Abstract
Background and aims
The mechanism by which vegetarian diets are associated with less inflammation is not clear. We investigated the role of BMI as a mediator in the relationship between vegetarian diet and concentrations of C-reactive protein (CRP), and the cytokines IL-6, IL-10 and TNF-α.
Methods and results
We used data from participants of the Adventist Health Study 2 (AHS-2) Calibration (n = 893) and Biological Manifestations of Religion (n = 478) sub-studies. Vegetarian diet variations were determined based on reported intake of animal products assessed by FFQ. Combining all participants, the proportion of non-vegetarians (NVs), partial vegetarians (PVs), lacto-ovo vegetarians (LOVs), and strict vegetarians (SVs) was 44%, 16%, 31%, and 9%, respectively. NV and PV participants were older than other dietary groups, and non-vegetarians had the highest BMI. Mediation analyses supported the mediating effect of BMI in associations of vegetarian diet with CRP (p < 0.001 each for PV, LOV and SV), and with IL-6 (p < 0.05 each for PV, LOV and SV). Mediation by BMI was not evident between vegetarian diet and the biomarkers IL-10 and TNF-α. A direct pathway was significant only in the association between strict vegetarians and CRP (p = 0.017).
Conclusion
The lower CRP and IL-6 concentrations among vegetarians may be mediated by BMI.
Keywords: Vegetarian, CRP, Cytokines, Biomarkers, BMI
Introduction
The cytokines and C-reactive protein are biomarkers with pro-inflammatory or anti-inflammatory properties, or both. Inflammatory biomarkers are influenced by genetic, environmental, and/or lifestyle factors, among which diet is thought to be particularly influential [1,2]. Vegetarian diets generally contain higher amounts of foods that are thought to be anti-inflammatory such as those of plant origin, and somewhat lower amounts of inflammation-related foods such as fried foods, white bread, and meats found in Western diets.
Reports comparing levels of inflammatory biomarkers among vegetarians and non-vegetarians are inconclusive. Among these, results for CRP, IL-6, interleukin-10 (IL-10), and TNF-α are mixed. The relationship between CRP and vegetarian diets differs depending on the particular population and study design [3–6]. Studies on IL-10 and TNF-α do not show a difference between vegetarians and non-vegetarians [7,8], while IL-6 is significantly lower among vegetarians in one study [8] but not in another [7]. In a 12-week intervention study, participants following a plant-based diet have significantly lower CRP and lower IL-6 levels, but no change in TNF-α after the intervention [9]. A recent meta-analysis shows that following a vegetarian diet for at least 2 years is associated with lower hs-CRP [10].
Vegetarian diets generally are associated with lower body weight in observational and randomized controlled studies [11]. Obesity is characterized by a state of chronic, low-grade inflammation, due to the release of cytokines such as IL-6 and TNF-α from adipocytes [12]. However, weight loss can have a favorable impact on inflammation by lowering CRP, IL-6 and TNF-α [13]. Based on these considerations, body mass index (BMI) may constitute a mechanism whereby vegetarian diet is related to inflammation. In this study we examined the role of BMI as a mediator in the associations between vegetarian diets and the inflammatory biomarkers which we measured (CRP, IL-6, IL-10, and TNF-α), and hypothesized that lower concentrations of inflammatory biomarkers associated with vegetarian diets are mediated by BMI.
Methods
Parent study
The Adventist Health Study-2 (AHS-2) is a cohort of 96,194 Seventh-day Adventist (SDA) men and women located throughout North America. AHS-2 was designed to examine associations between lifestyle and risk of colon, prostate, breast, and other cancers. Recruitment and selection methods have been described previously [14]. Briefly, members of SDA churches were recruited between 2002 and 2007. Subjects were enrolled if their age was at least 30 years, and they completed the baseline AHS-2 questionnaire. Approximately 27% of the cohort is Black, of US or Caribbean origin. Those remaining are of other races, primarily White. The institutional review board of Loma Linda University approved the study protocol, and all study participants provided written consent at the time of enrollment.
Study population
The study samples for this report include participants from the Biological Manifestations of Religion Sub-study (BioMRS) [15] and the AHS-2 Calibration Sub-study [14], as in these two sub-studies blood samples were collected. A random sample of AHS-2 cohort members was selected in 2006–2007 to participate in the Psychosocial Manifestations of Religion Sub-study (PsyMRS) [15]. A subset of the PsyMRS cohort also formed the BioMRS sub-study, which includes individuals who were age 50 + years at the time of enrollment in AHS-2 and resided within 50 miles of Loma Linda, or Los Angeles, CA. The Calibration Sub-study participants were randomly selected from the parent cohort by church and then subject-within-church [16]. Both Calibration and BioMRS participants attended a clinic at their local church where anthropometric measurements and fasting blood samples were collected.
Two analytic samples were used: 478 participants from BioMRS for analyses of IL-6, IL-10, and TNF-α and 1371 combined participants from Calibration and BioMRS for analyses of CRP. Demographic, lifestyle and FFQ data were obtained from the AHS-2 lifestyle questionnaire; anthropometric measures and blood samples were obtained during the calibration sub-study and BioMRS clinics.
Vegetarian diets
Dietary intake was assessed by a food frequency questionnaire, and vegetarian diets were determined according to the reported frequency of animal-based food consumption, which includes meats (red meats + poultry), fish, and dairy (dairy products + eggs). Strict vegetarians consumed meat, fish, and dairy never or rarely (<1 per month); lacto-ovo vegetarians ate meat and fish never or rarely, and dairy ≥ 1 per month; pesco vegetarians consumed meat never or rarely, fish ≥ 1 per month, and no limits on dairy; semi vegetarians ate meat 1/month to 1/week, combined meat and fish 1/month to 1 per week, and no limits on dairy; and non-vegetarians ate meat and fish >1 per week with no limits on dairy.
We performed guided multiple imputation [17] for the small amount of missing data in the dietary variables used to define dietary pattern, at the level of the individual food variables, and conditional on other covariates and the dependent variable. The FFQ was previously validated against six 24-h dietary recalls for intake of selected foods/food groups [18]. Correlations for red meat, poultry, fish, dairy and eggs were moderate to high. Sociodemographic and other factors.
Variables assessed at baseline included age, gender, race (White or Black), education (≤high school, some college, ≥Bachelor’s degree), cigarette smoking (ever or never), alcohol use (ever or never), use of non-steroidal anti-inflammatory drugs (NSAIDs, never used or current use), and cholesterol-lowering medication (never used or current use). Physical activity was scored as average minutes per week of combined vigorous and extremely vigorous activities, and then categorized as low (0 min/week), medium (>0–60 min/week), and high (>60 min/week). Body mass index was calculated as body weight/height squared (kg/m2).
Laboratory methods
Collection and processing of blood after an overnight fast followed standard protocols [19]. Serum was separated from the cells within 30 min of collection, placed on wet ice, and then shipped or transported to the central study laboratory at Loma Linda, CA for further processing. Blood was aliquoted to 0.5 mL samples and immediately stored in liquid nitrogen until analysis. Determination of C-reactive protein was performed separately for BioMRS and Calibration Study subjects, whereas IL-6, IL-10, and TNF-α were measured only in BioMRS participants.
For BioMRS samples, concentrations of CRP, IL-6, IL-10 and TNF-α were measured in duplicate samples using enzyme linked immunosorbent assay (ELISA) kits from R & D Systems (Minneapolis, MN, USA) for IL-6, Thermo Fisher Scientific (Waltham, MA, USA) for IL-10 and TNF-α, and Assaypro (St. Charles, MO, USA) for CRP. For IL-6, the detectable limit was 0.039 pg/mL, intra- and inter-assay CV were 6.9%, and 7.2%; for IL-10, these were 3 pg/mL, 8.7%, and 9.4%, and 2 pg/mL; for TNF-α, the minimum detectable concentration was 2 pg/mL, intra-assay CV 4.2%, and interassay CV 5.2%; and for CRP, these were 100 pg/mL, 5.5%, and 7.6%, respectively. For the Calibration study samples, high-sensitivity C-reactive protein (hs-CRP) concentration was determined in duplicate samples using latex particle enhanced immunoturbidimetric assay kits from Pointe Scientific, Inc. (Canton, MI, USA). The minimum detectable concentration, intra-assay coefficient of variation (CV) and inter-assay CV were 0.1 mg/L, 8.5%, and 10.5%, respectively.
Statistical analysis
We combined the pesco- and semi-vegetarians into the partial vegetarian dietary group to ensure adequate numbers in each group. Thus four dietary patterns were used: strict vegetarian (SV), lacto-ovo vegetarian (LOV), partial vegetarian (PV), and non-vegetarian (NV). Selected demographic and lifestyle characteristics were compared according to dietary pattern using ANOVA for continuous, and χ2 tests for categorical variables.
CRP was a combination of the high-sensitivity CRP collected in the Calibration and the ELISA CRP collected in the BioMRS sub-studies. Where respondents were in both studies (n = 9), we preferred the high sensitivity values, which were strongly correlated with the ELISA CRP (r = 0.7). Distributions of CRP were similar in both studies, with a narrower distribution for the high-sensitivity CRP. To account for differences between the CRP sources, study and study-diet pattern differences were explored. There was no significant modification of the diet-CRP association by study (p > 0.40 for every diet pattern). However, a lower mean CRP was observed in BioMRS (difference was 0.639 mg/L). This difference was added back on to the BioMRS to place both studies on the same scale. A logarithmic transformation was then applied to CRP, IL-6 and IL-10 to approximate normality. TNF-α had an irregular distribution that was not improved by logarithmic transformation, therefore TNF-α was categorized to zero (or below limit of detection), and the non-zero values were divided to two equal sized ordered categories. For this variable ordinal logistic regression was used.
Mediation analysis
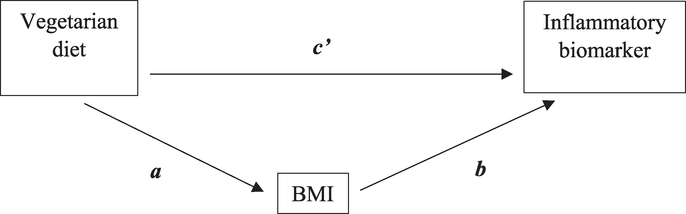
Mediation analyses were performed for all biomarkers. Indirect, direct, and total effects were calculated using the methods as previously described [20–22]. Fig. 1 illustrates the direct and indirect pathways. The indirect effect represents the effect through the mediator pathway, and is quantified by the product of the coefficient relating the main exposure variable (vegetarian diet) to the mediator (a) and the coefficient relating the mediator (BMI) with the inflammatory biomarker (b). The relative effect (c’) represents the effect through the direct pathway from the exposure to the outcome, while controlling for the mediator. The total effect is the sum of the indirect and the direct effects (ab + c’). Confidence intervals were approximated through bootstrapping with BCa correction. All models controlled for age, gender, race, education, cigarette smoke, alcohol use, physical activity, and use of NSAIDs and statins. Inclusion of energy intake did not change the estimates appreciably; therefore we report results from analyses excluding energy intake. Finally, we repeated the analyses in a sub-group of subjects without acute inflammatory status (CRP<10 mg/L). Estimates from sub-group analyses were similar to that of the full sample, therefore we report results from the full analytic sample. Analyses were carried out using SAS statistical software package release 9.3 (SAS Institute Inc., Cary, NC, USA) and R version 2.10.1 (http://www.r-project.org/). The mediation R package version 3.4.3 by Tingley et al. [23] was used for mediation analyses for CRP, IL-6 and IL-10. The same methods were used for TNF-α, but using instead a multinomial logistic regression.
Figure 1.
Mediation of BMI on the relationship between vegetarian diet and inflammation. This figure illustrates how mediation effects of BMI were estimated in the association between vegetarian diet and inflammatory biomarkers. Mediation occurs when part of the association between vegetarian diet and the biomarker is explained by BMI. In two separate regression models including all covariates, the association between vegetarian diet and BMI (path a) and the association between BMI and the biomarker independent of vegetarian diet (path b) are determined. The product of regression coefficients a and b (ab) represents the relative indirect ‘effect’ by BMI in the relation between vegetarian diet and the biomarker. The part of the association not explained by the mediator BMI is the relative direct ‘effect’ of vegetarian diet on the biomarker (path c’).
Results
Univariate analyses comparing selected demographic and lifestyle characteristics by vegetarian dietary pattern are presented in Table 1. Among the Calibration Sub-study subjects, the proportion of SVs, LOVs, PVs, and NVs was 9%, 28%, 16%, and 47%, respectively. Age was significantly associated with dietary pattern, with NVs being the youngest and SVs the oldest (p < 0.0001). Higher proportions of partial and non-vegetarians were Black, and there were more Whites than Blacks among SVs and LOVs (p < 0.0001). Participants were mostly never smokers, but rates of (predominately) past smoking differed among dietary groups (p = 0.0002). Most had never used cholesterol-lowering medications, but again this differed between dietary groups (p = 0.01) with greater use by non-vegetarians. SVs, LOVs, and PVs were more likely to have never consumed alcohol compared to non-vegetarians (p < 0.001).
Table 1.
Comparison of selected demographics and lifestyle characteristics according to vegetarian dietary patterns in the biological manifestations of religion sub-study (BioMRS) and adventist health Study-2 (AHS-2) calibration sub-study.
| Strict vegetarian | Lacto-ovo vegetarian | Partial vegetarian | Non vegetarian | p-value | |
|---|---|---|---|---|---|
| AHS-2 Calibration Sub-study | |||||
| n(%) | 83 (9.3) | 247 (27.7) | 143 (16.0) | 420 (47.0) | |
| Age in years, mean (SD) | 63.8 (12.7) | 60.1 (14.0) | 57.3 (12.8) | 56.3 (12.9) | <0.0001 |
| BMI (kg/m2) Gender(%) | 25.3 (5.8) | 25.9 (5.7) | 27.4 (5.6) | 30.1 (7.2) | <0.0001 0.16 |
| Gender(%) | 0.16 | ||||
| Females | 73.5 | 61.5 | 68.5 | 67.9 | |
| Males | 26.5 | 38.5 | 31.5 | 31.9 | |
| Race (%) | <0.0001 | ||||
| Non-black | 59.0 | 74.9 | 48.2 | 47.0 | |
| Blacks | 41.0 | 25.1 | 51.8 | 52.9 | 0.50 |
| Education (%) | |||||
| High school or less | 24.1 | 19.0 | 23.1 | 21.7 | |
| Some college | 39.8 | 37.7 | 38.5 | 42.4 | |
| Bachelor or above | 34.9 | 42.5 | 37.1 | 33.8 | |
| Alcohol use | <0.001 | ||||
| Ever | 27.7 | 26.3 | 39.9 | 51.2 | |
| Never | 69.9 | 71.7 | 58.7 | 47.1 | |
| Cigarette smoke | 0.0002 | ||||
| Ever | 9.6 | 11.7 | 16.1 | 23.3 | |
| Never | 90.4 | 88.3 | 83.9 | 76.4 | |
| Cholesterol-lowering medication | 0.01 | ||||
| Currently using | 7.2 | 10.5 | 10.5 | 17.1 | |
| Not using | 92.8 | 89.5 | 89.5 | 82.9 | |
| Physical Activity (%) | 0.04 | ||||
| 0 min/wk | 21.7 | 29.6 | 32.9 | 29.1 | |
| >0–60 min/wk | 49.4 | 47.4 | 44.1 | 38.1 | |
| >60 min/wk | 28.9 | 23.1 | 23.1 | 32.9 | |
| BioMRS | |||||
| n(%) | 32 (6.8) | 173 (36.9) | 77 (16.4) | 187 (39.9) | |
| Age in years, mean (SD) | 59.5 (11.6) | 66.9 (12.1) | 67.8 (11.7) | 61.9 (10.0) | <0.0001 |
| BMI (kg/m2) | 25.7 (5.4) | 25.7 (4.6) | 27.4 (5.3) | 29.5 (6.6) | <0.0001 |
| Gender (%) | 0.68 | ||||
| Females | 62.5 | 59.5 | 67.5 | 62.6 | |
| Males | 37.5 | 40.5 | 32.5 | 36.9 | |
| Race (%) | <0.0001 | ||||
| Non-black | 34.4 | 83.2 | 70.1 | 46.0 | |
| Blacks | 65.6 | 16.8 | 29.9 | 52.9 | |
| Education (%) | <0.0001 | ||||
| High school or less | 0.0 | 3.5 | 6.5 | 7.0 | |
| Some college | 34.4 | 17.9 | 28.6 | 46.0 | |
| Bachelor or above | 62.5 | 78.0 | 64.9 | 45.5 | |
| Alcohol use (%) | <0.0001 | ||||
| Ever | 25.0 | 17.3 | 33.8 | 38.0 | |
| Never | 75.0 | 82.1 | 64.9 | 59.9 | |
| Smoking (%) | 0.01 | ||||
| Ever | 9.4 | 6.9 | 14.3 | 18.7 | |
| Never | 90.6 | 93.1 | 85.7 | 80.8 | |
| NSAIDs (%) | 0.02 | ||||
| Currently using | 12.5 | 23.7 | 27.3 | 34.8 | |
| Not using | 7.5 | 76.3 | 72.7 | 65.2 | |
| Physical Activity (%) | 0.13 | ||||
| 0 min/wk | 34.4 | 32.4 | 39.0 | 24.6 | |
| >0–60 min/wk | 46.9 | 49.7 | 49.4 | 49.7 | |
| >60 min/wk | 18.8 | 17.9 | 11.7 | 25.1 | |
Comparison of demographic and lifestyle characteristics by dietary pattern was done using χ2 test, except age and BMI (ANOVA).
Selected characteristics among BioMRS participants are presented in the lower portion of Table 1. Blacks tended to be SVs and NVs, and more Whites than Blacks were LOVs and PVs (p < 0.0001). Participants were generally well educated, with SVs, LOVs and partial vegetarians having greater education than non-vegetarians (p < 0.0001). Partial vegetarians were the oldest and SVs the youngest (p < 0.0001). The majority of participants were never smokers, and among those who ever smoked, the highest proportion were non-vegetarians (p = 0.01); this was also the case among users of alcohol (p < 0.0001). The use of NSAIDS medication was more common among non-vegetarians (p < 0.02).
The mediating effects of BMI in the association between vegetarian diet and inflammatory biomarkers are shown in Table 2. As an example, compared to non-vegetarian the total effect (path c) of the association between strict vegetarian and CRP was β = −0.680 (95%CI−1.071, −0.290).This association was attenuated after adjusting for BMI (direct effect or path c’) (β = −0.395; 95% CI −0.795, 0), but remained significant. The magnitude of the mediating (indirect) effect of BMI (path ab) was β = −0.285 (95% CI −0.425, −0.170), which explained 42% of the association. For the lacto-ovo and partial vegetarian diets, the proportion of the indirect effect accounted by BMI was 67% and 52%, respectively. Overall, the indirect effect of BMI in the association between vegetarian diet (strict, lacto-ovo, and partial) and inflammation was significant for CRP and IL-6, but not for IL-10 and TNF-α. Except for the association between strict vegetarian and CRP, we found no evidence of a direct effect of vegetarian diet on any of the inflammatory biomarkers. The total effect for IL-6 was not significant due to an estimated direct effect that appeared to completely compensate for the significant indirect effect. However, this direct effect had a very large standard error and the corresponding true effect may well be non-compensating or null.
Table 2.
The mediating role of BMI in the associations between vegetarian diet and inflammatory biomarkers.
| Path | Effect | β | 95% CIa | |
|---|---|---|---|---|
| C-reactive protein | ||||
| Strict vegetarian | c | Total | −0.680 | −1.071, −0.290** |
| c’ | Direct | −0.395 | −0.795, 0.000* | |
| ab | Indirect | −0.285 | −0.425, −0.170** | |
| Lacto-ovo vegetarian | c | Total | −0.356 | −0.601, −0.100** |
| c’ | Direct | −0.118 | −0.366, 0.130 | |
| ab | Indirect | −0.238 | −0.337, −0.160** | |
| Partial vegetarian | c | Total | −0.316 | −0.627, −0.040* |
| c’ | Direct | −0.152 | −0.450, 0.120 | |
| ab | Indirect | −0.164 | −0.259, −0.080** | |
| IL-6 | ||||
| Strict vegetarian | c | Total | −0.009 | −0.477, 0.590 |
| c’ | Direct | 0.103 | −0.368, 0.680 | |
| ab | Indirect | −0.112 | −0.254, −0.020* | |
| Lacto-ovo vegetarian | c | Total | 0.016 | −0.258, 0.290 |
| c’ | Direct | 0.090 | −0.185, 0.350 | |
| ab | Indirect | −0.073 | −0.179, −0.020* | |
| Partial vegetarian | c | Total | 0.160 | −0.205, 0.530 |
| c’ | Direct | 0.203 | −0.162, 0.570 | |
| ab | Indirect | −0.043 | −0.140, 0.000* | |
| IL-10 | ||||
| Strict vegetarian | c | Total | −0.335 | −1.365, 0.330 |
| c’ | Direct | −0.295 | −1.294, 0.360 | |
| ab | Indirect | −0.040 | −0.197, 0.080 | |
| Lacto-ovo vegetarian | c | Total | −0.320 | −0.894, 0.230 |
| c’ | Direct | −0.295 | −0.874, 0.250 | |
| ab | Indirect | −0.025 | −1.230, 0.050 | |
| Partial vegetarian | c | Total | −0.371 | −0.916, 0.170 |
| c’ | Direct | −0.356 | −0.891, 0.190 | |
| ab | Indirect | −0.015 | −0.097, 0.030 | |
| TNF-αb | ||||
| OR | 95% CI | |||
| Strict vegetarian | c | Total | 0.098 | −0.904, 1.052 |
| c’ | Direct | 0.035 | −0.950, 1.011 | |
| ab | Indirect | 0.063 | −0.098, 0.315 | |
| Lacto-ovo vegetarian | c | Total | −0.061 | −0.632, 0.518 |
| c’ | Direct | −0.099 | −0.686, 0.499 | |
| ab | Indirect | 0.039 | −0.062, 0.191 | |
| Partial vegetarian | c | Total | −0.326 | −1.026, 0.370 |
| c’ | Direct | −0.349 | −1.056, 0.339 | |
| ab | Indirect | −0.023 | −0.029, 0.154 | |
Path c represent the total effect of vegetarian diet on inflammatory biomarker in standard regression coefficient (ß), which reflect the change in the biomarker per a specific vegetarian diet as compared to a non-vegetarian diet. Path ab represent the indirect effect, which is the portion of total effect that is explained by BMI. Path c’ represent the direct effect, which is the residual effect of vegetarian diet on inflammatory biomarker controlling for BMI. All regressions were adjusted for age, gender, race, education, cigarette smoke, alcohol use, physical activity, and use of NSAIDs and statins.
Confidence intervals were calculated using Bca method with 10,000 iterations. P-values were permutation p-values.
p<0.05
p<0.001
For TNF-α, effect estimates are odds ratios of different vegetarian diets having a higher TNF-α level, relative to the non-vegetarian diet.
Discussion
In this cross-sectional analysis, we found that the association between vegetarian diet (strict, lacto-ovo and partial) and the inflammatory biomarkers CRP and IL-6 was mediated by BMI through an indirect pathway. A significant direct effect was observed only in the relationship between strict vegetarian and CRP.
Results from mediation analyses for CRP and IL-6 were as hypothesized. The lower CRP concentrations associated with vegetarian diets were likely mediated by the much lower BMI values among the vegetarians. For IL-6, however, we found a strong mediating effect of BMI, but the direction was not clear. The appearance of no direct effect in this case was likely by chance, which could be detected with a larger sample size.
That BMI is lower in vegetarians is a well-known finding [24,25], although the explanation is not entirely clear. The dietary groups in this study do not vary greatly in intake of energy, but the qualitative aspects of the foods and nutrients consumed differ greatly [26,27]. This may have implications for nutrient absorption or metabolic rate. Montalcini et al., for example, report higher resting energy expenditure associated with vegetarian diets and vegetable fats [7].
Our finding of lower CRP and IL-6 in vegetarians compared to non-vegetarians is consistent with several other studies relating plant foods or healthy dietary patterns with inflammatory markers in an elderly US population, diabetic women, and healthy adults in Iran and Japan [28–31]. Concentrations of IL-6 and CRP have been reported by others as negatively associated with empirically derived healthy dietary patterns characterized by intake of such foods as whole grains, fruits, green leafy vegetables, cruciferous, fish, dairy, and cereal fiber [28,32–35], but positively associated with Western dietary patterns, which tend to be high in foods such as refined grains, red meat, high-fat dairy, sweets and desserts and low in fiber [34]. Studies in vegetarians have generally been small and results non-significant. In a population of Taiwanese females, CRP was actually higher among vegetarians compared to omnivores [3]. It is thought that the lower consumption of fresh vegetables among the Taiwanese compared to western vegetarians, and their intake of many deep-fried foods, refined soybean and grain products may have contributed to elevated inflammatory markers [34,36]. In other studies, vegetarians have blood levels of CRP, IL-6, IL-10, or TNF-α concentrations that do not differ from non-vegetarians, although these are small studies and/or involve a short duration intervention [4–7].
The antioxidant components of plant foods (fruits and vegetables in particular) are thought to contribute to their anti-inflammatory effect. Polyphenols are bioactive plant compounds with antioxidant properties, and intake of these has been found to have an inverse relationship with CRP [37], while the highest 1-yr change in urinary polyphenol excretion (a marker of polyphenol intake) was associated with lower concentrations of TNF-α and IL-6 [38]. The dietary inflammatory index (DII), a tool recently developed to assess the inflammatory potential of the overall diet [39], also demonstrates this relationship. Higher DII scores, which represent higher intake of foods with inflammatory qualities, appear to predict higher levels of CRP in longitudinal studies [40,41], or to be associated with higher IL-6 levels [42].
The lack of association between vegetarian diet and the cytokines IL-10, and TNF-α in our data is inconsistent with our hypotheses. Though not significant, the inverse association between vegetarian diets and IL-10 means that non-vegetarians had higher levels of IL-10, which may have inhibited TNF-α production in this group such that no difference in TNF-α could be detected. IL-10 is a cytokine with apparently mixed anti- and pro-inflammatory effects that is also thought to have a role in regulating inflammation [19,43] through a number of mechanisms. One such mechanism is inhibiting synthesis of other pro-inflammatory cytokines, including TNF-α [44]. A further partial explanation may be that the non-vegetarians in our study are still relatively low meat consumers (about 2 oz per day) [26].
The cross-sectional design of this study has some limitations. In particular, reverse causality cannot be excluded as a cause of some expected associations which we did not find, particularly as Adventists with conditions that may tend to elevate CRP or IL-6, are possibly more likely to change in the direction of vegetarianism, due to church teachings. We did not exclude participants with preclinical conditions; however, we adjusted for the use of cholesterol-lowering medications and NSAIDs as a marker of relevant existing medical conditions. We also recognize the potential measurement error inherent in both FFQ data and biomarkers which may attenuate the associations observed. Finally, we acknowledge the limited generalizability of our findings from this faith-based Adventist population to the general North American population, although we have no evidence of biological differences in response to environmental factors.
The study has the strength of a group of subjects whose diets span a wide range of use of animal and plant-based foods. Our CRP and IL-6 results are statistically significant, and are consistent with a previous limited study among BioMRS participants alone, which focused on ethnic differences in socio-demographic and health factors associated with inflammation [45]. Results from that study also suggest that a vegetarian diet is associated with CRP; however, the dietary pattern was determined from a limited 17-item dietary questionnaire, and was restricted to a single vegetarian category and non-vegetarian category only.
In summary, we provide evidence consistent with a hypothesis that the lower risk of inflammation-related disease among vegetarians may be related to lower CRP levels that are mediated by lower BMI values. Cautious interpretation of our findings is necessary due to the cross-sectional design; nevertheless, consistency with other studies suggest etiologic relationships.
Acknowledgments
Financial support
NCI Grant #U01CA152939; USDA 2010-38938-20924, and NIA Grant #5R01AG026348.
Footnotes
Disclosure of potential conflicts of interest
The authors declare that they have no conflict of interest.
References
- [1].Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol 2006;71: 1397–421. [DOI] [PubMed] [Google Scholar]
- [2].Wu X, Schauss AG. Mitigation of inflammation with foods. J Agr Food Chem 2012;60:6703–17. [DOI] [PubMed] [Google Scholar]
- [3].Chen CW, Lin CT, Lin YL, Lin TK, Lin CL. Taiwanese female vegetarians have lower lipoprotein-associated phospholipase A2 compared with omnivores. Yonsei Med J 2011;52:13–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Link LB, Hussaini NS, Jacobson JS. Change in quality of life and immune markers after a stay at a raw vegan institute: a pilot study. Complement Ther Med 2008;16:124–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mahon AK, Flynn MG, Stewart LK, McFarlin BK, Iglay HB, Mattes RD, et al. Protein intake during energy restriction: effects on body composition and markers of metabolic and cardiovascular health in postmenopausal women. J Am Coll Nutr 2007;26:182–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mezzano D, Munoz X, Martinez C, Cuevas A, Panes O, Aranda E, et al. Vegetarians and cardiovascular risk factors: hemostasis, inflammatory markers and plasma homocysteine. Thromb Haemostasis 1999;81:913–7. [PubMed] [Google Scholar]
- [7].Montalcini T, De Bonis D, Ferro Y, Care I, Mazza E, Accattato F, et al. High vegetable fats intake is associated with high resting energy expenditure in vegetarians. Nutrients 2015;7:5933–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yu X, Huang T, Weng X, Shou T, Wang Q, Zhou X, et al. Plasma n-3 and n-6 fatty acids and inflammatory markers in Chinese vegetarians. Lipids Health Dis 2014;13:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dod HS, Bhardwaj R, Sajja V, Weidner G, Hobbs GR, Konat GW, et al. Effect of intensive lifestyle changes on endothelial function and on inflammatory markers of atherosclerosis. Am J Cardiol 2010;105:362–7. [DOI] [PubMed] [Google Scholar]
- [10].Haghighatdoost F, Bellissimo N, Totosy de Zepetnek JO, Rouhani MH. Association of vegetarian diet with inflammatory biomarkers: a systematic review and meta-analysis of observational studies. Public Health Nutr 2017;20:2713–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Berkow SE, Barnard N. Vegetarian diets and weight status. Nutr Rev 2006;64:175–88. [DOI] [PubMed] [Google Scholar]
- [12].Engin A The pathogenesis of obesity-associated adipose tissue inflammation. Adv Exp Med Biol 2017;960:221–45. [DOI] [PubMed] [Google Scholar]
- [13].Forsythe LK, Wallace JM, Livingstone MB. Obesity and inflammation: the effects of weight loss. Nutr Res Rev 2008;21:117–33. [DOI] [PubMed] [Google Scholar]
- [14].Butler TL, Fraser GE, Beeson WL, Knutsen SF, Herring RP, Chan J, et al. Cohort profile: the adventist health Study-2 (AHS-2). Int J Epidemiol 2008;37:260–5. [DOI] [PubMed] [Google Scholar]
- [15].Lee JW, Morton KR, Walters J, Bellinger DL, Butler TL, Wilson C, et al. Cohort profile: the biopsychosocial religion and health study (BRHS). Int J Epidemiol 2009;38:1470–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jaceldo-Siegl K, Knutsen SF, Sabate J, Beeson WL, Chan J, Herring RP, et al. Validation of nutrient intake using an FFQ and repeated 24 h recalls in black and white subjects of the Adventist Health Study-2 (AHS-2). Public Health Nutr 2010;13:812–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fraser G, Yan R. Guided multiple imputation of missing data: using a subsample to strengthen the missing-at-random assumption. Epidemiology 2007;18:246–52. [DOI] [PubMed] [Google Scholar]
- [18].Jaceldo-Siegl K, Fan J, Sabate J, Knutsen SF, Haddad E, Beeson WL, et al. Race-specific validation of food intake obtained from a comprehensive FFQ: the Adventist Health Study-2. Public Health Nutr 2011;14:1988–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zhou X, Fragala MS, McElhaney JE, Kuchel GA. Conceptual and methodological issues relevant to cytokine and inflammatory marker measurements in clinical research. Curr Opin Clin Nutr 2010;13:541–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol 1986;51:1173–82. [DOI] [PubMed] [Google Scholar]
- [21].Hayes AF, Preacher KJ. Statistical mediation analysis with a multicategorical independent variable. Br J Math Stat Psychol 2014;67: 451–70. [DOI] [PubMed] [Google Scholar]
- [22].Rucker DD, Preacher KJ, Tormala ZL, Petty RE. Mediation analysis in Social Psychology: current practices and new recommendations. Soc Pers Psychol Compass 2011:359–71. [Google Scholar]
- [23].Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. Mediation: R package for causal mediation analysis. J Stat Softw 2014;59: 1–38.26917999 [Google Scholar]
- [24].Barnard ND, Levin SM, Yokoyama Y. A systematic review and meta-analysis of changes in body weight in clinical trials of vegetarian diets. J Acad Nutr Diet 2015;115:954–69. [DOI] [PubMed] [Google Scholar]
- [25].Spencer EA, Appleby PN, Davey GK, Key TJ. Diet and body mass index in 38000 EPIC-Oxford meat-eaters, fish-eaters, vegetarians and vegans. Int J Obes Relat Metab Disord 2003;27:728–34. [DOI] [PubMed] [Google Scholar]
- [26].Orlich MJ, Jaceldo-Siegl K, Sabate J, Fan J, Singh PN, Fraser GE. Patterns of food consumption among vegetarians and non-vegetarians. Br J Nutr 2014;112:1644–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rizzo NS, Jaceldo-Siegl K, Sabate J, Fraser GE. Nutrient profiles of vegetarian and nonvegetarian dietary patterns. J Acad Nutr Diet 2013;113:1610–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Esmaillzadeh A, Kimiagar M, Mehrabi Y, Azadbakht L, Hu FB, Willett WC. Dietary patterns and markers of systemic inflammation among Iranian women. J Nutr 2007;137:992–8. [DOI] [PubMed] [Google Scholar]
- [29].Gao X, Bermudez OI, Tucker KL. Plasma C-reactive protein and homocysteine concentrations are related to frequent fruit and vegetable intake in Hispanic and non-Hispanic white elders. J Nutr 2004;134:913–8. [DOI] [PubMed] [Google Scholar]
- [30].Nanri A, Yoshida D, Yamaji T, Mizoue T, Takayanagi R, Kono S. Dietary patterns and C-reactive protein in Japanese men and women. Am J Clin Nutr 2008;87:1488e96. [DOI] [PubMed] [Google Scholar]
- [31].Qi L, van Dam RM, Liu S, Franz M, Mantzoros C, Hu FB. Whole-grain, bran, and cereal fiber intakes and markers of systemic inflammation in diabetic women. Diabetes Care 2006;29:207–11. [DOI] [PubMed] [Google Scholar]
- [32].Ma Y, Hebert JR, Li W, Bertone-Johnson ER, Olendzki B, Pagoto SL, et al. Association between dietary fiber and markers of systemic inflammation in the Women’s Health Initiative Observational Study. Nutrition 2008;24:941–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chuang SC, Vermeulen R, Sharabiani MT, Sacerdote C, Fatemeh SH, Berrino F, et al. The intake of grain fibers modulates cytokine levels in blood. Biomarkers 2011;16:504–10. [DOI] [PubMed] [Google Scholar]
- [34].Nettleton JA, Steffen LM, Mayer-Davis EJ, Jenny NS, Jiang R, Herrington DM, et al. Dietary patterns are associated with biochemical markers of inflammation and endothelial activation in the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr 2006;83:1369–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].van Bussel BC, Henry RM, Schalkwijk CG, Ferreira I, Feskens EJ, Streppel MT, et al. Fish consumption in healthy adults is associated with decreased circulating biomarkers ofendothelial dysfunction and inflammation during a 6-year follow-up. J Nutr 2011;141:1719–25. [DOI] [PubMed] [Google Scholar]
- [36].Lopez-Garcia E, Schulze MB, Fung TT, Meigs JB, Rifai N, Manson JE, et al. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr 2004;80:1029–35. [DOI] [PubMed] [Google Scholar]
- [37].Pounis G, Bonaccio M, Di Castelnuovo A, Costanzo S, de Curtis A, Persichillo M, et al. Polyphenol intake is associated with low-grade inflammation, using a novel data analysis from the Moli-sani study. Thromb Haemost 2016;115:344–52. [DOI] [PubMed] [Google Scholar]
- [38].Medina-Remon A, Casas R, Tressserra-Rimbau A, Ros E, Martinez-Gonzalez MA, Fito M, et al. Polyphenol intake from a Mediterranean diet decreases inflammatory biomarkers related to atherosclerosis: a substudy of the PREDIMED trial. Br J Clin Pharmacol 2017;83: 114–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Shivappa N, Steck SE, Hurley TG, Hussey JR, Hebert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr 2014;17:1689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Shivappa N, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, et al. A population-based dietary inflammatory index predicts levels of C-reactive protein in the Seasonal Variation of Blood Cholesterol Study (SEASONS). Public Health Nutr 2014;17:1825–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wirth MD, Burch J, Shivappa N, Violanti JM, Burchfiel CM, Fekedulegn D, et al. Association of a dietary inflammatory index with inflammatory indices and metabolic syndrome among police officers. J Occup Environ Med 2014;56:986–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Shivappa N, Hebert JR, Rietzschel ER, De Buyzere ML, Langlois M, Debruyne E, et al. Associations between dietary inflammatory index and inflammatory markers in the Asklepios study. Br J Nutr 2015;113:665–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Lio D, Candore G, Crivello A, Scola L, Colonna-Romano G, Cavallone L, et al. Opposite effects of interleukin 10 common gene polymorphisms in cardiovascular diseases and in successful ageing: genetic background of male centenarians is protective against coronary heart disease. J Med Genet 2004;41:790–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB. Interleukin-10 and related cytokines and receptors. Annu Rev Immunol 2004;22:929–79. [DOI] [PubMed] [Google Scholar]
- [45].Paalani M, Lee JW, Haddad E, Tonstad S. Determinants of inflammatory markers in a bi-ethnic population. Ethnic Dis 2011;21: 142–9. [PMC free article] [PubMed] [Google Scholar]