Abstract
Objective:
To evaluate whether equal volumes of oral rehydration solution (ORS) or intravenous saline provide similar improvements in cardiovascular status during controlled orthostatic challenge when administered to patients with postural tachycardia syndrome (POTS) with orthostatic intolerance.
Study design:
We studied the neurovascular response to fluid loading during orthostatic stress using lower body negative pressure (LBNP) in 10 subjects with POTS with OI and 15 controls, and on subsequent days before, and 1 hour after intravenous saline or ingestion of ORS.
Results:
Subjects with POTS exhibited reduced tolerance (P < .0001) to LBNP compared with controls (Orthostatic Index of 35,715±3,469 vs. 93,980±7,977, respectively). In POTS, following ORS but not saline, cerebral blood flow velocity (CBFv) was significantly higher than no treatment (p<0.0005) at −45 mmHg. Although fluid loading did not confer any advantage in controls, subjects with POTS experienced a significant improvement in orthostatic tolerance following both saline (100±9.7 vs. 134.5±17.4, p<0.05) and OR S (100±9.7 vs. 155.6±15.7, p<0.001) when evaluated by normalized orthostatic index (p<0.001, compared with untreated baseline).
Conclusion:
Maintenance of CBFv may have resulted in improved short-term orthostatic tolerance exhibited by POTS subjects following ORS administration. ORS is a convenient, safe and effective therapy for short-term relief of OI.
Keywords: Reduced Orthostatic Tolerance, Oral Rehydration Solution, Intravenous Saline, Postural Tachycardia Syndrome, Lower Body Negative Pressure
The imposition of an orthostatic stress, such as standing up, causes a rapid gravitational displacement of approximately 500–700 ml of central blood volume into the splanchnic and lower extremity vascular beds.1 If uncompensated, this can result in orthostatic intolerance. Normal circulatory compensation for orthostasis occurs rapidly via the sympathetic and parasympathetic arms of the autonomic nervous system for appropriate heart rate (HR) and blood pressure (BP) control 2–6. The normal baroreflex response to decreased BP involves peripheral vasoconstriction and a reflex tachycardia7,8.
Orthostatic intolerance is accompanied by signs and symptoms that can include loss of consciousness, cognitive deficits, loss of vision or hearing, lightheadedness, headache, fatigue, nausea and abdominal pain, sweating and tremulousness9. Orthostatic intolerance is commonly seen in younger patients with postural tachycardia syndrome (POTS)10,11.
Patients with POTS experience chronic orthostatic intolerance plus excessive tachycardia when upright in the absence of hypotension. Symptoms occur daily, almost always interfere with work and/or school activities, and most patients are female12–14. Excessive tachycardia is defined by an increase of HR to >120 beats per minute during a 10-minute tilt or an increase of >30 beats per minute (bpm) in adults or an increase of >40 bpm in those under 19 years15.
Although the mechanisms of POTS are heterogenous, its effects resemble well-known forms of hypovolemia with reduced systemic venous return and reduced cardiac output16–19. A reduction in total blood volume has been reported in many cases20–22. Therefore, treatment has included attempts at repletion of blood volume using various substances including fludrocortisone, tested in adults, and erythropoietin. However, a study of orthostatic intolerance in patients with chronic fatigue syndrome (CFS) showed that low-dose mineralocorticoids did not mitigate symptoms compared with placebo23. In other cases, upright POTS patients demonstrated a reduction in central blood volume due to blood volume redistribution without a reduction in total blood volume; this results in reflex sympathetic excitation, vagal withdrawal and tachycardia22,24,25. Thus, reduced orthostatic central blood volume is common to both acute and chronic orthostatic intolerance.
More direct methods have been employed to mitigate the effects of orthostasis, including the administration of intravenous saline as this should improve all forms of orthostatic intolerance by increasing central blood volume and venous return. Thus, saline may prevent syncope26 and improve orthostatic intolerance and heart rate changes in POTS patients27. Enthusiasm for the use of intravenous (IV) saline is diminished because of the expense, need for repeated infusions or unacceptable risks (bruising and infection) of chronic central venous catheterization28. Aside from IV saline, alternatives such as oral salt and water have been recommended to reduce orthostatic intolerance symptoms29,30.
We investigated whether equal volumes of oral rehydration solution (ORS) or intravenous saline provide similar improvements in cardiovascular status and in orthostatic tolerance when administered to patients with orthostatic intolerance. We evaluated orthostatic tolerance using lower body negative pressure (LBNP)31–33 which is a controllable form of orthostatic challenge which has been used as a reversible simulation of central hypovolemia 34,35.
Methods
To test the hypothesis that increasing total blood volume with intravenous saline or ORS improves orthostatic intolerance and cardiorespiratory properties, we performed step-wise LBNP to measure changes in cardiorespiratory properties, BP and CO at different stages LBNP (–15, – 30, and –45 mmHg, each for 5 min and at −60mmHg for up to 50 min until orthostatic intolerance is achieved) to determine the threshold for orthostatic tolerance for each subject. This was repeated on 3 separate days before which either no fluids were administered, subjects drank 1 liter of ORS (World Health Organization formulation containing Na+= 90 mEq/l and glucose = saline administered over 30 minutes. The order of testing was assigned randomly. All orthostatic testing was performed 1 hour after fluid administration.
Subject Recruitment and Classification
Healthy control subjects and patients with orthostatic intolerance, aged 15 to 29 years old were recruited. We enrolled 15 healthy control subjects and 10 with a history of orthostatic intolerance and POTS. POTS cases had chronic, day-to-day symptoms of orthostatic intolerance for at least 3 months. POTS was confirmed by duplication of these symptoms associated with an excessive increase in HR for age during a clinical 70° upright tilt test30,36. Control subjects (N=15) were all free of orthostatic intolerance based on clinical history12. No subjects were taking neurally active, vasoactive or birth control drugs. Any prior medication was discontinued for at least 2 weeks prior to participation.
Protocol:
all experiment days began between 9–10 am in a room at 25° C. Subjects refrained from eating for at least 4 hours and eliminated caffeinated beverages for at least 12 hours prior to testing. Upon arrival, all subjects were placed in the lower body negative pressure (LBNP) chamber and tested as described below. An intravenous catheter was placed in the left antecubital vein for administration of intravenous fluids. We used a finger photoplethysmograph to assess beat-to-beat BP, EKG for heart rate and rhythm (Finometer, FMS, The Netherlands)37,38. Mean arterial pressure (MAP) was calculated from systolic BP (SBP) and diastolic BP as (SBP + 2*diastolic BP)/3. Cardiac output (CO), expressed as L/min, was intermittently measured using the inert gas rebreathing (Innocor, Innovision, DE) and measured continuously using the FMS ModelFlow arterial pulse wave algorithm. Transcranial Doppler ultrasound was used to assess changes in middle cerebral artery blood flow velocity (CBFV) by insonation at a depth of 5–6 cm using a 2-MHz probe (Neurovision; Multigon, Yonkers, NY). Blood pressure, EKG, TCD, ModelFlow and cardiac output were acquired continuously to computer through an A/D conversion system using custom computer software. Following instrumentation, all subjects rested for 30-minute while supine. After this rest period, we acquired 10 minutes of baseline cardiorespiratory properties.
LBNP:
Subjects are placed supine with their lower body (legs and hips up to the iliac crest), within a sealed airtight chamber– the LBNP tank. A snug rubber diaphragm makes an air-tight seal without compressing the abdomen. Suction is provided by a vacuum pump which rapidly produces desired negative pressure and controlled with a variable autotransformer calibrated against an electronic manometer. Graded LBNP is applied sequentially at –15, –30, and –45 mmHg, for 5 min at each stage and at −60mmHg for up to 50 min until the threshold for orthostatic intolerance is achieved. BP by oscillometry and CO by ModelFlow was measured for 2 min at every stage of lower LBNP and for 2 min every 10 min during −60mmHg LBNP, along with continuous measurements of HR, BP, respirations, IPG regional blood flow and changes in regional blood volume.
A priori stopping criteria (end test) were signs and symptoms of presyncope defined as a decrease in systolic BP to 80 mmHg; a decrease in systolic BP to 90 mmHg associated with orthostatic intolerance symptoms of lightheadedness, pallor, hyperpnea, nausea, sweating, or diaphoresis; or progressive symptoms of orthostatic intolerance accompanied by a request to discontinue the test. This strategy has produced orthostatic intolerance in healthy volunteers and permits nearly instant recovery by release of negative pressure32.
Data Collection and Analyses:
Multiple variables (eg, heart rate, blood pressure, cardiac output, cerebral blood flow velocity) were collected while supine and during LBNP. The total response to orthostatic stress for each subject, or orthostatic index (also referred to as the cumulative stress index), was calculated as the product of LBNP and time it took to reach the threshold of orthostatic intolerance 17,32. Because all controls and POTS were able to tolerate some exposure to −45 mmHg of LBNP, analysis of BP, CO and CBFv was performed using data collected for a 2-minute period prior to stopping at the end of exposure to this pressure Thus, data shown as −45 mmHg is the response following 10 minutes of supine rest and the cumulative exposure to 5 minutes at −15, −30 and at least 2 minutes at −45 mmHg pressure. These results are depicted graphically as “−45 mmHg.”
To investigate the response of each dependent variable during LBNP, we used a linear mixed model regression approach to account for the within-subject correlation across interventions and pressure challenge. We used a hierarchical approach to determine which post-hoc analyses were conducted. For each model, a 3-way interaction term (group x treatment x pressure) was evaluated, along with all lower-level interaction terms. If the 3-way interaction term was significant, then all post-hoc comparisons of interest were examined. If the 3-way interaction was not significant, then it was removed and the 2-way interaction terms were evaluated. Significant 2-way interaction terms subsequently guided post-hoc testing. Within each model, post-hoc testing was adjusted for multiple comparisons using the Sidak approach.
The Institutional Review Board of New York Medical College reviewed and approved this protocol. Each subject received a detailed description of all protocols and was given an opportunity to have questions answered. Signed informed consent was obtained from all adult participants; those younger than 18 assented to participate and their parent of legal guardian signed an informed consent.
RESULTS:
The demographics of the subjects are shown in the Table. The results are reported as Mean±SEM. There were no significant differences between controls and POTS in height, weight or body mass index, however POTS were younger than controls.
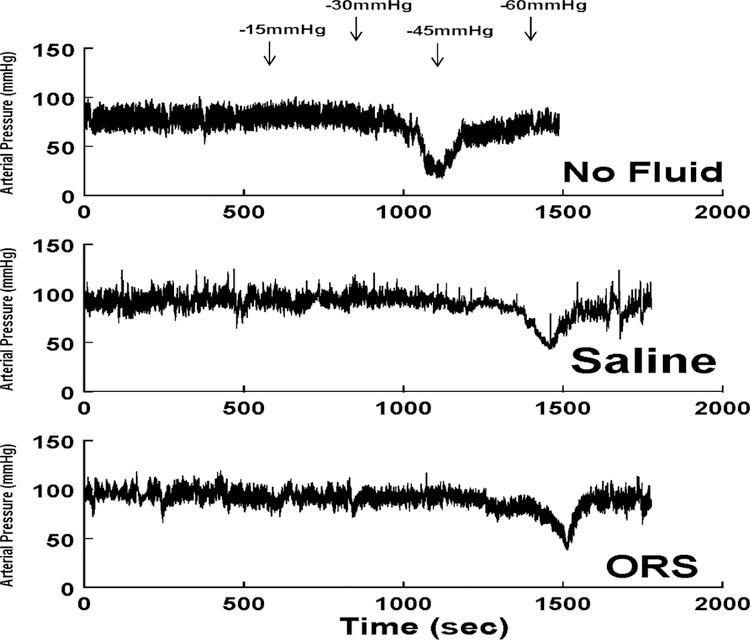
We compared the responses to the orthostatic challenge imposed by LBNP in the absence of supplemental fluid administration with that following oral rehydration solution ingestion and intravenous fluid infusion. Figure 1 shows these responses in a representative patient with orthostatic intolerance. The top panel shows the response on a day when no fluid was given (No Fluid) where fainting occurred while a −30mmHg negative pressure was generated by LBNP. Arrows are placed at times of transition in the top panel for convenience; −45 or −60mmHg pressures were not achieved for this patient during the during the No Fluid day. Both saline administration (Saline) and oral rehydration solution (ORS) increased initial blood pressure and prolonged the time to faint. With saline, the patient fainted at the transition to −60mmHg. After ORS the patient shown fainted subsequent to the transition to −60mmHg as well.
Figure 1.
The response of a representative patient with OI to the imposition of a controlled orthostatic challenge using LBNP. The top panel shows that in the absence of supplemental fluid administration (No Fluid), presyncope occurred during exposure to −30 mmHg negative pressure. Following IV saline (Saline, middle panel), and after ingestion of oral rehydration solution (ORS, lower panel), enhanced orthostatic tolerance was achieved as presyncope occurred during exposure to −60 mmHg negative pressure.
Next we determined the orthostatic tolerance of controls and POTS subjects and expressed this as the Orthostatic Index (the sum of the product of LBNP negative pressure and time at each pressure required to reach the threshold of orthostatic intolerance). As expected, POTS participants with a history of OI, exhibited a significantly reduced tolerance (p<0.001) to LBNP compared with controls (Orthostatic Index of 35,715±3,469 vs 93,980±7,977, respectively). POTS subjects were therefore significantly more susceptible to the influence an imposed orthostatic challenge than healthy controls.
We then evaluated the cardiovascular and circulatory changes that accompanied exposure to the orthostatic challenge imposed by LBNP while untreated, following infusion of 1 liter of saline, or following ingestion of 1 liter of ORS. Heart rate increased significantly upon exposure to −45 mmHg which was similar for both Control and POTS, whether no treatment, saline or ORS were administered (p = 0.38). On average, there was a similar significant (p < 0.001) increase for control (59.3±1.9 vs. 73.6±3.4 bpm, n=15) and POTS (67.5±4.3 vs. 80.6±6.0 bpm, n=10) comparing no orthostatic challenge (normal atmospheric pressure - baseline) with −45mmHg pressure, regardless of group or treatment.
When evaluating the changes in MAP resulting from the imposed orthostatic challenge, a significant 3-way interaction emerged (p = 0.016). Post-hoc testing indicated that in controls exposed to −45mmHg, MAP following IV saline, although higher than baseline, was not significantly increased (p = 0.055). However, in controls, only ORS significantly increased MAP (p < 0.001). Similar treatment and pressure effects were not evident in POTS subjects.
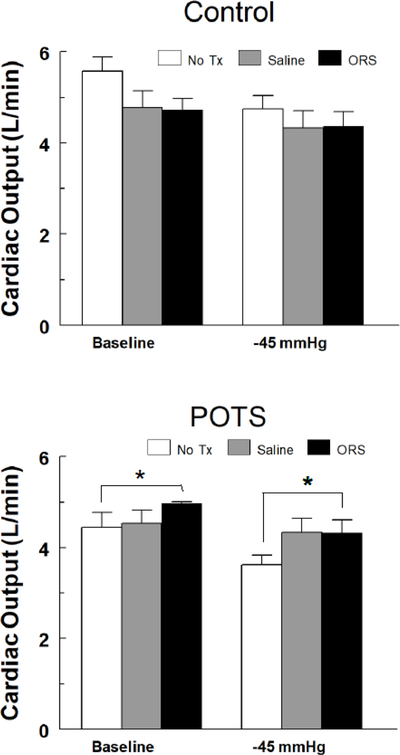
Measurements indicated that the CO of POTS subjects was significantly reduced compared with controls (4.4±0.3 vs. 5.6±0.3 respectively, p<0.001 ). For CO, shown in Figure 2, the 3-way interaction was not significant (p= 0.85), however, a significant 2-way interaction was calculated for group by treatment (p< 0.005), which was independent of pressure. Post-hoc testing indicated that for POTS, CO was significantly higher following ORS, compared with no treatment (No Tx, p<0.05), both at baseline and −45mmHg and saline had no significant effect. Treatment effects for controls were not significantly different.
Figure 2.
Changes in cardiac output (CO in L/min) in control (top panel) and POTS patients with OI (bottom panel) during imposition of a controlled orthostatic challenge using LBNP following no treatment (No TX – white bars), IV sal ine (Saline – gray bars) and ingestion of oral rehydration solution (ORS – black bars). Treatment effects for controls were not significantly different. For POTS, CO was significantly higher following ORS, compared with no treatment (* = No Tx, p<0.05), both at baseline and −45mmHg and saline had no significant effect.
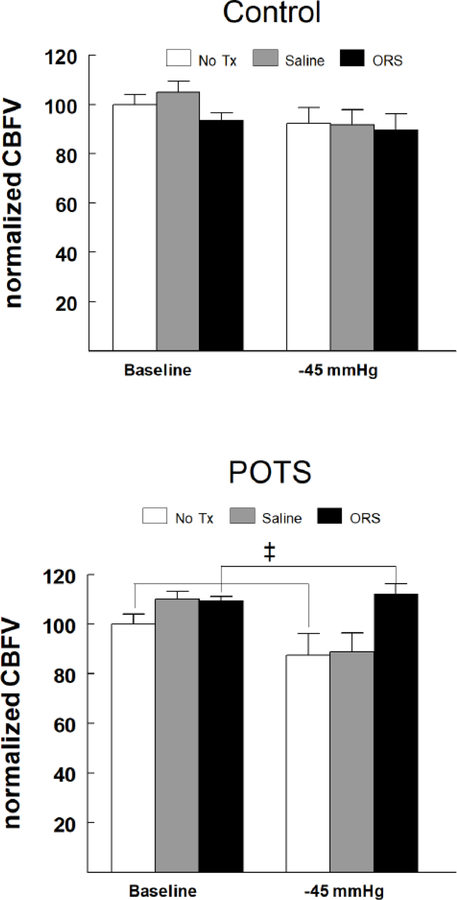
CBFv at baseline in controls and POTS was similar (76.6±2.5 cm/sec vs. 70.9±2.7 cm/sec, respectively (p=0.12). To appreciate the effect of fluid administration comparing control with POTS, CBFv was normalized to values measured while supine in the absence of LBNP. Figure 3 shows that in controls, normalized CBFv was not significantly reduced at −45 mmHg, nor did fluid administration result in any significant change. Interestingly however, a significant 2-way interaction was apparent for study group by treatments (p < 0.0005) as POTS responded differently than controls regardless of pressure. Post-hoc evaluation indicated that in POTS, CBFv following ORS was significantly higher than No TX (p<0.0005) as the normalized CBFV for POTS was about 13 percent higher. This effect was independent of pressure.
Figure 3.
Changes in normalized cerebral blood flow velocity (normalizedCBFV) in control (top panel) and POTS patients with OI (bottom panel) during imposition of a controlled orthostatic challenge using LBNP following no treatment (No TX – white bars), IV saline (Saline – gray bars) and ingestion of oral rehydration solution (ORS – black bars). Because there was no pressure effect, the comparisons reflect the combined effect (i.e., the weighted average) of the CBVv in the ORS group vs. No Tx. In controls, normalized CBFv was not significantly reduced at −45 mmHg, nor did fluid administration result in any significant change. In POTS, CBFv following ORS was significantly higher than No TX (ǂ = p<0.0005), which was independent of pressure.
To compare the influence of fluid administration (intravenous vs. oral) on the orthostatic challenge imposed by LBNP, we normalized the orthostatic intolerance for each group to that measured without treatment (Untreated) compared with I.V. saline or ORS. Figure 4 shows that although neither saline nor ORS increased orthostatic tolerance in untreated controls (p=0.46, N=15), both I.V. saline and ORS significantly improved orthostatic tolerance (p<0.05 and p<0.001, respectively, N=10) in POTS subjects. Thus ORS, in contrast to saline, afforded a beneficial effect from the imposed orthostatic stress.
Figure 4.
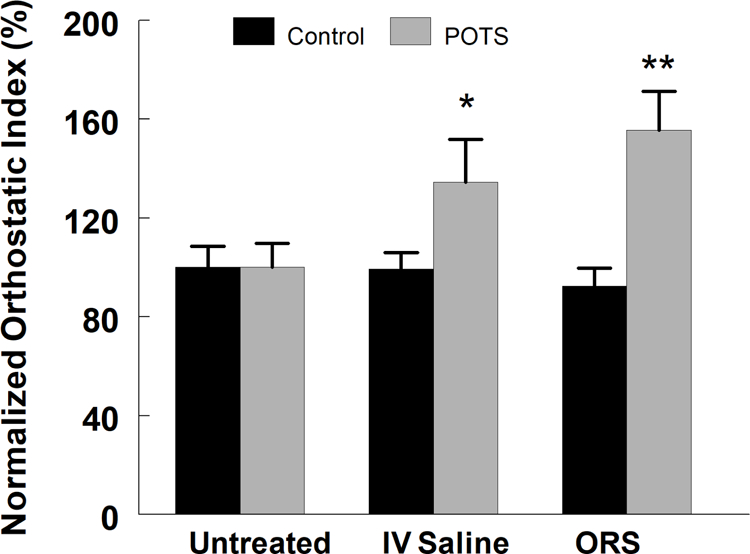
Changes in normalized orthostatic index (% of that measured without treatment in control (black bars) and POTS patients with OI (gray bars) measured following no treatment (Untreated), intravenous saline (IV Saline) and ingestion of oral rehydration solution (ORS). Neither saline nor ORS increased orthostatic tolerance in untreated controls (p=0.46, N=15); both I.V. saline and ORS significantly improved orthostatic tolerance (* = p<0.05 and ** = p<0.001, respectively, N=10) in POTS subjects.
DISCUSSION:
Previous studies have shown that infusion of IV saline improves orthostatic tolerance and autonomic symptoms in patients with orthostatic intolerance 26,27,39,40. Although there have been reports describing improvement of qualitative outcomes associated with syncope with long-term ORS usage41,42, the quantitative effects of ORS on orthostatic tolerance have not been reported. In this study, orthostatic tolerance was evaluated using LBNP which we have used previously to induce orthostatic stress32,43. LBNP, standing and head-up tilt (HUT) cause many of the changes of neurovascular physiology which can result in signs and symptoms of orthostatic intolerance. For example, both HUT and LBNP produce central hypovolemia and comparable unloading of the cardiopulmonary and arterial baroreceptors32,33,44,45. It has also been shown that cerebral hemodynamic responses to LBNP to −45 mmHg are similar to that which accompany blood loss up to 1,000 ml46.
The rapid beneficial effect of ORS may be caused by increasing blood volume given its efficacy in enhancing consistent and nearly complete fluid and salt absorption through the intestinal Na+-glucose co-transport (GLUT2, symporter) carrier. This effective enteral salt and water transport system has been employed to combat the catastrophic fluid loss of infectious diarrhea47. ORS in POTS may temporarily correct the central hypovolemia that has been reported in many subsets of patients with orthostatic intolerance and various types of syncope10,18,48. This may have resulted in part from the significantly higher CO that was measured following ORS, but not saline, in POTS patients.
In addition to facilitating the absorption of Na+ and water, the ability of ORS to mitigate orthostatic intolerance in susceptible subjects may also be due in part to the gastropressor effect that occurs through sympathetic nervous system activation mediated through gut or portal osmoreceptors in the afferent signaling response to oral water49. The gastropressor response is a control mechanism that can lead to a marked increase in blood pressure after water, but not saline ingestion. An acute response peaks within 20 to 40 minutes and resolves within 60 to 90 minutes. In the current study, although there was a similar increase in HR with orthostatic challenge in both controls and POTS independent of fluid supplementation, ingestion of ORS only caused an increase in MAP in control subjects but not in POTS, suggesting that they are less capable of mounting a functional pressor response.
Several studies have demonstrated the acute benefits of supplemental intravenous hydration in improving orthostatic intolerance, showing that hemodynamic and symptomatic tolerance to repeated tilt-table testing can be restored following acute IV administration of saline26,50 Although there are reports of benefit to chronic or repeated saline administration, potential benefits must be considered within the context of the associated risks of infection, thrombi and the difficulty of administration28.
Orthostatic intolerance is often accompanied by loss of cognition referred to as brain fog. Standing results in gravitational blood transfer, reducing central and increasing splanchnic vasculature and lower extremity blood volumes1,14,38. There is often a period of initial orthostatic hypotension during which BP and CBFV transiently decrease, sometimes markedly, reaching their nadir 10 to 20 s after standing. A reflex tachycardia results, and BP and CBFv are restored within 30 to 60 s but CBFv recovers to somewhat less than supine30,51. When the effects of low CBFv on CNS function were examined using graded incremental head-up tilt, and executive working memory evaluation using N-Back testing as an objective measure of cognitive impairment, executive memory function was progressively impaired in POTS patients with increasing angle of tilt. Thus increasing orthostatic stress combined with a cognitive challenge impairs the neurocognitive abilities of working memory, accuracy and information processing in CFS/POTS52.
Although measurements of cognitive ability were not performed in this study, preservation of CBFv likely supported improved cognitive abilities in the POTS subjects. In this study using LBNP as a controlled orthostatic stressor, although POTS had about 30% of the orthostatic tolerance of controls, normalized CBFv was best preserved by ORS in the POTS subjects and may have contributed in part to the significant increase in orthostatic tolerance afforded by this treatment.
Giving ORS to POTS patients produced effective, short-term mitigation of their orthostatic intolerance, presumably by facilitating rapid repletion of salt and water. Within the short time course of this investigation, ORS was at least as effective in increasing orthostatic tolerance than IV saline. This supports the use of ORS as an easy, safe, practical therapy to mitigate symptoms associated with orthostatic intolerance. Because ORS is inexpensive, safe and easily administered, it may be considered as an effective alternative to IV saline for rapid resolution of symptoms associated with orthostatic intolerance.
This study did not include evaluation of cognitive abilities during LBNP which would have provided additional information regarding orthostatic intolerance CBFv and executive function. In addition, we were not able to measure changes in blood volume during LBNP in the setting of fluid supplementation, either from saline of ORS. Obtaining subjective information about symptoms and other indices of wellness during imposition of LBNP, while desirable, would have been difficult to obtain. In addition, because LBNP imposes a negative pressure below the level of the iliac crest and is not gravitational, this orthostatic challenge does not replicate that of upright posture.
We only investigated the short-term use of fluids in subjects with orthostatic intolerance. Further investigations of long-term regular use of ORS are therefore warranted.
This study was also limited by the small number of participants. Although many physiological responses appear to have been influenced by the imposed maneuvers, not all reached statistical significance. It would have therefore been beneficial to compare the IV saline and the ORS arms in a larger cohort of age matched controls and patients.
Table 1.
Demographics of Study Subjects
| Age | Height | Weight | BMI (Kg/m2) | M/F | |
|---|---|---|---|---|---|
| Control (n=15) | 24.7±0.5 | 171.1±2.1 | 70.0±3.4 | 23.7±0.7 | 3/12 |
| POTS (n=10) | 19.5±1.4 | 168.6±3.0 | 62.6±4.2 | 22.0±1.2 | 1/9 |
Acknowledgments
Funded by the National Institute of Neurologic Disorders and Stroke (R21 NS 094644), the National Heart Lung and Blood Institute (RO1 HL 112736 and RO1 HL 134674), and the National Institutes of Health (NIH). The authors declare no conflicts of interest.
ABBREVIATIONS
- BP
blood pressure
- CBFV
cerebral blood flow velocity
- CFS
chronic fatigue syndrome
- CO
cardiac output
- HR
heart rate
- HUT
head up tilt
- IV
intravenous
- LBNP
lower body negative pressure
- MAP
mean arterial pressure
- ORS
oral rehydration solution
- POTS
postural tachycardia syndrome
- SBP
systolic blood pressure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Sheriff DD, Nadland IH, Toska K. Role of sympathetic responses on the hemodynamic consequences of rapid changes in posture in humans. J Appl Physiol (1985 ) 2010;108:523–532. [DOI] [PubMed] [Google Scholar]
- 2.Brack KE, Coote JH, Ng GA. Vagus nerve stimulation inhibits the increase in Ca2+ transient and left ventricular force caused by sympathetic nerve stimulation but has no direct effects alone--epicardial Ca2+ fluorescence studies using fura-2 AM in the isolated innervated beating rabbit heart. Exp Physiol 2010;95:80–92. [DOI] [PubMed] [Google Scholar]
- 3.Macarthur H, Wilken GH, Westfall TC, Kolo LL. Neuronal and non-neuronal modulation of sympathetic neurovascular transmission. Acta Physiol (Oxf) 2011;203:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raczak G, La Rovere MT, Mortara A, Assandri J, Prpa A, Pinna GD, et al. Arterial baroreflex modulation of heart rate in patients early after heart transplantation: lack of parasympathetic reinnervation. J Heart Lung Transplant 1999;18:399–406. [DOI] [PubMed] [Google Scholar]
- 5.Toda N, Ayajiki K, Okamura T. Cerebral blood flow regulation by nitric oxide: recent advances. Pharmacol Rev 2009;61:62–97. [DOI] [PubMed] [Google Scholar]
- 6.Von Euler US. Identification of the sympathomimetic ergone in adrenergic nerves of cattle (sympathin N) with levonoradrenaline. Acta Physiol Scand 1948;16:63–74. [Google Scholar]
- 7.Borst C, Wieling W, van Brederode JF, Hond A, de Rijk LG, Dunning AJ. Mechanisms of initial heart rate response to postural change. Am J Physiol 1982;243:H676–H681. [DOI] [PubMed] [Google Scholar]
- 8.Ewing DJ, Hume L, Campbell IW, Murray A, Neilson JM, Clarke BF. Autonomic mechanisms in the initial heart rate response to standing. J Appl Physiol Respir Environ Exerc Physiol 1980;49:809–814. [DOI] [PubMed] [Google Scholar]
- 9.Low PA, Opfer-Gehrking TL, McPhee BR, Fealey RD, Benarroch EE, Willner CL, et al. Prospective evaluation of clinical characteristics of orthostatic hypotension. Mayo Clin Proc 1995;70:617–622. [DOI] [PubMed] [Google Scholar]
- 10.Sheldon RS, Grubb BP, Olshansky B, Shen WK, Calkins H, Brignole M, et al. 2015 heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm 2015;12:e41–e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stewart JM. Common syndromes of orthostatic intolerance. Pediatrics 2013;131:968–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medow MS, Stewart JM, Sanyal S, Mumtaz A, Sica D, Frishman WH. Pathophysiology, diagnosis, and treatment of orthostatic hypotension and vasovagal syncope. Cardiol Rev 2008;16:4–20. [DOI] [PubMed] [Google Scholar]
- 13.Schondorf R, Low PA. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology 1993;43:132–137. [DOI] [PubMed] [Google Scholar]
- 14.Stewart JM. Chronic orthostatic intolerance and the postural tachycardia syndrome (POTS). J Pediatr 2004;145:725–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singer W, Sletten DM, Opfer-Gehrking TL, Brands CK, Fischer PR, Low PA. Postural tachycardia in children and adolescents: what is abnormal? J Pediatr 2012;160:222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barcroft H, McMichael JE, Scarpey-Schafer EP. Posthaemorrhagic fainting. Study by cardiac outputand forearm flow. Lancet 1944;1:489–491. [Google Scholar]
- 17.Fu Q, Witkowski S, Okazaki K, Levine BD. Evidence for unloading arterial baroreceptors during low levels of lower body negative pressure in humans. Am J Physiol Regul Integr Comp Physiol 2005;289:R109–116.15761188 [Google Scholar]
- 18.Stewart JM, Taneja I, Medow MS. Reduced body mass index is associated with increased angiotensin II in young women with postural tachycardia syndrome. Clin Sci (Lond) 2007;113:449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu Q, Vangundy TB, Galbreath MM, Shibata S, Jain M, Hastings JL, et al. Cardiac origins of the postural orthostatic tachycardia syndrome. J Am Coll Cardiol 2010;55:2858–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fouad FM, Tadena-Thome L, Bravo EL, Tarazi RC. Idiopathic hypovolemia. Ann Intern Med 1986;104:298–303. [DOI] [PubMed] [Google Scholar]
- 21.Raj SR, Biaggioni I, Yamhure PC, Black BK, Paranjape SY, Byrne DW, et al. Renin-aldosterone paradox and perturbed blood volume regulation underlying postural tachycardia syndrome. Circulation 2005;111:1574–1582. [DOI] [PubMed] [Google Scholar]
- 22.Raj SR, Robertson D. Blood volume perturbations in the postural tachycardia syndrome. Am J Med Sci 2007;334:57–60. [DOI] [PubMed] [Google Scholar]
- 23.Rowe PC, Calkins H, Debusk K, McKenzie R, Anand R, Sharma G, et al. Fludrocortisone acetate to treat neurally mediated hypotension in chronic fatigue syndrome: a randomized controlled trial. JAMA 2001;285:52–59. [DOI] [PubMed] [Google Scholar]
- 24.Grubb BP, Kosinski DJ, Boehm K, Kip K. The postural orthostatic tachycardia syndrome: a neurocardiogenic variant identified during head-up tilt table testing. Pacing Clin Electrophysiol 1997;20:2205–2212. [DOI] [PubMed] [Google Scholar]
- 25.Stewart JM, Taneja I, Medow MS. Reduced central blood volume and cardiac output and increased vascular resistance during static handgrip exercise in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol 2007;293:H1908–H1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burklow TR, Moak JP, Bailey JJ, Makhlouf FT. Neurally mediated cardiac syncope: autonomic modulation after normal saline infusion. J Am Coll Cardiol 1999;33:2059–2066. [DOI] [PubMed] [Google Scholar]
- 27.Jacob G, Shannon JR, Black B, Biaggioni I, Mosqueda-Garcia R, Robertson RM, et al. Effects of volume loading and pressor agents in idiopathic orthostatic tachycardia. Circulation 1997;96:575–580. [DOI] [PubMed] [Google Scholar]
- 28.Moak JP, Leong D, Fabian R, Freedenberg V, Jarosz E, Toney C, et al. Intravenous Hydration for Management of Medication-Resistant Orthostatic Intolerance in the Adolescent and Young Adult. Pediatr Cardiol 2016;37:278–282. [DOI] [PubMed] [Google Scholar]
- 29.Claydon VE, Hainsworth R. Salt supplementation improves orthostatic cerebral and peripheral vascular control in patients with syncope. Hypertension 2004;43:809–813. [DOI] [PubMed] [Google Scholar]
- 30.Stewart JM, Boris JR, Chelimsky G, Fischer PR, Fortunato JE, Grubb BP, et al. Pediatric Disorders of Orthostatic Intolerance. Pediatrics 2018;141. [DOI] [PMC free article] [PubMed]
- 31.Wolthuis RA, Hoffler GW, Johnson RL. Lower body negative pressure as an assay technique for orthostatic tolerance: I. The individual response to a constant level (−40 mm. Hg) of LBNP. Aerosp Med 1970;41:29–35. [PubMed] [Google Scholar]
- 32.Taneja I, Moran C, Medow MS, Glover JL, Montgomery LD, Stewart JM. Differential effects of lower body negative pressure and upright tilt on splanchnic blood volume. Am J Physiol Heart Circ Physiol 2007;292:H1420–H1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goswami N, Blaber AP, Hinghofer-Szalkay H, Convertino VA. Lower Body Negative Pressure: Physiological Effects, Applications, and Implementation. Physiol Rev 2019;99:807–851. [DOI] [PubMed] [Google Scholar]
- 34.Cooke WH, Ryan KL, Convertino VA. Lower body negative pressure as a model to study progression to acute hemorrhagic shock in humans. J Appl Physiol (1985 ) 2004;96:1249–1261. [DOI] [PubMed] [Google Scholar]
- 35.Murray RH, Thompson LJ, Bowers JA, Steinmetz EF, Albright CD. Hemodynamic effects of hypovolemia in normal subjects and patients with congestive heart failure. Circulation 1969;39:55–63. [DOI] [PubMed] [Google Scholar]
- 36.Medow MS, Stewart JM. The postural tachycardia syndrome. Cardiol Rev 2007;15:67–75. [DOI] [PubMed] [Google Scholar]
- 37.Stewart JM, Medow MS, Montgomery LD, Glover JL, Millonas MM. Splanchnic hyperemia and hypervolemia during Valsalva maneuver in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol 2005;289:H1951–H1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stewart JM, Medow MS, Glover JL, Montgomery LD. Persistent splanchnic hyperemia during upright tilt in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol 2006;290:H665–H673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaffney FA, Buckey JC, Lane LD, Hillebrecht A, Schulz H, Meyer M, et al. The effects of a 10-day period of head-down tilt on the cardiovascular responses to intravenous saline loading. Physiologist 1990;33:S171–S172. [PubMed] [Google Scholar]
- 40.Takenaka K, Suzuki Y, Uno K, Sato M, Komuro T, Haruna Y, et al. Effects of rapid saline infusion on orthostatic intolerance and autonomic tone after 20 days bed rest. Am J Cardiol 2002;89:557–561. [DOI] [PubMed] [Google Scholar]
- 41.Chu W, Wang C, Wu L, Lin P, Li F, Zou R. Oral rehydration salts: an effective choice for the treatment of children with vasovagal syncope. Pediatr Cardiol 2015;36:867–872. [DOI] [PubMed] [Google Scholar]
- 42.Li H, Wang Y, Liu P, Chen Y, Feng X, Tang C, et al. Body Mass Index (BMI) is Associated with the Therapeutic Response to Oral Rehydration Solution in Children with Postural Tachycardia Syndrome. Pediatr Cardiol 2016;37:1313–1318. [DOI] [PubMed] [Google Scholar]
- 43.Stewart JM, Sutton R, Kothari ML, Goetz AM, Visintainer P, Medow MS. Nitric oxide synthase inhibition restores orthostatic tolerance in young vasovagal syncope patients. Heart 2017;103:1711–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cooper VL, Hainsworth R. Carotid baroreceptor reflexes in humans during orthostatic stress. Exp Physiol 2001;86:677–681. [DOI] [PubMed] [Google Scholar]
- 45.Halliwill JR, Lawler LA, Eickhoff TJ, Joyner MJ, Mulvagh SL. Reflex responses to regional venous pooling during lower body negative pressure in humans. J Appl Physiol (1985 ) 1998;84:454–458. [DOI] [PubMed] [Google Scholar]
- 46.Rickards CA, Johnson BD, Harvey RE, Convertino VA, Joyner MJ, Barnes JN. Cerebral blood velocity regulation during progressive blood loss compared with lower body negative pressure in humans. J Appl Physiol (1985 ) 2015;119:677–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.World Health Organization. Department of Child Health and Adolescent Health and Development. World Health Organization. Reduced osmolarity oral rehydration salts (ORS) formulation - Report from a meeting of experts jointly organized by UNICEF and WHO (WHO/FCH/CAH/01.22). UNICEF and WHO Report 2001.
- 48.Raj SR. Postural tachycardia syndrome (POTS). Circulation 2013;127:2336–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raj SR, Biaggioni I, Black BK, Rali A, Jordan J, Taneja I, et al. Sodium paradoxically reduces the gastropressor response in patients with orthostatic hypotension. Hypertension 2006;48:329–334. [DOI] [PubMed] [Google Scholar]
- 50.Younoszai AK, Franklin WH, Chan DP, Cassidy SC, Allen HD. Oral fluid therapy. A promising treatment for vasodepressor syncope. Arch Pediatr Adolesc Med 1998;152:165–168. [DOI] [PubMed] [Google Scholar]
- 51.Carey BJ, Manktelow BN, Panerai RB, Potter JF. Cerebral autoregulatory responses to head-up tilt in normal subjects and patients with recurrent vasovagal syncope. Circulation 2001;104:898–902. [DOI] [PubMed] [Google Scholar]
- 52.Ocon AJ, Messer ZR, Medow MS, Stewart JM. Increasing orthostatic stress impairs neurocognitive functioning in chronic fatigue syndrome with postural tachycardia syndrome. Clin Sci (Lond) 2012;122:227–238. [DOI] [PMC free article] [PubMed] [Google Scholar]