Abstract
The intracellular mechanical link tethering the nucleus to the cytoskeleton has been suggested to be the linker of the nucleoskeleton and cytoskeleton (LINC) complex. Previous studies have reported that knockdown of nesprin-1, a component of the LINC complex that directly binds to actin filaments, suppresses cellular morphological response to mechanical stimuli. The relation between nesprin-1 knockdown and cellular morphological changes, however, remains unclear. In this study, we examined the mechanical role of nucleus–actin filament binding in morphological changes of fibroblasts exposed to cyclic stretching. After exposure to 10% cyclic stretching for 6 h, fibroblasts transfected with nesprin-1-specific small interfering RNA showed fewer elongated shapes compared with non-transfected cells. To further examine the mechanical role of the nucleus and nucleus-bound actin filaments, we applied cyclic stretching to fibroblasts treated with Trichostatin A (TSA), which decreases nuclear stiffness and thereby reduces nucleus-binding actin filament tension. TSA-treatment was found to induce more rounded cellular shapes than those of non-treated cells under both static and cyclic stretching conditions. These results suggest that the tension of nucleus-bound actin filaments plays an important role in the formation of elongated fibroblasts under cyclic stretching and that nesprin-1 knockdown causes a decrease of tension in nucleus-associated actin filaments.
Keywords: LINC complex, Nuclear stiffness, Intracellular mechanical balance, Cellular morphology
Introduction
The nucleus is the largest cellular organelle and its primary functions are maintaining hereditary information and controlling cellular activity. Alternations in nuclear components and function are therefore implicated in disease pathogenesis. Recently, nuclear mechanical properties have received increasing attention because structural alterations are features of laminopathies, genetic disorders caused by mutations in genes encoding nuclear membrane proteins.7,28 Yet, the elaborate mechanical role of the nucleus in the physiology and pathology of laminopathies at the cellular and tissue levels remain unclear.
There is increasing evidence that the linker of nucleoskeleton and cytoskeleton (LINC) complex is central to the mechanical binding of the nucleus to the cellular cytoskeleton. Formation of the LINC complex involves nesprins and SUN proteins at the nuclear envelope,6,24,30 and the nuclear–cytoskeleton binding may play an important role in cellular morphological and functional response to environmental stimuli.23,35
Mechanical forces are now recognized as important environmental stimuli in the development of cellular physiology and morphology. For example, fluid shear stress and mechanical cyclic strain induce cellular elongation and orientation concomitant with actin cytoskeletal reorganization, which is dependent on the direction of the stimuli, in cells such as vascular endothelial cells, fibroblasts, and macrophages.10,15,16,26 Mechanical forces acting on the cellular surface are transmitted through the actin cytoskeleton to the intracellular mechanosensor regions, including focal adhesions and intercellular junctions, where mechanical signals are sensed and transduced into biochemical signals, leading to intracellular structural changes and functional responses.13,27,29 In addition to mechanosensors, environmental mechanical forces are also transmitted from the actin cytoskeleton to the nucleus through the LINC complex.22 Indeed, previous studies have showed that knockdown of nesprin-1, which is a component of LINC complex and directly binds to the actin cytoskeleton, suppresses the morphological response of endothelial cells to mechanical stimuli.1,3 These results indicate that the nucleus–actin filament binding and force transmission to the nucleus are crucial to the morphological responses of cells to mechanical stimuli; however, detailed relationship between the nucleus–actin filament binding and cell morphological changes remains unclear.
Similar to endothelial cells used in many previous studies, fibroblasts also respond to mechanical stimuli by showing morphological changes. In order to examine the role of nesprin-1-mediated nucleus–actin filament binding in the cellular morphological response to mechanical stimuli, we explored morphological changes in nesprin-1-depleted fibroblasts exposed to cyclic stretching. While endothelial cells show cobblestone-like polarized shapes, fibroblasts show polarized elongated shapes under unstimulated conditions. Thus, it is clear that elongated shape of fibroblasts makes their polarity or shape in response to cyclic stretching easier to distinguish as compared to cells of other shapes (i.e. endothelial/cobble-stone).
Methods
Cell Culture and siRNA Transfection
Human skin fibroblasts (Riken Bioresource Center, Cell Bank, Japan) were cultured using MEMα (Wako, Japan) containing 10% heat-inactivated fetal bovine serum (Sigma-Aldrich, USA) and 100 U/mL penicillin/streptomycin (Wako). Fibroblasts were transfected with 100 nM small interfering RNA (siRNA) targeting human nesprin-1 (Dharmacon, USA) using Dharmafect1 transfection reagent (Dharmacon) according to a previously described method.1 Loss of localization of nesprin-1 to the nuclear envelope in siRNA-treated cells was confirmed by fluorescence microscopy (Fig. 1).
Figure 1.
Typical fluorescence images of nuclei (blue) and Nesprin-1 (green) in fibroblasts. WT, wild type; siNes1, cells treated with siRNA against Nesprin-1. Scale bars = 20 µm.
To evaluate the effect of transcription on cyclic stretching-induced morphological changes, 1 µg/mL Actinomycin D (ActD) (Wako) was added to the culture medium 1 h before cyclic stretching (see below).14 To decrease nuclear elasticity, cells were also cultured with 0.5 µg/mL Trichostatin A (TSA) (Wako) for 24 h prior to the experiment.21 ActD can bind to DNA and thereby inhibit transcription. TSA, an inhibitor of histone deacetylases, causes hyperacetylation of histones and leads to more relaxed chromatin structure.
Cyclic Stretching
Fibroblasts were seeded at a concentration of 0.25 × 104 cells/cm2 on a collagen-type I-coated silicone chamber (Strex, Japan) and were cultured for 24 h. The cells were then exposed to 10% simple uniaxial stretching (the substrate is allowed to compress in the orthogonal direction to the stretching) at 1 Hz using a previously described home-built cyclic stretching apparatus in a humidified CO2 incubator.26 Unstretched control cells were referred to as static in the present study.
Fluorescence Staining and Image Analysis
After cyclic stretching, the cells were fixed with 4% paraformaldehyde for 20 min at room temperature (RT), permeabilized with 0.1% Triton X-100 in PHEM buffer for 5 min, and blocked with 1% (w/v) Block Ace (DS Pharma Biomedical, Japan) for 60 min at RT. Then, the cells were incubated with a primary antibody for nesprin-1 (Abcam, UK) in the blocking solution for 60 min at RT, followed by incubation with Alexa Fluor 488-conjugated secondary antibody (Invitrogen). Actin filaments and nuclei were respectively stained with Alexa Fluor 546 phalloidin (Invitrogen) and Hoechst33342, trihydrochloride, trihydrate (Invitrogen). Fluorescence imaging of cells was performed with an inverted fluorescence microscope (Olympus, Japan).
To evaluate cellular morphology, the outline of each cell in the fluorescence microscopy images was traced and fitted to an equivalent ellipsoid with the ImageJ program (National Institutes of Health, USA) (Fig. 3a). The cell area was determined by measuring the area of the cell outline. Inverse aspect ratio of the cells was defined as the ratio between the minor and major axes of the ellipsoid. The inverse aspect ratio ranges from 0 to 1 and is equal to 1 for a perfect circle, with approaching 0 for elongated shapes. The angle between the major axis and the perpendicular direction of stretching was also measured and set as the cellular orientation angle. The mean resultant length (R) was calculated as a quantitative indicator for cell orientation.2,18 R is determined by following equation:
where n is the total cell number and θ i is the orientation angle of cell i. R approaches to 0 when orientation angles are uniformly distributed, and 1 when angles are highly aligned in one direction. Rayleigh test was used to determine whether the distribution has a significant direction. Nuclear morphology was also evaluated in the same way as those of cells. It should be noted that, when we analyzed the morphology of siRNA-transfected cells, we only traced cells expressing decreased levels of nesprin-1 on the fluorescence microscopy images.
Figure 3.
Results of cellular morphological analyses of non-treated wild type (WT) and small interfering RNA(siRNA)-treated (siNes1) fibroblasts cultured statically (Static) and exposed to 10% cyclic stretching for 1, 3, and 6 h. (a) Schematic diagram of the morphological parameters of a cell. (b) Cell areas and (c) inverse aspect ratios of fibroblasts. Results are expressed as mean + SD. *p < 0.01 (vs. static), **p < 0.001 (vs. static), † p < 0.001 (vs. 1 h), # p < 0.05 (vs. WT), § p < 0.01 (vs. WT). (d) Histograms of angles of cell orientation. R and p values are mean resultant lengths and the results of the Rayleigh test, respectively.
Statistics
Data are shown as mean + standard deviation (SD) for 150 cells and derived from three independent experiments. Comparisons among means of the experimental groups were made using two-way analysis of variance (ANOVA). A p value < 0.05 was considered statistically significant. If the difference was significant, then Bonferroni post hoc test was used for comparison among the groups.
Results and Discussion
Fluorescence microscopy images of fibroblasts exposed to cyclic stretching are shown in Fig. 2. Before cyclic stretching, no evident differences were present in cell shapes and actin filament structures between non-treated wild type fibroblasts (hereinafter referred to as WT cells) and fibroblasts treated with siRNA against nesprin-1 (siNes1 cells). Both WT and siNes1 cells contracted once and were round in shape after exposure to cyclic stretching for 1 h, and then elongated and oriented in a direction perpendicular to that of the cyclic stretching. Figures 3b–3d show the results of morphological analyses. The cell areas of WT and siNes1 cells showed a tendency to once decrease at around 1 h to 3 h after exposure to cyclic stretching (Fig. 3b). After 6 h, the cell area of WT cells was significantly larger than those of statically cultured WT cells and siNes1 cells exposed to cyclic stretching (p < 0.01). By exposure to cyclic stretching, the inverse aspect ratios of both WT and siNes1 cells increased at 1 h and then started to decrease and returned to the level of statically cultured cells after 6 h (Fig. 3c). Two-way ANOVA analysis of the data showed that the knockdown of nesprin-1 significantly influenced changes in the inverse aspect ratios (p < 0.0001), and there was no significant difference between exposure time and nesprin-1 knockdown. While there was no significant difference in the inverse aspect ratios between WT and siNes1 cells under static culture, the inverse aspect ratios of siNes1 cells exposed to cyclic stretching were significantly higher after 1 h (p < 0.05) and 6 h (p < 0.01) than those of WT cells. The results of the cell area and the inverse aspect ratio indicate that siNes1 cells were more round and less elongated in shape compared with WT cells after exposure to cyclic stretching for 6 h. Although cyclic stretching experiments for 24 and 48 h were also performed, no further changes in cellular morphology were observed compared with those after 6 h of cyclic stretching (data not shown). The perpendicular orientations of both WT and siNes1 cells to the direction of stretching were marginally significant at 1 h, and becoming increasingly significant at 3 h and 6 h after exposure to cyclic stretching (Fig. 3d). We could not detect any effects of nesprin-1 knockdown on cyclic stretching-induced reorientation of fibroblasts. These results indicate that siRNA-mediated knockdown of nesprin-1 suppresses cyclic stretching-induced polarized elongation, but that it does not affect the reorientation of fibroblasts.
Figure 2.
Typical fluorescence microscopy images of non-treated wild type (WT) and small interfering RNA(siRNA)-treated (siNes1) fibroblasts cultured statically (static) and exposed to 10% cyclic stretching for 1, 3, and 6 h (blue: Nucleus; green: Nesprin-1; red: F-actin). Scale bars = 100 µm.
Results of nuclear morphological analyses are shown in Fig. 4. Although nuclear areas showed similar time-course changes to cell areas, nuclear inverse aspect ratios both of WT and siNes1 cells did not changes by exposure to cyclic stretching (Figs. 4a and 4b). There was no significant effect of the nesprin-1 knockdown on nuclear areas and nuclear inverse aspect ratios. Exposure of cyclic stretching also caused nuclear orientation perpendicular to the direction of stretching both in WT and siNes1 cells (Fig. 4c).
Figure 4.
Results of nuclear morphological analyses of non-treated wild type (WT) and small interfering RNA(siRNA)-treated (siNes1) fibroblasts cultured statically (Static) and exposed to 10% cyclic stretching for 1, 3, and 6 h. (a) Nuclear areas and (b) nuclear inverse aspect ratios of WT and siNes1 cells. Results are expressed as mean + SD. *p < 0.01 (vs. static), **p < 0.001 (vs. static). (c) Histograms of angles of nuclear orientation. R and p values are mean resultant lengths and the results of the Rayleigh test, respectively.
Changes in transcriptional activity are, in general, involved in cellular responses to external stimuli, and the loss of nesprin-1-mediated nucleus–actin filament binding may influence gene expression, along with the cellular response to mechanical stimuli.35 On the other hand, cellular morphological changes in response to cyclic stretching are believed to involve the activation of mechanosensing protein complexes such as focal adhesions and ion channels,13,29 followed by mechanical and structural changes in actin filaments,9,31 which directly lead to changes in the activity of the modulators of actin cytoskeletal network and small GTPases, including RhoA and Rac1,17,32 independently of transcription. Nesprin-1 depletion could also cause mechanical changes in actin cytoskeletal structure of siNes1 cells different from those in WT cells. To clarify the effect of nesprin-1 knockdown on morphological changes in fibroblasts induced by cyclic stretching, we examined the involvement of transcriptional activity by treating the cells with ActD.
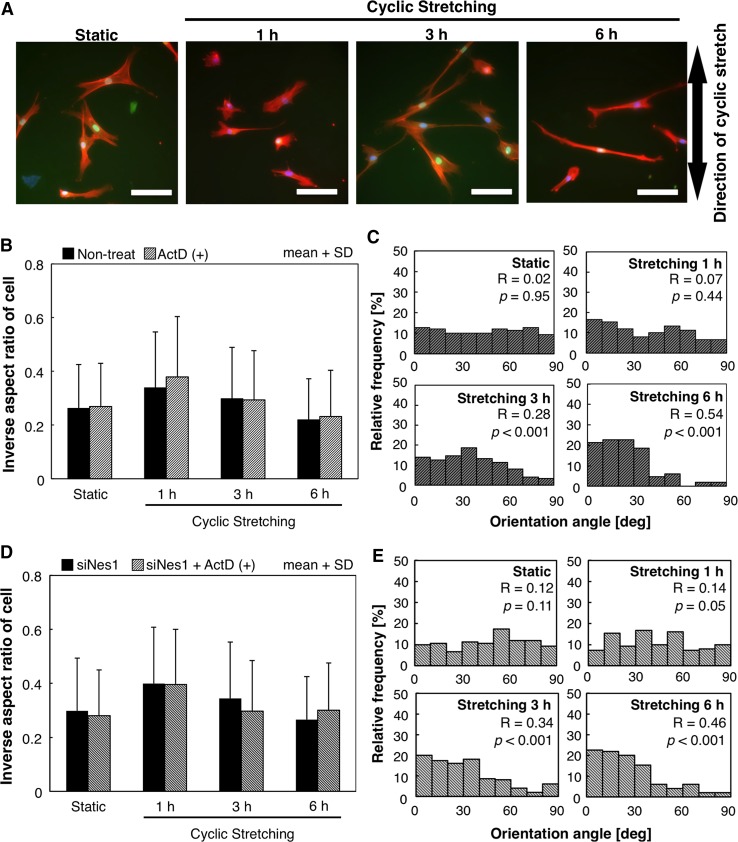
Figure 5 represents the results of ActD treatment. Under static conditions, the morphology and actin filament structures of fibroblasts treated with ActD were similar to those of non-treated cells (Fig. 5a). ActD-treated cells also showed contracted shapes 1 h after exposure to cyclic stretching; and after 6 h of exposure, the cells showed elongation and alignment perpendicular to the direction of cyclic stretching. There was no significant effect of ActD treatment on the inverse aspect ratios of WT cells compared with non-treated WT cells, and no detectable difference in the distribution of the orientation angle of cells between non-treated and ActD-treated cells (Figs. 5b and 5c). We additionally treated siNes1 cells with ActD, but could not detect any significant effect of ActD treatment on the cellular morphological response to cyclic stretching (Figs. 5d and 5e). Similarly, cell areas and nuclear morphology were also not affected by ActD treatment (data not shown). These results indicate that morphological changes in fibroblasts in response to cyclic stretching did not involve changes in their transcriptional activity. Loss of nesprin-1-mediated binding between the nucleus and actin filaments is, therefore, thought to induce alternations of mechanical conditions in actin filament structures, thereby suppressing cyclic stretching-induced polarized elongation.
Figure 5.
Results of actinomycin D (ActD) treatment of fibroblasts. (a) Typical fluorescence images of ActD-treated fibroblasts cultured statically (static) and exposed to 10% cyclic stretching for 1, 3, and 6 h (Blue: Nucleus, Green: Nesprin-1, Red: F-actin). Bars = 100 µm. (b) Inverse aspect ratios and (c) histograms of angles of cell orientation of ActD-treated wild fibroblasts. (d) Inverse aspect ratios and (e) histograms of angles of cell orientation of nesprin-1 knocked-down fibroblasts (siNes1) treated with ActD. Results are expressed as mean + SD (b and d). R and p values are mean resultant lengths and the results of the Rayleigh test, respectively (c and e).
Because depletion of nesprin-1 suppresses nucleus–actin filament binding, the tension present in actin filaments bound to the nucleus in WT cells is thought to be low or negligible in siNes1 cells. These differences in intracellular mechanical condition of actin filament structures may play a role in the effects of nesprin-1 knockdown on cellular morphological response. In order to confirm the role of such mechanical condition of nucleus-associated actin filament in morphological response of fibroblasts to cyclic stretching, TSA-treated cells were additionally used in our study. TSA treatment decreases nuclear elasticity21 and can lead to changes in nucleus-associated mechanical condition of actin filament structures. In preliminary atomic force microscopy-based indentation tests, we found a significant decrease in nuclear elasticity of TSA-treated fibroblasts compared with that of non-treated cells (non-treat: 17.6 ± 9.7 kPa, TSA-treated: 10.8 ± 4.9 kPa, mean ± SD, p < 0.001).
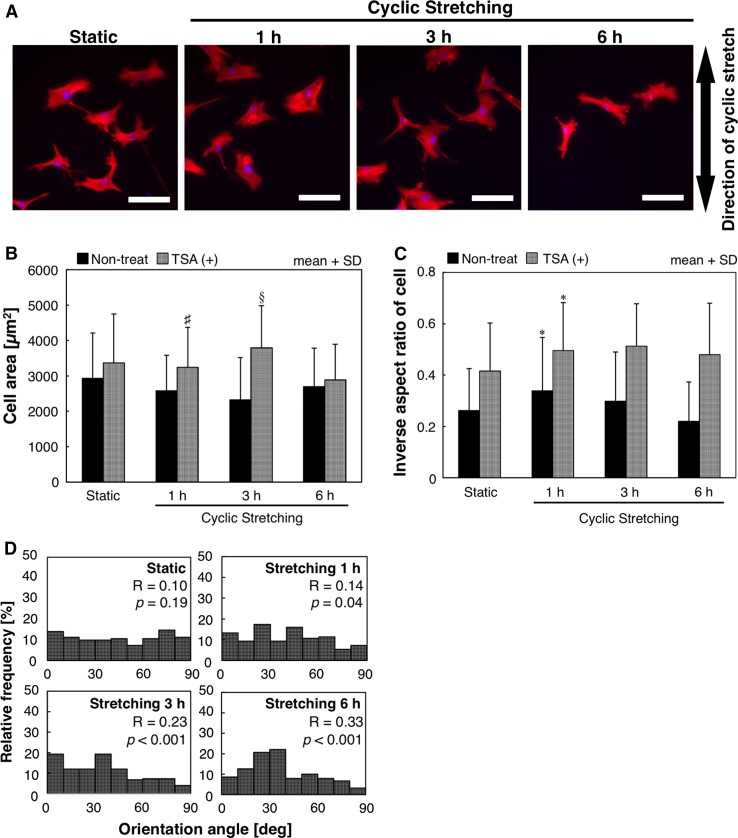
Unlike non-treated cells, TSA-treated cells were round in shape under static conditions and did not show cyclic stretching-induced elongation even after exposure for 6 h (Fig. 6a). Treatment of TSA with cells significantly increased cell areas (Fig. 6b) (p < 0.001) and nuclear areas (Fig. 7a) (p < 0.001), whereas the exposure time has no significant effect on them. The inverse aspect ratios of TSA-treated cells were significantly higher than those of non-treated cells under both static and stretching conditions (p < 0.001). The inverse aspect ratios of TSA-treated cells increased after an hour of exposure to cyclic stretching, which was similar to that of non-treated cells, and remained unchanged after 3 and 6 h (Fig. 6c). In contrast, inverse aspect ratios of nuclei were unaffected by treatment of TSA (Fig. 7b). TSA-treated cells and nuclei both showed orientation perpendicular to the direction of the cyclic stretching after 6 h of exposure (Figs. 6d and 7c). To check whether TSA treatment induced changes in the transcription activity and cellular morphological response, we used cells treated with both ActD and TSA for the experiments, which showed morphological changes similar to those of TSA-treated cells (data not shown). The TSA treatment was reported to cause an increase in nuclear areas12 and the nuclear size is found to have a close correlation with the size of the cell.25,34 Changes in cell and nuclear areas by TSA treatment in the present study is in agreement with the previous studies. However, TSA treatment did not have a significant effect on nuclear inverse aspect ratio and orientation, and time-course changes in the inverse aspect ratio of cells are different from those in cell and nuclear areas. Thus, these results indicate that the mechanical condition of actin filaments bound to the nucleus plays an important role in polarized elongation of fibroblasts.
Figure 6.
Results of Trichostatin A (TSA) treatment of fibroblasts. (a) Typical fluorescence images of fibroblasts cultured statically (static) and exposed to 10% cyclic stretching for 1, 3, and 6 h (Blue: Nucleus; Red: F-actin). Scale bars = 100 µm. (b) Cell areas and (c) inverse aspect ratios of TSA-treated fibroblasts. Results are expressed as mean + SD. *p < 0.01 (vs. static), # p < 0.05 (vs. non-treat), § p < 0.01 (vs. non-treat). (d) Histograms of angles of cell orientation. R and p values are mean resultant lengths and the results of the Rayleigh test, respectively.
Figure 7.
Results of nuclear morphological analyses of Trichostatin A (TSA) treated fibroblasts cultured statically (static) and exposed to 10% cyclic stretching for 1, 3, and 6 h. (a) Nuclear areas and (b) nuclear inverse aspect ratios of WT and TSA-treated cells. Results are expressed as mean + SD. § p < 0.001 (vs. non-treat). (c) Histograms of angles of nuclear orientation. R and p values are mean resultant lengths and the results of the Rayleigh test, respectively.
In summary, we showed that the mechanical condition of actin filaments bound to the nucleus in fibroblasts plays an important role in cyclic stretching-induced cellular elongation independently of transcription activity. Elongation of cells in response to cyclic stretching involves local activity changes of small GTPases, including RhoA and Rac1, at the cellular periphery along a direction perpendicular to that of stretching, which causes dynamic structural changes in actin filaments and formation and maturation of focal adhesions. These signaling and remodeling mechanisms underlying cyclic stretching-induced cellular morphological changes have been discussed and explained with respect to mechanical conditions of focal adhesions and actin filament structures bound to focal adhesions.8,31,32 The results of our study shed light on the role of the mechanical condition of the nucleus–actin filament binding in these mechanisms.
Similarly to non-treated cells, TSA-treated cells also showed an increase in their inverse aspect ratios 1 h after exposure to cyclic stretching, indicating that the cyclic stretching-induced contraction of fibroblasts occurs regardless of nuclear elasticity. By contrast, after being exposed to 3 and 6 h of cyclic stretching, the inverse aspect ratios of fibroblasts did not significantly change from those at 1 h. Because actin cytoskeletal structures in TSA-treated cells were similar to those in non-treated cells, it is possible that the nuclear mechanical properties play an important role in the elongation of fibroblasts exposed to cyclic stretching. As mentioned earlier, tension in actin cytoskeletal structure is closely related to cellular morphology. In physical terms, boundary conditions of actin filaments significantly affect maintenance and variation of their tensile force. A decrease in nuclear elasticity following TSA treatment can thus reduce tension in actin filaments bound to the nuclei, which seem to be implicated in the elongation of fibroblasts exposed to cyclic stretching. Likewise, knockdown of nesprin-1 may reduce or eliminate tension in actin filaments bound to the nuclei, resulting in the suppression of fibroblast elongation.
We expected knockdown of nesprin-1 would cause different shapes and morphological changes of nuclei in response to cyclic stretching compared with WT cells, because actin cytoskeletal structure and its tension are known to play an important role in the nuclear shape5,19 and nesprin-1 knockdown could release the nucleus from actin cytoskeletal tension. However, contrary to our expectation, there are no significant differences in nuclear area, inverse aspect ratio, and orientation between WT and siNes1 cells. In our results, no evident differences were present in actin filament structures between non-treated WT and siNes1 cells, which suggest that there is still perinuclear actin structure in siNes1 cells similar to WT cells. In addition, we previously observed nuclear deformation due to substrate stretching even in nesprin-1 knocked-down cells and thereby suggested that actin cortical layer beneath the cell membrane and perinuclear actin structure play an important role in nuclear deformation subjected to substrate stretching.1 Although we need further investigation of the mechanisms which regulates the nuclear shape, our results suggest that despite of nesprin-1 knockdown, actin cortical layer and perinuclear actin structure may preserve nuclear shapes similar to those in WT cells.
Chancellor et al. previously showed that nesprin-1 knockdown abolished the morphological response of endothelial cells to cyclic stretching, and treatment of Y27632, an inhibitor of RhoA effector Rho-associated kinases, recovers this response under cyclic stretching in nesprin-1-depleted cells.3 They also suggested that knockdown of nesprin-1 could affect RhoA/Rho kinase signaling activity. Greiner et al. showed that stabilization and disruption of microtubules by treating cells with taxiol and nocodazole, respectively, did not affect their perpendicular orientation to the stretching direction; however, it did interfere in cellular elongation under cyclic stretching.10 The effects of microtubule interference on the morphological response to cyclic stretching are very similar to those of nesprin-1 knockdown shown in the present study. Guanine nucleotide exchange factor-H1, a major RhoA activator, is known to associate with microtubules,4,33 and formation and disruption of microtubules are important in local RhoA activity. The cellular morphological changes caused by microtubule-interfering reagent treatment, as reported by Greiner et al., may be provoked by hampered local control of RhoA activity. However, we confirmed by fluorescent microscopy that the microtubule structure is intact in nesprin-1-depleted fibroblasts and WT cells (data not shown). GEF/RhoA activity is regulated by signaling from focal adhesions11 and focal adhesion activity has a close correlation with the forces generated by actin filaments bound to focal adhesions.8 Furthermore, actin filaments connected to the apical surface of the nucleus, referred to as the “actin cap,” may specifically associate with focal adhesions at the cell periphery.20 Considering this, changes in the tension generated by nucleus-associated actin filaments, induced by nesprin-1 knockdown and TSA-treatment, may cause changes in focal activity and adhesion at cell periphery, which reduces cellular elongation under cyclic stretching conditions.
Acknowledgments
The present study was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan (Nos. 15K01304 and 16K15837) and the interdepartmental research fund of Kawasaki University of Medial Welfare.
Conflict of interest
N. Sakamoto, M. Ogawa, K. Sadamoto, M. Takeuchi, and N. Kataoka declare that they have no conflicts of interest.
Ethical Standards
No human or animal studies were carried out by the authors for this article.
References
- 1.Anno T, Sakamoto N, Sato M. Role of nesprin-1 in nuclear deformation in endothelial cells under static and uniaxial stretching conditions. Biochem. Biophys. Res. Commun. 2012;424(1):94–99. doi: 10.1016/j.bbrc.2012.06.073. [DOI] [PubMed] [Google Scholar]
- 2.Bashur C, Dahlgren LA, Goldstein AG. Effect of fiber diameter and orientation on fibroblast morphology and proliferation on electrospun poly(D,L-lactic-co-glycolic acid) meshes. Biomaterials. 2006;27(33):5681–5688. doi: 10.1016/j.biomaterials.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Chancellor TJ, Lee J, Thodeti CK, Lele T. Actomyosin tension exerted on the nucleus through nesprin-1 connections influences endothelial cell adhesion, migration, and cyclic strain-induced reorientation. Biophys. J. 2010;99(1):115–123. doi: 10.1016/j.bpj.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang YC, Nalbant P, Birkenfeld J, Chang ZF, Bokoch GM. GEF-H1 couples nocodazole-induced microtubule disassembly to cell contractility via RhoA. Mol. Biol. Cell. 2008;19(5):2147–2153. doi: 10.1091/mbc.E07-12-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen B, Co C, Ho CC. Cell shape dependent regulation of nuclear morphology. Biomaterials. 2015;67:129–136. doi: 10.1016/j.biomaterials.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crisp M, Liu Q, Roux K, Rattner JB, Shanahan C, Burke B, Stahl PD, Hodzic D. Coupling of the nucleus and cytoplasm: role of the LINC complex. J. Cell Biol. 2006;172(1):41–53. doi: 10.1083/jcb.200509124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dahl KN, Scaffidi P, Islam MF, Yodh AG, Wilson KL, Misteli T. Distinct structural and mechanical properties of the nuclear lamina in Hutchinson-Gilford progeria syndrome. Proc. Natl. Acad. Sci. USA. 2006;103(27):10271–10276. doi: 10.1073/pnas.0601058103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deguchi S, Matsui TS, Iio K. The position and size of individual focal adhesions are determined by intracellular stress-dependent positive regulation. Cytoskeleton (Hoboken) 2011;68(11):639–651. doi: 10.1002/cm.20541. [DOI] [PubMed] [Google Scholar]
- 9.Deguchi S, Ohashi T, Sato M. Intracellular stress transmission through actin stress fiber network in adherent vascular cells. Mol. Cell Biomech. 2005;2(4):205–216. [PubMed] [Google Scholar]
- 10.Greiner AM, Chen H, Spatz JP, Kemkemer R. Cyclic tensile strain controls cell shape and directs actin stress fiber formation and focal adhesion alignment in spreading cells. PLoS ONE. 2013;8(10):e77328. doi: 10.1371/journal.pone.0077328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guilluy C, Garcia-Mata R, Burridge K. Rho protein crosstalk: another social network? Trends Cell Biol. 2011;21(12):718–726. doi: 10.1016/j.tcb.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guilluy C, Osborne LD, Van Langeghem L, Sharek L, Superfine R, Garcia-Mata R, Burridge K. Isolated nuclei adapt to force and reveal a mechanotransduction pathway in the nucleus. Nat. Cell Biol. 2014;16(4):376–381. doi: 10.1038/ncb2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayakawa K, Tatsumi H, Sokabe M. Actin stress fibers transmit and focus force to activate mechanosensitive channels. J. Cell Sci. 2008;121(Pt 4):496–503. doi: 10.1242/jcs.022053. [DOI] [PubMed] [Google Scholar]
- 14.Hoffman LM, Jensen CC, Chaturvedi A, Yoshigi M, Beckerle MC. Stretch-induced actin remodeling requires targeting of zyxin to stress fibers and recruitment of actin regulators. Mol. Biol. Cell. 2012;23(10):1846–1859. doi: 10.1091/mbc.E11-12-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang W, Sakamoto N, Miyazawa R, Sato M. Role of paxillin in the early phase of orientation of the vascular endothelial cells exposed to cyclic stretching. Biochem. Biophys. Res. Commun. 2012;418(4):708–713. doi: 10.1016/j.bbrc.2012.01.083. [DOI] [PubMed] [Google Scholar]
- 16.Kataoka N, Ujita S, Sato M. Effect of flow direction on the morphological responses of cultured bovine aortic endothelial cells. Med. Biol. Eng. Comput. 1998;36(1):122–128. doi: 10.1007/BF02522869. [DOI] [PubMed] [Google Scholar]
- 17.Katsumi A, Milanini J, Kiosses WB, del Pozo MA, Kaunas R, Chien S, Hahn KM, Schwartz MA. Effects of cell tension on the small GTPase Rac. J. Cell Biol. 2002;158(1):153–164. doi: 10.1083/jcb.200201105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaunas R, Nguyen P, Usami S, Chein S. Cooperative effects of Rho and mechanical stretch on stress fiber organization. Proc. Natl. Acad. Sci. USA. 2005;102(44):15895–15900. doi: 10.1073/pnas.0506041102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khatau SB, Hale CM, Stewart-Hutchinson PJ, Patel MS, Stewart CL, Searson PC, Hodzic D, Wirtz D. A perinuclear actin cap regulates nuclear shape. Proc. Natl. Acad. Sci. USA. 2009;106(45):19017–19022. doi: 10.1073/pnas.0908686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khatau SB, Kim DH, Hale CM, Bloom RJ, Wirtz D. The perinuclear actin cap in health and disease. Nucleus. 2010;1(4):337–342. doi: 10.4161/nucl.1.4.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krause M, Te Riet J, Wolf K. Probing the compressibility of tumor cell nuclei by combined atomic force-confocal microscopy. Phys. Biol. 2013;10(6):065002. doi: 10.1088/1478-3975/10/6/065002. [DOI] [PubMed] [Google Scholar]
- 22.Lombardi ML, Jaalouk DE, Shanahan CM, Burke B, Roux KJ, Lammerding J. The interaction between nesprins and sun proteins at the nuclear envelope is critical for force transmission between the nucleus and cytoskeleton. J. Biol. Chem. 2011;286:26743–26753. doi: 10.1074/jbc.M111.233700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meinke P, Schirmer EC. LINC’ing form and function at the nuclear envelope. FEBS Lett. 2015;589:2514–2521. doi: 10.1016/j.febslet.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 24.Mejat A, Misteli T. LINC complexes in health and disease. Nucleus. 2010;1(1):40–52. doi: 10.4161/nucl.1.1.10530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neumann FR, Nurse P. Nuclear size control in fission yeast. J. Cell Biol. 2007;179(4):593–600. doi: 10.1083/jcb.200708054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oya K, Sakamoto N, Ohashi T, Sato M. Combined stimulation with cyclic stretching and hypoxia increases production of matrix metalloproteinase-9 and cytokines by macrophages. Biochem. Biophys. Res. Commun. 2011;412(4):678–682. doi: 10.1016/j.bbrc.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 27.Prager-Khoutorsky M, Lichtenstein A, Krishnan R, Rajendran K, Mayo A, Kam Z, Geiger B, Bershadsky AD. Fibroblast polarization is a matrix-rigidity-dependent process controlled by focal adhesion mechanosensing. Nat. Cell Biol. 2011;13(12):1457–1465. doi: 10.1038/ncb2370. [DOI] [PubMed] [Google Scholar]
- 28.Scaffidi P, Gordon L, Misteli T. The cell nucleus and aging: tantalizing clues and hopeful promises. PLoS Biol. 2005;3(11):e395. doi: 10.1371/journal.pbio.0030395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shyy JY, Chien S. Role of integrins in endothelial mechanosensing of shear stress. Circ. Res. 2002;91(9):769–775. doi: 10.1161/01.RES.0000038487.19924.18. [DOI] [PubMed] [Google Scholar]
- 30.Stewart-Hutchinson PJ, Hale CM, Wirtz D, Hodzic D. Structural requirements for the assembly of LINC complexes and their function in cellular mechanical stiffness. Exp. Cell Res. 2008;314(8):1892–1905. doi: 10.1016/j.yexcr.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tondon A, Kaunas R. The direction of stretch-induced cell and stress fiber orientation depends on collagen matrix stress. PLoS ONE. 2014;9(2):e89592. doi: 10.1371/journal.pone.0089592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tzima E. Role of small GTPases in endothelial cytoskeletal dynamics and the shear stress response. Circ. Res. 2006;98(2):176–185. doi: 10.1161/01.RES.0000200162.94463.d7. [DOI] [PubMed] [Google Scholar]
- 33.Varma H, Yamamoto A, Sarantos MR, Hughes RE, Stockwell BR. Mutant huntingtin alters cell fate in response to microtubule depolymerization via the GEF-H1-RhoA-ERK pathway. J. Biol. Chem. 2010;285(48):37445–37457. doi: 10.1074/jbc.M110.125542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vishavkarma R, Raghavan S, Kuyyamudi C, Majumder A, Dhawan J, Pullarkat PA. Role of actin filaments in correlating nuclear shape and cell spreading. PLoS ONE. 2014;9(9):e107895. doi: 10.1371/journal.pone.0107895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat. Rev. Mol. Cell Biol. 2009;10(1):75–82. doi: 10.1038/nrm2594. [DOI] [PubMed] [Google Scholar]