Abstract
Objective:
Premature ejaculation (PE) is regarded as one of the most common male sexual dysfunctions. This review introduced several pharmaceutical and surgical methods for the management of PE. The definition, etiology, behavioral, and psychological therapy of PE were also discussed.
Data sources:
“Premature,” “ejaculation,” or “sexual dysfuction” were used as the medical subject headings (MeSH) to obtain relevant articles before June 2019 on Pubmed, Google Scholar and CNKI. Most articles used were written in English and several Chinese articles were also cited.
Study selection:
Full-text articles of retrospective/prospective/randomized controlled trials were analyzed. Animal experiments and letters were excluded.
Results:
There are four PE sub-types: lifelong PE, acquired PE, natural variable PE, and subjective PE. Behavioral therapy, psychotherapy, medication, topical anesthetics, and surgery are currently used for the treatment of PE. However, all the above treatments have limitations. Therefore, novel ways should be investigated to more efficiently control PE.
Conclusions:
The pharmaceutical therapy that is currently being used in clinical practice for the management of PE is still the main choice globally due to its good efficacy. Surgery may be a choice for patients who are resistant to medication. However, it should be performed cautiously.
Keywords: Premature ejaculation, Selective serotonin reuptake inhibitors, Penile dorsal nerve neurotomy
Introduction
Premature ejaculation (PE) is one of the most common types of ejaculatory dysfunction, affecting approximately 20% to 30% of the male population.[1] PE is also associated with distress, anxiety, and having a negative relationship with sexual partners.[2,3] Currently, several advancements have been made for the pharmaceutical and surgical management of PE. This review briefly introduces the definition, etiology, behavioral, and psychological therapy of PE. The pharmaceutical and surgical methods that are currently being used in clinical practice for the management of PE were emphasized.
Definitions and classification of PE
The definition of PE is controversial. The American Psychiatric Association has defined PE as a “persistent or recurrent ejaculation with minimal sexual stimulation prior or shortly after penetration and before the person wishes it.”[4] The World Health Organization defined PE as “the inability to delay ejaculation sufficiently to enjoy lovemaking, which is demonstrated by either an occurrence of ejaculation before or shortly after the beginning of intercourse, or an ejaculation that occurs in the absence of a sufficient erection to make intercourse possible.”[4] The American Urological Association has defined PE as “ejaculation that occurs sooner than desired, either before or shortly after penetration, causing distress to either one or both partners.”[5] These definitions were criticized because they are authority-based rather than evidence-based, and were conceptual and vague.[6] To develop a definition with a definite scientific criteria, the International Society for Sexual Medicine (ISSM) proposed that PE to be defined as “ejaculation that always or nearly always occurs prior to or within approximately 1 min of vaginal penetration from the first sexual experience (lifelong PE [LPE]; also called primary PE), or a clinically significant and bothersome reduction in latency time, often to about 3 min or less (acquired PE [APE]; also called secondary PE), or the inability to delay ejaculation on all or nearly all vaginal penetrations, and negative personal consequences, such as distress, bother, frustration, and/or the avoidance of sexual intimacy.”[7]
It is now widely accepted that three factors should be considered for the definition of PE: (1) Short time interval between penetration and ejaculation; (2) Lack of control over ejaculation; (3) Distress felt in one or both partners.[7] An important index, the intra-vaginal ejaculatory latency time (IELT) was also proposed to better obtain an objective evaluation of PE.[7]
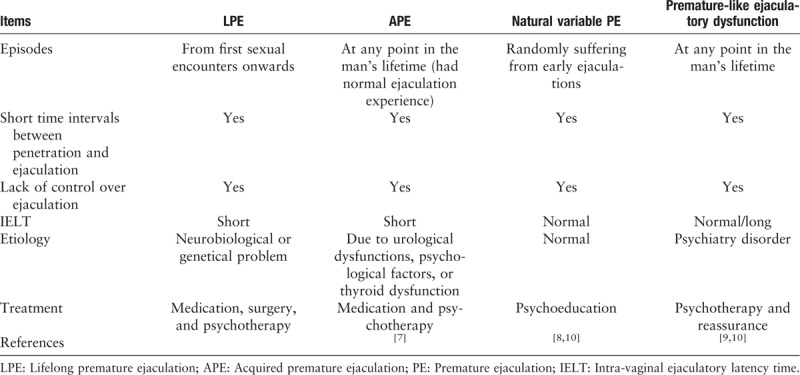
Although the definition proposed by ISSM was only suited for LPE and APE,[7] several other sub-types of PE have been described, such as natural variable PE[8]and premature-like ejaculatory dysfunction,[9,10] and can be differentiated by their corresponding IELT and etiology [Table 1].
Table 1.
Classification of premature ejaculation.
Physiology of ejaculation
The physiology of ejaculation is complicated and obscure. Currently, a two-pathway model is used to explain the physiology of ejaculation. Ejaculation is subjected to both central and peripheral control. For the peripheral control of ejaculation, the pudendal sensory nerves input the sexual stimulating signal, and it is then transferred to the spinal network. After the signal is processed by the spinal network, the sensory information is converted into both a secretory and motor signal output. Somatic, sympathetic, and parasympathetic fibers are involved in signal transduction. The integration and coordination of somatic, sympathetic, and parasympathetic signals are sequentially relayed to the muscles and structures of the pelvis and perineum to facilitate ejaculation.[11] For the central control of ejaculation, the spinal ejaculatory reflex is mediated by the cerebral network. Additionally, the cerebral network controls the final output from all ejaculatory stimuli. Serotonin, dopamine, acetylcholine, adrenaline, neuropeptide, oxytocin, γ-aminobutyric acid and nitric oxide have all been implicated in the regulation of the ejaculatory reflex. The most studied neurotransmitter is 5-hydroxytryptamine (5-HT). Fourteen different 5-HT receptor sub-types have been identified and each of them has a different neuroanatomical location and function. Some sub-types (5-HT1a) reduce ejaculatory latency and other sub-types (5-HT1b, 5-HT2c) prolong ejaculatory latency.[12–14]
Based on ejaculatory physiology, two possible factors may be involved in PE. Penile hypersensitivity, which increases sexual signal input, and may be responsible for peripheral PE.[15] An imbalance of 5-HT synaptic concentration and receptor sensitivity may also disturb the central ejaculatory control and cause central PE[12] [Figure 1].
Figure 1.
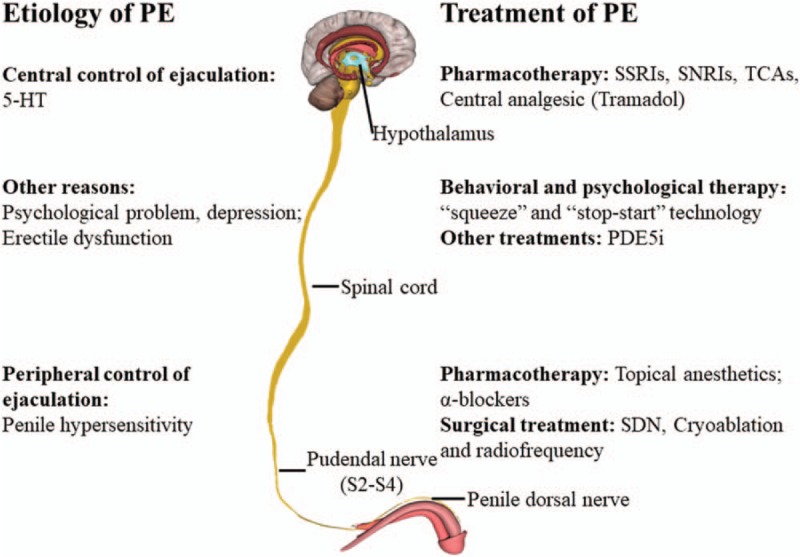
The etiology and treatment of PE. 5-HT: 5-Hydroxytryptamine; PDE5i: Phosphodiesterase type 5 inhibitors; PE: Premature ejaculation; SDN: Selective penile dorsal nerve neurotomy; SNRIs: Serotonin-norepinephrine reuptake inhibitors; SSRIs: Selective serotonin reuptake inhibitors; TCAs: Tricyclic anti-depressants.
The “central and peripheral control” model is used to explain the physiology of PE. For peripheral control, the sexual stimulating signal is sent to the pudendal sensory nerves and is transferred and processed in the spinal network. Subsequently, the signal is converted into both a secretory and motor signal output. Lastly, through the integration and co-ordination of the efferent nerve signals, the structures of the pelvis and perineum are triggered to facilitate ejaculation. Penile hypersensitivity, increased sexual signal input, may be responsible for peripheral PE. For this reason, topical anesthetics, α-blockers, and surgical interventions may be used to decrease penile hypersensitivity. For central control, the cerebral network controls the final output from all ejaculatory stimuli. Many neurotransmitters have been implicated in the regulation of the ejaculation process. 5-HT is the most studied. The imbalance of 5-HT synaptic concentration and receptor sensitivity may cause disturbances to the central ejaculatory control and induce central PE. Selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), tricyclic anti-depressant (TCAs), and tramadol target the central control of PE. There are various other reasons for PE, such as psychological problems and depression. Behavioral and psychological therapies are preferred for those reasons. Erectile dysfunction also can result in PE, and phosphodiesterase type 5 (PDE-5) inhibitors may be used to treat such patients.
Etiology of PE
Although PE is regarded as the most common male sexual dysfunction, its etiologies are still obscure. Previously it was recognized as a psychological problem without “organic” changes, but subsequent studies have suggested that PE may be influenced by various somatic disorders and/or neurobiological disturbances.[16] Several genetic studies have indicated that polymorphisms of the serotonin transporter or its promoters are associated with PE.[17,18] In addition, PE is complex with a variety of etiological factors such as depression,[19] erectile dysfunction,[20] metabolic syndrome,[21] chronic prostatitis,[22] and thyroid dysfunction.[23] These findings have further increased the difficulty in the understanding of PE.
Treatment of PE
Behavioral and psychotherapy, medication, topical anesthetics, and surgery are currently used for PE treatment [Figure 1]. Among them, surgery is mostly preferred in China and South Korea, and is gaining attention but not yet widely accepted in western countries.
Behavioral and psychological therapy in PE
Behavioral therapy mainly includes the “stop-start” technique and “penis squeezing” technique. The “stop-start” technique refers to the stimulation of the male to a would-be ejaculation and is then immediately stopped by the partner, this is repeated until the final ejaculation can only be completed with a significant level of stimulation. The “penis squeezing” technique involves squeezing the glans when there is an urge to ejaculate. Theoretically, both methods increase the threshold, delay ejaculation, and ultimately increase sexual self-confidence.[24] However, four randomized controlled studies using psychological therapy provided weak evidence regarding its efficacy in the management of PE.[25] Recently, Fu et al[3] reported that 30 mg dapoxetine improved the intra-vaginal ejaculation latency time (IELT) from 1.16 to 4.10 min. However, the combination of behavior therapy and 30 mg dapoxetine increased the IELT from 1.00 to 5.97 min, which was significantly better than a single drug treatment.
Pharmacotherapy
The peripheral ejaculatory reflex has three stages: first, during sexual activity, the glans of the penis is stimulated to trigger the pudendal sensory nerves, which is then transferred to the spinal network. After the signal is processed by the spinal network, the sensory information is converted to both a secretory and motor signal output, which induces contraction of the epididymis, vas deferens, seminal vesicles, prostate, and bladder neck, which in turn, rhythmically lead to an ejaculation through the distal urethra.[11] Additionally, the ejaculation process is also regulated by the central control. There are multiple treatment options for PE available. SSRIs, topical anesthetic, and PDE-5 inhibitors are widely used.
SSRIs
Currently, LPE is regarded as a neurobiological dysfunction related to disturbances in the neurotransmission of central serotonin and 5-HT post-synaptic receptor function. Thus, SSRIs are a mainstay in the treatment of LPE as advised by ISSM.[26] Although SNRIs and clomipramine (a tricyclic antidepressant) have shown to be clinically beneficial for the treatment of LPE,[27] most LPE patients are treated by SSRIs including dapoxitine (30–60 mg), fluoxetine (20 mg), paroxetine (20 mg), sertraline (50–100 mg), and citalopram (20 mg). These SSRIs are usually taken daily. After receiving treatment for 1 to 2 weeks, serotonin neurotransmission is increased and alterations in specific serotonin receptors will occur in the central nervous system, which is responsible for the central pathway to LPE. However, sex-related side effects such as reduced libido, erectile dysfunction, and non-sex related side effects such as nausea, fatigue, insomnia, constipation, and loss appetite have led to the suggestion that an “on-demand” SSRI with a short half-time should be developed for the treatment of LPE.[28]
Dapoxetine
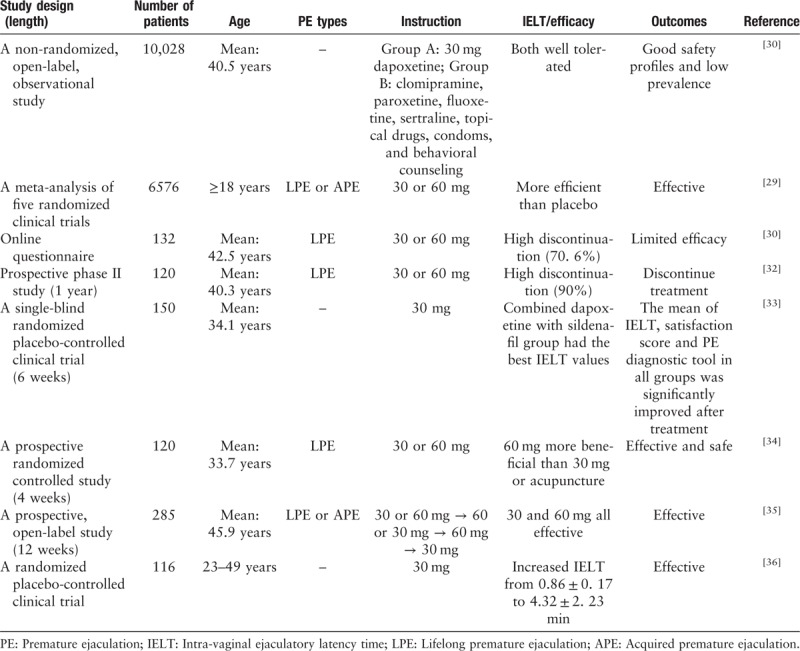
Dapoxetine is the first new generation of SSRIs, designed specifically for the treatment of LPE and APE.[29] It has the advantages of fast absorption, short half-life, and fast metabolism, etc. In several large global phase III clinical trials, dapoxetine 30 or 60 mg showed significant IELT improvement of more than 3.5-fold and presented with no apparent safety concerns.[30–32] Recently, a randomized placebo clinical trial[33] compared dapoxetine or paroxetine or sildenafil alone with dapoxetine in combination with sildenafil, and the result suggested that drug combination resulted in a better IELF. Furthermore, many studies have confirmed the efficacy of dapoxetine for the treatment of PE [Table 2].[29,34–36]
Table 2.
Efficacy of dapoxetine or its combination to delay ejaculation in patients with PE.
Dapoxetine may not be appropriate for every patient due to its high dropout rate.[30] In a recent study of 120 patients with PE who were being treated with dapoxetine initially, 90% patients discontinued the treatment within 1 year due to the reasons as follows; efficacy below expectations, cost, loss of interest in sex, and adverse effects.[37] Another research paper published by Park et al[38] showed that of 182 patients with PE treated with dapoxetine, only 9.9% of people maintained the treatment for 2 years.
Paroxetine
Paroxetine is another SSRIs drug used to treat depression and is currently also used to treat PE. Simsek et al[39] studied 150 patients with PE and compared the effects of paroxetine and dapoxetine in the treatment of PE. Their statistics showed that both dapoxetine and paroxetine increased IELT significantly, but the former was more effective. However, compared with dapoxetine, paroxetine had the lowest dropout rate. Yu et al[40] divided 142 patients with PE into three groups according to the pre-treatment IELT: group A: IELT ≤30 s, group B: 30 s < IELT ≤ 60 s, group C: 60 s < IELT ≤ 120 s, all groups were treated with 20 mg/d of paroxetine for 8 weeks. The results showed that: group A increased by 37.9-fold IELT, group B increased by 8.81-fold IELT, group C increased by 3.05-fold IELT, therefore paroxetine may be a better fit for patients with PE with shorter IELT. Furthermore, a meta-analysis by Zhang et al[41] based on 19 randomized controlled trials reported that paroxetine had better efficacy than placebo, fluoxetine, and escitalopram with better-tolerated side effects [Table 3].
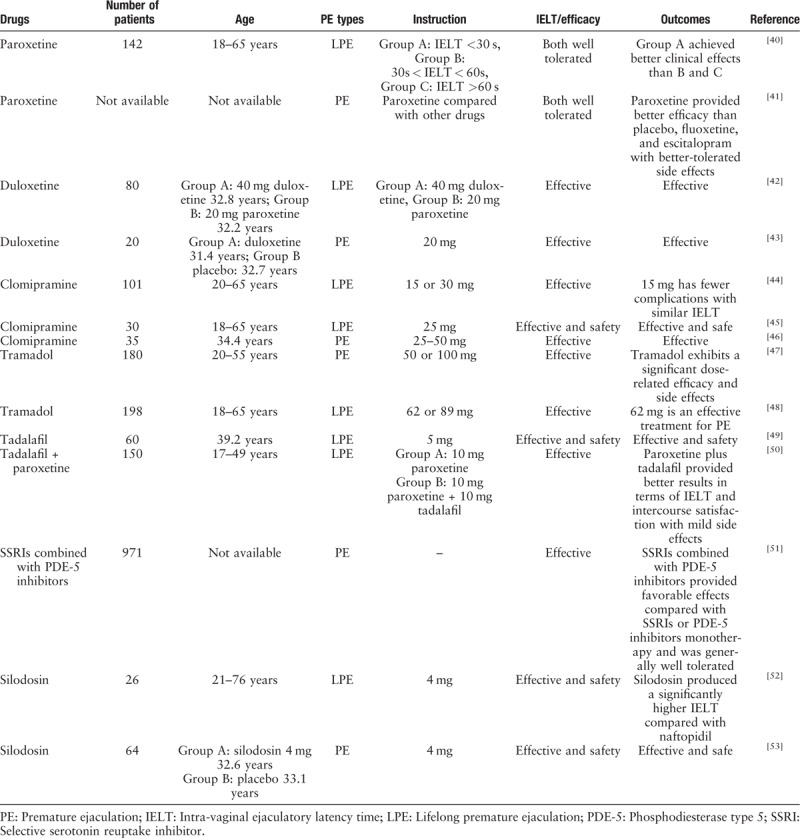
Table 3.
Efficacy of other drugs or their combination in the delaying of ejaculation in patients with PE.
Other SSRIs
Other SSRIs drugs include citalopram, sertraline, and so on. Related studies have confirmed their efficacy.[54,55] However, none have been approved for treatment in the European Union and needs more large randomized controlled trials to find the optimal dosages of these medications for the treatment of PE.
SNRIs
SNRIs are widely used in the treatment of depression and chronic pain syndromes.[56,57] It has also shown to be clinically beneficial in the treatment of LPE. Dutoxetine is a new orally administered, dual serotonin and norepinephrine re-uptake inhibitor, which can be used to treat PE. Ozcan et al[42] compared the efficacy of paroxetine 20 mg with duloxetine 40 mg once a day for a month for patients with PE. The result showed that the IELT of the two groups increased by 126% and 117%, respectively and there was no significant difference in efficacy. Furthermore, both the groups had mild and but generally tolerable adverse effects. Additionally, a study showed that dutoxetine can improve sexual desire and partner satisfaction.[43] However, more clinical trials are needed to determine its efficacy [Table 3].
Tricyclic anti-depressant
TCAs are similar to SSRIs and SNRIs, it treats PE by inhibiting the serotonin and norepinephrine transport, which results in an increased level of 5-HT and norepinephrine in the synaptic cleft. Many clinical trials have demonstrated that clomipramine, one of the TCAs, significantly improved IELT during treatment of PE [Table 3],[44–46] but the drug also has complications such as fatigue, nausea, dry mouth, low blood pressure, and dizziness.[58] Recently, a randomized, double-blind, placebo-controlled, fixed-dose, parallel-group clinical study found that clomipramine 15 mg/d may have a better risk-to-benefit ratio when compared with placebo and clomipramine 30 mg/day.[27]
Tramadol
Tramadol is a promising opioid analgesic for the treatment of PE, it works by binding the μ receptor and inhibiting serotonin and norepinephrine reuptake.[59] A randomized, double-blind clinical trial by Kurkar et al[47] showed that tramadol can prolong IELT compared with placebo. The patients were divided into three groups and were given 50 mg tramadol, 100 mg tramadol, and placebo, respectively, every patient received ten doses of each medication for 2 months [Table 3]. The result showed that the mean IELT was 72 at presentation, 82 for placebo, 150 for tramadol 50 mg, and 272 for tramadol 100 mg, indicating that tramadol may have a better therapeutic effect on PE with a larger dose. Another study showed that on-demand 62 mg tramadol was a safe and effective therapeutic dose in the treatment of PE and had fewer complications than the 89 mg tramadol group[48] [Table 3]. Additionally, unlike other types of therapies, tramadol addiction and abuse can occur. Therefore, SSRIs should be considered first before tramadol.
PDE-5 inhibitors
PDE-5 inhibitors can increase nitric oxide release, reduce anxiety, relax the vas deferens, seminal vesicles, prostate smooth muscle, urethral smooth muscle, and reduce central output.[58] It has been approved by the Food and Drug Administration (FDA) for the treatment of erectile dysfunction. Recently, it was discovered that it can be used to treat PE. Therefore, PDE-5 inhibitors may be the gold standard when patients with PE have erectile dysfunction.[60] Abu et al[60] conducted a placebo randomized controlled trial and found that after 6 weeks, oral tadalafil 5 mg was found to have a more satisfactory Arab index of PE than the control group [Table 3]. This result was also consistent with other reports[50,61] [Table 3]. Some scholars found that tadalafil combined with other drugs such as lidocaine or SSRIs had better clinical efficacy than single-use, but the incidence of drug-related complications also increased[51,62,63] [Table 3]. Therefore, if the complications are well tolerated, combination therapy may be a better choice for PE, if the complications cannot be tolerated and cause distress to the patients or partners, a single drug can be considered.
Alpha-blockers
Silodosin and naftopidil are both α1-adrenoceptor antagonists and is both effective and safe for the treatment of benign prostate hyperplasia (BPH) and lower urinary tract symptoms secondary to BPH.[64–66] The α1-adrenoceptor antagonists may be inhibiting contraction of the vas deferens, seminal vesicles, prostate smooth muscle, and so on, resulting in delay ejaculation[52] [Table 3]. A prospective multi-center clinical trial comparing silodosin with naftopidil in the treatment of patients with PE found that the IELT of the silodosin group was 7.6 min, which was higher than naftopidil group (4.1 min), and no significant clinical complications occurred.[52] Bhat et al[53] conducted a randomized controlled trial of silodosin for the treatment of PE, and also demonstrated the efficacy of silodosin [Table 3].
Topical anesthetics
Topical anesthetics have long been used in the treatment of PE. The rationale for using these agents is that they inhibit penile hypersensitivity. Therefore, the peripheral pathway may be blocked by topical agents. Currently, three topical anesthetics are recommended in the treatment of PE, including eutectic mixture of local anesthetics (EMLA), topical eutectic mixture for premature ejaculation (TEMPE, also known as PSD502), and severance secret (SS) cream. A systematic review and meta-analysis have shown that all of them increased the IELT significantly.[67]
EMLA is one of the topical anesthetics containing 2.5% lidocaine and 2.5% prilocaine. Although it is effective, EMLA should be applied for 20 min before coition and a condom should be used to prevent transferring to partner. The efficacy has been provided by Xia et al.[68]
TEMPE is the only topical anesthetic approved by the FDA for the treatment of LPE. Each injection comprises of 2.5 mg prilocaine and 7.5 mg lidocaine and it should be applied 10 to 15 min before initiating intercourse. Two large phase III clinical trials demonstrated its significance ability to delay ejaculation with generally well-tolerated side effects.[69,70]
SS cream is mainly made from a mixture of traditional medicines such as ginseng, cinnamon, scorpion venom, etc.[71] Some of these drugs have a local anesthetic effect and are, therefore, also used to treat PE.[72,73]
In conclusion, topical anesthetics appear to have the ability to increase IELT. They can also be administrated on demand conveniently. However, some female partners may complain of decreased vaginal sensation that causes them to fail to reach orgasm. Furthermore, randomized controlled trials are needed to evaluate the efficacy and safety of topical anesthetics for PE.
Other drugs
Several literatures have associated PE with sex steroid, pituitary, and thyroid hormones, which have all been advocated as potential candidates in the regulation of the ejaculatory process, but the exact mechanisms are still unknown and further studies are required to identify potential targets for treatment.[23] Serefoglu et al[74] found that the mice injected with botulinum toxin into the bulbar and corpus cavernosum had increased IELT significantly. The botulinum toxin may interfere with the contraction of muscles in ejaculation reflex to increase IELT. Other drugs such as caffeine,[75] DA8031 (a new type of SSRIs drug),[76] Modafinil,[77] Resiniferatoxin[78] (blocking penile sensory nerves) were also described, but more large-scale, multi-center, randomized controlled trials are needed to determine the advantages and disadvantages of these new drugs in the treatment of PE.
Surgical treatment
Although significant improvements in the treatment of LPE have been made through pharmacotherapy, complications still exist. Topical anesthetics may cause numbness in the patient and his partner(s) and their usage are not convenient.[79] SSRIs usually have to last for a long time and treatment cost is high. Additionally, sexual desire may also be inhibited by SSRIs. The side effects on sperm production, transportation, sperm cell membranes, and DNA also have been reported.[80,81] Is there a simple way to cure LPE? Surgery may be a choice for patients who are resistant to medication treatment.[82] The efforts to explore and find new surgical treatment for PE has never stopped. At least three surgical procedures have been reported in the treatment of PE.[83–86]
Selective penile dorsal nerve neurotomy
Zhang et al[87] reported that the number of terminal branches of the penile dorsal nerve among patients with LPE (average 7.16 branches) was higher compared to an average male (average 3.55 branches), this fact provided the “pathological basis” for selective penile dorsal nerve neurotomy (SDN). It was concluded that the number of dorsal nerve branches in patients with LPE is positively correlated with penile sensitivity. Theoretically, SDN may be the solution as it blocks hypersensitive peripheral sexual stimulation signals, inhibits penile hypersensitivity and central excitability, which may help improve ejaculation threshold, and extend IELT.[4] A clinical observation in China reported the effect of SDN in patients with LPE, it showed that the IELT was significantly increased after surgery (0.86 ± 0.32 min vs. 6.65 ± 3.90 min).[88] Through a simple surgical procedure [Figure 2], it provides a treatment similar to topical anesthetics. The effect is permanent, no oral medication is needed and no special preparation has to be considered before coitus. Korean doctors preferred to remove the collateral branch to reduce penile sensitivity and increase the IELT and patient satisfaction.[89] They reported that 81.8% of the patients were satisfied after cutting 2 to 3 lateral branches on both sides in 143 patients, the patients experienced an increase in IELT and a reduction of the vibration threshold without loss of sensation. Furthermore, a randomized controlled trial by Liu et al[90] showed that SDN was an effective method for patients with PE who were refractory to drugs or refused oral medication.
Figure 2.
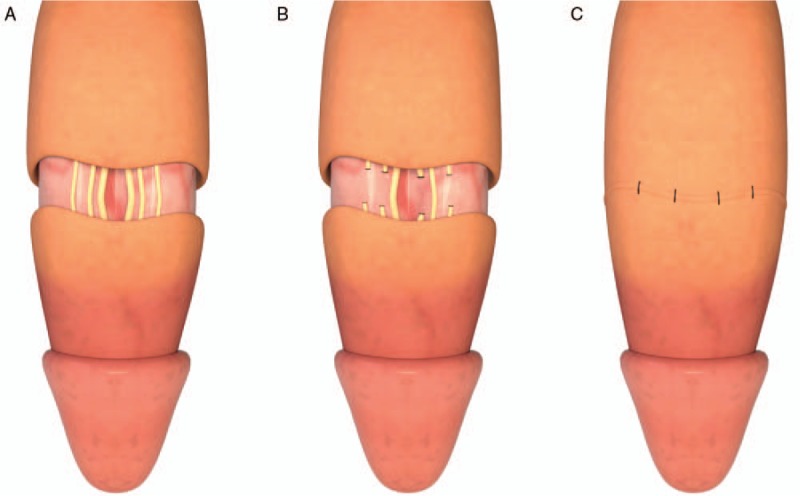
A brief surgical procedure of selective penile dorsal nerve neurotomy. (A) Check the patient's penis foreskin, circumcision can be performed if the foreskin is too long; cut the skin of the penis along the 0.8 to 1.5 cm below the coronal sulcus of the penis, make an incision into both the skin and the sub-cutaneous tissue, and separate 3 to 4 layers of penile fascia to reveal the deep vein of the penis. The dorsal nerve of the penis can be seen on the dorsal artery of the penis. The dorsal nerve of the penis is yellowish and elastic. (B) Retain 3 to 4 branches of the penile dorsal nerve, and remove 3 to 4 cm of remaining penile dorsal nerve branches. (C) Next, suture the surgical site and treat any bleeding, the skin and sub-cutaneous tissue of the suture area should be divided into two layers.
Currently, guidelines in China does not prohibit the application of a penile dorsal nerve neurotomy for the treatment of LPE as a second-line treatment.[91,92] But this surgery should only be applied to patients who meet the following criteria. A practical guideline published on National Journal of Andrology[92] suggested the indications for SDN in LPE are as follows: (1) IELT ≤2 min, lasting more than 6 months; (2) excluded any psychological factor related PE; (3) condition caused distress, anxiety, and other negative emotion changes; (4) penile hypersensitivity, without erectile dysfunction; (5) has strong intentions to undergo SDN; (6) refused behavioral, psychological, and drug treatments.
Cryoablation and radiofrequency
Cryoablation and pulsed radiofrequency are established types of minimally invasive techniques for the treatment of urinary tumor, which can kill tumor cells with precision.[93,94] Some scholars expanded their application based on the hypothesis that performing cryoablation or pulsed radiofrequency on the dorsal nerve reduces hypersensitive peripheral sexual input signals and inhibited penile hypersensitivity. A prospective study by David et al[83] evaluated the efficacy of percutaneous computed tomography (CT)-guided cryoablation in 24 patients on a unilateral dorsal penile nerve in the treatment of symptomatic PE. The report showed that IELT was increased from a baseline value of 54.7 ± 7.8 s to a maximum of 256.0 ± 104.0 s by day 7, to 182.5 ± 87.8 s by day 90, and 145.9 ± 86.5 s in 1 year. Although four patients experienced a decrease in erectile stiffness, two spontaneously recovered, and two recovered after taking an oral PDE-5 inhibitor. Pulsed radiofrequency (PRF) is often used to treat pain in a variety of areas, which regulates neural activity primarily through electric fields without causing nerve damage.[86,95] Similarly, PRF therapy can modulate the activity of the dorsal nerve of the penis and reduce its sensitivity. Basal et al[86] performed PRF for the treatment of PE. Fifteen patients with LPE had increased IELT from 18.5 ± 17.9 s to 139.9 ± 55.1 s after 3 weeks. During the follow-up period, no patients had any erection problem, penile hypoesthesia, or pain after the procedure. Therefore, compared with CT-guided cryoablation, PRF is described as a neuro-modulation technology with the merit of no radiation, no complications, and no nerve injury.
Glandular augmentation with hyaluronic acid gel
This approach refers to the injection of hyaluronic acid gel into the glans, to build a barrier artificially between the dorsal penile nerve and its external environment. Some authors reported the efficacy of this surgery, but a series of complications such as possible sensory loss were also reported.[96–98]
Controversies in surgical treatment with LPE
Most Chinese andrologists are opposed to performing surgery for the management of LPE due to insufficient supporting evidence. Furthermore, possible complications include a loss of sexual desire. However, in both China and Korea, some andrologists insist on performing surgeries as they believe that these procedures are effective for the treatment of LPE. They believe that surgeries are more convenient and are an effective alternative for patients with LPE who reject long-term drug usage. Till now, no large, multi-center, double-blind randomized controlled trials or long-term follow-up data support the safety and efficacy of the surgical treatment of LPE.[99] More high-quality clinical trials and long-term follow-up data are needed to determine the efficacy and safety of surgery for the management of LPE.
Summary
The causes of PE remain unclear. Previously it was recognized as a psychological problem without “organic” changes; however, subsequent studies have suggested that PE may be influenced by various somatic disorders, neurobiological disturbances, genetic changes, metabolism diseases, and penile hypersensitivity. Therefore, there are several methods in use for the treatment of PE. Currently, on-demand pharmacotherapy is still the main choice globally. However, a significant proportion of patients discontinue the treatment within a year due to the following reasons; efficacy below expectations, cost, loss of interest in sex, and adverse effects. Topical anesthetics are an effective alternative but may cause numbness in the patient and his partner(s). Behavioral and psychological therapies are non-invasive methods in the treatment of PE. A combination of an adjusted dosage of drugs taken while undergoing behavioral or psychological therapy may be a better choice for decreasing the complications and optimizing efficacy theoretically. But this combination should be evaluated by more high-quality studies. SDN is not recommended by the ISSM guideline for the treatment of PE due to insufficient evidence. However, SDN may be a reasonable choice with certain patients. In summary, all current treatment methods for PE have limitations and complication, and other novel methods such as drugs or surgery require further studies to determine their efficacy and safety for the treatment of PE.
Funding
This work was supported by a grant from the Zhejiang Provincial Foundation for Medical and Health Sciences (No. 2014KYB355).
Conflicts of interest
None.
Footnotes
How to cite this article: Hu QB, Zhang D, Ma L, Ng DM, Haleem M, Ma Q. Progresses in pharmaceutical and surgical management of premature ejaculation. Chin Med J 2019;132:2362–2372. doi: 10.1097/CM9.0000000000000433
Qin-Bo Hu and Dong Zhang contributed equally to this work.
References
- 1.Porsta H, Rosen RC, Gaynor L, Grupe S, Alexander J. The premature ejaculation prevalence and attitudes (PEPA) survey: prevalence, comorbidities, and professional help-seeking. Eur Urol 2007; 51:816–824. doi: 10.1016/j.eururo.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Kempeneers P, Andrianne R, Cuddy M, Blairy S. Sexual cognitions, trait anxiety, sexual anxiety, and distress in men with different subtypes of premature ejaculation and in their partners. J Sex Marital Ther 2018; 44:319–332. doi: 10.1080/0092623x.2017.1405299. [DOI] [PubMed] [Google Scholar]
- 3.Fu M, Peng X, Hu Y. Effect of premature ejaculation desensitisation therapy combined with dapoxetine hydrochloride on the treatment of primary premature ejaculation. Andrologia 2019; 51:e13135.doi: 10.1111/and.13135. [DOI] [PubMed] [Google Scholar]
- 4.Althof SE, Abdo CH, Dean J, Hackett G, Mccabe M, Mcmahon CG, et al. International society for sexual medicine's guidelines for the diagnosis and treatment of premature ejaculation. J Sex Med 2010; 7:2947–2969. doi: 10.1111/j.1743-6109.2010.01975.x. [DOI] [PubMed] [Google Scholar]
- 5.Montague DK, Jarow J, Broderick GA, Dmochowski RR, Heaton JP, Lue TF, et al. AUA guideline on the pharmacologic management of premature ejaculation. J Urol 2004; 172:290–294. doi: 10.1097/01.ju.0000132159.61156.ea. [DOI] [PubMed] [Google Scholar]
- 6.McMahon CG, Althof S, Waldinger MD, Porst H, Dean J, Sharlip I, et al. An evidence-based definition of lifelong premature ejaculation: report of the international society for sexual medicine ad hoc committee for the definition of premature ejaculation. BJU Int 2008; 102:338–350. doi: 10.1111/j.1464-410X.2008.07755.x. [DOI] [PubMed] [Google Scholar]
- 7.Serefoglu EC, McMahon CG, Waldinger MD, Althof SE, Shindel A, Adaikan G, et al. An evidence-based unified definition of lifelong and acquired premature ejaculation: report of the second international society for sexual medicine ad hoc committee for the definition of premature ejaculation. Sex Med 2014; 2:41–59. doi: 10.1002/sm2.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waldinger MD, Schweitzer DH. Changing paradigms from a historical DSM-III and DSM-IV view toward an evidence-based definition of premature ejaculation. Part II--proposals for DSM-V and ICD-11. J Sex Med 2006; 3:693–705. doi: 10.1111/j.1743-6109.2006.00276.x. [DOI] [PubMed] [Google Scholar]
- 9.Godpodinoff ML. Premature ejaculation: clinical subgroups and etiology. J Sex Marital Ther 1989; 15:130–134. doi: 10.1080/00926238908403817. [DOI] [PubMed] [Google Scholar]
- 10.Waldinger MD. Recent advances in the classification, neurobiology and treatment of premature ejaculation. Adv Psychosom Med 2008; 29:50–69. doi: 10.1159/000126624. [DOI] [PubMed] [Google Scholar]
- 11.Puppo V, Puppo G. Comprehensive review of the anatomy and physiology of male ejaculation: premature ejaculation is not a disease. Clin Anatomy 2016; 29:111–119. doi: 10.1002/ca.22655. [DOI] [PubMed] [Google Scholar]
- 12.Giuliano F, Clement P. Serotonin and premature ejaculation: from physiology to patient management. Eur Urol 2006; 50:454–466. doi: 10.1016/j.eururo.2006.05.055. [DOI] [PubMed] [Google Scholar]
- 13.Jannini EA, Carosa E, Pepe M, Lombardo F, Lenzi A. Update on pathophysiology of premature ejaculation: the bases for new pharmacological treatments. EAU-EBU Update Series 2006; 4:141–149. doi: 10.1016/j.eeus.2006.05.002. [Google Scholar]
- 14.Janssen PK, van Schaik R, Zwinderman AH, Olivier B, Waldinger MD. The 5-HT(1)A receptor C(1019)G polymorphism influences the intravaginal ejaculation latency time in Dutch Caucasian men with lifelong premature ejaculation. Pharmacol Biochem Behav 2014; 121:184–188. doi: 10.1016/j.pbb.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Gallo L. Patients affected by premature ejaculation due to glans hypersensitivity refuse circumcision as a potential definite treatment for their problem. Andrologia 2014; 46:349–355. doi: 10.1111/and.12083. [DOI] [PubMed] [Google Scholar]
- 16.Saitz TR, Serefoglu EC. Advances in understanding and treating premature ejaculation. Nat Rev Urol 2015; 12:629–640. doi: 10.1038/nrurol.2015.252. [DOI] [PubMed] [Google Scholar]
- 17.Safarinejad MR. Retraction statement: analysis of association between the 5-HTTLPR and STin2 polymorphisms in the serotonin-transporter gene and clinical response to a selective serotonin reuptake inhibitor (sertraline) in patients with premature ejaculation. BJU Int 2015; 115:E9.doi: 10.1111/bju.13062. [DOI] [PubMed] [Google Scholar]
- 18.Jern P, Eriksson E, Westberg L. A reassessment of the possible effects of the serotonin transporter gene linked polymorphism 5-HTTLPR on premature ejaculation. Arch Sex Behav 2013; 42:45–49. doi: 10.1007/s10508-012-9991-x. [DOI] [PubMed] [Google Scholar]
- 19.Mourikis I, Antoniou M, Matsouka E, Vousoura E, Tzavara C, Ekizoglou C, et al. Anxiety and depression among Greek men with primary erectile dysfunction and premature ejaculation. Ann Gen Psychiatry 2015; 14:34.doi: 10.1186/s12991-015-0074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brody S, Weiss P. Erectile dysfunction and premature ejaculation: interrelationships and psychosexual factors. J Sex Med 2015; 12:398–404. doi: 10.1111/jsm.12738. [DOI] [PubMed] [Google Scholar]
- 21.Salama N, Eid A, Swedan A, Hatem A. Increased prevalence of premature ejaculation in men with metabolic syndrome. Aging Male 2017; 20:89–95. doi: 10.1080/13685538.2016.1277515. [DOI] [PubMed] [Google Scholar]
- 22.Lee JH, Lee SW. Relationship between premature ejaculation and chronic prostatitis/chronic pelvic pain syndrome. J Sex Med 2015; 12:697–704. doi: 10.1111/jsm.12796. [DOI] [PubMed] [Google Scholar]
- 23.Sansone A, Romanelli F, Jannini EA, Lenzi A. Hormonal correlations of premature ejaculation. Endocrine 2015; 49:333–338. doi: 10.1007/s12020-014-0520-7. [DOI] [PubMed] [Google Scholar]
- 24.Castiglione F, Albersen M, Hedlund P, Gratzke C, Salonia A, Giuliano F. Current pharmacological management of premature ejaculation: a systematic review and meta-analysis. Eur Urol 2016; 69:904–916. doi: 10.1016/j.eururo.2015.12.028. [DOI] [PubMed] [Google Scholar]
- 25.Melnik T, Althof S, Atallah AN, Puga ME, Glina S, Riera R. Psychosocial interventions for premature ejaculation. Cochrane Database Syst Rev 2011; 10:Cd008195.doi: 10.1002/14651858.CD008195.pub2. [DOI] [PubMed] [Google Scholar]
- 26.Althof SE, McMahon CG, Waldinger MD, Serefoglu EC, Shindel AW, Adaikan PG, et al. An update of the international society of sexual medicine's guidelines for the diagnosis and treatment of premature ejaculation (PE). J Sex Med 2014; 11:1392–1422. doi: 10.1111/jsm.12504. [DOI] [PubMed] [Google Scholar]
- 27.Keating GM. Silodosin: a review of its use in the treatment of the signs and symptoms of benign prostatic hyperplasia. Drugs 2015; 75:207–217. doi: 10.1007/s40265-014-0344-z. [DOI] [PubMed] [Google Scholar]
- 28.Waldinger MD. Premature ejaculation: definition and drug treatment. Drugs 2007; 67:547–568. doi: 10.2165/00003495-200767040-00005. [DOI] [PubMed] [Google Scholar]
- 29.Yue FG, Dong L, Hu TT, Qu XY. Efficacy of dapoxetine for the treatment of premature ejaculation: a meta-analysis of randomized clinical trials on intravaginal ejaculatory latency time, patient-reported outcomes, and adverse events. Urology 2015; 85:856–861. doi: 10.1016/j.urology.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 30.Mirone V, Arcaniolo D, Rivas D, Bull S, Aquilina JW, Verze P. Results from a prospective observational study of men with premature ejaculation treated with dapoxetine or alternative care: the PAUSE study. Eur Urol 2014; 65:733–739. doi: 10.1016/j.eururo.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 31.Jern P, Johansson A, Piha J, Westberg L, Santtila P. Antidepressant treatment of premature ejaculation: discontinuation rates and prevalence of side effects for dapoxetine and paroxetine in a naturalistic setting. Int J Impot Res 2015; 27:75–80. doi: 10.1038/ijir.2014.37. [DOI] [PubMed] [Google Scholar]
- 32.Mondaini N, Fusco F, Cai T, Benemei S, Mirone V, Bartoletti R. Dapoxetine treatment in patients with lifelong premature ejaculation: the reasons of a “waterloo”. Urology 2013; 82:620–624. doi: 10.1016/j.urology.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 33.Abu El-Hamd M, Abdelhamed A. Comparison of the clinical efficacy and safety of the on-demand use of paroxetine, dapoxetine, sildenafil and combined dapoxetine with sildenafil in treatment of patients with premature ejaculation: a randomised placebo-controlled clinical trial. Andrologia 2018; 50:1.doi: 10.1111/and.12829. [DOI] [PubMed] [Google Scholar]
- 34.Sahin S, Bicer M, Yenice MG, Seker KG, Yavuzsan AH, Tugcu V. A prospective randomized controlled study to compare acupuncture and dapoxetine for the treatment of premature ejaculation. Urol Int 2016; 7:104–111. doi: 10.1159/000445253. [DOI] [PubMed] [Google Scholar]
- 35.Mcmahon C, Lee SW, Kim SW, Du GM, Kongkanand A, Tantiwongse K. The Asia-Pacific flexible dose study of dapoxetine and patient satisfaction in premature ejaculation therapy: the PASSION study. Sex Med 2016; 4:e18–e27. doi: 10.1016/j.esxm.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen XY, Qu YW, Wang SG. Efficacy and safety of dapoxetine in the treatment of premature ejaculation. Natl J Androl 2016; 22:411–414. doi: 10.13263/j.cnki.nja.2016.05.005. [PubMed] [Google Scholar]
- 37.Jiann BP, Huang YJ. Assessing satisfaction in men with premature ejaculation after dapoxetine treatment in real-world practice. Int J Clin Pract 2015; 69:1326–1333. doi: 10.1111/ijcp.12700. [DOI] [PubMed] [Google Scholar]
- 38.Park HJ, Park NC. Discontinuation of dapoxetine treatment in patients with premature ejaculation: a 2-year prospective observational study. Sex Med 2017; 5:e99–e105. doi: 10.1016/j.esxm.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simsek A, Sarilar O, Kirecci S, Kucuktopcu O, Ozgor F, Akbulut F, et al. Comparison of paroxetine and dapoxetine, a novel selective serotonin reuptake inhibitor in the treatment of premature ejaculation. Asian J Androl 2014; 191:725–727. doi: 10.4103/1008-682X.128467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu G, You W, Xi W, Gang Y, Jiang Y, Wei X. Association Between changes in serum 5-hydroxy-tryptamine concentrations and improvement in clinical symptoms in primary premature ejaculation with paroxetine treatment. Med Sci Monit 2015; 21:2521–2527. doi: 10.12659/MSM.894662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang D, Cheng Y, Wu K, Ma Q, Jiang J, Yan Z. Paroxetine in the treatment of premature ejaculation: a systematic review and meta-analysis. BMC Urol 2019; 19:2.doi: 10.1186/s12894-018-0431-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ozcan L, Polat EC, Otunctemur A, Ozbek E. Duloxetine, dual serotonin and norepinephrine reuptake inhibitor, versus paroxetine, selective serotonin reuptake inhibitor, in the treatment for premature ejaculation. Int Urol Nephrol 2015; 47:283–287. doi: 10.1007/s11255-014-0905-9. [DOI] [PubMed] [Google Scholar]
- 43.Athanasios Z, Polyanthi P, George K. The efficacy of duloxetine in the treatment of premature ejaculation. Int Urol Nephrol 2007; 39:115–118. doi: 10.1007/s11255-006-6659-2. [DOI] [PubMed] [Google Scholar]
- 44.Kim SW, Choi JB, Kim SJ, Kim KS, Kim CM, Lee DH, et al. Tolerability and adequate therapeutic dosage of oral clomipramine for the treatment of premature ejaculation: a randomized, double-blind, placebo-controlled, fixed-dose, parallel-grouped clinical study. Int J Impot Res 2018; 30:65–70. doi: 10.1038/s41443-017-0011-5. [DOI] [PubMed] [Google Scholar]
- 45.Waldinger MD, Zwinderman AH, Olivier B. On-demand treatment of premature ejaculation with clomipramine and paroxetine: a randomized, double-blind fixed-dose study with stopwatch assessment. Eur Urol 2004; 46:510–515. doi: 10.1016/j.eururo.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 46.Abdollahian E, Javanbakht A, Javidi K, Samari AA, Shakiba M, Sargolzaee MR. Study of the efficacy of fluoxetine and clomipramine in the treatment of premature ejaculation after opioid detoxification. Am J Addict 2006; 15:100–104. doi: 10.1080/10550490500419151. [DOI] [PubMed] [Google Scholar]
- 47.Kurkar A, Elderwy AA, Abulsorour S, Awad SM, Safwat AS, Altaher A. A randomized, double-blind, placebo-controlled, crossover trial of “on-demand” tramadol for treatment of premature ejaculation. Urol Ann 2015; 7:205–210. doi: 10.4103/0974-7796.150481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bar-Or D, Salottolo KM, Orlando A, Winkler JV. Group TOS. A randomized double-blind, placebo-controlled multicenter study to evaluate the efficacy and safety of two doses of the tramadol orally disintegrating tablet for the treatment of premature ejaculation within less than 2 minutes. Eur Urol 2012; 61:736–743. doi: 10.1016/j.eururo.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 49.Ozcan L, Polat EC, Onen E, Kocaaslan R, Otunctemur A, Cekmen M, et al. Effects of tadalafil 5 mg dosed once daily in men with premature ejaculation. Urol Int 2017; 98:210–214. doi: 10.1159/000445839. [DOI] [PubMed] [Google Scholar]
- 50.Moudi E, Kasaeeyan AA. Comparison between tadalafil plus paroxetine and paroxetine alone in the treatment of premature ejaculation. Nephrourol Mon 2016; 8:e32286.doi: 10.5812/numonthly.32286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bai Y, Pu C, Han P, Li J, Yuan H, Tang Y, et al. Selective serotonin reuptake inhibitors plus phosphodiesterase-5 inhibitors for premature ejaculation: a systematic review and meta-analysis. Urology 2015; 86:758–765. doi: 10.1016/j.urology.2015.06.045. [DOI] [PubMed] [Google Scholar]
- 52.Sato Y, Otani T, Amano T, Araki T, Kondou N, Matsukawa M, et al. Silodosin versus naftopidil in the treatment of premature ejaculation: a prospective multicenter trial. Int J Urol 2017; 24:626–631. doi: 10.1111/iju.13392. [DOI] [PubMed] [Google Scholar]
- 53.Bhat GS, Anuradha S. Effectiveness of ‘on demand’ silodosin in the treatment of premature ejaculation in patients dissatisfied with dapoxetine: a randomized control study. Cent European J Urol 2016; 69:280–284. doi: 10.5173/ceju.2016.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu G, Jiang HW, Fang J, Wen H, Gu B, Liu J, et al. An improved dosage regimen of sertraline hydrochloride in the treatment for premature ejaculation: an 8-week, single-blind, randomized controlled study followed by a 4-week, open-label extension study. J Clin Pharm Ther 2014; 39:84–90. doi: 10.1111/jcpt.12115. [DOI] [PubMed] [Google Scholar]
- 55.Dadfar MR, Baghinia MR. Salvage use of citalopram for treatment of fluoxetine-resistant premature ejaculation in recently married men: a prospective clinical trial. Urol J 2010; 7:40–44. doi: 10.1002/alr.21142. [PubMed] [Google Scholar]
- 56.Onutu AH. Duloxetine, an antidepressant with analgesic properties - a preliminary analysis. Rom J Anaesth Intensive Care 2015; 22:123–128. [PMC free article] [PubMed] [Google Scholar]
- 57.Papakostas GI. Antidepressants and their effect on cognition in major depressive disorder. J Clin Psychiatry 2015; 76:e1046.doi: 10.4088/JCP.13086tx5c. [DOI] [PubMed] [Google Scholar]
- 58.Martin C, Nolen H, Podolnick J, Wang R. Current and emerging therapies in premature ejaculation: where we are coming from, where we are going. Int J Urol 2017; 24:40–50. doi: 10.1111/iju.13202. [DOI] [PubMed] [Google Scholar]
- 59.Olivier JD, Esquivel Franco DC, Oosting R, Waldinger M, Sarnyai Z, Olivier B. Tramadol: effects on sexual behavior in male rats are mainly caused by its 5-HT reuptake blocking effects. Neuropharmacology 2017; 116:50–58. doi: 10.1016/j.neuropharm.2016.11.020. [DOI] [PubMed] [Google Scholar]
- 60.Abu El-Hamd M. Efficacy and safety of daily use of tadalafil in treatment of patients with premature ejaculation: a randomised placebo-controlled clinical trial. Andrologia 2018; 50:e13005.doi: 10.1111/and.13005. [DOI] [PubMed] [Google Scholar]
- 61.Karabakan M, Keskin E, Akdemir S, Bozkurt A. Effect of tadalafil 5 mg daily treatment on the ejaculatory times, lower urinary tract symptoms and erectile function in patients with erectile dysfunction. Int Braz J Urol 2017; 43:317–324. doi: 10.1590/S1677-5538.IBJU.2016.0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Men C, Yu L, Yuan H, Cui Y. Efficacy and safety of phosphodiesterase type 5 inhibitors on primary premature ejaculation in men receiving selective serotonin reuptake inhibitors therapy: a systematic review and meta-analysis. Andrologia 2016; 48:1066–1073. doi: 10.1111/and.12540. [DOI] [PubMed] [Google Scholar]
- 63.Martynst MJ, Cooper K, Ren S, Kaltenthaler E, Dickinson K, Cantrell A, et al. Phosphodiesterase-5 inhibitors for premature ejaculation: a systematic review and meta-analysis. Eur Urol Focus 2017; 3:119–129. doi: 10.1016/j.euf.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Buono R, Briganti A, Freschi M, Villa L, La CG, Moschini M, et al. Silodosin and tadalafil have synergistic inhibitory effects on nerve-mediated contractions of human and rat isolated prostates. Eur J Pharmacol 2014; 744:42–51. doi: 10.1016/j.ejphar.2014.09.030. [DOI] [PubMed] [Google Scholar]
- 65.Seki N, Takahashi R, Yamaguchi A, Ito K, Takayama K, Nanri K, et al. Non-inferiority of silodosin 4 mg once daily to twice daily for storage symptoms score evaluated by the International Prostate Symptom Score in Japanese patients with benign prostatic hyperplasia: a multicenter, randomized, parallel-group study. Int J Urol 2015; 22:311–316. doi: 10.1111/iju.12680. [DOI] [PubMed] [Google Scholar]
- 66.Takeshita H, Moriyama S, Arai Y, Washino S, Saito K, Chiba K, et al. Randomized crossover comparison of the short-term efficacy and safety of single half-dose silodosin and tamsulosin hydrochoride in men with lower urinary tract symptoms secondary to benign prostatic hyperplasia. Low Urin Tract Symptoms 2016; 8:38–43. doi: 10.1111/luts.12106. [DOI] [PubMed] [Google Scholar]
- 67.James MM, Cooper K, Ren K, Kaltenthaler E, Dickinson K, Cantrell A, et al. Topical anaesthetics for premature ejaculation: a systematic review and meta-analysis. Sex Health 2016; 13:114–123. doi: 10.1071/SH15042. [DOI] [PubMed] [Google Scholar]
- 68.Xia JD, Zhou LH, Han YF, Chen Y, Wang R, Dai YT. A reassessment of penile sensory pathways and effects of prilocaine-lidocaine cream in primary premature ejaculation. Int J Impot Res 2014; 26:186–190. doi: 10.1038/ijir.2014.5. [DOI] [PubMed] [Google Scholar]
- 69.Dinsmore WW, Wyllie MG. PSD502 improves ejaculatory latency, control and sexual satisfaction when applied topically 5 min before intercourse in men with premature ejaculation: results of a phase III, multicentre, double-blind, placebo-controlled study. BJU Int 2009; 103:940–949. doi: 10.1111/j.1464-410X.2009.08456.x. [DOI] [PubMed] [Google Scholar]
- 70.Carson C, Wyllie M. Improved ejaculatory latency, control and sexual satisfaction when PSD502 is applied topically in men with premature ejaculation: results of a phase III, double-blind, placebo-controlled study. J Sex Med 2010; 7:3179–3189. doi: 10.1111/j.1743-6109.2010.01913.x. [DOI] [PubMed] [Google Scholar]
- 71.Xin ZC, Choi YD, Seong DH, Choi HK. Sensory evoked potential and effect of SS-cream in premature ejaculation. Yonsei Med J 1995; 36:397–401. doi: 10.3349/ymj.1995.36.5.397. [DOI] [PubMed] [Google Scholar]
- 72.Mark KP, Kerner I. Event-level impact of promescent on quality of sexual experience in men with subjective premature ejaculation. Int J Impot Res 2016; 28:216–220. doi: 10.1038/ijir.2016.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sutton M, Hunn C, Summers-Colquitt RB, Chen SH, Kavoussi SK, Kavoussi KM, et al. Promescent has a cytotoxic impact on fresh human sperm in vitro. Urology 2018; 114:95–98. doi: 10.1016/j.urology.2017.12.027. [DOI] [PubMed] [Google Scholar]
- 74.Serefoglu EC, Hawley WR, Lasker GF, Grissom EM, Mandava SH, Sikka SC, et al. Effect of botulinum-A toxin injection into bulbospongiosus muscle on ejaculation latency in male rats. J Sex Med 2014; 11:1657–1663. doi: 10.1111/jsm.12553. [DOI] [PubMed] [Google Scholar]
- 75.Saadat SH, Ahmadi K, Panahi Y. The effect of on-demand caffeine consumption on treating patients with premature ejaculation: a double-blind randomized clinical trial. Curr Pharm Biotechnol 2015; 16:281–287. doi: 10.2174/1389201016666150118133045. [DOI] [PubMed] [Google Scholar]
- 76.Kang KK, Sung JH, Kim SH, Lee S. Effect of DA-8031, a novel oral compound for premature ejaculation, on male rat sexual behavior. Int J Urol 2014; 21:325–329. doi: 10.1111/iju.12256. [DOI] [PubMed] [Google Scholar]
- 77.Tuken M, Kiremit MC, Serefoglu EC. On-demand modafinil improves ejaculation time and patient reported outcomes in men with lifelong premature ejaculation. Urology 2016; 94:139–142. doi: 10.1016/j.urology.2016.04.036. [DOI] [PubMed] [Google Scholar]
- 78.Shi B, Li X, Chen J, Su B, Li X, Yang S, et al. Resiniferatoxin for treatment of lifelong premature ejaculation: a preliminary study. Int J Urol 2014; 21:923–926. doi: 10.1111/iju.12471. [DOI] [PubMed] [Google Scholar]
- 79.Giuliano F, Hellstrom WJG. The pharmacological treatment of premature ejaculation. BJU Int 2008; 102:668–675. doi: 10.1111/j.1464-410x.2008.07719.x. [DOI] [PubMed] [Google Scholar]
- 80.Koyuncu H, Serefoglu EC, Ozdemir AT, Hellstrom WJ. Deleterious effects of selective serotonin reuptake inhibitor treatment on semen parameters in patients with lifelong premature ejaculation. Int J Impot Res 2012; 24:171–173. doi: 10.1038/ijir.2012.12. [DOI] [PubMed] [Google Scholar]
- 81.Safarinejad MR. Sperm DNA damage and semen quality impairment after treatment with selective serotonin reuptake inhibitors detected using semen analysis and sperm chromatin structure assay. J Urol 2008; 180:2124–2128. doi: 10.1016/j.juro.2008.07.034. [DOI] [PubMed] [Google Scholar]
- 82.Serefoglu EC, Saitz TR. New insights on premature ejaculation: a review of definition, classification, prevalence and treatment. Asian J Androl 2012; 14:822–829. doi: 10.1038/aja.2012.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.David PJ, Snyder LL, Cherullo E, Passalacqua M, Pirasteh A, Corn D. Percutaneous CT-guided cryoablation of the dorsal penile nerve for treatment of symptomatic premature ejaculation. J Vasc Interv Radiol 2013; 24:214–219. doi: 10.1016/j.jvir.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 84.Shi WG, Wang XJ, Liang XQ, Liu ZQ, Huang MJ, Li SQ, et al. Selective resection of the branches of the two dorsal penile nerves for primary premature ejaculation. Natl J Androl 2008; 14:436–438. doi: 10.3969/j.issn.1009-3591.2008.05.012. [PubMed] [Google Scholar]
- 85.Kwak TI, Jin MH, Kim JJ, Moon DG. Long-term effects of glans penis augmentation using injectable hyaluronic acid gel for premature ejaculation. Int J Impot Res 2008; 20:425–428. doi: 10.1038/ijir.2008.26. [DOI] [PubMed] [Google Scholar]
- 86.Basal S, Goktas S, Ergin A, Yildirim I, Atim A, Tahmaz L, et al. A novel treatment modality in patients with premature ejaculation resistant to conventional methods: the neuromodulation of dorsal penile nerves by pulsed radiofrequency. J Androl 2010; 31:126–130. doi: 10.2164/jandrol.108.007344. [DOI] [PubMed] [Google Scholar]
- 87.Zhang HF, Zhang CY, Li XH, Fu ZZ, Chen ZY. Dorsal penile nerves and primary premature ejaculation. Chin Med J 2009; 122:3017–3019. doi: 10.3760/cma.j.issn.0366-6999.2009.24.020. [PubMed] [Google Scholar]
- 88.Zhou XJ, Zhang ZG, Hao L, Zhang WD, Dong BZ, Han CH. Elective microscopic resection of dorsal penile nerves for primary premature ejaculation: a clinical observation. Natl J Androl 2013; 19:1003–1006. [PubMed] [Google Scholar]
- 89.You HS. The partial neurectomy of the dorsal nerve of the penis for patient with premature ejaculation. Korean J Androl 2000; 18:143–148. [Google Scholar]
- 90.Liu Q, Li S, Zhang Y, Cheng Y, Fan J, Jiang L, et al. Anatomic basis and clinical effect of selective dorsal neurectomy for patients with lifelong premature ejaculation: a randomized controlled trial. J Sex Med 2019; 16:522–530. doi: 10.1016/j.jsxm.2019.01.319. [DOI] [PubMed] [Google Scholar]
- 91.Guo YL, Zhu JC, Jiang H, Zheng CH, Shang XJ, Gu XQ, et al. Guidelines for the Diagnosis and Treatment of Male Diseases in China and Expert Consensus. 2016; Beijing: People's Medical Publishing House, 1–22. [Google Scholar]
- 92.Zhang M, Zhang C, Jin B, Shang X, Bin B, Wang Y, et al. A practice guideline for PE of Chinese association of integrative medicine, branch of andrology (in Chinese). Natl J Androl 2018; 24:176–181. doi: 10.13263/j.cnki.nja.2018.02.016. [Google Scholar]
- 93.Glybochko PV, Aliaev IG, Amosov AV, Enikeev DV, Chinenov DV, Lumpov IS, et al. Cryoablation in urology. Urologiia 2014; 6:108–112. [PubMed] [Google Scholar]
- 94.Cazzato RL, Garnon J, Ramamurthy N, Koch G, Tsoumakidou G, Caudrelier J, et al. Percutaneous image-guided cryoablation: current applications and results in the oncologic field. Med Oncol 2016; 33:140.doi: 10.1007/s12032-016-0848-3. [DOI] [PubMed] [Google Scholar]
- 95.Slappendel R, Crul BJ, Braak GJ, Geurts JW, Booij LH, Voerman VF, et al. The efficacy of radiofrequency lesioning of the cervical spinal dorsal root ganglion in a double blinded randomized study: no difference between 40°C and 67°C treatments. Pain 1997; 73:159–163. doi: 10.1016/S0304-3959(97)00094-8. [DOI] [PubMed] [Google Scholar]
- 96.Littara A, Palmieri B, Rottigni V, Iannitti T. A clinical study to assess the effectiveness of a hyaluronic acid-based procedure for treatment of premature ejaculation. Int J Impot Res 2013; 25:117–120. doi: 10.1038/ijir.2013.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Abdallah H, Abdelnasser T, Hosny H, Selim O, Al-Ahwany A, Shamloul R. Treatment of premature ejaculation by glans penis augmentation using hyaluronic acid gel: a pilot study. Andrologia 2012; 44:650–653. doi: 10.1111/j.1439-0272.2011.01244.x. [DOI] [PubMed] [Google Scholar]
- 98.Du GM, Kwak TI, Kim JJ. Glans penis augmentation using hyaluronic acid gel as an injectable filler. World J Mens Health 2015; 33:50–61. doi: 10.5534/wjmh.2015.33.2.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Anaissie J, Yafi FA, Hellstrom WJ. Surgery is not indicated for the treatment of premature ejaculation. Transl Androl Urol 2016; 5:607–612. doi: 10.21037/tau.2016.03.10. [DOI] [PMC free article] [PubMed] [Google Scholar]


