sub-networks of invasion related genes (Blazejewski et al., 2015). The first composed of genes encoding micronemal proteins, which drive host-cell attachment and formation of the moving junction; the second composed of genes encoding rhoptry proteins, largely associated with modulation of host pathways. The dense connections within these networks illustrate the tight co-ordination in timing of expression associated with these genes.
Phospho-proteome networks
Protein phosphorylation is one of the most ubiquitously used post-translational mechanisms for regulation inside a cell. Recent advances in phosphopeptide enrichment and mass-spectrometric techniques have made it possible to study protein phosphorylation from a global perspective (Figure 3c; (Villen and Gygi, 2008)). A comparative study of both intracellular and extracellular forms of the invasive stages of P. falciparum (schizont) and T. gondii (tachyzoite), revealed 5,000 and 10,000 new phosphorylation sites respectively, including, unexpectedly, tyrosine residues (Treeck et al., 2011). The study further revealed that many parasite proteins are phosphorylated after secretion into the host cell, indicating novel routes for regulation of host pathways. More recent phospho-proteome studies in T. gondi and P. falciparum, focusing on calcium dependent protein kinase 3 and protein kinase G, are also beginning to expand on their respective roles in parasite egress by identifying novel phosphorylation sites in protein targets (Brochet et al., 2014, Treeck et al., 2014, Alam et al., 2015). Phospho-proteome analyses add an additional dimension to apicomplexan biology, allowing researchers to examine the impact of post-translational modifications on stage-specific development as well as host-parasite interactions, and offering additional routes for therapeutic intervention.
Protein-protein interaction networks
The generation of large-scale protein-protein interaction datasets are proving revolutionary for understanding the organization of complex biological processes (Butland et al., 2005, Krogan et al., 2006, Hu et al., 2009). In addition to aiding annotation efforts, such networks may be usefully exploited to identify proteins mediating critical roles in organization of complexes highlighting their potential for therapeutic intervention. To date, P. falciparum is the only apicomplexan for which protein-interaction data has been generated on a genome scale (LaCount et al., 2005). Applying a yeast-two hybrid approach, a network of ~2800 interactions between 1267 proteins was generated. Due to challenges in the expression of P. falciparum genes in a heterologous system, attempts have been made to improve on the quality of this initial dataset through integration of additional functional datasets (Hu et al., 2010, Ramaprasad et al., 2012); however such studies tend to be limited to functional, rather than physical interactions. Instead, an alternative strategy based on protein co-elution offers promise for deriving information on physical associations (Figure 3d). Avoiding technical challenges that arise with approaches such as tandem affinity purification, co-elution profiling has been successfully applied to soluble human complexes, (Havugimana et al., 2012), complexes conserved across 9 metazoans (Wan et al., 2015) and, significantly, the kinetoplastid, Trypanosoma brucei (Gazestani et al., 2016). These studies provide a valuable framework to generate similar protein-protein interaction networks for apicomplexans.
From genomes to populations
With falling costs in genome sequencing, attention is now focusing on examining strain level differences to gain insights into the emergence of genetic variation that impacts virulence and the emergence of resistance (Ellegren, 2014). An initial study of 16 geographically diverse isolates of P. falciparum revealed that while genes encoding housekeeping functions such as metabolic enzymes exhibited little variation, those encoding surface functions such as cytoadherence and antigenic variation displayed a rich diversity in sequence (Volkman et al., 2007). A recent study of 182 clinical isolates of P. vivax also revealed that the genes exhibiting the most variation are antigenic and involved in immune evasion, and additionally revealed a richer diversity than P. falciparum indicating larger and/or more stable population (Hupalo et al., 2016). More concerning, this study also revealed signals of recent evolution in response to antimalarial drug exposure. Similar concerns arose in a targeted analysis focusing on artemisinin resistance across 825 P. falciparum isolates (Miotto et al., 2013). In addition to revealing an unusual population structure associated with isolates from Western Cambodia, a hub of artemisinin resistance, artemisinin resistance was associated with multiple SNPs in kelch13, resulting in slow parasite clearance (Ariey et al., 2014). Genome-wide association studies also showed that nonsynonymous polymorphisms in genes encoding ferredoxin, apicoplast ribosomal protein S10, multidrug resistance protein 2, and the chloroquine resistance transporter were associated with artemisinin resistance and may act as predisposing factors, allowing the emergence of kelch13 variations, and thus serving as risk markers for new resistance-causing mutations (Miotto et al., 2015).
Population genomics studies have also been applied to T. gondii isolates to reveal 6 major clades based on specific gene markers, with three major types (designated type I, type II and type III) dominating in North America and Europe (Su et al., 2012). Analysis of SNP distributions across 10 isolates, further reveal the presence of haploblocks indicating the significant influence of recombination and admixture on the global population structure of T. gondii (Minot et al., 2012). Recently, a larger scale analysis of 64 T. gondii isolates, was able to recapitulate previously defined haplogroups (Lorenzi et al., 2016b). Through the application of chromosome painting in which chromosomal segments are coloured according to ancestry (Yahara et al., 2013), this study also revealed the extent to which shared inheritance of haploblocks shapes population structure. Further examination of these haploblocks revealed conserved regions enriched for specific secretory pathogenesis determinants (proteins involved in parasite-host interactions, host invasion and modulation), which undergo tandem amplifications and diversification, likely influencing host range and pathogenicity. Future population based studies offer the potential to associate phenotypic traits with specific genetic loci based on shared patterns of haploblock inheritance.
Conclusions and Future perspectives
The availability and analysis of apicomplexan genomes and related datasets provides unprecedented views of their biology and emergence as a successful parasitic phylum. Apicomplexans are hypothesised to have evolved through the endosymbiosis involving a red algal and an alveolate ancestor (Janouskovec et al., 2010). This event likely introduced vast genetic diversity into alveolate gene pool, driving the emergence of new species with diverse metabolic pathways and distinct life styles: parasitic apicomplexans, free-living ciliates and photosynthetic chromerids and dinoflagellates. Comparative genomic analyses suggest that the lineage-specific loss of components important for free-living lifestyle (metabolic pathways, endomembrane trafficking, flagellar apparatus for motility) happened progressively within the apicomplexan lineage (Woo et al., 2015). At the same time, genes encoding proteins driving parasite-specific processes such as invasion and host modulation, were either repurposed from pre-existing components associated with the free-living ancestor (e.g. components of the acto-myosin complex or inner membrane complex) or later acquired during adaptation to specific host niches (e.g. AP2 transcription factors or host-modulation machineries) (Janouskovec and Keeling, 2016).
From a practical perspective, genome analyses are proving essential to our understanding of the emergence of drug resistance as well as in the identification of novel drug targets. Key to this endeavour are systems-based approaches, based for example on the analysis of protein-interaction or metabolic networks that facilitate the elucidation of protein function in the context of the complex and/or pathway in which it operates. Although systems-based approaches provide important insights that cannot be gained from a reductionist approach, they come with the caveat that they incorporate automated functional annotations, which are likely to be erroneous especially for proteins with no known homologues. This underscores the need for community-wide efforts to generate and integrate functional datasets on various aspects of apicomplexan biology with the systems-based models, in order to improve the predictive validity of these models. We also anticipate greater emphasis on population-based studies that move beyond understanding the factors that govern population structure to reveal the mechanisms that allow these parasites to acquire resistance to therapeutics, in addition to virulence factors that drive pathogenesis.
Acknowledgements
The authors gratefully acknowledge Nirvana Nursimulu for helpful suggestions.
Footnotes
Declaration of interest:
The authors declare that they have no conflict of interest.
References
- Abrahamsen MS, Templeton TJ, Enomoto S, Abrahante JE, Zhu G, Lancto CA, Deng M, Liu C, Widmer G, Tzipori S, Buck GA, Xu P, Bankier AT, Dear PH, Konfortov BA, Spriggs HF, Iyer L, Anantharaman V, Aravind L & Kapur V, 2004. Complete genome sequence of the apicomplexan, Cryptosporidium parvum. Science, 304, 441–5. [DOI] [PubMed] [Google Scholar]
- Adl SM, Leander BS, Simpson AG, Archibald JM, Anderson OR, Bass D, Bowser SS, Brugerolle G, Farmer MA, Karpov S, Kolisko M, Lane CE, Lodge DJ, Mann DG, Meisterfeld R, Mendoza L, Moestrup O, Mozley-Standridge SE, Smirnov AV & Spiegel F, 2007. Diversity, nomenclature, and taxonomy of protists. Syst Biol, 56, 684–9. [DOI] [PubMed] [Google Scholar]
- Alam MM, Solyakov L, Bottrill AR, Flueck C, Siddiqui FA, Singh S, Mistry S, Viskaduraki M, Lee K, Hopp CS, Chitnis CE, Doerig C, Moon RW, Green JL, Holder AA, Baker DA & Tobin AB, 2015. Phosphoproteomics reveals malaria parasite Protein Kinase G as a signalling hub regulating egress and invasion. Nat Commun, 6, 7285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andenmatten N, Egarter S, Jackson AJ, Jullien N, Herman JP & Meissner M, 2013. Conditional genome engineering in Toxoplasma gondii uncovers alternative invasion mechanisms. Nat Methods, 10, 125–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakaki AK, Tian W & Skolnick J, 2006. High precision multi-genome scale reannotation of enzyme function by EFICAz. BMC Genomics, 7, 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois AC, Khim N, Kim S, Duru V, Bouchier C, Ma L, Lim P, Leang R, Duong S, Sreng S, Suon S, Chuor CM, Bout DM, Menard S, Rogers WO, Genton B, Fandeur T, Miotto O, Ringwald P, Le Bras J, Berry A, Barale JC, Fairhurst RM, Benoit-Vical F, Mercereau-Puijalon O & Menard D, 2014. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature, 505, 50–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arredondo SA, Cai M, Takayama Y, Macdonald NJ, Anderson DE, Aravind L, Clore GM & Miller LH, 2012. Structure of the Plasmodium 6-cysteine s48/45 domain. Proc Natl Acad Sci U S A, 109, 6692–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurrecoechea C, Barreto A, Brestelli J, Brunk BP, Cade S, Doherty R, Fischer S, Gajria B, Gao X, Gingle A, Grant G, Harb OS, Heiges M, Hu S, Iodice J, Kissinger JC, Kraemer ET, Li W, Pinney DF, Pitts B, Roos DS, Srinivasamoorthy G, Stoeckert CJ Jr., Wang H & Warrenfeltz S, 2013. EuPathDB: the eukaryotic pathogen database. Nucleic Acids Res, 41, D684–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaji S, Babu MM, Iyer LM & Aravind L, 2005. Discovery of the principal specific transcription factors of Apicomplexa and their implication for the evolution of the AP2-integrase DNA binding domains. Nucleic Acids Res, 33, 3994–4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannai H, Tamada Y, Maruyama O, Nakai K & Miyano S, 2002. Extensive feature detection of N-terminal protein sorting signals. Bioinformatics, 18, 298–305. [DOI] [PubMed] [Google Scholar]
- Bargieri D, Lagal V, Andenmatten N, Tardieux I, Meissner M & Menard R, 2014. Host cell invasion by apicomplexan parasites: the junction conundrum. PLoS Pathog, 10, e1004273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barta JR & Thompson RC, 2006. What is Cryptosporidium? Reappraising its biology and phylogenetic affinities. Trends Parasitol, 22, 463–8. [DOI] [PubMed] [Google Scholar]
- Battistuzzi FU, Schneider KA, Spencer MK, Fisher D, Chaudhry S & Escalante AA, 2016. Profiles of low complexity regions in Apicomplexa. BMC Evol Biol, 16, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke MS, Fentress SJ, Mashayekhi M, Li LX, Taylor GA & Sibley LD, 2012. The polymorphic pseudokinase ROP5 controls virulence in Toxoplasma gondii by regulating the active kinase ROP18. PLoS Pathog, 8, e1002992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict MN, Mundy MB, Henry CS, Chia N & Price ND, 2014. Likelihood-based gene annotations for gap filling and quality assessment in genome-scale metabolic models. PLoS Comput Biol, 10, e1003882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazejewski T, Nursimulu N, Pszenny V, Dangoudoubiyam S, Namasivayam S, Chiasson MA, Chessman K, Tonkin M, Swapna LS, Hung SS, Bridgers J, Ricklefs SM, Boulanger MJ, Dubey JP, Porcella SF, Kissinger JC, Howe DK, Grigg ME & Parkinson J, 2015. Systems-based analysis of the Sarcocystis neurona genome identifies pathways that contribute to a heteroxenous life cycle. MBio, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blunt DS, Khramtsov NV, Upton SJ & Montelone BA, 1997. Molecular karyotype analysis of Cryptosporidium parvum: evidence for eight chromosomes and a low-molecular-size molecule. Clin Diagn Lab Immunol, 4, 11–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnin A, Gut J, Dubremetz JF, Nelson RG & Camerlynck P, 1995. Monoclonal antibodies identify a subset of dense granules in Cryptosporidium parvum zoites and gamonts. J Eukaryot Microbiol, 42, 395–401. [DOI] [PubMed] [Google Scholar]
- Boucher LE & Bosch J, 2015. The apicomplexan glideosome and adhesins - Structures and function. J Struct Biol, 190, 93–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brochet M, Collins MO, Smith TK, Thompson E, Sebastian S, Volkmann K, Schwach F, Chappell L, Gomes AR, Berriman M, Rayner JC, Baker DA, Choudhary J & Billker O, 2014. Phosphoinositide metabolism links cGMP-dependent protein kinase G to essential Ca(2)(+) signals at key decision points in the life cycle of malaria parasites. PLoS Biol, 12, e1001806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen HE, Charnaud SC, Kalanon M, Riglar DT, Dekiwadia C, Kangwanrangsan N, Torii M, Tsuboi T, Baum J, Ralph SA, Cowman AF, De Koning-Ward TF, Crabb BS & Gilson PR, 2012. Biosynthesis, localization, and macromolecular arrangement of the Plasmodium falciparum translocon of exported proteins (PTEX). J Biol Chem, 287, 7871–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butland G, Peregrin-Alvarez JM, Li J, Yang W, Yang X, Canadien V, Starostine A, Richards D, Beattie B, Krogan N, Davey M, Parkinson J, Greenblatt J & Emili A, 2005. Interaction network containing conserved and essential protein complexes in Escherichia coli. Nature, 433, 531–7. [DOI] [PubMed] [Google Scholar]
- Callebaut I, Prat K, Meurice E, Mornon JP & Tomavo S, 2005. Prediction of the general transcription factors associated with RNA polymerase II in Plasmodium falciparum: conserved features and differences relative to other eukaryotes. BMC Genomics, 6, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell TL, De Silva EK, Olszewski KL, Elemento O & Llinas M, 2010. Identification and genome-wide prediction of DNA binding specificities for the ApiAP2 family of regulators from the malaria parasite. PLoS Pathog, 6, e1001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton JM, Adams JH, Silva JC, Bidwell SL, Lorenzi H, Caler E, Crabtree J, Angiuoli SV, Merino EF, Amedeo P, Cheng Q, Coulson RM, Crabb BS, Del Portillo HA, Essien K, Feldblyum TV, Fernandez-Becerra C, Gilson PR, Gueye AH, Guo X, Kang’a S, Kooij TW, Korsinczky M, Meyer EV, Nene V, Paulsen I, White O, Ralph SA, Ren Q, Sargeant TJ, Salzberg SL, Stoeckert CJ, Sullivan SA, Yamamoto MM, Hoffman SL, Wortman JR, Gardner MJ, Galinski MR, Barnwell JW & Fraser-Liggett CM, 2008. Comparative genomics of the neglected human malaria parasite Plasmodium vivax. Nature, 455, 757–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruthers VB & Tomley FM, 2008. Microneme proteins in apicomplexans. Subcell Biochem, 47, 33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalier-Smith T, 1993. Kingdom protozoa and its 18 phyla. Microbiol Rev, 57, 953–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checkley W, White AC Jr., Jaganath D, Arrowood MJ, Chalmers RM, Chen XM, Fayer R, Griffiths JK, Guerrant RL, Hedstrom L, Huston CD, Kotloff KL, Kang G, Mead JR, Miller M, Petri WA Jr., Priest JW, Roos DS, Striepen B, Thompson RC, Ward HD, Van Voorhis WA, Xiao L, Zhu G & Houpt ER, 2015. A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for cryptosporidium. Lancet Infect Dis, 15, 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L & Vitkup D, 2006. Predicting genes for orphan metabolic activities using phylogenetic profiles. Genome Biol, 7, R17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XM, O’hara SP, Huang BQ, Splinter PL, Nelson JB & Larusso NF, 2005. Localized glucose and water influx facilitates Cryptosporidium parvum cellular invasion by means of modulation of host-cell membrane protrusion. Proc Natl Acad Sci U S A, 102, 6338–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cilingir G, Broschat SL & Lau AO, 2012. ApicoAP: the first computational model for identifying apicoplast-targeted proteins in multiple species of Apicomplexa. PLoS One, 7, e36598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claudel-Renard C, Chevalet C, Faraut T & Kahn D, 2003. Enzyme-specific profiles for genome annotation: PRIAM. Nucleic Acids Res, 31, 6633–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clode PL, Koh WH & Thompson RC, 2015. Life without a Host Cell: What is Cryptosporidium? Trends Parasitol, 31, 614–24. [DOI] [PubMed] [Google Scholar]
- Coulson RM, Hall N & Ouzounis CA, 2004. Comparative genomics of transcriptional control in the human malaria parasite Plasmodium falciparum. Genome Res, 14, 1548–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counihan NA, Kalanon M, Coppel RL & De Koning-Ward TF, 2013. Plasmodium rhoptry proteins: why order is important. Trends Parasitol, 29, 228–36. [DOI] [PubMed] [Google Scholar]
- Cowper B, Matthews S & Tomley F, 2012. The molecular basis for the distinct host and tissue tropisms of coccidian parasites. Mol Biochem Parasitol, 186, 1–10. [DOI] [PubMed] [Google Scholar]
- Culvenor JG, Day KP & Anders RF, 1991. Plasmodium falciparum ring-infected erythrocyte surface antigen is released from merozoite dense granules after erythrocyte invasion. Infect Immun, 59, 1183–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale JM, Popescu L & Karp PD, 2010. Machine learning methods for metabolic pathway prediction. BMC Bioinformatics, 11, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Koning-Ward TF, Dixon MW, Tilley L & Gilson PR, 2016. Plasmodium species: master renovators of their host cells. Nat Rev Microbiol, 14, 494–507. [DOI] [PubMed] [Google Scholar]
- De Koning-Ward TF, Gilson PR, Boddey JA, Rug M, Smith BJ, Papenfuss AT, Sanders PR, Lundie RJ, Maier AG, Cowman AF & Crabb BS, 2009. A newly discovered protein export machine in malaria parasites. Nature, 459, 945–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Silva EK, Gehrke AR, Olszewski K, Leon I, Chahal JS, Bulyk ML & Llinas M, 2008. Specific DNA-binding by apicomplexan AP2 transcription factors. Proc Natl Acad Sci U S A, 105, 8393–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debarry JD & Kissinger JC, 2011. Jumbled genomes: missing Apicomplexan synteny. Mol Biol Evol, 28, 2855–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doliwa C, Escotte-Binet S, Aubert D, Sauvage V, Velard F, Schmid A & Villena I, 2013. Sulfadiazine resistance in Toxoplasma gondii: no involvement of overexpression or polymorphisms in genes of therapeutic targets and ABC transporters. Parasite, 20, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellegren H, 2014. Genome sequencing and population genomics in non-model organisms. Trends Ecol Evol, 29, 51–63. [DOI] [PubMed] [Google Scholar]
- Elsworth B, Matthews K, Nie CQ, Kalanon M, Charnaud SC, Sanders PR, Chisholm SA, Counihan NA, Shaw PJ, Pino P, Chan JA, Azevedo MF, Rogerson SJ, Beeson JG, Crabb BS, Gilson PR & De Koning-Ward TF, 2014. PTEX is an essential nexus for protein export in malaria parasites. Nature, 511, 587–91. [DOI] [PubMed] [Google Scholar]
- Fang X, Reifman J & Wallqvist A, 2014. Modeling metabolism and stage-specific growth of Plasmodium falciparum HB3 during the intraerythrocytic developmental cycle. Mol Biosyst, 10, 2526–37. [DOI] [PubMed] [Google Scholar]
- Flegr J, Prandota J, Sovickova M & Israili ZH, 2014. Toxoplasmosis--a global threat. Correlation of latent toxoplasmosis with specific disease burden in a set of 88 countries. PLoS One, 9, e90203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flueck C, Bartfai R, Niederwieser I, Witmer K, Alako BT, Moes S, Bozdech Z, Jenoe P, Stunnenberg HG & Voss TS, 2010. A major role for the Plasmodium falciparum ApiAP2 protein PfSIP2 in chromosome end biology. PLoS Pathog, 6, e1000784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner MJ, Bishop R, Shah T, De Villiers EP, Carlton JM, Hall N, Ren Q, Paulsen IT, Pain A, Berriman M, Wilson RJ, Sato S, Ralph SA, Mann DJ, Xiong Z, Shallom SJ, Weidman J, Jiang L, Lynn J, Weaver B, Shoaibi A, Domingo AR, Wasawo D, Crabtree J, Wortman JR, Haas B, Angiuoli SV, Creasy TH, Lu C, Suh B, Silva JC, Utterback TR, Feldblyum TV, Pertea M, Allen J, Nierman WC, Taracha EL, Salzberg SL, White OR, Fitzhugh HA, Morzaria S, Venter JC, Fraser CM & Nene V, 2005. Genome sequence of Theileria parva, a bovine pathogen that transforms lymphocytes. Science, 309, 134–7. [DOI] [PubMed] [Google Scholar]
- Gardner MJ, Hall N, Fung E, White O, Berriman M, Hyman RW, Carlton JM, Pain A, Nelson KE, Bowman S, Paulsen IT, James K, Eisen JA, Rutherford K, Salzberg SL, Craig A, Kyes S, Chan MS, Nene V, Shallom SJ, Suh B, Peterson J, Angiuoli S, Pertea M, Allen J, Selengut J, Haft D, Mather MW, Vaidya AB, Martin DM, Fairlamb AH, Fraunholz MJ, Roos DS, Ralph SA, Mcfadden GI, Cummings LM, Subramanian GM, Mungall C, Venter JC, Carucci DJ, Hoffman SL, Newbold C, Davis RW, Fraser CM & Barrell B, 2002. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature, 419, 498–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza-Leon M & Garcia LA, 2012. Ocular toxoplasmosis: clinical characteristics in pediatric patients. Ocul Immunol Inflamm, 20, 130–8. [DOI] [PubMed] [Google Scholar]
- Gazestani VH, Nikpour N, Mehta V, Najafabadi HS, Moshiri H, Jardim A & Salavati R, 2016. A Protein Complex Map of Trypanosoma brucei. PLoS Negl Trop Dis, 10, e0004533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginger ML, 2006. Niche metabolism in parasitic protozoa. Philos Trans R Soc Lond B Biol Sci, 361, 101–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg H & Abdel-Haleem AM, 2016. Malaria Parasite Metabolic Pathways (MPMP) Upgraded with Targeted Chemical Compounds. Trends Parasitol, 32, 7–9. [DOI] [PubMed] [Google Scholar]
- Gohil S, Kats LM, Sturm A & Cooke BM, 2010. Recent insights into alteration of red blood cells by Babesia bovis: moovin’ forward. Trends Parasitol, 26, 591–9. [DOI] [PubMed] [Google Scholar]
- Gold DA, Kaplan AD, Lis A, Bett GC, Rosowski EE, Cirelli KM, Bougdour A, Sidik SM, Beck JR, Lourido S, Egea PF, Bradley PJ, Hakimi MA, Rasmusson RL & Saeij JP, 2015. The Toxoplasma Dense Granule Proteins GRA17 and GRA23 Mediate the Movement of Small Molecules between the Host and the Parasitophorous Vacuole. Cell Host Microbe, 17, 642–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman CD & Mcfadden GI, 2008. Fatty acid synthesis in protozoan parasites: unusual pathways and novel drug targets. Curr Pharm Des, 14, 901–16. [DOI] [PubMed] [Google Scholar]
- Goodswen SJ, Kennedy PJ & Ellis JT, 2013. A review of the infection, genetics, and evolution of Neospora caninum: from the past to the present. Infect Genet Evol, 13, 133–50. [DOI] [PubMed] [Google Scholar]
- Goodwin S, Mcpherson JD & Mccombie WR, 2016. Coming of age: ten years of next-generation sequencing technologies. Nat Rev Genet, 17, 333–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gschloessl B, Guermeur Y & Cock JM, 2008. HECTAR: a method to predict subcellular targeting in heterokonts. BMC Bioinformatics, 9, 393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubbels MJ & Duraisingh MT, 2012. Evolution of apicomplexan secretory organelles. Int J Parasitol, 42, 1071–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding CR & Meissner M, 2014. The inner membrane complex through development of Toxoplasma gondii and Plasmodium. Cell Microbiol, 16, 632–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havugimana PC, Hart GT, Nepusz T, Yang H, Turinsky AL, Li Z, Wang PI, Boutz DR, Fong V, Phanse S, Babu M, Craig SA, Hu P, Wan C, Vlasblom J, Dar VU, Bezginov A, Clark GW, Wu GC, Wodak SJ, Tillier ER, Paccanaro A, Marcotte EM & Emili A, 2012. A census of human soluble protein complexes. Cell, 150, 1068–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriquez FL, Richards TA, Roberts F, Mcleod R & Roberts CW, 2005. The unusual mitochondrial compartment of Cryptosporidium parvum. Trends Parasitol, 21, 68–74. [DOI] [PubMed] [Google Scholar]
- Hiller NL, Bhattacharjee S, Van Ooij C, Liolios K, Harrison T, Lopez-Estrano C & Haldar K, 2004. A host-targeting signal in virulence proteins reveals a secretome in malarial infection. Science, 306, 1934–7. [DOI] [PubMed] [Google Scholar]
- Horvath S & Dong J, 2008. Geometric interpretation of gene coexpression network analysis. PLoS Comput Biol, 4, e1000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao CH, Luisa Hiller N, Haldar K & Knoll LJ, 2013. A HT/PEXEL motif in Toxoplasma dense granule proteins is a signal for protein cleavage but not export into the host cell. Traffic, 14, 519–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, Cabrera A, Kono M, Mok S, Chaal BK, Haase S, Engelberg K, Cheemadan S, Spielmann T, Preiser PR, Gilberger TW & Bozdech Z, 2010. Transcriptional profiling of growth perturbations of the human malaria parasite Plasmodium falciparum. Nat Biotechnol, 28, 91–8. [DOI] [PubMed] [Google Scholar]
- Hu P, Janga SC, Babu M, Diaz-Mejia JJ, Butland G, Yang W, Pogoutse O, Guo X, Phanse S, Wong P, Chandran S, Christopoulos C, Nazarians-Armavil A, Nasseri NK, Musso G, Ali M, Nazemof N, Eroukova V, Golshani A, Paccanaro A, Greenblatt JF, Moreno-Hagelsieb G & Emili A, 2009. Global functional atlas of Escherichia coli encompassing previously uncharacterized proteins. PLoS Biol, 7, e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung SS & Parkinson J, 2011. Post-genomics resources and tools for studying apicomplexan metabolism. Trends Parasitol, 27, 131–40. [DOI] [PubMed] [Google Scholar]
- Hung SS, Wasmuth J, Sanford C & Parkinson J, 2010. DETECT--a density estimation tool for enzyme classification and its application to Plasmodium falciparum. Bioinformatics, 26, 1690–8. [DOI] [PubMed] [Google Scholar]
- Hupalo DN, Luo Z, Melnikov A, Sutton PL, Rogov P, Escalante A, Vallejo AF, Herrera S, Arevalo-Herrera M, Fan Q, Wang Y, Cui L, Lucas CM, Durand S, Sanchez JF, Baldeviano GC, Lescano AG, Laman M, Barnadas C, Barry A, Mueller I, Kazura JW, Eapen A, Kanagaraj D, Valecha N, Ferreira MU, Roobsoong W, Nguitragool W, Sattabonkot J, Gamboa D, Kosek M, Vinetz JM, Gonzalez-Ceron L, Birren BW, Neafsey DE & Carlton JM, 2016. Population genomics studies identify signatures of global dispersal and drug resistance in Plasmodium vivax. Nat Genet, 48, 953–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huthmacher C, Hoppe A, Bulik S & Holzhutter HG, 2010. Antimalarial drug targets in Plasmodium falciparum predicted by stage-specific metabolic network analysis. BMC Syst Biol, 4, 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde JE, 2007. Targeting purine and pyrimidine metabolism in human apicomplexan parasites. Curr Drug Targets, 8, 31–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakalski M, Wakaguri H, Kischka TG, Nishikawa Y, Kawazu S, Matsubayashi M, Kawahara F, Tsuji N, Cao S, Sunaga F, Xuan X, Okubo K, Igarashi I, Tuda J, Mongan AE, Eshita Y, Maeda R, Makalowski W, Suzuki Y & Yamagishi J, 2015. DB-AT: a 2015 update to the Full-parasites database brings a multitude of new transcriptomic data for apicomplexan parasites. Nucleic Acids Res, 43, D631–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janouskovec J, Horak A, Obornik M, Lukes J & Keeling PJ, 2010. A common red algal origin of the apicomplexan, dinoflagellate, and heterokont plastids. Proc Natl Acad Sci U S A, 107, 10949–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janouskovec J & Keeling PJ, 2016. Evolution: Causality and the Origin of Parasitism. Curr Biol, 26, R174–7. [DOI] [PubMed] [Google Scholar]
- Janse CJ, Carlton JM, Walliker D & Waters AP, 1994. Conserved location of genes on polymorphic chromosomes of four species of malaria parasites. Mol Biochem Parasitol, 68, 285–96. [DOI] [PubMed] [Google Scholar]
- Jones SH, Lew AE, Jorgensen WK & Barker SC, 1997. Babesia bovis: genome size, number of chromosomes and telomeric probe hybridisation. Int J Parasitol, 27, 1569–73. [DOI] [PubMed] [Google Scholar]
- Kafsack BF, Rovira-Graells N, Clark TG, Bancells C, Crowley VM, Campino SG, Williams AE, Drought LG, Kwiatkowski DP, Baker DA, Cortes A & Llinas M, 2014. A transcriptional switch underlies commitment to sexual development in malaria parasites. Nature, 507, 248–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko I, Iwanaga S, Kato T, Kobayashi I & Yuda M, 2015. Genome-Wide Identification of the Target Genes of AP2-O, a Plasmodium AP2-Family Transcription Factor. PLoS Pathog, 11, e1004905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitonov VV & Jurka J, 2008. A universal classification of eukaryotic transposable elements implemented in Repbase. Nat Rev Genet, 9, 411–2; author reply 414. [DOI] [PubMed] [Google Scholar]
- Katzer F, Lizundia R, Ngugi D, Blake D & Mckeever D, 2011. Construction of a genetic map for Theileria parva: identification of hotspots of recombination. Int J Parasitol, 41, 669–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp LE, Yamamoto M & Soldati-Favre D, 2013. Subversion of host cellular functions by the apicomplexan parasites. FEMS Microbiol Rev, 37, 607–31. [DOI] [PubMed] [Google Scholar]
- Khaminets A, Hunn JP, Konen-Waisman S, Zhao YO, Preukschat D, Coers J, Boyle JP, Ong YC, Boothroyd JC, Reichmann G & Howard JC, 2010. Coordinated loading of IRG resistance GTPases on to the Toxoplasma gondii parasitophorous vacuole. Cell Microbiol, 12, 939–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A, Taylor S, Su C, Mackey AJ, Boyle J, Cole R, Glover D, Tang K, Paulsen IT, Berriman M, Boothroyd JC, Pfefferkorn ER, Dubey JP, Ajioka JW, Roos DS, Wootton JC & Sibley LD, 2005. Composite genome map and recombination parameters derived from three archetypal lineages of Toxoplasma gondii. Nucleic Acids Res, 33, 2980–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Thiessen PA, Bolton EE, Chen J, Fu G, Gindulyte A, Han L, He J, He S, Shoemaker BA, Wang J, Yu B, Zhang J & Bryant SH, 2016. PubChem Substance and Compound databases. Nucleic Acids Res, 44, D1202–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissinger JC & Debarry J, 2011. Genome cartography: charting the apicomplexan genome. Trends Parasitol, 27, 345–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono M, Herrmann S, Loughran NB, Cabrera A, Engelberg K, Lehmann C, Sinha D, Prinz B, Ruch U, Heussler V, Spielmann T, Parkinson J & Gilberger TW, 2012. Evolution and architecture of the inner membrane complex in asexual and sexual stages of the malaria parasite. Mol Biol Evol, 29, 2113–32. [DOI] [PubMed] [Google Scholar]
- Kono M, Prusty D, Parkinson J & Gilberger TW, 2013. A membranous system in the spotlight: The apicomplexan Inner Membrane Complex and its adaptation to an endoparasitic life style. Frontiers in Bioscience, In Press. [DOI] [PubMed] [Google Scholar]
- Koreny L, Obornik M & Lukes J, 2013. Make it, take it, or leave it: heme metabolism of parasites. PLoS Pathog, 9, e1003088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acacio S, Biswas K, O’reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM & Levine MM, 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet, 382, 209–22. [DOI] [PubMed] [Google Scholar]
- Krogan NJ, Cagney G, Yu H, Zhong G, Guo X, Ignatchenko A, Li J, Pu S, Datta N, Tikuisis AP, Punna T, Peregrin-Alvarez JM, Shales M, Zhang X, Davey M, Robinson MD, Paccanaro A, Bray JE, Sheung A, Beattie B, Richards DP, Canadien V, Lalev A, Mena F, Wong P, Starostine A, Canete MM, Vlasblom J, Wu S, Orsi C, Collins SR, Chandran S, Haw R, Rilstone JJ, Gandi K, Thompson NJ, Musso G, St Onge P, Ghanny S, Lam MH, Butland G, Altaf-Ul AM, Kanaya S, Shilatifard A, O’shea E, Weissman JS, Ingles CJ, Hughes TR, Parkinson J, Gerstein M, Wodak SJ, Emili A & Greenblatt JF, 2006. Global landscape of protein complexes in the yeast Saccharomyces cerevisiae. Nature, 440, 637–43. [DOI] [PubMed] [Google Scholar]
- Lacount DJ, Vignali M, Chettier R, Phansalkar A, Bell R, Hesselberth JR, Schoenfeld LW, Ota I, Sahasrabudhe S, Kurschner C, Fields S & Hughes RE, 2005. A protein interaction network of the malaria parasite Plasmodium falciparum. Nature, 438, 103–7. [DOI] [PubMed] [Google Scholar]
- Lamarque M, Besteiro S, Papoin J, Roques M, Vulliez-Le Normand B, Morlon-Guyot J, Dubremetz JF, Fauquenoy S, Tomavo S, Faber BW, Kocken CH, Thomas AW, Boulanger MJ, Bentley GA & Lebrun M, 2011. The RON2-AMA1 interaction is a critical step in moving junction-dependent invasion by apicomplexan parasites. PLoS Pathog, 7, e1001276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamarque MH, Roques M, Kong-Hap M, Tonkin ML, Rugarabamu G, Marq JB, Penarete-Vargas DM, Boulanger MJ, Soldati-Favre D & Lebrun M, 2014. Plasticity and redundancy among AMA-RON pairs ensure host cell entry of Toxoplasma parasites. Nat Commun, 5, 4098. [DOI] [PubMed] [Google Scholar]
- Le Roch KG, Zhou Y, Blair PL, Grainger M, Moch JK, Haynes JD, De La Vega P, Holder AA, Batalov S, Carucci DJ & Winzeler EA, 2003. Discovery of gene function by expression profiling of the malaria parasite life cycle. Science, 301, 1503–8. [DOI] [PubMed] [Google Scholar]
- Lee JM, Gianchandani EP & Papin JA, 2006. Flux balance analysis in the era of metabolomics. Brief Bioinform, 7, 140–50. [DOI] [PubMed] [Google Scholar]
- Lee JM & Sonnhammer EL, 2003. Genomic gene clustering analysis of pathways in eukaryotes. Genome Res, 13, 875–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesenfeld O, Parvanova I, Zerrahn J, Han SJ, Heinrich F, Munoz M, Kaiser F, Aebischer T, Buch T, Waisman A, Reichmann G, Utermohlen O, Von Stebut E, Von Loewenich FD, Bogdan C, Specht S, Saeftel M, Hoerauf A, Mota MM, Konen-Waisman S, Kaufmann SH & Howard JC, 2011. The IFN-gamma-inducible GTPase, Irga6, protects mice against Toxoplasma gondii but not against Plasmodium berghei and some other intracellular pathogens. PLoS One, 6, e20568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim DC, Cooke BM, Doerig C & Saeij JP, 2012. Toxoplasma and Plasmodium protein kinases: roles in invasion and host cell remodelling. Int J Parasitol, 42, 21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim L & Mcfadden GI, 2010. The evolution, metabolism and functions of the apicoplast. Philos Trans R Soc Lond B Biol Sci, 365, 749–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingelbach K & Joiner KA, 1998. The parasitophorous vacuole membrane surrounding Plasmodium and Toxoplasma: an unusual compartment in infected cells. J Cell Sci, 111 ( Pt 11), 1467–75. [DOI] [PubMed] [Google Scholar]
- Lorenzi H, Khan A, Behnke MS, Namasivayam S, Swapna LS, Hadjithomas M, Karamycheva S, Pinney D, Brunk BP, Ajioka JW, Ajzenberg D, Boothroyd JC, Boyle JP, Darde ML, Diaz-Miranda MA, Dubey JP, Fritz HM, Gennari SM, Gregory BD, Kim K, Saeij JP, Su C, White MW, Zhu XQ, Howe DK, Rosenthal BM, Grigg ME, Parkinson J, Liu L, Kissinger JC, Roos DS & David Sibley L, 2016a. Local admixture of amplified and diversified secreted pathogenesis determinants shapes mosaic Toxoplasma gondii genomes. Nat Commun, 7, 10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzi H, Khan A, Behnke MS, Namasivayam S, Swapna LS, Hadjithomas M, Karamycheva S, Pinney D, Brunk BP, Ajioka JW, Ajzenberg D, Boothroyd JC, Boyle JP, Darde ML, Diaz-Miranda MA, Dubey JP, Fritz HM, Gennari SM, Gregory BD, Kim K, Saeij JP, Su C, White MW, Zhu XQ, Howe DK, Rosenthal BM, Grigg ME, Parkinson J, Liu L, Kissinger JC, Roos DS & Sibley LD, 2016b. Local admixture of amplified and diversified secreted pathogenesis determinants shapes mosaic Toxoplasma gondii genomes. Nat Commun, 7, 10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrid-Aliste CJ, Dybas JM, Angeletti RH, Weiss LM, Kim K, Simon I & Fiser A, 2009. EPIC-DB: a proteomics database for studying Apicomplexan organisms. BMC Genomics, 10, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malleret B, Li A, Zhang R, Tan KS, Suwanarusk R, Claser C, Cho JS, Koh EG, Chu CS, Pukrittayakamee S, Ng ML, Ginhoux F, Ng LG, Lim CT, Nosten F, Snounou G, Renia L & Russell B, 2015. Plasmodium vivax: restricted tropism and rapid remodeling of CD71-positive reticulocytes. Blood, 125, 1314–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti M, Good RT, Rug M, Knuepfer E & Cowman AF, 2004. Targeting malaria virulence and remodeling proteins to the host erythrocyte. Science, 306, 1930–3. [DOI] [PubMed] [Google Scholar]
- Marti M & Spielmann T, 2013. Protein export in malaria parasites: many membranes to cross. Curr Opin Microbiol, 16, 445–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinsen ES, Perkins SL & Schall JJ, 2008. A three-genome phylogeny of malaria parasites (Plasmodium and closely related genera): evolution of life-history traits and host switches. Mol Phylogenet Evol, 47, 261–73. [DOI] [PubMed] [Google Scholar]
- Mazurie AJ, Alves JM, Ozaki LS, Zhou S, Schwartz DC & Buck GA, 2013. Comparative genomics of cryptosporidium. Int J Genomics, 2013, 832756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcconkey GA, Pinney JW, Westhead DR, Plueckhahn K, Fitzpatrick TB, Macheroux P & Kappes B, 2004. Annotating the Plasmodium genome and the enigma of the shikimate pathway. Trends Parasitol, 20, 60–5. [DOI] [PubMed] [Google Scholar]
- Mcdonald V & Shirley MW, 2009. Past and future: vaccination against Eimeria. Parasitology, 136, 1477–89. [DOI] [PubMed] [Google Scholar]
- Mcfadden GI & Yeh E, 2016. The apicoplast: now you see it, now you don’t. Int J Parasitol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckinney JD, Honer Zu Bentrup K, Munoz-Elias EJ, Miczak A, Chen B, Chan WT, Swenson D, Sacchettini JC, Jacobs WR Jr. & Russell DG, 2000. Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature, 406, 735–8. [DOI] [PubMed] [Google Scholar]
- Meissner M, Ferguson DJ & Frischknecht F, 2013. Invasion factors of apicomplexan parasites: essential or redundant? Curr Opin Microbiol, 16, 438–44. [DOI] [PubMed] [Google Scholar]
- Mercier C, Adjogble KD, Daubener W & Delauw MF, 2005. Dense granules: are they key organelles to help understand the parasitophorous vacuole of all apicomplexa parasites? Int J Parasitol, 35, 829–49. [DOI] [PubMed] [Google Scholar]
- Mercier C & Cesbron-Delauw MF, 2015. Toxoplasma secretory granules: one population or more? Trends Parasitol, 31, 60–71. [DOI] [PubMed] [Google Scholar]
- Minot S, Melo MB, Li F, Lu D, Niedelman W, Levine SS & Saeij JP, 2012. Admixture and recombination among Toxoplasma gondii lineages explain global genome diversity. Proc Natl Acad Sci U S A, 109, 13458–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miotto O, Almagro-Garcia J, Manske M, Macinnis B, Campino S, Rockett KA, Amaratunga C, Lim P, Suon S, Sreng S, Anderson JM, Duong S, Nguon C, Chuor CM, Saunders D, Se Y, Lon C, Fukuda MM, Amenga-Etego L, Hodgson AV, Asoala V, Imwong M, Takala-Harrison S, Nosten F, Su XZ, Ringwald P, Ariey F, Dolecek C, Hien TT, Boni MF, Thai CQ, Amambua-Ngwa A, Conway DJ, Djimde AA, Doumbo OK, Zongo I, Ouedraogo JB, Alcock D, Drury E, Auburn S, Koch O, Sanders M, Hubbart C, Maslen G, Ruano-Rubio V, Jyothi D, Miles A, O’brien J, Gamble C, Oyola SO, Rayner JC, Newbold CI, Berriman M, Spencer CC, Mcvean G, Day NP, White NJ, Bethell D, Dondorp AM, Plowe CV, Fairhurst RM & Kwiatkowski DP, 2013. Multiple populations of artemisinin-resistant Plasmodium falciparum in Cambodia. Nat Genet, 45, 648–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miotto O, Amato R, Ashley EA, Macinnis B, Almagro-Garcia J, Amaratunga C, Lim P, Mead D, Oyola SO, Dhorda M, Imwong M, Woodrow C, Manske M, Stalker J, Drury E, Campino S, Amenga-Etego L, Thanh TN, Tran HT, Ringwald P, Bethell D, Nosten F, Phyo AP, Pukrittayakamee S, Chotivanich K, Chuor CM, Nguon C, Suon S, Sreng S, Newton PN, Mayxay M, Khanthavong M, Hongvanthong B, Htut Y, Han KT, Kyaw MP, Faiz MA, Fanello CI, Onyamboko M, Mokuolu OA, Jacob CG, Takala-Harrison S, Plowe CV, Day NP, Dondorp AM, Spencer CC, Mcvean G, Fairhurst RM, White NJ & Kwiatkowski DP, 2015. Genetic architecture of artemisinin-resistant Plasmodium falciparum. Nat Genet, 47, 226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogi T & Kita K, 2010. Diversity in mitochondrial metabolic pathways in parasitic protists Plasmodium and Cryptosporidium. Parasitol Int, 59, 305–12. [DOI] [PubMed] [Google Scholar]
- Moreno SN & Li ZH, 2008. Anti-infectives targeting the isoprenoid pathway of Toxoplasma gondii. Expert Opin Ther Targets, 12, 253–63. [DOI] [PubMed] [Google Scholar]
- Moriya Y, Itoh M, Okuda S, Yoshizawa AC & Kanehisa M, 2007. KAAS: an automatic genome annotation and pathway reconstruction server. Nucleic Acids Res, 35, W182–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’hara SP & Chen XM, 2011. The cell biology of cryptosporidium infection. Microbes Infect, 13, 721–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberstaller J, Pumpalova Y, Schieler A, Llinas M & Kissinger JC, 2014. The Cryptosporidium parvum ApiAP2 gene family: insights into the evolution of apicomplexan AP2 regulatory systems. Nucleic Acids Res, 42, 8271–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pain A, Bohme U, Berry AE, Mungall K, Finn RD, Jackson AP, Mourier T, Mistry J, Pasini EM, Aslett MA, Balasubrammaniam S, Borgwardt K, Brooks K, Carret C, Carver TJ, Cherevach I, Chillingworth T, Clark TG, Galinski MR, Hall N, Harper D, Harris D, Hauser H, Ivens A, Janssen CS, Keane T, Larke N, Lapp S, Marti M, Moule S, Meyer IM, Ormond D, Peters N, Sanders M, Sanders S, Sargeant TJ, Simmonds M, Smith F, Squares R, Thurston S, Tivey AR, Walker D, White B, Zuiderwijk E, Churcher C, Quail MA, Cowman AF, Turner CM, Rajandream MA, Kocken CH, Thomas AW, Newbold CI, Barrell BG & Berriman M, 2008. The genome of the simian and human malaria parasite Plasmodium knowlesi. Nature, 455, 799–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pain A, Renauld H, Berriman M, Murphy L, Yeats CA, Weir W, Kerhornou A, Aslett M, Bishop R, Bouchier C, Cochet M, Coulson RM, Cronin A, De Villiers EP, Fraser A, Fosker N, Gardner M, Goble A, Griffiths-Jones S, Harris DE, Katzer F, Larke N, Lord A, Maser P, Mckellar S, Mooney P, Morton F, Nene V, O’neil S, Price C, Quail MA, Rabbinowitsch E, Rawlings ND, Rutter S, Saunders D, Seeger K, Shah T, Squares R, Squares S, Tivey A, Walker AR, Woodward J, Dobbelaere DA, Langsley G, Rajandream MA, Mckeever D, Shiels B, Tait A, Barrell B & Hall N, 2005. Genome of the host-cell transforming parasite Theileria annulata compared with T. parva. Science, 309, 131–3. [DOI] [PubMed] [Google Scholar]
- Painter HJ, Campbell TL & Llinas M, 2011. The Apicomplexan AP2 family: integral factors regulating Plasmodium development. Mol Biochem Parasitol, 176, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker ML, Penarete-Vargas DM, Hamilton PT, Guerin A, Dubey JP, Perlman SJ, Spano F, Lebrun M & Boulanger MJ, 2016. Dissecting the interface between apicomplexan parasite and host cell: Insights from a divergent AMA-RON2 pair. Proc Natl Acad Sci U S A, 113, 398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson J, Anthony A, Wasmuth J, Schmid R, Hedley A & Blaxter M, 2004. PartiGene--constructing partial genomes. Bioinformatics, 20, 1398–404. [DOI] [PubMed] [Google Scholar]
- Pelle KG, Jiang RH, Mantel PY, Xiao YP, Hjelmqvist D, Gallego-Lopez GM, A OTL, Kang BH, Allred DR & Marti M, 2015. Shared elements of host-targeting pathways among apicomplexan parasites of differing lifestyles. Cell Microbiol, 17, 1618–39. [DOI] [PubMed] [Google Scholar]
- Petersen I, Eastman R & Lanzer M, 2011a. Drug-resistant malaria: molecular mechanisms and implications for public health. FEBS Lett, 585, 1551–62. [DOI] [PubMed] [Google Scholar]
- Petersen TN, Brunak S, Von Heijne G & Nielsen H, 2011b. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods, 8, 785–6. [DOI] [PubMed] [Google Scholar]
- Pinney JW, Papp B, Hyland C, Wambua L, Westhead DR & Mcconkey GA, 2007. Metabolic reconstruction and analysis for parasite genomes. Trends Parasitol, 23, 548–54. [DOI] [PubMed] [Google Scholar]
- Plata G, Hsiao TL, Olszewski KL, Llinas M & Vitkup D, 2010. Reconstruction and flux-balance analysis of the Plasmodium falciparum metabolic network. Mol Syst Biol, 6, 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poukchanski A, Fritz HM, Tonkin ML, Treeck M, Boulanger MJ & Boothroyd JC, 2013. Toxoplasma gondii sporozoites invade host cells using two novel paralogues of RON2 and AMA1. PLoS One, 8, e70637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proellocks NI, Coppel RL & Waller KL, 2010. Dissecting the apicomplexan rhoptry neck proteins. Trends Parasitol, 26, 297–304. [DOI] [PubMed] [Google Scholar]
- Radke JB, Lucas O, De Silva EK, Ma Y, Sullivan WJ Jr., Weiss LM, Llinas M & White MW, 2013. ApiAP2 transcription factor restricts development of the Toxoplasma tissue cyst. Proc Natl Acad Sci U S A, 110, 6871–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman K & Chandra N, 2009. Flux balance analysis of biological systems: applications and challenges. Brief Bioinform, 10, 435–49. [DOI] [PubMed] [Google Scholar]
- Ramaprasad A, Pain A & Ravasi T, 2012. Defining the protein interaction network of human malaria parasite Plasmodium falciparum. Genomics, 99, 69–75. [DOI] [PubMed] [Google Scholar]
- Reid AJ, 2015. Large, rapidly evolving gene families are at the forefront of host-parasite interactions in Apicomplexa. Parasitology, 142 Suppl 1, S57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid AJ, Blake DP, Ansari HR, Billington K, Browne HP, Bryant J, Dunn M, Hung SS, Kawahara F, Miranda-Saavedra D, Malas TB, Mourier T, Naghra H, Nair M, Otto TD, Rawlings ND, Rivailler P, Sanchez-Flores A, Sanders M, Subramaniam C, Tay YL, Woo Y, Wu X, Barrell B, Dear PH, Doerig C, Gruber A, Ivens AC, Parkinson J, Rajandream MA, Shirley MW, Wan KL, Berriman M, Tomley FM & Pain A, 2014. Genomic analysis of the causative agents of coccidiosis in domestic chickens. Genome Res, 24, 1676–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid AJ, Vermont SJ, Cotton JA, Harris D, Hill-Cawthorne GA, Konen-Waisman S, Latham SM, Mourier T, Norton R, Quail MA, Sanders M, Shanmugam D, Sohal A, Wasmuth JD, Brunk B, Grigg ME, Howard JC, Parkinson J, Roos DS, Trees AJ, Berriman M, Pain A & Wastling JM, 2012. Comparative genomics of the apicomplexan parasites Toxoplasma gondii and Neospora caninum: Coccidia differing in host range and transmission strategy. PLoS Pathog, 8, e1002567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo I, Babbitt S, Muralidharan V, Butler T, Oksman A & Goldberg DE, 2010. Plasmepsin V licenses Plasmodium proteins for export into the host erythrocyte. Nature, 463, 632–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S, 2011. The apicomplexan plastid and its evolution. Cell Mol Life Sci, 68, 1285–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf A, Hernandez-Rivas R, Buffet P, Bottius E, Benatar C, Pouvelle B, Gysin J & Lanzer M, 1998. Antigenic variation in malaria: in situ switching, relaxed and mutually exclusive transcription of var genes during intra-erythrocytic development in Plasmodium falciparum. EMBO J, 17, 5418–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluter D, Daubener W, Schares G, Gross U, Pleyer U & Luder C, 2014. Animals are key to human toxoplasmosis. Int J Med Microbiol, 304, 917–29. [DOI] [PubMed] [Google Scholar]
- Schmuckli-Maurer J, Casanova C, Schmied S, Affentranger S, Parvanova I, Kang’a S, Nene V, Katzer F, Mckeever D, Muller J, Bishop R, Pain A & Dobbelaere DA, 2009. Expression analysis of the Theileria parva subtelomere-encoded variable secreted protein gene family. PLoS One, 4, e4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider AG & Mercereau-Puijalon O, 2005. A new Apicomplexa-specific protein kinase family: multiple members in Plasmodium falciparum, all with an export signature. BMC Genomics, 6, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schomburg I, Chang A, Placzek S, Sohngen C, Rother M, Lang M, Munaretto C, Ulas S, Stelzer M, Grote A, Scheer M & Schomburg D, 2013. BRENDA in 2013: integrated reactions, kinetic data, enzyme function data, improved disease classification: new options and contents in BRENDA. Nucleic Acids Res, 41, D764–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeber F & Steinfelder S, 2016. Recent advances in understanding apicomplexan parasites. F1000Res, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugasundram A, Gonzalez-Galarza FF, Wastling JM, Vasieva O & Jones AR, 2013. Library of Apicomplexan Metabolic Pathways: a manually curated database for metabolic pathways of apicomplexan parasites. Nucleic Acids Res, 41, D706–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P & Chitnis CE, 2013. Key molecular events during host cell invasion by Apicomplexan pathogens. Curr Opin Microbiol, 16, 432–7. [DOI] [PubMed] [Google Scholar]
- Shaw MK, 2003. Cell invasion by Theileria sporozoites. Trends Parasitol, 19, 2–6. [DOI] [PubMed] [Google Scholar]
- Shaw MK, Tilney LG & Musoke AJ, 1991. The entry of Theileria parva sporozoites into bovine lymphocytes: evidence for MHC class I involvement. J Cell Biol, 113, 87–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shears MJ, Botte CY & Mcfadden GI, 2015. Fatty acid metabolism in the Plasmodium apicoplast: Drugs, doubts and knockouts. Mol Biochem Parasitol, 199, 34–50. [DOI] [PubMed] [Google Scholar]
- Sheiner L, Vaidya AB & Mcfadden GI, 2013. The metabolic roles of the endosymbiotic organelles of Toxoplasma and Plasmodium spp. Curr Opin Microbiol, 16, 452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley LD, 2011. Invasion and intracellular survival by protozoan parasites. Immunol Rev, 240, 72–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley LD, Qiu W, Fentress S, Taylor SJ, Khan A & Hui R, 2009. Forward genetics in Toxoplasma gondii reveals a family of rhoptry kinases that mediates pathogenesis. Eukaryot Cell, 8, 1085–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh GP, Chandra BR, Bhattacharya A, Akhouri RR, Singh SK & Sharma A, 2004. Hyper-expansion of asparagines correlates with an abundance of proteins with prion-like domains in Plasmodium falciparum. Mol Biochem Parasitol, 137, 307–19. [DOI] [PubMed] [Google Scholar]
- Sinha A, Hughes KR, Modrzynska KK, Otto TD, Pfander C, Dickens NJ, Religa AA, Bushell E, Graham AL, Cameron R, Kafsack BF, Williams AE, Llinas M, Berriman M, Billker O & Waters AP, 2014. A cascade of DNA-binding proteins for sexual commitment and development in Plasmodium. Nature, 507, 253–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Chiasson MA, Nursimulu N, Hung SS, Wasmuth J, Grigg ME & Parkinson J, 2013. Metabolic reconstruction identifies strain-specific regulation of virulence in Toxoplasma gondii. Mol Syst Biol, 9, 708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan P, Beatty WL, Diouf A, Herrera R, Ambroggio X, Moch JK, Tyler JS, Narum DL, Pierce SK, Boothroyd JC, Haynes JD & Miller LH, 2011. Binding of Plasmodium merozoite proteins RON2 and AMA1 triggers commitment to invasion. Proc Natl Acad Sci U S A, 108, 13275–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart JM, Segal E, Koller D & Kim SK, 2003. A gene-coexpression network for global discovery of conserved genetic modules. Science, 302, 249–55. [DOI] [PubMed] [Google Scholar]
- Su C, Khan A, Zhou P, Majumdar D, Ajzenberg D, Darde ML, Zhu XQ, Ajioka JW, Rosenthal BM, Dubey JP & Sibley LD, 2012. Globally diverse Toxoplasma gondii isolates comprise six major clades originating from a small number of distinct ancestral lineages. Proc Natl Acad Sci U S A, 109, 5844–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhre K, 2007. Inference of gene function based on gene fusion events: the rosetta-stone method. Methods Mol Biol, 396, 31–41. [DOI] [PubMed] [Google Scholar]
- Tachibana S, Sullivan SA, Kawai S, Nakamura S, Kim HR, Goto N, Arisue N, Palacpac NM, Honma H, Yagi M, Tougan T, Katakai Y, Kaneko O, Mita T, Kita K, Yasutomi Y, Sutton PL, Shakhbatyan R, Horii T, Yasunaga T, Barnwell JW, Escalante AA, Carlton JM & Tanabe K, 2012. Plasmodium cynomolgi genome sequences provide insight into Plasmodium vivax and the monkey malaria clade. Nat Genet, 44, 1051–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talevich E & Kannan N, 2013. Structural and evolutionary adaptation of rhoptry kinases and pseudokinases, a family of coccidian virulence factors. BMC Evol Biol, 13, 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardieux I & Baum J, 2016. Reassessing the mechanics of parasite motility and host-cell invasion. J Cell Biol, 214, 507–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiburcio M, Sauerwein R, Lavazec C & Alano P, 2015. Erythrocyte remodeling by Plasmodium falciparum gametocytes in the human host interplay. Trends Parasitol, 31, 270–8. [DOI] [PubMed] [Google Scholar]
- Tomita T, Bzik DJ, Ma YF, Fox BA, Markillie LM, Taylor RC, Kim K & Weiss LM, 2013. The Toxoplasma gondii cyst wall protein CST1 is critical for cyst wall integrity and promotes bradyzoite persistence. PLoS Pathog, 9, e1003823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonkin ML, Roques M, Lamarque MH, Pugniere M, Douguet D, Crawford J, Lebrun M & Boulanger MJ, 2011. Host cell invasion by apicomplexan parasites: insights from the co-structure of AMA1 with a RON2 peptide. Science, 333, 463–7. [DOI] [PubMed] [Google Scholar]
- Torgerson PR & Mastroiacovo P, 2013. The global burden of congenital toxoplasmosis: a systematic review. Bull World Health Organ, 91, 501–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treeck M, Sanders JL, Elias JE & Boothroyd JC, 2011. The phosphoproteomes of Plasmodium falciparum and Toxoplasma gondii reveal unusual adaptations within and beyond the parasites’ boundaries. Cell Host Microbe, 10, 410–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treeck M, Sanders JL, Gaji RY, Lafavers KA, Child MA, Arrizabalaga G, Elias JE & Boothroyd JC, 2014. The calcium-dependent protein kinase 3 of toxoplasma influences basal calcium levels and functions beyond egress as revealed by quantitative phosphoproteome analysis. PLoS Pathog, 10, e1004197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tymoshenko S, Oppenheim RD, Agren R, Nielsen J, Soldati-Favre D & Hatzimanikatis V, 2015. Metabolic Needs and Capabilities of Toxoplasma gondii through Combined Computational and Experimental Analysis. PLoS Comput Biol, 11, e1004261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanee N, Roberts SB, Fong SS, Manque P & Buck GA, 2010. A genome-scale metabolic model of Cryptosporidium hominis. Chem Biodivers, 7, 1026–39. [DOI] [PubMed] [Google Scholar]
- Villen J & Gygi SP, 2008. The SCX/IMAC enrichment approach for global phosphorylation analysis by mass spectrometry. Nat Protoc, 3, 1630–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkman SK, Sabeti PC, Decaprio D, Neafsey DE, Schaffner SF, Milner DA Jr., Daily JP, Sarr O, Ndiaye D, Ndir O, Mboup S, Duraisingh MT, Lukens A, Derr A, Stange-Thomann N, Waggoner S, Onofrio R, Ziaugra L, Mauceli E, Gnerre S, Jaffe DB, Zainoun J, Wiegand RC, Birren BW, Hartl DL, Galagan JE, Lander ES & Wirth DF, 2007. A genome-wide map of diversity in Plasmodium falciparum. Nat Genet, 39, 113–9. [DOI] [PubMed] [Google Scholar]
- Walker R, Gissot M, Huot L, Alayi TD, Hot D, Marot G, Schaeffer-Reiss C, Van Dorsselaer A, Kim K & Tomavo S, 2013. Toxoplasma transcription factor TgAP2XI-5 regulates the expression of genes involved in parasite virulence and host invasion. J Biol Chem, 288, 31127–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walzer KA, Adomako-Ankomah Y, Dam RA, Herrmann DC, Schares G, Dubey JP & Boyle JP, 2013. Hammondia hammondi, an avirulent relative of Toxoplasma gondii, has functional orthologs of known T. gondii virulence genes. Proc Natl Acad Sci U S A, 110, 7446–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan C, Borgeson B, Phanse S, Tu F, Drew K, Clark G, Xiong X, Kagan O, Kwan J, Bezginov A, Chessman K, Pal S, Cromar G, Papoulas O, Ni Z, Boutz DR, Stoilova S, Havugimana PC, Guo X, Malty RH, Sarov M, Greenblatt J, Babu M, Derry WB, Tillier ER, Wallingford JB, Parkinson J, Marcotte EM & Emili A, 2015. Panorama of ancient metazoan macromolecular complexes. Nature, 525, 339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P, Equinet L, Packer J & Doerig C, 2004. Protein kinases of the human malaria parasite Plasmodium falciparum: the kinome of a divergent eukaryote. BMC Genomics, 5, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasmuth J, Daub J, Peregrin-Alvarez JM, Finney CA & Parkinson J, 2009. The origins of apicomplexan sequence innovation. Genome Res, 19, 1202–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasmuth JD, Pszenny V, Haile S, Jansen EM, Gast AT, Sher A, Boyle JP, Boulanger MJ, Parkinson J & Grigg ME, 2012. Integrated bioinformatic and targeted deletion analyses of the SRS gene superfamily identify SRS29C as a negative regulator of Toxoplasma virulence. MBio, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F, Wang W & Liu Q, 2013. Protein kinases of Toxoplasma gondii: functions and drug targets. Parasitol Res, 112, 2121–9. [DOI] [PubMed] [Google Scholar]
- White NJ, 2009. Malaria Edinburgh: Saunders Ltd. [Google Scholar]
- Who, 2016. World Malaria Report. World Health Organization, 1–186. [Google Scholar]
- Widmer G & Sullivan S, 2012. Genomics and population biology of Cryptosporidium species. Parasite Immunol, 34, 61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JD, Merino EF, Krai PM, Mclean KJ, Tripathi AK, Vega-Rodriguez J, Jacobs-Lorena M, Klemba M & Cassera MB, 2015. Isoprenoid precursor biosynthesis is the essential metabolic role of the apicoplast during gametocytogenesis in Plasmodium falciparum. Eukaryot Cell, 14, 128–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo YH, Ansari H, Otto TD, Klinger CM, Kolisko M, Michalek J, Saxena A, Shanmugam D, Tayyrov A, Veluchamy A, Ali S, Bernal A, Del Campo J, Cihlar J, Flegontov P, Gornik SG, Hajduskova E, Horak A, Janouskovec J, Katris NJ, Mast FD, Miranda-Saavedra D, Mourier T, Naeem R, Nair M, Panigrahi AK, Rawlings ND, Padron-Regalado E, Ramaprasad A, Samad N, Tomcala A, Wilkes J, Neafsey DE, Doerig C, Bowler C, Keeling PJ, Roos DS, Dacks JB, Templeton TJ, Waller RF, Lukes J, Obornik M & Pain A, 2015. Chromerid genomes reveal the evolutionary path from photosynthetic algae to obligate intracellular parasites. Elife, 4, e06974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Q, Muraoka WT, Shen Z, Sahin O, Wang H, Wu Z, Liu P & Zhang Q, 2013. Adaptive mechanisms of Campylobacter jejuni to erythromycin treatment. BMC Microbiol, 13, 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Widmer G, Wang Y, Ozaki LS, Alves JM, Serrano MG, Puiu D, Manque P, Akiyoshi D, Mackey AJ, Pearson WR, Dear PH, Bankier AT, Peterson DL, Abrahamsen MS, Kapur V, Tzipori S & Buck GA, 2004. The genome of Cryptosporidium hominis. Nature, 431, 1107–12. [DOI] [PubMed] [Google Scholar]
- Yahara K, Furuta Y, Oshima K, Yoshida M, Azuma T, Hattori M, Uchiyama I & Kobayashi I, 2013. Chromosome painting in silico in a bacterial species reveals fine population structure. Mol Biol Evol, 30, 1454–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B & Horvath S, 2005. A general framework for weighted gene co-expression network analysis. Stat Appl Genet Mol Biol, 4, Article17. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Ramachandran V, Kumar KA, Westenberger S, Refour P, Zhou B, Li F, Young JA, Chen K, Plouffe D, Henson K, Nussenzweig V, Carlton J, Vinetz JM, Duraisingh MT & Winzeler EA, 2008. Evidence-based annotation of the malaria parasite’s genome using comparative expression profiling. PLoS One, 3, e1570. [DOI] [PMC free article] [PubMed] [Google Scholar]