Abstract
Tight junctions (TJ) play a central role in the homeostasis of epithelial and endothelial tissues, by providing a semipermeable barrier to ions and solutes, by contributing to the maintenance of cell polarity, and by functioning as signaling platforms. TJ are associated with the actomyosin and microtubule cytoskeletons, and the crosstalk with the cytoskeleton is fundamental for junction biogenesis and physiology. TJ are spatially and functionally connected to adherens junctions (AJ), which are essential for the maintenance of tissue integrity. Mechano-sensing and mechano-transduction properties of several AJ proteins have been characterized during the last decade. However, little is known about how mechanical forces act on TJ and their proteins, how TJ control the mechanical properties of cells and tissues, and what are the underlying molecular mechanisms. Here I review recent studies that have advanced our understanding of the relationships between mechanical force and TJ biology.
Keywords: Tight junction, Mechanobiology, Actin, ZO-1
Introduction
Tight junctions (TJ) are localized at the apicolateral borders of vertebrate epithelial cells and in endothelial cells, and are part of a junctional complex with cadherin-based junctions (Fig. 1). The canonical functions of TJ are: (1) paracellular “barrier”, e.g., to form a selective filter for the passage of ions, water, and solutes across different compartments of the extracellular space. At the ultrastructural level, the TJ barrier to ions corresponds to a network of intramembrane strands (fibrils), formed between contacting cells by the trans-association of cis-polymers of transmembrane proteins belonging to the claudin family. Large solutes flow through transient breaks in the strands. Abnormal regulation of TJ barrier function allows the passage of pathogens and antigens through epithelial monolayers, and is implicated in disease (Anderson and Van Itallie 2009; Buckley and Turner 2018; Citi 2018). (2) “fence” function, e.g., to define the limit between the apical and lateral domains of the plasma membrane of polarized cells (Anderson and Van Itallie 2009; Zihni et al. 2016). The barrier and fence functions of TJ can be uncoupled (Mandel et al. 1993), and a polarized distribution of lipid and protein markers occurs even in the absence of TJ fibrils (Ikenouchi et al. 2012; Umeda et al. 2006), consistent with the notion that claudin strands are not directly responsible for the maintenance of apicobasal polarity. Thus, the molecular composition of the TJ intramembrane “fence” remains unclear. In addition to these canonical functions, TJ play multiple roles in the control of signaling pathways involved in the regulation of cytoskeletal organization, gene expression, and cell proliferation, through the interaction of their cytoplasmic scaffolding proteins and adaptor proteins with transcription factors, regulators of Rho family GTPases, and other signaling proteins (Braga 2017; Citi et al. 2014; Zihni et al. 2016).
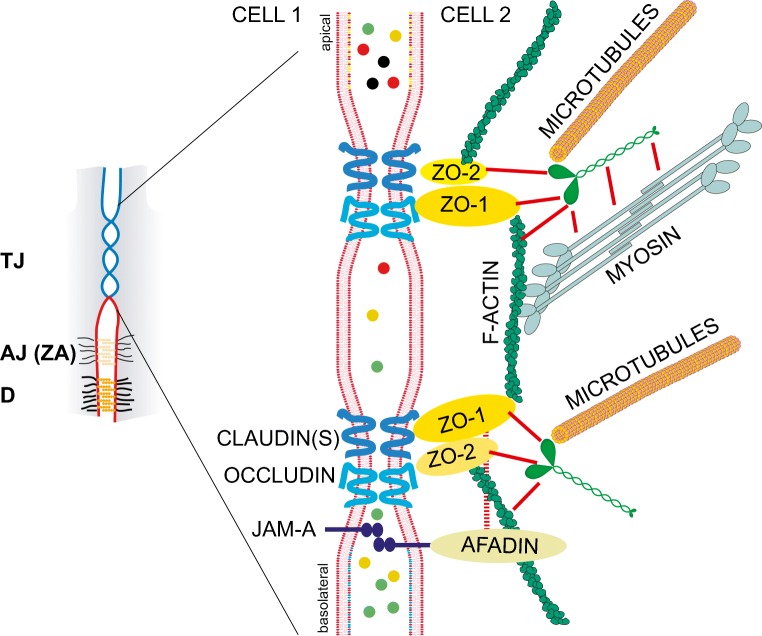
Fig. 1.
Scheme of tight junctions (TJ) and their interactions with the actin and microtubule cytoskeletons. The small scheme on the left illustrates the apical position of the TJ (also called zonulae occludentes or ZO) with respect to the more basolateral adherens junctions (AJ) and desmosomes (D). The circumferential AJ of polarized cells is also called zonula adhaerens (ZA). The larger scheme on the right is an expansion of the TJ region of the apical junctional complex of a polarized epithelial cell. During junction biogenesis, several TJ and AJ components are intermingled, and the spatial segregation between TJ and ZA occurs only in mature, fully polarized epithelial cells. The junctional membrane, selected transmembrane proteins of TJ (claudin, occludin, JAM-A), and the major cytoplasmic proteins involved in TJ-cytoskeleton interactions are schematically depicted in the larger scheme. Actin filaments interact with ZO proteins, afadin, and cingulin, and microtubules interact with cingulin. Additional interactions of cingulin with ZO proteins and myosin (Cordenonsi et al. 1999; D'Atri et al. 2002) are indicated by red lines. The colored circles in the space between cells represent solutes that are selectively blocked or not blocked by the TJ barrier, which forms a selective filter for the paracellular pathway. The barrier function of TJ is controlled by the expression of specific claudin isoforms and by the cytoskeleton (see text)
Topologically, TJ are always closely associated with circumferential adherens junctions (AJ), also known as zonulae adhaerentes (ZA), which are cadherin-based junctions that contain afadin and PLEKHA7. The reader is referred to excellent reviews for a more detailed description of the organization of the junctional complex in general and adherens junctions in particular, and their role in mechano-transduction (Brieher and Yap 2013; Charras and Yap 2018; Ladoux et al. 2015; Meng and Takeichi 2009; Sluysmans et al. 2017; Takeichi 2014). Importantly, some TJ proteins, such as ZO-1, are localized at AJ during TJ biogenesis, are localized at AJ in cells lacking TJ (Fesenko et al. 2000; Howarth et al. 1992; Itoh et al. 1993), and interact with AJ mechano-sensing proteins. For example, ZO-1 binds to α-catenin (Itoh et al. 1997; Maiers et al. 2013) and vinculin (Zemljic-Harpf et al. 2014). ZO proteins and other TJ components are localized along lateral contacts either in developing or mature epithelia (Fesenko et al. 2000; Paschoud et al. 2007; Rahner et al. 2001). Taken together, these and other observations suggest that TJ formation in polarized epithelia involves the spatial reorganization and segregation of TJ proteins from AJ/ZA, which enables the clustering of transmembrane ligands and assembly of TJ fibrils (Campbell et al. 2017). One unanswered question in TJ mechanobiology is to understand to what extent variable forces along lateral contacts, ZA, TJ, and the cytoplasm control these different pools of TJ proteins.
In this minireview, I will first summarize a few basic concepts about the molecular composition of TJ, and its linkages to the cytoskeleton. I will next discuss recently published work which addresses the question of how TJ may contribute to regulating cellular and tissue responses to mechanical force. Finally, I will address the issue of molecular mechanisms, which concerns the identity of the TJ components that are directly or indirectly implicated in the generation of and response to force, and the downstream targets and pathways of this regulation.
Molecular anatomy of TJ, and structural and functional association of TJ with the cytoskeleton
TJ are constituted by: (1) transmembrane proteins; (2) cytoplasmic scaffolding and adaptor proteins and associated signaling proteins; (3) cytoskeletal filaments; (4) membrane lipids.
The transmembrane TJ proteins comprise claudins, TAMPs (TJ-associated MARVEL proteins: occludin, tricellulin, marvelD3) and immunoglobulin-like cell-adhesion molecules (Ig-like CAMs) (Shen et al. 2011; Tsukita et al. 2019; Zihni et al. 2016). Claudins, of which at least 27 isoforms exist in human tissues, are composed of short cytoplasmic N- and C-termini, four membrane-spanning regions, and short and large extracellular loops. First, single molecule magnetic tweezer experiments show that force is required to disrupt the interaction between the C-terminal and the ZPSG regions of ZO-1, and this force is within a physiological range (5–25 pN). TAMPs also show four membrane-spanning regions, but have little sequence similarity to claudins. Claudin-based polymers form size- and charge-selective barriers and channels/pores for the paracellular flux of ions (Gunzel and Yu 2013; Krug et al. 2012; Tsukita et al. 2019). The passage of larger solutes and cells through the paracellular barrier is mediated by temporary breaks in the strand network, and is regulated by the cytoskeleton, by TJ-associated Ig-like adhesion molecules, and by TAMPs (Anderson and Van Itallie 2009; Luissint et al. 2016; Shen et al. 2011; Zihni et al. 2016). Ig-like CAMs include JAM-A, CAR (Coksackie and Adenovirus Receptor), ESAM, and angulins, and all contain one membrane-spanning region, and an extracellular region with Ig-like domains. Tricellulin and angulins are specifically accumulated at tricellular junctions (Furuse et al. 2014). Transmembrane TJ proteins are anchored and/or clustered at TJ through interaction of their C-terminal cytoplasmic tails with scaffolding molecules, and through cis-interactions.
The cytoplasmic scaffolding proteins of TJ comprise ZO proteins (ZO-1, ZO-2, ZO-3), the membrane-associated guanylate kinase inverted proteins (MAGI-1 and MAGI-3), the multi-PDZ protein MUPP1, and the polarity proteins PAR-3, PALS-1, and PATJ. Additional cytoplasmic TJ proteins include the angiomotin family proteins, and the cingulin family proteins (cingulin and paracingulin-JACOP) (reviewed in (Citi et al. 2012; Fanning et al. 2012; Guillemot et al. 2008; Shin et al. 2006; Tsukita et al.; Zihni et al. 2016)). The ZA-associated protein afadin/AF-6, which links JAM-A and nectins to the actin cytoskeleton (and is crucially important for epithelial morphogenesis) has also been alternatively considered as a TJ- or ZA-associated protein (Ikeda et al. 1999; Takai and Nakanishi 2003; Yamamoto et al. 1997) (Fig. 1). These proteins are implicated not only in scaffolding of TJ transmembrane proteins, organization of apico-basal polarity, and linkage to the cytoskeleton, but also in the regulation of signaling (Citi et al. 2014).
Interaction with the cytoskeleton is crucial for mechano-sensing and mechano-transduction. In this respect, the TJ proteins that interact with cytoskeletal polymers are the ZO proteins, which interact with actin filaments (Fanning et al. 1998; Fanning et al. 2002; Van Itallie et al. 2009), and cingulin, which interacts with actin filaments (D'Atri and Citi 2001), myosin (Cordenonsi et al. 1999) and microtubules (Mangan et al. 2016; Yano et al. 2013) (Fig. 1). Cingulin is recruited to TJ by ZO-1 (Cordenonsi et al. 1999; D'Atri et al. 2002; Umeda et al. 2004). Afadin is also an actin-binding ZA/TJ protein, it binds to ZO-1 and JAM-A, and it is involved in the regulation of barrier function (Sakakibara et al. 2018; Tanaka-Okamoto et al. 2011). Furthermore, it cooperates with ZO-1 in force regulation at junctions (Choi et al. 2011; Choi et al. 2016) (Fig. 1). ZO proteins are multidomain proteins comprising 3 N-terminal PDZ domains, a central region with SH3 and GUK domains, and a C-terminal region of varying length (reviewed in (Fanning and Anderson 2009)). Claudins and JAM-A interact with the PDZ1 and PDZ3 domains, respectively, and occludin interacts with the GUK domain of ZO-1. The second PDZ domain of ZO proteins is involved in heterodimerization with another ZO protein (Fanning and Anderson 2009). Depletion of both ZO-1 and ZO-2 abolishes the formation of claudin-based TJ fibrils, indicating that heterodimerization of ZO proteins is critical to their ability to nucleate claudin assembly into fibrils (Umeda et al. 2006). Actin filaments bind to the C-terminal regions of ZO-1 and ZO-2, which contain an actin-binding region (ABR) (Fanning et al. 1998). Cingulin is a homodimer, each subunit comprising a globular head domain, a coiled-coil rod region, and a small globular tail (Cordenonsi et al. 1999). While the head domain of cingulin interacts with ZO proteins, actin, and microtubules (Cordenonsi et al. 1999; D'Atri and Citi 2001; Mangan et al. 2016; Yano et al. 2013), its coiled-coil region binds to GEFs and GAPs of the Rho family GTPases (Aijaz et al. 2005; Guillemot et al. 2014) and to myosin (Cordenonsi et al. 1999).
Actin filaments and microtubules are the two major cytoskeletal polymers that are associated with TJ (reviewed in (Anderson and Van Itallie 2009; Buckley and Turner 2018; Fanning and Anderson 2009; Fanning et al. 2012; Turner 2000; Van Itallie et al. 2015; Vasileva and Citi 2018)). The presence and functional relevance of actin filaments in the submembrane cortex of TJ was demonstrated by early electron microscopy and pharmacological studies (Madara 1987; Meza et al. 1982). The circumferential actomyosin belt associated with apical junctions (TJ and ZA) is organized in sarcomeric-like units, with a periodic array of nonmuscle myosin II bipolar filaments, which overlap with the basal half of the TJ and the apical end of the ZA (Ebrahim et al. 2013). ZO-1 and ZO-2 are the main cytoplasmic scaffolds which connect TJ membrane proteins to actin filaments (Fig. 1). The tethering of claudins to actin through ZO-1 is dynamic and intermittent, probably to allow for adaptation to changes in cell shape or movement (Van Itallie et al. 2009). Regarding microtubules, there is so far limited evidence that they are involved in transmission of, and resistance to, force at apical junctions (Ko et al. 2019; Singh et al. 2018; Takeda et al. 2018). At vertebrate TJ, microtubules are anchored to cingulin, which organizes them into planar apical networks, and promotes the generation of the apical luminal membrane of epithelial cells (Mangan et al. 2016; Yano et al. 2013). Although cingulin binds to actin filaments and to myosin (see above), its role in the regulation of global actin filament organization and downstream signaling appears to be dependent mainly on its ability to sequester GEF-H1 at junctions (Aijaz et al. 2005; Guillemot et al. 2004; Guillemot et al. 2012; Paschoud and Citi 2008). Moreover, although microtubules are essential for efficient junction biogenesis and polarized cytoplasm organization (Vasileva and Citi 2018), their potential role in junctional mechano-sensing and mechano-transduction is only beginning to be investigated (De Pascalis and Etienne-Manneville 2017).
As of the present time, the role of lipids in TJ organization is still not well understood. Before the discovery of claudins, a model was proposed whereby TJ were formed by a specific organization of lipids into “inverted micelles” (Pinto da Silva and Kachar 1982). Although it is now clear that barrier and permeability to ions is controlled by claudin strands, there is evidence for a contribution of lipids to the structure and physiology of TJ. For example, cholesterol depletion results in loss of barrier function, and occludin endocytosis is regulated by its interaction with the lipid raft-associated protein caveolin-1 (Marchiando et al. 2010; Shigetomi et al. 2018). AJs mediate the formation of TJs by increasing the level of cholesterol in the PM (Shigetomi et al. 2018). Interestingly, tetraspanins are four-membrane-pass proteins that are unrelated to claudins and TAMPs, but have been implicated in barrier function of septate junctions in invertebrates (Izumi et al. 2016), and in targeting of transmembrane receptors to the ZA (Shah et al. 2018). Since tetraspanins are associated with cholesterol-rich membranes, and require cholesterol for their proper functioning, one hypothesis is that ZA tetraspanins, such as Tspan33 (Shah et al. 2018), help to generate and/or maintain a cholesterol-rich environment, which then promote the correct functioning of claudins and TAMPs. These hypotheses require further testing, and the role of lipids in the mechanics and dynamics of transmembrane TJ proteins is an interesting area for future investigations.
The impact of mechanical force on TJ function
Multiple physiological and pathophysiological inputs affect TJ barrier function through modulation of actomyosin-dependent force. Furthermore, tensile, compressive and shear forces within tissues and cells can be transduced to TJ protein complexes, to affect TJ functions.
When considering the regulation of TJ barrier function by actomyosin, it is necessary to clarify that the TJ paracellular barrier comprises two elements (Shen et al. 2011): (1) the “pore” pathway: a high-capacity, highly selective route, that is based on claudins, and regulates the flux of ions and water. This is measured by the instantaneous assessment of transepithelial electrical resistance (TEER); 2) the “leak” pathway”: a low-capacity route, which regulates the passage of larger molecules, depends on transient breaks within the network of TJ fibrils, and is measured by the passage of fluorescent tracers across epithelial monolayers within a longer time scale (typically 2 h). These two routes are regulated independently (Shen et al. 2011). While tissue-specific claudin isoform expression dictates the properties of an epithelium with regard to size- and charge-selectivity of the pore pathway (Gunzel and Yu 2013), the regulation of TJ transmembrane proteins by their scaffolding proteins and by cytoskeletal force impacts differently on pore and leak pathways. For example, depletion of ZO-1 destabilizes the barrier to large solutes, but does not disrupt the claudin-based pores (Van Itallie et al. 2009), presumably due to the functional redundancy between ZO-1 and ZO-2 in forming scaffolds for claudin polymers (Umeda et al. 2006). Similarly, physiological and pathological mechanisms mediated by the activation of myosin light chain kinase (MLCK) act primarily on the leak pathway (Shen et al. 2011; Turner et al. 1997; Wang et al. 2005). Inhibition of ROCK-mediated myosin light chain phosphorylation reduces permeability to ions, whereas it increases permeability to larger molecules in a ZO-1-dependent manner, suggesting that in ZO-1-depleted cells there are more frequent transient breaks in the contacts (Van Itallie et al. 2009). However, in cells treated with actin-disrupting drugs, and additionally when cadherin-based adhesion is compromised by removal of extracellular calcium, ZO-1 has also found to be required to stabilize the pore pathway (Van Itallie et al. 2009). Collectively, these results indicate that a homeostatic, finely tuned level of actomyosin contractility is required both to allow claudins fibrils to operate correctly, and to reinforce the actin cytoskeleton to prevent leaks. This is supported by recent evidence, showing that breaches in the barrier are repaired by transient, localized RhoA activation (Rho “flares”) (Stephenson et al. 2019). However, when actomyosin-mediated force is too high, more frequent breaks in the strands result in loss of barrier function of the leak pathway.
Cadherin-based AJ play an important role both in protecting TJ from the effects of excessive tension, and in promoting TJ formation. During cytokinesis of Xenopus epithelia, the tension from the ingressing contractile ring is transmitted primarily to the AJ, which are reinforced in a vinculin-dependent manner, allowing for the maintenance of TJ barrier function (Higashi et al. 2016). Vinculin was recently shown to be indispensable for the paracellular barrier to ions, by dampening mechanical fluctuations applied to the TJ (Konishi et al. 2019). In the upper layer of the stratified epithelium of the skin, enrichment of ZO-1 and development of the TJ barrier correlates with the development of high mechanical tension at AJ (Rubsam et al. 2017). Confirming the key role of cadherin-based junctions on TJ barrier regulation, a pathological increase in cadherin endocytosis weakens cell-cell adhesion, and causes leakage of bacterial antigens through the TJ barrier, contributing to inflammatory bowel disease (Mohanan et al. 2018). Earlier studies showed that TJ disruption following actin filament depolymerization or inhibition of cadherins is an active, energy-dependent process, that involves endocytic removal of occludin from TJ (Ivanov et al. 2004; Madara et al. 1986), which destabilizes the leak pathway (Shen et al. 2011). Loss of junctional occludin, but not ZO-1, is also observed in lung alveolar epithelial cells subjected to intense mechanical stretch or depleted of ATP (Cavanaugh et al. 2001). On the other hand, lung endothelial cells subjected to cyclic mechanical strain show altered barrier function, due to the disassembly of microtubules, which cross-talk with the actin cytoskeleton through the release of microtubule-associated GEF-H1, that promotes stress fiber formation (Birukova et al. 2004; Birukova et al. 2010).
TJ as regulators of cell and tissue mechanics
Information on the magnitude, direction, and distribution of forces and stresses in individual cells or groups of cells can be obtained by different biophysical techniques, including laser ablation and measurement of recoil velocity, FRET sensors, traction force microscopy, non-contact acoustic frequency-modulation, optical tweezers, magnetic twisting cytometry, and atomic force microscopy (AFM) (Sugimura et al. 2016). Thus, studies on cells depleted of one or more TJ proteins have allowed to investigate their role in the organization and contractility of the actomyosin cytoskeleton, cell migration, generation of membrane and cortical tension, and resistance to, and transmission of, monolayer force.
Early studies on MDCK and Eph4 cells depleted of/or knock-out (KO) for ZO-1 did not report major changes in epithelial morphology, junction integrity, and actin organization (McNeil et al. 2006; Umeda et al. 2004). However, cells lacking ZO-1 were later shown to have an altered morphology, characterized by a loss of tortuosity at the apical junction, and changes in apical actin localization and junctional tension (Tokuda et al. 2014; Tornavaca et al. 2015; Van Itallie et al. 2009). Moreover, targeting either ZO-1 or other TJ proteins alters cellular migration velocities, tractions, and intracellular tensions in spreading monolayers of mammary (MCF10A) cells grown on micropatterned sheets (Bazellieres et al. 2015). Downregulation of ZO-1 and ZO-3 leads to marked but opposite changes in monolayer mechanics, and ZO-1 is unique, since only its depletion results in a phenotype in which cells migrate and deform rapidly, while exerting strong cell-cell and cell-substrate forces (Bazellieres et al. 2015). Physiologically, most epithelial cells are present in the context of a stable, confluent epithelium. Recent analysis of such epithelia within the intestine of conditional ZO-1 KO mice indicates that ZO-1 is essential to maintain a uniform epithelial apical surface, and a normal shape and length of apical microvilli (Odenwald et al. 2018). In confluent MDCK cells, depletion of ZO-1, but not ZO-2, recapitulates the aberrant apical architecture induced by ZO-1 KO in vivo, resulting in the formation of cytoplasmic actomyosin rings, and increased cell height (Odenwald et al. 2018). In agreement, ZO-1 depletion in confluent MDCK cells results in changes in the AFM deflection and height images of the apical surfaces of the monolayer, as well as changes in the apical and basal actin filament organization (Bruckner and Janshoff 2018). However, mechanical parameters such as overall tension, comprising cortical tension and membrane tension, and apparent area compressibility modulus are not significantly affected by ZO-1 depletion, whereas depletion of cadherins leads to a considerable drop in cortical and membrane tension (Bruckner and Janshoff 2018). Data obtained using Xenopus gastrulae in vivo confirm the notion that ZO-1 is an important modulator of actomyosin organization and contractility. Using an actin fluorescent probe and a FRET tension-sensing cadherin construct it was shown that there is an increase in global tensile forces applied to AJ in cells depleted of either ZO-1 or GEF-H1 (Hatte et al. 2018). This generates a flattened contractile ring, a slower apical constriction of the ring, and cytokinesis defects (Hatte et al. 2018). Thus, it was proposed that GEF-H1 and ZO-1 are implicated in the negative regulation of global tensile forces applied to the AJ (Hatte et al. 2018).
Depletion of both ZO-1 and ZO-2 leads to an even more striking phenotype than depletion of ZO-1 alone. MDCK cells depleted of both ZO proteins show a dramatic thickening of the circumferential, peri-junctional actin cytoskeleton associated with apical junctions, with the formation of sarcomere-like arrays of actomyosin (Fanning et al. 2012). In mammary (Eph4) cells, depletion of both ZO proteins delays the formation of the circumferential actomyosin bundle associated with AJ, and prevents myosin II integration into AJ, suggesting that ZO-1 is required for the correct formation of the actomyosin belt of the ZA (Yamazaki et al. 2008). In agreement, laser-ablation experiments show an increase in recoil velocity in double knock-down cells, indicating an increase in epithelial tension (Choi et al. 2016). AFM measurements provide additional evidence that simultaneous depletion of both ZO-1 and ZO-2 elevates the apical epithelial tension and effective viscosity (Cartagena-Rivera et al. 2017). Moreover, at tricellular junctions, there is an increase in epithelial intercellular pulling forces, adhesion, and contractility in the absence of both ZO-1 and ZO-2 (Cartagena-Rivera et al. 2017).
Collectively, these studies show that ZO proteins, and more specifically ZO-1, are central players in the organization and contractility of cytoplasmic and peri-junctional actomyosin, in microvillar cytoskeleton dynamics, and in cortical and membrane tension. The function of ZO-1 appears to be, at least in part, to dissipate mechanical stresses that are otherwise generated at the ZA, and to regulate actin dynamics. In addition, ZO proteins fine tune the transmission of force from and to TJ transmembrane proteins, to adapt barrier function to the changing physiological requirements of the tissue.
Molecular mechanisms — I: mechanical adhesion by TJ transmembrane proteins
Transduction of force across cells in a monolayer requires adhesive junctions and adhesion receptors. At AJ, cadherins and Ig-like transmembrane proteins provide mechanical adhesion strength through homophylic interactions between their extracellular domains, and transduce force to their cytoplasmic interactors (Conway and Schwartz 2015; Leckband and de Rooij 2014). However, little is known about mechano-sensing and mechano-transduction by TJ membrane proteins. To sense and respond to mechanical forces, TJ transmembrane proteins must display adhesive properties.
The observation that exogenous expression of either occludin or different claudin isoforms in fibroblasts confers calcium-independent adhesion indicates that transmembrane TJ proteins are indeed cell adhesion molecules (Kubota et al. 1999; Van Itallie and Anderson 1997). However, antibody-induced cell-dissociation and cell re-aggregation assays indicate that the cell-cell adhesion activity of occludin is weaker than that of several members of the claudin family (claudin-1, claudin-2, and claudin-3), and these in turn provide a mechanically much weaker adhesion than that conferred by cadherins (Kubota et al. 1999). The same conclusion is supported by measurements of adhesive force using either the dual micropipette assay (Vedula et al. 2009) or AFM (Lim et al. 2008). Between 2 and 3 nN are required to separate fibroblasts expressing either claudin-1 or claudin-2, less than 1.5 nN is required when cells express either occludin or a mix of claudin-1 and claudin-2 (Vedula et al. 2009), whereas 29 nM is required to separate cells that express cadherins (Martinez-Rico et al. 2005). It should be noted that these measurements do not take into full account the potential effects of claudin clustering by scaffolding molecules, and their stabilization by the actin cytoskeleton, which likely do not occur in fibroblasts (Vedula et al. 2009). Finally, Ig-like adhesion molecules such as JAMs and CAR undergo homophylic interactions of cis-dimers (Ebnet et al. 2004), but the mechanical strength of their adhesion is not known.
Molecular mechanisms — II: regulation of actin-binding proteins, Rho GTPases, and junctional contractility
TJ proteins are both the targets and effectors of a number of signaling pathways, which impact on force generation by the cytoskeleton, and on the ability of cells to respond to force.
First, TJ proteins bind not only to actin filaments and microtubules, but to additional cytoskeletal proteins. Besides α-catenin and vinculin (Birukova et al. 2016; Maiers et al. 2013), ZO-1 also binds to the PDZ protein shroom2, which links it to myosin-VII (Etournay et al. 2007). ZO-1 and ZO-2 also bind to cortactin, which stabilizes the assembly of branched actin filaments at the plasma membrane (Katsube et al. 1998). ZO-1, ZO-2, and occludin bind to protein 4.1, which could provide a link between the spectrin and the actin cytoskeletons (Mattagajasingh et al. 2000). These interactions may be critical in the ability of ZO proteins to organize the cytoskeleton and mediate force transmission.
Rho family GTPases are essential in the regulation of multiple aspects of actin cytoskeleton assembly, dynamics, and contractility. Critically, several TJ proteins interact with regulators of Rho family GTPases pathways (GEFs and GAPs), although it is not always clear whether these interactions result in sequestration and inactivation, or activation (Braga 2017; Citi et al. 2014). For example, ZO-1 binds to the Cdc42 GEF Tuba (Otani et al. 2006), and to the Rho GEF ARHGEF11 (Itoh et al. 2012). Cingulin binds to the Rho GEFs GEF-H1 and p114RhoGEF (Aijaz et al. 2005; Terry et al. 2011), and to the Rac GTPase MgcRacGAP (Guillemot et al. 2014), paracingulin to the Cdc42 and Rac GAP SH3BP1 (Elbediwy et al. 2012). JAM-A, ZO-2, and MAGI-I functionally interact with Rap family GTPases (Birukova et al. 2011; Mino et al. 2000; Monteiro et al. 2013), and angiomotin with the Cdc42 GAP Rich1 (Wells et al. 2006). These interactions collectively control the spatially restricted and/or global activation and inactivation of Cdc42, Rac1, and RhoA at junctions, thus allowing dynamic remodeling of the cytoskeleton both during junction assembly, and at homeostasis (Braga 2017; Citi et al. 2014).
One of the major downstream effectors of RhoA is ROCK kinase, which in turn promotes increased myosin light chain phosphorylation. Increased MLCK activity and activation of peri-junctional myosin increases solute flux through the leak pathway, induced by physiological and pathological signals, such as sodium-dependent glucose transport, and stimulation by TNF, through occludin endocytosis (Marchiando et al. 2010; Shen et al. 2011; Van Itallie et al. 2010). FRAP studies show that ZO-1 interactions mediated by the actin-binding region (ABR) are critical for MLCK-dependent barrier regulation. The rate of ZO-1 exchange between junction-associated and cytoplasmic pools of ZO-1 also requires actomyosin function (Shen et al. 2008; Yu et al. 2010), and the presence of the ABR greatly enhances this localization of ZO-1 along a continuous circumferential belt (Fanning and Anderson 2009; Itoh et al. 2012). Thus, junctional actomyosin controls ZO-1, and in turn ZO-1 transmits the force generated by the circumferential junctional actomyosin arrays to its transmembrane ligands.
Molecular mechanisms — III: force-dependent conformational changes in ZO proteins
Using structured illumination microscopy, we showed that ZO-1 exists in either extended (stretched) or folded (auto-inhibited) conformations, depending on force and heterodimerization, resulting in modulation of its molecular interactions and downstream signaling (Spadaro et al. 2017). Either inhibiting myosin activity or disrupting the organization of actin filaments does not, alone, affect the conformation of junctional ZO-1 in WT cells. However, when disruption of actomyosin is coupled to depletion of ZO-2, ZO-1 assumes a “folded” conformation, indicating that either heterodimerization or actomyosin-dependent force are sufficient to stabilize the extended conformation of ZO-1. We discovered an intramolecular interaction between the C-terminal region of ZO-1, which comprises the ZU5 domain, and its central region, comprising PDZ3, SH3, and GUK domains (ZPSG) (Spadaro et al. 2017). The C-terminal region competitively inhibits the interaction of the ZPSG with the transcription factor ZONAB/DbpA and the TJ transmembrane protein occludin. Correspondingly, under the conditions where ZO-1 is in a folded conformation within cells, there is a loss of junctional DbpA and occludin, indicating that when ZO-1 undergoes the ZU5-ZPSG intramolecular interaction, the ZPSG is unable to bind to its junctional ligands. This correlates with proteasomal degradation and loss of signaling by DbpA, and increased sensitivity of the TJ barrier to disruption of actomyosin contractility (Spadaro et al. 2017). The intramolecular interaction occurs also in ZO-2, and cells lacking ZO-1 also show decreased junctional localization of DbpA and occludin, suggesting that ZO-2 also undergoes intramolecular folding in the absence of heterodimerization and force (Spadaro et al. 2017). Our observations suggest that the actin-linked ZO-1 pool, which exchanges slowly between the cytosol and the TJ and is sensitive to MLCK inhibition (Yu et al. 2010) is constituted by stretched molecules, whereas the pool that exchanges rapidly between the tight junction and the cytoplasm (Yu et al. 2010) may be at least in part in a folded conformation. The concept that a certain degree of tension is required to stabilize ZO-1 is supported by the observation that calyculin A, which increases tension, decreases the ZO-1 mobile fraction in Xenopus embryos (Higashi et al. 2016).
The idea that force, and not other signals, such as post-translational modifications or drug-dependent modulation of biochemical pathways, modifies ZO conformation, is supported by multiple lines of evidence (Spadaro et al. 2017). First, single molecule magnetic tweezer experiments show that force is required and sufficient to disrupt the interaction between the C-terminal and the ZPSG regions of ZO-1, and this force is within a physiological range (5–25 pN). Second, not one, but multiple different pharmacological tools, which inhibit either myosin-dependent force generation, or the integrity of actin filaments, similarly promote ZO-1 folding, when coupled to ZO-2 depletion. Third, the signaling outputs mediated by folded ZO proteins, e.g., degradation of DbpA/ZONAB transcription factor, resulting in stunted proliferation and growth, were observed in cysts grown in Matrigel, in the absence of any drugs. Thus, growth of cysts in a soft substrate is equivalent to treating cells grown on a stiff substrate (glass or plastic) with blebbistatin, and physiological forces developed at junctions and heterodimerization are sufficient to control ZO protein conformation, independently of any drug. Fourth, treating ZO-1-KO cysts with dATP, which stimulates actomyosin contractility, rescues normal DbpA localization, levels and activity, consistent with actomyosin-dependent force acting on ZO-2, to induce its stretching.
In summary, ZO-1 and ZO-2 are TJ mechano-sensors, and their force- and heterodimerization-dependent conformations control their interactions with ligands at junctions and downstream functions.
Conclusions and perspectives
TJ participate in the regulation of the mechanical properties of epithelial monolayers, although their contribution is quantitatively less important than that of cadherin-based ZA/AJ. The pore and leak pathways of the paracellular TJ barrier are finely tuned by the contractility of the actomyosin cytoskeleton, through ZO proteins, and the cross-talk with AJ and regulators and effectors of Rho family GTPases. ZO proteins are not only critical organizers of junctional actomyosin and actin dynamics but also mechano-sensors, which transduce mechanical cues into regulation of signaling to the nucleus and barrier function.
Much remains to be learned about TJ mechanobiology. Are there additional mechano-sensors at TJ, besides ZO proteins? Which additional protein interactions and signaling outputs to/from TJ are regulated by force? What are the conformational and dimerization states of ZO proteins from the time of their synthesis to their assembly as TJ scaffolds for claudins? Are there additional pathways that control ZO protein conformation? What mechanisms underlie the regulation of microvilli and junctional actin dynamics by ZO-1? Addressing these and other questions will provide exciting new avenues in TJ mechanobiology.
Acknowledgments
I am grateful to Sophie Sluysmans, Ekaterina Vasileva, Florian Rouaud, and Jimit Shah for comments on the manuscript. The Citi laboratory is supported by the Swiss National Foundation, the State of Geneva, and the Novartis Foundation.
Abbreviations
- ABR
actin binding region
- AFM
atomic force microscopy
- AJ
adherens junction
- CAM
cell adhesion molecule
- CAR
Coxsackie adenovirus receptor
- dATP
deoxyadenosine triphosphate
- DbpA
DNA binding protein A
- ESAM
endothelial cell-selective adhesion molecule
- FRAP
fluorescence recovery after photobleaching
- FRET
fluorescence resonance energy transfer
- GAP
GTPase activating protein
- GEF
guanine nucleotide exchange factor
- GUK
guanylate kinase (domain)
- JAM
junction associated molecule
- MAGI
membrane-associated guanylate kinase with Inverted orientation
- MDCK
Madin Darby Canine Kidney
- MLCK
myosin light chain kinase
- PALS
protein associated with Lin-7
- PAR
partition-defective (gene/protein)
- PATJ
Pals1-associated tight junction protein
- PDZ
PSD-95/disks large/zonula occludens-1 (domain)
- PLEKHA7
Pleckstrin Homology Domain Containing A7
- ROCK
Rho-associated protein Kinase
- SH3
Src homology-3
- TAMPS
TJ-associated MARVEL proteins
- TEER
trans-epithelial electrical resistance
- TJ
tight junction
- TNF
tumor necrosis factor
- ZA
zonula adhaerens
- ZO
zonula occludens
- ZO-1
zonula-occludens-1 (protein)
- ZO-2
zonula-occludens-2 (protein)
- ZO-3
zonula-occludens-3 (protein)
- ZONAB
ZO-1–associated nucleic acid binding protein
- ZPSG
ZO protein PDZ3-SH3-GUK (domains)
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aijaz S, D'Atri F, Citi S, Balda MS, Matter K. Binding of GEF-H1 to the tight junction-associated adaptor cingulin results in inhibition of Rho signaling and G1/S phase transition. Dev Cell. 2005;8:777–786. doi: 10.1016/j.devcel.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol. 2009;1(a002584):1–16. doi: 10.1101/cshperspect.a002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazellieres E, Conte V, Elosegui-Artola A, Serra-Picamal X, Bintanel-Morcillo M, Roca-Cusachs P, Munoz JJ, Sales-Pardo M, Guimera R, Trepat X. Control of cell-cell forces and collective cell dynamics by the intercellular adhesome. Nat Cell Biol. 2015;17:409–420. doi: 10.1038/ncb3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birukova AA, Smurova K, Birukov KG, Usatyuk P, Liu F, Kaibuchi K, Ricks-Cord A, Natarajan V, Alieva I, Garcia JG, Verin AD. Microtubule disassembly induces cytoskeletal remodeling and lung vascular barrier dysfunction: role of Rho-dependent mechanisms. J Cell Physiol. 2004;201:55–70. doi: 10.1002/jcp.20055. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Fu P, Xing J, Yakubov B, Cokic I, Birukov KG. Mechanotransduction by GEF-H1 as a novel mechanism of ventilator-induced vascular endothelial permeability. Am J Physiol Lung Cell Mol Physiol. 2010;298:L837–L848. doi: 10.1152/ajplung.00263.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birukova AA, Zebda N, Fu P, Poroyko V, Cokic I, Birukov KG. Association between adherens junctions and tight junctions via Rap1 promotes barrier protective effects of oxidized phospholipids. J Cell Physiol. 2011;226:2052–2062. doi: 10.1002/jcp.22543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birukova AA, Shah AS, Tian Y, Moldobaeva N, Birukov KG. Dual role of vinculin in barrier-disruptive and barrier-enhancing endothelial cell responses. Cell Signal. 2016;28:541–551. doi: 10.1016/j.cellsig.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga Vania. Signaling by Small GTPases at Cell–Cell Junctions: Protein Interactions Building Control and Networks. Cold Spring Harbor Perspectives in Biology. 2017;10(10):a028746. doi: 10.1101/cshperspect.a028746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brieher WM, Yap AS. Cadherin junctions and their cytoskeleton(s) Curr Opin Cell Biol. 2013;25:39–46. doi: 10.1016/j.ceb.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Bruckner BR, Janshoff A. Importance of integrity of cell-cell junctions for the mechanics of confluent MDCK II cells. Sci Rep. 2018;8:14117. doi: 10.1038/s41598-018-32421-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley Aaron, Turner Jerrold R. Cell Biology of Tight Junction Barrier Regulation and Mucosal Disease. Cold Spring Harbor Perspectives in Biology. 2017;10(1):a029314. doi: 10.1101/cshperspect.a029314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell HK, Maiers JL, DeMali KA. Interplay between tight junctions & adherens junctions. Exp Cell Res. 2017;358:39–44. doi: 10.1016/j.yexcr.2017.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartagena-Rivera AX, Van Itallie CM, Anderson JM, Chadwick RS. Apical surface supracellular mechanical properties in polarized epithelium using noninvasive acoustic force spectroscopy. Nat Commun. 2017;8:1030. doi: 10.1038/s41467-017-01145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh KJ, Jr, Oswari J, Margulies SS. Role of stretch on tight junction structure in alveolar epithelial cells. Am J Respir Cell Mol Biol. 2001;25:584–591. doi: 10.1165/ajrcmb.25.5.4486. [DOI] [PubMed] [Google Scholar]
- Charras G, Yap AS. Tensile forces and mechanotransduction at cell-cell junctions. Curr Biol. 2018;28:R445–R457. doi: 10.1016/j.cub.2018.02.003. [DOI] [PubMed] [Google Scholar]
- Choi W, Jung KC, Nelson KS, Bhat MA, Beitel GJ, Peifer M, Fanning AS. The single Drosophila ZO-1 protein Polychaetoid regulates embryonic morphogenesis in coordination with canoe/afadin and enabled. Mol Biol Cell. 2011;22:2010–2030. doi: 10.1091/mbc.E10-12-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi W, Acharya BR, Peyret G, Fardin MA, Mege RM, Ladoux B, Yap AS, Fanning AS, Peifer M. Remodeling the zonula adherens in response to tension and the role of afadin in this response. J Cell Biol. 2016;213:243–260. doi: 10.1083/jcb.201506115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citi S. Intestinal barriers protect against disease. Science. 2018;359:1097–1098. doi: 10.1126/science.aat0835. [DOI] [PubMed] [Google Scholar]
- Citi S, Pulimeno P, Paschoud S. Cingulin, paracingulin, and PLEKHA7: signaling and cytoskeletal adaptors at the apical junctional complex. Ann N Y Acad Sci. 2012;1257:125–132. doi: 10.1111/j.1749-6632.2012.06506.x. [DOI] [PubMed] [Google Scholar]
- Citi S, Guerrera D, Spadaro D, Shah J. Epithelial junctions and rho family GTPases: the zonular signalosome. Small GTPases. 2014;5:1–15. doi: 10.4161/21541248.2014.973760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway DE, Schwartz MA. Mechanotransduction of shear stress occurs through changes in VE-cadherin and PECAM-1 tension: implications for cell migration. Cell Adhes Migr. 2015;9:335–339. doi: 10.4161/19336918.2014.968498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordenonsi M, D'Atri F, Hammar E, Parry DA, Kendrick-Jones J, Shore D, Citi S. Cingulin contains globular and coiled-coil domains and interacts with ZO-1, ZO-2, ZO-3, and myosin. J Cell Biol. 1999;147:1569–1582. doi: 10.1083/jcb.147.7.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Atri F, Citi S. Cingulin interacts with F-actin in vitro. FEBS Lett. 2001;507:21–24. doi: 10.1016/s0014-5793(01)02936-2. [DOI] [PubMed] [Google Scholar]
- D'Atri F, Nadalutti F, Citi S. Evidence for a functional interaction between cingulin and ZO-1 in cultured cells. J Biol Chem. 2002;277:27757–27764. doi: 10.1074/jbc.M203717200. [DOI] [PubMed] [Google Scholar]
- De Pascalis C, Etienne-Manneville S. Single and collective cell migration: the mechanics of adhesions. Mol Biol Cell. 2017;28:1833–1846. doi: 10.1091/mbc.E17-03-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebnet K, Suzuki A, Ohno S, Vestweber D. Junctional adhesion molecules (JAMs): more molecules with dual functions? J Cell Sci. 2004;117:19–29. doi: 10.1242/jcs.00930. [DOI] [PubMed] [Google Scholar]
- Ebrahim S, Fujita T, Millis BA, Kozin E, Ma X, Kawamoto S, Baird MA, Davidson M, Yonemura S, Hisa Y, Conti MA, Adelstein RS, Sakaguchi H, Kachar B. NMII forms a contractile transcellular sarcomeric network to regulate apical cell junctions and tissue geometry. Curr Biol. 2013;23:731–736. doi: 10.1016/j.cub.2013.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbediwy A, Zihni C, Terry SJ, Clark P, Matter K, Balda MS. Epithelial junction formation requires confinement of Cdc42 activity by a novel SH3BP1 complex. J Cell Biol. 2012;198:677–693. doi: 10.1083/jcb.201202094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etournay R, Zwaenepoel I, Perfettini I, Legrain P, Petit C, El-Amraoui A. Shroom2, a myosin-VIIa- and actin-binding protein, directly interacts with ZO-1 at tight junctions. J Cell Sci. 2007;120:2838–2850. doi: 10.1242/jcs.002568. [DOI] [PubMed] [Google Scholar]
- Fanning AS, Anderson JM. Zonula occludens-1 and -2 are cytosolic scaffolds that regulate the assembly of cellular junctions. Ann N Y Acad Sci. 2009;1165:113–120. doi: 10.1111/j.1749-6632.2009.04440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998;273:29745–29753. doi: 10.1074/jbc.273.45.29745. [DOI] [PubMed] [Google Scholar]
- Fanning AS, Ma TY, Anderson JM. Isolation and functional characterization of the actin binding region in the tight junction protein ZO-1. FASEB J. 2002;16:1835–1837. doi: 10.1096/fj.02-0121fje. [DOI] [PubMed] [Google Scholar]
- Fanning AS, Van Itallie CM, Anderson JM. Zonula occludens-1 and -2 regulate apical cell structure and the zonula adherens cytoskeleton in polarized epithelia. Mol Biol Cell. 2012;23:577–590. doi: 10.1091/mbc.E11-09-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesenko I, Kurth T, Sheth B, Fleming TP, Citi S, Hausen P. Tight junction biogenesis in the early Xenopus embryo. Mech Dev. 2000;96:51–65. doi: 10.1016/s0925-4773(00)00368-3. [DOI] [PubMed] [Google Scholar]
- Furuse M, Izumi Y, Oda Y, Higashi T, Iwamoto N. Molecular organization of tricellular tight junctions. Tissue Barriers. 2014;2:e28960. doi: 10.4161/tisb.28960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemot L, Hammar E, Kaister C, Ritz J, Caille D, Jond L, Bauer C, Meda P, Citi S. Disruption of the cingulin gene does not prevent tight junction formation but alters gene expression. J Cell Sci. 2004;117:5245–5256. doi: 10.1242/jcs.01399. [DOI] [PubMed] [Google Scholar]
- Guillemot L, Paschoud S, Pulimeno P, Foglia A, Citi S. The cytoplasmic plaque of tight junctions: a scaffolding and signalling center. Biochimica et Biophysica Acta (BBA)-Biomembranes. 2008;1778:601–613. doi: 10.1016/j.bbamem.2007.09.032. [DOI] [PubMed] [Google Scholar]
- Guillemot L, Schneider Y, Brun P, Castagliuolo I, Pizzuti D, Martines D, Jond L, Bongiovanni M, Citi S. Cingulin is dispensable for epithelial barrier function and tight junction structure, and plays a role in the control of claudin-2 expression and response to duodenal mucosa injury. J Cell Sci. 2012;125:5005–5012. doi: 10.1242/jcs.101261. [DOI] [PubMed] [Google Scholar]
- Guillemot L, Guerrera D, Spadaro D, Tapia R, Jond L, Citi S. MgcRacGAP interacts with cingulin and paracingulin to regulate Rac1 activation and development of the tight junction barrier during epithelial junction assembly. Mol Biol Cell. 2014;25:1995–2005. doi: 10.1091/mbc.E13-11-0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunzel D, Yu AS. Claudins and the modulation of tight junction permeability. Physiol Rev. 2013;93:525–569. doi: 10.1152/physrev.00019.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatte Guillaume, Prigent Claude, Tassan Jean-Pierre. Tight junctions negatively regulate mechanical forces applied to adherens junctions in vertebrate epithelial tissue. Journal of Cell Science. 2017;131(3):jcs208736. doi: 10.1242/jcs.208736. [DOI] [PubMed] [Google Scholar]
- Higashi T, Arnold TR, Stephenson RE, Dinshaw KM, Miller AL. Maintenance of the epithelial barrier and remodeling of cell-cell junctions during cytokinesis. Curr Biol. 2016;26:1829–1842. doi: 10.1016/j.cub.2016.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth AG, Hughes MR, Stevenson BR. Detection of the tight junction-associated protein ZO-1 in astrocytes and other nonepithelial cell types. Am J Phys. 1992;262:C461–C469. doi: 10.1152/ajpcell.1992.262.2.C461. [DOI] [PubMed] [Google Scholar]
- Ikeda W, Nakanishi H, Miyoshi J, Mandai K, Ishizaki H, Tanaka M, Togawa A, Takahashi K, Nishioka H, Yoshida H, Mizoguchi A, Nishikawa S, Takai Y. Afadin: a key molecule essential for structural organization of cell-cell junctions of polarized epithelia during embryogenesis. J Cell Biol. 1999;146:1117–1132. doi: 10.1083/jcb.146.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikenouchi J, Suzuki M, Umeda K, Ikeda K, Taguchi R, Kobayashi T, Sato SB, Kobayashi T, Stolz DB, Umeda M. Lipid polarity is maintained in absence of tight junctions. J Biol Chem. 2012;287:9525–9533. doi: 10.1074/jbc.M111.327064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Nagafuchi A, Yonemura S, Yasuda-Kitani T, Tsukita S, Tsukita S. The 220kD protein colocalizing with cadherins in non-epithelial cells is identical to ZO-1, a tight junction-associated protein in epithelial cells: cDNA cloning and immunoelectron microscopy. J Cell Biol. 1993;121:491–502. doi: 10.1083/jcb.121.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Nagafuchi A, Moroi S, Tsukita S. Involvement of ZO-1 in cadherin-based cell adhesion through its direct binding to alpha catenin and actin filaments. J Cell Biol. 1997;138:181–192. doi: 10.1083/jcb.138.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Tsukita S, Yamazaki Y, Sugimoto H. Rho GTP exchange factor ARHGEF11 regulates the integrity of epithelial junctions by connecting ZO-1 and RhoA-myosin II signaling. Proc Natl Acad Sci U S A. 2012;109:9905–9910. doi: 10.1073/pnas.1115063109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov AI, McCall IC, Parkos CA, Nusrat A. Role for actin filament turnover and a myosin II motor in cytoskeleton-driven disassembly of the epithelial apical junctional complex. Mol Biol Cell. 2004;15:2639–2651. doi: 10.1091/mbc.E04-02-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi Y, Motoishi M, Furuse K, Furuse M. A tetraspanin regulates septate junction formation in Drosophila midgut. J Cell Sci. 2016;129:1155–1164. doi: 10.1242/jcs.180448. [DOI] [PubMed] [Google Scholar]
- Katsube T, Takahisa M, Ueda R, Hashimoto N, Kobayashi M, Togashi S. Cortactin associates with the cell-cell junction protein ZO-1 in both Drosophila and mouse. J Biol Chem. 1998;273:29672–29677. doi: 10.1074/jbc.273.45.29672. [DOI] [PubMed] [Google Scholar]
- Ko Clint S., Tserunyan Vardges, Martin Adam C. Microtubules promote intercellular contractile force transmission during tissue folding. The Journal of Cell Biology. 2019;218(8):2726–2742. doi: 10.1083/jcb.201902011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi Satoshi, Yano Tomoki, Tanaka Hiroo, Mizuno Tomoaki, Kanoh Hatsuho, Tsukita Kazuto, Namba Toshinori, Tamura Atsushi, Yonemura Shigenobu, Gotoh Shimpei, Matsumoto Hisako, Hirai Toyohiro, Tsukita Sachiko. Vinculin is critical for the robustness of the epithelial cell sheet paracellular barrier for ions. Life Science Alliance. 2019;2(4):e201900414. doi: 10.26508/lsa.201900414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug SM, Gunzel D, Conrad MP, Lee IF, Amasheh S, Fromm M, Yu AS. Charge-selective claudin channels. Ann N Y Acad Sci. 2012;1257:20–28. doi: 10.1111/j.1749-6632.2012.06555.x. [DOI] [PubMed] [Google Scholar]
- Kubota K, Furuse M, Sasaki H, Sonoda N, Fujita K, Nagafuchi A, Tsukita S. Ca(2+)-independent cell-adhesion activity of claudins, a family of integral membrane proteins localized at tight junctions. Curr Biol. 1999;9:1035–1038. doi: 10.1016/s0960-9822(99)80452-7. [DOI] [PubMed] [Google Scholar]
- Ladoux B, Nelson WJ, Yan J, Mege RM. The mechanotransduction machinery at work at adherens junctions. Integr Biol (Camb) 2015;7:1109–1119. doi: 10.1039/c5ib00070j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckband DE, de Rooij J. Cadherin adhesion and mechanotransduction. Annu Rev Cell Dev Biol. 2014;30:291–315. doi: 10.1146/annurev-cellbio-100913-013212. [DOI] [PubMed] [Google Scholar]
- Lim TS, Vedula SR, Hui S, Kausalya PJ, Hunziker W, Lim CT. Probing effects of pH change on dynamic response of Claudin-2 mediated adhesion using single molecule force spectroscopy. Exp Cell Res. 2008;314:2643–2651. doi: 10.1016/j.yexcr.2008.05.015. [DOI] [PubMed] [Google Scholar]
- Luissint AC, Parkos CA, Nusrat A. Inflammation and the intestinal barrier: leukocyte-epithelial cell interactions, cell junction remodeling, and mucosal repair. Gastroenterology. 2016;151:616–632. doi: 10.1053/j.gastro.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madara JL. Intestinal absorptive cell tight junctions are linked to the cytoskeleton. Am J Phys. 1987;253:C171–C175. doi: 10.1152/ajpcell.1987.253.1.C171. [DOI] [PubMed] [Google Scholar]
- Madara JL, Barenberg D, Carlson S. Effects of cytochalasin D on occluding junctions of intestinal absorptive cells: further evidence that the cytoskeleton may influence paracellular permeability and junctional charge selectivity. J Cell Biol. 1986;102:2125–2136. doi: 10.1083/jcb.102.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiers JL, Peng X, Fanning AS, DeMali KA. ZO-1 recruitment to alpha-catenin--a novel mechanism for coupling the assembly of tight junctions to adherens junctions. J Cell Sci. 2013;126:3904–3915. doi: 10.1242/jcs.126565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel LJ, Bacallao R, Zampighi G. Uncoupling of the molecular ‘fence’ and paracellular ‘gate’ functions in epithelial tight junctions. Nature. 1993;361:552–555. doi: 10.1038/361552a0. [DOI] [PubMed] [Google Scholar]
- Mangan AJ, Sietsema DV, Li D, Moore JK, Citi S, Prekeris R. Cingulin and actin mediate midbody-dependent apical lumen formation during polarization of epithelial cells. Nat Commun. 2016;7:12426. doi: 10.1038/ncomms12426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchiando AM, Shen L, Graham WV, Weber CR, Schwarz BT, Austin JR, 2nd, Raleigh DR, Guan Y, Watson AJ, Montrose MH, Turner JR. Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J Cell Biol. 2010;189:111–126. doi: 10.1083/jcb.200902153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Rico C, Pincet F, Perez E, Thiery JP, Shimizu K, Takai Y, Dufour S. Separation force measurements reveal different types of modulation of E-cadherin-based adhesion by nectin-1 and -3. J Biol Chem. 2005;280:4753–4760. doi: 10.1074/jbc.M412544200. [DOI] [PubMed] [Google Scholar]
- Mattagajasingh SN, Huang SC, Hartenstein JS, Benz EJ., Jr Characterization of the interaction between protein 4.1R and ZO-2: a possible link between the tight junction and the actin cytoskeleton. J Biol Chem. 2000;275:30573–30585. doi: 10.1074/jbc.M004578200. [DOI] [PubMed] [Google Scholar]
- McNeil E, Capaldo CT, Macara IG. Zonula occludens-1 function in the assembly of tight junctions in Madin-Darby canine kidney epithelial cells. Mol Biol Cell. 2006;17:1922–1932. doi: 10.1091/mbc.E05-07-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng W., Takeichi M. Adherens Junction: Molecular Architecture and Regulation. Cold Spring Harbor Perspectives in Biology. 2009;1(6):a002899–a002899. doi: 10.1101/cshperspect.a002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meza I, Sabenero M, Stefoni E, Cereijido M. Occluding junctions in MDCK cells: modulation of transepithelial permeability by the cytoskeleton. J Cell Biochem. 1982;18:407–421. doi: 10.1002/jcb.1982.240180403. [DOI] [PubMed] [Google Scholar]
- Mino A, Ohtsuka T, Inoue E, Takai Y. Membrane-associated guanylate kinase with inverted orientation (MAGI)-1/brain angiogenesis inhibitor 1-associated protein (BAP1) as a scaffolding molecule for rap small G protein GDP/GTP exchange protein at tight junctions. Genes Cells. 2000;5:1009–1016. doi: 10.1046/j.1365-2443.2000.00385.x. [DOI] [PubMed] [Google Scholar]
- Mohanan V, Nakata T, Desch AN, Levesque C, Boroughs A, Guzman G, Cao Z, Creasey E, Yao J, Boucher G, Charron G, Bhan AK, Schenone M, Carr SA, Reinecker HC, Daly MJ, Rioux JD, Lassen KG, Xavier RJ. C1orf106 is a colitis risk gene that regulates stability of epithelial adherens junctions. Science. 2018;359:1161–1166. doi: 10.1126/science.aan0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro AC, Sumagin R, Rankin CR, Leoni G, Mina MJ, Reiter DM, Stehle T, Dermody TS, Schaefer SA, Hall RA, Nusrat A, Parkos CA. JAM-A associates with ZO-2, afadin, and PDZ-GEF1 to activate Rap2c and regulate epithelial barrier function. Mol Biol Cell. 2013;24:2849–2860. doi: 10.1091/mbc.E13-06-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odenwald MA, Choi W, Kuo WT, Singh G, Sailer A, Wang Y, Shen L, Fanning AS, Turner JR (2018) The scaffolding protein ZO-1 coordinates actomyosin and epithelial apical specializations in vitro and in vivo. J Biol Chem [DOI] [PMC free article] [PubMed]
- Otani T, Ichii T, Aono S, Takeichi M. Cdc42 GEF tuba regulates the junctional configuration of simple epithelial cells. J Cell Biol. 2006;175:135–146. doi: 10.1083/jcb.200605012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschoud S, Citi S. Inducible overexpression of cingulin in stably transfected MDCK cells does not affect tight junction organization and gene expression. Mol Membr Biol. 2008;25:1–13. doi: 10.1080/09687680701474009. [DOI] [PubMed] [Google Scholar]
- Paschoud S, Bongiovanni M, Pache JC, Citi S. Claudin-1 and claudin-5 expression patterns differentiate lung squamous cell carcinomas from adenocarcinomas. Mod Pathol. 2007;20:947–954. doi: 10.1038/modpathol.3800835. [DOI] [PubMed] [Google Scholar]
- Pinto da Silva P, Kachar B. On tight-junction structure. Cell. 1982;28:441–450. doi: 10.1016/0092-8674(82)90198-2. [DOI] [PubMed] [Google Scholar]
- Rahner C, Mitic LL, Anderson JM. Heterogeneity in expression and subcellular localization of claudins 2, 3, 4 and 5 in rat liver, pancreas and gut. Gastroenterology. 2001;120:411–422. doi: 10.1053/gast.2001.21736. [DOI] [PubMed] [Google Scholar]
- Rubsam M, Mertz AF, Kubo A, Marg S, Jungst C, Goranci-Buzhala G, Schauss AC, Horsley V, Dufresne ER, Moser M, Ziegler W, Amagai M, Wickstrom SA, Niessen CM. E-cadherin integrates mechanotransduction and EGFR signaling to control junctional tissue polarization and tight junction positioning. Nat Commun. 2017;8:1250. doi: 10.1038/s41467-017-01170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara S, Maruo T, Miyata M, Mizutani K, Takai Y. Requirement of the F-actin-binding activity of l-afadin for enhancing the formation of adherens and tight junctions. Genes Cells. 2018;23:185–199. doi: 10.1111/gtc.12566. [DOI] [PubMed] [Google Scholar]
- Shah J, Rouaud F, Guerrera D, Vasileva E, Popov LM, Kelley WL, Rubinstein E, Carette JE, Amieva MR, Citi S. A dock-and-lock mechanism clusters ADAM10 at cell-cell junctions to promote alpha-toxin cytotoxicity. Cell Rep. 2018;25(2132–2147):e2137. doi: 10.1016/j.celrep.2018.10.088. [DOI] [PubMed] [Google Scholar]
- Shen L, Weber CR, Turner JR. The tight junction protein complex undergoes rapid and continuous molecular remodeling at steady state. J Cell Biol. 2008;181:683–695. doi: 10.1083/jcb.200711165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. Tight junction pore and leak pathways: a dynamic duo. Annu Rev Physiol. 2011;73:283–309. doi: 10.1146/annurev-physiol-012110-142150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi K, Ono Y, Inai T, Ikenouchi J. Adherens junctions influence tight junction formation via changes in membrane lipid composition. J Cell Biol. 2018;217:2373–2381. doi: 10.1083/jcb.201711042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol. 2006;22:207–235. doi: 10.1146/annurev.cellbio.22.010305.104219. [DOI] [PubMed] [Google Scholar]
- Singh A, Saha T, Begemann I, Ricker A, Nusse H, Thorn-Seshold O, Klingauf J, Galic M, Matis M. Polarized microtubule dynamics directs cell mechanics and coordinates forces during epithelial morphogenesis. Nat Cell Biol. 2018;20:1126–1133. doi: 10.1038/s41556-018-0193-1. [DOI] [PubMed] [Google Scholar]
- Sluysmans S, Vasileva E, Spadaro D, Shah J, Rouaud F, Citi S. The role of apical cell-cell junctions and associated cytoskeleton in mechanotransduction. Biol Cell. 2017;109:139–161. doi: 10.1111/boc.201600075. [DOI] [PubMed] [Google Scholar]
- Spadaro D, Le S, Laroche T, Mean I, Jond L, Yan J, Citi S. Tension-dependent stretching activates ZO-1 to control the junctional localization of its interactors. Curr Biol. 2017;27(3783–3795):e3788. doi: 10.1016/j.cub.2017.11.014. [DOI] [PubMed] [Google Scholar]
- Stephenson RE, Higashi T, Erofeev IS, Arnold TR, Leda M, Goryachev AB, Miller AL. Rho flares repair local tight junction leaks. Dev Cell. 2019;48(445–459):e445. doi: 10.1016/j.devcel.2019.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimura K, Lenne PF, Graner F. Measuring forces and stresses in situ in living tissues. Development. 2016;143:186–196. doi: 10.1242/dev.119776. [DOI] [PubMed] [Google Scholar]
- Takai Y, Nakanishi H. Nectin and afadin: novel organizers of intercellular junctions. J Cell Sci. 2003;116:17–27. doi: 10.1242/jcs.00167. [DOI] [PubMed] [Google Scholar]
- Takeda M, Sami MM, Wang YC. A homeostatic apical microtubule network shortens cells for epithelial folding via a basal polarity shift. Nat Cell Biol. 2018;20:36–45. doi: 10.1038/s41556-017-0001-3. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Dynamic contacts: rearranging adherens junctions to drive epithelial remodelling. Nat Rev Mol Cell Biol. 2014;15:397–410. doi: 10.1038/nrm3802. [DOI] [PubMed] [Google Scholar]
- Tanaka-Okamoto M, Hori K, Ishizaki H, Itoh Y, Onishi S, Yonemura S, Takai Y, Miyoshi J. Involvement of afadin in barrier function and homeostasis of mouse intestinal epithelia. J Cell Sci. 2011;124:2231–2240. doi: 10.1242/jcs.081000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry SJ, Zihni C, Elbediwy A, Vitiello E, San IVLC, Balda MS, Matter K. Spatially restricted activation of RhoA signalling at epithelial junctions by p114RhoGEF drives junction formation and morphogenesis. Nat Cell Biol. 2011;13:159–166. doi: 10.1038/ncb2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda S, Higashi T, Furuse M. ZO-1 knockout by TALEN-mediated gene targeting in MDCK cells: involvement of ZO-1 in the regulation of cytoskeleton and cell shape. PLoS One. 2014;9:e104994. doi: 10.1371/journal.pone.0104994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornavaca O, Chia M, Dufton N, Almagro LO, Conway DE, Randi AM, Schwartz MA, Matter K, Balda MS. ZO-1 controls endothelial adherens junctions, cell-cell tension, angiogenesis, and barrier formation. J Cell Biol. 2015;208:821–838. doi: 10.1083/jcb.201404140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S, Tanaka H, Tamura A. The claudins: from tight junctions to biological systems. Trends Biochem Sci. 2019;44:141–152. doi: 10.1016/j.tibs.2018.09.008. [DOI] [PubMed] [Google Scholar]
- Turner JR. ‘Putting the squeeze’ on the tight junction: understanding cytoskeletal regulation. Semin Cell Dev Biol. 2000;11:301–308. doi: 10.1006/scdb.2000.0180. [DOI] [PubMed] [Google Scholar]
- Turner JR, Rill BK, Carlson SL, Carnes D, Kerner R, Mrsny RJ, Madara JL. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am J Phys. 1997;273:C1378–C1385. doi: 10.1152/ajpcell.1997.273.4.C1378. [DOI] [PubMed] [Google Scholar]
- Umeda K, Matsui T, Nakayama M, Furuse K, Sasaki H, Furuse M, Tsukita S. Establishment and characterization of cultured epithelial cells lacking expression of ZO-1. J Biol Chem. 2004;279:44785–44794. doi: 10.1074/jbc.M406563200. [DOI] [PubMed] [Google Scholar]
- Umeda K, Ikenouchi J, Katahira-Tayama S, Furuse K, Sasaki H, Nakayama M, Matsui T, Tsukita S, Furuse M, Tsukita S. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell. 2006;126:741–754. doi: 10.1016/j.cell.2006.06.043. [DOI] [PubMed] [Google Scholar]
- Van Itallie CM, Anderson JM. Occludin confers adhesiveness when expressed in fibroblasts. J Cell Sci. 1997;110:1113–1121. doi: 10.1242/jcs.110.9.1113. [DOI] [PubMed] [Google Scholar]
- Van Itallie CM, Fanning AS, Bridges A, Anderson JM. ZO-1 stabilizes the tight junction solute barrier through coupling to the perijunctional cytoskeleton. Mol Biol Cell. 2009;20:3930–3940. doi: 10.1091/mbc.E09-04-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Itallie CM, Fanning AS, Holmes J, Anderson JM. Occludin is required for cytokine-induced regulation of tight junction barriers. J Cell Sci. 2010;123:2844–2852. doi: 10.1242/jcs.065581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Itallie CM, Tietgens AJ, Krystofiak E, Kachar B, Anderson JM. A complex of ZO-1 and the BAR-domain protein TOCA-1 regulates actin assembly at the tight junction. Mol Biol Cell. 2015;26:2769–2787. doi: 10.1091/mbc.E15-04-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasileva E, Citi S (2018) The role of microtubules in the regulation of epithelial junctions. Tissue Barriers:1–20 [DOI] [PMC free article] [PubMed]
- Vedula SRK, Lim TS, Kausalya PJ, Lane EB, Rajagopal G, Hunziker W, Lim CT. Quantifying forces mediated by integral tight junction proteins in cell-cell adhesion. Exp Mechanics. 2009;49:3–9. [Google Scholar]
- Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol. 2005;166:409–419. doi: 10.1016/s0002-9440(10)62264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells CD, Fawcett JP, Traweger A, Yamanaka Y, Goudreault M, Elder K, Kulkarni S, Gish G, Virag C, Lim C, Colwill K, Starostine A, Metalnikov P, Pawson T. A Rich1/Amot complex regulates the Cdc42 GTPase and apical-polarity proteins in epithelial cells. Cell. 2006;125:535–548. doi: 10.1016/j.cell.2006.02.045. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Harada N, Kano K, Taya S, Canaani E, Matsuura Y, Mizoguchi A, Ide C, Kaibuchi K. The Ras target AF-6 interacts with ZO-1 and serves as a peripheral component of tight junctions in epithelial cells. J Cell Biol. 1997;139:785–795. doi: 10.1083/jcb.139.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki Y, Umeda K, Wada M, Nada S, Okada M, Tsukita S, Tsukita S. ZO-1- and ZO-2-dependent integration of myosin-2 to epithelial zonula adherens. Mol Biol Cell. 2008;19:3801–3811. doi: 10.1091/mbc.E08-04-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano T, Matsui T, Tamura A, Uji M, Tsukita S. The association of microtubules with tight junctions is promoted by cingulin phosphorylation by AMPK. J Cell Biol. 2013;203:605–614. doi: 10.1083/jcb.201304194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Marchiando A, Weber C, Raleigh D, Wang Y, Shen L, Turner J. MLCK-dependent exchange and actin binding region-dependent anchoring of ZO-1 regulate tight junction barrier function. Proc Natl Acad Sci U S A. 2010;107:8237–8241. doi: 10.1073/pnas.0908869107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemljic-Harpf AE, Godoy JC, Platoshyn O, Asfaw EK, Busija AR, Domenighetti AA, Ross RS. Vinculin directly binds zonula occludens-1 and is essential for stabilizing connexin-43-containing gap junctions in cardiac myocytes. J Cell Sci. 2014;127:1104–1116. doi: 10.1242/jcs.143743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zihni C, Mills C, Matter K, Balda MS. Tight junctions: from simple barriers to multifunctional molecular gates. Nat Rev Mol Cell Biol. 2016;17:564–580. doi: 10.1038/nrm.2016.80. [DOI] [PubMed] [Google Scholar]