Abstract
Store-operated Ca2+ entry (SOCE) channels are highly selective Ca2+ channels activated by the endoplasmic reticulum (ER) sensors STIM1 and STIM2. Their direct interaction with the pore-forming plasma membrane ORAI proteins (ORAI1, ORAI2, and ORAI3) leads to sustained Ca2+ fluxes that are critical for many cellular functions. Mutations in the human ORAI1 gene result in immunodeficiency, anhidrotic ectodermal dysplasia, and enamel defects. In our investigation of the role of ORAI proteins in enamel, we identified enamel defects in a patient with an ORAI1 null mutation. Targeted deletion of the Orai1 gene in mice showed enamel defects and reduced SOCE in isolated enamel cells. However, Orai2−/− mice showed normal enamel despite having increased SOCE in the enamel cells. Knockdown experiments in the enamel cell line LS8 suggested that ORAI2 and ORAI3 modulated ORAI1 function, with ORAI1 and ORAI2 being the main contributors to SOCE. ORAI1-deficient LS8 cells showed altered mitochondrial respiration with increased oxygen consumption rate and ATP, which was associated with altered redox status and enhanced ER Ca2+ uptake, likely due to S-glutathionylation of SERCA pumps. Our findings demonstrate an important role of ORAI1 in Ca2+ influx in enamel cells and establish a link between SOCE, mitochondrial function, and redox homeostasis.
INTRODUCTION
Intracellular second messengers such as Ca2+ modulate a wide range of functions in cells (1). In mineralizing cells, of which enamel-forming cells are a primary example, Ca2+ is also critical for the mineralization of crystals. The mechanisms by which Ca2+ enters the enamel cells and the signaling pathways that are modulated by this process have remained poorly understood (2, 3). We have shown that store-operated Ca2+ entry (SOCE) through the Ca2+ release–activated Ca2+ (CRAC) channels is important for Ca2+ uptake in enamel cells (2, 4–6). CRAC channels are formed by the stromal interaction molecules STIM1 (and its homolog STIM2), which are found in the endoplasmic reticulum (ER), and the pore subunit of the channel known as ORAI1, which is found in the plasma membrane (7–10). Mutations in human STIM1 or ORAI1 genes cause CRAC channelopathy, including abnormal enamel mineralization (11). This enamel defect can be very severe, leading to near-complete loss of enamel in some children (12), but how this mineralization deficiency is caused remains poorly known.
CRAC channels are present in many types of cells (13–15). STIM1 and STIM2 are single-pass transmembrane proteins that function as ER Ca2+ sensors with a luminal EF-hand Ca2+ binding motif (16, 17). Loss of luminal ER Ca2+ induces conformational changes, including the formation of STIM1 clusters known as puncta (18). STIM1 traps ORAI proteins at the puncta and binds to its C terminus to activate the opening of the ORAI channel, thereby enabling sustained Ca2+ influx (19, 20). Given the dearth of dental samples from patients with STIM1 or ORAI1 mutations, we have developed murine models to study the function of CRAC channels in enamel. We and others reported that Stim1/Stim2-deficient mice show a dental phenotype similar to that observed in human patients with STIM1 mutations (6, 21). However, the role of ORAI proteins in enamel remains poorly understood.
The ORAI protein family contains three homologs (ORAI1, ORAI2, and ORAI3), and all are expressed in enamel cells (4, 22). ORAI proteins are relatively small (~300 amino acids) and form one of the most highly selective Ca2+ channels known (7). This high Ca2+ selectivity is associated with the properties of the first transmembrane domain, in which Glu106 acts as a selectivity filter (23, 24). All ORAI proteins contain four transmembrane domains (TM1 to TM4) and N and C termini located in the cytosol (25). The first of these TM domains in ORAI1 forms the pore of the channel (23). Although all three ORAI homologs exhibit characteristic features of CRAC channels (namely, high Ca2+ selectivity and low conductance), they have different functional properties including sensitivity to pharmacological inhibitors, inactivation kinetics (25, 26), and redox sensitivity (27, 28). ORAI1 remains the best characterized ORAI homolog and is the predominant channel that mediates SOCE in most cells (25). The crystal structure of Drosophila Orai has revealed a hexameric assembly of channel subunits (29), which is assumed to be the case for mammalian ORAI channels as well. Most studies have investigated channels formed by homomers of individual ORAI homologs. Overexpression studies have highlighted the possibility of ORAI1:ORAI3 heteromeric channels (27, 30), and we have reported using gene-targeted mice that endogenous ORAI1 and ORAI2 can form heteromeric channels (31). Whereas combined deletion of both genes abolishes SOCE in T cells and other murine immune cells, individual deletion of Orai1 and Orai2 reduces and enhances SOCE, respectively, suggesting that ORAI2 forms heteromeric channel with ORAI1 and attenuates its function. Similar observations have been made in mast cells (32).
Given that ORAI1 mutations can severely affect enamel (2, 11) and how little is known about the role of ORAI proteins in enamel, we investigated the dental phenotype of a patient with a mutation in ORAI1 and characterized Orai-deficient mice. Moreover, considering that Orai2 and Orai3 genes are also expressed in rodent enamel cells (4, 22), we investigated their roles in enamel using short hairpin RNA (shRNA) knockdowns of each Orai gene in the murine enamel cell line LS8 cells. We found that SOCE was reduced, but not absent, in primary enamel cells of Orai1-deficient mice and their enamel was abnormal. Orai2-deficient mice showed increased SOCE in the enamel cells, which did not result in obvious enamel defects. The differential effects of Orai1 and Orai2 deletion on SOCE in dental enamel cells were also apparent in the expression of enamel-specific genes. Lack of ORAI1 in enamel cells was associated with increased mitochondrial respiration, adenosine 5′-triphosphate (ATP) production, and changes in redox homeostasis that boosted the activity of the sarco-endoplasmic reticulum Ca2+–adenosine triphosphatase (ATPase) (SERCA) pump. These data provide evidence in favor of a dominant role for ORAI1, but not for ORAI2, in enamel cells and establish a link between Ca2+ influx through ORAI1 channels, mitochondrial function, and redox in enamel cells.
RESULTS
A human null mutation in ORAI1 causes localized enamel defects
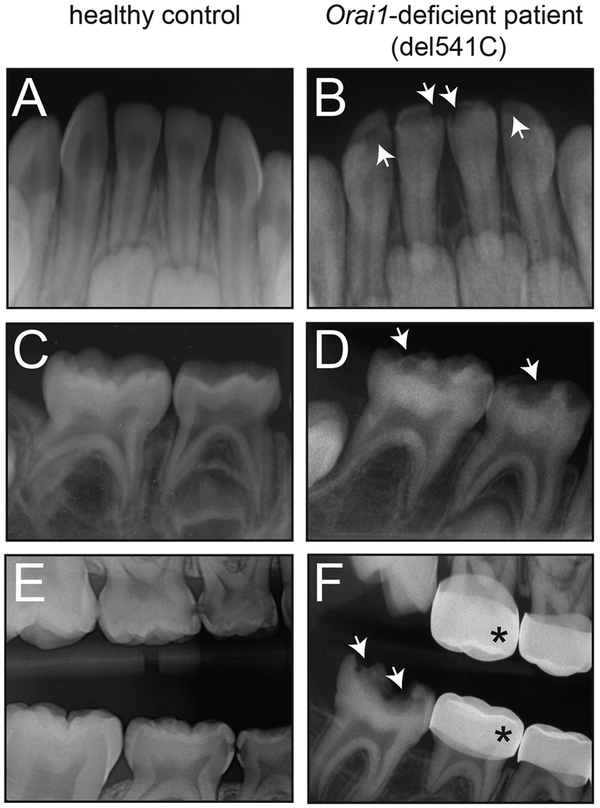
We investigated the tooth defects in a male patient born to consanguineous parents. This patient has a complex medical history that includes combined immunodeficiency (CID), ectodermal dysplasia with anhidrosis (EDA), and muscular hypotonia typical of CRAC channelopathy. The patient was reported to be homozygous for a null mutation in ORAI1 (del541C) that abolishes ORAI1 expression and SOCE (33). Compared to an age-matched healthy control (Fig. 1, A to C), at 8 months of age, the patient showed “pitted” enamel hypoplasia of the primary mandibular central incisors and hypomineralization of primary maxillary incisors (Fig. 1D). At around age 6, the patient returned presenting with hypoplasia of the primary molars, severe early childhood caries, and multiple dental abscesses (Fig. 1E). The patient required comprehensive dental treatment in the operating room under general anesthesia (Fig. 1F). At age 8, the patient presented with permanent molar incisor hypomineralization (MIH) with post-eruptive breakdown (PEB) and had to undergo extensive dental surgery for a second time. MIH with PEB is a condition that results from a major disturbance during tooth development. Because the patient’s teeth were actively developing and undergoing major mitotic activity at the time of the bone marrow transplantation and chemotherapy he received to cure his CID, it is possible that his treatment was responsible for an epigenetic or localized systemic insult at the time. Arguing against this etiology, however, is the fact that the hypoplasia and reduced radiolucency of the enamel of the primary mandibular incisors and molars, which begin forming in utero, were initially noted before the initiation of bone marrow transplantation or chemotherapy. The clinical presentation of this patient strongly suggests that the localized enamel defects on specific primary and permanent teeth are not caused by a post-eruptive insult but are due to a developmental defect.
Fig. 1. Amelogenesis imperfecta in a patient with del541C null mutation in the ORAI1 gene.
(A and B) Dental radiographs of an ~8-month-old healthy control compared with a patient homozygous for a del541C null mutation in ORAI1 (D and E) of similar age. ORAI1 null mutation was associated with “pitted” enamel with hypoplasia of the primary central and lateral incisors (D) and the primary mandibular molars (E). The pitted areas are indicated by white arrows. (C and F) Dental radiograph of the same patient at ~6 years of age (F) and an age-matched healthy control (C). Arrows indicate hypoplasia and associated dental caries of the mandibular permanent molar. The crowns of the primary molar teeth were covered with stainless steel crowns as indicated by asterisks.
SOCE is decreased in Orai1K14 but elevated in Orai2−/− murine enamel cells
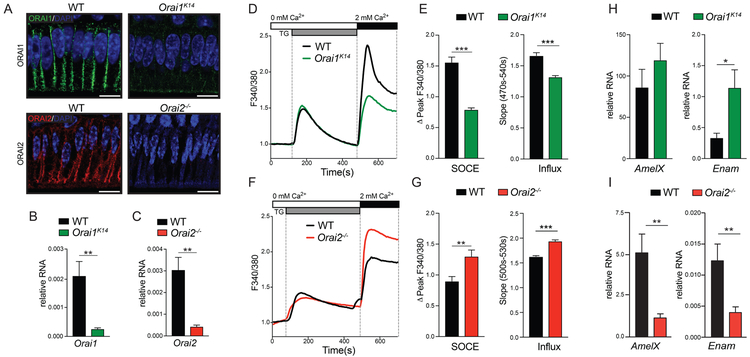
Because teeth of patients with ORAI1 mutations are not available for study, we analyzed the role of ORAI proteins in enamel in murine models with conditional deletion of Orai1 in ectodermal derived tissues using Orai1fl/fl K14-Cre (Orai1K14) mice and complete deletion of Orai2 (Orai2−/−) (31, 34). Immunofluorescence and reverse transcription polymerase chain reaction (RT-PCR) analysis revealed decreased expression of Orai1 and Orai2 in enamel cells of Orai1K14 and Orai2−/− mice, as expected (Fig. 2, A to C). We next isolated enamel organ (EO) cells of Orai1K14 and Orai2−/− mice to assess SOCE. EO cells were stimulated with thapsigargin to passively deplete Ca2+ from the ER by blocking SERCA pumps, which prevents SERCA from refilling the ER with Ca2+ and thus induces activation of STIM proteins and SOCE after the readdition of extracellular Ca2+ (6). Using this protocol, we measured a ~50% reduction of SOCE in primary EO cells of Orai1K14 mice and found that the rate of Ca2+ influx also significantly decreased (Fig. 2, D and E). By contrast, EO cells of Orai2−/− mice showed a ~30% increase in SOCE as well as an increased Ca2+ influx rate (Fig. 2, F and G). These findings suggest that ORAI1 is the main channel mediating SOCE in EO cells, whereas ORAI2 likely attenuates SOCE.
Fig. 2. Abnormal SOCE in primary ameloblasts from ORAI1- and ORAI2-deficient mice.
(A) Immunofluorescence staining for ORAI1 and ORAI2 of ameloblasts from WT, Orai1K14, and Orai2−/− mice. Nuclear staining by 4′,6-diamidino-2-phenylindole (DAPI) is shown in blue. Images are representative of n = 3 mice for each genotype. Scale bars, 20 μm. (B and C) Analysis of (B) Orai1 and (C) Orai2 gene expression in ameloblast cells isolated from Orai1K14 and Orai2−/− mice, respectively. Data represent the mean ± SEM of three independent experiments with ameloblasts obtained from five to seven mice for each genotype. (D to G) Measurements of [Ca2+]i (shown as F340/F380 ratio) in ameloblasts of WT, Orai1K14 (D and E), and Orai2−/− (F and G) mice after thapsigargin (TG) stimulation. The peaks of F340/F380 corrected for baseline levels (ΔPeak F340/F380) and the slope are quantified in (E) and (G). Data represent the mean ± SEM of three independent experiments, with ameloblasts obtained from four to five mice for each genotype. (H and I) RT-PCR analysis of enamel gene expression in ameloblasts of Orai1K14 (H) and Orai2−/− (I) mice. Data represent the mean ± SEM of three independent experiments, with ameloblasts obtained from five mice for each genotype. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 using unpaired Welch’s t test (B and H), Mann-Whitney test (C, E, and G), or Student’s t test (I).
ORAI1 deficiency alters enamel gene expression but does not cause ER stress
The correct development of enamel relies on the expression of a limited number of enamel matrix proteins (EMPs), which interact with the enamel crystals to guide and participate in the assembly of crystal growth (35). The EMPs amelogenin, ameloblastin, and enamelin are coded by the AmelX, Ambn, and Enam genes, respectively. To assess the possible effects of altered Ca2+ influx on the expression of these genes, we analyzed them in EO cells of Orai1K14 and Orai2−/− mice by RT-PCR. The expression of AmelX was slightly increased, and Enam was significantly increased in EO cells of Orai1K14 mice (Fig. 2H). By contrast, the expression of both AmelX and Enam was significantly decreased in enamel cells of Orai2−/− mice (Fig. 2I). No differences were detected in Ambn in either Orai1K14 (fig. S1A) or Orai2−/− (fig. S1B) mice. These data suggest that SOCE plays an important role in regulating gene expression in enamel genes. We have previously identified increased ER stress in Stim1/2-deficient enamel cells (6). We analyzed the expression of the ER stress marker Grp78 but found no differences in its expression in EO cells of Orai1K14 or Orai2−/− mice compared to control mice (fig. S2).
Orai1K14, but not Orai2−/−, mice have altered enamel structure
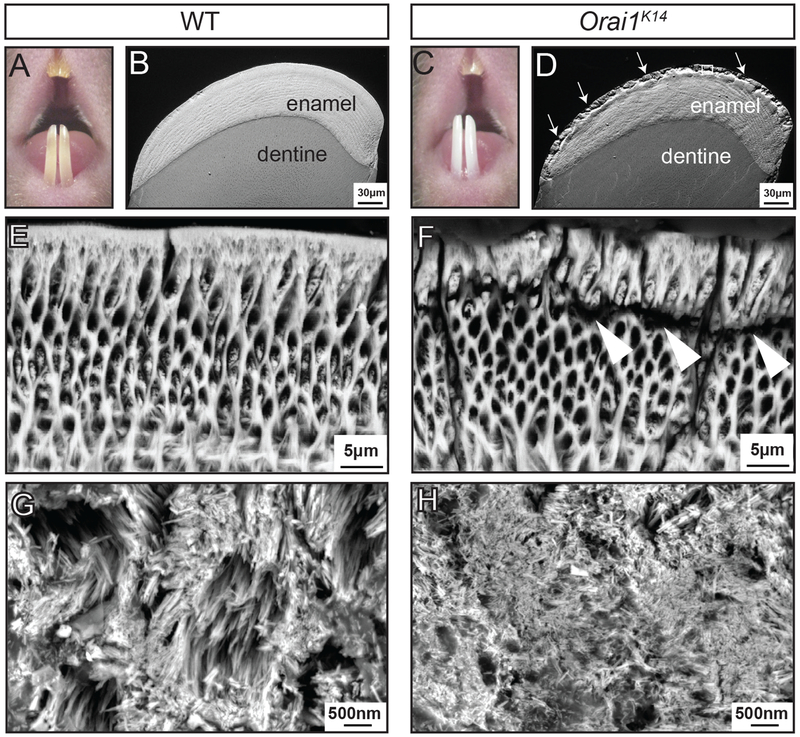
To further investigate how changes in intracellular Ca2+ levels in enamel cells of Orai1K14 or Orai2−/− mice affected the formation of their enamel, we analyzed the lower incisor enamel by scanning electron microscopy, including scanning electron microscopy in the backscattered electron (BSE) mode and in field-emission scanning electron microscopy (FE-SEM). BSE provides a gross assessment of mineralization differences between samples, and FE-SEM provides increased visual resolution. Sectioned and polished cross sections of the lower incisors of Orai1K14 mice imaged by scanning electron microscopy showed that the bulk of the tissue formed normally, as evidenced by the development of the rod-interrod patterning and normal thickness of the enamel layer (Fig. 3, A to D). BSE analyses did not show gross differences in mineralization between genotypes (Fig. 3, B and D). However, the outer enamel of Orai1K14 mice showed visible structural alterations when compared to age-matched wild-type (WT) littermates (Fig. 3, B and D). After acid etching the samples to increase topographic detail, cracks in the enamel separating the bulk of the enamel from the last formed outer (aprismatic) enamel layer were visualized (Fig. 3, E and F). Analysis of the enamel crystals by FE-SEM showed that Orai1K14 mice had smaller-sized crystals compared to control mice (Fig. 3, G and H). By contrast, gross examination of incisor enamel and cross-sectional analyses of tooth enamel did not show structural or mineralization differences between WT and Orai2−/− mice (fig. S3, A to D), suggesting that elevated Ca2+ influx did not severely disrupt enamel development or function.
Fig. 3. Conditional deletion of Orai1 in murine ameloblast cells causes enamel defects.
(A to D) Visual examination of teeth using a stereomicroscope reveals differences in the enamel of the incisors of WT (A) and Orai1K14 (C) mice. BSE-SEM micrographs of incisor cross sections showing enamel and underlying dentine. Images were taken 1 mm from the incisor tip of WT (B) and Orai1K14 (D) mice. (E and F) High-magnification micrographs of WT (E) and Orai1K14 (F) enamel after acid etching. (G and H) FE-SEM micrographs of WT (G) and Orai1K14 (H) enamel crystals. Images shown in (A) to (H) are representative of n = 4 mice for each genotype.
Deletion of individual ORAI homologs in LS8 enamel cells has variable effects on SOCE
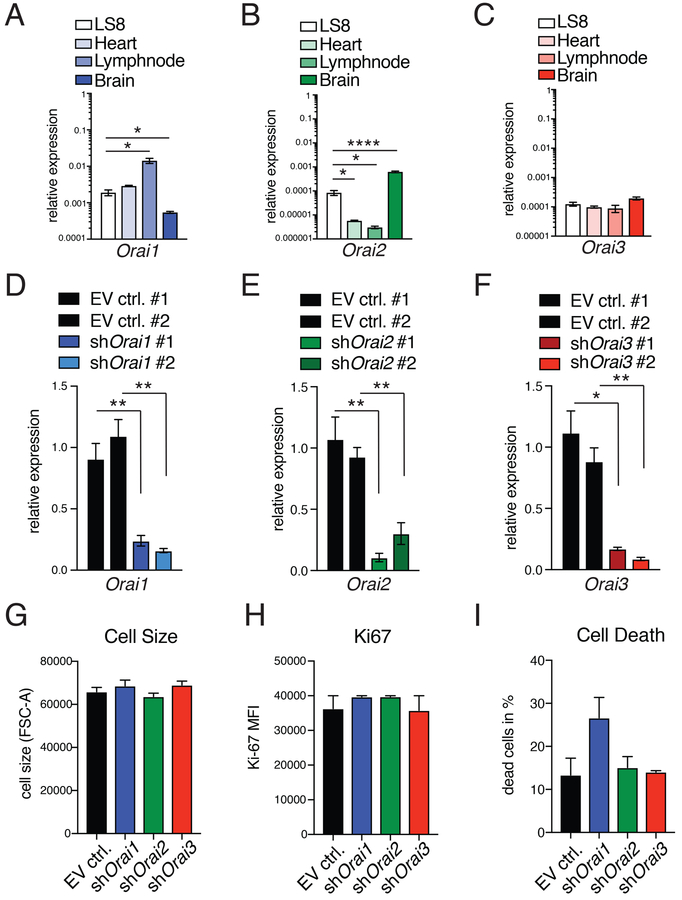
The mammalian ORAI protein family consists of ORAI1, ORAI2, and ORAI3 homologs. Given the difference in SOCE observed in primary enamel cells of Orai1- and Orai2-deficient mice and to determine how SOCE affects the function of enamel cells, we acutely deleted individual ORAI homologs in a murine enamel cell line. Among the limited number of enamel cell lines available (36), murine LS8 cells are one of the most widely used in enamel research (37, 38). Moreover, we have reported previously that LS8 cells express all three ORAI isoforms and are a good model to study SOCE (22). Relative to its expression in control tissues, Orai1 mRNA expression in LS8 cells was higher than that in brain and comparable to its expression in heart (Fig. 4A). Orai2 mRNA expression in LS8 cells was higher than that in heart or lymph nodes but significantly lower than in brain, whereas Orai3 mRNA expression in LS8 cells was comparable to that in other tissues (Fig. 4, B and C).
Fig. 4. Deletion of Orai1, Orai2, and Orai3 expression in the LS8 ameloblast cell line.
(A to C) Expression pattern of Orai1 (A), Orai2 (B), and Orai3 (C) in LS8 cells compared to other murine tissues, including heart, lymph node, and brain measured by RT-PCR. Data in (A) to (C) represent averages (±SEM) of a minimum of three independent experiments. (D to F) RT-PCR analyses of Orai1, Orai2, and Orai3 deletion in LS8 cells after transduction with empty vector (EV ctrl.) or two independent shRNAs each against Orai1, Orai2, and Orai3. Data represent averages (±SEM) of a minimum of three independent experiments. (G to I) Evaluation of effects of Orai1, Orai2, and Orai3 deletion on cell size measured by flow cytometry (FSC-A) (G), proliferation measured using Ki67 staining by flow cytometry (H), and cell death measured using annexin V staining by flow cytometry (I). MFI, mean fluorescence intensity. Data in (G) to (I) represent four independent experiments (mean ± SEM). For all panels, *P < 0.05, **P < 0.01, ****P < 0.0001 using unpaired Welch’s t test [(A) and (B) with LS8 versus heart and LS8 compared to lymph node, (D) with EV ctrl. #2 compared to shOrai1 #2 (F)] or an unpaired Student’s t test (E).
To analyze the individual contribution of each ORAI homolog to SOCE in these cells, we retrovirally transduced LS8 cells with green fluorescent protein (GFP)–expressing plasmids encoding for different shRNAs specific for each of the Orai genes (referred to here as shOrai1, shOrai2, and shOrai3). Transduction efficiency was analyzed 48 hours after transduction using flow cytometry. Puromycin-selected LS8 GFP+ cells were 100% for each control (fig. S4, A and B) and >99% GFP+ for each of the shOrai knockdowns (fig. S4, C to H). RT-PCR verified the down-regulation of Orai1, Orai2, or Orai3 in each of the shRNA-transduced cell lines (Fig. 4, D to F), and we also verified ORAI1 down-regulation by immunofluorescence compared to controls (fig. S5A) in shOrai1 cells (fig. S5B) and quantitated in fig. S5 (C and D). The expression of Orai1 was unchanged in shOrai2 cells (fig. S6). To determine whether Orai knockdown had an effect on LS8 enamel cells, we analyzed cell size, proliferation, and death by flow cytometry. No significant differences were found in any of these parameters (Fig. 4, G to I, and fig. S7, A to D).
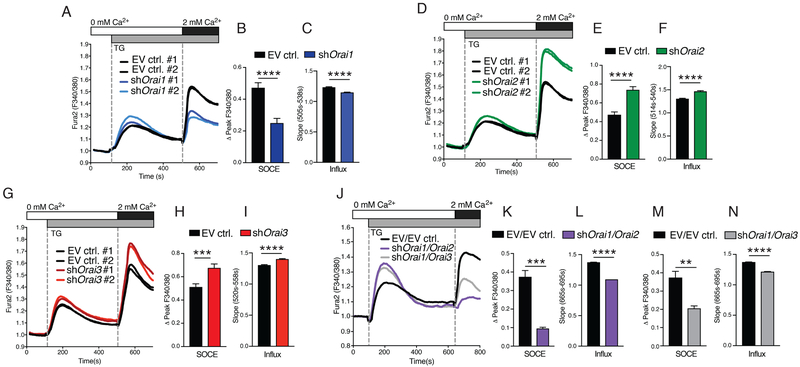
We next tested the effects of deleting individual ORAI homologs on SOCE in LS8 cells. Thapsigargin stimulation of cells lacking ORAI1 resulted in a ~50% reduction of SOCE and a concomitant reduction in the rate of Ca2+ influx (Fig. 5, A to C), in line with results obtained in primary EO cells. By contrast, SOCE was elevated by ~55% in LS8 cells lacking ORAI2 (Fig. 5, D to F) and by ~30% in ORAI3-deficient LS8 cells (Fig. 5, G and H). These results were similar using either of the shRNAs for each gene. The rate of Ca2+ influx of shOrai2 and shOrai3 cells was faster than in control cells (Fig. 5, F and I). Together, these data indicate that all ORAI subunits contribute to SOCE in enamel cells, raising the possibility that they form heteromeric channels.
Fig. 5. SOCE is mediated by ORAI1 and ORAI2 in LS8 cells.
(A to F) [Ca2+]i measurements of LS8 cells transduced with empty vector (EV ctrl.) and either shOrai1 (A to C), shOrai2 (D to F), or shOrai3 (G to I). SOCE was analyzed after thapsigargin stimulation and readdition of 2 mM Ca2+. Quantification of SOCE corrected for baseline Ca2+ levels as ΔPeak F340/F380 and pooling of values from both shRNAs (B, E, and H). All [Ca2+]i measurements were done using a FlexStation 3 plate reader. (J to M) [Ca2+]i measurements in LS8 cells twice transduced with empty vector control plasmids, both shOrai1 and shOrai2, or both shOrai1 and shOrai3. Quantification of peak and rate of SOCE levels was corrected for baseline Ca2+ levels (ΔPeak F340/F380) in shOrai1/shOrai2 (K and L) and shOrai1/shOrai3 (M and N) cells. Ca2+ measurements were performed as described in (A). All data represent three independent experiments (mean ± SEM). **P < 0.01, ***P < 0.001, ****P < 0.0001 using Mann-Whitney test (B, C, F, I, K, and L) or unpaired Student’s t test (E and H).
Combined deletion of ORAI1 and ORAI2 abolishes SOCE and implicates both proteins as critical CRAC channel subunits in enamel cells
To further investigate the possibility that different ORAI homologs form heteromeric complexes in enamel cells, we analyzed SOCE in LS8 cells deficient in ORAI1 and either ORAI2 or ORAI3. Stimulation of ORAI1/ORAI2-deficient cells with thapsigargin resulted in near-complete loss of SOCE (Fig. 5, J to L), whereas combined deletion of ORAI1/ORAI3 resulted in significantly reduced SOCE to a degree that was similar to the deletion of ORAI1 alone (Fig. 5, M and N). To address whether the residual SOCE observed in shOrai1/Orai2 cells after the readdition of external Ca2+ was the result of plasma membrane leaks, we switched the external solutions from 0 to 2 mM Ca2+ in cells without previous depletion of ER stores. This resulted in a small increase in [Ca2+]i of similar magnitude to that observed in the thapsigargin-stimulated shOrai1/Orai2 cells (fig. S8, A and B). In the presence of the CRAC channel blocker GSK-7975A, this increase in [Ca2+]i in shOrai1/Orai2 cells was not significantly different (fig. S8, C and D), suggesting that this residual influx is attributable to an unknown plasma membrane leak with little or no contribution from ORAI3. Together, these data indicate that SOCE in LS8 cells is mediated mainly by ORAI1 and ORAI2, which synergize by potentially forming heteromeric channel complexes as previously reported for T cells (31).
ORAI1-deficient cells have increased mitochondrial cellular respiration
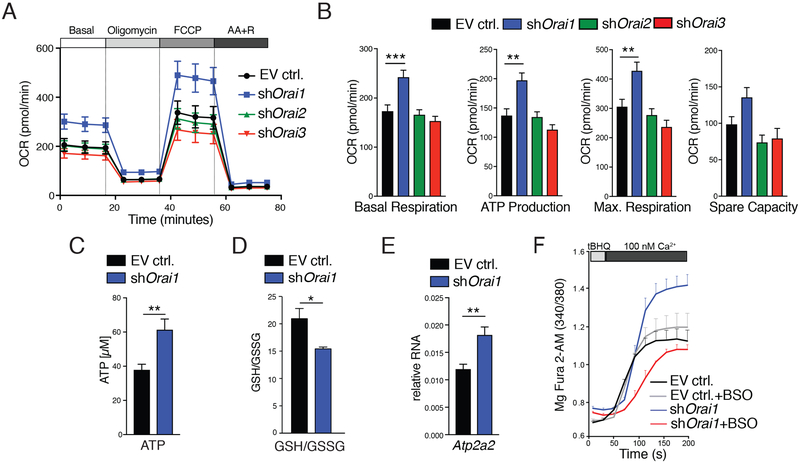
We have previously shown that inhibition of SOCE alters mitochondrial morphology and enhances mitochondrial reactive oxygen species (ROS) levels in unstimulated enamel cells from Stim1/2-deficient mice (6). We therefore evaluated whether ORAI-deficient LS8 cells also had altered mitochondrial function by the mitochondrial stress test, which measures oxygen consumption rate, an indicator of mitochondrial respiration (39). ORAI1-deficient LS8 cells had increased basal mitochondrial respiration compared to ORAI2-and ORAI3-deficient or mock-transduced cells (Fig. 6, A and B). Maximal respiration and ATP production were also increased in the absence of ORAI1 compared to cells lacking ORAI2 or ORAI3 (Fig. 6B). We further confirmed increased ATP levels in ORAI1-deficient cells compared to mock-transduced control cells using an independent luminescence-based method (Fig. 6C). In addition to enhanced mitochondrial respiration and ATP production, ORAI1-deficient LS8 cells also had an increased extracellular acidification rate presumably because of enhanced glycolysis and lactate production (fig. S9, A and B). These data indicate that ORAI1-deficient enamel cells are metabolically more active.
Fig. 6. Increased mitochondrial function and Ca2+ reuptake into the ER in ORAI1-deficient LS8 cells.
(A) Analysis of oxygen consumption rates (OCRs) in LS8 cells transduced with empty vector (EV ctrl.) or shRNAs against Orai1, Orai2, and Orai3 using a Seahorse flux analyzer. FCCP, carbonyl cyanide p-trifluoromethoxyphenylhydrazone; AA, antimycin A; R, rotenone. (B) Quantification of basal respiration, ATP production, maximal respiration, and spare respiratory capacity from experiments in (A). Data in (A) and (B) represent four independent experiments. (C) ATP measurements in LS8 cells transduced with empty vector or shOrai1. Luminescence intensities were measured using a FlexStation 3 plate reader. Data represent the mean ± SEM of five independent experiments. (D) Measurement of the GSH:GSSG ratio in empty vector and shOrai1 cells. Data represent the mean ± SEM of three independent experiments. (E) Relative mRNA expression of Atp2a2 (SERCA2) in control and shOrai1-transduced LS8 cells. Data represent the mean ± SEM of five independent experiments. (F) The refilling rate of ER Ca2+ was measured in Mag-Fura-2–loaded empty vector and shOrai1 cells after permeabilization. ER stores were depleted with the reversible SERCA inhibitor tBHQ in a solution containing 100 nM Ca2+ for 3 min without tBHQ to allow Ca2+ refilling (see also fig. S7). SERCA activity was also measured in cells pretreated with BSO to inhibit GSH synthesis (gray and red tracings) (see also fig. S9). Data represent averages (±SEM) of n = 3 independent experiments. **P < 0.01, ***P < 0.001 using Mann-Whitney test (B), Welch’s t test (C), or unpaired Student’s t test (E).
ORAI1-deficient cells have an altered redox potential that promotes S-glutathionylation of SERCA
Abnormal mitochondrial function may alter the redox state of cells. An important determinant of the redox state in cells is the ratio of reduced glutathione (GSH) to its oxidized form (GSSG) (40, 41), and changes in the GSH:GSSG ratio affect cell signaling (42, 43). ORAI1-deficient cells had a significantly lower GSH:GSSG ratio than control LS8 cells (Fig. 6D), indicating increased oxidative stress. S-glutathionylation, the coupling of GSH to exposed cysteines in proteins, prevents irreversible oxidation and is also a key posttranslational modification, altering protein function and cell signaling (43, 44). A principal target of S-glutathionylation is the SERCA pump (45), which transports cytosolic Ca2+ into the ER lumen to maintain ER Ca2+ homeostasis. Under intracellular oxidizing conditions, SERCA is S-glutathionylated, a modification that increases its activity (46). In ORAI1-deficient cells, the expression of SERCA2 (encoded by Atp2a) was significantly up-regulated compared to control cells (Fig. 6E). We reasoned that increased SERCA2 expression and SERCA S-glutathionylation in the presence of increased GSSG levels may result in enhanced SERCA activity and Ca2+ uptake into the ER. We measured ER Ca2+ levels in permeabilized LS8 cells transduced with EV control or shOrai1 using the ER Ca2+ indicator Mag-Fura-2. Passive depletion of ER Ca2+ stores using the reversible SERCA inhibitor 2,5-di-(tert-butyl)-1,4-benzohydroquinone (tBHQ) (47) triggered faster refilling in ORAI1-deficient cells than in control cells (Fig. 6F and fig. S10). To test whether enhanced store refilling was mediated by S-glutathionylation of SERCA, we inhibited GSH synthesis with l-buthionine sulfoximine (BSO) to prevent S-glutathionylation (48, 49). BSO-treated ORAI1-deficient cells showed a decrease in ER refilling kinetics compared to untreated ORAI1-deficient cells (Fig. 6F and fig. S10). BSO-treated and untreated control cells showed no statistically significant differences. Collectively, these data indicate that, in the absence of ORAI1, the intracellular environment becomes more oxidizing, which promotes S-glutathionylation of SERCA pumps and more efficient refilling of ER Ca2+ stores.
DISCUSSION
Patients with null mutations in ORAI1 suffer from EDA. Symptoms of EDA include amelogenesis imperfecta type III (11). Dental samples from these patients have been unavailable for structural analyses, and descriptions of their tooth defects have been limited to oral photographs of their teeth. The x-ray images of a patient with an ORAI1 null mutation (del541C) we report here indicate that his enamel defects on specific primary and permanent teeth are not caused by a multifactorial post-eruptive insult but rather are due to a disruption during the period of enamel formation causing hypomineralization (Fig. 1, A to F). However, this patient differs from other reported cases of ORAI1 deficiency (33, 50) in that he lacked a generalized dental defect. In genetic conditions such as amelogenesis imperfecta, there is either a generalized hypoplasia or hypomineralization in most teeth. This did not appear to be the case here because there was a mix of both hypomineralization and hypoplasia, and the defects were localized to particular areas of the dentition. The phenotype described here suggests that the effects of the del541C ORAI1 mutation cause a less severe enamel phenotype.
The structural and functional consequences of ORAI1 deficiency for enamel development are not well understood because of a lack of available patient tissues to study. Furthermore, the role of other ORAI homologs, namely, ORAI2 and ORAI3, in enamel development is unknown. We therefore used mice with conditional deletion of Orai1 (Orai1K14) and Orai2 null mice (Orai2−/−) to investigate how these genes regulate enamel development. Loss of ORAI1 decreased SOCE by ~50% in primary enamel cells (Fig. 2D), whereas deletion of ORAI2 increased SOCE in enamel cells by ~30% (Fig. 2F). These data are consistent with similar changes in SOCE in murine T cells and other immune cells that lack Orai1 or Orai2 expression (31). We also sought to determine whether changes in [Ca2+]i had downstream effects on genes that are important for enamel formation. We have previously shown that the activation of SOCE in LS8 cells induces increased expression of AmelX, Enam, and Ambn, whereas inhibiting SOCE with CRAC channel blockers in vitro results in decreased expression of these genes (22). Thus, we had anticipated that loss of SOCE in primary enamel cells would also affect the expression of enamel genes. Surprisingly, in our previous analyses of completely SOCE-deficient enamel cells from Stim1/Stim2K14 mice, we had found a nonsignificant decrease in the expression of these enamel genes (6). To better understand the link between SOCE and enamel gene expression, we analyzed these genes in Orai1- and Orai2-deficient primary enamel cells. Decreased SOCE in Orai1-deficient cells resulted in an overall increase in AmelX and Enam, whereas the opposite effect occurred in Orai2-deficient cells, which showed increased SOCE (Fig. 2, H and I). These results indicate that SOCE regulates enamel gene expression, but its effects appear to be complex. Whereas complete deletion of SOCE in the absence of STIM1 and STIM2 moderately decreases enamel gene expression, partial reduction of SOCE in ORAI1-deficient cells enhanced expression and a partial increase of SOCE in ORAI2-deficient enamel cells suppressed expression. For the highly expressed AmelX gene, we have previously found that a critical transcriptional activator, CEBPα, is unaffected by changes in SOCE in vitro (22). Therefore, the transcriptional factors controlling enamel gene expression downstream of SOCE remain elusive. Nonetheless, emerging data indicate that SOCE regulates other genes besides AmelX and Enam to regulate enamel development, including those that encode the exchanger SLC24A4 (6), which is critically involved in Ca2+ extrusion in ameloblasts (51, 52) and SERCA2 (6).
An important but complex role of [Ca2+]i in enamel formation was further evidenced by the structural defects in the dental enamel of ORAI1- and ORAI2-deficient mice. Whereas ORAI2 deficiency and the resulting [Ca2+]i increase did not alter enamel formation (fig. S3, C and D), loss of Orai1 expression and the concomitant decrease in [Ca2+]i affected enamel and crystal formation. The outer enamel layer of ORAI1-deficient mice was cracked (Fig. 3D), and the size of the formed enamel crystals appeared smaller than that of WT mice after etching (Fig. 3H). Thus, a decrease in SOCE negatively affects enamel formation, which is consistent with the severe enamel defects we had observed in Stim1/Stim2K14 mice (6). The more severe enamel defect in Stim1/Stim2K14 compared to Orai1K14 mice is likely due to abolished SOCE in the former (6), whereas SOCE was only partially decreased in ORAI1-deficient ameloblasts. The altered enamel of Orai1K14 mice with elevated expression of the enamel genes resembles some aspects of the enamel phenotype of amelogenin knock-in mice (53). Knock-in mice for M180, the principal splice form of amelogenin, do not show the altered enamel architecture (rod/interred patterning) seen in Orai1K14 mice, but they have structural alterations caused by altered mechanical properties (53). The smaller size of crystals after acid etching and the cracking of the outer enamel of Orai1K14 mice are consistent with a degree of structural abnormalities in the enamel of these mice resulting from reduced SOCE.
Each ORAI protein can function as a Ca2+ channel when overexpressed, although the biophysical properties differ between ORAI1, ORAI2, and ORAI3 (26, 54, 55). Overexpressed ORAI proteins can form heteromeric channels with potentially different properties compared to homomeric channels. We have reported evidence for the existence of endogenous heteromeric channels formed by ORAI1:ORAI2 in T cells and other immune cells in which ORAI2 acts as an inhibitor of Ca2+ influx through ORAI1 (31). Similar observations have been made in mast cells of ORAI2-deficient mice (32). We here report reduced SOCE in ORAI1-deficient enamel cells and increased SOCE in ORAI2-deficient cells (Fig. 5, A and D). Combined deletion of ORAI1 and ORAI2 (but not ORAI1 and ORAI3) almost completely abolished SOCE. Together, these data indicate that both ORAI1 and ORAI2 are required to mediate SOCE in enamel cells. In the absence of ORAI1, residual SOCE is mediated by ORAI2. In the absence of ORAI2, however, SOCE is mediated by homomeric ORAI1 channels, which conduct Ca2+ more efficiently than mixed ORAI1:ORAI2 channels. As discussed previously for T cells, we conclude that ORAI2 acts as an inhibitor of ORAI1 (31).
The potential role of ORAI3 in enamel development in vivo could not be assessed because Orai3−/− mice were not available. To further analyze the role of ORAI homologs in Ca2+ signaling in enamel cells, we compared the effects of ORAI1, ORAI2, and ORAI3 deletion in LS8 enamel cells. Similar to our findings in primary enamel cells of Orai1K14 and Orai2−/− mice, deletion of ORAI1 in LS8 cells reduced SOCE (~50%), whereas deletion of ORAI2 enhanced SOCE by ~55% (Fig. 5, A and D). Combined deletion of ORAI1 and ORAI2 in LS8 cells abolished SOCE, suggesting that both homologs are required for CRAC channel function. Surprisingly, deletion of ORAI3 also resulted in increased SOCE by ~30% (Fig. 5G), contrasting with previous reports in which Orai3 deletion does not change or mildly reduces SOCE (56–58). RNA interference (RNAi)–mediated deletion of ORAI3 in human embryonic kidney (HEK) 293 cells, Jurkat T cells (57), and human aortic smooth muscle cells (58, 59) does not affect SOCE (57), but in myoblasts and neuronal cells, ORAI3 deletion reduces SOCE (56, 60). Our data showing increased SOCE in ORAI3-deficient enamel cells suggest that ORAI3, like ORAI2, may act as an inhibitor of ORAI1, although this role may be specific for enamel cells. Combined deletion of ORAI1 and ORAI3 in LS8 cells reduced SOCE to levels similar to those seen with ORAI1 deletion alone, suggesting that ORAI1 and ORAI3 do not synergize to modulate SOCE and that ORAI2 homomers alone can mediate a limited influx of Ca2+. By contrast, small interfering RNA (siRNA)–mediated knockdown of ORAI1 and ORAI3 in murine neuronal cells abolishes SOCE (56). Our data suggest that, in contrast to other cell types, ORAI3 can act as an inhibitor of ORAI1. Overall, however, ORAI3 appears to play a lesser role in enamel cells compared to ORAI1 and ORAI2, which are the main channel subunits that store-operated Ca2+ entry in enamel cells. In addition, ER Ca2+ release was increased in both shOrai1/Orai2 and shOrai1/Orai3 cells relative to control cells, an effect that may be related to a previously reported role of ORAI2 in preventing Ca2+ overload of the ER (61).
We have previously shown that SOCE affects mitochondrial function in unstimulated enamel cells lacking Stim1 and Stim2 (6). Because the role of mitochondria in enamel cells is poorly understood, we investigated the impact of ORAI1 deletion and reduced SOCE on mitochondrial function. ORAI1-deficient enamel cells were more metabolically active and showed increased basal, maximal respiration and ATP production compared to WT control, ORAI2-deficient, or ORAI3-deficient cells (Fig. 6, A and B). We reasoned that the greater mitochondrial respiration of ORAI1-deficient enamel cells indicated an increased demand for ATP, for instance, for ATP-dependent Ca2+ mobilization through SERCA pumps that are abundant in enamel cells. SERCA transports cytosolic Ca2+ into the ER lumen (two Ca2+ per ATP) to replenish the loss of Ca2+ associated with the opening of the ER Ca2+ channels IP3R or RyR or through ER leak channels (62). We found that the expression of Atp2a2, which encodes SERCA2, was increased in ORAI1-deficient cells, consistent with similar findings in STIM1/2-deficient cells (6). In keeping with increased SERCA2 expression, we also found that the rate of refilling the ER with Ca2+ was faster in enamel cells lacking ORAI1 than in control cells (Fig. 6F). Because SERCA2 activity increases oxidizing conditions through S-glutathionylation (46), we further investigated this possibility in shOrai1 cells.
The tripeptide GSH is the main intracellular thiol antioxidant, but GSH can also form mixed disulfide bonds with “redox-sensitive” cysteines in proteins, a phenomenon known as protein S-glutathionylation (63, 64). The reversibility of protein glutathionylation makes this posttranslational modification a molecular mechanism by which GSH acts as a redox-dependent signaling molecule (64, 65). We thus tested whether ORAI1-deficient enamel cells had altered redox by measuring the GSH:GSSG ratio, a key indicator of oxidative stress (40). The GSH:GSSG ratio was lower in ORAI1-deficient cells (Fig. 6D), demonstrating that unstimulated shOrai1 cells experienced more oxidizing intracellular conditions. These data on the GSH:GSSG ratio of ORAI1-deficient cells are also consistent with increased ROS that we have reported previously in STIM1/2-deficient enamel cells (6). To further investigate whether SERCA is S-glutathionylated, we measured ER refilling kinetics in shOrai1 cells, in which GSH synthesis was inhibited with BSO. We found that Ca2+ refilling of the ER by SERCA was decreased in GSH-depleted shOrai1 cells compared to untreated shOrai1 cells. These data indicate that enhancement of SERCA activity in shOrai cells is dependent on GSH and that depleting GSH prevents S-glutathionylation. These data also suggest that, in cells lacking ORAI1, the enhanced activity of SERCA is promoted by intracellular oxidizing conditions. This finding adds to ongoing work on the effects of oxidation and S-glutathionylation in CRAC channel function, with at least one previous study reporting on the S-glutathionylation of STIM1 (66).
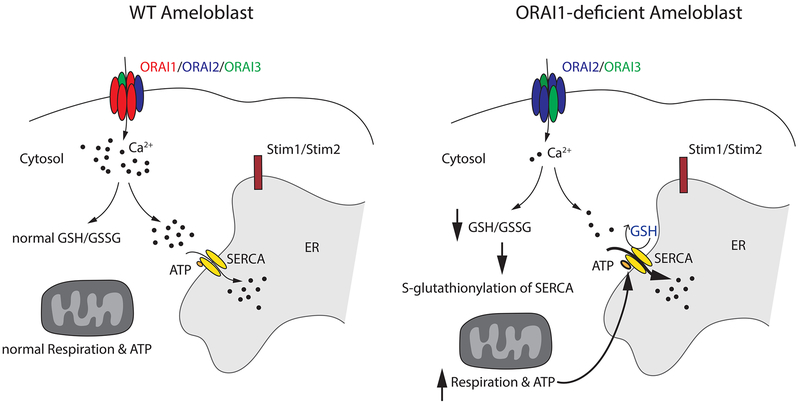
The picture that emerges from data presented here suggests that SOCE deficiency not only alters mitochondrial function but also alters redox homeostasis of enamel cells promoting posttranslational modifications of SERCA by S-glutathionylation and thus affecting Ca2+ homeostasis (Fig. 7). In summary, we showed that loss of SOCE by deletion of ORAI1 expression in a human patient and mice resulted in defective enamel mineralization. By contrast, ORAI2 deletion in mice did not affect enamel formation despite increased SOCE in ORAI2-deficient enamel cells. Deletion of the three ORAI homologs in enamel cells either alone or in combination showed that ORAI1 is the dominant ORAI family member responsible for SOCE in enamel cells and that ORAI2 modulates its function, potentially by forming heteromeric ORAI1:ORAI2 channel complexes. In the absence of ORAI1, mitochondrial respiration was increased. Lack of ORAI1 also promoted changes in redox homeostasis that boosted SERCA activity, which required increased ATP. Our findings provide new mechanistic insights into the role of ORAI channels and SOCE in enamel formation and the causes of amelogenesis imperfecta in ORAI1-deficient patients.
Fig. 7. Schematic representation of the effects of ORAI1 deficiency in enamel cells.
In WT ameloblasts, ORAI1, the predominant subunit, and, to some extent, also ORAI2 and ORAI3 modulate Ca2+ influx. Under normal redox conditions, these cells have physiological levels of GSH and GSSG, normal mitochondrial respiration, and normal ATP production; also, S-glutathionylation is not activated. In enamel cells lacking ORAI1, there is a marked reduction in Ca2+ influx. Residual Ca2+ influx is likely mediated by ORAI2 and possibly ORAI3 subunits. In these conditions, the GSH/GSSG ratio decreases, indicative of altered redox status, specifically increased oxidizing conditions. This might be associated with the observed increase in mitochondrial respiration and elevated ATP production. Changes in redox also affect the activity of SERCA through S-glutathionylation, stimulating its pumping action.
MATERIALS AND METHODS
Mice
Mice were generated as described previously (31, 67, 68). Briefly, Orai1fl/fl were crossed to K14-Cre (Jackson Laboratory, strain 004782) to generate a conditional mouse strain with deletion of Orai1 in ectoderm-derived cells, including ameloblasts (referred to as Orai1K14). Orai2−/− mice were generated using VGB6 embryonic stem cells (C57BL/6NTac) obtained from the Knockout Mouse Project (KOMP; www.komp.org) repository at University of California, Davis [project ID VG14962, Orai2tm1(KOMP)Vlcg]. All mice were maintained on a C57BL/6 genetic background and used between 6 and 16 weeks of age unless otherwise indicated. All animal procedures were conducted in accordance with the guidelines approved by the Institutional Animal Care and Use Committee of New York University Langone Medical Center and NYU College of Dentistry (protocol no. 140108–02).
Cell culture
Primary EO cells were isolated from the lower incisors of Orai1K14 and Orai2−/− mice, as described (6, 22). EO cells were digested with Liberase (0.25 mg/ml; Roche) for 30 min at 37°C, washed in Hanks’ balanced salt solution, and plated onto Cell-Tak (Corning)–coated coverslips in X-VIVO 15 medium (Lonza) supplemented with 10% fetal bovine serum (FBS), 1% penicillin/streptomycin, and 1% glutamine. Isolated EO cells were used within 24 hours after dissection. LS8 cells are an immortalized murine-derived enamel cell line (37) that have been used widely to study various aspects of enamel development and enamel gene expression (38) and are a good model to study Ca2+ dynamics in the context of enamel formation as they express SOCE channels (22). LS8 cells were maintained in standard cell culture conditions as described (37) and routinely tested for contamination.
Retroviral transduction of LS8 cells
shRNAs against Orai1, Orai2, Orai3, or Renilla (as negative control) were cloned into the shRNA scaffold of the pLMP-mirE11 retroviral backbone (expressing GFP and puromycin resistance) (shRNA sequences shown in table S1). Retroviral particles were produced by the Platinum-E retroviral packaging cell line [American Type Culture Collection (ATCC)]. Platinum-E cells were transfected by lipofection (GeneJET, Thermo Fisher Scientific) with pLMP or pLMPd retroviral constructs together with the amphotrophic packaging vector pCL-10A1. Two days after transfection, the supernatant was collected over 3 days and concentrated using Amicon Ultra-15 centrifugal filters (Merck Millipore). Subconfluent LS8 cells grown in 12-well plates were transduced by spin-infection (2500 rpm, 30°C, 90 min) with concentrated retroviral supernatant and polybrene (10 μg/ml) (Santa Cruz Biotechnology). Six hours after spin-infection, viral supernatant was removed from the LS8 cells and replaced by fresh medium. Transduction efficiency was analyzed 48 hours after transduction and after selection with puromycin (5 μg/ml) (Invitrogen) using flow cytometry. Puromycin-selected LS8 cells were typically >98% GFP+, and Orai1–3 knockdown was verified by quantitative RT-PCR (qRT-PCR). For the retroviral transduction of LS8 cells targeting Orai1/Orai2 and Orai1/Orai3, shRNAs against Orai1 or Renilla (as negative control) were cloned into the shRNA scaffold of the modified pLMPd-mirE11 vector (which expresses Ametrine and lacks a resistance gene). Retroviral particles were produced by the Platinum-E retroviral packaging cell line (ATCC). Platinum-E cells were transfected by lipofection (GeneJET, Thermo Fisher Scientific) with pLMP or pLMPd retroviral constructs together with the amphotrophic packaging vector pCL-10A1. Two days after transfection, the supernatant was collected over 3 days and concentrated using Amicon Ultra-15 centrifugal filters (Merck Millipore). Subconfluent LS8 cells, which were previously transduced with pLMP vectors (puromycin resistance/GFP positive) with shRNA targeting Orai1, Orai2, and Orai3, grown in 12-well plates were supertransduced by spin-infection (2500 rpm, 30°C, 90 min) with concentrated retroviral supernatant and polybrene (10 μg/ml) (Santa Cruz Biotechnology). Six hours after spin-infection, viral supernatant was removed from the LS8 cells and replaced by fresh medium. Transduction efficiency was analyzed 48 hours after transduction and after selection with puromycin (5 μg/ml) (Invivogen) using flow cytometry. Puromycin-selected LS8 cells were typically >99% GFP+, and Ametrine+, Orai1, Orai2, and Orai3 knockdown was verified by qRT-PCR.
Cell size, cell death, and proliferation rate of single shLS8 cells
To characterize the transduced LS8 cells, we analyzed cell size, cell death, and proliferation rate through flow cytometry. Analysis of cell size was performed by acquiring 20,000 cells using the forward scatter (FSC-A). Cell cycle analysis was performed by staining the cells with an anti-Ki67 phycoerythrin (PE) antibody before acquiring fluorescence intensities in the PE channel. Ki67 is expressed in all phases of cell cycle but G0; therefore, it is a suitable marker for cell proliferation. For cell viability, we used the APC Annexin Apoptosis Detection Kit with Propidium Iodide (PI) (BioLegend) and staining was performed according to the manufacturer’s protocol. Fluorescence intensity was analyzed using the allophycocyanin (APC) (annexin V, which detects apoptotic cells) compared to the PE (PI, which detects dead cells) channel. All flow cytometry analyses were acquired on an LSRII flow cytometer using FACSDiva software (BD Biosciences) and further analyzed with FlowJo (Tree Star).
[Ca2+]i measurements
Measurements of [Ca2+]i of single EO cells and LS8 cells were performed as described (6, 22). Briefly, single cells were plated overnight on a round microscope cover glass in X-VIVO 15 medium supplemented with 10% FBS. Cells were loaded with 1 μM Fura-2 AM (Invitrogen) for 20 min at room temperature and washed in Ca2+-free Ringer solution [155 mM NaCl, 4.5 mM KCl, 3 mM MgCl2, 5 mM Na-Hepes, and 10 mM d-glucose (pH 7.4)]. To increase cell purity in primary EO cells, we labeled fibroblasts using a PE-conjugated anti-CD90 antibody (1:500 dilution; BioLegend) and excluded the latter from further analysis. In EO and LS8 cells, ER store depletion was stimulated with either 1.25 μM thapsigargin (Sigma) or 0.5 μM ionomycin in Ca2+-free Ringer solution, followed by readdition of 2 mM extracellular Ca2+ Ringer solution to stimulate Ca2+ flux by SOCE. Fluorescence intensities at 510 nm were recorded every 5 s after excitation at 340 and 380 nm using a Nikon 2000 U Eclipse microscope or a FlexStation 3 plate reader. The ratio of F340 and F380 values correlating with [Ca2+]i was calculated and graphed.
Immunofluorescence
Orai1K14 and Orai2−/− mice were perfused with 4% paraformaldehyde, and mandibles were isolated, decalcified (10% EDTA) for 2 weeks, and embedded in paraffin to obtain thin sections (5 μm thick). Immunofluorescence staining was performed using an antigen retrieval step as described (69). The following primary antibodies (all rabbit-raised) were incubated at 4°C overnight: polyclonal rabbit anti-ORAI1 (1:75; S.F., New York University Medical Center) and monoclonal rabbit anti-ORAI2 [1:75; Abcam; clone EPR10043(2)]. The next day, sections were washed twice for 5 min each with phosphate-buffered saline before incubation for 1 hour with biotin-labeled anti-rabbit immunoglobulin G (1:500 dilution; Vector Laboratories) and washing and incubation with streptavidin Alexa Fluor 488 (1:800 dilution; Life Technologies). Samples were embedded using Fluoromount mounting medium (Novus) containing DAPI (Thermo Fisher Scientific). Images were obtained using a Nikon Eclipse 2000TE or a Leica TCS SP5 II confocal microscope and edited using Icy BioImage analysis.
Real-time PCR
Total RNA was isolated using the RNeasy Micro Kit (Qiagen) as indicated by the manufacturer followed by reverse transcription using the iScript cDNA Synthesis Kit (Bio-Rad Laboratories). For qRT-PCR, we used the SsoAdvanced Universal SYBR Green qPCR Supermix (Bio-Rad) and performed the experiments in a CFX Connect thermocycler (Bio-Rad). Gapdh functioned as a housekeeping gene in both LS8 and primary mouse EO cells. All primers were used at 0.25 nM. Relative quantification of gene expression was determined by the 2–ΔΔCT method. All primers used in this study can be found in table S1.
Scanning electron microscopy
Mandibles of 6- to 8-week-old WT and Orai1K14 and Orai2−/− mice were extracted and cleaned of soft tissues, dehydrated at 4°C in ethanol series, and embedded in polymethyl methacrylate (PMMA) resin, and the erupted incisor was cut with a circular diamond saw about 2 to 3 mm from the tip and was polished. Detailed protocols were previously reported (6). BSE imaging was performed to assess mineralization differences in a Zeiss EVO-50 microscope without a conductive coating in the following settings: 50 Pa, 15 kV, and 200 pA. WT and corresponding Orai1K14 and Orai2−/− samples were analyzed in parallel, setting contrast and brightness arbitrarily to the range of WT samples. To determine microstructural differences, samples were acid-etched (phosphoric acid, 37% for 5 s), rinsed in deionized water, and imaged in BSE. FE-BSE imaging was conducted in a Zeiss Gemini 300 in nano-VP mode using BSD1 detectors at a 7.4-mm working distance. A total of three Orai1K14 and three Orai2−/− mice were analyzed plus a corresponding three WT mice per group.
Mitochondrial respiration
We used the Mitochondrial Stress Test Kit (Agilent) to analyze mitochondrial oxygen consumption in LS8 cells following the manufacturer’s instructions. The mitochondrial stress test reveals four parameters: basal respiration, ATP turnover, maximal respiration, and spare respiratory capacity [the capacity of a cell to generate ATP through oxidative phosphorylation in response to increased demand for energy (39)]. To assess each function, a number of drugs were sequentially used (39): oligomycin, an ATP synthase inhibitor; FCCP, a mitochondrial uncoupler; and rotenone and antimycin A, which are inhibitors of the electron transport chain complex I and III, respectively. Respiratory reserve is the difference between the maximal and the basal respiration rates. In brief, shLS8 cells were seeded 24 hours ahead in an XFe24-well microplate (Agilent) at 2500 cells per well in complete Dulbecco’s modified Eagle’s medium (10% FBS, 1% penicillin/streptomycin, and 1% glutamine). In parallel, a cartridge plate was hydrated with 1 ml of XF Calibrant (Agilent) per well and kept overnight in a non-CO2 incubator. The following day, cells were washed several times with XF Base medium (Agilent) containing 1 mM Na-pyruvate, 2 mM l-glutamine, and 10 mM glucose at pH 7.4. Each well was refilled with exactly 500 μl of complete XF base medium, and cells were equilibrated for 1 hour in a non-CO2 incubator. Oligomycin, FCCP, and rotenone/antimycin A (Agilent) were serially added in a Seahorse XFe24 analyzer. All compounds were prepared in the desired stock solution and loaded into the compound plate. Cell plate and compound plate were loaded into a Seahorse XFe analyzer, and OCR was analyzed. After the run, protein content of each well was analyzed by bicinchoninic acid (BCA) and data were normalized before analyzing basal respiration, ATP production, maximal respiration, and respiratory reserve.
ATP determination
ATP was quantified by using a luciferase-based kit (Molecular Probes). Briefly, cells were permeabilized with cold methanol for 15 min at 4°C and then loaded with the standard reaction solution (Molecular Probes) containing the firefly luciferase and d-luciferin. Oligomycin (25 μM), a selective inhibitor of F0/F1-ATPase, and ATP (50 μM) were used as positive controls 15 min before the permeabilization. Luminescence was measured in a FlexStation 3 plate reader (Molecular Devices), and the integration time of the luminometer was set at 1000 ms with normal gain. A total of five independent samples per cell type were analyzed.
GSH/GSSG determination
The GSH/GSSG was quantified by using a luciferase-based kit (GSH/GSSG-Glo Assay, Promega), following the manufacturer’s instruction. This assay was performed on empty vector and shOrai1 LS8 cells in the presence and absence of H2O2 (500 μM, 15 min) as positive control. The luminescence signal was recorded with a SpectraMax 5 plate reader (Molecular Devices), and the integration time of the luminometer was set at 500 ms with normal gain. A minimum of three independent experiments was performed.
SERCA refilling
Cells were plated on 25-mm optical borosilicate poly-l-lysine–coated sterile cover glasses (Sigma) at 80% confluence and loaded with 1 μM Mag-Fura-2 AM in Ringer solution for 60 min at 37°C. For permeabilization, cells were perfused (5 ml/min) with 2 mM Ca2+ Ringer solution for 1 min followed by perfusion with 0.01 saponin for 1 min in intracellular-like medium containing 100 nM Ca2+ and 5 μM of the reversible SERCA inhibitor tBHQ. The solution was then switched to intracellular-like medium without saponin for 3 min to allow ER Ca2+ refilling. The intracellular-like medium contained 130 mM KCl, 1 mM KH2PO4, 1 mM MgCl2, 1 mM ATP, 5 mM Na-succinate, 5 mM Na-pyruvate, 20 mM Na-Hepes (pH 7.0), and Ca2+ buffered at 100 nM (with titrated 0.6 mM EGTA and EGTA-Ca2+ solutions). Fluorescence intensities were recorded as indicated above. For GSH inhibition, cells were pretreated with 2 mM BSO for 2 hours before cell permeabilization.
Statistics
All statistical analyses were done using Prism7 (GraphPad Software) and confirmed using SAS (SAS Institute). Tests for normality (Shapiro-Wilk test) and equal variance (Brown-Forsythe test) were done before performing group comparisons using two-tailed un-paired Student’s (with Welch’s correction in the event of unequal variance) or nonparametric Mann-Whitney tests. Regression analysis was done using Pearson correlation. Differences with P values of <0.05 were considered significant: *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Supplementary Material
Fig. S1. Unchanged expression of Ambn in ORAI-deficient cells.
Fig. S2. Enamel cells of Orai1K14 mice do not show increased expression of an unfolded protein response marker.
Fig. S3. Normal dental phenotype in ORAI2-deficient mice.
Fig. S4. Transduction efficiency in LS8 cells.
Fig. S5. Immunofluorescence analysis showing down-regulation of ORAI1 in GFP-positive shOrai1 cells.
Fig. S6. Orai1 expression is not significantly altered in shOrai2 cells.
Fig. S7. Apoptosis and cell death.
Fig. S8. A small Ca2+ leak is present in shOrai1/Orai2 cells.
Fig. S9. Increased extracellular acidification rate in shOrai1 cells.
Fig. S10. Quantification of velocity (slope) of ER Ca2+ refilling.
Fig. S10. Quantification of velocity (slope) of ER Ca2+ refilling.
Table S1. Primers and shRNA sequences used in this study.
Acknowledgments:
We thank N. Demaurex, B. Niemeyer, M. Muniswamy, and D. Townsend for previous discussions and M. Snead for the use of LS8 cells.
Funding: This work was funded by the National Institute of Dental and Craniofacial Research (NIH/NIDCR) awards to R.S.L. (DE025639 and DE027679) and by NIH grants to S.F. (AI097302, AI130143, and AI107448).
Footnotes
Competing interests: S.F. is a cofounder of CalciMedica. The other authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper or the Supplementary Materials. The mouse strains described in this study are available from S.F. and require a material transfer agreement.
SUPPLEMENTARY MATERIALS
REFERENCES AND NOTES
- 1.Berridge MJ, Lipp P, Bootman MD, The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol 1, 11–21 (2000). [DOI] [PubMed] [Google Scholar]
- 2.Nurbaeva MK, Eckstein M, Feske S, Lacruz RS, Ca2+ transport and signalling in enamel cells. J. Physiol 595, 3015–3039 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hubbard MJ, Calcium transport across the dental enamel epithelium. Crit. Rev. Oral Biol. Med 11, 437–466 (2000). [DOI] [PubMed] [Google Scholar]
- 4.Nurbaeva MK, Eckstein M, Concepcion AR, Smith CE, Srikanth S, Paine ML, Gwack Y, Hubbard MJ, Feske S, Lacruz RS, Dental enamel cells express functional SOCE channels. Sci. Rep 5, 15803 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lacruz RS, Enamel: Molecular identity of its transepithelial ion transport system. Cell Calcium 65, 1–7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eckstein M, Vaeth M, Fornai C, Vinu M, Bromage TG, Nurbaeva MK, Sorge JL, Coelho PG, Idaghdour Y, Feske S, Lacruz RS, Store-operated Ca2+ entry controls ameloblast cell function and enamel development. JCI Insight 2, e91166 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prakriya M, Lewis RS, Store-operated calcium channels. Physiol. Rev 95, 1383–1436 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel S-H, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A, A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 441, 179–185 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Deng X, Zhou Y, Hendron E, Mancarella S, Ritchie MF, Tang XD, Baba Y, Kurosaki T, Mori Y, Soboloff J, Gill DL, STIM protein coupling in the activation of Orai channels. Proc. Natl. Acad. Sci. U.S.A 106, 7391–7396 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gudlur A, Zhou Y, Hogan PG, STIM-ORAI interactions that control the CRAC channel. Curr. Top. Membr 71, 33–58 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Lacruz RS, Feske S, Diseases caused by mutations in ORAI1 and STIM1. Ann. N. Y. Acad. Sci 1356, 45–79 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eckstein M, Aulestia FJ, Nurbaeva MK, Lacruz RS, Altered Ca2+ signaling in enamelopathies. Biochim. Biophys. Acta 1865, 1778–1785 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shim AH-R, Tirado-Lee L, Prakriya M, Structural and functional mechanisms of CRAC channel regulation. J. Mol. Biol 427, 77–93 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feske S, CRAC channelopathies. Pflugers Arch. 460, 417–435 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Putney JW, Steinckwich-Besançon N, Numaga-Tomita T, Davis FM, Desai PN, D’Agostin DM, Wu S, Bird GS, The functions of store-operated calcium channels. Biochim. Biophys. Acta 1864, 900–906 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE Jr., T. Meyer, STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol 15, 1235–1241 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Veliçelebi G, Stauderman KA, STIM1, an essential and conserved component of store-operated Ca2+ channel function. J. Cell Biol 169, 435–445 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park CY, Hoover PJ, Mullins FM, Bachhawat P, Covington ED, Raunser S, Walz T, Garcia KC, Dolmetsch RE, Lewis RS, STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell 136, 876–890 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parekh AB, On the activation mechanism of store-operated calcium channels. Pflügers Arch. 453, 303–311 (2006). [DOI] [PubMed] [Google Scholar]
- 20.Putney JW Jr., New molecular players in capacitative Ca2+ entry. J. Cell Sci 120, 1959–1965 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furukawa Y, Haruyama N, Nikaido M, Nakanishi M, Ryu N, Oh-Hora M, Kuremoto K, Yoshizaki K, Takano Y, Takahashi I, Stim1 regulates enamel mineralization and ameloblast modulation. J. Dent. Res 96, 1422–1429 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Nurbaeva MK, Eckstein M, Snead ML, Feske S, Lacruz RS, Store-operated Ca2+ entry modulates the expression of enamel genes. J. Dent. Res 94, 1471–1477 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeung PS-W, Yamashita M, Prakriya M, Pore opening mechanism of CRAC channels. Cell Calcium 63, 14–19 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoth M, Niemeyer BA, The neglected CRAC proteins: Orai2, Orai3, and STIM2. Curr. Top. Membr 71, 237–271 (2013). [DOI] [PubMed] [Google Scholar]
- 25.Frischauf I, Fahrner M, Jardín I, Romanin C, The STIM1: Orai interaction. Adv. Exp. Med. Biol 898, 25–46 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Lis A, Peinelt C, Beck A, Parvez S, Monteilh-Zoller M, Fleig A, Penner R, CRACM1, CRACM2, and CRACM3 are store-operated Ca2+ channels with distinct functional properties. Curr. Biol 17, 794–800 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shuttleworth TJ, Orai3—The ‘exceptional’ Orai? J. Physiol 590, 241–257 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bogeski I, Kummerow C, Al-Ansary D, Schwarz EC, Koehler R, Kozai D, Takahashi N, Peinelt C, Griesemer D, Bozem M, Mori Y, Hoth M, Niemeyer BA, Differential redox regulation of ORAI ion channels: A mechanism to tune cellular calcium signaling. Sci. Signal 3, ra24 (2010). [DOI] [PubMed] [Google Scholar]
- 29.Hou X, Pedi L, Diver MM, Long SB, Crystal structure of the calcium release-activated calcium channel Orai. Science 338, 1308–1313 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schindl R, Frischauf I, Bergsmann J, Muik M, Derler I, Lackner B, Groschner K, Romanin C, Plasticity in Ca2+ selectivity of Orai1/Orai3 heteromeric channel. Proc. Natl. Acad. Sci. U.S.A 106, 19623–19628 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vaeth M, Yang J, Yamashita M, Zee I, Eckstein M, Knosp C, Kaufmann U, Jani PK, Lacruz RS, Flockerzi V, Kacskovics I, Prakriya M, Feske S, ORAI2 modulates store-operated calcium entry and T cell-mediated immunity. Nat. Commun 8, 14714 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsvilovskyy V, Solís-López A, Schumacher D, Medert R, Roers A, Kriebs U, Freichel M, Deletion of Orai2 augments endogenous CRAC currents and degranulation in mast cells leading to enhanced anaphylaxis. Cell Calcium 71, 24–33 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Lian J, Cuk M, Kahlfuss S, Kozhaya L, Vaeth M, Rieux-Laucat F, Picard C, Benson MJ, Jakovcevic A, Bilic K, Martinac I, Stathopulos P, Kacskovics I, Vraetz T, Speckmann C, Ehl S, Issekutz T, Unutmaz D, Feske S, ORAI1 mutations abolishing store-operated Ca2+ entry cause anhidrotic ectodermal dysplasia with immunodeficiency. J. Allergy Clin. Immunol 142, 1297–1310.e11 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Concepcion AR, Vaeth M, Wagner II LE, Eckstein M, Hecht L, Yang J, Crottes D, Seidl M, Shin HP, Weidinger C, Cameron S, Turvey SE, Issekutz T, Meyts I, Lacruz RS, Cuk M, Yule DI, Feske S, Store-operated Ca2+ entry regulates Ca2+-activated chloride channels and eccrine sweat gland function. J. Clin. Invest 126, 4303–4318 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lacruz RS, Habelitz S, Wright JT, Paine ML, Dental enamel formation and implications for oral health and disease. Physiol. Rev 97, 939–993 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klein O, Duverger O, Shaw W, Lacruz RS, Joester D, Moradian-Oldak J, Pugach MK, Wright JT, Millar SE, Kulkarni AB, Bartlett JD, Diekwisch TGH, DenBesten P, Simmer JP, Meeting report: A hard look at the state of enamel research. Int. J. Oral Sci 9, e3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen LS, Couwenhoven RI, Hsu D, Luo W, Snead ML, Maintenance of amelogenin gene expression by transformed epithelial cells of mouse enamel organ. Arch. Oral Biol 37, 771–778 (1992). [DOI] [PubMed] [Google Scholar]
- 38.Sarkar J, Simanian EJ, Tuggy SY, Bartlett JD, Snead ML, Sugiyama T, Paine ML, Comparison of two mouse ameloblast-like cell lines for enamel-specific gene expression. Front. Physiol 5, 277 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pelletier M, Billingham LK, Ramaswamy M, Siegel RM, Extracellular flux analysis to monitor glycolytic rates and mitochondrial oxygen consumption. Methods Enzymol. 542, 125–149 (2014). [DOI] [PubMed] [Google Scholar]
- 40.Rebrin I, Sohal RS, Pro-oxidant shift in glutathione redox state during aging. Adv. Drug Deliv. Rev 60, 1545–1552 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiong Y, Uys JD, Tew KD, Townsend DM, S-glutathionylation: From molecular mechanisms to health outcomes. Antioxid. Redox Signal 15, 233–270 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghezzi P, Regulation of protein function by glutathionylation. Free Radic. Res 39, 573–580 (2005). [DOI] [PubMed] [Google Scholar]
- 43.Holmstrom KM, Finkel T, Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol 15, 411–421 (2014). [DOI] [PubMed] [Google Scholar]
- 44.Dalle-Donne I, Rossi R, Colombo G, Giustarini D, Milzani A, Protein S-glutathionylation: A regulatory device from bacteria to humans. Trends Biochem. Sci 34, 85–96 (2009). [DOI] [PubMed] [Google Scholar]
- 45.Townsend DM, S-glutathionylation: Indicator of cell stress and regulator of the unfolded protein response. Mol. Interv 7, 313–324 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adachi T, Weisbrod RM, Pimentel DR, Ying J, Sharov VS, Schöneich C, Cohen RA, S-glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat. Med 10, 1200–1207 (2004). [DOI] [PubMed] [Google Scholar]
- 47.Aulestia FJ, Redondo PC, Rodríguez-García A, Rosado JA, Salido GM, Alonso MT, García-Sancho J, Two distinct calcium pools in the endoplasmic reticulum of HEK-293T cells. Biochem. J 435, 227–235 (2011). [DOI] [PubMed] [Google Scholar]
- 48.Grek CL, Townsend DM, Tew KD, The impact of redox and thiol status on the bone marrow: Pharmacological intervention strategies. Pharmacol. Ther 129, 172–184 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grek CL, Zhang J, Manevich Y, Townsend DM, Tew KD, Causes and consequences of cysteine S-glutathionylation. J. Biol. Chem 288, 26497–26504 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCarl C-A, Picard C, Khalil S, Kawasaki T, Röther J, Papolos A, Kutok J, Hivroz C, LeDeist F, Plogmann K, Ehl S, Notheis G, Albert MH, Belohradsky BH, Kirschner J, Rao A, Fischer A, Feske S, ORAI1 deficiency and lack of store-operated Ca2+ entry cause immunodeficiency, myopathy, and ectodermal dysplasia. J. Allergy Clin. Immunol 124, 1311–1318 e7 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang S, Choi M, Richardson AS, Reid BM, Seymen F, Yildirim M, Tuna E, Gençay K, Simmer JP, Hu JC, STIM1 and SLC24A4 are critical for enamel maturation. J. Dent. Res 93, 94S–100S (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu P, Lacruz RS, Smith CE, Smith SM, Kurtz I, Paine ML, Expression of the sodium/calcium/potassium exchanger, NCKX4, in ameloblasts. Cells Tissues Organs 196, 501–509 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Snead ML, Zhu D-H, Lei Y, Luo W, Bringas PO Jr., Sucov HM, Rauth RJ, Paine ML, White SN, A simplified genetic design for mammalian enamel. Biomaterials 32, 3151–3157 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DeHaven WI, Smyth JT, Boyles RR, Putney JW Jr., Calcium inhibition and calcium potentiation of Orai1, Orai2, and Orai3 calcium release-activated calcium channels. J. Biol. Chem 282, 17548–17556 (2007). [DOI] [PubMed] [Google Scholar]
- 55.Mercer JC, DeHaven WI, Smyth JT, Wedel B, Boyles RR, Bird GS, Putney JW Jr., Large store-operated calcium selective currents due to co-expression of Orai1 or Orai2 with the intracellular calcium sensor, Stim1. J. Biol. Chem 281, 24979–24990 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wei D, Mei Y, Xia J, Hu H, Orai1 and Orai3 mediate store-operated calcium entry contributing to neuronal excitability in dorsal root ganglion neurons. Front. Cell. Neurosci 11, 400 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gwack Y, Srikanth S, Feske S, Cruz-Guilloty F, Oh-hora M, Neems DS, Hogan PG, Rao A, Biochemical and functional characterization of Orai proteins. J. Biol. Chem 282, 16232–16243 (2007). [DOI] [PubMed] [Google Scholar]
- 58.Baryshnikov SG, Pulina MV, Zulian A, Linde CI, Golovina VA, Orai1, a critical component of store-operated Ca2+ entry, is functionally associated with Na+/Ca2+ exchanger and plasma membrane Ca2+ pump in proliferating human arterial myocytes. Am. J. Physiol. Cell Physiol 297, C1103–C1112 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bisaillon JM, Motiani RK, Gonzalez-Cobos JC, Potier M, Halligan KE, Alzawahra WF, Barroso M, Singer HA, Jourd’heuil D, Trebak M, Essential role for STIM1/Orai1-mediated calcium influx in PDGF-induced smooth muscle migration. Am. J. Physiol. Cell Physiol 298, C993–C1005 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Darbellay B, Arnaudeau S, König S, Jousset H, Bader C, Demaurex N, Bernheim L, STIM1- and Orai1-dependent store-operated calcium entry regulates human myoblast differentiation. J. Biol. Chem 284, 5370–5380 (2009). [DOI] [PubMed] [Google Scholar]
- 61.Bandara S, Malmersjö S, Meyer T, Regulators of calcium homeostasis identified by inference of kinetic model parameters from live single cells perturbed by siRNA. Sci. Signal 6, ra56 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vandecaetsbeek I, Vangheluwe P, Raeymaekers L, Wuytack F, Vanoevelen J, The Ca2+ pumps of the endoplasmic reticulum and Golgi apparatus. Cold Spring Harb. Perspect. Biol 3, a004184 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ghezzi P, Protein glutathionylation in health and disease. Biochim. Biophys. Acta 1830, 3165–3172 (2013). [DOI] [PubMed] [Google Scholar]
- 64.Fratelli M, Goodwin LO, Ørom UA, Lombardi S, Tonelli R, Mengozzi M, Ghezzi P, Gene expression profiling reveals a signaling role of glutathione in redox regulation. Proc. Natl. Acad. Sci. U.S.A 102, 13998–14003 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Townsend DM, Lushchak VI, Cooper AJL, A comparison of reversible versus irreversible protein glutathionylation. Adv. Cancer Res 122, 177–198 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hawkins BJ, Irrinki KM, Mallilankaraman K, Lien Y-C, Wang Y, Bhanumathy CD, Subbiah R, Ritchie MF, Soboloff J, Baba Y, Kurosaki T, Joseph SK, Gill DL, Madesh M, S-glutathionylation activates STIM1 and alters mitochondrial homeostasis. J. Cell Biol 190, 391–405 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Concepcion AR, Vaeth M, Wagner LE, Eckstein M, Hecht L, Yang J, Crottes D, Seidl M, Shin HP, Weidinger C, Cameron S, Turvey SE, Issekutz T, Meyts I, Lacruz RS, Cuk M, Yule DI, Feske S, Store-operated Ca2+ entry regulates Ca2+-activated chloride channels and eccrine sweat gland function. J. Clin. Invest 126, 4303–4318 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Somasundaram A, Shum AK, McBride HJ, Kessler JA, Feske S, Miller RJ, Prakriya M, Store-operated CRAC channels regulate gene expression and proliferation in neural progenitor cells. J. Neurosci 34, 9107–9123 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vaeth M, Eckstein M, Shaw PJ, Kozhaya L, Yang J, Berberich-Siebelt F, Clancy R, Unutmaz D, Feske S, Store-operated Ca2+ entry in follicular T cells controls humoral immune responses and autoimmunity. Immunity 44, 1350–1364 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Unchanged expression of Ambn in ORAI-deficient cells.
Fig. S2. Enamel cells of Orai1K14 mice do not show increased expression of an unfolded protein response marker.
Fig. S3. Normal dental phenotype in ORAI2-deficient mice.
Fig. S4. Transduction efficiency in LS8 cells.
Fig. S5. Immunofluorescence analysis showing down-regulation of ORAI1 in GFP-positive shOrai1 cells.
Fig. S6. Orai1 expression is not significantly altered in shOrai2 cells.
Fig. S7. Apoptosis and cell death.
Fig. S8. A small Ca2+ leak is present in shOrai1/Orai2 cells.
Fig. S9. Increased extracellular acidification rate in shOrai1 cells.
Fig. S10. Quantification of velocity (slope) of ER Ca2+ refilling.
Fig. S10. Quantification of velocity (slope) of ER Ca2+ refilling.
Table S1. Primers and shRNA sequences used in this study.