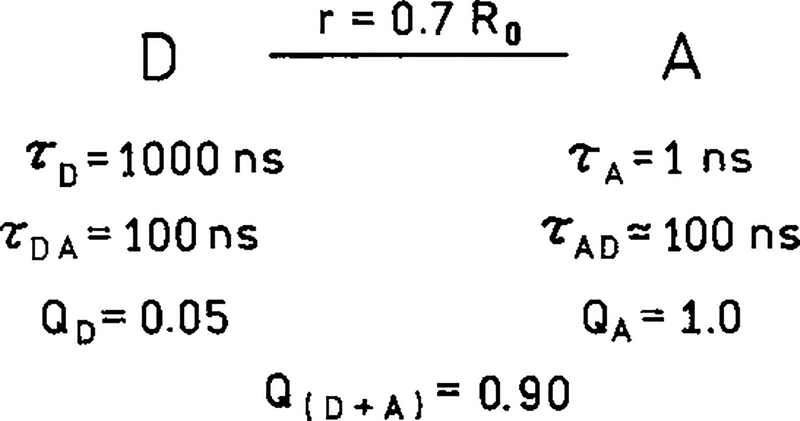Abstract
We describe an approach to creating a new class of luminophores which display both long wavelength emissions exceeding 600 nm and long lifetimes. These luminophores are based on resonance energy transfer (RET) from a long lifetime donor to a short lifetime but long wavelength acceptor. We demonstrated the possibility of obtaining these desirable spectral properties using donors and acceptors noncovalently bound to DNA. The donor was a ruthenium (Ru) metal–ligand complex in which one of the diimine ligands intercalated into double-helix DNA. The acceptors were either nile blue, TOTO-3, or TO-PRO-3. Upon binding of the acceptor to donor-labeled DNA, we found that the acceptor quantum yield was remarkably enhanced so that the wavelength-integrated intensities of the donor and acceptor bound to DNA were many-fold greater than the intensity of the donor and acceptor alone when separately bound to DNA. The origin of this effect is efficient energy transfer from the donor. Under these conditions the effective overall quantum yield approaches that of the acceptor. Importantly, the increased quantum yield can be obtained while maintaining usefully long apparent acceptor lifetimes of 30 to 80 ns. The effect of an increased quantum yield from a low quantum yield donor may find use in assays to detect macromolecular binding interactions. These results suggest the synthesis of covalently linked donor–acceptor pairs with the desirable spectral properties of long wavelength emission, high quantum yield, and moderately long lifetimes for gated detection.
There is considerable interest in the development and use of fluorophores with red or near infrared (NIR)3 emission (1, 2). Long wavelength excitation and emission are valuable because the background autofluorescence from biological samples and optical components typically decreases at longer incident wavelengths. Red and NIR probes are now routinely used in protein labeling (2, 3), chromatography (4, 5), measurements in blood (6, 7), noninvasive medical testing (8–10), and DNA analysis (11–13). An advantage of the presently available red-NIR fluorophores is their high extinction coefficient, which in turn allows high sensitivity detection. However, the high extinction coefficients are also a disadvantage because a high probability for absorption also results in a high rate of emission, which results in short decay times (14). Consequently, most known red-NIR fluorophores display lifetimes below 4 ns, and often below 1 ns. These fluorophores typically display small Stokes’ shifts, and scattered light is most difficult to eliminate at wavelengths close to the excitation wavelength. Also, the autofluorescence from samples is typically on the ns timescale, and the signal-to-background ratio cannot be significantly improved by gated detection after the excitation pulse. Hence, the signals detected with red or NIR probes can be affected by scattered light and/or sample autofluorescence.
In the present paper we report a new approach to obtaining luminophores with long emission maxima, large Stokes’ shifts and high effective quantum yields. These luminophores are typified in Scheme 1, which shows a long lifetime donor (D) which is covalently linked to an acceptor (A), with spectral properties such that resonance energy transfer occurs with moderate to high efficiency. In this case the D-to-A distance is assumed to be 0.7 R0, where R0 is the Förster distance. This separation results in approximately 90% transfer. The donor is assumed to be a luminescent transition metal–ligand complex (MLC). Many such MLCs are known, and they can display a wide range of absorption and emission wavelengths and long decay times ranging from 100 ns to 10 μs (15, 16). In recent years these complexes have been developed for use as luminescent probes (17, 18) for studies of protein dynamics, immunoassays, and chemical sensing (19–24).
SCHEME 1.
A potential long wavelength, long lifetime luminophore based on a long lifetime donor (D) and a short lifetime acceptor (A).
While the MLCs are useful luminophores, they possess several disadvantages. The extinction coefficients are low, typically near 10,000 M−1 cm−1, and the emission spectra are broad. Broad emission spectra result in significant spectral overlap of the emission spectra of various MLCs, and an inability to use measurements at multiple emission wavelengths to resolve multiple species in macroscopic or microscopic samples. Broad emission spectra also reduce sensitivity because the autofluorescence over the wide range of wavelengths contributes to the measured intensity.
We have now realized that most of the disadvantages of the MLCs could be overcome by the use of a tandem probes in which a long lifetime MLC is the donor and a high quantum yield but short lifetime fluorophore is the acceptor (Scheme 1). In such luminophores RET will result in excitation of the acceptor throughout the donor decay. Assume the donor lifetime in the absence of acceptor is τD = 1000 ns. The presence of a single acceptor at a distance of 0.7 R0 will reduce the lifetime to about 100 ns. For the moment assume that the acceptor does not absorb at the donor excitation wavelength . In this case the acceptor is excited solely by RET from the donor. Since the acceptor lifetime is short (τD = 1 ns), the acceptor intensity will closely follow the donor intensity. Hence the acceptor will display the same decay time as the donor and the acceptor decay time (τAD) will be near 100 ns. Most acceptors will display some absorption at the donor excitation wavelength. In this case the acceptor emission will typically display two decay times, a nanosecond component due to directly excited acceptor and long decay time near 100 ns due to RET from the donor. The long lifetime emission acceptor can be readily isolated with gated detection, which is readily accomplished with photomultiplier tubes (PMTs) (25–27). Gated detection is frequently used in immunoassay based on the lanthanides (28, 29).
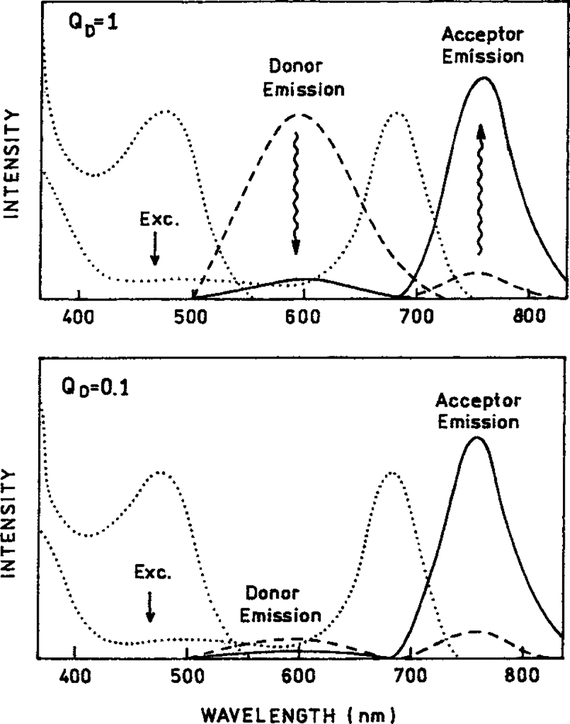
An important advantage of such a RET probe (Scheme 1) is an increase in the effective quantum yield of the long lifetime luminophore. This effect is illustrated in Scheme 2. Suppose the donor and acceptor both display quantum yields of unity (QD = QA = 1.0). In this case (top) RET quenches the donor and results in an equivalent increase in the emission intensity of the acceptor. The integrated or total intensity of the donor and acceptor remains the same in the presence or absence of RET.
SCHEME 2.
Intuitive description of resonance energy transfer from a high quantum yield donor (QD = 1.0, top) and a low quantum yield donor (QD = 0.1, bottom). For both panels ∈A/∈D = 0.1. For the low quantum yield donor RET results in an increase in the overall quantum efficiency of the tandem luminophore.
A surprisingly different result is obtained if the donor displays a low quantum yield. For example, the commonly used ruthenium MLCs have quantum yields of 0.05 or less. In this case the donor emission without RET is much weaker (Scheme 2, bottom). However, RET to a nearby acceptor still results in the same increased intensity of the acceptor. More specifically, the transfer efficiency can approach unity even though the donor quantum yield is low. A favorable result of efficient RET from the donor is that the wavelength integrated intensity of the D–A pair can be almost 20-fold larger than that of the donor or acceptor alone. More specifically, for 100% transfer efficiency, the overall quantum yield becomes the quantum yield of the acceptor. These considerations suggest that tandem RET probes based on MLC donors can be used to create long lifetime probes, with red-NIR emission, with the added advantage of an increased effective quantum yield. Additionally, the modular design of these probes allows practical and rational adjustment of the spectral properties including the excitation and emission wavelengths and the decay times.
Given the simplicity of this idea we examined the literature for suggestions of designing luminophores using low quantum yield donors. There are numerous primary reports and review articles on RET, and the concept of using the acceptor emission to measure the transfer efficiency is not new (30–33). Additionally, Selvin and coworkers have already noted the usefulness of measuring the long lifetime acceptor emission with lanthanide donors to selectively detect D–A pairs (34) and to provide a long decay time for the acceptor (35, 36). Donors and acceptors with short decay times have been covalently linked for use in DNA sequencing (37, 38) and as high-affinity dyes which bind noncovalently to DNA (39, 40). The novelty of the present report is recognition that the effective quantum yield of the luminophore could be increased by rapid energy transfer. Such an increase in effective quantum yield was not important for the biochemical uses of RET (30–33) because most organic donors have good quantum yields. An increased effective quantum yield of the donor has not been important for RET with the lanthanides because the chelated lanthanide donors often display quantum yields near unity. The value of the RET-enhanced quantum yield only becomes apparent because of the development of the long lifetime MLC probes and their low quantum yields. In retrospect, the possibility of increasing the effective quantum yield of the donor was observed by the enhancement of lanthanide emission when bound to essentially nonluminescent DNA or nucleotides (41–43). However, to the best of our knowledge, the practical utility of the RET-enhanced effective quantum yield with low quantum yield donors has not been previously reported.
THEORY
The theory and application of RET have been described in numerous reviews (44–46). Here we discuss those aspects of RET needed to demonstrate the occurrence of the RET enhanced quantum yield. The rate of energy transfer from a donor to an acceptor is given by
| [1] |
where τD is the donor lifetime in the absence of acceptor, r is the donor-to-acceptor distance, and R0 is the Förster distance at which RET is 50% efficient. The value of R0 is dependent on the spectral properties of the donor and acceptor and can be accurately calculated from the spectral properties of the donor and acceptor.
Consider the donor–acceptor pair (Scheme 1) with the hypothetical spectral properties shown in Scheme 2. The donor is assumed to have a lifetime τD = 1000 ns in the absence of RET, and the acceptor a lifetime of τA = 1 ns when directly excited. The efficiency of energy transfer is given by the ratio of the transfer rate to the total rate of donor deactivation, which is the reciprocal of the lifetime in the presence of RET (τDA). Hence the transfer efficiency (E) from the donor is given by
| [2] |
The transfer efficiency as measured using this donor can be determined experimentally from the relative intensities of the donor (ED) in the absence (FD) and presence of acceptor (FDA):
| [3] |
The transfer efficiencies can also be calculated from the donor decay times in the absence (τD) or presence (τDA) of acceptors:
| [4] |
Equation [4] is valid only when the donor decay is a single exponential. For the D–A pair shown in Scheme 2 the donor will display a lifetime (τDA) of 100 ns in the presence of acceptor. This decay time is the reciprocal of the sum of the deactivation rates of the donor,
| [5] |
The acceptor is expected to show two decay times, one due to directly excited acceptors (τA = 1 ns) and one due to RET from the donor (τAD). This long component will have a decay time essentially equivalent to that of the donor (τAD = 100 ns) because the decay time of the acceptor is much shorter than that of the donor. Hence one can obtain a long lifetime component from the acceptor, which if directly excited would display a short lifetime.
It is known that the transfer efficiency can be measured using the intensity of the acceptor emission (30). The transfer efficiency as seen from the acceptor (EA) is given by
| [6] |
where ∈A and ∈D are the molar extinction coefficients of the donor (∈D) and acceptor (∈A) at the excitation wavelength, and FA and FAD are the acceptor intensities in the absence and presence of the donor, respectively. The use of ∈A and ∈D is only appropriate for a one-to-one ratio of donor to acceptor. In the present report the donors and acceptors are not covalently linked and are not present in a one-to-one molar ratio. In this case Eq. [6] becomes
| [7] |
where ODD and ODA refers to the optical density of the donor or acceptor at the donor excitation wavelength.
In the present report we used donors and acceptors noncovalently bound to DNA, so that Eq. [7] is appropriate. However, it is easier to consider a single D–A pair. For a covalently linked D–A pair assume that the acceptor absorbs 10-fold less at the excitation wavelength than the donor, that is, ∈A/∈D = 0.1. This is possible because long-wavelength probes frequently show weak absorption between their long wavelength and short wavelength absorption maxima. Assume additionally that the transfer efficiency is EA = 90%. Substitution of these assumed values into Eq. [6] yields FAD/FD = 10. Hence RET results in a 10-fold enhancement of the acceptor emission (Scheme 2, bottom).
What is the origin of the larger overall quantum yield for the tandem luminophore when the donor quantum yield is low? This can be understood by considering the factors which determine the intensity of the donor and acceptor. One can readily show that the total emission from a tandem luminophore FT = FD + FA is given by
| [8] |
where QD and QA are the quantum yields of the donors and acceptors, respectively, and E is the actual transfer efficiency, and k is a constant containing the excitation intensity, concentration and instrumental factors. This expression assumes the sample is diluted so that the amount of light absorbed is directly proportional to the concentration. In the absence of energy transfer the total intensity is given by
| [9] |
as expected for a mixture of two noninteracting fluorophores. Suppose the transfer efficiency is 100%. In this case the overall quantum yield of the tandem luminophore becomes
| [10] |
This result shows that all the photons absorbed by the donor are transferred to the acceptor, and the effective quantum yield becomes that of the acceptor. Examination of Eq. [8] shows that as the transfer efficiency increases the absorbed energy is shifted from the low quantum yield donor to the high quantum acceptor, irrespective of the quantum yield of the donor in the absence of energy transfer.
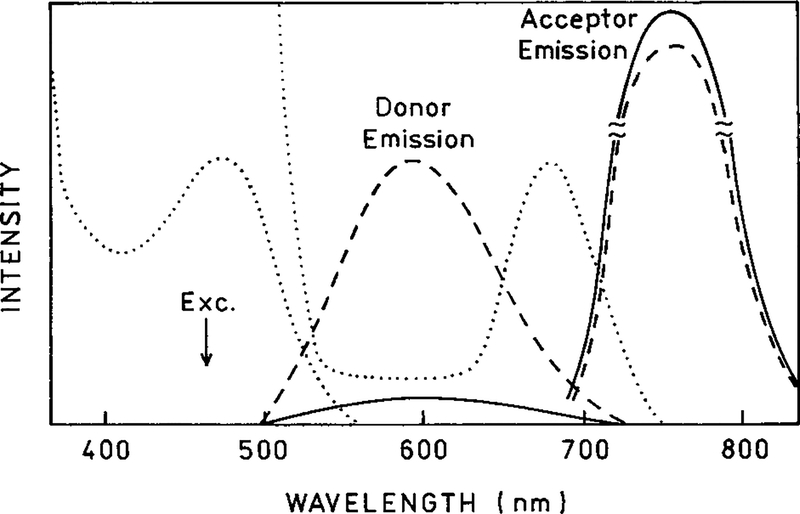
Given the magnitude of this effect one may wonder why such large enhancements of the acceptor emission have not been reported. One explanation is that acceptors frequently absorb as strongly as the donors at the donor excitation wavelengths. For instance, suppose ∈A/∈D = 1.0. In this case a transfer efficiency of 90% results in a 1.9-fold or 90% enhancement of the acceptor emission. A common occurrence is when the acceptor absorbs more strongly than the donor. Suppose ∈A/∈D = 10 (Scheme 3). In this case a transfer efficiency of 90% results in FAD/FA = 1.09, so the acceptor enhancement is only 9% (Eq. [6]). Also, when working with typical organic fluorophores the quantum yields of the donors and acceptors are usually comparable so there is a shift in emission wavelength without an overall increase in the total intensity (Scheme 2, top).
SCHEME 3.
Effect of RET on the acceptor emission when the acceptor absorbs more strongly than the donor.
It is informative to consider the time-dependent decays of the donor and acceptor in the presence of energy transfer. These expressions can be obtained using the known solution for a reversible excited state reaction (47–51) and setting the reverse transfer rate to zero. Assuming that the donor emission and the acceptor emission can be observed separately, the time-dependent changes of the donor and acceptor are then given by
| [11] |
| [12] |
In these expressions αD and βA are the time zero amplitudes of the intensity decays, respectively, and CD and CA are constants which depend on the sample concentration and the experimental apparatus. Also, we have assumed that the donor and directly excited acceptors display a single exponential decay. The pre-exponential factors for the acceptor decay are equal and opposite and are given by
| [13] |
In this expression λA is the radiative decay rate of the acceptor and ΓD and ΓA are the decay rates of the donor in the absence of acceptors, and the directly excited acceptor, respectively. These values are the reciprocals of the decay times in the absence of energy transfer, and .
Suppose the rate of transfer is very rapid (τDA → 0 or kT → ∞). Then the acceptor decay becomes that would be observed if the acceptor were directly excited:
| [14] |
The more interesting result is when the decay time of the donor is much longer than that of the acceptor; that is, τD ⪢ τA. Assuming the acceptor displays a reasonable quantum yield, the acceptor decay becomes
| [15] |
Following decay of the short component with a decay time τA the acceptor decay displays a lifetime equivalent to the donor decay time in the presence of energy transfer,
| [16] |
where C is a constant.
MATERIALS AND METHODS
Materials.
CT-DNA, Tris–HCl, and EDTA were obtained from Sigma (St. Louis, MO). Ru–BD was synthesized by the method described previously (52, 53). AO, EB, TOTO-3, and TO-PRO-3 were purchased from Molecular Probes (Eugene, OR) and NB was from Aldrich (Milwaukee, WI). All reagents were used without further purification and water was deionized with a Milli-Q system. To convert CT-DNA into linear fragments comparable in length to one persistent length, about 5 mg/ml solution of CT-DNA was sonicated approximately 10 min while submerged in an ice bath. The sonicated DNA solution was centrifuged for 1 h at 75,000g to remove titanium particles and undissolved DNA. All experiments were undertaken at room temperature in 2 mM Tris–HCl, pH 8.0, containing 0.1 mM EDTA.
Absorption and steady-state fluorescence measurement.
AO, EB, and Ru-BD served as donors and NB, TOTO-3, and TO-PRO-3 were used as acceptors. About 5–10 mM stock solutions of AO, Ru-BD, and NB were prepared in dimethylformamide (DMF) and about a 10 mM stock solution of EB were made in DMSO. The final DMF concentration in all solutions was less than 1% (v/v). The concentration of DNA was quantified using a molar extinction coefficient of 13,300 M−1 cm−1 (expressed as bp) at 260 nm. The DNA concentration was 1 mM bp while the concentrations of AO, EB, and Ru–BD were 5, 10, and 20 μM, respectively. Concentrations of the probes were determined using the extinction coefficients in Table 1. The highest acceptor concentrations of Ru–BD/NB, Ru–BD/TOTO-3 and Ru–BD/TO PRO-3 D–A pairs were 120, 60, and 120 μM, respectively. Because TOTO-3 and TO-PRO-3 were supplied as 1 mM stock solutions in DMSO, the maximum percentages of DMSO in the Ru–BD/TOTO-3 and Ru–BD/TO-PRO-3 D–A pairs were 6 and 12% (v/v), respectively. In preliminary experiments, we found that DMSO increased the steady-state fluorescence intensity of Ru–BD (data not shown). Hence, we added aliquots of DMSO to obtain 6 and 12% (v/v) DMSO in all Ru–BD/TOTO-3 and Ru–BD/TO-PRO-3 D–A pairs, respectively, to equalize the effect of DMSO. UV–visible absorption spectra were measured with a Hewlett-Packard 8453 diode array spectrophotometer with ±1 nm resolution. Steady-state fluorescence measurements were carried out using an Aminco SLM AB2 spectrofluorometer (Spectronic Instruments, Inc., Rochester, NY) under magic angle conditions. The excitation wavelengths of AO, EB, and RuBD were 470, 518, and 440 nm, respectively.
TABLE 1.
Quantum Yields (Q), Decay Times (τ), and Molar Extinction Coefficients (∈/λmax) of Fluorophores in DNA
| Probe | Donor/acceptor | Qa | 〈τ〉 (ns)b | ∈/λex (M−1 cm−1/nm) | ∈/λmax (M−1 cm−1/nm) |
|---|---|---|---|---|---|
| AO | Donor | 0.392 | 5.0 | 23,300/470 | 53,000/500 |
| EB | Donor | 0.219 | 21.9 | 5,200/518 | 5,200/518 |
| Ru-BD | Donor | 0.008 | 84.0 | 13,000/440 | 13,000/440 |
| NB | Acceptor | 0.004 | 0.32 | 1,180/440 | 42,900/656 |
| TOTO-3 | Acceptor | 0.06 | 2.3 | 2,240/440 | 154,000/642c |
| TO-PRO-3 | Acceptor | 0.11 | 1.8 | 200/440 | 102,000/642c |
The following compounds were used as quantum yield references: in the case of AO, 3-aminofluoranthene in DMSO (Q = 0.32); EB in methanol (Q = 0.06) for EB; 4-dicyanomethylene-2-methyl-6-(p-dimethylaminostyryl)4H-pyran in methanol (Q = 0.38) in the case of RuBD and NB; and fluorescein in 0.1 M NaOH (Q = 0.92) for TOTO-3 and TO-PRO-3.
Mean lifetime calculated using 〈τ〉 = Σ fiτi, where fi is the fractional steady state contribution of each component to the total emission.
From Molecular Probes, Inc.
Frequency-domain fluorescence measurements.
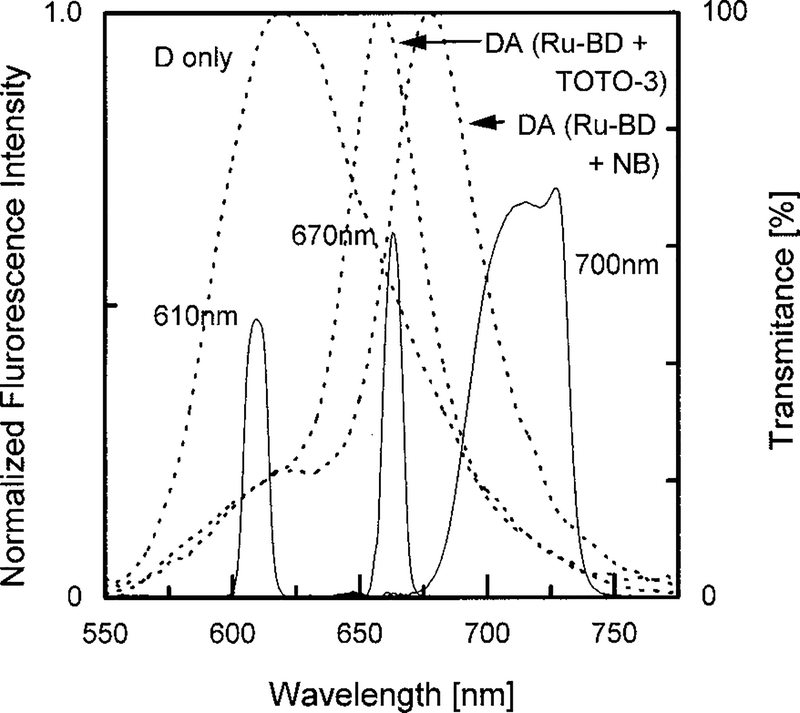
Measurements were performed using the instruments described previously (54) and modified with a data acquisition card from ISS, Inc. (Urbana, IL) (55). The excitation source was a blue LED LNG992CFBW (Panasonic, Japan) with luminous intensity of 1500 mcd, and an LED driver LDX-3412 (ILX Lightwave, Boseman, MO) provided 30 mA of current at frequencies from 1 to 9.3 MHz. A 450RD55 interference filter (Omega Optical, Inc., Brattleboro, VT) and a 4–96 color glass filter (Corning Glass Work, Corning, NY) were used to isolate the excitation wavelength. Rhodamine B in water was utilized as a lifetime standard. The transmission curves of the filters for isolating the emission from the donor, D–A pairs, and acceptors are shown below (Fig. 6).
FIG. 6.
Transmission spectra of the emission filters used for measuring the frequency-domain intensity decays (—). The dashed lines show representative emission spectra of the donor alone (D) and donor plus acceptor (DA) samples.
RESULTS
Steady-State Spectra
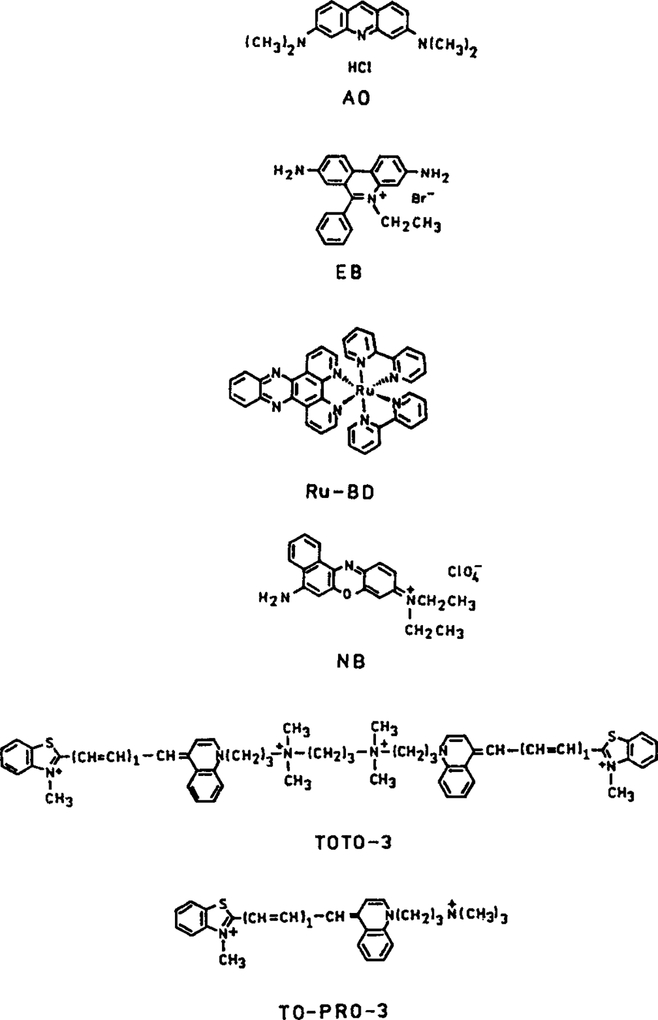
We use DNA with noncovalently bound donors and acceptors to test the possibility of creating long lifetime luminophores with high quantum yields. We chose three donors, AO, EB, and Ru-BD. These structures are shown in Fig. 1. When bound to DNA the quantum yields decrease in this respective order (Table 1). As acceptors, we selected nile blue (NB), TOTO-3, and TO-PRO-3 (Fig. 1), which display increasing quantum yields in the listed order. We chose to examine dyes noncovalently bound to DNA because this approach allowed us to select donors and acceptors with various quantum yields, without the need for chemical synthesis. Also, this approach allowed us to adjust the concentrations of donors and acceptors to observe trends in the spectra. Based on the theory described above, we expect the largest overall increase in the total emission of the tandem luminophore to occur with RET between the lowest quantum yield donor (Ru–BD) and the highest quantum yield acceptor (TO-PRO-3).
FIG. 1.
Chemical structures of the donors and acceptors used in this report.
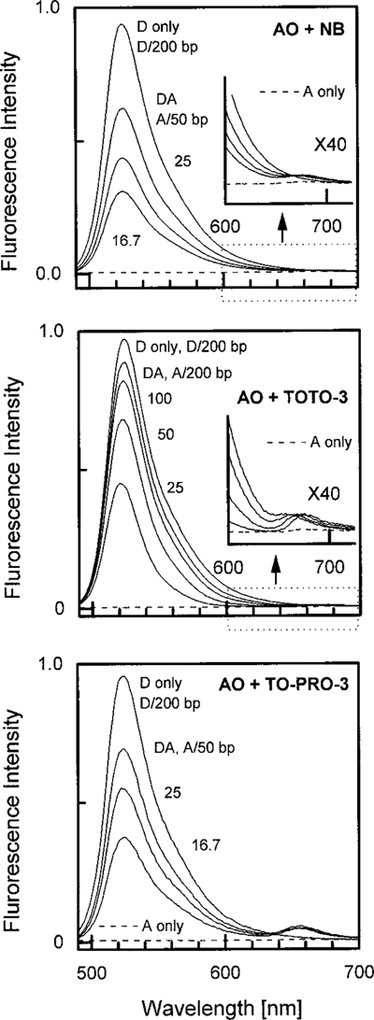
Figure 2 shows the emission spectra of AO bound to DNA with increasing amounts of acceptor. With the high quantum yield AO donor the NB acceptor emission is almost undetectable (Fig. 2, top inset). The quantum yield of the TOTO-3 acceptor is higher than that of NB, and the quantum yield of TO-PRO-3 is higher still. As may be expected, the acceptor emission becomes more easily detectable as the acceptor quantum yields increase. In each case the observed acceptor emission is due to RET from the donor. No significant acceptor emission was found for the acceptors bound to DNA in the absence of donor (dashed lines). An interesting aspect of Fig. 2 is that it demonstrates an effect opposite to that emphasized in this paper. RET from a high quantum yield donor (AD) to a low quantum yield acceptor (NB) decreases the total emission from the donor and acceptor.
FIG. 2.
Emission spectra of the acridine orange donor [Donor] = 5 μM bound to DNA in the presence of the acceptors nile blue (top), TOTO-3 (middle), or TO-PRO-3 (bottom). The insets show the acceptor region with the amplitude increased by a factor of 40. The dashed lines show the emission of acceptor alone with DNA, but without donor, at the highest acceptor concentration used in the figure. The donor is present at 1 donor per 200 base pairs. The number for the donor–acceptor (DA) pair is the number of base pairs for each acceptor.
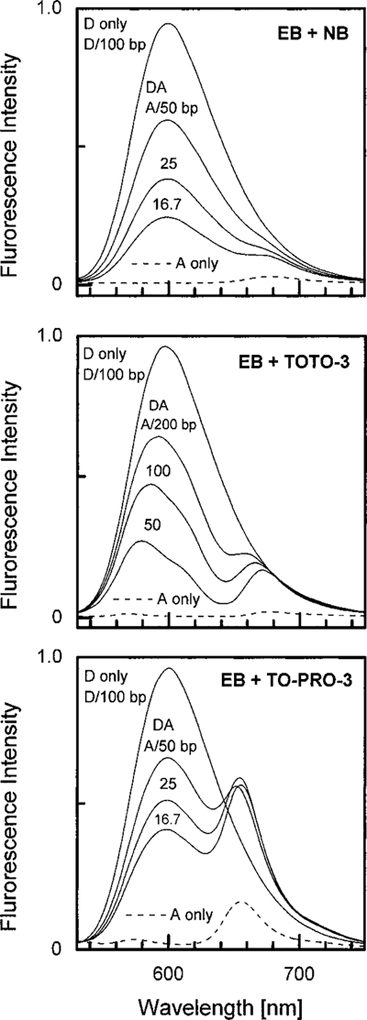
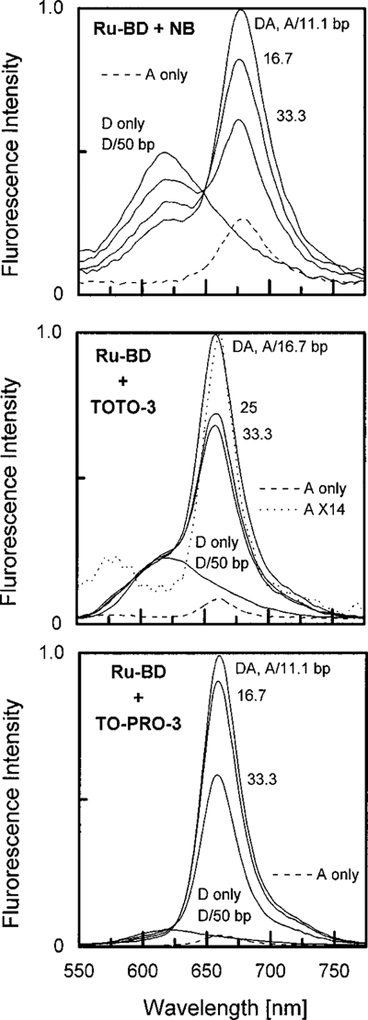
Figures 3 and 4 show emission spectra with the same acceptors, but with EB and Ru–BD as the donors. Examination of these spectra shows that the enhancement of the acceptor emission is larger for Ru–BD than for EB. Also, the largest enhancements are seen for TO-PRO-3, the acceptor with the highest quantum yield (Fig. 4, bottom). In this case the acceptor emission is increased many-fold by energy transfer from the Ru–BD donor. Also, the emission from the D–A system is considerably larger than that of the donor alone bound to DNA, or the acceptor alone bound to DNA (dashed line). This effect is the opposite of that found for the AO/NB D–A pair. In this case the weakly fluorescent NB received most of the energy by RET, but still emits with its own low quantum yield. For the Ru–BD/TO-PRO-3 D–A pair the strongly fluorescent TO-PRO-3 receives most of the energy absorbed by the donor, in spite of the low intrinsic quantum yield of the donor.
FIG. 3.
Emission spectra of ethidium bromide donor [Donor] = 10 μM bound to DNA in the presence of the acceptors nile blue (top), TOTO-3 (middle), or TO-PRO-3 (bottom). The dashed lines show the emission of the acceptor alone with DNA, but without donor, at the highest used acceptor concentration. The donor is present at 1 donor per 100 base pairs. The number for the donor–acceptor (DA) pair is the number of base pairs for each acceptor.
FIG. 4.
Emission spectra of Ru–BD([Ru(bpy)2 dppz]2+) donor [Donor] = 20 μM bound to DNA in the presence of the acceptors nile blue (top), TOTO-3 (middle), and TO-PRO-3 (bottom). The dashed lines show the emission spectra of the acceptor alone with DNA, but without donor, at the highest used acceptor concentration. One donor is present per 50 base pairs. The number for the donor–acceptor (DA) pair is the number of base pairs for each acceptor.
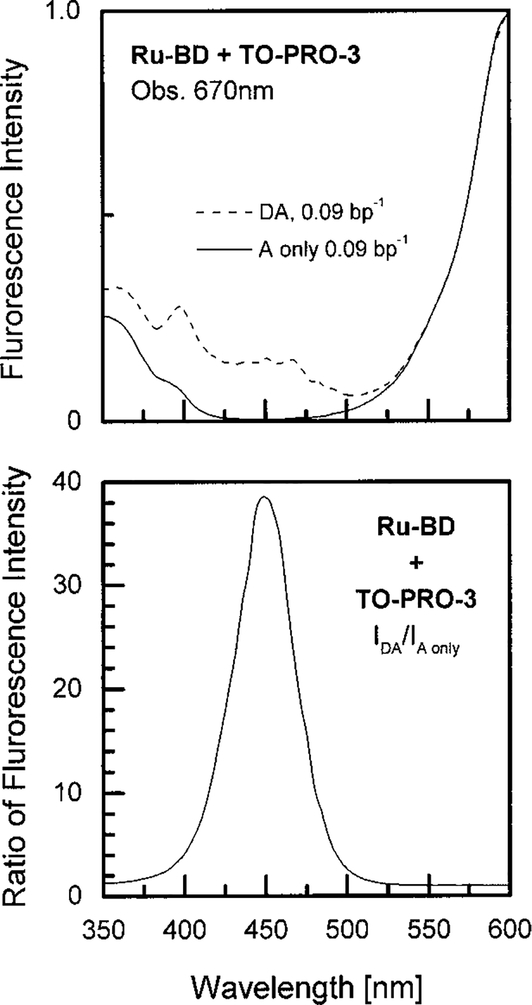
While we expected to see an increased overall quantum yield due to RET from Ru–BD, we were surprised by the magnitude of the effect. In the absence of energy transfer the intensity of the acceptor is proportional to ∈AQA, where ∈A refers to the extinction coefficient of the acceptor at the donor excitation wavelength. If transfer is 100% effective the intensity of the acceptor is proportional to (∈A + ∈D)/∈A. According to Table 1 this ratio is near 4. Examination of Fig. 4 (bottom) indicates that the acceptor enhancement is greater than 4. To further this effect we examined the excitation spectra of the D–A pair, and the acceptor alone, when bound to DNA. On the same relative scale the acceptor alone displays essentially no emission upon excitation at 450 nm (Fig. 5). The lower panel shows the ratio of these excitation spectra, which become close to 40 at 450 nm. This ratio is larger than expected from the extinction coefficients listed in Table 1. It appears that excitation of TO-PRO-3 near 450 nm results in less emission than predicted by its absorption spectrum. This effect could be due to the presence of nonfluorescent absorbing impurities or absorption of nonfluorescent conformers of TO-PRO-3 at 450 nm. It is known that this class of dyes display weak fluorescence in water or when there is torsional motions about the central methine bridge (56). Irrespective of the origin of this low intensity, the acceptor enhancement seen in Fig. 4 is consistent with the excitation spectrum for this D–A pair.
FIG. 5.
Uncorrected excitation spectra of Ru–BD and TO-PRO-3 bound to DNA (- - -) and of TO-PRO-3 alone bound to DNA (—). (Bottom) Ratio of the two excitation spectra.
Time-Resolved Decays
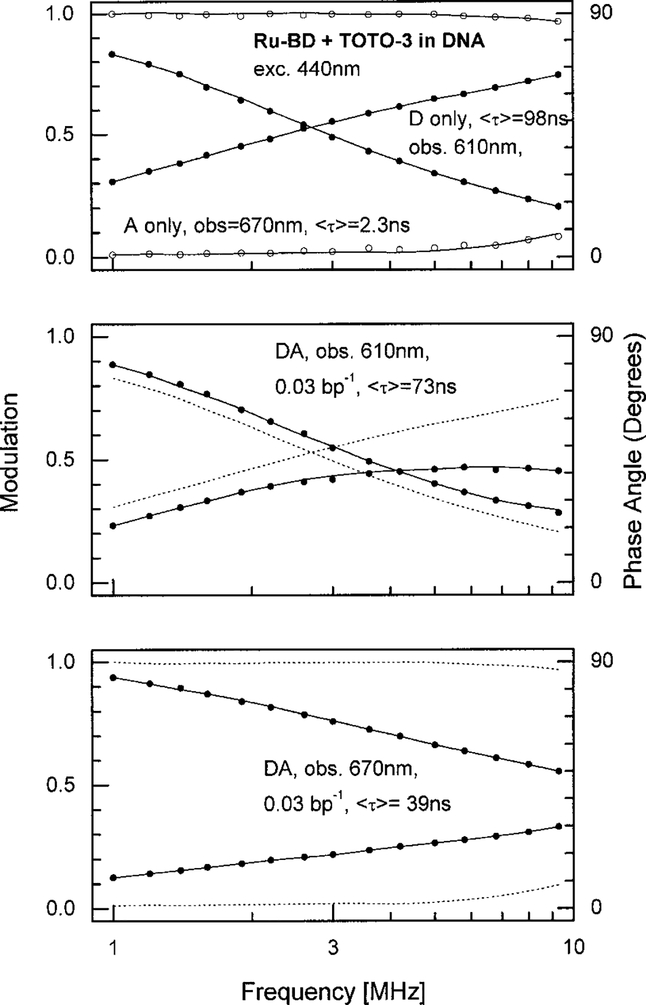
Following demonstration of the enhanced acceptor emission we questioned whether the acceptors would display adequately long lifetimes. Frequency-domain intensity decays were measured through filters selected to isolate the desired emission wavelengths (Fig. 6). Observation at 610 nm results in selective observation of the donor emission, and observation at 670 or 700 nm selects the acceptor emission.
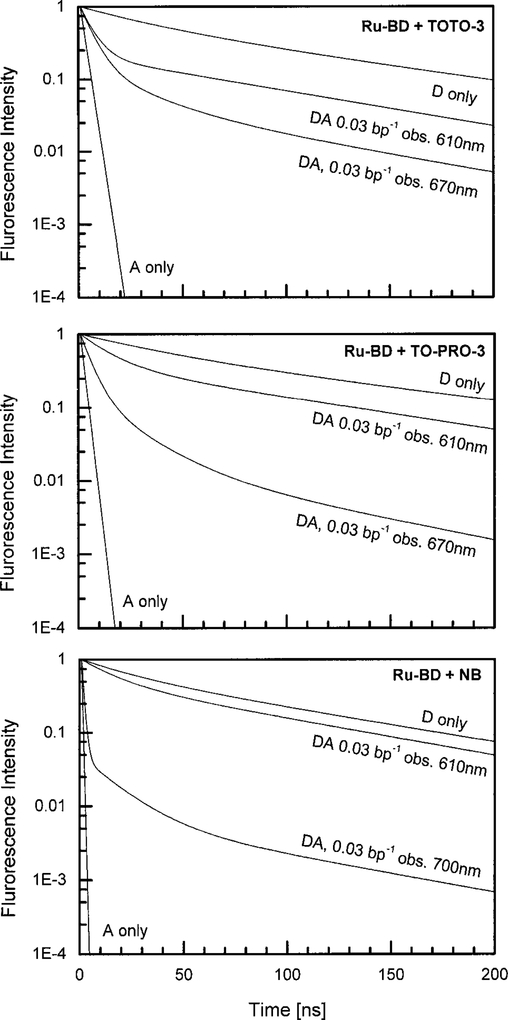
Figures 7–9 show the frequency-domain data for three D–A pairs. In these data Ru–BD is always the donor. The acceptor is NB, TOTO-3, or TO-PRO-3, respectively. In the absence of acceptors, the mean Ru–BD lifetime is near 100 ns (Table 2). The Ru–BD lifetime is only moderately decreased by the acceptor. For instance, for any of the acceptors, a ratio of 0.03 acceptors per base pair results in a mean donor lifetime near 70 ns. This was initially surprising given the 2-fold or larger quenching of the Ru–BD intensity by these acceptor concentrations. However, we can explain this difference in intensity and lifetime quenching as due to a range of D-to-A distances in the labeled DNA. More specifically, most of the acceptor emission results from the more closely spaced D–A pairs. In contrast, the observed donor emission in the presence of acceptors is increased by the higher intensities of those donors most distant from acceptors, which are also the donors with the longer lifetimes.
FIG. 7.
Frequency-domain intensity decays of Ru–BD bound to DNA in the absence and presence of the nile blue acceptor. The solid dots represent the phase or modulation values and the solid lines the best multiexponential fits to the data. In the middle and bottom panels the dotted lines represent the donor-alone and acceptor-alone frequency responses, respectively.
FIG. 9.
Frequency-domain intensity decay of Ru–BD bound to DNA in the absence and presence of the TO-PRO-3 acceptor. See legend to Fig. 7.
TABLE 2.
Multiexponential Intensity Decay Analyses of the Ru–BD Donor and Acceptors Bound to Calf Thymus DNA
| na | 〈τ〉b | αi | fi | τi | ||
|---|---|---|---|---|---|---|
| Donor/acceptor Ru-BD/NBc | ||||||
| Ru-BD | 2 | 84 | 0.36 | 0.13 | 24 | 0.91 |
| 0.64 | 0.87 | 93 | ||||
| NB | 1 | 0.32 | 1.00 | 1.00 | 0.32 | 0.85 |
| DA Obs. 610 nm | 2 | 75 | 0.50 | 0.15 | 16 | 1.40 |
| 0.50 | 0.85 | 86 | ||||
| DA Obs. 700 nm. | 3 | 30 | 0.95 | 0.51 | 1.9 | 0.90 |
| 0.04 | 0.21 | 22 | ||||
| 0.01 | 0.28 | 87 | ||||
| Donor/acceptor Ru-BD/TOTO-3c | ||||||
| Ru-BD | 2 | 98 | 0.42 | 0.18 | 33 | 0.99 |
| 0.58 | 0.82 | 111 | ||||
| TOTO-3 | 1 | 2.3 | 1.00 | 1.00 | 2.3 | 1.30 |
| DA Obs. 610 nm | 2 | 73 | 0.79 | 0.19 | 5.8 | 1.07 |
| 0.21 | 0.81 | 90 | ||||
| DA Obs. 670 nm. | 3 | 39 | 0.83 | 0.41 | 5.6 | 1.02 |
| 0.12 | 0.24 | 23 | ||||
| 0.05 | 0.35 | 88 | ||||
| Donor/acceptor Ru-BD/TO-PRO-3c | ||||||
| Ru-BD | 2 | 114 | 0.42 | 0.17 | 38 | 1.02 |
| 0.58 | 0.83 | 130 | ||||
| TO-PRO-3 | 1 | 1.8 | 1.00 | 1.00 | 1.8 | 1.02 |
| DA Obs. 610 nm | 2 | 83 | 0.62 | 0.19 | 14 | 0.81 |
| 0.38 | 0.81 | 99 | ||||
| DA Obs. 670 nm. | 3 | 24 | 0.83 | 0.48 | 5.1 | 1.05 |
| 0.15 | 0.33 | 19 | ||||
| 0.02 | 0.19 | 78 | ||||
Number of decay times in the multiexponential fit.
〈τ〉 = Σ τifi where fi is the steady state contribution of each component.
All acceptor concentrations are 0.03 bp−1.
The bottom panels of Figs. 7–9 show the frequency response observed for the longer wavelength regions dominated by the acceptor emission. In each case the mean decay times are near 30 ns for observation at the acceptor emission wavelengths. While the frequency responses are multiexponential, we did not find visually obvious contributions from the directly excited acceptors with their 0.3- to 2.3-ns lifetimes. The apparent acceptor lifetimes are shorter than the apparent donor lifetimes because the acceptor emission is enriched for the shorter distances D–A pairs which have a shorter donor lifetime.
It is informative to examine the intensity decays in the time-domain reconstructed from the frequency-domain data (Fig. 10). The decays of the directly excited acceptors are short, and emission from the directly excited acceptors will not be observed if the detection is off-gated for the first 10–20 ns following the excitation pulse. The donor decays, even in the presence of acceptors, are long lived. Also, following a brief transition period out to 10–40 ns, the acceptor decay rates are comparable to that of the quenched donors. This long lived emission from the donors can be used for biophysical or analytical purposes.
FIG. 10.
Time-domain intensity decay of Ru–BD and acceptor complexes with DNA.
An important conclusion from these experiments is that the apparent acceptor decays are adequately long for off-gating of the autofluorescence from biological samples. Hence the use of MLC-acceptor pairs provides an opportunity to obtain luminophores which display long lifetimes, high quantum yields, and long emission wavelengths.
DISCUSSION
In the preceding sections we presented data suggesting an approach to obtaining long wavelength emission while retaining desirably long lifetimes. By consideration of the well known characteristics of Förster transfer, one can predict that suitable designed D-A pairs will display even more favorable properties. For instance, the acceptor decay times for the DNA bound probes were shorter than the donor decay times. We explained this effect as due to a range of donor-to-acceptor distances for the probes randomly bound to DNA. It is well known that unique D-to-A distances can be obtained with polyproline spacers (57) or with double-stranded DNA as the spacer (58, 59). In such cases the donor decay times will decrease in proportion to the transfer efficiency, and the acceptor decay times will be similar to the donor decay times. The results for a donor and acceptor separated by a single distance are expected to be comparable to that shown in Scheme 1, where a 1-μs decay time donor, with 90% transfer efficiency, results in a luminophore with a 100-ns lifetime. Since metal–ligand complexes are known with decay times as long as 42 μs (60, 61), one can predict 4-μs decay time luminophores with 90% transfer.
Another advantage of these RET probes is that the emission spectra of red and NIR fluorophores are typically narrow on the wavelength scale, whereas the emission spectra of the MLCs are broad. Since autofluorescence from biological samples is typically distributed broadly on the wavelength scale, the concentration of the emission into a narrow spectral range by the acceptor should improve detectability of these luminophores.
Another possible application of this phenomena is for the study of macromolecular association reactions, such as protein–protein interactions, DNA hybridization (62–64), fluorescence in situ hybridization (FISH) (65), or the use of molecular beacons (66, 67). As an example, suppose it was necessary to test for binding of donor-labeled oligonucleotides to a mixture of acceptor-labeled oligonucleotides. When using a RuMLC donor and one of the acceptors used in this report, most of the species labeled with donor or acceptor alone will display little emission. In contrast the D–A pairs due to macromolecular association will be brightly fluorescent. Additionally, the acceptor emission will be long lived. Using time-gated detection brightly fluorescent spots may become apparent against a background of weakly stained chromatin and/or short decay time. These spectral properties could be useful for detection of oligonucleotide hybridization on DNA arrays (68, 69). Such arrays are becoming widely used for analysis of gene expression (70–72).
In summary, we have described a generic approach to obtaining an unusual combination of spectral properties by using appropriate D–A pairs. This approach can be used to create D–A pairs which act as a single luminophore, or this effect can be used to detect interactions in samples containing species labeled with the donor or acceptor. This approach may also be useful in studies of macromolecular folding, as illustrated by the use of RET to study ribozyme structures (73, 74). One can also imagine long lifetime donors linked to pH, Ca2+, or other analyte-sensitive fluorophores (75, 76). If the analyte sensitive fluorophore displays distinct emission spectra with and without bound analyte, then there will be a long lived component in the emission with the spectral characteristics of each form. Finally, one can imagine the use of the enhanced emission and inhibition of the enhancement for use in macromolecular binding assays in high throughput screening (77, 78). There appear to be numerous applications of our approach in biochemical and biomedical research.
FIG. 8.
Frequency-domain intensity decay of Ru–BD bound to DNA in the absence and presence of the TOTO-3 acceptor. See legend to Fig. 7.
ACKNOWLEDGMENTS
This work was supported by the NIH, NCRR RR-08119, and GM-35154. The authors also thank Drs. Ignacy Gryczynski, Zygmunt Gryczynski, and Badri Maliwal for their valuable suggestions.
Footnotes
Abbreviations used: A, acceptor; AO, acridine orange; bp, base pair; bpy, 2,2′-bipyridine; CT-DNA, calf thymus DNA; D, donor; DA, donor–acceptor; dppz, dipyrido [3,2-a:2′,3′-c]phenazine; EB, ethidium bromide; LED, light-emitting diode; MLC, metal–ligand complex; NB, nile blue A perchlorate; RET, resonance energy transfer; Ru–BD, [Ru(bpy)2(dppz)](PF6)2; NIR, near infrared; PMT, photomultiplier tube; DMSO, dimethylsulfoxide; FISH, fluorescence in situ hybridization.
REFERENCES
- 1.Thompson RB (1994) Red and near-infrared fluorometry, in Topics in Fluorescence Spectroscopy (J. R. Lakowicz, Ed.), Vol. 4, pp. 151–222. Plenum Press, New York. [Google Scholar]
- 2.Daehne S, Resch-Genger U, and Wolfbeis OS (1998) Near-Infrared Dyes for High Technology Applications, pp. 458, Kluwer Academic, Boston. [Google Scholar]
- 3.Southwick PL, Ernst LA, Tauriello EW, Parker SR, Mujumdar RB, Mujumdar SW, Clever HA, and Waggoner AS (1990) Cyanine dye labeling reagents-carboxymethylindocyanine succinimidyl esters. Cytometry 11, 418–430. [DOI] [PubMed] [Google Scholar]
- 4.Rahavendran SV, and Karnes HT (1996) An oxazine reagent for derivatization of carboxylic acid analytes suitable for liquid chromatographic detection using visible diode laser induced fluorescence. J. Pharm. Biomed. Anal 15, 83–98. [DOI] [PubMed] [Google Scholar]
- 5.Rahavendran SV, and Karnes HT (1996) Application of rhodamine 800 for reversed phase liquid chromatographic detection using visible diode laser induced fluorescence. Anal. Chem 68, 3763–3768. [DOI] [PubMed] [Google Scholar]
- 6.Kessler MA, and Wolfbeis OS (1992) Laser-induced fluorometric determination of albumin using longwave absorbing molecular probes. Anal. Biochem 200, 254–259. [DOI] [PubMed] [Google Scholar]
- 7.Abugo OO, Nair R, and Lakowicz JR (2000) Fluorescence properties of rhodamine 800 in whole blood and plasma. Anal. Biochem 279, 142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dorshow RB, Bugaj JE, Burleigh BD, Duncan JR, Johnson MA, and Jones WB (1998) Noninvasive fluorescence detection of hepatic and renal function. J. Biomed. Opt 3(3), 340–345. [DOI] [PubMed] [Google Scholar]
- 9.Kanda M, and Niwa S (1992) Development of a noninvasive monitoring instrument for serum Indocyanine Green dye concentration. Appl. Opt 31(31), 6668–6675. [DOI] [PubMed] [Google Scholar]
- 10.Bollinger A, Saesseli B, Hoffmann U, and Franzeck UK (1991) Intravital detection of skin capillary aneurysms by videomicroscopy with Indocyanine Green in patients with progressive systemic sclerosis and related disorders. Circulation 83, 546–551. [DOI] [PubMed] [Google Scholar]
- 11.Middendorf L, Amen J, Bruce R, Draney D, DeGraff D, Gewecke J, Grone D, Humphrey P, Little G, Lugade A, Narayanan N, Oommen A, Osterman H, Peterson R, Rada J, Raghavachari R, and Roemer S (1998) Near-infrared fluorescence instrumentation for DNA analysis, in Near-Infrared Dyes for High Technology Applications (Daehen S, Ed.), pp. 21–54. Kluwer Academic, Netherlands. [Google Scholar]
- 12.Flanagan JH, Romero SE, Legendre BL, Hammer RP, and Soper A (1997) Heavy-atom modified near IR fluorescent dyes for DNA sequencing applications: Synthesis and photophysical characterization. SPIE 2980, 328–337. [Google Scholar]
- 13.Owens CV, Davidson YY, Kar S, and Soper SA (1997) High-resolution separation of DNA restriction fragments using capillary electrophoresis with near-IR, diode-based, laser-in-duced fluorescence detection. Anal. Chem 69, 1256–1261. [Google Scholar]
- 14.Strickler SJ, and Berg RA (1962) Relationship between absorption intensity and fluorescence lifetime of molecules. J. Chem. Phys 37, 814–822. [Google Scholar]
- 15.Kalayanasundarm K (1992) Photochemistry of Polypyridine and Porphyrin Complexes, Academic Press, New York. [Google Scholar]
- 16.Juris A, Balzani V, Barigelletti F, Campagna S, Belser P, and Von Zelewsky A (1988) Ru(II) polypyridine complexes: Photophysics, photochemistry, electrochemistry, and chemiluminescence. Coord. Chem. Rev 84, 85–277. [Google Scholar]
- 17.Demas JN, and DeGraff BA (1991) Design and applications of highly luminescent transition metal complexes. Anal. Chem 63, 829A–837A. [Google Scholar]
- 18.Demas JN, and DeGraff BA (1994) Design and applications of highly luminescent transition metal complexes, in Topics in Fluorescence Spectroscopy (Lakowicz JR, Ed.), Vol. 4, pp. 71–107, Plenum Press, New York. [Google Scholar]
- 19.Terpetschnig E, Szmacinski H, Malak H, and Lakowicz JR (1995) Metal–ligand complexes as a new class of long lived fluorophores for protein hydrodynamics. Biophys. J 68, 342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szmacinski H, Terpetschnig E, and Lakowicz JR (1996) Synthesis and evaluation of Ru-complexes as anisotropy probes for protein hydrodynamics and immunoassays of high-molecular weight antigens. Biophys. Chem 62, 109–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo X-Q, Castellano FN, Li L, and Lakowicz JR (1998) Use of a long lifetime Re(I) complex in fluorescence polarization immunoassays of high-molecular weight analytes. Anal. Chem 70, 632–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murtaza Z, and Lakowicz JR (1999) Long-lifetime and long-wavelength osmium(II) metal compounds containing polypyridine ligands. Excellent red fluorescent dyes for biophysics and sensors. SPIE 3602, 309–315. [Google Scholar]
- 23.Grigg R, and Norbert WDJA (1992) Luminescent pH sensors based on di(2,29-bipyridyl)(5,59-diaminomethyl-2–2′-bi-pyridyl)-ruthenium(II) complexes. J. Chem. Soc. Chem. Commun 1992, 1300–1302. [Google Scholar]
- 24.Lippitsch ME, and Wolfbeis OS (1988) Fiber-optics oxygen sensor with the fluorescence decay time as the information carrier. Anal. Chim 205, 1–6. [Google Scholar]
- 25.Haugen GR, Wallin BW, and Lytle FE (1979) Optimization of data-acquisition rates in time-correlated single-photon fluorimetry. Rev. Sci. Instrum 50, 64–72. [DOI] [PubMed] [Google Scholar]
- 26.Barisas BG, and Lauther MD (1980) Grid-gated photomultiplier photometer with subnanosecond time response. Rev. Sci. Instrum 51, 74–78. [Google Scholar]
- 27.James DR, Siemiarczuk A, and Ware WR (1992) Stroboscopic optical boxcar technique for the determination of fluorescence lifetimes. Rev. Sci. Instrum 63, 1710–1716. [Google Scholar]
- 28.Lovgren T, Hemmila I, Pettersson K, and Halonen P (1985) Time-resolved fluorescence fluorometry in immunoassays, in Alternative Immunoassays (Collins WP, Ed.), pp. 203–217. Wiley, New York. [Google Scholar]
- 29.Diamandis EP (1988) Immunoassays with time-resolved fluorescence spectroscopy: Principles and applications. Clin. Biochem 21, 139–150. [DOI] [PubMed] [Google Scholar]
- 30.Cheung HC (1991) Resonance energy transfer, in Topics in Fluorescence Spectroscopy (Lakowicz JR, Ed.), Vol. 2, pp. 127–176, Plenum Press, New York. [Google Scholar]
- 31.Clegg RM (1992) Fluorescence resonance energy transfer and nucleic helical geometry of double-stranded DNA in solution by fluorescence resonance energy transfer. Proc. Natl. Acad. Sci 211, 353–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clegg RM, Murchie AI, Zechel A, and Lilley DM (1993) Observing the helical geometry of double-stranded DNA in solution by fluorescence resonance energy transfer. Proc. Natl. Acad. Sci. USA 90, 2994–2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Root DD (1997) In situ molecular association of dystrophin with actin revealed by sensitized emission immuno-resonance energy transfer. Proc. Natl. Acad. Sci. USA 94, 5685–5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Selvin PR (1996) Lanthanide-based resonance energy transfer. IEEE J. Sel. Top. Quantum Electron. 2(4), 1077–1087. [Google Scholar]
- 35.Chen J, and Selvin PR (2000) Lifetime- and color-tailored fluorophores in the micro- and millisecond time regime. J. Am. Chem. Soc 122, 657–660. [Google Scholar]
- 36.Selvin PR, and Hearst JE (1994) Luminescence energy transfer using a terbium chelate: Improvements on fluorescence energy transfer. Proc. Natl. Acad. Sci. USA 91, 10024–10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ju J, Ruan C, Fuller CW, Glazer AN, and Mathies RA (1995) Fluorescence energy transfer dye-labeled primers for DNA sequencing and analysis. Proc. Natl. Acad. Sci. USA 92, 4347–4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ju J, Glazer AN, and Mathies RA (1996) Energy transfer primers: A new fluorescence labeling paradigm for DNA sequencing and analysis. Nat. Med 2(2), 246–249. [DOI] [PubMed] [Google Scholar]
- 39.Benson SC, Mathies RA, and Glazer AN (1993) Heterodimeric DNA-binding dyes designed for energy transfer. Stability and application of the DNA complexes. Nucleic Acids 21, 5720–5726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benson SC, Zeng Z, and Glazer AN (1995) Fluorescence energy-transfer cyanine heterodimers with high affinity for double-stranded DNA. Anal. Biochem 231, 247–255. [DOI] [PubMed] [Google Scholar]
- 41.Klakamp SL, and Horrocks DeW., W. (1992) Lanthanide ion luminescence as a probe of DNA structure. 1. Guanine-containing oligomers and nucleotides. J. Inorg. Biochem 46, 175–192. [DOI] [PubMed] [Google Scholar]
- 42.Klakamp SL, and Horrocks DeW., W. (1992) Lanthanide ion luminescence as a probe of DNA structure. 2. Non-guanine containing oligomers and nucleotides. J. Inorg. Biochem 46, 193–205. [DOI] [PubMed] [Google Scholar]
- 43.Fu PKL, and Turro C (1999) Energy transfer from nucleic acids to Tb(III): Selective emission enhancement by single DNA mismatches. J. Am. Chem. Soc 121(1), 1–7. [Google Scholar]
- 44.Stryer L (1978) Fluorescence energy transfer as a spectroscopic ruler. Annu. Rev. Biochem 47, 819–846. [DOI] [PubMed] [Google Scholar]
- 45.Clegg RM (1996) Fluorescence resonance energy transfer, in Fluorescence Imaging Spectroscopy and Microscopy (Wang XF and Herman B, Eds.), pp. 179–252, Wiley, New York. [Google Scholar]
- 46.Lakowicz JR (1999) Principles of Fluorescence Spectroscopy, 2nd ed., Chaps. 13–15, pp. 367–443, Kluwer Academic/Plenum Publishers, New York. [Google Scholar]
- 47.Laws JR, and Brand L (1979) Analysis of two-state excited-state reactions. The fluorescence decay of 2-naphthol. J. Physiol. Chem 83, 795–802. [Google Scholar]
- 48.Gafni A, and Brand L (1978) Excited state proton transfer reactions of acridine studied by nanosecond fluorometry. Chem. Phys. Letts 58, 346–350. [Google Scholar]
- 49.Lakowicz JR, and Balter A (1982a) Theory of phase-modulation fluorescence spectroscopy for excited state processes. Biophys. Chem 16, 99–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lakowicz JR, and Balter A (1982) Analysis of excited state process by phase-modulation fluorescence spectroscopy. Biophys. Chem 16, 117–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lakowicz JR (1983) Principles of Fluorescence Spectroscopy, pp. 496 Plenum Press, New York. [Google Scholar]
- 52.Malak H, Gryczynski I, Lakowicz JR, Meyers GJ, and Castellano FN (1997) Long-lifetime metal-ligand complexes as luminescent probes for DNA. J. Fluoresc 7(2), 107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lakowicz JR, Malak H, Gryczynski I, Castellano FN, and Meyer GJ (1995) DNA dynamics observed with long lifetime metal–ligand complexes. Biospectroscopy 1, 163–168. [Google Scholar]
- 54.Lakowicz JR, and Maliwal BP (1985) Construction and performance of a variable-frequency phase-modulation fluorometer. Biophys. Chem 21, 61–78. [DOI] [PubMed] [Google Scholar]
- 55.Feddersen BA, Piston DW, and Gratton E (1989) Digital parallel acquisition in frequency domain fluorimetry. Rev. Sci. Instrum 60(9), 2929–2936. [Google Scholar]
- 56.Netzel TL, Nafisi K, Zhao M, Lenhard JR, and Johnson I (1995) Base-content dependence of emission enhancements, quantum yields, and lifetimes for cyanine dyes to double-strand DNA: Photophysical properties of monomeric and bichromophoric DNA stains. J. Phys. Chem 99, 17936–17947. [Google Scholar]
- 57.Stryer L, and Haugland RP (1967) Energy transfer: A spectroscopic ruler. Proc. Natl. Acad. Sci. USA 58, 719–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parkhurst KM, and Parkhurst LJ (1996) Detection of point mutations in DNA by fluorescence energy transfer. J. Biomed. Optics 1, 435–441. [DOI] [PubMed] [Google Scholar]
- 59.Hochstrasser RA, Chen SM, and Millar DP (1992) Distance distribution in a dye-linked oligonucleotide determined by time-resolved fluorescence energy transfer. Biophys. Chem 45, 133–141. [DOI] [PubMed] [Google Scholar]
- 60.Harriman A, Hissler M, Khatyr A, and Ziessel R (1999) A ruthenium(II) tris-(2,29-bipyridine) derivative possessing a triplet lifetime of 42 μs. Chem. Commun 735–736. [Google Scholar]
- 61.Simon JA, Curry SL, Schmehl RH, Schatz TR, Piotrowiak P, Jin X, and Thummel RP (1997) Intramolecular electron energy transfer in ruthenium(II) diimine donor/pyrene acceptor complexes linked by a single C–C bond. J. Am. Chem. Soc 119, 11012–11022. [Google Scholar]
- 62.Morrison LE (1995). Detection of energy transfer and fluorescence quenching, in Nonisotopic Probing, Blotting, and Sequencing (Kricka LJ, Ed.), pp. 429–471, Academic Press, New York. [Google Scholar]
- 63.Parkhurst KM, and Parkhurst LJ (1996). Detection of point mutations in DNA by fluorescence energy transfer. J. Biomed. Opt 1, 435–441. [DOI] [PubMed] [Google Scholar]
- 64.Ota N, Hirano K, Warashina M, Andrus A, Mullah B, Hatanaka K, and Taira K (1998). Determination of interactions between structured nucleic acids by fluorescence resonance energy transfer (FRET): Selection of target sites for functional nucleic acids. Nucleic Acids Res. 26(3), 735–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kostrikis L, Tyagi S, Mhlanga MM, Ho DD, and Kramer FR (1998). Spectral genotyping of human alleles. Science 279, 1228–1229. [DOI] [PubMed] [Google Scholar]
- 66.Tyagi S, Bratu DP, and Kramer FR (1998) Multicolor molecular beacons discrimination. Nat. Biotechnol 16, 49–52. [DOI] [PubMed] [Google Scholar]
- 67.Steemers FJ, Ferguson JA, and Walt DR (2000) Screening unlabeled DNA targets with randomly ordered fiber-optic gene arrays. Nat. Biotechnol 18, 91–94. [DOI] [PubMed] [Google Scholar]
- 68.Brown PO, and Botstein D (1999) Exploring the new world of the genome with DNA microarrays. Nat. Genet. Suppl 21, 33–37. [DOI] [PubMed] [Google Scholar]
- 69.Cheung VG, Morley M, Aguilar F, Massimi A, Kucherlapati R, and Childs G (1999) Making and reading microarrays. Nat. Genet. Suppl 21, 15–19. [DOI] [PubMed] [Google Scholar]
- 70.Hacia JG, Brody LC, and Collins FS (1998) Applications of DNA chips for genomic analysis. Mol. Psychiatry 3, 483–492. [DOI] [PubMed] [Google Scholar]
- 71.Harrington CA, Rosenow C, and Retief J (2000) Monitoring gene expression using DNA microarrays. Curr. Opin. Microbiol 3, 285–291. [DOI] [PubMed] [Google Scholar]
- 72.Abramowitz S (1999) DNA analysis in microfabricated formats. J. Biomed. Microdevices 1, 107–112. [DOI] [PubMed] [Google Scholar]
- 73.Walter NG, Burke JM, and Millar DP (1999) Stability of hairpin ribozyme tertiary structure is governed by the interdomain junction. Nat. Struct. Biol 6(6), 544–549. [DOI] [PubMed] [Google Scholar]
- 74.Walter NG, Hampel KJ, Brown KM, and Burke JM (1998) Tertiary structure formation in the hairpin ribozyme monitored by fluorescence resonance energy transfer. EMBO J. 17(8), 2378–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Czarnik AW (Ed.) (1993) Fluorescent Chemosensors for Ion and Molecule Recognition, American Chemical Society, Washington, DC. [Google Scholar]
- 76.Lakowicz JR (Ed.) (1994) Topics in Fluorescence Spectroscopy, Vol. 4, pp. 501, Plenum Press, New York. [Google Scholar]
- 77.Pope AJ, Haupts UM, and Moore KJ (1999) Homogeneous fluorescence readouts for miniaturized high-throughput screening: Theory and practice. DDT 4(8), 350–362. [DOI] [PubMed] [Google Scholar]
- 78.Mere L, Bennett T, Coassin P, England P, Hamman B, Rink T, Zimmerman S, and Negulescu P (1999) Miniaturized FRET assays and microfluidics: Key components for ultra-high-throughput screening. DDT 4(8), 363–367. [DOI] [PubMed] [Google Scholar]