Abstract
BACKGROUND AND PURPOSE:
Metabolic syndrome (MetS), a prothrombotic state, is associated with an increased risk of atrial fibrillation (AF) and stroke. The CHA2DS2-VASc score does not account for the MetS components of prehypertension, prediabetes, abdominal obesity, elevated triglycerides and low high- density lipoprotein (HDL). Data are limited on the association of MetS with stroke in AF, independent of CHA2DS2-VASc variables. Our aim was to identify MetS components associated with ischemic stroke in participants with AF in the Atherosclerosis Risk in Communities (ARIC) Study.
METHODS:
We included 1,172 participants with incident AF within 5 years of measurement of MetS components. MetS was defined by ATP criteria and International Diabetes Federation criteria. Incident ischemic stroke was physician adjudicated. Multivariable Cox proportional hazards regression was used to assess the association of MetS components with stroke.
RESULTS:
After a median follow-up of 14.8 years, there were 113 ischemic stroke cases. Of the individual MetS components, low HDL was borderline associated with increased stroke risk (HR, 1.48; 95% CI, 0.99–2.21) after adjustment for CHA2DS2-VASc variables while the remaining MetS variables were not associated with stroke risk. The presence of ≥3 components of MetS was not significantly associated with ischemic stroke after adjustment for CHA2DS2-VASc variables (HR, 1.38; 95% CI, 0.91–2.11). The risk of stroke increased by 13% for each additional component of MetS; however, this association was borderline significant (HR, 1.13 95% CI, 0.99–1.28).
CONCLUSION:
The presence of MetS was not significantly associated with ischemic stroke after adjustment for CHA2DS2-VASc variables. Consideration of MetS is unlikely to improve stroke prediction in AF.
Keywords: atrial fibrillation, metabolic syndrome, ischemic stroke, hypertension, diabetes, obesity, hypertriglyceridemia, high-density lipoprotein
Subject Terms: atrial fibrillation, ischemic stoke
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia in the general population and it is associated with a 5-fold increased risk of ischemic stroke and a 2-fold increased risk of cardiac mortality.1,2 Given the significant morbidity and mortality associated with stroke in AF, the CHADS2 and CHA2DS2-VASc scores have been established as validated stroke risk stratification schemes in patients with AF.3,4 The CHADS2 score includes congestive heart failure, hypertension, age >75 years, diabetes mellitus and prior stroke or transient ischemic attack (TIA). Although simple to use, the CHADS2 score has modest predictive ability and known limitations with classifying low and intermediate risk patients.5 The CHA2DS2-VASc score, which was developed to address the limitations of the CHADS2 score, includes additional risk factors of vascular disease, age between 65 and 74 years, and female sex and allows for more comprehensive stroke risk assessment.
Metabolic syndrome is a constellation of cardiovascular risk factors and is defined as the presence of any three of the following five traits including abdominal obesity, elevated triglycerides, reduced high-density lipoprotein (HDL) cholesterol, prehypertension or hypertension and insulin resistance or diabetes. Metabolic syndrome is considered to be both a proinflammatory and prothrombotic state as adults with metabolic syndrome have been shown to have decreased serum concentrations of antioxidants and elevated C-reactive protein, fibrinogen and plasminogen activator inhibitor-1 concentrations.6,7 Additionally, metabolic syndrome has been shown to be independently associated with an increased risk of new-onset AF and ischemic stroke.8,9 The association between metabolic syndrome and thromboembolic risk in patients with AF was evaluated in a study based in Taiwan which found that every component of metabolic syndrome was associated with increased risk of thromboembolism, range of HR: 1.35 to 1.75 .10 Furthermore, considering components of the metabolic syndrome improved model discrimination of thromboembolic events over the CHADS2 score alone (ROC area: 0.729 vs. 0.670, P = 0.034).10
While overlap exists between metabolic syndrome and CHA2DS2-VASc with regards to hypertension and diabetes, the CHA2DS2-VASc score does not include prehypertension, prediabetes, abdominal obesity, elevated triglycerides and low HDL. It remains unknown whether the components of metabolic syndrome would improve risk prediction of stroke, after accounting for the CHA2DS2-VASc score in the US population. The objective of this study was to assess the association between the metabolic syndrome and the risk of ischemic stroke in participants with AF in the Atherosclerosis Risk in Communities (ARIC) Study, a community-based prospective cohort study.
Methods
The data, analytic methods, and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure in accordance with ARIC policies. Data are maintained by ARIC through the University of North Carolina Collaborative Studies Coordinating Center. Further information is available on the ARIC website.
Study Population and follow-up
The ARIC study is a community-based prospective cohort study of cardiovascular disease with participants sampled from 4 US communities: suburbs of Minneapolis, Minnesota; Washington County, Maryland; Forsyth County, North Carolina; and Jackson, Mississippi. The study included 15,792 men and women 45–64 years of age at enrollment (1987–1989) who underwent a baseline visit at the time of enrollment (visit 1) and then attended subsequent exams in 1990–1992, 1993–1995, 1996–1998 and 2011–2013, 2016–2017 (visits 2–6), with visit 7 ongoing. Study visits included a clinical exam, fasting laboratory measurements and an electrocardiogram (ECG) which was centrally read. Additional details on outcome ascertainment procedures, study design and population statistics have been previously described.11 The ARIC study’s research protocol was approved by institutional review boards at each participating center, and all participants provided informed consent.
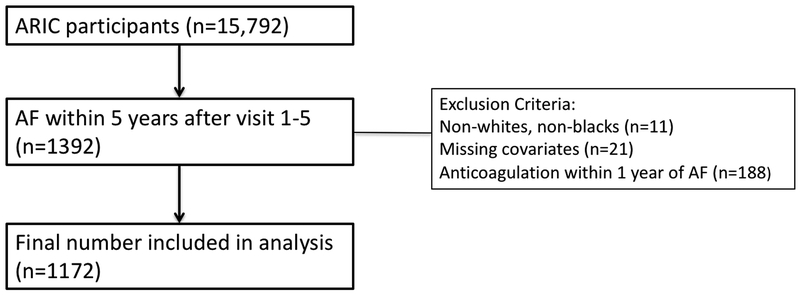
For our analysis, we considered 1,392 participants who developed AF within a 5 year time period after presentation for study clinic visits 1–5. We excluded those with missing covariates (n=21), on anticoagulation within 1 year after AF diagnosis (n=188) and race other than white or black, along with non-whites in the Minneapolis and Washington County field centers, due to small numbers (n=11), which would not contribute meaningfully to the analysis (Figure 1). The final analytic cohort included 1,172 participants.
Figure 1.
Flowchart of study participants.
Ascertainment of Atrial Fibrillation
Details of AF ascertainment in ARIC have previously been described.12,13 Briefly, AF was ascertained from resting 12-lead ECGs obtained during the baseline and 4 follow-up visits as well as hospital discharge records using International Classification of Disease 9th revision, Clinical Modification [ICD-9-CM] 427.31 or 427.32 diagnosis codes. All study visit ECGs coded by ECG software as AF were visually confirmed by either a cardiologist or a trained coder.
Components of Metabolic Syndrome
Measurements of components of metabolic syndrome were obtained at visits 1–5. Metabolic syndrome was defined by current ATP III criteria.14 Abdominal obesity was defined as a waist circumference in men ≥102 cm (40 in) and in women ≥88 cm (35 in). Elevated triglycerides was defined as ≥1.7 mmol/L (150 mg/dL) or drug treatment for elevated triglycerides. Low high-density lipoprotein (HDL) cholesterol was defined as <1 mmol/L (40 mg/dL) in men and <1.3 mmol/L (50 mg/dL) in women or drug treatment for low HDL cholesterol. Elevated fasting plasma glucose was defined as fasting plasma glucose (FPG) ≥5.6 mmol/L or 100 mg/dL (also known as prediabetes), drug treatment for elevated blood glucose, or a diagnosis of diabetes. Diabetes was defined as FPG ≥7.0 mmol/L (126 mg/dL), non-fasting glucose ≥11.1 mmol/L (200 mg/dl) or drug treatment for diabetes. There was a small number of participants (n=36) who were not fasting and had a blood glucose of ≥5.6 mmol/L (100 mg/dL); however, all of these participants met criteria for inclusion in the elevated fasting plasma group based on either drug treatment for elevated blood glucose or a diagnosis of diabetes. Elevated blood pressure was defined as blood pressure ≥130/85 mmHg (also known as prehypertension) or drug treatment for elevated blood pressure. Hypertension was defined as systolic blood pressure >140 mmHg and/or diastolic blood pressure >90 mmHg or drug treatment for hypertension. Participants were considered to have metabolic syndrome if ≥3 components of metabolic syndrome were present.
An additional analysis defined metabolic syndrome by the International Diabetes Federation criteria: BMI >30 mg/m2 is used to define central obesity rather than waist circumference. The definitions for elevated triglycerides, low HDL, elevated fasting plasma glucose and elevated blood pressure, as detailed above, are the same for both ATP and International Diabetes Federation criteria.
Ascertainment of Ischemic Stroke
ARIC participants were contacted annually by phone in order to identify deaths and hospitalizations within the previous year. Hospital records and death certificates were then obtained for physician review of all potential cases of stroke. Further classification of stroke was adjudicated by a panel of physicians with assistance of a computerized algorithm utilizing validated criteria from the National Survey of Stroke by the National Institute of Neurological Disorders. If the physician panel was unable to reach a consensus on a stroke diagnosis, further review and adjudication was determined by a neurologist or cardiologist.
Strokes were classified as definite or probable thrombotic stroke, definite or probable cardioembolic stroke, definite or probable subarachnoid hemorrhage, definite or probable brain hemorrhage, and possible stroke of undetermined type or cryptogenic stroke. All definite thrombotic strokes were further sub-typed as definite thrombotic lacunar and definite thrombotic non-lacunar strokes. The primary endpoint in our study was definite ischemic stroke, which included all definite thrombotic strokes and all definite cardioembolic strokes. Further details on stroke identification and specific classification criteria in the ARIC study have been previously described.15,16
Statistical Analyses
Metabolic syndrome components were assessed at each of the 5 study visits, and the measurements from the visit preceding development of AF were used for this analysis. The components of metabolic syndrome were modeled as dichotomous variables, with an additional analysis modeling HDL as a continuous variable. In order to estimate the association between metabolic syndrome components with incident ischemic stroke, we calculated hazard ratios (HRs) and 95% confidence intervals (CIs) using multivariable Cox proportional hazard models. The first multivariable model was adjusted for age, sex, race/study center. The second model additionally adjusted for CHA2DS2-VASc variables of heart failure, hypertension, diabetes, coronary artery disease, previous myocardial infarction, peripheral artery disease and history of stroke or transient ischemic attack and also adjusted for use of anticoagulation. A third model additionally adjusted for the CHADS2 variables of heart failure, hypertension, diabetes and history of stroke or transient ischemic attack as well as for use of anticoagulation.
The reference group for comparison of participants with elevated blood pressure comprised participants without hypertension or prehypertension. The reference group for comparison of participants with elevated fasting plasma glucose comprised participants without diabetes or prediabetes. The CHA2DS2-VASc and CHADS2 scores, which are the basis for adjustments used in models 2 and 3 respectively, overlap with the definition of metabolic syndrome with regards to hypertension and diabetes. As a result of these overlapping definitions, we did not include hypertension in the adjustments for the second and third models when assessing for the association of elevated BP with stroke. Similarly, we did not include diabetes in the second and third models when assessing for the association of elevated fasting plasma glucose and stroke.
In addition to assessing the association of individual metabolic syndrome components with incident ischemic stroke, we also performed analysis evaluating the risk of stroke associated with meeting the criteria for the diagnosis of metabolic syndrome, based on both ATP criteria and International Diabetes Federation criteria. For this analysis, the second and third models did not include hypertension or diabetes given the aforementioned overlap of those within the CHA2DS2-VASc score and the definition of metabolic syndrome. We also performed analysis evaluating the association between the number of components of metabolic syndrome and stroke and also assessed the risk of stroke for one additional component of metabolic syndrome, modeled as a discrete continuous variable. Furthermore, we conducted an additional analysis to evaluate the association of metabolic syndrome components with embolic stroke. Lastly, we performed a sensitivity analysis by fitting the Fine-Gray model, a proportional hazards model for the subdistribution of competing risks.17
Results
Baseline Characteristics
Baseline characteristics of the 1,172 study participants, all of whom had AF, stratified by metabolic syndrome are summarized in Table 1. There were 514 (44%) female participants and 194 (17%) were black. Compared with participants without metabolic syndrome, those with metabolic syndrome were older, had a higher CHA2DS2-VASc score, and had higher prevalence of MI, stroke/TIA, and heart failure.
Table 1.
Clinical Characteristics at Time of Atrial Fibrillation Diagnosis, Atherosclerosis Risk in Communities (ARIC) Study
| All | No MetS | MetS | ||
|---|---|---|---|---|
| Characteristic | N=1172 | N=340 | N=832 | P-value |
| Age (Years) | 71 +/− 9 | 68.6 +/− 8 | 71.6 +/−9 | <0.0001 |
| Female sex | 514 (44) | 143 (42) | 371 (45) | 0.43 |
| Previous MI | 254 (22) | 49 (14) | 205 (25) | <0.0001 |
| Heart failure | 374 (32) | 87 (26) | 287 (35) | 0.003 |
| PAD | 125 (11) | 32 (9) | 93 (11) | 0.37 |
| Past stroke/TIA | 163 (14) | 35 (10) | 133 (16) | 0.02 |
| CHA2DS2VASc | 3.4 +/− 1.8 | 2.5 +/− 1.6 | 3.7 +/− 1.7 | <0.0001 |
| Black race | 194 (17) | 48 (14) | 146 (18) | 0.15 |
| Metabolic syndrome variables | ||||
| High waist circumference | 780 (67) | 121 (36) | 659 (79) | <0.0001 |
| High triglycerides | 624 (53) | 26 (8) | 598 (72) | <0.0001 |
| Low HDL | 704 (60) | 52 (15) | 652 (78) | <0.0001 |
| Elevated fasting plasma glucose | 795 (68) | 113 (33) | 682 (82) | <0.0001 |
| Diabetes | 351 (30) | 23 (7) | 338 (39) | <0.0001 |
| Elevated blood pressure | 955 (81) | 190 (56) | 765 (92) | <0.0001 |
| Hypertension | 819 (70) | 156 (46) | 663 (80) | <0.0001 |
Data are presented as n (%) or mean +/− standard deviation
Abbreviations: Myocardial Infarction (MI), Peripheral Arterial Disease (PAD), Transient
Ischemic Attack (TIA), High-density lipoprotein (HDL).
Elevated blood glucose - Fasting plasma glucose (FPG) ≥100 mg/dL (5.6 mmol/L) or drug treatment for elevated blood glucose and includes participants with diabetes
Elevated blood pressure - Blood pressure ≥130/85 mmHg or drug treatment for elevated blood pressure and includes participants with hypertension
Association of Metabolic Syndrome Components with Ischemic Stroke in AF
There were 113 incident ischemic stroke events occurring among 1,172 participants with AF after a median follow-up of 14.8 years. Of the 113 ischemic stroke events, 59 were classified as embolic and 21 were classified as thrombotic. Of the 21 thrombotic stroke events, 12 were non-lacunar and only 9 were lacunar. Table 2 lists the results of our Cox proportional hazards models for ischemic stroke. Of all the components of the metabolic syndrome, low HDL was the only component that was associated with an increased risk of stroke in AF after adjustment for age, sex, race/study center (model 1, Table 2), HR (95% CI), 1.58 (1.07–2.34). However, after adjustment for CHA2DS2-VASc and CHADS2 variables (models 2 and 3), the association between low HDL and ischemic stroke was attenuated, HR (95% CI), 1.48 (0.99–2.21), P value=0.05 and HR (95% CI) 1.47 (0.99–2.22) P value=0.06 (models 2 and 3 respectively, Table 2). Supplemental Table I lists the results of our Cox proportional hazards models for subgroup analysis of embolic stroke. Low HDL was borderline associated with embolic stroke, HR (95% CI), 1.67 (0.97–2.87), HR (95% CI), 1.60 (0.92–2.78) HR (95% CI), 1.61 (0.93–2.81) (models 1, 2 and 3 respectively, Supplemental Table I in supplemental material) while the other components of the metabolic syndrome were not associated with increased risk of embolic stroke. Supplemental Table II lists the results of our proportional hazards models for the subdistribution of competing risks for models 2 and 3, which did not materially change the aforementioned results in Table 2 (Supplemental Table II in supplemental material). When HDL was modeled as a continuous variable, hazard ratios per standard deviation increment in HDL did not reach statistical significance for models 1–3 (Supplemental Table III in supplemental material).
Table 2.
Association of Metabolic Syndrome Components with Ischemic Stroke in Participants with Atrial Fibrillation, Atherosclerosis Risk in Communities (ARIC) Study
| Individual variables | Absent | HR (95% CI), Model 1 | P-value | HR (95% CI), Model 2 | P-Value | HR (95% CI), Model 3 | P-Value |
|---|---|---|---|---|---|---|---|
| High Waist Circumference | 1 (Ref) | 0.95 (0.64–1.42) | 0.81 | 0.84 (0.56–1.27) | 0.41 | 0.81 (0.55–1.25) | 0.37 |
| High Triglycerides | 1 (Ref) | 1.33 (0.92–1.94) | 0.13 | 1.22 (0.83–1.80) | 0.31 | 1.20 (0.82–1.77) | 0.36 |
| Low HDL | 1 (Ref) | 1.58 (1.07–2.34) | 0.02 | 1.48 (0.99–2.21) | 0.06 | 1.47 (0.99–2.20) | 0.06 |
| Elevated blood pressure | 1 (Ref) | 1.35 (0.83–2.20) | 0.23 | 1.28 (0.77–2.12)* | 0.34 | 1.24 (0.75–2.05)* | 0.40 |
| Elevated fasting plasma glucose | 1 (Ref) | 1.18 (0.79–1.77) | 0.42 | 1.13 (0.75–1.71)** | 0.55 | 1.12 (0.75–1.69)** | 0.58 |
Model 1: Cox proportional hazards model adjusted for age, sex, race/center
Model 2: Cox proportional hazards model adjusted for age, sex, race/center and the remaining CHA2DS2-VASc
variables of stroke/transient ischemic attack, heart failure, hypertension, diabetes,
myocardial infarction, peripheral artery disease and also anticoagulation use.
Model 3: Cox proportional hazards model adjusted for age, sex, race/center and the remaining CHADS2
variables of stroke/transient ischemic attack, heart failure, hypertension, diabetes and also anticoagulation use.
Ref indicates reference and HDL, High-density lipoprotein.
Ref for prehypertension includes participants without prehypertension or HTN
Ref for prediabetes includes participants without prediabetes or diabetes
Not adjusted for hypertension
Not adjusted for diabetes
When comparing participants without metabolic syndrome to those meeting diagnostic criteria with three or more of any of the components, metabolic syndrome was not found to be associated with ischemic stroke after adjustment for age, sex, race/study center (model 1), HR (95% CI), 1.36 (0.90–2.07) (Table 3). Utilizing BMI to define central obesity (based on International Diabetes Federation criteria) also did not demonstrate a significant association between the diagnosis of metabolic syndrome and stroke (Table 4).
Table 3.
Association of Metabolic Syndrome (ATP Criteria) with Ischemic Stroke in Participants with Atrial Fibrillation, Atherosclerosis Risk in Communities (ARIC) Study
| No metabolic syndrome | Metabolic syndrome (≥3 components) | |
|---|---|---|
| N | 340 | 832 |
| # incident stroke | 31 | 82 |
| HR (95% CI), Model 1 | 1(ref) | 1.36 (0.90–2.07) |
| HR (95% CI), Model 2 | 1(ref) | 1.38 (0.91–2.11) |
Model 1: Cox proportional hazards model adjusted for age, sex, race/center
Model 2: Cox proportional hazards model adjusted for age, sex, race/center and the remaining CHA2DS2-VASc
variables of stroke/transient ischemic attack, heart failure, myocardial infarction, peripheral artery disease and also anticoagulation use.
Ref indicates reference
Table 4.
Association of Metabolic Syndrome (IDF Criteria) with Ischemic Stroke in Participants with Atrial Fibrillation, Atherosclerosis Risk in Communities (ARIC) Study*
| No metabolic syndrome | Metabolic syndrome (≥3 components) | |
|---|---|---|
| N | 421 | 751 |
| # incident stroke | 40 | 73 |
| HR (95% CI), Model 1 | 1(ref) | 1.29 (0.88–1.90) |
| HR (95% CI), Model 2 | 1(ref) | 1.29 (0.87–1.91) |
Using BMI to define central obesity (per International Diabetes Federation criteria)
Model 1: Cox proportional hazards model adjusted for age, sex, race/center
Model 2: Cox proportional hazards model adjusted for age, sex, race/center and the remaining CHA2DS2-VASc
variables of stroke/transient ischemic attack, heart failure, myocardial infarction and peripheral artery disease
Ref indicates reference
Next, we considered whether having more components of the metabolic syndrome was associated with a higher risk of ischemic stroke. We did not observe a significant association between increasing number of metabolic syndrome components with higher risk of stroke after adjustment for CHA2DS2-VASc variables (Table 5). When modeled as a discrete continuous variable, we observed that the risk of stroke increased by 13% for each additional component of metabolic syndrome; this association, however, was borderline significant. (Table 5).
Table 5.
Association of Number of Metabolic Syndrome Components with Ischemic Stroke in Participants with Atrial Fibrillation, Atherosclerosis Risk in Communities (ARIC) Study
| Number of components | 0–1 | 2 | 3 | 4 | 5 | Per 1 additional component |
|---|---|---|---|---|---|---|
| N | 145 | 195 | 269 | 266 | 297 | |
| # incident stroke | 11 | 20 | 28 | 24 | 30 | |
| HR (95% CI), Model 1 | 1(ref) | 1.29 (0.62–2.70) | 1.50 (0.74–3.02) | 1.42 (0.69–2.91) | 1.91 (0.95–3.82) | 1.12 (0.99–1.27) |
| HR (95% CI), Model 2 | 1(ref) | 1.26 (0.60–2.64) | 1.51 (0.75–3.06) | 1.40 (0.68–2.88) | 1.93 (0.96–3.90) | 1.13 (0.99–1.28) |
Model 1: Cox proportional hazards model adjusted for age, sex, race/center
Model 2: Cox proportional hazards model adjusted for age, sex, race/center and the remaining CHA2DS2-VASc
variables of stroke/transient ischemic attack, heart failure, myocardial infarction, peripheral artery disease and also anticoagulation use.
Ref indicates reference
Discussion
In this prospective community-based cohort study, metabolic syndrome was not shown to be associated with ischemic stroke in participants with AF. Using a demographically adjusted model, low HDL was the only metabolic syndrome component that was shown to be associated with increased risk of ischemic stroke; the association, however, was borderline significant after adjustment for the CHA2DS2-VASc variables. This finding is consistent with a previously established association between low HDL and higher stroke risk in chronic AF.18 The results of our study are further supported by our competing risk analysis, which found a borderline significant association between low HDL and ischemic stroke. Additional analysis assessing for risk of embolic stroke, which is a mechanism that is more commonly responsible for stroke in AF, also did not significantly change our results.19
Despite the association of low HDL and increased stroke risk, our findings indicate that a constellation of 3 or more of any components of metabolic syndrome did not confer an increased risk of stroke. Hence, consideration of metabolic syndrome is unlikely to provide additional information on stroke risk assessment beyond the CHA2DS2-VASc score. Additionally, our study found that components of metabolic syndrome which overlap with the CHA2DS2-VASc score, namely elevated blood pressure and elevated fasting plasma glucose, were not shown to be associated with an increased risk of ischemic stroke. One possible explanation for this finding is that there are subgroups within risk profiles that may confer a higher risk of stroke. For instance, Patti et al. found that patients with AF and diabetes on insulin therapy had a significantly higher risk of thromboembolic events than diabetic patients who were not being treated with insulin.20 Given the inclusion of a broad spectrum of disease from prediabetes to advanced diabetes requiring insulin, patients fitting the metabolic syndrome criteria for elevated fasting plasma glucose likely have varying degrees of stroke risk.
Our results are also in contrast to the findings of a study including 721 Taiwanese patients with AF, which found metabolic syndrome components to be associated with greater thromboembolic risk.10 This study by Tsai and colleagues demonstrated a graded association between increasing number of components of metabolic syndrome and higher risk of thromboembolic events and found that the proposed CHADS2 –Metabolic syndrome score was superior to the CHADS2 score in predicting thromboembolic risk.10 In our study, we observed that the risk of ischemic stroke increased by 12% for one additional component of the metabolic syndrome, but the association was borderline significant.
There are several differences between our study and the study conducted by Tsai and colleagues which provide potential explanations for these discrepant findings. One possible explanation is the different racial compositions of both studies: ARIC is comprised of black and white participants and patients in the study by Tsai and colleagues are Han Chinese. Racial differences may be important in regards to the prognostic significance of metabolic syndrome and its components. For example, Tsai et al. found BMI >30 to be associated with increased risk of thromboembolic events HR (95% CI), 1.44 (1.15–1.80).10 Previous studies indicate that metabolic responses to central obesity—with regards to cardiovascular risk factors such as hypertension, hyperglycemia and dyslipidemia—may be greater in South and East Asians than in Caucasians at given BMIs.21 The difference in metabolic risk at a fixed level of obesity, which is felt to be related in part to a higher percentage of body fat accumulation in Asians than Caucasians, may explain why our study did not find an association between BMI >30, or increased waist circumference, and stroke.21
Another potential explanation for conflicting results can be attributed to different primary endpoints. Our study looked strictly at ischemic stroke as a primary endpoint while the other study assessed for thromboembolic events, including ischemic stroke as well as peripheral embolic events and acute coronary syndrome. Lastly, our study excluded participants on anticoagulation, a potential confounding variable, while 56.9% and 13.5% in the other study’s participants were on aspirin and warfarin, respectively.
The strengths of our study include a community-based sample with extensive and rigorous measurement of covariates and long follow-up. Our study has several limitations. First, with a relatively small sample size of 1,172 participants with 113 total stroke events occurring over a nearly 15 year follow up period, it is possible that the study was underpowered to detect an association between metabolic syndrome and stroke. Secondly, incident AF was identified mostly from hospitalization discharges and we could have missed asymptomatic AF or AF managed exclusively in an outpatient setting. However, we and others have previously shown that the validity of AF ascertainment using hospitalizations is acceptable13,22 and that incidence rates of AF in ARIC are consistent with other population-based studies.13,22
Conclusion
In conclusion, our report—from a community based-cohort study with close to 15 years of follow-up—suggests that the metabolic syndrome is not significantly associated with ischemic stroke in AF after adjustment for CHA2DS2-VASc variables. Given the contrasting findings between our study and that of Tsai and colleagues, future studies could be pursued with larger sample sizes including individuals of different races and greater numbers of stroke events. In additional, further research is warranted to identify novel risk factors for stroke-related AF.
Supplementary Material
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
Sources of Funding
The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I).
Dr. Chen receives grant support from the National Heart, Lung and Blood Institute (R01HL126637 and R01HL141288). Dr. Alonso was supported by American Heart Association grant 16EIA26410001. Ms. Rooney was supported by the National Heart, Lung and Blood Institute grant T32HL007779.
Footnotes
Conflicts of Interest
None
References:
- 1.Wolf PA, Dawber TR, Thomas HE Jr., Kannel WB. Epidemiologic Assessment of Chronic Atrial Fibrillation and Risk of Stroke: the Framingham Study. Neurology. 1978;28(10):973–977. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Wolf PA, D′Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of Atrial Fibrillation on the Risk of Death: The Framingham Heart Study. Circulation. 1998;98:946–52. [DOI] [PubMed] [Google Scholar]
- 3.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial fibrillation. JAMA. 2001;285(22):2864–2870. [DOI] [PubMed] [Google Scholar]
- 4.Lip GY. Stroke in Atrial Fibrillation: Epidemiology and Thromboprophylaxis. Journal of Thrombosis Haemostasis, 2011;9:344–351. [DOI] [PubMed] [Google Scholar]
- 5.Olesen JB, Torp-Pedersen C, Hansen ML, Lip GY. The Value of the CHA2DS2-VASc Score for Refining Stroke Risk Stratification in Patients with Atrial Fibrillation with a CHADS2 Score 0–1: A Nationwide Cohort Study. Journal of Thrombosis and Haemostasis. 2012;107:1172–9. [DOI] [PubMed] [Google Scholar]
- 6.Ford ES, Mokdad AH, Giles WH, Brown DW. The Metabolic Syndrome and Antioxidant Concentrations: Findings from the Third National Health and Nutrition Examination Survey. Diabetes. 2003;52: 2346–2352. [DOI] [PubMed] [Google Scholar]
- 7.Lau DC, Yan H, Dhillon B. Metabolic Syndrome: A Marker of Patients at High Cardiovascular Risk. Canadian Journal of Cardiology. 2006; 22: pp. 85B–90B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watanabe H, Tanabe N, Watanabe T, Darbar D, Roden DM, Sasaki S, et al. Metabolic Syndrome and Risk of Development of Atrial Fibrillation: the Niigata Preventive Medicine Study. Circulation. 2008; 117:1255–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodriguez-Colon SM, Mo J, Duan Y, Liu J, Caulfield JE, Jin X, et al. Metabolic Syndrome Clusters and the Risk of Incident Stroke: The Atherosclerosis Risk in Communities (ARIC) Study. Stroke. 2009;40:200–205. [DOI] [PubMed] [Google Scholar]
- 10.Tsai CT, Chang SH, Chang SN, Hwang JJ, Wu CK, Wang YC, et al. Additive Effect of the Metabolic Syndrome Score to the Conventional CHADS2 Score for the Thromboembolic Risk Stratification of Patients with Atrial Fibrillation. Heart Rhythm. 2014;11:352–7. [DOI] [PubMed] [Google Scholar]
- 11.The ARIC Investigators. The Atherosclerosis Risk In Communities (ARIC) Study: Design and Objectives. American Journal of Epidemiology. 1989;129:687–702. [PubMed] [Google Scholar]
- 12.Soliman EZ, Prineas RJ, Case LD, Zhang Z, Goff DC. Ethnic Distribution of ECG Predictors of Atrial Fibrillation and its Impact on Understanding the Ethnic Distribution of Ischemic Stroke in the Atherosclerosis Risk in Communities (ARIC) Study. Stroke. 2009;40:1204–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, et al. Incidence of Atrial Fibrillation in Whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 2009;158: 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106:3143–421 [PubMed] [Google Scholar]
- 15.Rosamond WD, Folsom AR, Chambless LE, Wang C, Mcgovern PG, Howard G, et al. Stroke Incidence and Survival Among Middle-Aged Adults. Stroke. 1999;30:736–743. [DOI] [PubMed] [Google Scholar]
- 16.ARIC Manual 3: Surveillance component procedures. https://www2.cscc.unc.edu/aric/sites/default/files/public/manuals/Manual3_Ver%206.6_20151112_0.pdf. (accessed July 1, 2018).
- 17.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 18.Aronow WS, Ahn C, Kronzon I, Gutstein H. Risk Factors for New Thromboembolic Stroke in Patients ≥62 Years of Age with Chronic Atrial Fibrillation. American Journal of Cardiology 1998;82(1):119–121. [DOI] [PubMed] [Google Scholar]
- 19.Pistoia F, Sacco S, Tiseo C, Degan D, Ornello R, Carolei A. The Epidemiology of Atrial Fibrillation and Stroke. Cardiology Clinics. 2016;34(2):255–68. [DOI] [PubMed] [Google Scholar]
- 20.Patti G, Lucerna M, Cavallari I, Ricottini E, Renda G, Pecan L, et al. Insulin-Requiring Versus Noninsulin-Requiring Diabetes and Thromboembolic Risk in Patients with Atrial Fibrillation: PREFER in AF. JACC. 2017;69: 409–19. [DOI] [PubMed] [Google Scholar]
- 21.Pan WH, Yeh WT. Epidemiology of Metabolic Syndrome in Asia. Asia Pacific Journal of Clinical Nutrition. 2008;17(S1):37–42. [PubMed] [Google Scholar]
- 22.Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, et al. Incidence of and Risk Factors for Atrial Fibrillation in Older Adults. Circulation. 1997;96:2455–2461. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.