Abstract
Objective
To determine the prevalence of functional gastrointestinal disorders, the demographic and clinical characteristics associated with the presence of functional gastrointestinal disorders, and the effects of these disorders with health-related quality of life (HRQOL) in a large, diverse population of persons with MS.
Methods
In 2014, we surveyed participants in the North American Research Committee on Multiple Sclerosis registry regarding functional gastrointestinal disorders using the Rome III questionnaire. Participants also reported their sociodemographic characteristics, disability status using Patient Determined Disease Steps, the presence of comorbid depression and anxiety, health behaviors, and HRQOL using the RAND-12. We determined the prevalence of each gastrointestinal disorder using the Rome III criteria. Using multivariable logistic regression models, we assessed the factors associated with the presence of each bowel disorder. Using linear regression, we evaluated the association between functional gastrointestinal disorders and HRQOL.
Results
Of 6,312 eligible respondents, 76.5% were female, with a mean (SD) age of 58.3 (10.2) years. Forty-two percent of respondents (n = 2,647) had a functional gastrointestinal disorder, most often irritable bowel syndrome (IBS), which affected 28.2% of participants. The prevalence of all functional gastrointestinal disorders increased with greater disability, and the prevalence of IBS increased with longer disease duration. After adjusting for sociodemographic and clinical characteristics, functional gastrointestinal disorders were associated with lower physical and mental HRQOL (both p < 0.0001).
Conclusions
Functional gastrointestinal disorders are common in MS and are associated with reduced HRQOL.
Functional gastrointestinal disorders are common in the general population, affecting up to 1 in 3 people.1 Functional gastrointestinal disorders are characterized by persistent symptoms referable to the middle or lower abdomen in the absence of other diseases or structural abnormalities, which account for the symptoms.2 Symptoms may include abdominal pain, diarrhea, constipation, bloating, and irregular stool. The Rome III criteria classified these disorders as irritable bowel syndrome (IBS), functional bloating, functional constipation, functional diarrhea, and functional bowel disorders not otherwise specified.3 Functional dyspepsia affects the upper gastrointestinal tract, and symptoms may include early satiety, epigastric pain, and postprandial fullness.4 These functional gastrointestinal disorders associated with reduced health-related quality of life (HRQOL), increased health care utilization and impaired work productivity.5–7
MS is a disease of the CNS, which causes a plethora of symptoms, including bowel dysfunction. Little is known about the prevalence of functional gastrointestinal disorders in MS; most previous studies have focused on IBS, suggesting that 9.40%–19.3% of persons with MS are affected.8–11 Only 1 study has reported the prevalence of other functional gastrointestinal disorders in MS,9 and the clinical and demographic characteristics associated with these disorders remain poorly understood. The contribution of comorbid functional gastrointestinal disorders to HRQOL in MS is also unknown.
Therefore, we aimed to determine the prevalence of functional gastrointestinal disorders, demographic and clinical characteristics associated with the presence of functional gastrointestinal disorders, and the effects of these disorders with HRQOL in a large, diverse population of persons with MS.
Methods
Study population
The North American Research Committee on Multiple Sclerosis (NARCOMS) registry is a self-report registry for persons with MS, which has been enrolling participants since 1996. Participants share information regarding their sociodemographic characteristics and clinical aspects of their MS at enrollment and update this information semiannually through surveys. Participants may complete questionnaires on paper or online.
Standard protocol approvals, registrations, and patient consents
Participants allow the use of their de-identified information for research purposes. At the time of the spring 2014 survey, the NARCOMS registry was approved by the Institutional Review Board of the University of Alabama at Birmingham.
Participant characteristics
For this study, we obtained information from the enrollment and spring 2014 questionnaires. We obtained date of birth, sex, race, education level, region of residence, and ages at MS symptom onset and diagnosis from the enrollment questionnaire. We categorized race as white or nonwhite and education level as <high school, high school/GED, and postsecondary (associate's degree, bachelor's degree, postgraduate education, and technical degree). From the spring 2014 questionnaire, we obtained annual household income, marital status, disability status, and health behaviors. Annual household income was reported as ≤$50,000, $15,001–$30,000, $30,001–$50,000, $50,001–$100,000, >$100,000 and “I do not wish to answer”. Marital status was categorized as single (never married, divorced, widowed, or separated) and married (married or cohabiting). Disease duration was categorized based on time from symptom onset (<10, 10–19, ≥20 years).
Participants reported disability status using Patient Determined Disease Steps (PDDS), a single-item measure with 9 potential responses ranging from 0 (normal) to 8 (bedridden). The PDDS is highly correlated with the Expanded Disability Status Scale score.12 We categorized PDDS as mild (0–1), moderate (2–4), and severe (5–8).13
With respect to health behaviors, we captured smoking status, physical activity, and alcohol intake. Using a question from the Behavioral Risk Factor Surveillance Survey, participants reported their current smoking status as No, Yes Some days, and Yes Every day; we collapsed these responses to yes/no.14 They also reported whether they had participated in any physical activity or exercise during the past month (yes/no). We captured alcohol intake using 1 question from the Alcohol Use Disorders Identification Test.15 The question was modified to ask about alcohol intake over the past 6 months instead of the past 12 months and was reported as never, monthly or less, 2 to 4 times a month, 2 to 3 times a week, or 4 or more times a week.
Gastrointestinal symptoms
In the spring 2014 survey, participants responded to 26 questions from the Rome III Diagnostic Questionnaire regarding adult functional gastrointestinal disorders aimed at identifying IBS, functional bloating, functional constipation, functional diarrhea, and functional dyspepsia.16 The Rome III criteria were the prevailing diagnostic criteria at the time the survey was conducted. Participants also reported whether a physician had ever diagnosed them (yes/no) with inflammatory bowel disease, depression, or anxiety.
Health-related quality of life
We measured HRQOL using the RAND-12, a shortened version of the RAND-36, which has been used in the MS population.17 The RAND scoring has been proposed to better assess mental health in MS than the scoring used for the Short Form-12 and Short Form-36.17 The RAND-12 generates aggregate physical component (PCS-12) and mental component (MCS-12); standardized scores range from 0 (worst HRQOL) to 100 (best HRQOL), with a mean of 50 and SD of 10.
Statistical analysis
We excluded individuals who reported inflammatory bowel disease (IBD, n = 194) from the analysis because IBD is an exclusion to diagnosing functional bowel disorder. We also excluded individuals who did not provide complete responses to the questions regarding functional gastrointestinal disorders (n = 1,415).
We summarized the characteristics of the respondents using mean (SD), median (interquartile range), and frequency (percent). We examined correlations between continuous variables using Spearman correlations. First, we report the prevalence of functional gastrointestinal disorders overall and stratified by sex, age (18–34, 35–54, and ≥55 years), and disease duration (<10, 10–19, and ≥20 years) and disability status (mild, moderate, and severe). Second, we examined factors associated with the presence or absence of functional gastrointestinal disorders using a series of binary logistic regression models. Covariates included in the models were sex, age (as defined above), disease duration (as defined above), race, educational level, income, smoking status, alcohol intake, physical activity, disability status, depression, and anxiety disorder. We used standard methods to assess model assumptions and assessed model fit using the Hosmer Lemeshow Goodness of Fit Statistic.18 We report the c-statistic for each model.
Third, we examined the association between the presence of any functional bowel disorder and HRQOL (PCS-12 and MCS-12) using multivariable linear regression, adjusting for all the covariates described above. Model assumptions were assessed using standard methods. Statistical analyses were conducted using SAS V9.4 (SAS Institute Inc., Cary, NC).
Data availability
The data sets generated and analyzed during the current study are held by the NARCOMS registry (narcoms.org).
Results
Participants
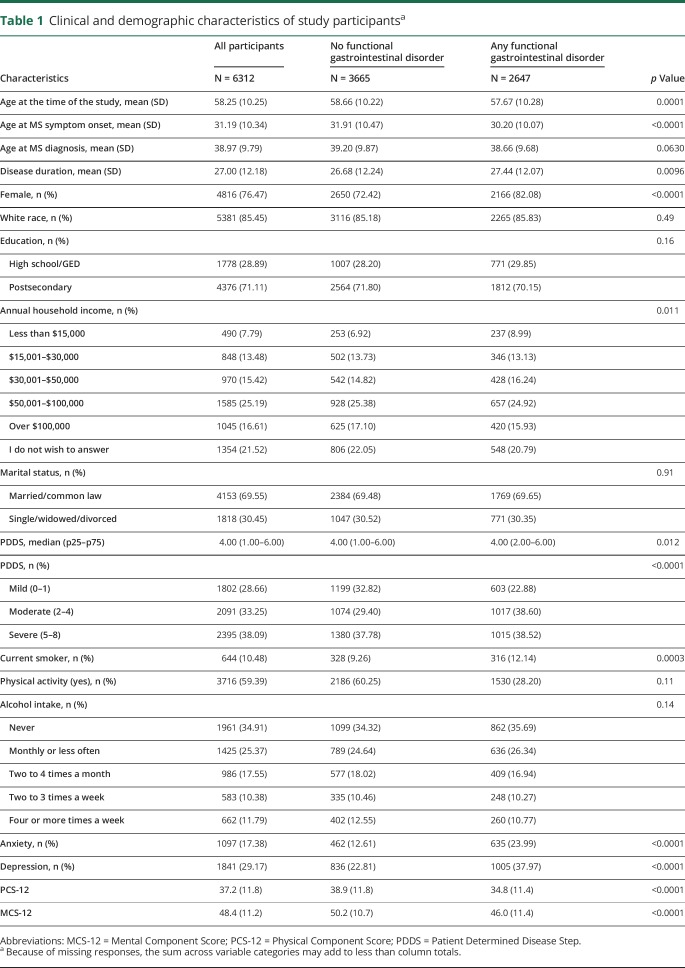
Of 13,609 participants to whom the survey was sent, 7,921 (58.2%) responded. Compared with responders, nonresponders were younger at MS symptom onset (mean [SD] 30.30 [10.4] vs 31.1 [9.8], p < 0.0001), more likely to be nonwhite (1505 [26.4%] vs 1131 [14.3%], p < 0.0001), and were more likely to have attained ≤high school education (1893 [34.3%] vs 2304 [29.9%], p < 0.0001). Sex and disability status at enrollment did not differ between responders and nonresponders. After applying the exclusion criteria, 6,312 of the responders were eligible for the analyses. Participants who were eligible were less likely to be female, more likely to have attained more than a high school education, and had a lower level of disability than those who were ineligible (all p < 0.0001).
Over three-quarters of participants were women, most were white, of mean (SD) age 58.2 (10.2) years at the time of the survey (table 1). Age was moderately correlated with disease duration (r = 0.56; 95% confidence interval [CI]: 0.55, 0.58), but more weakly correlated with disability status (r = 0.34; 95% CI: 0.32, 0.36). Disability and disease duration were weakly correlated (r = 0.34; 95% CI: 0.32, 0.36).
Table 1.
Clinical and demographic characteristics of study participantsa
Functional gastrointestinal disorders
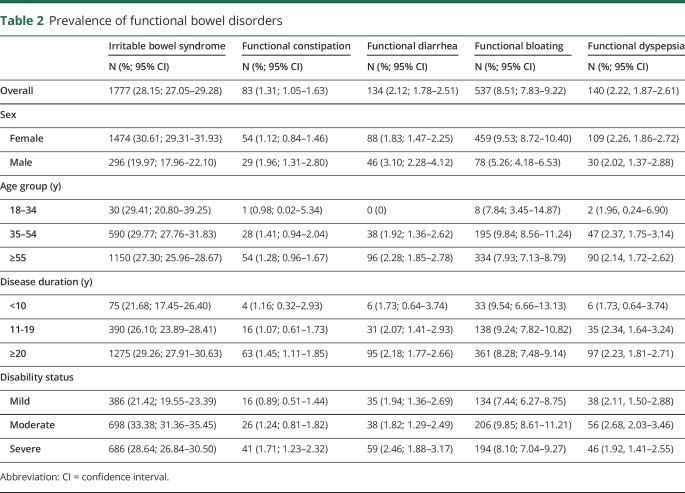
Overall, 2,647 participants (41.9%) met the criteria for a functional bowel disorder. Compared with participants with no functional bowel disorder, those with any disorder were younger, had an earlier age at symptom onset, were more like to be female, to have a lower annual household income, more disability, be more likely to smoke, and more likely to report a diagnosis of anxiety or depression (table 2). Functional bloating affected 8.5% of participants, whereas functional constipation and diarrhea were uncommon. Stratified analyses indicated that women had a higher prevalence of IBS or functional bloating than men, whereas men had a higher prevalence of functional constipation or diarrhea than women. The prevalence of each disorder consistently increased with disease duration and was higher in participants with moderate or severe disability than those with mild disability.
Table 2.
Prevalence of functional bowel disorders
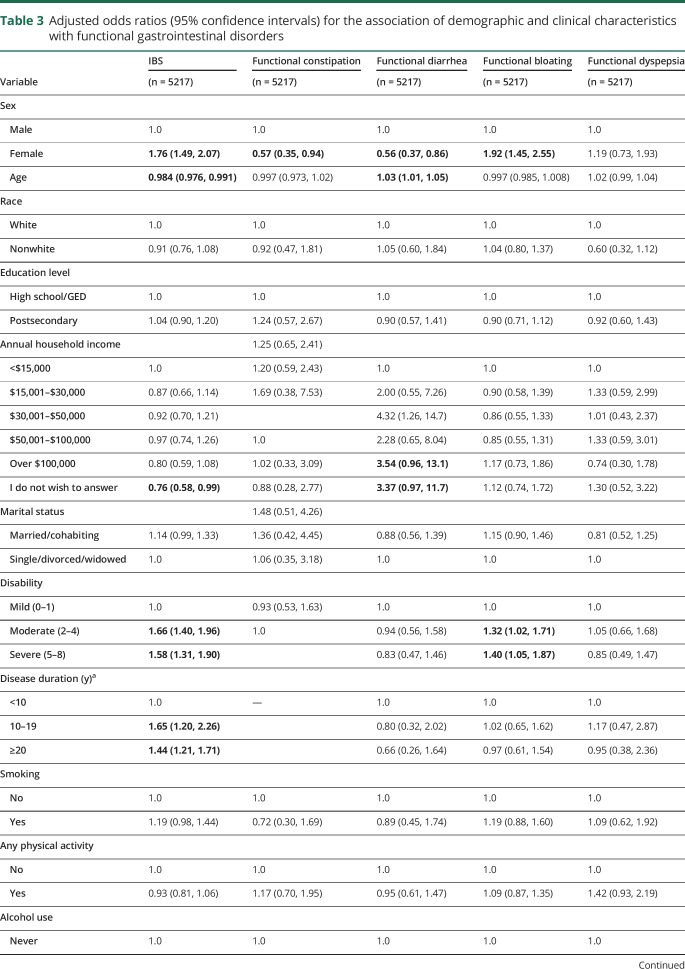
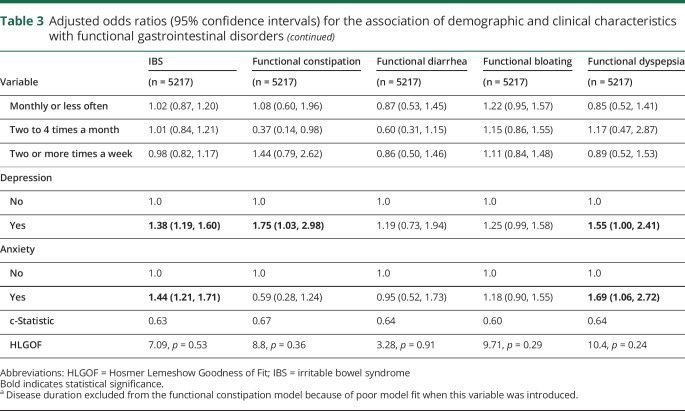
On multivariable analysis, women had increased odds of IBS and functional bloating but decreased odds of functional constipation or diarrhea. Older age was associated with reduced odds of IBS, but increased odds of functional diarrhea. Longer disease duration was associated with increased odds of IBS, but was not associated with the odds of the other functional gastrointestinal disorders. Severe, compared with mild, disability was associated with increased odds of IBS, functional diarrhea, and functional bloating. Depression was associated with increased odds of IBS, functional diarrhea, and functional dyspepsia. Anxiety was associated with increased odds of IBS and functional dyspepsia. Race, level of education, marital status, smoking status, physical activity, and amount of alcohol intake were not associated with increased odds of having any of the functional gastrointestinal disorders (table 3).
Table 3.
Adjusted odds ratios (95% confidence intervals) for the association of demographic and clinical characteristics with functional gastrointestinal disorders
Health-related quality of life
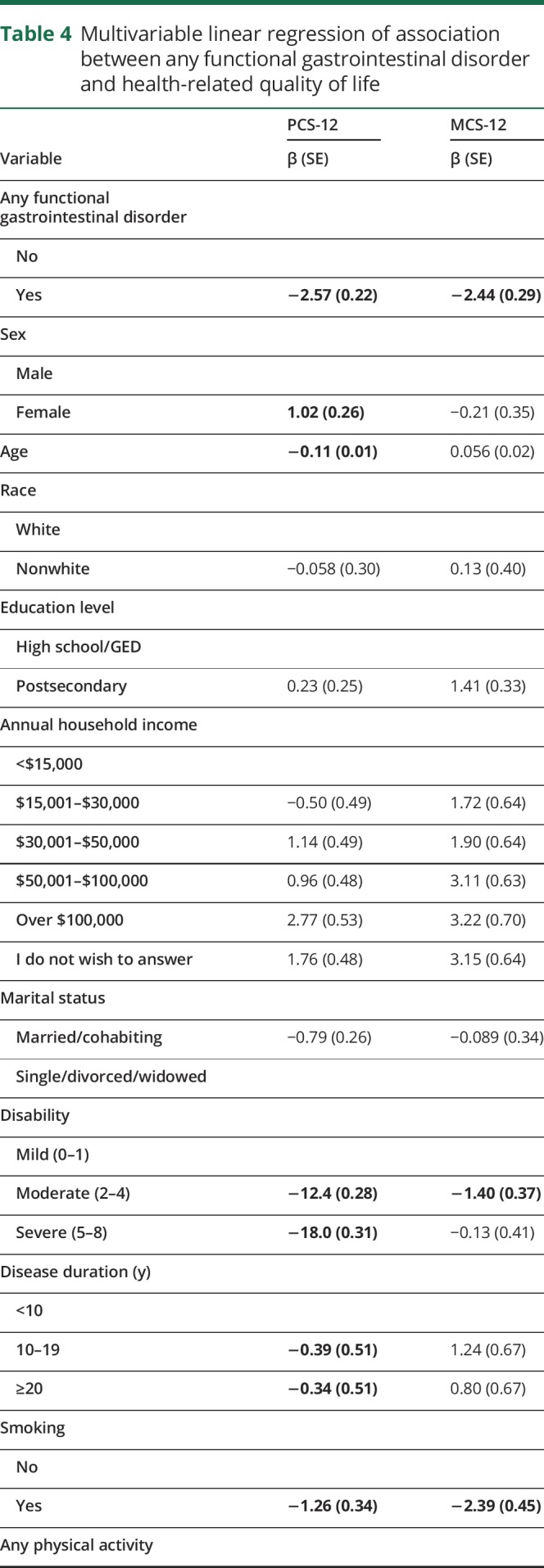
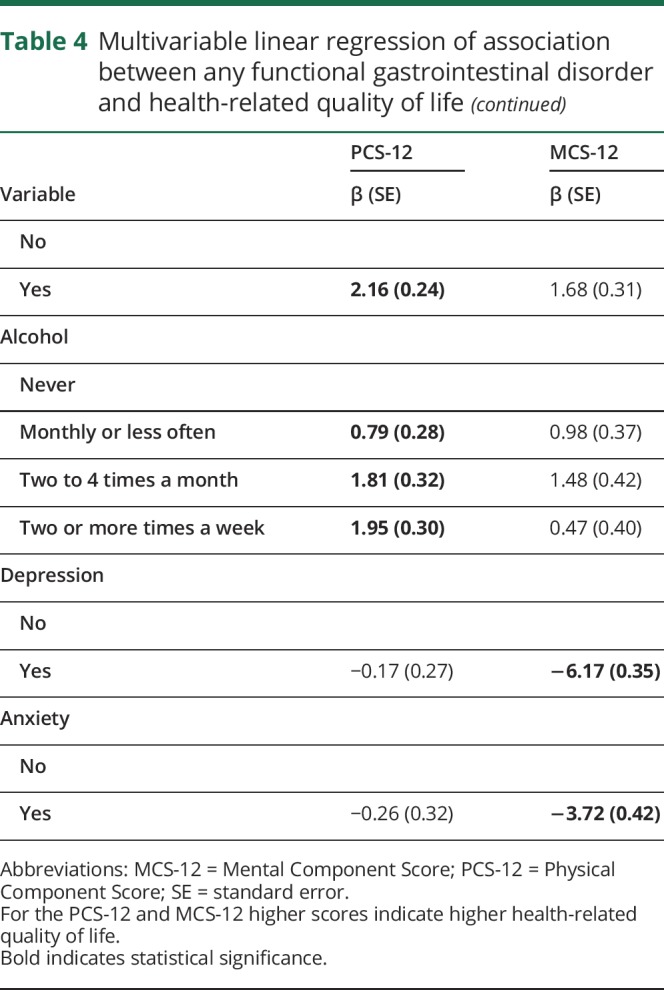
After adjustment for sociodemographic and clinical characteristics and health behaviors, participants with any functional gastrointestinal disorder reported lower physical HRQOL on average [SD] (PCS-12 35.5 [0.30]) than participants without a functional gastrointestinal disorder (38.1 [0.29], p < 0.0001, table 4). Similarly, participants with any functional gastrointestinal disorder reported lower mental HRQOL (42.9 [0.40]) than those without a functional gastrointestinal disorder (45.4 [0.38], p < 0.0001, table 4).
Table 4.
Multivariable linear regression of association between any functional gastrointestinal disorder and health-related quality of life
Discussion
In this large cross-sectional study, we found that 2 in 5 participants with MS had a functional gastrointestinal disorder, most often IBS, followed by functional bloating. In North America, the most common functional gastrointestinal disorder is IBS, which is estimated to affect 12% of the general population.19 This estimate is substantially lower than we observed. Only a few studies have examined the prevalence of IBS among persons with MS.8–11 These studies used a variety of methods including surveys and administrative data. The sole population-based study relied on administrative data and found a prevalence of 12.2%,11 which was nearly two-fold higher than in age, sex, and geographically matched controls. However, the administrative definition was insensitive (39.1%) despite high specificity (96.6%) and also would only capture individuals who sought medical care for their symptoms, potentially underestimating the prevalence of IBS. Another study, conducted by Levinthal et al.,9 also examined the prevalence of other functional gastrointestinal disorders among 218 persons with MS using the Rome III questionnaire. In that study, 19.3% of participants had IBS, over 30% had functional constipation, and >15% had functional dyspepsia; the prevalence of functional diarrhea was not reported. These estimates differ from ours, identifying a lower prevalence of IBS, but higher prevalence of the other functional gastrointestinal disorders. The reason for these differences is uncertain but may reflect differences in the characteristics of the study populations. On average, our sample was 10 years older, with a disease duration that was about 14 years longer than the sample in the Levinthal study. Disability status may have differed as well, but the disability measures used in the 2 studies are not directly comparable. These differences are relevant, given that age, disability, and disease duration influenced the prevalence of functional gastrointestinal disorders in our sample.
Female sex was associated with a higher prevalence of IBS, and functional bloating, but a lower prevalence of functional constipation and diarrhea. Our findings regarding female sex and functional constipation and diarrhea should be viewed with caution, given the small number of participants affected. Greater disability and disease duration were also associated with a higher prevalence of IBS, whereas comorbid anxiety and depression were associated with a higher prevalence of most of the functional gastrointestinal disorders. Women are recognized to be at increased risk of IBS.20 In the previous study by Levinthal et al.,9 greater severity of gastrointestinal symptoms was associated with greater impairment of physical function as assessed by the MSIS-29, which would be consistent with our findings regarding disability. In systemic lupus erythematosus, the prevalence of functional gastrointestinal disorders has also been reported to be higher with longer disease duration. Depression and anxiety disorders are associated with functional gastrointestinal disorders in the general population21 and may precede or follow the development of gastrointestinal symptoms.22 Treatment of psychiatric comorbidities in functional gastrointestinal disorders is associated with reduced severity of gastrointestinal symptoms.23
The presence of a comorbid functional gastrointestinal disorder was associated with reduced HRQOL, although the effects were modest, with effect sizes at the minimum threshold for clinical importance (0.2).24 Although we were unable to identify other studies that evaluated the effects of functional gastrointestinal disorders on HRQOL in MS, some studies have evaluated the effect of bowel symptoms on HRQOL. In a study of 56 persons with MS from Hordaland County, Norway, the presence of bowel symptoms including flatus, constipation, and incontinence was associated with lower HRQOL scores as measured using the Short Form-36.25,26 Similarly, a study of 223 persons with MS found that more severe bowel symptoms, as assessed using the Bowel Control Scale, were correlated with lower physical and mental HRQOL as measured using the Short Form-36.25 Satisfaction with treatment of bowel symptoms is modest, but treatment of such symptoms has been reported to improve HRQOL,27 emphasizing the clinical importance of recognizing and addressing gastrointestinal symptoms and disorders.
The reasons for the high prevalence of functional gastrointestinal disorders in MS are unknown, but several reasons can be postulated. First, MS-related immune dysregulation may play a role. A case-control study of 23,471 persons captured in the Health Improvement Network database found that the prevalence of any autoimmune disease was higher among persons with a functional gastrointestinal disorder (odds ratio [OR] 1.35; 95% CI: 1.12–1.63).28 The odds of any immune-mediated rheumatologic disorder were higher among persons with functional dyspepsia (OR 1.44; 95% CI: 1.15–1.80). In a study of 86 persons with systemic lupus erythematosus, 76.7% had at least 1 functional gastrointestinal disorder, most often functional dyspepsia.29 Several studies suggest that immune dysregulation and inflammation may underlie these disorders in some cases. In IBS, altered cytokine profiles and T-cell population and increased immune activation30,31 are recognized. Altered immune activation has also been reported in functional dyspepsia.32 Second, psychiatric comorbidity may increase the risk of functional gastrointestinal disorders. The prevalence of depression and anxiety disorders is higher in the MS population than in the general population.33 Also, depression and anxiety disorders are associated with functional gastrointestinal disorders in the general population, which is postulated to reflect dysregulation of the gut-brain axis.34
We acknowledge several study limitations. Study participants are volunteers, and the response rate was 58%, which is slightly lower than the mean response rate of 60% in the medical literature.35 Responders differed with respect to race and educational status from nonresponders, and responders who were eligible for inclusion differed from those with incomplete responses with respect to sex (less likely to be female), education (higher education level), and disability at enrollment (less disability). Thus, our findings may not be representative of the entire MS population. Specifically, those eligible for the analysis had a lower proportion of the characteristics that are positively associated with functional gastrointestinal disorders, which could reduce the estimated prevalence compared with the larger MS population. Although our sample averaged 58 years old, this falls within the range of the peak age-specific prevalence of MS in the United States.36 Like several other studies, we relied on a questionnaire to establish diagnoses of functional gastrointestinal disorders. Although we excluded self-reported inflammatory bowel disease, participants may have had other underlying disorders that could account for their gastrointestinal symptoms; thus, our estimates of functional gastrointestinal disorders may be slightly elevated. Strengths of the study included the large sample size and evaluation of the association of a broad range of demographic factors, health behaviors, and clinical characteristics with functional gastrointestinal disorders.
Our findings indicate that functional gastrointestinal disorders are common in MS and increase in frequency with increasing disability and disease duration. The association of anxiety and depression with functional gastrointestinal disorders suggests that effectively diagnosing and treatment psychiatric comorbidities may reduce the burden of functional gastrointestinal disorders. These disorders are associated with reduced HRQOL, suggesting that improved management of these disorders may offer a means of improving HRQOL in persons with MS.
Acknowledgment
NARCOMS is a project of the Consortium of Multiple Sclerosis Centers (CMSC). NARCOMS is funded in part by the CMSC and the Foundation of the CMSC. The study was also supported in part by the Waugh Family Chair in Multiple Sclerosis and Research Manitoba Chair (to RAM). The funding source(s) had no role in the study design, collection, analysis or interpretation of the data, or in the decision to submit the article for publication.
Appendix. Authors
Study funding
NARCOMS is supported in part by the Consortium of Multiple Sclerosis Centers (CMSC) and The Foundation of the CMSC. Ruth Ann Marrie is supported by the Waugh Family Chair in Multiple Sclerosis and a Manitoba Research Chair from Research Manitoba.
Disclosure
T. Tyry and A. Salter report no disclosures. R. Fox receives consultant fees from Actelion, Biogen, Genentech, Novartis, and Teva. He has served on advisory committees of Biogen and Novartis. He also receives research support from Biogen (clinical trial contracts) and Novartis (research study support). G. Cutter serves on Data and Safety Monitoring Boards for AMO Pharmaceuticals, Apotek, Gilead Pharmaceuticals, Horizon Pharmaceuticals, Modigenetech/Prolor, Merck, Merck/Pfizer, Opko Biologics, Neurim, Sanofi-Aventis, Reata Pharmaceuticals, Receptos/Celgene, Teva Pharmaceuticals, NHLBI (Protocol Review Committee), and NICHD (OPRU oversight committee). He also serves on consulting or advisory boards of Atara Biotherapeutics, Bioeq GmBH, Cerespir Inc, Consortium of MS Centers (grant), Genzyme, Genentech, Innate Therapeutics, Janssen Pharmaceuticals, Klein-Buendel Incorporated, MedImmune, Medday, Nivalis, Novartis, Opexa Therapeutics, Roche, Savara Inc., Somahlution, Teva Pharmaceuticals, Transparency Life Sciences, and TG Therapeutics. R. A. Marrie receives research funding from the CIHR, the National MS Society, the MS Society of Canada, the MS Scientific Research Foundation, Research Manitoba, the Consortium of MS Centers, Crohn's and Colitis Canada, and the Waugh Family Chair in Multiple Sclerosis. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Koloski NA, Talley NJ, Boyce PM. Epidemiology and health care seeking in the functional GI disorders: a population-based study. Am J Gastroenterol 2002;97:2290–2299. [DOI] [PubMed] [Google Scholar]
- 2.Häuser W, Layer P, Henningsen P, Kruis W. Functional bowel disorders in adults. Dtsch Arztebl Int 2012;109:83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology 2006;130:1480–1491. [DOI] [PubMed] [Google Scholar]
- 4.Talley NJ, Goodsall T, Potter M. Functional dyspepsia. Aust Prescr 2017;40:209–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dean BB, Aguilar D, Barghout V, et al. Impairment in work productivity and health-related quality of life in patients with IBS. Am J Manag Care 2005;11:S17–S26. [PubMed] [Google Scholar]
- 6.Paré P, Gray J, Lam S, et al. Health-related quality of life, work productivity, and health care resource utilization of subjects with irritable bowel syndrome: baseline results from LOGIC (Longitudinal Outcomes Study of Gastrointestinal Symptoms in Canada), a naturalistic study. Clin Ther 2006;28:1726–1731. [DOI] [PubMed] [Google Scholar]
- 7.Longstreth GF, Wilson A, Knight K, et al. Irritable bowel syndrome, health care use, and costs: a U.S. managed care perspective. Am J Gastroenterol 2003;98:600–607. [DOI] [PubMed] [Google Scholar]
- 8.Horton M, Rudick RA, Hara-Cleaver C, Marrie RA. Validation of a self-report comorbidity questionnaire for multiple sclerosis. Neuroepidemiology 2010;35:83–90. [DOI] [PubMed] [Google Scholar]
- 9.Levinthal DJ, Rahman A, Nusrat S, O'Leary M, Heyman R, Bielefeldt K. Adding to the burden: gastrointestinal symptoms and syndromes in multiple sclerosis. Mult Scler Int 2013;2013:319201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marrie R, Horwitz R, Cutter G, Tyry T, Campagnolo D, Vollmer T. Comorbidity, socioeconomic status and multiple sclerosis. Mult Scler 2008;14:1091–1098. [DOI] [PubMed] [Google Scholar]
- 11.Marrie RA, Yu BN, Leung S, et al. The utility of administrative data for surveillance of comorbidity in multiple sclerosis: a validation study. Neuroepidemiology 2013;40:85–92. [DOI] [PubMed] [Google Scholar]
- 12.Marrie RA, Goldman MD. Validity of performance scales for disability assessment in multiple sclerosis. Mult Scler 2007;13:1176–1182. [DOI] [PubMed] [Google Scholar]
- 13.Marrie RA, Salter A, Tyry T, Cutter GR, Cofield S, Fox RJ. High hypothetical interest in physician-assisted death in multiple sclerosis. Neurology 2017;88:1528–1534. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System Survey Questionnaire. Atlanta, Georgia: U.S. Department of Health and Human Services; 1995. [Google Scholar]
- 15.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med 1998;158:1789–1795. [DOI] [PubMed] [Google Scholar]
- 16.Whitehead WE, Validation working team committee in association with the Rome questionnaire committee. Development and validation of the Rome Iii diagnostic questionnaire. In: Drossman DA, editor, Rome Iii: The Functional Gastrointestinal Disorders, 3rd ed McLean: Degnon Associates Inc.; 2006:835–853. [Google Scholar]
- 17.Nortvedt MW, Riise T, Myhr KM, Nyland HI. Performance of the SF-36, SF-12, and RAND-36 summary scales in a multiple sclerosis population. Med Care 2000;38:1022–1028. [DOI] [PubMed] [Google Scholar]
- 18.Hosmer DW, Lemeshow S. Applied Logistic Regression. New York: John Wiley & Sons; 1989. [Google Scholar]
- 19.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol 2012;10:712–e4. [DOI] [PubMed] [Google Scholar]
- 20.Lovell RM, Ford AC. Effect of gender on prevalence of irritable bowel syndrome in the community: systematic review and meta-analysis. Am J Gastroenterol 2012;107:991–1000. [DOI] [PubMed] [Google Scholar]
- 21.Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA 2015;313:949–958. [DOI] [PubMed] [Google Scholar]
- 22.Jones MP, Tack J, Van Oudenhove L, et al. Mood and anxiety disorders precede development of functional gastrointestinal disorders in patients but not in the population. Clin Gastroenterol Hepatol 2017;15:1014–e4. [DOI] [PubMed] [Google Scholar]
- 23.Stasi C, Caserta A, Nisita C, et al. The complex Interplay between gastrointestinal and psychiatric symptoms in irritable bowel syndrome: a longitudinal assessment. J Gastroenterol Hepatol 2019;34:713–719. [DOI] [PubMed] [Google Scholar]
- 24.Samsa G, Edelman D, Rothman ML, Williams GR, Lipscomb J, Matchar D. Determining clinically important differences in health status measures: a general approach with illustration to the health utilities Index mark Ii. Pharmacoeconomics 1999;15:141–155. [DOI] [PubMed] [Google Scholar]
- 25.Vitkova M, Rosenberger J, Krokavcova M, et al. Health-related quality of life in multiple sclerosis patients with bladder, bowel and sexual dysfunction. Disabil Rehabil 2014;36:987–992. [DOI] [PubMed] [Google Scholar]
- 26.Nortvedt MW, Riise T, Frugård J, et al. Prevalence of bladder, bowel and sexual problems among multiple sclerosis patients two to five years after diagnosis. Mult Scler 2007;13:106–112. [DOI] [PubMed] [Google Scholar]
- 27.Wang G, Marrie RA, Fox RJ, et al. Treatment satisfaction and bothersome bladder, bowel, sexual symptoms in multiple sclerosis. Mult Scler Relat Disord 2018;20:16–21. [DOI] [PubMed] [Google Scholar]
- 28.Ford AC, Talley NJ, Walker MM, Jones MP. Increased prevalence of autoimmune diseases in functional gastrointestinal disorders: case–control study of 23 471 primary care patients. Aliment Pharmacol Ther 2014;40:827–834. [DOI] [PubMed] [Google Scholar]
- 29.García-Carrasco M, Mendoza-Pinto C, Autrán-Limón MA, et al. Prevalence of functional gastrointestinal disorders in adults with systemic lupus erythematosus. Lupus 2018;27:788–793. [DOI] [PubMed] [Google Scholar]
- 30.Liebregts T, Adam B, Bredack C, et al. Immune activation in patients with irritable bowel syndrome. Gastroenterology 2007;132:913–920. [DOI] [PubMed] [Google Scholar]
- 31.Bashashati M, Rezaei N, Andrews CN, et al. Cytokines and irritable bowel syndrome: where do we stand? Cytokine 2012;57:201–209. [DOI] [PubMed] [Google Scholar]
- 32.Burns G, Carroll G, Mathe A, et al. Evidence for local and systemic immune activation in functional dyspepsia and the irritable bowel syndrome: a systematic review. Am J Gastroenterol Epub 2018 Nov 14. [DOI] [PubMed]
- 33.Marrie RA, Fisk JD, Tremlett H, et al. Differences in the burden of psychiatric comorbidity in MS vs the general population. Neurology 2015;85:1972–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moser G, Fournier C, Peter J. Intestinal microbiome-gut-brain axis and irritable bowel syndrome. Wien Med Wochenschr 2018;168:62–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Asch DA, Jedrziewski MK, Christakis NA. Response rates to mail surveys published in medical journals. J Clin Epidemiol 1997;50:1129–1136. [DOI] [PubMed] [Google Scholar]
- 36.Wallin MT, Culpepper WJ, Campbell JD, et al. The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology 92:e1029–e1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated and analyzed during the current study are held by the NARCOMS registry (narcoms.org).