Abstract
Background:
Low physical fitness, obesity, and the combination of the two in adolescence may be related to risk for disability in adulthood, but this has rarely been studied.
Objective:
To examine individual and combined associations of cardiorespiratory fitness and obesity in male adolescents with later receipt of a disability pension due to all and specific causes.
Design:
Population-based cohort study.
Setting:
Sweden.
Participants:
1 079 128 Swedish adolescents aged 16 to 19 years who were conscripted into the military between 1972 and 1994.
Measurements:
Cardiorespiratory fitness and body mass index (BMI) were measured at conscription and were related to information on later receipt of a disability pension obtained from the Social Insurance Agency.
Results:
Over a median follow-up of 28.3 years, 54 304 men were granted a disability pension. Low cardiorespiratory fitness was strongly associated with later receipt of a disability pension due to all causes (hazard ratio, 3.74 [95% CI, 3.55 to 3.95] for lowest vs. highest fitness decile) and specific causes (psychiatric, musculoskeletal, injuries, nervous system, circulatory, and tumors). Obesity was associated with greater risk for receipt of a disability pension due to all and specific causes, with the greatest risks observed for class II and III obesity. Compared with being unfit, being moderately or highly fit was associated with attenuated risk for receipt of a disability pension across BMI categories.
Limitation:
The cohort did not include women, had data on smoking and alcohol intake only in a subsample, and lacked repeated measures of exposures and covariates.
Conclusion:
Low cardiorespiratory fitness, obesity, and the combination of the two were strongly associated with later chronic disability due to a wide range of diseases and causes. Although additional well-designed studies are required, these findings support the importance of high cardiorespiratory fitness and healthy body weight during adolescence to prevent later chronic disease.
Primary Funding Source:
Karolinska Institutet.
Noncommunicable diseases are global public health issues that lead to premature death and disability (1, 2). Psychiatric and musculoskeletal disorders, cardiovascular diseases, cancer, and injuries are a major burden on societies (1, 3–5). Thus, identification of early and potentially modifiable risk factors for later chronic disease is of great public health importance. An objective approach to studying severe chronic diseases is to use receipt of a disability pension as a health outcome. In many countries, disability pensions are granted to working-aged persons who are likely to never work full-time again because of a chronic disease or injury diagnosed by a physician. In addition to serving as an important indicator of chronic disease, disability pensions are associated with high societal costs (6) and thus have not only clinical but also economic consequences.
Low cardiorespiratory fitness in childhood and adolescence has been associated with increased risk for death and disability later in life (7–9). However, few studies have examined associations of cardiorespiratory fitness in youth with later receipt of a disability pension. A study of 49 321 Swedish military conscripts aged 18 to 20 years showed that low cardiorespiratory fitness was associated with higher risk for receipt of a disability pension due to all causes, although it did not examine specific causes (10). Therefore, large studies that are powered to examine the association of cardiorespiratory fitness with receipt of a disability pension for specific causes are needed.
Obesity in youth has been found to be related to impaired health and premature death (11). Although previous studies have reported that obesity is associated with increased risk for receipt of a disability pension (6, 12), the association between severe obesity (body mass index [BMI] ≥40.0 kg/m2) in youth and later chronic disability (as indicated by receipt of a disability pension) is unknown. High cardiorespiratory fitness has been shown to potentially attenuate the negative effects of obesity (13, 14). However, the combined association of cardiorespiratory fitness and obesity with receipt of a disability pension has yet to be examined.
The aim of this study was to examine the associations of cardiorespiratory fitness and obesity with receipt of a disability pension in a large sample (>1 million) of Swedish male adolescents. Given the high power of the study, we examined individual and combined associations of cardiorespiratory fitness and obesity with receipt of a disability pension due to all and specific causes.
METHODS
Study Design and Population
This prospective cohort study used data from the Swedish Military Service Conscription Registry, which was linked to several other national registries using the men’s unique personal identification numbers as described previously (12). The Cause of Death Register and the Registry of the Total Population were used to identify men who died or emigrated during follow-up. The study was approved by the Regional Ethical Review Board, Stockholm, Sweden.
The cohort comprised male adolescents who were born in Sweden between 1951 and 1976, were conscripted into the military between 1972 and 1994, and were followed until 31 December 2012. Military conscription was mandatory for the study participants; only 2% to 3% of Swedish adolescents were exempted from conscription because of incarceration or severe medical conditions (15). In this study, we included male adolescents who were aged 16 to 19 years at conscription and had complete data on the exposures, outcomes, and covariates. In accordance with previous studies (12), we excluded males with extreme values for height (≤150 or ≥210 cm), weight (≤40 or ≥150 kg), or BMI (≤15 or ≥60 kg/m2). The overall cohort consisted of 1 125 739 adolescents, of whom 1 083 561 had complete data on cardiorespiratory fitness (the primary exposure). Another 4433 had missing or extreme values for the other exposures, outcomes, and covariates, leaving 1 079 128 adolescents (95.9%) for the analyses.
Cardiorespiratory Fitness, BMI, and Covariates
Cardiorespiratory fitness was measured in conscripts with a normal electrocardiogram using an electrically braked ergometer cycle test, as described previously (16). The initial resistance was determined by weight and was increased by 25 W for men who reported regular exercise. After a 5-minute warm-up period with a pulse between 120 and 170 beats/min, the resistance was further increased by 25 W/min until volitional exhaustion. The final work rate was retained and used in the analysis. Weight was measured using analogue or digital scales, and height was assessed using wall-mounted stadiometers according to standardized procedures (7). Body mass index was calculated as weight in kilograms divided by height in meters squared and was classified according to World Health Organization criteria (17). Year of conscription, conscription center (6 centers), and age at conscription were obtained from the Military Service Conscription Registry, and information on the occupations of participants’ parents was retrieved from the Population Housing Censuses. Childhood socioeconomic status was defined as the highest-level occupation of either parent.
Disability Pension
Data on the date and cause of receipt of a disability pension were retrieved from the Social Insurance Agency for 1972 to 2012. During the study years, the cause of receipt of a disability pension had to be confirmed by a certificate from a physician using International Classification of Diseases (ICD) codes (ICD-8, ICD-9, or ICD-10), and the work capacity had to be reduced by at least 25%. The codes were then used to classify the cause as psychiatric (for example, affective and nonaffective disorders), musculoskeletal (for example, dorsalgia and soft tissue disorders), nervous system (for example, multiple sclerosis), circulatory (for example, cerebrovascular and ischemic heart diseases), injuries, or tumors (Appendix Table 1, available at Annals.org).
Statistical Analysis
We used Cox proportional hazards regression models to calculate hazard ratios (HRs) with 95% CIs and to estimate cumulative incidences. Men were followed until they were granted a disability pension, died, or emigrated or until the end of follow-up (31 December 2012), whichever occurred first.
We first analyzed the associations of cardiorespiratory fitness and BMI with later receipt of a disability pension due to all and specific causes. We created 2 models: the first included basic covariates (conscription year, conscription center, age at conscription, and childhood socioeconomic status), and the second included these covariates plus BMI or cardiorespiratory fitness. Analyses involving receipt of a disability pension due to all and psychiatric causes were also adjusted for any mental hospitalization before conscription and any psychiatric diagnosis at conscription. In the analyses that included BMI categories, we examined the association of severe obesity with receipt of a disability pension due to all causes as well as psychiatric and musculoskeletal causes (which were most prevalent) because there were too few cases for the remaining causes.
We also assessed the combined association of cardiorespiratory fitness and BMI categories with later receipt of a disability pension, with adjustment for the aforementioned basic covariates. In these analyses, we investigated receipt of a disability pension due to all, psychiatric, and musculoskeletal causes. Adolescents in the first 2 deciles were classified as unfit, and fit adolescents were further divided into moderately fit (deciles 3 to 8) and highly fit (deciles 9 and 10) categories. The proportional hazards assumption for the exposures (fitness, BMI, and both combined) in the Cox regression was examined using a log-minus-log plot, and we found no evidence that the assumption was violated.
Statistical analyses were conducted using SPSS Statistics, version 22 (IBM).
Role of the Funding Source
Karolinska Institutet had no role in the design or conduct of the study, management of the data, interpretation of the results, or the decision to submit the manuscript for publication.
RESULTS
Descriptive Statistics
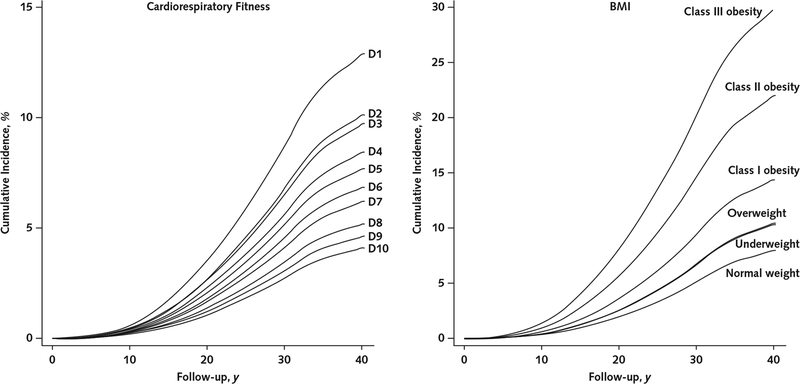
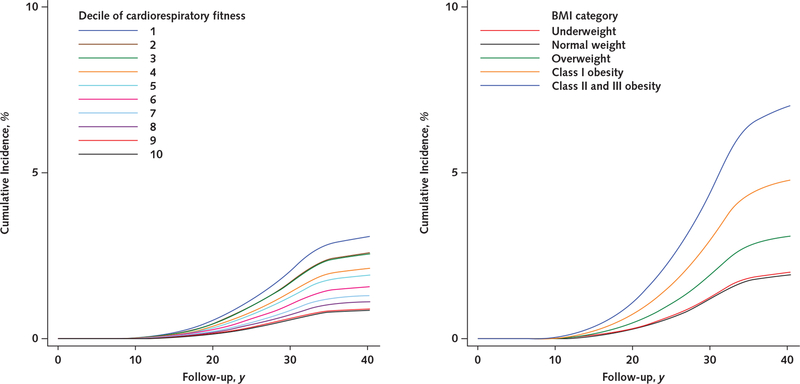
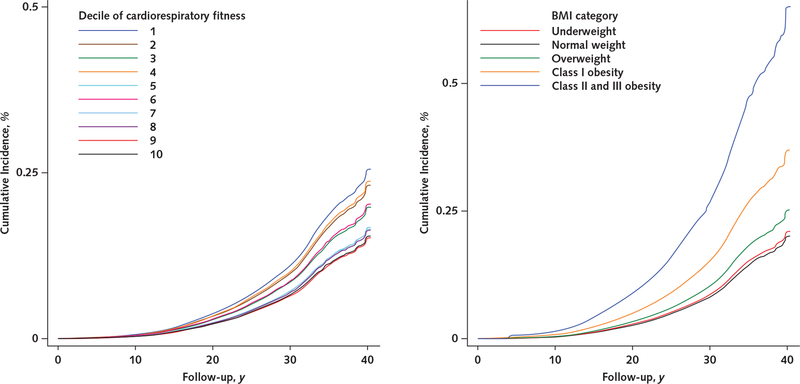
The Table shows descriptive data on the 1 079 128 participants. Over a median follow-up of 28.3 years (30.6 million person-years), 54 304 men were granted a disability pension. Figure 1 shows the unadjusted cumulative incidences of receipt of a disability pension due to all causes by cardiorespiratory fitness level (left panel) and BMI category (right panel). The cumulative incidence was consistently higher in adolescents with lower cardiorespiratory fitness and a higher BMI during follow-up. Additional data on cumulative incidences by cardiorespiratory fitness level and BMI category are shown in Appendix Figures 1 to 6 and Appendix Tables 2 and 3 (available at Annals.org).
Table.
Descriptive Data on Study Participants (n = 1 079 128)
| Characteristic | Value |
|---|---|
| Mean age at conscription (SD), y | 18.32 (0.44) |
| Mean height (SD), cm | 179.3 (6.5) |
| Mean weight (SD), kg | 69.9 (10.3) |
| Mean cardiorespiratory fitness (SD), W* | 275.2 (52.0) |
| BMI at conscription (SD), kg/m2 | 21.7 (2.8) |
| BMI category, n (%) | |
| Underweight (<18.5 kg/m2) | 87 575 (8.1) |
| Normal weight (18.5–24.9 kg/m2) | 879 367 (81.5) |
| Overweight (25.0–29.9 kg/m2) | 94 253 (8.7) |
| Mild (class I) obesity (30.0–34.9 kg/m2) | 15 027 (1.4) |
| Moderate (class II) obesity (35.0–39.9 kg/m2) | 2517 (0.2) |
| Severe (class III) obesity (≥40.0 kg/m2) | 389 (<0.01) |
| Childhood socioeconomic status, n (%)† | |
| High-level nonmanual workers | 92 361 (8.6) |
| Intermediate-level nonmanual workers | 228 669 (21.2) |
| Low-level nonmanual workers | 166 293 (15.4) |
| Self-employed or farmers | 79 483 (7.4) |
| Skilled workers | 352 448 (32.7) |
| Unskilled workers | 129 429 (12.0) |
| Other | 30 445 (2.8) |
| Cause of receipt of disability pension, n (%) | |
| All | 54 304 (5.0) |
| Psychiatric | 25 200 (2.3) |
| Musculoskeletal | 12 337 (1.1) |
| Injuries | 5387 (0.5) |
| Nervous system | 3222 (0.3) |
| Circulatory | 2289 (0.2) |
| Tumors | 968 (0.1) |
| Other | 4901 (0.5) |
| Mental hospitalization before conscription, n (%) | 9337 (0.9) |
| Psychiatric diagnosis at conscription, n (%)‡ | 53 126 (4.9) |
BMI = body mass index; ICD = International Classification of Diseases.
Measured by ergometer cycle test. Decile 1 = ≤211 W, decile 2 = 212 to 229 W, decile 3 = 230 to 241 W, decile 4 = 242 to 254 W, decile 5 = 255 to 270 W, decile 6 = 271 to 284 W, decile 7 = 285 to 300 W, decile 8 = 301 to 320 W, decile 9 = 321 to 343 W, and decile 10 = ≥344 W.
Based on highest-level occupation of parents.
Any diagnosis in psychiatric chapter of ICD (ICD-10: Chapter F; ICD-9: 290 to 319; ICD-8: 290 to 315).
Figure 1.
Unadjusted cumulative incidences of receipt of a disability pension due to all causes, by cardiorespiratory fitness level (left) and BMI category (right).
Cumulative incidences were estimated from unadjusted Cox regression models. BMI = body mass index; D = decile.
Cardiorespiratory Fitness and Receipt of a Disability Pension
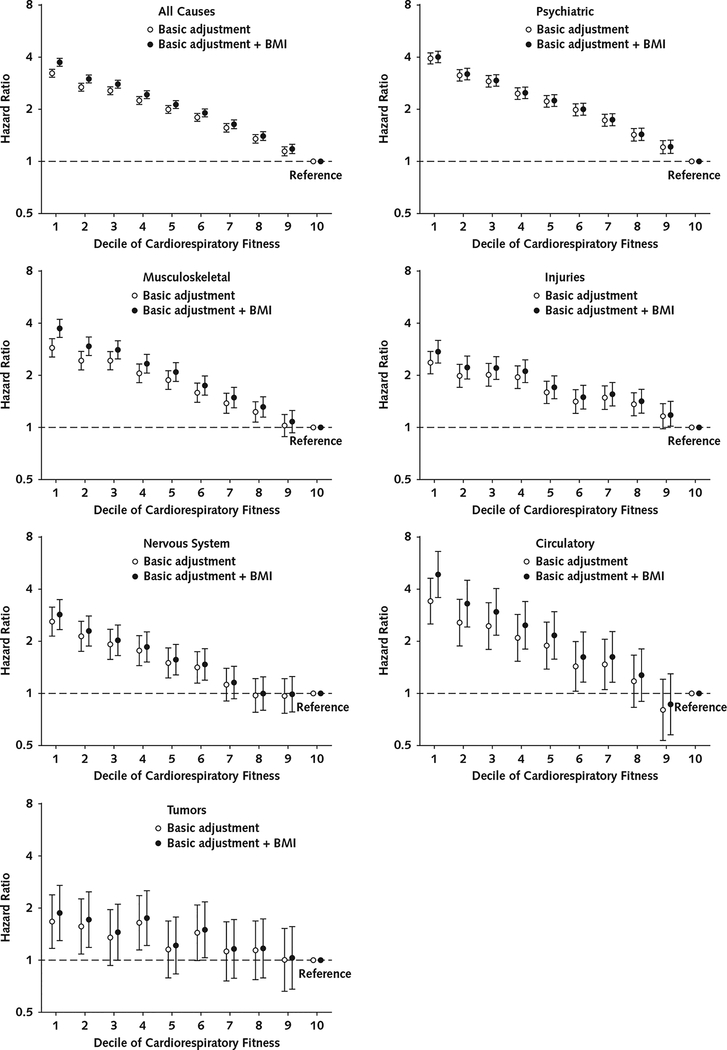
Figure 2 shows the associations of cardiorespiratory fitness with receipt of a disability pension due to all causes and specific causes. Higher cardiorespiratory fitness was associated with lower risk for receipt of a disability pension in a dose-response manner, although there was a steep decrease in risk between the first and second deciles. In the fully adjusted models, compared with adolescents in the highest decile, those in the lowest decile had a 3.74-fold (95% CI, 3.55- to 3.95-fold) higher risk for receipt of a disability pension due to all causes. They also had a statistically significantly increased risk for receipt of a disability pension due to all specific causes (psychiatric: HR, 4.01 [CI, 3.72 to 4.32]; musculoskeletal: HR, 3.72 [CI, 3.29 to 4.20]; injuries: HR, 2.74 [CI, 2.35 to 3.18]; nervous system: HR, 2.86 [CI, 2.35 to 3.48]; circulatory: HR, 4.87 [CI, 3.58 to 6.61]; tumors: HR, 1.89 [CI, 1.31 to 2.73]).
Figure 2.
Association of cardiorespiratory fitness with later receipt of a disability pension due to all and specific causes (n = 1 079 128).
Cox proportional hazards regression models were used to estimate hazard ratios with 95% CIs. Basic adjusted models included conscription year, conscription center, age at conscription, and childhood socioeconomic status as covariates. Models for receipt of a disability pension due to all or psychiatric causes were also adjusted for any mental hospitalization before conscription and for any psychiatric diagnosis at conscription. Error bars indicate 95% CIs. BMI = body mass index.
BMI and Receipt of a Disability Pension
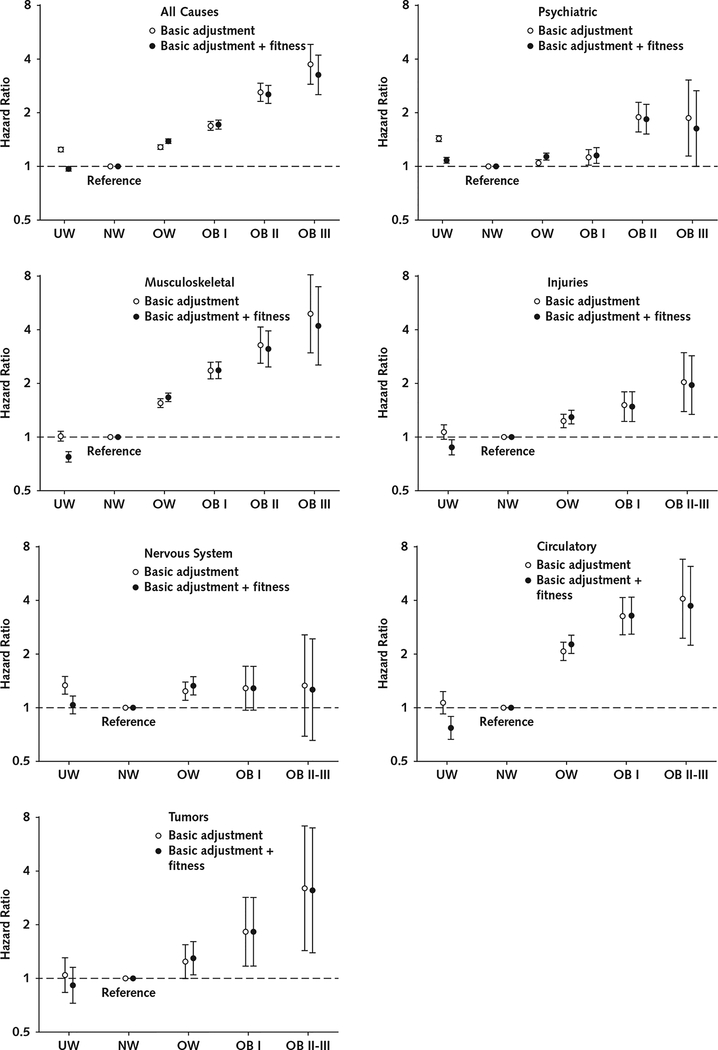
Overall, there were J-shaped associations between BMI categories and risk for receipt of a disability pension in the models that were adjusted for basic covariates. However, these associations were more linear when they were also adjusted for cardiorespiratory fitness (Figure 3). In the adjusted analyses, compared with normal weight, severe obesity was associated with greater risk for receipt of a disability pension due to all causes (HR, 3.21 [CI, 2.49 to 4.15]), psychiatric causes (HR, 1.63 [CI, 1.00 to 2.67]), and musculoskeletal causes (HR, 4.11 [CI, 2.48 to 6.82]).
Figure 3.
Association of BMI with later receipt of a disability pension due to all and specific causes (n = 1 079 128).
Cox proportional hazards regression models were used to estimate hazard ratios with 95% CIs. Basic adjusted models included conscription year, conscription center, age at conscription, and childhood socioeconomic status as covariates. Models for receipt of a disability pension due to all or psychiatric causes were also adjusted for any mental hospitalization before conscription and for any psychiatric diagnosis at conscription. Error bars indicate 95% CIs. BMI = body mass index; OB I = class I obesity; OB II = class II obesity; OB III = class III obesity; NW = normal weight; OW = overweight; UW = underweight.
Combined Association of Cardiorespiratory Fitness and BMI With Receipt of a Disability Pension
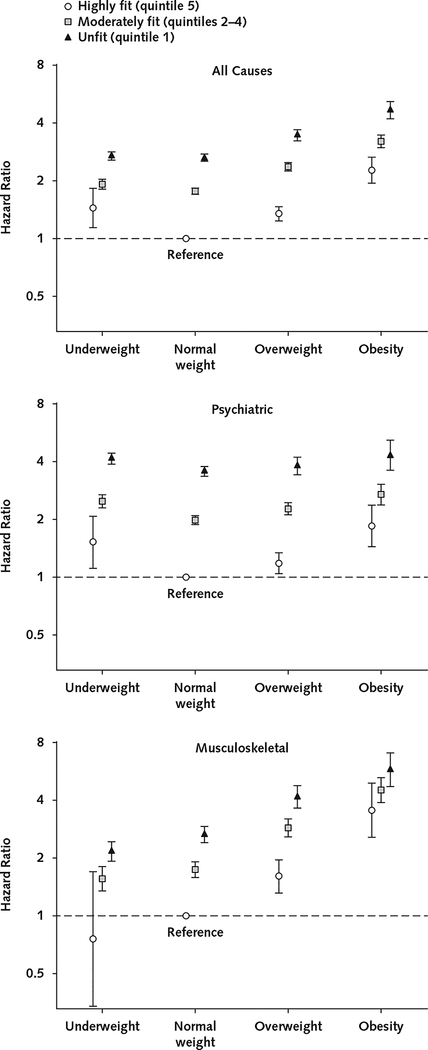
Figure 4 shows the combined associations of cardiorespiratory fitness and BMI with receipt of a disability pension due to all, psychiatric, and musculoskeletal causes. Higher cardiorespiratory fitness attenuated risk for receipt of a disability pension in all BMI categories. For example, highly fit adolescents with obesity had similar risk for receipt of a disability pension due to all causes (HR, 2.27 [CI, 1.94 to 2.66]) compared with unfit adolescents with normal weight (HR, 2.64 [CI, 2.53 to 2.76]), whereas the risk was considerably higher for unfit adolescents with obesity (HR, 4.67 [CI, 4.21 to 5.17]). Furthermore, highly fit adolescents with obesity had lower risk for receipt of a disability pension due to psychiatric causes (HR, 1.86 [CI, 1.45 to 2.39]) than unfit adolescents with either obesity (HR, 4.34 [CI, 3.63 to 5.19]) or normal weight (HR, 3.59 [CI, 3.38 to 3.80]).
Figure 4.
Combined associations of cardiorespiratory fitness and BMI with later receipt of a disability pension due to all causes and the 2 most prevalent causes (psychiatric and musculoskeletal).
Cox proportional hazards regression models were used to estimate hazard ratios with 95% CIs. Models were adjusted for conscription year, conscription center, age at conscription, and childhood socioeconomic status as covariates. Models for receipt of a disability pension due to all or psychiatric causes were also adjusted for any mental hospitalization before conscription and for any psychiatric diagnosis at conscription. Error bars indicate 95% CIs. BMI = body mass index.
Sensitivity Analyses
We performed a sensitivity analysis in a subset of males (n = 34 966) with data on smoking and alcohol consumption, which were available in the early years of the Swedish Military Service Conscription Registry (Appendix Table 4, available at Annals.org). Low cardiorespiratory fitness and obesity remained strong risk factors for receipt of a disability pension due to all causes, even after adjustment for smoking and alcohol consumption at conscription.
We then examined the robustness of our findings to unmeasured confounding by calculating E-values for our main outcomes (Appendix Table 5, available at Annals.org), as described by VanderWeele and Ding (18). Values were generally high, and the E-value for the association of cardiorespiratory fitness with receipt of a disability pension due to all causes was 6.94.
We also examined the association of cardiorespiratory fitness and BMI with receipt of a disability pension due to specific psychiatric causes (substance abuse, nonaffective disorders, affective disorders, and personality disorders) (Appendix Table 6, available at Annals.org). Higher cardiorespiratory fitness was strongly associated with lower risk for receipt of a disability pension for all psychiatric causes, although the association with substance abuse was particularly strong.
Finally, in 2 sensitivity analyses, we excluded participants with a prior mental hospitalization and psychiatric diagnosis at conscription and excluded those who were granted a disability pension within the first 10 years of follow-up. Both analyses yielded estimates that were similar to our main results. For example, in fully adjusted models comparing the lowest and highest fitness deciles, HRs were 3.92 (CI, 3.71 to 4.15) and 3.67 (CI, 3.47 to 3.88), respectively, compared with 3.74 (CI, 3.55 to 3.95) in our main analysis.
DISCUSSION
In this population-based cohort study of more than 1 million male adolescents, low cardiorespiratory fitness was a strong risk factor for later chronic disability, as indicated by receipt of a disability pension. Higher cardiorespiratory fitness was associated with lower risk for receipt of a disability pension for all examined causes, particularly all causes, psychiatric causes, musculoskeletal causes, and circulatory diseases. Another novel finding was that severe obesity was strongly related to increased risk for receipt of a disability pension, especially due to all and musculoskeletal causes. Finally, higher cardiorespiratory fitness attenuated risk for receipt of a disability pension in all BMI categories. Of note, highly fit adolescents with obesity had lower risk for disability due to psychiatric causes than unfit adolescents with normal weight.
Previous studies have examined the association between cardiorespiratory fitness and disability. A relatively small Finnish study (n = 1307) reported that low cardiorespiratory fitness in middle age was associated with increased risk for receipt of a disability pension (19), and low cardiorespiratory fitness has also been found to be a risk factor for future absence from work due to illness (20–22). Of note, the largest study to date (n = 49 321) showed that low cardiorespiratory fitness in adolescence was a risk factor for receipt of a disability pension due to all causes but did not examine specific causes (10). We expand the literature by demonstrating in a cohort of more than 1 million male adolescents that low cardiorespiratory fitness in youth is a strong risk factor for later disability due to a wide range of diseases and causes, regardless of BMI.
Our results may also be compared with those of studies that analyzed cardiorespiratory fitness in relation to mortality and other indicators of morbidity besides receipt of a disability pension (7–9, 15, 23–29). These studies, some of which also used data from the Swedish Military Service Conscription Registry (7, 15, 23–25), have shown that low cardiorespiratory fitness is associated with increased mortality and morbidity due to a wide variety of diseases, such as cardiovascular disease, type 2 diabetes, cancer, and depression. This is consistent with our finding that low cardiorespiratory fitness is associated with receipt of a disability pension due to a wide range of diseases and causes, including circulatory and psychiatric diseases as well as tumors. Although the mechanisms by which cardiorespiratory fitness may influence health are not fully understood, higher cardiorespiratory fitness has been linked to more favorable cardiometabolic risk profiles (lower blood pressure, insulin resistance, and healthier lipid profile), lower concentrations of inflammatory markers, and better cognitive function, which may influence both physical and mental health (28, 30).
Studies have consistently identified obesity as a risk factor for receipt of a disability pension (6, 12). However, the association between severe obesity and later receipt of a disability pension has not been previously assessed. In our study, the risk for receipt of a disability pension associated with severe obesity was considerably higher than for moderate and mild obesity, highlighting the importance of severe obesity as a strong risk factor for later chronic disability. Although obesity in youth (11, 31) and adulthood (32) is recognized to be associated with impaired health, evidence suggests that the negative effects of obesity can be attenuated by being fit (the “fat but fit” paradox) (13, 14). Indeed, fit adolescents with obesity had lower risk for receipt of a disability pension than unfit adolescents with obesity in the current study. This study also showed that being moderately or highly fit attenuated risk for receipt of a disability pension in all BMI categories. Therefore, our results indicate that cardiorespiratory fitness is an important marker for later health, regardless of BMI.
This study has several strengths, including a long follow-up and a population-based sample of more than 1 million male adolescents that enabled analysis of specific causes of receipt of a disability pension as well as combined analyses of cardiorespiratory fitness and BMI. Furthermore, the main study variables (cardiorespiratory fitness, BMI, and receipt of a disability pension) were measured using objective and standardized procedures. Finally, the use of a young cohort with little preexisting disease decreases risk for reverse causation (low cardiorespiratory fitness due to disease) while also providing support for early prevention of chronic disease.
A major limitation of this study is that we did not have data on smoking status and alcohol intake for all adolescents and lacked repeated measures of covariates, cardiorespiratory fitness, and BMI during follow-up. This, coupled with the fact that the determinants of fitness in adolescents are not fully understood, led to considerable risk for residual confounding by such factors as socioeconomic status. To address this risk, we conducted 2 sensitivity analyses. The first showed that low cardiorespiratory fitness and obesity remained strong risk factors for receipt of a disability pension after additional adjustment for smoking and alcohol consumption in a subset of adolescents (Appendix Table 4). In the second, we calculated E-values to assess the robustness of our main results to unmeasured confounding (Appendix Table 5). Values were generally high, and the E-value for the association of cardiorespiratory fitness with receipt of a disability pension due to all causes was 6.94. This indicates that an unmeasured confounder would have to be associated with both cardiorespiratory fitness and receipt of a disability pension by a risk ratio of 6.94 to fully explain away the association of cardiorespiratory fitness (lowest vs. highest fitness decile) with receipt of a disability pension (18). Although a single confounder of that magnitude is unlikely, we cannot exclude the possibility of residual confounding. Therefore, it is important for future studies to consider repeated measures of key variables, such as socioeconomic status, smoking, alcohol consumption, physical activity, other health behaviors, fitness, and BMI, to further elucidate the role of cardiorespiratory fitness and BMI in receipt of a disability pension. Another limitation of this study is that it included only male adolescents, which limits the generalizability of the results to female adolescents. Although a previous study in women indicated that low cardiorespiratory fitness is related to later absence from work due to illness (33), additional studies are needed in women. Finally, it should be noted that although our study outcome is receipt of a disability pension, the underlying cause of the disability must be diagnosed and certified by a physician.
Our study has implications for public health and clinical care. We found evidence of a strong association between physical fitness and risk for later receipt of a disability pension due to a wide range of diseases and causes. However, it should be noted that cardiorespiratory fitness is influenced by not only physical activity but also other environmental factors and genetics (34–36), with the latter having been found to be a strong influence in a previous study that used data from the Swedish Military Service Conscription Registry (36). Nevertheless, our findings indicate that promoting fitness in adolescence may be important to counteract later chronic disabilities. The findings showed that adolescents classified as unfit (those in the first quintile) had the greatest risk for later receipt of a disability pension. Although cardiorespiratory fitness data are available only as the final work rate (in watts achieved), a crude estimation of the corresponding maximum oxygen consumption (VO2max) values can be calculated using the cutoff for work rate (229 W) and the average weight in the first quintile (37). Of note, the estimated cutoff for being unfit in our data (VO2max approximately 41 mL/kg per minute) is very similar to the cutoff (42 mL/kg per minute for male adolescents) proposed in a recent meta-analysis to identify children and adolescents with increased cardiovascular disease risk (38). Furthermore, our results underscore a recent position of the American Heart Association that cardiorespiratory fitness should be considered a clinical vital sign that is easily measurable in clinical settings (28). Therefore, increasing cardiorespiratory fitness in youth may have a large effect on public health and thus should be an important focus during clinical care.
In addition to low cardiorespiratory fitness, obesity (especially severe obesity) was a strong risk factor for later disability, and adolescents who were obese and unfit had the greatest risk. Our data indicated that such high-risk persons may benefit from improved cardiorespiratory fitness and/or decreased body weight. Most adolescents in this study were conscripted at a time when obesity was uncommon. However, the secular increases in pediatric obesity (39) and decreases in cardiorespiratory fitness (40, 41) are likely to result in later impaired health and, consequently, greater prevalence of disability in contemporary adolescents. Thus, although additional well-designed studies are required, our results show the importance of high cardiorespiratory fitness and a healthy body weight in all adolescents.
In conclusion, this population-based cohort study of more than 1 million male adolescents indicated that low cardiorespiratory fitness and obesity are strongly associated with receipt of a disability pension due to a wide range of diseases and causes later in life. Although additional well-designed studies are required to provide further evidence, these findings emphasize the importance of high cardiorespiratory fitness and healthy body weight during adolescence.
Grant Support:
This study was supported by a grant from the Karolinska Institutet to Dr. Ortega (2018–02043). Dr. Pontus Henriksson was supported by grants from the Henning and Johan Throne-Holst Foundation and the Strategic Research Area Health Care Science, Karolinska Institutet/Umeå University. Dr. Hanna Henriksson was supported by grants from the Swedish Society of Medicine and the County Council of Östergötland, Sweden. Dr. Shiroma was supported by the intramural research program at the National Institute on Aging. Dr. Ortega was supported by a visiting grant from the Henning and Johan Throne-Holst Foundation and by grants from the European Union’s Horizon 2020 Research and Innovation Programme under grant agreement no. 667302; the University of Granada, Plan Propio de Investigación 2016, Excellence actions: Units of Excellence, Unit of Excellence on Exercise and Health; the SAMID III network, RETICS, funded by the PNI+D+I 2017–2021 (Spain), ISCIII Sub-Directorate General for Research Assessment and Promotion, the European Regional Development Fund (ref. RD16/0022); and the EXERNET Research Network on Exercise and Health in Special Populations (DEP2005–00046/ACTI).
Appendix Table 1.
ICD Codes Used in the Study
| Category | ICD-10 | ICD-9 | ICD-8 |
|---|---|---|---|
| Circulatory | Chapter I | 390 to 398, 401 to 405, 410 to 417, 420 to 438, 440 to 448, 451 to 459 | 390 to 392, 400 to 404, 410 to 414, 420 to 429, 430 to 438, 440 to 448, 450 to 458 |
| Psychiatric | Chapter F | 290 to 319 | 290 to 315 |
| Musculoskeletal | Chapter M | 710 to 739 | 710 to 718, 720 to 738 |
| Nervous system | Chapter G | 320 to 326, 330 to 337, 340 to 359 | 320 to 324, 330 to 358 |
| Tumors | Chapter C; D00 to 48 | 140 to 165, 170 to 176, 179 to 208, 210 to 239 | 140 to 163, 170 to 174, 180 to 228, 230 to 239 |
| Injuries | Chapters S, T, V, W, X, and Y | 800 to 848, 850 to 854, 860 to 887, 890 to 897, 900 to 999, Chapter E | Chapters E and N |
ICD = International Classification of Diseases.
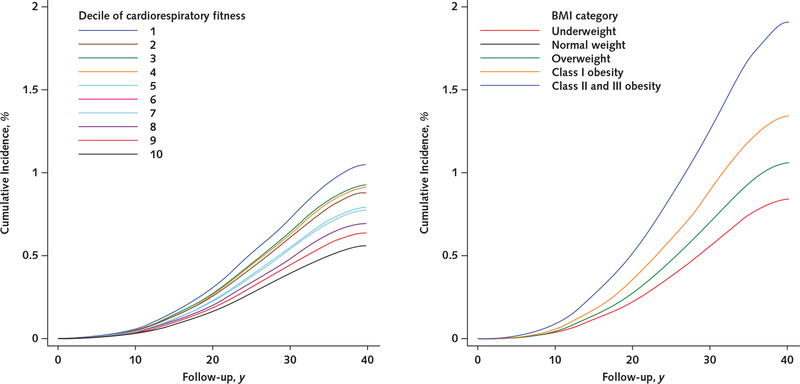
Appendix Figure 1.
Unadjusted cumulative incidences of receipt of a disability pension due to psychiatric causes, by cardiorespiratory fitness level (left) and BMI category (right).
Cumulative incidences were estimated from unadjusted Cox regression models. BMI = body mass index.
Appendix Figure 2.
Unadjusted cumulative incidences of receipt of a disability pension due to musculoskeletal causes, by cardiorespiratory fitness level (left) and BMI category (right).
Cumulative incidences were estimated from unadjusted Cox regression models. BMI = body mass index.
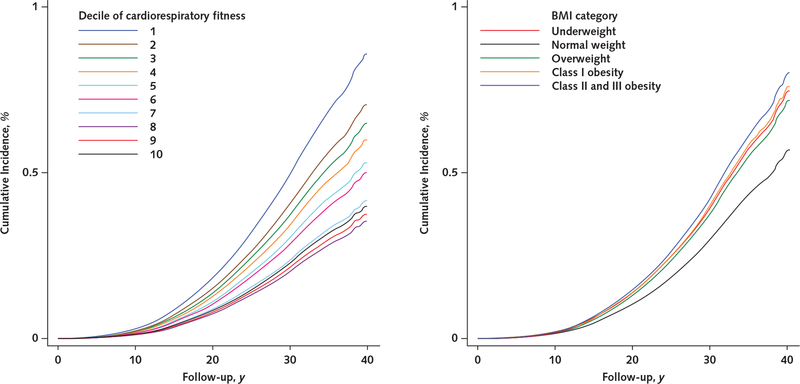
Appendix Figure 3.
Unadjusted cumulative incidences of receipt of a disability pension due to injuries, by cardiorespiratory fitness level (left) and BMI category (right).
Cumulative incidences were estimated from unadjusted Cox regression models. BMI = body mass index.
Appendix Figure 4.
Unadjusted cumulative incidences of receipt of a disability pension due to nervous system causes, by cardiorespiratory fitness level (left) and BMI category (right).
Cumulative incidences were estimated from unadjusted Cox regression models. BMI = body mass index.
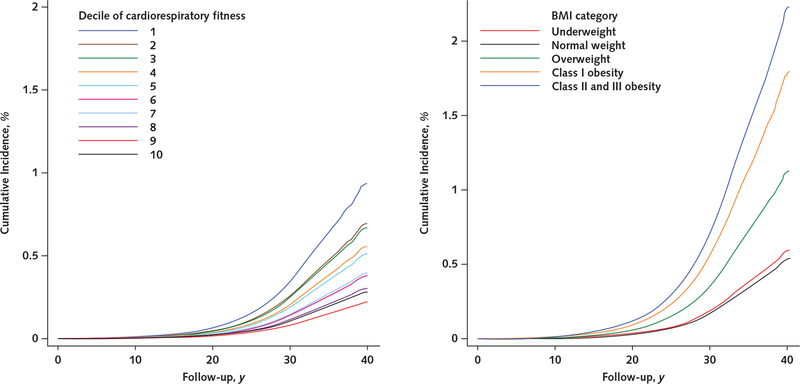
Appendix Figure 5.
Unadjusted cumulative incidences of receipt of a disability pension due to circulatory causes, by cardiorespiratory fitness level (left) and BMI category (right).
Cumulative incidences were estimated from unadjusted Cox regression models. BMI = body mass index.
Appendix Figure 6.
Unadjusted cumulative incidences of receipt of a disability pension due to tumors, by cardiorespiratory fitness level (left) and BMI category (right).
Cumulative incidences were estimated from unadjusted Cox regression models. BMI = body mass index.
Appendix Table 2.
Unadjusted Cumulative Incidence of Receipt of a Disability Pension, by Decile of Cardiorespiratory Fitness
| Disability Cause | Decile of Cardiorespiratory Fitness | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| All | ||||||||||
| Cases, n | 12 009 | 8471 | 7217 | 6286 | 5668 | 4242 | 3378 | 3021 | 2139 | 1873 |
| Incidence per 10 000 person-years | 31.7 | 24.7 | 23.0 | 19.5 | 16.8 | 14.8 | 12.3 | 10.3 | 8.4 | 7.2 |
| Cumulative incidence per 1000 persons during follow-up | ||||||||||
| 10 y | 4.9 | 2.9 | 3.6 | 3.0 | 3.8 | 3.2 | 3.3 | 2.8 | 3.0 | 2.6 |
| 20 y | 31.8 | 22.3 | 24.4 | 21.3 | 22.1 | 20.3 | 19.9 | 16.3 | 15.7 | 13.6 |
| 30 y | 88.2 | 68.6 | 66.7 | 57.9 | 52.0 | 45.7 | 39.1 | 32.9 | 26.0 | 22.1 |
| 40 y | 144.6 | 113.5 | 105.6 | 90.2 | 78.5 | 70.2 | 58.7 | 49.2 | 42.2 | 39.6 |
| Psychiatric | ||||||||||
| Cases, n | 5689 | 3737 | 3193 | 2738 | 2638 | 2001 | 1701 | 1465 | 1103 | 935 |
| Incidence per 10 000 person-years | 15.0 | 10.9 | 10.2 | 8.5 | 7.8 | 7.0 | 6.2 | 5.0 | 4.3 | 3.6 |
| Cumulative incidence per 1000 persons during follow-up | ||||||||||
| 10 y | 3.7 | 2.2 | 2.5 | 2.1 | 2.5 | 2.3 | 2.3 | 2.0 | 2.0 | 1.5 |
| 20 y | 20.4 | 13.5 | 14.3 | 12.3 | 12.6 | 11.9 | 11.6 | 9.1 | 8.8 | 7.1 |
| 30 y | 45.6 | 32.6 | 31.8 | 26.6 | 25.0 | 21.9 | 19.6 | 15.9 | 13.3 | 10.9 |
| 40 y | 65.3 | 48.2 | 43.6 | 36.6 | 33.6 | 30.2 | 26.2 | 21.1 | 18.0 | 17.3 |
| Musculoskeletal | ||||||||||
| Cases, n | 2720 | 2099 | 1826 | 1514 | 1329 | 914 | 641 | 593 | 362 | 339 |
| Incidence per 10 000 person-years | 7.2 | 6.1 | 5.8 | 4.7 | 3.9 | 3.2 | 2.3 | 2.0 | 1.4 | 1.3 |
| Cumulative incidence per 1000 persons during follow-up | ||||||||||
| 10 y | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.1 | 0.1 | 0.2 | 0.2 |
| 20 y | 4.6 | 3.6 | 4.2 | 3.4 | 3.6 | 3.2 | 3.0 | 2.6 | 2.3 | 2.2 |
| 30 y | 20.3 | 17.4 | 17.2 | 14.2 | 12.8 | 10.5 | 7.9 | 6.9 | 4.6 | 4.3 |
| 40 y | 36.6 | 30.2 | 29.3 | 23.8 | 20.3 | 16.4 | 12.9 | 10.7 | 9.7 | 8.5 |
| Injuries | ||||||||||
| Cases, n | 931 | 714 | 670 | 667 | 576 | 429 | 425 | 409 | 302 | 264 |
| Incidence per 10 000 person-years | 2.5 | 2.1 | 2.1 | 2.1 | 1.7 | 1.5 | 1.6 | 1.4 | 1.2 | 1.0 |
| Cumulative incidence per 1000 persons during follow-up | ||||||||||
| 10 y | 0.3 | 0.2 | 0.3 | 0.3 | 0.5 | 0.4 | 0.4 | 0.4 | 0.4 | 0.5 |
| 20 y | 2.3 | 1.7 | 2.3 | 2.2 | 2.5 | 2.2 | 2.7 | 2.3 | 2.3 | 2.1 |
| 30 y | 7.0 | 5.9 | 6.3 | 6.5 | 5.5 | 4.9 | 5.0 | 4.5 | 3.7 | 3.1 |
| 40 y | 12.2 | 10.3 | 10.4 | 9.8 | 7.8 | 6.8 | 7.0 | 6.4 | 5.3 | 3.9 |
| Nervous system | ||||||||||
| Cases, n | 679 | 508 | 411 | 381 | 330 | 262 | 188 | 172 | 142 | 149 |
| Incidence per 10 000 person-years | 1.8 | 1.5 | 1.3 | 1.2 | 1.0 | 0.9 | 0.7 | 0.6 | 0.6 | 0.6 |
| Cumulative incidence per 1000 persons during follow-up | ||||||||||
| 10 y | 0.2 | 0.1 | 0.3 | 0.2 | 0.2 | 0.1 | 0.2 | 0.1 | 0.2 | 0.2 |
| 20 y | 1.5 | 1.5 | 1.2 | 1.3 | 1.2 | 1.1 | 0.9 | 0.8 | 1.0 | 1.0 |
| 30 y | 4.8 | 4.0 | 3.8 | 3.4 | 3.0 | 2.8 | 2.2 | 1.9 | 1.8 | 1.9 |
| 40 y | 9.4 | 7.5 | 6.7 | 6.1 | 5.1 | 5.0 | 4.1 | 3.5 | 3.5 | 2.5 |
| Circulatory | ||||||||||
| Cases, n | 609 | 411 | 334 | 272 | 228 | 139 | 111 | 90 | 44 | 51 |
| Incidence per 10 000 person-years | 1.6 | 1.2 | 1.1 | 0.8 | 0.7 | 0.5 | 0.4 | 0.3 | 0.2 | 0.2 |
| Cumulative incidence per 1000 persons during follow-up | ||||||||||
| 10 y | 0.1 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 |
| 20 y | 0.4 | 0.3 | 0.4 | 0.4 | 0.5 | 0.2 | 0.4 | 0.3 | 0.2 | 0.3 |
| 30 y | 3.1 | 2.5 | 2.3 | 2.0 | 1.7 | 1.3 | 1.1 | 0.9 | 0.6 | 0.6 |
| 40 y | 10.6 | 7.3 | 6.8 | 5.4 | 4.6 | 3.6 | 3.6 | 2.2 | 1.9 | 3.1 |
| Tumors | ||||||||||
| Cases, n | 182 | 150 | 111 | 132 | 89 | 90 | 61 | 64 | 45 | 44 |
| Incidence per 10 000 person-years | 0.5 | 0.4 | 0.4 | 0.4 | 0.3 | 0.3 | 0.2 | 0.2 | 0.2 | 0.2 |
| Cumulative incidence per 1000 persons during follow-up | ||||||||||
| 10 y | 0.0 | 0.0 | 0.0 | 0.1 | 0.1 | 0.0 | 0.1 | 0.1 | 0.0 | 0.1 |
| 20 y | 0.3 | 0.3 | 0.3 | 0.3 | 0.4 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| 30 y | 1.2 | 1.0 | 0.8 | 1.1 | 0.8 | 0.7 | 0.7 | 0.6 | 0.6 | 0.5 |
| 40 y | 2.7 | 2.5 | 2.2 | 2.3 | 1.4 | 2.3 | 1.4 | 1.5 | 0.9 | 1.1 |
Appendix Table 3.
Unadjusted Cumulative Incidence of Receipt of a Disability Pension, by BMI Category
| Disability Cause | BMI Category | |||||
|---|---|---|---|---|---|---|
| Underweight | Normal Weight | Overweight | Class I Obesity | Class II Obesity* | Class III Obesity* | |
| All | ||||||
| Cases, n | 5763 | 41 560 | 5480 | 1158 | 284 | 59 |
| Incidence per 10 000 person-years | 22.5 | 16.6 | 21.2 | 29.0 | 44.2 | 61.3 |
| Cumulative incidence per 1000 persons during follow-up | ||||||
| 10 y | 4.6 | 3.1 | 3.8 | 5.3 | 10.0 | 12.9 |
| 20 y | 27.7 | 19.4 | 25.0 | 36.1 | 60.1 | 90.2 |
| 30 y | 66.2 | 50.2 | 64.9 | 88.8 | 135.8 | 174.1 |
| 40 y | 109.5 | 84.8 | 109.8 | 149.0 | 213.4 | 298.5 |
| Psychiatric | ||||||
| Cases, n | 3091 | 19 459 | 2143 | 385 | 122 | |
| Incidence per 10 000 person-years | 12.1 | 7.8 | 8.3 | 9.6 | 16.5 | |
| Cumulative incidence per 1000 persons during follow-up | ||||||
| 10 y | 3.5 | 2.2 | 2.5 | 3.4 | 6.9 | |
| 20 y | 18.3 | 11.5 | 13.0 | 16.1 | 30.7 | |
| 30 y | 37.2 | 24.5 | 26.3 | 30.2 | 49.5 | |
| 40 y | 54.4 | 36.0 | 37.7 | 43.8 | 72.9 | |
| Musculoskeletal | ||||||
| Cases, n | 1041 | 9393 | 1477 | 339 | 87 | |
| Incidence per 10 000 person-years | 4.1 | 3.8 | 5.7 | 8.5 | 11.8 | |
| Cumulative incidence per 1000 persons during follow-up | ||||||
| 10 y | 0.2 | 0.1 | 0.4 | 0.3 | 1.4 | |
| 20 y | 3.3 | 3.0 | 5.1 | 8.7 | 14.0 | |
| 30 y | 12.4 | 11.8 | 18.4 | 28.1 | 36.9 | |
| 40 y | 22.5 | 21.5 | 32.9 | 47.4 | 73.5 | |
| Injuries | ||||||
| Cases, n | 468 | 4223 | 562 | 107 | 27 | |
| Incidence per 10 000 person-years | 1.8 | 1.7 | 2.2 | 2.7 | 3.7 | |
| Cumulative incidence per 1000 persons during follow-up | ||||||
| 10 y | 0.4 | 0.4 | 0.5 | 0.9 | 0.7 | |
| 20 y | 2.1 | 2.2 | 3.0 | 4.1 | 5.7 | |
| 30 y | 5.7 | 5.3 | 6.9 | 8.2 | 13.4 | |
| 40 y | 9.4 | 8.6 | 11.0 | 14.1 | 13.4 | |
| Nervous system | ||||||
| Cases, n | 350 | 2502 | 312 | 49 | 9 | |
| Incidence per 10 000 person-years | 1.4 | 1.0 | 1.2 | 1.2 | 1.2 | |
| Cumulative incidence per 1000 persons during follow-up | ||||||
| 10 y | 0.2 | 0.2 | 0.2 | 0.1 | 0.0 | |
| 20 y | 1.5 | 1.1 | 1.3 | 1.5 | 1.5 | |
| 30 y | 3.9 | 3.0 | 3.8 | 3.7 | 4.1 | |
| 40 y | 7.7 | 5.7 | 7.0 | 7.5 | 6.9 | |
| Circulatory | ||||||
| Cases, n | 204 | 1668 | 328 | 74 | 15 | |
| Incidence per 10 000 person-years | 0.8 | 0.7 | 1.3 | 1.9 | 2.0 | |
| Cumulative incidence per 1000 persons during follow-up | ||||||
| 10 y | 0.0 | 0.0 | 0.1 | 0.1 | 0.3 | |
| 20 y | 0.3 | 0.3 | 0.6 | 1.6 | 1.5 | |
| 30 y | 1.6 | 1.6 | 3.4 | 5.2 | 7.4 | |
| 40 y | 6.4 | 5.3 | 10.1 | 14.0 | 12.8 | |
| Tumors | ||||||
| Cases, n | 86 | 763 | 93 | 20 | 6 | |
| Incidence per 10 000 person-years | 0.3 | 0.3 | 0.4 | 0.5 | 0.8 | |
| Cumulative incidence per 1000 persons during follow-up | ||||||
| 10 y | 0.1 | 0.1 | 0.0 | 0.1 | 0.3 | |
| 20 y | 0.3 | 0.3 | 0.4 | 0.6 | 1.5 | |
| 30 y | 0.9 | 0.8 | 1.0 | 1.5 | 2.2 | |
| 40 y | 2.1 | 2.0 | 2.3 | 3.2 | 4.9 | |
BMI = body mass index.
Data on class II and III obesity are combined for all specific causes.
Appendix Table 4.
Associations of Cardiorespiratory Fitness and BMI With Later Receipt of a Disability Pension due to All Causes, With Adjustment for Smoking and Alcohol Consumption*
| Variable | Hazard Ratio (95% CI) | ||
|---|---|---|---|
| Basic Adjustment‡ | Basic Adjustment + BMI | Basic Adjustment + BMI + Smoking + Alcohol Consumption | |
| Cardiorespiratory fitness level† | |||
| 1 | 3.40 (1.61–7.17) | 4.36 (2.06–9.21) | 4.70 (2.22–9.93) |
| 2 | 1.82 (1.24–2.66) | 2.20 (1.50–3.22) | 2.03 (1.38–2.97) |
| 3 | 1.55 (1.35–1.79) | 1.82 (1.58–2.11) | 1.62 (1.40–1.87) |
| 4 | 1.48 (1.33–1.65) | 1.68 (1.50–1.88) | 1.52 (1.36–1.70) |
| 5 | 1.39 (1.25–1.53) | 1.52 (1.37–1.69) | 1.37 (1.23–1.52) |
| 6 | 1.27 (1.14–1.42) | 1.34 (1.20–1.49) | 1.22 (1.10–1.36) |
| 7 | 1.10 (0.97–1.25) | 1.14 (1.00–1.29) | 1.06 (0.93–1.20) |
| 8 | 1.12 (0.99–1.28) | 1.16 (1.01–1.32) | 1.10 (0.96–1.25) |
| 9 | Reference | Reference | Reference |
| BMI category | |||
| Class II or III obesity | 2.88 (1.44–5.76) | 2.71 (1.35–5.43) | 2.75 (1.37–5.50) |
| Class I obesity | 1.55 (1.17–2.07) | 1.55 (1.16–2.06) | 1.55 (1.17–2.07) |
| Overweight | 1.39 (1.24–1.55) | 1.44 (1.29–1.61) | 1.43 (1.28–1.59) |
| Normal weight | Reference | Reference | Reference |
| Underweight | 1.06 (0.97–1.15) | 0.93 (0.85–1.02) | 0.95 (0.87–1.04) |
BMI = body mass index.
Analyses were performed in a subset of adolescents (n = 34 966) who were conscripted between 1969 and 1973 because data on smoking and alcohol consumption were collected only during these years. These adolescents had complete data on cardiorespiratory fitness, BMI, and basic confounders as well as smoking (0, 1 to 5, 6 to 10, 11 to 20, or >20 cigarettes per day) and alcohol consumption (yes vs. no), as described in a previous study that used data from the Swedish Military Service Conscription Registry (10).
Presented as stanine (standard nine) scores, as described previously (10).
Basic adjusted models included conscription year, conscription center, age at conscription, and childhood socioeconomic status as covariates.
Appendix Table 5.
Robustness to Unmeasured Confounding (Reported as E-Values) for Assessment of the Association of Cardiorespiratory Fitness and BMI With Receipt of a Disability Pension*
| Disability Cause | Cardiorespiratory Fitness | BMI | ||
|---|---|---|---|---|
| For Effect Estimate† | For CI Limit Closest to Null‡ | For Effect Estimate† | For CI Limit Closest to Null‡ | |
| All | 6.94 | 6.56 | 5.87 | 4.42 |
| Psychiatric | 7.48 | 6.90 | 2.64 | 1.00 |
| Musculoskeletal | 6.90 | 6.03 | 7.69 | 4.40 |
| Injuries | 4.92 | 4.13 | 3.33 | 2.01 |
| Nervous system | 5.17 | 4.13 | 1.79 | 1.00 |
| Circulatory | 9.21 | 6.62 | 7.08 | 4.01 |
| Tumors | 3.19 | 1.95 | 5.59 | 2.08 |
BMI = body mass index.
E-values are for the associations of cardiorespiratory fitness (decile 1 vs. decile 10) and BMI (class III obesity vs. normal weight for all causes and psychiatric and musculoskeletal causes, and class II and III obesity vs. normal weight for remaining causes) with receipt of a disability pension due to all or specific causes and were calculated according to the method of VanderWeele and Ding (18).
The E-value for the effect estimate is the minimum strength of the association on the risk scale that an unmeasured confounder would need to have with both the exposure and the outcome to fully explain away the observed association.
The E-value for the 95% CI limit closest to the null is the minimum strength of the association on the risk scale that an unmeasured confounder would need to have with both the exposure and the outcome in order for the CI of the observed association to include the null value.
Appendix Table 6.
Associations of Cardiorespiratory Fitness and BMI With Later Receipt of a Disability Pension due to Specific Psychiatric Causes (n = 1 079 128)*
| Variable | Hazard Ratio (95% CI) | ||||
|---|---|---|---|---|---|
| All Psychiatric Causes (n = 25 200) | Substance Abuse† (n = 2592) | Nonaffective Disorders† (n = 4475) | Affective Disorders† (n = 11 761) | Personality Disorders† (n = 3313) | |
| Decile of cardiorespiratory fitness | |||||
| 1 | 4.01 (3.72–4.32) | 9.28 (6.42–13.40) | 2.08 (1.77–2.44) | 3.55 (3.18–3.97) | 5.82 (4.64–7.30) |
| 2 | 3.18 (2.95–3.44) | 7.04 (4.86–10.19) | 1.83 (1.55–2.15) | 2.82 (2.52–3.16) | 4.34 (3.45–5.47) |
| 3 | 2.93 (2.71–3.16) | 5.65 (3.89–8.20) | 1.86 (1.59–2.18) | 2.68 (2.39–3.00) | 3.91 (3.10–4.92) |
| 4 | 2.48 (2.30–2.68) | 4.69 (3.22–6.82) | 1.66 (1.41–1.94) | 2.30 (2.05–2.57) | 3.18 (2.52–4.02) |
| 5 | 2.24 (2.07–2.41) | 3.96 (2.72–5.77) | 1.50 (1.28–1.75) | 2.12 (1.90–2.37) | 2.86 (2.27–3.61) |
| 6 | 2.00 (1.84–2.16) | 3.57 (2.43–5.24) | 1.34 (1.14–1.58) | 1.89 (1.69–2.12) | 2.41 (1.89–3.01) |
| 7 | 1.73 (1.60–1.88) | 2.54 (1.70–3.78) | 1.36 (1.16–1.60) | 1.70 (1.51–1.91) | 1.96 (1.54–2.51) |
| 8 | 1.43 (1.31–1.55) | 1.79 (1.18–2.72) | 1.22 (1.04–1.44) | 1.36 (1.20–1.53) | 1.55 (1.20–1.99) |
| 9 | 1.21 (1.11–1.32) | 1.94 (1.27–2.96) | 1.05 (0.88–1.24) | 1.19 (1.05–1.35) | 1.47 (1.14–1.91) |
| 10 | Reference | Reference | Reference | Reference | Reference |
| BMI categories | |||||
| Class II or III obesity | 1.81 (1.52–2.17) | 0.74 (0.31–1.78) | 0.57 (0.27–1.20) | 2.53 (2.00–3.18) | 1.13 (0.63–2.05) |
| Class I obesity | 1.15 (1.04–1.27) | 1.09 (0.79–1.51) | 0.62 (0.45–0.85) | 1.24 (1.07–1.43) | 1.48 (1.16–1.88) |
| Overweight | 1.13 (1.08–1.18) | 1.12 (0.97–1.29) | 0.86 (0.76–0.96) | 1.20 (1.13–1.28) | 1.13 (1.00–1.28) |
| Normal weight | Reference | Reference | Reference | Reference | Reference |
| Underweight | 1.08 (1.04–1.13) | 1.00 (0.89–1.13) | 1.13 (1.03–1.25) | 1.08 (1.01–1.14) | 1.09 (0.98–1.21) |
BMI = body mass index; ICD = International Classification of Diseases.
Models were adjusted for conscription year, conscription center, age at conscription, childhood socioeconomic status, any mental hospitalization before conscription, and any psychiatric diagnosis at conscription. Models with cardiorespiratory fitness as the exposure were further adjusted for BMI, and models with BMI as the exposure were further adjusted for cardiorespiratory fitness.
Use of ICD codes for specific psychiatric causes of receipt of a disability pension was based on Kark and colleagues (42) (substance abuse: ICD-8 codes 291 and 303 to 304, ICD-9 codes 291 to 292 and 303 to 305, and ICD-10 codes F10 to F19; nonaffective disorders [including schizophrenia]: ICD-8 codes 295 and 297, ICD-9 codes 295 and 297, and ICD-10 codes F20 to F29; affective disorders: ICD-8 codes 296, 298, 300, and 305, ICD-9 codes 296, 298, 300, 306, 308 to 309, and 311, and ICD-10 codes F30 to F49; personality disorders: ICD-8 codes 301 to 302, ICD-9 codes 301 to 302, and ICD-10 codes F60 to F69).
Footnotes
Reproducible Research Statement: Study protocol, statistical code, and data set: Not available.
Disclosures: Authors have disclosed no conflicts of interest. Forms can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M18-1861.
Current author addresses and author contributions are available at Annals.org.
References
- 1.Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800. doi: 10.1016/S0140-6736(15)60692-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016; 388:1459–544. doi: 10.1016/S0140-6736(16)31012-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1545–602. doi: 10.1016/S0140-6736(16)31678-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJ, et al. Burden of depressive disorders by country, sex, age, and year: findings from the Global Burden of Disease Study 2010. PLoSMed.2013;10:e1001547[PMID:24223526]doi: 10.1371/journal.pmed.1001547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382:1575–86. doi: 10.1016/S0140-6736(13)61611-6 [DOI] [PubMed] [Google Scholar]
- 6.Neovius K, Johansson K, Rössner S, Neovius M. Disability pension, employment and obesity status: a systematic review. Obes Rev. 2008;9:572–81. doi: 10.1111/j.1467-789X.2008.00502.x [DOI] [PubMed] [Google Scholar]
- 7.Högström G, Nordström A, Nordström P. Aerobic fitness in late adolescence and the risk of early death: a prospective cohort study of 1.3 million Swedish men. Int J Epidemiol. 2016;45:1159–68. [DOI] [PubMed] [Google Scholar]
- 8.Ortega FB, Ruiz JR, Castillo MJ, Sjöström M. Physical fitness in childhood and adolescence: a powerful marker of health. Int J Obes (Lond). 2008;32:1–11. [DOI] [PubMed] [Google Scholar]
- 9.Ruiz JR, Castro-Piñero J, Artero EG, Ortega FB, Sjöström M, Suni J, et al. Predictive validity of health-related fitness in youth: a systematic review. Br J Sports Med. 2009;43:909–23. doi: 10.1136/bjsm.2008.056499 [DOI] [PubMed] [Google Scholar]
- 10.Rabiee R, Agardh E, Kjellberg K, Falkstedt D. Low cardiorespiratory fitness in young adulthood and future risk of disability pension: a follow-up study until 59 years of age in Swedish men. J Epidemiol Community Health. 2015;69:266–71. doi: 10.1136/jech-2014-204851 [DOI] [PubMed] [Google Scholar]
- 11.Kelly AS, Barlow SE, Rao G, Inge TH, Hayman LL, Steinberger J, et al. ; American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young, Council on Nutrition, Physical Activity and Metabolism, and Council on Clinical Cardiology. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American Heart Association. Circulation. 2013;128:1689–712. doi: 10.1161/CIR.0b013e3182a5cfb3 [DOI] [PubMed] [Google Scholar]
- 12.Neovius M, Kark M, Rasmussen F. Association between obesity status in young adulthood and disability pension. Int J Obes (Lond). 2008;32:1319–26. doi: 10.1038/ijo.2008.70 [DOI] [PubMed] [Google Scholar]
- 13.Ortega FB, Lavie CJ, Blair SN. Obesity and cardiovascular disease. Circ Res. 2016;118:1752–70. doi: 10.1161/CIRCRESAHA.115.306883 [DOI] [PubMed] [Google Scholar]
- 14.Ortega FB, Ruiz JR, Labayen I, Lavie CJ, Blair SN. The fat but fit paradox: what we know and don’t know about it [Editorial]. Br J Sports Med. 2018;52:151–3. doi: 10.1136/bjsports-2016-097400 [DOI] [PubMed] [Google Scholar]
- 15.Crump C, Sundquist J, Winkleby MA, Sundquist K. Interactive effects of physical fitness and body mass index on the risk of hypertension. JAMA Intern Med. 2016;176:210–6. doi: 10.1001/jamainternmed.2015.7444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Svedenkrans J, Kowalski J, Norman M, Bohlin K. Low exercise capacity increases the risk of low cognitive function in healthy young men born preterm: a population-based cohort study. PLoS One. 2016;11:e0161314. doi: 10.1371/journal.pone.0161314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation WHO Technical Report Series, no. 894. Geneva: World Health Organization; 2000. [PubMed] [Google Scholar]
- 18.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167:268–74. doi: 10.7326/M16-2607 [DOI] [PubMed] [Google Scholar]
- 19.Karpansalo M, Lakka TA, Manninen P, Kauhanen J, Rauramaa R, Salonen JT. Cardiorespiratory fitness and risk of disability pension: a prospective population based study in Finnish men. Occup Environ Med. 2003;60:765–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kristensen P, Corbett K, Mehlum IS, Bjerkedal T. Impact of aerobic fitness on musculoskeletal sickness absence 5–15 years later: a cohort study of 227,201 male Norwegian employees. Occup Environ Med. 2012;69:250–5. doi: 10.1136/oemed-2011-100144 [DOI] [PubMed] [Google Scholar]
- 21.Kyröläinen H, Häkkinen K, Kautiainen H, Santtila M, Pihlainen K, Häkkinen A. Physical fitness, BMI and sickness absence in male military personnel. Occup Med (Lond). 2008;58:251–6. doi: 10.1093/occmed/kqn010 [DOI] [PubMed] [Google Scholar]
- 22.Strijk JE, Proper KI, van Stralen MM, Wijngaard P, van Mechelen W, van der Beek AJ. The role of work ability in the relationship between aerobic capacity and sick leave: a mediation analysis. Occup Environ Med. 2011;68:753–8. doi: 10.1136/oem.2010.057646 [DOI] [PubMed] [Google Scholar]
- 23.Åberg MA, Waern M, Nyberg J, Pedersen NL, Bergh Y, Åberg ND, et al. Cardiovascular fitness in males at age 18 and risk of serious depression in adulthood: Swedish prospective population-based study. Br J Psychiatry. 2012;201:352–9. doi: 10.1192/bjp.bp.111.103416 [DOI] [PubMed] [Google Scholar]
- 24.Crump C, Sundquist J, Winkleby MA, Sieh W, Sundquist K. Physical fitness among Swedish military conscripts and long-term risk for type 2 diabetes mellitus: a cohort study. Ann Intern Med. 2016;164: 577–84. doi: 10.7326/M15-2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersen K, Rasmussen F, Held C, Neovius M, Tynelius P, Sundström J. Exercise capacity and muscle strength and risk of vascular disease and arrhythmia in 1.1 million young Swedish men: cohort study. BMJ. 2015;351:h4543. doi: 10.1136/bmj.h4543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Papasavvas T, Bonow RO, Alhashemi M, Micklewright D. Depression symptom severity and cardiorespiratory fitness in healthy and depressed adults: a systematic review and meta-analysis. Sports Med. 2016;46:219–30. doi: 10.1007/s40279-015-0409-5 [DOI] [PubMed] [Google Scholar]
- 27.Rethorst CD, Wipfli BM, Landers DM. The antidepressive effects of exercise: a meta-analysis of randomized trials. Sports Med. 2009; 39:491–511. doi: 10.2165/00007256-200939060-00004 [DOI] [PubMed] [Google Scholar]
- 28.Ross R, Blair SN, Arena R, Church TS, Després JP, Franklin BA, et al. ; American Heart Association Physical Activity Committee of the Council on Lifestyle and Cardiometabolic Health. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. 2016;134:e653–e699. [DOI] [PubMed] [Google Scholar]
- 29.Schmid D, Leitzmann MF. Cardiorespiratory fitness as predictor of cancer mortality: a systematic review and meta-analysis. Ann Oncol. 2015;26:272–8. doi: 10.1093/annonc/mdu250 [DOI] [PubMed] [Google Scholar]
- 30.Silverman MN, Deuster PA. Biological mechanisms underlying the role of physical fitness in health and resilience. Interface Focus. 2014;4:20140040. doi: 10.1098/rsfs.2014.0040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reilly JJ, Kelly J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. Int J Obes (Lond). 2011;35:891–8. doi: 10.1038/ijo.2010.222 [DOI] [PubMed] [Google Scholar]
- 32.Global BMI Mortality Collaboration, Di Angelantonio E, Bhupathiraju ShN, Wormser D, Gao P, Kaptoge S, et al. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388:776–86. doi: 10.1016/S0140-6736(16)30175-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rasmussen CD, Andersen LL, Clausen T, Strøyer J, Jørgensen MB, Holtermann A. Physical capacity and risk for long-term sickness absence: a prospective cohort study among 8664 female health care workers. J Occup Environ Med. 2015;57:526–30. doi: 10.1097/JOM.0000000000000395 [DOI] [PubMed] [Google Scholar]
- 34.Costa AM, Breitenfeld L, Silva AJ, Pereira A, Izquierdo M, Marques MC. Genetic inheritance effects on endurance and muscle strength: an update. Sports Med. 2012;42:449–58. doi: 10.2165/11650560-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 35.Bouchard C, Sarzynski MA, Rice TK, Kraus WE, Church TS, Sung YJ, et al. Genomic predictors of the maximal O2 uptake response to standardized exercise training programs. J Appl Physiol (1985). 2011;110:1160–70. doi: 10.1152/japplphysiol.00973.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nordström P, Sievänen H, Gustafson Y, Pedersen NL, Nordström A. High physical fitness in young adulthood reduces the risk of fractures later in life in men: a nationwide cohort study. J Bone Miner Res. 2013;28:1061–7. doi: 10.1002/jbmr.1829 [DOI] [PubMed] [Google Scholar]
- 37.Kokkinos P, Kaminsky LA, Arena R, Zhang J, Myers J. A new generalized cycle ergometry equation for predicting maximal oxygen uptake: The Fitness Registry and the Importance of Exercise National Database (FRIEND). Eur J Prev Cardiol. 2018;25:1077–82. doi: 10.1177/2047487318772667 [DOI] [PubMed] [Google Scholar]
- 38.Ruiz JR, Cavero-Redondo I, Ortega FB, Welk GJ, Andersen LB, Martinez-Vizcaino V. Cardiorespiratory fitness cut points to avoid cardiovascular disease risk in children and adolescents; what level of fitness should raise a red flag? A systematic review and metaanalysis. Br J Sports Med. 2016;50:1451–8. doi: 10.1136/bjsports-2015-095903 [DOI] [PubMed] [Google Scholar]
- 39.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014; 384:766–81. doi: 10.1016/S0140-6736(14)60460-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tomkinson GR, Léger LA, Olds TS, Cazorla G. Secular trends in the performance of children and adolescents (1980–2000): an analysis of 55 studies of the 20m shuttle run test in 11 countries. Sports Med. 2003;33:285–300. [DOI] [PubMed] [Google Scholar]
- 41.Tomkinson GR, Olds TS. Secular changes in pediatric aerobic fitness test performance: the global picture. Med Sport Sci. 2007;50: 46–66. [DOI] [PubMed] [Google Scholar]
- 42.Kark M, Neovius M, Rasmussen F. Obesity status and risk of disability pension due to psychiatric disorders. Int J Obes (Lond). 2010; 34:726–32. doi: 10.1038/ijo.2009.298 [DOI] [PubMed] [Google Scholar]