Abstract
Background:
Evidence supports the notion that early-life stress and trauma impacts cortical development and increases vulnerability to depression. However, it remains unclear whether common stressful life events in community-dwelling adolescents has similar consequences for cortical development.
Methods:
232 adolescent females (mean age 15.29±0.65 years) were assessed with the Stressful Life Events Schedule (SLES; a semi-structured interview of stressors in the previous 9 months) and underwent an MRI scan. FreeSurfer 5.3.0 was utilized to perform whole-brain surface-based morphometry. Dysphoria was assessed at time of imaging and prospectively at three 9-month follow-ups using the Inventory of Depression and Anxiety Symptoms (IDAS-II).
Results:
90% of girls reported at least 1 stressful life event in the 9 months prior to imaging. Greater burden of recent life stress was associated with smaller left precuneus and left post-central cortical thickness, and left superior frontal and right inferior parietal volume (all p<0.05 after multiple comparisons correction). Furthermore, left precuneus thickness in the stress-associated cluster significantly predicted dysphoria for 27-months after imaging controlling for prior dysphoria (β=−0.11, p=0.004). Left precuneus cortical thickness accounted for 17.0% of the association between stress and dysphoric mood for 27 months following imaging (β=0.04, p=0.05).
Conclusions:
Consistent with evidence from imaging studies of trauma-exposed youth and preclinical stress models, heavy burden of recent common life stress in community-dwelling adolescent girls was associated with altered frontal/parietal cortical morphology. Stress-linked precuneus cortical thickness represents a candidate prospective biomarker of adolescent depression.
Keywords: adolescent development, life stress, depression, cortical thickness, volume, dysphoric mood
INTRODUCTION
Stress-induced changes in cognitive and biological processes are central to leading paradigms of depression(1), (e.g. diathesis-stress, stress generation, and learned helplessness(2–4)). In such models, stress exposure is internalized within the central nervous system, shaping development deleteriously, thereby increasing risk for and maintenance of depressive symptoms. The phenotypic link between stress and adolescent depression is well supported; life stress potently predicts depressive symptoms, such as dysphoria and onset of depressive disorders(5). However, progress in identifying the neurobiological mechanisms linking stress exposure to course of adolescent depression has been slow, likely hindering the development of targeted prevention and treatment programs. Identifying and understanding the neurobiological vectors along which stress increases vulnerability for depression represents a key target for the next generation of translational research.
Two prominent hypotheses have been proposed to account for stress-induced neurodevelopmental adaptations. The neurotoxicity hypothesis(6), or the glucocorticoid cascade hypothesis(7), is based largely on preclinical investigations that utilized severe, chronic stress models to track inhibition of dendritic arborization and glucocorticoid-induced synaptic loss and atrophy(8, 9). This has been reported in the hippocampus, as well as amygdala and prefrontal cortex(6, 8, 9). The stress acceleration hypothesis(10) posits that stress-related grey matter (GM) loss represents accelerated maturation, especially in circuits implicated in emotion processing(10, 11). Neural sensitivity to stress then varies as a function of the linear and nonlinear negative trajectories in GM volume, thickness, and surface area across the cerebral cortex during development, which may reflect, for example, experience-based synaptic pruning and expansion of cerebral white matter due to axonal myelination(12–16).
Such hypotheses were largely developed from preclinical studies, which benefit from experimentally-controlled models of stress exposure. In humans, laboratory stress experiments are necessarily brief (e.g. public speaking evaluation(17)). Such stress inductions in humans increase cortisol production, which helps bridge human studies with preclinical stress models(18, 19). However, laboratory studies have limited utility for investigating long-term consequences of stress exposure on neural development in humans. An alternative approach is to combine neuroimaging with careful phenotyping of naturally-occurring stressors(20). In adults, several studies link reduced GM volume in frontal and temporal lobes and the hippocampus with early life stress, chronic life stress, and recent stressful life events(21–23). In youth, imaging studies report that exposure to severe or extreme life events, such as trauma, neglect, or onset of post-traumatic stress disorder (PTSD), is associated with reduced GM volume, particularly in frontal and temporal lobes(24–30). One study in children found that cumulative early life stress occurring more than one year before imaging was associated with smaller prefrontal, temporal, and precuneus volumes(31). Altogether, exposure to extreme and/or distal stressors, such as trauma or higher burden of cumulative early life stress, appears sufficient to alter GM maturation, particularly in cognitive-affective substrates necessary for healthy adaptation(24, 31).
Middle-to-late adolescence is notable for increased autonomy and exposure to common stressful life events (e.g. dissolution of relationships, financial struggles, and health problems)(32). That the predominance of structural imaging studies in youth have focused on extreme or traumatic stressors limits generalizability to the general population of adolescents, many of whom are exposed to common stressors. Thus, a critical gap in the literature is whether common stressful life events are sufficient to alter ongoing neuromaturation. This developmental period is also associated with increases in depression symptoms, especially in girls, where the rate of depressive disorders reaches 2:1 relative to boys(33). Imaging studies have reported several GM correlates of depression in adults and adolescents, especially in the superior frontal and parietal cortices and hippocampus(34–37). Of note, these imaging studies did not assess the mediative role of stress exposure on cortical structure. One study in adolescent females linked early life stress to accelerated pituitary gland development, but this was unrelated to depressive symptoms(38). Thus, a second critical gap in the literature is lack of integration of stress-linked alterations in cortical morphology with depression in youth.
The first aim of this study was to assess the relationship between recent stressful life events and cortical structure in a community-dwelling sample of 232 girls with the Stressful Life Events Schedule (SLES) and surface-based morphometry. The second aim of this study was to test the link between stress-sensitive cortical markers and dysphoric mood for up to 27 months post-imaging. Then, we characterized the mediative role of stress-linked regions in accounting for the depressogenic effects of stress.
METHODS and MATERIALS
Participants
Participants were a subset of the multi-wave Adolescent Development of Emotions and Personality Traits (ADEPT; R01MH093479) study at Stony Brook University. Exclusion criteria at Wave 1 included intellectual disability, inability to complete questionnaires, lack of English fluency, lack of a biological parent consenting, and a lifetime history of Depressive Disorders (DD; e.g., major depressive disorder (MDD) or Dysthymia). The aim of the ADEPT project was to identify predictors and consequences of first-onset DD. Other psychopathologies are well-known predictors of first-onset DD(39–41) and thus, were not excluded. Absence of lifetime DD was confirmed first by phone screen (depression module of the Patient Health Questionnaire; PHQ-9(42)) and then in-person (Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children, Present and Lifetime Version; K-SADS-PL(43)). Adolescents provided written assent and biological parents provided written permission. The study was approved by Stony Brook University’s Committee on Research Involving Human Subjects.
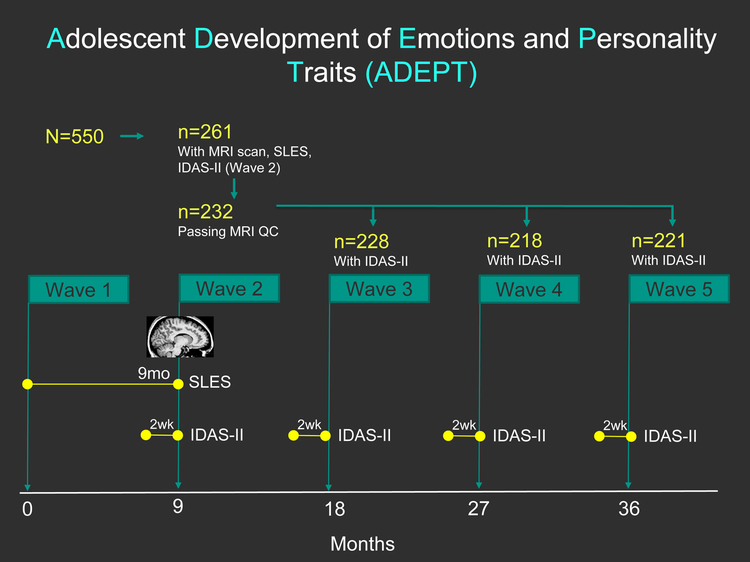
ADEPT enrolled 550 females (13.5–15.5 years-old) and included 5 assessment Waves at 9-month intervals (Figure 1). A single MRI session was funded proximal to Wave 2 (R01MH093479-S1). All participants were invited for imaging, yielding a sample of 261 (reduction in sample due to refusal, attrition, and contraindications (e.g. braces, claustrophobia)).
Figure 1 Caption:
Study timeline for the Adolescent Development of Emotions and Personality Traits (ADEPT) as relevant to this analysis. Abbreviations: SLES: Stressful Life Events Schedule, IDAS-II: Inventory of Depressive and Anxiety Symptoms II. MRI: magnetic resonance imaging, QC: quality control.
Clinical Measures
The SLES, a semi-structured interview designed for adolescents(44), was administered at Wave 2. Trained interviewers asked about exposure to 77 stressful events “since we last saw you”, which spanned from Wave 1 to 2 (Figure 1). Descriptions of endorsed events, dates of onset and offset, real-life impact, and exposure frequency were recorded using uniform SLES-provided probes. A consensus team of trained interviewers reviewed each endorsed event and assigned an objective threat rating from 1 (‘little to no effect’) to 4 (‘great effect’) per SLES-provided anchors to ensure uniformity and generalizability(44). Each objective threat rating was squared to provide larger weight to more impactful/severe events and then summed into a total SLES score(45). This procedure shows excellent test-retest reliability (ICC = 0.93 in the range of 5–15 days apart(44)).
The 99-item, self-report Inventory of Depressive and Anxiety Symptoms (IDAS-II)(46), which contains 18 factor-analytically-derived scales, was completed at Waves 1–5 (Figure 1). Analysis focused on IDAS-II Dysphoria given a priori interest in the core emotional and cognitive symptoms of depression. This 10-item scale is comprised of items, such as “I felt depression” and “I blamed myself for things”, that are rated from 1 (Not at all) to 5 (Extremely) based on how the participant “felt or experienced things during the past two weeks, including today” and then summed(46, 47). Ipsative mean imputation was used if ≥9 items were endorsed. The IDAS-II displays excellent 1-week test-retest reliability (r = 0.75 to 0.84(48)).
Due to disapproval during MRI inspection(49) (details included in Image Acquisition and Processing), 29 girls were removed yielding N=232 participants for analysis. Retention at Waves 3–5 was excellent (n=228, n=218, n=221, respectively). Information on temporal sequencing of the IDAS-II, SLES, and MRI at Wave 2 is provided in supplemental material.
Image Acquisition and Processing
T1w structural MP-RAGE images were obtained at Stony Brook University on a 3T Siemens TRIO Tim (TR/TE=1900/2.53ms, FOV=350×263×350mm, IPAT factor 2, Flip angle=9°, slice oversampling=18.2%, voxel resolution=1mm3 isotropic, and duration=4:30min). A validated manual inspection quality control procedure(49) excluded 29 participants due to poor FreeSurfer segmentation (total N=232 approved MRIs). In brief, FreeSurfer 5.3.0’s standard, automated cortical reconstruction pipeline (http://surfer.nmr.mgh.harvard.edu/) was used to generate surface models on a Linux-based computing cluster. The surface models were inflated, registered to a spherical surface atlas(50), and underwent a systematic inspection process(49). The pial and white matter surface models, overlaid on the T1w image, were inspected for fidelity to visible tissue class boundaries. Cases where inaccurate tissue delineation persisted for ≥6 consecutive coronal and axial slices were deemed inaccurate and disapproved. All technicians were blinded. This validated QC procedure was shown to significantly boost reliability of structural metrics (ICC=0.81 with approved scans relative to ICC=0.75 with the whole sample) and thus, statistical power (49).
Statistics: Relationship between Brain Structure and Stress
Surface-based morphometry (SBM) analyses were performed in FreeSurfer. Cortical thickness, volume, and surface area maps were registered to a common spherical atlas and smoothed with a 10mm Gaussian kernel(50). General linear models were used to examine the vertex-wise correlations with total SLES, controlling for age. Right and left hemispheres were examined separately. Monte Carlo Null-Z simulation cluster analyses with 10,000 iterations and cluster-forming threshold of p<0.001 were used to correct for multiple comparisons (“mri_glmfit-sim”)(51, 52). In short, the family-wise error significance threshold was set at p<0.05 and through a combination of probability and cluster-size thresholding, cluster-wise probability p-values were obtained for resulting clusters(53). The Freesurfer flag “−−2spaces” was additionally used to account for left and right hemispheres. Clusters remaining significant after multiple comparisons correction were defined per the Desikan-Killiany atlas(54). Unadjusted average volume, cortical thickness, or surface area cluster values were extracted if the cluster survived multiple comparisons correction.
Region-wise subcortical volumes were examined separately from Freesurfer’s automatic subcortical segmentation(55). Nine unilateral regions were tested: accumbens area, amygdala, caudate, hippocampus, pallidum, putamen, thalamus, cerebellar white matter, cerebellar cortex. Linear regressions were fit for each region entered separately with the predictors SLES total load and age.
Statistics: Relationships between stress-linked morphology and depressive symptomatology
Linear mixed effects regression models were fit for each stress-linked cluster with IDAS-II Dysphoria at Waves 3–5 as the model outcome (SAS 9.1.3; SAS Institute Inc., Cary, NC, USA). Participant was modeled as a random effect and the stress-linked structural metric, age at imaging, Wave, and Wave2 were time-invariant covariates. If a stress-linked cluster was significantly associated with IDAS-II Dysphoria (Waves 3–5), then we re-ran analysis with Wave 2 Dysphoria (concurrent to imaging) as an additional covariate to determine if the effect was over and above current symptomatology. All models utilized robust regression (Huber sandwich estimator) to correct model standard errors in the case of outliers(56), and beta estimates were standardized. The IDAS-II Dysphoria scale was of a priori interest, but specificity was examined in exploratory analyses using the remaining 17 IDAS-II scales (see Supplementary Analysis 5).
Statistics: Mediation Analysis
Stress-linked clusters found to significantly predict IDAS-II Dysphoria (Waves 3–5) in the linear mixed models were retained for mediation analysis (independent variable = SLES total load, mediator = cluster extracted from Freesurfer, dependent variable = mean IDAS-II Dysphoria from Waves 3–5)(57) (Mplus 7.11 with bootstrapping for confidence intervals(58)). The proportion of variance explained by the mediator was computed as previously described(59).
RESULTS
Participants
Table 1 contains demographic characteristics. The 232 females were 15.29±0.65 years-old (range=14.10–15.37) at the time of imaging and were largely Caucasian (87.9%), reflecting the catchment area around Stony Brook, NY. The 9-month burden of stressful life events varied considerably (mean total SLES=8.54±7.84; median=6, range=0–47, interquartile range=3–15). The N=232 MRI-imaged sample did not differ from the non-imaged sample in total SLES (t=0.55, p=0.58). The total number of distinct stressful events recorded per participant (3.59±2.59 events) is shown in Table 2. 90.1% reported experiencing ≥1 stressful event. Health and Other/Non-Romantic Relationships were common categories for events. Anxiety disorders were the most common type of DSM-IV diagnosis (23.28%) (Table 1), which was not associated with total or category-wise stressful life event burden (see Supplemental Analysis 2). Thus, lifetime history of DSM-IV Anxiety Disorders is unlikely to complicate interpretation of results and was not further considered.
Table 1:
Sample Characteristics
| n | (%) | |
|---|---|---|
| Demographics | ||
| Hispanic | 25 | 10.80% |
| Caucasian | 204 | 87.90% |
| Lifetime Psychopathology | ||
| Any Diagnosis (#, %) | 61 | 26.29% |
| Any Anxiety Disorder (#, %) | 54 | 23.28% |
| Generalized Anxiety Disorder | 5 | 2.16% |
| Specific Phobia | 30 | 12.93% |
| Social Phobia | 24 | 10.34% |
| Separation Anxiety | 5 | 2.16% |
| Panic Disorder | 3 | 1.29% |
| Obsessive Compulsive Disorder | 2 | 0.86% |
| Post-Traumatic Stress Disorder | 0 | 0.00% |
| Any Behavioral Disorder (#, %) | 11 | 4.74% |
| Conduct Disorder | 0 | 0.00% |
| Oppositional Defiant Disorder | 5 | 2.16% |
| Attention Deficit Disorder | 6 | 2.59% |
| Bulimia Nervosa/Anorexia Nervosa (#, %) | 0 | 0% |
| Eating Disorder Not Otherwise Specified (NOS) | 5 | 2.16% |
| Any Substance Use Disorder (#, %) | 0 | 0% |
Note: Abbreviations: K-SADS-PL: Kiddie Schedule for Affective Disorders and Schizophrenia for School-Aged Children, Present and Lifetime Version; DSM-IV: Diagnostic and Statistical Manual of Mental Disorders;
Table 2.
Stressful Life Events
| Frequency of events | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Stressful Life Events | Mean | SD | Median | Range | 0 | 1 | 2 | 3 | 4 | 5 | 5+ |
| SLES Total | 3.59 | 2.59 | 3 | [0,12] | 23 | 33 | 36 | 33 | 29 | 26 | 52 |
| Education | 0.41 | 0.66 | 0 | [0, 3] | 158 | 57 | 14 | 3 | 0 | 0 | 0 |
| Work | 0.23 | 0.53 | 0 | [0, 3] | 189 | 33 | 9 | 1 | 0 | 0 | 0 |
| Money | 0.12 | 0.32 | 0 | [0, 1] | 205 | 27 | 0 | 0 | 0 | 0 | 0 |
| Housing | 0.12 | 0.36 | 0 | [0, 2] | 208 | 21 | 3 | 0 | 0 | 0 | 0 |
| Crime | 0.07 | 0.31 | 0 | [0, 2] | 218 | 11 | 3 | 0 | 0 | 0 | 0 |
| Health | 1.03 | 1 | 1 | [0, 5] | 82 | 85 | 48 | 12 | 4 | 1 | 0 |
| Death | 0.32 | 0.52 | 0 | [0, 2] | 163 | 63 | 6 | 0 | 0 | 0 | 0 |
| Romantic Relationships | 0.29 | 0.6 | 0 | [0, 3] | 180 | 40 | 9 | 3 | 0 | 0 | 0 |
| Other Relationships | 1.01 | 1.07 | 1 | [0, 5] | 92 | 75 | 43 | 15 | 6 | 1 | 0 |
SLES: Stressful Life Events Schedule
Cortical Morphology Correlates with Life Stress
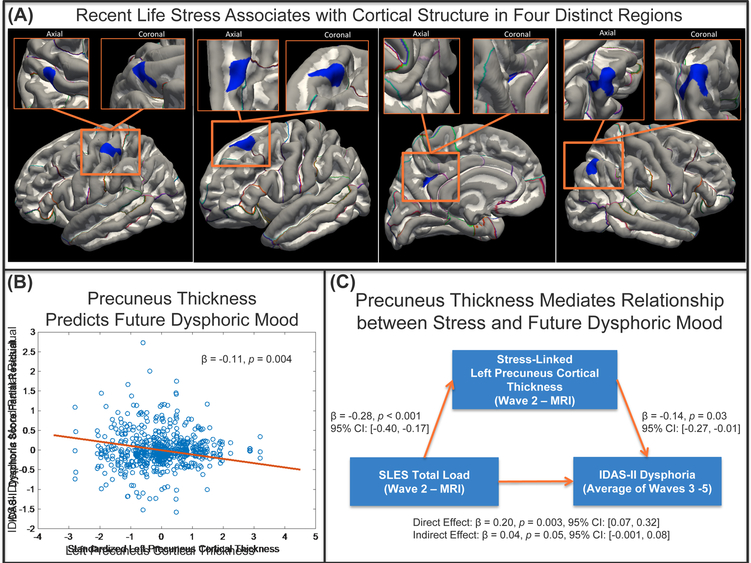
When controlling for age and after multiple comparisons correction, four clusters significantly correlated with SLES total load (Figure 2.A). Higher SLES total load was associated with thinner clusters in left precuneus (p=0.037) and left post-central (p=0.035) cortices. Higher SLES total load was also associated with smaller volumetric clusters in left superior frontal (p=0.006) and right inferior parietal (p=0.001) cortices (Table 3 and Figure 2.A). SLES total load was not significantly associated with surface area or subcortical volume (p-values>0.05). Supplementary Analysis 3 presents the association between the four extracted clusters and SLES.
Figure 2 Caption:
(A) Surface-based morphometry results surviving multiple comparisons correction for the stress analysis (cluster-wise p < 0.05, corrected). (Left): Thinner left post-central associated with more stress (Left-Center): Smaller left superior frontal associated with more stress (Right-Center): Thinner left precuneus associated with more stress (Right): Smaller right inferior parietal associated with more stress. (B) Partial residual for the IDAS-II Dysphoria scores across Waves 3–5 as estimated from the linear mixed model accounting for age, Wave, Wave2, IDAS-II dysphoria score at the time of imaging (Wave 2), and the stress-linked left precuneus cortical thickness estimate. Resulting model standardized beta for the fixed effect of precuneus cortical thickness shown and resulting p-value. (C) Mediation results shown for x = Stressful Life Events Schedule (SLES) total load, M = stress-linked left precuneus cortical thickness, and y = IDAS-II dysphoria score over Waves 3–5. Standardized model beta, p-values, and 95% confidence intervals (CIs) shown.
Table 3:
Surface-Based Morphometry Stress Association Results
| Region | Measure | Cluster-Wise Probability | Size (mm2) | MNI Coordinates [X, Y, Z] | Direction of Effect |
|---|---|---|---|---|---|
| Left Precuneus | Cortical Thickness | 0.037 | 173.12 | [−13.2, −53.1, 34.8] | more stress, thinner |
| Left Post-Central | Cortical Thickness | 0.035 | 174.12 | [−50.0, −22.6, 54.2] | more stress, thinner |
| Left Superior Frontal | Volume | 0.0058 | 283.24 | [−18.5, 30.4, 50.1] | more stress, smaller volume |
| Right Inferior Parietal | Volume | 0.001 | 339.09 | [40.4, −65.3, 46.4] | more stress, smaller volume |
SBM analyses were repeated covarying for the duration between the MRI and SLES assessments. The results were unchanged.
Abbreviations: MNI = Montreal Neurological Institute;
Note: Results were largely unchanged when additionally covarying for number of days between the SLES and MRI.
Stress-Associated Morphology Predicts Dysphoric Mood
IDAS-II Dysphoria showed modest stability across 9-month follow-ups (r’s=0.41–0.64; scores = 15.26±6.35 (Wave 2), 14.98±6.31 (Wave 3), 15.74±7.14 (Wave 4), 14.54±6.44 (Wave 5)) (trajectories of IDAS-II Dysphoria across Waves shown in Supplementary Analysis 4). During the post-imaging follow-up (Waves 3–5), first onset of MDD or Dysthymia was observed in 11.36% of participants (25 of 220; 12 participants with incomplete data). Wave 2 SLES total load was significantly correlated with IDAS-II Dysphoria (Wave 1: r=0.16, p=0.015; Wave 2: r=0.24, p<0.001; Wave 3: r=0.25, p<0.001; Wave 4: p=0.14, p=0.035; Wave 5: r=0.18, p=0.007).
We examined whether dysphoria levels assessed prior to imaging could underlie the association between Wave 2 SLES and the four clusters identified with SBM. Thus, SBM analyses were repeated with Wave 1 IDAS-II Dysphoria (assessed 9-months before imaging and before the start of the SLES assessment window) and Wave 2 IDAS-II Dysphoria (assessed concurrent to imaging and at the end of the SLES assessment window), respectively (see Supplementary Analysis 1 (available online)). In summary, the left superior frontal and right inferior parietal volume clusters remained associated with SLES, whereas left precuneus thickness remained associated with SLES when controlling for remote, but not current dysphoria. The post-central thickness cluster was no longer associated with SLES in both analyses.
We next examined whether these clusters predicted future IDAS-II Dysphoria (Waves 3–5). The covariates (Wave, Wave2, and age) were not significantly related to IDAS-II Dysphoria (p > 0.05). The stress-linked left precuneus cortical thickness cluster prospectively predicted IDAS-II Dysphoria (β=−0.169, t=−3.620, p=0.0004). This effect remained significant when controlling for Wave 2 IDAS-II Dysphoria (β=−0.110, t=−2.930, p=0.004; Figure 2.B), indicating that prediction of 27-month increases in dysphoria is over and above level of dysphoria at time of imaging.. The other three clusters were not significant predictors of IDAS-II Dysphoria. Specificity analyses (Supplementary Analysis 5) revealed that stress-linked precuneus thickness predicted increased levels of other mood and anxiety symptom dimensions at Waves 3–5 including lassitude, mania, social anxiety, and ill temperament (p<0.01) and additionally panic, appetite gain, traumatic intrusions, and suicidality (p<0.05).
Mediation Analysis
In mediation analysis, left precuneus cortical thickness accounted for approximately 17.0% of the association between SLES total load and mean IDAS-II Dysphoria (β=0.040, p=0.053; Figure 2.C). Controlling for Wave 2 IDAS-II Dysphoria yielded a minor reduction of the precuneus’ mediative effect (β=0.030, p=0.084), but increased the variance accounted for to 24.2%.
DISCUSSION
Despite the prominent role of depressogenic stress in translational models, evidence connecting stress-sensitive cortical markers to depression in human adolescents has been elusive(1, 5). In this project, we examined stress-sensitive cortical markers in a typically-developing cohort of adolescent girls during a developmental period notable for neural maturation, exposure to stress, and increased rates of depression(33). Then, we tested prognostic value using longitudinal follow-up of depression symptoms over 27 months.
Our first finding was that portions of frontal and parietal lobes (precuneus, post-central, inferior parietal, and superior frontal cortices) were associated with 9-month exposure to stressful life events. Similar results (smaller/thinner prefrontal and precuneus cortices) have been reported in relation to remote events in 12 year-olds(31), youth exposed to stimulus deprivation(60), childhood sexual abuse(61), temporally-distal traumatic life events(31), and diagnosis of PTSD(28). Our second main finding was that left precuneus thickness predicted subthreshold increases in dysphoric mood, the cardinal symptom of depressive disorders, for up to 27-months following imaging. Further, precuneus thickness mediated a modest amount of the depressogenic effects of stress.
Importantly, this identifies stress-linked precuneus thickness as a viable candidate biomarker for the depressogenic effects of stress. Ultimately, longitudinal, multimodal neuroimaging is needed to pinpoint the molecular mechanisms through which recent life stress alters cortical structure in vulnerable regions, especially using tools such as positron emission tomography (PET) to target levels of neuroinflammation following stress exposure. Such information is critical for translating findings such as these to the clinic where articulated mechanisms can drive the development of effective interventions.
One intriguing aspect of these results is that the community-dwelling cohort was not recruited based on risk status (e.g., not recruited based on trauma or neglect) and was rated at, or near, normative levels of stress exposure and dysphoria. For instance, mean SLES scores (8.5±7.8) were comparable to adolescent controls(44) and mean Wave 2 IDAS-II Dysphoria (15.3±6.4) placed the sample slightly above the 47th percentile of norms(62). Given the characteristics of the present cohort, these results suggest a model by which high concentrations of recent life stress impacts similar regions as implicated with severe stressors, neglect, and trauma.
A corollary of the stress acceleration hypothesis is that ongoing neural development dynamically shapes regional sensitivities to stress. Thus, these regions may be sensitive to recent life stress during mid-adolescence in particular, while a comparable study in an older cohort might detect effects in prefrontal and cingulate regions(12–16). Indeed, parietal and frontal lobe thickness peak at 10 and 11 years old, respectively, while the temporal lobe peaks at 17 years in females(12). The medial prefrontal and cingulate cortices, which are implicated in stress and depression literatures(63–66), are the last to develop, and appear to follow a complex cubic trajectory(16). The age of the sample may also account for the absence of effects in subcortical regions, such as hippocampal volume, which appears stable during this period(15, 67). Previous studies have identified subcortical regions, especially in the hippocampus, as sensitive to stress(8, 64, 66). This includes studies of perceived stress in adolescence and recent life stress in adulthood(21, 68), as well as youth with post-traumatic stress symptoms(69). However, null results have also been reported in youth with distal life stress(31) and in a meta-analysis that concluded hippocampal volume was reduced in adults, but not youth, with childhood PTSD(67). Thus, hippocampal volume may be sensitive to trauma but not stressful life events, or the effects do not emerge until adulthood.
A second corollary of the stress acceleration hypothesis is that programmed developmentally sensitive periods are necessary for healthy adaptation. Premature activation by stress may alter developmental programming and increase vulnerability to develop psychopathology(10). The four stress-linked regions share physical connection via the occipitofrontal fasciculus(70), implicating maturation of this particular fiber pathway in vulnerability to stress (more so than other tracts for this cohort). In addition, these regions are part of functional connectivity networks that increase following trauma(71) and during recovery from experimentally-induced social stress(72). Thus, altered maturation of these regions, stemming from repeated stress exposure, may impact reactivity to and recovery from later stress exposure. In addition, mistiming of precuneus maturation may alter structural connections with limbic structures and areas of the frontal cortex(71), or alter functional connectivity in circuitry involved in emotional learning, reactivity, and self-referential processes(10, 64, 73, 74). Cognitive functions of the precuneus include first-person perspective taking and an experience of agency(73, 74), as well as complex cognitive functions (e.g. coping styles and self-regulation(10)). Interestingly, in adults with MDD, hypogyrification in the precuneus cortex was associated with default mode network hyperconnectivity(75).
Dysphoria was not predicted by the other three stress-linked regions, but other consequences are plausible given their varied behavioral, cognitive, and affective functions. The inferior parietal cortex (posterior section of the inferior parietal lobule; angular gyrus) has been implicated in semantic and number processing, memory retrieval, attention, and social cognition(76), as well as propensity for self-referential thoughts(77). Interestingly, depressed individuals exposed to experimentally-induced stress show hyperconnectivity between the inferior parietal and prefrontal cortex relative to non-depressed individuals(78). Further, the prefrontal cortex may have top-down control over emotional responses through modulation of limbic activity. Therefore, disrupted maturation of this region could eventually impact emotional processing(79). The superior frontal gyrus is a key component of working memory networks(80), and through connections with middle and inferior frontal gyri(81), is thought to support high-level executive functions(80). The identified portion of the post-central gyrus (mid-to-superior portion) selectively encodes fear/anger emotions over happiness/ surprise (i.e., emotion-predictive patterns)(82). Thus, altered maturation in the post-central gyrus could impact emotion encoding and prediction.
These findings support the hypothesis that reduced cortical GM could serve as a viable biomarker for depression, especially in high-risk youth(35, 83, 84). Case-control studies of depressed adolescents have reported reduced superior frontal morphologic properties(35), and adolescents with accelerated frontal and parietal thinning exhibit higher depression severity(37). Thinning in the superior frontal and inferior parietal gyri was also correlated with genetic risk for depression(83). Our results contribute to this literature by suggesting that recent life stress may have underappreciated, and similar impacts, on cortical structure in subthreshold symptomatology, as observed for depressive disorders and familial risk for depression.
Strengths of this study include a semi-structured stress interview and consensus team derived objective-stress ratings. This rigorous approach prevents same-reporter bias from creating spurious correlations between stress and dysphoria. The study also repeatedly assessed dysphoria up to 27-months post-imaging. Additionally, MRI data was closely screened using a validated quality-control procedure.
However, there were also limitations. First, our study examined females of a narrow age range. The parent project aimed to minimize heterogeneity, focusing on a population at great risk of first depression onset. Diverse samples are needed to replicate these results in different age groups and explore gender differences. Importantly, whether these results generalize to clinical populations of adolescents is unclear. In addition, the clinical significance of precuneus thickness is unclear because it did not predict first-onset of DSM-IV MDD or Dysthymia (n=25; Supplemental Analysis 6). Increases in subthreshold symptoms are a robust predictor of risk for first-onset depression(85). Thus, longer follow-up may be necessary to capture associations between stress-linked precuneus thickness and transition from subthreshold symptoms to disorder onset. Indeed, the cohort is not yet through the period of highest risk for onset and relatively few have converted. Second, participants were assessed for stress and depression ecologically and completed one assessment of MR imaging. Thus, the causal link between stress and cortical structure requires further investigation using longitudinal neuroimaging.
Third, it is unclear why effects vary by gray matter parameter on a region-by-region basis. Preclinical studies suggest that inter-individual variability in cortical volume is more closely related to surface area and mostly independent of cortical thickness(86–88). Twin studies suggest that the genetic and environmental factors are distinct for surface area and cortical thickness(89). Further, regional gray matter properties may be sensitive to distinct pathophysiological processes as they each mature along distinct developmental trajectories(15). A comprehensive model of the neurodevelopmental mechanisms underlying individual differences in each aspect of gray matter is needed to reconcile this phenomenon.
Fourth, the study was not well-suited to identify consequences of early adversity, trauma, or neglect on cortical morphology. Exclusion of lifetime history of DSM-IV MDD or Dysthymia at Wave 1 may have led to enrollment of few, if any, high-risk youth. Indeed, none met criteria for DSM-IV PTSD at Wave 1. In addition, stressful life events were first assessed at Wave 2 and covered the period since Wave 1. This prevented direct comparison of effects of earlier stressful life events with those of more recent stressful life events. That cortical effects of stress at Wave 2 were largely independent of Wave 1 Dysphoria (except in the post-central) may be informative, given that Wave 1 Dysphoria should be elevated in those who experienced a high burden of early stressful life events. Future studies would benefit from direct comparison of community-dwelling youth exposed to normative stressful life events and youth selected for significant early adversity.
In this study, recent life stress was associated with subtle differences in grey matter morphology in adolescent females. This is novel evidence that common stressful life events may impact adolescent development, and contrasts with the notion that only particularly harsh, traumatic exposures alter neurodevelopment. The stressogenic effects on precuneus thickness represents a novel biomarker for understanding the depressogenic effects of stress. Imaging modalities such as PET are necessary to interrogate the molecular mechanisms of stress-induced deviations from normative neurodevelopment patterns. Such studies could eventually make it feasible to ameliorate or block stress-induced neurodevelopmental structural changes via targeted therapies and early intervention strategies.
Supplementary Material
Table 4:
Bivariate Pearson’s correlations among extracted stress-linked clusters
| 2 | 3 | 4 | 5 | Mean | SD | ||
|---|---|---|---|---|---|---|---|
| 1. Left precuneus CT | 0.26*** | 0.25*** | 0.20** | −0.05 | 2.57 | 0.21 | |
| 2. Left post-central CT | - | 0.23*** | 0.12 | −0.04 | 2.50 | 0.27 | |
| 3. Left superior frontal volume | - | 0.27*** | −0.05 | 2.50 | 0.49 | ||
| 4. Right inferior parietal volume | - | −0.16* | 1.87 | 0.35 | |||
| 5. Age | - | 15.29 | 0.65 |
Abbreviations: CT = cortical thickness; SD = standard deviation;
p<0.05,
p<0.01,
p<0.001
KEY RESOURCES TABLE
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| Software; Algorithm | FreeSurfer 5.3.0 | http://surfer.nmr.mgh.harvard.edu/ | RRID: SCR_001847 | |
| Software; Algorithm | Mplus 7.11 | https://www.statmodel.com | RRID: SCR_015578 | |
ACKNOWLEDGMENTS
We would like to thank the Center for Understanding Biology using Imaging Technology (CUBIT) image analysts and Alexandra de la Plante at Stony Brook University for their work in MRI importing, processing, and quality control. This study was supported by the National Institute of Mental Health R01MH093479 awarded to Dr. Roman Kotov. This analysis was presented in part at the 2018 Society of Biological Psychiatry (SOBP) annual meeting in oral abstract form (DOI: https://doi.org/10.1016/j.biopsych.2018.02.288).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
The authors report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Slavich GM, Irwin MR (2014): From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychological bulletin. 140:774–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hammen C (2006): Stress generation in depression: reflections on origins, research, and future directions. Journal of clinical psychology. 62:1065–1082. [DOI] [PubMed] [Google Scholar]
- 3.Monroe SM, Simons AD (1991): Diathesis-stress theories in the context of life stress research: implications for the depressive disorders. Psychological bulletin. 110:406–425. [DOI] [PubMed] [Google Scholar]
- 4.Abramson LY, Seligman ME, Teasdale JD (1978): Learned helplessness in humans: Critique and reformulation. Journal of abnormal psychology. 87:49. [PubMed] [Google Scholar]
- 5.Kendler KS, Karkowski LM, Prescott CA (1999): Causal relationship between stressful life events and the onset of major depression. The American journal of psychiatry. 156:837–841. [DOI] [PubMed] [Google Scholar]
- 6.Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, et al. (2002): Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nature neuroscience. 5:1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sapolsky RM, Krey LC, McEwen BS (1986): The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocrine reviews. 7:284–301. [DOI] [PubMed] [Google Scholar]
- 8.Lupien SJ, McEwen BS, Gunnar MR, Heim C (2009): Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 10:434–445. [DOI] [PubMed] [Google Scholar]
- 9.Sapolsky RM (2000): Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 57:925–935. [DOI] [PubMed] [Google Scholar]
- 10.Callaghan BL, Tottenham N (2016): The Stress Acceleration Hypothesis: Effects of early-life adversity on emotion circuits and behavior. Curr Opin Behav Sci. 7:76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tyborowska A, Volman I, Niermann HCM, Pouwels JL, Smeekens S, Cillessen AHN, et al. (2018): Early-life and pubertal stress differentially modulate grey matter development in human adolescents. Scientific reports. 8:9201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giedd JN (2004): Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences. 1021:77–85. [DOI] [PubMed] [Google Scholar]
- 13.Group BDC (2011): Total and regional brain volumes in a population-based normative sample from 4 to 18 years: the NIH MRI Study of Normal Brain Development. Cerebral cortex. 22:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. (2004): Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 101:8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ostby Y, Tamnes CK, Fjell AM, Westlye LT, Due-Tonnessen P, Walhovd KB (2009): Heterogeneity in subcortical brain development: A structural magnetic resonance imaging study of brain maturation from 8 to 30 years. The Journal of neuroscience : the official journal of the Society for Neuroscience. 29:11772–11782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, et al. (2008): Neurodevelopmental trajectories of the human cerebral cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 28:3586–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirschbaum C, Pirke K-M, Hellhammer DH (1993): The ‘Trier Social Stress Test’–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 28:76–81. [DOI] [PubMed] [Google Scholar]
- 18.Reschke-Hernández AE, Okerstrom KL, Bowles Edwards A, Tranel D (2017): Sex and stress: Men and women show different cortisol responses to psychological stress induced by the Trier social stress test and the Iowa singing social stress test. Journal of neuroscience research. 95:106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hostinar CE, McQuillan MT, Mirous HJ, Grant KE, Adam EK (2014): Cortisol responses to a group public speaking task for adolescents: variations by age, gender, and race. Psychoneuroendocrinology. 50:155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaidyanathan U, Vrieze SI, Iacono WG (2015): The Power of Theory, Research Design, and Transdisciplinary Integration in Moving Psychopathology Forward. Psychol Inq. 26:209–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papagni SA, Benetti S, Arulanantham S, McCrory E, McGuire P, Mechelli A (2011): Effects of stressful life events on human brain structure: a longitudinal voxel-based morphometry study. Stress. 14:227–232. [DOI] [PubMed] [Google Scholar]
- 22.Cohen RA, Grieve S, Hoth KF, Paul RH, Sweet L, Tate D, et al. (2006): Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biological psychiatry. 59:975–982. [DOI] [PubMed] [Google Scholar]
- 23.Gianaros PJ, Jennings JR, Sheu LK, Greer PJ, Kuller LH, Matthews KA (2007): Prospective reports of chronic life stress predict decreased grey matter volume in the hippocampus. NeuroImage. 35:795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackowski AP, de Araujo CM, de Lacerda AL, Mari Jde J, Kaufman J (2009): Neurostructural imaging findings in children with post-traumatic stress disorder: brief review. Psychiatry and clinical neurosciences. 63:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Bellis MD, Keshavan MS, Shifflett H, Iyengar S, Beers SR, Hall J, et al. (2002): Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biological psychiatry. 52:1066–1078. [DOI] [PubMed] [Google Scholar]
- 26.De Bellis MD, Zisk A (2014): The biological effects of childhood trauma. Child Adolesc Psychiatr Clin N Am. 23:185–222, vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Bellis MD, Hooper SR, Chen SD, Provenzale JM, Boyd BD, Glessner CE, et al. (2015): Posterior structural brain volumes differ in maltreated youth with and without chronic posttraumatic stress disorder. Dev Psychopathol. 27:1555–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morey RA, Haswell CC, Hooper SR, De Bellis MD (2016): Amygdala, Hippocampus, and Ventral Medial Prefrontal Cortex Volumes Differ in Maltreated Youth with and without Chronic Posttraumatic Stress Disorder. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 41:791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Association AP, Association AP (2000): DSM-IV-TR: Diagnostic and statistical manual of mental disorders, text revision. Washington, DC: American Psychiatric Association. 75:78–85. [Google Scholar]
- 30.Weathers FW, Keane TM (2007): The Criterion A problem revisited: controversies and challenges in defining and measuring psychological trauma. J Trauma Stress. 20:107–121. [DOI] [PubMed] [Google Scholar]
- 31.Hanson JL, Chung MK, Avants BB, Rudolph KD, Shirtcliff EA, Gee JC, et al. (2012): Structural variations in prefrontal cortex mediate the relationship between early childhood stress and spatial working memory. The Journal of neuroscience : the official journal of the Society for Neuroscience. 32:7917–7925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rudolph KD, Hammen C (1999): Age and gender as determinants of stress exposure, generation, and reactions in youngsters: A transactional perspective. Child development. 70:660–677. [DOI] [PubMed] [Google Scholar]
- 33.Hankin BL, Abramson LY, Moffitt TE, Silva PA, McGee R, Angell KE (1998): Development of depression from preadolescence to young adulthood: emerging gender differences in a 10-year longitudinal study. Journal of abnormal psychology. 107:128–140. [DOI] [PubMed] [Google Scholar]
- 34.Frodl T, Meisenzahl EM, Zetzsche T, Hohne T, Banac S, Schorr C, et al. (2004): Hippocampal and amygdala changes in patients with major depressive disorder and healthy controls during a 1-year follow-up. The Journal of clinical psychiatry. 65:492–499. [DOI] [PubMed] [Google Scholar]
- 35.Schmaal L, Hibar DP, Samann PG, Hall GB, Baune BT, Jahanshad N, et al. (2016): Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Molecular psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmaal L, Veltman DJ, van Erp TG, Samann PG, Frodl T, Jahanshad N, et al. (2016): Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Molecular psychiatry. 21:806–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bos MGN, Peters S, van de Kamp FC, Crone EA, Tamnes CK (2018): Emerging depression in adolescence coincides with accelerated frontal cortical thinning. J Child Psychol Psychiatry. 59:994–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ganella DE, Allen NB, Simmons JG, Schwartz O, Kim JH, Sheeber L, et al. (2015): Early life stress alters pituitary growth during adolescence-a longitudinal study. Psychoneuroendocrinology. 53:185–194. [DOI] [PubMed] [Google Scholar]
- 39.Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A (2003): Prevalence and development of psychiatric disorders in childhood and adolescence. Archives of general psychiatry. 60:837–844. [DOI] [PubMed] [Google Scholar]
- 40.Keenan K, Hipwell AE (2005): Preadolescent clues to understanding depression in girls. Clin Child Fam Psychol Rev. 8:89–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salk RH, Hyde JS, Abramson LY (2017): Gender differences in depression in representative national samples: Meta-analyses of diagnoses and symptoms. Psychological bulletin. 143:783–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kroenke K, Spitzer RL, Williams JB (2001): The PHQ-9: validity of a brief depression severity measure. Journal of general internal medicine. 16:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. (1997): Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 36:980–988. [DOI] [PubMed] [Google Scholar]
- 44.Williamson DE, Birmaher B, Ryan ND, Shiffrin TP, Lusky JA, Protopapa J, et al. (2003): The stressful life events schedule for children and adolescents: development and validation. Psychiatry Res. 119:225–241. [DOI] [PubMed] [Google Scholar]
- 45.Swartz JR, Williamson DE, Hariri AR (2015): Developmental change in amygdala reactivity during adolescence: effects of family history of depression and stressful life events. The American journal of psychiatry. 172:276–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watson D, O’Hara MW, Naragon-Gainey K, Koffel E, Chmielewski M, Kotov R, et al. (2012): Development and validation of new anxiety and bipolar symptom scales for an expanded version of the IDAS (the IDAS-II). Assessment. 19:399–420. [DOI] [PubMed] [Google Scholar]
- 47.Watson D, O’Hara MW, Simms LJ, Kotov R, Chmielewski M, McDade-Montez EA, et al. (2007): Development and validation of the Inventory of Depression and Anxiety Symptoms (IDAS). Psychol Assess. 19:253–268. [DOI] [PubMed] [Google Scholar]
- 48.Chmielewski M, Clark LA, Bagby RM, Watson D (2015): Method matters: Understanding diagnostic reliability in DSM-IV and DSM-5. Journal of abnormal psychology. 124:764–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iscan Z, Jin TB, Kendrick A, Szeglin B, Lu H, Trivedi M, et al. (2015): Test-retest reliability of freesurfer measurements within and between sites: Effects of visual approval process. Human brain mapping. 36:3472–3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fischl B, Sereno MI, Tootell RB, Dale AM (1999): High-resolution intersubject averaging and a coordinate system for the cortical surface. Human brain mapping. 8:272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wagner G, Schultz CC, Koch K, Schachtzabel C, Sauer H, Schlosser RG (2012): Prefrontal cortical thickness in depressed patients with high-risk for suicidal behavior. Journal of psychiatric research. 46:1449–1455. [DOI] [PubMed] [Google Scholar]
- 52.Hayasaka S, Nichols TE (2003): Validating cluster size inference: random field and permutation methods. NeuroImage. 20:2343–2356. [DOI] [PubMed] [Google Scholar]
- 53.Ward BD (2000): AlphaSim: Simultaneous Inference for fMRI Data. Biophysics Research Institute: Medical College of Wisconsin. [Google Scholar]
- 54.Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. (2006): An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 31:968–980. [DOI] [PubMed] [Google Scholar]
- 55.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. (2002): Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 33:341–355. [DOI] [PubMed] [Google Scholar]
- 56.Wang J, Lu Z, Cohen AS (2015): The sensitivity analysis of two-level hierarchical linear models to outliers Quantitative Psychology Research: Springer, pp 307–320. [Google Scholar]
- 57.Baron RM, Kenny DA (1986): The moderator–mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of personality and social psychology. 51:1173. [DOI] [PubMed] [Google Scholar]
- 58.Preacher KJ, Hayes AF (2004): SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavior research methods, instruments, & computers. 36:717–731. [DOI] [PubMed] [Google Scholar]
- 59.Ditlevsen S, Christensen U, Lynch J, Damsgaard MT, Keiding N (2005): The mediation proportion: a structural equation approach for estimating the proportion of exposure effect on outcome explained by an intermediate variable. Epidemiology. 16:114–120. [DOI] [PubMed] [Google Scholar]
- 60.Hubel DH, Wiesel TN (1998): Early exploration of the visual cortex. Neuron. 20:401–412. [DOI] [PubMed] [Google Scholar]
- 61.Andersen SL, Tomada A, Vincow ES, Valente E, Polcari A, Teicher MH (2008): Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci. 20:292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nelson GH, O’Hara MW, Watson D (2018): National norms for the expanded version of the inventory of depression and anxiety symptoms (IDAS-II). Journal of clinical psychology. 74:953–968. [DOI] [PubMed] [Google Scholar]
- 63.Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, et al. (2006): Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cerebral cortex. 16:313–320. [DOI] [PubMed] [Google Scholar]
- 64.McEwen BS, Nasca C, Gray JD (2016): Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 41:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, et al. (2006): Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. The Journal of neuroscience : the official journal of the Society for Neuroscience. 26:7870–7874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Romeo RD (2017): The impact of stress on the structure of the adolescent brain: implications for adolescent mental health. Brain research. 1654:185–191. [DOI] [PubMed] [Google Scholar]
- 67.Gogtay N, Nugent TF 3rd, Herman DH, Ordonez A, Greenstein D, Hayashi KM, et al. (2006): Dynamic mapping of normal human hippocampal development. Hippocampus. 16:664–672. [DOI] [PubMed] [Google Scholar]
- 68.Piccolo LR, Noble KG, Pediatric Imaging N, Study G (2018): Perceived stress is associated with smaller hippocampal volume in adolescence. Psychophysiology. 55:e13025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carrion VG, Wong SS (2012): Can traumatic stress alter the brain? Understanding the implications of early trauma on brain development and learning. Journal of adolescent health. 51:S23–S28. [DOI] [PubMed] [Google Scholar]
- 70.Makris N, Papadimitriou GM, Sorg S, Kennedy DN, Caviness VS, Pandya DN (2007): The occipitofrontal fascicle in humans: a quantitative, in vivo, DT-MRI study. NeuroImage. 37:1100–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lanius RA, Bluhm RL, Coupland NJ, Hegadoren KM, Rowe B, Theberge J, et al. (2010): Default mode network connectivity as a predictor of post-traumatic stress disorder symptom severity in acutely traumatized subjects. Acta psychiatrica Scandinavica. 121:33–40. [DOI] [PubMed] [Google Scholar]
- 72.Veer IM, Oei NY, Spinhoven P, van Buchem MA, Elzinga BM, Rombouts SA (2011): Beyond acute social stress: increased functional connectivity between amygdala and cortical midline structures. NeuroImage. 57:1534–1541. [DOI] [PubMed] [Google Scholar]
- 73.Kjaer TW, Nowak M, Lou HC (2002): Reflective self-awareness and conscious states: PET evidence for a common midline parietofrontal core. NeuroImage. 17:1080–1086. [PubMed] [Google Scholar]
- 74.Lou HC, Luber B, Crupain M, Keenan JP, Nowak M, Kjaer TW, et al. (2004): Parietal cortex and representation of the mental self. Proceedings of the National Academy of Sciences of the United States of America. 101:6827–6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nixon N, Liddle P, Nixon E, Worwood G, Liotti M, Palaniyappan L (2014): Biological vulnerability to depression: linked structural and functional brain network findings. The British Journal of Psychiatry. 204:283–289. [DOI] [PubMed] [Google Scholar]
- 76.Seghier ML (2013): The angular gyrus: multiple functions and multiple subdivisions. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 19:43–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cheng W, Rolls ET, Qiu J, Liu W, Tang Y, Huang CC, et al. (2016): Medial reward and lateral non-reward orbitofrontal cortex circuits change in opposite directions in depression. Brain : a journal of neurology. 139:3296–3309. [DOI] [PubMed] [Google Scholar]
- 78.Sinha R, Lacadie CM, Constable RT, Seo D (2016): Dynamic neural activity during stress signals resilient coping. Proceedings of the National Academy of Sciences of the United States of America. 113:8837–8842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ochsner KN, Silvers JA, Buhle JT (2012): Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences. 1251:E1–E24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.du Boisgueheneuc F, Levy R, Volle E, Seassau M, Duffau H, Kinkingnehun S, et al. (2006): Functions of the left superior frontal gyrus in humans: a lesion study. Brain : a journal of neurology. 129:3315–3328. [DOI] [PubMed] [Google Scholar]
- 81.Li W, Qin W, Liu H, Fan L, Wang J, Jiang T, et al. (2013): Subregions of the human superior frontal gyrus and their connections. NeuroImage. 78:46–58. [DOI] [PubMed] [Google Scholar]
- 82.Kragel PA, LaBar KS (2016): Somatosensory Representations Link the Perception of Emotional Expressions and Sensory Experience. eNeuro. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hao X, Talati A, Shankman S, Liu J, Kayser J, Tenke CE, et al. (2017): Stability of Cortical Thinning in Persons at Increased Familial Risk for Major Depressive Disorder Across 8 Years. Biological Psychiatry Cognitive Neuroscience and Neuroimaging. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Peterson BS, Warner V, Bansal R, Zhu H, Hao X, Liu J, et al. (2009): Cortical thinning in persons at increased familial risk for major depression. Proceedings of the National Academy of Sciences of the United States of America. 106:6273–6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jinnin R, Okamoto Y, Takagaki K, Nishiyama Y, Yamamura T, Okamoto Y, et al. (2017): Detailed course of depressive symptoms and risk for developing depression in late adolescents with subthreshold depression: a cohort study. Neuropsychiatr Dis Treat. 13:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Im K, Lee J-M, Lyttelton O, Kim SH, Evans AC, Kim SI (2008): Brain size and cortical structure in the adult human brain. Cerebral cortex. 18:2181–2191. [DOI] [PubMed] [Google Scholar]
- 87.Pakkenberg B, Gundersen HJG (1997): Neocortical neuron number in humans: effect of sex and age. Journal of Comparative Neurology. 384:312–320. [PubMed] [Google Scholar]
- 88.Storsve AB, Fjell AM, Tamnes CK, Westlye LT, Overbye K, Aasland HW, et al. (2014): Differential longitudinal changes in cortical thickness, surface area and volume across the adult life span: regions of accelerating and decelerating change. Journal of Neuroscience. 34:8488–8498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, et al. (2009): Distinct genetic influences on cortical surface area and cortical thickness. Cerebral cortex. 19:2728–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.