Summary:
Tissue-resident macrophages require specific milieus for the maintenance of defining gene expression programs. Expression of the transcription factor GATA6 is required for the homeostasis, function and localization of peritoneal cavity-resident macrophages. Gata6 expression is maintained in a non-cell autonomous manner and is elicited by the vitamin A metabolite, retinoic acid. Here, we found that the GATA6 transcriptional program is a common feature of macrophages residing in all visceral body cavities. Retinoic acid-dependent and - independent hallmark genes of GATA6+ macrophages were induced by mesothelial and fibroblastic stromal cells that express the transcription factor Wilms’ tumor 1 (Wt1), which drives the expression of two rate-limiting enzymes in retinol metabolism. Depletion of Wt1+ stromal cells reduced the frequency of GATA6+ macrophages in the peritoneal, pleural and pericardial cavities. Thus, Wt1+ mesothelial and fibroblastic stromal cells constitute essential niche components supporting the tissue-specifying transcriptional landscape and homeostasis of cavity-resident macrophages.
Keywords: Macrophages, Mesothelial cells, Fibroblasts, WT1, Retinoic acid
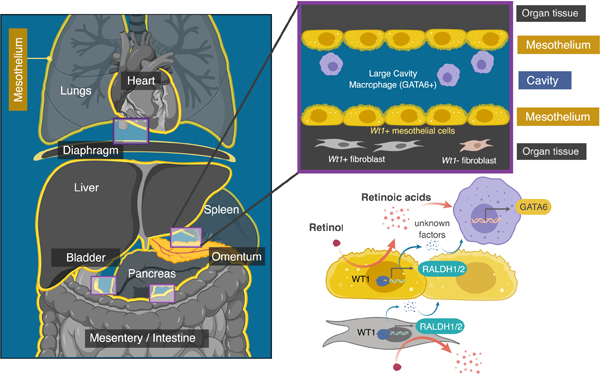
Graphical Abstract
eTOC blurb:
Buechler et al. show that large cavity macrophages (LCMs), which rely on the retinoic-acid-responsive transcription factor GATA6, are found in all visceral cavity spaces. Wt1+ mesothelial and fibroblastic stromal cells maintain the LCM transcriptome in part via the generation of retinoic acid, and are required for LCM homeostasis in vivo.
Introduction:
Tissue-resident macrophages are yolk-sac-derived cells that self-renew in situ with minimal replenishment from hematopoietic cells to maintain tissue equilibrium and provide immune surveillance (Bain et al., 2016; Davies et al., 2013; Guilliams and Scott, 2017; Jenkins et al., 2011; Sieweke and Allen, 2013). Tissue microenvironments elicit lineage-defining transcription factor expression in pre-macrophages during embryogenesis and maintain these transcriptional programs in adulthood (Gosselin et al., 2014; Lavin et al., 2014; Mass et al., 2016). However, the sources of key ‘tissue-identity’ cues for macrophage specification and lineage maintenance are largely uncharacterized (Okabe and Medzhitov, 2016).
CD11b+MHCII-ICAM2+F4/80+ large peritoneal macrophages rely on the transcription factor GATA6 for their homeostasis, function and localization (Gautier et al., 2014; Okabe and Medzhitov, 2014; Rosas et al., 2014). GATA6 expression by these cells is required for their essential roles in tissue repair (J. Wang and Kubes, 2016), pathogen clearance (Gundra et al., 2017) and for the production of IgA and natural antibodies by B cells (Okabe and Medzhitov, 2014; Zeng et al., 2018). Macrophage Gata6 expression is maintained in a non-cell autonomous manner, as these cells lose expression of this key gene in vitro or outside of their physiological niche in vivo (Gosselin et al., 2014; Lavin et al., 2014).
Retinoic acid (RA), a derivative of bio-inactive retinol (or vitamin A), is a pivotal driver of GATA6 expression by resident macrophages inhabiting the peritoneal cavity (Okabe and Medzhitov, 2014). However, the retinol-metabolizing cells in the peritoneal niche have not been identified. Investigations as to whether macrophage GATA6 expression is restricted to peritoneal macrophages remain limited.
The peritoneum, which surrounds the abdominal contents, is one of three distinct fluid-filled cavities in the visceral spaces of the body, the others being the pleural cavity around the lungs and the pericardial cavity around the heart. Pleural macrophages have, like peritoneal macrophages, been reported to express Gata6 (Bain et al., 2016; Rosas et al., 2014). Yolk-sac-derived cardiac cavity macrophages require the expression of the transcription factor Wilms’ Tumor 1 (WT1) in the epicardium, a mesothelial tissue lining the medial aspect of the pericardial cavity, for seeding in this space (Stevens et al., 2016). WT1 has multiple functions, including the induction of two rate-limiting enzymes in retinol metabolism: retinal dehydrogenases 1 and 2 (RALDH1 & 2) (Guadix et al., 2011; Klattig et al., 2007).
Wt1 is highly expressed in mesothelial cells, which construct overlapping cellular junctions to form the perimeter of peritoneal, pleural and pericardial tissues (Mutsaers, 2004) and a subset of cavity tissue-resident mesenchymal fibroblasts (Chau et al., 2014). Mesothelial cells and a portion of cavity-tissue fibroblasts may be developmentally linked as mesothelial cells have been shown to undergo mesothelial-to-mesenchymal transition (Mutsaers et al., 2015). Here, we investigated a two-tiered hypothesis that unified these discrete observations: first, that GATA6 expression governs the homeostasis of all cavity-resident macrophages and second, that tissue-resident Wt1+ stromal cells in peritoneal, pleural and pericardial organs release key factors to maintain this program.
Results:
Resident cavity macrophages express and depend on GATA6
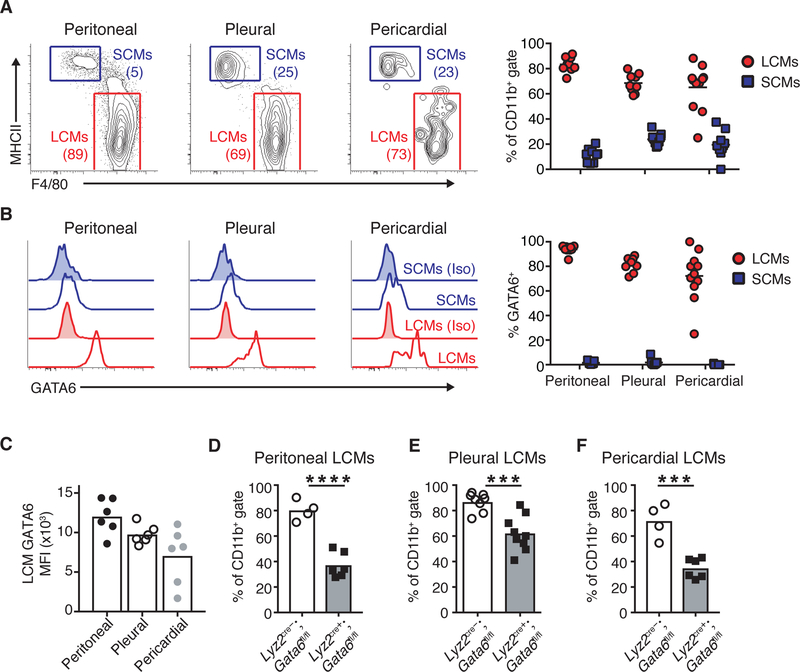
To investigate this hypothesis, we assayed cells from the peritoneal, pleural and pericardial spaces for the presence of GATA6+ resident macrophages. In all cavity spaces, we detected CD11b+MHCII- ICAM2+F4/80+GATA6+ cells, termed ‘large cavity macrophages’ (LCMs) and CD11b+MHCII+ICAM2-F480-GATA6- ‘small cavity macrophages’ (SCMs) (Figure 1A). We observed parity in the frequency of LCMs across cavities and found that a majority of these cells expressed GATA6 (Figure 1A–B and S1A). Peritoneal LCMs expressed the highest level of GATA6 on a per-cell basis compared to pleural and pericardial LCMs, indicating possible heterogeneity within LCM populations that varies across cavities (Figure 1C). F4/80+ cells derived by dissociating cavity tissues did not express GATA6 (Figure S1B), reinforcing that expression of this transcription factor is restricted to LCMs.
Figure 1.
Resident cavity macrophages express and depend on GATA6. (A) Representative gating and quantification of large cavity macrophages (LCMs) and small cavity macrophages (SCMs) and in peritoneal, pleural and pericardial cavities. Numbers are frequency of cells in gate. (B) Intracellular staining and quantification of GATA6 levels in LCMs and SCMs in peritoneal, pleural and pericardial cavities. Unshaded histograms represent isotype staining and shaded histograms are GATA6 staining. (C) LCM GATA6 mean fluorescence intensity (MFI) in peritoneal, pleural and pericardial cavities. (D-F) Frequency of LCMs in peritoneal (C), pleural (D) and pericardial cavities (E) in Lyz2cre-;Gata6fl/fl or Lyz2cre+;Gata6fl/fl mice. (A-C) Cells were gated as CD45+CD19-Gr1-SiglecF- CD11b+. (D-F) Cells were gated as CD11b+ICAM2+. Data are representative or from of ≥3 (A-E) or 1 (F) experiments. Each dot represents 1 mouse. Mean values are shown. *p<0.05, **p<0.005, ***p<0.0005, ****p<0.0001, as determined by unpaired students T-test.
To ascertain whether GATA6 is essential for resident macrophages in cavity spaces, we examined mice lacking GATA6 only in the myeloid compartment (Lyz2cre+;Gata6floxed (fl/fl)) (Gautier et al., 2014). Cytofluorimetric analysis showed that the frequency of LCMs was significantly reduced in all visceral cavities of Lyz2cre+;Gata6fl/fl mice compared to Lyz2cre-;Gata6fl/fl control mice (Figure 1 D–F). Consistent with expressing the highest levels of GATA6, peritoneal LCMs exhibited more dependence on this transcription factor for homeostasis compared to pleural and pericardial LCMs. These data demonstrate that GATA6 is required for stability of LCMs in all visceral body cavities. Therefore, we aimed to determine the source of RA that is required to maintain LCM GATA6 transcription.
Podoplanin+platelet-derived growth factor alpha- and podoplanin+platelet-derived growth factor alpha+ non-hematopoietic, -endothelial, cells at cavity surfaces exhibit robust retinoid metabolism and express Wt1.
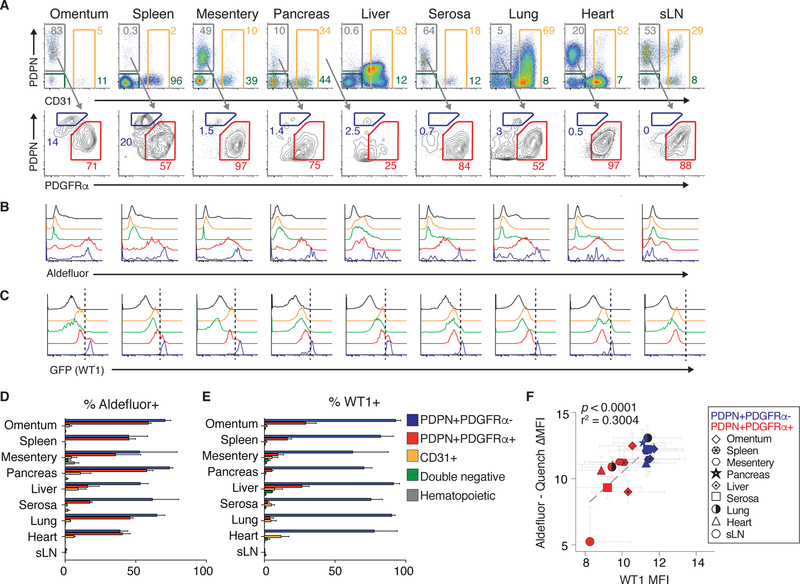
To this end, an array of cell types within visceral cavity tissues were assayed for retinol metabolism capacity using the cytofluorimetric aldefluor assay, which assesses RALDH enzymatic activity. Skin-draining lymph nodes were included in this analysis as negative controls as these tissues are not in body cavities (Figure 2A). RALDH enzymatic activity was largely restricted to non-immune and non-endothelial stromal cells expressing the transmembrane glycoprotein, podoplanin (PDPN), a surface marker associated with mesothelial cells and fibroblasts (Figure 2B, D). The PDPN+ stromal gate in cavity tissues was further subdivided by the fibroblast marker, platelet-derived growth factor receptor α (PDGFRα) (Fletcher et al., 2010) (Figure 2A).
Figure 2.
Podoplanin (PDPN)+platelet-derived growth factor alpha (PDGFRα)- and PDPN+PDGFRα+ non-hematopoietic, -endothelial, -epithelial cells at cavity surfaces exhibit robust retinoid metabolism and express Wt1. (A) Representative gating for CD45-EpCAM-CD31-PDPN+PDGFRα- cells, CD45-EpCAM-CD31- PDPN+PDGFRα+ cells, CD45-EpCAM-PDPN-CD31+ endothelial cells and CD45- EpCAM-PDPN-CD31- double negative cells in cavity tissues and sLN. (B, C) Representative gating for aldefluor activity (B) and GFP (Wt1) (C). (D, E) Quantification of aldefluor positive (D) and GFP (Wt1) (E) positive cells across tissues. (B-E) in CD45-EpCAM-CD31-PDPN+PDGFRα- cells (blue), CD45- EpCAM-CD31-PDPN+PDGFRα+ cells (red), CD45-EpCAM-PDPN−/+CD31+ endothelial cells (orange) and CD45-EpCAM-PDPN-CD31- double negative cells (green) and SSClowCD45+ hematopoietic cells (black) in cavity tissues and sLN. (F) Relationship between aldefluor activity (Log2 transformation of aldefluor mean fluorescence intensity (MFI)) minus quench MFI) and WT1 MFI (Log2 transformation) across cavity tissues and sLN in CD45-EpCAM-CD31- PDPN+PDGFRα- cells (blue; PDPN+PDGFRα- in legend), CD45-EpCAM-CD31- PDPN+PDGFRα+ cells (red; PDPN+PDGFRα+ in legend). (A-F) Data are from three experiments with one mouse per experiment. Mean values + SEM are shown in histograms. (F) p value and r-squared values determined by linear regression.
Wt1eGFP.cre mice, in which Wt1 drives expression of a eGFP.cre fusion protein, (B. Zhou et al., 2008) were used to track Wt1 expression at the single cell level in vivo. eGFP was found to be highly expressed in CD45-CD31-EpCam- PDPN+PDGFRα- cells and a subset of CD45-CD31-EpCam-PDPN+PDGFRα+ cells, but not in immune cells or other cell types in these tissues (Figure 2C, E). We observed a strong correlation between Wt1 expression and RALDH enzymatic activity in CD45-CD31-EpCam-PDPN+PDGFRα- and CD45-CD31- EpCam-PDPN+PDGFRα+ cells, with CD45-CD31-EpCam-PDPN+PDGFRα- exhibiting the highest expression of both (Figure 2F). These data suggest that the Wt1-RALDH pathway is active in CD45-CD31-EpCam-PDPN+PDGFRα-and a subset of CD45-CD31-EpCam-PDPN+PDGFRα+ cells, which we refer to collectively as Wt1+ stroma.
Mesothelial cells and cavity-associated fibroblasts comprise Raldh½-expressing Wt1+ stroma
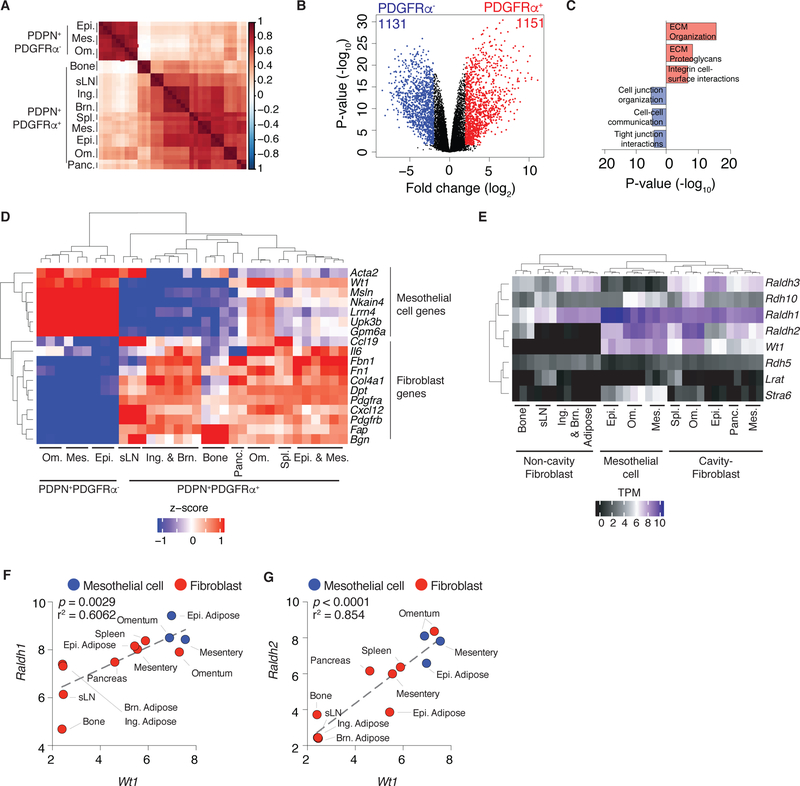
To reveal the identity of the two cell populations with active Wt1-RALDH, that compose Wt1+ stroma we utilized RNAseq and morphological analyses on FACS-sorted cells. RNAseq revealed that CD45-EpCAM-CD31-PDPN+PDGFRα- cells cluster separately from CD45-EpCAM-CD31-PDPN+PDGFRα+ cells and suggest these cells may have tissue-specific transcriptional programs (Figure 3A). CD45-EpCAM-CD31-PDPN+PDGFRα- cells exhibited a gene signature suggesting specialization in creating cell-cell junctions (Figure 3B–C). The presence of eccentric and round nuclei, fine chromatin and inconspicuous nucleoli in these cells indicated that these were mesothelial cells (Figure S2A). CD45-EpCAM-CD31-PDPN+PDGFRα+ cells expressed genes associated with extracellular matrix deposition, consistent with a fibroblastic identity (Figure 3B–C). Finally, evaluating the expression of hallmark mesothelial genes, such as Msln, Gpm6a and Upk3b, (Kanamori-Katayama et al., 2011; Rinkevich et al., 2012), and canonical fibroblastic genes including Fbn1, Dpt and Bgn (Rinkevich et al., 2015) we confirmed that CD45-EpCAM-CD31-PDPN+PDGFRα- and CD45- EpCAM-CD31-PDPN+PDGFRα+ cells were mesothelial cells and fibroblasts, respectively (Figure 3D). All cavity-associated tissues contained mesothelial cells and fibroblasts, though the number and frequency of these cells varied across sites (Figures 2A and S2B–C).
Figure 3.
Mesothelial cells and cavity-associated fibroblasts comprise Raldh½-expressing Wt1+ stroma. (A) Sample similarity heatmap showing spearman correlation between samples calculated from normalized, log transformed, mean-centered expression of top 2500 most variable transcripts. (B) Volcano plot depicting significantly different genes between omental, mesentery and epididymal adipose CD45-EpCAM-CD31-PDPN+PDGFRα- and CD45-EpCAM- CD31-PDPN+PDGFRα+ cells (FDR ≤ 0.05, Log2 FC ≥ 2). (C) Geneset enrichment of differentially expressed genes between CD45-EpCAM-CD31-PDPN+PDGFRα- cells (1131) and CD45-EpCAM-CD31-PDPN+PDGFRα+ cells (1151) evaluated using Reactome 2016. (D) Heatmap showing expression of a subset of differentially genes in comparison of CD45-EpCAM-CD31-PDPN+PDGFRα- cells and CD45-EpCAM-CD31-PDPN+PDGFRα+ cells that are hallmark mesothelial and fibroblastic genes. Significant genes were defined as FDR ≤ 0.05, Log2 FC ≥ 2. Wt1 was not differentially expressed but included in the heatmap. (E) Expression of genes associated with retinol metabolism in mesothelial cells and cavity and non-cavity fibroblasts. (F-G) Correlation between Raldh1 (F) and Raldh2 (G) and Wt1 in FACS-sorted mesothelial cells (blue) and fibroblasts (red). Gene expression across biological replicates (transcripts per million (TPM) + 5 (Log2)) were averaged to generate data points. (A, D-E) Ing. = inguinal, Brn. = brown, Om. = omentum, Epi. = epididymal, Mes. = mesentery, Panc. = pancreas, Spl. = spleen, sLN = skin draining lymph node. (A-G) Three independent biological replicates were sequenced for each cell type, aside from spleen and pancreas (n=2). (F, G) p value and r-squared values determined by linear regression.
Mesothelial cells and cavity-tissue fibroblasts expressed genes associated with retinol metabolism (Figure 3E). Clear relationships between Wt1 and Raldh1 and Raldh2 were observed in these cells (Figure 3F–G). Wt1 expression did not correlate with Raldh3 in these cell types (Figure S2D). Collectively, Wt1+ stroma is composed of mesothelial cells and a subset of cavity-associated fibroblasts that exhibit an active Wt1-Raldh½ axis.
Wt1+ mesothelial and fibroblastic stromal cells metabolize retinol and maintain GATA6 expression by large cavity macrophages.
To definitively measure retinol conversion into retinoic acids, mass spectrometry was employed to identify retinol-derived metabolites. Wt1+ stromal cells isolated from the omentum, a fatty, mesothelial organ that has been implicated in peritoneal macrophage homeostasis (Okabe and Medzhitov, 2014), rapidly converted retinol to all-trans retinoic acid (ATRA), a ligand for the retinoic acid receptors (RARα, RARβ, RARγ) and 9/13cis RA, an activator of retinoid X receptors (RXRα, RXRβ and RXRγ) (Figure 4A–B). RAs derived from Wt1+ stroma was active as conditioned media from these cells elicited RARα signaling (Figure S3A).
Figure 4.
Wt1+ mesothelial cells and fibroblastic stromal cells metabolize retinol and maintain GATA6 in LCM. (A) Representative chromatograms of LC-LC/MS analysis of omental Wt1+ stromal cells and CD45+CD31+ cells supplemented with retinol. Omental Wt1+ stroma (red), omental CD45+CD31+ cells (blue) (B) Quantification of LC-LC/MS for all-trans retinoic acid (ATRA) (ng/mL per number of cells plated; left) and 9/13cis retinoic acid (9/13cis RA) (ng/mL per number of cells plated; right). (C) Representative gating for GATA6 co-culture assay (left; gated on live cells; numbers represent frequencies) and GATA6 expression levels on LCMs after culture without (black) or with (red) omental Wt1+ stromal cells (right; gated on live LCMs; numbers represent frequencies). Unshaded histograms represent isotype staining and shaded histograms are GATA6 staining. (D-E) Quantification of the frequency (D) or MFI (E) of GATA6+ LCMs after culture with autologous omental Wt1+ stromal cells. Each line represents one mouse. (F) GATA6 expression of peritoneal LCM after co-culture with omental fibroblasts or omental mesothelial cells. (H) Co-culture of Wt1+ stromal cells from the omentum (Om.), mesentery (Mes.), pancreas (Panc.) and skin draining lymph node (sLN) and peritoneal LCMs. Cells were cultured for 4–6 days at a ratio of 2–11:1 (Peritoneal lavage: Wt1+ stromal cell/PDPN+ cell (sLN)) for 5 – 6 days. Non-hematopoietic cells purified by MACS. (H) GATA6 expression of peritoneal LCM or lung F4/80+ cells after co-culture with omental Wt1+ stroma. (A-B) Data are from 7 (Omental Wt1+ stromal cells) and 8 (Omental CD45+CD31+ cells) individual mice. (C-H) GATA6+ is determined by frequency positive minus isotype positive. (C-E) Data are representative of (C) or from (D-H) ≥3 experiments. (F-G) 5 mice were pooled for FACS-sorted to generate cells and cultured at a ratio of 1.6–1.8:1 (LCM or lung macrophage:Wt1+ stromal cell or mesothelial cell/fibroblast). *p<0.05, **p<0.005, ***p<0.0005, ****p<0.0001, as determined by paired student’s t-test (D-E), one-way ANOVA with Dunnett’s multiple comparison post-test (H). Mean values (D-E) or mean values ± SEM (B), + SEM (F-H) are shown.
We next explored whether vitamin A metabolites from Wt1+ stroma influenced the expression of GATA6 in LCMs. To this end, peritoneal lavage exudates, which contain a mixture of immune cells including LCMs, SCMs and B cells, were cultured with or without omental Wt1+ stroma. GATA6 expression by LCMs was then evaluated by intracellular flow cytometry (Figure 4C). Consistent with previous studies, LCM GATA6 expression was lost when peritoneal lavage cells were cultured alone (Gosselin et al., 2014; Rosas et al., 2014). This result indicates that local immune cells, including LCMs themselves, do not provide the crucial factors for maintaining LCM GATA6 expression (Figure 4C). In contrast, peritoneal lavage exudates cultured with omental Wt1+ stromal cells exhibited LCMs with >5x GATA6 compared to peritoneal lavage cells cultured alone (Figure 4C–E). GATA6 was not expressed by non-LCM peritoneal cells, which include SCM, co-cultured with omental Wt1+ stroma (Figure S3B). We observed no difference in the ability to promote GATA6 expression in LCM between omental mesothelial cells and fibroblasts (Figure 4F), consistent with their high expression of Wt1 and Raldh enzymes (Figure 2B–C, 3E). Wt1+ stroma were unable to elicit GATA6 expression from lung macrophages, suggesting that non-LCM are refractory to programming by Wt1+ stroma in vitro (Figure 4G).
Consistent with their co-expression of Wt1 and RALDH enzymatic activity, Wt1+ stromal cells from other cavity organs maintained GATA6 expression in LCMs. In contrast, PDPN+ fibroblasts from a non-cavity tissue (skin-draining LN) were unable to induce GATA6 expression in peritoneal LCMs (Figure 4H), which is consistent with their lack of Wt1-Raldh½ gene expression and RALDH enzymatic activity (Figures 2B–C and 3E) (Hammerschmidt et al., 2008; Molenaar et al., 2011). In sum, these results suggest that Wt1+ stromal cells from visceral cavity tissues are sufficient to induce expression of the lineage-defining transcription factor GATA6 in LCMs.
Wt1+ stroma programs the transcriptional landscape of large cavity macrophages.
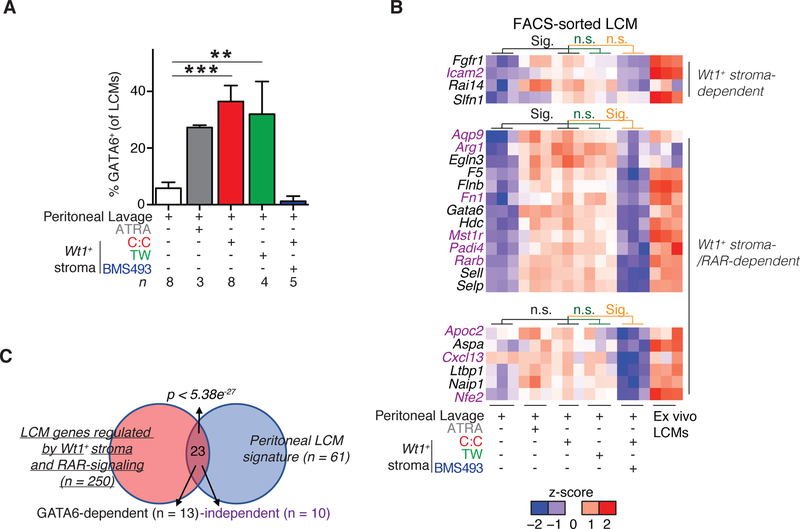
Next, we investigated the mechanism by which Wt1+ stroma maintains GATA6 expression in LCMs. ATRA was added to samples with peritoneal lavage cells as a positive control and this metabolite maintained GATA6 expression by LCMs, as previously published (Gosselin et al., 2014; Okabe and Medzhitov, 2014) (Figure 3D). To determine if direct contact was required, peritoneal lavage cells were separated from omental Wt1+ stromal cells by a semi-permeable membrane that permitted the transfer of media between cells but prevented cell-cell contact. In the transwell, LCM expression of GATA6 was comparable to co-cultures that permitted direct contact between LCMs and Wt1+ stroma and to the ATRA positive control (Figure 5A). Addition of retinoic acid receptor (RAR) inhibitor BMS493 to the co-culture diminished LCM GATA6 expression (Figure 5A) while having no effect on expression of Raldh family members in omental Wt1+ stromal cells (Figure S3C). This relationship was direct and did not require accessory cells or secondary signaling between omental Wt1+ stroma and non-macrophage peritoneal lavage cells as FACS-sorted peritoneal LCMs expressed GATA6 in a RAR-dependent manner when cultured with FACS-sorted omental Wt1+ stromal cells (Figure S3D–F). Thus, soluble factor(s) derived from omental Wt1+ stroma, including RAs, can directly program GATA6 expression in LCMs via a RAR-signaling dependent mechanism.
Figure 5.
Wt1+ stroma programs the transcriptional landscape of large cavity macrophages. (A) Frequency of GATA6+ LCMs in co-culture assay with conditions or treatments as noted. n denotes number of experiments. (B) Heatmap of peritoneal LCM hallmark genes expression in co-cultures. Significant genes exhibited FDR ≤ 0.05, Log2 FC ≥ 2 and were expressed ≥ 1 TPM in all three ex vivo peritoneal macrophage samples. Genes in black are dependent on GATA6-expression; genes in purple are regulated independently of GATA6. (C) Overlap of LCM genes regulated by Wt1+ stromal cells in vitro compared to hallmark LCM genes (Supplemental Table 1). (A) GATA6+ is determined by frequency positive minus isotype positive. (A-B) Co-culture with cell contact (C:C), co-culture with 0.4uM transwell membrane separation (TW), all trans retinoic acid (ATRA), or BMS493. Cells were cultured for 4–6 days at a ratio of 2–11:1 (Peritoneal lavage:Wt1+ stromal cells) for 5 – 6 days. **p<0.005, ***p<0.0005, as determined one-way ANOVA with Dunnett’s multiple comparison post-test (A) or hypergeometric test (C). Mean values or mean values + SEM (A) are shown.
We next asked if Wt1+ stromal cells turned on GATA6 expression at the RNA level in LCMs and more broadly influenced the peritoneal LCM transcriptome. Relative to other macrophage populations, peritoneal LCMs express a well-defined set of unique genes, with some of these being GATA6-dependent and others GATA6- and retinoic acid-independent (Gautier et al., 2014; 2012; Gundra et al., 2014; Okabe and Medzhitov, 2014; Rosas et al., 2014) (Table S1). Comparative RNAseq analysis on peritoneal LCMs FACS-sorted from cultures with and without omental Wt1+ stromal cells and ex vivo peritoneal LCMs (Figure S4A–B) revealed 165 genes in peritoneal LCMs that are regulated by omental Wt1+ stromal cells (Figure S4C–F). We identified an additional 85 genes that exhibited diminished expression after RAR inhibition, underscoring the importance of RA signaling in the programming of peritoneal LCMs by Wt1+ stroma (Figure S4G), and collectively yielding 250 genes of interest. 23 of the 250 genes identified in our RNAseq analysis were canonical peritoneal LCM genes (p < 5.38e−27) (Figure 5B–C). Expression of peritoneal LCM hallmark genes such as Gata6, Aspa and Arg1 required RAR signaling. Other LCM genes like Rai14 and Icam2 were induced by Wt1+ stromal cells independently of RAR signaling (Figure 5B and Table S1). Collectively, these data suggest that Wt1+ stromal cells can program LCM identity via RAR and GATA6 signaling but also, to a lesser degree, by means independent of these factors.
Wt1+ stromal niche controls cavity macrophage homeostasis in vivo.
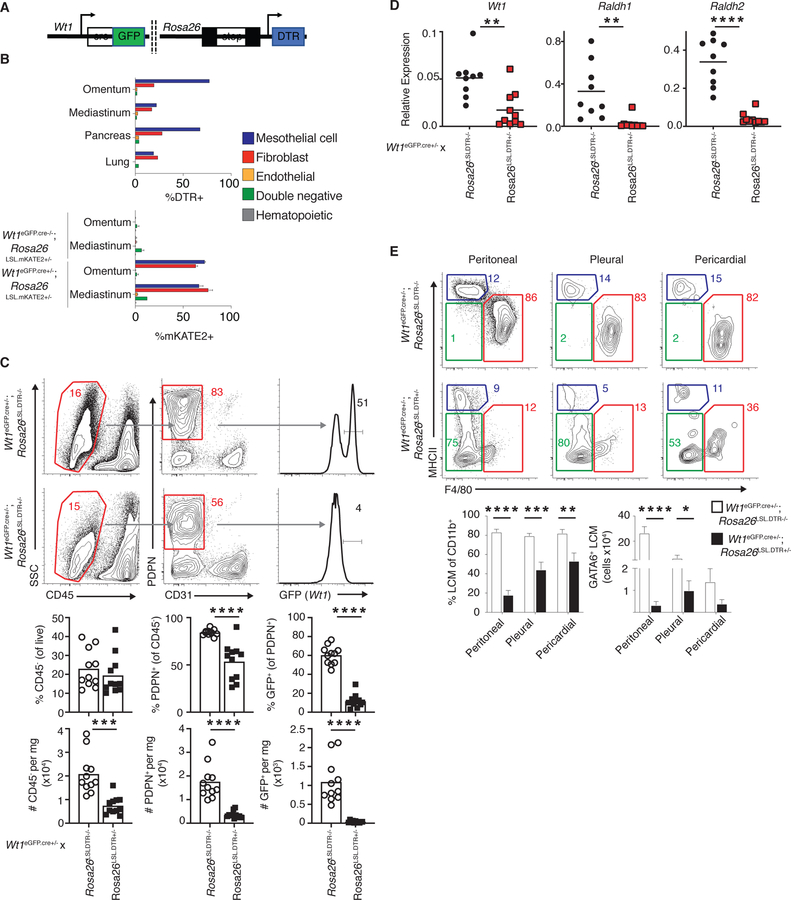
To determine whether GATA6 maintenance in LCMs was dependent on Wt1+ stroma in vivo, a mouse model that enabled selective depletion of Wt1-expressing cells was generated. This was achieved by crossing mice with the gene encoding the simian diphtheria toxin receptor (DTR) downstream of a loxP flanked transcriptional stop element in the ubiquitous Rosa26 locus to Wt1eGFP.cre mice (Wt1eGFP.cre;Rosa26LSL.DTR mice) (Figure 6A). In Wt1eGFP.cre+;Rosa26LSL.DTR mice, cells of the visceral cavity organs assayed, including the omentum and mediastinum, a fatty, mesothelial tissue in the pleural-pericardial space, pancreas and lung, with an active Wt1 promoter (mesothelial cells and cavity-tissue fibroblasts) expressed the simian DTR and were thereby sensitive to DT-induced apoptosis (Figure 6B and S5A). We also crossed Wt1eGFP.cre mice to animals in which mKATE2 is downstream of a loxP flanked transcriptional stop element in the Rosa26 locus (Wt1eGFP.cre;Rosa26LSL.mKATE2 mice mice). Using these animals, we observed that only Wt1eGFP.cre-positive cells were mKATE2-expressing in the omentum and mediastinum, reinforcing that only mesothelial cells and cavity-tissue fibroblasts were susceptible to DT-mediated apoptosis in Wt1eGFP.cre+;Rosa26LSL.DTR model (Figure 6B and S5A). Importantly, LCM do not express Wt1 RNA or DTR in this system and are therefore are not susceptible to direct toxin-mediated death (Figure S5B, C).
Figure 6.
Wt1+ stromal niche controls cavity macrophage homeostasis in vivo. (A) Schematic of Wt1eGFP.cre;Rosa26LSL.DTR mouse. DTR expression is achieved in Wt1-expressing cells following cre-mediated excision of a loxP-flanked transcriptional “stop” sequence. (B) DTR expression Wt1eGFP.cre;Rosa26LSL.DTR mice (top). mKATE2 expression in Wt1eGFP.cre−/−;Rosa26LSL.mKATE2+/− and Wt1eGFP.cre+/−;Rosa26LSL.mKATE2+/− mice (bottom). (C) Representative gating and quantification (frequency and number per milligram (mg)/tissue) of non-hematopoietic cells in the omenta of Wt1eGFP.cre+/−;Rosa26LSL.DTR−/− and Wt1eGFP.cre+/−;Rosa26LSL.DTR+/− mice 24 hours after DT-administration. (D) Abundance of Wt1, Raldh1 and Raldh2 transcripts in whole omentum 24 hours after DT-administration in Wt1eGFP.cre+/−;Rosa26LSL.DTR−/− and Wt1eGFP.cre+/− ;Rosa26LSL.DTR+/− mice. (E) Representative gating and quantification (frequency of CD11b+ gate and number of GATA6+ LCMs) of cavity LCMs Wt1eGFP.cre+/− ;Rosa26LSL.DTR−/− and Wt1eGFP.cre+/−;Rosa26LSL.DTR+/− mice 24 hours after DT- administration. LCMs were gated as Live CD45+Gr1-SiglecF-CD11b+MHCII- F4/80+. GATA6+ is determined by frequency positive minus isotype positive. (C-F) Data are from 3 (C) or 4 (E) experiments or representative of 2–4 (B, D) experiments. Each dot represents one mouse. *p<0.05, **p<0.005, ***p<0.0005, ****p<0.0001, as determined by unpaired student’s t-test. Mean (B (top), C, D) or mean +SEM values (B (bottom) E) are shown.
A single injection of DT caused an extensive loss of Wt1+ stromal cells in the omentum, the candidate organ we used to assess efficiency of our system, 24 hours after injection (Figure 6C). CD31+ endothelial cells were not affected by DT administration (Figure 6C). DT administration also elicited a significant diminishment of Wt1, Raldh1 and Raldh2 in whole omentum lysates (Figure 6D), demonstrating that the ablation of Wt1+ stroma is accompanied by a loss in retinol metabolizing genes in whole tissues. RAs are challenging to assay at physiological levels in vivo but exogenous administration of RA has demonstrated this metabolite has an in vivo half-life of 30–40 minutes in tissues (Jing et al., 2017). Therefore, we speculate that loss of Raldh family member genes impairs availability of bioactive retinoid species in cavity spaces.
Depletion of Wt1+ stromal cells resulted in a profound diminishment in the frequency of LCMs in the peritoneal, pleural and pericardial cavities, with the degree of loss correlating with basal GATA6 expression as shown in Figure 1 (Figure 6E). We also observed a loss in the number of GATA6+ LCMs in all cavities upon DT treatment, though these data were not significant in the pericardial space due to the variance in the number of cells isolated from this site (Figure 6E and Figure S5D). Of note, LCMs exhibited diminished GATA6 upon DT administration and no other CD11b+ myeloid cells including SCMs or CD11b+F4/80-MHCII- inflammatory macrophages (Gautier et al., 2013), expressed GATA6 (Figure S5E), consistent with diminished GATA6-sustaining RAs in cavity spaces. Loss of LCMs coincided with inflammation as read out by the appearance of inflammatory macrophages (Figure 6E and S5F). In sum, we observe a loss of LCM in Wt1eGFP.cre+;Rosa26LSL.DTR mice upon DT-mediated Wt1+ stromal deletion and diminished GATA6 expression by residual LCM which is accompanied by a concomitant diminishment of retinol metabolism machinery in a key organ. Overall, these data suggest that LCM are lost in the Wt1eGFP.cre+;Rosa26LSL.DTR model due to diminished RA availability resulting from a loss of Wt1+ stroma.
Since adipocytes are abundant in the omentum, and some other cavity tissues, and located just below the surface-lining mesothelial-fibroblastic network (Kenny et al., 2014), we tested whether they might contribute to LCM homeostasis. We enumerated GATA6+ peritoneal LCMs in Adiponectincre;Rosa26LSL.DTA lipodystrophic mice which lack adipocytes (Wu et al., 2018). LCM abundance was unaffected by the absence of adipocytes (Figure S5G). Overall, these data demonstrate the essential role of Wt1+ mesothelial and fibroblastic stromal cells in maintaining LCM homeostasis and GATA6 expression in vivo.
Discussion
Here, we revealed that tissue-resident macrophages, which expressed the transcription factor GATA6 were found within the peritoneal, pleural and pericardial visceral body cavity spaces, not just the peritoneum as previously believed. These cells all required GATA6 expression for homeostasis. Based on the shared surface phenotype and mutual reliance on GATA6, we termed these cells LCMs. Wt1+ stromal cells, a heterogeneous population of mesothelial cells and fibroblasts from visceral cavity tissues, maintained LCM GATA6 expression in vitro and sustained a significant portion of the LCM transcriptome. Importantly, Wt1+ stromal cells proved essential for LCM GATA6 expression and homeostasis in cavity spaces.
These data suggest a model in which Wt1+ stroma secrete multiple forms of RA that sustains LCM GATA6 expression, and therefore homeostasis, across body cavities. This conceptual framework is in accordance with data showing that Wt1 is expressed by embryonic day (E) 8.5 (Chau et al., 2014) and tissue-resident macrophage specification occurs between E10 and E12 (Mass et al., 2016). The distribution of Wt1+ stroma across visceral tissues may provide a means for LCMs to survey the entirety of the cavity space in which they reside while maintaining expression of GATA6. However, mesothelial organs, such as the omentum in the peritoneum and mediastinum in the pleural-pericardial space may play key roles in LCM maintenance and development. This paradigm raises new questions with respect to LCMs and the mesothelial-fibroblastic stromal cells that support them.
The tissue-resident macrophage microenvironment and the cellular and molecular factors that constitute essential niches across organs are still being described (Guilliams and Scott, 2017). It has been shown that factors derived from endothelial cells (Mondor et al., 2019), epithelial cells (Schneider et al., 2014), neurons (Y. Wang et al., 2012), red blood cells (Haldar et al., 2014) or even pathogens and toll-like receptor signals (Akilesh et al., 2019) can provoke macrophage polarization and constitute key niche factors, depending on the context. The data presented here show that Wt1+ stromal cells in cavity spaces are niche-defining for LCMs. The findings presented here suggest more complexity within macrophage-stromal interactions than has been previously described in vitro (X. Zhou et al., 2018). Yet, the complete mechanistic underpinning(s) of a reciprocal stromal cell-macrophage relationship in the steady state and in the context of inflammation requires increased study.
While GATA6 has been accurately implicated as the master transcription factor for LCMs, it is still unclear how this transcription factor, or downstream genes, act to regulate localization of LCMs. These cells are free-floating and in continuous motion within the cavity space in the steady state (Zhang et al., 2019), whereas other tissue-resident macrophages are sessile and reside directly within a stromal niche (Uderhardt et al., 2019). The cavity space localization of GATA6+ macrophages, and the tight regulation of GATA6 expression by Wt1+ stromal cells in a contact-independent manner raises more questions about the physiological role of LCMs and their supportive stromal cells. Wt1+ stromal cells may exhibit directional release of RA into cavity spaces to maintain LCMs much like the polarity observed in mucus-secreting intestinal epithelial cells (Peterson and Artis, 2014). Yet, precisely how RA is secreted by cells remains to be defined.
GATA6+ LCMs traffic into surrounding organs to facilitate tissue repair (J. Wang and Kubes, 2016) or pathogen clearance (Gundra et al., 2017) so these cells must integrate multiple environmental signals to determine their ultimate localization. Whether in cavities or in tissues, GATA6 expression by LCM depends on RA species and we propose that Wt1+ mesothelial cells provide these factors in the steady state and that LCMs receive these metabolites from Wt1+ fibroblasts below the mesothelial layer upon ingress in tissues. Precisely how LCM integrate RA species, both ATRA and 9/13cis RA, and how they interact in vivo with the Wt1+ mesothelial-fibroblastic niche are still outstanding questions. It is also tempting to speculate as to whether LCMs must receive contact-dependent signals from stromal cells before initiating a tissue-repair transcriptional program.
The immunologic functions of non-hematopoietic stromal cells are just beginning to be realized (Buechler and Turley, 2018). These cells have been previously implicated in directing and localizing immune cells in healthy tissues (Cremasco et al., 2014; Kolodin et al., 2015) or excluding immune cells from tumors (Mariathasan et al., 2018), but our finding that Wt1+ stromal cells operate to homeostatically exclude LCMs from body organs defines a new function. Just as inflammation perturbs LCMs, cavity stromal cells have been shown to undergo mesothelial-to-mesenchymal transition during injury (Mutsaers et al., 2015). This phenomenon may play a role in LCM tissue translocation and repair, though this remains to be studied. We propose that further evaluation of cavity stromal cells will continue to reveal new insights into tissue homeostasis as well as in fibrosis, infection, cancer, sterile injury, wound healing and tissue regeneration.
STAR Methods:
KEY RESOURCES TABLE
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources should be directed to and will be fulfilled by the Lead Contact, Shannon Turley (Turley.Shannon@gene.com). The mouse lines described below may require a Material Transfer Agreement (MTA) with the providing scientists.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mice and in vivo treatments
Mice were bred and maintained in groups of 1–5 animals per cage at Genentech or Washington University in St. Louis. Males and females from 6–20 weeks old were used. All experiments were performed under Genentech or Washington University in St. Louis approved protocols.
Wildtype (Jackson Laboratory (JAX) colony 00064), Wt1eGFP.cre (JAX colony 010911), constitutive GFP expressing (JAX colony 006567; used in some co-culture experiments) or IL6−/− mice (JAX 002650; used in one experiment to assess frequency of pleural resident macrophages) were obtained from JAX. The loxP/cre conditional reporter allele harboring mKATE2 was generated at Genentech. Wt1eGFP.cre mice were also bred at Genentech. Wt1eGFP.cre;Rosa26LSL.DTR and Wt1eGFP.cre;Rosa26LSL.mKATE2 were interbred and maintained on C57Bl6 at Genentech. Adiponectincre;Rosa26LSL.DTA, Lyz2cre;GATA6f/f mice and controls were bred and maintained at Washington University in St. Louis.
Diphtheria toxin (DT) (EMD Millipore, cat. 322326) was administered to the genotypes indicated at 0.006mg/kg intraperitoneally.
METHODS DETAILS
Cavity lavages and cavity cell isolation
Peritoneal lavage was performed using 10mL of PBS or 5mL 2mM EDTA + 2% BSA in PBS. Pleural lavage was performed using 3mL PBS or 2mM EDTA + 2% BSA in PBS. Lavages were typically performed on deceased mice though some pericardial procedures were accomplished under general anesthesia using an operating microscope. For pericardial lavage procedures, a midline laparotomy was made along the linea alba terminating at the xiphoid process. Bilateral incisions were then made lateral to the xiphoid process, extending through the rib cage to toward the axilla. The ribcage was lifted to expose the thoracic cavity and the diaphragm was meticulously dissected off the lower ribs from lateral to medial. Then, either a syringe filled with 30–50uL of HBSS with a 24-gauge Vialon angiocath (BD Insyte) tip was inserted into the pericardial sac between the sternum and heart, the pericardial space was gently filled with the buffer and witnessed for expansion and this was then removed and was repeated up to at least five times or an insulin syringe was inserted into the pericardial sac after removing excess fluid from the surrounding space with a kim-wipe and 300uL was injected into the sac and as much volume was retrieved as possible. These approaches were adapted for mice from a similar procedure used in rats (McLarty et al., 2011).
Tissue digestion and stromal cell isolation
For cavity tissue cell isolation, tissues and skin draining lymph nodes (sLN; composed of pooled inguinal, axial, brachial and cervical LN) were isolated and digested as previously described (Fletcher et al., 2010; Malhotra et al., 2012), with minor modifications. Briefly, tissues, aside from the omentum and mediastinum, were gently minced with scissors prior to initiating the digestion protocol. Bone was isolated from muscle and tendons, then epiphyses were removed with scissors and bones were placed in 500uL Eppendorf tube that had been punctured at the graduate end with an 18G syringe. Decapped bones in tubes were placed in a 1.5mL Eppendorf tube and spun for 30 seconds at 14000 x g. Marrow free bones were then gently crushed in 5mL media and swirled. Media was decanted and this was repeated. Then bones were put in digest media and finely minced with scissors. After isolation from mouse (sLNs, omentum and mediastinum) and mincing (epididymal adipose, inguinal adipose, brown adipose, pancreas) or removal and crushing (bone) cells were placed in a 15mL conical tube with 5mL digestion media (RPMI + 2% FBS with 100mg/mL Dispase (Life Tech., cat. 17105041, 100mg/mL Collagenase P (Roche, cat. 11249002001, and 50mg/mL DNase I (Roche, cat. 10104159001) and agitated. Tubes were placed in a 37C water bath for 15 mins, and 5mL fractions were removed and filtered (70uM) into RPMI supplemented with 2% FCS (VWR) three times. Cells were then incubated with Ack for 2–5 minutes to removed red blood cells. The resulting single cell suspension was used for experiments. All RNAseq experiments isolated cells in this manner. For figure 2, an improved digest protocol was used to isolate mesothelial cells and fibroblasts more efficiently from the non-adipose cavity tissues. This approach differed from normal digest protocol in that 2x more collagenase was used. Additionally, after a single cell suspension was obtained, the cells were layered on top of a 26% optiprep (Sigma, cat. D1556; diluted in PBS) gradient in 15mL conical tubes and spun at 1500 x g for 15 minutes with slow acceleration and the brake off. Cells in suspension were isolated with a transfer pipette. The cells were treated with Ack as above if pellet was bright red or directly used for experiments.
FACS identification and sorting:
Cells were labeled with the following mAbs purchased from eBioscience, BioLegend, or BD Biosciences at 1:200 for 20–30 min, unless otherwise noted. Prior to cell surface staining with the following fluorescently labeled antibodies, cells were blocked with Fc block (2.4G2; 1:500–1:1000). Surface staining for experiments was performed as below, unless otherwise noted: CD45 (30-F11), EPCAM (G8.8), CD31 (390), CD11b (M1/70; 1:200 or 1:400), F4/80 (BM8), ICAM2 (3C4), PDGFRα (AP5), PDPN (8.1.1; 1:800), CD19 (6D5), Ly6C (HK1.4; 1:400), Ly6G (1A8; 1:400), MHCII (M5/114.15.2), SIGLEC F (E50–2440), GR1 (8C5; 1:400). For DTR staining, cells were stained with human DTR biotinylated antibody (R&D Systems; catalog name: Human HB-EGF; BAF259: 1:100) with other surface stain markers. The antibodies were washed off and streptavidin-conjugated BV395 was used at 1:400 for 20 minutes. For intracellular staining cells were surface stained as above, washed, and then fixed and permeabilized using the Foxp3/Transcription Factor Staining Buffer Set, per the manufacturer’s directions (ThermoFisher, cat. 00–5523-00). Cells were then incubated with GATA6 (PE conjugate [50ug/mL], CST cat. 26452S at 1:50; or Rabbit isotype PE [100ug/mL], CST cat. 5742S 1:100) for 30 minutes on ice. Live cells were identified by washing after Fc block and incubation with Fixable Viability Dye Violet (Invitrogen, cat. L34955, 1:1000) prior to surface staining or incubation with calcein blue (Invitrogen, cat. C1429, 1:1000) after surface staining. Data were acquired on an Fortessa, Symphony or LSRII (BD Biosciences) and analyzed using FlowJo (Tree Star).
Aldefluor assay
Cells were isolated as above. Once a single cell suspension was obtained, cells were plated and resuspended in aldefluor assay buffer, as part of the aldefluor kit (STEMCELL, cat. 01700) in FACS tubes (500uL) or 96 well U bottom plates (200uL) with Fc block. Then, 10uL (FACS tubes) or 4uL (96 well plate) of assay buffer was aliquoted into quench tubes. 2.5uL (FACS tubes) or 1uL (96 well plates) of DEAB buffer was aliquoted into quench tubes. 5uL (FACS tubes) or 2uL (96 well plate) aldefluor reagent was then added to cells as rapidly as possible. Cells were then mixed and ½ of the volume was added to quench tubes with DEAB. Samples were incubated at 37C for 15–20 minutes, then spun down and surfaced stained as above on ice for 20–30 minutes before FACS analysis.
GATA6 co-culture assay
Cells were isolated as above. Once a single cell suspension was obtained, tissue-derived cells were counted and Fc blocked. To derive Wt1+ stromal cells, CD45+ and CD31+ cells were typically depleted via MACS per the manufacturer’s directions from cavity tissues (Miltenyi Biotec). CD45-CD31- cells were checked for purity by flow cytometry and were >90% PDPN+. 10,000 or 50,000 tissue-derived Wt1+ stromal cells were plated in a 24-well or 96-well flat bottom plates, respectively. 40,000 (96-well) to 200,000 (24-well) peritoneal lavage cells were then plated with or without Wt1+ stromal cells. Overall, for peritoneal exudate:Wt1+ stroma experiments, cells were cultured at a ratio of 2–11:1 (Peritoneal lavage:Wt1+ stoma cell). Cells were plated in complete RPMI supplemented with or without 10ng/mL MCSF (R&D, cat. 416-ML) and the media was changed every three days. For FACS sorting experiments, bulk omental Wt1+ stromal cells or FACS-separated mesothelial cells and fibroblasts, were co-cultured with CD11b+F480+MHCII- peritoneal LCMs at ratios of 1 omental Wt1+ stromal cell to 1–2 peritoneal LCMs or lung macrophage. For experiments with FACS-sorted macrophages, media was supplemented with 100ng/mL MCSF. A 0.4uM semi-permeable membrane (Costar, cat. 3413) was used to separate Wt1+ stroma and peritoneal lavage cells; Wt1+ stromal cells were plated in the well and peritoneal lavage cells were aliquoted into the transwell membrane. For pharmacological agents in these assays, ATRA (Tocris, cat. 0695) was used at a final concentration of 1uM, BMS493 (Tocris, cat. 3509) was used at a final concentration of 1uM. These agents were included at the start of culture and replaced during media changes. ATRA, when used, was also added 24 hours before the end of the experiment. Cultures incubated for 5–7 days before analysis or cell sorting.
Mass spectrometry
Wt1+ stromal cells and the CD31+CD45+ fraction were isolated by MACS, as described above. Cells were then plated in near equivalent numbers (55,000 – 190,000 cells/well of a 24-well plate) for 48 hours in complete RPMI with 10ng/mL MCSF. Media was then replaced and supplemented with 500nM retinol. After 24 hours, supernatant was collected and media with retinol was again added to the cells. This process was repeated after 12, 6, and 3 hours. Supernatant was frozen, then 1.5ml acetonitrile was added to 400ml of thawed supernatant to remove protein. The supernatant was then dried using nitrogen gas and then reconstituted into 100ul of dichloromethane (Honeywell Burdick & Jackson, >99.5%): Methanol (HPLC grade, Fisher Chemical), (1:1, v:v ). The samples were then analyzed with liquid-mass spectrum (LC-MS/MS). HPLC separation of retinoids was performed on a reverse-phase column (Metasil AQ 3 C18 150×2.0mm). The temperatures of the column oven and autosampler were set at 40°C and 15°C, respectively. The injection volume was 5ml. The LC flow rate was set at 0.3 ml/min. The LC gradient started with 70% mobile phase A (60% methanol, 40% water, 10mM ammonium formate, 0.2% Formic acid) and 30% mobile phase B (60% methanol, 40% chloroform, 10mM ammonium formate, 0.2% Formic acid) for the initial 1 min. Mobile phase B was increased to 60% within 9 min, then it was further increased to 100% in 30 seconds and maintained at 100% for 2 min. The gradient was finally returned to the initial condition within 0.5 min. The column was re-equilibrated with the initial condition for 3 min before the next injection.
The liquid chromatograph was coupled to a 6500+ QTRAP mass spectrometer (Applied Biosystems, Foster City, CA) operated under positive ionization mode with the following source settings: turbo-ion-spray source at 250°C, N2 nebulization at 70 psi, N2 heater gas at 80 psi, curtain gas at 35 psi, collision-activated dissociation gas pressure was held at medium, turbo ion-spray voltage at 5,500 V, declustering potential at 31V, entrance potential at 10V, collision energy at 19 V and collision cell exit potential at 14 V. Sample analyses was performed in multiple reaction monitoring (MRM) mode with dwell time of 0.15 s. The transition for ATRA and 9/13 cis RA was 301.1 > 123.2. The retention times for ATRA and 9/13 cis RA were 8.01 min and 8.35min, respectively. The quantitation of ATRA and 9/13 cis RA was performed based on their standard curves, derived from 9-cis-Retinoic acid (Santa Cruz Biotechnology, Inc.), all-trans retinoic acid (Santa Cruz Biotechnology, Inc).
RNAseq analysis
For ex vivo RNAseq on fibroblasts and mesothelial cells, cells were isolated and stained as described above. Each sample was derived by pooling lysed cells from three independent experiments in which 2,500 to 50,000 cells were sorted. In all cases, samples were sorted at >94% purity directly into TRIZOL (Invitrogen, cat. 15596026). RNA was isolated as described (Painter et al., 2011) or at Expression Analysis, Inc. All samples had RIN scores of 7.7 or greater, as determined with a BioAnalyzer (Agilent).
For in vitro GATA6 co-culture experiments, between 2,000 and 30,000 cells were FACS sorted directly into RLT Buffer (QIAGEN). All samples had RIN scores of 9.5 or greater as determined with a BioAnalyzer.
Paired-end RNA-seq libraries were constructed from 747 pg (fibroblasts and mesothelial cells) and 1.2 ng (GATA6 co-culture experiments) of RNA using the SMARTer Low Input RNA Kit (Takara). Libraries were then sequenced on an Illumina HiSeq yielding, on average, 38 million (fibroblasts and mesothelial cells) and 39 million (in vitro LCM) read pairs (2×50bp) per sample. We then used salmon version 0.6 (Patro et al., 2017) to quantitate transcript-level expression using the GENCODE M9 mouse transcript models with “--biasCorrect” enabled and the “-l IU” library type. These data were imported into R using the tximport package (Soneson et al., 2015) to get gene level expression estimates, and limma/voom (Law et al., 2014) was used for differential expression analysis. A hypergeometric test was used to determine whether omental Wt1+ stroma significantly influenced macrophage identity where overlap-1 = 23–1, list1 = 250, PopSize-list1 = 17063–250, list2 = 63. Geneset enrichment analysis was performed using the Enrichr platform (Kuleshov et al., 2016).
Cytospin and Wright-Giemsa stains
Immediately after FACS sorting, cells washed with PBS and cytospun onto slides (Cytospin 3, Shandon). Samples were dried and fixed with 100% methanol for 35 seconds, stained with Wright Stain (Sigma) for 4 minutes and stained with working Wright-Giemsa stain (4:1 Wright:Giemsa (Sigma) dye in buffer) for 14 minutes then washed.
RARα luciferase assays
RARα-luciferase reporter cells were purchased from BPS bioscience (cat. 60503). Cells were grown according to the manufacturer’s directions. These cells were then incubated with conditioned media derived from FACS-sorted mesothelial cells or fibroblasts cultured for 5 days or 1uM ATRA for 24 hours Luciferase was quantified using a SpectraMax M5 (Molecular Devices), per manufacturer’s directions.
qPCR, RNA Extraction and cDNA Synthesis
Total RNA was extracted from omental tissue using the RNeasy Plus Mini Kit (Qiagen, cat. 74134) and protocol. RNA concentration was quantified by optical density and normalized across samples. Equal amounts of cDNA were synthesized using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, cat. 4368814) with an RNase Inhibitor (Applied Biosystems, cat. N8080119) and according to the manufactures protocol. Each cDNA sample was diluted 1:200 in RNase free water prior to use in qPCR.
qPCR was conducted using TaqMan Gene Expression Assay Probes for the genes Wt1 (Mm01337048_m1), Aldh1a1 (Mm00657317_m1), Aldh1a2 (Mm00501306_m1) and Gapdh (Mm99999915_g1). Each TaqMan probe was diluted 1:10 in TaqMan Fast Advanced Master Mix (ThermoFisher, cat. 4444557) to create a TaqMan probe working solution. All qPCR reactions were carried out in a MicroAmp optical 384-well reaction plate. qPCR was performed using the QuantStudio 5 (Applied Biosystems) under the following cycling conditions: 1 cycle of 50°C for 2 minutes and 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. The average CT value for each gene was calculated and normalized to Gapdh.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical analysis was performed in Prism 8 (GraphPad Software) or R studio. Statistical significance was evaluated by Student’s t test or ANOVA with post test, unless otherwise noted. *p<0.05, **p<0.005, ***p<0.0005, ****p<0.0001. n represents number of replicates.
DATA AND SOFTWARE AVAILABILITY
RNAseq datasets are available at the NCBI GEO under GSE130813 (FACS-sorted fibroblasts from lymph node, spleen, bone, brown adipose, inguinal adipose, omentum, mesentery, pancreas and FACS-sorted mesothelial cells from omentum, mesentery and epididymal adipose) and GSE130815 (In vitro LCM, Wt1+ stromal cells and ex vivo LCM).
Supplementary Material
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Fc Block | BD | Cat#553142 |
| CD45 (30-F11) | BD | Cat#564279 |
| EPCAM (G8.8) | Biolegend | Cat#118216 |
| CD31 (MEC13.3) | BD | Cat#565097 |
| CD11b (M1/70; 1:200–1:600) | Biolegend/BD | Cat#101255/Cat#564443 |
| F4/80 (BM8) | eBioscience | Cat#17–4801-82 |
| ICAM2 (3C4) | Biolegend | Cat#105612 |
| PDGFRa (APA5) | Biolegend/BD | Cat#135906/Cat#740269 |
| PDPN (8.1.1; 1:800) | Biolegend | Cat#127410 |
| CD19 (6D5) | Biolegend | Cat#115520 |
| Ly6C (HK1.4; 1:400) | Ebioscience | Cat#53–5932-82 |
| Ly6G (1A8; 1:400) | Biolegend | Cat#127624 |
| MHCII (M5/114.15.2) | Ebioscience | Cat#25–5321-82 |
| SIGLEC F (E50–2440) | BD | Cat#565527 |
| GR1 (RB68C5; 1:400). | Biolegend | Cat#108424 |
| Human HB-EGF (DTR; 1:100) | R&D Systems | Cat#BAF259 |
| Streptavidin BUV396 (1:400) | BD | Cat#564176 |
| GATA6 PE (1:50) | CST | Cat#26452S |
| Isotype PE (1:100) | CST | Cat#5742S |
| Fixable Violet Dead Cell Stain Kit | Thermofisher | Cat#L34963 |
| Calcein Blue, AM | Thermofisher | Cat#C1429 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| All trans retinoic acid | Tocris | Cat#0695 |
| Retinol | Sigma | Cat#95144 |
| BMS493 | Tocris | Cat#3509 |
| MCSF | R&D systems | Cat#416-ML |
| Dichloromethane | Honeywell Burdick & Jackson | Cat#494453 |
| Methanol | Fisher Chemical | Cat#10675112 |
| RNase Inhibitor | Applied Biosystems | Cat#N8080119 |
| TaqMan Fast Advanced Master Mix | ThermoFisher | Cat#4444557 |
| Diphtheria toxin | EMD Millipore | Cat#322326 |
| Giemsa stain, modified | Sigma | Cat#GS500 |
| Wright stain | Sigma | Cat#WSGD128 |
| RNase Inhibitor | Applied Biosystems | Cat#N8080119 |
| TRIZOL | Invtirogen | Cat#15596026 |
| Collagenase P | Roche | Cat#11249002001 |
| Dispase | Life Technologies | Cat#17105041 |
| DNAse | Roche | Cat#10104159001 |
| Optiprep | Sigma | Cat#D1556 |
| RLT Buffer | QIAGEN | Cat#79216 |
| Critical Commercial Assays | ||
| High Capacity cDNA Reverse Transcription Kit | Applied Biosystems | Cat#4368814 |
| RNeasy Plus Mini Kit | Qiagen | Cat#74134 |
| SMARTer Ultra Low Input RNA Kit for Sequencing | Clontech | Cat#634848 |
| Aldefluor Assay | STEMCELL Technologies | Cat#01700 |
| Deposited Data | ||
| FACS-sorted fibroblasts from lymph node, spleen, bone, brown adipose, inguinal adipose, omentum, mesentery, pancreas and FACS-sorted mesothelial cells from omentum, mesentery and epididymal adipose | GSE130813 | |
| In vitro LCM, Wt1+ stromal cells and ex vivo LCM | GSE130815 | |
| Experimental Models: Cell Lines | ||
| RARα-luciferase reporter cells | BPS bioscience | Cat#60503 |
| Experimental Models: Organisms/Strains | ||
| Mouse: C57BL/6J | Jackson Laboratory | Cat#00064 |
| Mouse: Wt1eGFP.cre | Jackson Laboratory / Genentech | Cat#010911 |
| Mouse: Constitutive GFP+ | Jackson Laboratory | Cat#006567 |
| Mouse: IL6−/− | Jackson Laboratory | Cat#002650 |
| Mouse: Lyz2cre;Gata6fl/fl | Gautier et al., 2014 | N/A |
| Mouse: Adiponectincre;Rosa26LSL.DTA | Wu et al., 2018 | N/A |
| Mouse: Wt1eGFP.cre;Rosa26LSL.DTR | This paper | N/A |
| Mouse: Wt1eGFP.cre;Rosa26LSL.mKATE2 | This paper | N/A |
| Oligonucleotides | ||
| Aldh1a1 | ThermoFisher | Cat#Mm00657317_m1 |
| Aldh1a2 | ThermoFisher | Cat#Mm00501306_m1 |
| Gapdh | ThermoFisher | Cat#Mm99999915_g1 |
| Wt1 | ThermoFisher | Cat#Mm01337048_m1 |
| Software and Algorithms | ||
| Transcript-level gene expression | Patro et al., 2017 | Salmon |
| Differential expression analysis | Law et al., 2014 | limma/voom |
| FlowJo v9.8.1 | FlowJo, LLC | www.flowjo.com |
| Prism 8.1.0 | GraphPad Software | www.graphpad.com |
| Other | ||
| Transwell inserts (0.4µM) | Costar | Cat#3413 |
Highlights:
Large cavity macrophages (LCMs) reside in peritoneal, pleural and pericardial spaces
LCMs express the retinoic-acid-responsive transcription factor GATA6
Wt1+ stromal cells generate retinoic acid and maintain LCM GATA6 expression
Genetic ablation of Wt1+ stroma elicits loss of LCM across cavity spaces
Acknowledgements:
We acknowledge members of the Turley and Randolph labs for their useful discussions and assistance in the laboratory. We thank facility staff at Genentech and Washington University in St. Louis for vivarium maintenance and core facility assistance and NWL and SL for management of sequencing files. We also express gratitude to AMB for her careful review of this manuscript. This work was supported by Genentech, NIH grants R37 AI049653 and DP1DK109668 to GJR, K99 HL138163 to JWW and T32 DK077653 to EJO.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests:
MBB, CCL, CXD, JEC, QL, WS, MRJ and SJT are employees and/or stockholders of Genentech/Roche.
References:
- Akilesh HM, Buechler MB, Duggan JM, Hahn WO, Matta B, Sun X, Gessay G, Whalen E, Mason M, Presnell SR, Elkon KB, Lacy-Hulbert A, Barnes BJ, Pepper M, Hamerman JA, 2019. Chronic TLR7 and TLR9 signaling drives anemia via differentiation of specialized hemophagocytes. Science 363, eaao5213. doi: 10.1126/science.aao5213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain CC, Hawley CA, Garner H, Scott CL, Schridde A, Steers NJ, Mack M, Joshi A, Guilliams M, Mowat AMI, Geissmann F, Jenkins SJ, 2016. Long-lived self-renewing bone marrow-derived macrophages displace embryo-derived cells to inhabit adult serous cavities. Nature Communications 7, 1–14. doi: 10.1038/ncomms11852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buechler MB, Turley SJ, 2018. A short field guide to fibroblast function in immunity. Semin Immunol 35, 48–58. doi: 10.1016/j.smim.2017.11.001 [DOI] [PubMed] [Google Scholar]
- Chau Y-Y, Bandiera R, Serrels A, Martínez-Estrada OM, Qing W, Lee M, Slight J, Thornburn A, Berry R, McHaffie S, Stimson RH, Walker BR, Chapuli RM, Schedl A, Hastie N, 2014. Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat Cell Biol 16, 367–375. doi: 10.1038/ncb2922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremasco V, Woodruff MC, Onder L, Cupovic J, Nieves-Bonilla JM, Schildberg FA, Chang J, Cremasco F, Harvey CJ, Wucherpfennig K, Ludewig B, Carroll MC, Turley SJ, 2014. B cell homeostasis and follicle confines are governed by fibroblastic reticular cells. Nat Immunol 15, 973–981. doi: 10.1038/ni.2965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies LC, Jenkins SJ, Allen JE, Taylor PR, 2013. Tissue-resident macrophages. Nat Immunol 14, 986–995. doi: 10.1038/ni.2705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher AL, Lukacs-Kornek V, Reynoso ED, Pinner SE, Bellemare-Pelletier A, Curry MS, Collier AR, Boyd RL, Turley SJ, 2010. Lymph node fibroblastic reticular cells directly present peripheral tissue antigen under steady-state and inflammatory conditions. J Exp Med 207, 689–697. doi: 10.1038/ni.1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier EL, Ivanov S, Lesnik P, Randolph GJ, 2013. Local apoptosis mediates clearance of macrophages from resolving inflammation in mice. Blood 122, 2714–2722. doi: 10.1182/blood-2013-01-478206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier EL, Ivanov S, Williams JW, Huang SCC, Marcelin G, Fairfax K, Wang PL, Francis JS, Leone P, Wilson DB, Artyomov MN, Pearce EJ, Randolph GJ, 2014. Gata6 regulates aspartoacylase expression in resident peritoneal macrophages and controls their survival. J Exp Med 211, 1525–1531. doi: 10.1016/j.cmet.2006.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier EL, Shay T, Miller J, Greter M, Ivanov S, Helft J, Chow A, Elpek KG, Gordonov S, Mazloom AR, Ma’ayan A, Chua W-J, Hansen TH, Turley SJ, Jakubzick C, Randolph GJ, Best AJ, Knell J, Goldrath A, Brown B, Merad M, Jojic V, Koller D, Cohen N, Brennan P, Brenner M, Regev A, Fletcher A, Elpek K, Bellemare-Pelletier A, Malhotra D, Turley S, Jianu R, Laidlaw D, Collins J, Narayan K, Sylvia K, Kang J, Gazit R, Garrison BS, Rossi DJ, Kim F, Rao TN, Wagers A, Shinton SA, Hardy RR, Monach P, Bezman NA, Sun JC, Kim CC, Lanier LL, Heng T, Kreslavsky T, Painter M, Ericson J, Davis S, Mathis D, Benoist C, 2012. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol 13, 1118–1128. doi: 10.1038/ni.2419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin D, Link VM, Romanoski CE, Fonseca GJ, Eichenfield DZ, Spann NJ, Stender JD, Chun HB, Garner H, Geissmann F, Glass CK, 2014. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell 159, 1327–1340. doi: 10.1016/j.cell.2014.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadix JA, Ruiz-Villalba A, Lettice L, Velecela V, Munoz-Chapuli R, Hastie ND, Perez-Pomares JM, Martinez-Estrada OM, 2011. Wt1 controls retinoic acid signalling in embryonic epicardium through transcriptional activation of Raldh2. Development 138, 1093–1097. doi: 10.1242/dev.044594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilliams M, Scott CL, 2017. Does niche competition determine the origin of tissue-resident macrophages? Nat Rev Immunol 1–10. doi: 10.1038/nri.2017.42 [DOI] [PubMed] [Google Scholar]
- Gundra UM, Girgis NM, Gonzalez MA, San Tang M, Van Der Zande HJP, Lin J-D, Ouimet M, Ma LJ, Poles J, Vozhilla N, Fisher EA, Moore KJ, Loke P, 2017. Vitamin A mediates conversion of monocyte-derived macrophages into tissue-resident macrophages during alternative activation. Nat Immunol 57, 361–15. doi: 10.1038/ni.3734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundra UM, Girgis NM, Ruckerl D, Jenkins S, Ward LN, Kurtz ZD, Wiens KE, Tang MS, Basu-Roy U, Mansukhani A, Allen JE, Loke P, 2014. Alternatively activated macrophages derived from monocytes and tissue macrophages are phenotypically and functionally distinct. Blood 123, e110–e122. doi: 10.1182/blood-2013-08-520619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldar M, Kohyama M, So AY-L, KC W, Wu X, Briseño CG, Satpathy AT, Kretzer NM, Arase H, Rajasekaran NS, Wang L, Egawa T, Igarashi K, Baltimore D, Murphy TL, Murphy KM, 2014. Heme-Mediated SPI-C Induction Promotes Monocyte Differentiation into Iron-Recycling Macrophages. Cell 156, 1223–1234. doi: 10.1016/j.cell.2014.01.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt SI, Ahrendt M, Bode U, Wahl B, Kremmer E, Förster R, Pabst O, 2008. Stromal mesenteric lymph node cells are essential for the generation of gut-homing T cells in vivo. J Exp Med 205, 2483–2490. doi: 10.1084/jem.20080039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins SJ, Rückerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, Macdonald AS, Allen JE, 2011. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science 332, 1284–1288. doi: 10.1126/science.1204351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing J, Nelson C, Paik J, Shirasaka Y, Amory JK, Isoherranen N, 2017. Physiologically Based Pharmacokinetic Model of All-trans-Retinoic Acid with Application to Cancer Populations and Drug Interactions. J. Pharmacol. Exp. Ther 361, 246–258. doi: 10.1124/jpet.117.240523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamori-Katayama M, Kaiho A, Ishizu Y, Okamura-Oho Y, Hino O, Abe M, Kishimoto T, Sekihara H, Nakamura Y, Suzuki H, Forrest ARR, Hayashizaki Y, 2011. LRRN4 and UPK3B Are markers of primary mesothelial cells. PLoS ONE 6, e25391. doi: 10.1371/journal.pone.0025391.s006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny HA, Chiang C-Y, White EA, Schryver EM, Habis M, Romero IL, Ladanyi A, Penicka CV, George J, Matlin K, Montag A, Wroblewski K, Yamada SD, Mazar AP, Bowtell D, Lengyel E, 2014. Mesothelial cells promote early ovarian cancer metastasis through fibronectin secretion. J Clin Invest 124, 4614–4628. doi: 10.1172/JCI74778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klattig J, Sierig R, Kruspe D, Makki MS, Englert C, 2007. WT1-mediated gene regulation in early urogenital ridge development. Sex Dev 1, 238–254. doi: 10.1159/000104774 [DOI] [PubMed] [Google Scholar]
- Kolodin D, van Panhuys N, Li C, Magnuson AM, Cipolletta D, Miller CM, Wagers A, Germain RN, Benoist C, Mathis D, 2015. Antigen- and cytokine-driven accumulation of regulatory T cells in visceral adipose tissue of lean mice. Cell Metabolism 21, 543–557. doi: 10.1016/j.cmet.2015.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuleshov MV, Jones MR, Rouillard AD, Fernandez NF, Duan Q, Wang Z, Koplev S, Jenkins SL, Jagodnik KM, Lachmann A, McDermott MG, Monteiro CD, Gundersen GW, Ma’ayan A, 2016. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Research 44, W90–7. doi: 10.1093/nar/gkw377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, Jung S, Amit I, 2014. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 159, 1312–1326. doi: 10.1016/j.cell.2014.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law CW, Chen Y, Shi W, Smyth GK, 2014. voom: Precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol 15, R29. doi: 10.1186/gb-2014-15-2-r29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra D, Fletcher AL, Astarita J, Lukacs-Kornek V, Tayalia P, Gonzalez SF, Elpek KG, Chang SK, Knoblich K, Hemler ME, Brenner MB, Carroll MC, Mooney DJ, Turley SJ, Immunological Genome Project Consortium, 2012. Transcriptional profiling of stroma from inflamed and resting lymph nodes defines immunological hallmarks. Nat Immunol 13, 499–510. doi: 10.1038/ni.2262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, Kadel EE, Koeppen H, Astarita JL, Cubas R, Jhunjhunwala S, Banchereau R, Yang Y, Guan Y, Chalouni C, Ziai J, Şenbabaoğlu Y, Santoro S, Sheinson D, Hung J, Giltnane JM, Pierce AA, Mesh K, Lianoglou S, Riegler J, Carano RAD, Eriksson P, Höglund M, Somarriba L, Halligan DL, van der Heijden MS, Loriot Y, Rosenberg JE, Fong L, Mellman I, Chen DS, Green M, Derleth C, Fine GD, Hegde PS, Bourgon R, Powles T, 2018. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554, 544–548. doi: 10.1038/nature25501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mass E, Ballesteros I, Farlik M, Halbritter F, Günther P, Crozet L, Jacome-Galarza CE, Händler K, Klughammer J, Kobayashi Y, Gomez-Perdiguero E, Schultze JL, Beyer M, Bock C, Geissmann F, 2016. Specification of tissue-resident macrophages during organogenesis. Science 353, aaf4238. doi: 10.1126/science.aaf4238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLarty JL, Meléndez GC, Spencer WJ, Levick SP, Brower GL, Janicki JS, 2011. Isolation of functional cardiac immune cells. JoVE doi: 10.3791/3020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar R, Knippenberg M, Goverse G, Olivier BJ, de Vos AF, O’Toole T, Mebius RE, 2011. Expression of retinaldehyde dehydrogenase enzymes in mucosal dendritic cells and gut-draining lymph node stromal cells Is controlled by dietary vitamin A. The Journal of Immunology 186, 1934–1942. doi: 10.4049/jimmunol.1001672 [DOI] [PubMed] [Google Scholar]
- Mondor I, Baratin M, Lagueyrie M, Saro L, Henri S, Gentek R, Suerinck D, Kastenmuller W, Jiang JX, Bajénoff M, 2019. Lymphatic Endothelial Cells Are Essential Components of the Subcapsular Sinus Macrophage Niche. Immunity 1–26. doi: 10.1016/j.immuni.2019.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutsaers SE, 2004. The mesothelial cell. The International Journal of Biochemistry & Cell Biology 36, 9–16. doi: 10.1016/S1357-2725(03)00242-5 [DOI] [PubMed] [Google Scholar]
- Mutsaers SE, Birnie K, Lansley S, Herrick SE, Lim C-B, Prêle CM, 2015. Mesothelial cells in tissue repair and fibrosis. Front. Pharmacol 6, 1–12. doi: 10.3389/fphar.2015.00113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe Y, Medzhitov R, 2016. Tissue biology perspective on macrophages. Nat Immunol 17, 9–17. doi: 10.1038/ni.3320 [DOI] [PubMed] [Google Scholar]
- Okabe Y, Medzhitov R, 2014. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell 157, 832–844. doi: 10.1016/j.cell.2014.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter MW, Davis S, Hardy RR, Mathis D, Benoist C, The Immunological Genome Project Consortium, 2011. Transcriptomes of the B and T Lineages Compared by Multiplatform Microarray Profiling. J Immunol 186, 3047–3057. doi: 10.4049/jimmunol.1002695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patro R, Duggal G, Love MI, Irizarry RA, Kingsford C, 2017. Salmon provides fast and bias-aware quantification of transcript expression. Nat Meth 14, 417–419. doi: 10.1038/nmeth.4197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson LW, Artis D, 2014. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol 14, 141–153. doi: 10.1038/nri3608 [DOI] [PubMed] [Google Scholar]
- Rinkevich Y, Mori T, Sahoo D, Xu P-X, Bermingham JR, Weissman IL, 2012. Identification and prospective isolation of a mesothelial precursor lineage giving rise to smooth muscle cells and fibroblasts for mammalian internal organs, and their vasculature. Nat Cell Biol 14, 1251–1260. doi: 10.1038/ncb2610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinkevich Y, Walmsley GG, Hu MS, Maan ZN, Newman AM, Drukker M, Januszyk M, Krampitz GW, Gurtner GC, Lorenz HP, Weissman IL, Longaker MT, 2015. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science 348, aaa2151. doi: 10.1126/science.aaa2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas M, Davies LC, Giles PJ, Liao C-T, Kharfan B, Stone TC, O’Donnell VB, Fraser DJ, Jones SA, Taylor PR, 2014. The transcription factor Gata6 links tissue macrophage phenotype and proliferative renewal. Science 344, 645–648. doi: 10.1126/science.1251414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C, Nobs SP, Kurrer M, Rehrauer H, Thiele C, Kopf M, 2014. Induction of the nuclear receptor PPAR-γ by the cytokine GM-CSF is critical for the differentiation of fetal monocytes into alveolar macrophages. Nat Immunol 15, 1026–1037. doi: 10.1038/ni.3005 [DOI] [PubMed] [Google Scholar]
- Sieweke MH, Allen JE, 2013. Beyond stem cells: self-renewal of differentiated macrophages. Science 342, 1242974. doi: 10.1126/science.1242974 [DOI] [PubMed] [Google Scholar]
- Soneson C, Love MI, Robinson MD, 2015. Differential analyses for RNA-seq: transcript-level estimates improve gene-level inferences. F1000Res 4, 1521. doi: 10.12688/f1000research.7563.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens SM, Gise von A, VanDusen N, Zhou B, Pu WT, 2016. Epicardium is required for cardiac seeding by yolk sac macrophages, precursors of resident macrophages of the adult heart. Developmental Biology 413, 153–159. doi: 10.1016/j.ydbio.2016.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uderhardt S, Martins AJ, Tsang JS, Lämmermann T, Germain RN, 2019. Resident Macrophages Cloak Tissue Microlesions to Prevent Neutrophil-Driven Inflammatory Damage. Cell 177, 541–555.e17. doi: 10.1016/j.cell.2019.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Kubes P, 2016. A reservoir of mature cavity macrophages that can rapidly invade visceral organs to affect tissue repair. Cell 165, 668–678. doi: 10.1016/j.cell.2016.03.009 [DOI] [PubMed] [Google Scholar]
- Wang Y, Szretter KJ, Vermi W, Gilfillan S, Rossini C, Cella M, Barrow AD, Diamond MS, Colonna M, 2012. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat Immunol 13, 753–760. doi: 10.1038/ni.2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Hutson I, Akk AM, Mascharak S, Pham CTN, Hourcade DE, Brown R, Atkinson JP, Harris CA, 2018. Contribution of adipose-derived factor D/Adipsin to complement alternative pathway activation: lessons from lipodystrophy. The Journal of Immunology 200, 2786–2797. doi: 10.4049/jimmunol.1701668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Czepielewski RS, Jarjour NN, Erlich EC, Esaulova E, Saunders BT, Grover SP, Cleuren AC, Broze GJ, Edelson BT, Mackman N, Zinselmeyer BH, Randolph GJ, 2019. Expression of factor V by resident macrophages boosts host defense in the peritoneal cavity. J Exp Med 96, jem.20182024. doi: 10.1084/jem.20182024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Ma Q, Rajagopal S, Wu SM, Domian I, Rivera-Feliciano J, Jiang D, Gise von A, Ikeda S, Chien KR, Pu WT, 2008. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature 454, 109–113. doi: 10.1038/nature07060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Franklin RA, Adler M, Jacox JB, Bailis W, Shyer JA, Flavell RA, Mayo A, Alon U, Medzhitov R, 2018. Circuit design features of a stable two-cell system. Cell 172, 1–14. doi: 10.1016/j.cell.2018.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNAseq datasets are available at the NCBI GEO under GSE130813 (FACS-sorted fibroblasts from lymph node, spleen, bone, brown adipose, inguinal adipose, omentum, mesentery, pancreas and FACS-sorted mesothelial cells from omentum, mesentery and epididymal adipose) and GSE130815 (In vitro LCM, Wt1+ stromal cells and ex vivo LCM).