Abstract
Background
Aging is associated with sarcopenia (low muscle mass) and dynapenia (low muscle strength) leading to disability and mortality. Widely used previous cut‐points for sarcopenia were established from dated, small, or pooled cohorts. We aimed to identify cut‐points of low strength as a determinant of impaired physical performance and cut‐points of low appendicular lean mass (ALM) as a predictor of low strength in a single, large, and contemporary cohort of community‐dwelling older adults and compare these criteria with others.
Methods
Cross‐sectional analyses were conducted on baseline data from 4725 and 4363 community‐dwelling men and women (65–86 years, 96.8% Caucasian) of the Canadian longitudinal study on aging comprehensive cohort. Physical performance was evaluated from gait speed, timed up‐and‐go, chair rise, and balance tests; a weighted‐sum score was computed using factor analysis. Strength was measured by handgrip dynamometry; ALM, by dual‐energy X‐ray absorptiometry and ALM index (ALMI; kg/m2), was calculated. Classification and regression tree analyses determined optimal sex‐specific cut‐points of ALMI predicting low strength and of strength predicting impaired physical performance (score < 1.5 SD below the sex‐specific mean).
Results
Modest associations were found between ALMI and strength and between strength and physical performance score in both sexes. ALMI was not an independent predictor of physical performance score. Cut‐points of <33.1 and <20.4 kg were found to define dynapenia in men and in women, respectively, corresponding to 21.5% and 24.0% prevalence rates. Sarcopenia cut‐points were <7.76 kg/m2 in men and <5.72 kg/m2 in women; prevalence rates of 21.7% and 13.7%. Overall, 8.3% of men and 5.5% of women had sarco‐dynapenia. Sarcopenic were older and had lower fat mass and body mass index (BMI) than non‐sarcopenic participants. While the agreement between current criteria and the updated European Working Group for Sarcopenia in Older Persons recommendations was fair, we found only slight agreement with the Foundation for the National Institute of Health sarcopenia project. Older persons identified with sarcopenia as per the Foundation for the National Institute of Health criteria (using ALM/BMI as the index) have higher BMI and fat mass compared with non‐sarcopenic and have normal ALMI as per our criteria.
Conclusions
The proposed function‐derived cut‐points established from this single, large, and contemporary Canadian cohort should be used for the identification of sarcopenia and dynapenia in Caucasian older adults. We advise on using criteria based on ALMI in the diagnosis of sarcopenia. The modest agreement between sarcopenia and dynapenia denotes potential distinct health implications justifying to study both components separately.
Keywords: Sarcopenia, Dynapenia, Physical performance, Muscle mass, Strength, Aging
Introduction
The population is aging therefore increasing the prevalence of poor health outcomes. Muscle mass and strength decline at a rate of 0.5–1% and 2–3% per year, respectively, after the age of 50 years1 leading to increased disability, loss of autonomy, morbidity, decreased quality of life, and mortality.2 Sarcopenia, originally defined as the age‐related loss of muscle mass by Rosenberg,3 was attributed an International Classification of Disease‐10th revision code in 2016 recognizing the condition as a disease.4 Although the term sarcopenia is now consensually used to define the combined entities, that is, the loss of muscle mass and strength2, some authors argue that sarcopenia (low muscle mass) should be considered separately from dynapenia (low muscle strength).5 Recently, Bulow et al. have called for rejuvenating the term sarcopenia arguing that adding muscle strength and physical function, that is, gait speed, to the definition of sarcopenia for the condition to be clinically relevant resulted in a tautology.6, 7 Four major groups have been working towards a consensus for defining sarcopenia: the European Working Group on Sarcopenia in Older People (EWGSOP), the International Working Group on Sarcopenia (IWGS), the Asian Working Group on Sarcopenia, and the Foundation for the National Institute of Health (FNIH) sarcopenia project. While the former three endorsed cut‐points determined arbitrarily for low lean mass, strength, and gait speed from previously published work,2, 8, 9, 10 the FNIH determined empirical cut‐points of low lean mass relative to body mass index (BMI) and strength from data of pooled cohorts.11 Despite these collective efforts, an ongoing debate subsists. The lack of consensus to define sarcopenia and the use of different cut‐points prevent standard diagnosis in clinical settings and hinder comparison of research studies. Presently suggested cut‐points carry important limitations, namely, that they were derived from cohorts of limited sample sizes,12, 13, 14 from up to 20‐year‐old data11, 12, 13, 14, 15, 16, 17 when evaluation methods and the population have evolved over the last decades, for example, with increased prevalence of obesity, or from the aggregation of cohorts introducing important heterogeneity with regard to assessment methods.11, 17
To address these limitations from present diagnostic cut‐points and based on the hypothesis that low strength is a predictor of low gait speed and that lean mass is a predictor of low strength as demonstrated by the FNIH project,18, 19 our aim was twofold: (i) to define cut‐points for dynapenia, that is, low strength, to identify persons at risk of limited overall physical performance and for sarcopenia, that is, low lean mass, as a predictor of dynapenia, and (ii) determine the prevalence of each condition separately and combined, (sarco‐dynapenia), from the largest and contemporary Canadian longitudinal study on aging (CLSA). These cut‐points were to be clinically relevant and literal to their original definitions.
Methods
Subjects
Baseline data were from the large, nationally representative CLSA of 51 338 community‐dwelling men and women aged 45–86 years, recruited from 2011 to 2015. Participants from 11 cities across Canada (Victoria, BC; Vancouver, BC; Surrey, BC; Calgary, AB; Winnipeg, MB; Hamilton, ON; Ottawa, ON; Montreal, QC; Sherbrooke, QC; Halifax, NS; and St‐John's, NFLD) were randomly selected based on age and sex strata in each province through the Canadian Community Health Survey, provincial health care registries, and from random digit dialling and will be followed every 3 years for ≥20 years.20 Subjects were excluded from the sample frame if they were residents of one of the three territories, if they lived on a First Nation reserve, in institutions, or were full‐time members of the Canadian Armed Forces. Individuals were not eligible if they were unable to communicate in English or French or had cognitive impairment that precluded the ability to provide informed consent, at baseline. The study was approved by the CLSA research site ethics boards, and all participants of the CLSA study provided informed consent to use their data in research.21 This present study was approved by the McGill University Health Centre Ethics Board (REB 16‐068‐MUHC).
Cross‐sectional analyses were performed on data from the comprehensive cohort participants (n = 30 097, living within 25–50 km from CLSA collection data sites). This specific cohort provided core information by phone interview, and additional information from face‐to‐face interview questionnaires through a computer‐assisted personal interview software and from site‐based visits of neuropsychological, physical function, body composition, and clinical assessments performed by trained individuals.20 For the current study, participants who were aged <65 years, who had multiple sclerosis, Alzheimer's disease, effects from stroke or transient ischaemic attack, Parkinson's disease, surgery within the last 3 months, polio, unstable heart condition within the last 3 months, pulmonary embolism within the last 6 weeks, chemotherapy within the last 4 weeks, dialysis and missing or improper dual energy X‐ray absorptiometry (DXA) measurement, grip strength, or physical tests results were excluded. The total analytic sample was 9088 participants (4725 men and 4363 women) (Online Resource, Supporting Information, Figure S1).
Body composition
Lean soft tissue mass (lean mass) and fat mass were measured by DXA (Hologic Discovery A™ densitometer) of the whole body according to standard procedures. Appendicular lean mass (ALM; kg) was calculated as the sum of the upper and lower limbs lean mass. ALM index (ALMI) was obtained by dividing ALM over height squared (kg/m2) as previously suggested by Baumgartner et al.12 Weight over 204 kg, height over 1.88 m, exposition to an X‐ray with contrast material, or participation in a nuclear medicine study within the last 7 days before the DXA were contraindications to receiving the scan. There are risks for erroneous ALM assessment for some individuals who unproperly fit within the scanning area of the DXA bed. For this reason, only those with a valid DXA weight measure were retained in the analyses. Using a Bland and Altman plot, a participant's DXA weight was considered to be valid when the value of the difference between their DXA and scale weight was within the 95% confidence interval of the population mean difference.22
Anthropometry
Anthropometric evaluation included body weight (140–10 Healthweight Digital Physician Scale) and standing height (Seca 213 stadiometer) measured to the nearest 0.1 kg and 0.1 cm, respectively, following standard procedures; for each, the average of two measurements was used. BMI was calculated by dividing the weight (kg) by the height (m) squared.
Muscle strength
Maximum muscle strength was measured by hand‐held dynamometry (kg; Tracker Freedom® Wireless Grip). Participants were tested on their dominant hand and were assessed while sitting on a chair without arm rests, feet flat on the floor, arms close to the body with the elbow flexed at 90°, and the non‐dominant hand supporting the device. Participants were instructed to squeeze the dynamometer as hard as they could, three times with a 15 s rest between trials. The highest value was used for the current analyses. This assessment has shown excellent test–retest reliability [intraclass correlation coefficient (ICC) = 0.99].23
Physical performance tests
Four metre walk test
Gait speed was used to assess mobility. Studies have found gait speed to be strongly associated with adverse outcomes including disability, falls, and mortality.24, 25 Subjects were asked to walk a 4 m distance at their regular pace. Participants were allowed one trial before the actual test. Gait speed was calculated as 4 m divided by the time to walk this distance in seconds (m/s).
Timed up‐and‐go
Timed up‐and‐go (TUG) test is a valid measure of mobility, balance, and the ability to perform activities of daily living26 and is also associated with mortality.27 Participants were seated in an arm chair and were timed on their ability to stand up from the chair, walk a 3 m course, turn around, walk back to the chair, and sit down again (in seconds). The use of daily living assistive devices was permitted for both the 4 m walk and TUG tests. Excellent test–retest reliability, with an ICC of 0.99, was reported for this test.26
Chair rise test
This test is used to measure performance of lower extremity and balance.28 Participants were asked to rise from a chair and sit back down, five times, as quickly as possible, with their arms crossed on their chest. The time was recorded from the initial sitting position, with their back rested on the back of the chair and their knees bent at a 90° angle, to the fifth and final standing position (in seconds). The average time per sit‐to‐stand was used for analyses. In community‐dwelling older adults, test–retest reliability ranges from good to high (ICC: 0.890 and 0.957).29, 30
Standing balance test
Standing balance is a valid and reliable test31 and is used as a predictor of falls.32 Positioned at a 1 m distance from the wall, participants were instructed to stand in balance on one foot, for as long as possible with a maximum time of 60 s. The test was repeated on the other leg and the shortest time recorded was used for analyses.
Physical performance score adjusted for body mass index
A physical performance score including the four tests, (TUG, gait speed, chair rise, and balance tests) was created using the weighted‐sum score method. Physical performance tests measured as time were natural log‐transformed for normalization of the distributions (all except gait speed). All test results were then scaled to Z scores and entered in a factor analysis to obtain the loading value of each test. For every subject, a physical performance score was calculated; the Z score test result of all four physical tests was multiplied by its associated loading value before summation. This method accounts for the weight each test has in measuring the physical performance factor.33 Given the well‐known relationship between BMI and mobility disability, the residual‐based method was used to adjust participants' physical performance score for BMI.34, 35 A binary variable was created to classify participants as having limited physical performance using <1.5 SD below the sex‐specific mean of the physical performance score adjusted for BMI.
Potential covariates
Covariates included sex, age, race/ethnicity, smoking, number of medications, and four self‐reported and ascertained chronic diseases,36 namely, heart disease, kidney disease, chronic obstructive pulmonary disease, and type 2 diabetes that were associated with low lean mass, strength, or physical performance. Physical activity level was evaluated by the Physical Activity Scale for Elderly.37 The abbreviated Seniors in the Community Risk Evaluation for Eating and Nutrition version II, an 11‐item tool to evaluate weight change, meal intake, and its risk factors, was used to evaluate the risk of poor nutritional state.38
Statistical analysis
Analyses were conducted separately by sex because of recognized differences in lean mass and strength between men and women. Baseline characteristics are presented as means ± standard deviations (SD). Relationships between physical performance score, handgrip strength, and ALMI were examined using path analyses. Functionally derived cut‐points were identified using classification and regression tree (CART) analysis, which model is represented by a binary tree. It uses recursive partitioning to optimally classify individuals with a condition (binary‐dependent variable) from one or several factors (independent variables) by computing all possible factors splits while optimizing purity. It is a non‐parametric method that can be performed for prediction purposes and establishing diagnostic tests.39 In this study, CART analysis was carried out in two steps: (i) the first analysis was performed to identify cut‐points for dynapenia (low handgrip strength) as a predictor of physical performance impairment and (ii) using the cut‐points found in (i), a second model was performed to derive cut‐points for sarcopenia (low lean mass) as a predictor of dynapenia. Considering the large sample size, split sample was used for internal validation of the predictive model (80% random training data set and remaining 20% test data set).40 Analyses were performed using the Gini index as a cost function to maximize homogeneity in groups generated from the split, with regard to the outcome variable. CART analyses were previously used to derive cut‐points of low lean mass and strength.18, 19, 41 Agreement between cut‐points was examined using positive and negative per cent agreement (PPA and NPA) and Cohen's kappa coefficient (κ) as recommended by the Food and Drug Administration US federal agency in the absence of a diagnostic gold standard.42 Sensitivity analysis to evaluate prediction capacity of the cut‐points according to subgroup characteristics of the population was conducted using logistic regression models.
To allow comparison with previous sarcopenia definitions, the present cut‐points for low strength and lean mass were combined (sarco‐dynapenia) and applied to our study population. Characteristics of sarco‐dynapenic and non‐sarco‐dynapenic participants were compared using Mann–Whitney U test for non‐normally and independent t‐test for normally distributed variables. Differences in prevalence between current cut‐points and the ones from other cohorts were compared with the use of chi‐square tests. P < 0.05 was accepted as significant. Data analyses were performed using IBM® SPSS Statistics, Amos and Decision trees (version 24, Chicago, Il, USA).
Results
Baseline characteristics of participants
Characteristics of the 9088 participants (48% women) are summarized in Table 1, by sex. The mean age was 72.7 ± 5.5 in men and 72.5 ± 5.5 years in women and were comparable between sexes. The majority of participants were Caucasian (96.7%) and 3.3% were Asian, African‐American, Hispanic, or other ethnicities. The use of prescribed medications was low in both men and women, but women were taking more medications than men (from Mann–Whitney U test; P < 0.001). The mean BMI was in the overweight category range (25–29.9 kg/m2).
Table 1.
Baseline characteristics of the Canadian longitudinal study on aging participants by sex, 2011–2015
| Men (n = 4725) | Women (n = 4363) | |
|---|---|---|
| Age, year | 72.7 ± 5.5 | 72.5 ± 5.5 |
| Caucasian, % | 96.0 | 97.5 |
| Anthropomorphic measurements height, cm | 1.74 ± 0.07 | 1.60 ± 0.06 |
| Weight, kg | 83.9 ± 13.5 | 70.1 ± 13.5 |
| BMI, kg/m2 | 27.8 ± 4.0 | 27.5 ± 5.1 |
| Current smoker, % | 5 | 5 |
| Nutritional risk (SCREEN II‐AB; range 0–48) | 39.6 ± 5.5 | 39.0 ± 5.9 |
| Medication number (range 0–11) | 0.8 ± 0.9 | 1.0 ± 1.0 |
| PASE score (range 0–629) | 129 ± 59 | 111 ± 53 |
| Body composition | ||
| ALM, kg | 25.91 ± 3.75 | 17.24 ± 2.86 |
| ALM index, kg/m2 | 8.57 ± 1.02 | 6.74 ± 0.98 |
| Fat mass, kg | 25.02 ± 7.59 | 29.0 ± 8.89 |
| Strength | ||
| Maximum grip strength, kg | 39.8 ± 8.4 | 23.9 ± 5.1 |
| Physical performance | ||
| BMI‐adjusted physical performance, Z score | 0.17 ± 2.14 | −0.18 ± 2.16 |
| TUG, s | 9.9 ± 1.9 | 10.0 ± 2.0 |
| Gait speed, m/s | 0.95 ± 0.19 | 0.92 ± 0.18 |
| Balance (range 0–60 s) | 28.6 ± 23.1 | 25.1 ± 22.3 |
| Chair rise average time, s | 2.8 ± 0.8 | 2.9 ± 0.8 |
Values are mean ± SD. ALM, appendicular lean mass; BMI, body mass index; PASE, Physical Activity Scale for Elderly; SCREEN II, Seniors in the Community Risk Evaluation for Eating and Nutrition; TUG, timed up‐and‐go.
As expected, ALM, ALMI, and handgrip strength were higher in men than in women. Women had a lower physical performance score.
Associations between appendicular lean mass index, handgrip strength, and physical performance
To investigate the relationship between ALMI, handgrip strength, and physical performance, we used path analyses including all three variables in a first model and adjusting for the covariates fat mass and age in a second model (Figure 1). Results are reported as standardized β (std β) coefficient and R 2. We found positive associations between ALMI and handgrip strength (std β = 0.30 in men and std β = 0.22 in women; all P < 0.001) and between handgrip strength and physical performance score (std β = 0.22 and std β = 0.22; all P < 0.001) that remained after adjustment for covariates. Considered independently of strength, ALMI and physical performance were inversely associated (std β = −0.05 in men, P < 0.001; and std β = −0.19 in women, P = 0.001); however, this association did not remain after adjusting for age and fat mass, given the opposite effects of fat mass on ALMI and physical performance.
Figure 1.
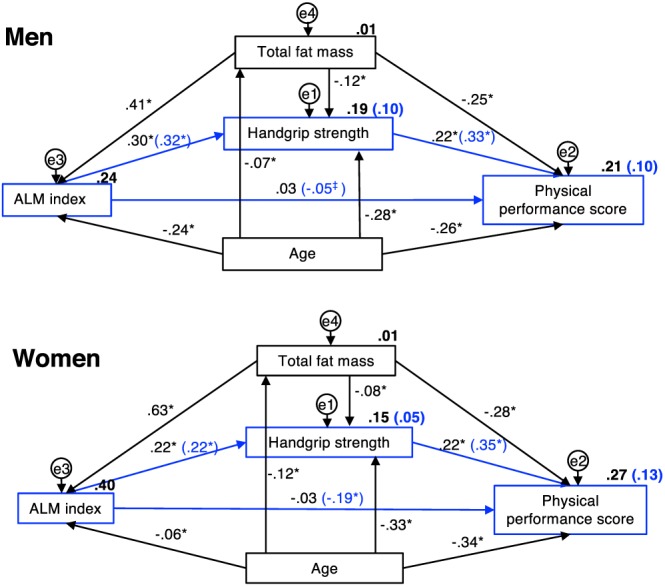
Associations between appendicular lean mass index, handgrip strength, and physical performance. This model was constructed based on the hypothesis that ALMI predicts handgrip strength and that handgrip strength predicts physical performance. Physical performance score is not adjusted for body mass index as fat mass was included in the model. Values along the arrows are expressed as standardized beta coefficient and R 2 (in bold) not adjusted for covariates (model 1, in blue) and adjusted for fat mass and age (model 2, in black). * P value <0.001; ‡ P value <0.05. ALMI, appendicular lean mass index.
Cut‐points for low strength and low appendicular lean mass, and prevalence rates
The significant association of ALMI with strength and strength with physical performance along with the absence of an independent relationship between ALMI and physical performance in the adjusted model justified the identification of cut‐points of low strength as a predictor of physical performance and of low ALMI as a predictor of low strength. To avoid obtaining numerous cut‐points by age groups and to simplify the clinical use of our criteria, age was not included in the statistical models. From CART analyses performed in random sex‐specific training samples (80% of total sex‐specific sample), the optimum splits for handgrip strength to predict limited physical performance were <33.1 kg and <20.4 kg in men and in women, respectively (Figure 2). For low ALM as a predictor of low strength, cut‐points identified were <7.76 kg/m2 in men and <5.72 kg/m2 in women (Figure 3). When applied to their respective test data sets, cut‐points were shown to have excellent validity as supported by highly similar PPA and NPA measures in the training compared with test data sets (data not shown), given this very large study population.
Figure 2.
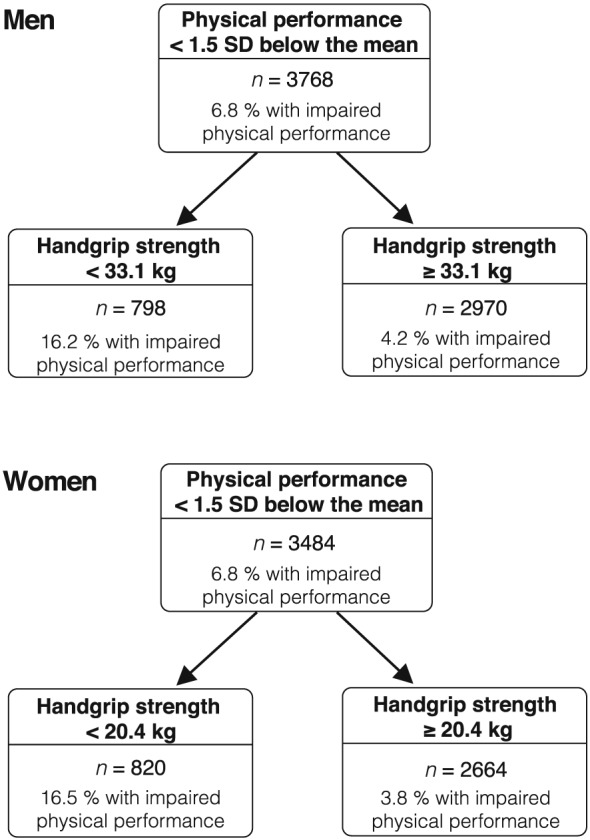
Classification and regression tree results illustrating the handgrip strength cut‐points as predictors of impaired physical performance in men and in women. Results in training samples representing 80% of the total study population.
Figure 3.
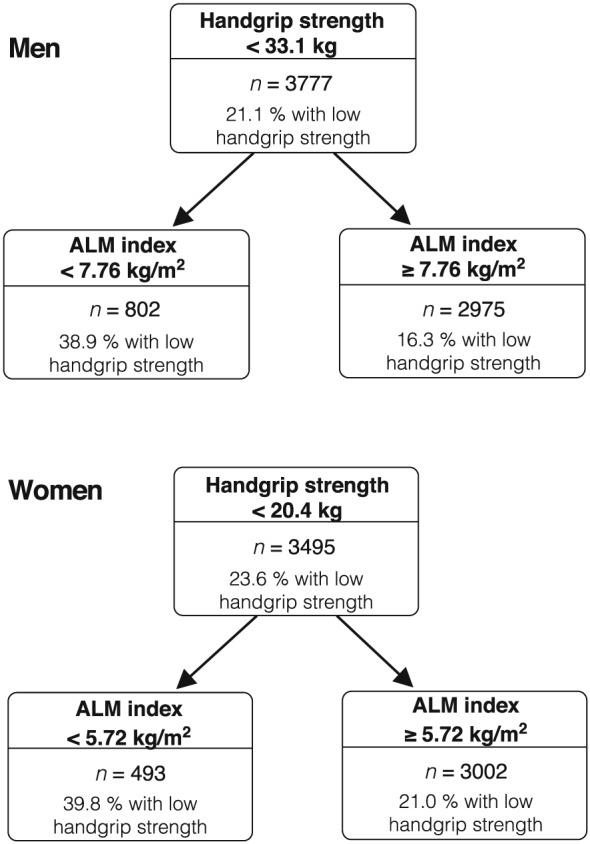
Classification and regression tree results illustrating the ALM index cut‐points as predictors of low handgrip strength in men and in women. Results in training samples representing 80% of the total study population. ALM, appendicular lean mass.
The prevalence of impaired physical performance in the cohort was 6.9% (Supporting Information, Figure S2). Table 2 presents the prevalence rates and sensitivity analysis for both low strength and low ALM cut‐points overall and across different population subgroups. Classifying participants as per the aforementioned criteria, 21.5% of men had low strength and were 4.51 (95% CI: 3.58, 5.68) folds more likely of having impaired physical performance compared with those with normal strength. The prevalence of low strength in women was 24.0% and these persons had 4.66 (95% CI: 3.67, 5.92) folds greater odds for impaired physical performance compared with women with normal strength. The prevalence rates of low ALM were 21.7% and 13.7% in men and in women, respectively. Participants with low ALM had 3.02 (95% CI: 2.59, 3.52 in men) and 2.46 (95% CI: 2.05, 2.95 in women) folds greater odds of having low strength than participants with normal ALM. Overall, 8.3% of men and 5.5% of women had both low strength and lean mass. Agreement measures including PPA, NPA, and Cohen's κ are described in the Online Resource, Supporting Information, Tables S1 and S2.
Table 2.
Sensitivity analysis for strength as a predictor of limited physical performance and for low ALM as a predictor of low strength across subgroups in the Canadian longitudinal study on aging cohort, 2011–2015
| N | Prevalence low HGS (%) | OR (95% CI) for impaired physical performance | P for interactiona | OR (95% CI) for low HGS | |||
|---|---|---|---|---|---|---|---|
| Low HGS | Prevalence low ALM (%) | Low ALM | P for interactionb | ||||
| Men | |||||||
| Overall | 4725 | 21.5 | 4.51 (3.58, 5.68) | 21.7 | 3.02 (2.59, 3.52) | ||
| Age | |||||||
| 65–74 | 2878 | 14.8 | 4.50 (2.87, 7.04) | 0.162 | 16.3 | 2.71 (2.14, 3.43) | 0.777 |
| ≥75 | 1847 | 32.0 | 3.09 (2.34, 4.07) | 30.0 | 2.59 (2.10, 3.19) | ||
| Nutritional risk (SCREEN II‐AB) | |||||||
| Yes | 1332 | 24.2 | 3.09 (2.34, 4.07) | 0.304 | 22.1 | 3.79 (2.87, 5.00) | 0.058 |
| No | 3393 | 20.5 | 4.50 (2.89, 7.04) | 21.5 | 2.74 (2.28, 3.30) | ||
| Heart disease | |||||||
| Yes | 1029 | 25.4 | 4.79 (3.17, 7.24) | 0.640 | 21.4 | 2.79 (2.04, 3.84) | 0.558 |
| No | 3696 | 20.4 | 4.25 (3.21, 5.62) | 21.8 | 3.11 (2.61, 3.71) | ||
| Kidney disease | |||||||
| Yes | 173 | 28.9 | 2.74 (0.97, 7.76) | 0.339 | 28.9 | 4.47 (2.19, 9.10) | 0.264 |
| No | 4552 | 21.2 | 4.61 (3.64, 5.84) | 21.4 | 2.95 (2.52, 3.45) | ||
| COPD | |||||||
| Yes | 273 | 26.0 | 3.55 (1.54, 8.20) | 0.565 | 30.4 | 3.39 (1.92, 5.99) | 0.667 |
| No | 4452 | 21.2 | 4.58 (3.61, 5.82) | 21.1 | 2.98 (2.54, 3.49) | ||
| Type 2 diabetes | |||||||
| Yes | 630 | 27.6 | 4.21 (2.57, 6.90) | 0.853 | 19.7 | 4.01 (2.66, 6.05) | 0.163 |
| No | 4095 | 20.6 | 4.44 (3.41, 5.76) | 22.0 | 2.93 (2.48, 3.45) | ||
| Women | |||||||
| Overall | 4363 | 24.1 | 4.66 (3.67, 5.92) | 13.7 | 2.46 (2.05, 2.95) | ||
| Age | |||||||
| 65–74 | 2706 | 15.4 | 5.43 (3.39, 8.70) | 0.013 | 11.5 | 2.44 (1.86, 3.21) | 0.483 |
| ≥75 | 1657 | 38.2 | 2.69 (2.02, 3.58) | 17.3 | 2.14 (1.65, 2.76) | ||
| Nutritional risk (SCREEN II‐AB) | |||||||
| Yes | 1427 | 27.4 | 2.69 (2.02, 3.58) | 0.867 | 14.0 | 2.07 (1.51, 2.81) | 0.168 |
| No | 2936 | 22.4 | 5.43 (3.39, 8.70) | 13.5 | 2.70 (2.16, 3.37) | ||
| Heart disease | |||||||
| Yes | 519 | 28.7 | 3.01 (1.77, 5.11) | 0.081 | 12.7 | 2.52 (1.49, 4.26) | 0.947 |
| No | 3844 | 23.4 | 5.11 (3.90, 6.70) | 13.8 | 2.47 (2.03, 2.99) | ||
| Kidney disease | |||||||
| Yes | 142 | 31.0 | 3.30 (1.25, 8.67) | 0.480 | 16.9 | 0.70 (0.26, 1.91) | 0.012 |
| No | 4221 | 23.8 | 4.72 (3.69, 6.05) | 13.6 | 2.58 (2.15, 3.10) | ||
| COPD | |||||||
| Yes | 318 | 27.4 | 3.31 (1.64, 6.66) | 0.314 | 15.7 | 2.22 (1.18, 4.15) | 0.736 |
| No | 4045 | 23.8 | 4.85 (3.75, 6.26) | 13.5 | 2.48 (2.05, 3.00) | ||
| Type 2 diabetes | |||||||
| Yes | 376 | 27.4 | 4.48 (2.41, 8.34) | 0.909 | 8.0 | 2.93 (1.38, 6.24) | 0.663 |
| No | 3987 | 23.8 | 4.66 (3.59, 6.05) | 14.2 | 2.47 (2.05, 2.98) | ||
ALMI, appendicular lean mass index; COPD, chronic obstructive pulmonary diseases; HGS, handgrip strength; SCREEN II‐AB, abbreviated Seniors in the Community Risk Evaluation for Eating and Nutrition, version II, score < 38 was considered as at risk of poor nutritional state.
Interaction for absence/presence of low HGS and subgroup characteristics in the prediction of impaired physical performance.
Interaction for absence/presence of low ALM and subgroup characteristics in the prediction of low HGS.
The prevalence rates of low strength and low ALM varied between age groups and between presence or absence of chronic diseases. Reflective of the path analysis associations (Figure 1, model 1), low strength cut‐points more strongly predicted impaired physical performance compared with low ALM as a predictor of low strength in all subgroups except in men at risk of poor nutritional state or with a kidney disease. Cut‐points for low strength and low ALM both had higher prediction of their respective outcome in younger (65–74 years) than older (≥75 years) seniors. In men, the likelihood of impaired physical performance associated with low strength ranged from 2.74 (95% CI: 0.97, 7.76) in individuals with a kidney disease to 4.79 (95% CI: 3.17, 7.24) in those with a heart disease. In women, low strength better predicted impaired physical performance in younger participants and in those without a risk of poor nutritional state 5.43 (95% CI: 3.39, 8.70). The likelihood of having low strength associated with low ALM ranged from 2.74 (95% CI: 2.28, 3.30) in men without a risk of poor nutritional state to 4.47 (95% CI: 2.19, 9.10) in men with a kidney disease; and in women, from non‐significant 0.70 (95% CI: 0.26, 1.91) in persons with a kidney disease to 2.93 (95% CI: 1.38, 6.24) in those with type 2 diabetes. Interactions between classification (low and normal strength or ALM) and characteristics groups, for example, presence/absence of a heart disease, were all non‐significant with the exception of age groups and low handgrip strength, and between presence/absence of a kidney disease and low ALMI in women. Sensitivity analysis by BMI was not performed as the physical performance score was already adjusted for this variable.
Characteristics of sarco‐dynapenic and non‐sarco‐dynapenic participants applying the Canadian longitudinal study on aging criteria
To allow comparison with previous studies using the broader definition of sarcopenia including both low ALM and strength, individuals with both conditions were identified and defined as having sarco‐dynapenia. By definition, sarco‐dynapenic participants had lower ALMI, handgrip strength, and physical performance score than non‐sarco‐dynapenic. They were also older, were less physically active, and had lower weight, BMI, and fat mass (Table 3).
Table 3.
Baseline characteristics of men and women by absence or presence of sarco‐dynapenia applying Canadian longitudinal study on aging cut‐points, 2011–2015
| Men | Women | |||
|---|---|---|---|---|
| Non‐sarco‐dynapenic (n = 4335) | Sarco‐dynapenic (n = 390) | Non‐sarco‐dynapenic (n = 4123) | Sarco‐dynapenic (n = 240) | |
| Prevalence, % | 91.7 | 8.3 | 94.5 | 5.5 |
| Age, year | 72.4 ± 5.4 | 76.5 ± 5.4** | 72.4 ± 5.5 | 75.5 ± 5.5** |
| Caucasian, %b | 96.4 | 92.8* | 97.6 | 96.3 |
| Weight, kg | 85.0 ± 13.2 | 71.5 ± 9.7** | 71.0 ± 13.3 | 55.3 ± 7.2** |
| BMI, kg/m2 | 28.1 ± 4.0 | 24.6 ± 2.8** | 27.7 ± 5.1 | 22.5 ± 2.8** |
| Nutritional risk (SCREEN II‐AB; 0–48) | 39.7 ± 5.5 | 38.5 ± 6.0** | 39.0 ± 5.9 | 38.8 ± 6.2 |
| Medication number (0–11) | 0.8 ± 0.9 | 1.0 ± 0.9** | 1.0 ± 1.0 | 1.1 ± 1.0 |
| PASE score (0–629) | 131 ± 59 | 104 ± 57** | 112 ± 53 | 94 ± 49** |
| Body composition | ||||
| ALM, kg | 26.35 ± 3.53 | 20.96 ± 2.21** | 17.48 ± 2.74 | 13.14 ± 1.21** |
| ALM index, kg/m2 | 8.70 ± 0.97 | 7.20 ± 0.47** | 6.82 ± 0.95 | 5.34 ± 0.31** |
| Total fat mass, kg | 25.3 ± 7.6 | 21.5 ± 6.2** | 29.4 ± 8.9 | 22.0 ± 5.6** |
| Muscle strength | ||||
| Maximal handgrip strength, kg | 40.9 ± 7.9 | 28.3 ± 4.1** | 24.3 ± 4.9 | 17.1 ± 2.7** |
| Physical performance | ||||
| TUG, s | 9.8 ± 1.9 | 10.8 ± 2.2** | 9.9 ± 2.0 | 10.6 ± 2.2** |
| Gait speed, m/sa | 0.96 ± 0.19 | 0.88 ± 0.18** | 0.93 ± 0.18 | 0.87 ± 0.18** |
| Average chair rise time, s | 2.8 ± 0.7 | 3.1 ± 0.1** | 2.9 ± 0.8 | 3.0 ± 0.8 |
| Balance (0–60 s) | 29.5 ± 23.1 | 18.5 ± 19.9** | 25.4 ± 22.3 | 19.8 ± 20.6** |
| BMI‐adjusted physical performance, Z score | 0.26 ± 2.14 | −1.07 ± 2.25** | −0.13 ± 2.22 | −0.88 ± 2.26** |
Values are mean ± SD. ALM, appendicular lean mass; BMI, body mass index; PASE, Physical Activity Scale for Elderly; SCREEN II‐AB, abbreviated Seniors in the Community Risk Evaluation for Eating and Nutrition, version II; TUG, timed up‐and‐go. Mann–Whitney U test unless otherwise specified.
Independent t‐test.
Chi‐square test.
P‐value <0.05.
P‐value <0.001.
Comparison with previous sarcopenia criteria
The cut‐points for low ALM and strength identified in this study were compared with the EWGSOP2 recommendations and the FNIH criteria. The EWGSOP2 endorses ALM/ht2 (kg/m2) ≤ 7.0 for men and ≤6.0 for women as criteria for low ALM and <27 kg for men and <16 kg for women for low handgrip strength.10 The FNIH criteria for low ALM, as defined by an index of ALM (kg) divided by BMI (kg/m2), are <0.789 for men and <0.512 for women,18 and criteria for low handgrip strength are <26 kg for men and <16 kg for women.19 The IWGS defines sarcopenia as the combination of low ALM and low gait speed (<1.0 m/s).8 Because our cut‐points for low strength are derived from limited physical performance, including a measure of gait speed would be redundant and thus, we did not compare our criteria to those suggested by the IWGS. The current population being mainly Caucasian, the Asian criteria9 were also not studied.
Our cut‐points agreement with the EWGSOP2 was fair with κ of 0.15 and 0.43 in men and in women, respectively. PPA of 100% in men and 76.2% in women were found, and 92.4% and 96.2% for NPA (data not shown). Comparison between our combined cut‐points and the FNIH sarcopenia project's criteria showed PPA of only 49.2% in men and 26.9% in women, NPA of 92.3% and 94.8%, and κ of 0.12 and 0.08. This observed disagreement was even more pronounced for the comparison of low ALM cut‐points alone (PPA = 33.8% and 20.3%; NPA = 79.7% and 86.9%; κ = 0.09 and 0.06). We thus examined the CLSA participants characteristics applying the ALM/BMI cut‐points determined by the FNIH.
Characteristics of participants with low appendicular lean mass applying the Canadian longitudinal study on aging and the Foundation for the National Institute of Health criteria
Characteristics of participants by presence or absence of low ALM as per the CLSA criteria and as per the FNIH cut‐points are displayed in Table 4 for men and Table 5 for women. The prevalence rates of low ALM with the CLSA criteria were 21.7% in men and 13.7% in women. Participants characterized as sarcopenic were older and had lower weight and BMI, ALMI, fat mass, strength, and physical performance score than non‐sarcopenic participants. Applying the FNIH sarcopenia project criteria to the CLSA cohort, 10% of men and 8% of women were identified with low ALM/BMI (Online Resource, Supporting Information, Figure S3). Sarcopenic participants as per the FNIH criteria had lower handgrip strength and physical performance score, and they were older and less physically active compared with those with normal ALM/BMI; however, they had only slightly lower ALM and ALMI (in men) but higher weight, BMI, and fat mass compared with participants with normal ALM/BMI. In women, ALMI was not different between individuals with low and normal ALM/BMI. Because of the use of the same ALMI and fair agreement between cut‐points, we did not examine participants characteristics applying the EWGSOP2 criteria.
Table 4.
Descriptive statistics between men with presence or absence of low ALM applying the new Canadian and the FNIH cut‐points, in the Canadian longitudinal study on aging cohort
| Men | Canadian cut‐points | FNIH cut‐points | ||||
|---|---|---|---|---|---|---|
| Non‐sarcopenic (n = 3701) | Sarcopenic (n = 1024) | P a | Non‐sarcopenic (n = 4254) | Sarcopenic (n = 471) | P a | |
| Prevalence, % | 78.3 | 21.7 | 90.0 | 10.0 | ||
| Age, year | 72.2 ± 5.3 | 74.8 ± 5.7 | <0.001 | 72.6 ± 5.5 | 74.2 ± 5.6 | <0.001 |
| Caucasian, % | 96.5 | 94.4 | 0.002c | 96.5 | 92.6 | <0.001c |
| Weight, kg | 87.0 ± 12.8 | 72.6 ± 9.5 | <0.001 | 83.7 ± 13.3 | 86.1 ± 15.4 | 0.001 |
| BMI, kg/m2 | 28.7 ± 3.8 | 24.3 ± 2.7 | <0.001 | 27.4 ± 4.6 | 31.2 ± 4.6 | <0.001 |
| Nutritional risk (SCREEN II‐AB; 0–48) | 39.8 ± 5.4 | 39.2 ± 5.9 | 0.027 | 39.8 ± 5.4 | 38.0 ± 6.1 | <0.001 |
| Medication number (0–11) | 0.8 ± 0.9 | 0.8 ± 0.9 | 0.713 | 0.8 ± 0.9 | 1.1 ± 1.0 | <0.001 |
| PASE score (0–629) | 132 ± 60 | 118 ± 57 | <0.001 | 131 ± 59 | 108 ± 56 | <0.001 |
| Body composition | ||||||
| ALM, kg | 27.1 ± 3.3 | 21.8 ± 2.1 | <0.001 | 26.2 ± 3.6 | 23.0 ± 3.4 | <0.001 |
| ALM index, kg/m2 | 8.93 ± 0.83 | 7.26 ± 0.41 | <0.001 | 8.59 ± 1.00 | 8.35 ± 1.10 | <0.001 |
| Total fat mass, kg | 26.0 ± 7.7 | 21.3 ± 6.0 | <0.001 | 24.4 ± 7.2 | 31.0 ± 8.6 | <0.001 |
| Muscle strength | ||||||
| Maximal handgrip strength, kg | 41.0 ± 8.3 | 35.6 ± 7.4 | <0.001 | 40.5 ± 8.2 | 33.7 ± 7.6 | <0.001b |
| Physical performance | ||||||
| Gait speed, m/s | 0.96 ± 0.19 | 0.93 ± 0.19 | <0.001b | 0.96 ± 0.19 | 0.87 ± 0.18 | <0.001b |
| TUG, s | 9.8 ± 1.9 | 10.2 ± 2.0 | <0.001 | 9.8 ± 1.9 | 10.8 ± 2.3 | <0.001 |
| Chair time average, s | 2.8 ± 0.7 | 2.9 ± 0.8 | <0.001 | 2.8 ± 0.7 | 2.9 ± 0.9 | <0.001 |
| Balance, s | 29.4 ± 23.1 | 25.8 ± 22.6 | <0.001 | 29.8 ± 23.1 | 17.4 ± 19.3 | <0.001 |
| BMI‐adjusted physical performance score | 0.37 ± 2.09 | −0.57 ± 2.18 | <0.001b | 0.26 ± 2.12 | −0.65 ± 2.19 | <0.001b |
Values are mean ± SD. ALM, appendicular lean mass; BMI, body mass index; FNIH, Foundation for the National Institute of Health; PASE, Physical Activity Scale for Elderly; SCREEN II‐AB, abbreviated Seniors in the Community Risk Evaluation for Eating and Nutrition, version II; TUG, timed up‐and‐go.
From Mann–Whitney U test unless otherwise specified.
Independent t‐test.
Chi‐square test.
Table 5.
Descriptive statistics between women with presence or absence of low ALM applying the new Canadian and the FNIH cut‐points, in the Canadian longitudinal study on aging cohort
| Women | Canadian cut‐points | FNIH cut‐points | ||||
|---|---|---|---|---|---|---|
| Non‐sarcopenic (n = 3767) | Sarcopenic (n = 596) | P a | Non‐sarcopenic (n = 4013) | Sarcopenic (n = 350) | P a | |
| Prevalence, % | 86.3 | 13.7 | 92.0 | 8.0 | ||
| Age, year | 72.4 ± 5.5 | 73.6 ± 5.8 | <0.001 | 72.5 ± 5.5 | 72.9 ± 5.7 | 0.294 |
| Caucasian, % | 97.5 | 97.3 | 0.754c | 97.8 | 94.3 | <0.001c |
| Weight, kg | 72.3 ± 12.9 | 56.0 ± 7.0 | <0.01 | 69.8 ± 13.4 | 74.0 ± 14.0 | <0.001 |
| BMI, kg/m2 | 28.3 ± 4.9 | 22.2 ± 2.7 | <0.001 | 27.0 ± 4.9 | 32.1 ± 5.4 | <0.001 |
| Nutritional risk (SCREEN II‐AB; 0–48) | 39.0 ± 5.9 | 38.9 ± 6.1 | 0.765 | 39.2 ± 5.8 | 36.7 ± 6.4 | <0.001 |
| Medication number (0–11) | 1.0 ± 1.0 | 1.0 ± 1.0 | 0.467 | 1.0 ± 1.0 | 1.5 ± 1.1 | <0.001 |
| PASE score (0–629) | 112 ± 53 | 106 ± 49 | 0.035 | 112 ± 53 | 96 ± 51 | <0.001 |
| Body composition | ||||||
| ALM, kg | 17.8 ± 2.6 | 13.6 ± 1.3 | <0.001 | 17.4 ± 2.8 | 15.3 ± 2.6 | <0.001 |
| ALM index, kg/m2 | 6.96 ± 0.87 | 5.36 ± 0.30 | <0.001 | 6.75 ± 0.98 | 6.67 ± 1.06 | 0.120 |
| Total fat mass, kg | 30.1 ± 8.8 | 21.9 ± 5.5 | <0.001 | 28.5 ± 8.7 | 35.4 ± 9.0 | <0.001 |
| Muscle strength | ||||||
| Maximal handgrip strength, kg | 24.3 ± 5.1 | 21.5 ± 4.7 | <0.001 | 24.2 ± 5.1 | 20.8 ± 4.5 | <0.001b |
| Physical performance | ||||||
| Gait speed, m/s | 0.92 ± 0.18 | 0.92 ± 0.19 | 0.932b | 0.93 ± 0.18 | 0.84 ± 0.17 | <0.001b |
| TUG, s | 10.0 ± 2.0 | 10.0 ± 2.0 | 0.802 | 9.9 ± 2.0 | 10.9 ± 2.4 | <0.001 |
| Chair time average, s | 2.9 ± 0.8 | 3.0 ± 0.9 | 0.909 | 2.9 ± 0.8 | 2.9 ± 0.9 | 0.604 |
| Balance, s | 24.9 ± 22.2 | 26.2 ± 22.6 | 0.172 | 25.9 ± 22.4 | 15.4 ± 17.7 | <0.001 |
| BMI‐adjusted physical performance score | −0.10 ± 2.14 | −0.68 ± 2.25 | <0.001b | −0.14 ± 2.14 | −0.73 ± 2.30 | <0.001b |
Values are mean ± SD. ALM, appendicular lean mass; BMI, body mass index; FNIH. Foundation for the National Institute of Health; PASE, Physical Activity Scale for Elderly; SCREEN II‐AB, abbreviated Seniors in the Community Risk Evaluation for Eating and Nutrition, version II; TUG, timed up‐and‐go.
From Mann–Whitney U test unless otherwise specified.
Independent t‐test.
Chi‐square test.
Discussion
Our study showed that greater strength is independently associated with better physical performance and that higher ALMI is independently associated with greater strength, but not with physical performance after adjusting for fat mass and age. From baseline data of the largest Canadian‐representative cohort of older adults, we established sex‐specific empirical cut‐point values to define low strength and low ALMI. Cut‐points of handgrip strength <33.1 kg in men and <20.4 kg in women best predicted the risk of limited physical performance, and the optimum cut‐points of ALMI to identify those at risk of low strength were <7.76 kg/m2 in men and <5.72 kg/m2 in women. These cut‐points had good predictive capacity across different subgroups of the population. Although the CLSA cut‐points agreed with those for sarco‐dynapenia endorsed by the EWGSOP2, poor agreement was found with those of the FNIH sarcopenia project primarily because of the use of a different ALMI, that is, ALM/BMI. To our knowledge, our findings represent the most recent cut‐points for sarcopenia and dynapenia in a contemporary database (recruitment between 2011 and 2015) and the first established in a single large national‐representative population addressing generalizability and methodological issues encountered with previously derived cut‐points.
Association between appendicular lean mass index, strength, and physical performance
Previous studies have examined the relationship between lean mass or sarcopenia and physical limitations and found that strength43 and body fat mass16, 44, 45, 46 were important mediators of the latter. Indeed, in the Health ABC cohort (n = 3075, aged 70–79, African‐American and Caucasian ethnicities), Visser et al. showed greater muscle cross‐sectional area (by computed tomography imaging) to be associated with lower extremity physical function at baseline47 and with incident physical limitations over a 2.5 year follow‐up,43 both independently of body fat mass. While authors did not adjust cross‐sectional results for strength, longitudinal findings were no longer significant after strength was included in the models. Also in the Health ABC cohort, Delmonico et al. reported a protective effect of low ALM/ht2 on incident persistent lower extremity limitation in both sexes before adjustment and found associations to be attenuated after correcting for body fat mass although, again, not adjusted for strength (n = 2976).44 In sarcopenic men as defined by low ALM/ht2 (20% below the mean of the distribution), greater odds for mobility limitations were reported by Dufour et al. after adjusting for co‐morbidities and BMI among other covariates (OR = 6.3, 95% CI: 2.5–16.1).45 Further, these groups investigated the capacity of sarcopenia, relative to fat mass, at predicting physical performance limitations and found the integration of fat mass as part of the index for sarcopenia to be superior compared with ALMI and concluded that sarcopenia should account for fat mass in its definition to allow better identification of individuals at risk of disability. Adding to these findings, we observed the cross‐sectional ALMI‐physical performance relationship to be completely mediated by fat mass and strength. Accounting for strength in our first model, we found that increased ALMI was associated with lower physical performance. Fat mass being positively associated with ALMI, but negatively linked with physical performance, the association between ALMI and physical performance disappeared after adjustment for fat mass suggesting that individuals carrying more weight perform less in any weight‐bearing physical tests. This would explain the higher odds of physical limitations obtained when using ALM relative to fat mass as the predictor. We confirmed that strength and fat mass are drivers of the relationship between ALMI and physical performance in the CLSA cohort. Albeit ALM can be manipulated, for example, through adjustment for fat mass, to become a clinically relevant predictor of functional limitations, the absence of a relationship challenges its use for that purpose and emphasizes that ALM and physical performance are two different conditions. We also observed modest, but stronger associations between ALMI and strength, and strength and physical performance that denotes potential distinct underlying mechanisms for low ALM and low strength but also likely distinct health implications of these conditions. As defended by Clark and Manini, neurologic factors would mostly be responsible for strength along with architectural changes and muscle mass, whereas growth factors, sex‐hormones, inflammation status, physical activity, and genetic background would be determinants of muscle mass, justifying to consider low strength and low ALM as two separate conditions, namely dynapenia and sarcopenia, respectively.5
Comparison to existing criteria
Our cut‐point values for low strength <33.1 kg in men and <20.4 kg in women are higher than those endorsed by the EWGSOP2, that is, <27 kg in men and <16 kg in women that increases the sensitivity of finding persons at risk of impaired physical performance. The latter cut‐points were determined using a sex‐specific T‐score of ≤−2.5 SD based on the mean grip strength of participants aged 32 years (from four pooled British cohorts, 1990–2012, n = 20 108, aged 16–90 years).17 Our values for low strength are rather concordant with those of a T‐score ≤ −2.0 SD, 32 kg and 19 kg for men and for women, also reported in this cohort. The EWGSOP2 recommended cut‐points for low lean mass of <7.0 kg/m2 for men and <6.0 kg/m2 for women were found by Gould et al. from a cohort of young Australian adults (1993–2006, n = 682, aged 20–39 years) using T‐scores ≤ −2.0 SD (<6.94 kg/m2) and ≤−1.0 SD (<6.07 kg/m2) in men and in women, respectively.14 Although the T‐score approach is logical considering that individuals who fall at the extreme left of the ALM distribution have low ALM relative to the rest of the population, it was not derived to identify persons with greater odds of low strength or mobility limitations. Our function‐derived empirical cut‐points for low ALM, <7.76 kg/m2 in men and <5.72 kg/m2 in women showed both fair positive and negative agreement with the EWGSOP2 recommendations that strengthens our argument that our diagnostic values are not only representative of low ALM relative to the mean of younger adults but also as a predictor of dynapenia. However, greater agreement was found in women than in men possibly because of the EWGSOP2 selection of a higher T‐score cut‐point for women (≤−1.0 SD vs. −2.0 SD for men).
Using CART, the FNIH recently established cut‐points for handgrip strength, <26 and <16 kg for men and for women, to predict slowness as defined by gait speed <0.8 m/s.19 From these values, cut‐points were derived for ALM using an index of ALM (kg) divided by BMI (kg/m2) of <0.789 for men and <0.512 for women, as predictors of weakness.18 These values were determined from eight pooled American cohorts and a set of clinical trials including older adults aged ≥65 years recruited between 1992 and 2007 (n = 20 847 for low strength criteria and n = 11 270 for low ALM criteria; 89.9–90.5% Caucasian). Of note, although pooling numerous cohorts provides a large sample size, important limitations pertain to this method, for example, standardization of measurements especially DXA, cross‐calibration of instruments, and selection bias from clinical trials. Not surprisingly, we observed poor agreement of our combined criteria for sarcopenia and dynapenia with the FNIH sarcopenia project's, mainly owed to the use of different ALM indices, that is, ALM/BMI in contrast to ALM/ht2. To better understand the discrepancy, we examined the characteristics of CLSA individuals with low vs. normal ALM as per the FNIH definition and our definition. Interestingly, the FNIH criteria for low ALM identified seniors with a mean BMI falling within the obese category (men: 31.2 ± 4.6 kg/m2 and women: 32.1 ± 5.4 kg/m2), normal mean ALMI (8.35 ± 4.6 kg/m2 and 6.67 ± 1.06 kg/m2), lower strength, although not low as per our criteria or the FNIH's, and having lower physical performance compared with those with normal ALM/BMI. Many of these persons identified as having low ALM are simply obese, or sarcopenic‐obese or ‘true’ sarcopenic. Therefore, although the FNIH criteria predict physical performance well, they are not discriminant to identify body composition characteristics. They would also have a limited use to assess ALM changes in a prospective study design as the ratio would be largely influenced by BMI changes over time. For instance, a loss of ALM and body fat mass (decreased BMI) would be erroneously interpreted as the absence of change in muscle mass.
Prevalence of sarcopenia, dynapenia, and sarco‐dynapenia
From heterogenous definitions used to identify sarco‐dynapenia, the prevalence of the condition was estimated to range from 5% to 13% in older adults aged 60–70 years and higher prevalence of 11–50% was found in those aged above 80 years.48 The EWGSOP and IWGS also reported substantial prevalence of sarco‐dynapenia in several older population settings (age ≥ 50 years): free‐living (1–29%), long‐term care (14–33%), and acute hospital care (10%) settings.49 In the current CLSA cohort, 6.9% had sarco‐dynapenia (both low strength and ALM), a prevalence that falls within previously reported range for this condition. Importantly, very low prevalence rates for sarco‐dynapenia were obtained in the CLSA cohort when applying the EWGSOP2 recommended cut‐points (0.7% in men and 2.3% in women) and also applying the FNIH's (1.4% in men and 1.2% in women).
The FNIH reported prevalence rates in free‐living conditions for low strength of 5.3% and 17.9% in men and in women respectively applying their cut‐points.19 In men and in women of our study, 21.5% and 24.1% had low strength applying our cut‐points. From their CART model, the FNIH obtained an initial split of data for handgrip strength at 31.8 kg in men and 20.0 kg in women but chose to use the values resulting from a second split of the data (within each lower sex‐specific groups) for more conservative cut‐points, explaining the apparent lower prevalence reported by the FNIH. When applying the FNIH criteria to the CLSA cohort, we found lower prevalence of low strength, 4.3% in men and 6.2% in women, compared with the prevalence obtained in the cohorts from which criteria were established. Regarding low ALM/BMI, the FNIH reported 20.2% and 16.7% prevalence rates18 in men and in women and quite similarly, 21.7% and 13.7% had low ALM using the CLSA‐derived criteria. Interestingly, the FNIH criteria resulted in roughly twofolds lower prevalence rates for low ALM when applied to the CLSA cohort (10% in men and 8% in women). It confirms that applying the same cut‐points to different populations leads to divergent prevalence possibly because of disparities in characteristics such as ethnicities, prevalence of obesity that drastically increased over the past decades,50 level of physical activity, and presence of other chronic diseases.
Other health implications of low appendicular lean mass: illnesses and surgery recovery, and survival
Function‐derived ALM cut‐points identify persons at risk for impaired mobility and daily life activities, but low ALM should also be considered in other contexts. Skeletal muscle mass is the largest reservoir of amino acid of the body,51 is a highly metabolically active tissue52 and has important endocrine and immune functions.51 Low muscle mass contributes to frailty and reflects decreased physiological reserves that may promote unfavourable outcomes under physiological stress such as after surgery53 or during illnesses54 because of poorer intrinsic adaptive mechanisms. Previous studies showed lean mass and muscle area to be protective of all‐cause mortality reducing the incidence by 9–20% independently of indicators of obesity (central obesity and fat tissue mass), chronic diseases, and strength.55, 56, 57 Low ALM/BMI has also been associated with mortality,58 but it may be possibly explained by factors that are different than those explaining the association between low ALMI and mortality. For example, a cross‐sectional study in Australian (n = 1005, mean age 62) and Korean (n = 376, mean age 58) cohorts showed greater likelihood of having the metabolic syndrome attributed to higher waist circumference, blood pressure, and triglycerides in participants with lower ALM/BMI, while opposite results were observed for sarcopenia as defined by low ALMI (kg/ht2).59 The presence of chronic diseases may describe the association between low ALM/BMI and mortality reported by Balogun et al. as results were only adjusted for age.58 Contrastingly, in other clinical conditions such as cancer or hospitalization, higher fat mass and normal ALM as observed with the FNIH criteria may confer a survival benefit.60 Thus, adjusting or normalizing ALM for fat mass, weight, or BMI may fail to recognize older adults with ‘true’ low muscle mass, that is, relative to height and impede prediction of clinical outcomes. This reinforces the importance of clearly defining sarcopenia to inform research directions.
Strengths and limitations
As we aimed to determine clinically relevant and simple to apply sex‐specific cut‐points, these are binary which limits their predictive capacity . This would be enhanced by considering other factors involved but they would complexify the use of cut‐points. Although handgrip strength was shown to be a valid proxy for overall muscle strength,61 a recent study reported moderate to poor correlation between handgrip and knee extension strength62 questioning the use of handgrip strength as the sole measure of overall muscle strength. Yet, it is an easy to perform measurement that permits to identify older adults at risk of impaired physical performance. Associations being examined in cross‐sectional data do not permit causal interpretation. The use of cut‐points to predict the incidence of decline in physical performance will be tested with follow‐up data. Further, the CLSA population mostly comprising Caucasian older adults may preclude the application of the present cut‐points to populations of other ethnicities. On the other hand, the new sarcopenia and dynapenia cut‐points are derived from the largest single contemporary cohort in aging, from the use of precise and accurate reference standard for measuring muscle mass and DXA63, 64 and the use of objective, valid, and reliable physical performance measures using standardized procedures, thus ensuring homogeneity in assessments. As well, the creation of a score that encompasses four tests that assess mobility, balance, and physical function altogether represents a novel and robust approach to evaluate physical performance. Another strength is the empirical method to determine cut‐points for low strength and ALM, as well as its application as two separate diagnosis that removes complexity to the term sarcopenia and clarifies its definition.
Conclusion
In conclusion, ALMI is not independently associated with physical performance cross sectionally; strength and fat mass are drivers of this hypothesized relationship. Therefore, combining lean mass, strength, and physical performance within one single sarcopenia definition is not supported and confuses the clinical picture. We reinforce that low ALM should be referred to as sarcopenia and low strength as dynapenia and strongly support the use of ALM/ht2 as an index of lean mass as opposed to normalizing ALM for body fat mass measures. Our functionally derived cut‐points from a single, large, and contemporary cohort are clinically relevant for identifying low strength as a predictor of limited physical performance and low ALM as a predictor of low strength, with a realistic 5–8% prevalence of sarco‐dynapenia. These cut‐points aim to guide researchers and health professionals in the identification of these two conditions in older Caucasian individuals to help design interventions targeted for muscle mass or strength and of clinical outcomes in addition to mobility and function. Further studies investigating the implications of these newly derived cut‐points for low ALMI in hospitalization, length of stay, recovery post‐surgery, ability to respond to treatment, and survival rates are therefore warranted.
Conflict of interest
A.‐J.T., S.S.W., E.R., J.A.M., and S.C. declare that they have no conflict of interest.
Supporting information
Table S1. Agreement of low handgrip strength cut‐points with impaired physical performance
Table S2. Agreement of low lean mass cut‐points with low handgrip strength
Table S3. Agreement of the CLSA with the FNIH criteria for sarcopenia (low lean mass)
Table S4. Agreement of the CLSA with the FNIH criteria for sarco‐dynapenia
Figure S1. Flow of participants in the CLSA cohort
Figure S2. Prevalence rates of impaired physical performance, low strength and low lean mass
Figure S3. Prevalence rates of low gait speed, strength and lean mass reported by the FNIH Sarcopenia Project and applying the FNIH criteria to the CLSA
Acknowledgements
This research was made possible using the data collected by the Canadian longitudinal study on aging (CLSA) led by Drs. Parminder Raina, Christina Wolfson, and Susan Kirkland. The CLSA was and is funded by the Government of Canada through the Canadian Institutes of Health Research (CIHR). We thank Raman Agnihothram for the biostatistics consultation. E.R. received funding from the Strategy for Patient‐Oriented Research (SPOR) through the CIHR. A.‐J.T. was awarded a PhD Graduate Excellence Fellowship by McGill University and a PhD fellowship from the Research Institute of the McGill University Health Centre (RI‐MUHC). The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle.65
Tessier A.‐J., Wing S. S., Rahme E., Morais J. A., and Chevalier S. (2019) Physical function‐derived cut‐points for the diagnosis of sarcopenia and dynapenia from the Canadian longitudinal study on aging, Journal of Cachexia, Sarcopenia and Muscle, 10: 985–999. 10.1002/jcsm.12462.
References
- 1. Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 2006;61:1059–1064. [DOI] [PubMed] [Google Scholar]
- 2. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr 1997;127:990S–991S. [DOI] [PubMed] [Google Scholar]
- 4. Anker SD, Morley JE, von Haehling S. Welcome to the ICD‐10 code for sarcopenia. J Cachexia Sarcopenia Muscle 2016;7:512–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clark BC, Manini TM. Sarcopenia =/= dynapenia. J Gerontol A Biol Sci Med Sci 2008;63:829–834. [DOI] [PubMed] [Google Scholar]
- 6. Bulow J, Ulijaszek SJ, Holm L. Rejuvenation of the term sarcopenia. J Appl Physiol (1985) 2019;126:255–256. [DOI] [PubMed] [Google Scholar]
- 7. Langer HT, Mossakowski AA, Baar K, Alcazar J, Martin‐Rincon M, Alegre LM, et al. Commentaries on viewpoint: rejuvenation of the term sarcopenia. J Appl Physiol (1985) 2019;126:257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc 2011;12:249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2014;15:95–101. [DOI] [PubMed] [Google Scholar]
- 10. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McLean RR, Kiel DP. Developing consensus criteria for sarcopenia: an update. J Bone Miner Res 2015;30:588–592. [DOI] [PubMed] [Google Scholar]
- 12. Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998;147:755–763. [DOI] [PubMed] [Google Scholar]
- 13. Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, Di Iorio A, et al. Age‐associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol (1985) 2003;95:1851–1860. [DOI] [PubMed] [Google Scholar]
- 14. Gould H, Brennan SL, Kotowicz MA, Nicholson GC, Pasco JA. Total and appendicular lean mass reference ranges for Australian men and women: the Geelong osteoporosis study. Calcif Tissue Int 2014;94:363–372. [DOI] [PubMed] [Google Scholar]
- 15. Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci 2014;69:547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Newman AB, Kupelian V, Visser M, Simonsick E, Goodpaster B, Nevitt M, et al. Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc 2003;51:1602–1609. [DOI] [PubMed] [Google Scholar]
- 17. Dodds RM, Syddall HE, Cooper R, Benzeval M, Deary IJ, Dennison EM, et al. Grip strength across the life course: normative data from twelve British studies. PLoS ONE 2014;9:e113637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cawthon PM, Peters KW, Shardell MD, McLean RR, Dam TT, Kenny AM, et al. Cutpoints for low appendicular lean mass that identify older adults with clinically significant weakness. J Gerontol A Biol Sci Med Sci 2014;69:567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alley DE, Shardell MD, Peters KW, McLean RR, Dam TT, Kenny AM, et al. Grip strength cutpoints for the identification of clinically relevant weakness. J Gerontol A Biol Sci Med Sci 2014;69:559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Raina PS, Wolfson C, Kirkland SA, Griffith LE, Oremus M, Patterson C, et al. The Canadian longitudinal study on aging (CLSA). Can J Aging 2009;28:221–229. [DOI] [PubMed] [Google Scholar]
- 21. Kirkland SA, Griffith LE, Menec V, Wister A, Payette H, Wolfson C, et al. Mining a unique Canadian resource: the Canadian longitudinal study on aging. Can J Aging 2015;34:366–377. [DOI] [PubMed] [Google Scholar]
- 22. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307–310. [PubMed] [Google Scholar]
- 23. Abizanda P, Navarro JL, Garcia‐Tomas MI, Lopez‐Jimenez E, Martinez‐Sanchez E, Paterna G. Validity and usefulness of hand‐held dynamometry for measuring muscle strength in community‐dwelling older persons. Arch Gerontol Geriatr 2012;54:21–27. [DOI] [PubMed] [Google Scholar]
- 24. Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, et al. Gait speed and survival in older adults. JAMA 2011;305:50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Abellan van Kan G, Rolland Y, Andrieu S, Bauer J, Beauchet O, Bonnefoy M, et al. Gait speed at usual pace as a predictor of adverse outcomes in community‐dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging 2009;13:881–889. [DOI] [PubMed] [Google Scholar]
- 26. Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991;39:142–148. [DOI] [PubMed] [Google Scholar]
- 27. Bergland A, Jorgensen L, Emaus N, Strand BH. Mobility as a predictor of all‐cause mortality in older men and women: 11.8 year follow‐up in the Tromso study. BMC Health Serv Res 2017;17:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lord SR, Murray SM, Chapman K, Munro B, Tiedemann A. Sit‐to‐stand performance depends on sensation, speed, balance, and psychological status in addition to strength in older people. J Gerontol A Biol Sci Med Sci 2002;57:M539–M543. [DOI] [PubMed] [Google Scholar]
- 29. Bohannon R, Shove ME, Barreca SR, Masters LM, Sigouin CS. Five‐repetition sit‐to‐stand test performance by community‐dwelling adults: a preliminary investigation of times, determinants, and relationship with self‐reported physical performance. Isokinetics and Exercise Science 2007;15:77–81. [Google Scholar]
- 30. Tiedemann A, Shimada H, Sherrington C, Murray S, Lord S. The comparative ability of eight functional mobility tests for predicting falls in community‐dwelling older people. Age Ageing 2008;37:430–435. [DOI] [PubMed] [Google Scholar]
- 31. Bohannon RW, Leary, KM . Standing balance and function over the course of acute rehabilitation. Arch Phys Med Rehabil 1995:76(11):994–996. [DOI] [PubMed] [Google Scholar]
- 32. Thorbahn L, Newton R: Use of the Berg Balance Test to predict falls in elderly persons. 1996. [DOI] [PubMed]
- 33. DiStefano C, Zhu M, Mîndrila D. Understanding and using factor scores: considerations for the applied researcher. Pract Assess Res Eval 2009;14. [Google Scholar]
- 34. Marsh AP, Rejeski WJ, Espeland MA, Miller ME, Church TS, Fielding RA, et al. Muscle strength and BMI as predictors of major mobility disability in the lifestyle interventions and independence for elders pilot (LIFE‐P). J Gerontol A Biol Sci Med Sci 2011;66A:1376–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997;65:1220S–1228S, discussion 1229S‐1231S. [DOI] [PubMed] [Google Scholar]
- 36. Raina PS, Wolfson C, Kirkland SA, Keshavarz H, Griffith LE, Patterson C, et al. Ascertainment of chronic diseases in the Canadian longitudinal study on aging (CLSA), systematic review. Can J Aging 2009;28:275–285. [DOI] [PubMed] [Google Scholar]
- 37. Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol 1993;46:153–162. [DOI] [PubMed] [Google Scholar]
- 38. Keller HH, Goy R, Kane SL. Validity and reliability of SCREEN II (Seniors in the community: risk evaluation for eating and nutrition, Version II). Eur J Clin Nutr 2005;59:1149–1157. [DOI] [PubMed] [Google Scholar]
- 39. Song YY, Lu Y. Decision tree methods: applications for classification and prediction. Shanghai Arch Psychiatry 2015;27:130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Steyerberg EW, Harrell FE Jr, Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol 2001;54:774–781. [DOI] [PubMed] [Google Scholar]
- 41. Duchowny KA, Peterson MD, Clarke PJ. Cut points for clinical muscle weakness among older Americans. Am J Prev Med 2017;53:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Food and Drug Administration, Center for Devices and Radiological Health : Statistical guidance on reporting results from studies evaluating diagnostic tests—guidance for industry and FDA staff; in services USDoHaH (ed), 2007.
- 43. Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well‐functioning older persons. J Gerontol A Biol Sci Med Sci 2005;60:324–333. [DOI] [PubMed] [Google Scholar]
- 44. Delmonico MJ, Harris TB, Lee JS, Visser M, Nevitt M, Kritchevsky SB, et al. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J Am Geriatr Soc 2007;55:769–774. [DOI] [PubMed] [Google Scholar]
- 45. Dufour AB, Hannan MT, Murabito JM, Kiel DP, McLean RR. Sarcopenia definitions considering body size and fat mass are associated with mobility limitations: the Framingham Study. J Gerontol A Biol Sci Med Sci 2013;68:168–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 2002;50:889–896. [DOI] [PubMed] [Google Scholar]
- 47. Visser M, Kritchevsky SB, Goodpaster BH, Newman AB, Nevitt M, Stamm E, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc 2002;50:897–904. [DOI] [PubMed] [Google Scholar]
- 48. von Haehling S, Morley JE, Anker SD. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle 2010;1:129–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cruz‐Jentoft AJ, Landi F, Schneider SM, Zuniga C, Arai H, Boirie Y, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014;43:748–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hruby A, Hu FB. The epidemiology of obesity: a big picture. Pharmacoeconomics 2015;33:673–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr 2006;84:475–482. [DOI] [PubMed] [Google Scholar]
- 52. Wang Z, Ying Z, Bosy‐Westphal A, Zhang J, Schautz B, Later W, et al. Specific metabolic rates of major organs and tissues across adulthood: evaluation by mechanistic model of resting energy expenditure. Am J Clin Nutr 2010;92:1369–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wagner D, DeMarco MM, Amini N, Buttner S, Segev D, Gani F, et al. Role of frailty and sarcopenia in predicting outcomes among patients undergoing gastrointestinal surgery. World J Gastrointest Surg 2016;8:27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bagshaw SM, McDermid RC. The role of frailty in outcomes from critical illness. Curr Opin Crit Care 2013;19:496–503. [DOI] [PubMed] [Google Scholar]
- 55. Spahillari A, Mukamal KJ, DeFilippi C, Kizer JR, Gottdiener JS, Djousse L, et al. The association of lean and fat mass with all‐cause mortality in older adults: the Cardiovascular Health Study. Nutr Metab Cardiovasc Dis 2016;26:1039–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Srikanthan P, Karlamangla AS. Muscle mass index as a predictor of longevity in older adults. Am J Med 2014;127:547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Reinders I, Murphy RA, Brouwer IA, Visser M, Launer L, Siggeirsdottir K, et al. Muscle quality and myosteatosis: novel associations with mortality risk: the Age, Gene/Environment Susceptibility (AGES)‐Reykjavik Study. Am J Epidemiol 2016;183:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Balogun S, Winzenberg T, Wills K, Scott D, Jones G, Aitken D, et al. Prospective associations of low muscle mass and function with 10‐year falls risk, incident fracture and mortality in community‐dwelling older adults. J Nutr Health Aging 2017;21:843–848. [DOI] [PubMed] [Google Scholar]
- 59. Scott D, Park MS, Kim TN, Ryu JY, Hong HC, Yoo HJ, et al. Associations of low muscle mass and the metabolic syndrome in Caucasian and Asian middle‐aged and older adults. J Nutr Health Aging 2016;20:248–255. [DOI] [PubMed] [Google Scholar]
- 60. Martin L, Senesse P, Gioulbasanis I, Antoun S, Bozzetti F, Deans C, et al. Diagnostic criteria for the classification of cancer‐associated weight loss. J Clin Oncol 2015;33:90–99. [DOI] [PubMed] [Google Scholar]
- 61. Bohannon RW, Magasi SR, Bubela DJ, Wang YC, Gershon RC. Grip and knee extension muscle strength reflect a common construct among adults. Muscle Nerve 2012;46:555–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yeung SSY, Reijnierse EM, Trappenburg MC, Hogrel JY, McPhee JS, Piasecki M, et al. Handgrip strength cannot be assumed a proxy for overall muscle strength. J Am Med Dir Assoc 2018;19:703–709. [DOI] [PubMed] [Google Scholar]
- 63. Buckinx F, Landi F, Cesari M, Fielding RA, Visser M, Engelke K, et al. Pitfalls in the measurement of muscle mass: a need for a reference standard. J Cachexia Sarcopenia Muscle 2018;9:269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hangartner TN, Warner S, Braillon P, Jankowski L, Shepherd J. The official positions of the international society for clinical densitometry: acquisition of dual‐energy X‐ray absorptiometry body composition and considerations regarding analysis and repeatability of measures. J Clin Densitom 2013;16:520–536. [DOI] [PubMed] [Google Scholar]
- 65. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2017. J Cachexia Sarcopenia Muscle 2017;8:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Agreement of low handgrip strength cut‐points with impaired physical performance
Table S2. Agreement of low lean mass cut‐points with low handgrip strength
Table S3. Agreement of the CLSA with the FNIH criteria for sarcopenia (low lean mass)
Table S4. Agreement of the CLSA with the FNIH criteria for sarco‐dynapenia
Figure S1. Flow of participants in the CLSA cohort
Figure S2. Prevalence rates of impaired physical performance, low strength and low lean mass
Figure S3. Prevalence rates of low gait speed, strength and lean mass reported by the FNIH Sarcopenia Project and applying the FNIH criteria to the CLSA


