Abstract
Historically, it had been widely accepted that the female mammalian ovary contained a limited number of oocytes that would reduce over time, without the possibility of replenishment. However, recent studies have suggested that female germline stem cells (FGSCs) could replenish the oocyte-pool in adults. The aim of this study was to isolate FGSCs from porcine ovaries and differentiate them into oocyte-like cells (OLCs). The FGSCs were successfully isolated from porcine ovarian tissue and cultured in vitro, in DMEM/F-12 medium supplemented with growth factors (EGF, FGF, GDNF, etc.) and a supplement (N21). These cells possessed spherical morphology and expressed specific germline characteristics (Vasa, Stella, Oct4, c-kit). By evaluating different conditions for in vitro differentiation of FGSCs, co-culturing the isolated FGSCs with MEF cells, under three-dimensional (3D) cell cultures, were shown to be optimal. FGSCs could successfully be differentiated into OLCs and reached about 70 µm in diameter, with a large number of surrounding somatic cells. Importantly, OLCs contained large nuclei, about 25–30 µm, with filamentous chromatin, similar to oocyte morphology, and expressed oocyte-specific markers (Gdf9, Zp2, SCP3, etc.) at the same level as oocytes. In conclusion, we successfully isolated FGSCs from porcine ovarian tissue and differentiated them into oocyte-like cells. This will provide a valuable model for studying a new, alternative source of oocytes.
Keywords: Co-culture, Differentiation, Female germline stem cells, Oocyte-like cells, 3D cell cultures
It has widely been accepted that female mammals were born with a fixed number of oocyte-containing follicles, which then became exhausted with age [1]. This differs from males who can produce sperm throughout their life, due to a source of constantly proliferating spermatogonial stem cells (SSCs), which can differentiate and allows male mammals a long-lasting reproductive period [2, 3]. In other words, no germline stem cells exist in the postnatal female mammalian ovary which can differentiate and produce new oocytes in order to offset their consumption by ovulation. This explanation was widely accepted due to shorter reproductive period of mammalian females, compared to males.
In 2004, a study proposed that the mouse ovary had an unexpected ability to rejuvenate the follicle pool, which led to the suggestion of a source of cells for oogenesis during the reproductive period [4]. This opened much debate on whether germline stem cells really exist in the postnatal ovary or not. In 2009, the first successful result in isolating and purifying female germline stem cells (FGSCs), from the ovary of neonatal and adult mice, by using magnetic bead sorting technique against Vasa protein, a germline-specific marker, was reported. These cells could be differentiated into oocyte-like cells in vitro [5]. Zhang et al. discovered FGSCs in mice and transplanted different genes into them. These FGSCs were then transferred into the ovaries of infertile, female mice, resulting in the creation of transgenic mice, after mating with normal male mice [6]. Recently, scientists have shown that FGSCs are a useful tool for the genetic manipulation of animals, by creating transgenic rats [7]. Scientists have suggested that the ability to produce transgenic animals in this way, would be an excellent tool for biological reproduction in the future. The benefits of this technology are significant in certain areas such as animal cloning in agriculture, creation of domestic animals with high commercial values, biotechnology-based pharmaceuticals from transgenic animals, including the production of animal medicine and specific proteins for clinical treatment. Interestingly, these studies presented important evidence supporting the existence of germline stem cells in postnatal female mammals and opened a new direction in the study of stem cells in the human ovary.
Stem cells are considered to have multiple applications for cell therapy. Ovarian failure can be caused by chemotherapy, radiation, genetic induction, or hormonal stress [8, 9]. Additionally, ovarian dysfunction, before the age of 40, is commonly caused by premature ovarian failure, in 1% of young women [10]. Sterility from oocyte loss can also be caused by some diseases, such as polycystic ovary syndrome [11]. Some progress has been made in solving this problem through assisted reproductive technologies, which found that FGSCs play a role in fertility, providing future application for clinical therapies. In 2012, remarkably, FGSCs were able to be isolated from the cortex of adult human female ovaries and differentiated into oocyte-like cells in vitro [12]. Further, these isolated FGSCs formed into follicles containing oocytes when transplanted into immunodeficient mice, indicating that FGSCs could be cultivated for months and spontaneously generate oocytes [13]. Nevertheless, a new question arises: Whether the egg cells were created from female germline stem cells or not? If FGSCs do exist in the ovary, why does the phenomenon of menopause occur only in females? The origin, ability, and roles of these cells in female reproduction should be clearly understood before the application of this treatment in humans. In 2014, our group successfully established porcine FGSCs and demonstrated that these FGSCs, derived from primordial germ cells (PGCs), had been retained but deactivated in the adult ovary [14]. It was previously thought that these PGCs were only existed during the fetal period and that all of them would turn into oocytes before the individual was born. In addition, other research has shown a similar pattern of gene expression profiles in FGSCs and male germline stem cells [15].
The differentiation ability of FGSCs in mammalian ovaries remains a controversial issue between reproductive biologists and stem cell researchers. Many groups have been studied for the differentiation potential of FGSCs [16,17,18]. However, there is still no success in creating offspring from FGSCs in large mammals, except for mice and rats [6, 7]. Therefore, research on a mammalian model other than mice needs to be done and a porcine model is compelling for the study of medical applications due to the different mechanisms underlying biological processes in mouse eggs compared with human eggs. Recently, cryopreservation of ovarian cortical tissue has been developed for woman with cancer [19]. Thus, isolation and cultivation of FGSCs from this tissue, before or after cryopreservation, may be useful for clinical fertility therapies. The pioneering work from our group has been successful in establishing porcine FGSCs and sustaining them, long term [14]. In this study, we have further researched on the differentiation potential of FGSCs into oocyte-like cells (OLCs), by using freshly isolated FGSCs, from porcine ovarian tissue.
Materials and Methods
Ethics statement
The treatment of porcine tissue used in this study followed the guidelines of International University – Vietnam National University, Ho Chi Minh City.
Isolation and culture of FGSCs
Porcine ovaries (10–12 samples per experiment) were collected from a slaughterhouse and washed in phosphate buffer saline (PBS). FGSCs were isolated using a previously described method [14]. Briefly, the ovarian cortical tissue (0.1–0.5 mm thick) was cut, minced and resuspended in DMEM/F12 medium (Sigma-Aldrich, St. Louis, MO, USA), containing 1 mg/ml collagenase (Sigma-Aldrich), for 20 min and 0.25% Trypsin-EDTA (Sigma-Aldrich) for 10 min at 37°C. The enzyme was deactivated by adding 10% fetal bovine serum (FBS) (Sigma-Aldrich) and cells were passed through a 40 μm strainer (Biologix, Abingdon, UK) to remove large, undigested tissue fragments. The dispersed cells were then precultured for 8 h in order to reduce fibroblast and somatic cell contamination. Most fibroblasts and somatic cells adhered to the surface of the dish, due to the different adherence characteristics of primary culture cells to a gelatin-coated surface, while FGSCs remained in solution, after 8 h. The remaining, floating, ovarian cells were collected and cultured in a 4-well, gelatin-coated plate (Nunc) with a density of 1–2 × 104 ml–1. The cells were cultured in DMEM/F12 that was modified as previously described [14]. Two different media for FGSC cultures, with growth factors and supplements, were evaluated. The first medium included DMEM/F12 with 10% FBS, while the second medium, used as a serum-free condition, included DMEM/F12, with FBS being replacement by N21 and growth factors. The N21 Supplement (AR008; R&D Systems, Minnesota, USA) is a serum-free media supplement containing 21 different components, specially optimized for the long-term survival and culture of stem cells [20]. Detailed compositions of each medium are shown in Supplementary Table 1 (online only). Cells were maintained at 37°C in an atmosphere of 5% CO2, in humidified air.
Isolation of oocyte from ovaries for control group
Cortical slices (0.1–1.5 mm thick) were cut from the ovarian surface using a surgical blade. Under a microscope, follicles of different sizes (0.1 mm, 0.5 mm, and 1 mm) were dissected from the tissue. Oocyte-granulosa cell complexes from the follicles were collected in HEPES solution. The oocytes from different size follicles were denuded by mouth pipetting, mounted on a slide, fixed in acetic alcohol and stained with 1% orcein. The diameters of the oocytes, chromatin, and nucleolar structure were examined using an inverted microscope (Eclipse Ti, Nikon, Tokyo, Japan) and NIS-Elements Microscope Imaging Software.
Oocytes from other groups were used for immunostaining to compare the expression of some markers and the morphology with OLCs differentiated from FGSCs.
In vitro differentiation of FGSCs
FGSCs were collected 1 week after isolation, for in vitro differentiation, and 4 differentiation conditions were evaluated.
Condition 1: FGSCs were plated on a 0.1% (w/v) gelatin-coated, non-feeder well of a 4-well plate, in the differentiation medium.
Condition 2: FGSCs were plated on a mitomycin C-treated (10 μg/ml, Sigma-Aldrich), mitotically inactivated, mouse embryonic fibroblast (MEF) feeder layer, in a well of a 4-well plate, in the differentiation medium.
Condition 3: FGSCs were co-cultured with granulosa cells, collected from porcine follicle ovaries, in an ultra low attachment dish (Corning, New York, USA), in the differentiation medium.
Condition 4: FGSCs were co-cultured with mitomycin C-treated (10 μg/ml), mitotically inactivated, MEF cells, in an ultra-low attachment dish, in the differentiation medium.
The differentiation medium was modified based on previous studies [14]. The detailed composition of the differentiation medium is shown in Supplementary Table 2 (online only).All cultures were maintained at 37°C in an atmosphere of 5% CO2, in humidified air. Half of the culture medium was changed every other day.
Immunofluorescence staining and image acquisition
For the staining of isolated FGSCs, after culturing in 4-well plates, cells were washed with PBS, fixed in 4% paraformaldehyde and treated as described previously [21]. The primary antibodies were rabbit polyclonal anti-Vasa (Santa Cruz Biotechnology, CA, USA), mouse monoclonal anti-Oct4 (Santa Cruz Biotechnology), rabbit polyclonal anti-Oct4 (Abcam, Cambridge, UK), mouse monoclonal anti-Stella (Millipore, CA, USA), and mouse monoclonal anti-CD117 (c-kit) (BD Bioscience, Franklin Lakes, NJ, USA). The secondary antibodies were either Alexa-Fluor-488-labeled goat anti-rabbit IgG or Alexa-Fluor-568-labeled goat anti-mouse IgG (Invitrogen, California, USA).
For the staining of OLCs and porcine oocytes, individual cells were collected from supernatants, fixed, and used for immunostaining. The primary antibodies were rabbit polyclonal anti-Vasa (Santa Cruz Biotechnology), mouse monoclonal anti-Zp2 (Santa Cruz Biotechnology), mouse monoclonal anti-GDF9 (Santa Cruz Biotechnology), rabbit polyclonal anti-SCP3 (Abnova Corporation, CA, USA), mouse monoclonal anti-DAZL (Santa Cruz Biotechnology), rabbit anti-acetyl-histone H3 at lysine 9 (Ac-H3-K9; Sigma-Aldrich), and goat anti-dimethyl-histone H3 at lysine 4 (Abcam). The secondary antibodies were Alexa-Fluor-568-labeled donkey anti-goat IgG (Invitrogen) and others as mention above.
Finally, DNA was counterstained with 2 µg/ml 4,6-diamidino-2-phenylindole (DAPI; Molecular Probes). Images were obtained using an inverted microscope (Nikon Eclipse Ti) and a Nikon digital camera, using fluorescein optics for TRITC and FITC, and ultraviolet optics for DAPI.
Collection and culture of granulosa cells
Granulosa cells were isolated using a previously described method [22]. Briefly, healthy pig antral follicles, 4–6 mm in diameter, were everted in HEPES-199 to collect mural granulosa cells, using two pairs of fine forceps. Granulosa cells were dispersed by vigorously aspirating them in and out of a pipette, and the suspension was then transferred to a tube. After centrifugation at 500 g for 3 min, the pellet was resuspended in PBS. This process was repeated twice and the resulting pellet of granulosa cells was resuspended in a small amount of Dulbecco’s modified Eagle’s medium (DMEM, Sigma Chemical, Missouri, USA), supplemented with 10% FBS. These cells were seeded on a 0.1% gelatin (Sigma Chemical) coated dish and cultured in an atmosphere of 5% CO2 humidified air, at 38.5°C for 2 days. After culture, the granulosa cells were collected by trypsinization. Briefly, the culture medium was removed, and the cells were thoroughly rinsed twice with PBS. Trypsin solution (Sigma Chemical) was added to cover the cultured granulosa cells for 1 min, then 2 ml of serum supplemented DMEM was added. Granulosa cells were dispersed and transferred to a tube. After three rounds of washing and centrifugation, granulosa cells were co-cultured with FGSCs.
Statistical analysis
Each experiment was repeated at least three times, with more than 50 samples (FGSCs or OLCs or oocytes) examined in each group. All data are presented as mean ± SEM. Statistical tests and graph generation were performed using Microsoft® Excel (Microsoft) and P < 0.05 was considered to indicate a significant difference.
Results
Growth factor and serum-free supplement promoted the proliferation of FGSCs
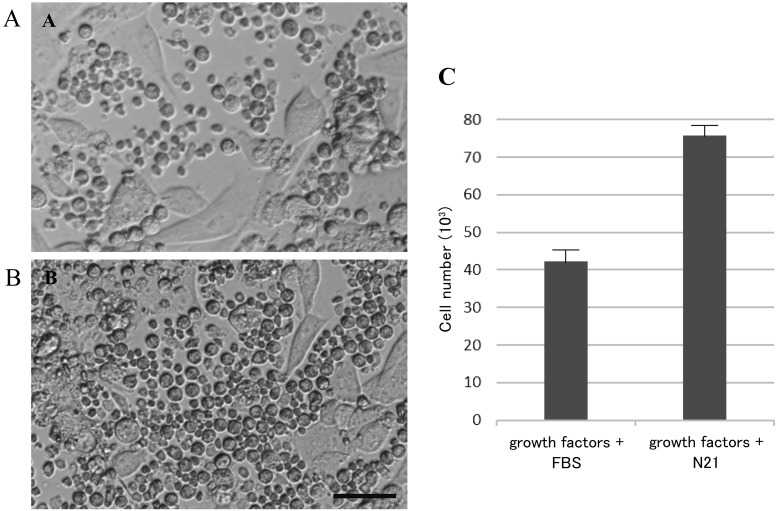
DMEM/F12 supplemented with FBS, or serum-free N21, were initially used to explore the ideal conditions for FGSC isolation and culture. The cells were cultured in 2 different media for 7 days, on gelatin-coated dishes (Fig. 1). The results indicate that the serum-free N21 medium significantly enhanced the proliferation of FGSCs. Although medium containing DMEM supplemented with 10% FBS and growth factors, such as EGF, FGF, and GDNF (Supplementary Table 1), also promoted cell growth, FBS stimulated the proliferation of flat ovarian somatic cells, which led to interfere the growth of FGSCs, and decreased their number. On the other hand, the quantity of FGSC cultured in serum-free medium had significantly increased. Therefore, DMEM/F12 supplemented with serum-free N21 and growth factors (Supplementary Table 1), was considered to be the most effective medium for FGSC development.
Fig. 1.
FGSCs proliferation after 1 week in different culture media. Proliferation of FGSCs after 1 week of culture in DMEM/F12 with supplements as (A) FBS and growth factors; (B) Free serum supplement (N21), and growth factors. (C) Cells number after 1 week of culture under 2 different media. Note the improved growth of FGSCs in DMEM/F12 supplied with N21, versus DMEM/F12 supplied with 10% FBS. Error bars indicate S.E.M. Scale bar = 50 µm.
Morphological and characterization of FGSCs after isolation
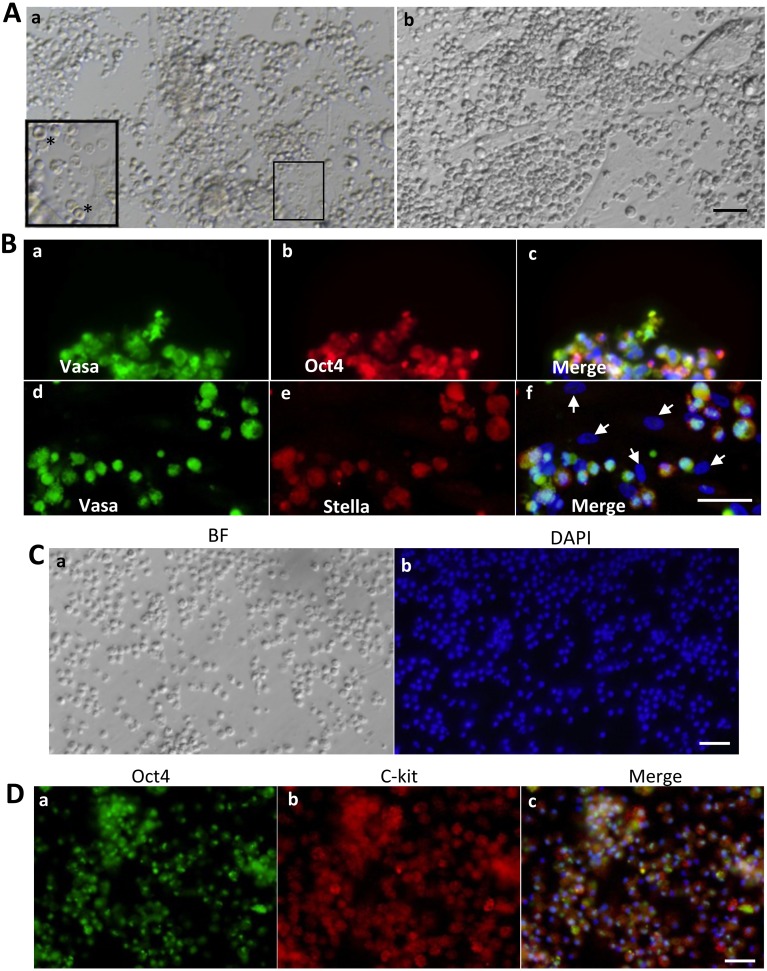
After the first day, the FGSCs were observed as spherically shaped, shiny, dark colored cells with a diameter of about 7 µm, which were easily distinguished from red blood cells, which have a typical biconcave disc morphology (Fig. 2Aa). Some flat somatic cells (such as epithelial cells and fibroblasts) were still present, attached to the bottom of the culture substrate. Because of that, the primary culture without MEF was chosen. After 1 week of culture, FGSCs increased in both number and size, to a round shape of about 10 µm diameter, forming groups of cells that clustered around the ovarian cell colonies (Fig. 2Ab). Immunofluorescence results showed that the group of cells expressed both germ cell markers (Vasa) and stem cell markers (Oct4), in most cells (Fig. 2Ba, b). In addition, some DAPI-positive cells, which did not form groups, were negative for both germ cell markers (Stella) and stem cell markers (Oct4) (Fig. 2Bf, arrows). This confirmed that some ovarian somatic cells still remained in the culture dish, 1 week after isolation. Taken together, these results indicate that the isolated FGSCs, from porcine ovaries, have the characteristics of germline stem cells, as reported previously [14].
Fig. 2.
Representative morphology and immunofluorescence analysis of FGSCs. (A) After 1 day in culture, FGSCs appeared dark and shiny with round shape (a). FGSCs increased in number and maintained ovoid shape after 1 week in culture (b). (B) After 1 week in culture, small FGSCs showed cytoplasmic localization of Vasa (a, d), Oct4 (b), and Stella (e). (C) Representative morphology of FGSCs in vitro culture for 1 month (a). Note the high nucleus-cytoplasmic ratio of FGSCs (b). (D) FGSCs could be cultured in vitro for months without the loss of germ cell markers, Oct4 (a) and c-kit (b). Scale bars: 50 µm (A, B, C, D).
The proliferative potential and germline specific protein expression of isolated FGSCs were further confirmed after a long period in in vitro culture. These cells maintained a size of 10–12 µm, were round in shape, and continuously grew and formed clusters of cells (Fig. 2Ca). Importantly, these cells maintained proliferative potential, by forming groups of cells, and expressed identifying germline markers Oct4 and c-kit (Fig. 2D). Although the FGSCs continued to grow, most of the ovarian somatic cells gradually disappeared after more than a month in culture. Therefore, the FGSCs were transferred onto mitomycin C-treated MEF feeder layers, for long-term culture.
The growth and development of OLCs in various differentiation conditions
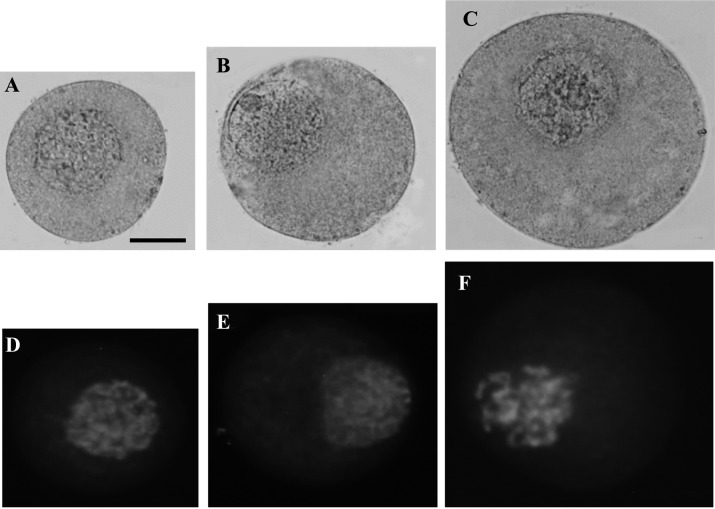
The chromatin morphology of growing oocytes, collected from porcine ovaries, was compared with OLCs derived from FGSCs. Growing oocytes were collected from three sizes of follicles: 0.1 mm, 0.5 mm, and 1 mm in diameter, as a control group. When the follicle increased in size to 0.1 mm, 0.5 mm, and 1 mm, the diameter of the oocyte also increased significantly (ranging from 59.42 ± 2.63 µm, 80.23 ± 3.96 µm to 107.45 ± 4.10 µm, respectively, Fig. 3A, B, C). In both 0.1 mm and 0.5 mm follicle groups, the oocytes contained diffuse chromatin in the nucleoplasm with a filamentous configuration and a nucleolar-like body (approximately 5 µm in diameter) (Fig. 3A, B). Filamentous configuration is defined as the stage when filamentous chromatin is decondensed and distributed throughout the germinal vesicle (GV) [23]. In the third group of follicles, 1 mm in size, the oocytes also contained a nucleolar-like body. However, the GV chromatin had a transitional, filamentous-reticular configuration (Fig. 3C). This pattern of transition of chromatin between the 0.1 mm, 0.5 mm, and 1 mm groups is similar to the pattern of transition between oocytes in secondary and pre-antral follicles [24]. Nuclei of oocytes maintained the same size, about 25 µm in diameter, during the growth phase of oocytes. This was confirmed by both orcein and DAPI staining (Fig. 3A–F)
Fig. 3.
Typical pattern of nucleus and chromosomes morphology of growing oocytes in 3 groups. Oocytes isolated from follicle (A) 0.1 mm, (B) 0.5 mm, (C) 1 mm were collected from porcine ovaries, denuded of cumulus cells and fixed for staining. Oocytes were stained by 1% Aceto-orcein solution and observed under microscope. In the 0.1 mm and 0.5 mm follicle size groups, the oocytes contained diffuse chromatin in the nucleoplasm with a filamentous configuration and a nucleolar-like body. In 0.5–1 mm group, the oocytes also contained a nucleolar-like body, but the GV chromatin had a transitional, filamentous-reticular configuration. (D, E, F) The typical patterns of filamentous chromatin in growing oocytes were counterstained by DAPI. Nuclei of oocytes maintained the same size, about 25 µm in diameter, during growth phase. Scale bar: 20 µm
To study the differentiation potential of FGSCs, isolated FGSCs after 1 week of culture were used. Since the ovary contains a high ratio of somatic cells to oocytes, it was essential to provide the required microenvironment for oocyte growth and differentiation, proper oocyte function, acting through bidirectional communication with surrounding ovarian somatic cells [25]. For that reason, using FGSCs 1 week after isolation, where ovarian somatic cells still remained in the culture, was necessary to study in vitro differentiation into oocyte-like cells.
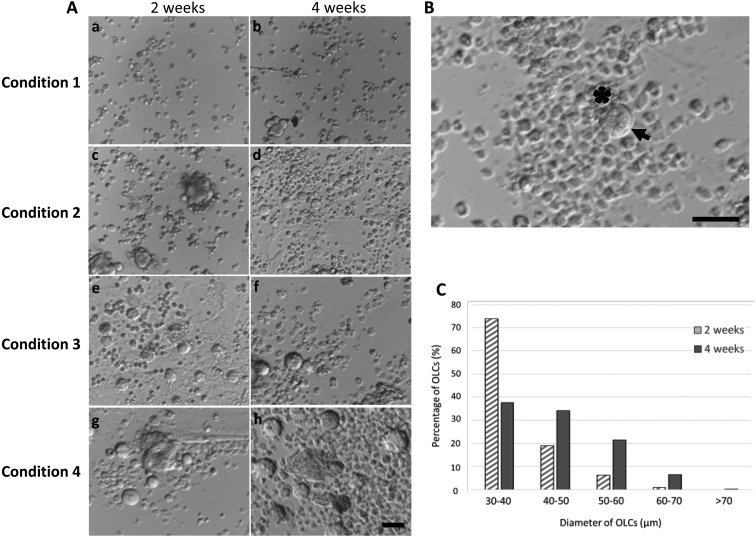
After 2 weeks of differentiation, the diameter of FGSCs increased to become OLCs, under 4 conditions. Under the first condition, FGSCs were cultured in gelatin-coated dishes in differentiation medium. Although all the FGSCs were exposed to the same culture medium, only ~0.07% developed into OLCs, with all of the OLCs increasing in cell diameter to around 30 µm (Fig. 4A; Table 1). After 4 weeks of culture, most of them still remain in the same size, only few OLC reached the diameter 40–50 µm (8.89%) (Table 2). These results suggest that a gelatin-coated dish was not adequate for the differentiation of FGSCs.
Fig. 4.
In vitro differentiated OLCs from FGSCs. (A) Various conditions were evaluated for in vitro differentiation of FGSCs. In condition 1, no significant change of the cells occurred after 2 weeks (a) and 4 weeks (b) of culture. In condition 2, the cells started to increase in diameter to form small OLCs after 2 weeks (c) and 4 weeks in culture (d). In condition 3, the number of OLCs was significantly increased after 2 weeks (e) and became larger in diameter after 4 weeks (f). In condition 4, many of the small OLCs grew larger in size, some OLCs growing larger than 70 µm in diameter (h). (B) Morphology of an OLC after 4 weeks in vitro differentiation, under condition 4. An OLC (arrow) with a diameter of 60 µm with somatic cells surrounding OLCs (asterisk). (C) Comparison of the number of OLCs between different diameter groups after in vitro culture under condition 4. Scale bars: 50 µm (A), 100 µm (B).
Table 1. Oocyte like cells (OLCs) increase in diameter after 2 weeks of in vitro culture and differentiation under various conditions.
| Condition | No. of total OLCs counted | No. (%) of OLCs at the diameter of |
||||
|---|---|---|---|---|---|---|
| 30–40 µm | 40–50 µm | 50–60 µm | 60–70 µm | > 70 µm | ||
| Condition 1 | 34 | 34 (100.00) a | 0 (0) a | 0 (0) a | 0 (0) a | 0 (0) a |
| Condition 2 | 125 | 102 (81.60) b | 23 (18.40) b | 0 (0) a | 0 (0) a | 0 (0) a |
| Condition 3 | 273 | 211 (77.29) c | 52 (19.05) b | 9 (3.30) b | 1 (0.37) c | 0 (0) a |
| Condition 4 | 306 | 226 (73.86) d | 58 (18.95) b | 19 (6.21) c | 3 (0.98) d | 0 (0) a |
a–d Within a column, the value associated with each different superscript differs significantly (P < 0.05). FGSCs after 1 week from isolation were differentiated to OLCs for 2 weeks, as mentioned in Materials and Methods. The experiments were repeated 5 times. In each experiment, the number of differentiated OLCs, from the total number of FGSCs, was counted for 5 times. The number of OLCs that developed from FGSCs (5 × 104) in condition 1, 2, 3, 4 were 34, 125, 273, 306 and correspond to 0.07%, 0.25%, 0.55%, 0.6%, respectively.
Table 2. Oocyte like cells (OLCs) increase in diameter after 4 weeks of in vitro culture for differentiation under various conditions.
| Condition | No. of total OLCs counted | No. (%) of OLCs at the diameter of |
||||
|---|---|---|---|---|---|---|
| 30–40 µm | 40–50 µm | 50–60 µm | 60–70 µm | > 70 µm | ||
| Condition 1 | 45 | 41 (91.11) a | 4 (8.89) a | 0 (0) a | 0 (0) a | 0 (0) a |
| Condition 2 | 176 | 147 (83.52) b | 29 (16.48) b | 0 (0) a | 0 (0) a | 0 (0) a |
| Condition 3 | 379 | 170 (44.85) c | 136 (35.88) c | 70 (18.47) b | 3 (0.79) b | 0 (0) a |
| Condition 4 | 372 | 140 (37.63) d | 127 (34.14) c | 80 (21.51) c | 24 (6.45) c | 1 (0.27) b |
a–d Within a column, the value associated with each different superscript differs significantly (P < 0.05). FGSCs after 1 week from isolation were differentiated to OLCs for 4 weeks as mentioned in Materials and Methods. The experiments were repeated 5 times. In each experiment, the number of differentiated OLCs, from the total number of FGSCs, was counted for 5 times. The number of OLCs that developed from FGSCs (5 × 104) in condition 1, 2, 3, 4 were 45, 176, 379, 372 and correspond to 0.09%, 0.35%, 0.76%, 0.74%, respectively.
MEF feeder layers had a beneficial effect on the survival and growth rate of preantral follicles [26]. Therefore, under the second condition, FGSCs were cultured on mitotically deactivated MEF feeder layers, for in vitro differentiation. This condition increased the number of FGSCs that developed into OLCs (0.25%) and they reached between 40–50 µm after two weeks (18%) (Table 1). Although it also increased the number of FGSCs that developed into OLCs, the diameter of OLCs were maintained after 4 weeks of differentiation (Table 2) (Fig. 4A). Therefore, MEF feeder layers had a positive effect on the initial differentiation of OLCs, compared to gelatin-coated dishes, but they do not contribute to long-term growth.
Recently, new approaches have emphasized that three-dimensional (3D) cell growth could mimic the in vivo environment [27] and induce specific differentiation of stem cells [28]. Previous studies showed that the presence of ovarian granulosa cells has an essential impact on primordial germ cell development and oocyte differentiation in vitro and in vivo, via cell-cell interactions [29]. In addition, MEF not only provided a substrate for stem cells to grow on but also secreted critical growth factors, cytokines, and nutrients [30, 31]. Based on the information above, we further optimized in vitro differentiation conditions using FGSCs that were co-cultured in a 3-D low attachment dish under differentiation medium with granulosa cells isolated from porcine ovaries (condition 3) or MEF cells (condition 4).
Under these conditions, the diameter of the OLCs reached 50–70 µm after 2 weeks of differentiation; however, this phenomenon was not observed under the first and second conditions (Table 1). Under conditions 3 and 4, the number of OLCs that developed from FGSC was significantly higher than under conditions 1 and 2 ( 0.55% and 0.6% vs 0.07% and 0.25%, respectively, Table 1). These results indicated that 3-D culture in low attachment dishes was most suitable for the in vitro differentiation and growth of OLCs. Furthermore, co-culture with granulosa cells or MEF enhanced the differentiation potential of OLCs.
After 4 weeks of culture, the number of OLCs that developed from FGSC was higher in both conditions 3 and 4 (0.76% and 0.74%, respectively) (Fig. 4A and Table 2). In addition, OLCs that increased in size to more than 60 µm were significantly higher under condition 4, compared to condition 3 (6.45% and 0.79%, respectively) (Table 2). In addition, the OLCs under the 4th condition were observed with a large number of somatic cells surrounding them (Fig. 4B). Furthermore, after long term differentiation culture under condition 4, many of the small OLCs grew larger in size (Fig. 4C). These results showed that co-culturing the FGSCs together with MEF cells, in 3-D low attachment dishes, increased the development rate and the diameter of OLCs.
OLCs expressed oocyte-specific markers
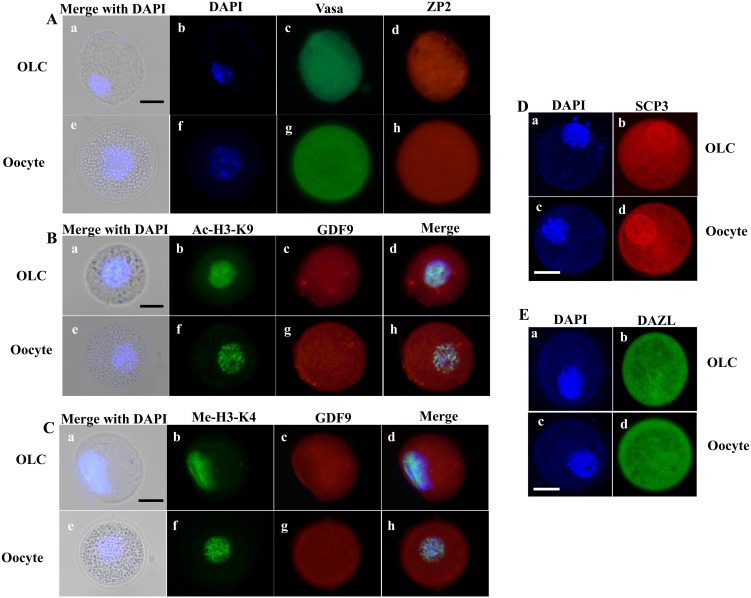
After in vitro differentiation for 4 weeks, OLCs were subjected to immunostaining to determine the expression of oocyte related genes, including Vasa, ZP2, GDF9, DAZL, SCP3, the acetylation level of histone H3 at K9 (Ac-H3-K9) and the methylation of histone H3 at K4 (Me-H3-K4).
The results showed that after in vitro differentiation, not only did the OLCs increase in size, but their nuclei also increased in size (approximately 25 µm in diameter), compared to growing oocytes isolated from ovaries (Fig. 5, bright field). This nuclei size was similar to that of growing oocytes collected from secondary follicles of porcine ovaries as we show in Fig. 3.
Fig. 5.
Expression of oocyte-specific markers in OLCs after differentiation. (A) Dual immunofluorescent of two oocyte-specific-markers, Vasa and Zp2, were expressed at the same level throughout the cytoplasm in both OLC and oocyte. The DNA was counterstained with DAPI. (B, C) GDF9, another of the oocyte markers, was distributed at the same level throughout the cytoplasm in both OLC and oocyte. In addition, Acetylation of histone H3 at lysine 9 (Ac-H3-K9) and Methylation of histone H3 at lysin 4 (Me-H3-K4) were expressed in similar levels in both OLC and oocyte. (D, E) The synaptonemal complex protein (SCP3), a meiosis-specific protein structure, and DAZL, a germ cell-specific RNA-binding protein, were highly expressed in OLCs, similar to oocytes extracted from ovaries. Scale bars: 30 µm.
ZP2 protein is a component of zona pellucida [32], which was clearly detected and localized surrounding the OLCs (Fig. 5A). Compared with the oocyte, OLCs had similar expression of both oocyte specific markers, Vasa and ZP2. Growth differentiation factor 9 (GDF9) is an oocyte derived growth factor, in the transforming growth factor β (TGF-β) superfamily. It is highly expressed in the oocyte and has a pivotal influence on the surrounding somatic cells, particularly granulosa, cumulus, and theca cells [33]. GDF9 is essential for the overall process of folliculogenesis, oogenesis, and ovulation and, thus, plays a major role in female fertility. Here, the OLC expressed GDF9 throughout the cytoplasm, similar to oocytes (Fig. 5B, C). High levels of Ac-H3-K9 are commonly known as a specific histone modification during the oocyte’s growth stage [34]. Immunofluorescent staining showed a high level of Ac-H3-K9 in OLCs, with expression similar to that of oocytes from an ovary (Fig. 5B). Moreover, it has been suggested that Me-H3-K4 plays a crucial role in structuring accessible chromatin domains in higher eukaryotes. K4 methylation is being associated with transcriptionally active chromatin, the main activity during the oocyte growth stage [35]. The methylation of H3-K4 was also detected in OLCs, with a signal level similar to the control oocyte (Fig. 5C). Finally, synaptonemal complex protein 3 (SCP3), a component of the synaptonemal complex which is a meiosis-specific protein structure essential for synapsis of homologous chromosomes, and DAZL, a germ cell-specific RNA-binding protein [36, 37] were both highly expressed in OLCs, similar to the oocytes taken from ovaries. Taken together, these results show that the OLCs have the same morphology and characteristic of oocytes from secondary follicles, from porcine ovaries.
Discussion
Confirming the presence of ovarian germline stem cells in the adult ovary is important to reproductive biology. It could suggest new strategies to promote ovarian regeneration, delay menopause, and cure ovarian dysfunction, and FGSCs have previously been suggested for use in therapy for ovarian disorders [38, 39]. However, to better understand ovarian physiology and develop effective strategies for treatment of ovarian dysfunction through FGSCs, the isolation, characterization and differentiation mechanisms of FGSCs needs to be further explored.
We found that DMEM/F12 exhibited a significant effect on the FGSCs proliferation and formation of cell groups. Furthermore, the FGSCs were effectively grown under leukemia inhibitory factor (LIF), epidermal growth factor (EGF), glial cell-derived neurotrophic factor (GDNF), and fibroblast growth factor (FGF). LIF had profound effects on the survival and maintenance of stem cells [40], while GDNF, EGF and FGF played vital roles in the growth of germline stem cells [41]. While the conditions containing FBS caused an overgrowth of ovarian somatic cells and interfered with growing FGSCs, the serum-free medium, with N21 supplement, significantly promoted the growth of FGSCs, in a concentration-dependent manner. N21 Supplement is a serum-free, media supplement containing different components specially optimized for the long-term survival and culture of stem cells [20]. The quantity of FGSCs cultured in serum-free medium was remarkably increased, making serum-free medium the most effective condition for the in vitro culture of FGSCs.
The FGSCs were next characterized by immunofluorescence analysis. The Oct4 gene encodes a transcription factor that plays a key role in the self-renewal of embryonic stem cell development and stem cell pluripotency [36]. An Oct4 marker was used in order to examine the differentiation ability of the isolated FGSCs. In normal development, Vasa, also known as Ddx4 homolog, should be expressed in migratory primordial germ cells, in the region of the gonadal ridge. Vasa protein is present in fetal and adult gonadal germ cells, in both males and females, and most abundant in spermatocytes and mature oocytes [42]. Stella protein is specifically, and highly, expressed in primordial germ cells [43]. These genes are considered highly specific markers of germ cells. In our results, the newly isolated FGSCs expressed these markers, Vasa, Stella and Otc4. Furthermore, the isolated FGSCs could be cultured in vitro for a long period of time, without the loss of germline stem cell markers Oct4 and c-Kit. The tyrosine-kinase receptor, c-Kit, is essential for the maintenance of primordial germ cells (PGCs) in both males and females [44]. This demonstrated that these cells retained the characteristics of germ cells with, multipotent potential.
To study the differentiation potential of FGSCs, freshly isolated FGSCs after 1 week of culture were used because some ovarian somatic cells still remained together with the FGSCs in the culture dish, as mentioned in the above results. Previous studies showed that the presence of ovarian somatic cells has an essential impact on oocyte development and growth, both in vivo and in vitro, through cell-cell interaction and paracrine factors [45, 46].
This study also evaluated various in vitro differentiation conditions for FGSCs. Two-dimensional (2D) cell culture has been used for some time now, but growing cells in flat layers on plastic surfaces does not mimic the in vivo condition accurately. Three-dimensional (3D) cell culture allows biological cells to grow and interact with their surroundings, in an artificial environment. Cells grown in a 3D model have proven to be more physiologically relevant, and have shown improvements in several studies of biological mechanisms [47]. A 3D culture system was used in order to allow somatic cells to be able to surround and directly communicate with FGSCs in every direction. Previous studies showed that the presence of somatic cells is critical for primordial germ cell development, through the secretion of different factors that induce differentiation of PGCs into gonocytes. Furthermore, direct cell-cell contact between somatic cells and gonocytes, due to the action of anti-apoptotic proteins, is also vital to their differentiation [48]. Our study demonstrated a significant increase in the percentage of large OLCs in fibroblastic cell co-culture groups, compared to granulosa cell co-culture groups. Previous studies showed that oviductal cells collected during the interestrual stage had no beneficial effect on meiotic resumption and maturation of dog oocytes in vitro [49] whereas, co-culture with cells from the estrus stage improved the meiotic capability of the oocytes [50]. This variable may reduce the ability of granulosa cells to induce OLC differentiation. By contrast, the MEF cells were all homogenous and well-characterized. These fibroblast cells secrete several cytokines, such as leukemia inhibitory factor, bFGF, stem cell factor, insulin-like growth factor and bone morphogenetic proteins, which are all critical for early follicle development. MEF paracrine factors stimulate follicle growth, allowing the follicle to survive, transition to the secondary stage and produce oocytes responsive to in vitro maturation [51]. Furthermore, MEF co-culture systems were also shown to have more of an effect on mouse embryo development, compared to ovarian cell co-culture systems [52]. The results show that, when the 1-week old FGSCs are co-cultured with MEF cells in differentiation medium enhanced with surrounding somatic cells, FGSCs possess greater potential to differentiate into oocyte-like cells.
The oocyte-like cells expressed multiple oocyte-specific markers, Vasa, GDF9, SCP 3 and Zp2. GDF9 plays a critical role as a growth and differentiation factor during early folliculogenesis, as well as playing a role as an oocyte-secreted, paracrine factor to regulate several, crucial granulosa cell enzymes. These enzymes are involved in cumulus expansion and maintenance of an optimal oocyte microenvironment, processes that are essential for normal ovulation, fertilization, and female reproduction [53]. While GDF9 is required for normal folliculogenesis, SCP3, a meiosis-specific protein, and ZP2 glycoprotein, a component of the zona pellucida, are expressed only in oocytes [31]. Besides that, the OLCs expressed high levels of acetylation of histone H3 at K9 and methylation of histone H3 at K4, which are common changes in small immature oocytes released from secondary follicles. This indicated that the OLCs shared similar characteristics to small oocytes and had high potential for further growth and development.
In summary, the FGSCs have successfully been isolated from porcine ovarian tissue, cultured under serum-free medium, supplemented with growth factors, and differentiated into large numbers of OLCs, with similar characteristics to oocytes released from secondary follicles. While studies into producing fertilizable oocytes from FGSCs are ongoing in our laboratory, this study provides a valuable model for a potentially new, alternative source of oocytes.
Conflicts of interest
The authors declare no conflict of interest in the research.
Supplementary
Acknowledgments
This research is funded by the Vietnam National Foundation for Science and Technology Development (NAFOSTED), under Grant number “106-NN.99-2015.90; by Vietnam Ministry of Science and Technology, under Grant No. ĐTĐL.CN-49/16; by Vietnam National University Ho Chi Minh City (VNU-HCM), under Grant No. B2017-28-04.
References
- 1.Zuckerman S. The number of oocytes in the mature ovary. Recent Prog Horm Res 1951; 6: 63–108. [Google Scholar]
- 2.Brinster RL. Male germline stem cells: from mice to men. Science 2007; 316: 404–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo Y, Hai Y, Gong Y, Li Z, He Z. Characterization, isolation, and culture of mouse and human spermatogonial stem cells. J Cell Physiol 2014; 229: 407–413. [DOI] [PubMed] [Google Scholar]
- 4.Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature 2004; 428: 145–150. [DOI] [PubMed] [Google Scholar]
- 5.Zou K, Yuan Z, Yang Z, Luo H, Sun K, Zhou L, Xiang J, Shi L, Yu Q, Zhang Y, Hou R, Wu J. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat Cell Biol 2009; 11: 631–636. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Yang Z, Yang Y, Wang S, Shi L, Xie W, Sun K, Zou K, Wang L, Xiong J, Xiang J, Wu J. Production of transgenic mice by random recombination of targeted genes in female germline stem cells. J Mol Cell Biol 2011; 3: 132–141. [DOI] [PubMed] [Google Scholar]
- 7.Zhou L, Wang L, Kang JX, Xie W, Li X, Wu C, Xu B, Wu J. Production of fat-1 transgenic rats using a post-natal female germline stem cell line. Mol Hum Reprod 2014; 20: 271–281. [DOI] [PubMed] [Google Scholar]
- 8.Wang XF, Zhang L, Wu QH, Min JX, Ma N, Luo LC. Biological mechanisms of premature ovarian failure caused by psychological stress based on support vector regression. Int J Clin Exp Med 2015; 8: 21393–21399. [PMC free article] [PubMed] [Google Scholar]
- 9.Meattini I, Saieva C, Meacci F, Scotti V, De Luca Cardillo C, Desideri I, Baldazzi V, Mangoni M, Scoccianti S, Detti B, Simontacchi G, Nori J, Orzalesi L, Sanchez L, Casella D, Bernini M, Fambrini M, Bianchi S, Livi L. Impact of age on cytotoxic-induced ovarian failure in breast cancer treated with adjuvant chemotherapy and triptorelin. Future Oncol 2016; 12: 625–635. [DOI] [PubMed] [Google Scholar]
- 10.Hernández-Angeles C, Castelo-Branco C. Early menopause: A hazard to a woman’s health. Indian J Med Res 2016; 143: 420–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dumesic DA, Oberfield SE, Stener-Victorin E, Marshall JC, Laven JS, Legro RS. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocr Rev 2015; 36: 487–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White YA, Woods DC, Takai Y, Ishihara O, Seki H, Tilly JL. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat Med 2012; 18: 413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woods DC, Tilly JL. Isolation, characterization and propagation of mitotically active germ cells from adult mouse and human ovaries. Nat Protoc 2013; 8: 966–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bui HT, Van Thuan N, Kwon DN, Choi YJ, Kang MH, Han JW, Kim T, Kim JH. Identification and characterization of putative stem cells in the adult pig ovary. Development 2014; 141: 2235–2244. [DOI] [PubMed] [Google Scholar]
- 15.Xie W, Wang H, Wu J. Similar morphological and molecular signatures shared by female and male germline stem cells. Sci Rep 2014; 4: 5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fereydouni B, Salinas-Riester G, Heistermann M, Dressel R, Lewerich L, Drummer C, Behr R. Long-term oocyte-like cell development in cultures derived from neonatal marmoset monkey ovary. Stem Cells Int 2016; 2016: 2480298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silvestris E, Cafforio P, D’Oronzo S, Felici C, Silvestris F, Loverro G. In vitro differentiation of human oocyte-like cells from oogonial stem cells: single-cell isolation and molecular characterization. Hum Reprod 2018; 33: 464–473. [DOI] [PubMed] [Google Scholar]
- 18.de Souza GB, Costa J, da Cunha EV, Passos J, Ribeiro RP, Saraiva M, van den Hurk R, Silva J. Bovine ovarian stem cells differentiate into germ cells and oocyte-like structures after culture in vitro. Reprod Domest Anim 2017; 52: 243–250. [DOI] [PubMed] [Google Scholar]
- 19.Kristensen SG, Andersen CY. Cryopreservation of ovarian tissue: opportunities beyond fertility preservation and a positive view into the future. Front Endocrinol (Lausanne) 2018; 9: 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Valk J, Brunner D, De Smet K, Fex Svenningsen A, Honegger P, Knudsen LE, Lindl T, Noraberg J, Price A, Scarino ML, Gstraunthaler G. Optimization of chemically defined cell culture media--replacing fetal bovine serum in mammalian in vitro methods. Toxicol In Vitro 2010; 24: 1053–1063. [DOI] [PubMed] [Google Scholar]
- 21.Bui HT, Kwon DN, Kang MH, Oh MH, Park MR, Park WJ, Paik SS, Van Thuan N, Kim JH. Epigenetic reprogramming in somatic cells induced by extract from germinal vesicle stage pig oocytes. Development 2012; 139: 4330–4340. [DOI] [PubMed] [Google Scholar]
- 22.Bui HT, Van Thuan N, Wakayama T, Miyano T. Chromatin remodeling in somatic cells injected into mature pig oocytes. Reproduction 2006; 131: 1037–1049. [DOI] [PubMed] [Google Scholar]
- 23.Bui HT, Hwang KC, Kim JH, Van Thuan N, Wakayama T, Miyano T. Effect of vanadate on the chromatin configuration in pig GV-oocytes. J Reprod Dev 2009; 55: 367–372. [DOI] [PubMed] [Google Scholar]
- 24.Comizzoli P, Pukazhenthi BS, Wildt DE. The competence of germinal vesicle oocytes is unrelated to nuclear chromatin configuration and strictly depends on cytoplasmic quantity and quality in the cat model. Hum Reprod 2011; 26: 2165–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parvari S, Yazdekhasti H, Rajabi Z, Gerayeli Malek V, Rastegar T, Abbasi M. Differentiation of mouse ovarian stem cells toward oocyte-like structure by coculture with granulosa cells. Cell Reprogram 2016; 18: 419–428. [DOI] [PubMed] [Google Scholar]
- 26.Kim C-H, Cheon Y-P, Lee Y-J, Lee K-H, Kim S-H, Chae H-D, Kang B-M. The effect of fibroblast co-culture on in vitro maturation of mouse preantral follicles. Dev Reprod 2013; 17: 269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee J, Cuddihy MJ, Kotov NA. Three-dimensional cell culture matrices: state of the art. Tissue Eng Part B Rev 2008; 14: 61–86. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Ye K. Three-dimensional differentiation of embryonic stem cells into islet-like insulin-producing clusters. Tissue Eng Part A 2009; 15: 1941–1952. [DOI] [PubMed] [Google Scholar]
- 29.Kidder GM, Vanderhyden BC. Bidirectional communication between oocytes and follicle cells: ensuring oocyte developmental competence. Can J Physiol Pharmacol 2010; 88: 399–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moscatelli D, Presta M, Joseph-Silverstein J, Rifkin DB. Both normal and tumor cells produce basic fibroblast growth factor. J Cell Physiol 1986; 129: 273–276. [DOI] [PubMed] [Google Scholar]
- 31.Chen S, Choo A, Chin A, Oh SK. TGF-beta2 allows pluripotent human embryonic stem cell proliferation on E6/E7 immortalized mouse embryonic fibroblasts. J Biotechnol 2006; 122: 341–361. [DOI] [PubMed] [Google Scholar]
- 32.Wassarman PM. Zona pellucida glycoproteins. J Biol Chem 2008; 283: 24285–24289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Otsuka F, McTavish KJ, Shimasaki S. Integral role of GDF-9 and BMP-15 in ovarian function. Mol Reprod Dev 2011; 78: 9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bui HT, Van Thuan N, Kishigami S, Wakayama S, Hikichi T, Ohta H, Mizutani E, Yamaoka E, Wakayama T, Miyano T. Regulation of chromatin and chromosome morphology by histone H3 modifications in pig oocytes. Reproduction 2007; 133: 371–382. [DOI] [PubMed] [Google Scholar]
- 35.Kageyama S, Liu H, Kaneko N, Ooga M, Nagata M, Aoki F. Alterations in epigenetic modifications during oocyte growth in mice. Reproduction 2007; 133: 85–94. [DOI] [PubMed] [Google Scholar]
- 36.Di Carlo AD, Travia G, De Felici M. The meiotic specific synaptonemal complex protein SCP3 is expressed by female and male primordial germ cells of the mouse embryo. Int J Dev Biol 2000; 44: 241–244. [PubMed] [Google Scholar]
- 37.Anderson RA, Fulton N, Cowan G, Coutts S, Saunders PTK. Conserved and divergent patterns of expression of DAZL, VASA and OCT4 in the germ cells of the human fetal ovary and testis. BMC Dev Biol 2007; 7: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiong J, Lu Z, Wu M, Zhang J, Cheng J, Luo A, Shen W, Fang L, Zhou S, Wang S. Intraovarian transplantation of female germline stem cells rescue ovarian function in chemotherapy-injured ovaries. PLoS One 2015; 10: e0139824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Erler P, Sweeney A, Monaghan JR. Regulation of injury-induced ovarian regeneration by activation of oogonial stem cells. Stem Cells 2017; 35: 236–247. [DOI] [PubMed] [Google Scholar]
- 40.Hall J, Guo G, Wray J, Eyres I, Nichols J, Grotewold L, Morfopoulou S, Humphreys P, Mansfield W, Walker R, Tomlinson S, Smith A. Oct4 and LIF/Stat3 additively induce Krüppel factors to sustain embryonic stem cell self-renewal. Cell Stem Cell 2009; 5: 597–609. [DOI] [PubMed] [Google Scholar]
- 41.Brown AC, Adams D, de Caestecker M, Yang X, Friesel R, Oxburgh L. FGF/EGF signaling regulates the renewal of early nephron progenitors during embryonic development. Development 2011; 138: 5099–5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Castrillon DH, Quade BJ, Wang TY, Quigley C, Crum CP. The human VASA gene is specifically expressed in the germ cell lineage. Proc Natl Acad Sci USA 2000; 97: 9585–9590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sato M, Kimura T, Kurokawa K, Fujita Y, Abe K, Masuhara M, Yasunaga T, Ryo A, Yamamoto M, Nakano T. Identification of PGC7, a new gene expressed specifically in preimplantation embryos and germ cells. Mech Dev 2002; 113: 91–94. [DOI] [PubMed] [Google Scholar]
- 44.Rossi P, Sette C, Dolci S, Geremia R. Role of c-kit in mammalian spermatogenesis. J Endocrinol Invest 2000; 23: 609–615. [DOI] [PubMed] [Google Scholar]
- 45.Chang HM, Qiao J, Leung PC. Oocyte-somatic cell interactions in the human ovary-novel role of bone morphogenetic proteins and growth differentiation factors. Hum Reprod Update 2016; 23: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Makabe S, Naguro T, Stallone T. Oocyte-follicle cell interactions during ovarian follicle development, as seen by high resolution scanning and transmission electron microscopy in humans. Microsc Res Tech 2006; 69: 436–449. [DOI] [PubMed] [Google Scholar]
- 47.Senbon S, Hirao Y, Miyano T. Interactions between the oocyte and surrounding somatic cells in follicular development: lessons from in vitro culture. J Reprod Dev 2003; 49: 259–269. [DOI] [PubMed] [Google Scholar]
- 48.Antoni D, Burckel H, Josset E, Noel G. Three-dimensional cell culture: a breakthrough in vivo. Int J Mol Sci 2015; 16: 5517–5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hewitt DA, England GC. Synthetic oviductal fluid and oviductal cell coculture for canine oocyte maturation in vitro. Anim Reprod Sci 1999; 55: 63–75. [DOI] [PubMed] [Google Scholar]
- 50.Bogliolo L, Zedda MT, Ledda S, Leoni G, Naitana S, Pau S. Influence of co-culture with oviductal epithelial cells on in vitro maturation of canine oocytes. Reprod Nutr Dev 2002; 42: 265–273. [DOI] [PubMed] [Google Scholar]
- 51.Tagler D, Tu T, Smith RM, Anderson NR, Tingen CM, Woodruff TK, Shea LD. Embryonic fibroblasts enable the culture of primary ovarian follicles within alginate hydrogels. Tissue Eng Part A 2012; 18: 1229–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hajializadeh N, Babaei H, Nematollahi-Mahani SN, Azizollahi S. The development of mouse early embryos in vitro in fibroblasts and cumulus cells co-cultures supplemented with retinoic acid. Iran J Vet Res 2008; 9: 1–8. [Google Scholar]
- 53.Bukovsky A, Svetlikova M, Caudle MR. Oogenesis in cultures derived from adult human ovaries. Reprod Biol Endocrinol 2005; 3: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.