Abstract
Objective
To evaluate the efficacy of high-flow nasal cannula (HFNC) therapy compared with conventional oxygen therapy (COT) or noninvasive ventilation (NIV) for the treatment of acute respiratory failure (ARF) in emergency departments (EDs).
Method
We comprehensively searched 3 databases (PubMed, EMBASE, and the Cochrane Library) for articles published from database inception to 12 July 2019. This study included only randomized controlled trials (RCTs) that were conducted in EDs and compared HFNC therapy with COT or NIV. The primary outcome was the intubation rate. The secondary outcomes were the mortality rate, intensive care unit (ICU) admission rate, ED discharge rate, need for escalation, length of ED stay, length of hospital stay, and patient dyspnea and comfort scores.
Result
Five RCTs (n = 775) were included. There was a decreasing trend regarding the application of HFNC therapy and the intubation rate, but the difference was not statistically significant (RR, 0.53; 95% CI, 0.26–1.09; p=0.08; I2 = 0%). We found that compared with patients who underwent COT, those who underwent HFNC therapy had a reduced need for escalation (RR, 0.41; 95% CI, 0.22–0.78; p=0.006; I2 = 0%), reduced dyspnea scores (MD −0.82, 95% CI −1.45 to −0.18), and improved comfort (SMD −0.76 SD, 95% CI −1.01 to −0.51). Compared with the COT group, the HFNC therapy group had a similar mortality rate (RR, 1.25; 95% CI, 0.79–1.99; p=0.34; I2 = 0%), ICU admission rate (RR, 1.11; 95% CI, 0.58–2.12; p=0.76; I2 = 0%), ED discharge rate (RR, 1.04; 95% CI, 0.63–1.72; p=0.87; I2 = 0%), length of ED stay (MD 1.66, 95% CI −0.95 to 4.27), and hospital stay (MD 0.9, 95% CI −2.06 to 3.87).
Conclusion
Administering HFNC therapy in ARF patients in EDs might decrease the intubation rate compared with COT. In addition, it can decrease the need for escalation, decrease the patient's dyspnea level, and increase the patient's comfort level compared with COT.
1. Background
Acute respiratory failure (ARF) is a critical condition faced in emergency departments (EDs). It can result from many conditions, such as cardiogenic pulmonary edema, pneumonia, or acute exacerbation of chronic obstructive pulmonary disease and has a high mortality rate [1]. Conventional oxygen therapy (COT), including a nasal cannula, face mask, venturi mask, and nonrebreathing mask, can be provided to correct hypoxemia. However, the maximal flow rate of COT devices is 15 L/min, which is not enough for patients with ARF. Thus, escalating oxygen therapy to noninvasive ventilation (NIV, e.g., biphasic positive airway pressure) or invasive ventilation may be needed.
Some studies have demonstrated that intubation in ARF patients is associated with an increased complication rate and mortality rate when compared with NIV [2–4]. Even so, NIV is associated with some disadvantages, such as gastric distension, vomiting, claustrophobia, possible nasal skin damage, and difficulty in speaking and coughing, and may lead to treatment failure [5]. According to a previous report, the NIV failure rate in ARF patients ranges from 5% to 60%, depending on numerous factors [6]. Another investigation revealed that up to 25% of chronic obstructive pulmonary disease acute exacerbation patients do not tolerate NIV for several reasons [7]. Therefore, using an ideal NIV device for patients not only improves comfort and dyspnea levels but also decreases intubation and mortality rates potentially.
In recent years, many studies have shown clinical benefits associated with high-flow nasal cannula (HFNC) therapy in ARF patients [8], the oxygen support of preoxygenation [9], acute pulmonary edema [10], the maintenance of oxygenation during bronchoscopy [11], and the prevention of reintubation [12] because an HFNC can provide warmed, humidified, and up to 100% oxygen. When compared to COT and NIV, HFNC therapy has some potential advantages. First, an HFNC can deliver a constant and wide FiO2 range according to the patient's needs. Second, a maximum flow of 60 L/min can generate positive end-expiratory pressure, resulting in the elimination of some airway dead space, improving oxygenation [13]. Third, inspired warm and humidified oxygen can optimize mucosal functions, maximize mucociliary clearance and help expectoration [14]. Finally, using an HFNC can decrease the interruption of oxygen therapy (e.g., during eating, drinking, or talking) and increase patient compliance, resulting in potentially improved outcomes [15]. Previous systematic reviews analyzing heterogeneous study methods (combining observational and randomized controlled trial (RCT) data [16]) and populations (combined ICU and ED populations [17]; those with ARF, and postextubation and postoperation populations [18, 19]) may cause controversial results. Thus, clarifying the use of HFNCs for ARF patients in EDs is necessary. In this study, we conducted a recent systematic review and meta-analysis to evaluate the differences between using HFNC therapy and COT or NIV in ARF patients in EDs.
2. Methods
This study design followed the Cochrane Handbook for Systematic Reviews of Interventions guidelines [20] and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statements [21].
3. Eligibility Criteria
3.1. Types of Studies
Only RCTs were eligible. We excluded retrospective studies, observational studies, before-after studies, crossover studies, case reports, abstract publications, and conference presentations.
3.2. Types of Participants
We included adult patients (>18 years old) with ARF due to any cause admitted to ED. “ARF” was defined as an SpO2 <92% in room air, a PaO2/FiO2 (ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen) <300, clinical symptoms and signs (including a respiratory rate >24 breaths per minute, the use of accessory muscles to breath, or shortness of breath at rest) or “author” definitions. Postoperation, postextubation, and ICU patients were all excluded.
3.3. Types of Interventions
Studies comparing HFNC therapy with COT and/or other NIV devices were included. There was no oxygen flow or concentration restriction for the intervention group (HFNC) or the comparison group (COT and/or other NIV devices).
3.4. Types of Outcome Measures
Our primary outcome was the intubation rates of both of the groups. The secondary outcomes were the mortality rate, ICU admission rate, ED discharge rate, need for escalation, length of ED stay, length of hospital stay, and patient dyspnea and comfort scores. We also considered 2 subgroup analyses according to the intervention device (HFNC versus COT and NIV versus COT) and treatment duration (HFNC ≤2 hours versus COT and HFNC >2 hours versus COT).
3.5. Search Methods for Identification of Studies
We comprehensively searched 3 databases (PubMed, EMBASE, and the Cochrane Library) for articles published from database inception to 12 July 2019. The following key words or medical subject headings (MeSH) terms were used: high-flow nasal cannula, high-flow nasal, high-flow oxygen therapy, or high-flow therapy, and emergency department, emergency room, emergency unit, or emergency service. To avoid the loss of possible studies, we also reviewed the references of the identified articles. No language restriction was applied.
3.6. Data Extraction, Quality Assessment, and Grading of the Quality of Evidence
Two authors (CCH and HML) extracted the data from the reviewed articles independently. We used an unweighted kappa score to test interrater reliability. If any disagreement occurred, it was resolved by discussion, consensus, or consultation with a third author (CJL). The following data were collected for each eligible study: authors, publication year, study design, study group, intervention/control detail, and outcome data.
The risk of bias was independently assessed by two authors (CCH and HML) according to the Cochrane Handbook for Systematic Reviews of Interventions guidelines, chapter 8 [20]. There were 7 domains that were assessed for each study: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. Each domain was rated as having low risk (green), unclear risk (yellow), or high risk (red).
We also used the Grading of Recommendation, Assessment, Development, and Evaluation (GRADE) method [22] to evaluate the quality of evidence, which was classified as very low, low, moderate, or high, for the primary and secondary outcomes.
3.7. Statistical Analysis
All data were analyzed by Review Manager (RevMan, Version 5.3, Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration, 2014). Because some of the included studies presented results as medians, interquartile ranges, or minimum/maximum values, we use the Wan et al. method to estimate the sample mean and standard deviation [23]. We expressed dichotomous data as risk ratios (RRs) and continuous data as mean differences (MDs) or standardized mean differences (SMDs). For all of the results, 95% confidence intervals (CIs) were calculated. To evaluate heterogeneity, we used chi-square and I2 tests. If the heterogeneity was nonsignificant (I2 < 50%), we applied fixed-effects models; otherwise, we applied random-effects models for analysis. In addition, we performed a visual inspection of the funnel plot to assess publication bias, and a sensitivity analysis was conducted by repeating the analysis after removing one RCT at a time. Finally, the results were presented in forest plots.
4. Result
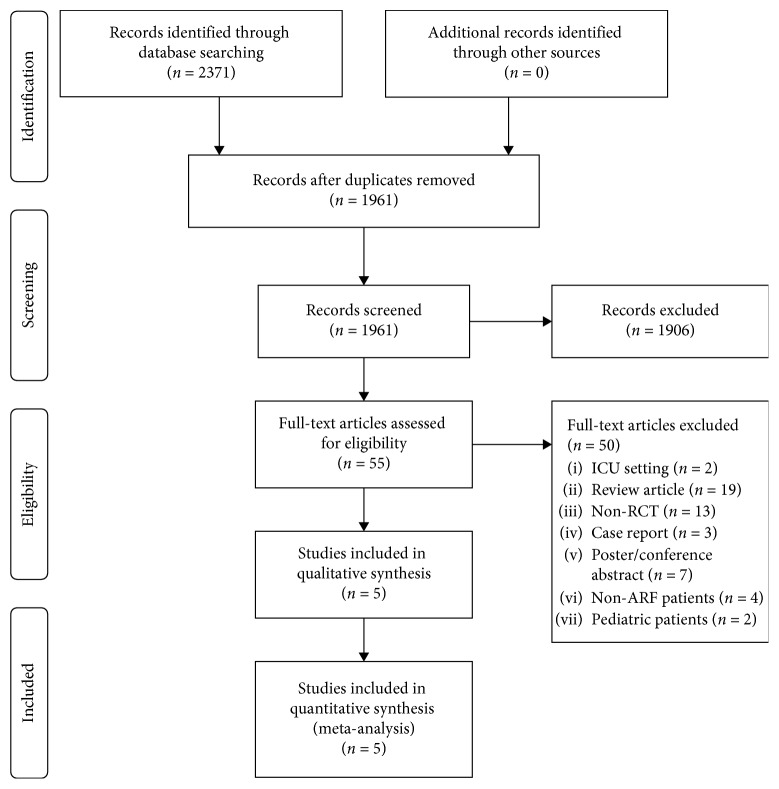
A total of 2371 potentially eligible studies were initially identified. After screening titles and abstracts, 55 full-text studies were retrieved for eligibility screening. Then, we excluded studies conducted in ICU settings, review articles, nonrandomized controlled trials, case reports, conference abstracts, studies involving non-ARF patients, and studies involving pediatric patients. Finally, 5 RCTs [24–28] including 775 patients were entered into our meta-analysis (Figure 1). The interrater reliability of study screening (kappa score, 0.76; 95% CI, 0.68–0.86) and risk of bias assessment (kappa statistic 0.77, 95% CI: 0.57–0.98) were good. The mean age ranged from 63.4 to 73.7 years old. Of the 5 RCTs, 1 RCT [28] compared HFNC therapy with NIV and the others [24–27] compared HFNC therapy with COT (nasal cannula, face mask, venturi mask, or nonrebreathing mask). The flow in the HFNC group was 35 L/min or more at initiation, and the duration of therapy ranged from 1 hour to 72 hours. The main causes of ARF were chronic obstructive pulmonary disease (COPD), pneumonia, and cardiac-related disease in our included studies. The basic characteristics of the included studies are shown in Table 1. Owing to the lack of an NIV group in the other studies, we were not able to analyze the RCT [28] that compared HFNC with NIV. In addition, there was no event (intubation) in one RCT [25], and the data in the included studies were too insufficient to obtain valuable conclusions. Because of the above 2 reasons, we were not able to perform subgroup analyses.
Figure 1.
PRISMA flow diagram.
Table 1.
The basic characteristics of the included studies.
| Study, year | Design | Setting | Age, years∗ | Cause of ARF | Sample size | Duration of therapy | ||
|---|---|---|---|---|---|---|---|---|
| HFNC | COT | NIV | ||||||
| Bell et al. [24] | RCT | ED | 73.7 ± 17.5 | COPD, respiratory tract infection, cardiac related, pulmonary embolism, asthma | 48 | 52 | — | 2 h |
| Rittayamai et al. [25] | RCT | ED | 64.6 ± 15.1 | CHF, pneumonia, asthma, COPD, others | 20 | 20 | — | 1 h |
| Jones et al. [26] | RCT | ED | 73.5 ± 16.2 | COPD, pneumonia, asthma, others | 165 | 138 | — | 5 h |
| Makdee et al. [27] | RCT | ED | 70 ± 15 | Cardiogenic pulmonary edema | 63 | 65 | — | 2.9 h (0.2–9.3 h) |
| Doshi et al. [28] | RCT | ED | 63.4 ± 14 | COPD, CHF, pneumonia, asthma | 104 | — | 100 | 72 h |
RCT, randomized controlled trial; ED, emergency department; COPD, chronic obstructive pulmonary disease; CHF, congestive heart failure; h, hours. ∗Mean ± standard deviation (SD).
4.1. Risk of Bias of the Included Studies
The risk of selection bias in our included studies was all low, except for 1 RCT [25] that had an unclear risk of bias. The risk of performance bias was all high because it was impossible to blind patients and personnel in the clinical setting when comparing HFNC to COT. The other risk of bias results is shown in Figure 2.
Figure 2.
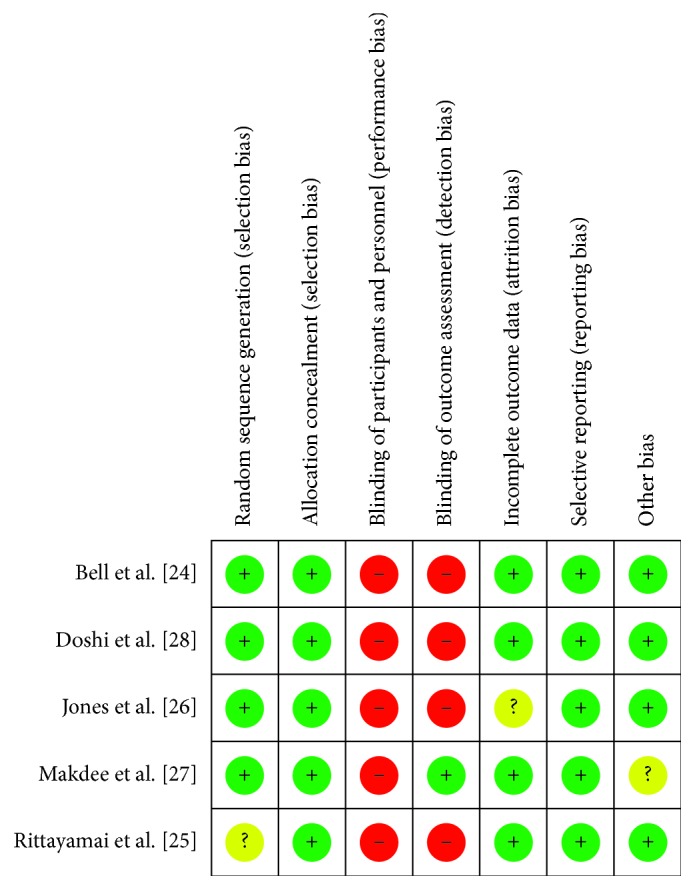
Risk of bias summary.
4.2. Quality Assessment
Table 2 summarizes all outcomes and the quality of evidence of the articles included in this meta-analysis. The intubation rate, length of ED/hospital stay, and patient dyspnea level were of low quality, and the others were of moderate quality. A visual inspection of the funnel plot revealed no publication bias (Additional ).
Table 2.
The summary of outcomes and GRADE quality assessments.
| Quality assessment | No of patients | Effect | Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other consideration | HFNC | COT | Relative RR (95% CI) | Absolute (95% CI) | ||
| Intubation rate | ||||||||||||
| 4 | RCTs | Seriousa | Not serious | Not serious | Seriousb | None | 10/296 (3.4%) | 17/275 (6.2%) | 0.53(0.26–1.09) | −32 per 1000 (from −69 to 4) | ⊕⊕Low | Critical |
|
| ||||||||||||
| Mortality rate | ||||||||||||
| 2 | RCTs | Not serious | Not serious | Not serious | Seriousb | None | 36/228 (15.8%) | 24/203 (11.8%) | 1.25(0.79–1.99) | 32 per 1000 (from −32 to 95) | ⊕⊕⊕Moderate | Critical |
|
| ||||||||||||
| ICU admission rate | ||||||||||||
| 2 | RCTs | Not serious | Not serious | Not serious | Seriousb | None | 17/213 (8%) | 15/190 (7.9%) | 1.11(0.58–2.12) | 8 per 1000 (from −43 to 59) | ⊕⊕⊕Moderate | Critical |
|
| ||||||||||||
| ED discharge rate | ||||||||||||
| 2 | RCTs | Not serious | Not serious | Not serious | Seriousb | None | 23/111 (20.7%) | 23/117 (19.7%) | 1.04(0.63–1.72) | 8 per 1000 (from −93 to 109) | ⊕⊕⊕Moderate | Critical |
|
| ||||||||||||
| Need for escalation | ||||||||||||
| 4 | RCTs | Seriousa | Not serious | Not serious | Not serious | None | 13/296 (4.4%) | 29/275 (10.5%) | 0.41(0.22–0.78) | −62 per 1000 (from −105 to −19) | ⊕⊕⊕Moderate | Critical |
|
| ||||||||||||
| Length of ED stay (hour) | ||||||||||||
| 3 | RCTs | Not serious | Seriousc | Not serious | Seriousb | None | 276 | 255 | — | MD 1.66 hours (from −0.95 to 4.27) | ⊕⊕Low | Critical |
|
| ||||||||||||
| Length of hospital stay (day) | ||||||||||||
| 2 | RCTs | Not serious | Seriousc | Not serious | Seriousb | None | 228 | 203 | — | MD 0.9 days (from −2.06 to 3.87) | ⊕⊕Low | Critical |
|
| ||||||||||||
| Patient dyspnea score | ||||||||||||
| 2 | RCTs | Seriousd | Seriousc | Not serious | Not serious | None | 83 | 85 | — | MD −0.82 point (from −1.45 to −0.18) | ⊕⊕Low | Critical |
|
| ||||||||||||
| Patient comfort score | ||||||||||||
| 3 | RCTs | Seriousd | Not serious | Not serious | Not serious | None | 131 | 137 | — | SMD −0.76 SD (from −1.01 to −0.51) | ⊕⊕⊕Moderate | Critical |
RCT, randomized controlled trial; HFNC, high-flow nasal cannula; COT, conventional oxygen therapy; CI, confidence interval; RR, risk ratio; MD, mean difference; SMD, standardized mean difference. aAll inclusion trials lacked blinding (performance bias), so escalation or intubation may be subjective. bInsufficient evidence of clear benefit or harm because of a wide CI. cSignificant heterogeneity among the included trials (I2 > 50%). dSubjective outcome. ⊕, very low quality; ⊕⊕, low quality; ⊕⊕⊕, moderate quality; ⊕⊕⊕⊕, high quality.
4.3. Primary Outcome
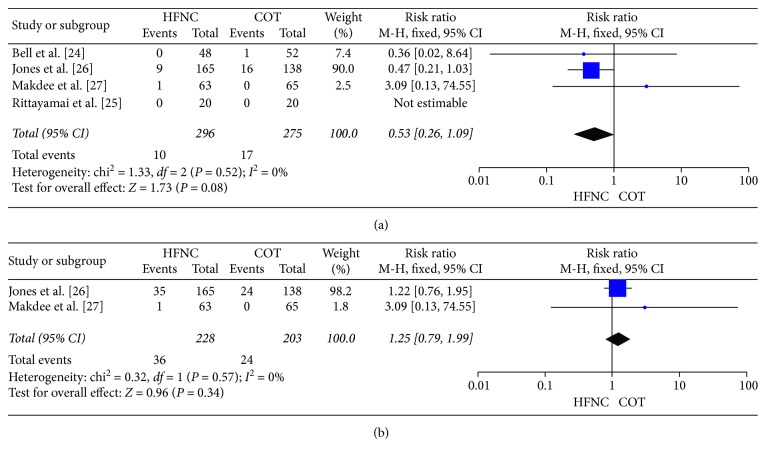
Four RCTs including 571 patients reported the intubation rates for both groups. Ten of 296 (3.38%) patients in the HFNC group were intubated, and 17 of 275 (6.18%) patients in the COT group were intubated. There was a decreasing trend of HFNC therapy and intubation rate, but it was not statistically significant (RR, 0.53; 95% CI, 0.26–1.09; p=0.08; I2 = 0%) (Figure 3(a)).
Figure 3.
Intubation rate and mortality rate. (a) Intubation rate: HFNC group versus COT group. (b) Mortality rate: HFNC group versus COT group.
4.4. Secondary Outcomes
4.4.1. Mortality, ICU Admission Rate, and ED Discharge Rate
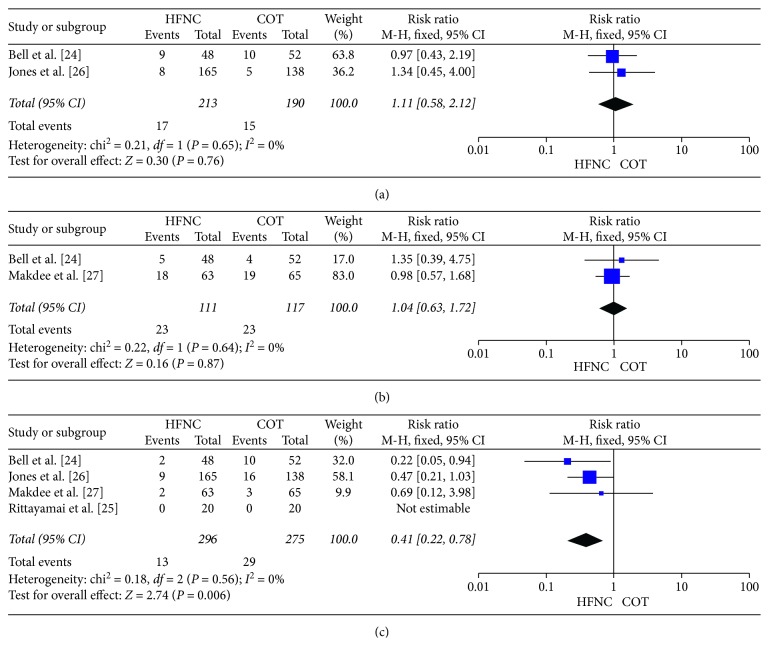
We did not observe a difference in the mortality rate (RR, 1.25; 95% CI, 0.79–1.99; p=0.34; I2 = 0%), ICU admission rate (RR, 1.11; 95% CI, 0.58–2.12; p=0.76; I2 = 0%), or ED discharge rate (RR, 1.04; 95% CI, 0.63–1.72; p=0.87; I2 = 0%) between the HFNC and COT groups (Figures 3(b), 4(a), and 4(b)).
Figure 4.
Other secondary outcomes. (a) ICU admission rate: HFNC group versus COT group. (b) ED discharge rate: HFNC group versus COT group. (c) Need for escalation: HFNC group versus COT group.
4.4.2. Need for Escalation
If the patient could not tolerate initial therapy (HFNC therapy or COT) or therapy failed, patient oxygenation required escalation to avoid hypoxia. The escalation strategies in our included studies were similar. Two RCTs [26, 27] escalated to NIV or invasive ventilation in both the HFNC and COT groups. One RCT [24] escalated to NIV or invasive ventilation in the HFNC group and HFNC, NIV or invasive ventilation in the COT group. No escalation was needed in 1 RCT [25]. HFNC therapy decreased the need for escalation compared to COT (RR, 0.41; 95% CI, 0.22–0.78; p=0.006; I2 = 0%) (Figure 4(c)).
4.4.3. Length of ED Stay and Hospital Stay
The length of ED stay (MD 1.66, 95% CI −0.95 to 4.27) and hospital stay (MD 0.9, 95% CI −2.06 to 3.87) were similar in both the HFNC and COT groups (Table 2).
4.4.4. Dyspnea Score
The dyspnea score in the HFNC group was significantly lower than that in the COT group (MD −0.82, 95% CI −1.45 to −0.18) (Table 2). Two of the 4 RCTs reported measurable dyspnea scores. One RCT [25] used a numerical rating scale ranging from 0 to 10, and the other RCT [27] used a visual analog scale ranging from 0 to 10. Of the included RCTs, 1 RCT [24] defined patient dyspnea as a reduction in the respiratory rate >20% from baseline and a reduction in the Borg score. Both arms showed a significant decrease in the dyspnea level in the HFNC group (reduction in the respiratory rate >20% from baseline: HFNC 32/48 (66.7%), COT 20/52 (38.5%), p=0.005; reduction in the Borg score: HFNC 36/48 (75%), COT 29/52 (55.8%), p=0.044).
4.4.5. Comfort Score
Because different scoring systems, including a 5-point Likert scale, numerical rating scale, and visual analog scale, were used in different RCTs, we calculated SMDs to evaluate patient comfort levels. Three of the 4 RCTs reported measurable comfort scores, and patients in the HFNC group were more comfortable than those in the COT group (SMD −0.76 SD, 95% CI −1.01 to −0.51) (Table 2).
5. Discussion
The most important result of this meta-analysis is that HFNC therapy for ARF patients in EDs can reduce the need for escalation oxygen therapy compared with COT. This result was similar to that in recent meta-analyses [17, 29, 30]. Although there were no differences found in the mortality rate, there was a decreasing trend between HFNC therapy and intubation rate in ARF patients, despite the lack of statistical significance. On the other hand, we did not observe an influence of HFNC therapy on the ICU admission rate, ED discharge rate or length of ED/hospital stay. A meta-analysis by Maitra et al. [31], which included 5 trials (n = 759), revealed no difference in the requirement of increased respiratory support between the HFNC therapy and COT groups. However, the study enrolled not only ARF patients but also postcardiac operation patients, and the heterogeneity was high. In addition, Rochwerg et al. [17], Monro–Somerville et al. [19], Bocchile et al. [30] who conducted 3 previous meta-analyses including 7 trials (n = 1647), 8 trials (n = 1567), and 6 trials (n = 839), respectively, demonstrated that HFNC therapy significantly reduced the intubation rate compared to COT. In our study, we did not observe an apparently reduced intubation rate in the HFNC group, probably because of the low patient number.
The other important result of this meta-analysis is that treating ARF patients with HFNC therapy resulted in lower patient dyspnea scores and higher patient comfortable than treating ARF patients with COT. All of our included RCTs revealed that HFNC therapy was better than COT regarding dyspnea and comfort scores, except for 1 RCT [26]. This RCT by Jones et al., which used a 5-point Likert scale to evaluate patient dyspnea and comfort levels, present the results by combining the best 2/other 3 or worst 2/other 3 categories for positive and negative questions, respectively, so we were not able to quantitatively pool these data. HFNC therapy can provide warmed, humidified, and 100% oxygen, which may explain the reduction in the need for escalation oxygen therapy compared to COT. Other features such as removing airway dead space, improving oxygenation, optimizing mucosal functions, maximizing mucociliary clearance, promoting expectoration, and decreasing interruptions to oxygen therapy are also possible reasons for the decreasing trend in the intubation rate, improved patient dyspnea scores, and improved comfort levels associated with HFNC therapy.
In this study, we noted that some targeted studies are heterogeneous with their methods and case mix. For example, three RCTs [24–26] included all kinds of ARF patients (COPD, pneumonia, cardiac-related disease, and others) while 1 RCT [27] only included cardiogenic pulmonary edema patients. In addition, initial FiO2 (ranged from 28% to 100%), flow rate (ranged from 35 L/min to 50 L/min) of HFNC, duration of therapy (ranged from 1 hour to 9.3 hours), and authors' definitions of ARF were all different. Although the inclusion studies were heterogeneous, most of the outcomes did not reveal statistical heterogeneity (I2 or inconsistency). The FiO2 and the flow rate needed to titrate to clinical demand in all including patients. Moreover, most improvements in respiratory effort and oxygenation were already obtained at the flow rate of 30 L/min [32]. These are possible reasons to explain consistency of most outcomes despite different cause of ARF and initial settings.
HFNC also plays an important role in acute exacerbations of chronic obstructive pulmonary disease (AECOPD). AECOPD with respiratory failure, a kind of type II respiratory failure, is caused by airflow obstruction or increasing dead space. As previously mentioned, HFNC can generate about 2–4 cm H2O positive end-expiratory pressure [33], resulting in decreasing PaCO2 level and improving oxygenation by elimination of some airway dead space [13]. Furthermore, titrated oxygen therapy with target saturation of 88–92% can significantly reduce mortality, hypercapnia, and respiratory acidosis in AECOPD [34]. HFNC is able to deliver a constant and wide FiO2 range oxygen, so it can titrate with target saturation of 88–92% depending on clinical needs. According to the 2019 Global Initiative for Chronic Obstructive Lung Disease (GOLD) guideline, HFNC may be an alternative to standard oxygen therapy or noninvasive positive pressure ventilation in AECOPD [35]. There are also some investigations demonstrate using HFNC in COPD patients can decrease PaCO2 level [36], respiratory effort, and improving oxygenation [37]. Thus, applying HFNC to AECOPD patients is reasonable respiratory support.
There was a decreasing trend between HFNC therapy and intubation rate in ARF patients, but there were no differences found in the mortality rate. There was a concern about that delay intubation could increase mortality in ARF patients treating with HFNC. Because HFNC could improve respiratory effort and patients “looked” better initially, they would not be intubated in a timely manner. Kang et al. illustrated failure of HFNC therapy may delay intubation and increase mortality [38]. However, the “delay” was defined as >48 hours after HFNC therapy, which was far from our included RCTs (ranged from 1 hour to 9.3 hours). In addition, there were various etiologies of ARF, such as COPD, pneumonia, pulmonary edema, asthma, pulmonary embolism, and other causes, in the included RCTs. Not every disease initially benefited equally from HFNC therapy. Messika et al. demonstrated that increased breathing frequency, an increased Simplified Acute Physiology Score (SAPS) II score, and decreased PaO2/FiO2 were associated with HFNC treatment failure [39].
To the best of our knowledge, this is the first meta-analysis regarding the use of HFNC for ARF patients in EDs. The advantages of this analysis include performing a comprehensive article search in 3 databases (PubMed, EMBASE, and the Cochrane Library). In addition, only RCTs involving HFNC therapy, COT or NIV for de novo acute hypoxemic respiratory failure patients in EDs were included in our meta-analysis. Moreover, we used the GRADE method to evaluate the quality of evidence for primary and secondary outcomes. There were also several limitations to this study. Firstly, the number of RCTs comparing HFNC to COT or NIV for patients with ARF in EDs were too few to include in our meta-analysis, so we were not able to perform subgroup analyses, and the low patient number increased the risk of bias. Secondly, our quality of evidence for outcomes was low to moderate and was affected by serious risk of bias, inconsistencies, and imprecision. Finally, we did not have enough power to evaluate publication bias by inspection of a funnel plot because of the small sample size. Therefore, further large-sample studies are warranted to clarify the role of HFNC therapy for ARF patients in EDs.
6. Conclusion
In this meta-analysis, we demonstrated that HFNC therapy for ARF patients in the ED might decrease the intubation rate compared to COT. In addition, it can decrease the need for escalation, decrease the patient's dyspnea score, and increase the patient's comfort level compared to COT. Further high-quality and large-sample studies are warranted to confirm the role of HFNC therapy for ARF patients in EDs.
Contributor Information
Han-Ping Wu, Email: arthur1226@gmail.com.
Yuan-Jhen Syue, Email: shueshue@cgmh.org.tw.
Data Availability
All data are available for all users. We comprehensively searched 3 databases (PubMed, EMBASE, and the Cochrane Library) for articles published from database inception to 12 July 2019. Only randomized controlled trials (RCTs) were conducted in EDs and HFNC therapy was compared to COT or NIV.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Supplementary Materials
A visual inspection of the funnel plot revealed no publication bias.
References
- 1.Ray P., Birolleau S., Lefort Y., et al. Acute respiratory failure in the elderly: etiology, emergency diagnosis and prognosis. Critical Care. 2006;10(3):p. R82. doi: 10.1186/cc4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antonelli M., Conti G., Rocco M., et al. A comparison of noninvasive positive-pressure ventilation and conventional mechanical ventilation in patients with acute respiratory failure. New England Journal of Medicine. 1998;339(7):429–435. doi: 10.1056/nejm199808133390703. [DOI] [PubMed] [Google Scholar]
- 3.Azevedo L. C. P., Caruso P., Silva U. V. A., et al. Outcomes for patients with cancer admitted to the ICU requiring ventilatory support. Chest. 2014;146(2):257–266. doi: 10.1378/chest.13-1870. [DOI] [PubMed] [Google Scholar]
- 4.Confalonieri M., Potena A., Carbone G., Porta R. D., Tolley E. A., Umberto Meduri G. Acute respiratory failure in patients with severe community-acquired pneumonia. American Journal of Respiratory and Critical Care Medicine. 1999;160(5):1585–1591. doi: 10.1164/ajrccm.160.5.9903015. [DOI] [PubMed] [Google Scholar]
- 5.Nava S., Hill N. Non-invasive ventilation in acute respiratory failure. The Lancet. 2009;374(9685):250–259. doi: 10.1016/s0140-6736(09)60496-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ozyilmaz E., Ugurlu A. O., Nava S. Timing of non-invasive ventilation failure: causes, risk factors, and potential remedies. BMC Pulmonary Medicine. 2014;14(1) doi: 10.1186/1471-2466-14-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Confalonieri M., Garuti G., Cattaruzza M. S., et al. A chart of failure risk for noninvasive ventilation in patients with COPD exacerbation. European Respiratory Journal. 2005;25(2):348–355. doi: 10.1183/09031936.05.00085304. [DOI] [PubMed] [Google Scholar]
- 8.Frat J. P., Thille A. W., Mercat A., et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. New England Journal of Medicine. 2015;372(23):2185–2196. doi: 10.1056/nejmoa1503326. [DOI] [PubMed] [Google Scholar]
- 9.Doyle A. J., Stolady D., Mariyaselvam M., et al. Preoxygenation and apneic oxygenation using transnasal humidified rapid-insufflation ventilatory exchange for emergency intubation. Journal of Critical Care. 2016;36:8–12. doi: 10.1016/j.jcrc.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 10.Haywood S. T., Whittle J. S., Volakis L. I., et al. HVNI vs. NIPPV in the treatment of acute decompensated heart failure: subgroup analysis of a multi-center trial in the ED. American Journal of Emergency Medicine. 2019;S0735-6757(19):30151–30152. doi: 10.1016/j.ajem.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Chung S. M., Choi J. W., Lee Y. S., et al. Clinical effectiveness of high-flow nasal cannula in hypoxaemic patients during bronchoscopic procedures. Tuberculosis and Respiratory Diseases. 2019;82(1):81–85. doi: 10.4046/trd.2017.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hernández G., Vaquero C., Colinas L., et al. Effect of postextubation high-flow nasal cannula vs. noninvasive ventilation on reintubation and postextubation respiratory failure in high-risk patients. JAMA. 2016;316(15):1565–1574. doi: 10.1001/jama.2016.14194. [DOI] [PubMed] [Google Scholar]
- 13.El-Khatib M. F. High-flow nasal cannula oxygen therapy during hypoxemic respiratory failure. Respiratory Care. 2012;57(10):1696–1698. doi: 10.4187/respcare.02072. [DOI] [PubMed] [Google Scholar]
- 14.Spoletini G., Alotaibi M., Blasi F., Hill N. S. Heated humidified high-flow nasal oxygen in adults. Chest. 2015;148(1):253–261. doi: 10.1378/chest.14-2871. [DOI] [PubMed] [Google Scholar]
- 15.Frat J. P., Brugiere B., Ragot S., et al. Sequential application of oxygen therapy via high-flow nasal cannula and noninvasive ventilation in acute respiratory failure: an observational pilot study. Respiratory Care. 2015;60(2):170–178. doi: 10.4187/respcare.03075. [DOI] [PubMed] [Google Scholar]
- 16.Ni Y. N., Luo J., Yu H., et al. Can high-flow nasal cannula reduce the rate of endotracheal intubation in adult patients with acute respiratory failure compared with conventional oxygen therapy and noninvasive positive pressure ventilation? Chest. 2017;151(4):764–775. doi: 10.1016/j.chest.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Rochwerg B., Granton D., Wang D. X., et al. High flow nasal cannula compared with conventional oxygen therapy for acute hypoxemic respiratory failure: a systematic review and meta-analysis. Intensive Care Medicine. 2019;45(5):563–572. doi: 10.1007/s00134-019-05590-5. [DOI] [PubMed] [Google Scholar]
- 18.Nedel W. L., Deutschendorf C., Moraes Rodrigues Filho E. High-flow nasal cannula in critically ill subjects with or at risk for respiratory failure: a systematic review and meta-analysis. Respiratory Care. 2017;62(1):123–132. doi: 10.4187/respcare.04831. [DOI] [PubMed] [Google Scholar]
- 19.Monro-Somerville T., Sim M., Ruddy J., Vilas M., Gillies M. A. The effect of high-flow nasal cannula oxygen therapy on mortality and intubation rate in acute respiratory failure. Critical Care Medicine. 2017;45(4):e449–e456. doi: 10.1097/ccm.0000000000002091. [DOI] [PubMed] [Google Scholar]
- 20.Higgins J. P. T., Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. London, UK: Cochrane; 2011. Preparing a cochrane review. [Google Scholar]
- 21.Moher D., Liberati A., Tetzlaff J., Altman D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of Internal Medicine. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 22.Guyatt G. H., Oxman A. D., Vist G. E., et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Medical Research Methodology. 2014;14(1):p. 135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bell N., Hutchinson C. L., Green T. C., Rogan E., Bein K. J., Dinh M. M. Randomised control trial of humidified high flow nasal cannulaeversusstandard oxygen in the emergency department. Emergency Medicine Australasia. 2015;27(6):537–541. doi: 10.1111/1742-6723.12490. [DOI] [PubMed] [Google Scholar]
- 25.Rittayamai N., Tscheikuna J., Praphruetkit N., Kijpinyochai S. Use of high-flow nasal cannula for acute dyspnea and hypoxemia in the emergency department. Respiratory Care. 2015;60(10):1377–1382. doi: 10.4187/respcare.03837. [DOI] [PubMed] [Google Scholar]
- 26.Jones P. G., Kamona S., Doran O., Sawtell F., Wilsher M. Randomized controlled trial of humidified high-flow nasal oxygen for acute respiratory distress in the emergency department: the HOT-ER study. Respiratory Care. 2016;61(3):291–299. doi: 10.4187/respcare.04252. [DOI] [PubMed] [Google Scholar]
- 27.Makdee O., Monsomboon A., Surabenjawong U., et al. High-flow nasal cannula versus conventional oxygen therapy in emergency department patients with cardiogenic pulmonary edema: a randomized controlled trial. Annals of Emergency Medicine. 2017;70(4):465–472. doi: 10.1016/j.annemergmed.2017.03.028. [DOI] [PubMed] [Google Scholar]
- 28.Doshi P., Whittle J. S., Bublewicz M., et al. High-velocity nasal insufflation in the treatment of respiratory failure: a randomized clinical trial. Annals of Emergency Medicine. 2018;72(1):73–83. doi: 10.1016/j.annemergmed.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Xu Z., Li Y., Zhou J., et al. High-flow nasal cannula in adults with acute respiratory failure and after extubation: a systematic review and meta-analysis. Respiratory Research. 2018;19(1):p. 202. doi: 10.1186/s12931-018-0908-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bocchile R. L. R., Cazati D. C., Timenetsky K. T., Neto A. S. The effects of high-flow nasal cannula on intubation and re-intubation in critically ill patients: a systematic review, meta-analysis and trial sequential analysis. Revista Brasileira de Terapia Intensiva. 2018;30(4):487–495. doi: 10.5935/0103-507x.20180070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maitra S., Som A., Bhattacharjee S., Arora M. K., Baidya D. K. Comparison of high-flow nasal oxygen therapy with conventional oxygen therapy and noninvasive ventilation in adult patients with acute hypoxemic respiratory failure: a meta-analysis and systematic review. Journal of Critical Care. 2016;35:138–144. doi: 10.1016/j.jcrc.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 32.Mauri T., Alban L., Turrini C., et al. Optimum support by high-flow nasal cannula in acute hypoxemic respiratory failure: effects of increasing flow rates. Intensive Care Medicine. 2017;43(10):1453–1463. doi: 10.1007/s00134-017-4890-1. [DOI] [PubMed] [Google Scholar]
- 33.Ritchie J. E., Williams A. B., Gerard C., Hockey H. Evaluation of a humidified nasal high-flow oxygen system, using oxygraphy, capnography and measurement of upper airway pressures. Anaesthesia and Intensive Care. 2011;39(6):1103–1110. doi: 10.1177/0310057x1103900620. [DOI] [PubMed] [Google Scholar]
- 34.Austin M. A., Wills K. E., Blizzard L., Walters E. H., Wood-Baker R. Effect of high flow oxygen on mortality in chronic obstructive pulmonary disease patients in prehospital setting: randomised controlled trial. BMJ. 2010;341(2):p. c5462. doi: 10.1136/bmj.c5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh D., Agusti A., Anzueto A., et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. European Respiratory Journal. 2019;53(5) doi: 10.1183/13993003.00164-2019.1900164 [DOI] [PubMed] [Google Scholar]
- 36.Bräunlich J., Seyfarth H. J., Wirtz H. Nasal high-flow versus non-invasive ventilation in stable hypercapnic COPD: a preliminary report. Multidisciplinary Respiratory Medicine. 2015;10(1):p. 27. doi: 10.1186/s40248-015-0019-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chatila W., Nugent T., Vance G., Gaughan J., Criner G. J. The effects of high-flow vs. low-flow oxygen on exercise in advanced obstructive airways disease. Chest. 2004;126(4):1108–1115. doi: 10.1378/chest.126.4.1108. [DOI] [PubMed] [Google Scholar]
- 38.Kang B. J., Koh Y., Lim C. M., et al. Failure of high-flow nasal cannula therapy may delay intubation and increase mortality. Intensive Care Medicine. 2015;41(4):623–632. doi: 10.1007/s00134-015-3693-5. [DOI] [PubMed] [Google Scholar]
- 39.Messika J., Ben Ahmed K., Gaudry S., et al. Use of high-flow nasal cannula oxygen therapy in subjects with ARDS: a 1-year observational study. Respiratory Care. 2015;60(2):162–169. doi: 10.4187/respcare.03423. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A visual inspection of the funnel plot revealed no publication bias.
Data Availability Statement
All data are available for all users. We comprehensively searched 3 databases (PubMed, EMBASE, and the Cochrane Library) for articles published from database inception to 12 July 2019. Only randomized controlled trials (RCTs) were conducted in EDs and HFNC therapy was compared to COT or NIV.